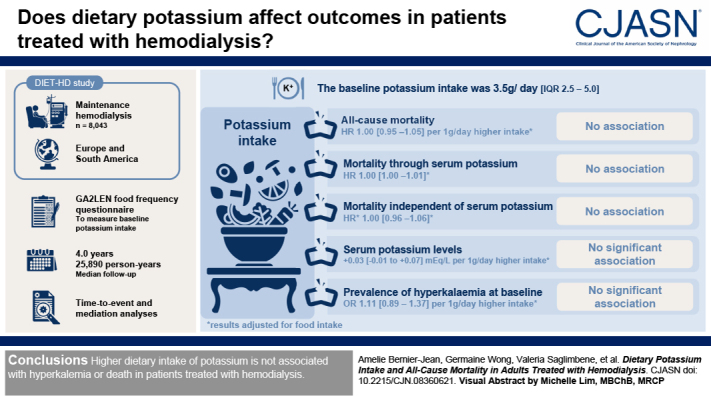
Visual Abstract
Keywords: hemodialysis, nutrition, mortality, potassium, dietary, cohort studies, diet
Abstract
Background and objectives
Dietary potassium restriction in people receiving maintenance hemodialysis is standard practice and is recommended in guidelines, despite a lack of evidence. We aimed to assess the association between dietary potassium intake and mortality and whether hyperkalemia mediates this association.
Design, setting, participants, & measurements
A total of 8043 adults undergoing maintenance hemodialysis in Europe and South America were included in the DIETary intake, death and hospitalization in adults with end-stage kidney disease treated with HemoDialysis (DIET-HD) study. We measured baseline potassium intake from the Global Allergy and Asthma European Network food frequency questionnaire and performed time-to-event and mediation analyses.
Results
The median potassium intake at baseline was 3.5 (interquartile range, 2.5–5.0) g/d. During a median follow-up of 4.0 years (25,890 person-years), we observed 2921 (36%) deaths. After adjusting for baseline characteristics, including cardiac disease and food groups, dietary potassium intake was not associated with all-cause mortality (per 1 g/d higher dietary potassium intake: hazard ratio, 1.00; 95% confidence interval [95% CI], 0.95 to 1.05). A mediation analysis showed no association of potassium intake with mortality, either through or independent of serum potassium (hazard ratio, 1.00; 95% CI, 1.00 to 1.00 and hazard ratio, 1.01; 95% CI, 0.96 to 1.06, respectively). Potassium intake was not significantly associated with serum levels (0.03; 95% CI, −0.01 to 0.07 mEq/L per 1 g/d higher dietary potassium intake) or the prevalence of hyperkalemia (≥6.0 mEq/L) at baseline (odds ratio, 1.11; 95% CI, 0.89 to 1.37 per 1 g/d higher dietary potassium intake). Hyperkalemia was associated with cardiovascular death (hazard ratio, 1.23; 95% CI, 1.03 to 1.48).
Conclusions
Higher dietary intake of potassium is not associated with hyperkalemia or death in patients treated with hemodialysis.
Introduction
Adequate intake of fruits and vegetables is encouraged in the general population to prevent noncommunicable diseases, such as cardiovascular disease and cancer (1). In contrast, people treated with maintenance hemodialysis are advised to limit intake of potassium-rich fruits and vegetables to prevent hyperkalemia, arrhythmia, and death (2,3). Limiting potassium intake to 2–2.5 g/d for patients undergoing hemodialysis is widely recommended, but based on little scientific evidence (4–7).
Recently, the lower certainty of the evidence supporting dietary potassium restriction has been recognized, together with the potentially detrimental effects of depriving patients on hemodialysis of the potential benefits associated with a potassium-replete diet, including better quality of life from fewer dietary restrictions (8).
We aimed to assess the association between dietary potassium intake and mortality in a large, multinational cohort of patients treated with maintenance hemodialysis and to define the extent to which serum potassium levels mediate any association between potassium intake and death.
Materials and Methods
Study Design
This is a substudy and mediation analysis of the DIETary intake, death and hospitalization in adults with end-stage kidney disease treated with HemoDialysis (DIET-HD) study, a prospective, multinational study evaluating the relationship between diet and adverse health outcomes in adults undergoing maintenance hemodialysis (9).
Study Population
Adults aged ≥18 years receiving hemodialysis treatments from a private dialysis provider network in Europe (France, Germany, Italy, Hungary, Poland, Portugal, Romania, Spain, Sweden, and Turkey) and South America (Argentina) were recruited between January 2014 and 2015. We excluded participants unable to complete a food frequency questionnaire (FFQ), those with limited life expectancy, or those expected to undergo kidney transplantation within 6 months. The study was in accordance with the Declaration of Helsinki. All relevant institutional ethics committees approved the study. All participants provided written informed consent.
Baseline Characteristics
Using data linkage, we extracted from the dialysis provider database information regarding sociodemographic characteristics, physical activity, education, living arrangements, smoking status, comorbidities, medication, BP, dialysis prescription, and vascular access. Blood samples were collected routinely before the midweek dialysis treatment. Sera were analyzed by indirect ion-selective electrodes on DxC 700 AU and AU 680 Chemistry Analyzers for European participants and on an AU5800 Chemistry Analyzer for South American participants (Beckman Coulter, Brea, CA). We recorded the blood results collected within 1 month of the FFQ completion.
Dietary Assessment
Participants completed the Global Allergy and Asthma European Network (GA2LEN) FFQ (Supplemental Appendix 1), which was designed and validated to assess the usual dietary intake of the past 12 months using a single standardized instrument. It comprises 32 food groups, including several whole fruits and vegetables, fruit juices, and many processed food items. It was designed to facilitate international comparisons across countries and has an intraclass correlation of 0.77–0.8 for potassium intake (10,11). FFQs with <80% responses recorded or with implausible total energy intake (defined as farther than three SDs from the mean after log transformation) were excluded. Daily food intake was calculated in grams per day from standard food portion sizes (12). Potassium intake was calculated from the latest available McCance and Widdowson food composition tables (13). The FFQ did not account for potassium additives, and the potassium intake measured in this study, therefore, reflects the intake of potassium that is naturally present in food items.
Outcomes
The outcomes of interest were all-cause mortality, cardiovascular mortality, and noncardiovascular mortality occurring before March 23, 2019, as recorded prospectively by the treating physician according to standardized definitions (14).
Statistical Analyses
We conducted the analysis in three steps. First, we assessed the association between dietary potassium intake and mortality in all eligible participants. Second, we conducted a mediation analysis within a subgroup of participants for which a measurement of serum potassium was available at baseline to assess the role of serum potassium as a mediator of the relationship between dietary potassium intake and mortality (Figure 1). Third, we conducted sensitivity analyses to assess the robustness of our findings to measurement error and unmeasured confounders. We assessed the potential effects of not adjusting for residual kidney function in greater detail because it is associated with a considerably lower risk of mortality (hazard ratio [HR] between 0.44 [15] to 0.70 [16]), making it a strong unmeasured confounder in our analysis. The details of the methods for each analysis are available in Supplemental Appendix 2.
Figure 1.
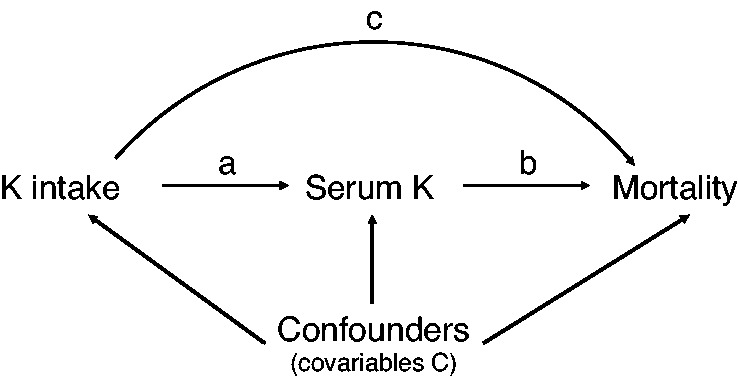
Simplified directed acyclic graph of the potential causal relationship between dietary potassium intake and mortality. The direct effect is represented by the arrow (c) and the indirect effect by the arrows (a) and (b). The measured potential confounders are included in the set of covariables C.
Cox proportional hazard models are most commonly used to assess time-to-event outcomes. However, they will produce biased estimates for mediation analysis of common outcomes (17). Because mortality was frequent in our cohort, we used accelerated time failure models to analyze the mortality outcomes. Accelerated time failure models directly regress the log of the survival time over the explanatory variables (18). They rely on the assumption that the exposure variable has a multiplicative effect on survival time that is constant through time and that the survival time follows a predefined distribution (19). Accelerated time failure models provide estimates called acceleration factors, which can be understood as the coefficient of that variable’s effect on survival time, such that an acceleration factor of two represents a doubling of the time to death and a mortality benefit. Acceleration factors and HRs, therefore, move in opposite directions and, when a Weibull distribution is fitted, acceleration factors and HRs can be converted into one another (20).
Models were sequentially adjusted, first for a set of potential confounders C selected on the basis of expert knowledge (age, sex, smoking status, body mass index, physical activity, presence of a life partner, Charlson comorbidity index, cardiac disease, diabetes, cancer, status on the transplant waiting list, vascular access, intradialytic weight loss, number of years on hemodialysis, weekly time on hemodialysis, Kt/V, serum albumin, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and total energy intake) and then for each food group (vegetables, fruits, grains, legumes and nuts, fish and white meat, red meat and meat products, dairy products, eggs, and sweets and sweetened drinks). The analysis adjusted for food groups was designed a priori to distinguish associations of dietary potassium intake from associations with fruit and vegetable intake, which were previously shown to be associated with lower mortality in this cohort (21) and with which dietary potassium intake is likely to be correlated. We conducted two post hoc exploratory analyses to further investigate the role of fiber and alkali intake (assessed using the net endogenous acid production estimated from the Remer and Manz equation [22]) and to distinguish the effect of dietary potassium derived from unprocessed plant sources (defined as whole fruits, whole vegetables, whole grain cereal products, and legumes and nuts) versus other sources of dietary potassium. We considered missing values for food groups as no intake (<0.1% missing) and imputed missing values for the remaining covariates using a multiple imputation chained equation (Supplemental Figure 1). Significance was set at 0.05, and hypothesis tests were two sided. We used the survival package (23) in R version 3.5.1 (July 2, 2018) for all analyses, except for the mediation analysis, for which we used the macro by Valeri and VanderWeele (24) in SAS (University Edition 3.8 Basic Edition).
Results
Baseline Characteristics
A total of 9690 patients completed the FFQ. Of these, 8043 participants (83%) had complete dietary data and outcome linkage and were included in analyses (Figure 2). Table 1 provides baseline characteristics per quartile of potassium intake.
Figure 2.
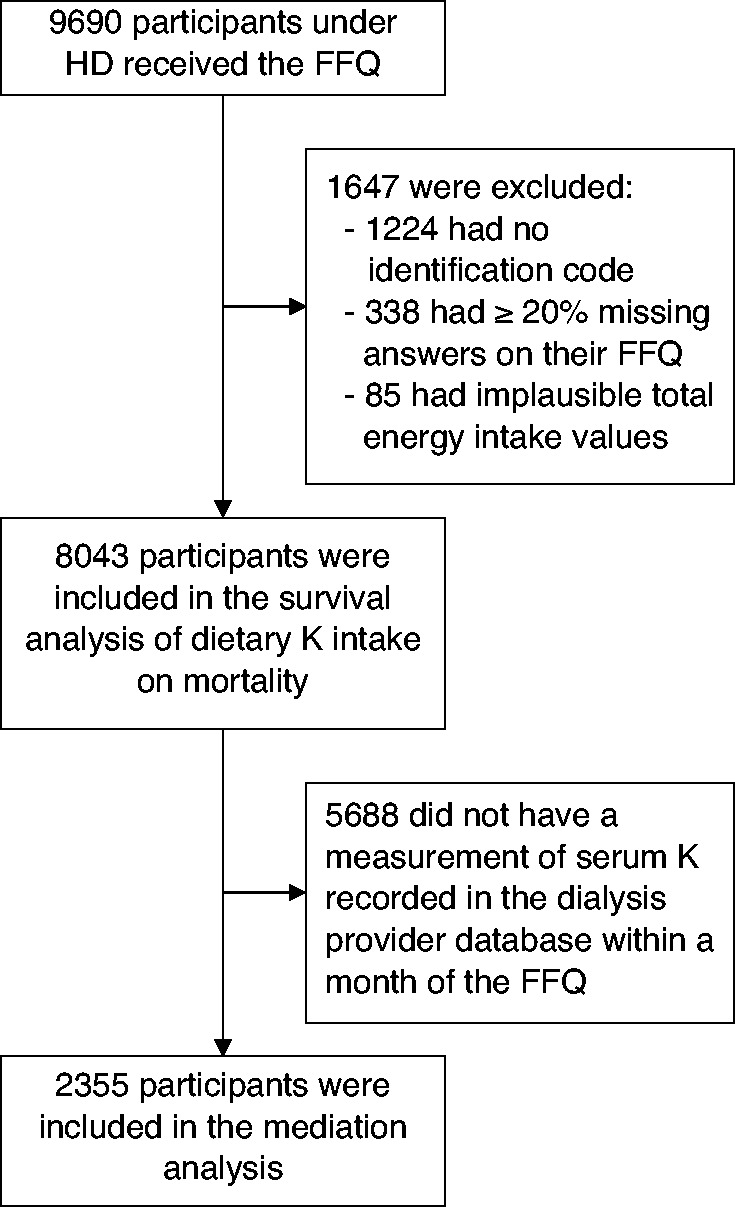
Flow chart of the study participants. FFQ, food frequency questionnaire; HD, hemodialysis; K, potassium.
Table 1.
Baseline characteristics of the participants to the DIETary intake, death and hospitalization in adults with end-stage kidney disease treated with HemoDialysis (DIET-HD) study
| Baseline Characteristics | Quartiles of Potassium Intake | Total | Missing (%) | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Quartile limits, g/d | <2.49 | 2.49–3.52 | 3.53–5.02 | >5.02 | ||
| Quartile, g/d, mean (SD) | 1.84 (0.46) | 3.00 (0.29) | 4.19 (0.43) | 7.18 (2.35) | ||
| N | 2011 | 2011 | 2010 | 2011 | 8043 | |
| Demographics | ||||||
| Age, yr | 0 | |||||
| <60 | 756 (38) | 681 (34) | 744 (37) | 843 (42) | 3024 (38) | |
| 60–80 | 1009 (50) | 1035 (52) | 985 (49) | 959 (48) | 3988 (50) | |
| >80 | 246 (12) | 295 (15) | 281 (14) | 209 (10) | 1031 (13) | |
| Men | 1113 (55) | 1151 (57) | 1175 (59) | 1219 (61) | 4658 (58) | 0 |
| Ethnicity | 8 | |||||
| White/Hispanic | 1733 (94) | 1753 (96) | 1785 (96) | 1821 (97) | 7092 (96) | |
| Black | 51 (3) | 49 (3) | 45 (2) | 29 (2) | 174 (2) | |
| Others | 51 (3) | 31 (2) | 31 (2) | 31 (2) | 144 (2) | |
| Body mass index, kg/m2 | 2 | |||||
| <18.5 | 99 (5) | 95 (5) | 86 (4) | 84 (4) | 364 (5) | |
| 18.5–25 | 850 (43) | 856 (43) | 811 (41) | 780 (40) | 3297 (42) | |
| 25–30 | 647 (33) | 639 (32) | 685 (35) | 679 (35) | 2650 (34) | |
| >30 | 366 (19) | 383 (19) | 375 (19) | 411 (21) | 1535 (20) | |
| Occupation status | 23 | |||||
| Working | 160 (12) | 178 (12) | 205 (13) | 149 (9) | 692 (11) | |
| Retired | 977 (71) | 1166 (75) | 1229 (75) | 1237 (78) | 4609 (75) | |
| Unemployed | 238 (17) | 207 (13) | 210 (13) | 208 (13) | 863 (14) | |
| Having a life partner | 930 (65) | 996 (66) | 1109 (69) | 1054 (70) | 4089 (68) | 25 |
| Secondary level education | 538 (40) | 642 (43) | 755 (47) | 727 (46) | 2662 (44) | 25 |
| Country | 0 | |||||
| Argentina | 497 (25) | 312 (16) | 226 (11) | 155 (8) | 1190 (15) | |
| France | 59 (3) | 63 (3) | 48 (2) | 50 (3) | 220 (3) | |
| Germany | 77 (4) | 59 (3) | 30 (2) | 11 (1) | 177 (2) | |
| Hungary | 72 (4) | 101 (5) | 143 (7) | 205 (10) | 521 (7) | |
| Italy | 143 (7) | 159 (8) | 151 (8) | 90 (5) | 543 (7) | |
| Poland | 124 (6) | 129 (6) | 117 (6) | 56 (3) | 426 (5) | |
| Portugal | 461 (23) | 557 (28) | 455 (23) | 302 (15) | 1775 (22) | |
| Romania | 131 (7) | 157 (8) | 263 (13) | 441 (22) | 992 (12) | |
| Spain | 190 (9) | 242 (12) | 303 (15) | 306 (15) | 1041 (13) | |
| Sweden | 6 (0) | 14 (1) | 19 (1) | 12 (1) | 51 (1) | |
| Turkey | 251 (13) | 218 (11) | 255 (13) | 383 (19) | 1107 (14) | |
| Lifestyle factors | ||||||
| Former or current smoker | 427 (31) | 533 (34) | 559 (34) | 537 (34) | 2056 (33) | 23 |
| Performs physical activity daily | 168 (12) | 197 (13) | 280 (17) | 272 (17) | 917 (15) | 24 |
| Dialysis | ||||||
| Etiology of kidney disease | 0 | |||||
| Diabetes | 497 (25) | 466 (23) | 485 (24) | 511 (25) | 1959 (24) | |
| Hypertension | 415 (21) | 384 (19) | 375 (19) | 379 (19) | 1553 (19) | |
| Glomerular disease | 562 (28) | 672 (33) | 659 (33) | 705 (35) | 2598 (32) | |
| Others | 537 (27) | 489 (24) | 491 (24) | 416 (21) | 1933 (24) | |
| >5 yr on HD | 766 (38) | 754 (38) | 727 (36) | 743 (37) | 2990 (37) | 0 |
| >12 h of HD per week | 278 (14) | 300 (15) | 325 (17) | 296 (15) | 1199 (15) | 2 |
| Kt/V≥1.4 | 1735 (89) | 1754 (89) | 1749 (90) | 1682 (87) | 6920 (89) | 3 |
| Dialyzed through AVF | 1577 (79) | 1585 (79) | 1619 (81) | 1686 (84) | 6467 (81) | 0.2 |
| Listed for transplant | 341 (17) | 376 (19) | 378 (19) | 382 (19) | 1477 (18) | 0.2 |
| Intradialytic weight loss, kg, mean (SD) | 3.0 (1.3) | 3.1 (1.3) | 3.1 (1.3) | 3.1 (1.4) | 3.1 (1.3) | 4 |
| Clinical characteristics | ||||||
| Charlson index >5 | 1119 (56) | 1164 (58) | 1198 (60) | 1082 (54) | 4563 (57) | 0 |
| Cardiac disease | 747 (44) | 778 (44) | 874 (50) | 859 (52) | 3258 (47) | 15 |
| Hypertension | 1519 (84) | 1576 (86) | 1574 (85) | 1490 (85) | 6159 (85) | 10 |
| Diabetes | 558 (31) | 594 (32) | 603 (33) | 567 (33) | 2322 (32) | 10 |
| Cancer | 225 (11) | 281 (14) | 280 (14) | 254 (13) | 1040 (13) | 0 |
| GI disease | 389 (19) | 449 (22) | 457 (23) | 461 (23) | 1756 (22) | 0 |
| Pulmonary disease | 219 (11) | 226 (11) | 260 (13) | 231 (12) | 936 (12) | 0 |
| Predialysis systolic BP, mm Hg, mean (SD) | 130 (22) | 130 (22) | 131 (22) | 134 (21) | 131 (22) | 16 |
| Receiving ACEI or ARB | 548 (27) | 625 (31) | 640 (32) | 627 (31) | 2440 (30) | 0 |
| >4 different drug classes | 429 (21) | 539 (27) | 640 (32) | 635 (32) | 2243 (28) | 0 |
| Predialysis blood results, mean (SD) | ||||||
| Potassium, mEq/L | 4.9 (0.7) | 5.0 (0.7) | 5.0 (0.7) | 5.1 (0.8) | 5.0 (0.7) | 71 |
| >5.5 | 138 (25) | 188 (27) | 172 (28) | 147 (31) | 645 (27) | 71 |
| >6 | 47 (9) | 75 (11) | 66 (11) | 48 (10) | 236 (10) | 71 |
| Calcium, mg/dl | 8.9 (0.7) | 9.0 (0.7) | 9.0 (0.7) | 8.9 (0.7) | 8.9 (0.7) | 3 |
| Phosphate, mg/dl | 4.6 (1.4) | 4.6 (1.4) | 4.7 (1.4) | 4.8 (1.4) | 4.7 (1.4) | 3 |
| Intact PTH, pg/ml | 455 (443) | 410 (366) | 419 (366) | 418 (375) | 427 (392) | 42 |
| Albumin, g/dl | 3.9 (0.4) | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.4) | 24 |
| Hemoglobin, g/dl | 11.0 (1.3) | 11.1 (1.3) | 11.1 (1.2) | 11.0 (1.3) | 11.1 (1.3) | 3 |
| Dietary assessment | ||||||
| Total energy intake (×103 kcal/d), median (IQR) | 1.2 (0.9–1.4) | 1.7 (1.4–2.0) | 2.1 (1.8–2.5) | 3.0 (2.5–3.7) | 1.9 (1.4–2.5) | 0 |
| Total energy intake per kg of body weight (kcal/kg), median (IQR) | 16.9 (12.4–21.9) | 24.7 (19.7–30.1) | 30.4 (24.5–37.5) | 43.2 (33.4–56.6) | 27.3 (19.6–37.5) | 2 |
| NPCR, mean (SD) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 3 |
| Fiber intake (g/d), median (IQR) | 7.1 (5.2–9.4) | 10.7 (8.2–13.5) | 13.9 (10.7–17.4) | 20.8 (15.6–27.1) | 12.0 (8.1–17.5) | 0 |
| Estimated NEAP, mean (SD)a | 49.2 (13.0) | 51.3 (18.8) | 49.8 (24.2) | 40.3 (46.7) | 47.7 (29.0) | 1 |
| Food groups (servings per day), median (IQR) | ||||||
| Cereals | 1.4 (0.8–2.4) | 2.1 (1.2–2.9) | 2.5 (1.5–3.6) | 3.2 (2.0–4.6) | 2.3 (1.2–3.3) | 0 |
| Fruits | 1.3 (0.7–2.1) | 2.1 (1.4–3.3) | 2.9 (1.9–4.6) | 5.1 (3.1–8.7) | 2.5 (1.4–4.4) | 0 |
| Vegetables | 1.9 (1.1–2.9) | 3.2 (2.1–4.4) | 4.4 (3.0–5.9) | 6.8 (4.8–9.6) | 3.6 (2.2–5.9) | 0 |
| Legumes and nuts | 0.1 (0.1–0.3) | 0.3 (0.1–0.4) | 0.4 (0.1–0.6) | 0.5 (0.3–1.1) | 0.3 (0.1–0.6) | 0 |
| Dairy | 0.8 (0.4–1.4) | 1.3 (0.7–2.1) | 1.6 (0.9–2.6) | 2.1 (1.1–3.3) | 1.4 (0.6–2.4) | 0 |
| Fish and white meat | 0.4 (0.1–0.6) | 0.6 (0.3–1.1) | 0.7 (0.4–1.3) | 1.0 (0.5–1.7) | 0.6 (0.3–1.1) | 0 |
| Red meat and meat | 0.6 (0.4–1.0) | 1.0 (0.6–1.6) | 1.2 (0.7–2.0) | 1.9 (1.0–3.1) | 1.1 (0.6–1.9) | 0 |
| Eggs | 0.1 (0.1–0.4) | 0.2 (0.1–0.4) | 0.4 (0.1–0.6) | 0.5 (0.2–0.9) | 0.3 (0.1–0.6) | 0.1 |
| Sweets and sweet drinks | 1.7 (0.8–2.9) | 2.4 (1.1–3.7) | 2.6 (1.4–4.1) | 3.4 (1.9–5.4) | 2.4 (1.2–4.1) | 0 |
All values are presented as n (%), unless otherwise indicated. HD, hemodialysis; AVF, arteriovenous fistula; GI, gastrointestinal; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; PTH, parathyroid hormone; IQR, interquartile range; NPCR, normalized protein catabolic rate; NEAP, net endogenous acid production.
Calculated from the Remer and Manz equation (22).
The median (interquartile range) potassium intake was 3.5 (2.5–5.0) g/d, and 1151 (14%) and 2024 (25%) participants consumed 2 g and ≤2.5 g/d, respectively (Supplemental Figure 2). There were more men in the higher quartile of potassium intake. Vegetables contributed more than any other food group to the dietary potassium intake (median [interquartile range] of individual intake, 26% [19%–33%]), followed by fruits and red meat (Supplemental Figure 3). After adjusting for total energy intake, the intake of vegetables, fruits, and legumes and nuts were significantly correlated with potassium intake (Supplemental Figure 4).
Survival Model of the Association between Potassium Intake and Mortality (n=8043)
During a median follow-up of 4.0 years (25,890 person-years), 2921 (36%) deaths occurred, including 1316 (45%) from cardiovascular causes. A total of 1033 participants (19%) were censored before March 2019.
Adjustment for the Set of Potential Confounders.
After adjustment for baseline characteristics, including the presence of cardiac disease and intradialytic weight change, higher dietary potassium intake was associated with a lower risk of noncardiovascular mortality but not of all-cause or cardiovascular mortality. For each gram per day of potassium intake, the time to noncardiovascular death was longer by a factor of 1.06 (95% confidence interval [95% CI], 1.02 to 1.11; P=0.01), which corresponded to an HR of 0.93 (95% CI, 0.88 to 0.98; Table 2).
Table 2.
Mortality acceleration factors and hazard ratios associated with 1 g/d higher dietary potassium intake or 1 mEq/L higher in serum potassium levels
| Analyses | N | All-Cause Mortality | Cardiovascular Mortality | Noncardiovascular Mortality | |||
|---|---|---|---|---|---|---|---|
| Acceleration Factor (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | Acceleration Factor (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | Acceleration Factor (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | ||
| Dietary potassium | |||||||
| Unadjusted | 8043 | 1.03 (1.02 to 1.05) | 0.97 (0.95 to 0.98) | 1.01 (0.98 to 1.03) | 0.99 (0.97 to 1.02) | 1.05 (1.03 to 1.07) | 0.94 (0.92 to 0.97) |
| Adjusted for Ca,b | 8043 | 1.01 (1.00 to 1.03) | 0.98 (0.97 to 1.00) | 1.01 (0.96 to 1.06) | 0.99 (0.94 to 1.05) | 1.06 (1.02 to 1.11) | 0.93 (0.88 to 0.98) |
| Adjusted for C and food groupsa,b | 8043 | 1.00 (0.96 to 1.04) | 1.00 (0.95 to 1.05) | 0.95 (0.90 to 1.02) | 1.05 (0.98 to 1.13) | 1.04 (0.98 to 1.10) | 0.96 (0.89 to 1.02) |
| Mediation analysisc | 2355 | ||||||
| Direct effect d | 0.99 (0.91 to 1.08) | 1.01 (0.96 to 1.06) | 1.04 (0.87 to 1.22) | 0.98 (0.91 to 1.07) | 0.97 (0.88 to 1.07) | 1.02 (0.97 to 1.08) | |
| Indirect effect e | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | 0.99 (0.98 to 1.00) | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) | |
| Total effect | 0.99 (0.90 to 1.08) | 1.01 (0.96 to 1.06) | 1.04 (0.86 to 1.21) | 0.98 (0.91 to 1.07) | 0.98 (0.88 to 1.07) | 1.01 (0.98 to 1.08) | |
| Serum potassium | |||||||
| Unadjusted | 2355 | 1.03 (0.95 to 1.12) | 0.97 (0.88 to 1.06) | 0.89 (0.76 to 1.05) | 1.12 (0.95 to 1.32) | 1.09 (1.00 to 1.20) | 0.90 (0.80 to 1.01) |
| Adjusted for Ca,b | 2355 | 0.97 (0.89 to 1.05) | 1.04 (0.94 to 1.15) | 0.83 (0.70 to 0.97) | 1.23 (1.03 to 1.47) | 1.03 (0.94 to 1.13) | 0.96 (0.85 to 1.09) |
| Adjusted for C, potassium intake, and food groupsa,b | 2355 | 0.98 (0.90 to 1.06) | 1.03 (0.93 to 1.14) | 0.82 (0.70 to 0.97) | 1.23 (1.03 to 1.48) | 1.04 (0.95 to 1.14) | 0.95 (0.84 to 1.07) |
Set of covariates C included: age, sex, smoking status, body mass index, physical activity, presence of a life partner, Charlson comorbidity index, history of cardiac disease, history of diabetes, history of cancer, listed for transplant, type of vascular access, body weight decrease during hemodialysis session, number of minutes of hemodialysis per week, hemodialysis vintage, Kt/V, receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, serum albumin, and total energy intake.
Clustered by country of origin.
Adjusted for C, the intake of each food group, and country of origin. The mediation analysis was not clustered by country of origin because the statistical software did not implement random effects.
Effect of dietary potassium on mortality not through serum potassium levels.
Effect of dietary potassium on mortality through serum potassium levels.
Adjustment for the Set of Potential Confounders and Food Groups.
After the analysis was further adjusted for intake of each food group in servings per day, dietary potassium was not associated with all-cause mortality (per gram per day: HR, 1.00; 95% CI, 0.95 to 1.05; P=0.94), cardiovascular mortality (per gram per day: HR, 1.05; 95% CI, 0.98 to 1.13; P=0.16), or noncardiovascular mortality (per gram per day: HR, 0.96; 95% CI, 0.89 to 1.02; P=0.19; Figure 3, Table 2). The intake of fruits (per serving per day: HR, 0.98; 95% CI, 0.97 to 1.00; P=0.03) and vegetables (per serving per day: HR, 0.98; 95% CI, 0.97 to 1.00; P=0.04) was associated with a lower risk of all-cause mortality (Supplemental Table 1).
Figure 3.
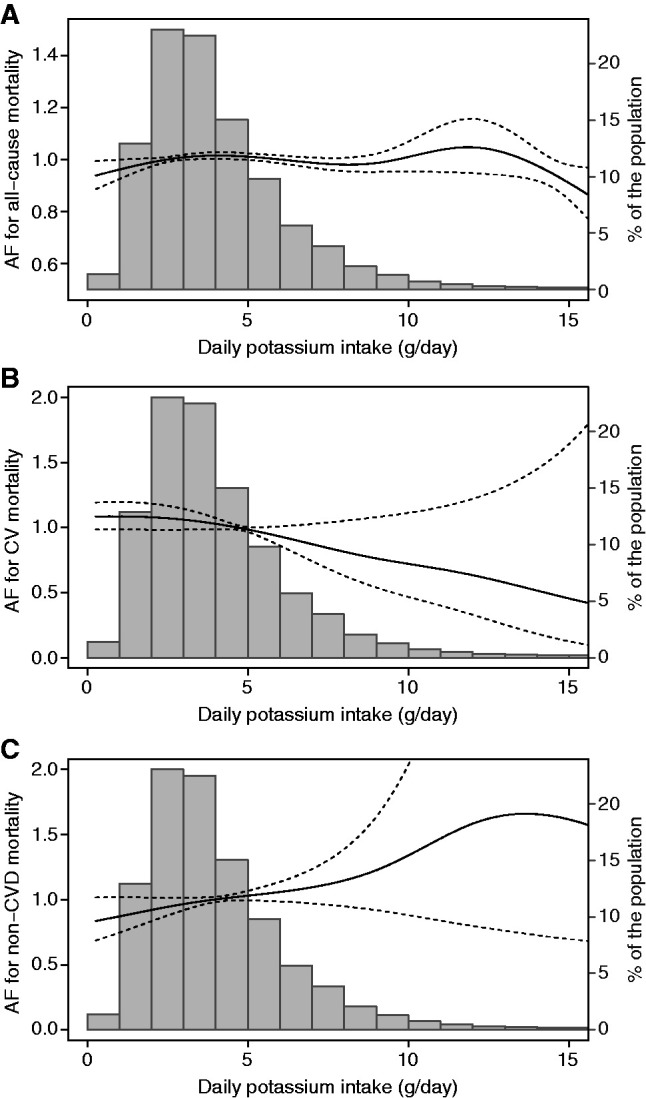
Association of dietary potassium intake (grams per day) with (A) all-cause mortality, (B) cardiovascular mortality, and (C) noncardiovascular mortality. Models adjusted for the age, sex, smoking status, body mass index, physical activity, presence of a life partner, Charlson comorbidity index, history of cardiac disease, history of diabetes, history of cancer, listed for transplant, type of vascular access, body weight decrease during HD session, number of minutes of HD per week, HD vintage, Kt/V, receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, serum albumin, total energy intake, and daily intake of each food group. AF, acceleration factor; CV, cardiovascular; CVD, cardiovascular disease.
The association between dietary potassium and mortality was not modified by the presence of cardiac disease, diabetes, hyperkalemia >5.5 mEq/L, or hyperkalemia >6.0 mEq/L at baseline (for interaction, P=0.81, 0.53, 0.52, and 0.25, respectively). Furthermore, the association was not modified by whether measurement of serum potassium was available at baseline (for interaction, P=0.33). We found similar results when dietary potassium was modeled as quartiles (Supplemental Table 2), as energy-adjusted potassium using the regression method, or as potassium density (Supplemental Table 3). Accounting for competing risks had little effect on the cumulative incidence function (Supplemental Figures 5 and 6).
Mediation Analysis
Baseline Serum Potassium Levels.
Predialysis baseline serum potassium levels were available for 2355 participants (29%). The likelihood of baseline serum potassium measurement was unbalanced toward certain geographic locations (Portugal [1708 participants; 73%] and Spain [5540 participants; 23%]), lower educational attainment, lower physical activity, higher prevalence of cancer and gastrointestinal disease, and higher consumption of fish and white meat (Supplemental Table 4). Mean (SD) predialysis baseline serum potassium was 5.02 (0.74) mEq/L. A total of 645 participants (27%) had levels >5.5 mEq/L, and 236 (10%) had levels >6.0 mEq/L.
Association between Dietary Potassium Intake and Serum Potassium Levels at Baseline.
Baseline serum potassium correlated poorly with dietary potassium intake (Spearman correlation coefficient=0.06; Supplemental Figure 7). After adjustment for the set of covariates, we found no cross-sectional association between dietary and serum potassium at baseline (per gram per day of dietary potassium intake: B=0.02; 95% CI, −0.01 to 0.05; P=0.22). After further adjustment for food groups, dietary potassium intake remained not associated with baseline serum potassium levels (per gram per day: B=0.03; 95% CI, −0.01 to 0.07; P=0.17). Dietary potassium intake was not associated with the prevalence of hyperkalemia at baseline, defined as ≥5.5 mEq/L (per gram per day: odds ratio, 1.05; 95% CI, 0.95 to 1.15) or ≥6.0 mEq/L (per gram per day: odds ratio, 1.04; 95% CI, 0.90 to 1.20; Table 3).
Table 3.
Cross-sectional associations of dietary potassium intake with serum potassium levels
| Analyses | N | Difference in Serum Potassium in mEq/L (95% Confidence Interval) | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Association of dietary potassium intake (g/d) with serum potassium level (or concentration) in mEq/L, continuous outcome | |||
| Unadjusted | 2355 | 0.011 (−0.003 to 0.020) | |
| Adjusted for Ca | 2355 | 0.02 (−0.01 to 0.05) | |
| Adjusted for C and food groupsa | 2355 | 0.03 (−0.01 to 0.07) | |
| Association of dietary potassium intake (g/d) with hyperkalemia, binary outcome | |||
| Defined as ≥5.5 mEq/L | |||
| Adjusted for C a | 2355 | 1.05 (0.95 to 1.15) | |
| Adjusted for C and food groups a | 2355 | 1.05 (0.92 to 1.21) | |
| Defined as ≥6.0 mEq/L | |||
| Adjusted for C a | 2355 | 1.04 (0.90 to 1.20) | |
| Adjusted for C and food groups a | 2355 | 1.11 (0.89 to 1.37) | |
Set of covariables C included: age, sex, smoking status, body mass index, physical activity, presence of a life partner, Charlson comorbidity index, history of cardiac disease, history of diabetes, history of cancer, listed for transplant, type of vascular access, body weight decrease during hemodialysis session, number of minutes of hemodialysis per week, hemodialysis vintage, Kt/V, receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, serum albumin, and total energy intake.
Association between Baseline Serum Potassium Level and Mortality.
During a median follow-up of 3.8 years (7273 person-years), 803 deaths occurred (33% cardiovascular) (Supplemental Figures 8 and 9). After adjustment for the set of covariates, high serum potassium at baseline was not associated with all-cause mortality (per mEq/L of serum potassium: HR, 1.04; 95% CI, 0.94 to 1.15), but was associated with a higher risk of cardiovascular death (per mEq/L of serum potassium: HR, 1.23; 95% CI, 1.03 to 1.47). Results were similar after further adjustment for food groups (Table 2). We did not observe a U-shaped relationship between serum potassium levels and all-cause and cause-specific mortality.
Estimates of the Direct, Indirect, and Total Effect.
After adjustment for the set of potential confounders and food groups, dietary potassium was not associated with mortality either through (indirect effect) or independent of (direct effect) serum potassium (Figure 1, Table 2). There was no evidence of an interaction between dietary potassium and serum potassium (P=0.64).
Assessment of Robustness to Unmeasured Confounding and Measurement Error.
First, adjustment for an extended set of covariates, identified by stepwise forward selection, resulted in similar results (Supplemental Table 5). Second, assuming that individuals with significant residual kidney function have more liberal diets and higher potassium intake, we calculated that not adjusting for residual kidney function would overestimate the acceleration factor for the direct effect and underestimate the acceleration factor for the indirect effect (Supplemental Table 6, B and C). A scenario where potassium intake is protective through increased serum potassium appears implausible. Third, for all levels of correlation between the error term of the outcome model and the error term of the mediator model, the CI for the direct and indirect effect always included 1.0, which signifies that an unmeasured confounder of the mediator-outcome relationship was unlikely to bias our results significantly (Supplemental Figure 10). Finally, using Simulation-Extrapolation, we found that even large nondifferential measurement errors of potassium intake and serum potassium levels would not change our conclusions (Supplemental Figure 11, Supplemental Table 7). A more extensive discussion of the sensitivity analyses' results is available in Supplemental Appendix 3.
Post Hoc Exploratory Analyses.
Further adjustment for fiber and alkali intake on the analysis adjusted for the set of covariables C provided similar findings (Supplemental Table 8). Upon dividing the potassium intake between whole plant sources versus other sources, potassium intake from whole plant sources was associated with a lower risk of all-cause and cause-specific mortality (Supplemental Table 9). Similarly to our analysis of the total potassium intake, this association was absent after adjusting for food groups.
Discussion
In this large cohort of adults receiving maintenance hemodialysis, we found no evidence of an independent association between the food-derived potassium intake and all-cause or cause-specific mortality (Supplemental Appendix 3). Furthermore, we did not find a significant association between dietary potassium intake and baseline serum potassium levels. In a mediation analysis, we found no evidence of an association of dietary potassium with mortality either through or independent of serum potassium. In line with previous literature, higher baseline serum potassium levels were associated with a greater risk of cardiovascular mortality (25–30).
Two prior longitudinal studies of potassium intake in adults receiving hemodialysis treatments have reported conflicting results. The Nutritional and Inflammatory Evaluation in Dialysis (NIED) study (224 participants) reported a 2.4-fold (95% CI, 1.1- to 7.5-fold) higher risk of death among the highest quartile of potassium intake (31). Contrastingly, the Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study (415 participants) found a 2.7 (95% CI, 1.4 to 5.0) higher risk of death among those in the lowest quartile of potassium intake (32). In our substantially larger population of 8043 adults (25,890 person-years) treated with hemodialysis, before adjusting for food groups, we found a lower risk of noncardiovascular death and a trend toward lower all-cause mortality among the participants with the highest potassium intake, in accordance with the MADRAD study (32). In post hoc analysis, this lower mortality risk was associated with the potassium intake from whole plant sources only. After adjustment for food groups, including the intake of fruits and vegetables, this association was no longer present. The potential benefits of a potassium-rich diet are inseparable from diets rich in fruits and vegetables, and healthy dietary patterns associated with potassium intake are likely responsible for the association observed in our study. In the NIED study, potassium mostly came from beef, chicken, Mexican food, hamburgers, legumes, fresh fruits, fruit juices, fried potatoes, cheeseburgers, and canned fruits (31). In contrast, the primary sources of potassium in our cohort were vegetables, fresh fruits, red meat, potatoes, milk, and bread. Thus, potassium intake may have acted as a proxy for less healthy dietary patterns and explained the higher risk of death observed in the NIED cohort.
Our findings concur with several studies reporting either weak or no associations between dietary and serum potassium levels in adults undergoing hemodialysis. The NIED study found FFQ-measured potassium intake to explain only 2% of the variance in serum potassium (31). The BalanceWise study found no correlation between potassium intake from three 24-hour dietary recalls and serum potassium (33). A cross-sectional analysis of 212 people with CKD (117 of whom were receiving hemodialysis) found 3-day food record–measured potassium intake not to be associated with the serum potassium levels or the prevalence of hyperkalemia (34). Finally, in the MADRAD study, serum potassium levels and the dialysate potassium concentration were similar across tertile of dietary potassium intake (32).
It is important to note that neither our study, nor those mentioned above, measured potassium additives, which can substantially increase the potassium content of foods (35,36). Therefore, it is crucial to interpret our findings as relating solely to the potassium naturally occurring in food items and not to artificially added potassium salts—the safety of which is more concerning because they are likely more bioavailable (37,38).
In two studies conducted in the 1940s, oral potassium chloride and oral potassium bicarbonate loading led to increased serum potassium in four out of five and nine out of ten individuals, respectively, with kidney disease (39,40). Considering that the amount of potassium ingested in these experiments exceeded what can usually be achieved through diet alone and that, unlike potassium salts, dietary potassium is coingested with carbohydrates and fiber, which cause an intracellular potassium shift from insulin secretion (41,42) and an increase in colonic potassium secretion (33,43,44), respectively, there will likely be a point at which dietary potassium intake will lead to hyperkalemia. Still, the current approach to hyperkalemia focuses disproportionally on the restriction of potassium-rich foods (3,45), particularly fruits and vegetables (3), thereby eclipsing interventions that may reduce serum potassium levels with less influence on patients’ quality of life (8).
Dietary restrictions significantly contribute to the burden of hemodialysis (46). Over half of patients on hemodialysis report feeling deprived and finding the prescribed diet bland and tasteless (47). Furthermore, strict dietary restrictions can lead to malnutrition and, potentially, lower quality of life among patients on hemodialysis (48). Dietary restrictions are, therefore, not without the potential for harm and must be supported by quality evidence.
This study had considerable strengths. DIET-HD is the most extensive assessment of the association between dietary potassium and mortality in adults undergoing hemodialysis. We recruited participants across many countries and considered multiple potential confounders. Furthermore, we conducted extensive sensitivity analyses and found our results robust against potential unmeasured confounders and measurement error.
This study had limitations. FFQs are subject to recall bias, and food composition tables can be inaccurate (8). Furthermore, although the FFQ was validated in a sample of European adults, it was not validated in patients on hemodialysis per se (35,36,49). Repeated FFQs would have improved the reliability of the dietary assessment, and medications that may alter potassium absorption, such as laxatives and potassium binders, were not considered. Collectively, these limitations may have led to measurement error and misclassification of the exposures. The FFQ assessed the average potassium intake in the year before completion, and this study was, therefore, not designed to assess the potential immediate consequences of an acute increase in dietary potassium intake. Serum potassium assessment was limited to a single measurement at baseline, which was only available for a subgroup of the study population. However, interaction with whether a serum potassium measurement was available was NS. Although sensitivity analyses suggested that residual kidney function and dialysate potassium concentration were unlikely to bias our results significantly, their complex interactions with dietary intake may not be fully considered. Finally, the external validity of our results to populations with distinct dietary habits, and to countries with differing life expectancies on hemodialysis, may be limited.
In conclusion, in this large cohort of adults treated with maintenance hemodialysis, we found no evidence that a higher dietary potassium intake was associated with higher mortality. Our results suggest that stringent dietary potassium restrictions may not be needed for most patients on hemodialysis. A diet more liberal in fruits and vegetables may provide health benefits and may lessen the burden of unduly stringent dietary restrictions. At this point, randomized controlled trials are warranted to determine whether diets proven to improve health outcomes in other populations are safe and advantageous for adults receiving maintenance hemodialysis.
Disclosures
A. Bernier-Jean was supported by a scholarship from the NHMRC grant GNT1151246 for the completion of this study. J.C. Craig reports serving as coordinating editor of Cochrane Kidney and Transplant, on the editorial boards of Diagnostic and Prognostic Research and Journal of Clinical Epidemiology, and as vice president of Flinders University. J. Hegbrant reports having ownership interest in Diaverum AB and Triomed AB, being employed by JBA Medical AB, and serving as a scientific advisor or member of Redsense Medical AB. D.W. Johnson reports serving as a scientific advisor or member of American Journal of Kidney Disease, Australian and New Zealand Society of Nephrology (councillor), CJASN, Cochrane Kidney and Transplant Group, International Society of Nephrology (past councillor), International Society of Peritoneal Dialysis (as immediate past president), National Health and Medical Research Council (NHMRC) Academy, and Peritoneal Dialysis International; having other interests in/relationships with Amgen (accommodation sponsorship), Australian and New Zealand Society of Nephrology (as president), and Kidney Health Australia (advisor); having consultancy agreements with AstraZeneca, AWAK, Bayer, and Lilly; receiving research funding from Baxter and Fresenius; receiving honoraria from Baxter, Fresenius, and Ono; and serving on a speakers bureau for Baxter Healthcare and Fresenius Medical Care. A. Teixeira-Pinto reports serving as a scientific advisor or member of the American Health Association. M. Tonelli reports serving as a scientific advisor or member of American Journal of Kidney Diseases, Kidney Diseases, Kidney Disease Improving Global Outcomes, and Kidney International; receiving honoraria from AstraZeneca (lecture fees); receiving a lecture fee from B. Braun in 2019 (the fee was donated to charity); and having other interests in/relationships with Canadian Institutes of Health Research. All remaining authors have nothing to disclose.
Funding
This work was supported by the provider of renal services Diaverum, which funded overhead costs for study coordinators in each contributing country and material printing. A. Bernier-Jean was supported by a scholarship from the NHMRC grant GNT1151246 for the completion of this study.
Supplementary Material
Acknowledgments
We would like to thank Dr. Louisa Hills Smith and Professor Tyler J. VanderWeele for their assistance in assessing the applicability of their method for sensitivity analysis to our analysis.
The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of the NHMRC.
A. Bernier-Jean, G. Wong, V. Saglimbene, J.C. Craig, and G.F.M. Strippoli were responsible for conceptualization and design; M. Ruospo, P. Natale, and M. Tonelli were responsible for data acquisition; A. Bernier-Jean, G. Wong, V. Saglimbene, A. Teixeira-Pinto, and G.F.M. Strippoli were responsible for data analysis; all authors were responsible for data interpretation; G.F.M. Strippoli, J.C. Craig, and G. Wong provided study supervision and mentorship; and each author contributed important intellectual content during manuscript drafting or revision and gave final approval of the version to be submitted.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Should We Let Dialysis Patients Eat Their Fruits and Veggies?,” on pages 1781–1783.
Data Sharing Statement
Individual participant data that underlie the results reported in this article after deidentification are available to researchers who provide a methodologically sound proposal. Proposals should be directed to Professor G.F.M. Strippoli at gfmstrippoli@gmail.com. To gain access, data requestors will need to sign a data access agreement.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08360621/-/DCSupplemental.
Supplemental Appendix 1. The GA2LEN Food frequency questionnaire.
Supplemental Appendix 2. Statistical methods.
Supplemental Appendix 3. Supplemental discussion for the results of the sensitivity analyses.
Supplemental Figure 1. Patterns of missingness for the imputed variables.
Supplemental Figure 2. Boxplots of the dietary potassium intake in gram per day by country.
Supplemental Figure 3. Boxplots of the relative contribution of each food group to the total potassium intake.
Supplemental Figure 4. Correlation between the energy-adjusted intake from each food group and the energy-adjusted potassium intake.
Supplemental Figure 5. Comparison of cumulative incidence function of all-cause mortality with and without adjustment for the competing risk of transplantation.
Supplemental Figure 6A. Comparison of cumulative incidence function of cardiovascular mortality with and without adjustment for the competing risk of noncardiovascular mortality.
Supplemental Figure 6B. Comparison of cumulative incidence function of noncardiovascular mortality with and without adjustment for the competing risk of cardiovascular mortality.
Supplemental Figure 7. Correlation between dietary potassium intake (g/d) and serum potassium levels (mEq/L).
Supplemental Figure 8. Cubic splines of dietary (exposure) and serum (mediator) potassium with mortality outcomes without adjustment for covariates.
Supplemental Figure 9. Association of baseline serum potassium levels (mEq/L) with (a) all-cause mortality, (b) cardiovascular mortality, and (c) noncardiovascular mortality.
Supplemental Figure 10. Sensitivity analysis for the direct (ADE) and the indirect (ACME) effect using the correlation between error terms.
Supplemental Figure 11. Plot of the SIMEX extrapolation curve for the assessment of measurement error.
Supplemental Table 1. Mortality acceleration factors and hazard ratios associated with all the variables (C and food groups).
Supplemental Table 2. Mortality acceleration factors and hazard ratios associated with quartiles of dietary potassium intake.
Supplemental Table 3. Mortality acceleration factors and hazard ratios associated with energy-adjusted potassium intake and potassium density.
Supplemental Table 4. Baseline characteristics of the DIET-HD participants with and without a measurement of serum potassium.
Supplemental Table 5. Mediation analysis adjusted for the extended set of covariables (sensitivity analysis).
Supplemental Table 6A. Estimates of the total, direct, and indirect effect for all-cause mortality corrected for an unmeasured confounder.
Supplemental Table 6B. Estimates of the total, direct, and indirect effect for cardiovascular mortality corrected for an unmeasured confounder.
Supplemental Table 6C. Estimates of the total, direct, and indirect effect for noncardiovascular mortality corrected for an unmeasured confounder.
Supplemental Table 7. Estimates of the total, direct, and indirect effects corrected for nondifferential measurement error using Simulation-Extrapolation (SIMEX).
Supplemental Table 8. Exploratory analyses adjusted for the set of covariables C and fiber and alkali intake.
Supplemental Table 9. Exploratory analysis of the association of dietary potassium intake (g/d) from unprocessed plant sources versus other sources of dietary potassium with mortality.
References
- 1.World Health Organization : Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Geneva, Switzerland, World Health Organization, 2003 [Google Scholar]
- 2.National Kidney Foundation : Dietary guidelines for adults starting on hemodialysis, 2019.. Available at: https://www.kidney.org/atoz/content/dietary_hemodialysis. Accessed April 2, 2020
- 3.Picard K, Griffiths M, Mager DR, Richard C: Handouts for low-potassium diets disproportionately restrict fruits and vegetables. J Ren Nutr 31: 210–214, 2021 [DOI] [PubMed] [Google Scholar]
- 4.James G, Jackson H: European guidelines for the nutritional care of adult renal patients. EDTNA ERCA J 29: 23–43, 2003 [Google Scholar]
- 5.Daugirdas JT, Blake PG, Ing TS: Handbook of Dialysis, Philadelphia, Lippincott Williams & Wilkins, 2001 [Google Scholar]
- 6.Ash S, Campbell K, MacLaughlin H, McCoy E, Chan M, Anderson K, Corke K, Dumont R, Lloyd L, Meade A, Montgomery-Johnson R, Tasker T, Thrift P, Trotter B: Evidence based practice guidelines for the nutritional management of chronic kidney disease. Nutr Diet 63: S33–S45, 2006 [Google Scholar]
- 7.Academy of Nutrition and Dietetics : Chronic Kidney Disease Guideline: Executive Summary of Recommendations, 2010. Available at: https://www.andeal.org/topic.cfm?menu=5303&pcat=3927&cat=3929. Accessed April 2, 2020
- 8.Clase CM, Carrero J-J, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R, Participants C, Ashuntantang GE, Bakker SJL, Bakris GL, Bhandari S, Burdmann EA, Campbell KL, Charytan DM, Clegg DJ, Cuppari L, Goldsmith D, Hallan SI, He J, Herzog CA, Hoenig MP, Hoorn EJ, Leipziger JG, Leonberg-Yoo AK, Lerma EV, Lopez-Almaraz JE, Małyszko J, Mann JFE, Marklund M, McDonough AA, Nagahama M, Navaneethan SD, Pitt B, Pochynyuk OM, de Moraes TP, Rafique Z, Robinson BM, Roger SD, Rossignol P, Singer AJ, Smyth A, Sood MM, Walsh M, Weir MR, Wingo CS: Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 97: 42–61, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Ruospo M, Campbell KL, Garcia Larsen V, Saglimbene V, Natale P, Gargano L, Craig JC, Johnson DW, Tonelli M, Knight J, Bednarek-Skublewska A, Celia E, Del Castillo D, Dulawa J, Ecder T, Fabricius E, Frazão JM, Gelfman R, Hoischen SH, Schön S, Stroumza P, Timofte D, Török M, Hegbrant J, Wollheim C, Frantzen L, Strippoli GFM; DIET-HD Study investigators : Nutrition and dietary intake and their association with mortality and hospitalisation in adults with chronic kidney disease treated with haemodialysis: Protocol for DIET-HD, a prospective multinational cohort study. BMJ Open 5: e006897, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Larsen V, Luczynska M, Kowalski ML, Voutilainen H, Ahlström M, Haahtela T, Toskala E, Bockelbrink A, Lee HH, Vassilopoulou E, Papadopoulos NG, Ramalho R, Moreira A, Delgado L, Castel-Branco MG, Calder PC, Childs CE, Bakolis I, Hooper R, Burney PG; GA2LEN-WP 1.2 ‘Epidemiological and Clinical Studies’ : Use of a common food frequency questionnaire (FFQ) to assess dietary patterns and their relation to allergy and asthma in Europe: Pilot study of the GA2LEN FFQ. Eur J Clin Nutr 65: 750–756, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Brussaard JH, Johansson L, Kearney J; EFCOSUM Group : Rationale and methods of the EFCOSUM project. Eur J Clin Nutr 56: S4–S7, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Food Standards Agency : Food Portion Sizes (Maff Handbook), London, The Stationery Office (TSO), 2002 [Google Scholar]
- 13.Roe M, Pinchen H, Church S, Finglas P: McCance and Widdowson’s The Composition of Foods Seventh Summary Edition and updated Composition of Foods Integrated Dataset. Nutr Bull 40: 36–39, 2015 [Google Scholar]
- 14.United States Renal Data System : ESRD Analytical Methods, 2017. Available at: https://www.usrds.org/media/1684/v2_c13_esrdmethods_17.pdf. Accessed January 9, 2017
- 15.Shemin D, Bostom AG, Laliberty P, Dworkin LD: Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 38: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J: Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 56: 348–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderweele TJ: Explanation in Causal Inference, Cary, NC, Oxford University Press, 2015 [Google Scholar]
- 18.Wei LJ: The accelerated failure time model: A useful alternative to the Cox regression model in survival analysis. Stat Med 11: 1871–1879, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Swindell WR: Accelerated failure time models provide a useful statistical framework for aging research. Exp Gerontol 44: 190–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein JP, Moeschberger ML: Survival Analysis: Techniques for Censored and Truncated Data, New York, Springer-Verlag, 2003 [Google Scholar]
- 21.Saglimbene VM, Wong G, Ruospo M, Palmer SC, Garcia-Larsen V, Natale P, Teixeira-Pinto A, Campbell KL, Carrero JJ, Stenvinkel P, Gargano L, Murgo AM, Johnson DW, Tonelli M, Gelfman R, Celia E, Ecder T, Bernat AG, Del Castillo D, Timofte D, Török M, Bednarek-Skublewska A, Duława J, Stroumza P, Hoischen S, Hansis M, Fabricius E, Felaco P, Wollheim C, Hegbrant J, Craig JC, Strippoli GFM: Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 14: 250–260, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parmenter BH, Slater GJ, Frassetto LA: Accuracy and precision of estimation equations to predict net endogenous acid excretion using the Australian food database. Nutr Diet 74: 308–312, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM, Lumley T, Elizabeth A, Cynthia C: Survival: Survival Analysis, 2020. Available at: https://cran.r-project.org/web/packages/survival/survival.pdf. Accessed April, 3, 2020
- 24.Valeri L, VanderWeele TJ: SAS macro for causal mediation analysis with survival data. Epidemiology 26: e23–e24, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Kim T, Rhee CM, Streja E, Soohoo M, Obi Y, Chou JA, Tortorici AR, Ravel VA, Kovesdy CP, Kalantar-Zadeh K: Racial and ethnic differences in mortality associated with serum potassium in a large hemodialysis cohort. Am J Nephrol 45: 509–521, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Kang E, Yoo KD, Choi Y, Kim DK, Joo KW, Yang SH, Kim Y-L, Kang S-W, Yang CW, Kim NH, Kim YS, Lee H: Lower serum potassium associated with increased mortality in dialysis patients: A nationwide prospective observational cohort study in Korea. PLoS One 12: e0171842, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB: Serum potassium levels and mortality in hemodialysis patients: A retrospective cohort study. Am J Nephrol 44: 179–186, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K: Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2: 999–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Iseki K, Uehara H, Nishime K, Tokuyama K, Yoshihara K, Kinjo K, Shiohira Y, Fukiyama K: Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis 28: 541–548, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Unruh ML, Evans IV, Fink NE, Powe NR, Meyer KB; Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study : Skipped treatments, markers of nutritional nonadherence, and survival among incident hemodialysis patients. Am J Kidney Dis 46: 1107–1116, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD: Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis 56: 338–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narasaki Y, Okuda Y, Kalantar SS, You AS, Novoa A, Nguyen T, Streja E, Nakata T, Colman S, Kalantar-Zadeh K, Nguyen DV, Rhee CM: Dietary potassium intake and mortality in a prospective hemodialysis cohort. J Ren Nutr 31: 411–420, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St-Jules DE, Goldfarb DS, Sevick MA: Nutrient non-equivalence: Does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr 26: 282–287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos CI, González-Ortiz A, Espinosa-Cuevas A, Avesani CM, Carrero JJ, Cuppari L: Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol Dial Transplant 36: 2049–2057, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Parpia AS, Goldstein MB, Arcand J, Cho F, L’Abbé MR, Darling PB: Sodium-reduced meat and poultry products contain a significant amount of potassium from food additives. J Acad Nutr Diet 118: 878–885, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Parpia AS, L’Abbé M, Goldstein M, Arcand J, Magnuson B, Darling PB: The impact of additives on the phosphorus, potassium, and sodium content of commonly consumed meat, poultry, and fish products among patients with chronic kidney disease. J Ren Nutr 28: 83–90, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Picard K: Potassium additives and bioavailability: Are we missing something in hyperkalemia management? J Ren Nutr 29: 350–353, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Naismith DJ, Braschi A: An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int J Food Sci Nutr 59: 438–450, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Winkler AW, Hoff HE, Smith PK: The toxicity of orally administered potassium salts in renal insufficiency. J Clin Invest 20: 119–126, 1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keith NM, Osterberg AE: The tolerance for potassium in severe renal insufficiency; A study of 10 cases. J Clin Invest 26: 773–783, 1947 [PubMed] [Google Scholar]
- 41.Allon M, Dansby L, Shanklin N: Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am J Med 94: 475–482, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Alvestrand A, Wahren J, Smith D, DeFronzo RA: Insulin-mediated potassium uptake is normal in uremic and healthy subjects. Am J Physiol 246: E174–E180, 1984 [DOI] [PubMed] [Google Scholar]
- 43.Hayes CP Jr, McLeod ME, Robinson RR: An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 80: 207–216, 1967 [PubMed] [Google Scholar]
- 44.Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI: Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 45.National Kidney Foundation : Hyperkalemia: Survey of Awareness and Experience among Adults with CKD: A Report of Findings, 2017. Available at: https://www.kidney.org/sites/default/files/HyperkalemiaReport1.pdf. Accessed June 7, 2020
- 46.Palmer SC, Hanson CS, Craig JC, Strippoli GFM, Ruospo M, Campbell K, Johnson DW, Tong A: Dietary and fluid restrictions in CKD: A thematic synthesis of patient views from qualitative studies. Am J Kidney Dis 65: 559–573, 2015 [DOI] [PubMed] [Google Scholar]
- 47.St-Jules DE, Woolf K, Pompeii ML, Sevick MA: Exploring problems in following the hemodialysis diet and their relation to energy and nutrient intakes: The BalanceWise Study. J Ren Nutr 26: 118–124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalantar-Zadeh K, Tortorici AR, Chen JLT, Kamgar M, Lau WL, Moradi H, Rhee CM, Streja E, Kovesdy CP: Dietary restrictions in dialysis patients: Is there anything left to eat? Semin Dial 28: 159–168, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherman RA, Mehta O: Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin J Am Soc Nephrol 4: 1370–1373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


