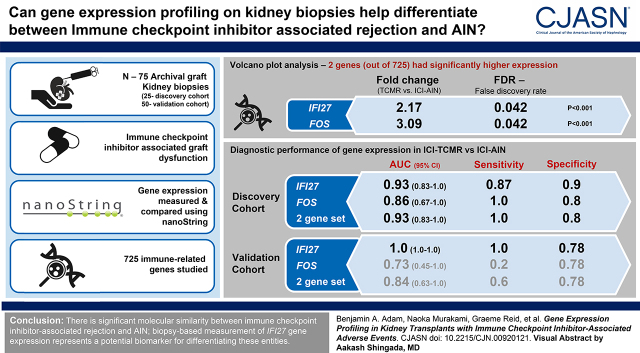
Visual Abstract
Keywords: kidney transplantation, drug nephrotoxicity, acute rejection, gene expression, histopathology, cancer, immune checkpoint inhibitors, gene expression profiling, allografts
Abstract
Background and objectives
Immune checkpoint inhibitors are increasingly used to treat various malignancies, but their application in patients with kidney transplants is complicated by high allograft rejection rates. Immune checkpoint inhibitor–associated rejection is a novel, poorly understood entity demonstrating overlapping histopathologic features with immune checkpoint inhibitor–associated acute interstitial nephritis, which poses a challenge for diagnosis and clinical management. We sought to improve the understanding of these entities through biopsy-based gene expression analysis.
Design, setting, participants, & measurements
NanoString was used to measure and compare the expression of 725 immune-related genes in 75 archival kidney biopsies, including a 25-sample discovery cohort comprising pure T cell–mediated rejection and immune checkpoint inhibitor–associated acute interstitial nephritis and an independent 50-sample validation cohort comprising immune checkpoint inhibitor–associated acute interstitial nephritis, immune checkpoint inhibitor–associated T cell–mediated rejection, immune checkpoint inhibitor–associated crescentic GN, drug-induced acute interstitial nephritis, BK virus nephropathy, and normal biopsies.
Results
Significant molecular overlap was observed between immune checkpoint inhibitor–associated acute interstitial nephritis and T cell–mediated rejection. Nevertheless, IFI27, an IFN-α–induced transcript, was identified and validated as a novel biomarker for differentiating immune checkpoint inhibitor–associated T cell–mediated rejection from immune checkpoint inhibitor–associated acute interstitial nephritis (validation cohort: P<0.001, area under the receiver operating characteristic curve =100%, accuracy =86%). Principal component analysis revealed heterogeneity in inflammatory gene expression patterns within sample groups; however, immune checkpoint inhibitor–associated T cell–mediated rejection and immune checkpoint inhibitor–associated acute interstitial nephritis both demonstrated relatively more molecular overlap with drug-induced acute interstitial nephritis than T cell–mediated rejection, suggesting potential dominance of hypersensitivity mechanisms in these entities.
Conclusions
These results indicate that, although there is significant molecular similarity between immune checkpoint inhibitor–associated rejection and acute interstitial nephritis, biopsy-based measurement of IFI27 gene expression represents a potential biomarker for differentiating these entities.
Introduction
Immune checkpoint inhibitors have demonstrated robust efficacy in the treatment of many cancers but have also been associated with numerous immune-related adverse events (AEs) affecting almost every organ system (1,2). Immune-related AEs involving the kidneys include acute interstitial nephritis (AIN) (3,4) and transplant rejection (5 –7), the underlying mechanisms of which remain poorly understood (8,9).
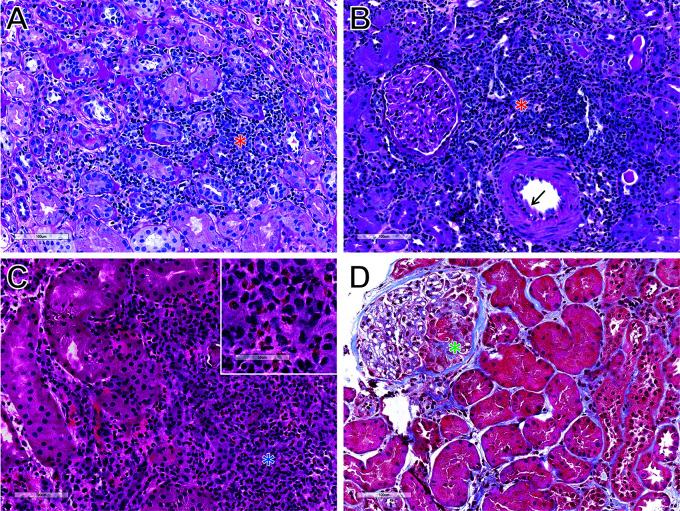
Although the use of immune checkpoint inhibitors in patients with kidney transplants is complicated by high rates of immune-related AEs, they are being increasingly utilized due to their excellent anticancer efficacy. Determining the etiology of kidney transplant dysfunction in the setting of immune checkpoint inhibitor treatment is important because rejection is associated with worse prognosis and may require more immunosuppression than AIN, at the expense of blunting the antitumor immune response (8). However, differentiating immune checkpoint inhibitor–associated acute interstitial nephritis (ICI-AIN) from rejection, particularly T cell–mediated rejection (TCMR), is often challenging due to their overlapping clinical and histopathologic features. In contrast to the eosinophil-rich inflammation typically seen in AIN due to other medications (e.g., antibiotics and proton pump inhibitors), ICI-AIN often demonstrates a lymphocyte-predominant tubulointerstitial infiltrate indistinguishable from Banff grade 1 acute TCMR (Figure 1) (10,11). There is thus a need to develop novel tools to differentiate these entities so that they can be further studied and more effectively treated in the future.
Figure 1.
Representative histology. (A) Immune checkpoint inhibitor–associated acute interstitial nephritis (ICI-AIN; periodic acid–Schiff [PAS] stain). (B) T cell–mediated rejection (TCMR; PAS stain). (C) Antibiotic drug–associated acute interstitial nephritis (Drug-AIN; hematoxylin and eosin stain). (D) Immune checkpoint inhibitor–associated crescentic GN (ICI-GN; Masson trichrome stain). ICI-AIN and TCMR exhibit overlapping histologic appearances characterized by lymphocyte-predominant tubulointerstitial inflammation (red asterisks). Mild intimal arteritis is also present in this case of TCMR (arrow). In contrast, Drug-AIN classically displays an eosinophil-rich interstitial infiltrate (blue asterisk and Inset). This case of ICI-GN shows cellular crescent formation (green asterisk) without significant tubulointerstitial inflammation.
Gene expression profiling has revolutionized the classification of kidney transplant rejection (12,13) and has been used for biomarker discovery in nonkidney immune-related AEs (14). We hypothesized that biopsy-based molecular analysis could similarly improve our understanding of kidney immune-related AEs, including the possibility that immune checkpoint inhibitor–associated T cell–mediated rejection (ICI-TCMR) may consist of concomitant alloimmune and hypersensitivity components. The feasibility of this approach is facilitated by the recent development of the novel NanoString nCounter gene expression system (NanoString Technologies, Seattle, WA), which allows for reliable assessment of formalin-fixed paraffin-embedded tissue (15,16) and, thus, retrospective analysis of biopsy-proven ICI-AIN and rejection, examples of which are relatively infrequent and difficult to prospectively collect. The objectives of this study were to (1) evaluate the molecular differences and similarities between pure (native kidney) ICI-AIN and pure (nonimmune checkpoint inhibitor–associated) TCMR, (2) assess the utility of these gene expression signatures for differentiating ICI-AIN and rejection, and (3) compare alloimmune and hypersensitivity patterns of gene expression in these entities.
Materials and Methods
Study Cohort
This study was approved by the participating institutions’ review boards. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
A total of 75 archival formalin-fixed paraffin-embedded human kidney biopsies were recruited from multiple institutions (Arkana Laboratories, Brigham and Women’s Hospital, Imperial College London, Nantes University Hospital, the University of Alberta, and the University of Minnesota), including a 25-sample discovery cohort and a 50-sample validation cohort. The discovery cohort consisted of ten native kidney biopsies from patients with diagnoses of ICI-AIN and 15 kidney transplant biopsies from patients not on immune checkpoint inhibitors with pure acute TCMR (Figure 1, A and B, Table 1). The validation cohort included nine additional native kidney biopsies from patients with ICI-AIN, five kidney transplant biopsies from patients on immune checkpoint inhibitor therapy with Banff grade 2 acute TCMR (11), one native kidney biopsy from a patient with immune checkpoint inhibitor–associated crescentic GN (ICI-GN), 15 native kidney biopsies from patients with nonimmune checkpoint inhibitor drug-associated acute interstitial nephritis (Drug-AIN), ten kidney transplant biopsies with BK virus nephropathy (BKVN), and ten histologically normal implantation/time 0 kidney transplant biopsies (normal) (Figure 1, C and D, Table 1). Of note, the requirement for Banff grade 2 acute TCMR (i.e., intimal arteritis) (11) in the ICI-TCMR group was considered to represent evidence of an alloimmune process, with or without potential concurrent ICI-AIN.
Table 1.
Clinical and histological characteristics of discovery and validation cohorts
| Feature a | Discovery Cohort | Validation Cohort | ||
| ICI-AIN, n=10 | TCMR, n=15 | ICI-AIN, n=9 | ICI-TCMR, n=5 | |
| Clinical features (at time of biopsy) | ||||
| Patient age, yr | 62±10 (52–76) | 51±12 (29–65) | 65±9 (49–75) | 70±4 (65–74) |
| Sex (women) | 4 (40) | 7 (47) | 3 (33) | 0 (0) |
| Cancer type | ||||
| Bladder cancer | 0 (0) | N/A | 2 (22) | 0 (0) |
| Colorectal cancer | 1 (10) | N/A | 0 (0) | 0 (0) |
| Lung cancer | 1 (10) | N/A | 4 (44) | 1 (20) |
| Melanoma | 5 (50) | N/A | 1 (11) | 2 (40) |
| Renal cell carcinoma | 2 (20) | N/A | 1 (11) | 0 (0) |
| Squamous cell carcinoma | 1 (10) | N/A | 1 (11) | 2 (40) |
| Immune checkpoint inhibitor regimen | ||||
| PD-1 inhibitor only | 6 (60) | N/A | 8 (88) | 5 (100) |
| PD-1 + CTLA-4 inhibitor | 4 (40) | N/A | 1 (11) | 0 (0) |
| No. of cycles | 4.1±1.3 (2–6) | N/A | 6.4±2.7 (3–10) | 3.8±2.0 (2–6) |
| Immune-related adverse events | ||||
| Acute interstitial nephritis | 10 (100) | N/A | 9 (100) | 0 (0) b |
| Hypophysitis | 1 (10) | N/A | 0 (0) | 0 (0) |
| Thyroiditis | 1 (10) | N/A | 0 (0) | 0 (0) |
| Peripheral neuropathy | 0 (0) | N/A | 1 (11) | 0 (0) |
| Pneumonitis | 0 (0) | N/A | 1 (11) | 0 (0) |
| Cause of kidney failure | ||||
| Diabetes | N/A | 6 (40) | N/A | 1 (20) |
| GN | N/A | 3 (20) | N/A | 0 (0) |
| Hypertension | N/A | 2 (13) | N/A | 2 (40) |
| Polycystic kidney disease | N/A | 3 (20) | N/A | 2 (40) |
| Reflux/obstructive nephropathy | N/A | 1 (7) | N/A | 0 (0) |
| Immunosuppression regimen | ||||
| Azathioprine | N/A | 2 (13) | N/A | 0 (0) |
| Calcineurin inhibitor | N/A | 13 (87) | N/A | 1 (20) |
| Corticosteroid | N/A | 15 (100) | N/A | 4 (80) |
| Mycophenolate | N/A | 13 (87) | N/A | 0 (0) |
| mTOR inhibitor | N/A | 2 (13) | N/A | 4 (80) |
| Baseline serum creatinine, mg/dl | 1.1±0.4 (0.6–1.6) | 1.3±0.4 (0.8–2.2) | 1.1±0.2 (0.9–1.6) | 1.4±0.8 (0.6–2.3) |
| Peak serum creatinine, mg/dl | 3.0±1.7 (1.4–6.9) | 3.0±2.0 (1.2–7.9) | 3.2±1.5 (1.8–5.8) | 4.5±2.4 (2.5–7.5) |
| Time post-transplant, mo | N/A | 8±9 (1–33) | N/A | 90±91 (18–191) |
| Presence of donor-specific antibodies | N/A | 0 (0) | N/A | 0 (0) |
| Biopsy features | ||||
| Diagnosis | ||||
| Acute interstitial nephritis | 10 (100) | 0 (0) | 9 (100) | 0 (0) b |
| Acute TCMR only | N/A | 15 (100) | N/A | 2 (40) |
| Mixed acute TCMR + active ABMR | N/A | 0 (0) | N/A | 3 (60) |
| Banff acute TCMR grade | ||||
| 1A | N/A | 3 (20) | N/A | 0 (0) |
| 1B | N/A | 3 (20) | N/A | 0 (0) |
| 2A | N/A | 7 (47) | N/A | 3 (60) |
| 2B | N/A | 2 (13) | N/A | 2 (40) |
| 3 | N/A | 0 (0) | N/A | 0 (0) |
| Global glomerulosclerosis, % | 12±13 (0–26) | 2±5 (0–17) | 11±11 (0–31) | 13±12 (0–25) |
| Interstitial fibrosis and tubular atrophy, % | 31±20 (12–60) | 12±13 (0–50) | 37±29 (5–75) | 24±14 (10–40) |
| Banff lesion scores | ||||
| Interstitial inflammation | N/A | 2.3±0.7 (1–3) | N/A | 2.2±0.8 (1–3) |
| Total interstitial inflammation | N/A | 2.3±0.7 (1–3) | N/A | 2.3±1.0 (1–3) |
| Tubulitis | N/A | 2.4±0.7 (1–3) | N/A | 2.0±1.4 (0–3) |
| Arteritis | N/A | 0.7±0.7 (0–2) | N/A | 1.4±0.5 (1–2) |
| Glomerulitis | N/A | 0.3±0.7 (0–2) | N/A | 1.6±1.1 (0–3) |
| Peritubular capillaritis | N/A | 1.2±1.0 (0–3) | N/A | 1.4±0.5 (1–2) |
| Interstitial fibrosis | N/A | 0.8±0.9 (0–3) | N/A | 1.3±1.0 (0–2) |
| Tubular atrophy | N/A | 0.9±0.8 (0–3) | N/A | 1.5±0.6 (1–2) |
| Transplant glomerulopathy | N/A | 0.1±0.5 (0–2) | N/A | 1.0±1.2 (0–2) |
| Mesangial matrix expansion | N/A | 0.3±0.5 (0–1) | N/A | 0.5±0.6 (0–1) |
| Arterial fibrointimal thickening | N/A | 0.7±0.9 (0–2) | N/A | 2.0±0.0 (2–2) |
| Arteriolar hyalinosis | N/A | 0.2±0.4 (0–1) | N/A | 2.8±0.5 (2–3) |
| C4d positive | N/A | 0 (0) | N/A | 3 (60) |
ICI-AIN, immune checkpoint inhibitor–associated acute interstitial nephritis; TCMR, T cell–mediated rejection; N/A, not applicable; mTOR, mammalian target of rapamycin; ABMR, antibody-mediated rejection.
aData are presented as count (percentage) for categorical data and mean ± SD (range) for continuous/ordinal data.
bICI-AIN could not be definitively excluded in these cases but was considered less likely than rejection.
Gene Expression Analyses
RNA extraction and gene expression analyses were performed as previously described (16 –18). Briefly, three to five consecutive 20-µm sections were obtained from each formalin-fixed paraffin-embedded block, and RNA was isolated using the RNeasy FFPE Kit (Qiagen, Toronto, ON, Canada). RNA concentration and purity were measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Gene expression was quantified using a NanoString nCounter FLEX Analysis System (NanoString Technologies), as per the manufacturer’s recommendations. We utilized the nCounter PanCancer Immune Profiling Panel, a 770-gene set designed to comprehensively profile the innate and adaptive immune response, including markers for all major immune signaling pathways and immune cell types (https://www.nanostring.com/products/gene-expression-panels/gene-expression-panels-overview/hallmarks-cancer-gene-expression-panel-collection/pancancer-immune-profiling-panel). Thirty cancer/testis antigens contained in this gene set were excluded from analysis due to absence of expression. We added 25 genes previously reported to be associated with TCMR and/or BKVN (Supplemental Table 1) (18,19). This resulted in 765 genes being analyzed, including 725 experimental genes and 40 housekeeping genes. Quality control assessment and data normalization were performed using nSolver Analysis Software Version 4.0 (NanoString Technologies).
Statistical Analyses
All postnormalization statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Categorical data are presented as count (percentage), and continuous and ordinal data are presented as mean ± SD (range). Class comparison analyses were performed using the Mann–Whitney U test for ordinal and continuous data; log2 normalized counts were used for individual gene analyses, and mean log2 normalized counts were used for gene set analyses. Volcano plot analysis was performed using linear regression with a false discovery rate (FDR) threshold of 0.05. Receiver operating characteristic curve analysis was used to assess diagnostic performance. Validation cohort performance was evaluated using diagnostic thresholds derived from the discovery cohort, as defined by the Youden J statistic (20). Principal component analysis and heat map analysis with hierarchical clustering were used to compare gene expression patterns between sample groups. Statistical significance was considered at P=0.05 or FDR<0.05, as appropriate.
Results
Baseline Characteristics
Relevant clinical and pathologic characteristics for the discovery and validation cohorts are presented in Table 1. The nonimmune checkpoint inhibitor–associated TCMR group had relatively younger patient age, less interstitial fibrosis and tubular atrophy, and shorter time post-transplant compared with the immune checkpoint inhibitor–treated groups. However, baseline serum creatinine, peak serum creatinine, global glomerulosclerosis, immune checkpoint inhibitor regimen, cancer type, cause of kidney failure, and Banff grades of acute TCMR (11) were similar between respective groups. Four nonkidney immune-related AEs were documented, including one instance each of hypophysitis, thyroiditis, peripheral neuropathy, and pneumonitis. Most Banff histology scores were similar between the kidney transplant groups; however, there was relatively more glomerulitis, transplant glomerulopathy, arteriosclerosis, arteriolar hyalinosis, and C4d positivity in ICI-TCMR versus TCMR. No donor-specific antibodies were identified in any of the patients included in this study.
Differential Gene Expression in T Cell–Mediated Rejection versus Immune Checkpoint Inhibitor–Associated Acute Interstitial Nephritis
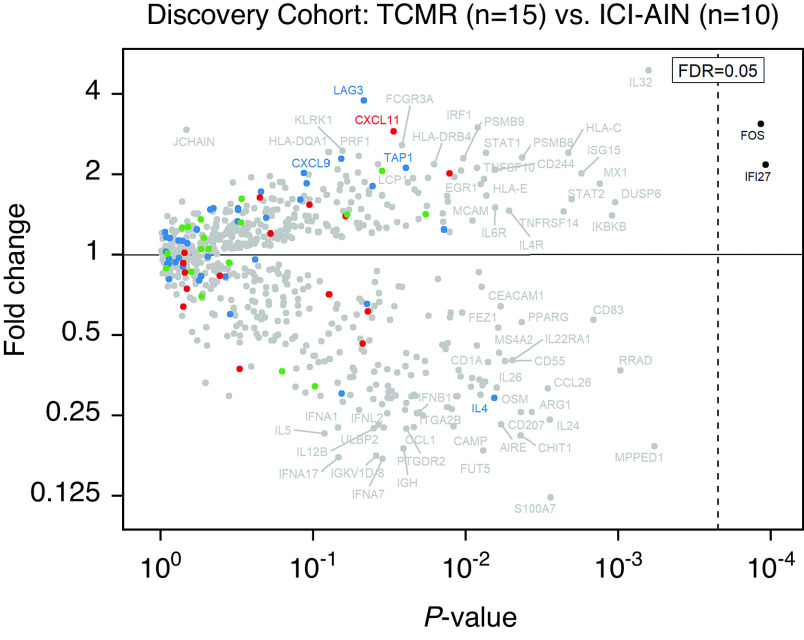
First, we asked whether there are molecular differences that can distinguish pure ICI-AIN and pure acute TCMR. Analysis of 39 previously reported TCMR-associated genes (21) demonstrated no significant differential expression between the TCMR and ICI-AIN groups in the discovery cohort (P=0.68) (Supplemental Figure 1). We, therefore, performed an unbiased volcano plot analysis to identify novel discriminatory transcripts within the full set of 725 experimental genes (Figure 2). After correcting for multiple comparisons, only two genes, IFI27 and FOS, demonstrated statistically significantly higher expression in TCMR versus ICI-AIN (FDR=0.04 for both) (Table 2). Of note, there was no significant difference in IFI27 or FOS expression between Banff grades 1 and 2 within the TCMR sample group (P=0.39) (Supplemental Figure 2), suggesting that increased expression in these samples was not simply a reflection of immune response severity. No genes had significantly higher expression in ICI-AIN versus TCMR (FDR≥0.11) (Table 3).
Figure 2.
Differential gene expression in the discovery cohort. The volcano plot shows fold change (y axis) versus linear regression P value (x axis) between TCMR and ICI-AIN for 725 immune-related genes. Blue, red, and green points represent genes previously associated with TCMR, antibody-mediated rejection, and the immune checkpoint pathway, respectively. After correction for multiple comparisons, only two genes, IFI27 and FOS, demonstrate statistically significantly higher expression in TCMR compared with ICI-AIN (false discovery rate [FDR] <0.05). No genes demonstrate significantly higher expression in ICI-AIN versus TCMR.
Table 2.
Top 20 genes with relatively higher expression in T cell–mediated rejection (n=15) versus immune checkpoint inhibitor–associated acute interstitial nephritis (n=10) in the discovery cohort
| Gene Symbol | Gene Name | Functional Association | Fold Change (TCMR versus ICI-AIN) | Unadjusted P Value | FDR |
| IFI27 | IFN-α inducible protein 27 | Immune response (IFN-α pathway) | 2.2 | <0.001 | 0.04 |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit | Cell proliferation (TGF-β pathway) | 3.2 | <0.001 | 0.04 |
| IL32 | IL-32 | Immune response (T cells, NK cells, TNF) | 4.9 | <0.001 | 0.11 |
| DUSP6 | Dual-specificity phosphatase 6 | Cell proliferation (MAPK pathway) | 1.6 | 0.001 | 0.11 |
| IKBKB | Inhibitor of NF-κB kinase subunit-β | Immune response (NF-κB pathway) | 1.4 | 0.001 | 0.11 |
| MX1 | MX dynamin like GTPase 1 | Immune response (IFN-α/β/γ pathway) | 1.8 | 0.001 | 0.12 |
| ISG15 | ISG15 ubiquitin-like modifier | Immune response (IFN-α/β/γ pathway) | 2.0 | 0.002 | 0.13 |
| STAT2 | Signal transducer and activator of transcription 2 | Immune response (IFN-α/β pathway) | 1.6 | 0.002 | 0.13 |
| HLA-C | MHC, class I, C | Immune response (antigen presentation) | 2.4 | 0.002 | 0.13 |
| TNFRSF14 | TNF receptor superfamily member 14 | Immune response (TNF superfamily) | 1.5 | 0.002 | 0.13 |
| PSMB8 | Proteasome 20S subunit-β8 | Immune response (IFN-γ pathway) | 2.3 | 0.004 | 0.13 |
| IL4R | IL-4 receptor | Immune response (Th2 cells, IgE) | 1.5 | 0.005 | 0.13 |
| IL6R | IL-6 receptor | Immune response (B cells, T cells) | 1.5 | 0.006 | 0.13 |
| CD244 | CD244 molecule | Immune response (NK cells) | 2.1 | 0.007 | 0.13 |
| MCAM | Melanoma cell adhesion molecule | Endothelial adhesion | 1.7 | 0.007 | 0.13 |
| STAT1 | Signal transducer and activator of transcription 1 | Immune response (IFN-α/γ pathway) | 2.4 | 0.007 | 0.13 |
| HLA-E | MHC, class I, E | Immune response (NK cells) | 1.9 | 0.008 | 0.13 |
| EGR1 | Early growth response 1 | Cell proliferation (TGF-β pathway) | 1.8 | 0.008 | 0.13 |
| IRF1 | IFN regulatory factor 1 | Immune response (IFN-α/β pathway) | 3.0 | 0.008 | 0.13 |
| TNFSF10 | TNF superfamily member 10 | Immune response (TNF superfamily) | 2.1 | 0.009 | 0.13 |
TCMR, T cell–mediated rejection; ICI-AIN, immune checkpoint inhibitor–associated acute interstitial nephritis; FDR, false discovery rate; MAPK, mitogen-activated protein kinase.
Table 3.
Top 20 genes with relatively higher expression in immune checkpoint inhibitor–associated acute interstitial nephritis (n=10) versus T cell–mediated rejection (n=15) in discovery cohort
| Gene Symbol | Gene Name | Functional Association | Fold Change (ICI-AIN versus TCMR) | Unadjusted P Value | FDR |
| MPPED1 | Metallophosphoesterase domain containing 1 | Cell functions (hydrolase activity) | 5.2 | <0.001 | 0.11 |
| RRAD | Ras-related glycolysis inhibitor and calcium channel regulator | Cell functions (calcium regulation) | 2.7 | <0.001 | 0.11 |
| CD83 | CD83 molecule | Immune response (antigen presentation) | 1.8 | 0.001 | 0.12 |
| S100A7 | S100 calcium binding protein A7 | Cell proliferation | 8.1 | 0.003 | 0.13 |
| IL24 | IL-24 | Apoptosis | 4.1 | 0.003 | 0.13 |
| CCL26 | C-C motif chemokine ligand 26 | Immune response (eosinophils, basophils) | 3.2 | 0.003 | 0.13 |
| ARG1 | Arginase 1 | Cell functions (urea cycle) | 3.9 | 0.004 | 0.13 |
| PPARG | Peroxisome proliferator–activated receptor-γ | Cell functions (fatty acid oxidation) | 1.8 | 0.004 | 0.13 |
| CD207 | CD207 molecule | Immune response (Langerhans cells) | 3.9 | 0.004 | 0.13 |
| CHIT1 | Chitinase 1 | Immune response (macrophages) | 4.8 | 0.004 | 0.13 |
| IL22RA1 | IL-22 receptor subunit-α1 | Immune response (Th17 cells) | 2.5 | 0.005 | 0.13 |
| CD55 | CD55 molecule (Cromer blood group) | Complement cascade regulation | 2.5 | 0.006 | 0.13 |
| AIRE | Autoimmune regulator | Immune regulation | 4.3 | 0.006 | 0.13 |
| CEACAM1 | CEA cell adhesion molecule 1 | Cell adhesion, immune regulation | 1.6 | 0.006 | 0.13 |
| MS4A2 | Membrane spanning 4-domains A2 | Immune response (IgE, mast cells) | 2.3 | 0.006 | 0.13 |
| FEZ1 | Fasciculation and elongation protein-ζ1 | Cell functions (axonal development) | 1.9 | 0.006 | 0.13 |
| OSM | Oncostatin M | Immune regulation | 3.1 | 0.006 | 0.13 |
| IL4 | IL-4 | Immune response (IgE) | 3.4 | 0.007 | 0.13 |
| CD1A | CD1a molecule | Immune response (antigen presentation) | 2.5 | 0.007 | 0.13 |
| IL26 | IL-26 | Immune response (T cells) | 3.0 | 0.008 | 0.13 |
TCMR, T cell–mediated rejection; ICI-AIN, immune checkpoint inhibitor–associated acute interstitial nephritis; FDR, false discovery rate.
Despite only two genes meeting the threshold for statistical significance in volcano plot analysis, those most strongly associated with TCMR and ICI-AIN, as defined by unadjusted P values, were further examined to elucidate trends in functional association. Sixteen of the top 20 genes with relatively higher expression in TCMR versus ICI-AIN were primarily associated with immune response, with three of the remaining four involved in cell proliferation (FOS, DUSP6, and EGR1) and one involved in endothelial adhesion (MCAM). Consistent with previously reported gene expression signatures in TCMR (13,19), the most common pathways and effector cell types represented by the 16 immune response genes included IFN signaling (IFI27, MX1, ISG15, STAT2, PSMB8, STAT1, and IRF1), T cell and NK cell functions (IL32, IL4R, IL6R, CD244, and HLA-E), and TNF pathway (IL32, TNFRSF14, and TNFSF10) (Table 2).
The top 20 genes with relatively higher expression in ICI-AIN versus TCMR demonstrated a less specific pattern of functional association, with nine genes primarily involved in immune response, three genes primarily involved in immune regulation (AIRE, CEACAM1, and OSM), one gene primarily involved in cell proliferation (S100A7), one gene primarily involved in apoptosis (IL24), and one gene primarily involved in complement cascade regulation (CD55). The remaining five were primarily involved in normal cell functions, including hydrolase activity (MPPED1), calcium regulation (RRAD), urea cycle (ARG1), fatty acid oxidation (PPARG), and axonal development (FEZ1). Three of the nine immune genes represented allergic response components, including IgE, mast cells, and eosinophils (CCL26, MS4A2, IL4), whereas three were associated with antigen presentation and Langerhans cells (CD83, CD207, and CD1A): one with macrophages (CHIT1), one with Th17 cells (IL22RA1), and one with T cells (IL26) (Table 3). Pathway analysis was not performed due to the limited scope of the gene set used in this study.
Diagnostic Performance of Gene Expression in Immune Checkpoint Inhibitor–Associated T Cell–Mediated Rejection versus Immune Checkpoint Inhibitor–Associated Acute Interstitial Nephritis
Next, we evaluated the potential utility of gene expression for differentiating immune checkpoint inhibitor–associated rejection and AIN. As anticipated, both IFI27 and FOS demonstrated excellent performance in the discovery cohort (TCMR versus ICI-AIN), both individually (P<0.001 and P=0.002, respectively) and as a two-gene set (P<0.001) (Figure 3A). IFI27 displayed an area under the receiver operating characteristic curve (AUC) of 93% (95% confidence interval, 84% to 100%), and FOS had an AUC of 86% (95% confidence interval, 67% to 100%) (Figure 3B).
Figure 3.
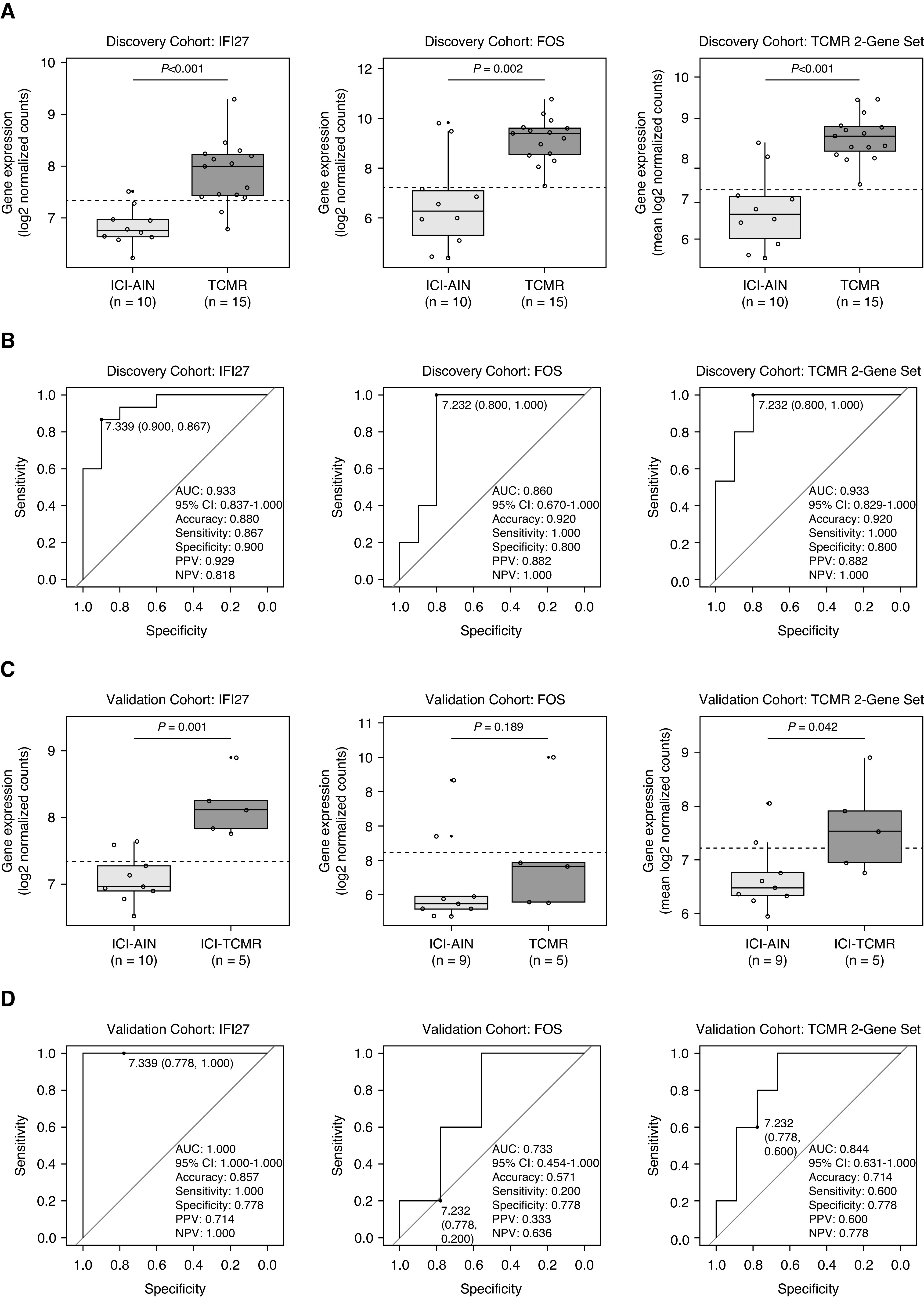
Diagnostic performance of IFI27 and FOS gene expression in the discovery and validation cohorts. (A) Discovery cohort box plots and (B) receiver operating characteristic (ROC) curves demonstrate excellent discrimination between TCMR and ICI-AIN with IFI27, FOS, and average two-gene set expression (accuracy 88%–92%). Diagnostic thresholds derived from the discovery cohort were then assessed in a separate validation cohort comprising immune checkpoint inhibitor–associated T cell–mediated rejection (ICI-TCMR) and ICI-AIN. (C) Validation cohort box plots and (D) ROC curves reveal similarly excellent diagnostic performance with IFI27 (accuracy 86%) but suboptimal performance with FOS (accuracy 57%). AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval; NPV, negative predictive value: PPV, positive predictive value.
Using the discovery cohort–derived diagnostic thresholds, these genes were then evaluated in a separate cohort of ICI-AIN and ICI-TCMR cases (Figure 3, C and D). In this validation cohort, IFI27 again demonstrated very good diagnostic performance (P<0.001, AUC=100%, accuracy =86%, sensitivity =100%, specificity =78%, positive predictive value [PPV] =71%, negative predictive value [NPV] =100%). However, FOS displayed suboptimal diagnostic performance in the validation cohort (P=0.19, AUC=73%, accuracy =57%, sensitivity =20%, specificity =78%, PPV =33%, NPV =64%), as did the two-gene set (P=0.04, AUC=84%, accuracy =71%, sensitivity =60%, specificity =78%, PPV=60%, NPV=78%). These findings were confirmed with volcano plot analysis of the validation cohort (Supplemental Figure 3).
Given the promising validation cohort performance with IFI27, this gene was further evaluated in an expanded cohort of validation sample groups, including Drug-AIN, ICI-GN, BKVN, and normal (Figure 4). IFI27 expression was similar between discovery cohort TCMR and validation cohort ICI-TCMR (P=0.40), both of which had significantly higher expression than all other sample groups (P=0.008). There were no significant differences in IFI27 gene expression between normal, ICI-AIN, Drug-AIN, BKVN, and ICI-GN (P=0.09).
Figure 4.
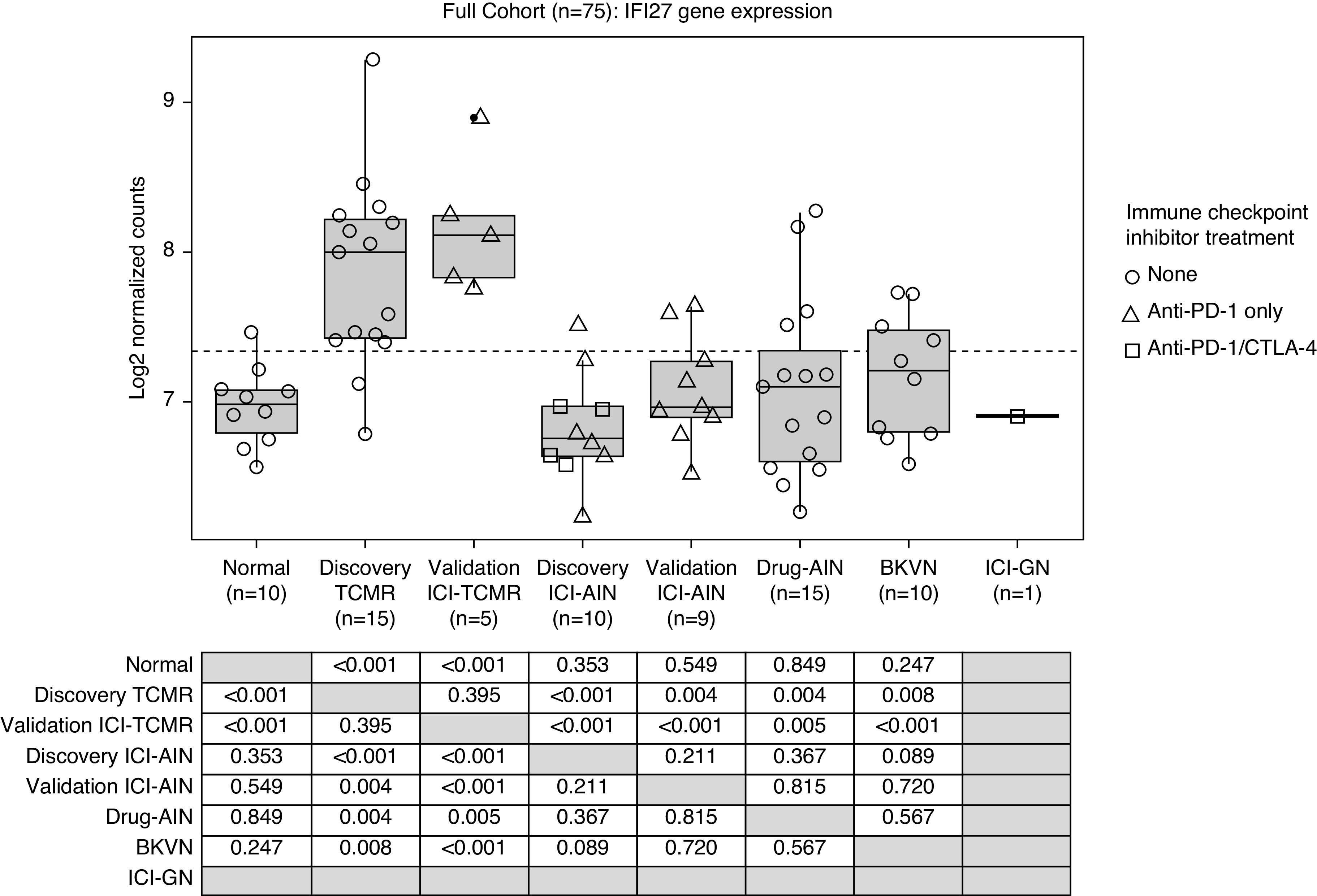
IFI27 gene expression in the full cohort. IFI27 expression is similar between ICI-TCMR and nonimmune checkpoint inhibitor–associated TCMR (validation ICI-TCMR versus discovery TCMR; P=0.40), both of which demonstrate significantly higher expression than all other groups (P=0.008). There is no significant difference in IFI27 gene expression between normal, ICI-AIN, Drug-AIN, BK virus nephropathy (BKVN), and ICI-GN biopsies (P=0.09).
Alloimmune and Hypersensitivity Gene Expression Patterns in Immune Checkpoint Inhibitor–Associated T Cell–Mediated Rejection and Immune Checkpoint Inhibitor–Associated Acute Interstitial Nephritis
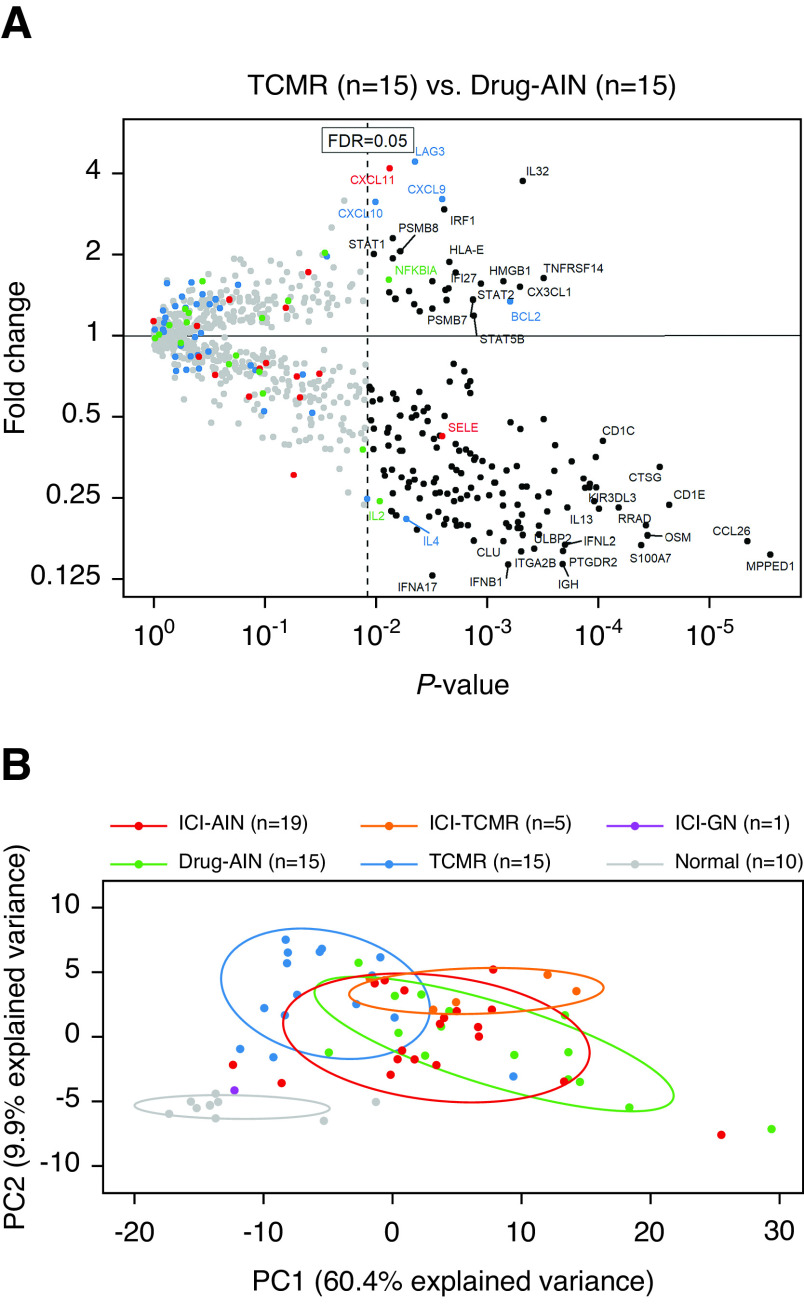
To further explore the differences and similarities in inflammatory gene expression between immune checkpoint inhibitor–associated rejection and AIN versus nonimmune checkpoint inhibitor–associated rejection and AIN, transcriptional signatures of alloimmune and hypersensitivity responses were evaluated in these entities. Differential expression analysis between TCMR and Drug-AIN was first performed for all 725 experimental genes to identify transcripts most representative of these pathophysiologic processes (Figure 5A). Thirty genes were found to be statistically significantly higher (FDR<0.05) in TCMR (Supplemental Table 2), and 140 were higher in Drug-AIN (Supplemental Table 3). Principal component analysis evaluating the expression of these 170 genes in the full study cohort demonstrated significant overlap between non-normal sample groups, as well as significant heterogeneity within them (Figure 5B). Overall, however, ICI-TCMR and ICI-AIN both displayed relatively more overlap with Drug-AIN than TCMR, suggesting potential dominance of hypersensitivity mechanisms in these entities.
Figure 5.
Alloimmune and hypersensitivity gene expression patterns in immune checkpoint inhibitor–associated adverse events. (A) The volcano plot shows fold change (y axis) versus linear regression P value (x axis) between TCMR and Drug-AIN for 725 immune-related genes. Blue, red, and green points represent genes previously associated with TCMR, antibody-mediated rejection, and immune checkpoint inhibitor pathways, respectively. After correction for multiple comparisons, 30 genes are statistically significantly higher in TCMR, and 140 are higher in Drug-AIN (FDR<0.05). (B) Principal component (PC) analysis evaluating the expression of these 170 genes in the full study cohort demonstrates significant overlap between non-normal sample groups; however, ICI-TCMR and ICI-AIN both display relatively more overlap with Drug-AIN than TCMR, suggesting that their gene expression profiles are more reflective of a hypersensitivity than an alloimmune phenomenon.
Discussion
Current data indicate that 40%–50% of patients with kidney transplants on immune checkpoint inhibitors will experience rejection (8,22). Although AIN in native kidneys is generally responsive to corticosteroid therapy, immune checkpoint inhibitor–associated rejection is often severe, and up to 60%–80% of patients will lose their graft despite corticosteroid pulse treatment (22,23). The first step in advancing our ability to manage this complication is to improve the diagnosis of such cases.
In this study, we identified IFI27 as a novel biomarker for differentiating ICI-TCMR from ICI-AIN. IFI27 (IFN-α–inducible protein 27), also known as ISG12A (IFN-stimulated gene 12a protein), is one of the most highly induced genes by IFN-α (24). The protein it encodes is involved in type 1 IFN-induced apoptosis (24 –26), inhibition of vascular response to injury (27), and innate immune response to hepatitis C virus (28,29). IFI27 has previously been demonstrated to be upregulated in TCMR in small bowel transplants (30). Although it has not specifically been reported to be upregulated in kidney transplant TCMR, several related IFN-α–induced genes have been, including IFIH1, IFIT1–4, IRF1/8/7, and ISG15/G1P2 (31 –33). IFN-inducible genes have also consistently been shown to be associated with antibody-mediated rejection in kidney and heart transplants, although not IFI27 in particular (16,34). The reason for IFI27 being identified for the first time in this study may be due to the novel direct comparison with AIN and/or improved sensitivity for this transcript with the NanoString platform.
Of note, there was no significant difference in IFI27 gene expression between Banff grades 1 and 2 acute TCMR in our study, suggesting that its expression represents underlying pathophysiologic mechanisms rather than immune response severity. In addition, higher expression in rejection versus BKVN biopsies further supports its association with pathophysiology rather than post-transplant status and immunosuppression use. However, despite this promising preliminary validation, further work at both the gene and protein level is warranted to confirm the clinical utility of IFI27. The feasibility of translating this biomarker to a more rapid, affordable, and less invasive methodology, such as urine- or blood-based quantitative RT-PCR, should also be considered.
Despite the discovery of IFI27 as a potential novel biomarker, the observation that almost all genes evaluated in this study were not significantly differentially expressed between ICI-AIN and TCMR suggests that there is a high degree of inflammatory molecular overlap between these entities, mirroring their histologic similarities. The top relatively differentially expressed genes did reflect patterns of molecular heterogeneity in keeping with their purported pathophysiologic mechanisms, although the possibility of this being influenced by other cancer treatments or malignancy itself cannot be excluded. Many of the top relatively upregulated transcripts in TCMR were associated with types 1 and 2 IFN signaling (including IFI27), T cell and NK cell functions, and TNF superfamily members, consistent with previously reported molecular signatures of kidney transplant rejection (13,19,31,32). The top relatively upregulated genes in ICI-AIN included transcripts associated with allergic response components (i.e., IgE, mast cells, and eosinophils) consistent with the hypersensitivity phenomena believed to occur in Drug-AIN (35). Indeed, Cortazar et al. (4) reported the use of nephrotoxic drugs (i.e., proton pump inhibitors) as a risk factor for ICI-AIN, suggesting that immune checkpoint inhibitors may unleash the hypersensitivity reaction to these medications and lead to mononuclear cell infiltration. Interestingly, principal component analysis performed in this study demonstrated ICI-AIN and ICI-TCMR to have relatively more molecular overlap with Drug-AIN than TCMR, suggesting a dominant hypersensitivity (rather than alloimmune) mechanism in ICI-AIN and ICI-TCMR. The clinical significance of this preliminary finding is uncertain, however, particularly given the poor response to steroid treatment in immune checkpoint inhibitor–associated rejection compared with AIN (23). Further analysis of the novel comparison between TCMR and AIN presented in this study is ongoing and will be the focus of a separate manuscript.
The nonimmune checkpoint inhibitor–associated TCMR group in our study demonstrated relatively younger patient age, less interstitial fibrosis and tubular atrophy, and shorter time post-transplant compared with the immune checkpoint inhibitor–treated groups. This most likely reflects the fact that older individuals tend to develop the malignancies for which immune checkpoint inhibitors are indicated. Of note, mast cell transcripts have previously been reported to be associated with interstitial fibrosis and tubular atrophy (36) and were identified among the top relatively upregulated genes in ICI-AIN versus TCMR (e.g., MS4A2) in our analysis. Therefore, it is possible that differences in gene expression between these groups reflect the extent of parenchymal scarring, rather than underlying etiology, although identification of other nonmast cell–specific hypersensitivity genes among the top upregulated transcripts in ICI-AIN (e.g., CCL26 and IL4) argues against this (37,38). It is also possible that longer lifetime exposure to immunosuppression in the ICI-TCMR group may have resulted in an attenuated alloimmune molecular response, which will require the recruitment of more closely matched TCMR and ICI-TCMR cohorts in the future to fully delineate.
The strengths of this study include the stringently selected cases of pure (native kidney) ICI-AIN and pure (nonimmune checkpoint inhibitor–associated) TCMR included in the discovery cohort, which allowed us to exclude the possibility of concomitant rejection in the former and ICI-AIN in the latter. Limitations include the relatively small number of ICI-TCMR cases, which reflects the rarity of biopsy-proven examples of these entities, particularly with residual tissue available for molecular analysis. We endeavored to mitigate this by soliciting cases from multiple international collaborators but were ultimately able to acquire only five samples with sufficient residual material. The possibility of misdiagnosis in our study cohort, particularly with the ICI-TCMR cases, cannot be definitively excluded. However, the five examples of this entity that were included were labeled as such on the basis of the presence of intimal arteritis (in addition to tubulointerstitial inflammation), which would not be expected in pure ICI-AIN and was thus taken as evidence of an alloimmune process. Another limitation is that a maximum of only 800 genes could be analyzed with the NanoString nCounter platform, which is significantly less than the tens of thousands possible with microarrays and RNA sequencing (39). In particular, only 39 previously reported TCMR-associated transcripts were assessed. Nevertheless, previous studies have demonstrated the highly stereotyped nature of inflammatory molecular signals in kidney transplant biopsies (12,40), and analysis of a carefully selected panel of representative genes, as in this study, is likely adequate.
In conclusion, this study provides a novel analysis of gene expression in ICI-AIN and rejection. Despite observing significant molecular overlap between these entities, we identified IFI27 as a potential biomarker for discriminating them. However, on a broader inflammatory gene expression level, we also observed significant heterogeneity within these entities, with both exhibiting relatively more similarity with traditional AIN than TCMR. Further investigation of these novel findings is warranted, with these results providing a framework for advancing our understanding of the molecular mechanisms underlying immune checkpoint inhibitor–related AKI.
Disclosures
B.A. Adam reports employment with Alberta Precision Laboratories. C.L. Boils reports employment with Arkana Laboratories. P.D. Hill reports employment with Imperial College Healthcare; receiving honoraria from Genzyme, Otsuka, Roche, and Sanofi; and serving as the chairman of the West London Hospitals Holiday Dialysis Trust. M. Mengel reports consultancy agreements with Novartis and Vitaeris Inc.; ownership interest with Roche; receiving honoraria from CSL Behring, Novartis, and Vitaeris; and serving as a scientific advisor or member of the American Journal of Transplantation editorial board and Novartis. N. Murakami reports receiving honoraria from Natera Inc. G. Reid reports employment with the Manchester University National Health Service (NHS) Foundation Trust. K. Renaudin reports receiving honoraria from GSK and MSD. L.V. Riella reports receiving research funding from Brystol-Meyers Squibb, CareDx, Natera Inc., and Visterra; receiving honoraria from CareDx; and serving as a scientific advisor or member of CareDx. C. Roufosse reports consultancy agreements with Achillion, Rigel Pharmaceutical, and UCB. A. Weins reports consultancy agreements with Expansion Technologies, Inc. and Goldfinch Bio. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank Ms. Shalawny Miller and Ms. Kim Formenti for outstanding technical support.
B.A. Adam, M. Mengel, N. Murakami, A.G. Murray, L.V. Riella, and K. Wen designed the study; B.A. Adam, C.L. Boils, L. Bu, K. Du, P.D. Hill, R. Jasim, N. Murakami, G. Reid, K. Renaudin, C. Roufosse, and A. Weins acquired the data and carried out the experiments; B.A. Adam, M. Mengel, and N. Murakami analyzed the data and drafted the manuscript; and all authors critically revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Gene Expression Profiling in Kidney Transplant Recipients on Immune Checkpoint Inhibitors: More than Meets the Eye,” on pages 1315–1317.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00920121/-/DCSupplemental.
Supplemental Figure 1. Expression of T cell–mediated rejection 39-gene set and GZMB in the discovery cohort.
Supplemental Figure 2. IFI27 and FOS gene expression in the discovery cohort T cell–mediated rejection according to Banff grade.
Supplemental Figure 3. Differential gene expression in the validation cohort.
Supplemental Table 1. Twenty-five custom genes analyzed with the NanoString PanCancer Immune Profiling Panel.
Supplemental Table 2. Significantly differentially expressed genes in T cell–mediated rejection (n=15) versus nonimmune checkpoint inhibitor drug–associated acute interstitial nephritis (n=15).
Supplemental Table 3. Significantly differentially expressed genes in nonimmune checkpoint inhibitor drug–associated acute interstitial nephritis (n=15) versus T cell–mediated rejection (n=15).
References
- 1.Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wahab N, Shah M, Suarez-Almazor ME: Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 11: e0160221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, Cortazar FB, Leaf DE, Mooradian MJ, Villani AC, Sullivan RJ, Reynolds K, Sise ME: The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1692–1700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE: Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J Am Soc Nephrol 31: 435–446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boils CL, Aljadir DN, Cantafio AW: Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant 16: 2496–2497, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Deltombe C, Garandeau C, Renaudin K, Hourmant M: Severe allograft rejection and autoimmune hemolytic anemia after anti-PD1 therapy in a kidney transplanted patient. Transplantation 101: e291, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Murakami N, Motwani S, Riella LV: Renal complications of immune checkpoint blockade. Curr Probl Cancer 41: 100–110, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Cortazar FB, Riella LV, Leaf DE: Immune checkpoint inhibitor nephrotoxicity: Update 2020. Kidney360 1: 130–140, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perazella MA, Shirali AC: Nephrotoxicity of cancer immunotherapies: Past, present and future. J Am Soc Nephrol 29: 2039–2052, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M: The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant 20: 2318–2331, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halloran PF, Famulski KS, Reeve J: Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol 12: 534–548, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, Famulski KS: Review: The transcripts associated with organ allograft rejection. Am J Transplant 18: 785–795, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, Panting L, Jure-Kunkel M, Ji RR: Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 11: 75, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K: Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26: 317–325, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Adam B, Afzali B, Dominy KM, Chapman E, Gill R, Hidalgo LG, Roufosse C, Sis B, Mengel M: Multiplexed color-coded probe-based gene expression assessment for clinical molecular diagnostics in formalin-fixed paraffin-embedded human renal allograft tissue. Clin Transplant 30: 295–305, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Adam BA, Smith RN, Rosales IA, Matsunami M, Afzali B, Oura T, Cosimi AB, Kawai T, Colvin RB, Mengel M: Chronic antibody-mediated rejection in nonhuman primate renal allografts: Validation of human histological and molecular phenotypes. Am J Transplant 17: 2841–2850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam BA, Kikic Z, Wagner S, Bouatou Y, Gueguen J, Drieux F, Reid G, Du K, Bräsen JH, D’Agati VD, Drachenberg CB, Farkash EA, Brad Farris A, Geldenhuys L, Loupy A, Nickeleit V, Rabant M, Randhawa P, Regele H, Mengel M: Intragraft gene expression in native kidney BK virus nephropathy versus T cell-mediated rejection: Prospects for molecular diagnosis and risk prediction. Am J Transplant 20: 3486–3501, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Reeve J, Sellarés J, Mengel M, Sis B, Skene A, Hidalgo L, de Freitas DG, Famulski KS, Halloran PF: Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant 13: 645–655, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Youden WJ: Index for rating diagnostic tests. Cancer 3: 32–35, 1950 [DOI] [PubMed] [Google Scholar]
- 21.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, Duong van Huyen JP, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manohar S, Thongprayoon C, Cheungpasitporn W, Markovic SN, Herrmann SM: Systematic review of the safety of immune checkpoint inhibitors among kidney transplant patients. Kidney Int Rep 5: 149–158, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, Abdelrahim M, Khairallah P, Shirazian S, Kukla A, Owoyemi IO, Alhamad T, Husami S, Menon M, Santeusanio A, Blosser CD, Zuniga SC, Soler MJ, Moreso F, Mithani Z, Ortiz-Melo D, Jaimes EA, Gutgarts V, Lum E, Danovitch GM, Cardarelli F, Drews RE, Bassil C, Swank JL, Westphal S, Mannon RB, Shirai K, Kitchlu A, Ong S, Machado SM, Mothi SS, Ott PA, Rahma O, Hodi FS, Sise ME, Gupta S, Leaf DE, Devoe CE, Wanchoo R, Nair VV, Schmults CD, Hanna GJ, Sprangers B, Riella LV, Jhaveri KD; Immune Checkpoint Inhibitors in Solid Organ Transplant Consortium: A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 100: 196–205, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosebeck S, Leaman DW: Mitochondrial localization and pro-apoptotic effects of the interferon-inducible protein ISG12a. Apoptosis 13: 562–572, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Gytz H, Hansen MF, Skovbjerg S, Kristensen AC, Hørlyck S, Jensen MB, Fredborg M, Markert LD, McMillan NA, Christensen EI, Martensen PM: Apoptotic properties of the type 1 interferon induced family of human mitochondrial membrane ISG12 proteins. Biol Cell 109: 94–112, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Liu N, Zuo C, Wang X, Chen T, Yang D, Wang J, Zhu H: miR-942 decreases TRAIL-induced apoptosis through ISG12a downregulation and is regulated by AKT. Oncotarget 5: 4959–4971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papac-Milicevic N, Breuss JM, Zaujec J, Ryban L, Plyushch T, Wagner GA, Fenzl S, Dremsek P, Cabaravdic M, Steiner M, Glass CK, Binder CJ, Uhrin P, Binder BR: The interferon stimulated gene 12 inactivates vasculoprotective functions of NR4A nuclear receptors. Circ Res 110: e50–e63, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Xue B, Yang D, Wang J, Xu Y, Wang X, Qin Y, Tian R, Chen S, Xie Q, Liu N, Zhu H: ISG12a restricts hepatitis C virus infection through the ubiquitination-dependent degradation pathway. J Virol 90: 6832–6845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Jiao B, Yao M, Shi X, Zheng Z, Li S, Chen L: ISG12a inhibits HCV replication and potentiates the anti-HCV activity of IFN-α through activation of the Jak/STAT signaling pathway independent of autophagy and apoptosis. Virus Res 227: 231–239, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Bradley SP, Banner B, Elias G, Pahari MP, Brown M, Rastellini C, Cicalese L: Genetic expression profile during acute cellular rejection in clinical intestinal transplantation. Transplantation 86: 998–1001, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Famulski KS, Einecke G, Reeve J, Ramassar V, Allanach K, Mueller T, Hidalgo LG, Zhu LF, Halloran PF: Changes in the transcriptome in allograft rejection: IFN-gamma-induced transcripts in mouse kidney allografts. Am J Transplant 6: 1342–1354, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Famulski KS, Einecke G, Sis B, Mengel M, Hidalgo LG, Kaplan B, Halloran PF: Defining the canonical form of T-cell-mediated rejection in human kidney transplants. Am J Transplant 10: 810–820, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Halloran PF, de Freitas DG, Einecke G, Famulski KS, Hidalgo LG, Mengel M, Reeve J, Sellares J, Sis B: The molecular phenotype of kidney transplants. Am J Transplant 10: 2215–2222, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Loupy A, Duong Van Huyen JP, Hidalgo L, Reeve J, Racapé M, Aubert O, Venner JM, Falmuski K, Bories MC, Beuscart T, Guillemain R, François A, Pattier S, Toquet C, Gay A, Rouvier P, Varnous S, Leprince P, Empana JP, Lefaucheur C, Bruneval P, Jouven X, Halloran PF: Gene expression profiling for the identification and classification of antibody-mediated heart rejection. Circulation 135: 917–935, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Koda R, Watanabe H, Tsuchida M, Iino N, Suzuki K, Hasegawa G, Imai N, Narita I: Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: A case report. BMC Nephrol 19: 48, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mengel M, Reeve J, Bunnag S, Einecke G, Sis B, Mueller T, Kaplan B, Halloran PF: Molecular correlates of scarring in kidney transplants: The emergence of mast cell transcripts. Am J Transplant 9: 169–178, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Moledina DG, Wilson FP, Pober JS, Perazella MA, Singh N, Luciano RL, Obeid W, Lin H, Kuperman M, Moeckel GW, Kashgarian M, Cantley LG, Parikh CR: Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4: e127456, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moledina DG, Wilson FP, Kukova L, Obeid W, Luciano R, Kuperman M, Moeckel GW, Kashgarian M, Perazella MA, Cantley LG, Parikh CR: Urine interleukin-9 and tumor necrosis factor-α for prognosis of human acute interstitial nephritis [published online ahead of print October 28, 2020]. Nephrol Dial Transplant 10.1093/ndt/gfaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, Clahsen-van Groningen MC, Dagobert J, Demetris AJ, Duong van Huyen JP, Gueguen J, Issa F, Robin B, Rosales I, Von der Thüsen JH, Sanchez-Fueyo A, Smith RN, Wood K, Adam B, Colvin RB: Banff 2019 Meeting Report: Molecular diagnostics in solid organ transplantation-Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant 20: 2305–2317, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller TF, Einecke G, Reeve J, Sis B, Mengel M, Jhangri GS, Bunnag S, Cruz J, Wishart D, Meng C, Broderick G, Kaplan B, Halloran PF: Microarray analysis of rejection in human kidney transplants using pathogenesis-based transcript sets. Am J Transplant 7: 2712–2722, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.