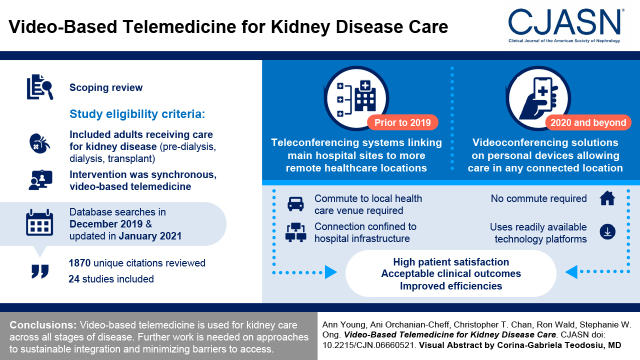
Visual Abstract
Keywords: virtual care, chronic kidney disease, review, telemedicine, health services delivery model, digital health, communications media
Abstract
Background and objectives
Video-based telemedicine provides an alternative health care delivery model for patients with CKD. The objective was to provide an overview of the available evidence on the implementation and outcomes of adopting video-based telemedicine in nephrology.
Design, setting, participants, & measurements
MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and CINAHL were searched in December 2019 and again in January 2021 for studies using video-based telemedicine for adults across the spectrum of kidney disease. Study types included peer-reviewed clinical trials, observational studies, and descriptive studies available in full text. Search results were independently screened by two authors, who then independently reviewed and extracted data from the eligible studies. Results were synthesized in tabular format, summarizing study characteristics by area within nephrology; the video-based interventions used; and clinical, health care utilization, and patient-reported outcomes.
Results
After reviewing 1870 unique citations, 24 studies were included (four randomized controlled trials, six cohort studies, five pre-post intervention studies, seven case series, and two qualitative studies). Video-based technology was used to facilitate care across all stages of CKD. Although earlier studies used a range of institution-specific technologies that linked main hospital sites to more remote health care locations, more recent studies used technology platforms that allowed patients to receive care in a location of their choice. Video-based care was well received, with the studies reporting high patient satisfaction and acceptable clinical outcomes.
Conclusions
Video-based telemedicine is being used for kidney care and has evolved to be less reliant on specialized telemedicine equipment. As its use continues to grow, further primary studies and systematic reviews of outcomes associated with the latest innovations to video-based care in nephrology can address knowledge gaps, such as approaches to sustainable integration and minimization of barriers to access.
Introduction
CKD is a complex health condition, the management of which is often burdensome on patients, caregivers, and the health care system. Because of its progressive nature, CKD requires long-term follow-up with close surveillance of clinical and laboratory parameters and, in advanced stages, support by a multidisciplinary team (1). Moreover, CKD care is resource intensive. A recent review found that the annual health care costs per patient across developed countries for those with stages 4–5 CKD ranged from $5367 to $53,186 USD and from $20,110 to $100,593 USD for those with kidney failure (2). Access to CKD care due to geographic barriers, late referrals to nephrologists, and other health care system constraints have been associated with significant morbidity and mortality (3).
Capacity and gaps within the health care system became widely evident during the coronavirus disease 2019 (COVID-19) pandemic. Restrictions imposed to limit virus spread severely limited ambulatory, in-person CKD care to streamline resources to acute care needs. Pandemic-related restrictions stimulated the accelerated implementation of alternative health care delivery models. Virtual care is defined as “any interaction between patients and/or members of their circle of care, occurring remotely, using any forms of communication or information technologies with the aim of facilitating or maximizing the quality and effectiveness of patient care” (4). The incorporation of telephone and video-based visits quickly became an accepted and, in some cases, preferred health care delivery model. Although virtual care was rapidly implemented to provide seamless access to nephrology services under the pressures of the COVID-19 pandemic, little was known on how to do this most effectively and how this would affect our patients.
The objective of this scoping review was to provide an overview of the available evidence on the implementation and outcomes of adopting video-based telemedicine in nephrology relative to traditional, in-person care.
Materials and Methods
Reporting of this scoping review was in accordance with the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (5), along with guidance specific to the PRISMA Extension for Scoping Reviews (6).
Eligibility Criteria
Studies were included if they involved adults receiving care for kidney disease using video-based telemedicine. The population included patients with predialysis CKD, those receiving dialysis (all modalities), and kidney transplant recipients. The intervention had to be video based and synchronous (i.e., live, two-way audiovisual link between a patient and a care provider [7]) involving at least ten patients. Comparisons were made with the existing standard of care. Study types included clinical trials and observational and descriptive studies available in full text. Case reports, study protocols, editorials or commentaries, and other reviews were excluded.
Information Sources
A comprehensive search strategy was initially developed for Ovid MEDLINE using a combination of database-specific subject headings and text words for the two main concepts of telemedicine and kidney diseases. Additional text words were generated through consultation with subject specialists on the team. The MEDLINE search strategy was then customized for each database. Limits were applied for English language and human adults. No restrictions were placed for date. Conference and book materials were excluded where applicable.
The following databases were searched on December 13, 2019 and again on January 5, 2021: Ovid MEDLINE; Ovid MEDLINE e-published ahead of print and in-process and other nonindexed citations; Ovid EMBASE; Cochrane Database of Systematic Reviews (Ovid); Cochrane Central Register of Controlled Trials (Ovid); and CINAHL with full text (December 13, 2019)/CINAHL complete (January 5, 2021; EBSCO). Supplemental Table 1 shows database search strategies.
Selection of Sources of Evidence
All citations from the search strategy were imported into Zotero 5.0, and duplicates were removed. Two authors (A.Y. and S.W.O.) independently screened the titles and abstracts of each unique citation to exclude clearly nonrelevant citations. Any potentially relevant studies were retrieved in full text for more detailed evaluation to determine final eligibility. Full text articles were evaluated independently by the same authors. Disagreements on article eligibility were resolved by discussion and consensus.
Data Charting Process
A data extraction form was developed to summarize the information from the included studies. A.Y. independently extracted data from the included studies. S.W.O. independently verified the accuracy of the extracted data. Data items of interest included study characteristics (e.g., study design, publication year, and origin); nephrology population; and setting, duration, and delivery of the video-based intervention.
Synthesis of Results
Results were synthesized in tables, summarizing key characteristics of included studies by area within nephrology; descriptions of the video-based interventions; and study outcomes subcategorized into clinical, health care utilization, efficiency, and patient- or physician-reported outcomes. Cost outcomes were described when available. As this scoping review was conducted to provide an overview of the existing evidence, the methodologic quality of the included studies and associated risk of bias were not critically appraised (6).
Results
Selection of Sources of Evidence
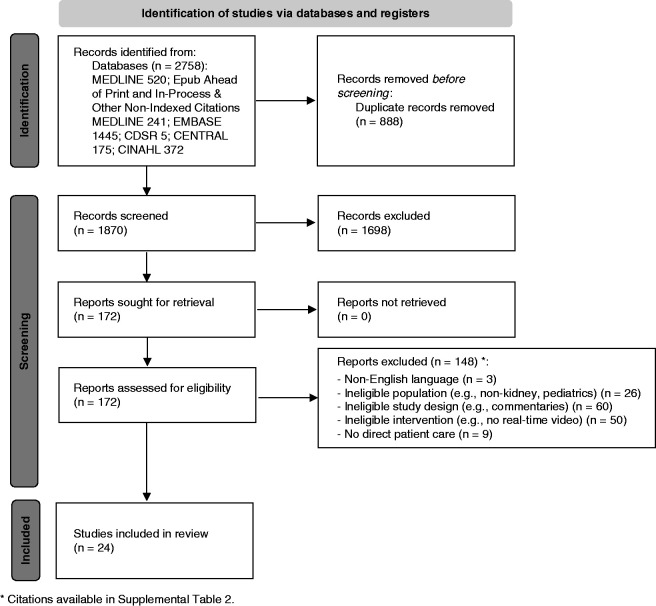
Study selection is summarized in Figure 1 (5). There were in total 1870 unique citations after removal of duplicates. Many were excluded on the basis of title and abstract, leaving 172 studies that were reviewed in full text for eligibility. Of these, 148 studies were excluded with reasons (Supplemental Table 2).
Figure 1.
PRISMA flow diagram predicting the flow of information through the different phases of this scoping review. CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Characteristics of Sources of Evidence
There were 24 studies included in this review, with characteristics summarized in Table 1 (8–31). There were seven studies in patients with CKD, nine studies in peritoneal dialysis and hemodialysis populations, and eight studies in kidney transplant recipients. The studies were published between 1997 and 2020 and were conducted across ten countries. Most studies were case series (n=7), cohort (n=6), or pre-post design (n=5). There were four randomized controlled trials (RCTs), two of which had studied the same sample of individuals. Study sizes were variable, with as few as 11 patients in a case series (21) to over 2500 patients in a pre-post design (18). There were two qualitative studies that administered questionnaires and semistructured interviews.
Table 1.
Characteristics of included studies by area within nephrology
| Study and Year (Reference) | Study Origin | Study Design | Study Size | Study Time Frame |
|---|---|---|---|---|
| CKD | ||||
| Tan et al. 2018 (8) | New York, United States | Cohort study | 228 (E=112, C=116) | April 2011 to August 2014 |
| Venuthurupalli et al. 2018 (9) | Queensland, Australia | Cohort study | 1051 (E=234, C=817) | September 2011 to December 2016 |
| Narva 2017 et al. (10) | New Mexico, United States | Case series | 1870 patient visits | 2007 to May 2016 |
| AlAzab and Khader 2016 (11) | Mafraq and Ma'an, Jordan | Pre-post | 64 | September 2013 to January 2014 |
| Ishani et al. 2016 (12) | Minnesota, United States | RCT | 601 (E=451, C=150) | March 2012 to October 2012 |
| Ladino et al. 2016 (13) | Florida, United States | Case series | 101 | 2013–2015 |
| Campbell et al. 2012 (14) | Ontario, Canada | Case series | 99 | March 2009 to July 2009 |
| Peritoneal dialysis | ||||
| Viglino et al. 2020 (15) | Cuneo, Italy | Case series | 15 | January 2009 to December 2018 |
| Gallar et al. 2007 (16) | Madrid, Spain | Cohort | 57 (E=25, C=32) | September 2003 to August 2005 |
| Hemodialysis | ||||
| Michel et al. 2021 (17) | Poindimie and Wallis and Futuna Islands, France | Cohort study | 80 (E=40; C=40) | January 2012 to December 2017 |
| Singh et al. 2020 (18) | Singapore | Pre-post | 2589 | Pre: November 2019 to January 2020/post: February 2020 to April 2020 |
| Ditchburn and Marshall 2017 (19) | Lancashire and Cumbria, United Kingdom | Qualitative | 10 | 2013 |
| Berman et al. 2011 (20) | Hawaii, United States | Cohort study | 44 (E=19, C=25) | Not specified |
| Sicotte et al. 2011 (21) | Quebec, Canada | Pre-post | 11 | May 2005 to December 2006 |
| Whitten and Buis 2008 (22) | Michigan, United States | Pre-post | 34 | April 2005 to March 2006 |
| Mitchell and Disney 1997 (23) | Adelaide, South Australia | Qualitative | 18 | 1995–1996 |
| Kidney transplantation (pretransplant) | ||||
| Alshaer et al. 2020 (24) | London, United Kingdom | Case series | 44 | Not specified |
| Chang et al. 2020 (25) | New York, United States | Case series | 109 | March 2020 to April 2020 |
| Forbes et al. 2018 (26) | Tennessee, United States | Cohort study | 302 (E=143, C=159) | May 2013 to May 2016 |
| Forbes et al. 2018 (27) | Tennessee, United States | Pre-post | 158 | Pre: October 2013 to January 2015/post: February 2015 to May 2016 |
| Kidney transplantation (post-transplant) | ||||
| Andrew et al. 2018 (28) | Melbourne, Australia | Case series | 45 | May 2016 for 2 years |
| Kaier et al. 2017 (29) | Freiburg, Germany | RCT | 46 (E=23, C=23) | October 2011 to March 2012 |
| Schmid et al. 2017 (30) | Freiburg, Germany | RCT | 46 (E=23, C=23) | October 2011 to March 2012 |
| Leimig et al. 2008 (31) | California, United States | RCT | 106 (E=53; C=53) | August 2005 to October 2006 |
E, experimental; C, control; RCT, randomized controlled trial.
Description of Video-Based Telemedicine Interventions
Details of the video-based telemedicine interventions used in each study are summarized in Table 2. Most studies used teleconferencing systems with audio and video capability, typically set up in community hospitals/clinics with connection to hub hospitals. Patients receiving care by video-based telemedicine often traveled to a local venue to mitigate a longer commute to the hub hospital. Physical examination could often be facilitated over videoconferencing by nursing or support staff, and allied health (e.g., diabetes educators, dietitians) may have also participated. Shared information technology systems between sites often allowed for viewing of the patient’s medical record, including laboratory values and dialysis parameters. Five studies described the use of telemonitoring systems that were placed in a patient’s home, which included an audio and video connection as well as other peripheral monitoring devices (e.g., scale, pulse oximeter, BP monitor) connected to the hub center (12,15,20,29,30). More recent studies used web-based videoconferencing solutions (e.g., Microsoft Teams) on personal devices with a microphone and webcam, connecting patients to their nephrologists from a location of their choice (18,24,28). Anyone with a link to the appointment could join virtually, including other family members or the patient’s primary care provider. Studies that followed patients longitudinally often included a mix of virtual and in-person care.
Table 2.
Details of the video-based telemedicine intervention
| Study and Year (Reference) | Intervention |
|---|---|
| CKD | |
| Tan et al. 2018 (8) | I8500 Mobile Telemedicine Station (Global Med, Scottsdale, AZ) connected to high-speed internet with stethoscope, headphone, and digital camera Local technician beside patient facilitates physical findings to the remote physician |
| Venuthurupalli et al. 2018 (9) | Cisco Jabber Video for TelePresence (Cisco Systems Australia Pty Ltd., Sydney, Australia) Patients attended the nearest Queensland Health facility for follow-up Clinical examination done by CKD nurse practitioner or local nursing support |
| Narva 2017 et al. (10) | 2007: Tandberg Codec 6000 videoconference system (at NIH in Bethesda, MD) with broadband connection to a Polycom MGC100 video bridge (at Technology Support Centre in Sioux Falls, SD) to a Polycom VSX 7000 videoconference system (at Zuni Indian Hospital, Pueblo, NM). System upgraded to Cisco TelePresence System EX90 in 2016 (encrypted connection between NIH to Zuni) Clinic managed by an RN case manager who is with the patient during the entire visit Diabetes educators, dietitians, and primary clinicians available for consultation |
| AlAzab and Khader 2016 (11) | Cisco CTS500 videoconferencing connected patients from two remote hospitals in Jordan with specialists at Prince Hamzah Hospital in Amman over broadband internet connection Two nurses available in each remote hospital; general examination camera, electronic stethoscope, and vital signs monitor available |
| Ishani et al. 2016 (12) | LifeView (AmericanTeleCare) video monitoring device and peripherals (BP cuff, scale, glucometer, pulse oximeter, stethoscope, and web camera) in patient’s home Interactive video conferencing between nurses and patients (routine and acute care) |
| Ladino et al. 2016 (13) | Real-time videoconferencing at four telenephrology clinic locations Patients must have lived >40 miles from the nephrology clinic at the Miami VAMC Vital signs were obtained by the telehealth nurse |
| Campbell et al. 2012 (14) | Tandberg Intern cart, 880 Tandberg Codec camera, and an electronic stethoscope (AMD). The remote sites used Polycom IDOC cart, QDX Codec camera, and AMD stethoscope Initial nephrology consultation in Ottawa and follow-up via telemedicine (patients traveled to their local community hospital) A specialist nurse and a nephrologist located in Ottawa, Ontario, Canada, and two nurses at each remote site. Vital signs and medication review conducted prior to connecting with the physician |
| PD | |
| Viglino et al. 2020 (15) | Custom Videodialysis system—remote station at the patient’s home (video camera, a monitor, a microphone), control station in the center (webcam, a hands-free phone, a high-resolution monitor, and a personal computer) Nursing staff members monitor multiuser connection, acquisition and recording of dialysis parameters, performance of the CAPD or APD dialysis procedure, filling in of the dialysis sheets, exit site care, assessing any dialysis and/or clinical issues, and adherence to medication/diet 1-hour sessions; nurse-patient ratio is 1:6 |
| Gallar et al. 2007 (16) | Patients on PD received alternating monthly teleconsultation and hospital consultation with additional visits by telemedicine as needed Videoconference unit in patient’s home (Falcon, Vcon)—built in camera connected to television; in hospital—personal computer with a videoconference card (Cruiser, Vcon), webcam, and software to control the patient’s camera. Connection via three integrated services digital network lines PD nurse reviewed patient’s technique and catheter exit site and assessed for peritonitis |
| Hemodialysis | |
| Michel et al. 2021 (17) | Main center in Noumea, New Caledonia. Wallis Island is 2100 km from Noumea, and there are only two flights per week between them; thus, Wallisian patients were followed by monthly teleconsultation (using assisted video conferencing) and a quarterly in-person visit Shared electronic medical records; real-time interventions as needed |
| Singh et al. 2020 (18) | Virtual patient rounds via videoconferencing (Microsoft Teams on an iPad) between nephrologist (in hospital) and patients and dialysis nursing staff physically at remote dialysis centers. Teleconsultation rounds monthly and ad hoc, if needed Laboratory and dialysis reports were reviewed on an electronic medical record |
| Ditchburn and Marshall 2017 (19) | Video as a service: Remote access to patients by live video link using Polycom video technology through a rotating pan, tilt, zoom (PTZ) camera and a high-definition screen at the patient’s home connected to the main nephrology unit Initial investment of £50,000 (60,000 euros) for videoconferencing equipment (including four high-definition cameras, large television screen at the training center, associated technical consultancy, and connectivity costs) |
| Berman et al. 2011 (20) | VitelCare Turtle 500 home monitoring unit with a BP monitor, scale, pulse oximeter, glucose monitor, video camera, and headset Trained personnel installed the equipment and provided instruction in the home Video camera and headset provide videoconferencing capability (at arranged times) |
| Sicotte et al. 2011 (21) | Remote clinical team of nephrologists and nurses provides specialized clinical supervision by communicating with local care teams and patients In Chibougamau, the remote nephrologist communicated directly with each patient by videoconference. In Chisasibi, videoconferencing was used for telereview only (no direct contact between the distant team and patients) |
| Whitten and Buis 2008 (22) | Wireless videoconferencing equipment mounted on a mobile cart for patients to connect with their nephrologists while receiving dialysis (no details of the equipment used) Patients assessed 3–4 times per month |
| Mitchell and Disney 1997 (23) | Equipment consisted of 4 “roll-about” units (System 4000; PictureTel), 5 desktop units (PCS 50andPCS 100; PictureTel), 4 miniature probe cameras (PCXC/999P; Sony), 2 video cassette recorders, 2 document cameras, and patient and staff headphones. The videoconferencing equipment operated at 128 kbit/s and provided full CIF resolution video (352 pixels × 288 lines) at 15 frames per second squared Used on the dialysis ward (next to dialysis chair) or in private interview rooms for outpatient clinics |
| Kidney transplantation (pretransplant) | |
| Alshaer et al. 2020 (24) | Virtual consultations using Attend Anywhere Patients were sent a text or email prior to their clinical appointment with instructions to check their weight and BP before they attended, as well as the instructions for logging in and connecting to their video clinic appointments |
| Chang et al. 2020 (25) | Kidney transplant program at the Columbia University Irving Medical Center rapidly implemented a telehealth program that offers virtual visits for ambulatory patients (no additional details) |
| Forbes et al. 2018 (26) | Telehealth visit at Veterans Affairs facilities using a Cisco Telepresence EX90 System (teleconference display with camera plus touch control tablet with integrated phone handset) Visit included transplant physician, patient, and support person(s) at the remote center Nurses in patient’s remote location could obtain vital signs and perform limited physical examination |
| Forbes et al. 2018 (27) | Forbes et al. (26) |
| Kidney transplantation (post-transplant) | |
| Andrew et al. 2018 (28) | Skype for Business—May 20 to July 16, 2017; Health Direct Videocall—August 2017 onward Patients able to access from any device with a microphone and webcam (all patients had a test call prior to visit) GP or other health care providers can also join virtually In-person reviews interspersed with telehealth |
| Kaier et al. 2017 (29) | Telemedically supported post-transplant case management Daily questionnaires about their physical condition and vital data via an interactive web-based telemonitor; video teleconferences between the patient and the Transplantation Center Freiburg if anomalous values occurred |
| Schmid et al. 2017 (30) | Telemedically supported case management with daily questions answered on an interactive terminal and data transferred through a safe web-based connection Prompt real-time video consultations as needed Team including a transplant nurse case manager and two senior transplant physicians |
| Leimig et al. 2008 (31) | Patients traveled to one of three satellite telemedicine sites located 19, 90, or 120 miles from the standard care clinic Sites equipped with a television monitor, a Polycom H.323 video conference camera, an analog stethoscope with headphones, a handheld close examination camera, and an otoscope. Telehealth infrastructure used dedicated point-to-point T-1 lines capable of transmission speeds of 1.544 Mbps Telehealth visits were conducted in the same pattern as the standard care visit but via a live interactive session. Nurse available at satellite sites to assist with physical examination |
NIH, National Institutes of Health; RN, registered nurse; VAMC, Veterans Affairs Medical Center; PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; APD, automated peritoneal dialysis; CIF, common intermediate format; GP, general practitioner.
Outcomes in Patients with Nondialysis-Dependent CKD
A summary of reported outcomes associated with video-based telemedicine is provided in Table 3. Among the seven studies that used video-based telemedicine for patients with CKD, outcomes could be broadly categorized as clinical, health care utilization, efficiency, patient centered, and economic (8–14). There were few differences in reported clinical outcomes, including mortality, need and timing of dialysis, change in kidney function, BP, or other biochemical markers. Emergency room visits and hospitalization were no more frequent in the telemedicine group compared with in-person care (12). All studies reported improved efficiency with telemedicine, including a reduction in the waiting time to see a specialist, fewer clinic cancellations, and reduction in travel time and distance traveled. This was balanced against a slight increase in hours spent by clerical staff per patient (14). Overall, patient satisfaction was high for video-based care (>95%), with most patients perceiving it to be the same level of care compared with being seen in person. There were improvements in quality of life noted after receiving telemedicine-based care (11). Most of the cost savings were due to a reduction in travel costs. The studies did not describe the setup or licensing costs associated with telemedicine-capable clinics as they were often part of a hospital-wide telemedicine initiative.
Table 3.
Summary of outcomes studied
| Outcome | Main Results | ||
|---|---|---|---|
| CKD | Dialysis | Kidney Transplant | |
| Clinical outcomes | |||
| Composite clinical outcome | ↔ in death, kidney failure, doubling of Cr over 2 years (8) ↔ in death, hospitalization, ER visit, or admission at 1 year (E: 46.2% versus C: 46.7%) (12) |
||
| Mortality | ↓ (E: 4.5 versus C: 5.3 cases per 100 patient-years) (9) ↔ (E: 2.9% versus C: 2.0%) (12) |
↔ death (E: 8.3% versus C: 6.5% per 100 person-years (17) ↔ death (pre 2.77%, post 1.92%) (18) |
|
| Kidney function/need for KRT/dialysis issues | ↓ KRT (E: 5.1% versus C: 9.9%) (9) ↔ KRT (E: 2.4 versus C: 1.3%); ↔ time to KRT (12) ↔ Cr (study start: 197 mmol/L to study end: 174 mmol/L), P=0.47 (13) |
↔ peritonitis (low in all assisted PD); 6% ↑ in the use of PD; 7.6% ↓ in drop out from PD to HD (15) ↔ vascular access complications (17) ↔ satellite to urban center transfers (21) |
|
| BP and other physiologic markers |
↔ % patients with systolic BP ≤140 mm Hg (12) ↓ systolic BP 12 mm Hg (P<0.001), ↓ diastolic BP 6 mm Hg (P=0.004); better K+ control (P=0.008); ↔ HCO3 (P=0.79); ↔ PO4 (P=0.91) (13) |
↔ (BP, interdialytic weight gain, Hb, albumin, Ca2+, PO4) (17) ↔ mean systolic BP: pre 157 versus post 155; mean diastolic BP: pre 85 versus post 80; ↔ in Hb, HbA1c, Kt/V, PO4, PTH, Gluc (21) Most met or exceeded clinical targets (Hb, calcium/phosphate, albumin, and urea reduction) (22) |
↔ infection or rejection (31) |
| Health care utilization | |||
| ER visits | ↔ (E: 36.4 versus C: 38.7%) (12) | ↓ (E: 0.0003 versus C: 0.0019 per study day) (20) | 26% had ED visit within 3 mo of TM visit (during COVID-19) (25) |
| Hospitalizations | ↓ (E: 1.63 versus C: 2.25 per patient) (9) ↔ (E: 29.8 versus C: 26.7%) (12) |
↓ (E: 2.2 versus C: 5.7 day per patient per year) (16) ↓ (E: 3.0 versus C: 5.5 per patient) (17) ↓ (pre 22 versus post 16 ppy, P<0.001); more dialysis-related admissions (18) ↓ (E: 0.0018 versus C: 0.0056 per study day) (20) |
34% had admission within 3 mo of TM visit (during COVID-19) (25) ↓ unplanned admissions, E: 0 versus C: 2, P=0.002 (30) ↔ hospitalizations (31) |
| Efficiency measures | |||
| Time savings | 21 hours to attend in-person clinic if TM not an option; ↓ travel time; ↓ waiting time to see specialist (11) | PD teleconsultation 22 min (SD 9) versus hospital consultation 33 min (SD 8) (16) | ↓ time from referral to visit (E: 51.4 versus C: 87.9 days, P<0.001) (26) ↑ patients seen within 30 days of referral with ↑ TM use (27) |
| Nursing hours/clerical staff hours | Fewer clinic cancellations (8) >90% clinic attendance with TM (9) Nurse manager and strong ancillary staff critical for TM referral clinic (10) ↔ nursing hours per patient; slight ↑ clerical staff hours (14) |
One nurse for up to 6 patients (15) “More efficient use of staff time” (19) |
|
| Distance traveled | ↓ Six-fold (8) ↓ with telemedicine visits (9) |
↓ unnecessary travel for nurses and maintenance visits by technicians (19) | ↓ (E: 593.8 versus C: 932.4 miles) (26) ↓ travel time and distance (28) |
| Technical issues | Loss of audio or video or access to electronic health record delayed or cancelled patient care; lack of bandwidth interrupted video link (10) | Technical problems related to ISDN lines in (19% of) teleconsultations (16) Average 7.7 technical issues per month, ↓ over course of study (20) |
−9% (virtual visits) failed due to technical issues (25) |
| Patient- or physician-reported outcome measures | |||
| General impression/satisfaction | Mean satisfaction score 96.8% on the basis of 15-item questionnaire (11) ≥95% patients agreed/strongly agreed: “satisfied with session,” “willing to use video conferencing again,” “[felt] as confident about the doctor’s assessment as [they] would with an in-person assessment” (14) |
97% patients perceived teleconsultation as being similar to hospital consultation (16) E versus C: ↔ no difference in quality of dialysis care delivered (17) TM “Useful, encouraged adherence to the dialysis therapy and could nurture confidence in patients” (19) Positive perceptions; patients leaned toward using TM only when providers were not available. Providers had mixed results on when telemedicine should be used (22) TM clinically feasible, enabled a variety of procedures, expanded services (23) |
91% “no different, better or significantly better,” 63% “more convenient,” 77% “safer” (during COVID-19), 63% “shorter wait time” (24) TM feasible and effective for care of transplant recipients during COVID-19 … those who required next level care were triaged appropriately (25) 95% felt they received the same standard of care as a face-to-face consultation (28) |
| Quality of life | ↑ SF-8 score from 33.1 to 45.0 (P=0.02) after 2 mo (11) | SF-36 (E: 63.9 versus C: 59.1); SF-36 after 6–9 mo (E: 60.76 versus C: 59.5) (20) | ↑ disease-specific QoL (↓ cardiac/kidney symptoms, steroid side effects); both groups ↓ psychologic distress (30) |
| Patient engagement | >98% intend to continue with TM (9) 96.2% completed ≥1 video visit (12) “Less stressful,” “appreciated the ease of access to care” (14) |
TM instilled confidence; enabled them to stay independent; no dropouts from VD-assisted PD (15) ↑ patient empowerment (20) |
88% would continue with video-based visits (24) Less post-transplant nonadherence in E group (E 17.4% versus C: 56.5%) (30) |
| Health care provider experience | I was “able to provide the same level of care as in an in-person clinic” (score: 4.5/5); “able to get enough relevant information to assist me in clinical decision making” (score: 4.8/5) (14) | In all cases, the (PD) catheter exit site could be evaluated, as well as the dialysis fluid appearance, presence of edema, and exact medication received (16) “Quicker way to solve problems,” able to remotely see and advise the patient at the moment the problem was happening (19) |
“Video clinics offer a possibility of reviewing patients safely,” “suitable for follow-ups, rather than first-time appointments” (24) |
| Cost | |||
| Various categories as indicated | ↓ Travel costs—E: USD $8.29 versus C: $18.99; P<0.001 (8) “Significant economic advantages” due to ↓ travel costs with TM visits (9) ↓ (∼$72 USD savings compared with in-person visit) (11) |
“Considerable savings” with VD-assisted PD compared with nurse-assisted PD at home (15) E: $198 versus C: $177 per visit; E had savings in transportation (16) ↓ (E: $114 versus C: $322 per study day) (20) |
↓ for E versus C: visit costs (USD $138.62 versus $176, P<0.001), dialysis costs (USD $99.18 versus $199.00, P<0.001), travel costs (USD $287.21 versus $473.93, P<0.001), total costs per patient (USD $656.11 versus $1108.91, P<0.001) (18) ↓ petrol costs (28) ↓ E: €5,504.21 versus C: €10,449.28 (due to ↓ in medical service use) (29) Savings of €3417 per inpatient (30) |
Results highlighted in bold indicate outcome data for randomized clinical trials; ↑ indicates increase/higher, ↓ indicates decrease/lower, and ↔ indicates no change/difference. Cr, creatinine; ER, emergency room; E, experimental; C, control; PD, peritoneal dialysis; HD, hemodialysis; K+, potassium; HCO3, bicarbonate; PO4, phosphate; Hb, hemoglobin; Ca2+, calcium; HbA1c, glycosylated hemoglobin; PTH, parathyroid hormone; Gluc, glucose; ED, emergency department; TM, telemedicine; COVID-19, coronavirus disease 2019; ppy, per person year; ISDN, integrated services digital network; SF-8, short form 8; SF-36, short form 36; QoL, quality of life; VD, video dialysis; USD, US dollars.
Outcomes in Patients on Dialysis
There were two studies using video-based telemedicine in patients on peritoneal dialysis (15) and seven studies in patients on hemodialysis (17–21). There were no differences between patients receiving video-based telemedicine compared with in-person care across the reported clinical outcomes (mortality, vascular access complications, peritonitis, and BP). Video-assisted peritoneal dialysis may have facilitated the use of home dialysis (more incident cases, fewer dropouts) (15). Video-based care was associated with fewer emergency room visits and hospital admissions. Patients reported increased confidence, empowerment, and independence. Perceived quality of life may be slightly higher in the video-based care group (20). The economic implications of virtual care in the dialysis population were not well studied.
Outcomes in the Kidney Transplant Population
Of the eight studies in kidney transplant recipients, clinical end points for patients receiving video-based care were reportedly equivocal. Unplanned hospital admissions were lower in one study (30); one study reported significant numbers of emergency room visits and hospitalizations after receiving video-based transplant follow-up, but this may have been affected by the COVID-19 pandemic (25). Patients with transplants may live far from their nearest transplant center; accordingly, distance traveled, visit costs, and travel costs were reduced for those receiving telemedicine-supported care. Virtual appointments seemed to improve access to transplant assessment, with shorter wait times after referral (26,27). Pretransplant care by telemedicine was felt to be at least the same as the standard of care provided with face-to-face consultation (24,28).
Discussion
This review provides an overview of studies on video-based telemedicine for care across the spectrum of kidney disease. Although earlier studies used institution-specific technologies that linked main hospital sites to more remote health care locations, recent studies saw the use of consumer-based platforms on personal devices that further removed geographic barriers. This review highlights a broad range of outcomes following video-based telemedicine relative to in-person medical care, many of which are important to patients and providers. Studies reported acceptable clinical outcomes, generally fewer emergency room visits and hospitalizations, improved efficiencies, and high patient satisfaction. Nevertheless, the quality of the studies in this scoping review was not formally appraised; study designs, statistical power, and publication biases may have affected the reporting of these largely positive outcomes.
Early and consistent access to nephrology care has been shown to improve clinical outcomes and reduce economic costs (32,33). Telehealth utilization was increasing over the past decade but, until recently, still accounted for a small proportion of ambulatory care. From 2010 to 2015, it accounted for 1.5 outpatient private health insurance claims per 10,000 outpatient visits in the United States (34). On March 11, 2020, the World Health Organization declared the novel coronavirus outbreak (COVID-19) a global pandemic (35). This triggered health care systems to dramatically shift the prioritization of health care resources from elective and nonurgent care to the most acute and critically ill patients to limit the spread of disease. This forced a paradigm shift in the way many health care institutions delivered outpatient medical care. Technology-assisted medicine rapidly became a dominant model for chronic disease management to minimize disruptions in access to care during pandemic restrictions (36). In Ontario, Canada, where virtual care accounted for only 2% of total ambulatory visits in 2019, an increase in video visit use by 40% was seen between 2019 and 2020 (36).
The rapid uptake of virtual models during the pandemic did not have the benefit of years of preplanning to ensure a controlled and smooth integration into clinical workflows. Yet, the nature of CKD care makes it particularly amenable to virtual care given that relevant history, review of laboratory investigations, and counseling can all be conducted via virtual platforms. The main obstacle of virtual care is the lack of a physical examination. This can partly be mitigated by observing patients’ demeanor in real time, patient-assisted clinical examinations (e.g., pressing thumb into pretibial area to assess edema [37]), and ambulatory BP monitoring. Moreover, because virtual care does not rely on physical clinic space with defined schedules, virtual care may enhance kidney care delivery through easier access for urgent evaluations and more frequent care for higher-risk patients.
This review highlights changes to telemedicine integration over time. Most of the studies showed the implementation of institutional telemedicine systems that ensured the privacy and security of connections within the confines of hospital infrastructure. More recent studies reflected the use of consumer-based platforms (e.g., Zoom or Microsoft Teams), which facilitated visits in patient’s homes or another location of their choice. Third-party platforms flourished in popularity during the pandemic but were challenged with ensuring that their telehealth solutions were compliant with local privacy legislation (38). Studies on video-based support in peritoneal dialysis were limited in this review. Consumer-based applications open more opportunities to study virtual models beyond defined settings (e.g., hospital-based videoconference rooms), allowing for the possibility of synchronous video-based interactions with patients connected to dialysis machines in the hospital or at home. This may be further facilitated by integrating video-based telemedicine within electronic medical record systems; although such technology is available and being used, none of the studies in this review described this. Facilitating ambulatory visits in patient’s preferred location is likely associated with less costly infrastructure, more scalability, and further reductions in geographic barriers, but these newer approaches require further study.
Long-term policies around funding virtual health care will need to be addressed. Cost considerations in the included studies focused primarily on savings associated with care delivery (e.g., reduced travel and visit-associated costs). One study estimated significant savings due to the reduction in medical service use (29). Areas that were rarely described included infrastructure costs (which may be moderated by using personal technology and third-party platforms) and human resource costs. Prior to the pandemic, a barrier to virtual care uptake in universal single-payer systems, like Canada, was the limited remuneration options for virtual medical services by fee-for-service physicians who would otherwise be publicly funded (39). As such, virtual care was occurring primarily in the private, noninsured sector. However, temporary changes during the pandemic allowed physicians to be paid for virtual care on par with in-person care, including telephone calls and third-party videoconferencing platforms (40). Accessibility to virtual care will be partially tied to provider remuneration (41). Moreover, as the latest technologies are streamlined into routine health care, the “digital divide” will become more pronounced, negatively affecting those without access to broadband internet connections, those without access to video-capable devices, and those with limited technology literacy (42). The effect of socioeconomic disparities on virtual care provision was not addressed in the studies in this review. Expansion of telemedicine programs will require flexible solutions for those without video-capable personal technology, perhaps increasing access to video-capable satellite centers or continued acceptance and remuneration of audio-only telemedicine solutions.
This review captured studies in nephrology that broadly considered the use of video-based technology for care provision. Each study applied custom-built telemedicine solutions unique to their center; some may now be considered legacy telemedicine systems or, even if current, may not inform current practices at any given center. Many of the studies that reported on clinical end points (e.g., need for dialysis, mortality) were likely underpowered to detect significant differences. The reported efficiency and experiential outcomes were overwhelmingly positive. Although it may be that video-based telemedicine is at least comparable with in-person care, the possibility of publication bias, with negative experiences underpublished, cannot be ignored. As future research in this theme continues, all outcomes should be reported to best guide the integration of virtual care.
Video-based telemedicine has been used for the care of patients with kidney disease for some time and has evolved in recent years to be less reliant on specialized telemedicine equipment with greater flexibility to allow for patient care in a wider variety of locations. Video-based care was well received, with studies reporting high patient satisfaction and acceptable clinical outcomes; however, many of the studies were small, and there may be reporting biases. With the ongoing evolution in the delivery of video-based telemedicine and the rapid uptake of virtual care spurred by the COVID-19 pandemic, there is a unique opportunity to further evaluate the successes and shortcomings of video-based kidney care.
A national poll conducted by Abacus Data for the Canadian Medical Association in May 2020 found that almost half of those polled with complex needs would prefer a virtual method as a first point of contact with their doctor following the conclusion of the pandemic (43). Moving forward, further research will help guide the sustainable integration of video-based telemedicine in nephrology. When there are fewer restrictions on in-person care, the environment will be more amenable to longer-term, prospective, controlled studies as patients become more familiar with telemedicine. The effect on patient-centered outcomes due to omission of the physical examinations and in-person interactions will become more evident over time, as will the disparities due to various social determinants of health and the “digital divide.” These will warrant careful scrutiny. On the other hand, having the capacity and convenience of video-based telemedicine in any location offers a growing opportunity to study this model of care in other clinical environments (e.g., in-center hemodialysis, home dialysis). Finally, economic analyses are needed to determine the direct and indirect cost savings relative to in-person care and guide policies on how to optimally reimburse health care providers to ensure cost-effective virtual care provision.
Disclosures
C.T. Chan reports consultancy agreements with DaVita, Dialco, Medtronic, and Quanta; receiving research funding from Medtronic through their external grant program; serving as a scientific advisor or member of DaVita, Medtronic, and Quanta; and serving as an associate editor of CJASN. R. Wald reports receiving research funding from Baxter; serving as on the editorial boards of CJASN, Kidney360, and Kidney Medicine; and being a contributor of UpToDate. All remaining authors have nothing to disclose.
Funding
This work was supported through funds from the Toronto General and Western Hospital Foundation. A. Young is supported by the University of Toronto Clinician Investigator Program, with salary support through post-doctoral research fellowships from the Ontario Ministry of Health and Long-Term Care the Canadian Institutes of Health Research Canadian Society of Nephrology, and Kidney Foundation of Canada Kidney Research Scientist Core Education and National Training Program.
Supplementary Material
Acknowledgments
We thank Mr. Caleb Nault from the University Health Network Library Services for his technical assistance with retrieving articles.
Supporters played no role in the design and conduct of the study or in the collection, management, analysis, or interpretation of the data.
Because Dr. Christopher T. Chan is an associate editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
C.T. Chan, S.W. Ong, A. Orchanian-Cheff, and A. Young drafted the protocol; S.W. Ong and A. Young provided study selection; S.W. Ong and A. Young extracted data from studies; S.W. Ong and A. Young carried out the analysis; C.T. Chan, S.W. Ong, R. Wald, and A. Young interpreted the analysis; C.T. Chan, S.W. Ong, A. Orchanian-Cheff, R. Wald, and A. Young drafted the final review; all authors participated in the planning, execution, or analysis of the study; all authors have read and approved the final submitted version; and all authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of the review to the protocol and analysis plan, both of which are available on request.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06660521/-/DCSupplemental.
Supplemental Table 1. Database search strategies.
Supplemental Table 2. Reports assessed for eligibility in full text and those excluded.
References
- 1.Eckardt K-U, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Elshahat S, Cockwell P, Maxwell AP, Griffin M, O’Brien T, O’Neill C: The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS One 15: e0230512, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smart NA, Titus TT: Outcomes of early versus late nephrology referral in chronic kidney disease: A systematic review. Am J Med 124: 1073–80.e2, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Shaw J, Jamieson T, Agarwal P, Griffin B, Wong I, Bhatia RS: Virtual care policy recommendations for patient-centred primary care: Findings of a consensus policy dialogue using a nominal group technique. J Telemed Telecare 24: 608–615, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D: The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE: PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 169: 467–473, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Tuckson RV, Edmunds M, Hodgkins ML: Telehealth. N Engl J Med 377: 1585–1592, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Mehrotra A, Nadkarni GN, He JC, Langhoff E, Post J, Galvao-Sobrinho C, Thode HC Jr., Rohatgi R: Telenephrology: Providing healthcare to remotely located patients with chronic kidney disease. Am J Nephrol 47: 200–207, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Venuthurupalli SK, Rolfe A, Fanning J, Cameron A, Hoy WE; NHMRC CKD.CRE and the CKD.QLD Collaborative : Chronic Kidney Disease, Queensland (CKD.QLD) Registry: Management of CKD with telenephrology. Kidney Int Rep 3: 1336–1343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narva AS, Romancito G, Faber T, Steele ME, Kempner KM: Managing CKD by telemedicine: The Zuni Telenephrology Clinic. Adv Chronic Kidney Dis 24: 6–11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AlAzab R, Khader Y: Telenephrology application in rural and remote areas of Jordan: Benefits and impact on quality of life. Rural Remote Health 16: 3646, 2016 [PubMed] [Google Scholar]
- 12.Ishani A, Christopher J, Palmer D, Otterness S, Clothier B, Nugent S, Nelson D, Rosenberg ME; Center for Innovative Kidney Care : Telehealth by an interprofessional team in patients with CKD: A randomized controlled trial. Am J Kidney Dis 68: 41–49, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Ladino MA, Wiley J, Schulman IH, Sabucedo AJ, Garcia D, Cardona JM, Valdes A, Pedraza F, Echeverriet al. RJ: Tele-nephrology: A feasible way to improve access to care for patients with kidney disease who reside in underserved areas. Telemed J E Health 22: 650–654, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Campbell M, Akbari A, Amos S, Keyes C: Feasibility of providing nephrology services to remote communities with videoconferencing. J Telemed Telecare 18: 13–16, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Viglino G, Neri L, Barbieri S, Tortone C: Videodialysis: A pilot experience of telecare for assisted peritoneal dialysis. J Nephrol 33: 177–182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallar P, Vigil A, Rodriguez I, Ortega O, Gutierrez M, Hurtado J, Oliet A, Ortiz M, Mon C, Herrero JC, Lentisco C: Two-year experience with telemedicine in the follow-up of patients in home peritoneal dialysis. J Telemed Telecare 13: 288–292, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Michel LM, Baroux N, Frimat L, Quirin N: Telenephrology and on-site nephrology: Comparable adequate dialysis care to patients living in remote Pacific Islands. J Telemed Telecare 27: 562–571, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Singh T, Ngoh CL, Wong K, Khan BA: Impact of telemedicine on hospitalisation and mortality rates in community-based haemodialysis centres in Singapore during the COVID-19 pandemic. Ann Acad Med Singap 49: 756–763, 2020 [PubMed] [Google Scholar]
- 19.Ditchburn JL, Marshall A: Renal telemedicine through video-as-a-service delivered to patients on home dialysis: A qualitative study on the renal care team members’ experience. J Ren Care 43: 175–182, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Berman SJ, Wada C, Minatodani D, Halliday T, Miyamoto R, Lindo J, Jordan PJ: Home-based preventative care in high-risk dialysis patients: A pilot study. Telemed J E Health 17: 283–287, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Sicotte C, Moqadem K, Vasilevsky M, Desrochers J, St-Gelais M: Use of telemedicine for haemodialysis in very remote areas: The Canadian First Nations. J Telemed Telecare 17: 146–149, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Whitten P, Buis L: Use of telemedicine for haemodialysis: Perceptions of patients and health-care providers, and clinical effects. J Telemed Telecare 14: 75–78, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JG, Disney AP: Clinical applications of renal telemedicine. J Telemed Telecare 3: 158–162, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Alshaer I, Teles J, Imedi A: Virtual renal transplant clinics: Patients’ and clinicians’ experiences. J. Kidney Care 5: 260–263, 2020 [Google Scholar]
- 25.Chang JH, Diop M, Burgos YL, Blackstock DM, Fernandez HE, Morris HK, Dube GK, Crew RJ, Mohan S, Husain SA, Cohen DJ, Tsapepas DS: Telehealth in outpatient management of kidney transplant recipients during COVID-19 pandemic in New York. Clin Transplant 34: e14097, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Forbes RC, Rybacki DB, Johnson TB, Hannah-Gillis A, Shaffer D, Hale DA: A cost comparison for telehealth utilization in the kidney transplant waitlist evaluation process. Transplantation 102: 279–283, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Forbes RC, Broman KK, Johnson TB, Rybacki DB, Hannah Gillis AE, Hagemann Williams M, Shaffer D, Feurer ID, Hale DA: Implementation of telehealth is associated with improved timeliness to kidney transplant waitlist evaluation. J Telemed Telecare 24: 485–491, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Andrew N, Barraclough KA, Long K, Fazio TN, Holt S, Kanhutu K, Hughes PD: Telehealth model of care for routine follow up of renal transplant recipients in a tertiary centre: A case study. J Telemed Telecare 26: 232–238, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Kaier K, Hils S, Fetzer S, Hehn P, Schmid A, Hauschke D, Bogatyreva L, Jänigen B, Pisarski P: Results of a randomized controlled trial analyzing telemedically supported case management in the first year after living donor kidney transplantation - A budget impact analysis from the healthcare perspective. Health Econ Rev 7: 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid A, Hils S, Kramer-Zucker A, Bogatyreva L, Hauschke D, De Geest S, Pisarski P: Telemedically supported case management of living-donor renal transplant recipients to optimize routine evidence-based aftercare: A single-center randomized controlled trial. Am J Transplant 17: 1594–1605, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Leimig R, Gower G, Thompson DA, Winsett RP: Infection, rejection, and hospitalizations in transplant recipients using telehealth. Prog Transplant 18: 97–102, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Obialo CI, Ofili EO, Quarshie A, Martin PC: Ultralate referral and presentation for renal replacement therapy: Socioeconomic implications. Am J Kidney Dis 46: 881–886, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Stack AG: Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 41: 310–318, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J: Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health 25: 132–136, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Cucinotta D, Vanelli M: WHO declares COVID-19 a pandemic. Acta Biomed 91: 157–160, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia RS, Chu C, Pang A, Tadrous M, Stamenova V, Cram P: Virtual care use before and during the COVID-19 pandemic: A repeated cross-sectional study. CMAJ Open 9: E107–E114, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benziger CP, Huffman MD, Sweis RN, Stone NJ: The telehealth ten: A guide for a patient-assisted virtual physical examination. Am J Med 134: 48–51, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoom.us : Zoom and PIPEDA/PHIPA Compliance Guide, 2020. Available at: https://zoom.us/docs/doc/PIPEDA_PHIPA%20Canadian%20Public%20Information%20Compliance%20Guide.pdf. Accessed April 12, 2021
- 39.Canadian Medical Association; The College of Family Physicians of Canada; Royal College of Physicians and Surgeons of Canada : Virtual care: Recommendations for scaling up virtual medical services, 2020. Available at: https://www.cma.ca/sites/default/files/pdf/virtual-care/ReportoftheVirtualCareTaskForce.pdf. Accessed April 12, 2021
- 40.Ontario Ministry of Long-Term Care : Ontario Health Insurance Plan Bulletin Number: 4755, 2020. Available at: https://www.health.gov.on.ca/en/pro/programs/ohip/bulletins/4000/bul4755.pdf. Accessed April 12, 2021
- 41.Mehrotra A, Bhatia RS, Snoswell CL: Paying for telemedicine after the pandemic. JAMA 325: 431–432, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam K, Lu AD, Shi Y, Covinsky KE: Assessing telemedicine unreadiness among older adults in the United States during the COVID-19 pandemic. JAMA Intern Med 180: 1389–1391, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abacus Data CMA : What Canadians think about virtual health care, 2020. Available at: https://www.cma.ca/sites/default/files/pdf/virtual-care/cma-virtual-care-public-poll-june-2020-e.pdf. Accessed April 12, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.