Abstract
Sonogashira coupling represents an indispensable tool for the preparation of organic materials that contain C(sp)–C(sp2) bonds. Improving the efficiency and generality of this methodology has long been an important research subject in materials science. Here, we show that a high-temperature ball-milling technique enables the highly efficient palladium-catalyzed Sonogashira coupling of solid aryl halides that bear large polyaromatic structures including sparingly soluble substrates and unactivated aryl chlorides. In fact, this new protocol provides various materials-oriented polyaromatic alkynes in excellent yield within short reaction times in the absence of bulk reaction solvents. Notably, we synthesized a new luminescent material via the mechanochemical Sonogashira coupling of poorly soluble Vat Red 1 in a much higher yield compared to those obtained using solution-based conditions. The utility of this method was further demonstrated by the rapid synthesis of a fluorescent metal–organic framework (MOF) precursor via two sequential mechanochemical Sonogashira cross-coupling reactions. The present study illustrates the great potential of Sonogashira coupling using ball milling for the preparation of materials-oriented alkynes and for the discovery of novel functional materials.
Using a high-temperature ball-milling technique, a practical mechanochemical protocol for the Sonogashira cross-coupling of polyaromatic halides was achieved, which provides efficient access to materials-oriented aromatic alkynes.
Introduction
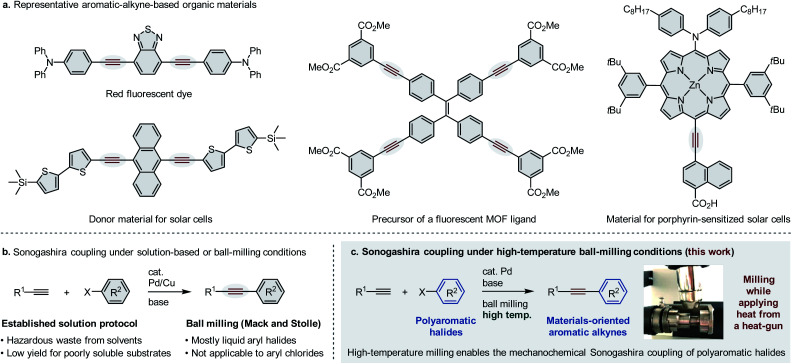
Aromatic alkynes have long been privileged structures in functional molecules and have found broad applications in organic fluorescent materials,1 electroluminescent materials,2 and donor–acceptor materials in organic solar cells3 (Fig. 1a). The carbon–carbon triple bond can extend the π-conjugation throughout a molecule to modify its optoelectronic properties and can also act as a linear spacer with a low torsion barrier and torsion angle to meet specific topological requirements.4 Therefore, the development of efficient synthetic methods for these compounds is of great importance to the discovery of novel organic materials. Cross-coupling of aryl halides with terminal alkynes, i.e., the so-called Sonogashira coupling, has been widely employed as the most powerful and general method for the construction of C(sp)–C(sp2) bonds (Fig. 1b).5 Continuous efforts have been devoted to overcome the limitations of these solution-based protocols, including simplification and optimization of the catalytic system,5g,6 discovery of effective additives,7 and extension of the substrate scope.8 However, the limitations of this solution-based methodology have frequently been mentioned in recent years. From an environmental perspective, the hazardous waste derived from organic solvents is becoming a critical issue, especially in large-scale industrial processes. Moreover, from a synthetic perspective, solution-based reactions of poorly soluble substrates that bear large polycyclic systems often require large amounts of organic solvent to dissolve the reactants, resulting in a significant decrease in the reaction rate. As such, prolonged reaction times are often necessary to obtain synthetically acceptable yields. Therefore, the development of a Sonogashira coupling protocol that does not require solvents to overcome the aforementioned shortcomings would be highly desirable for the further development of novel organic materials.
Fig. 1. Sonogashira cross-coupling for the synthesis of materials-oriented aromatic alkynes. (a) Representative aromatic alkynes found in functional organic materials. (b) Conventional solution-based protocol and a ball-milling approach for Sonogashira coupling reactions. (c) The first practical Sonogashira coupling of poorly soluble polyaromatic halides for the synthesis of materials-oriented aromatic alkynes enabled by high-temperature ball milling.
Recently, ball-milling techniques have emerged as a practical tool to carry out organic transformations under solventless conditions,9 and mechanochemical Sonogashira coupling reactions have already been reported (Fig. 1b).10,11 In 2009, Mack and co-workers reported the first example of a palladium/copper co-catalyzed classical Sonogashira coupling via ball milling.10 Interestingly, the copper catalyst can be replaced by copper milling balls. Later, in 2010, the Stolle group reported a copper- and ligand-free protocol for Sonogashira coupling via ball milling.11 Although these pioneering studies are remarkable, these methods focus mostly on liquid aryl halides, and the scope is limited to structurally simple aryl iodides and electron-deficient aryl bromides. Thus, despite their significant synthetic potential, the applicability of mechanochemical Sonogashira coupling reactions to solid polyaromatic halides for the preparation of materials-oriented aromatic alkynes has not yet been explored systematically.
We have previously reported extremely fast and efficient solid-state Suzuki–Miyaura cross-coupling reactions via a high-temperature ball-milling technique with a heat gun.12 This protocol is applicable to a wide range of aryl halides that bear large polycyclic π-conjugated systems and that are virtually unreactive under either room-temperature ball-milling conditions or conventional solution-based conditions. In this article, we report that mechanochemical Sonogashira coupling reactions of solid polyaromatic halides under high-temperature ball-milling conditions proceed with excellent efficiency, thus providing practical access to materials-oriented aromatic alkynes in excellent yield with short reaction times (Fig. 1c). In fact, using the mechanochemical Sonogashira coupling presented herein, a poorly soluble pigment, Vat Red 1, was successfully converted into a strongly luminescent molecule in good yield. Furthermore, the developed method allowed the synthesis of a precursor of a ligand for luminescent metal–organic frameworks (MOFs)1d in much better yield than a solution-based method. Thus, we anticipate that the present mechanochemical palladium-catalyzed Sonogashira cross-coupling reactions may potentially find broad applications in the preparation of materials-oriented aromatic alkynes.
Results and discussion
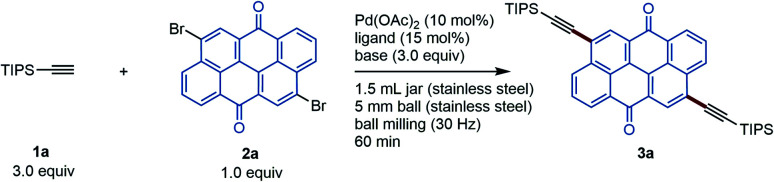
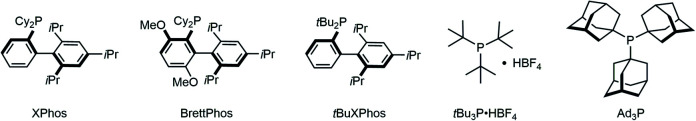
All mechanochemical reactions were conducted in a Retsch MM400 mill (stainless-steel milling jar; 30 Hz; stainless-steel balls). In order to develop a powerful Sonogashira cross-coupling protocol using mechanochemistry, we focused on Pd(OAc)2/XPhos, which is a high-performance catalytic system for copper-free Sonogashira cross-coupling reactions developed by Buchwald and co-workers.8a Initially, we selected the poorly soluble pigment Vat Orange 3 (2a) as a model substrate (Table 1). This choice was motivated by Morin's report on the synthesis of 3a in moderate yield (70%) via a solution-based Sonogashira coupling between triisopropylsilyl (TIPS) acetylene (1a) and 2a, which required a prolonged reaction time (72 h) due to the poor solubility of 2a.13 First, we investigated the coupling reaction between 1a and 2a in the presence of Pd(OAc)2 (10 mol%), XPhos (15 mol%), and Et3N as a base under ball-milling conditions at room temperature. Unfortunately, the formation of 3a was not observed under these conditions (entry 1). To accelerate the mechanochemical cross-coupling with 2a, we decided to carry out the reaction at a higher temperature. Specifically, we used a commercially available, temperature-controllable heat gun, which was placed directly above the ball-milling jar (for details, see the ESI†). Pleasingly, 3a was obtained in 47% yield when the internal temperature was increased to 80 °C using a heat gun at a pre-set temperature of 150 °C (entry 2); the internal temperature was confirmed using thermography. Further increasing the temperature did not improve the reactivity (34%, entry 3). Next, we attempted liquid-assisted grinding (LAG),9t where sub-stoichiometric liquid additives are used, to improve the yield of 3a. Unless otherwise noted, the following reactions with liquid additives are all characterized by a 0.40 ratio between the volume (μL) of liquid added to the weight (mg) of the reactant. Although small amounts of dioxane, toluene, and n-PrOH did not improve the reactivity (entries 4–6), we found that H2O dramatically promoted the formation of the coupling product, providing 3a in 76% yield (entry 7). Other organic bases, namely, 1,4-diazabicyclo[2.2.2]octane (DABCO) or tetramethylethylenediamine (TMEDA), also provided good yields of 3a (72% and 74% yield, entries 8 and 9). We also investigated the effect of other phosphine ligands on this reaction. The use of other Buchwald ligands such as BrettPhos and tBuXPhos provided a lower yield or no product (entries 10 and 11). The reaction using tBu3P·HBF4 proceeded smoothly to give 3a in good yield (77%, entry 12). We found that the reaction using Ad3P, which is more electron-donating than tBu3P,14 afforded 3a in the highest yield (80%, entry 13). The use of Pd(PPh3)4/CuI, which is the optimal catalytic system under the conditions reported by Mack,10 resulted in no reaction for this poorly soluble substrate (entry 14). When we used Pd(PPh3)4 as a single catalyst, which is the optimal catalytic system under the conditions reported by Stolle,112a also remained unreacted (entry 15).
Optimization of the coupling reaction between 1a and 2a to generate 3aa.
| |||||
|---|---|---|---|---|---|
| Entry | Catalytic system | Base | Additive (0.40 μL mg−1) | Internal temp. (°C) | Yieldb (%) |
| 1 | Pd(OAc)2/XPhos | Et3N | None | 30 | <1 |
| 2 | Pd(OAc)2/XPhos | Et3N | None | 80 | 47 |
| 3 | Pd(OAc)2/XPhos | Et3N | None | 120 | 34 |
| 4 | Pd(OAc)2/XPhos | Et3N | Dioxane | 80 | 43 |
| 5 | Pd(OAc)2/XPhos | Et3N | Toluene | 80 | 26 |
| 6 | Pd(OAc)2/XPhos | Et3N | nPrOH | 80 | 39 |
| 7 | Pd(OAc)2/XPhos | Et3N | H2O | 80 | 76 |
| 8 | Pd(OAc)2/XPhos | DABCO | H2O | 80 | 72 |
| 9 | Pd(OAc)2/XPhos | TMEDA | H2O | 80 | 74 |
| 10 | Pd(OAc)2/BrettPhos | Et3N | H2O | 80 | 20 |
| 11 | Pd(OAc)2/tBuXPhos | Et3N | H2O | 80 | <1 |
| 12c | Pd(OAc)2/tBu3P·HBF4 | Et3N | H2O | 80 | 77 |
| 13 | Pd(OAc)2/Ad3P | Et3N | H2O | 80 | 80 |
| 14d | Pd(PPh3)4/CuI | Et3N | H2O | 80 | <1 |
| 15e | Pd(PPh3)4 | Et3N | H2O | 80 | <1 |
| |||||
Conditions: 1a (0.45 mmol), 2a (0.15 mmol), Pd(OAc)2 (0.015 mmol), ligand (0.0225 mmol), base (0.45 mmol), liquid (0.4 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL).
Determined via1H NMR analysis of the crude reaction mixture with an internal standard.
3.5 equiv. of Et3N was used.
Pd(PPh3)4 (0.015 mmol), CuI (0.006 mmol).
Pd(PPh3)4 (0.015 mmol).
With the optimized conditions in hand, we explored the scope of solid aryl bromides in the present Sonogashira cross-coupling reaction under the high-temperature ball-milling conditions (Table 2). Although Ad3P was chosen as the optimal ligand, XPhos provided better conversion in some cases (Table 2, upper part). Therefore, both ligands were used to investigate the substrate scope. Using high-temperature ball milling, reactions between aryl bromides that bear large π-conjugated systems (2a–2d) and TIPS acetylene (1a) proceeded smoothly to give doubly alkynylated π-extended aryl alkynes in moderate to excellent yield (39–95%). 9,10-Bis(2′-naphthyl)anthracene (BNA) is a blue- and white-emitting OLED material with excellent electroluminescence performance.15 Notably, alkynylated BNA (3e) was synthesized in quantitative yield via our method. Several tetrabrominated polycyclic aromatic compounds (2f–2h) were also tested in this mechanochemical Sonogashira reaction. Four-fold alkynylation allows the simultaneous extension of π-conjugated systems in four directions through one step. This synthetic strategy has been widely employed in the design of functional materials. Various polyaromatic units, namely, pyrene,16 spirobifluorene (SF),17 and tetraphenylethylene (TPE)18 were chosen as cores to construct functional organic molecules that feature diverse topologies and properties. Alkynylpyrene has been developed as a biomolecular probe with a high fluorescence quantum yield,16a and also features unique stimuli-responsive emission.16f X-shaped SFs that bear acetylene linkers have proven to be promising non-fullerene acceptors for solar cells.17 Tetrakis(triisopropylsilyl-4-ethynylphenyl)ethene (3h) exhibits remarkable solid-state mechanofluorochromism owing to the aggregation-induced emission (AIE) properties of the TPE unit.18a The present protocol facilitates the synthesis of tetraalkynylated products (3f–3h) based on these cores in excellent yield (83–92%).
Substrate scope of the Sonogashira coupling under the high-temperature ball-milling conditionsa.
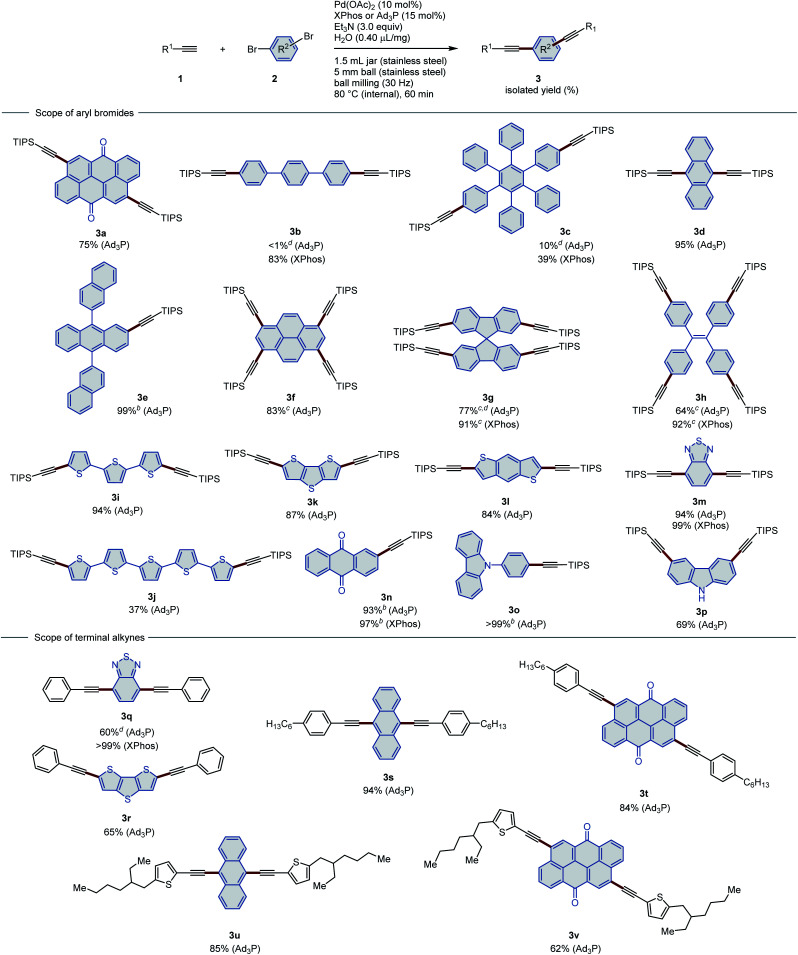
|
Conditions: 1 (0.45 mmol), 2 (0.15 mmol), Pd(OAc)2 (0.015 mmol), ligand (0.0225 mmol), Et3N (0.45 mmol), H2O (0.4 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL).
5 mol% of Pd(OAc)2, 7.5 mol% of ligand, and 1.5 equiv. of Et3N were used.
20 mol% of Pd(OAc)2, 30 mol% of ligand, and 6.0 equiv. of Et3N were used.
Determined via1H NMR analysis of the crude reaction mixture with an internal standard.
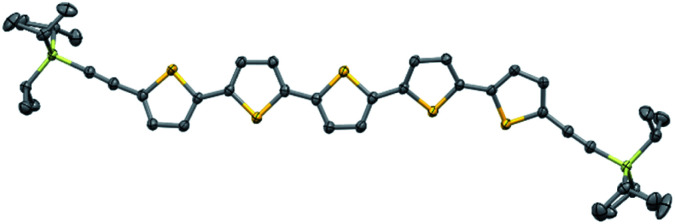
In addition to polycyclic aromatic hydrocarbons (PAHs), brominated aromatic heterocycles are also suitable substrates for this mechanochemical Sonogashira coupling reactions. Oligothiophene derivatives are among the most highly investigated materials for OLEDs and organic semiconductors owing to their extraordinary optoelectrical properties and efficient charge transfer.19 A substrate that bears three linked thiophene units (2i) reacted smoothly to afford the corresponding product (3i) in 94% yield. Functionalization of longer oligothiophene chains is rather challenging due to their significantly decreased solubility. However, substrate 2j with five linked thiophene units is also suitable for this mechanochemical Sonogashira coupling and provides 3j in moderate yield (37%). Notably, this is the first reported synthetic route to 3j. Single crystals of 3j were easily obtained by recrystallization from CHCl3/MeOH, and its structure was unambiguously determined using X-ray crystallography (Fig. 2). Fused heteroaromatic compounds based on thiophene units play an important role in organic electronics.20 Double alkynylation of dithieno[3,2-b:2′,3′-d]thiophene (2k) and benzo[1,2-b:4,5-b′]dithiophene (2l) proceeded smoothly to give the corresponding silylalkyne products (3k and 3l) in good yield (84–87%). Nitrogen- and oxygen-containing conjugated units, including 2,1,3-benzothiadiazole (2m), anthraquinone (2n), and N-substituted carbazole (2o), were all tolerated under these conditions, resulting in the quantitative formation of the expected coupling products (97–99%). Unprotected carbazole (2p) moieties can also participate in this reaction to give dialkynylated product 3p in an acceptable yield (69%).
Fig. 2. X-ray crystal structure of 3j with thermal ellipsoids at 50% probability; all hydrogen atoms are omitted for clarity.
Subsequently, several terminal aryl alkynes were selected in order to showcase the generality of this method (Table 2, lower part). Direct introduction of an aromatic ethynyl group to a polycyclic core can extend the conjugation of the π-system to a great extent, leading to significant modification of the properties of the original materials. Two known OLED materials, namely, a D–A–D chromophore based on 2,1,3-benzothiadiazole (3q)21 and a D–π–D chromophore based on dithieno[3,2-b:2′,3′-d]thiophene (3r),22 were successfully synthesized using our method. The moderate to high yields (99% and 65%) indicate the high reactivity of phenylacetylene (1b). It should be noted here that in a previous report,22 the solution-based synthesis of 3r by the palladium/copper co-catalyzed Sonogashira coupling provided only 24% yield even after prolonged reaction time (48 h), which emphasizes the superior efficiency of our mechanochemical protocol (65%, 60 min). The reactivity of 4-hexylphenyl acetylene (1c) was tested with 9,10-dibromoanthracene (2d) and Vat Orange 3 (2a). Both attempts provided the corresponding products (3s and 3t) in excellent yield (94% and 84%, respectively). Substituted ethynyl thiophene (1d) also proved to be a suitable coupling partner, affording the desired products (3u and 3v) in moderate to good yield (85% and 62%, respectively).
After the investigation of the scope of aryl bromides, we turned our attention to unactivated aryl chlorides. In comparison with aryl bromides and iodides, the intrinsic inertness of aryl chlorides presents a challenging barrier to the use of these highly accessible and economical compounds in cross-coupling. Although elegant methods have been established to achieve Sonogashira coupling of aryl chlorides in solution,8a,b the development of a mechanochemical analogue remains unexplored. Inspired by Buchwald's contribution describing the copper-free Sonogashira reactions of aryl chlorides,8a we speculated that a similar catalytic system might be successful in the ball-milling strategy. After an intensive optimization study (for details, see the ESI†), Pd(OAc)2/BrettPhos was determined to be the best catalytic system, and DABCO was chosen as the base.
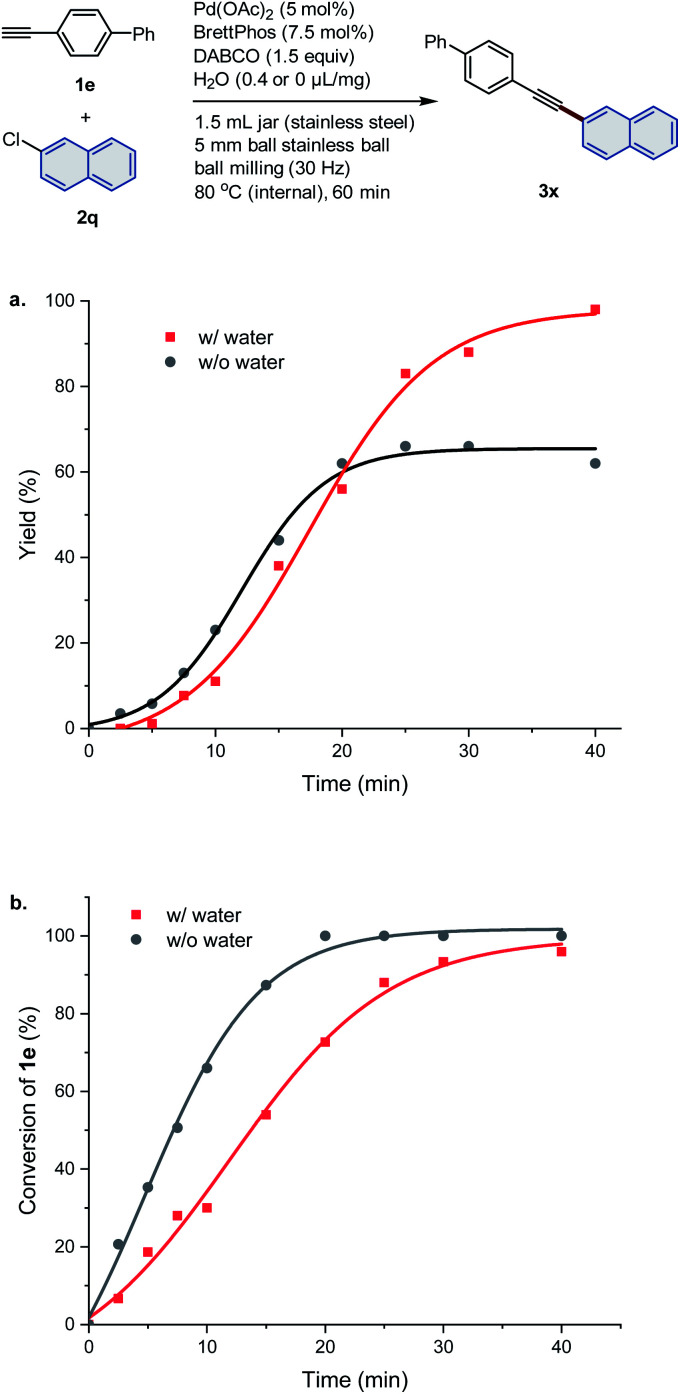
Similar to reactions of aryl bromides, the addition of water as the LAG agent significantly improved the yield of the coupling product. In order to gain insight into the acceleration effect of water as an additive, a comparative kinetic study was conducted (Fig. 3). Solid-state cross-coupling between 4-ethynyl-1,1′-biphenyl (1e) and 2-chloronaphthalene (2q) was chosen as a model reaction. As periodic sampling of the mechanochemical reaction runs would require stopping the mill and opening the jar, each data point was obtained from an individual reaction. We found that both systems showed sigmoidal kinetics (Fig. 3a). Under the conditions with H2O (Fig. 3a, red line), the reaction was slow during the first 10 min but was rapidly completed within 40 min. In contrast, under the H2O-free conditions (Fig. 3a, black line), the reaction stopped after 20 min, and the yield did not reach beyond 60%. Subsequently, we investigated the conversion rate of the alkyne 1e (Fig. 3b). For the H2O-free conditions (Fig. 3b, black line), the conversion of 1e increased faster than the conditions with H2O (Fig. 3b, red line), and full conversion was observed after 20 min while the product yield remained around 60% (Fig. 3a, black line). These results suggest the presence of competing side reactions, such as homocoupling and oligomerization of 1e. Conversely, the addition of H2O could suppress such side reactions of the alkyne 1e, leading to the quantitative formation of Sonogashira coupling product 3x. However, the details of the mechanism of this water-enhanced reactivity are still unclear.
Fig. 3. Comparative kinetic study on the influence of water. (a) Time-dependent plot of the yield of target product 3x. (b) Time-dependent plot of the conversion of alkyne substrate 1e.
After 1 h of high-temperature ball milling (internal temperature: 80 °C), alkynylated products were obtained for aryl chloride substrates (Table 3). Both TIPS acetylene (1a) and solid aryl alkyne 4-ethynyl-1,1′-biphenyl (1e) reacted smoothly with chloronaphthalene (2q and 2r) to afford the corresponding aromatic internal alkynes in high yield (91–99%). The functional-group compatibility of this method was further demonstrated by (4-chlorophenyl)methanol (2s) and 4-chloroaniline (2t). Unprotected hydroxyl and amine groups remained unchanged after heated ball milling, affording the corresponding products (3aa and 3ab) in 72% and 90% yield, respectively. However, the reactivity of 9,10-dichloroanthracene (2u) is significantly lower than that of its brominated analogue, resulting in only 45% yield. Heteroatom-containing 2,5-dichlorothiophene (2v) is also a suitable substrate. Both silyl and aryl alkynes are compatible coupling partners, producing good yields (86–87%).
Sonogashira coupling with aryl chlorides under the high-temperature ball-milling conditionsa.
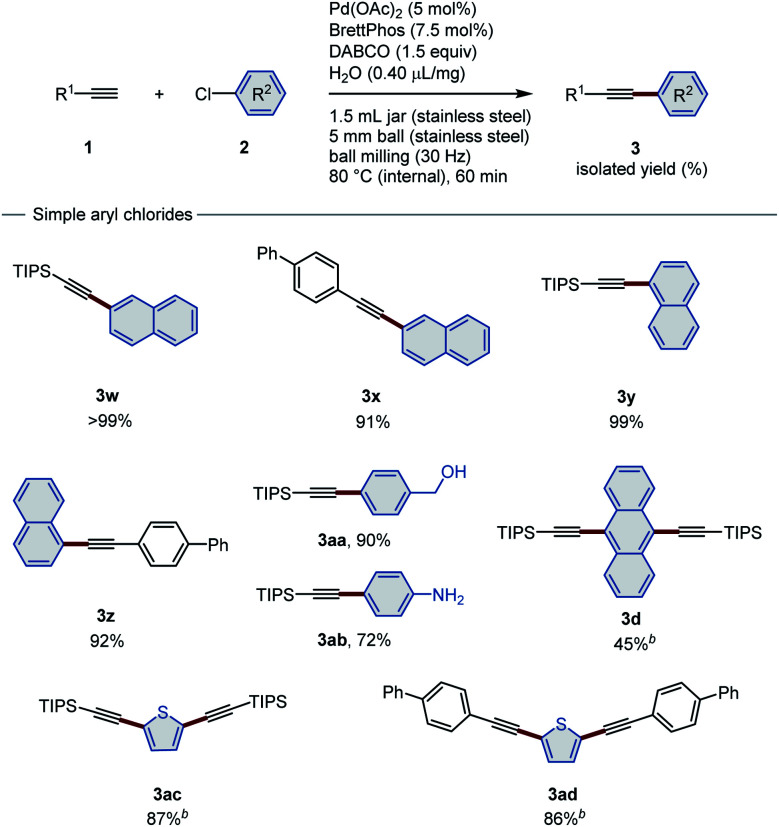
|
Conditions: 1 (0.45 mmol), 2 (0.30 mmol), Pd(OAc)2 (0.015 mmol), BrettPhos (0.0225 mmol), DABCO (0.45 mmol), H2O (0.4 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL).
10 mol% of Pd(OAc)2, 15 mol% of BrettPhos, and 3.0 equiv. of DABCO were used.
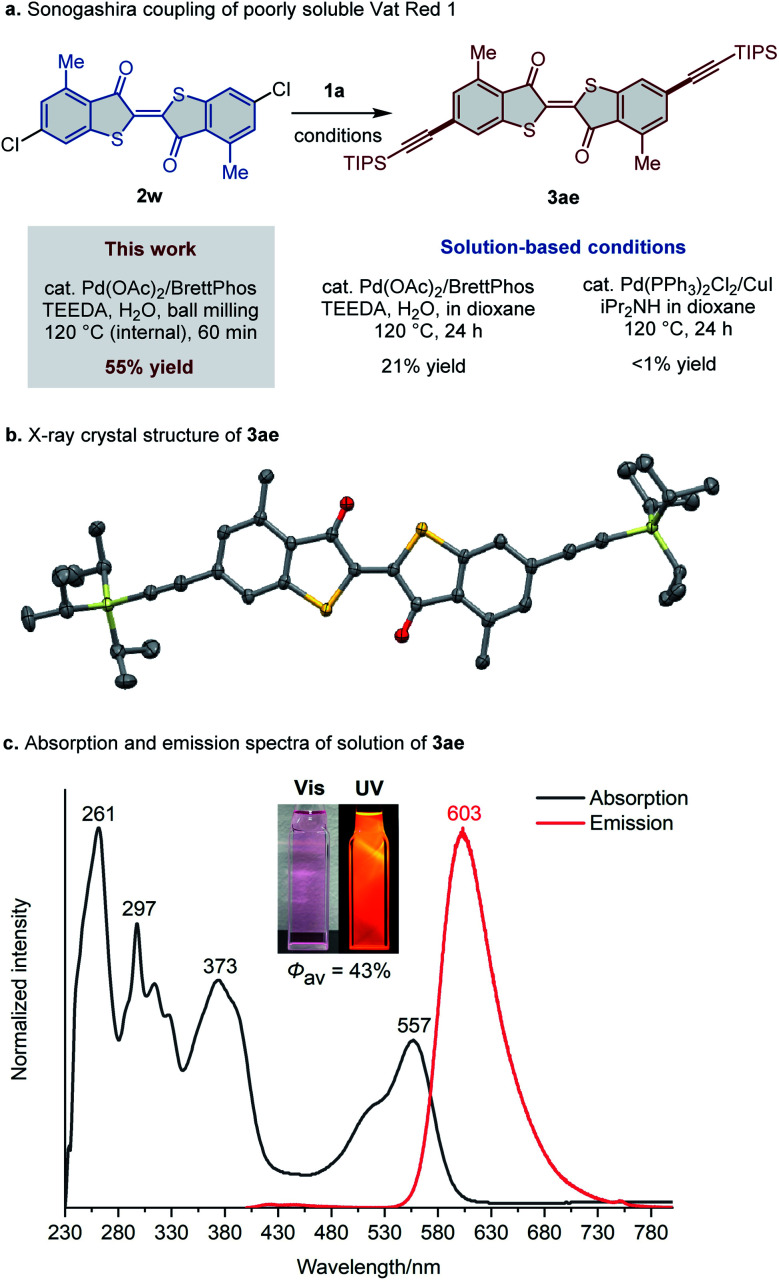
Next, we investigated the applicability of the developed protocol to poorly soluble aryl chlorides. Vat Red 1 (2w) is a pigment derived from thioindigo23 that has a solubility of 1.0 × 10−4 M in toluene at room temperature,12 which is lower than those of the iconic poorly soluble molecules pentacene and phthalocyanine (4.7 × 10−4 M and 1.9 × 10−4 M in toluene at room temperature, respectively).12 After slight modifications of the reaction conditions, the desired doubly alkynylated product 3ae was obtained in 55% yield via the cross-coupling of 2w with 1a under the high-temperature ball-milling conditions (Fig. 4a). Notably, the solution-based reactions using the same catalytic system, or the conventional palladium/copper co-catalytic system, provided a significantly lower yield of 3ae (21%) or resulted in no product formation even after prolonged reaction time (24 h), respectively. These results clearly demonstrate the advantages of the high-temperature ball-milling approach in the Sonogashira couplings of poorly soluble substrates. The molecular structure of 3ae was confirmed by single-crystal X-ray diffraction analysis (Fig. 4b; for details, see the ESI†). In order to further demonstrate the synthetic utility of our method, a large-scale reaction of Vat Red 1 (2w) and 1a was conducted in two batches using 10 mL stainless-steel jars. Only a slight decrease in the yield (51%) was observed compared to the small-scale reaction, which afforded approximately 900 mg of 3ae (for details, see the ESI†). We also noticed that 3ae, which was synthesized for the first time, exhibits strong photoluminescence in chloroform (Fig. 4c).24 A dilute solution (10−5 M) of 3ae in chloroform shows strong orange emission under ultraviolet (UV) irradiation (Φav = 43%; λex = 375 nm). We expect that the newly developed Sonogashira coupling conditions will allow the discovery of novel luminescent materials from poorly soluble aryl halides that cannot be prepared under conventional solution-based conditions.
Fig. 4. High-temperature ball milling for the Sonogashira coupling of poorly soluble aryl chlorides. (a) Sonogashira coupling of poorly soluble Vat Red 1 (2w) enabled by a high-temperature ball-milling approach. (b) X-ray crystal structure of 3ae with thermal ellipsoids at 50% probability; all hydrogen atoms are omitted for clarity. (c) Absorption and emission spectra of solutions of 3ae (c = 1.0 × 10−5 M; λex = 373 nm) in CHCl3.
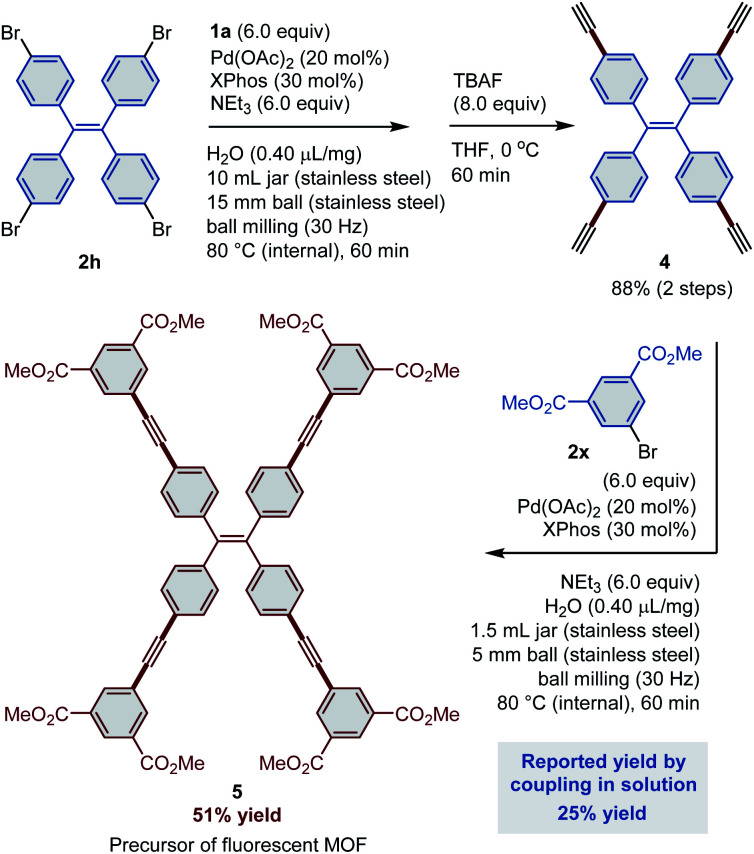
The stepwise sequence of Sonogashira coupling–desilylation–Sonogashira coupling is an established way to introduce an ethynyl linkage between two functional moieties. Compound 5,1d which is a precursor of a well-designed fluorescent MOF ligand, was chosen as a representative target to show the applicability of mechanochemical approaches to this strategy (Scheme 1). In the first step, mechanochemical Sonogashira coupling between 1a and 1,1,2,2-tetrakis(4-bromophenyl)ethene (2h) was conducted on a 500 mg scale. After 1 h of high-temperature ball milling, the crude mixture was directly treated with a solution of tetrabutylammonium fluoride (TBAF). The desired terminal alkyne 4 was obtained in 88% overall yield. Subsequently, 4 was subjected to a second mechanochemical solid-state Sonogashira coupling with dimethyl 5-bromoisophthalate (2x), which afforded the target molecule 5 in 51% yield. Scale-up of the reaction led to a slightly diminished yield (44%; for details, see the ESI†). Notably, the reported solution-based method furnishes 5 in only 25% yield,1d highlighting the advantage of the solid-state ball-milling protocol.
Scheme 1. Sequential Sonogashira coupling under the high-temperature ball-milling conditions for the synthesis of 5.
Conclusions
Using a high-temperature ball-milling technique, we have developed the first practical mechanochemical protocol for the Sonogashira cross-coupling of polyaromatic halides, which readily provides access to materials-oriented aromatic alkynes in excellent yield with short reaction times. In comparison to previous mechanochemical attempts, this novel method features a much broader substrate scope, which includes poorly soluble aryl halides that bear large polycyclic conjugated systems. Notably, the developed protocol allowed the synthesis of a new luminescent organic material derived from a poorly soluble pigment that is an unsuitable substrate in conventional solution-based approaches due to its extremely low solubility. The utility of this method was further demonstrated by the rapid synthesis of a fluorescent MOF precursor via two sequential mechanochemical Sonogashira cross-coupling reactions, which gave a better yield than solution-based reactions. We expect that this method will inspire a new synthetic strategy for the design and preparation of novel functional materials containing C(sp)–C(sp2) bonds.
Data availability
All experimental data is available in the ESI.†
Author contributions
K. K. and H. I. conceived and designed the study. Y. G., K. K. and H. I. co-wrote the paper. Y. G. performed the chemical experiments and analyzed the data. C. F. performed the X-ray crystallography experiments and analyzed the obtained data. T. S. performed the preliminary experimental studies. All authors discussed the results and commented on the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 17H06370, 18H03907, 20H04795, and 21H01926; by the JST via CREST grant JPMJCR19R1; by FOREST grant JPMJFR201I; as well as by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan. We are grateful to Dr M. Jin for his assistance with analyzing the absorption and emission spectra and the X-ray data of 3j and 3ae.
Electronic supplementary information (ESI) available. CCDC 2106264 and 2106265. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc05257h
Notes and references
- (a) Kato S.-i. Matsumoto T. Shigeiwa M. Gorohmaru H. Maeda S. Ishi-i T. Mataka S. Chem.–Eur. J. 2006;12:2303. doi: 10.1002/chem.200500921. [DOI] [PubMed] [Google Scholar]; (b) Yen W.-N. Lo S.-S. Kuo M.-C. Mai C.-L. Lee G.-H. Peng S.-M. Yeh C.-Y. Org. Lett. 2006;8:4239. doi: 10.1021/ol061478w. [DOI] [PubMed] [Google Scholar]; (c) Wu Y.-T. Bandera D. Maag R. Linden A. Baldridge K. K. Siegel J. S. J. Am. Chem. Soc. 2008;130:10729. doi: 10.1021/ja802334n. [DOI] [PubMed] [Google Scholar]; (d) Shustova N. B. Cozzolino A. F. Dincă M. J. Am. Chem. Soc. 2012;134:19596. doi: 10.1021/ja3103154. [DOI] [PubMed] [Google Scholar]; (e) Shang C. Wang G. Liu K. Jiang Q. Liu F. Chou P.-T. Fang Y. Angew. Chem., Int. Ed. 2020;59:8579. doi: 10.1002/anie.201914070. [DOI] [PubMed] [Google Scholar]
- (a) Zhao Z. Li J.-H. Chen X. Wang X. Lu P. Yang Y. J. Org. Chem. 2009;74:383. doi: 10.1021/jo802237c. [DOI] [PubMed] [Google Scholar]; (b) Slodek A. Filapek M. Schab-Balcerzak E. Grucela M. Kotowicz S. Janeczek H. Smolarek K. Mackowski S. Malecki J. G. Jedrzejowska A. Szafraniec-Gorol G. Chrobok A. Marcol B. Krompiec S. Matussek M. Eur. J. Org. Chem. 2016:4020. doi: 10.1002/ejoc.201600532. [DOI] [Google Scholar]; (c) Usta H. Alimli D. Ozdemir R. Dabak S. Zorlu Y. Alkan F. Tekin E. Can A. ACS Appl. Mater. Interfaces. 2019;11:44474. doi: 10.1021/acsami.9b12971. [DOI] [PubMed] [Google Scholar]; (d) Wang Z. Peng Z. Huang K. Lu P. Wang Y. J. Mater. Chem. C. 2019;7:6706. doi: 10.1039/C9TC01059A. [DOI] [Google Scholar]
- (a) Lu H.-P. Mai C.-L. Tsia C.-Y. Hsu S.-J. Hsieh C.-P. Chiu C.-L. Yeh C.-Y. Diau E. W.-G. Phys. Chem. Chem. Phys. 2009;11:10270. doi: 10.1039/B917271H. [DOI] [PubMed] [Google Scholar]; (b) Colella S. Mazzeo M. Grisorio R. Fabiano E. Melcarne G. Carallo S. Angione M. D. Torsi L. Suranna G. P. della Sala F. Mastrorilli P. Gigli G. Chem. Commun. 2010;46:6273. doi: 10.1039/C0CC01229G. [DOI] [PubMed] [Google Scholar]; (c) Zhang A. Li C. Yang F. Zhang J. Wang Z. Wei Z. Li W. Angew. Chem., Int. Ed. 2017;56:2694. doi: 10.1002/anie.201612090. [DOI] [PubMed] [Google Scholar]; (d) Li G. Zhang Y. Liu T. Wang S. Li D. Li J. Li F. Yang L.-M. Luo Z. Yang C. Yan H. Hao P. Shang Q. Tang B. J. Mater. Chem. C. 2018;6:11111. doi: 10.1039/C8TC02823K. [DOI] [Google Scholar]; (e) Wang H. Li M. Liu Y. Song J. Li C. Bo Z. J. Mater. Chem. C. 2019;7:819. doi: 10.1039/C8TC05332D. [DOI] [Google Scholar]
- (a) Yang L. Yao Z. Liu J. Wang J. Wang P. ACS Appl. Mater. Interfaces. 2016;8:9839. doi: 10.1021/acsami.6b02075. [DOI] [PubMed] [Google Scholar]; (b) Mu Y. Wu H. Dong G. Shen Z. Li S. Zhang M. J. Mater. Chem. A. 2018;6:21493. doi: 10.1039/C8TA08363K. [DOI] [Google Scholar]
- For selected reviews on Sonogashira cross-coupling reactions, see: ; (a) Negishi E.-i. Anastasia L. Chem. Rev. 2003;103:1979. doi: 10.1021/cr020377i. [DOI] [PubMed] [Google Scholar]; (b) Chinchilla R. Nájera C. Chem. Rev. 2007;107:874. doi: 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]; (c) Chinchilla R. Nájera C. Chem. Soc. Rev. 2011;40:5084. doi: 10.1039/C1CS15071E. [DOI] [PubMed] [Google Scholar]; (d) Chinchilla R. Nájera C. Chem. Rev. 2014;114:1783. doi: 10.1021/cr400133p. [DOI] [PubMed] [Google Scholar]; (e) Wang D. Gao S. Org. Chem. Front. 2014;1:556. doi: 10.1039/C3QO00086A. [DOI] [Google Scholar]; (f) Kanwal I. Mujahid A. Rasool N. Rizwan K. Malik A. Ahmad G. Shah S. A. A. Rashid U. Nasir N. M. Catalysts. 2020;10:443. doi: 10.3390/catal10040443. [DOI] [Google Scholar]; (g) Mohajer F. Heravi M. M. Zadsirjan V. Poormohammad N. RSC Adv. 2021;11:6885. doi: 10.1039/D0RA10575A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Isfahani A. L. Mohammadpoor-Baltork I. Mirkhani V. Khosropour A. R. Moghadam M. Tangestaninejad S. Eur. J. Org. Chem. 2014:5603. doi: 10.1002/ejoc.201402503. [DOI] [PubMed] [Google Scholar]; (b) Kozell V. McLaughlin M. Strappaveccia G. Santoro S. Bivona L. A. Aprile C. Gruttadauria M. Vaccaro L. ACS Sustainable Chem. Eng. 2016;4:7209. doi: 10.1021/acssuschemeng.6b02170. [DOI] [Google Scholar]; (c) Zhu J. Lindsay V. N. G. ACS Catal. 2019;9:6993. doi: 10.1021/acscatal.9b02420. [DOI] [Google Scholar]; (d) Jin B. Gallou F. Reilly J. Lipshutz B. H. Chem. Sci. 2019;10:3481. doi: 10.1039/C8SC05618H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Martek B. A. Gazvoda M. Urankar D. Košmrlj J. Org. Lett. 2020;22:4938. doi: 10.1021/acs.orglett.0c01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Nakamura K. Okubo H. Yamaguchi M. Synlett. 1999:549. doi: 10.1055/s-1999-2684. [DOI] [Google Scholar]; (b) Mori A. Kawashima J. Shimada T. Suguro M. Hirabayashi K. Nishihara Y. Org. Lett. 2000;2:2935. doi: 10.1021/ol0061586. [DOI] [PubMed] [Google Scholar]; (c) Eberhard M. R. Wang Z. Jensen C. M. Chem. Commun. 2002:818. doi: 10.1039/B200453B. [DOI] [PubMed] [Google Scholar]; (d) Elangovan A. Wang Y.-H. Ho T.-I. Org. Lett. 2003;5:1841. doi: 10.1021/ol034320+. [DOI] [PubMed] [Google Scholar]
- (a) Gelman D. Buchwald S. L. Angew. Chem., Int. Ed. 2003;42:5993. doi: 10.1002/anie.200353015. [DOI] [PubMed] [Google Scholar]; (b) Huang H. Liu H. Jiang H. Chen K. J. Org. Chem. 2008;73:6037. doi: 10.1021/jo800994f. [DOI] [PubMed] [Google Scholar]; (c) Tian Z.-Y. Wang S.-M. Jia S.-J. Song H.-X. Zhang C.-P. Org. Lett. 2017;19:5454. doi: 10.1021/acs.orglett.7b02764. [DOI] [PubMed] [Google Scholar]; (d) He J. Yang K. Zhao J. Cao S. Org. Lett. 2019;21:9714. doi: 10.1021/acs.orglett.9b03815. [DOI] [PubMed] [Google Scholar]; (e) Li X. Liu L. Huang T. Tang Z. Li C. Li W. Zhang T. Li Z. Chen T. Org. Lett. 2021;23:3304. doi: 10.1021/acs.orglett.1c00768. [DOI] [PubMed] [Google Scholar]
- For recent reviews on organic synthesis using mechanochemistry, see: ; (a) James S. L. Adams C. J. Bolm C. Braga D. Collier P. Friščić T. Grepioni F. Harris K. D. M. Hyett G. Jones W. Krebs A. Mack J. Maini L. Orpen A. G. Parkin I. P. Shearouse W. C. Steed J. W. Waddell D. C. Chem. Soc. Rev. 2012;41:413. doi: 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]; (b) Wang G.-W. Chem. Soc. Rev. 2013;42:7668. doi: 10.1039/C3CS35526H. [DOI] [PubMed] [Google Scholar]; (c) Do J.-L. Friščić T. ACS Cent. Sci. 2017;3:13. doi: 10.1021/acscentsci.6b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hernández J. G. Bolm C. J. Org. Chem. 2017;82:4007. doi: 10.1021/acs.joc.6b02887. [DOI] [PubMed] [Google Scholar]; (e) Hernández J. G. Chem.–Eur. J. 2017;23:17157. doi: 10.1002/chem.201703605. [DOI] [PubMed] [Google Scholar]; (f) Achar T. K. Bose A. Mal P. Beilstein J. Org. Chem. 2017;13:1907. doi: 10.3762/bjoc.13.186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Do J.-L. Friščić T. Synlett. 2017;28:2066. doi: 10.1055/s-0036-1590854. [DOI] [Google Scholar]; (h) Tan D. Friščić T. Eur. J. Org. Chem. 2018:18. doi: 10.1002/ejoc.201700961. [DOI] [Google Scholar]; (i) Howard J. L. Cao Q. Browne D. L. Chem. Sci. 2018;9:3080. doi: 10.1039/C7SC05371A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Andersen J. Mack J. Green Chem. 2018;20:1435. doi: 10.1039/C7GC03797J. [DOI] [Google Scholar]; (k) Rightmire N. R. Hanusa T. P. Dalton Trans. 2016;45:2352. doi: 10.1039/C5DT03866A. [DOI] [PubMed] [Google Scholar]; (l) Leonardi M. Villacampa M. Menéndez J. C. Chem. Sci. 2018;9:2042. doi: 10.1039/C7SC05370C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Tan D. Loots L. Friščić T. Chem. Commun. 2016;52:7760. doi: 10.1039/C6CC02015A. [DOI] [PubMed] [Google Scholar]; (n) Tan D. García F. Chem. Soc. Rev. 2019;48:2274. doi: 10.1039/C7CS00813A. [DOI] [PubMed] [Google Scholar]; (o) Bolm C. Hernández J. G. Angew. Chem., Int. Ed. 2019;58:3285. doi: 10.1002/anie.201810902. [DOI] [PubMed] [Google Scholar]; (p) Friščić T. Mottillo C. Titi H. M. Angew. Chem., Int. Ed. 2020;59:1018. doi: 10.1002/anie.201906755. [DOI] [PubMed] [Google Scholar]; (q) Kubota K. Ito H. Trends Chem. 2020;2:1066. doi: 10.1016/j.trechm.2020.09.006. [DOI] [Google Scholar]; (r) Egorov I. N. Santra S. Kopchuk D. S. Kovalev I. S. Zyryanov G. V. Majee A. Ranu B. C. Rusinov V. L. Chupakhin O. N. Green Chem. 2020;22:302. doi: 10.1039/C9GC03414E. [DOI] [Google Scholar]; (s) Porcheddu A. Colacino E. De Luca L. Delogu F. ACS Catal. 2020;10:8344. doi: 10.1021/acscatal.0c00142. [DOI] [Google Scholar]; (t) Ying P. Yu J. Su W. Adv. Synth. Catal. 2021;363:1246. doi: 10.1002/adsc.202001245. [DOI] [Google Scholar]; (u) Ardila-Fierro K. J. Hernández J. G. ChemSusChem. 2021;14:2145. doi: 10.1002/cssc.202100478. [DOI] [PubMed] [Google Scholar]
- Fulmer D. A. Shearouse W. C. Medonza S. T. Mack J. Green Chem. 2009;11:1821. doi: 10.1039/B915669K. [DOI] [Google Scholar]
- Thorwirth R. Stolle A. Ondruschka B. Green Chem. 2010;12:985. doi: 10.1039/C000674B. [DOI] [Google Scholar]
- Seo T. Toyoshima N. Kubota K. Ito H. J. Am. Chem. Soc. 2021;143:6165. doi: 10.1021/jacs.1c00906. [DOI] [PubMed] [Google Scholar]
- Giguère J.-B. Verolet Q. Morin J.-F. Chem.–Eur. J. 2013;19:372. doi: 10.1002/chem.201202878. [DOI] [PubMed] [Google Scholar]
- Chen L. Ren P. Carrow B. P. J. Am. Chem. Soc. 2016;138:6392. doi: 10.1021/jacs.6b03215. [DOI] [PubMed] [Google Scholar]
- (a) Zhang X. H. Liu M. W. Wong O. Y. Lee C. S. Kwong H. L. Lee S. T. Wu S. K. Chem. Phys. Lett. 2003;369:478. doi: 10.1016/S0009-2614(02)02042-0. [DOI] [Google Scholar]; (b) Raghunath P. Reddy M. A. Gouri C. Bhanuprakash K. Rao V. J. J. Phys. Chem. A. 2006;110:1152. doi: 10.1021/jp0555753. [DOI] [PubMed] [Google Scholar]
- (a) Maeda H. Maeda T. Mizuno K. Fujimoto K. Shimizu H. Inouye M. Chem.–Eur. J. 2006;12:824. doi: 10.1002/chem.200500638. [DOI] [PubMed] [Google Scholar]; (b) Wang Q. Liu H. Lu H. Xu Z. Lai G. Li Z. Mack J. Shen Z. Dyes Pigm. 2013;99:771. doi: 10.1016/j.dyepig.2013.07.003. [DOI] [Google Scholar]; (c) Cappellari M. V. Mangione M. I. Spanevello R. A. Marzari G. Morales G. M. Fungo F. J. Electrochem. Soc. 2018;165:G163. doi: 10.1149/2.0881814jes. [DOI] [Google Scholar]; (d) Maeda H. Shoji T. Segi M. Tetrahedron Lett. 2017;58:4372. doi: 10.1016/j.tetlet.2017.10.008. [DOI] [Google Scholar]; (e) Kim H. M. Lee Y. O. Lim C. S. Kim J. S. Cho B. R. J. Org. Chem. 2008;73:5127. doi: 10.1021/jo800363v. [DOI] [PubMed] [Google Scholar]; (f) Xu F. Nishida T. Shinohara K. Peng L. Takezaki M. Kamada T. Akashi H. Nakamura H. Sugiyama K. Ohta K. Orita A. Otera J. Organometallics. 2017;36:556. doi: 10.1021/acs.organomet.6b00781. [DOI] [Google Scholar]
- Wu X.-F. Fu W.-F. Xu Z. Shi M. Liu F. Chen H.-Z. Wan J.-H. Russell T. P. Adv. Funct. Mater. 2015;25:5954. doi: 10.1002/adfm.201502413. [DOI] [Google Scholar]
- (a) Ramachandran E. Dhamodharan R. J. Mater. Chem. C. 2017;5:10469. doi: 10.1039/C7TC03211K. [DOI] [Google Scholar]; (b) Xie Y. Tu J. Zhang T. Wang J. Xie Z. Chi Z. Peng Q. Li Z. Chem. Commun. 2017;53:11330. doi: 10.1039/C7CC04663D. [DOI] [PubMed] [Google Scholar]; (c) De J. Haseeb M. M. A. Yadav R. A. K. Gupta S. P. Bala I. Chawla P. Kesavan K. K. Jou J.-H. Pal S. K. Chem. Commun. 2020;56:14279. doi: 10.1039/D0CC05813K. [DOI] [PubMed] [Google Scholar]
- For selected reviews on oligothiophene-based materials, see: ; (a) Fichou D. J. Mater. Chem. 2000;10:571. doi: 10.1039/A908312J. [DOI] [Google Scholar]; (b) Mishra A. Ma C.-Q. Bäuerle P. Chem. Rev. 2009;109:1141. doi: 10.1021/cr8004229. [DOI] [PubMed] [Google Scholar]; (c) Zade S. S. Zamoshchik N. Bendikov M. Acc. Chem. Res. 2011;44:14. doi: 10.1021/ar1000555. [DOI] [PubMed] [Google Scholar]; (d) Zhang L. Colella N. S. Cherniawski B. P. Mannsfeld S. C. B. Briseno A. L. ACS Appl. Mater. Interfaces. 2014;6:5327. doi: 10.1021/am4060468. [DOI] [PubMed] [Google Scholar]
- For selected reviews on fused heteroaromatic materials based on thiophene, see: ; (a) Li M. Ni W. Wan X. Zhang Q. Kan B. Chen Y. J. Mater. Chem. A. 2015;3:4765. doi: 10.1039/C4TA06452F. [DOI] [Google Scholar]; (b) Cinar M. E. Ozturk T. Chem. Rev. 2015;115:3036. doi: 10.1021/cr500271a. [DOI] [PubMed] [Google Scholar]; (c) Yao H. Ye L. Zhang H. Li S. Zhang S. Hou J. Chem. Rev. 2016;116:7397. doi: 10.1021/acs.chemrev.6b00176. [DOI] [PubMed] [Google Scholar]; (d) Xue Z. Chen S. Gao N. Xue Y. Lu B. Watson O. A. Zang L. Xu J. Polym. Rev. 2020;60:318. doi: 10.1080/15583724.2019.1673404. [DOI] [Google Scholar]; (e) Tang H. Yan C. Huang J. Kan Z. Xiao Z. Sun K. Li G. Lu S. Matter. 2020;3:1403. doi: 10.1016/j.matt.2020.09.001. [DOI] [Google Scholar]
- DaSilveira Neto B. A. Lopes A. S. Ebeling G. Gonçalves R. S. Costa V. E. U. Quina F. H. Dupont J. Tetrahedron. 2005;61:10975. doi: 10.1016/j.tet.2005.08.093. [DOI] [Google Scholar]
- Wang C.-Z. Do J.-H. Akther T. Feng X. Horsburgh L. Elsegood M. R. J. Redshaw C. Yamato T. Tetrahedron. 2017;73:307. doi: 10.1016/j.tet.2016.11.077. [DOI] [Google Scholar]
- For a review on the application of thioindigo dyes, see: ; Hosseinnezhad M. Moradian S. Gharanjig K. Dyes Pigm. 2015;123:147. doi: 10.1016/j.dyepig.2015.07.016. [DOI] [Google Scholar]
- For a study on the visible spectra of thioindigo dyes, see: ; Jacquemin D. Preat J. Wathelet V. Fontaine M. Perpète E. A. J. Am. Chem. Soc. 2006;128:2072. doi: 10.1021/ja056676h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All experimental data is available in the ESI.†