Abstract
Glucocorticoids and other immunosuppressants still represent the cornerstone drugs for the management of SLE and lupus nephritis. The refined use of these drugs over the years has allowed us to obtain stable disease remission and improvement of long-term kidney and patient survival. Nevertheless, a prolonged use of immunosuppressive agents may be accompanied by severe and even life-threatening side effects. Theoretically, a transient or even definitive withdrawal of immunosuppression could be useful to prevent iatrogenic morbidities. For many years, however, the risk of SLE reactivation has held clinicians back from trying to interrupt therapy. In this review, we report the results of the attempts to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. The available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy. A slow and gradual reduction of treatment under medical surveillance is needed to prevent flares of activity. After therapy withdrawal, around one-quarter of patients may have kidney or systemic flares. However, most flares may respond to therapy if rapidly diagnosed. The other patients can enter stable remission for even 20 years or more. The use of antimalarials can help in maintaining the remission after the withdrawal of the immunosuppressive therapy. A repeated kidney biopsy could be of help in deciding to stop therapy, but given the few available data, it cannot be considered essential.
Keywords: lupus nephritis, systemic lupus erythematosus, immunosuppression, immunology and pathology
Introduction
The last European League Against Rheumatism-European Dialysis and Transplant Association-European Renal Association (EULAR-EDTA-ERA) guidelines recommended to initiate therapy with mycophenolate or low-dose intravenous cyclophosphamide, both combined with intravenous methylprednisolone pulses followed by low-dose oral prednisone. A combination of mycophenolate with a calcineurin inhibitor or high-dose cyclophosphamide is an alternative for patients with nephrotic-range proteinuria and adverse prognostic factors. Hydroxychloroquine is recommended with regular ophthalmologic monitoring for class 3 or 4 lupus nephritis. For subsequent treatment, mycophenolate or azathioprine is recommended with no or low-dose (<7.5-mg/d) prednisone. In nonresponding disease, switch of initial regimens or rituximab should be prescribed (1). Therefore, in comparison with a recent past, minimization or elimination of glucocorticoids, whenever possible, is recommended to prevent long-term complications of immunosuppressive therapy. However, a survey from 30 countries showed that clinicians are cautious in treatment reduction when patients have persistent serologic abnormalities of SLE and previous organ involvement (2). On the other hand, even low doses of prednisone between 6 and 12 mg/d cause a higher risk of organ damage (3), and the long-term use of antiproliferative drugs, such as mycophenolate and azathioprine, may expose to disquieting side effects such as opportunistic infections, bone marrow toxicity, and malignancy. As a matter of fact, despite a consistent improvement in prognosis with patient survival rates of 99% and 98% at 5 and at 10 years, respectively (4,5), class 3 or 4 lupus nephritis is associated with a six-fold higher mortality compared with the general population, and patients with lupus who develop kidney failure have a 26-fold excess in the risk of death (5). Thus, management of lupus nephritis remains unsatisfactory. In this narrative review, the results of immunosuppressive therapy interruption will be reviewed.
Results in Lupus Nephritis
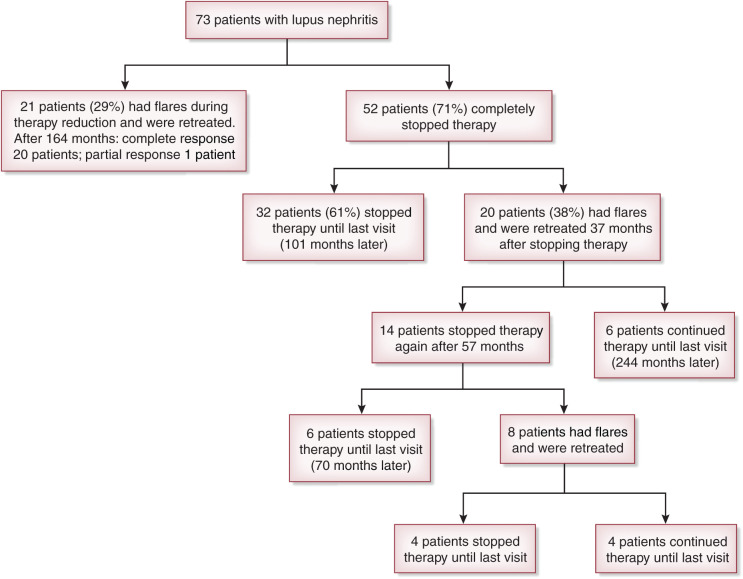
Until recently, discontinuation of immunosuppression in lupus nephritis was considered impractical for the fear that it could trigger flares and irreversible kidney damage (Table 1). This concern was confirmed by the rapid increase of disease activity and serum creatinine when treatment was abruptly discontinued (6). However, a few years later, other reports showed that withdrawal of immunosuppressive therapy was successful if drugs were discontinued with a slow and progressive tapering under strict medical surveillance (7–9). The largest trial of immunosuppressive therapy interruption in lupus nephritis was reported by Moroni et al. (10), who gradually withdrew treatment in 73 of 161 patients (45%). Before reduction of immunosuppressive drugs, all of the patients were in stable clinical remission, defined by normal serum creatinine, proteinuria <0.5 g/24 h, inactive urine sediment, and no clinical signs of extrarenal activity of SLE for at least 12 months. Twenty-one participants (11%) developed flares during therapy reduction. Treatment reinforcement obtained remission in 20 of 21 patients. The last patient was lost to follow-up at achievement of partial remission. In the remaining 52 participants (26%), immunosuppressive therapy was completely withdrawn. During the subsequent follow-up, 32 patients did not resume immunosuppressive therapy; the other 20 patients had at least one flare, in a median of 37 months after withdrawing therapy, and had to be retreated. After a median follow-up of 24 years, ten of these 20 patients were without therapy (0.8 flares per year) at the last observation, four patients (two without flares and two with flares) died, and two patients who had flares doubled serum creatinine (2.5 and 3 mg/dl, respectively) (Figure 1). Compared with patients with new flares, those who never developed flares received significantly longer treatment (98.1 versus 31.0 months; P=0.01), had longer remission before immunosuppression withdrawal (52.8 versus 12.0 months; P≤0.001), and continued hydroxychloroquine after stopping immunosuppressive therapy (52% versus 10%; P=0.004). Reduced C3 and C4 complement fractions and high antidouble-stranded DNA antibody titers at the time of withdrawal of therapy did not predict the development of flares. According to a recent study (11), changes in serologic activity are not necessarily associated with the development of new flares. Nevertheless, if after cessation of therapy, patients develop a rapid decrease in complement fractions or higher levels of antidouble-stranded DNA antibodies, strict monitoring is recommended.
Table 1.
Rate of recurrence of SLE flare after withdrawal of therapy in patients with SLE and in patients with lupus nephritis
| Authors | Type of Study | No. of Patients with SLE (% of Patients with Lupus Nephritis) | Criteria for Withdraw Therapy | Drug Withdrawn for Study | No. (%) of Patients Who Stopped the Study Drug | No. (%) of Patients Taking No Other Immunosuppressant after Stopping the Study Druga | Follow-Up after Stop Therapy, mo/yr | No. of Patients (%) Who Had Flares | Flare Rate, no./yr |
|---|---|---|---|---|---|---|---|---|---|
| Lupus nephritis | |||||||||
| Barber et al. (9) 2006 | R | 16 (100) | ≥3 years therapy + complete remission | Steroids + IS | 16 (100) | 16 (100) | 120 | 0 | 0 |
| Moroni et al. (10) 2013 | R | 198 (100) | Clinical remission ≥12 months | Steroids + IS | 73 (36.9) attempted, 52 (26.3) succeeded | 52 (100) | 288 | 20 (38.5) | 0.83 |
| Galbraith et al. (15) 2014 | Pilot | 15 (100) | Class 3 or 4 lupus nephritis | Steroids | 7 (46.7) | NA | 12 | 1 (14) without steroid | 1 |
| Zen et al. (11) 2021 | R | 238 (100) | Stable remission | IS | 83 (34.8) | NA | 116.5 | 19 (22.8) | 1.96 |
| SLE | |||||||||
| Mathian et al. (16) 2020 | RCT | 124 (37.9) | Clinical remission ≥12 months | Steroids | 63 (50.8) | 47 (75) | 12 | 17 (27) | 17 |
| Goswami et al. (13) 2019 | R | 148 (52.7) | Remission = SLEDAI-2K=0 | Steroids | 148 (100) | 64 (43.2) | 17.9 | 31 (20.9) | 20.8 |
| Tani et al. (12) 2019 | R | 148 (53.3) | Complete or clinical remission | Steroids | 91 (61.5) attempted, 77 (52) succeeded | 41 (53) | 48 | 18 (23.3) | 4.5 |
| Zen et al. (14) 2017 | P | 319 (53) | 105 patients clinical remission; 34 patient poor adherence | IS | 139 (43.5) | 63 (45.3) | 91 | 26 (24.8) versus 23 (67.6) | 3.4 |
| Total | 1206 (69.6) | 585 (48.5) | 284 (56.6) | 705.4/58.8 | 155 (26.5) | 2.77b |
R, retrospective; IS, nonsteroidal immunosuppressant agent; NA, not available; RCT, randomized controlled trial; SLEDAI-2K, SLE disease activity index; P, prospective.
Except antimalarial immunosuppressors.
The rate flare per year reduced to 1.36 if 23 patients from the study by Zen et al. (14) are included. Those patients voluntarily stopped therapy for poor adherence to prescriptions.
Figure 1.
An attempt to interrupt immunosuppressive therapy completely was made in 73 of 161 patients (45%) with lupus nephritis. The participants (class 3, 4, or 5 at kidney biopsy) had to be in stable and complete kidney remission. Immunosuppressive drugs were slowly tapered off, and patients were followed for a mean period of 286 (183–312) months.
These data showed that a gradual discontinuation of therapy is feasible in patients who had received therapy for at least 5 years and are in complete clinical and kidney remission for at least 3 years.
Results in Systemic Lupus Erythematosus
Recently, attempts at discontinuing immunosuppressive therapy were made in patients with SLE with or without kidney involvement (Table 1). In a retrospective study, withdrawal of glucocorticoids was made in 91 of 310 patients with SLE (29%). About 50% of patients did not receive immunosuppressive drugs other than glucocorticoids. Seventy-seven patients (25%) could successfully stop glucocorticoids and were followed for 6 years. Eighteen flares (23%) occurred after a median of 1 year in comparison with flares in 70% of 219 patients who continued glucocorticoids. In patients who stopped glucocorticoids, 13 flares were mild and four were kidney flares. Lower-activity SLE activity index scores (median of two [zero to two] versus two [one to four]) and longer time from the last flare (6.0 versus 0.93 years; P<0.001) were associated with lower likelihood of flare after glucocorticoid withdrawal (12). In another study, steroid therapy was gradually tapered off in 148 patients with SLE who were previously in remission; of these participants, 78 had lupus nephritis. Patients received glucocorticoids for a median of 5 years before discontinuation. No other immunosuppressive agent was given in 43% of them. During a median duration of follow-up of 17.9 months, flares developed in 31 patients (21%). Most flares (93%) occurred within the first year in patients who received glucocorticoids ≤8 years versus only 7% flares in patients treated with steroids for >8 years (P=0.009). In multivariable Cox analysis, duration of disease (hazard ratio [HR], 0.89; 95% confidence interval [95% CI], 0.84 to 0.94; P<0.001), duration of glucocorticoids before withdrawal (4.7 versus 6.8 years; HR, 1.00; 95% CI, 1.00 to 1.00; P<0.001), and a second immunosuppressive drug (HR, 1.89; 95% CI, 1.25 to 2.87; P=0.003) were significantly associated with flare-free survival (13). A further attempt was done in 139 (44%) of 319 eligible patients with SLE; 53% had kidney involvement and 45% were not receiving glucocorticoids. Reasons for discontinuation were poor adherence/intolerance in 34 and remission in 105 patients. Remission was defined by SLE activity index equal to zero, stable immunosuppression, prednisone <5 mg/d, and antimalarial therapy. The mean follow-up after drug withdrawal was 91 months. SLE flares occurred in 25% of patients in remission versus 68% of those with poor adherence/intolerance after median follow-up of 57 and 8 months, respectively. These differences were significant. Negative antidouble-stranded DNA antibodies and normal complement fractions did not exert an additional protective effect over clinical remission on flare. Therapy with antimalarials and the duration of remission before discontinuation were independent protective factors against flare. The authors concluded that SLE flares are not uncommon after immunosuppressive therapy discontinuation, even in patients on remission. However, antimalarial therapy and durable remission significantly reduced the risk of flare (14). A small pilot trial included 15 patients in whom prednisone was tapered down to ≤20 mg/d. Participants had class 3 or 4 (plus or minus membranous) lupus nephritis and achieved at least a partial response by cyclophosphamide or mycophenolate. After 3 weeks, prednisone was decreased to 5–7.5 mg/d in eight patients, and that dose was maintained. Prednisone was completely withdrawn in the 17th week in seven patients who were followed for a median of 12 months. Relapse occurred in one patient of the withdrawal group (14%) and in 50% of the low-dose prednisone group. However, the small number of participants and the short duration of follow-up cannot allow us to draw firm conclusions (15).
For a randomized controlled trial, 124 patients with inactive disease during the year before the study who were receiving prednisone 5 mg/d at randomization were admitted to the study. Of them, 61 were randomized to the maintenance treatment, and 63 were randomized to the rapid steroid withdrawal. During a follow-up of 12 months, only four patients (7%) in the maintenance group experienced a flare versus 17 (27%) in the withdrawal group, the difference being significant (risk ratio [RR], 0.2; 95% CI, 0.1 to 0.7; P=0.003). Maintenance of 5 mg prednisone was significantly superior with respect to time to first flare (HR, 0.2; 95% CI, 0.1 to 0.6; P=0.002), occurrence of mild/moderate flares (RR, 0.2; 95% CI, 0.1 to 0.8; P=0.01), and occurrence of moderate/severe flares (RR, 0.1; 95% CI, 0.1 to 0.9; P=0.01). Adverse events were similar in the two groups. The authors concluded that maintenance of long-term low-dose prednisone in patients with SLE and inactive disease prevents relapse (16). Unfortunately, only 21 patients in the maintenance group and 26 in the withdrawal had lupus nephritis. The critical points of the study are the short duration of remission before the interruption of immunosuppressive therapy, the abrupt withdrawal of prednisone, and the lack of placebo.
Repeated Kidney Biopsy
A randomized controlled trial assessed the role of repeated biopsy in predicting flares after immunosuppressive therapy interruption (17) (Table 2). Eligible patients had to be in clinical remission for at least 12 months and had to have received at least 36 months of immunosuppression. Clinical remission was defined by proteinuria ≤500 mg/d, normal kidney function, and inactive urinary sediment. Of 44 enrolled patients, 36 received a second biopsy. Therapy was tapered off over 6 months, and patients were followed for 24 months after repeated biopsy. Nephritic flares occurred in 11 (30%) patients, and all but one showed residual histologic activity on the second biopsy. Duration of SLE and activity index at the second biopsy were independent predictors of flare. The authors concluded that patients in both clinical and histologic remissions may be candidates for therapy withdrawal (17). Despite these impressive results, some issues remain. The above reported studies in lupus nephritis and in SLE suggest that remission longer than 12 months before therapy withdrawal is necessary to reduce flares’ occurrence. However, it is possible or even likely that a longer period of therapy is needed to heal histologic lesions. In a study, complete histologic remission (defined as activity index =0) was documented in 93% of 29 patients with lupus nephritis who received a repeated biopsy >48 months after sustained complete remission (18). Altogether, the small numbers of patients enrolled in these studies do not provide a definitive answer about the need of repeated kidney biopsy. Thus, kidney biopsy may give important pieces of information but is not indispensable to decide whether treatment may be stopped in lupus nephritis.
Table 2.
Repeated kidney biopsy as a predictor of kidney flares and of safety withdrawal of therapy
| Authors | No. (%) of Patients Recruited/ Completed Study | Months of Therapy/ Remission before Repeated Kidney Biopsy | No. (%) of Patients with an Activity Index >0 at Repeated Kidney Biopsy | No. (%) of Patients Who Stopped Therapy | Months after Stopping Therapy | No. (%) of Patients Who Developed Kidney Flares | No. (%) of Flares in Patients with Activity Index =0 | Flare Rate: no./yr | Predictors of Flares |
|---|---|---|---|---|---|---|---|---|---|
| De Rosa et al. (17) 2018 | 44/36 | 36/≥12 | 16 (44.4)a | 36 (100) | 24 | 11 (30.5) | 1 (9%) | 5.5 | AI>2 at R.B., duration of SLE |
| Malvar et al. (19) 2019 | 76/55 | 42/≥12 | 20 (26.6) | 55 (72.4), all patients with AI=0 | 50b | 7 (12.7) | 7 (100) | 1.55 | None |
| Parodis et al. (21) 2020 | 42/42 | 24/NA | 10 (23.8) | 0 | 107.7 | 11 (26.2) | NA | 1.23 | AI and high proteinuria at R.B. |
AI, activity index; R.B., repeated kidney biopsy; NA, not available.
AI was one or two in nine of 16 patients and three or five in seven patients.
Mycophenolate was withdrawn in 6 months; glucocorticoids were continued beyond year 2 of treatment only if needed for extrarenal SLE activity.
Malvar et al. (19) moved one step further. They discontinued treatment only in the case of a normal histology at kidney biopsies performed during therapy. Biopsy was performed in 75 of 220 patients (35%) with class 3 or 4 lupus nephritis who had received immunosuppressive therapy for at least 42 months and had been in remission for at least 12 months. Treatment was withdrawn only if biopsy showed an activity index of zero. Nephritic flares developed within 50 months from the last biopsy in seven of 75 patients (9%) who stopped therapy due to absent histologic activity. This flare rate of 1.5/yr was significantly lower than reported flare rates. No patient developed kidney failure. Four patients developed CKD. The authors concluded that repeated biopsy is safe and may improve the flare rate compared with conventional clinical management (19). However, therapy interruption on the basis of an activity index of zero does not eliminate the likelihood of exacerbation. Patients with activity index greater than or equal to one, severe chronic damage at basal biopsy, rapid progression of kidney disease, and/or poor response to initial therapy were excluded from the study. This negative selection could have influenced the low kidney progression and relapse rate (20). In another study of 42 patients with class 3 or 4 lupus nephritis who received a per-protocol repeat biopsy 24 months after basal biopsy, 11 (26%) relapsed after a median time of 17.9 months from repeated biopsy despite the continuation of therapy. Activity index greater than two and urinary protein-creatinine ratio >1 g/g at repeated biopsy were independent predictors of subsequent kidney relapses (21). The ongoing Per-protocol Repeat Kidney Biopsy in Incident Cases of Lupus Nephritis (REBIOLUP), a prospective multicenter trial aimed to investigate how the prognostic information obtained from control biopsy should assist clinical decisions, may better define the time and the role of repeated biopsy in lupus nephritis (22).
In summary, complete discontinuation of immunosuppressive therapy, including mycophenolate monotherapy, if the patient has been free of relapses for 4 or more years and is in a complete remission is feasible in a selected group of patients. However, it may be burdened by flares of lupus activity in one-third to one-quarter of patients (Figure 2). Flares occur particularly in the period of drug tapering and in the first years after therapy withdrawal. Thus, regular monitoring is mandatory. The prompt reintroduction of glucocorticoids and other immunosuppressants can induce the remission of the kidney and systemic manifestations. The good news is that the three-quarters of patients who do not develop flares enter stable and long-lasting remission with significant improvement in iatrogenic morbidity and quality of life. Before considering immunosuppression withdrawal, some points should be considered. (1) The longer the duration of immunosuppressive therapy is before discontinuation, the better the results are. Hence, it is better to wait for at least 5–6 years before stopping therapy. (2) The ideal candidate for treatment discontinuation should be in complete remission. Persistent clinical signs of lupus activity may be associated with kidney or extrarenal flares after therapy discontinuation. Repeated kidney biopsy may be useful in excluding persistent histologic activity, but it is not mandatory (Table 3). (3) Drugs should be tapered off very gradually under strict surveillance. During an attempt to discontinue treatment, the most delicate phase is represented by treatment reduction (23). (4) Hydroxychloroquine may help to prevent extrarenal flares. (5) In case of kidney flares, a rapid reinstitution of treatment is needed to recover remission and prevent kidney function deterioration. If these cautions are respected, patients with lupus nephritis may discontinue glucocorticoids and other immunosuppressive drugs.
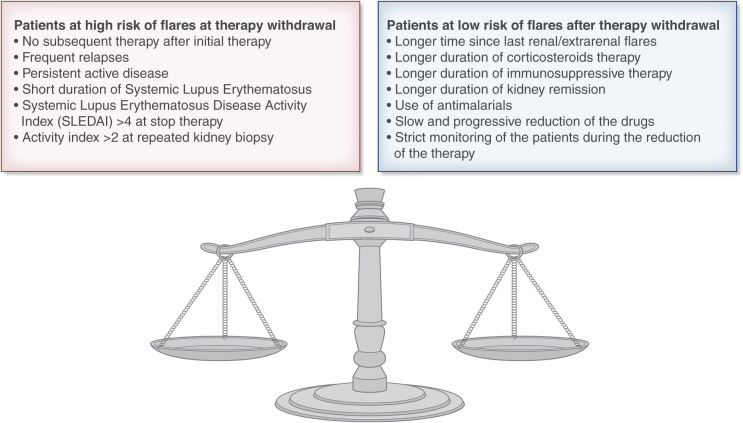
Figure 2.
The characteristics of patients who are at high risk and at low risk for stopping immunosuppression.
Table 3.
Predictors of flares after withdrawal of immunosuppressive therapy
| Authors | Clinical Predictors | Therapy Predictors | Histologic Predictors |
|---|---|---|---|
| De Rosa et al. (17) 2018 | Short duration of SLE at repeated kidney biopsy | Activity index >2 at repeated kidney biopsy | |
| Goswami et al. (13) 2019 | Short duration of SLE at withdrawal of therapy (<8 years) | Short duration of steroid before stop (4.7 versus 6.8 yr),a no treatment with IS; only for kidney flares: lower initial dose of steroids, presence of AIHA | |
| Tani et al. (12) 2019 | SLEDAI>4 at stop therapy; short time from last flare (0.93 versus 6 yr) | ||
| Zen et al. (14) 2017 | Short duration of remission at stop therapy | No use of antimalarial therapy | |
| Moroni et al. (10) 2013 | Short duration of remission at stop therapy (1 versus 4.4 yr) | Short duration of therapy before stop (2.6 versus 8.2 yr); no use of antimalarial therapy | |
| Zen et al. (11) 2021 | Younger age at discontinuation of therapy; remission <3 yr at discontinuation of therapy | No use of antimalarials after discontinuation of therapy |
IS, immunosuppressor; AIHA, autoimmune hemolytic anemia; SLEDAI, SLE disease activity index.
The probability of not having a flare is 3.2 times lower for each additional year of steroids administered after remission activity index.
Benefits of Immune Suppression Withdrawal
Although anti-inflammatory and immunosuppressive drugs may control the disease activity, they can cause side effects and lead to severe comorbidity that can strongly influence life expectancy and quality of life. Usually, inflammatory manifestations and infections are prevalent in the early years after diagnosis of SLE. In the long term, the activity of kidney disease tends to be quenched, but the risks of cardiovascular disease, chronic organ damage according to the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (24), malignancy, and thrombotic events increase.
In SLE, atherosclerosis occurs early in the disease course and progressively aggravates over the time (Figure 3). The atherosclerotic risk cannot be fully accounted for by the higher prevalence of traditional risk factors, but nontraditional risk factors, including immune dysregulation and metabolic disturbances, can also play an important role (25–27). The chronic use of glucocorticoids may aggravate the effects of those risk factors (28,29). Possible mechanisms include hypertension, diabetes mellitus, dyslipidemia, and imbalances in thrombosis and fibrinolysis (30). In SLE, a higher risk of cardiovascular disease is associated with glucocorticoids even at low doses (<5 mg/d prednisone). This risk is higher if high doses and prolonged courses are used (31). On the other hand, in a long-term study, patients with lupus nephritis who were able to interrupt glucocorticoids and any immunosuppressive therapy had significantly less frequent hypertension (33% versus 67%; P<0.001) and fewer cardiovascular events (12% versus 28%; P=0.04) in comparison with patients who never stopped therapy (10). An important contribution to cardiovascular disease may be given by the prolonged use of calcineurin inhibitors, which may cause hypertension, dyslipidemia, and diabetes (32).
Figure 3.
The risks of continued immunosuppression versus the risks of stopping therapy.
Organ damage is an important issue in patients with SLE. The kidney is a frequent target, especially in patients with lupus nephritis. However, many other organs and systems may be involved, including eyes, central and peripheral nervous systems, lungs, the gastrointestinal system, the musculoskeletal system, and skin. Large analyses of patients with SLE showed that the damage depends on different variables, but hypertension and glucocorticoids are among the strongest predictors of an accrued organ damage (33–36). Patients who interrupted steroid treatment had significantly lower risk of chronic kidney insufficiency (4% versus 28%) and no case of kidney failure in comparison with 13% in patients who never stopped therapy. Flares and chronic organ damage were extremely rare in patients who stopped therapy (8). Moreover, the withdrawal of glucocorticoids reduces the risk of osteopenia and fracture, which are 2.16 times higher in patients who received a dosage of prednisone >7.5 mg/d (37).
In SLE, there is higher risk of hematologic malignancies and other nonhormone-sensitive cancers (38–41). Reduced immunosurveillance may explain the higher risk of malignancy, but high doses or long-term use of cyclophosphamide may exert a direct oncogenic effect, leading to higher rates of bladder cancer, leukemia, lymphoma, and skin malignancies (42–44). Azathioprine is considered as an oncogenic drug as it is incorporated into the DNA of the genome (45). A higher risk of cancer for azathioprine was found in patients with inflammatory bowel disease (46,47). The US Food and Drug Administration warned against the higher risk of lymphoma and skin malignancy. Instead, mycophenolate mofetil is considered an immunosuppressant with antitumor effects by the US Food and Drug Administration. Calcineurin inhibitors can lead to a higher risk of cancer, which is dose and time dependent. This effect is mainly related to their interference with the immune surveillance. Tacrolimus appears to have fewer oncogenic effects than cyclosporin, but it may be associated with a higher risk of tumors when used at high doses (48,49). Few data are available about the use of voclosporin in lupus nephritis (50). However, there is a stable pharmacodynamic profile and a fast elimination of metabolites, resulting in low exposure. Whether this fast elimination will result in a reduced oncogenicity is still unknown.
Regarding severe acute respiratory syndrome coronavirus 2 infections, patients not taking immunosuppressive drugs have a similar risk of symptomatic infection as the general population (51). On the other hand, the antibody response to the mRNA severe acute respiratory syndrome coronavirus 2 vaccine of kidney transplant recipients was reduced by immunosuppressive therapy (52).
A prominent role in the late complications of SLE is represented by thrombotic events (53). Persistent hypertension, smoking, nephrotic syndrome, and use of glucocorticoids and/or calcineurin inhibitors together with advanced age and the presence of antiphospholipid antibodies can lead to a higher risk of thrombotic events in the long term (54–56). One can speculate that the elimination of glucocorticoids and calcineurin inhibitors may prevent thrombotic events in patients with SLE, but no randomized trials are available to demonstrate this hypothesis.
In conclusion, most of the serious complications of lupus nephritis originate in the early phases when the disease is particularly active and immunosuppressive therapy is aggressive. However, the persistent use of immunosuppressive drugs, even at low doses, may perpetuate and worsen the deleterious effects of these complications and can be responsible for frailty and the poor quality of life of patients (57,58). In the long term, patients with lupus nephritis usually show a low disease activity. This may facilitate a gradual withdrawal of immunosuppressive therapy in selected patients, which can prevent the development of life-threatening and invalidating iatrogenic complications.
Disclosures
G. Moroni reports consultancy agreements with Glaxo. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
Present address: Dr. Gabriella Moroni, Department of Biomedical Sciences, Humanitas University, Milan, Italy, and IRCCS Humanitas Research Hospital, Milan, Italy.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT: 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 79: 713–723, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Ngamjanyaporn P, McCarthy EM, Sergeant JC, Reynolds J, Skeoch S, Parker B, Bruce IN: Clinicians approaches to management of background treatment in patients with SLE in clinical remission: Results of an international observational survey. Lupus Sci Med 4: e000173, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thamer M, Hernán MA, Zhang Y, Cotter D, Petri M: Prednisone, lupus activity, and permanent organ damage. J Rheumatol 36: 560–564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C: The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant 22: 2531–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Yap DYH, Tang CSO, Ma MKM, Lam MF, Chan TM: Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 27: 3248–3254, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Aptekar RG, Decker JL, Steinberg AD: Exacerbation of SLE nephritis after cyclophosphamide withdrawal. N Engl J Med 286: 1159–1160, 1972 [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli C, Moroni G, Banfi G: Discontinuation of therapy in diffuse proliferative lupus nephritis. Am J Med 85: 275, 1988 [PubMed] [Google Scholar]
- 8.Moroni G, Gallelli B, Quaglini S, Banfi G, Rivolta E, Messa P, Ponticelli C: Withdrawal of therapy in patients with proliferative lupus nephritis: Long-term follow-up. Nephrol Dial Transplant 21: 1541–1548, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Barber CE, Geldenhuys L, Hanly JG: Sustained remission of lupus nephritis. Lupus 15: 94–101, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Moroni G, Longhi S, Giglio E, Messa P, Ponticelli C: What happens after complete withdrawal of therapy in patients with lupus nephritis. Clin Exp Rheumatol 31[Suppl 78]: S75–S81, 2013 [PubMed] [Google Scholar]
- 11.Zen M, Fuzzi E, Loredo Martinez M, Depascale R, Fredi M, Gatto M, Larosa M, Saccon F, Iaccarino L, Doria A: Immunosuppressive therapy withdrawal after remission achievement in patients with lupus nephritis [published online ahead of print April 28, 2021]. Rheumatology (Oxford) 10.1093/rheumatology/keab373 [DOI] [PubMed] [Google Scholar]
- 12.Tani C, Elefante E, Signorini V, Zucchi D, Lorenzoni V, Carli L, Stagnaro C, Ferro F, Mosca M: Glucocorticoid withdrawal in systemic lupus erythematosus: Are remission and low disease activity reliable starting points for stopping treatment? A real-life experience. RMD Open 5: e000916, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami RP, Sit H, Ghosh P, Sircar G, Ghosh A: Steroid-free remission in lupus: Myth or reality; An observational study from a tertiary referral centre. Clin Rheumatol 38: 1089–1097, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Zen M, Saccon F, Gatto M, Montesso G, Larosa M, Benvenuti F, Iaccarino L, Doria A: Prevalence and predictors of flare after immunosuppressant discontinuation in patients with systemic lupus erythematosus in remission. Rheumatology (Oxford) 59: 1591–1598, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Galbraith L, Manns B, Hemmelgarn B, Walsh M: The Steroids In the Maintenance of remission of Proliferative Lupus nephritis (SIMPL) pilot trial. Can J Kidney Health Dis 1: 30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathian A, Pha M, Haroche J, Cohen-Aubart F, Hié M, Pineton de Chambrun M, Boutin THD, Miyara M, Gorochov G, Yssel H, Cherin P, Devilliers H, Amoura Z: Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: A randomised clinical trial. Ann Rheum Dis 79: 339–346, 2020 [DOI] [PubMed] [Google Scholar]
- 17.De Rosa M, Azzato F, Toblli JE, De Rosa G, Fuentes F, Nagaraja HN, Nash R, Rovin BH: A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int 94: 788–794, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Das U, Patel R, Guditi S, Tadure G: Correlation between the clinical remission and histological remission in repeat biopsy findings of quiescent proliferative lupus nephritis. Lupus 30: 876–883, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Malvar A, Alberton V, Lococo B, Ferrari M, Delgado P, Nagaraja HN, Rovin BH: Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int 97: 156–162, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Jayne D, Bajema IM: “In my beginning is my end”: Usefulness of repeat kidney biopsies in lupus nephritis. Kidney Int 97: 27–29, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Parodis I, Adamichou C, Aydin S, Gomez A, Demoulin N, Weinmann-Menke J, Houssiau FA, Tamirou F: Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology (Oxford) 59: 3424–3434, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Parodis I, Tamirou F, Houssiau FA: Prediction of prognosis and renal outcome in lupus nephritis. Lupus Sci Med 7: e000389, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grootscholten C, Berden JH: Discontinuation of immunosuppression in proliferative lupus nephritis: Is it possible? Nephrol Dial Transplant 21: 1465–1469, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, Hanly JG, Isenberg DA, Kalunian K, Nived O, Petri M, Sanchez-Guerrero J, Snaith M, Sturfelt G: The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 40: 809–813, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Bruce IN, Urowitz MB, Gladman DD, Ibañez D, Steiner G: Risk factors for coronary heart disease in women with systemic lupus erythematosus: The Toronto Risk Factor Study. Arthritis Rheum 48: 3159–3167, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Giannelou M, Mavragani CP: Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J Autoimmun 82: 1–12, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Kaplan MJ: Cardiovascular disease in systemic lupus erythematosus: An update. Curr Opin Rheumatol 30: 441–448, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Kostopoulou M, Nikolopoulos D, Parodis I, Bertsias G: Cardiovascular disease in systemic lupus erythematosus: Recent data on epidemiology, risk factors and prevention. Curr Vasc Pharmacol 18: 549–565, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Karp I, Abrahamowicz M, Fortin PR, Pilote L, Neville C, Pineau CA, Esdaile JM: Recent corticosteroid use and recent disease activity: Independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum 59: 169–175, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Sholter DE, Armstrong PW: Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol 16: 505–511, 2000 [PubMed] [Google Scholar]
- 31.Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J: Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: A population-based cohort study. PLoS Med 17: e1003432, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponticelli C, Glassock RJ: Prevention of complications from use of conventional immunosuppressants: A critical review. J Nephrol 32: 851–870, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Mok CC, Ho LY, Cheung MY, Yu KL, To CH: Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: A 2-year prospective study. Scand J Rheumatol 38: 121–127, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Prasad R, Ibañez D, Gladman D, Urowitz M: Anti-dsDNA and anti-Sm antibodies do not predict damage in systemic lupus erythematosus. Lupus 15: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Petri M, Purvey S, Fang H, Magder LS: Predictors of organ damage in systemic lupus erythematosus: The Hopkins Lupus Cohort. Arthritis Rheum 64: 4021–4028, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, Gladman DD, Bae SC, Sanchez-Guerrero J, Romero-Diaz J, Gordon C, Wallace DJ, Clarke AE, Bernatsky S, Ginzler EM, Isenberg DA, Rahman A, Merrill JT, Alarcón GS, Fessler BJ, Fortin PR, Petri M, Steinsson K, Dooley MA, Khamashta MA, Ramsey-Goldman R, Zoma AA, Sturfelt GK, Nived O, Aranow C, Mackay M, Ramos-Casals M, van Vollenhoven RF, Kalunian KC, Ruiz-Irastorza G, Lim S, Kamen DL, Peschken CA, Inanc M, Urowitz MB: Factors associated with damage accrual in patients with systemic lupus erythematosus: Results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 74: 1706–1713, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, Petri M: Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-The Hopkins Lupus Cohort. Lupus Sci Med 2: e000066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y: The risks of cancer development in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Arthritis Res Ther 20: 270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernatsky S, Kale M, Ramsey-Goldman R, Gordon C, Clarke AE: Systemic lupus and malignancies. Curr Opin Rheumatol 24: 177–181, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Ladouceur A, Tessier-Cloutier B, Clarke AE, Ramsey-Goldman R, Gordon C, Hansen JE, Bernatsky S: Cancer and systemic lupus erythematosus. Rheum Dis Clin North Am 46: 533–550, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Chan K, Clarke AE, Ramsey-Goldman R, Foulkes W, Tessier Cloutier B, Urowitz MB, Gladman D, Nived O, Romero-Diaz J, Petri M, Ginzler E, Fortin PR, Bae SC, Wallace DJ, Yelin EH, Bernatsky S: Breast cancer in systemic lupus erythematosus (SLE): Receptor status and treatment. Lupus 27: 120–123, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N, Baslund B: Malignancies in Wegener’s granulomatosis: Incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 35: 100–105, 2008 [PubMed] [Google Scholar]
- 43.Shang W, Ning Y, Xu X, Li M, Guo S, Han M, Zeng R, Ge S, Xu G: Incidence of cancer in ANCA-associated vasculitis: A meta-analysis of observational studies. PLoS One 10: e0126016, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponticelli C, Escoli R, Moroni G: Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev 17: 1022–1027, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Penn I: The price of immunotherapy. Curr Probl Surg 18: 681–751, 1981 [DOI] [PubMed] [Google Scholar]
- 46.Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A: Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol 177: 1296–1305, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Zheng KYC, Guo CG, Wong IOL, Chen L, Chung HY, Cheung KS, Leung WK: Risk of malignancies in patients with inflammatory bowel disease who used thiopurines as compared with other indications: A territory-wide study. Therap Adv Gastroenterol 13: 1756284820967275, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carenco C, Assenat E, Faure S, Duny Y, Danan G, Bismuth M, Herrero A, Jung B, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP: Tacrolimus and the risk of solid cancers after liver transplant: A dose effect relationship. Am J Transplant 15: 678–686, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Lichtenberg S, Rahamimov R, Green H, Fox BD, Mor E, Gafter U, Chagnac A, Rozen-Zvi B: The incidence of post-transplant cancer among kidney transplant recipients is associated with the level of tacrolimus exposure during the first year after transplantation. Eur J Clin Pharmacol 73: 819–826, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, Kaplan J, Lisk L, Navarra S, Parikh SV, Randhawa S, Solomons N, Huizinga RB: Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397: 2070–2080, 2021 [DOI] [PubMed] [Google Scholar]
- 51.Saadoun D, Vieira M, Vautier M, Baraliakos X, Andreica I, da Silva JAP, Sousa M, Luis M, Khmelinskii N, Gracía JMA, Castrejon I, Gonzalez JCN, Scirè CA, Silvagni E, Bortoluzzi A, Penn H, Hamdulay S, Machado PM, Fautrel B, Cacoub P, Resche-Rigon M, Gossec L: SARS-CoV-2 outbreak in immune-mediated inflammatory diseases: The Euro-COVIMID multicentre cross-sectional study. Lancet Rheumatol 3: e481–e488, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, Katchman E, Halperin T, Turner D, Goykhman Y, Shibolet O, Levy S, Houri I, Baruch R, Katchman H: Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus [published online ahead of print April 18, 2021]. Am J Transplant 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO, Chwalinska-Sadowska H, de Ramón E, Fernández-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR; European Working Party on Systemic Lupus Erythematosus : Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82: 299–308, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Calvo-Alén J, Toloza SMA, Fernández M, Bastian HM, Fessler BJ, Roseman JM, McGwin G Jr., Vilá LM, Reveille JD, Alarcón GS; LUMINA Study Group : Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXV. Smoking, older age, disease activity, lupus anticoagulant, and glucocorticoid dose as risk factors for the occurrence of venous thrombosis in lupus patients. Arthritis Rheum 52: 2060–2068, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Burgos PI, Alarcón GS: Thrombosis in systemic lupus erythematosus: Risk and protection. Expert Rev Cardiovasc Ther 7: 1541–1549, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Dhar JP, Andersen J, Essenmacher L, Ager J, Sokol RJ: Thrombophilic patterns of coagulation factors in lupus. Lupus 18: 400–406, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Katz PP, Andrews J, Yazdany J, Schmajuk G, Trupin L, Yelin E: Is frailty a relevant concept in SLE? Lupus Sci Med 4: e000186, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jolly M, Toloza S, Goker B, Clarke AE, Navarra SV, Wallace D, Weisman M, Mok CC: Disease-specific quality of life in patients with lupus nephritis. Lupus 27: 257–264, 2018 [DOI] [PubMed] [Google Scholar]