ABSTRACT
Insulin stimulates adipose tissue to extract fatty acids from circulation and sequester them inside adipose cells. How fatty acids are transported across the capillary endothelial barrier, and how this process is regulated, remains unclear. We modeled the relationship of adipocytes and endothelial cells in vitro to test the role of insulin in fatty acid transport. Treatment of endothelial cells with insulin did not affect endothelial fatty acid uptake, but endothelial cells took up more fatty acids when exposed to medium conditioned by adipocytes treated with insulin. Manipulations of this conditioned medium indicated that the secreted factor is a small, hydrophilic, non-proteinaceous metabolite. Factor activity was correlated with lactate concentration, and inhibition of lactate production in adipocytes abolished the activity. Finally, lactate alone was sufficient to increase endothelial uptake of both free fatty acids and lipids liberated from chylomicrons, and to promote transendothelial transport, at physiologically relevant concentrations. Taken together, these data suggest that insulin drives adipocytes to secrete lactate, which then acts in a paracrine fashion to promote fatty acid uptake and transport across the neighboring endothelial barrier.
KEY WORDS: Adipose tissue, Endothelium, Fatty acids, Lactate, Paracrine, Fatty acid uptake, Lipid droplet, Chylomicrons
Summary: Transport of lipids across vessel walls is vital for tissue function. We show that adipocytes regulate this process by paracrine secretion of lactate, linking carbohydrate metabolism to control of fat influx.
INTRODUCTION
Adipocytes are specialized to protect other tissues from toxic exposure to fatty acids (FAs). The majority of fat cells in most mammals exist within white adipocyte tissue (WAT) depots, which sequester circulating fat by storing them within lipid droplets as neutral triglycerides (Vishvanath and Gupta, 2019). Animals that generate more adipocytes in response to obesogenic conditions are typically protected from lipotoxicity and insulin resistance (Ghaben and Scherer, 2019).
Adipocytes store lipids in the fed state and release them in the fasted state, the latter of which occurs via the lipolysis of triglycerides. Lipid storage is promoted by insulin, which stimulates both FA synthesis and uptake while suppressing lipolysis (Cignarelli et al., 2019). If adipocytes fail to suppress lipolysis in response to insulin (i.e. they are insulin resistant), unfettered release of FAs into the bloodstream overwhelms other organs such as the liver, where FAs contribute to improper hepatic glucose production, and skeletal muscle, where excess accumulation of lipid impairs insulin signaling (Morigny et al., 2016).
Every adipocyte within each WAT bed is neighbored by capillaries composed of endothelial cells (ECs). Vascularization of adipose tissue is critical for normal function and proper expansion of the depot in response to obesogenic diets (Tran et al., 2012). Capillaries in adipose tissue are continuous, providing a tight barrier to nutrient and hormone exchange (Jansson et al., 1990a; Qvisth et al., 2007; Rosdahl et al., 1998). Infusion experiments using synthetic PEGylated insulin, which cannot transverse continuous endothelial barriers, demonstrate selective action on the liver over peripheral tissues like skeletal muscle and adipose tissue (Moore et al., 2014), and electron microscopy of capillary ECs in human adipose tissue shows them to be closely adherent via adherens and tight junctions (Yazdani et al., 2019). Thus, the vasculature in adipose tissue creates a barrier to transport of metabolites, likely including FAs.
These observations led us to question how insulin might stimulate FA transport across the endothelial barrier. Mice lacking the insulin receptor in ECs have normal FA metabolism and insulin sensitivity (Vicent et al., 2003). In contrast, mice lacking the insulin receptor in adipocytes have impaired adipose tissue function, including promotion of fat uptake and storage, and suppression of fat release (Sakaguchi et al., 2017). We and others have shown previously that, in skeletal muscle, muscle cells secrete factors that activate FA uptake across the endothelial barrier (Hagberg et al., 2010; Jang et al., 2016). We thus hypothesized that insulin-stimulated adipocytes secrete a factor that induces FA uptake and transport in ECs.
RESULTS
Insulin-stimulated adipocytes secrete a factor that induces endothelial FA uptake
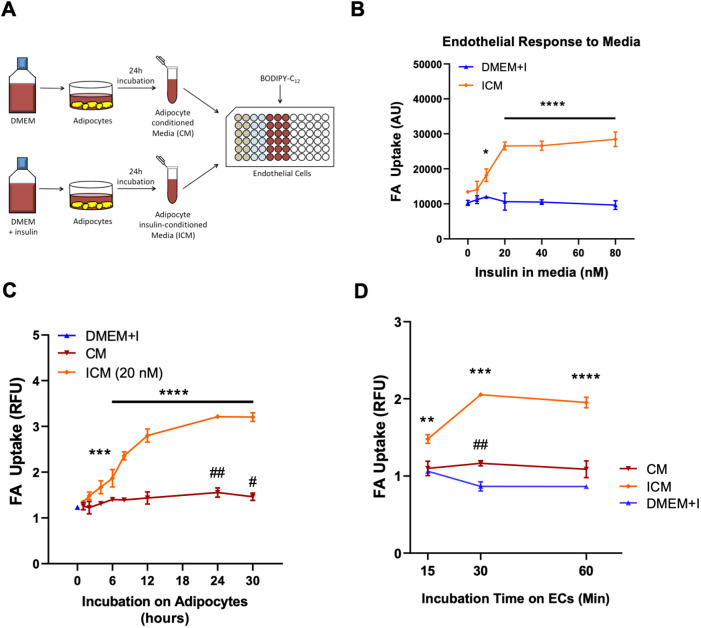
We modeled adipocytes in cell culture by isolating, immortalizing and differentiating preadipocytes taken from mouse inguinal WAT (iWAT; Wada et al., 2016). After full maturation, these adipocytes were treated with or without insulin, after which the conditioned medium (CM) and insulin-conditioned medium (ICM) were collected, centrifuged to remove any cellular debris and incubated with primary human ECs (specifically endothelial colony-forming cells; ECFCs). The uptake of FAs into the ECs was then measured using the fluorescent long-chain FA analog BODIPY-C12 (Fig. 1A). Conditioned medium from adipocytes treated with insulin strongly stimulated uptake of FAs by ECs. Dose-response experiments showed that 20 nM insulin treatment of adipocytes maximized the stimulation of endothelial FA uptake in response to ICM, as compared to controls treated with unconditioned medium containing matching concentrations of insulin (Fig. 1B). Time-course studies revealed that although just 3 h of insulin treatment was sufficient to produce an ICM with significant effect, a minimum of 24 h treatment was required to produce ICM that had maximal stimulatory effect on the ECs (Fig. 1C). CM produced only mild induction of FA uptake. Importantly, insulin did not alter endothelial FA uptake directly, even with concentrations as high as 80 nM.
Fig. 1.
Insulin stimulates FA uptake and transport in endothelium indirectly by signaling to adipocytes. (A) Diagram showing the experimental workflow. (B) Conditioned media produced by adipocytes in the presence of increasing concentrations of insulin (ICM) stimulate endothelial FA uptake. DMEM+I, non-conditioned media with equivalent concentration of added insulin. (C) Conditioned media produced by incubating adipocytes with 0 (CM) or 20 nM insulin (ICM) for the indicated times stimulate endothelial FA uptake. (D) ICM maximally induced endothelial cell FA uptake within 30 min of incubation upon ECs. In B–D, data are mean±s.e.m, n=3 biological replicates for every condition. Each experiment was replicated at least twice. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 for ICM; #P<0.05 and ##P<0.01 for CM (one-way ANOVA with Dunnett's test for multiple comparisons). Statistical comparisons are relative to (B) the 0 nM insulin condition, (C) DMEM+I at time zero, and (D) DMEM+I at the corresponding times. AU, arbitrary units; RFU, relative fluorescence units.
Using this optimized protocol of 24-h 20 nM insulin treatment of adipocytes, we carried out another time-course experiment, this time varying how long the CM and ICM were incubated with the ECs. ICM induced its maximal effect on FA uptake within 30 min (Fig. 1D). Taken together, these experiments demonstrated that adipocytes secrete, in response to insulin, a factor that strongly promotes FA uptake by ECs; that adipocytes continuously secrete the active factor for several hours; and that ECs respond to the active factor rapidly (within minutes). They also revealed the optimal conditions for further experiments to identify the secreted factor.
The main active metabolite in ICM is a small, hydrophilic metabolite that is different from 3-hydroxyisobutyrate
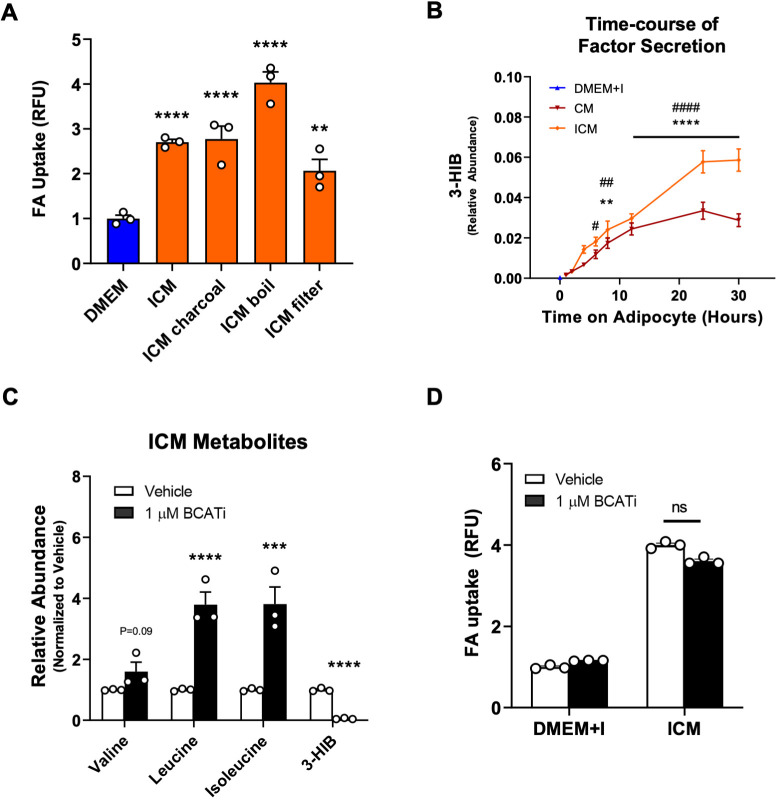
To determine the nature of the active factor contained within ICM, we subjected the ICM to charcoal stripping to remove hydrophobic candidates, boiling to denature and inactivate proteins, and filtering to remove any molecule larger than 3 kDa. None of these treatments decreased the potency of ICM to promote FA uptake in ECs, indicating that the secreted factor is small, hydrophilic and likely not a protein (Fig. 2A).
Fig. 2.
The main active metabolite in adipocyte-conditioned medium is a small, hydrophilic metabolite that is different from 3-HIB. (A) Treatment of ICM with charcoal, boiling and filtering did not appreciably reduce the potency of ICM. (B) 3-HIB concentrations in adipocyte CM and ICM at the indicated times. n=4 biological replicates for the 1–12 h timepoints, and n=6 for 24 h and 30 h timepoints. DMEM+I, non-conditioned medium with equivalent concentration of added insulin. (C) Inhibition of BCAT increased levels of BCAAs and decreased levels of 3-HIB in ICM. (D) Inhibition of BCAT in adipocytes did not suppress the ability of ICM to induce FA uptake (ns, not significant). Data in A–D are means±s.e.m. n=3 biological replicates for each condition in A, C and D. Each experiment was replicated at least twice. **P<0.01, ***P<0.001, ****P<0.0001 (for ICM compared to DMEM control in A, CM compared to DMEM+I control in B, and BCATi compared to vehicle control in C); #P<0.05, ##P<0.01, ####P<0.0001 for ICM compared to DMEM+I control in B (one-way ANOVA with Dunnett's test for multiple comparisons). RFU, relative fluorescence units.
Previously, we have described how muscle cells secrete 3-hydroxyisobutyrate (3-HIB), a valine catabolic product, to stimulate endothelial FA uptake and transport (Jang et al., 2016). We therefore hypothesized that 3-HIB may also be the active metabolite in ICM. This hypothesis was supported by the observation that the capacity of ICM to induce endothelial FA uptake followed similar kinetics to that of the secretion of 3-HIB into ICM (Fig. 2B). We observed that 3-HIB reached a maximum concentration of 125 µM in ICM (Fig. S1A). To test this hypothesis directly, we inhibited BCAT2, the first enzyme in the catabolism of branched chain amino acids (BCAAs), including valine (Deng et al., 2016). To confirm inhibitor (BCATi) activity, we co-treated adipocytes with BCATi and insulin, and then measured metabolite levels in the medium using gas chromatography–mass spectrometry (GC–MS). BCATi prevented BCAA consumption from the medium by the adipocytes, and concomitantly prevented 3-HIB secretion (Fig. 2C). However, ICM from BCATi-treated adipocytes induced ECs to take up FAs as efficiently as ICM from cells not given BCATi (Fig. 2D). Thus, depleting ICM of 3-HIB did not reduce its efficacy.
The active factor in adipocyte-conditioned medium is lactate, which alone is necessary and sufficient to increase endothelial FA uptake and transport
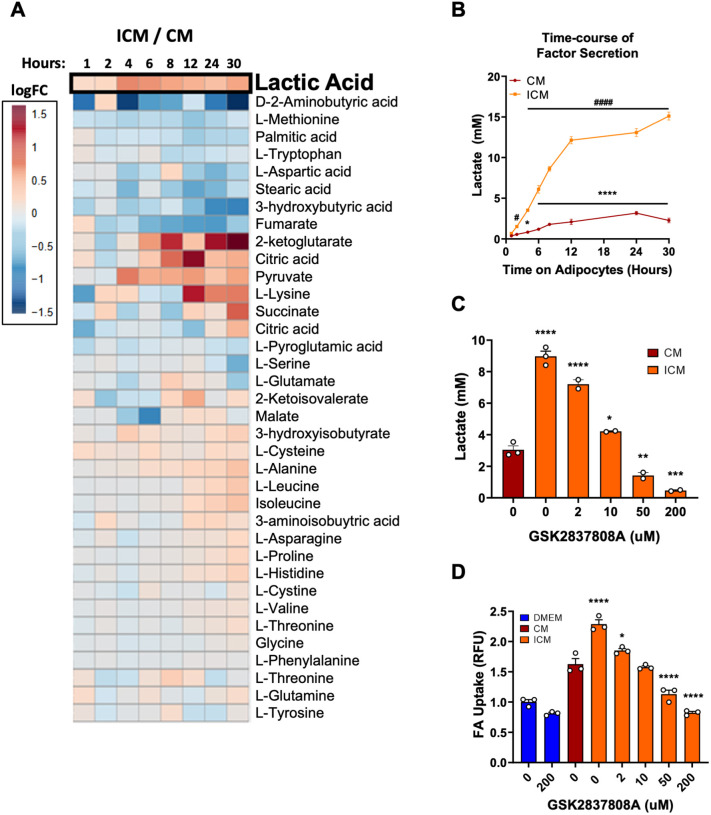
To identify the active factor, we performed targeted metabolomics on media taken from adipocytes that underwent a time course of insulin treatment (i.e. ICM) and on media taken from vehicle-treated cells (CM). Over the span of 30 h of incubation, the levels of several metabolites increased more in ICM than CM (Fig. 3A). We focused our attention on lactate. Direct quantifications of media concentrations showed that lactate was excreted into adipocyte media in millimolar concentrations over time (Fig. 3B). Moreover, the kinetics of lactate appearance in ICM closely matched the kinetics of ICM activity to promote FA uptake in ECs, approaching a maximal level at around 24 h (Figs 1C and 3B). We next treated adipocytes with GSK2837808A, a potent inhibitor of lactate dehydrogenase A (LDHA), the enzyme that produces lactate (Billiard et al., 2013). As expected, the inhibitor reduced the levels of lactate secreted into the medium by adipocytes in a dose-dependent manner (Fig. 3C). Strikingly, providing these media from GSK2837808A-treated adipocytes to ECs resulted in a mirrored dose-dependent decrease in endothelial FA uptake (Fig. 3D). Thus, the capacity of ICM to induce endothelial FA uptake is dependent on the concentration of lactate in ICM.
Fig. 3.
The capacity of ICM to induce endothelial FA uptake is dependent on its lactate concentration. (A) Heatmap of metabolite ratios for ICM versus CM over the course of 30 h. The data are represented in terms of log2 of the fold change (FC). n=4 biological replicates for the 1–12 h timepoints, and n=6 for 24 h and 30 h timepoints. (B) Lactate concentration in CM and ICM at the indicated times. n=4 biological replicates for the 1–12 h timepoints, and n=6 for 24 h and 30 h timepoints. (C) Lactate concentration in CM and ICM after treatment with the LDHA inhibitor GSK2837808A at the indicated concentrations. n=2 or 3 biological replicates per condition. (D) ICM from adipocytes treated with GSK2837808A exhibit reduced capacity to induce endothelial FA uptake. n=3 biological replicates per condition. Data in B–D are mean±s.e.m. Each experiment was replicated at least twice. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 for CM compared to time zero in B and for ICM compared to CM in C,D; #P<0.05 and ####P<0.0001 for ICM compared to time zero in B (one-way ANOVA with Dunnett's test for multiple comparisons). RFU, relative fluorescence units.
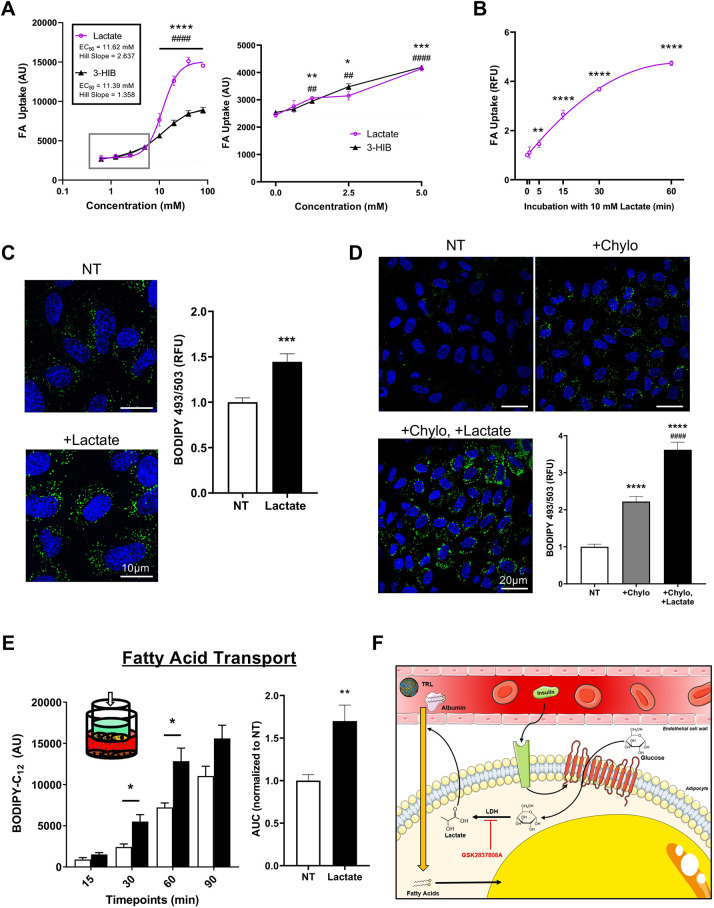
To test whether lactate is sufficient to stimulate endothelial FA uptake, we treated ECs with lactate alone. We observed strong induction of fat uptake by lactate – indeed, stronger than that by 3-HIB, though both displayed an EC50 of 11–12 mM (Fig. 4A). At least 2.5 mM lactate was required for a statistically significant increase in uptake of ∼25%. Lactate significantly stimulated FA uptake within 5 min, approaching maximal activity after 60 min (Fig. 4B). To test if lactate alters lipid homeostasis over a longer time period in ECs, we incubated ECs (specifically human umbilical vein endothelial cells, HUVECs) with oleic acid in the presence or absence of lactate for 6 h. Staining with free BODIPY indicated a higher lipid droplet content in ECs treated with lactate, consistent with increased fatty acid uptake and incorporation into the triglyceride pool (Fig. 4C).
Fig. 4.
Lactate alone is sufficient to increase endothelial FA uptake. (A) Dose-dependent induction of EC FA uptake by lactate or 3-HIB. The dotted lines indicate the curves fit as four-parameter nonlinear models with variable slope, where one curve was fit for responses to lactate and another for responses to 3-HIB. An enlarged view of the boxed region is shown in the right hand graph. n= 3 biological replicates. (B) EC FA uptake after exposure to 10 mM lactate for the indicated time. n=3 biological replicates. (C) Left: representative images of ECs treated for 6 h with 100 µM oleic acid in the presence (+Lactate) or absence (NT) of 10 mM lactate (blue, DAPI staining; green, BODIPY 493/503, marking lipid droplets). Right: quantification of BODIPY signal. n=4 biological replicates, for each of which three different fields of view were imaged. (D) ECs were serum-starved for 2 h before being treated with 10 U/ml LPL for 1 h either without additions (NT), with 25 µg/ml chylomicrons (+Chylo) or with 25 µg/ml chylomicrons and 10 mM lactate (+Chylo +Lactate). Blue, DAPI; green, BODIPY 493/503. Bottom right: quantification of BODIPY signal. n=4 biological replicates, for each of which five different fields of view were imaged. (E) Inset: schematic of the Transwell assay, indicating confluent monolayer of bEnd.3 endothelial cells (yellow), preadipocytes in the bottom chamber (brown), and BODIPY-C12 (green) added to the top chamber. Left: transport of BODIPY-C12 across the endothelial barrier was measured by sampling the bottom chamber at the indicated timepoints. White bars, no lactate (NT); black bars, 10 mM lactate. Right: area under the curve (AUC) quantification. n=6 biological replicates. (F) Diagram summarizing experiment results and model for insulin-stimulated endothelial transport of bloodborne fatty acids from the capillary lumen to the underlying adipocytes. (TRL, triglyceride-rich lipoprotein). Data in A–E are mean±s.e.m. Each experiment was replicated at least twice. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 (compared to no treatment); ##P<0.01, ####P<0.0001 (compared to no treatment in A, and to +Chylo in E). Statistics were by one-way ANOVA with Dunnett's test for multiple comparisons. AU, arbitrary units; RFU, relative fluorescence units. Scale bars: 10 µm (C) and 20 µm (D).
These experiments demonstrate that capacity of lactate to induce uptake of free FAs. However, the majority of blood-borne fat is contained within triglyceride-rich lipoprotein (TRL) particles, such as chylomicrons. These interact with lipoprotein lipase (LPL) on the endothelial luminal surface to hydrolyze and deposit FAs into the ECs. To test whether lactate also plays a role in regulating this form of fat uptake, we conducted an experiment where we pre-treated HUVECs with LPL, followed by addition of chylomicrons with or without lactate, and then we stained the cells with free BODIPY to measure total neutral lipid content. Just as with uptake of free FAs, the ECs co-treated with lactate took up more lipid from the chylomicrons (Fig. 4D).
Finally, to test whether lactate could also promote FA transport across an endothelial monolayer, we employed a Boyden chamber-type Transwell assay with a tight monolayer of bEnd.3 cells (a mouse brain-derived endothelial cell line) between two chambers (described in the Materials and Methods) (Fig. 4E, left inset). Measurements of the migration of BODIPY-C12 from the upper to the lower chamber over time demonstrated that lactate is sufficient to induce transport of FA across an endothelial monolayer (Fig. 4E).
Taken together, our data suggest a model of regulated FA transport in vivo whereby insulin stimulates adipocytes to secrete lactate, which acts upon the neighboring endothelium to transport blood-borne FAs into the underlying adipose tissue (Fig. 4F). We propose that this process is likely an integral part of the promotion of post-prandial transport of FAs into adipose tissue for storage.
A significant portion of glucose taken up by adipocytes is metabolized and then released as lactate. In vitro, insulin stimulates adipocytes to take up glucose and convert over half of it into secreted lactate (Krycer et al., 2020). In vivo, the concentration of lactate within the interstitial fluid of human adipose tissue is ∼0.5–1 mM and doubles after a glucose load or during hyperinsulinemic clamp (Hagstrom et al., 1990; Jansson et al., 1990b; Qvisth et al., 2007, 2008). This change in lactate concentration predicts a 1.5-fold change in endothelial FA uptake in the post-prandial state, based on our studies. Consistent with this prediction, after a hyperinsulinemic clamp in pigs, FA uptake into adipose tissue increases 1.5-fold in subcutaneous adipose depots and 3-fold in visceral adipose depots (Guiducci et al., 2007). ECs themselves are unlikely to influence interstitial lactate concentrations because the mass of adipocytes is much greater, and ECs likely secrete much of their lactate directly into the plasma. Moreover, ECs secrete far less lactate as compared to adipocytes, at least in our in vitro work (Fig. S1B). These data are thus consistent with the physiological model that post-prandial secretion of lactate from adipocytes signals to the endothelium to increase fat uptake in vivo.
Lactate may also modulate capillary FA transport in other physiological states. During exercise, human plasma lactate concentrations rise from 0.5–1.5 mM to 6–10 mM upon moderate exercise and to 15–25 mM upon intense exercise (Goodwin et al., 2007; Sung et al., 2016). The EC50 of lactate-stimulated FA uptake is 11.6 mM, indicating that lactate concentrations achieved with exercise are well within range to modulate FA uptake (Fig. 4A). This promotion of FA transport could occur both in paracrine fashion at the muscle capillaries, as well as in an endocrine fashion at the adipose capillaries, thereby coordinating systemic transport of FAs released from adipose tissues to their consumption by exercising muscle.
Lactate-regulated capillary FA transport may also impact disease states. Insulin-resistant adipose tissue takes up less fat as compared to healthy adipose tissue, contributing to the failure of adipose tissue to sequester lipids from other tissues (Ye et al., 2004). Obese, insulin-resistant subjects have elevated adipose interstitial concentrations of lactate (∼2 mM) that, importantly, do not change in response to insulin or glucose load (Jansson et al., 1994; Qvisth et al., 2007). Lactate signaling to FA uptake could thus be constitutively activated in unhealthy adipose tissue, or alternatively this might preload the endothelium with lipid, which might then slow the uptake of fatty acid from plasma. Testing these notions in vivo will require methods to specifically impair lactate secretion from adipocytes and to accurately quantify sub-tissue-level fat transport.
Our data adds regulation of endothelial FA transport to a growing list of paracrine and endocrine functions attributed to lactate. For example, lactate activation of GPR81 (also known as HCAR1) in adipocytes inhibits lipolysis (Liu et al., 2009). Lactylation of histones has recently been described, a process that can affect gene expression (Zhang et al., 2019a). Lactate can interact directly with mitochondria and suppress type I interferon production (Zhang et al., 2019b). Most recently, lactate has been shown to modulate transport of Mg2+ from the endoplasmic reticulum to mitochondria to modulate mitochondrial metabolism (Daw et al., 2020). The mechanism for lactate-induced endothelial FA transport is unclear but may involve rapid changes in protein signal transduction, membrane fluidity and permeability, and/or mitochondrial ATP (Ibrahim et al., 2020).
In conclusion, we have identified a novel paracrine relationship between adipocytes and their neighboring endothelium, in which adipose glucose metabolism is linked to endothelial FA uptake and transport. It will be of great interest to test the importance of these mechanisms in vivo, especially under the conditions of obesity, insulin resistance and type 2 diabetes.
MATERIALS AND METHODS
Isolation and culture of primary human endothelial cells
Endothelial colony-forming cells (ECFCs) were obtained from human umbilical cord blood as previously described (Lin and Melero-Martin, 2012). Briefly, cord blood was drawn from the umbilical vein. Mononuclear cells were separated by using Ficoll-Paque solution (GE Healthcare) and plated in a dish coated with 1% gelatin (BD Biosciences). After the cells reached 80% confluency, the CD31-positive cell fraction was purified by using Dynabead-conjugated anti-CD31 (Invitrogen) capture. These were cultured on 100-mm dishes coated with 0.1% gelatin (dissolved in PBS filtered through a 0.22 μm filter). Medium used was EBM-2 (Lonza) with EGM-2 SingleQuots supplements (Lonza), 10% FBS (MilliporeSigma) and 1% penicillin-streptomycin antibiotic solution (Thermo Fisher Scientific) (hereafter termed 10% EGM-2). ECFCs were used between passage 4 and 16. Informed consent was obtained for all tissue donation and experiments have been conducted according to the principles expressed in the Declaration of Helsinki.
Culture of bEnd.3 cells
Cells were obtained from ATCC and cultured with DMEM-GlutaMAX (Thermo Fisher Scientific) supplemented with 10% FBS, and 1% penicillin-streptomycin. For the bEnd.3 cells, dishes were coated with 0.1% gelatin prior to plating.
Culture and differentiation of adipocytes
Primary fibroblastic preadipocyte cells were harvested from mouse inguinal white fat as described previously (Wada et al., 2016). Preadipocytes were cultured in DMEM/F-12 (Thermo Fisher Scientific) supplemented with 10% FBS and 1% penicillin-streptomycin either 6-well or 12-well dishes, with the medium changed every 48 h until the cells were 100% confluent. Cells were kept confluent for an additional 48 h before differentiation was started by replacing media with DMEM/F-12 supplemented with 10% FBS, 1% penicillin-streptomycin, 20 nM insulin (MilliporeSigma), 500 nM dexamethasone (MilliporeSigma), 500 μM 3-isobutyl-1-methylxanthine (MilliporeSigma) and 1 μM rosiglitazone (MilliporeSigma). After 48 h, this differentiation induction medium was replaced with DMEM/F-12 supplemented with 10% FBS, 1% penicillin-streptomycin and 20 nM insulin. This medium was replaced every 24 h for 48 h prior to treatment. All procedures of animal experiments were approved by the University of Pennsylvania Animal Care and Use Committee.
Cell contamination
All cell lines were confirmed free of mycobacterium contamination every 6 months.
Treatments on adipocytes
Two hours before planned treatment (for example, insulin or inhibitors), fully differentiated adipocytes were given a fresh change of medium (DMEM/F-12 supplemented with 10% FBS, 1% 1% penicillin-streptomycin and 20 nM insulin). All drugs and inhibitors were diluted in DMEM/F-12 medium with or without 20 nM insulin and placed onto cells. When appropriate, inhibitors of lactate dehydrogenase A (GSK2837808A; Cayman Chemical Company) or BCAT2 (BCATi; synthesized by Icagen, Inc.) were added to the medium. After the allotted treatment time, the conditioned medium was collected, centrifuged at 4°C and 16,000 g, and the supernatant was frozen at −80°C.
Manipulation of conditioned media
Where indicated, conditioned media were manipulated as follows before being used in the FA uptake assay. To denature proteins, the medium was boiled at 85°C for 30 min, cooled to room temperature, and then used in the FA uptake assay. To strip the medium of hydrophobic compounds, the medium was mixed with activated charcoal (Sigma, C4386), centrifuged at 16,000 g for 10 min, and the supernatant was saved for FA uptake assay. Media were filtered according to the manufacturer's instructions.
Fatty acid uptake assay of adherent cells
ECFCs were plated onto Corning 96-well black-walled, clear-bottom plates (Millipore Sigma, CLS3603) at 40,000 cells/well and cultured until confluent. For the assay, the ECFCs were treated with conditioned media obtained from the treated differentiated adipocytes for varying times depending on the experiment, or with varying concentrations of 3-HIB or lactate (in PBS), after which they were given 2 μM BODIPY-C12 (Thermo Fisher Scientific, complexed with 1 μM BSA) for 4 min. After two washes with 0.1% BSA (in PBS), cells were given 0.08% Trypan Blue to quench extracellular fluorescence. Intracellular fluorescence was then swiftly measured using a Spectramax microplate reader or Synergy H1 Multi-Mode microplate reader (bottom read; excitation, 488 nm; emission, 520 nm). Signal from wells containing cells given no BODIPY-C12 was used as background noise and subtracted from experimental values.
Fatty acid transport assay
bEnd.3 cells were seeded at 50,000 cells/Transwell onto 0.4 μm inserts (Millipore Sigma, CLS3413) that were pre-coated with 0.1% gelatin. Two days later, another 50,000 cells/well were seeded into the same inserts and allowed to grow for another two days. In order to ascertain that a tight monolayer of cells had formed, 70 kDa Texas Red-labeled dextran was added to the lower chamber of these Transwells, as well as to ‘empty’ Transwells (whose inserts had been coated with gelatin but were not seeded with cells). Analyzing the fluorescence of the dextran on a Spectramax microplate reader (bottom read; excitation, 595 nm; emission, 615 nm) from medium periodically sampled from the upper chamber over 1 hour indicated the Texas Red-labeled dextran accumulated in the upper chamber of the empty Transwells but not the seeded Transwells.
For the transport experiment, bEnd.3 cells were plated onto the inserts (i.e. the upper chamber) as described above, and preadipocytes were plated in the bottom chambers (seeded at 80% confluency). 20 mM lactate (MilliporeSigma) was given for 30 min to some of these bottom wells. Then, the medium in the top chamber was replaced with serum-free medium containing 20 μM BODIPY-C12 (with 10 μM BSA), while the bottom chamber was given only 10 μM BSA with or without lactate. The bottom chambers were sampled periodically over an hour, and each sample was kept in a black-walled 96-well plate (in the dark and on ice) until conclusion of the assay. Finally, the BODIPY-C12 signal from these samples was measured using the Synergy H1 Multi-Mode microplate reader (bottom read; excitation, 488 nm; emission, 520 nm).
LPL-mediated FA uptake
HUVECs (Lonza) were seeded in glass coverslips pre-coated with 0.1% gelatin and cultured in the same medium used for ECFCs (10% EGM-2) at full confluency for 2–3 days. Cells were serum-starved for 2 h prior to experiment to minimize the presence of lipid droplets. Then, cells were pre-treated with LPL (Sigma, 62335; 10 U/ml) for 1 h at 37°C and washed with serum-free EGM-2 to remove unbound LPL before incubation with chylomicrons. Cells were exposed to chylomicrons (Biovision, 7285; 25 µg/ml) in the presence and absence of 10 mM lactate for 1 h at 37°C. Following treatment, cells in glass coverslips were washed with PBS, fixed in 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and subjected to staining with BODIPY 493/503 (Thermo Fisher Scientific).
Extraction of metabolites from conditioned media for mass spectrometry
The extraction of metabolites to run through a GC–MS machine required 50 μl of conditioned medium to be added to 0.4 μl of 4 M HCl, 8.35 μl of 5.625 mM norvaline and 244 ml of cold methanol. Samples were kept on dry ice for 15 min before centrifuging (14,000 g, 4°C, 15 min). Equal volumes of supernatant from each sample were collected, partially dried in a speed-vac, and then completely dried in a chemical hood overnight. Dried metabolites were derivatized at 70°C for 90 min with equal parts acetonitrile (Sigma, 34998) and MTBSTFA (Regis Technologies, 77377-52-7) (67 μl total). After cooling to room temperature, samples were centrifuged (room temperature, 10,000 g, 5 min) and the supernatant was transferred to vials for GC–MS analysis.
Gas chromatography–mass spectrometry
One microliter of the sample was injected via automatic liquid sampler (Agilent 7693A) into an Agilent 7890B gas chromatograph (GC) coupled with an Agilent 5977B mass selective detector (MSD) (Agilent Technologies). The GC was operated in splitless injection mode with helium as the carrier gas at a flow rate of 1.2 ml/min. The GC column was a 30 m×250 µm×0.25 µm HP-5ms Ultra Inert column (Agilent, 19091S-433UI). The inlet temperature was 250°C, and, after 3 min at 100°C, the oven temperature program was increased as follows: 4°C/min to 230°C then 20°C/min to 300°C and hold 5 min. The transfer line temperature was 250°C, and the MSD source and quadrupole temperatures were 230°C and 150°C, respectively. After a 6 min solvent delay, the MSD was operated in electron ionization mode and scan mode with a mass range of 50–550 atomic mass units at 2.9 scans/s. Agilent MassHunter Quantitative Analysis software was used for quantification of chromatograms. The relative abundance of each metabolite was normalized to the internal standard norvaline.
Enzymatic quantification of lactate concentration
Lactate production was measured using a YSI 2950 Bioanalyzer with 30 μl sample acquisitions. Media samples were collected, spun down and frozen prior to YSI analysis. Media sampling was conducted over a 48-h time period for the LDH inhibition experiment and over 1–30 h for the insulin incubation time course. Metabolite concentrations were normalized to 15 µM lactate standards. Samples were analyzed in technical duplicate for each biological replicate (n=3 for the LDH inhibition experiment and n=4 for the insulin incubation time course).
Statistics
Data are presented as mean±s.e.m., unless otherwise indicated in the figure legend. For comparisons of two groups, an unpaired, two-tailed Student's t-test was used, with significance accepted as P<0.05. For multiple comparisons (i.e. time-course or dose-response experiments), an ordinary one-way ANOVA with Dunnett's correction for multiple comparisons was used.
Supplementary Material
Acknowledgements
We would like to acknowledge Shogo Wada for his assistance in harvesting and establishing the adipocyte cell line used in this paper. We also thank Icagen, Inc. (a Ligand subsidiary) for synthesis of the BCAT inhibitor.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.I., M.D.N., K.L., R.M., Z.A.; Methodology: A.I., M.D.N., K.L., M.N., B.K., M.R.B., R.M.; Validation: A.I., M.D.N., K.L., M.N., B.K., M.R.B., Z.A.; Formal analysis: A.I., M.D.N., K.L., B.K.; Resources: Z.A.; Data curation: A.I., M.D.N., K.L., M.N., B.K., M.R.B., Z.A.; Writing - original draft: A.I., M.D.N.; Writing - review & editing: A.I., M.D.N., K.L., Z.A.; Visualization: Z.A.; Supervision: K.E.W., Z.A.; Project administration: A.I., Z.A.; Funding acquisition: A.I., M.D.N., B.K., K.E.W., Z.A.
Funding
This work was supported by funding from the National Institutes of Health: DK111091 to A.I., T32-GM07229 to M.D.N., DK107667 and R01 DK114103 to Z.A., and R01 CA248315 to Z.A. and K.E.W. This work was also supported by an American Diabetes Association fellowship, 1-18-PDF-153 to B.K. Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.258964
References
- Billiard, J., Dennison, J. B., Briand, J., Annan, R. S., Chai, D., Colón, M., Dodson, C. S., Gilbert, S. A., Greshock, J., Jing, J.et al. (2013). Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 1, 19. 10.1186/2049-3002-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarelli, A., Genchi, V. A., Perrini, S., Natalicchio, A., Laviola, L. and Giorgino, F. (2019). Insulin and insulin receptors in adipose tissue development. Int. J. Mol. Sci. 20, 759. 10.3390/ijms20030759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw, C. C., Ramachandran, K., Enslow, B. T., Maity, S., Bursic, B., Novello, M. J., Rubannelsonkumar, C. S., Mashal, A. H., Ravichandran, J., Bakewell, T. M.et al. (2020). Lactate elicits ER-mitochondrial Mg2+ dynamics to integrate cellular metabolism. Cell 183, 474-489.e17. 10.1016/j.cell.2020.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H., Zhou, J., Sundersingh, F., Messer, J. A., Somers, D. O., Ajakane, M., Arico-Muendel, C. C., Beljean, A., Belyanskaya, S. L., Bingham, R.et al. (2016). Discovery and optimization of potent, selective, and in vivo efficacious 2-aryl benzimidazole BCATm inhibitors. ACS Med. Chem. Lett. 7, 379-384. 10.1021/acsmedchemlett.5b00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaben, A. L. and Scherer, P. E. (2019). Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242-258. 10.1038/s41580-018-0093-z [DOI] [PubMed] [Google Scholar]
- Goodwin, M. L., Harris, J. E., Hernández, A. and Gladden, L. B. (2007). Blood lactate measurements and analysis during exercise: a guide for clinicians. J. Diabetes Sci. Technol. 1, 558-569. 10.1177/193229680700100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci, L., Grönroos, T., Järvisalo, M. J., Kiss, J., Viljanen, A., Naum, A. G., Viljanen, T., Savunen, T., Knuuti, J., Ferrannini, E.et al. (2007). Biodistribution of the fatty acid analogue 18F-FTHA: plasma and tissue partitioning between lipid pools during fasting and hyperinsulinemia. J. Nucl. Med. 48, 455-462. [PubMed] [Google Scholar]
- Hagberg, C. E., Falkevall, A., Wang, X., Larsson, E., Huusko, J., Nilsson, I., van Meeteren, L. A., Samen, E., Lu, L., Vanwildemeersch, M.et al. (2010). Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917-921. 10.1038/nature08945 [DOI] [PubMed] [Google Scholar]
- Hagstrom, E., Arner, P., Ungerstedt, U. and Bolinder, J. (1990). Subcutaneous adipose tissue: a source of lactate production after glucose ingestion in humans. Am. J. Physiol. Endocrinol. Metab. 258, E888-E893. 10.1152/ajpendo.1990.258.5.E888 [DOI] [PubMed] [Google Scholar]
- Ibrahim, A., Yucel, N., Kim, B. and Arany, Z. (2020). Local mitochondrial ATP production regulates endothelial fatty acid uptake and transport. Cell Metab. 32, 309-319.e7. 10.1016/j.cmet.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, C., Oh, S. F., Wada, S., Rowe, G. C., Liu, L., Chan, M. C., Rhee, J., Hoshino, A., Kim, B., Ibrahim, A.et al. (2016). A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 22, 421-426. 10.1038/nm.4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson, P. A., Smith, U. and Lönnroth, P. (1990a). Interstitial glycerol concentration measured by microdialysis in two subcutaneous regions in humans. Am. J. Physiol. 258, E918-E922. 10.1152/ajpendo.1990.258.6.E918 [DOI] [PubMed] [Google Scholar]
- Jansson, P.-A., Smith, U. and Lönnroth, P. (1990b). Evidence for lactate production by human adipose tissue in vivo. Diabetologia 33, 253-256. 10.1007/BF00404805 [DOI] [PubMed] [Google Scholar]
- Jansson, P. A., Larsson, A., Smith, U. and Lönnroth, P. (1994). Lactate release from the subcutaneous tissue in lean and obese men. J. Clin. Invest. 93, 240-246. 10.1172/JCI116951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krycer, J. R., Quek, L.-E., Francis, D., Fazakerley, D. J., Elkington, S. D., Diaz-Vegas, A., Cooke, K. C., Weiss, F. C., Duan, X., Kurdyukov, S.et al. (2020). Lactate production is a prioritized feature of adipocyte metabolism. J. Biol. Chem. 295, 83-98. 10.1074/jbc.RA119.011178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, R.-Z. and Melero-Martin, J. M. (2012). Fibroblast growth factor-2 facilitates rapid anastomosis formation between bioengineered human vascular networks and living vasculature. Methods 56, 440-451. 10.1016/j.ymeth.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C., Wu, J., Zhu, J., Kuei, C., Yu, J., Shelton, J., Sutton, S. W., Li, X., Yun, S. J., Mirzadegan, T.et al. (2009). Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled Receptor, GPR81. J. Biol. Chem. 284, 2811-2822. 10.1074/jbc.M806409200 [DOI] [PubMed] [Google Scholar]
- Moore, M. C., Smith, M. S., Sinha, V. P., Beals, J. M., Michael, M. D., Jacober, S. J. and Cherrington, A. D. (2014). Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 63, 494-504. 10.2337/db13-0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigny, P., Houssier, M., Mouisel, E. and Langin, D. (2016). Adipocyte lipolysis and insulin resistance. Biochimie 125, 259-266. 10.1016/j.biochi.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Qvisth, V., Hagström-Toft, E., Moberg, E., Sjöberg, S. and Bolinder, J. (2007). Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am. J. Physiol. Endocrinol. Metab. 292, E709-E714. 10.1152/ajpendo.00104.2006 [DOI] [PubMed] [Google Scholar]
- Qvisth, V., Hagström-Toft, E., Enoksson, S. and Bolinder, J. (2008). Catecholamine regulation of local lactate production in Vivo in skeletal muscle and adipose tissue: role of β-adrenoreceptor subtypes. J. Clin. Endocrinol. Metab. 93, 240-246. 10.1210/jc.2007-1313 [DOI] [PubMed] [Google Scholar]
- Rosdahl, H., Hamrin, K., Ungerstedt, U. and Henriksson, J. (1998). Metabolite levels in human skeletal muscle and adipose tissue studied with microdialysis at low perfusion flow. Am. J. Physiol. 274, E936-E945. 10.1152/ajpendo.1998.274.5.E936 [DOI] [PubMed] [Google Scholar]
- Sakaguchi, M., Fujisaka, S., Cai, W., Winnay, J. N., Konishi, M., O'Neill, B. T., Li, M., García-Martín, R., Takahashi, H., Hu, J.et al. (2017). Adipocyte dynamics and reversible metabolic syndrome in mice with an inducible adipocyte-specific deletion of the insulin receptor. Cell Metab. 25, 448-462. 10.1016/j.cmet.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, D. J., So, W.-Y., Choi, D.-H. and Jeong, T. T. (2016). Blood lactate levels after all-out exercise depend on body fat percentage in korean college students. Iran J. Public Health 45, 817-819. [PMC free article] [PubMed] [Google Scholar]
- Tran, K.-V., Gealekman, O., Frontini, A., Zingaretti, M. C., Morroni, M., Giordano, A., Smorlesi, A., Perugini, J., De Matteis, R., Sbarbati, A.et al. (2012). The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222-229. 10.1016/j.cmet.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent, D., Ilany, J., Kondo, T., Naruse, K., Fisher, S. J., Kisanuki, Y. Y., Bursell, S., Yanagisawa, M., King, G. L. and Kahn, C. R. (2003). The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Invest. 111, 1373-1380. 10.1172/JCI15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath, L. and Gupta, R. K. (2019). Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Invest. 129, 4022-4031. 10.1172/JCI129191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, S., Neinast, M., Jang, C., Ibrahim, Y. H., Lee, G., Babu, A., Li, J., Hoshino, A., Rowe, G. C., Rhee, J.et al. (2016). The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 30, 2551-2564. 10.1101/gad.287953.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani, S., Jaldin–Fincati, J. R., Pereira, R. V. S. and Klip, A. (2019). Endothelial cell barriers: transport of molecules between blood and tissues. Traffic 20, 390-403. 10.1111/tra.12645 [DOI] [PubMed] [Google Scholar]
- Ye, J.-M., Dzamko, N., Cleasby, M. E., Hegarty, B. D., Furler, S. M., Cooney, G. J. and Kraegen, E. W. (2004). Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 47, 1306-1313. 10.1007/s00125-004-1436-1 [DOI] [PubMed] [Google Scholar]
- Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui, C., Weng, Y., Liu, W., Kim, S., Lee, S., Perez-Neut, M.et al. (2019a). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575-580. 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Wang, G., Xu, Z.-G., Tu, H., Hu, F., Dai, J., Chang, Y., Chen, Y., Lu, Y., Zeng, H.et al. (2019b). Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176-189.e15. 10.1016/j.cell.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.