Abstract
Fatigue is a commonly reported and debilitating symptom among patients with CKD, yet little is known about its epidemiology, pathogenesis, and treatment. Various measurement tools have been used in published studies to identify and quantify fatigue. These include several single-item measures embedded in longer questionnaires for assessing depression, quality of life, or symptom burden in patients with kidney disease. Approximately 70% of patients with CKD report fatigue, with up to 25% reporting severe symptoms. Patient-reported fatigue is associated with death, dialysis initiation, and hospitalization among individuals with CKD. The pathophysiology is multifactorial and likely includes decreased oxygen delivery and increased reliance on anaerobic metabolism, thus generating lactic acidosis in response to exertion; the effects of chronic metabolic acidosis and hyperphosphatemia on skeletal muscle myocytes; protein-energy wasting and sarcopenia; and depression. Physical activity has been shown to improve fatigue in some small but promising trials, and so should be recommended, given the additional benefits of exercise. Targeting higher hemoglobin levels with erythropoiesis-stimulating agents may improve fatigue, but potential adverse cardiovascular effects preclude their use to solely treat fatigue without the presence of another indication. Current guidelines recommend cautious individualization of hemoglobin targets for those at low cardiovascular risk who still experience fatigue or functional limitation despite a hemoglobin level of 10 g/dl. Sodium bicarbonate supplementation for the treatment of metabolic acidosis may also improve functional status. Selective serotonin reuptake inhibitors have not been consistently shown to improve fatigue in patients with kidney disease, but an ongoing trial will evaluate the effect of alternative antidepressant drug and behavioral activation therapy on fatigue in patients with CKD. Overall, more research is needed to further clarify underlying mechanisms of fatigue and identify effective, targeted treatments for patients with CKD.
Keywords: fatigue, chronic kidney disease, sarcopenia, protein-energy wasting, depression, functional impairment, anemia
Introduction
Fatigue has been increasingly recognized as an important symptom in patients with chronic medical illnesses, including kidney failure requiring maintenance dialysis, with a recent prioritization of identification, quantification, and further study of fatigue in patients receiving hemodialysis (1). Contemporary studies have also identified fatigue as a debilitating symptom in patients with nondialysis CKD (2 –4). The purpose of this review is to consolidate known information about the identification, epidemiology, pathophysiology, and potential treatments of fatigue in patients with CKD.
Defining, Identifying, and Measuring Fatigue
Definition
Concrete definitions of fatigue are elusive. It is a complex, multidimensional, subjective experience that encompasses both physical and psychologic symptoms. It is usually described by patients as extreme and persistent tiredness, weakness, exhaustion, or lack of energy that is out of proportion to their degree of exertion, and may interfere with physical functioning (5 –8). Physical symptoms of fatigue include muscle weakness or poor endurance, whereas psychologic symptoms include a sense of increased effort or diminished cognitive endurance (7). Fatigue in CKD, as in other chronic diseases, is multifactorial and may manifest differently between individual patients.
Identification and Measurement
There is currently no consensus about the best tools to measure fatigue in patients with CKD. Table 1 provides a summary of fatigue scales used in studies of patients with CKD, and designates whether these scales were also used in studies of patients with kidney failure. Most available questionnaires use Likert scales to generate a score to quantify fatigue. They range from one to 13 questions, with some scales assessing various components of fatigue and others asking one global question about the presence and severity of fatigue, tiredness, or energy level. Of the measures studied in patients with CKD, the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) is the only multi-item survey designed to ascertain multiple dimensions of fatigue (9). Most of the other surveys are single items embedded within longer questionnaires designed to ascertain depression, quality of life, or symptom burden. Briefer measures have the benefit of being simple, fast, and easy to administer, and can be obtained when assessing other important symptoms. It is unclear whether the isolated use of fatigue items from these questionnaires reflects the complex nature of fatigue, or whether these various measures elicit different aspects (e.g., physical versus psychologic) of this multifaceted symptom.
Table 1.
Fatigue measurement tools that have been used in studies of patients with CKD
| Measurement Tool | No. of Fatigue Questions | Scoring | Comments |
| Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) (9) | 13 | Each item is a five-point scale; total score 0–52, with lower scores indicating worse fatigue | Scale is designed to assess fatigue, rather than being a single-item of a scale designed for other purposes. Score ≤44 frequently used to indicate fatigue. Scale has also been used in patients with kidney failure |
| CKD Symptom Burden Index (10) fatigue item | 1 | Presence (yes or no) and ten-point scales for severity, distress, or frequency; total score for each domain 0–10, with higher score indicating worse symptoms | Single item of a questionnaire designed to assess the burden of multiple symptoms among patients with CKD stages 4 and 5; derived from the Dialysis Symptom Index. Scale has also been used in patients with kidney failure |
| Patient Outcome Scale (4,11) | 1 | Each item is a five-point Likert scale from 1 (not at all bothersome) to 5 (very bothersome) | Single item of a 17-item questionnaire that was originally created for palliative care patients and modified for patients with kidney failure |
| Dialysis Symptom Index (12) | 1 | Presence (yes or no) and five-point Likert scale to assess fatigue severity, with a higher score indicating more severe fatigue | Single item of a questionnaire designed to assess the burden of 30 symptoms among patients with kidney failure on dialysis. Scale has also been used in patients with kidney failure. |
| Memorial Symptom Assessment Scale Short Form (13) lack of energy item | 1 | Likert scale from 0 (not at all) to 4 (very much) referring to the amount fatigue distressed the patient | Single item of a questionnaire evaluating the burden of 32 symptoms |
| Edmonton Symptom Assessment Scale (14) fatigue item | 1 | Symptom severity rated from 0 to 10 on a visual analog scale | Single item of a questionnaire evaluating eight symptoms |
| Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR16) (15) fatigue item | 1 | Likert scale from 0 (no fatigue) to 3 (severe fatigue) | Single item of a validated depression scale (16); score ≥1 associated with death, dialysis initiation, or hospitalization among patients with CKD (2) |
| Beck Depression Inventory (BDI) (17) fatigue item | 1 | Likert scale from 0 (no fatigue) to 3 (severe fatigue) | Single item of a validated depression scale (16) |
| 12-Item Short Form Health Survey (SF-12) (18) vitality scale | 1 | Six-point Likert scale with higher scores indicating more severe fatigue | Fatigue score is derived from one item from a questionnaire designed to evaluate eight domains of quality of life |
| 36-Item Short Form Health Survey (SF-36) (19) vitality scale | 4 | Each item is a six-point Likert scale; total score 0–100, with higher scores indicating worse fatigue | Fatigue score is derived from four items from a questionnaire designed to evaluate eight domains of quality of life. Scale has also been used in patients with kidney failure |
Given the variability in these measures, larger studies are needed to validate an easily administered tool that can be reliably used for both clinical care and research. As has been prioritized for patients with kidney failure, a short, simple metric that reflects fatigue severity and its effect on ability to participate in activities, and is shown to be associated with adverse outcomes, may have the greatest value (1,20). The Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) group recently developed and validated a novel, three-item fatigue measure in patients on maintenance hemodialysis, which has not yet been studied in patients with nondialysis CKD (21). Until more research identifies an optimal measure, we suggest using the FACIT-F for screening in patients with CKD, given that this questionnaire is fatigue-specific, easy to administer, studied in this patient population (22,23), and currently being used in National Institutes of Health–sponsored clinical trials of patients with kidney disease (24,25). Alternatively, single-item measures, such as the fatigue question on the 16-item self-reported Quick Inventory of Depressive Symptomatology Self Report (QIDS-SR16) or the 12-item Short Form Health Survey (SF-12), may be useful for identifying fatigue because of associations with clinically meaningful outcomes, as detailed below.
Epidemiology
Prevalence and Severity
Fatigue is among the most common and most distressing symptoms for patients with kidney disease (26), who may experience more fatigue than healthy people as early as CKD stages 2–3. Fatigue affects 20%–91% of patients with CKD, and the prevalence increases with advancing CKD stages (27 –29). This variability in prevalence is likely due to differences in samples and the instruments used (Figure 1) (2 –4,22,23,26,29 –33). The majority of reported fatigue appears to be mild to moderate, with 5%–24% of patients reporting severe fatigue (Figure 1). The study reporting the lowest prevalence defined fatigue to be present if a patient scored ≤24 points on the FACIT-F (23), which is stricter than the cutoff of ≤44 used in other studies and likely identified only individuals with severe fatigue (22). The study reporting the highest prevalence sampled individuals with CKD stage 5 who declined to initiate dialysis, and measured fatigue on a scale that was not used in other studies (31). In sum, approximately two-thirds to three-quarters of patients with CKD experience fatigue, with as many as one in four reporting severe symptoms.
Figure 1.
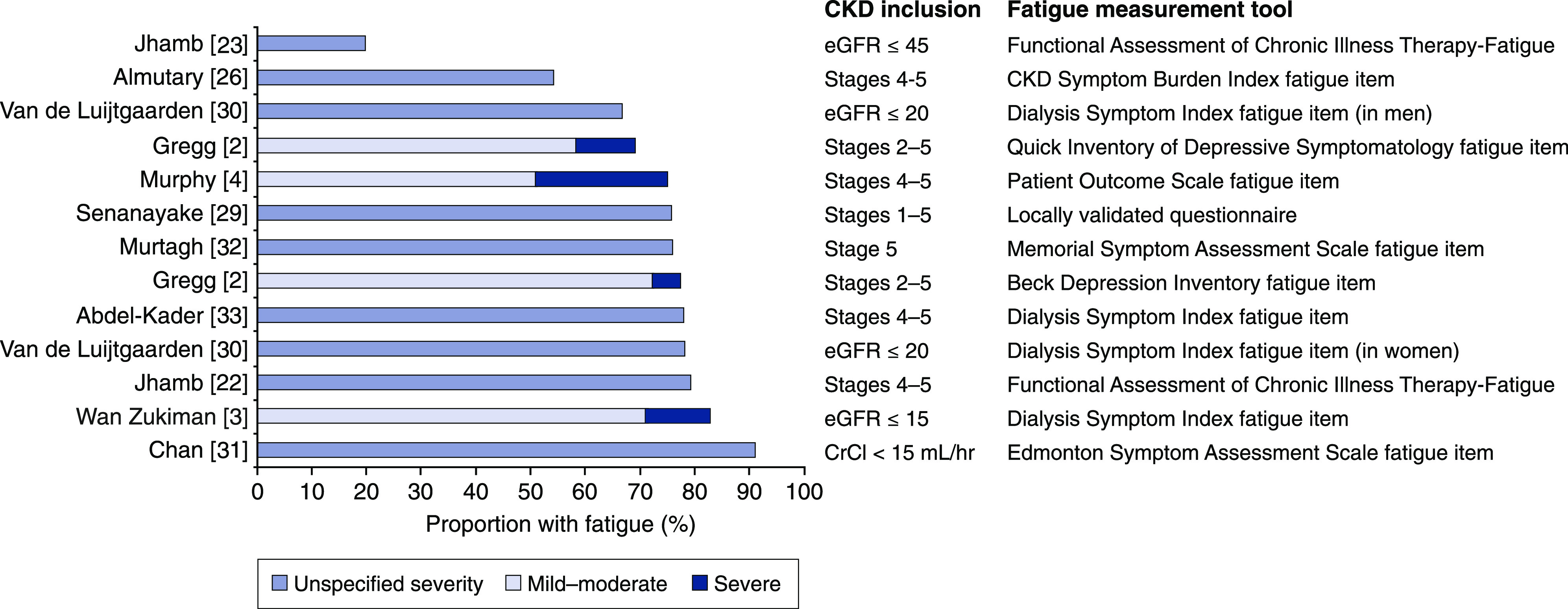
Fatigue is prevalent in approximately two-thirds to three-quarters of patients with CKD. When reported, symptom severity is denoted. CKD inclusion refers to the inclusion criteria for the samples with CKD that were included in each study; eGFR units are ml/min per 1.73 m2. CrCl, creatinine clearance.
Association with Death, Hospitalization, and Kidney Failure
Fatigue is associated with mortality in patients with kidney failure (34 –37). Only two prospective cohort studies investigated the associations of fatigue with clinically important outcomes in patients with CKD. In 433 individuals with CKD stages 4 and 5, there was a marginal association between fatigue and dialysis initiation within 6 months (38). A prospective study of 266 consecutively recruited outpatients with CKD stages 2–5 reported that the presence of any fatigue versus none, as measured by the fatigue item on the QIDS-SR16 depression questionnaire, and fatigue severity measured by the SF-12 were independently associated with a composite of death, hospitalization, or dialysis initiation (2).
Pathophysiology
Studies investigating the pathophysiology and correlates of fatigue in patients with kidney disease suggest that multiple abnormalities contribute to fatigue. Cross-sectional associations of fatigue with demographic characteristics, comorbidities, and laboratory variables have been demonstrated in some studies (Table 2). Studies of the pathophysiology of muscular fatigue have also investigated potential causative factors, such as impaired oxygen delivery, altered cardiovascular response to exertion, and metabolic acidosis (Figure 2).
Table 2.
Studies of fatigue prevalence and associated factors in patients with nondialysis CKD
| Study | Sample | Fatigue Scale and Definition | Clinical Factors Associated with Fatigue |
| Mujais et al. (28) | CKD stages 3–5 (n=1186) | SF-36 fatigue subscale, used as a continuous variable | Female sex, diabetes, history of myocardial infarction, hypoalbuminemia. Increase in hemoglobin over follow-up was associated with improved fatigue |
| Pagels et al. (27) | CKD stages 2–5 (n=535) | SF-36 fatigue subscale, used as a continuous variable | Advancing CKD stage |
| Jhamb et al. (23) | CKD (n=87) and kidney failure (n=86) | FACIT-F, high fatigue if ≤24 | Cardiovascular disease, benzodiazepine use, lower hemoglobin and albumin, depression, poor sleep quality, daytime sleepiness, restless leg syndrome |
| Manavalan et al. (39) | CKD stages 3–5 (n=162) and kidney failure (n=42) | SF-36 fatigue subscale, used as a continuous variable | Advancing CKD stage |
| Oh et al. (40) | CKD stages 1–5 (n=1844) | SF-36 fatigue subscale, used as a continuous variable | Vitamin D deficiency |
| van de Luijtgaarden et al. (30) | CKD with eGFR newly ≤20 ml/min per 1.73 m2 within 6 mo (n=1479) | Dialysis Symptom Index, cutoff not specified to define fatigue | Female sex |
| Gregg et al. (2) | CKD stages 2–5 (n=266) | QIDS-SR16 fatigue item, fatigue present if ≥1 | Employment, antidepressant medication use |
| BDI fatigue item, fatigue present if ≥1 | Employment, no. of comorbidities, depression, antidepressant medication use, lower hemoglobin | ||
| SF-12 fatigue item, used as a continuous variable | Employment, diabetes mellitus, number of comorbidities, depression, alcohol or drug abuse, β-blocker, antidepressant medication use, lower hemoglobin, lower albumin | ||
| Wang et al. (41) | CKD stages 1–5 (n=1163) | SF-12 fatigue item, used as a continuous variable | Depression |
| Gregg et al. (42) | CKD stages 3b–5 and major depressive disorder (n=201) | QIDS-SR16 fatigue item, used as a continuous variable | hsCRP |
| SF-36 fatigue subscale, used as a continuous variable | Not associated with inflammatory biomarkers | ||
| Nixon et al. (43) | CKD stages 4 and 5 (n=60) and kidney failure (n=30) | SF-36 fatigue subscale, used as a continuous variable | Frailty |
SF-36, 36-item Short-Form Health Survey; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology Self-Report; BDI, Beck Depression Inventory; SF-12, 12-item Short-Form Health Survey; hsCRP, high-sensitivity C-reactive protein.
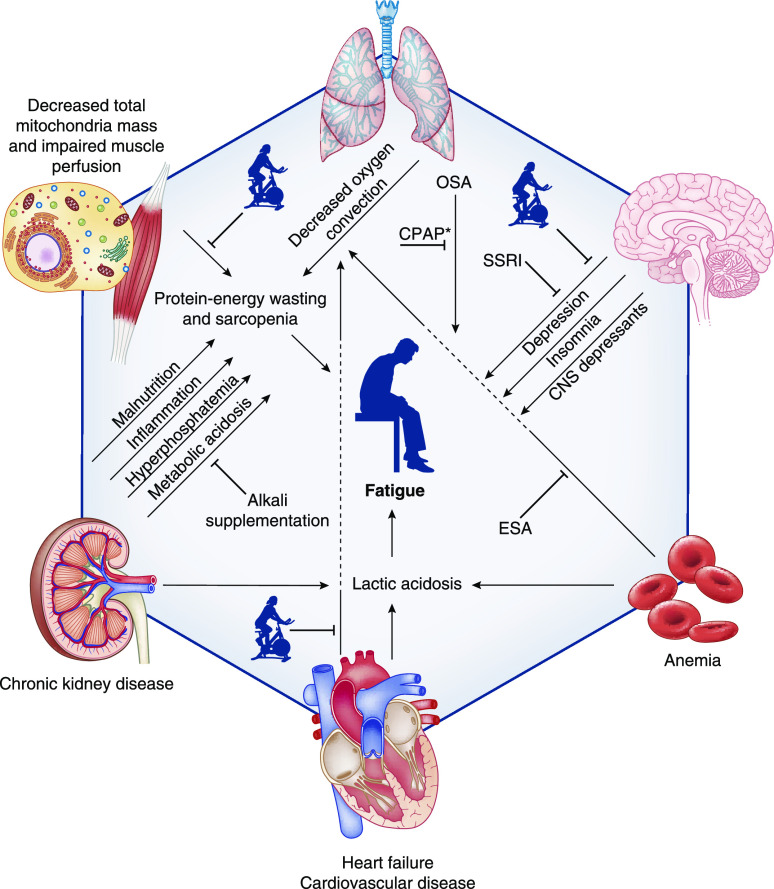
Figure 2.
Pathogenesis and treatment of fatigue in patients with CKD. The icon of a person on a stationary bike represents physical activity. *Treatment of OSA. Figure 2 was created with BioRender.com. CNS, central nervous system; CPAP, continuous positive airway pressure; ESA, erythropoietin-stimulating agent; OSA, obstructive sleep apnea; SSRI, selective serotonin reuptake inhibitor.
Oxygen Delivery and Lactic Acidosis
Patients with CKD develop lactic acidosis more quickly than healthy controls in response to low levels of physical activity, which may contribute to muscular fatigue (44,45). Patient-reported fatigue parallels higher plasma lactate levels and higher respiratory exchange ratio in patients with CKD, suggesting that increased anaerobic respiration contributes to fatigue pathophysiology (44). Alterations in oxygen transport from the lungs to the skeletal muscle capillaries (oxygen convection), the movement of oxygen from the intravascular to the intracellular space (oxygen conductance), and oxygen utilization in mitochondria have each been studied as possible contributors to lactic acidosis and fatigue.
Anemia and Oxygen Convection.
The combination of anemia and poor compensatory cardiovascular mechanisms leads to decreased oxygen convection, contributing to exertional lactic acidosis and fatigue in patients with CKD, leading to decreased arterial oxygen carrying capacity and impaired total body oxygen delivery. It is, therefore, unsurprising that anemia is one of the most commonly identified risk factors associated with fatigue (Table 2). In patients with CKD stages 2–5, a decrease in hemoglobin of 1 g/dl was associated with increased severity of fatigue on the SF-12, and 19% greater odds of fatigue as measured by the Beck Depression Inventory (2). In other observational studies, hemoglobin was lower in those with high fatigue than those without (11.2 versus 12.0 g/dl; P=0.05) (23), and increased hemoglobin over time was associated with improvement in patient-reported fatigue (28). However, unlike what would be expected in otherwise healthy people with anemia, individuals with CKD may not demonstrate compensatory increases in cardiac output or oxygen extraction to account for decreased arterial oxygen content (44). In patients on maintenance hemodialysis, treatment with an erythropoietin-stimulating agent (ESA) led to an increase in hemoglobin, but a decrease in blood flow to exercising skeletal muscle, indicating a blunting of the hyperdynamic cardiovascular response seen with more severe anemia (46). The common use of β-blockers in this patient population may preclude an increase in heart rate in response to exertion. Another possibility is that systolic and diastolic cardiac dysfunction, common in patients with CKD, may contribute to the inability to increase cardiac output and oxygen delivery in the setting of mild anemia. In patients with an eGFR of 25–60 ml/min per 1.73 m2, global longitudinal strain (a measure of systolic function) correlated with patient-reported fatigue and was independently associated with poorer physical functioning as measured by the 6-minute walk test and the get-up-and-go test (47).
Skeletal muscle blood flow and capillary recruitment may also play a role in decreased oxygen delivery to skeletal muscle. Although it has been hypothesized that inadequate capillary density may contribute to decreased oxygen delivery, muscle biopsy specimens in patients with kidney failure show that the number and morphology of capillaries within skeletal muscles are near normal (48). Insulin resistance may play an important role, as insulin increases capillary recruitment in skeletal muscle to increase glucose uptake in those tissues (49). In animal models, acute insulin resistance has been shown to decrease muscle blood flow and myogenic activity (50). In humans, insulin resistance is associated with a diminished microvascular vasodilatory response (51 –53). Given that insulin-resistant states, such as obesity and diabetes, are frequent causes of CKD, it is likely that this contributes to the pathophysiology of muscle fatigue in this population.
Oxygen Conductance.
Among patients with kidney failure, low muscle oxygen conductance contributes to overall low maximal oxygen uptake (54). Once oxygen arrives in the muscle capillaries, it must dissociate from hemoglobin and move to the mitochondria where it can be used for aerobic respiration. In patients with kidney disease, peak oxygen uptake increases with ESA treatment, but less than expected when compared with a healthy but sedentary control group (46). The end result is that with exercise, net oxygen extraction in individuals with kidney failure treated with an ESA remains similar to that seen before treatment initiation, and lower than that seen in healthy controls, indicating that alterations in oxygen conductance limit aerobic respiration despite improvement in anemia and oxygen convection.
Oxygen Utilization.
Data on muscle mitochondrial metabolism in patients with CKD are conflicting. Two small studies in patients with kidney failure showed that muscle mitochondrial oxidative capacity is not limited (54), and that mitochondrial content is preserved, respiratory activity is conserved, and mitochondrial respiratory chain complexes are intact (55). However, another study demonstrated that patients with nondialysis CKD had decreased mitochondrial mass on skeletal muscle biopsy, and decreased gene expression of transcription factors involved in mitochondrial biogenesis compared with non-CKD controls, likely contributing to overall decreased oxygen utilization (56).
Skeletal Muscle Function
Metabolic Acidosis.
Because of their impaired ability to excrete acid as GFR declines, patients with CKD develop chronic metabolic acidosis, which is associated with functional impairment (57,58). Chronic metabolic acidosis depletes skeletal muscle, bones, and other peripheral tissues of bicarbonate to buffer the elevated acid content, and contributes to ubiquitin-mediated proteolysis in skeletal muscle (59). Chronic metabolic acidosis was associated with more rapid development of exertional muscle fatigue in patients with CKD (60). Although it follows that this would be a result of an inability to buffer lactic acid that is produced in response to exercise, studies have not shown decreased interstitial pH in exercising muscle cells in patients with CKD with versus without acidosis (60). Further studies about the effects of metabolic acidosis on muscle biology, bone remodeling, and oxygen delivery and utilization may improve understanding of this potentially important contributor to fatigue.
Protein-Energy Wasting.
Protein-energy wasting, which encompasses the chronic inflammation, malnutrition, protein catabolism, and metabolic and hormonal abnormalities that accompany worsening kidney function, is thought to contribute to fatigue (61). Sarcopenia, the loss of skeletal muscle mass, is one of the most concerning results of protein-energy wasting and is a strong correlate of poor physical functioning (62). Although not necessarily a feature of kidney disease per se, patients with secondary hyperparathyroidism or other systemic disease may also develop alterations in muscle morphology, such as intramuscular fibrosis and fat infiltration, which may contribute to fatigue (48,62). IGF is also decreased in patients with kidney failure, which may contribute to sarcopenia (63).
Protein deficiency and malnutrition are thought to contribute to sarcopenia and protein-energy wasting, and hypoalbuminemia has been associated with fatigue (2,23,28). In patients on maintenance hemodialysis, fatigue is associated with elevated inflammatory biomarkers such as IL-6 and high-sensitivity C-reactive protein (37,64,65). In the only study that investigated the associations of fatigue with inflammatory biomarkers in patients with CKD stages 3b–5 and major depressive disorder, fatigue ascertained by the QIDS-SR16 depression questionnaire was associated with higher plasma high-sensitivity C-reactive protein, but not with IL-6 or prealbumin (42).
Hyperphosphatemia.
Hyperphosphatemia is thought to contribute to muscular fatigue via its effects on intracellular calcium. Elevated intracellular inorganic phosphate decreases the available amount of calcium ions by binding them and precipitating as calcium phosphate in the sarcoplasmic reticulum (66 –68). The unavailability of calcium ions required for excitation-contraction coupling can contribute to a sensation of fatigue and poor exercise tolerance (67).
Other Associated Factors
Depression.
Depression and fatigue commonly coexist in the general population and among patients on maintenance hemodialysis (69 –73). In fact, one of the nine diagnostic domains of major depressive disorder is the presence of fatigue for at least 2 weeks. Depressive symptoms, depression presence, and antidepressant medication use correlate with fatigue presence and severity in patients with CKD, but these relationships vary depending on the metric used to quantify fatigue (2,23,41). Fatigue and depression may be related by a syndrome connecting depression, inflammation, and atherosclerotic cardiovascular risk, but the pathophysiology of this is not well understood (74).
Obstructive Sleep Apnea.
Obstructive sleep apnea (OSA) is a known cause of fatigue. Several studies reported strong associations between kidney disease and OSA (75), which is prevalent in approximately 40%–64% of patients with CKD (76 –78). Increased sympathetic tone, hypertension, hypoxia, and fluid overload all contribute to the development of OSA in patients with CKD (79).
Potential Treatments
Because of the multifaceted pathophysiology of fatigue and associations with the presence of chronic disease, inflammation, and depression, there are several potential targets for the treatment of fatigue in patients with CKD. So far, clinical trials have focused on physical activity and available treatments for known correlates of fatigue, such as anemia and depression.
Physical Activity
Small clinical trials investigated the effect of physical activity on fatigue. In a 12-week trial of aerobic versus combined aerobic and weight training among 36 patients with CKD stages 3b–5, the FACIT-F fatigue score improved from baseline in both groups, with no difference between groups in improvement (80). In 40 participants with CKD stages 3–4 who were randomized to home-based exercise, center-based exercise, or control, there was improvement in the SF-36 vitality score in the home- and center-based physical activity groups compared with the control group (81). A third trial showed that a 12-week supervised rehabilitation exercise intervention resulted in improvements in both 6-minute walk test and patient-reported fatigue among 59 individuals with CKD stages 3–4, compared with usual care in 48 controls (82). Given the many other benefits of physical activity on cardiovascular profile and mental health, these studies, although small, suggest that exercise may be an effective intervention to improve fatigue and physical functioning.
Erythropoiesis-Stimulating Agents
Few randomized controlled trials suggest that treatment with ESAs to raise hemoglobin may improve fatigue in patients with CKD, which may be because of the central role decreased oxygen convection plays in the setting of anemia. The Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta trial showed improvement in fatigue among those randomized to a hemoglobin target of 13–15 g/dl versus 11–12.5 g/dl (83). The Correction of Hemoglobin and Outcomes in Renal Insufficiency trial showed that targeting a hemoglobin level of 13.5 g/dl versus 11.3 g/dl was not associated with any benefit to fatigue (84). Finally, the Trial to Reduce Cardiovascular Events with Aranesp Therapy showed a modest benefit in patient-reported fatigue, with an improvement from baseline on the FACIT-F of 4.2±10.5 points in the darbepoetin group versus 2.8±10.3 points in the placebo group (P<0.001); an improvement that may or may not be clinically relevant (85). Given that these trials also revealed potentially higher risk of adverse cardiovascular events with ESA use (83 –85), indiscriminate use of ESAs to treat fatigue in patients with kidney disease would not be prudent. However, an individualized higher hemoglobin target has been suggested to be useful for younger, active patients who remain limited by symptoms such as fatigue, despite a hemoglobin level of 10 g/dl (86 –88).
Alkali Supplementation
Some studies suggested an improvement in functional status and muscle strength with sodium bicarbonate therapy to treat metabolic acidosis. In one clinical trial of 20 adults with an eGFR of 15–45 ml/min per 1.73 m2 and serum bicarbonate 20–24 mEq/L, supplementation with 1 mEq/kg per day of oral sodium bicarbonate was associated with dose-dependent improvement in lower extremity muscle strength (89). Two other trials also showed that sodium bicarbonate supplementation targeting plasma bicarbonate levels of ≥23 or 24–26 mEq/L was associated with an increase in midarm muscle circumference compared with placebo (90,91). However, other studies failed to show improvements in SF-36 vitality score; midarm muscle circumference; hand-grip strength; sit-to-stand time; or muscle insulin signaling, proteolysis, or inflammation with alkali therapy, when compared with placebo controls (92,93). Despite the remaining question of efficacy for fatigue, sodium bicarbonate supplementation should be considered in patients with CKD who require treatment for metabolic acidosis (90).
Vitamin D Supplementation
To our knowledge, only one study has evaluated the effects of vitamin D supplementation on fatigue and functional status in patients with CKD. A total of 97 participants were randomized to cholecalciferol or placebo, and despite favorable effects on vitamin D, calcium, and parathyroid hormone levels, there were no observed differences between groups in phosphate level, hand-grip strength, or fatigue scores (94).
Selective Serotonin Reuptake Inhibitors
Given the associations of depression with fatigue in patients with CKD, it would follow that treatment of depression may improve fatigue in this patient population. In the CKD Antidepressant Sertraline Trial, a randomized, double-blinded trial of antidepressant therapy in 201 patients with nondialysis CKD, treatment with sertraline versus placebo did not result in improvement in fatigue, measured by the SF-36 vitality score (95). In another 12-week trial of patients on maintenance hemodialysis, the SF-36 vitality subscale was better in those treated with sertraline versus cognitive behavioral therapy (96). However, there was no control arm, and the findings were attenuated toward the null in the multiple imputation analyses (96). An ongoing randomized controlled trial is investigating whether treatment of patients with CKD stages 3b–5 and major depressive disorder with behavioral activation therapy or antidepressant drug bupropion, with augmentation to the combination of both in nonresponders, will improve depression and fatigue (24).
Future Areas of Study
To our knowledge, no interventional studies evaluated the effect of anti-inflammatory therapies, such as cytokine or IL inhibitors, on fatigue in patients with CKD. Other factors that have not been well studied but may be associated with fatigue include cardiovascular disease, OSA, obesity, iron deficiency, restless leg syndrome, pain, disrupted sleep quality, and the presence of other medical or psychiatric comorbidities prevalent in patients with CKD, such as congestive heart failure, diabetes mellitus, and anxiety disorders, to name a few (2,22,23). Further studies of these conditions and targeted interventions, such as sleep aides and hygiene, continuous positive airway pressure therapy, physical therapy, or pain control, would be needed to determine whether they may have a role in the management of fatigue among patients with CKD. Furthermore, it remains unclear whether preventive interventions given in early stages of CKD, such as sodium-glucose cotransporter-2 inhibitors, with the goal of improving long-term kidney and cardiovascular outcomes, may prevent the development of fatigue.
Conclusions
Fatigue is a commonly reported and debilitating symptom among patients with CKD and may begin affecting quality of life as early as CKD stage 2. There is no consensus on the best tool to identify or measure fatigue in this population, and assorted instruments have shown variable associations with clinical parameters and outcomes across studies. The pathophysiology of fatigue in CKD is multifactorial and includes multiple contributors to decreased oxygen delivery and lactic acidosis, chronic metabolic acidosis, protein-energy wasting, hyperphosphatemia, depression, and OSA. The approach to a patient with fatigue should begin with an assessment of factors where one might be able to intervene. These include recognition and treatment of anemia, sleep disorders, and depression. Targeting higher hemoglobin levels with ESAs has associated risks that preclude their indiscriminate use other than on an individualized basis. Interventions targeting depression have not yet proven convincingly beneficial for improving fatigue, but studies are ongoing. Sodium bicarbonate supplementation to treat metabolic acidosis may help improve fatigue and functional status. Physical activity is worth recommending in light of the additional benefits of exercise. Overall, more research is needed to further clarify underlying mechanisms of fatigue and identify effective, targeted treatments in patients with CKD.
Disclosures
M. Bossola reports employment with Università Cattolica Sacro Cuore. L.P. Gregg reports employment with Baylor College of Medicine, Michael E. DeBakey Veterans Affairs Medical Center, and Veterans Affairs Health Services Research and Development Center for Innovations in Quality, Effectiveness and Safety. S.S. Hedayati reports employment with University of Texas Southwestern; honoraria from the American College of Physicians for participation in Nephrology Medical Knowledge Self Assessment Program and the American Society of Nephrology Post-Graduate Education Program; and serving as a scientific advisor or member of American Heart Association study sections, American College of Physicians Medical Knowledge Self Assessment Program Nephrology Committee, and American Society of Nephrology In-Training Examination Committee. M. Ostrosky-Frid reports employment with the University of Texas Southwestern Medical Center.
Funding
M. Ostrosky-Frid was supported by the University of Texas Internal Medicine Physician Scientist Training Program. S.S. Hedayati is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK124379-01 and the Yin Quan-Yuen Distinguished Professorship in Nephrology at the University of Texas Southwestern Medical Center. The study was supported in part by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ju A, Unruh M, Davison S, Dapueto J, Dew MA, Fluck R, Germain M, Jassal SV, Obrador G, O’Donoghue D, Josephson MA, Craig JC, Viecelli A, O’Lone E, Hanson CS, Manns B, Sautenet B, Howell M, Reddy B, Wilkie C, Rutherford C, Tong A; SONG-HD Fatigue Workshop Collaborators: Establishing a core outcome measure for fatigue in patients on hemodialysis: A Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop report. Am J Kidney Dis 72: 104–112, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Gregg LP, Jain N, Carmody T, Minhajuddin AT, Rush AJ, Trivedi MH, Hedayati SS: Fatigue in nondialysis chronic kidney disease: Correlates and association with kidney outcomes. Am J Nephrol 50: 37–47, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Zukiman WZH, Yaakup H, Zakaria NF, Shah SAB: Symptom prevalence and the negative emotional states in end-stage renal disease patients with or without renal replacement therapy: A cross-sectional analysis. J Palliat Med 20: 1127–1134, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Murphy EL, Murtagh FE, Carey I, Sheerin NS: Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: Use of a short patient-completed assessment tool. Nephron Clin Pract 111: c74–c80, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Menting J, Tack CJ, Bleijenberg G, Donders R, Droogleever Fortuyn HA, Fransen J, Goedendorp MM, Kalkman JS, Strik-Albers R, van Alfen N, van der Werf SP, Voermans NC, van Engelen BG, Knoop H: Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychol 37: 530–543, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Franssen PM, Bültmann U, Kant I, van Amelsvoort LG: The association between chronic diseases and fatigue in the working population. J Psychosom Res 54: 339–344, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Finsterer J, Mahjoub SZ: Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care 31: 562–575, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Mücke M, Mochamat, Cuhls H, Peuckmann-Post V, Minton O, Stone P, Radbruch L: Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev 2015: CD006788, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster K, Cella D, Yost K: The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes 1: 79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almutary H, Bonner A, Douglas C: Arabic translation, adaptation and modification of the Dialysis Symptom Index for chronic kidney disease stages four and five. BMC Nephrol 16: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hearn J, Higginson IJ; Palliative Care Core Audit Project Advisory Group: Development and validation of a core outcome measure for palliative care: The palliative care outcome scale. Qual Health Care 8: 219–227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, Switzer GE: Development of a symptom assessment instrument for chronic hemodialysis patients: The Dialysis Symptom Index. J Pain Symptom Manage 27: 226–240, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT: The Memorial Symptom Assessment Scale Short Form (MSAS-SF). Cancer 89: 1162–1171, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K: The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 7: 6–9, 1991 [PubMed] [Google Scholar]
- 15.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM: The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol Med 34: 73–82, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Validation of depression screening scales in patients with CKD. Am J Kidney Dis 54: 433–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571, 1961 [DOI] [PubMed] [Google Scholar]
- 18.Ware J Jr, Kosinski M, Keller SD: A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care 34: 220–233, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Ware JE Jr, Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30: 473–483, 1992 [PubMed] [Google Scholar]
- 20.Ju A, Unruh ML, Davison SN, Dapueto J, Dew MA, Fluck R, Germain M, Jassal SV, Obrador G, O’Donoghue D, Tugwell P, Craig JC, Ralph AF, Howell M, Tong A: Patient-reported outcome measures for fatigue in patients on hemodialysis: A systematic review. Am J Kidney Dis 71: 327–343, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Ju A, Teixeira-Pinto A, Tong A, Smith AC, Unruh M, Davison SN, Dapueto J, Dew MA, Fluck R, Germain MJ, Jassal SV, Obrador GT, O’Donoghue D, Viecelli AK, Strippoli G, Ruospo M, Timofte D, Sharma A, Au E, Howell M, Costa DSJ, Anumudu S, Craig JC, Rutherford C: Validation of a core patient-reported outcome measure for fatigue in patients receiving hemodialysis: The SONG-HD fatigue instrument. Clin J Am Soc Nephrol 15: 1614–1621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhamb M, Abdel-Kader K, Yabes J, Wang Y, Weisbord SD, Unruh M, Steel JL: Comparison of fatigue, pain, and depression in patients with advanced kidney disease and cancer-symptom burden and clusters. J Pain Symptom Manage 57: 566–575.e3, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jhamb M, Liang K, Yabes J, Steel JL, Dew MA, Shah N, Unruh M: Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: Are sleep disorders a key to understanding fatigue? Am J Nephrol 38: 489–495, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ClinicalTrials.gov: Combination of Novel Therapies for CKD Comorbid Depression (CONCORD). Identifier NCT04422652. Available at: https://clinicaltrials.gov/ct2/show/NCT04422652. Accessed September 10, 2020 [Google Scholar]
- 25.ClinicalTrials.gov: Technology Assisted Stepped Collaborative Care Intervention (TASSCI). Identifier NCT03440853. Available at: https://clinicaltrials.gov/ct2/show/NCT03440853. Accessed September 10, 2020 [Google Scholar]
- 26.Almutary H, Bonner A, Douglas C: Which patients with chronic kidney disease have the greatest symptom burden? A comparative study of advanced CKD stage and dialysis modality. J Ren Care 42: 73–82, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S: Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, Mendelssohn D, Finkelstein FO: Health-related quality of life in CKD patients: Correlates and evolution over time. Clin J Am Soc Nephrol 4: 1293–1301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senanayake S, Gunawardena N, Palihawadana P, Bandara P, Haniffa R, Karunarathna R, Kumara P: Symptom burden in chronic kidney disease; a population based cross sectional study. BMC Nephrol 18: 228, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Luijtgaarden MWM, Caskey FJ, Wanner C, Chesnaye NC, Postorino M, Janmaat CJ, Rao A, Torino C, Klinger M, Drechsler C, Heimburger O, Szymczak M, Evans M, Dekker FW, Jager KJ; EQUAL Study Investigators : Uraemic symptom burden and clinical condition in women and men of ≥65 years of age with advanced chronic kidney disease: Results from the EQUAL study. Nephrol Dial Transplant 34: 1189–1196, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Chan KY, Li CW, Wong H, Yip T, Sham MK, Cheng HW, Teo KC, Kwok WC, Chan TM: Effect of erythropoiesis-stimulating agents on hemoglobin level, fatigue and hospitalization rate in renal palliative care patients. Int Urol Nephrol 46: 653–657, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, Higginson IJ: Symptoms in advanced renal disease: A cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 10: 1266–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XH, Zhang BL, Gu YH, Zhan XL, Guo LL, Jin HM: Association of sleep disorders, chronic pain, and fatigue with survival in patients with chronic kidney disease: A meta-analysis of clinical trials. Sleep Med 51: 59–65, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Jhamb M, Pike F, Ramer S, Argyropoulos C, Steel J, Dew MA, Weisbord SD, Weissfeld L, Unruh M: Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am J Nephrol 33: 515–523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossola M, Di Stasio E, Antocicco M, Panico L, Pepe G, Tazza L: Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron 130: 113–118, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Jhamb M, Argyropoulos C, Steel JL, Plantinga L, Wu AW, Fink NE, Powe NR, Meyer KB, Unruh ML; Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study: Correlates and outcomes of fatigue among incident dialysis patients. Clin J Am Soc Nephrol 4: 1779–1786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Goeij MC, Ocak G, Rotmans JI, Eijgenraam JW, Dekker FW, Halbesma N: Course of symptoms and health-related quality of life during specialized pre-dialysis care. PLoS One 9: e93069, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manavalan M, Majumdar A, Harichandra Kumar KT, Priyamvada PS: Assessment of health-related quality of life and its determinants in patients with chronic kidney disease. Indian J Nephrol 27: 37–43, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh TR, Kim CS, Bae EH, Ma SK, Han SH, Sung SA, Lee K, Oh KH, Ahn C, Kim SW; Representatives of the KNOW-CKD Investigator Group: Association between vitamin D deficiency and health-related quality of life in patients with chronic kidney disease from the KNOW-CKD study. PLoS One 12: e0174282, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang WL, Liang S, Zhu FL, Liu JQ, Wang SY, Chen XM, Cai GY: The prevalence of depression and the association between depression and kidney function and health-related quality of life in elderly patients with chronic kidney disease: A multicenter cross-sectional study. Clin Interv Aging 14: 905–913, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregg LP, Carmody T, Le D, Bharadwaj N, Trivedi MH, Hedayati SS: Depression and the effect of sertraline on inflammatory biomarkers in patients with non-dialysis CKD. Kidney360 1: 436–446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixon AC, Bampouras TM, Pendleton N, Mitra S, Brady ME, Dhaygude AP: Frailty is independently associated with worse health-related quality of life in chronic kidney disease: A secondary analysis of the Frailty Assessment in Chronic Kidney Disease study. Clin Kidney J 13: 85–94, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macdonald JH, Fearn L, Jibani M, Marcora SM: Exertional fatigue in patients with CKD. Am J Kidney Dis 60: 930–939, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Parrish AE: The effect of minimal exercise on the blood lactate in azotemic subjects. Clin Nephrol 16: 35–39, 1981 [PubMed] [Google Scholar]
- 46.Marrades RM, Roca J, Campistol JM, Diaz O, Barberá JA, Torregrosa JV, Masclans JR, Cobos A, Rodríguez-Roisin R, Wagner PD: Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J Clin Invest 97: 2092–2100, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnasamy R, Hawley CM, Stanton T, Howden EJ, Beetham KS, Strand H, Leano R, Haluska BA, Coombes JS, Isbel NM: Association between left ventricular global longitudinal strain, health-related quality of life and functional capacity in chronic kidney disease patients with preserved ejection fraction. Nephrology (Carlton) 21: 108–115, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Campistol JM: Uremic myopathy. Kidney Int 62: 1901–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Clough GF, Egginton S: Vasomotion and insulin-mediated capillary recruitment--Part of the explanation? J Physiol 587: 3407–3408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman JMB, Dwyer RM, St-Pierre P, Richards SM, Clark MG, Rattigan S: Decreased microvascular vasomotion and myogenic response in rat skeletal muscle in association with acute insulin resistance. J Physiol 587: 2579–2588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lind L: Endothelium-dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 196: 795–802, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Carmassi F, De Negri F, Fioriti R, De Giorgi A, Giannarelli C, Fruzzetti F, Pedrinelli R, Dell’Omo G, Bersi C: Insulin resistance causes impaired vasodilation and hypofibrinolysis in young women with polycystic ovary syndrome. Thromb Res 116: 207–214, 2005 [DOI] [PubMed] [Google Scholar]
- 53.de Jongh RT, Serné EH, IJzerman RG, Jørstad HT, Stehouwer CD: Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc Res 75: 256–262, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Sala E, Noyszewski EA, Campistol JM, Marrades RM, Dreha S, Torregrossa JV, Beers JS, Wagner PD, Roca J: Impaired muscle oxygen transfer in patients with chronic renal failure. Am J Physiol Regul Integr Comp Physiol 280: R1240–R1248, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Miró O, Marrades RM, Roca J, Sala E, Masanés F, Campistol JM, Torregrosa JV, Casademont J, Wagner PD, Cardellach F: Skeletal muscle mitochondrial function is preserved in young patients with chronic renal failure. Am J Kidney Dis 39: 1025–1031, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Watson EL, Baker LA, Wilkinson TJ, Gould DW, Graham-Brown MPM, Major RW, Ashford RU, Philp A, Smith AC: Reductions in skeletal muscle mitochondrial mass are not restored following exercise training in patients with chronic kidney disease. FASEB J 34: 1755–1767, 2020 [DOI] [PubMed] [Google Scholar]
- 57.Yenchek R, Ix JH, Rifkin DE, Shlipak MG, Sarnak MJ, Garcia M, Patel KV, Satterfield S, Harris TB, Newman AB, Fried LF; Health, Aging, and Body Composition Study: Association of serum bicarbonate with incident functional limitation in older adults. Clin J Am Soc Nephrol 9: 2111–2116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abramowitz MK, Hostetter TH, Melamed ML: Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis 58: 29–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raphael KL: Metabolic acidosis in CKD: Core curriculum 2019. Am J Kidney Dis 74: 263–275, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Sprick JD, Morison DL, Fonkoue IT, Li Y, DaCosta D, Rapista D, Choi H, Park J: Metabolic acidosis augments exercise pressor responses in chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 317: R312–R318, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregg LP, Carmody T, Le D, Martins G, Trivedi M, Hedayati SS: A systematic review and meta-analysis of depression and protein-energy wasting in kidney disease. Kidney Int Rep 5: 318–330, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkinson TJ, Gould DW, Nixon DGD, Watson EL, Smith AC: Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant 34: 1344–1353, 2019 [DOI] [PubMed] [Google Scholar]
- 63.Macdonald JH, Phanish MK, Marcora SM, Jibani M, Bloodworth LLO, Holly JMP, Lemmey AB: Muscle insulin-like growth factor status, body composition, and functional capacity in hemodialysis patients. J Ren Nutr 14: 248–252, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Bossola M, Di Stasio E, Giungi S, Rosa F, Tazza L: Fatigue is associated with serum interleukin-6 levels and symptoms of depression in patients on chronic hemodialysis. J Pain Symptom Manage 49: 578–585, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Bossola M, Luciani G, Giungi S, Tazza L: Anorexia, fatigue, and plasma interleukin-6 levels in chronic hemodialysis patients. Ren Fail 32: 1049–1054, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Amann M, Calbet JAL: Convective oxygen transport and fatigue. J Appl Physiol (1985) 104: 861–870, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Dutka TL, Cole L, Lamb GD: Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol 289: C1502–C1512, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Dahlstedt AJ, Katz A, Westerblad H: Role of myoplasmic phosphate in contractile function of skeletal muscle: Studies on creatine kinase-deficient mice. J Physiol 533: 379–388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corfield EC, Martin NG, Nyholt DR: Co-occurrence and symptomatology of fatigue and depression. Compr Psychiatry 71: 1–10, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Jhamb M, Weisbord SD, Steel JL, Unruh M: Fatigue in patients receiving maintenance dialysis: A review of definitions, measures, and contributing factors. Am J Kidney Dis 52: 353–365, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bossola M, Luciani G, Tazza L: Fatigue and its correlates in chronic hemodialysis patients. Blood Purif 28: 245–252, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Balconi M, Angioletti L, De Filippis D, Bossola M: Association between fatigue, motivational measures (BIS/BAS) and semi-structured psychosocial interview in hemodialytic treatment. BMC Psychol 7: 49, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brys ADH, Lenaert B, Van Heugten CM, Gambaro G, Bossola M: Exploring the diurnal course of fatigue in patients on hemodialysis treatment and its relation with depressive symptoms and classical conditioning. J Pain Symptom Manage 57: 890–898.e4, 2019 [DOI] [PubMed] [Google Scholar]
- 74.Choi MJ, Seo JW, Yoon JW, Lee SK, Kim SJ, Lee YK, Noh JW, Koo JR: The malnutrition-inflammation-depression-arteriosclerosis complex is associated with an increased risk of cardiovascular disease and all-cause death in chronic hemodialysis patients. Nephron Clin Pract 122: 44–52, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M: A systematic review on the association between obstructive sleep apnea and chronic kidney disease. Sleep Med Rev 53: 101337, 2020 [DOI] [PubMed] [Google Scholar]
- 76.Fernandes JFR, Barreto Silva MI, Loivos CP, Menna Barreto APM, Meira VDS, Kaiser SE, Bregman R, Klein MRST: Obstructive sleep apnea in non-dialyzed chronic kidney disease patients: Association with body adiposity and sarcopenia. Nutrition 57: 282–289, 2019 [DOI] [PubMed] [Google Scholar]
- 77.Fleischmann G, Fillafer G, Matterer H, Skrabal F, Kotanko P: Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant 25: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Nicholl DDM, Ahmed SB, Loewen AHS, Hemmelgarn BR, Sola DY, Beecroft JM, Turin TC, Hanly PJ: Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 141: 1422–1430, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Abuyassin B, Sharma K, Ayas NT, Laher I: Obstructive sleep apnea and kidney disease: A potential bidirectional relationship? J Clin Sleep Med 11: 915–924, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilkinson TJ, Watson EL, Gould DW, Xenophontos S, Clarke AL, Vogt BP, Viana JL, Smith AC: Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: A secondary analysis of the ‘ExTra CKD’ trial. Clin Kidney J 12: 113–121, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aoike DT, Baria F, Kamimura MA, Ammirati A, Cuppari L: Home-based versus center-based aerobic exercise on cardiopulmonary performance, physical function, quality of life and quality of sleep of overweight patients with chronic kidney disease. Clin Exp Nephrol 22: 87–98, 2018 [DOI] [PubMed] [Google Scholar]
- 82.Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC: Effects of a renal rehabilitation exercise program in patients with CKD: A randomized, controlled trial. Clin J Am Soc Nephrol 9: 2052–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Fishbane S, Spinowitz B: Update on anemia in ESRD and earlier stages of CKD: Core curriculum 2018. Am J Kidney Dis 71: 423–435, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, Szczech L: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62: 849–859, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf. Accessed March 19, 2021 [Google Scholar]
- 89.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH: Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8: 714–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS: Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol Dial Transplant 35: 121–129, 2020 [DOI] [PubMed] [Google Scholar]
- 92.Navaneethan SD, Shao J, Buysse J, Bushinsky DA: Effects of treatment of metabolic acidosis in CKD: A systematic review and meta-analysis. Clin J Am Soc Nephrol 14: 1011–1020, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melamed ML, Horwitz EJ, Dobre MA, Abramowitz MK, Zhang L, Lo Y, Mitch WE, Hostetter TH: Effects of sodium bicarbonate in CKD stages 3 and 4: A randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis 75: 225–234, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westerberg PA, Sterner G, Ljunggren Ö, Isaksson E, Elvarson F, Dezfoolian H, Linde T: High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD stages 3-4: Results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 33: 466–471, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hedayati SS, Gregg LP, Carmody T, Jain N, Toups M, Rush AJ, Toto RD, Trivedi MH: Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: The CAST randomized clinical trial. JAMA 318: 1876–1890, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, Dember LM, Diaz-Linhart Y, Dubovsky A, Greene T, Grote N, Kutner N, Trivedi MH, Quinn DK, Ver Halen N, Weisbord SD, Young BA, Kimmel PL, Hedayati SS: Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: A randomized clinical trial. Ann Intern Med 170: 369–379, 2019 [DOI] [PubMed] [Google Scholar]