Visual Abstract
Keywords: acute renal failure, genetic renal disease, COVID-19, apolipoprotein L1, acute kidney injury
Abstract
Background and objectives
Black Americans have a higher incidence of kidney disease compared with populations that do not have recent African ancestry. Two risk variants in the APOL1 are responsible for a portion of this higher risk. We sought to assess the odds of AKI conferred by APOL1 risk alleles in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Design, setting, participants, & measurements
Black Americans who tested positive for coronavirus disease 2019 (COVID-19) were genotyped to determine APOL1 risk allele status. We assessed the incidence of AKI, persistent AKI, and AKI requiring KRT within 21 days of the PCR-based diagnosis. Outcomes were adjusted for age, sex, body mass index, hypertension, eGFR, and use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Results
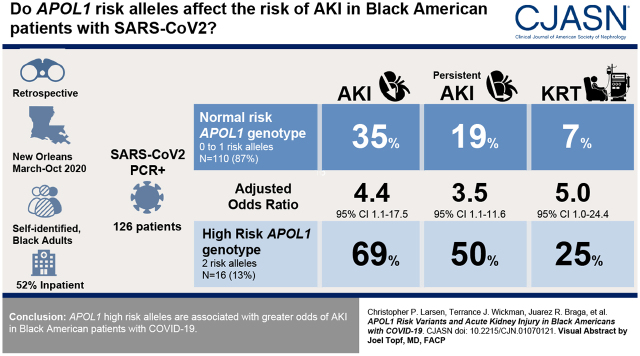
In total, 126 cases of SARS-CoV-2 infection were included within a 5-month period, with 16 (13%) and 110 (87%) cases with two and zero/one APOL1 high-risk alleles, respectively. AKI occurred in 11 (69%) patients with two APOL1 high-risk alleles and 39 (35%) patients with zero/one high-risk alleles (adjusted odds ratio, 4.41; 95% confidence interval, 1.11 to 17.52; P=0.04). Persistent AKI occurred in eight (50%) patients with two APOL1 high-risk alleles and 21 (19%) of those with zero/one high-risk alleles (adjusted odds ratio, 3.53; 95% confidence interval, 1.8 to 11.57; P=0.04). AKI KRT occurred in four (25%) of those with two APOL1 high-risk alleles and eight (7%) of those with zero/one high-risk alleles (adjusted odds ratio, 4.99; 95% confidence interval, 1.02 to 24.4, P=0.05).
Conclusions
APOL1 high-risk alleles are associated with greater odds of AKI in Black American patients with COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19) was initially recognized as a pulmonary disease, with treatment and research efforts focused on diffuse alveolar damage and respiratory failure (1). Awareness of the systemic manifestations of COVID-19 has steadily grown as the pandemic has progressed, and the kidney has emerged as one of the extrapulmonary organs most commonly affected. A recent meta-analysis that examined 21,531 patients in 51 studies found the pooled incidence of AKI to be 12%, with lower rates in Asia (7%) compared with Europe (23%) and North America (35%) (2). Development of AKI while hospitalized with COVID-19 is a serious complication as it has been shown to be associated with a higher mortality rate (3). AKI in the setting of COVID-19 represents an important public health concern that needs focused prevention and intervention to mitigate adverse outcomes.
Black Americans are well known to have higher incidence rates of CKD compared with populations that do not have recent African ancestry (4). Two coding risk variants in the APOL1 gene, termed G1 and G2, are responsible for a portion of the higher incidence of progressive nondiabetic kidney disease in Black Americans relative to European Americans (5,6). The clinicopathologic spectrum of APOL1-associated kidney disease includes FSGS, nondiabetic kidney failure often attributed to systemic hypertension, and collapsing glomerulopathy in the setting of systemic diseases including SLE (7,8) and HIV (4,9). More recently, the occurrence of collapsing glomerulopathy in Black American patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been reported, an entity that has been named COVID-19–associated nephropathy (10–12). Other reports have shown that Black Americans have a significantly higher risk of AKI in the setting of COVID-19 (3). However, it is unclear if it is the presence of APOL1 risk alleles that influences kidney outcomes among Black American individuals with COVID-19. Therefore, we designed this study to assess if the presence of two APOL1 risk alleles was associated with a higher likelihood of developing AKI and proteinuria among those with SARS-CoV-2 infection.
Materials and Methods
Study Design and Participants
This was a single-center retrospective study cohort of individuals with SARS-CoV-2 infection between March 13, 2020 and October 1, 2020 in New Orleans, Louisiana, United States. To create the study cohort, we included self-identified Black American adults who tested positive for SARS-CoV-2 RNA by PCR by nasopharyngeal swab. Individuals younger than 18 years of age, with presence of kidney failure on long-term dialysis, with a history of dialysis, and with a kidney transplant were excluded.
Standard procedure at the Central Laboratory at Ochsner Medical Center is to store blood specimens for 6 days after being collected. After that, the specimens are routinely discarded. The Ochsner Biorepository Unit has an institutionally approved research protocol that allows access to those blood specimens with a waiver of consent as long as they are retrieved from the laboratory right before disposal. Thus, whole-blood specimens were made available to the research team only on the sixth day postcollection before being discarded. Our research personnel was only available to screen and collect specimens once weekly. Thus, the remainder of the days of the week, the specimens were discarded without undergoing screening by the research personnel. The research personnel had no knowledge about the origin or number of specimens made available at any given day. All blood specimens that met the inclusion/exclusion criteria were deidentified, assigned a sequential alpha numeric code unique to the study, and logged into shared electronic database. Samples were stored under refrigerated conditions (2°C–4°C) until shipment to Arkana Laboratories.
Definitions
A baseline serum creatinine was determined for each study participant by a physician unaware of the APOL1 status by computing the average of the serum creatinine values obtained within 18 months prior to the date of the positive SARS-CoV-2 PCR test. For those in which a baseline serum creatinine was not available within the 18-month prestudy interval, the research team utilized the serum creatinine values obtained during the course of the acute illness and at subsequent follow-up to retrospectively determine a baseline as indicated by the lowest consistent value of serum creatinine registered. AKI was defined as an increase in serum creatinine ≥0.3 mg/dl above baseline that was known or presumed to have occurred within 48 hours or an increase to 1.5 times baseline that was known or presumed to have occurred within the last 7 days. Persistent AKI was defined as absence of recovery within 3 days, whereas recovery was defined as return to baseline serum creatinine or at least a 50% reduction. New-onset overt proteinuria was defined as an increase from baseline in dipstick proteinuria of 2+ or a urine protein-creatinine ratio ≥1.0 g/g.
Main Exposure
The main exposure for the study was the presence of APOL1 risk alleles. DNA was extracted from peripheral blood and genotyped for APOL1 risk alleles on a ViiA 7 Real-Time PCR System (Thermo Fisher, Waltham, MA) using TaqMan assays as previously described (8). Accordingly, individuals were categorized in two groups: (1) those with two APOL1 risk alleles and (2) those with zero or one risk allele.
Outcomes
The primary outcome was defined before data acquisition as the development of AKI within 21 days of a positive SARS-CoV-2 PCR. Secondary outcomes included AKI requiring KRT, persistent AKI, and new-onset proteinuria.
Statistical Methods
A descriptive analysis was performed comparing baseline characteristics according to the number of APOL1 risk alleles. Continuous variables were expressed as median (25th, 75th percentiles) and compared with the Kruskal–Wallis test. Categorical variables were expressed as the absolute number (proportion) and compared using the chi-squared statistic. Logistic regression models were used to evaluate the relationship between allele group differences and outcomes of interest. Allele group differences were tested using a recessive (two versus zero or one risk allele) model. Models were adjusted for patient characteristics; were selected on the basis of clinical knowledge and prior studies; and included age, sex, body mass index, hypertension, CKD, use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), and proteinuria. Calibration was assessed using the Hosmer–Lemeshow statistic. Adjusted odds ratios (ORs) with associated 95% confidence limits were calculated for the group with two risk alleles using the zero/one group as the reference. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) statistical software.
Ethics
The study was approved by the institutional board review and conducted following the principles of the Declaration of Helsinki.
Results
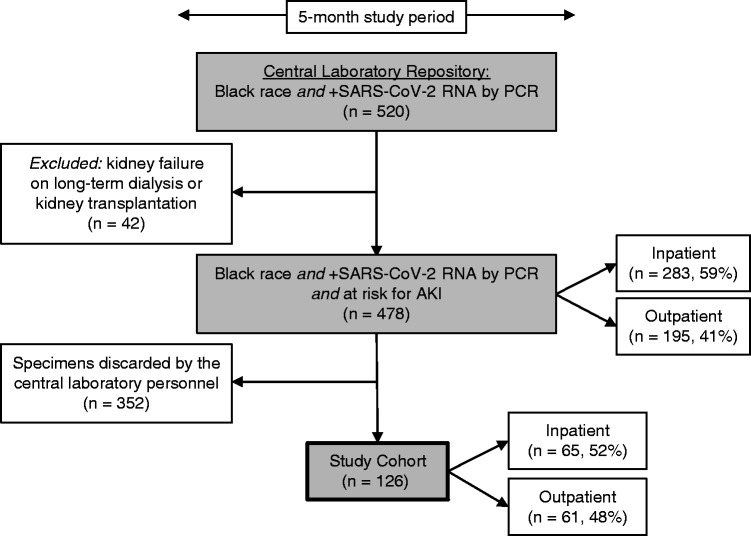
From a pool of 478 eligible blood specimens within the study period, 352 were discarded without undergoing screening by our research personnel. Thus, blood specimens from 126 Black American patients with documented SARS-CoV-2 infection were arbitrarily extracted and included in the study cohort (Figure 1). The prevalence of the high-risk APOL1 genotype in the cohort was 13%, similar to the known prevalence of the high-risk APOL1 genotype in Black Americans (13). Among patients with two APOL1 risk alleles, seven (44%) were G1/G1, seven (44%) were G1/G2, and two (13%) were G2/G2. When comparing the baseline characteristics of patients with two APOL1 risk alleles with those with zero or one risk allele, there were no significant differences in age, sex, body mass index, previous history of hypertension, diabetes mellitus, CKD, prior use of ACEis/ARBs, or rate of inpatient status. However, the baseline eGFR was significantly higher in the zero– to one–risk allele group (Table 1).
Figure 1.
Flow chart illustrating the methodology followed to establish the study cohort. The cohort of 126 patients constitutes an arbitrary sample obtained from a pool of 478 patients with coronavirus disease 2019 at risk for AKI during the study period. Because of limited availability of the research personnel, 352 specimens were discarded by the central laboratory without undergoing screening. By virtue of their disease state, patients with kidney failure on long-term dialysis were not at risk for AKI and thus were excluded. Patients with kidney transplantation were also excluded to minimize confounding factors. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline characteristics of Black Americans with severe acute respiratory syndrome coronavirus 2 infection between March 13, 2020 and October 1, 2020 at Ochsner Medical Center in New Orleans, Louisiana, United States
| Baseline Characteristics | Overall Study Cohort, n=126 | Number of APOL1 High-Risk Alleles | |
|---|---|---|---|
| 0 or 1, n=110; 87% | 2, n=16; 13% | ||
| Age, median (25th–75th) | 60 (47–73) | 58 (47–73) | 62 (59–78) |
| Body mass index, median (25th–75th) | 32.9 (26.0–39.1) | 32.6 (25.8–39.4) | 34.1 (27.7–38.1) |
| Men, n (%) | 52 (41) | 45 (41) | 7 (44) |
| Hypertension, n (%) | 83 (66) | 70 (64) | 13 (81) |
| eGFR, median (25th–75th%) | 85 (63–103) | 90 (66–106) | 68 (50–89) |
| Use of ACEi/ARB, n (%) | 34 (27) | 31 (28) | 3 (19) |
| Inpatient status, n (%) | 65 (52) | 54 (49) | 11 (69) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
The primary outcome, AKI, occurred in 11 (69%) individuals in the group with two APOL1 high-risk alleles (including five with stage 1 AKI, three with stage 2 AKI, and three with stage 3 AKI) and in 39 (35%) in the group with zero or one risk allele (including 17 with stage 1 AKI, eight with stage 2 AKI, and 14 with stage 3 AKI) (Table 2). After adjustment for age, sex, body mass index, hypertension, eGFR, and use of ACEi/ARB, the presence of two APOL1 risk alleles was independently associated with higher odds of developing AKI (adjusted OR, 4.41; 95% confidence interval [95% CI], 1.11 to 17.52; P=0.04) as compared with the group with zero or one APOL1 risk allele (Table 2). APOL1 polymorphism did not associate with AKI in a dominant fashion as the presence of one risk allele was not associated with higher odds of developing AKI (adjusted OR, 0.65; 95% CI, 0.25 to 1.70; P=0.40) as compared with those who had no risk alleles.
Table 2.
Association of high-risk APOL1 genotype with AKI
| Outcome | Overall Study Cohort, n=126 | No. of APOL1 High-Risk Alleles | Adjusted Odds Ratioa (95% Confidence Interval) | P Value | |
|---|---|---|---|---|---|
| 0 or 1, n=110; 87% | 2, n=16; 13% | ||||
| Primary composite outcome, n (%) | |||||
| AKI | 50 (40) | 39 (35) | 11 (69) | 4.41 (1.11 to 17.52) | 0.04 |
| Secondary outcomes, n (%) | |||||
| Persistent AKI | 29 (23) | 21 (19) | 8 (50) | 3.53 (1.08 to 11.57) | 0.04 |
| AKI requiring KRT | 12 (10) | 8 (7) | 4 (25) | 4.99 (1.02 to 24.40) | 0.05 |
The primary exposure was two APOL1 high-risk alleles. Unadjusted odds ratio estimates are AKI: odds ratio, 4.01; 95% confidence interval, 1.30 to 12.36; P=0.02; persistent AKI: odds ratio, 4.24; 95% confidence interval, 1.43 to 12.60; P=0.01; and AKI KRT: odds ratio, 4.25; 95% confidence interval, 1.11 to 16.25; P=0.03.
Adjusted for age, sex, body mass index, hypertension, eGFR, and use of angiotensin receptor blocker/angiotensin-converting enzyme inhibitor.
Analyses of secondary outcomes revealed that Black Americans with two APOL1 risk alleles had greater odds of requiring KRT (adjusted OR, 4.99; 95% CI, 1.02 to 24.4; P=0.05) and acquiring persistent AKI (adjusted OR, 3.53; 95% CI, 1.8 to 11.57; P=0.04) compared with individuals with zero or one risk allele (Table 2).
In individuals with AKI, presence of overt proteinuria was assessed, but missing data precluded full analyses (Figure 2). Among 46 individuals with AKI who had assessment of proteinuria, five of 11 (45%) patients with two APOL1 risk alleles presented with new-onset overt proteinuria compared with 12 of 35 (34%; P=0.51) for those with zero or one APOL1 risk allele. There was no significant difference in mortality during the study period, with seven (6%) deaths in patients with zero or one risk allele and one (6%) death in patients with two APOL1 high-risk alleles.
Figure 2.
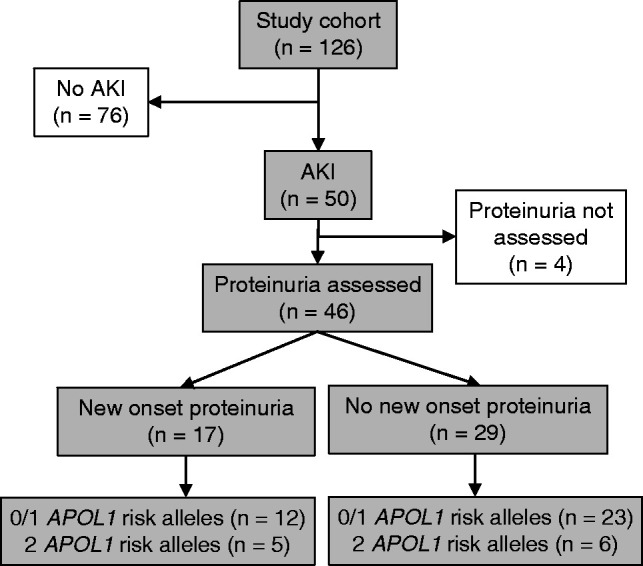
Schematic depicting the proportion of patients in the cohort with AKI who had proteinuria assessed at the time of coronavirus disease 2019 diagnosis and showing the distribution of zero to one and two APOL1 high-risk alleles.
Nine patients in the cohort lacked baseline serum creatinine value. After exclusion of those participants from the analyses, a significant association of APOL1 high-risk alleles with AKI, persistent AKI, and AKI KRT was still observed (Supplemental Table 1). Additional sensitivity analysis was performed by only including patients with inpatient status, and the results revealed nonsignificant association of APOL1 risk alleles with all outcomes except for AKI KRT under model 3, where the adjusted OR was significant (adjusted OR, 9.01; 95% CI, 1.12 to 72.71; P=0.04) (Supplemental Table 2).
Discussion
The APOL1 high-risk genotype accounts for the higher burden of kidney disease in Black Americans. However, the fact remains that most people homozygous for APOL1 risk alleles will never be diagnosed with kidney disease (14). In those who do develop disease, there is a broad clinical and pathologic spectrum that ranges from what resembles arterionephrosclerosis with slowly progressive kidney disease to the most aggressive form of disease, collapsing glomerulopathy, which has a high risk for irreversible kidney damage over a period of months (5). It is not possible to definitively predict which individuals who are homozygous for APOL1 risk alleles will eventually develop kidney disease. However, there are certain associated diseases that are believed to act as “second hits,” tipping genetically susceptible individuals into disease (8,9,15). We show here that COVID-19 may act as an environmental second hit leading to AKI in patients with two APOL1 high-risk alleles. In this study, the group with two risk alleles had four-fold higher odds of developing AKI compared with those who had zero or one risk allele and five-fold higher odds of developing AKI KRT. Given the widespread nature of SARS-CoV-2 infection, the higher burden of COVID-19 in minority populations, and the relatively high incidence of the presence of two risk alleles in the Black American population, the second hit caused by COVID-19 could have profound effects on the future incidence of kidney failure in this population. Because the APOL1 genotyping assay is relatively inexpensive and currently available in the clinic, it could be used to identify a population at higher risk of morbidity from kidney injury in the COVID-19 pandemic (16).
There is abundant evidence that APOL1 risk variants lead to kidney disease, but the precise pathogenic mechanism by which they do so is not well understood. This work has proved challenging due in large part to the fact that APOL1 is a recently evolved gene that is present in the genome of humans and only a few primates, making the creation of animal models of disease difficult. Cell culture experiments indicate that the APOL1 risk variants induce a toxic gain of function, as cells overexpressing the kidney risk variants show more cytotoxicity than those overexpressing wild-type APOL1 (17,18). A mouse model with transgenic podocyte expression of APOL1 also supports a toxic gain of function for risk alleles as the mice with G1 or G2 risk alleles develop proteinuria and histopathologic injury that resembles human kidney disease, whereas mice with transgenic G0 do not (19). In this transgenic model, there is evidence that the pathogenesis of disease is driven by abnormal endosomal trafficking and autophagic flux with resulting inflammatory-mediated cell death and glomerular scarring. APOL1 risk alleles have also been shown to induce cytotoxicity through cellular potassium depletion and subsequent initiation of stress-activated protein kinase pathways (20). Lastly, overexpression of APOL1 G1 and G2 risk alleles has been shown to lead to impairment of mitochondrial function induced by a greater propensity of the APOL1 protein with risk variants to oligomerize and activate opening of the mitochondrial permeability transition pore (21,22). Thus, there are multiple mechanistic pathways by which APOL1 risk variants induce cell injury, but many questions remain as to the role that each of these plays in human APOL1-associated nephropathy.
The recent increase in reports of collapsing glomerulopathy in the setting of COVID-19 has led to speculation that this virus is a strong APOL1 second hit similar to that which was reported for HIV in the 1980s (10). It has been hypothesized that COVID-19–associated nephropathy is driven by activation of IFN pathways (12). This concept is not new as APOL1-associated collapsing glomerulopathy in humans is well known to occur in association with second hits that have an elevated IFN state, including SLE and viral infections such as HIV, cytomegalovirus, and parvovirus B19 (8,9,23). Additionally, APOL1-associated collapsing glomerulopathy is known to occur in association with exogenous IFN therapy and has been reported in a child who also had a rare genetic condition that results in an increased IFN state, stimulator of IFN genes–associated vasculopathy with onset in infancy, during a flare of his disease (18,24). The precise mechanism by which APOL1 nephropathy is IFN driven is yet unknown, but it is supported by in vitro experimental work that has shown that IFN markedly upregulates the APOL1 gene (18,19). Additionally, an APOL1-transgenic mouse model in which APOL1 expression was driven by the endogenous human APOL1 regulatory elements also supports the role of IFN in driving APOL1 expression and kidney disease. In this model, administration of IFN-γ upregulated APOL1 expression in both G0 and G1 mice but only induced proteinuria in the mice that expressed the APOL1 G1 risk allele. The proteinuria in these mice could be prevented by treatment with an antisense oligonucleotide targeting and preventing APOL1 expression (25). Thus, there is experimental, animal, and human data to support the role for IFN pathways driving kidney disease in susceptible individuals with two APOL1 risk alleles.
A limitation of this study is that the patients with AKI did not undergo kidney biopsy to determine the pathologic lesions causing the injury. Most patients were thought to have AKI due to acute tubular injury at the time of clinical presentation. However, in retrospect, the onset and persistence of proteinuria in many of these patients suggest that glomerular disease, such as collapsing glomerulopathy, or other less aggressive forms of podocyte insult might have been a driver of the kidney injury. APOL1-associated nephropathy is most commonly thought of as a primarily glomerular disease with secondary tubular injury. However, the possibility of APOL1-induced tubular injury has not been well studied, and it is conceivable that it plays an under-recognized role in the APOL1 nephropathy disease phenotype. Acute tubular injury is commonly present in biopsies with APOL1-associated collapsing glomerulopathy, and retrospective patient-control studies have shown that certain tubular lesions, including microcystic tubular dilation and thyroidization-type tubular atrophy, are significantly more common in APOL1-associated kidney disease (8,26). The known expression of APOL1 in human kidney tubular cells (27), combined with abundant evidence suggesting local kidney production of the APOL1 kidney-risk variant protein drives development of nephropathy, raises the possibility that tubular injury could be an underappreciated phenotype in the APOL1 nephropathy spectrum (28). Other studies have failed to show an association of APOL1 risk variants with AKI (29). However, a recent retrospective analysis of Black Americans who underwent cardiac surgery suggested that homozygosity for APOL1 risk variants may be associated with a rise in serum creatinine (30). This knowledge combined with the results from this study support the need for further investigation to determine the role of APOL1 risk alleles in acute tubular injury.
In conclusion, APOL1 risk alleles raise the odds of AKI in patients with COVID-19. This means that the 13% of Black Americans who are homozygous for APOL1 risk alleles are in jeopardy of morbidity from kidney injury when infected with SARS-CoV-2. Although we do not have long-term follow-up data on this cohort, COVID-19 may result in a higher rate of progressive CKD in patients with two risk alleles. This could result in the Black American community bearing an even larger portion of the burden of the current pandemic than is already appreciated (31).
Disclosures
J.M. Arthur reports employment with the Department of Veterans Affairs, receiving honoraria from Travere Pharmaceuticals, and serving as a scientific advisor or member of Kidney360. L.R. Buckner reports employment with Ochsner Medical Center. R.S. Haun and C.P. Larsen report employment with Arkana Laboratories. J.C.Q. Velez reports employment with Ochsner Health; consultancy agreements with Bayer, Mallinckrodt Pharmaceuticals, and Travere; receiving honoraria from Bayer, Mallinckrodt, Otsuka, and Travere; serving as a scientific advisor or member of the Mallinckrodt Advisory Board and the Travere Advisory Board; and being a member of a speakers bureau for Otsuka Pharmaceuticals. T.J. Wickman reports employment with Ochsner Clinic Foundation. All remaining authors have nothing to disclose.
Funding
The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH), the NIH, and Translational Research Institute grant KL2 TR003108.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ms. L.R. Buckner, Ms. A.E. Hasty, Dr. C.P. Larsen, Mr. L.A. Matute-Trochez, and Dr. J.C.Q. Velez designed the study; Dr. R.S. Haun carried out the APOL1 genotyping; Dr. J.M. Arthur, Dr. J.R. Braga, Dr. J.C.Q. Velez, and Dr. T.J. Wickman analyzed the data; Dr. C.P. Larsen and Dr. J.C.Q. Velez drafted the original manuscript; and all authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “APOL1 Kidney Risk Variants and Acute Kidney Injury in Those with COVID-19: True Association or Red Herring?,” on pages 1779–1780.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01070121/-/ DCSupplemental.
Supplemental Table 1. Logistic regression analyses after exclusion of individuals lacking baseline serum creatinine value (n=115).
Supplemental Table 2. Logistic regression analyses for a subgroup of individuals with inpatient status (n=65).
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Tian S, Guo H: Acute kidney injury and renal replacement therapy in COVID-19 patients: A systematic review and meta-analysis. Int Immunopharmacol 90: 107159, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimkar A, Naaraayan A, Hasan A, Pant S, Durdevic M, Suarez CN, Elenius H, Hambardzumyan A, Lakshmi K, Mandel M, Jesmajian S: Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from COVID-19. Mayo Clin Proc Innov Qual Outcomes 4: 687–695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Easterling RE: Racial factors in the incidence and causation of end-stage renal disease (ESRD). Trans Am Soc Artif Intern Organs 23: 28–33, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcon GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP; Lupus Nephritis–End‐Stage Renal Disease Consortium : End-stage renal disease in African Americans with lupus nephritis associates with APOL1. Arthritis Rheum 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, Chughtai A, Xie L, Gimenez JM, Sandow TA, Lusco MA, Yang H, Acheampong E, Rosales IA, Colvin RB, Fogo AB, Velez JCQ: AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol 31: 1688–1695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR: COVID-19-associated collapsing focal segmental glomerulosclerosis: A report of 2 cases. Kidney Med 2: 493–497, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velez JCQ, Caza T, Larsen CP: COVAN is the new HIVAN: The re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol 16: 565–567, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limou S, Nelson GW, Kopp JB, Winkler CA: APOL1 kidney risk alleles: Population genetics and disease associations. Adv Chronic Kidney Dis 21: 426–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, Limou S, Sezgin E, Nelson GW, Fogo AB, Goetsch S, Kopp JB, Winkler CA, Naicker S: APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol 26: 2882–2890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BI, Larsen CP: Apolipoprotein L1 gene testing comes of age. Kidney360 1: 58–61, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC: APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, Boustany-Kari CM, Pullen SS, Miner JH, Hu CA, Rohacs T, Inoue K, Ishibe S, Saleem MA, Palmer MB, Cuervo AM, Kopp JB, Susztak K: Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, Schlöndorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR: APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L, Chou JW, Snipes JA, Bharadwaj MS, Craddock AL, Cheng D, Weckerle A, Petrovic S, Hicks PJ, Hemal AK, Hawkins GA, Miller LD, Molina AJ, Langefeld CD, Murea M, Parks JS, Freedman BI: APOL1 renal-risk variants induce mitochondrial dysfunction. J Am Soc Nephrol 28: 1093–1105, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SS, Lannon H, Dias L, Zhang JY, Alper SL, Pollak MR, Friedman DJ: APOL1 kidney risk variants induce cell death via mitochondrial translocation and opening of the mitochondrial permeability transition pore. J Am Soc Nephrol 30: 2355–2368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlinson L, Boriskin Y, McPhee I, Holwill S, Rice P: Acute cytomegalovirus infection complicated by collapsing glomerulopathy. Nephrol Dial Transplant 18: 187–189, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Abid Q, Best Rocha A, Larsen CP, Schulert G, Marsh R, Yasin S, Patty-Resk C, Valentini RP, Adams M, Baracco R: APOL1-associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI). Am J Kidney Dis 75: 287–290, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghajan M, Booten SL, Althage M, Hart CE, Ericsson A, Maxvall I, Ochaba J, Menschik-Lundin A, Hartleib J, Kuntz S, Gattis D, Ahlström C, Watt AT, Engelhardt JA, Monia BP, Magnone MC, Guo S: Antisense oligonucleotide treatment ameliorates IFN-γ-induced proteinuria in APOL1-transgenic mice. JCI Insight 4: e126124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen CP, Beggs ML, Saeed M, Ambruzs JM, Cossey LN, Messias NC, Walker PD, Freedman BI: Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol 28: 95–102, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI: Localization of APOL1 protein and mRNA in the human kidney: Nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Divers J, Freedman BI: Mechanisms of injury in APOL1-associated kidney disease. Transplantation 103: 487–492, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, Kao WH, Coresh J: Explaining the racial difference in AKI incidence. J Am Soc Nephrol 25: 1834–1841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Privratsky JR, Li YJ, Haynes C, Podgoreanu M, Mathew J, Shah SH, Stafford-Smith M: Apolipoprotein L1 (APOL1) coding variants are associated with creatinine rise after cardiac surgery. J Cardiothorac Vasc Anesth 34: 3314–3320, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price-Haywood EG, Burton J, Fort D, Seoane L: Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med 382: 2534–2543, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.