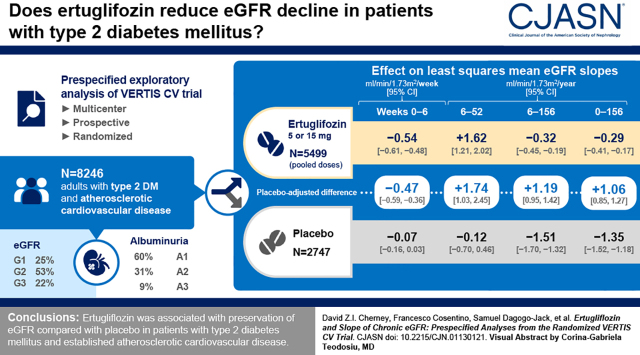
Visual Abstract
Keywords: renal protection, renal function decline, glomerular filtration rate, diabetic nephropathy, type 2 diabetes mellitus, clinical trial, sodium-glucose cotransporter 2 inhibitor
Abstract
Background and objectives
A reduction in the rate of eGFR decline, with preservation of ≥0.75 ml/min per 1.73 m2 per year, has been proposed as a surrogate for kidney disease progression. We report results from prespecified analyses assessing effects of ertugliflozin versus placebo on eGFR slope from the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes (VERTIS CV) trial (NCT01986881).
Design, setting, participants, & measurements
Patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease were randomized to placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg (1:1:1). The analyses compared the effect of ertugliflozin (pooled doses, n=5499) versus placebo (n=2747) on eGFR slope per week and per year by random coefficient models. Study periods (weeks 0–6 and weeks 6–52) and total and chronic slopes (week 0 or week 6 to weeks 104, 156, 208, and 260) were modeled separately and by baseline kidney status.
Results
In the overall population, for weeks 0–6, the least squares mean eGFR slopes (ml/min per 1.73 m2 per week [95% confidence interval (95% CI)]) were −0.07 (−0.16 to 0.03) and −0.54 (−0.61 to −0.48) for the placebo and ertugliflozin groups, respectively; the difference was −0.47 (−0.59 to −0.36). During weeks 6–52, least squares mean eGFR slopes (ml/min per 1.73 m2 per year [95% CI]) were −0.12 (−0.70 to 0.46) and 1.62 (1.21 to 2.02) for the placebo and ertugliflozin groups, respectively; the difference was 1.74 (1.03 to 2.45). For weeks 6–156, least squares mean eGFR slopes (ml/min per 1.73 m2 per year [95% CI]) were −1.51 (−1.70 to −1.32) and −0.32 (−0.45 to −0.19) for the placebo and ertugliflozin groups, respectively; the difference was 1.19 (0.95 to 1.42). During weeks 0–156, the placebo-adjusted difference in least squares mean slope was 1.06 (0.85 to 1.27). These findings were consistent by baseline kidney status.
Conclusions
Ertugliflozin has a favorable placebo-adjusted eGFR slope >0.75 ml/min per 1.73 m2 per year, documenting the kidney function preservation underlying the clinical benefits of ertugliflozin on kidney disease progression in patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease.
Clinical Trial registry name and registration number:
US National Library of Medicine, ClinicalTrials.gov NCT01986881. Date of trial registration: November 13, 2013.
Introduction
Beyond reducing blood pressure and albuminuria, and metabolic risk parameters including glycated hemoglobin (HbA1c) and weight, some sodium-glucose cotransporter 2 (SGLT2) inhibitors have demonstrated reductions in the risk of clinical CKD progression in people with and without type 2 diabetes mellitus (1,2). Clinical protection against CKD progression with SGLT2 inhibitors has been attributed to several glucose-lowering independent factors (3–6). CKD is closely associated with cardiovascular morbidity and mortality (7). Kidney function loss, reflected by the rate of decline in estimated glomerular filtration (eGFR slope), is a predictor of CKD risk and a surrogate for kidney failure (8,9). Attenuation of changes in eGFR slope has been used as a surrogate in clinical studies assessing the potential for kidney protection by pharmacologic agents (1,2,10,11). The use of eGFR slopes, rather than dichotomous categoric outcomes (8), is potentially advantageous owing to it being a continuous variable with greater potential for discrimination and the ability of novel therapies to demonstrate an effect on eGFR slope over relatively brief periods of time. Results from previous modeling analyses demonstrate that preservation of eGFR slope by ≥0.75 ml/min per 1.73 m2 per year over 3 years predicts clinically relevant delay of CKD progression with at least 96% probability (9).
In previous work involving the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes (VERTIS CV) study population, we reported that although ertugliflozin had a nonsignificant 21% relative risk reduction in the risk of events of doubling of serum creatinine (i.e., corresponding to a decline in eGFR by 57%), the risk of events of a sustained 40% decline from baseline in eGFR was significantly reduced by 35% (12). Ertugliflozin also preserved eGFR by approximately 3 ml/min per 1.73 m2 compared with placebo after 5 years, and lowered albuminuria, especially in patients with microalbuminuria or macroalbuminuria at baseline (while still on study drug). The aim of the current prespecified exploratory analysis was to elucidate the effect of ertugliflozin treatment on eGFR slope compared with placebo in the VERTIS CV study.
Materials and Methods
The VERTIS CV study was an event-driven study comparing two doses of ertugliflozin (5 mg and 15 mg) with placebo. The design, primary results, and full study protocol of the VERTIS CV study have been previously published (13,14).
Study Population
The full details of study eligibility criteria have been previously described (13,14). The study recruited patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease who had a baseline eGFR≥30 ml/min per 1.73 m2. The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies, with all participants providing written, informed consent. The trial was conducted in accordance with the Declaration of Helsinki and was consistent with Good Clinical Practice and applicable regulatory requirements.
eGFR Slope Analyses
In this prespecified exploratory analysis, the slopes for changes in eGFR per week or per year were analyzed by random coefficient models (eGFR was calculated using the Modification of Diet in Renal Disease formula). A central laboratory measurement for serum creatinine (to estimate the GFR) was performed at baseline; weeks 0, 6, 12, 18, 26, 39, and 52; and every 4 months up to 5 years. Least squares mean differences between ertugliflozin (observations from both doses were pooled for all analyses) and placebo for the weekly or yearly eGFR slopes were assessed for four periods:
-
1)
acute eGFR “dip” period: weekly slope from week 0 (baseline) to week 6;
-
2)
post–eGFR “dip” readjustment period: yearly slope from week 6 to 52;
-
3)
chronic slope: yearly slopes from week 6 to weeks 104, 156, 208, and 260; and
-
4)
total yearly slope from week 0 (baseline) to weeks 52, 104, 156, 208, and 260.
Chronic slope was investigated to omit the period when the known hemodynamic effects of SGLT2 inhibitors may confound the effect of ertugliflozin on longer-term kidney function decline. By contrast, total slope was used as a measure of long-term eGFR decline that also included the initial, reversible hemodynamic eGFR “dip” (and may therefore underestimate the magnitude of kidney function preservation).
Weekly and yearly eGFR slopes were assessed for the overall population and by three baseline kidney status classification schemes: eGFR, urinary albumin-to-creatinine ratio (UACR), and the Kidney Disease Improving Global Outcomes in Chronic Kidney Disease (KDIGO CKD) risk categories, which combine eGFR and UACR. A full description of the subgroups can be viewed in the Supplemental Material.
Statistical Analyses
The slope analyses were performed on the basis of the full analysis set (randomized participants who received one or more doses of blinded study medication and had one or more measurements of the analysis end point). Data collected after initiation of glycemic rescue therapy were included; however, data obtained >2 days after the last dose of study medication were excluded from the analyses of the eGFR. Weekly and yearly eGFR slopes were analyzed by generalized random coefficient models. The models included the eGFR value as a response variable, with treatment, time, baseline HbA1c, baseline eGFR, and treatment-by-time interaction as linear covariates. Time was treated as a continuous variable. The model enabled individual participant slopes to vary by random effects of intercept and time. An unstructured covariance matrix was used to model the correlation of random effects. Missing data were not imputed. The random effects model used a likelihood-based estimation, which produced unbiased estimates for data missing at random. Treatment-by-subgroup interaction was tested by generalized random coefficient models with treatment, time, subgroup, and treatment-by-subgroup interaction as linear covariates. All analyses were exploratory and prespecified; they were defined in a separate kidney statistical analysis plan that was completed before database lock. The analyses were performed using SAS version 9.4.
Results
Baseline Characteristics
A total of 8246 patients were randomized and followed for a median of 3.0 years. Of these, 2747 received placebo and 5499 received ertugliflozin (5 mg or 15 mg; pooled for this analysis). Patient demographic and baseline clinical characteristics for the overall population and by baseline kidney status have been previously reported and are summarized in Table 1 by treatment group for the overall population (12). Baseline eGFR values were available for 2747 and 5498 patients for the placebo and ertugliflozin groups, respectively. Of the study participants, 2048 (24.8%), 4390 (53.2%), and 1807 (21.9%) had eGFR G1, G2, and G3 at baseline, respectively. In subgroups defined by baseline albuminuria status, 4783 (59.6%), 2492 (31.0%), and 755 (9.4%) study participants had normoalbuminuria, microalbuminuria, and macroalbuminuria, respectively. At baseline, 3916 (48.8%), 2568 (32.0%), and 1548 (19.3%) patients were assigned to the KDIGO CKD low-risk, moderate-risk, and high-/very high-risk categories, respectively.
Table 1.
Baseline demographic and disease characteristics of the overall population from the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes clinical trial (intention to treat)
| Characteristic | Placebo | Ertugliflozin, Pooled | Total |
| (n=2747) | (n=5499) | (n=8246) | |
| Female sex, n (%) | 844 (31) | 1633 (30) | 2477 (30) |
| Age, yr | 64±8 | 64±8 | 64±8 |
| HbA1c, % | 8.2±0.9 | 8.2±1.0 | 8.2±1.0 |
| Duration of type 2 diabetes mellitus, yr | 13±8 | 13±8 | 13±8 |
| Hemoglobin, g/dl | 14.0±1.4 | 14.0±1.4 | 14.0±1.4 |
| BMI, kg/m2 | 32.0±5.5 | 31.9±5.3 | 32.0±5.4 |
| eGFR, ml/min per 1.73 m2 (MDRD) | 76±21 | 76±21 | 76±21 |
| UACR, mg/g | 19 (6–67) | 18 (6–69) | 19 (6–68) |
| Systolic BP, mmHg | 133±14 | 133±14 | 133±14 |
| Glucose-lowering agents, n (%) | |||
| Insulin | 1344 (49) | 2556 (46) | 3900 (47) |
| Biguanides | 2124 (77) | 4168 (76) | 6292 (76) |
| Antihypertensive agents, n (%) | |||
| Any antihypertensive | 2632 (96) | 5221 (95) | 7853 (95) |
| RAAS inhibitor | 2239 (82) | 4447 (81) | 6686 (81) |
| Diuretic | 1196 (44) | 2346 (43) | 3542 (43) |
| Loop diuretic | 426 (16) | 826 (15) | 1252 (15) |
| Mineralocorticoids receptor antagonists | 224 (8) | 450 (8) | 674 (8) |
| Antiplatelet or antithrombotic drugs, n (%) | 2446 (89) | 4880 (89) | 7326 (89) |
| Lipid-lowering agents, n (%) | 2313 (84) | 4655 (85) | 6968 (85) |
| eGFR category, n (%) a | |||
| eGFR G1 (eGFR≥90 ml/min per 1.73 m2) | 678 (25) | 1370 (25) | 2048 (25) |
| eGFR G2 (eGFR≥60 and <90 ml/min per 1.73 m2) | 1461 (53) | 2929 (53) | 4390 (53) |
| eGFR G3 (eGFR<60 ml/min per 1.73 m2) | 608 (22) | 1199 (22) | 1807 (22) |
| UACR category, n (%) b | |||
| Normoalbuminuria | 1597 (60) | 3186 (60) | 4783 (60) |
| Microalbuminuria | 845 (31) | 1647 (31) | 2492 (31) |
| Macroalbuminuria | 242 (9) | 513 (10) | 755 (9) |
| KDIGO CKD risk category, n (%) c | |||
| Low risk of CKD | 1307 (49) | 2609 (49) | 3916 (49) |
| Moderate risk of CKD | 859 (32) | 1709 (32) | 2568 (32) |
| High/very high risk of CKD | 517 (19) | 1031 (19) | 1548 (19) |
Values are mean±SD or median (interquartile range) unless otherwise stated. Table adapted from ref. 12. HbA1c, glvcated hemoglobin; BMI, body mass index; MDRD, Modification of Diet in Renal Disease; UACR, urinary albumin-to-creatinine ratio; RAAS, renin-angiotensin-aldosterone system; KDIGO CKD, Kidney Disease Improving Global Outcomes in Chronic Kidney Disease.
Participants required a baseline eGFR value for classification: n=2747 for placebo; n=5498 for ertuglifozin, pooled; n=8245 total.
Participants required a baseline UACR value for classification: n=2684 for placebo; n=5346 for ertugliflozin, pooled; n=8030 total.
Participants required baseline eGFR and UACR values for classification: n=2683 for placebo; n=5349 for ertuglifozin, pooled; n=8032 total.
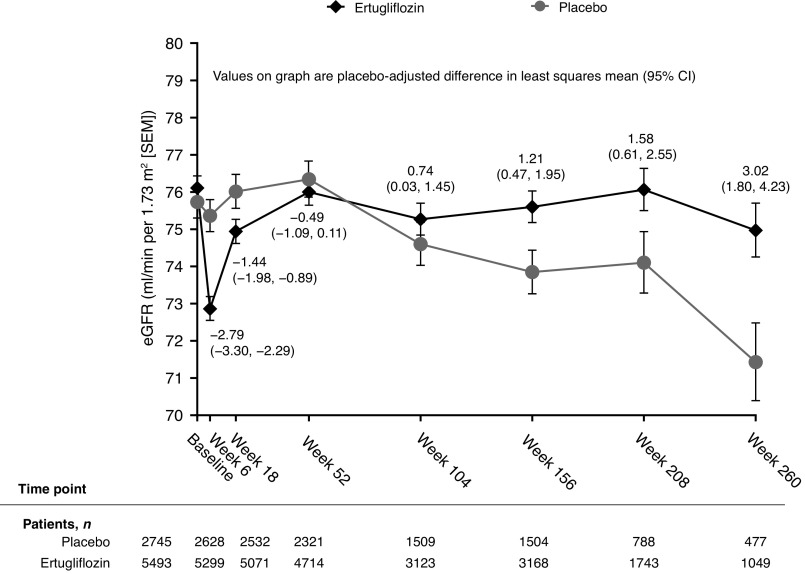
Overall eGFR Over Time
Mean eGFR over time has been previously reported and is displayed in Figure 1 (12). After the initial decrease from baseline in eGFR in the ertugliflozin group at week 6, there was an increase in eGFR toward baseline that continued up to week 52, followed by an attenuation in the decline of eGFR over time compared with placebo. The placebo-adjusted least squares mean differences from baseline in eGFR (ml/min per 1.73 m2 [95% confidence interval (95% CI)]) with ertugliflozin were −2.79 (−3.30 to −2.29) at week 6, −0.49 (−1.09 to 0.11) at week 52, 1.21 (0.47 to 1.95) at week 156, and 3.02 (1.80 to 4.23) at week 260.
Figure 1.
Mean eGFR over time in the overall population using the MDRD equation. Analysis was performed on the full analysis set. Figure from ref. 12 under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/). 95% CI, 95% confidence interval; MDRD, Modification of Diet in Renal Disease.
Acute eGFR Dip Period—Weekly Changes in eGFR for Weeks 0–6
During the acute period (from week 0 to 6), least squares mean eGFR slopes (ml/min per 1.73 m2 per week [95% CI]) were −0.07 (−0.16 to 0.03) and −0.54 (−0.61 to −0.48) for the placebo and ertugliflozin groups, respectively (Figure 2A); placebo-adjusted least squares mean difference in eGFR slope (ml/min per 1.73 m2 per week [95% CI]) was −0.47 (−0.59 to −0.36) (P<0.001). Similar findings were observed for the placebo-adjusted differences in slopes in subgroups defined by baseline kidney status (Table 2). There were differences when separately assessing the ertugliflozin and placebo groups by baseline eGFR and the KDIGO CKD risk categories. In patients taking placebo, a negative eGFR slope was observed in the eGFR G1 and KDIGO CKD low-risk subgroups, whereas eGFR slope was neutral in the eGFR G2 and KDIGO CKD moderate-risk subgroups and a positive eGFR slope was observed in the eGFR G3 and KDIGO high-/very high-risk subgroups. In patients taking ertugliflozin, all acute eGFR slopes were negative; however, the weekly eGFR slope was larger in the eGFR G1 subgroup and lowest in the eGFR G3 subgroup, with no overlap of the 95% CIs observed, suggestive of a significant difference (Table 2).
Figure 2.
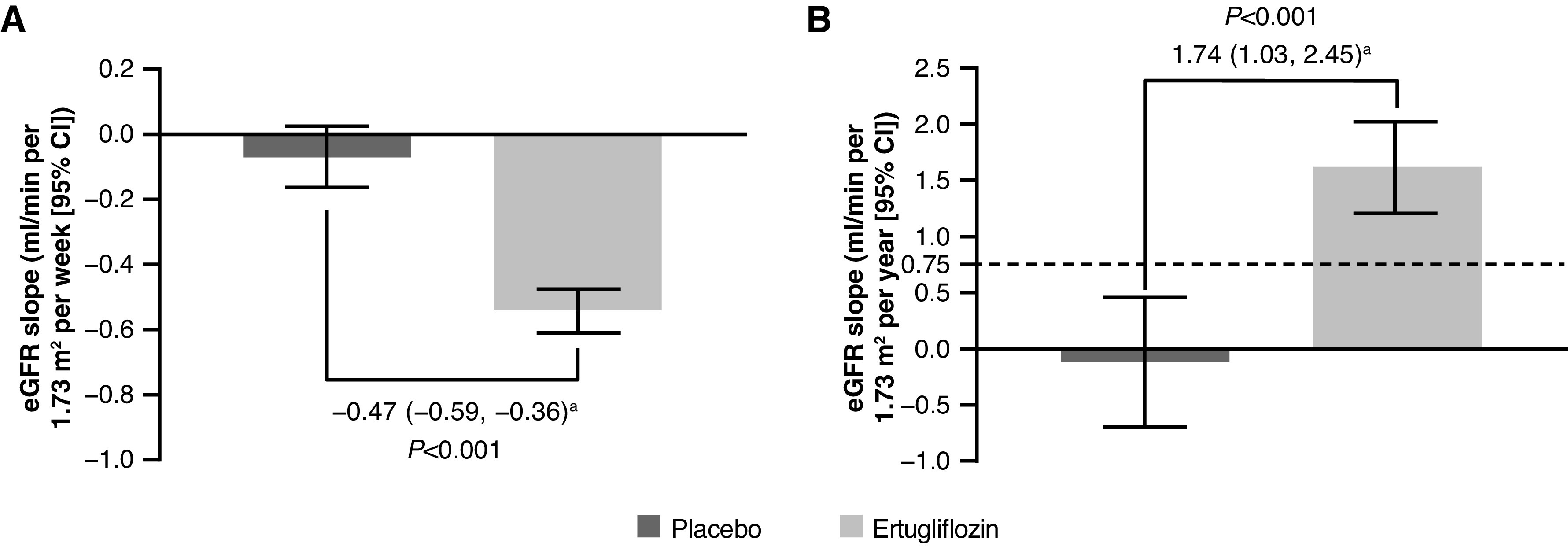
Weekly eGFR slope during the acute eGFR dip period (weeks 0–6) by treatment group and yearly eGFR slope during the post–acute eGFR dip readjustment period (weeks 6–52) by treatment group. (A) Weekly eGFR slope (weeks 0–6) and (B) yearly eGFR slope (weeks 6–52). Preservation of ≥0.75 ml/min per 1.73 m2 per year on eGFR slope predicts protection against CKD (9). 95% CI, 95% confidence interval; LSM, least squares mean. Analysis was performed on the full analysis set. aPlacebo-adjusted difference in LSM (95% CI).
Table 2.
eGFR slope during the acute “dip” period (per week) and the readjustment period (per year), by baseline kidney status
| Time Period |
eGFR G1
(eGFR≥90 ml/min per 1.73 m2) |
eGFR G2
(eGFR≥60 and <90 ml/min per 1.73 m2) |
eGFR G3
(eGFR<60 ml/min per 1.73 m2) |
||||||
| Placebo | Ertugliflozin | Difference a | Placebo | Ertugliflozin | Difference a | Placebo | Ertugliflozin | Difference a | |
| Weeks 0–6b | −0.61 | −1.13 | −0.52 | −0.01 | −0.42 | −0.41 | 0.38 | −0.18 | −0.57 |
| (−0.86 to −0.36) | (−1.30 to −0.96) | (−0.82 to −0.22) | (−0.10 to 0.08) | (−0.48 to −0.35) | (−0.52 to −0.30) | (0.26 to 0.50) | (−0.27 to −0.10) | (−0.72 to −0.42) | |
| Weeks 6–52c | −0.14 | 1.97 | 2.11 | −0.27 | 1.49 | 1.76 | 0.25 | 1.49 | 1.25 |
| (−1.58 to 1.31) | (0.95 to 3.00) | (0.33 to 3.89) | (−1.00 to 0.47) | (0.98 to 2.00) | (0.87 to 2.66) | (−0.77 to 1.26) | (0.76 to 2.22) | (−0.01 to 2.50) | |
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | |||||||
| Weeks 0–6b | −0.09 | −0.51 | −0.42 | −0.03 | −0.55 | −0.52 | −0.05 | −0.68 | −0.63 |
| (−0.22 to 0.04) | (−0.60 to −0.41) | (−0.58 to −0.26) | (−0.20 to 0.13) | (−0.67 to −0.43) | (−0.72 to −0.32) | (−0.3 to 0.21) | (−0.85 to −0.50) | (−0.94 to −0.32) | |
| Weeks 6–52c | 0.03 | 2.18 | 2.16 | −0.05 | 1.13 | 1.18 | −1.53 | −0.36 | 1.16 |
| (−0.71 to 0.77) | (1.66 to 2.71) | (1.25 to 3.06) | (−1.13 to 1.04) | (0.36 to 1.90) | (−0.15 to 2.51) | (−3.59 to 0.54) | (−1.78 to 1.05) | (−1.34 to 3.66) | |
| KDIGO CKD Low Risk | KDIGO CKD Moderate Risk | KDIGO CKD High/Very High Risk | |||||||
| Weeks 0–6b | −0.23 | −0.60 | −0.37 | −0.00 | −0.51 | −0.51 | 0.22 | −0.42 | −0.64 |
| (−0.37 to −0.08) | (−0.70 to −0.50) | (−0.55 to −0.19) | (−0.18 to 0.17) | (−0.63 to −0.39) | (−0.72 to −0.30) | (0.03 to 0.41) | (−0.56 to −0.29) | (−0.88 to −0.41) | |
| Weeks 6–52c | −0.08 | 2.12 | 2.20 | −0.08 | 1.53 | 1.62 | −0.35 | 0.38 | 0.73 |
| (−0.92 to 0.76) | (1.53 to 2.71) | (1.17 to 3.23) | (−1.16 to 0.99) | (0.78 to 2.29) | (0.30 to 2.93) | (−1.59 to 0.89) | (−0.49 to 1.25) | (−0.78 to 2.25) | |
Analysis was performed on the full analysis set population. KDIGO CKD, Kidney Disease Improving Global Outcomes in Chronic Kidney Disease.
Difference versus placebo (least squares mean [95% confidence interval]).
eGFR slope (least squares mean), ml/min per 1.73 m2 per week (95% confidence interval).
eGFR slope (least squares mean), ml/min per 1.73 m2 per year (95% confidence interval).
Post–Acute eGFR Dip Readjustment Period—Yearly Changes in eGFR for Weeks 6–52
During weeks 6–52, least squares mean eGFR slopes (ml/min per 1.73 m2 per year [95% CI]) were −0.12 (−0.70 to 0.46) and 1.62 (1.21 to 2.02) for the placebo and ertugliflozin groups, respectively (Figure 2B); placebo-adjusted least squares mean difference in eGFR slope (ml/min per 1.73 m2 per year [95% CI]) was 1.74 (1.03 to 2.45) (P<0.001). Similar findings were observed in subgroups defined by baseline kidney function category (Table 2).
Chronic eGFR Slopes
Chronic yearly eGFR slopes (from week 6 to weeks 104, 156, 208, and 260) by treatment group are summarized in Figure 3 and Supplemental Table 1. For all reported periods, the rate of eGFR decline (defined by eGFR slope; ml/min per 1.73 m2 per year) with ertugliflozin was slower than with placebo. The placebo-adjusted least squares mean chronic eGFR slopes (ml/min per 1.73 m2 per year [95% CI]) were 1.43 (1.07 to 1.78), 1.19 (0.95 to 1.42), 1.03 (0.84 to 1.22), and 1.02 (0.84 to 1.20), for the week 6 to weeks 104, 156, 208, and 260 periods, respectively, with all P values <0.001. Similar findings were observed in subgroups defined by baseline kidney status (Figure 3, Supplemental Table 2), in which all placebo-adjusted least squares mean differences in chronic eGFR slopes were statistically significant and >0.75 ml/min per 1.73 m2 per year.
Figure 3.
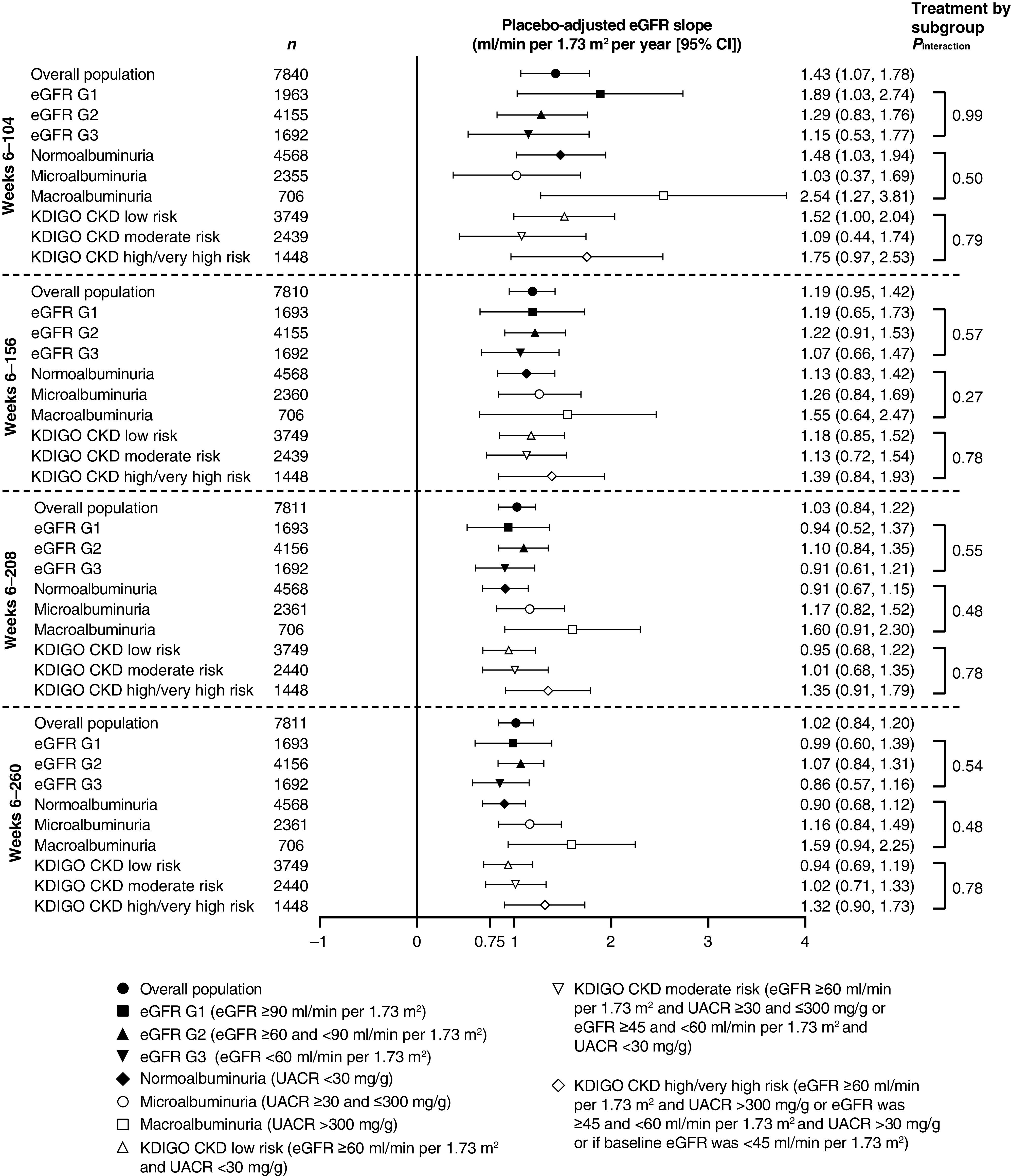
Placebo-adjusted chronic yearly slopes from week 6 in the overall population and by baseline kidney function. Preservation of ≥0.75 ml/min per 1.73 m2 per year on eGFR slope predicts protection against CKD (9). Analysis was performed on the full analysis set. 95% CI, 95% confidence interval; KDIGO CKD, Kidney Disease Improving Global Outcomes in Chronic Kidney Disease; UACR, urinary albumin-to-creatinine ratio.
Total eGFR Slopes
Total eGFR slopes (from week 0 to weeks 52, 104, 156, 208, and 260) by treatment group are summarized in Supplemental Table 1. For all reported periods, the rate of eGFR decline (defined by eGFR slope; ml/min per 1.73 m2 per year) with ertugliflozin was slower than with placebo. The placebo-adjusted least squares mean chronic eGFR slopes (ml/min per 1.73 m2 per year [95% CI]) were 0.89 (0.33 to 1.46), 1.13 (0.83 to 1.43), 1.06 (0.85 to 1.27), 0.96 (0.79 to 1.13), and 0.96 (0.80 to 1.11), for the week 0 to weeks 52, 104, 156, 208, and 260 periods, respectively, with all P values <0.003. Similar findings were generally observed in subgroups defined by baseline kidney status category (Figure 4, Supplemental Table 2), in which placebo-adjusted chronic eGFR slopes were statistically significant and >0.75 ml/min per 1.73 m2 per year for many of the subgroups, with the exception of the microalbuminuria subgroup during week 0 to weeks 52 and 104, the macroalbuminuria subgroup during week 0 to week 52, and for most time periods from week 0 for the eGFR G3 subgroup.
Figure 4.
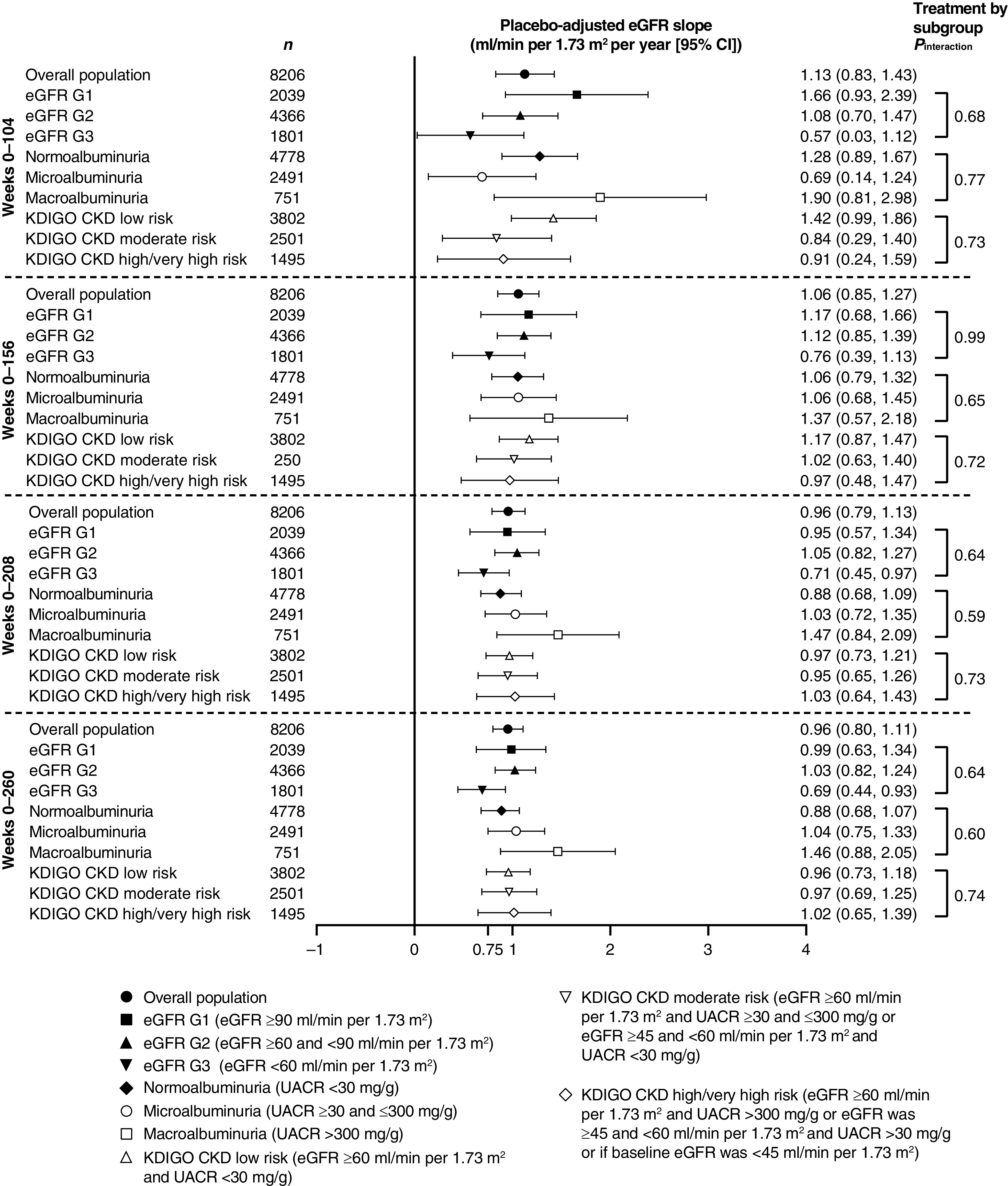
Placebo-adjusted total yearly slopes from week 0 in the overall population and by baseline kidney function. Preservation of ≥0.75 ml/min per 1.73 m2 per year on eGFR slope predicts protection against CKD (9). Analysis was performed on the full analysis set. 95% CI, 95% confidence interval; KDIGO CKD, Kidney Disease Improving Global Outcomes in Chronic Kidney Disease; UACR, urinary albumin-to-creatinine ratio.
Discussion
The identification of reasonable clinical surrogates for kidney protection is a major nephrology research priority. Recent trials have used the risk of reaching a sustained significant percentage decline in eGFR over time as a surrogate for clinical end points in kidney outcome studies. There is an emerging consensus that a ≥40% decline in eGFR is the most appropriate measure under most circumstances (8). However, these surrogates may not be applicable to all populations, to all interventions, or in early stages of kidney disease (9). Another method to assess significant kidney function loss over time involves the use of eGFR slope as a surrogate for kidney failure. Using eGFR slope as a surrogate for CKD progression in clinical studies has been supported by National Kidney Foundation working groups (8) and has the added benefit of allowing for a smaller sample size (15). In analyses by Levey, Inker, and others, a treatment effect of ≥0.75 ml/min per 1.73 m2 per year on slope over 3 years in sufficiently powered studies predicts a clinical benefit on CKD progress with at least 96% probability (8,9). Therefore, beyond assessing the effect of ertugliflozin on more traditional definitions of kidney function loss, which have been reported elsewhere (12), we examined the effect of ertugliflozin on acute and chronic slopes. In this analysis from the VERTIS CV study, ertugliflozin had a favorable effect on eGFR change over time compared with placebo, reflected by a greater preservation of kidney function during the chronic treatment period after 6 weeks.
SGLT2 inhibitors induce a characteristic and reversible acute ”dip” in eGFR after initiation of treatment. In a mechanistic study, this effect occurred within 24 hours, in conjunction with an increase in natriuresis, after a single dose of the SGLT2 inhibitor empagliflozin (16). The initial rapid rate of change has been most closely linked with an acute hemodynamic effect on renal tubuloglomerular feedback, secondary to acute blockade of tubular sodium reabsorption in the S1 segment of the proximal tubule, leading to afferent vasoconstriction under the influence of adenosine (6,17,18). Our earliest observation was consistent with these mechanisms and previous observations; treatment with ertugliflozin induced an expected greater degree of weekly eGFR decline from week 0 to 6. The initial mean eGFR “dip” associated with SGLT2 inhibitor treatment is commonly observed in clinical studies and in practice (19). After the initial eGFR change, from week 6 to 52, eGFR values returned toward baseline with ertugliflozin. Although the mechanisms responsible for this subsequent increase in eGFR over time are not fully understood, it might represent an adaptation in downstream sodium reabsorption pathways. These may include tubular sodium-glucose cotransporter 1 or sodium-hydrogen exchanger bioactivity, leading to a new state of tubuloglomerular feedback equilibrium and afferent redilatation (20–22).
On the basis of these well-established acute hemodynamic effects of SGLT2 inhibitors, the most informative period related to long-term benefits of these therapies is the chronic slope, after the initial change (week 6 in VERTIS CV), and for 3 or more years (8,9). Data from VERTIS CV meet criteria outlined for the use of eGFR slope as a reasonable surrogate measure of kidney protection (i.e., study duration and sample size). Importantly, the treatment effect of ertugliflozin on eGFR slope slowed the rate of decline in both chronic and total eGFR slope, compared with placebo, by a meaningful amount, as defined by a threshold of ≥0.75 ml/min per 1.73 m2 per year (9). Even when examined according to subgroups by eGFR, UACR, and KDIGO CKD risk category, chronic eGFR slope decline was significantly reduced with ertugliflozin in all investigated subgroups, compared with placebo.
The effect of ertugliflozin on attenuating eGFR decline was consistently beneficial in analyses from 2 to up to 5 years of follow-up and was consistent with previous studies with ertugliflozin. In a pooled analysis of two phase 3 studies from the ertugliflozin clinical development program, 2-year comparator-adjusted chronic eGFR slopes (from week 6) were between 1.56 and 2.60 ml/min per 1.73 m2 per year (3). Similar data around eGFR slope have been reported with other SGLT2 inhibitors, supporting the concept that kidney protection is broadly seen with these therapies, including in dedicated diabetic kidney disease cohort trials, such as the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial (1), and in previous cardiovascular outcome trials. In the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program (median follow-up period of 20.9 months), the placebo-adjusted chronic eGFR slope was +1.2 ml/min per 1.73 m2 per year from week 13 onwards with canagliflozin (23). In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) study, from week 4 to the last value on treatment (median follow-up period of 3.1 years), chronic eGFR slopes were −1.46 and 0.23 ml/min per 1.73 m2 per year in the placebo and empagliflozin groups, respectively (10). Similar benefits were recently reported in the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced), in which total slope was better preserved with empagliflozin compared with placebo, with a placebo-adjusted eGFR slope of 1.73 ml/min per 1.73 m2 per year over a median follow-up of 16 months (11). These protective effects were emphasized by the observation that after an off-drug washout period at the end of the trial, eGFR rebounded almost back to baseline, augmenting the placebo-adjusted preservation of eGFR in patients treated with empagliflozin (24). Most recently, in the Dapagliflozin and Prevention of Adverse Outcomes in CKD study, from week 2 to month 30, reductions in eGFR were reduced with dapagliflozin compared with placebo, with a between-group difference of 1.92 ml/min per 1.73 m2 per year (2). The current analyses from VERTIS CV further illustrate the potential for kidney function benefits achieved with SGLT2 inhibition, even in a cohort that had a relatively low overall risk for CKD progression. Protection against CKD progression may be especially important in light of the close relationship between kidney function loss and the development of heart failure. As SGLT2 inhibitors preserve eGFR and reduce heart failure progression, the effects in the kidney may lead to better salt and water homeostasis, thereby keeping patients out of the hospital (25,26).
Our analysis does have limitations. First, we report prespecified exploratory end points that were not controlled for type 1 error. Although eGFR slope is an important surrogate marker of long-term kidney risk, it does have limitations due to variability of the measurement in response to factors such as hydration and changes to medication. We minimized the effect of measurement variability with the use of a large sample size and further recognize that eGFR variability would have biased our analysis toward null. An assessment of eGFR after discontinuation of study drug was not performed in this study, because in other studies of SGLT2 inhibitors (including a study with ertugliflozin), eGFR increases upon cessation of the medication (10,23,27). Finally, we had insufficient power to adequately assess slope in some of the subgroups, especially those with advanced kidney disease at baseline, who made up <20% of the overall patient population.
In conclusion, ertugliflozin was associated with clinically relevant preservation of eGFR compared with placebo in the VERTIS CV study cohort involving patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease, a benefit that may contribute to end-organ protection with SGLT2 inhibitors.
Disclosures
C.P. Cannon reports grants and personal fees from Pfizer Inc. and grants and personal fees from Merck & Co., Inc. during the conduct of the study. C.P. Cannon reports grants and personal fees from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, and Janssen; grants from Daiichi Sankyo and Novo Nordisk; and personal fees from Aegerion, Alnylam, Amarin, Applied Clinical Therapeutics, Ascendia, Corvidia, Eli Lilly, HLS Therapeutics, Innovent, Kowa, Lexicon, Rhoshan, and Sanofi, outside the submitted work. D.Z.I. Cherney has received consulting fees or speaking honorarium, or both, from Abbvie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim-Lilly, Bristol Myers Squibb, Janssen, JNJ, MAZE, Merck & Co., Inc., Mitsubishi-Tanabe, Novo Nordisk, Otsuka, Prometic, and Sanofi; has received operating funds from AstraZeneca, Boehringer Ingelheim-Lilly, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi; and has served as a scientific advisor or member of AstraZeneca, Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, and Sanofi. F. Cosentino has received fees from Abbott, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Lilly, Merck Sharp & Dohme, Novo Nordisk, and Pfizer; research grants from the King Gustav V and Queen Victoria Foundation, the Swedish Heart & Lung Foundation, and the Swedish Research Council; and serves as Deputy Editor of the European Heart Journal and Consulting Editor of the Cardiovascular Research Journal. S. Dagogo-Jack has led clinical trials for AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk, Inc.; has received fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Merck & Co., Inc. and Sanofi; holds equity interests in Jana Care, Inc. and Aerami Therapeutics; and serves on editorial boards of the American Journal of the Medical Sciences, BMJ Diabetes Research & Care, and Frontiers in Endocrinology. R. Frederich is an employee and shareholder of Pfizer Inc. and may own shares/stock options in Pfizer Inc. and may own stock in Bristol Myers Squibb. M. Maldonado is an employee of MSD UK. He may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. D.K. McGuire has had leadership roles in clinical trials for AstraZeneca, Boehringer Ingelheim, CSL Behring, Eisai, Esperion, GlaxoSmithKline, Janssen, Lexicon, Lilly USA, Merck & Co., Inc., Novo Nordisk, and Sanofi USA and has received consultancy fees from Afimmune, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Lexicon, Lilly USA, Merck & Co., Inc., Metavant, Novo Nordisk, Metavant, Pfizer, and Sanofi. C-C. Liu, J. Liu, and A. Pong are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. R. Pratley is an employee of AdventHealth Translational Research Institute; has received (directed to his institution) speaker fees from Novo Nordisk; speaker fees and consulting fees from Merck & Co., Inc.; and grants from Hanmi Pharmaceuticals Co., Janssen, Lexicon Pharmaceuticals, Metavention, Novo Nordisk, Poxel SA, and Sanofi. R. Pratley has received consulting fees from AstraZeneca, Corcept Therapeutics Incorporated, Glytec LLC, Janssen, Mundipharma, Novo Nordisk, Pfizer, Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries. Except for services for Sanofi US Services Inc. on 2/12/2018 and 6/25/2018 (which were paid to R. Pratley personally), all payments are made directly to his employer.
Funding
The VERTIS CV study and these analyses were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, in collaboration with Pfizer Inc., New York, NY.
Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes study. The authors would like to personally thank Ira Gantz, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co Inc., Kenilworth, NJ, for technical assistance and advice. Medical writing and/or editorial assistance was provided by Dr. Moamen Hammad and Dr. Ian Norton, both of Scion, London, UK. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and Pfizer Inc., New York, NY.
Some of the results from these analyses were presented at the 56th annual meeting of the European Association for the Study of Diabetes, at the annual meeting of the American Society of Nephrology 2020 (Kidney Week), at Diabetes UK 2021 and the Spanish Society of Diabetes 2021 (XXXII Congreso De La Sociedad Española de Diabetes 2021).
The sponsor was involved in the study design; collection, analysis, and interpretation of data; and data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Are All AGLT2 Inhibitors Created Equal?,” on pages 1309–1311.
Contributor Information
Collaborators: VERTIS CV Investigators, Jose Maria Pozzi, Laura Maffei, Lucrecia Nardone, Horacio Sessa, Daniela Garcia Brasca, Diego Aizenberg, Claudia Emilce Baccaro, Elizabeth Silvana Gelersztein, Fabian Calella Pedro Rosario, Ricardo Leon de la Fuente, Silvia Gorban de Lapertosa, Natacha Maldonado, Adrian Cruciani, Ines Bartolacci, Eduardo Hasbani, Gustavo Frechtel, Nelson Rodriguez Papini, Alejandro Pereyra, Oscar Montana, Cesar Zaidman, Maria Mansilla, Bronte Ayres, Richard Simpson, Sarah Glastras, Maged William, Timothy Davis, David Colquhoun, Joseph Proietto, Adam Roberts, Anthony Roberts, Bronwyn Stuckey, Gary Wittert, Georgia Soldatos, Parind Vora, Zumreta Kusljugic, Azra Avdagic, Azra Durak-Nalbantic, Kanita Ibrahimpasic, Bosanko Horozic, Muhamed Spuzic, Valentina Soldat-Stankovic, Dragan Stevanovic, Damir Koco, Zaim Jatic, Ibrahim Terzic, Ljiljana Markovic Potkonjak, Besim Prnjavorac, Aleksandar Radanovic, Muhamed Salihbasic, Dimitar Dimitrov, Anastas Stoikov, Velichka Damyanova, Darina Vasileva, Kiril Kirilov, Ivailo Lefterov, Lyudmila Lyubenova, Dimitar Raev, Veska Tsanova, Mitko Mitkov, Rositsa Shumkova, Kostadin Kichukov, Tatyana Simeonova-Nikolova, Jean-Marie Ekoe, Shekhar A. Pandey, Richard Dumas, Vincent Woo, Kevin Saunders, James Conway, Laurie Breger, Christian Constance, Michael O'Mahony, Patrice Perron, Claude Garceau, Michael Csanadi, Ronald Bourgeois, Timothy Salter, Andre Frechette, Ariel Diaz, Thomas Ransom, Gregorio Sanchez-Vallejo, Sandra Isabel Barrera Silva, Jose Accini Mendoza, Luis Garcia Ortiz, Hernan Yupanqui Lozno, Dora Molina de Salazar, Julian Coronel Arroyo, Luis Alejandro Orozco Linares, Claudia Patricia Lenis Rendon, Pocanic Darko, Srecko Tusek, Sonja Slosic Weiss, Canecki-Varzic Silvija, Altabas Velimir, Zukanovic Sidbela, Tatjana Cikac, Jasna Vucak, Ljiljana Cenan, Zrinka Boljkovac, Srecko Margetic, Ksenija Kranjcevic, Renata Cop, Vera Adamkova, Kyselova Pavlina, Bedrich Wasserburger, Tomas Hrdina, Katerina Smolenakova, Chagunava Ketevan, Zurab Pagava, Merab Mamatsashvili, Koba Burnadze, Vakhtang Chumburidze, Zaza Lominadze, Lali Nikoleishvili, Bondo Kobulia, Nana Koberidze, Nodar Emukhvari, Zviad Kipiani, David Metreveli, Salome Glonti, Elene Giorgadze, Tamaz Shaburishvili, Gulnara Chapidze, Julieta Gulua, Irakli Gogorishvili, Ekaterine Berukashvili, Shalva Petriashvili, Givi Kurashvili, Moses Elisaf, Stavros Bousboulas, Nikolaos Tentolouris, Konstantinos Tsioufis, Sotiria Lymperi, Andrea Luk, Kelvin Kai Hang Yiu, Chiu Chi Tsang, Vincent Tok Fai Yeung, Alex Pui-Wai Lee, Kathryn Choon Beng Tan, Ronald Ching Wan Ma, Krisztina Beatrix Wudi, Zoltan Bujtor, Zsuzsanna Feher, Laszlo Koranyi, Gyorgy Paragh, Szilard Vasas, Zsolt Pauker, Nora Kesmarki, Janos Takacs, Laszlo Nagy, Csaba Salamon, Zsuzsanna Papp, Csaba Hajdu, Galina Abramov, Osamah Hussein, Victor Vishlitzky, Dror Dicker, Mahmud Darawsha, Baruch Itzhak, Dov Gavish, Julio Wainstein, Basil Lewis, Eliezer Klainman, Amos Katz, Mazen Elias, Rosane Ness-Abramof, Tony Hayek, Emanuela Orsi, Giuseppe Lembo, Piermarco Piatti, Agostino Consoli, Sergio Berti, Giuseppe Pugliese, Giuseppe Derosa, Giuseppe Paolisso, Paolo Piero Limone, Hak Chul Jang, Moon-Kyu Lee, Nam Hoon Kim, Bong-Soo Cha, Kyong Soo Park, Kun Ho Yoon, Chul Woo Ahn, Kwan Woo Lee, Sei Hyun Baik, Valdis Pirags, Sigita Pastare, Jurate Kavaliauskiene, Audrone Augusteniene, Antanas Navickas, Vaidotas Urbanavicius, Roma Kavaliauskiene, Ausra Sirutaviciene, Guillermo Antonio Llamas Esperon, Juan Villagordoa Mesa, Pedro Alberto Garcia Hernandez, Rosa Luna Ceballos, Carlos Aguilar Salinas, Guillermo Gonzalez Galvez, Guillermo Melendez-Mier, Raul Aguilar Orozco, Harry Crijns, Klaas Hoogenberg, Christine Voors-Pette, Helen Lunt, Russell Scott, John Baker, Michael Williams, Jane Elizabeth Kerr, Marian Denopol, Araceli Panelo, Louie Tirador, Grace Aquitania, Gregorio Rogelio, Florence Amorado-Santos, Ramoncito Habaluyas, Allyn Sy Rosa, Ellen Palomares, Geraldine Ebo, Chela Marie Romero, Joanna Gladczak, Jaroslaw Hawryluk, Edward Franek, Monika Lukaszewicz, Aleksandra Madej-Dmochowska, Anna Jeznach-Steinhagen, Grzegorz Sokolowski, Jan Ruxer, Agnieszka Karczmarczyk, Ewa Krzyzagorska, Iwona Wozniak, Anna Olech-Cudzik, Marek Dwojak, Izabela Anand, Krzysztof Strojek, Ewa Czernecka, Karolina Antkowiak-Piatyszek, Aleksandra Rozanska, Waldemar Gadzinski, Piotr Mader, Monika Kuligowska-jakubowska, Malgorzata Arciszewska, Andrzej Stankiewicz, Katarzyna Cypryk, Andrzej Wittek, Joanna Sawer-Szewczyk, Adam Pieniazek, Gabriela Negrisanu, Noemi Pletea, Daniela Zaharie, Ella Pintilei, Adriana Cif, Livia Duma, Iosif Szilagyi, Ildiko Halmagyi, Magdalena Morosanu, Mircea Munteanu, Adrian Albota, Alexandrina Popescu, Dan Anton Enculescu, Nicolae Hancu, Valerica Nafornita, Lacramioara Croitoru, Lucia Luana Sebestyen, Elena Pavlysh, Konstantin Nikolaev, Alexandr Vishnevsky, Olga Reshetko, Liudmila Kvitkova, Olga Barbarash, Tatiana Rodionova, Oxana Shaydyuk, Olga Ershova, Petr Chizhov, Svetlana Berns, Elena Rechkova, Sergey Yakushin, Olga Zanozina, Yuri Lukyanov, Elena Vorobeva, Dmitry Zateyshchikov, Nelly Verbovaya, Olga Bolshakova, Oleg Khrustalev, Leonid Strongin, Irina Bondar, Alsu Zalevskaya, Ekaterina Filippova, Galina Chumakova, Alexey Repin, Alexey Panov, Teodora Beljic Zivkovic, Milan Petakov, Mirjana Sumarac-Dumanovic, Georgina Pudar Brankovic, Aleksandra Kendereski, Stevo Stojic, Djuro Macut, Jana Babikova, Miriam Teplanova, Livia Tomasova, Emil Martinka, Beata Lachova, Gabriela Ivancova, Jana Rociakova, Zbynek Schroner, Viera Donicova, Adriana Ilavska, Katarina Raslova, South Africa, Lawrence Distiller, Heidi Siebert, Aysha Badat, Suzanne Blignaut, Mahomed Sarvan, Thirumani Govender, Mashra Gani, Hendrik Nortje, Mohamed Mookadam, Puvanesveri Naiker, Douwe de Jong, Louis Van Zyl, Mary Seeber, Dorothea Urbach-Bolus, Junaid Bayat, Warren Boyd, Margaretha du Toit, Zelda Punt, Susan Arnold, Christo Van Dyk, Leslie Ivan Robertson, Hester Johanna Kotze, Luts Anders, Lundvall Martin, Lindholm Carl-Johan, Tengmark Bengt-Olov, Curiac Dan, Bosson Peter, Koskinen Pekka, Ali Hajimirsadeghi, Chen-Ling Huang, Ju-Ying Jiang, Te- Lin Hsia, Kwo-Chang Ueng, Wei-Shiung Yang, Yung-Chuan Lu, Chwen-Yi Yang, Wayne Huey-Herng Sheu, Chern-En Chiang, Hung-I Yeh, Sompongse Suwanwalaikorn, Natapong Kosachunhanun, Suchai Sritippayawan, Sirakarn Tejavanija, Songkwan Silaruks, Mustafa Araz, Ramazan Sari, Dilek Sensoz Berker, Muyesser Sayki Arslan, Olga Godlevska, Ivan Chopey, Zinaida Teliatnikova, Petro Kuskalo, Orest Abrahamovych, Borys Mankovskyi, Ivan Fushtey, Galyna Myshanych, Susanna Tykhonova, Vira Tseluyko, Olena Koval, Oleksandr Parkhomenko, Oleksandr Prokhorov, Myroslava Vayda, Larysa Martymianova, Viktoriia Zharinova, Lyudmyla Prystupa, Larysa Pererva, Oleksandr Kovalov, Lyubov Sokolova, Volodymyr Botsyurko, Vitaliy Maslyanko, Maryna Vlasenko, Tetyana Khomazyuk, Anna Kulyk, Volodymyr Synenko, Oleksandr Karpenko, Yuriy Mostovoy, Olga Gyrina, Maryna Dolzhenko, Oleksandra Donets, Inna Sorokina, Yaroslav Malynovsky, Olena Lysunets, Roman Petrovskyy, Svitlana Panina, Anthony Avornyo, Susannah Eyre, John Ryan, Glenn Cardwell, Anthony Robinson, Rebecca Clark, Basil Issa, Devinda Weeraratne, Rory McCrimmon, Thomas Jones, Sam Philip, Ronnie Beboso, Melanie Davies, Christina Kyriakidou, Manish Saxena, Hawys Thomas, Madhu Gowda, Imrozia Arif, Babatunde Oyesile, John Cecil, Jonathan Mark Edwin Brunskill, Olakunle Akinboboye, Laura Akright, Chander Arora, Eric Ball, Sabrina Benjamin, Matthew Budoff, Harold Cathcart, Nizar Daboul, Paul Denker, Andrew Garner, Serge Jabbour, Naseem Jaffrani, Robert Jeanfreau, Thomas Knutson, Michael Lillestol, Donald McNeil, Samer Nakhle, William Randall, Jyothi Mallepalli, Jay Schmidt, Jorge Serje, James Shoemaker, Daniel Storey, Alberta Warner, Severino Pimentel, Luis Quintero, Juan Galvez, David Bernard, Humberto Cruz, Philip O'Donnell, Hemant Thawani, Ehab Sorial, Arthur Schwartzbard, John Agaiby, Bernard Garcia, Clancy Cone, Ankur Doshi, Ali Iranmanesh, Ildiko Lingvay, Kimberly Smith, Barry McLean, Joel Neutel, Adeniyi Odugbesan, Teresa Sligh, Kurt Lesh, Kathryn Rohr, Dilawar Ajani, Jerome Daniel, George Cornett, Gary Dykstra, Sharan Mahal, Michael Adams, David Ramstad, Ahmed Arif, Earl Martin, Ramon Sastre, Sunny Srivastava, Lawrence Feld, Kelvin Bush, Vinay Shah, Sarah Konigsberg, Mohammed Allaw, Richard Kelly, Brian MacGillivray, Michael McGuire, Debra Weinstein, Madhusudan Budhraja, Naeem Tahirkheli, Josefa Binker, Marc Bernstein, Nabil Andrawis, Shuaib Abdullah, Tom Christensen, Misal Khan, Mahfouz El Shahawy, Devjit Tripathy, Nandkishore Ranadive, Paul Grena, Betty Villafuerte, Charles Cook, Jose Bautista, Isam Marar, Christian Kaunzinger, Neville Bittar, Juan Frias, Rajesh Davit, Harold Bays, Robert Cohen, William Garvey, Stephen Smith, Jose-Luis Ruiz, Aaron Hartman, James Diener, Ronald Chochinov, John Hunter, Stephen Aronoff, Larry Reed, John Evans, Jeremy Ackermann, James Haaksma, Aurelio Torres-Consuegra, Jalil Khan, Kabir Yousuf, Lidia Bermudez, Benjamin Lumicao, Jorge Torres Olmeda, Evan Kessler, Michael Winnie, Robert Lending, Naveed Razzaque, Michael McCartney, Francisco Munoz, Robert Buynak, Lilia Rodriguez Ables, Sashi Makam, Josefina Lozano, Henry Paez, Ashwini Gore, Amer Al-Karadsheh, Bassem Masri, Sushma Gorrela, Jessie Louise Al-Amin, Firas Alani, Jason Haffizulla, Ron Hsieh, Luis Castillo Hernandez, Merle Turner, William French, Preetham Jetty, Mae Sheikh-Ali, Richard Leggett, Richard Perlman, Gary Goldstein, Edgardo A. Osea, Anthony James Bartkowiak, Michael Paul Gimness, Alexander John Higgins, Sohail Mohyuddin Khan, Ali R Rahimi, Anil Vallabhdas Shah, Joseph Leonard Lillo, Israel Alejandro Hartman, Edwin J. Whitney, Janice G. Johnston, Mario R. Juarez, Michael J. Perley, Brian Howard Kahn, Maged A. Amine, Michael Anthony Pace, Shirin Sabina Peters, Horacio Augusto Hidalgo, Jr., Perry Krichmar, and Rabi Ranjan Sinha
Data Sharing Statement
The data sharing policy of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01130121/-/DCSupplemental.
Supplemental Summary 1. VERTIS CV Investigators.
Supplemental Table 1. Chronic and total yearly eGFR slope in the overall population.
Supplemental Table 2. Chronic and total yearly eGFR slope analyses by baseline kidney status.
References
- 1.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators: Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Cherney DZI, Heerspink HJL, Frederich R, Maldonado M, Liu J, Pong A, Xu ZJ, Patel S, Hickman A, Mancuso JP, Gantz I, Terra SG: Effects of ertugliflozin on renal function over 104 weeks of treatment: A post hoc analysis of two randomised controlled trials. Diabetologia 63: 1128–1140, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Raalte DH, Cherney DZI: Sodium glucose cotransporter 2 inhibition and renal ischemia: Implications for future clinical trials. Kidney Int 94: 459–462, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI: The new biology of diabetic kidney disease—Mechanisms and therapeutic implications. Endocr Rev 41: 202–231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, Vervloet MG, Wong MG, Gansevoort RT, Heerspink HJL; DIAMOND investigators: Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8: 582–593, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Cherney DZI, Repetto E, Wheeler DC, Arnold SV, MacLachlan S, Hunt PR, Chen H, Vora J, Kosiborod M: Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol 51: 74–82, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, Greene T, Tighiouart H, Matsushita K, Ballew SH, Sang Y, Vonesh E, Ying J, Manley T, de Zeeuw D, Eckardt KU, Levin A, Perkovic V, Zhang L, Willis K: Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 75: 84–104, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, Simon AL, Ying J, Beck GJ, Wanner C, Floege J, Li PK, Perkovic V, Vonesh EF, Greene T: GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30: 1735–1745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, von Eynatten M, Groop PH; EMPA-REG OUTCOME Investigators: Empagliflozin and kidney function decline in patients with type 2 diabetes: A slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 29: 2755–2769, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators: Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Cherney DZI, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, Shih WJ, Frederich R, Maldonado M, Pong A, Cannon CP; VERTIS CV Investigators: Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: An analysis from the randomised VERTIS CV trial. Diabetologia 64: 1256–1267, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Charbonnel B, Shih WJ, Gallo S, Masiukiewicz U, Golm G, Cosentino F, Lauring B, Terra SG; VERTIS-CV Investigators: Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J 206: 11–23, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators: Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 383: 1425–1435, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Greene T, Ying J, Vonesh EF, Tighiouart H, Levey AS, Coresh J, Herrick JS, Imai E, Jafar TH, Maes BD, Perrone RD, Del Vecchio L, Wetzels JFM, Heerspink HJL, Inker LA: Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: A statistical simulation. J Am Soc Nephrol 30: 1756–1769, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Laffel L, Tamborlane WV, Simons G, Hantel S, von Eynatten M, George J, Marquard J, Cherney DZI: Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care 41: e129–e130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Rajasekeran H, Lytvyn Y, Bozovic A, Lovshin JA, Diamandis E, Cattran D, Husain M, Perkins BA, Advani A, Reich HN, Kulasingam V, Cherney DZI: Urinary adenosine excretion in type 1 diabetes. Am J Physiol Renal Physiol 313: F184–F191, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Oshima M, Neal B, Toyama T, Ohkuma T, Li Q, de Zeeuw D, Heerspink HJL, Mahaffey KW, Fulcher G, Canovatchel W, Matthews DR, Perkovic V: Different eGFR decline thresholds and renal effects of canagliflozin: Data from the CANVAS Program. J Am Soc Nephrol 31: 2446–2456, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layton AT, Vallon V, Edwards A: Modeling oxygen consumption in the proximal tubule: Effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V: Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R: Macula densa SGLT1-NOS1-tubuloglomerular feedback pathway, a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 30: 578–593, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B: Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6: 691–704, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, Schnee J, Wanner C, Packer M: Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: Insights from the EMPEROR-Reduced trial. Circulation 26: 310–321, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI: Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136: 1643–1658, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, Jiang ZW, Golm G, Engel SS, Lauring B: Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: The VERTIS RENAL randomized study. Diabetes Ther 9: 49–66, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]