Abstract
Background
Acute pain frequently occurs after surgical procedures. Nicotine has been explored as an adjunctive medication for management of postoperative pain.
Objectives
To assess the effect of transdermal or intranasal nicotine administration on postoperative pain, opioid analgesic use, and opioid‐related adverse events.
Search methods
We searched MEDLINE (1966 to 20 March 2014), the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 3), EMBASE (1980 to 20 March 2014), and also databases of ongoing trials (www.controlled‐trials.com/ and http://clinicaltrials.gov/). We re‐ran the search on 28 April 2015. We will assess the one study of interest when we update the review.
Selection criteria
We included randomized, placebo‐controlled clinical trials that evaluated the effects of perioperative (pre‐, intra‐, or postoperative) administration of nicotine on postoperative pain, opioid use, and opioid‐related adverse events. We excluded all other studies.
Data collection and analysis
Two authors independently screened all titles and abstracts for eligibility and documented reasons for exclusion. In case of disagreement, a third author decided on the inclusion or exclusion of a trial report. When additional information was needed in order to decide if a trial should be included, one of the authors contacted the corresponding author of the trial in question.
Main results
Nine trials (666 participants) evaluated nicotine for postoperative pain. Nicotine may reduce postoperative pain scores at 24 hours by a small amount compared with placebo (eight trials, mean difference ‐0.88 on a 0 to 10 scale, 95% confidence interval (CI) ‐1.58 to ‐0.18; low quality evidence). The effect on pain at one hour and 12 hours postoperatively was less certain (very low quality evidence). Statistical heterogeneity was substantial and not adequately explained by stratification of trials according to type of surgical procedure, smoking status, mode of nicotine administration, timing of administration, or assessed risk of bias. Excluding one trial at high risk of bias resulted in similar findings. The effect of nicotine on postoperative opioid use was uncertain due to small number of participants in the studies. Nicotine probably increases the risk of postoperative nausea (seven trials, RR 1.24, 95% CI 1.03 to 1.50; moderate quality evidence). Three trials assessed sedation but the effect is very uncertain due to the very low quality of evidence. We found no evidence that nicotine increased the risk of vomiting (seven studies, risk difference (RD) 0.03, 95% CI ‐0.04 to 0.09; low quality evidence). The results from one single small trial were insufficient to establish whether nicotine led to an earlier hospital discharge (very low quality evidence).
Authors' conclusions
Based on evidence of generally low quality, nicotine may reduce postoperative pain at 24 hours compared with placebo, but the effects were relatively small (less than 1 point on a 10 point pain scale) and there was substantial heterogeneity in the results of our analyses. Nicotine does not appear to reduce postoperative use of opioids or opioid‐related adverse events but probably increases the risk of nausea. More research is needed to determine the effectiveness of nicotine for postoperative pain and to understand the optimal timing, dose, and method of delivery of nicotine.
Plain language summary
Nicotine for postoperative pain
Review question
This Cochrane review examines whether nicotine given prior to, during, or immediately after surgery results in less pain, use of opioids, and side effects from opioids.
Background study characteristics
Major surgery is usually associated with significant pain. The mainstay of treatment for pain following major surgery is opioid medications (strong pain killers such as morphine). However, opioids are not always entirely effective and are associated with side effects including sleepiness (sedation), shallow breathing (respiratory depression), feeling sick (nausea), and being sick (vomiting). Co‐administered medications, like paracetamol, may help improve postoperative pain control and reduce the need for opioids.
We included nine clinical trials with a total of 666 participants. We searched several databases to March 2014, to find placebo‐controlled, randomized trials (clinical studies where people are randomly put into one of two or more treatment groups, one of which includes a pretend (placebo) group) of nicotine for postoperative pain. We also contacted study authors for additional data. Not all studies reported all of the symptoms (outcomes) listed above, so what we can say about some outcomes is limited. We re‐ran the search on 28 April 2015. We will assess the one study of interest when we update this review.
Key results
Our results indicated that there is low quality evidence that nicotine use results in slightly lower postoperative pain scores 24 hours after surgery. At one hour and 12 hours postoperatively the effect was less certain. Nicotine appeared not to reduce use of opioids at 60 minutes or 24 hours, neither was there evidence that it reduced sedation or vomiting. Nicotine was associated with higher risk of nausea than placebo, and this may limit its use. There was not enough data to evaluate the effects of nicotine use on other side effects associated with opioids, including respiratory depression, or the effects of nicotine use on length of hospital stay following surgery.
Quality of the evidence
We downgraded the quality of the evidence to low or very low quality largely because of problems with the way that the studies were designed, which could have exaggerated the results, because there was insufficient data in many of the analyses to be certain about the size of the average effect and because the results of some of the studies varied substantially.
Summary of findings
Summary of findings for the main comparison. Transdermal or intranasal nicotine versus placebo for the treatment of postoperative pain.
| Main outcomes: Transdermal or intranasal nicotine versus placebo for the treatment of postoperative pain | ||||||
|
Patient or population: people being treated for postoperative pain
Settings: postsurgical inpatients
Intervention: transdermal or intranasal nicotine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Main outcomes: transdermal or intranasalnicotine versus placebo | |||||
| Pain at 60 minutes | The mean pain at 60 minutes in the control groups was 3.1 to 6.5 points | The mean pain at 60 minutes in the intervention groups was 0.14 lower (0.94 lower to 0.65 higher) | MD ‐0.14 (‐0.94 to 0.65) | 442 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
| Pain at 12 hours | The mean pain at 12 hours in the control groups was 1.7 to 1.9 points | The mean pain at 12 hours in the intervention groups was 0.14 lower (0.98 lower to 0.98 higher) | MD ‐0.00 (‐0.98 to 0.98) | 175 (2 studies) | ⊕⊝⊝⊝ very low1, 3, 4 | ‐ |
| Pain at 24 hours | The mean pain at 24 hours in the control groups was 0.6 to 5.3 points | The mean pain at 24 hours in the intervention groups was 0.88 lower (1.58 to 0.18 lower) | MD ‐0.88 (‐1.58 to ‐0.18) | 562 (8 studies) | ⊕⊕⊝⊝ low1, 4 | ‐ |
| Hourly morphine equivalents at 60 minutes | The mean hourly morphine equivalents at 60 minutes in the control groups was 0.5 to 1.3 mg morphine equivalents | The mean hourly morphine equivalents at 60 minutes in the intervention groups was 0.08 lower (0.4 lower to 0.24 higher) | MD ‐0.08 (‐0.40 to 0.24) | 168 (4 studies) | ⊕⊕⊝⊝ low1, 3 | ‐ |
| Hourly morphine equivalents at 24 hours | The mean hourly morphine equivalents at 24 hours in the control groups was 30.2 to 51.6 mg morphine equivalents | The mean hourly morphine equivalents at 24 hours in the intervention groups was 6.06 lower (12.91 lower to 0.79 higher) | MD ‐6.06 (‐12.91 to 0.79) | 168 (4 studies) | ⊕⊕⊝⊝ low1,3 | ‐ |
| Sedation score | The mean sedation score in the control groups was ‐1 to 19.21 | The mean sedation score in the intervention groups was 0.13 standard deviations lower (0.88 lower to 0.62 higher) | SMD ‐0.13 (‐0.88 to 0.62) | 148 (3 studies) | ⊕⊕⊝⊝ very low1,2,3 | ‐ |
| Nausea | Study population | RR 1.24 (1.03 to 1.5) | 592 (7 studies) | ⊕⊕⊕⊝ moderate1 | ‐ | |
| 379 per 1000 | 469 per 1000 (390 to 568) | |||||
| 400 per 1000 | 496 per 1000 (412 to 600) | |||||
| Vomiting | Study population | RD 0.03 (‐0.04 to 0.09) | 602 (7 studies) | ⊕⊕⊝⊝ low1,3 | ‐ | |
| 150 per 1000 | 176 per 1000 (110 to 241) | |||||
| 65 per 1000 | 76 per 1000 (47 to 104) | |||||
| Time to hospital discharge | The mean time to hospital discharge in the control groups was 45.5 hours | The mean time to hospital discharge in the intervention groups was 1.2 hours longer (6.19 shorter to 8.59 longer) | MD 1.20 (‐6.19 to 8.59) | 90 (1 study) | ⊕⊕⊝⊝ very low1,5 | ‐ |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. CI: confidence interval; MD: mean difference; SMD: standardized mean difference; RD: risk difference; RR: risk ratio. | ||||||
1 Downgraded one level due to serious risk of bias: methodological limitations present in most studies. 2 Downgraded one level due to serious inconsistency: statistical heterogeneity was 50‐75%. 3 Downgraded one level due to serious imprecision: the confidence interval around the effect includes benefit and harm.
4 Downgraded one level due to serious inconsistency: statistical heterogeneity was 75‐100%.
5 Downgraded two levels due very serious imprecision: evidence comes from one small study and the confidence interval around the effect included a clinically meaningful effect with intervention or control.
Background
Many medications are available for management of postoperative pain. Opioids are the most commonly used class of systemic medications for postoperative pain (Vadivelu 2010), but they are not always entirely effective and can result in adverse events such as excessive sedation, respiratory depression, nausea, vomiting, constipation, and rash. One medication that has been explored for adjunctive postoperative pain management is nicotine. There have been few studies on nicotine for postoperative pain, and results of individual studies have been variable in showing benefits. In this systematic review, we synthesized the evidence on intranasal and transdermal nicotine for postoperative pain, explored potential reasons for inconsistent results between studies, and highlighted areas for further research.
Description of the condition
Acute pain frequently occurs after surgical procedures, due to tissue damage as a result of surgery and related inflammation. In one study (250 participants), 82% of participants reported some pain after surgery and 39% of these participants reported severe to extreme pain (Apfelbaum 2003). In another study (200 participants), the rate of moderate to extreme pain at some stage during the first 24 hours after surgery was 88% (Svensson 2000). Management of postoperative pain is an important component of surgical care, but research indicates that many people report suboptimal postoperative pain control (Owen 1990).
Description of the intervention
The usual clinical practice in the management of postoperative pain is multimodal analgesia. This refers to the use of combinations of pain medications (such as opioids and paracetamol (acetaminophen), non‐steroidal anti‐inflammatory drugs, gabapentin or pregabalin, or others) and routes of delivery (such as regional, intravenous, and epidural) as well as non‐pharmacological modalities, in order to decrease requirements for opioids and associated adverse effects such as sedation, nausea, vomiting, constipation, and itching. Multimodal analgesia may include pre‐emptive systemic medications before or during surgery, or before fully awakening from anaesthesia, as well as management after the person has resumed consciousness.
One specific strategy in postoperative multimodal pain management is to augment pain medications with additional medications. These additional medications in combination with opioids may reduce pain and the total amount of opioid needed. Some potential agents that can be used this way include capsaicin, N‐methyl‐D‐aspartate receptor antagonists, or gabapentinoids (Vadivelu 2010).
Nicotine is available in several forms, including transdermal (patch) and inhaled formulations. Nicotine may reduce pain both by directly reducing pain and by improving the overall treatment of pain even though not providing direct pain relief though potential mechanisms are not completely understood. Research indicates that given alone, nicotine increases pain thresholds in people undergoing the cold pressor test (submersion of a hand in cold water), though results are inconsistent with heat or electrical stimulation tests (Shi 2010). Nicotine may decrease the risk of respiratory depression, either by reducing the dose of opioid required for adequate pain control or directly as a respiratory stimulant. Other stimulants have been shown to decrease respiratory depression (Miller 1962). There is some evidence that nicotinic agonists may block hyperalgesia associated with some inhaled anaesthetics (Flood 2002; Yan 2009).
One of the adverse effects of nicotine, particularly in nicotine‐naive people, can be nausea and vomiting. In contrast, there is some evidence that current smokers or users of snuff are less likely to have postoperative nausea and vomiting, perhaps due to nicotinic effects (Brattwall 2010).
Several factors may influence the pain‐relieving effects of nicotine including the history of current or former smoking, the route of administration (e.g. transdermal or intranasal), timing of nicotine administration (e.g. preoperative, intraoperative, or postoperative), sex, and age. For example, some evidence suggests that female smokers have lower pain sensitivity than female non‐smokers (Girdler 2005). There may also be underlying genetic variability in response to nicotine (Campbell 2006).
How the intervention might work
There are several hypotheses about how nicotine might directly affect the pain system. Most involve the nicotinic cholinergic receptor system. One theory is that nicotine stimulates the alpha‐4 and beta‐2 nicotinic receptors and thus stimulates spinal noradrenaline (norepinephrine) release, leading to pain relief. It has also been suggested that the pressor activity of nicotine on the cardiovascular system may result in decreased pain. There may be an anti‐inflammatory effect of nicotine through the alpha‐7 cholinergic receptor (Benowitz 2008; Shi 2010). Chronic smokers experience upregulation and desensitization of nicotine receptors, which may result in attenuated effects of nicotine in this population.
The opioid system may also be involved in the pain‐relieving effects of nicotine. Some studies have found that animals treated with naloxone or deficient in the mu opioid receptor do not experience pain relief with nicotine, or have an attenuated response to pain (Campbell 2006).
Why it is important to do this review
Although some studies have evaluated effects of nicotine patches or inhaled nicotine in people undergoing surgery, results from individual studies are somewhat mixed (Cheng 2008; Flood 2004; Habib 2008; Hong 2008; Olson 2009; Turan 2008). The purpose of this review was to synthesize the literature and, if appropriate, to combine the studies to provide pooled estimates of effect and increase the power to detect effects. If there was significant statistical heterogeneity in pooled estimates, another goal of this review was to evaluate whether type of surgery; smoking status; sex; differences in study quality; or differences in the dose, timing, or mode of delivery of nicotine may help explain the divergent results.
Objectives
To assess the effect of transdermal or intranasal nicotine administration on postoperative pain, opioid analgesic use, and opioid‐related adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized, placebo‐controlled clinical trials that evaluated the effect of perioperative (pre‐, intra‐, or postoperative) administration of intranasal or transdermal nicotine on postoperative pain or opioid analgesic use. We excluded all other studies.
Types of participants
We included participants undergoing any minor or major, elective inpatient or outpatient surgery that had been randomized to receive either nicotine or placebo for postsurgical pain. We included smoking or non‐smoking men and women of all ages, as well as children.
Types of interventions
Interventions of interest included placement of a transdermal nicotine patch or use of intranasal nicotine spray for postoperative pain control one or more times before, during, or after surgery. Nicotine was included if administered either solely or as an adjuvant to other pain treatments, and whether nicotine was given pre‐, intra‐, or postoperatively. Comparison groups received a placebo.
Types of outcome measures
Primary outcomes
Postoperative pain scores at rest, as reported by participant on a numerical rating scale or categorical rating scale at 60 minutes, 12 hours, and 24 hours.
Postoperative hourly morphine equivalents. Morphine equivalents are a way of uniformly assessing the amount given in milligrams per kilogram per hour across various opioids.
Secondary outcomes
Sedation as reported and scaled by the participant.
Nausea as reported by the participant.
Vomiting as reported by participant.
Time to hospital discharge.
Search methods for identification of studies
Electronic searches
We searched MEDLINE (1966 to 20 March 2014), and adapted the search strategy (found in Appendix 1) for the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 3, see Appendix 2) and EMBASE (Ovid SP, 1980 to 20 March 2014, see Appendix 3). We assessed retrieved studies for free‐text terms or MeSH terms, nicotine, or pain that have not been previously included in the search and incorporated them into the final searches.
We excluded studies not fully published (e.g. studies published only as conference abstracts) because of the difficulty evaluating methods and because results often change between an initial abstract publication and final publication.
We did not impose a language restriction.
We re‐ran the search on 28 April 2015. We will assess the one study of interest when we update the review.
Searching other resources
We screened the reference lists of relevant trials and review papers. We asked the corresponding authors of the studies that we located if they knew of additional relevant unpublished studies (none were identified). We also searched the following clinical trial registries: www.controlled‐trials.com/ and clinicaltrials.gov/.
Data collection and analysis
Selection of studies
Two authors (AM and TD) independently screened all titles and abstracts for eligibility and documented reasons for exclusion. In case of disagreement, a third author (RC) decided on inclusion or exclusion (see Appendix 4). When we needed additional information in order to decide if a trial should be included, one of the authors (AM) contacted the corresponding author of the trial in question. We compiled a list of eligible trials and their unique identifiers on an electronic version of the data abstraction form (see Appendix 4).
Data extraction and management
Two authors (AM and TD) independently extracted data. Data abstraction included the following variables for each arm of each study: mean age in years, sex, smoking status; surgery type by category (gynaecological, male pelvic, or mixed/other); nicotine dose (in micrograms); timing of nicotine administration (only pre‐ or intraoperatively (or both) or involving postoperative administration); route of nicotine administration (intranasal or transdermal); and the following outcomes: pain at 60 minutes, 12 hours, and 24 hours; cumulative morphine dose at 60 minutes, 12 hours, and 24 hours; hourly morphine use at 60 minutes and 24 hours; time to hospital discharge; any reported nausea; any reported vomiting; sedation score; and participant satisfaction. For continuous outcomes, we abstracted mean values as well as standard deviations. We combined nicotine trials that just gave nicotine postoperatively with trials that gave nicotine pre‐ or intraoperatively. We were unable to assess respiratory depression or constipation because trials did not report these outcomes. We resolved any discrepancies in data abstraction by discussion. If we needed additional information on outcomes to enable our analyses, one of the authors (AM) contacted the corresponding author of the trial in question.
Assessment of risk of bias in included studies
Two authors (AM and RC) independently assessed the methodological quality of the eligible trials. We resolved any disagreements by discussion with a third author (TD). We performed the assessments as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and by Jüni (Jüni 2001). Appendix 4 shows the form used to assess risk of bias.
Random sequence generation
We considered sequence generation at low risk of bias if the investigators described a random component in the sequence generation process, such as referring to a random number table, computer generation randomization sequence, or tossing a coin. We considered random sequence generation at high risk of bias if the investigators used a non‐random approach, such as a sequence generated by odd or even date of birth, alternating allocation, or by some rule based on hospital or clinic record number. We considered random sequence generation at unclear risk of bias if there was insufficient information about the sequence generation process to permit an informed judgement.
Allocation concealment
We considered allocation concealment at low risk of bias if the process used prevented investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study, such as the use of centralized allocation or sequential sealed opaque envelopes with allocation assignments. We considered allocation concealment at high risk of bias if the participants could possibly foresee assignments and thus potentially introduce selection bias. Examples of inadequate allocation concealment include day of the week or alternating allocation. We considered allocation concealment at unclear risk of bias if insufficient information about the methods was reported to permit an informed judgement.
Blinding of participants and outcomes
We considered blinding at low risk of bias if participants or personnel were blinded and it was unlikely that the blinding could have been broken; or if participants or personnel were not blinded but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias. If a trial reported that it was double‐blinded, we assumed that participants and the personnel providing the intervention were blinded, unless there was information to the contrary.
We considered blinding at high risk of bias if: there was incomplete blinding; the outcome or outcome measurement was likely to have been influenced by the lack of blinding; or there was blinding of participants and personnel attempted but it was likely that the blinding could have been broken; or participants and personnel were not blinded and the non‐blinding of others was likely to introduce bias. We considered blinding at unclear risk of bias if insufficient information about blinding was provided to permit an informed judgement.
Incomplete outcome data
We considered the completeness of outcome data at low risk of bias if any one of the following was true: there were no missing outcome data; the reasons for missing data were unlikely to be related to the outcomes; the reasons for missing data were similar and balanced across groups; there was no clinically relevant impact on the intervention effect estimate (proportion of missing outcomes compared with observed event risk for dichotomous outcome data) or the plausible effect size (difference in means or standardized difference in means for continuous data); or missing data were not large, and appropriate methods were used to impute missing data.
We considered the completeness of outcome data at high risk of bias if: the reasons for missing data were likely to be related to the outcomes; there were enough missing data to induce clinically relevant bias in the observed effect size (proportion of missing outcomes compared with observed event risk for dichotomous outcome data) or the plausible effect size (difference in means or standardized difference in means) for continuous data; the analysis was performed on an on‐treatment basis and there was substantial departure from the intervention received from that assigned at randomization; or there was inappropriate use of imputed data.
We considered the risk of bias unclear if insufficient information was given about the completeness of outcome data to permit judgement, or the study did not address the particular outcome of interest.
Selective reporting
We considered selective outcome reporting at low risk of bias if the study protocol was available and all of the pre‐specified outcomes of interest in the review were reported in the pre‐specified way, or if the study protocol was not available but it was clear that the published reports include all major expected outcomes, including those that were pre‐specified.
We considered selective reporting to be at high risk of bias if any of the following were present: not all of the pre‐specified primary outcomes were reported; outcome(s) were reported using measurements, analysis methods, or subsets of the data that were not pre‐specified; outcome(s) were not pre‐specified, unless clear justification for their reporting was provided; outcome(s) of interest in the review were reported incompletely; or the study failed to include results for a key outcome that would be expected to have been reported.
We considered selective reporting at unclear risk of bias if insufficient information was given about selective outcome reporting to permit an informed judgement.
Other bias
We assessed other factors that might contribute to the risk of bias, including similarity of baseline groups, avoidance or similarity of co‐interventions, and similarity of timing of outcome assessment (van Tudler 2003).
We displayed the results of our risk of bias assessment for each domain by creating a 'Risk of bias' graph (Figure 1) and a 'Risk of bias' summary figure (Figure 2) using Review Manager 5 software (RevMan 2014). Based on the assessment of risk of bias, two authors (AM and RC) rated the overall quality of each study as good, fair, or poor, based on the number and seriousness of methodological shortcomings. A third author (TD) resolved discrepancies in risk of bias assessments and overall quality rating.
1.
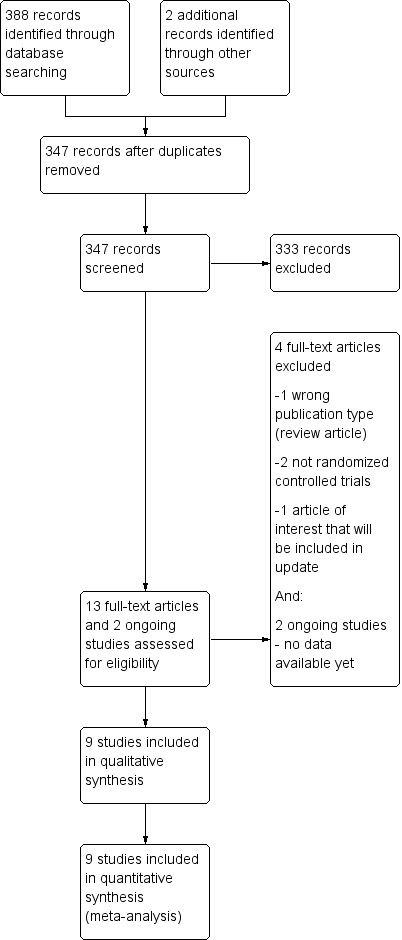
Study flow diagram. Note: We re‐ran the search on 28 April 2015 and found one additional study of interest. We will assess this study when we update the review.
2.
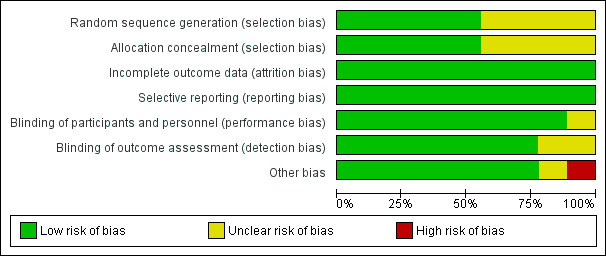
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We intended to analyse pain scores as dichotomous and continuous outcomes, but the trials only reported continuous data. Other continuous variables were opioid use (in milligram morphine equivalents) and sedation scores. For continuous variables, we reported the absolute mean difference (MD) (for outcomes measured using similar scales) or standardized mean difference (SMD) (for outcomes measured using different scales). For dichotomous variables (nausea, vomiting), we used risk ratios (RRs) unless the rates were similar in the control groups, in which case we presented risk differences (RDs).
Unit of analysis issues
In randomized trials that first randomized people to a type of anaesthesia (e.g. propofol or isoflurane) and then to nicotine or placebo, we compared the nicotine and placebo groups for the meta‐analysis irrespective of the type of anaesthesia.
Dealing with missing data
We analysed results based on intention‐to‐treat (ITT), that is, based on the groups to which the participants were allocated. If ITT analyses were not available, one of the authors (AM) contacted the corresponding author of the trial to ask for the missing data. If trials imputed data, we planned to perform the primary analysis using the imputed results and record the method of imputation, and carry out sensitivity analyses without imputation. For continuous data where standard deviations were missing, we planned to impute using a best case, worst case technique, or other appropriate methods.
Assessment of heterogeneity
We used the Chi2 test to evaluate the statistical significance of heterogeneity in meta‐analyses and the I2 statistic to describe the percentage of variability in variance across studies due to heterogeneity rather than chance (Higgins 2002). We considered an I2 statistic greater than 50% to represent substantial heterogeneity.
Assessment of reporting biases
We did not have enough studies to create a meaningful funnel plot or conduct statistical analyses for small sample size study effects (Sterne 2011). Instead, we assessed for reporting bias by comparing pre‐specified to reported outcomes, querying authors of included studies regarding unpublished trials. Risk of selective outcomes reporting was assessed using the Cochrane 'Risk of bias' tool (see Appendix 4). We were unable to assess publication bias formally using graphical or statistical methods due to the small numbers of trials available for each analysis.
Data synthesis
We conducted meta‐analyses, if possible. The main comparisons were made for the two primary outcomes (postoperative pain scores, hourly morphine use), as well as secondary outcomes (sedation, nausea, vomiting, time to hospital discharge).
We calculated a pooled intervention effect across studies under the assumption that the studies were estimating an intervention effect that followed a distribution across studies (random‐effects model meta‐analysis). We combined RRs for dichotomous outcomes and RDs when the control rate was similar across trials. For studies reporting continuous outcomes, we combined MDs or an effect size (if trials used different measures to assess an outcome). For postoperative pain scores, the preferred outcome for meta‐analyses was pain measured on a 0 to 10 numerical rating scale. For studies that used other numerical rating scales to measure postoperative pain (e.g. 0 to 100 or 0 to 20), we transformed these to a 0 to 10 scale in order to include the data in the meta‐analysis.
We performed the meta‐analyses using the DerSimonian‐Laird, random‐effects model. As described above, the choice of reporting MD versus SMD for continuous variables and RR versus RD for dichotomous variables was made after looking at the data.
Subgroup analysis and investigation of heterogeneity
The small number of trials limited the usefulness of subgroup analyses. However, we examined the following variables in subgroup analyses as potential sources of heterogeneity.
Type of surgery (gynaecological, male pelvic, or other).
Route of administration of nicotine.
Smoking status (restricted to smokers or non‐smokers).
We also examined the following variables, not originally listed in our protocol, in subgroup analyses as potential sources of heterogeneity.
Sex (male or female).
Individual nicotine dose (not cumulative) (5 mg or less, between 5 and 10 mg, or 10 mg or greater).
Timing of nicotine administration (pre‐ or intraoperatively only or including postoperative administration).
Study quality (good, fair, or poor).
We planned to examine the following variables in meta‐regression as potential sources of heterogeneity.
Mean age.
Proportion of males.
Dose of nicotine.
Smoking status (proportion of smokers).
Opioids administered during surgery or postoperatively in morphine equivalents (for outcomes other than postoperative opioid use).
However, because there were fewer than 10 studies, we did not perform meta‐regression, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Sensitivity analysis
The usefulness of sensitivity analyses was limited by a relatively small number of trials. However, we examined the meta‐analyses for outliers and performed sensitivity analyses by excluding them. We also performed sensitivity analysis by excluding poor‐quality trials.
'Summary of findings' tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with the comparison of nicotine versus placebo on specific outcomes (pain scores at rest, hourly morphine equivalents, sedation, nausea, vomiting, time to hospital discharge) in our review and constructed a 'Summary of findings' table using the GRADE software.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We conducted the search on 20 March 2014 (see Appendix 1; Appendix 2; Appendix 3 for full search strategies). We identified 388 studies. We identified two ongoing studies on searches of clinical trials registries (www.controlled‐trials.com/ and www.clinicaltrials.gov). We contacted the authors of the two trials (NCT00790829; NCT01194089), but no data were available yet. After removal of duplicates, there were 347 unique citations for screening. Of those 347 citations, 12 studies appeared to meet inclusion criteria on initial screen and we obtained full‐texts of the articles for further review. After review of full‐text articles and ongoing trials, nine articles met our inclusion criteria (See Figure 1).
We re‐ran the search on 28 April 2015 and found one additional study of interest (Weingarten 2015). We will assess that study when we update the review.
Additional data from primary authors
We obtained additional data from primary authors for four studies (Cheng 2008; Flood 2004; Hong 2008; Turan 2008). Pamela Flood, MD provided pain scores and morphine equivalents at 30 and 60 minutes and one, three, and five days for the Hong 2008 study. Dr Flood also provided the mean and standard deviations for pain scores at 30 minutes, 60 minutes, one day, three days, and five days and morphine equivalents used at these times points for the Cheng 2008 study, and clarified that there were 10 participants per group in the Flood 2004 study. Alparslan Turan, MD provided mean and standard deviation for pain scores at 30 minutes, 60 minutes, one day, three days, and five days as well as the cumulative morphine dose used at each of those time points for the Turan 2008 study.
Included studies
All nine included studies were randomized trials (Cheng 2008; Czarnetzki 2011; Flood 2004; Habib 2008; Hong 2008; Jankowski 2011; Olson 2009; Turan 2008; Yagoubian 2011). Eight were parallel group trials and one was a cross‐over trial (Yagoubian 2011). Sample sizes ranged from 20 to 118 (total n = 666) and duration of follow‐up ranged from one to seven postoperative days.
Four trials focused on gynaecological surgery and included only women (Cheng 2008; Flood 2004; Jankowski 2011; Turan 2008), one trial focused on prostate surgery in men (Habib 2008), and the remainder included both men and women undergoing various surgeries (elective inpatient surgery (Czarnetzki 2011), general surgery (Hong 2008), pelvic or abdominal surgeries (Olson 2009), or third molar extraction (Yagoubian 2011)).
Doses of nicotine ranged from 3 to 17 mg per dose. Nicotine was administered only pre‐ or intra‐operatively, or both, in two trials (Cheng 2008; Yagoubian 2011), only postoperatively in two trials (Flood 2004; Jankowski 2011), and continuously through both time periods in five trials (Cheng 2008; Czarnetzki 2011; Habib 2008; Hong 2008; Olson 2009). Nicotine was administered as a patch in five trials (Czarnetzki 2011; Habib 2008; Hong 2008; Olson 2009; Turan 2008), and as an inhaler in four trials (Cheng 2008; Flood 2004; Jankowski 2011; Yagoubian 2011). Seven studies excluded smokers (Cheng 2008; Czarnetzki 2011; Flood 2004; Habib 2008; Hong 2008; Jankowski 2011; Yagoubian 2011), and one study restricted enrolment to smokers (Olson 2009). One study excluded participants with a history of postoperative nausea and vomiting (Jankowski 2011).
All of the trials were single centre studies. Seven trials were conducted in the USA (Cheng 2008; Flood 2004; Habib 2008; Hong 2008; Jankowski 2011; Olson 2009; Yagoubian 2011), one in Switzerland (Czarnetzki 2011), and one in Turkey (Turan 2008).
Excluded studies
We excluded three studies after full‐text review. One was not a randomized controlled trial (Ionescu 2007), one was an editorial (Benowitz 2008), and one was an abstract of a review (Souzdalnitski 2009) (See Characteristics of excluded studies table).
Ongoing studies
Two studies are ongoing and therefore no data are available yet (NCT00790829; NCT01194089) (see Characteristics of ongoing studies table).
Studies awaiting classification
We re‐ran our search on 28 April 2015. We found one study, which is awaiting classification (Weingarten 2015) (see Characteristics of studies awaiting classification table).
Risk of bias in included studies
See Table 1 for an overall assessment of the risk of bias assessment of included studies for each comparison and outcome. See also the 'Risk of bias' graph (see Figure 2) and 'Risk of bias' summary (see Figure 3) for an overview of risk of bias. Details about assessments for specific risk of bias criteria are described below.
3.
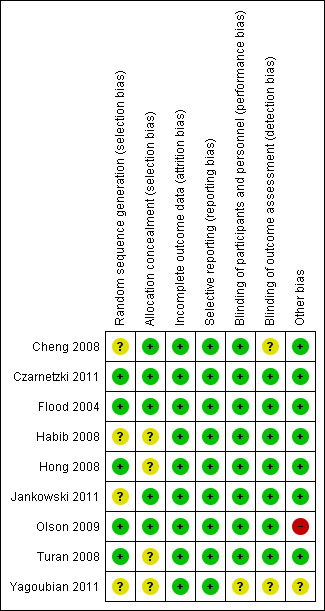
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Based on the risk of bias assessments, we rated three trials overall as good quality (Czarnetzki 2011; Flood 2004; Jankowski 2011), five as fair quality (Cheng 2008; Habib 2008; Hong 2008; Olson 2009; Turan 2008), and one as poor quality (Yagoubian 2011). The poor quality trial had unclear blinding of participants and study personnel, outcomes assessors, and unclear similarity of intervention groups at baseline (Yagoubian 2011).
Allocation
Three trials reported computerized randomization (Czarnetzki 2011; Olson 2009; Turan 2008), and two reported use of randomization tables (Flood 2004; Hong 2008). The method of random sequence generation was unclear in four trials (Cheng 2008; Habib 2008; Jankowski 2011; Yagoubian 2011).
Two trials reported use of numbered, opaque sealed envelopes for allocation concealment (Cheng 2008; Olson 2009), and two used an opaque container (Czarnetzki 2011; Flood 2004), and one used identical syringes (Jankowski 2011). The method of allocation concealment was unclear in four studies (Habib 2008; Hong 2008; Turan 2008; Yagoubian 2011).
Blinding
Seven trials reported blinded outcomes assessment; in the other two, use of blinded outcome assessment was unclear (Cheng 2008; Yagoubian 2011).
All but one of the studies reported blinding of study participants and personnel. In one other trial, it was unclear whether either personnel or participants were blinded (Yagoubian 2011).
Incomplete outcome data
We did not detect incomplete outcome data in any of the studies.
Selective reporting
We did not detect selective reporting in any of the studies. No trial was available only as an abstract.
Other potential sources of bias
One trial reported significant baseline differences between intervention groups (Olson 2009), and in one trial it was unclear if groups were similar at baseline (Yagoubian 2011). All studies described ITT analysis, withdrawals, co‐interventions were avoided or similar, and timing of outcome assessments were similar.
Effects of interventions
See: Table 1
Primary outcome: postoperative pain scores
There was no difference between nicotine and placebo in postoperative pain score at 60 minutes (six trials, MD ‐0.14, 95% CI ‐0.94 to 0.65; Chi2 test = 17.31, degrees of freedom (df) = 5 (P value = 0.72); I2 statistic = 71%, Analysis 1.1) or at 12 hours (two trials, MD ‐0.00, 95% CI ‐0.98 to 0.98, Chi2 test = 4.67, df = 1 (P value = 1.00); I2 statistic = 79%, Analysis 1.2). Sensitivity and subgroup analyses showed no differences when stratified by type of surgery, route of administration, nicotine dose, timing of nicotine, gender, or overall quality.
1.1. Analysis.
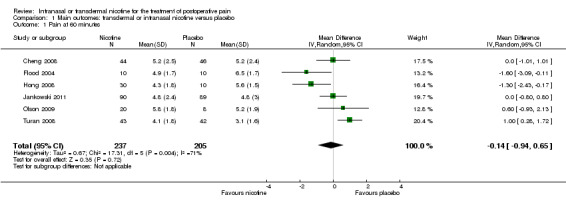
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 1 Pain at 60 minutes.
1.2. Analysis.
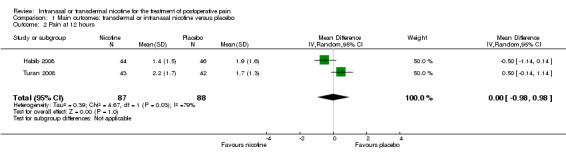
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 2 Pain at 12 hours.
For pain at 60 minutes, there was a statistically significant difference in the effect of nicotine on pain between studies that recruited smokers and studies recruiting a mix of smokers and non‐smokers, with a more favourable effect with placebo in the studies recruiting smokers (P value = 0.004). However, results should be interpreted with caution because neither trial enrolled only smokers, and there was substantial statistical heterogeneity in the subgroup of trials that focused on non‐smokers (I2 statistic = 53%).
At 24 hours, nicotine was associated with lower pain score than placebo, with a difference of slightly less than 1 on a 0 to 10 point scale (eight trials, MD ‐0.88; 95% CI ‐1.58 to ‐0.18; Chi2 test = 79.23, df = 7 (P value < 0.00001); Figure 4, Analysis 1.3). Results were characterized by a high degree of statistical heterogeneity (I2 statistic = 91%). The Flood 2004 study appeared to be an outlier, reporting a substantially stronger effect for nicotine (MD ‐3.40, 95% CI ‐4.32 to ‐2.48) than the other trials (MD ranged from ‐1.30 to 0.20). Excluding this trial resulted in a difference that was no longer statistically significant (seven trials, MD ‐0.53, 95% CI ‐1.12 to 0.06; Chi2 = 43.85, df = 56 (P value < 0.00001)), but did not eliminate statistical heterogeneity (I2 statistic = 86%). Subgroup analyses showed no clear differences when trials were stratified by type of surgery, route of administration, smoking status, nicotine dose, timing of nicotine, gender, or overall study quality. For a complete overview, see Table 1.
4.
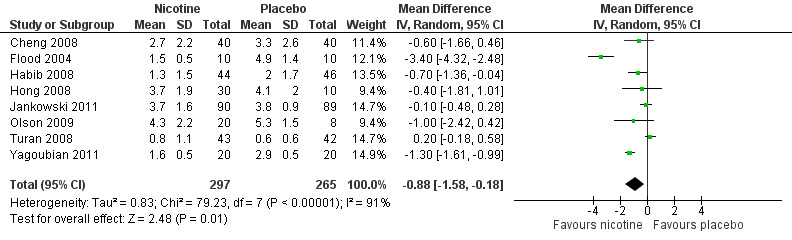
Forest plot of comparison: 1 Main outcomes: Transdermal or intranasal nicotine versus placebo, outcome: 1.3 Pain at 24 hours.
1.3. Analysis.
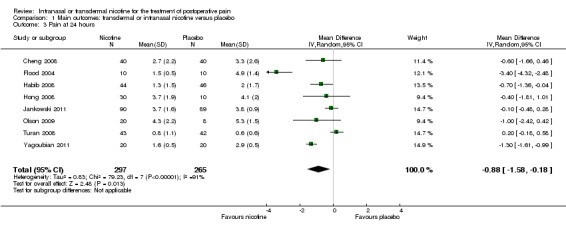
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 3 Pain at 24 hours.
Primary outcome: postoperative opioid use
There was no difference between nicotine and placebo in mean hourly morphine use at 60 minutes (four trials, MD ‐0.08, 95% CI ‐0.40 to 0.24; Chi2 test = 3.41, df = 3 (P value = 0.33); I2 statistic = 12%; Analysis 1.4) or 24 hours (four trials, MD ‐6.06, 95% CI ‐12.91 to 0.79; Chi2 test = 1.10, df = 3 (P value = 0.78); I2 statistic = 0%; Analysis 1.5). There were also no differences in sensitivity or subgroup analyses. For a complete overview, see Table 1.
1.4. Analysis.
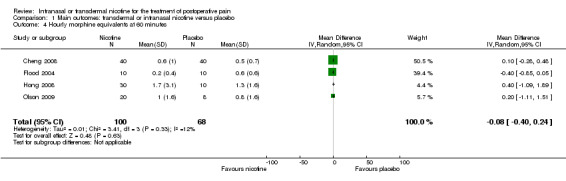
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 4 Hourly morphine equivalents at 60 minutes.
1.5. Analysis.
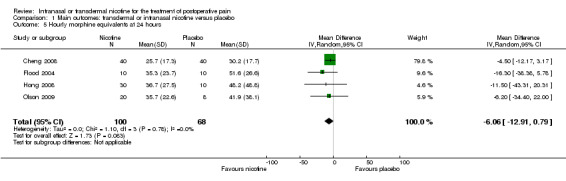
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 5 Hourly morphine equivalents at 24 hours.
Secondary outcomes: adverse effects ‐ sedation, nausea, vomiting
There was no difference between nicotine and placebo in sedation scores (three trials, SMD ‐0.13, 95% CI ‐0.88 to 0.62; Chi2 test = 7.68, df = 2 (P value = 0.02); I2 statistic = 74%; Figure 5, Analysis 1.6).
5.
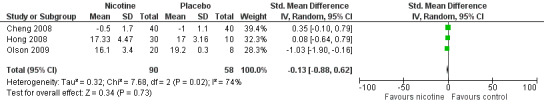
Forest plot of comparison: 1 Main outcomes: Transdermal or intranasal nicotine versus placebo, outcome: 1.6 Sedation score.
1.6. Analysis.
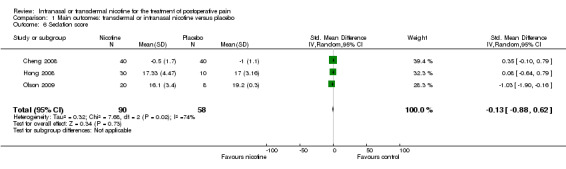
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 6 Sedation score.
Nicotine was associated with higher risk of nausea than placebo (seven trials, RR 1.24, 95% CI 1.03 to 1.50; Chi2 test = 2.63, df = 6 (P value = 0.85); I2 statistic = 0%; Analysis 1.7), but there was no difference in risk of vomiting (seven trials, RD 0.03, 95% CI ‐0.04 to 0.09; Chi2 test = 9.83, df = 6 (P value = 0.13); I2 statistic = 39%; Analysis 1.8). There were also no differences in risk of nausea or vomiting in sensitivity or subgroup analyses.
1.7. Analysis.
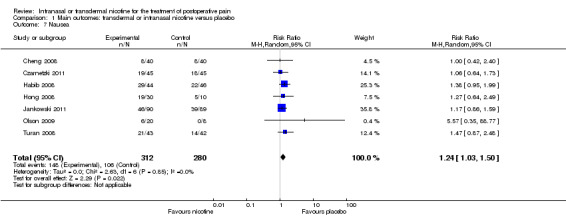
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 7 Nausea.
1.8. Analysis.
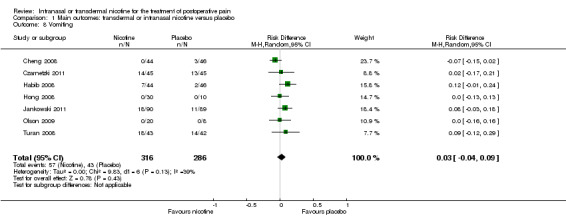
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 8 Vomiting.
Secondary outcome: time to hospital discharge
Only one study reported time to hospital discharge. Results did not favour either nicotine or placebo (Analysis 1.9).
1.9. Analysis.
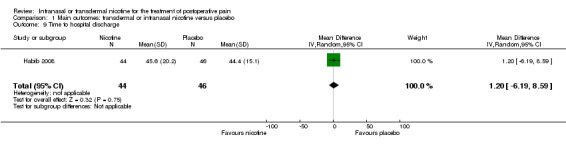
Comparison 1 Main outcomes: transdermal or intranasal nicotine versus placebo, Outcome 9 Time to hospital discharge.
Discussion
Summary of main results
Our systematic review of nine published randomized controlled trials found that nicotine was associated with less pain than placebo at 24 postoperative hours, but associated with no difference in pain scores at earlier time points. The difference at 24 hours was less than 1 point on a 10‐point pain scale, which is lower than typically considered clinically meaningful, and results were characterized by substantial statistical heterogeneity. There was no difference between nicotine and placebo in postoperative opioid use. Nicotine was associated with increased risk of nausea versus placebo.
Overall completeness and applicability of evidence
The review was limited by the relatively small sample sizes available for many of the main outcomes of interest (range 90 to 602 participants) decreasing the precision of the estimates. The small number of trials (nine) limited the usefulness of subgroup and sensitivity analyses. Several trials did not report some outcomes, such as opioid use prior to 24 hours, sedation, and time to hospital discharge. Four of the trials focused on women undergoing gynaecological surgery and one of the trials on men undergoing prostate surgery, which might introduce heterogeneity and limit applicability to other surgical procedures. Seven trials excluded smokers or recent smokers and only one trial restricted enrolment to smokers (the others enrolled a mix of smokers and non‐smokers), precluding strong conclusions regarding the effects of nicotine for postoperative pain in smokers. In addition, morphine equivalents (opioid consumption) may be an insensitive measurement of pain relief (Kissin 2009; McQuay 2008).
Quality of the evidence
We assessed three of the nine studies as overall 'good' quality, based on our assessments of domains related to risk of bias and we downgraded the quality of evidence for all of our analyses accordingly (Table 1).
Some analyses were characterized by a high degree of statistical heterogeneity (variation in study outcomes between studies). One statistical measure used to characterize heterogeneity is the I2 statistic ‐ the larger the value, the more variability in study outcomes. Outcomes with a high degree of statistical heterogeneity included pain at 60 minutes (I2 statistic = 71%), 12 hours (I2 statistic = 79%), 24 hours (I2 statistic = 91%), and sedation scores (I2 statistic = 74%). In general, statistical heterogeneity was not significantly reduced by exclusion of poor‐quality or outlier trials, or by stratification of trials according to type of surgery, timing of administration, smoking status, and other factors. In addition, the small numbers of trials limited the usefulness of subgroup analyses. Results based on analyses with substantial statistical heterogeneity should be interpreted with caution.
Using the GRADE system, we assigned overall scores as very low to low, based on the presence of methodological limitations, imprecision, and inconsistency. This suggests that further research is likely to have an important impact in estimates of effects.
Potential biases in the review process
Two authors (TD and AM) extracted data from the studies. Two authors (TD and AM) independently ranked risk of bias with a third author (RC) resolving any disagreements. AM drafted the review but all authors contributed to the final product. We were unable to assess publication bias formally using graphical or statistical methods due to the small numbers of trials available for each analysis. As discussed above, two studies did meet our inclusion criteria but were currently recruiting participants and so no data were available yet (NCT00790829; NCT01194089).
Agreements and disagreements with other studies or reviews
We found three other reviews on this topic. One was published as an abstract (Souzdalnitski 2009). They looked at 11 studies, four of which were randomized controlled trials. The abstract did not state which four randomized controlled trials were used. Similar to our study, using a random‐effects model, they found that nicotine was associated with less postoperative pain than placebo at 24 hours (P value = 0.031). They also reported a non‐statistically significant trend to towards less opioid consumption (P value = 0.054). Another review also reported results consistent with ours; it found that six out of seven of the studies they looked at supported nicotine as a treatment for postoperative pain in nicotine‐naive participants, but with an increased incidence of postoperative nausea (Vibe Nielsen 2012). All seven of their included studies were in our review and they were all randomized controlled trials (Flood 2004; Habib 2008; Hong 2008; Jankowski 2011; Olson 2009; Turan 2008; Yagoubian 2011).
Mishriky 2014 also completed a review using the same studies as included in this review. Mishriky 2014 found that there was no difference in pain reduction in the pooled analysis at any time point, and that there was significant heterogeneity much of which appears from the forest plot to be caused by the Flood 2004 study. As we did, Mishriky 2014 found similarly that when the Flood 2004 study was removed there was still no difference in pain at 24 hours in the pooled studies.
Mishriky 2014 used cumulative morphine consumption at 24 hours and our study used hourly morphine equivalents at 24 hours. Mishriky 2014 found a significant reduction in cumulative opioid consumption at 24 hours with the administration of nicotine (MD ‐4.85 mg, 95% CI ‐9.40 to ‐0.30 (P value = 0.04), I2 statistic = 24%), but we did not find a significant difference in hourly morphine equivalents at 24 hours. Similar to our finding, Mishriky 2014 found no difference in pain or opiate use when looking at subgroups (participants receiving transdermal patch versus nasal spray, women versus men, and non‐smokers versus smokers).
In agreement with our review, Mishriky 2014 also found increased nausea in the nicotine group (at one hour: five trials, RR 1.26, 95% CI 1.03 to 1.55; I2 statistic = 0%; at 24 hours: seven trials, RR 1.14, 95% CI 1.03 to 1.26; I2 statistic = 0%). We found no difference in the risk of vomiting and Mishriky 2014 were not willing to conclude anything about vomiting due to there being wide CIs.
Authors' conclusions
Implications for practice.
Based on evidence of generally low quality, nicotine may reduce postoperative pain at 24 hours compared with placebo, but the effects were relatively small (less than 1 point on a 10‐point pain scale) and there was substantial heterogeneity in the results of our analyses. Nicotine does not appear to reduce postoperative use of opioids or opioid‐related adverse events but probably increases the risk of nausea.
Implications for research.
Further research is likely to have an important impact on estimates of effect of nicotine for postoperative pain. Research is needed to determine optimal timing and route of nicotine administration and to understand better how population characteristics (such as smoking status or gender) affect estimates of benefits and harms. More research is needed to determine the effects of smoking status on the effectiveness of nicotine for postoperative pain and to understand the optimal timing, dose, and method of delivery of nicotine.
Notes
We would like to thank Andrew Smith (content editor), Marialena Trivella (statistical editor), Pamela Flood and Sheena Derry (peer reviewers), and Janet Durhane Wong‐Rieger (consumer representative) for their help and editorial advice during the preparation of the protocol (Matthews 2012) for the systematic review.
Acknowledgements
The authors would like to thank Andrew Hamilton at Oregon Health and Science University and Karen Hovhannisyan for their help in developing the search strategies. We would also like to thank Jane Cracknell (Managing Editor Cochrane Anaesthesia, Critical and Emergency Care Group).
We would like to thank Andy Smith (Content Editor), Cathal Walsh (Statistical Editor), Toby Lasserson (Senior Editor, Cochrane Editorial Unit) and Ashraf S Habib and Sheena Derry (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank those who provided us with additional data including Shelby Davis, Alparslan Turan, and Pamela Flood.
Appendices
Appendix 1. Ovid MEDLINE search strategy
1. exp Nicotine/ or exp Nicotinic Agonists/ or exp Receptors, Nicotinic/ or nicotin*.af. 2. exp Analgesics/ or exp Analgesia/ or analges*.mp. or exp Pain, Postoperative/ or exp pain/dt or exp Surgical Procedures, Operative/ or su.fs. or (pain* adj5 ((post or follow* or after*) adj3 (surger* or surgic* or operat* or procedur*))).mp. 3. 1 and 2 4. (randomized controlled trial.pt. or controlled clinical trial.pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals.sh not (humans.sh and animals.sh)) 5. 3 and 4
Appendix 2. Search strategy for CENTRAL
#1 MeSH descriptor Nicotine explode all trees #2 MeSH descriptor Nicotinic Agonists explode all trees #3 MeSH descriptor Receptors, Nicotinic explode all trees #4 nicotin* #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Analgesics explode all trees #7 MeSH descriptor Analgesia explode all trees #8 MeSH descriptor Pain, Postoperative explode all trees #9 MeSH descriptor Pain, this term only #10 MeSH descriptor Surgical Procedures, Operative explode all trees #11 (pain* NEAR ((post or follow* or after*) NEAR (surger* or surgic* or operat* or procedur*))) #12 (#6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 (#5 AND #12)
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp nicotine/ or exp nicotinic agent/ or exp nicotinic receptor/ or nicotin*.af. 2. analgesic agent/ or analgesia/ or analges*.ti,ab. or postoperative pain/ or pain/dt or surgery/ae, co, su or (pain* adj3 ((post or follow* or after*) adj3 (surger* or surgic* or operat* or procedur*))).ti,ab. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Data abstraction form
Date:
Reviewer: TD / AM / RC / RF Other:__________________
To Do List:
1.
2.
3.
4.
Additional data from primary authors: __ received ___ attached
Study Selection, Quality Assessment & Data Extraction Form
| Study ID | First author | Journal/Conference Proceedings etc | Year |
| |
Study ID: last name of first author and the year of the primary reference for the study.
Study eligibility
| RCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Study ID | Author(s) | Journal/Conference Proceedings etc | Year |
Study ID: last name of first author and the year of the primary reference for the study .
Participants and trial characteristics
| Participant characteristics | ||
| Nicotine | Placebo | |
| Mean Age (years) | ||
| Mean weight (kg) | ||
| Mean duration of surgery (minutes) | ||
| Mean IO fentanyl dose (ug/min) | ||
| Sex (%) | ||
| Race (%) | ||
Trial characteristics
seeAppendix 1, usually just completed by one reviewer
Risk of Bias / Quality Rating
| Sequence Generation | |
| State here method used to generate allocation, whether it generates comparable groups, and reasons for grading | Grade (circle) |
| |
Adequate (Random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Allocation Concealment Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation, if allocations could have been foreseen in advance, and reasons for grading | Grade (circle) |
| Low Risk | |
| High Risk | |
| Unclear | |
| Blinding | |
| Intervention blinded e.g. "identical appearance" or "same colour and smell" | Yes / No |
| Person responsible for participants care | Yes / No |
| Participant | Yes / No |
| Outcome assessor | Yes / No |
| Other (please specify) | Yes / No |
| Completeness of Outcome Data | ||
| Data is complete? | Attrition and exclusions reported? | |
| Pain score at rest (primary) | Yes / No | Yes / No |
| Cumulative morphine equivalents (primary) | Yes / No | Yes / No |
| Hourly morphine equivalents (primary) | Yes / No | Yes / No |
Comments (reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors):
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
| Other Factors | |
| Baseline groups similar | Yes / No |
| Co‐interventions avoided or similar | Yes / No |
| Timing of outcome assessment similar | Yes / No |
| Is there evidence of selective outcome reporting (please specify) | Yes / No |
| Other sources of bias (please specify) | Yes / No |
|
Overall Quality Rating Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| Comments: | Good |
| Fair | |
| Poor | |
Data extraction
| Outcomes relevant to your review | |
| Reported in paper (circle) | |
| Pain score at rest (primary) | Yes / No |
| Cumulative morphine equivalents (primary) | Yes / No |
| Hourly morphine equivalents (primary) | Yes / No |
| Time to hospital discharge (secondary) | Yes / No |
| PostOp nausea (secondary) | Yes / No |
| PostOp vomiting (secondary) | Yes / No |
| Sedation (secondary) | Yes / No |
| For Continuous data | |||||||
| Code of paper |
Outcomes |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Pain score at rest | |||||||
| Cumulative morphine equivalents | |||||||
| Hourly morphine equivalents | |||||||
| Time to hospital discharge | |||||||
| Sedation | |||||||
| Patient satisfaction | |||||||
| Other | |||||||
| For Dichotomous data | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| PostOp nausea | |||
| PostOp vomiting | |||
| Sedation | |||
| Other | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
| Trial characteristics | |
| Further details | |
| Inclusion criteria | |
| Exclusion criteria | |
| Eligible/enrolled/analysed | |
| Type of surgery(s) (female pelvic, male pelvic, GI, kidney, other) | |
| Other postop analgesia (PCA, oral opioids, NSAIDs, nurse rescue, other) | |
| Country and setting | |
| Number of participants in each intervention group | Nicotine: Placebo: |
| Rout of administration (patch, inhaler) | |
| Dose | |
| Timing of nicotine dose (1 = pre‐ or inter‐operation only, 2 = postop only, 3 = both) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Data and analyses
Comparison 1. Main outcomes: transdermal or intranasal nicotine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 60 minutes | 6 | 442 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.94, 0.65] |
| 2 Pain at 12 hours | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.98, 0.98] |
| 3 Pain at 24 hours | 8 | 562 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.58, ‐0.18] |
| 4 Hourly morphine equivalents at 60 minutes | 4 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.24] |
| 5 Hourly morphine equivalents at 24 hours | 4 | 168 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐12.91, 0.79] |
| 6 Sedation score | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.88, 0.62] |
| 7 Nausea | 7 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.50] |
| 8 Vomiting | 7 | 602 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.09] |
| 9 Time to hospital discharge | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
Comparison 2. Subgroups: transdermal or intranasal nicotine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 60 minutes by type of surgery | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Type of surgery is gynaecological | 4 | 374 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.89, 0.90] |
| 1.2 Type of surgery is mixed/other | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐2.28, 1.43] |
| 2 Pain at 60 minutes by route of administration | 6 | 442 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.94, 0.65] |
| 2.1 Route of administration is patch | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐1.40, 1.63] |
| 2.2 Route of administration is inhaler | 3 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.19, 0.49] |
| 3 Pain at 60 minutes by smokers or mix of smokers/non‐smokers | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Non‐smokers only | 4 | 329 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.38, 0.19] |
| 3.2 Mix of smokers and non‐smokers | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.27, 1.58] |
| 4 Pain at 60 minutes by nicotine dose | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 5 mg | 6 | 398 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.70, 0.73] |
| 4.2 5‐15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐3.00, 2.48] |
| 4.3 ≥ 15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐2.27, 0.13] |
| 5 Pain at 60 minutes by timing of nicotine administration | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Pre‐ or intraoperative | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.01, 1.01] |
| 5.2 Postoperative | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.22, 0.88] |
| 5.3 Both | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐1.40, 1.63] |
| 6 Pain at 60 minutes by gender | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Women only | 4 | 374 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.89, 0.90] |
| 6.2 Men and women | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐2.28, 1.43] |
| 7 Pain at 60 minutes by overall quality | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Good | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.22, 0.88] |
| 7.2 Fair | 4 | 243 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.95, 1.14] |
| 8 Pain at 12 hours by type of surgery | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Type of surgery is gynaecological | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.14, 1.14] |
| 8.2 Type of surgery is male pelvic | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.14, 0.14] |
| 9 Pain at 12 hours by route of administration | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Route is patch | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.98, 0.98] |
| 10 Pain at 12 hours by smokers or mix of smokers/non‐smokers | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Non‐smokers only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.14, 0.14] |
| 10.2 Mix of smokers and non‐smokers | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.14, 1.14] |
| 11 Pain at 12 hours by nicotine dose | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 ≤ 5 mg | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.14, 1.14] |
| 11.2 5‐15 mg | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.14, 0.14] |
| 12 Pain at 12 hours by timing of nicotine administration | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Pre‐ or intraoperative | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Pre‐ or intraoperative and postoperative | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.98, 0.98] |
| 13 Pain at 12 hours by gender | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Women only | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.14, 1.14] |
| 13.2 Men and women | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.14, 0.14] |
| 14 Pain at 12 hours by overall quality | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Fair | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.98, 0.98] |
| 15 Pain at 24 hours type of surgery | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Type of surgery is gynaecological | 4 | 364 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐2.08, 0.26] |
| 15.2 Type of surgery is male pelvic | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.36, ‐0.04] |
| 15.3 Type of surgery is mixed/other | 3 | 108 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.54, ‐0.95] |
| 16 Pain at 24 hours by route of administration | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Route is patch | 4 | 243 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.97, 0.30] |
| 16.2 Route is inhaler | 4 | 319 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.41, ‐0.21] |
| 17 Pain at 24 hours by smokers or mix of smokers/non‐smokers | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Non‐smokers only | 6 | 449 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.89, ‐0.27] |
| 17.2 Mix of smokers and non‐smokers | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.30, 0.91] |
| 18 Pain at 24 hours by nicotine dose | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 ≤ 5 mg | 7 | 438 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.53, 0.12] |
| 18.2 5‐15 mg | 3 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.44, 0.06] |
| 18.3 ≥ 15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐3.28, 0.63] |
| 19 Pain at 24 hours by timing of nicotine administration | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Pre‐ or intraoperative | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.72, ‐0.56] |
| 19.2 Postoperative | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐4.96, 1.51] |
| 19.3 Both | 4 | 243 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.97, 0.30] |
| 20 Pain at 24 hours by gender | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Women only | 4 | 364 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐2.08, 0.26] |
| 20.2 Men and women | 4 | 198 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.45, ‐0.68] |
| 21 Pain at 24 hours by overall quality | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Good | 2 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐4.96, 1.51] |
| 21.2 Fair | 5 | 323 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.90, 0.18] |
| 21.3 Poor | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.61, ‐0.99] |
| 22 Hourly morphine at 60 minutes by type of surgery | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Type of surgery is gynaecological | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.62, 0.35] |
| 22.2 Type of surgery is mixed/other | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.27] |
| 23 Hourly morphine at 60 minutes by route of administration | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Route is patch | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.27] |
| 23.2 Route is inhaler | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.62, 0.35] |
| 24 Hourly morphine at 60 minutes smokers or mix of smokers/non‐smokers | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Non‐smokers only | 3 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.50, 0.32] |
| 24.2 Mix of smokers and non‐smokers | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.11, 1.51] |
| 25 Hourly morphine at 60 minutes by nicotine dose | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 ≤ 5 mg | 3 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.51, 0.28] |
| 25.2 5‐15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.03, 0.75] |
| 25.3 ≥ 15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.39, 0.90] |
| 26 Hourly morphine at 60 minutes timing of nicotine administration | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.1 Pre‐ or intraoperative | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.28, 0.48] |
| 26.2 Postoperative | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.85, 0.05] |
| 26.3 Both | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.27] |
| 27 Hourly morphine at 60 minutes by gender | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 27.1 Women only | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.62, 0.35] |
| 27.2 Men and women | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.27] |
| 28 Hourly morphine at 60 minutes by overall quality | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 Good | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.62, 0.35] |
| 28.2 Fair | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.27] |
| 29 Hourly morphine at 24 hours by type of surgery | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.1 Type of surgery is gynaecological | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐13.02, 1.47] |
| 29.2 Type of surgery is mixed/other | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐8.53 [‐29.63, 12.57] |
| 30 Hourly morphine at 24 hours by route of administration | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 30.1 Route is patch | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐8.53 [‐29.63, 12.57] |
| 30.2 Route is inhaler | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐13.02, 1.47] |
| 31 Hourly morphine at 24 hours by smokers or mix of smokers/non‐smokers | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 31.1 Non‐smokers only | 3 | 140 | Mean Difference (IV, Random, 95% CI) | ‐6.05 [‐13.12, 1.01] |
| 31.2 Mix of smokers and non‐smokers | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐6.20 [‐34.40, 22.00] |
| 32 Hourly morphine at 24 hours by nicotine dose | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 32.1 ≤ 5 mg | 4 | 129 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐12.92, 0.96] |
| 32.2 5‐15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐5.51 [‐28.90, 17.88] |
| 32.3 ≥ 15 mg | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐25.02, 25.48] |
| 33 Hourly morphine at 24 hours by timing of nicotine administration | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 33.1 Pre‐ or intraoperative | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐4.62 [‐12.02, 2.78] |
| 33.2 Postoperative | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐16.30 [‐38.38, 5.78] |
| 33.3 Both | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐11.5 [‐43.31, 20.31] |
| 34 Hourly morphine at 24 hours by gender | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 34.1 Women only | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐13.02, 1.47] |
| 34.2 Men and women | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐8.53 [‐29.63, 12.57] |
| 35 Hourly morphine at 24 hours overall quality | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.1 Good | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐13.02, 1.47] |
| 35.2 Fair | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐8.53 [‐29.63, 12.57] |
| 36 Sedation by type of surgery | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 36.1 Type of surgery is gynaecological | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.10, 0.79] |
| 36.2 Type of surgery is mixed/other | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.54, 0.64] |
| 37 Sedation by route of administration | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 37.1 Route is patch | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.54, 0.64] |
| 37.2 Route is inhaler | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.10, 0.79] |
| 38 Sedation by smokers or mix of smokers/non‐smokers | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 38.1 Non‐smokers only | 2 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.10, 0.65] |
| 38.2 Mix of smokers and non‐smokers | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.90, ‐0.16] |
| 39 Sedation by nicotine dose | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 39.1 ≤ 5 mg | 3 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.80, 0.75] |
| 39.2 5‐15 mg | 2 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.72, 0.24] |
| 39.3 ≥ 15 mg | 2 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.41, ‐0.12] |
| 40 Sedation by timing of nicotine administration | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 40.1 Pre‐ or intraoperative | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.10, 0.79] |
| 40.2 Both | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.54, 0.64] |
| 41 Sedation by gender | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 41.1 Women only | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.10, 0.79] |
| 41.2 Men and women | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.54, 0.64] |
| 42 Sedation by overall quality | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 42.1 Fair | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.88, 0.62] |
| 43 Nausea by type of surgery | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 43.1 Type of surgery is gynaecological | 3 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.94, 1.57] |
| 43.2 Type of surgery is male pelvic | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.95, 1.99] |
| 43.3 Type of surgery is mixed/other | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.78, 1.73] |
| 44 Nausea by route of administration | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 44.1 Route is patch | 5 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.03, 1.67] |
| 44.2 Route is inhaler | 2 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.86, 1.54] |
| 45 Nausea by smokers or mix of smokers/non‐smokers | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 45.1 Non‐smokers only | 5 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.99, 1.47] |
| 45.2 Mix of smokers and non‐smokers | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.92, 2.57] |
| 46 Nausea by nicotine dose | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 46.1 ≤ 5 mg | 4 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.45, 1.68] |
| 46.2 5‐15 mg | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.01, 1.95] |
| 46.3 ≥ 15 mg | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.79, 1.79] |
| 47 Nausea by timing of nicotine administration | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 47.1 Pre‐ or intraoperative | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.42, 2.40] |
| 47.2 Postoperative | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 47.3 Both | 5 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.03, 1.67] |
| 48 Nausea by gender | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 48.1 Women only | 3 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.94, 1.57] |
| 48.2 Men and women | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.67] |
| 49 Nausea by overall quality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 49.1 Good | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.47] |
| 49.2 Fair | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.05, 1.77] |
| 50 Vomiting by type of surgery | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 50.1 Type of surgery is gynaecological | 3 | 354 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.11, 0.15] |
| 50.2 Type of surgery is male pelvic | 1 | 90 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [‐0.01, 0.24] |
| 50.3 Type of surgery is mixed/other | 3 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.08, 0.09] |
| 51 Vomiting by route of administration | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 51.1 Route is patch | 5 | 333 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 51.2 Route is inhaler | 2 | 269 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.16, 0.17] |
| 52 Vomiting by smokers or mix of smokers/non‐smokers | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 52.1 ≤ 5 mg | 5 | 378 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 52.2 5‐15 mg | 3 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.03, 0.16] |
| 52.3 ≥ 15 mg | 3 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
| 53 Vomiting by nicotine dose | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 53.1 ≤ 5 mg | 5 | 378 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 53.2 5‐15 mg | 3 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.03, 0.16] |
| 53.3 ≥ 15 mg | 3 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
| 54 Vomiting by timing of nicotine administration | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 54.1 Pre‐ or intraoperative | 1 | 90 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.15, 0.02] |
| 54.2 Postoperative | 1 | 179 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.03, 0.18] |
| 54.3 Both | 5 | 333 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 55 Vomiting by gender | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 55.1 Women only | 3 | 354 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.11, 0.15] |
| 55.2 Men and women | 4 | 248 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.03, 0.12] |
| 56 Vomiting by overall quality | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 56.1 Good | 2 | 269 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.03, 0.16] |
| 56.2 Fair | 5 | 333 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.07, 0.10] |
| 57 Time to hospital discharge by type of surgery | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 57.1 Type of surgery is male pelvic | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 58 Time to hospital discharge by route of administration | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 58.1 Route is patch | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 59 Time to hospital discharge by smokers or mix of smokers and non‐smokers | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 59.1 Non‐smokers only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 60 Time to hospital discharge by nicotine dose | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 60.1 5‐15 mg | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 61 Time to hospital discharge timing of nicotine administration | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 61.1 Pre‐ or intraoperative | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 62 Time to hospital discharge by gender | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 62.1 Men and women | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
| 63 Time to hospital discharge by overall quality | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 63.1 Fair | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐6.19, 8.59] |
2.1. Analysis.
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 1 Pain at 60 minutes by type of surgery.
2.2. Analysis.
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 2 Pain at 60 minutes by route of administration.
2.3. Analysis.
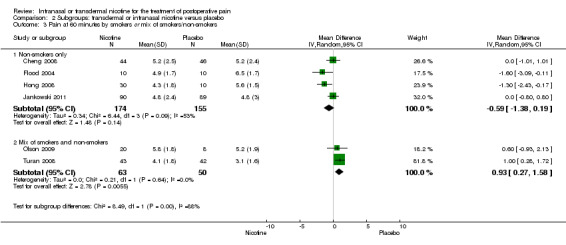
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 3 Pain at 60 minutes by smokers or mix of smokers/non‐smokers.
2.4. Analysis.
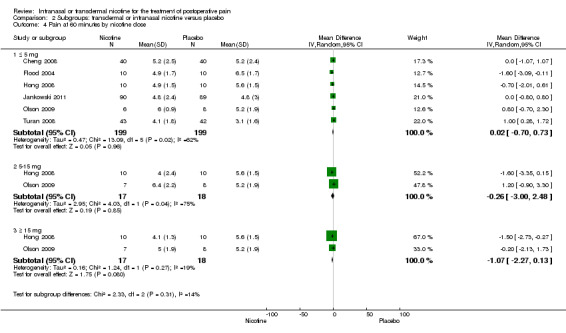
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 4 Pain at 60 minutes by nicotine dose.
2.5. Analysis.
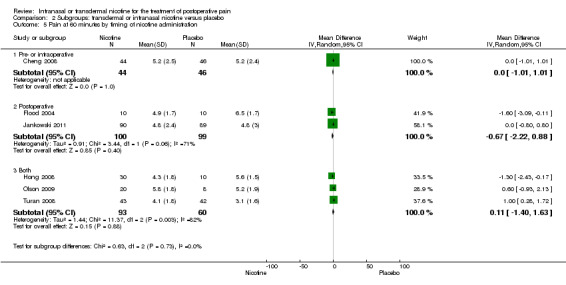
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 5 Pain at 60 minutes by timing of nicotine administration.
2.6. Analysis.
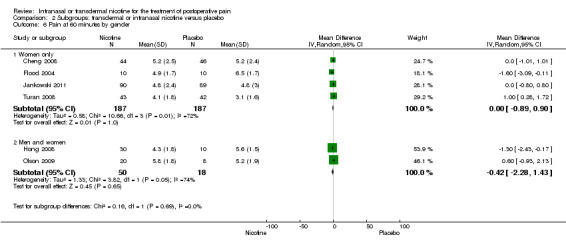
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 6 Pain at 60 minutes by gender.
2.7. Analysis.
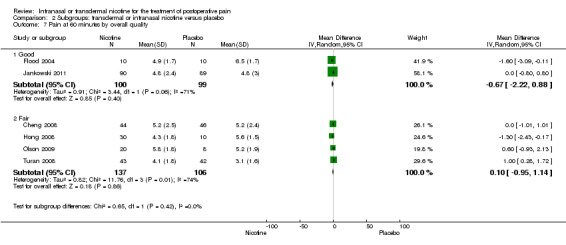
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 7 Pain at 60 minutes by overall quality.
2.8. Analysis.
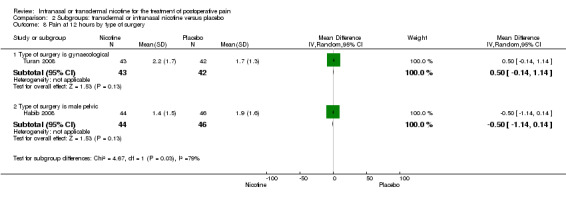
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 8 Pain at 12 hours by type of surgery.
2.9. Analysis.
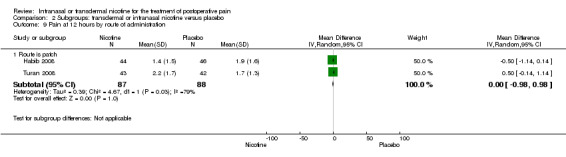
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 9 Pain at 12 hours by route of administration.
2.10. Analysis.
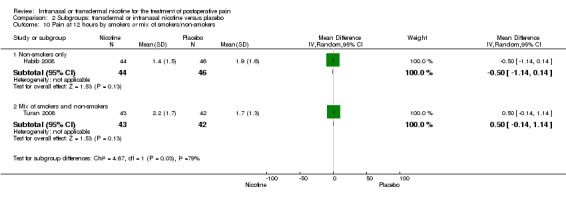
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 10 Pain at 12 hours by smokers or mix of smokers/non‐smokers.
2.11. Analysis.
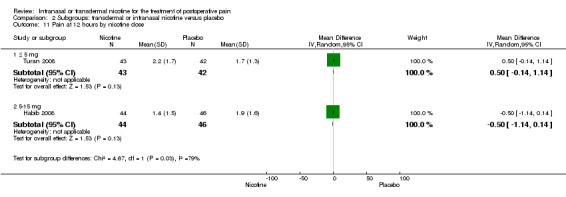
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 11 Pain at 12 hours by nicotine dose.
2.12. Analysis.
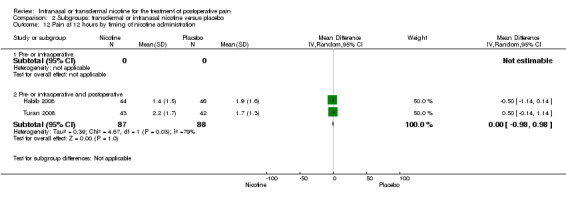
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 12 Pain at 12 hours by timing of nicotine administration.
2.13. Analysis.
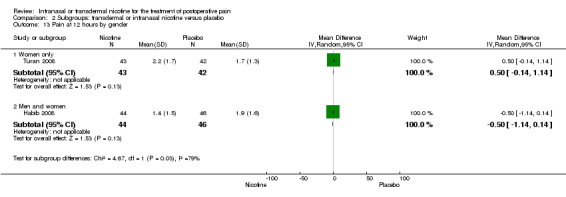
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 13 Pain at 12 hours by gender.
2.14. Analysis.
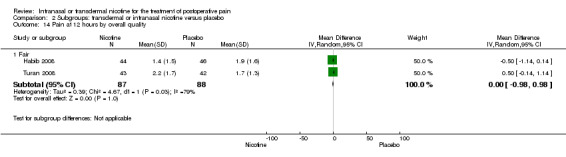
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 14 Pain at 12 hours by overall quality.
2.15. Analysis.
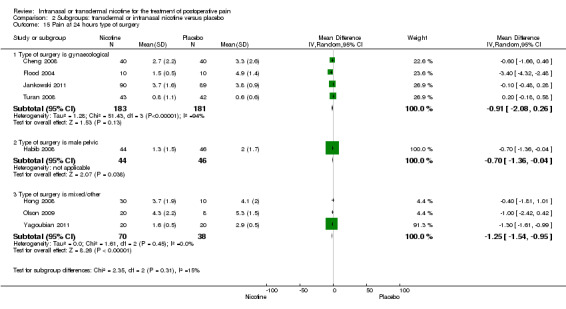
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 15 Pain at 24 hours type of surgery.
2.16. Analysis.
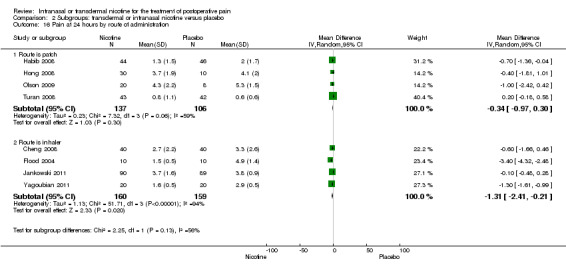
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 16 Pain at 24 hours by route of administration.
2.17. Analysis.
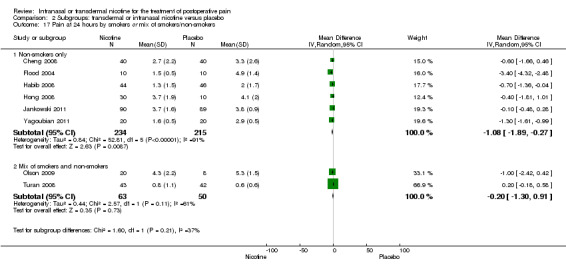
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 17 Pain at 24 hours by smokers or mix of smokers/non‐smokers.
2.18. Analysis.
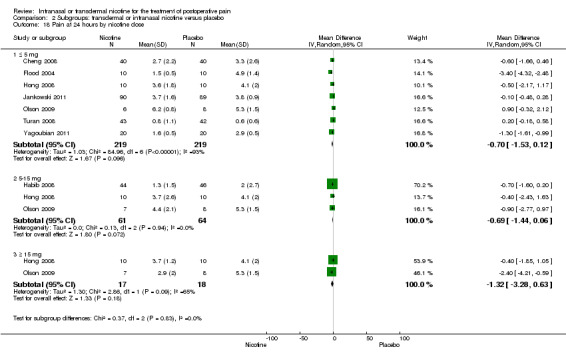
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 18 Pain at 24 hours by nicotine dose.
2.19. Analysis.
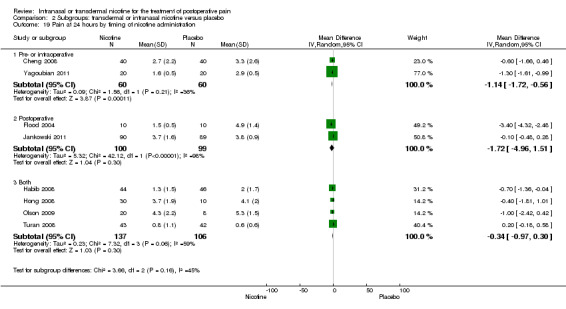
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 19 Pain at 24 hours by timing of nicotine administration.
2.20. Analysis.
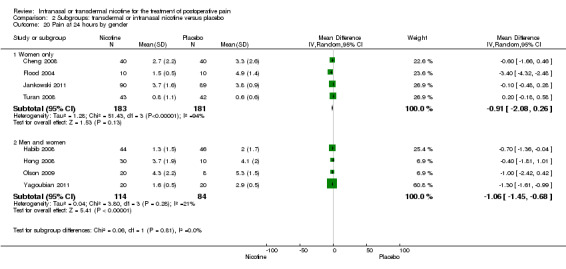
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 20 Pain at 24 hours by gender.
2.21. Analysis.
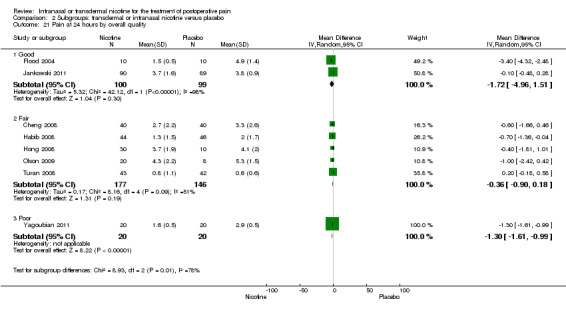
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 21 Pain at 24 hours by overall quality.
2.22. Analysis.
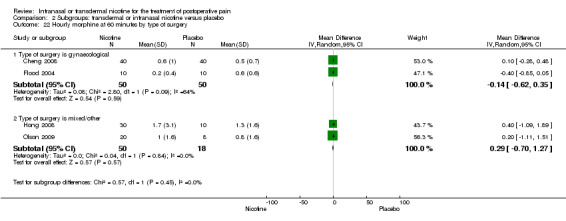
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 22 Hourly morphine at 60 minutes by type of surgery.
2.23. Analysis.
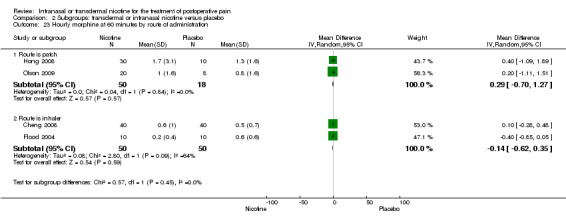
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 23 Hourly morphine at 60 minutes by route of administration.
2.24. Analysis.
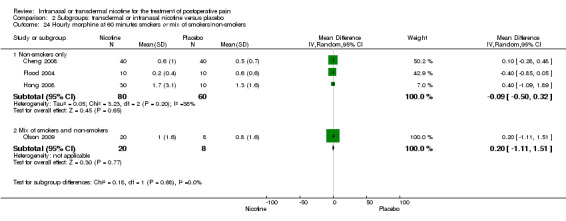
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 24 Hourly morphine at 60 minutes smokers or mix of smokers/non‐smokers.
2.25. Analysis.
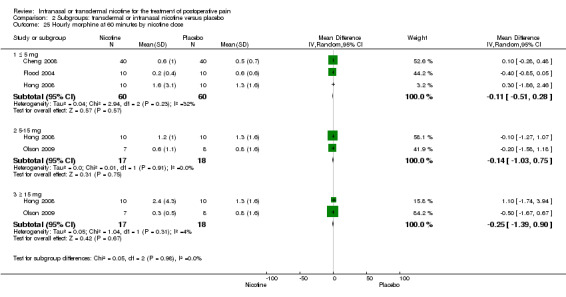
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 25 Hourly morphine at 60 minutes by nicotine dose.
2.26. Analysis.
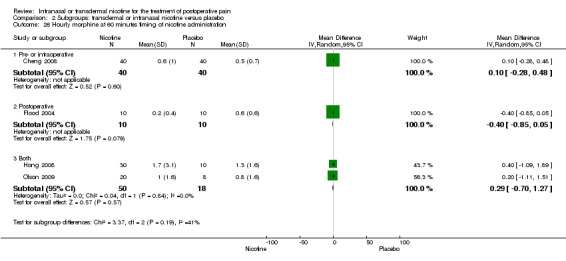
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 26 Hourly morphine at 60 minutes timing of nicotine administration.
2.27. Analysis.
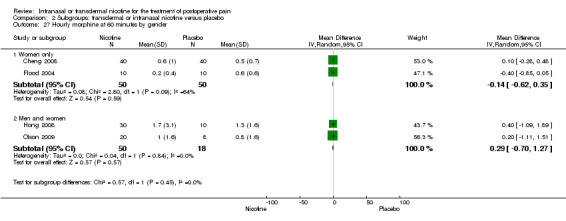
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 27 Hourly morphine at 60 minutes by gender.
2.28. Analysis.
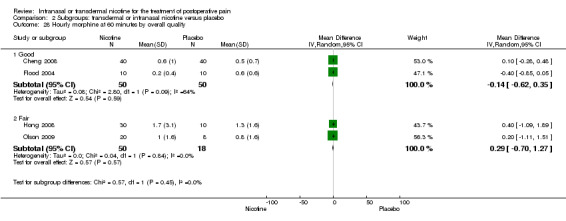
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 28 Hourly morphine at 60 minutes by overall quality.
2.29. Analysis.
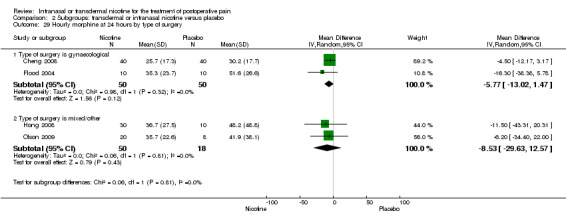
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 29 Hourly morphine at 24 hours by type of surgery.
2.30. Analysis.
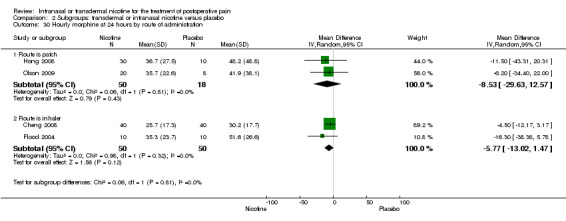
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 30 Hourly morphine at 24 hours by route of administration.
2.31. Analysis.
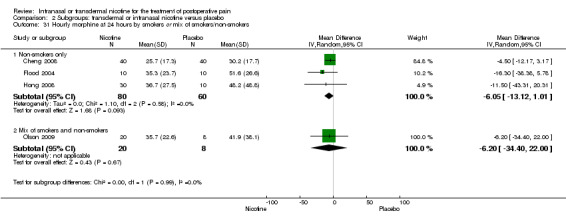
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 31 Hourly morphine at 24 hours by smokers or mix of smokers/non‐smokers.
2.32. Analysis.
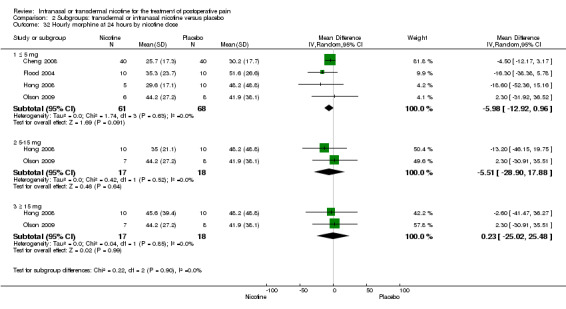
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 32 Hourly morphine at 24 hours by nicotine dose.
2.33. Analysis.
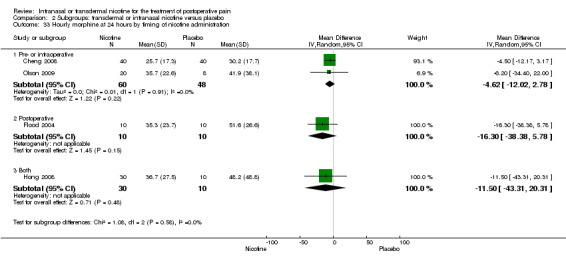
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 33 Hourly morphine at 24 hours by timing of nicotine administration.
2.34. Analysis.
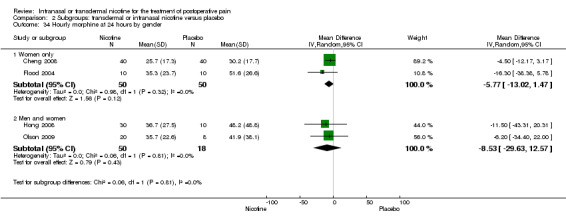
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 34 Hourly morphine at 24 hours by gender.
2.35. Analysis.
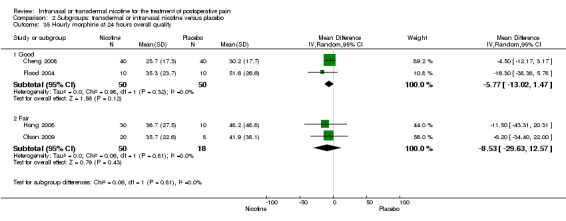
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 35 Hourly morphine at 24 hours overall quality.
2.36. Analysis.
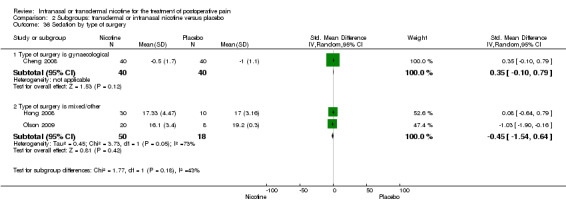
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 36 Sedation by type of surgery.
2.37. Analysis.
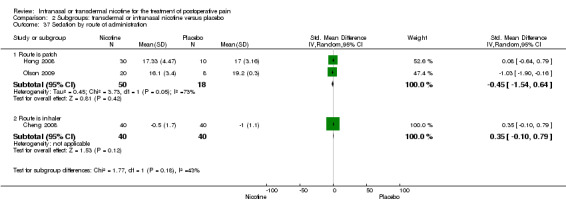
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 37 Sedation by route of administration.
2.38. Analysis.
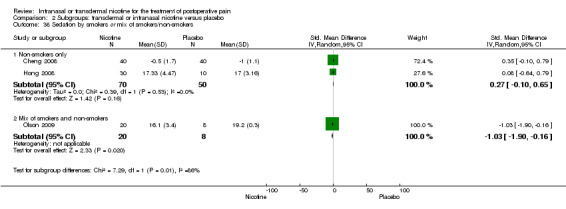
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 38 Sedation by smokers or mix of smokers/non‐smokers.
2.39. Analysis.
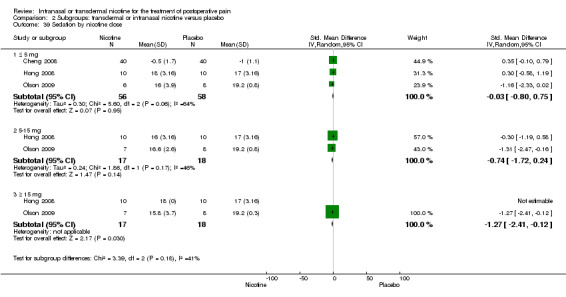
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 39 Sedation by nicotine dose.
2.40. Analysis.
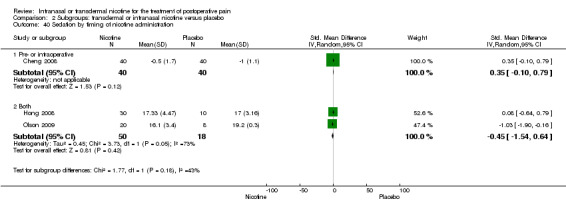
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 40 Sedation by timing of nicotine administration.
2.41. Analysis.
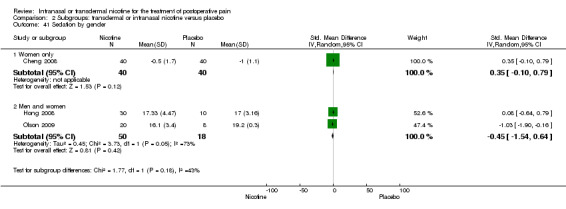
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 41 Sedation by gender.
2.42. Analysis.
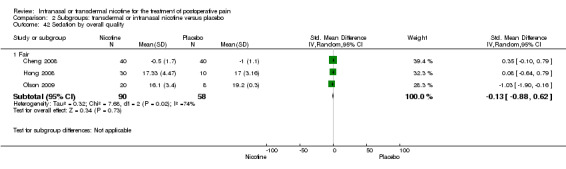
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 42 Sedation by overall quality.
2.43. Analysis.
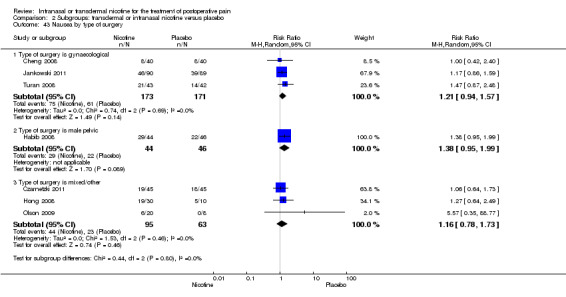
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 43 Nausea by type of surgery.
2.44. Analysis.
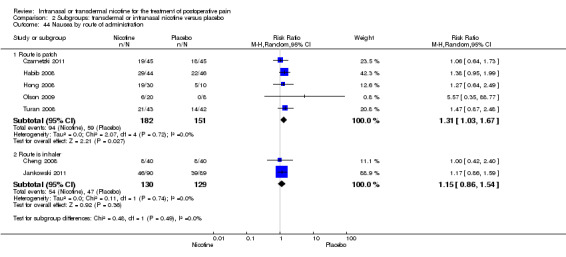
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 44 Nausea by route of administration.
2.45. Analysis.
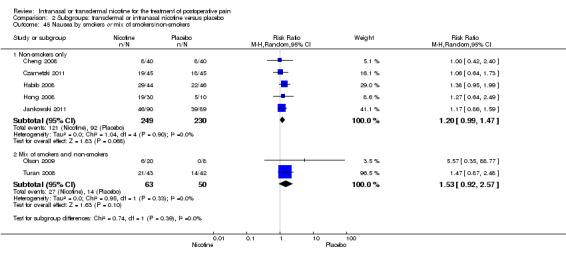
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 45 Nausea by smokers or mix of smokers/non‐smokers.
2.46. Analysis.
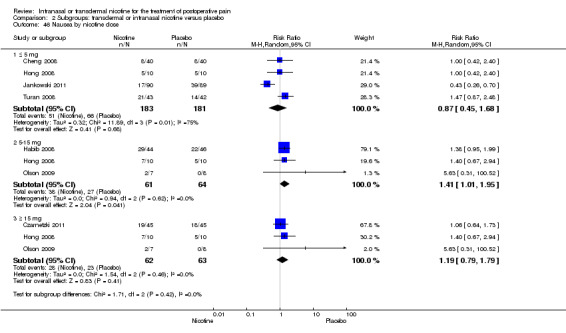
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 46 Nausea by nicotine dose.
2.47. Analysis.
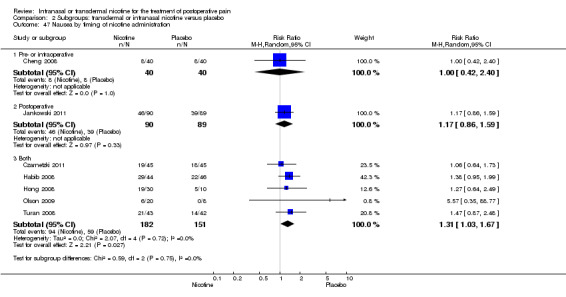
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 47 Nausea by timing of nicotine administration.
2.48. Analysis.
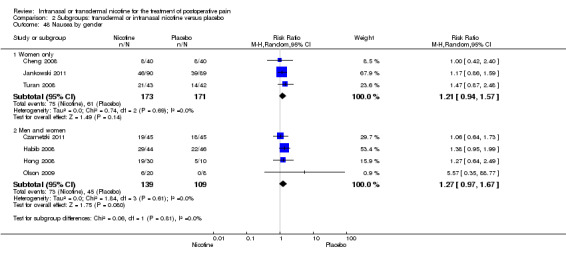
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 48 Nausea by gender.
2.49. Analysis.
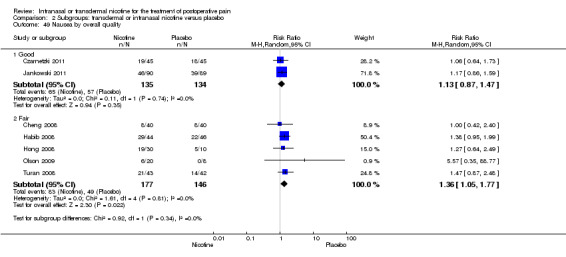
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 49 Nausea by overall quality.
2.50. Analysis.
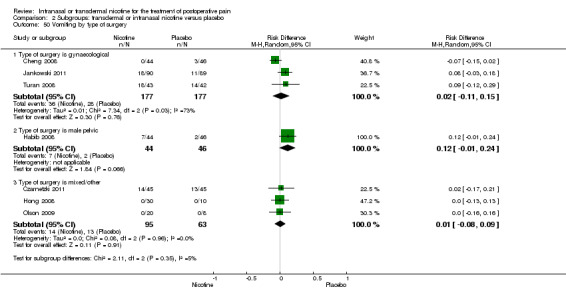
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 50 Vomiting by type of surgery.
2.51. Analysis.
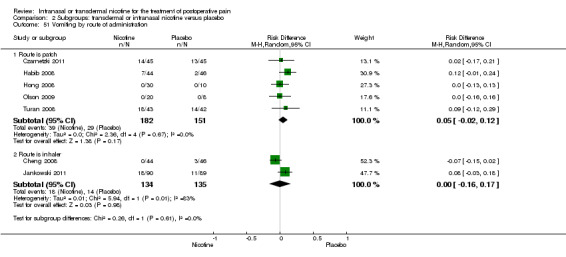
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 51 Vomiting by route of administration.
2.52. Analysis.
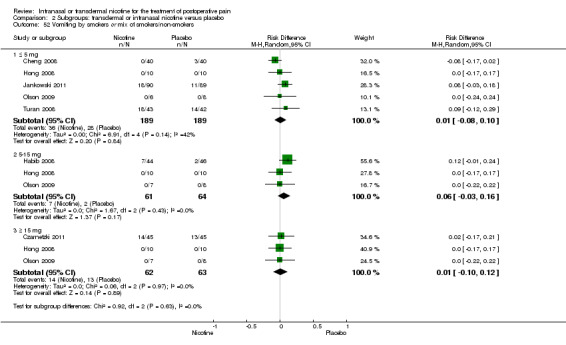
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 52 Vomiting by smokers or mix of smokers/non‐smokers.
2.53. Analysis.
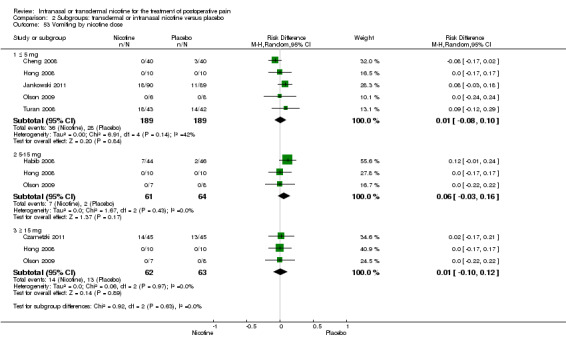
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 53 Vomiting by nicotine dose.
2.54. Analysis.
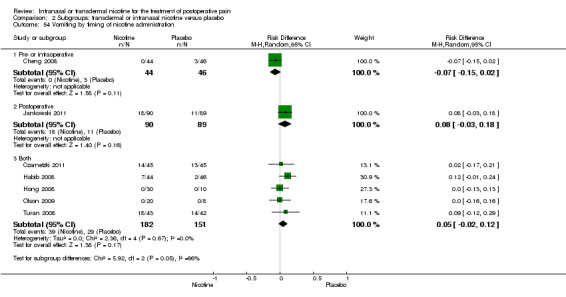
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 54 Vomiting by timing of nicotine administration.
2.55. Analysis.
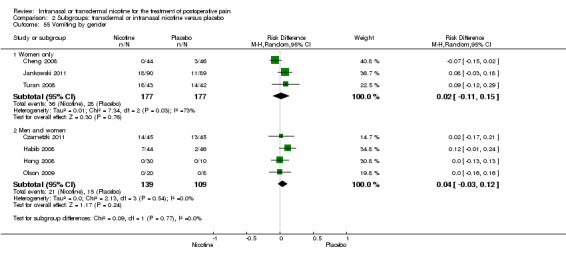
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 55 Vomiting by gender.
2.56. Analysis.
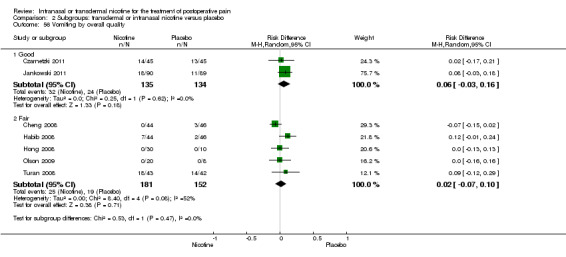
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 56 Vomiting by overall quality.
2.57. Analysis.
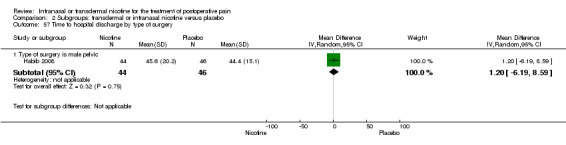
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 57 Time to hospital discharge by type of surgery.
2.58. Analysis.
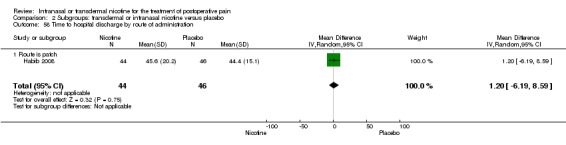
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 58 Time to hospital discharge by route of administration.
2.59. Analysis.
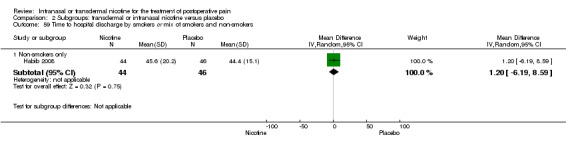
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 59 Time to hospital discharge by smokers or mix of smokers and non‐smokers.
2.60. Analysis.
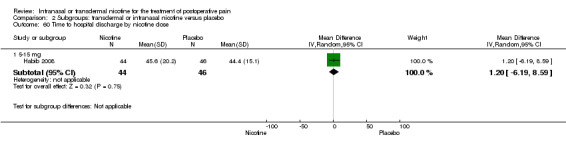
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 60 Time to hospital discharge by nicotine dose.
2.61. Analysis.
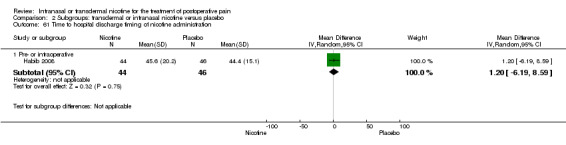
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 61 Time to hospital discharge timing of nicotine administration.
2.62. Analysis.
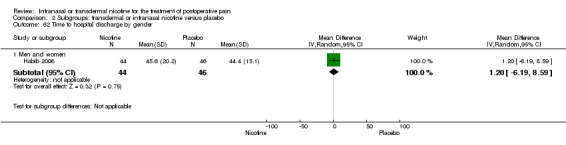
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 62 Time to hospital discharge by gender.
2.63. Analysis.
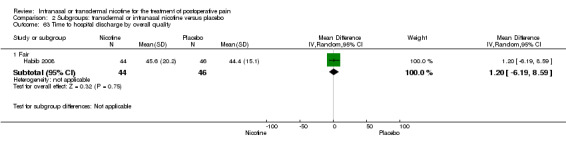
Comparison 2 Subgroups: transdermal or intranasal nicotine versus placebo, Outcome 63 Time to hospital discharge by overall quality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheng 2008.
| Methods | Randomized controlled trial conducted from July 2003 to July 2005 | |
| Participants | Inclusion criteria: women aged >18 years undergoing open hysterectomy or myomectomy. Exclusion criteria: history of tobacco use within the year prior to study entry, uncontrolled hypertension or other CVD, respiratory disease |
|
| Interventions | Participants were anaesthetized with isoflurane or propofol. Within each anaesthetic group, the participants were further randomly assigned to receive nasal spray either nicotine 3 mg nasal spray or saline placebo once at the conclusion of surgery (postoperatively) | |
| Outcomes | Primary: pain at 60 minutes post‐surgery, pain at 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 24 hours Secondary: sedation, nausea, vomiting |
|
| Notes | Additional data provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described as evidenced by the following quote from the paper: "Subjects were randomly assigned to receive one of two standard anaesthetic regimens", but method of sequence generation was not reported. "Subjects in both anaesthetic arms were further randomly assigned to receive a nasal spray containing either nicotine 3 mg or saline placebo at the conclusion of surgery" |
| Allocation concealment (selection bias) | Low risk | Quote: "The clinical anaesthesiologists was familiarized with both anaesthetic protocols by the research coordinator and then provided with a sealed envelope containing the general anaesthetic protocol assignment. Neither the patient nor the study coordinator was aware of the assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | No evidenced of selective reporting in the paper. Quote: "The primary outcome variable was NAS [numerical analogue score]", which was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Neither the patient nor the study coordinator was aware of the assignment," but it is unclear if the postoperative nurse was blinded |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups are similar, co‐interventions were avoided or similar, and timing of outcome assessments are similar |
Czarnetzki 2011.
| Methods | Randomized controlled trial conducted over 24 months | |
| Participants | Inclusion criteria: non‐smokers or ex‐smokers for at least 2 years; ASA physical status I or II Exclusion criteria: use of nicotine replacement therapy; need for prolonged postoperative intubation or nasogastric tube; dermal hypersensitivity to nicotine or 1 of the components of the patch; systemic cutaneous disease; unstable angina; recent MI; severe arrhythmia; recent cerebral vascular accident; parkinsonism; renal or hepatic failure; diabetes; uncontrolled arterial hypertension; hyperthyroidism; gastroduodenal ulcer; pregnant or breastfeeding |
|
| Interventions | Participants were randomized to either a nicotine 17.5 mg patch with a mean delivery rate of nicotine 7 mg per 24 hours or matching placebo patch administered at the time of induction of anaesthesia and left in place for 24 hours after surgery or until first PONV episode (perioperatively) | |
| Outcomes | Primary: none Secondary: nausea, vomiting |
|
| Notes | Conducted in Switzerland. Funded by institutional funds from the University of Geneva, Geneva, Switzerland. The nicotine and placebo patches were provided by LTS Lohmann Terapie‐Systeme AG, Andernach, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study medications were randomized in blocks of ten (five nicotine and five placebo) using a computer program by the pharmacy of Geneva University Hospitals" |
| Allocation concealment (selection bias) | Low risk | Quote: "... and were kept concealed in a neutral opaque cover" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason for withdrawals reported in Figure 3 |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting in the paper. Quote: "The primary endpoint of the study was the cumulative incidence of PONV (i.e. any nausea and/or vomiting symptoms) within 24h", which was reported Secondary outcomes were also specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to the study drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators were blinded to the study drug |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups were similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Flood 2004.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: women aged 18‐50 years undergoing myomectomy or hysterectomy Exclusion criteria: smoking within 1 year of study entry, pre‐existing pain syndromes, hypertension, history of CVD |
|
| Interventions | Participants were randomized to either nasal spray nicotine 3 mg or saline placebo once at the conclusion of surgery (postoperatively) | |
| Outcomes | Primary: pain at 60 minutes post‐surgery, pain at 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 24 hours Secondary: none | |
| Notes | Additional data provided by authors. Supported by grant K08 GM00695 (to Dr. Flood) National Institute of General Medical Studies, Rockville, MD and department funding from the Department of Anesthesiology, Columbia University, New York, New York | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... prepared by the research pharmacy according to a random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the completion of surgery, the anesthesiologist was given an opaque sealed container with either a nicotine nasal spray (3 mg Nicotrol NS; Pharmacia, Peapack, NJ) or saline nasal spray" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason withdrawals reported in the bottom of p. 1418, first column |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting in the paper. Quote: "We assessed the analgesic activity of nicotine administered in a nasal spray in women after uterine surgery," was the primary outcome and was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None of the study personnel knew which group the participant was assigned to |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups are similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Habib 2008.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: non‐smoking men aged 18‐75 years undergoing radical retropubic prostatectomy Exclusion criteria: current smokers or non‐smokers for < 5 years, chronic pain, regular use of analgesics, uncontrolled hypertension, ischaemic heart disease, peripheral vascular disease, arrhythmia, diabetes, asthma, hyperthyroidism, phaeochromocytoma |
|
| Interventions | Participants were randomized to nicotine 7 mg/24 hour or identical placebo patch placed 30‐60 minutes preoperatively and left in place for 24 hours (perioperatively) | |
| Outcomes | Primary: pain at 12 and 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 12 and 24 hours Secondary: nausea, vomiting, time to hospital discharge |
|
| Notes | Additional data provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized in equal numbers to receive either a nicotine patch releasing 7 mg/24 h or an identical placebo patch", but the method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Data were collected by study personnel unaware of the patients’ randomization every 30 min for 2 h in the PACU [post‐anaesthesia care unit], and at 6, 12, and 24 h postoperatively," but the actual method of allocation concealment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Quote: "Ninety‐six patients were enrolled in the study. Six were subsequently excluded: the patch fell off intraoperatively in two patients, and a PCA was not prescribed for four patients" |
| Selective reporting (reporting bias) | Low risk | No evidenced of selective reporting in the paper. Quote: "The primary objective of this study was therefore to assess the 24 h morphine‐sparing effect of the preoperative administration of a 7 mg nicotine patch in this patient population", which was reported as the primary outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None of the study personnel knew which group the participant was assigned to |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups are similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Hong 2008.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: non‐smokers aged > 18 years undergoing general surgery (including pelvic and abdominal) with planned overnight stay and use of postoperative PCA Exclusion criteria: current or recent (within 6 months) smoker, uncontrolled hypertension, myocardial disease, stroke, respiratory disease, pregnancy, chronic pain, use of chronic pain medications, spinal or epidural anaesthesia during surgery |
|
| Interventions | Participants were randomized to nicotine patch in 1 of 3 doses (5 mg/16 hours, 10 mg/16 hours, or 15 mg/16 hours) or identical placebo patch applied before induction and removed the night of surgery (perioperatively) | |
| Outcomes | Primary: pain at 60 minutes and 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 60 minutes and 24 hours Secondary: nausea, vomiting, sedation | |
| Notes | Additional data provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Ten patients (5 men and 5 women) were assigned by a computer‐generated randomization table to each of four treatment groups: 0, 5, 10, and 15 mg of nicotine" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patient, investigator, and health care staff were blinded to the treatment group", but it does not report how allocation concealment was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Quote: "Forty patients were enrolled in the study. In the control group, one patient was not followed after 2 days postoperatively because of a second surgery for placement of a gastrostomy tube. Another patient in the control group was not followed after 3 days postoperatively because of reexploration for bleeding. One patient in the 15 mg nicotine group was not followed after 3 days, also because of reoperation for bleeding" |
| Selective reporting (reporting bias) | Low risk | No evidenced of selective reporting. Quote "This double‐blind, randomized, prospective, placebo controlled trial was designed to evaluate the effects of nicotine patches on postoperative pain", which was reported as the primary outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient, investigator, and health care staff were blinded to the treatment group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient, investigator, and health care staff were blinded to the treatment group" |
| Other bias | Low risk | Describes ITT analysis, withdrawals, baseline groups were similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Jankowski 2011.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: people aged > 18 years having an elective abdominal or vaginal gynaecological procedure, ASA physical status class ≤ 4; BMI < 35 kg/m2 and non‐smoking status (defined as no tobacco use for at least 1 year and < 100 cigarettes/life) Exclusion criteria: known allergy or contraindication to any of the study medications (nicotine, morphine, fentanyl, or ketorolac); pregnancy or lactation; participant or physician preference for regional anaesthesia for either surgery or postoperative pain control; calculated creatinine clearance below the age‐adjusted norms; CVD (e.g. angina, uncontrolled hypertension, and cardiac dysrhythmias); pain requiring daily preoperative use of opioids or scheduled prescription for non‐opioid analgesics; history of nausea within 24 hours of surgery; and any scheduled use of antiemetic drugs during the study period |
|
| Interventions | Participants were randomized to nicotine 3 mg nasal spray or saline placebo once at the conclusion of surgery (postoperatively) | |
| Outcomes | Primary: pain at 60 minutes and 24 hours post‐surgery, cumulative morphine at 24 hours Secondary: nausea, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not explicitly stated. Quote: "Patients were randomised in a double‐blind fashion using sealed envelopes provided by the Division of Biostatistics. The randomisation included two stratification factors: type of procedure (abdominal vs. vaginal) and history of motion sickness or PONV (positive history vs. no history). Within each stratum, randomisation was performed using blocks of size n equal to 2 to ensure that treatment arms remain balanced within strata over time" |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately after the end of the operation, but before emergence from anaesthesia, patients received either nicotine nasal spray (Nicotrol NS; Pharmacia, Peapack, New Jersey, USA) or a placebo moisturising nasal spray (isotonic saline solution). These were prepared by the research pharmacist in identical syringes and were delivered to the operating room labelled ‘study drug’ so that the anaesthesia provider was blinded to the group assignment. The double‐blind strategy was maintained with identical unlabelled packaging and dispensing of nicotine nasal spray and placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data completely reported. Quote: "For the 21 patients who were excluded, the reasons are provided in Fig. 1. In all cases, the decision to exclude a patient was made after randomisation but prior to administration of the study drug and without knowledge of the patient’s treatment assignment" |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Quote: "The purpose of this study was to determine whether a single administration of intranasal nicotine has an opioid sparing effect in non‐smoking women undergoing gynaecological surgery. The secondary aim was to characterise the effects of intranasal nicotine on postoperative nausea and vomiting (PONV), with the hypothesis that intranasal nicotine will not increase PONV, a mechanism that may be mediated through nicotine‐induced opioid sparing", and these outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | None of the study personnel knew which group the participant was assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None of the study personnel knew which group the participant was assigned to |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups were similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Olson 2009.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: smokers aged > 18 years, ASA status I or II, planned overnight hospital stay, anticipated use of PCA Exclusion criteria: uncontrolled hypertension, myocardial disease, stroke, respiratory disease, chronic pain, use of chronic pain medications |
|
| Interventions | Nicotine patch in 1 of 3 doses (5 mg/16 hours, 10 mg/16 hours, or 15 mg/16 hours) or identical placebo patch applied on hour before induction and removed 24 hours after application (perioperatively) | |
| Outcomes | Primary: pain at 60 minutes and 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 60 minutes and 24 hours Secondary: nausea, vomiting, sedation | |
| Notes | Additional data provided by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All participants were assigned by a computer generated randomisation table to 1 of 4 treatment groups: 0, 5, 10, and 15 mg of nicotine by patch, delivered over 16 h" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patches were provided in a sealed opaque envelope. Placebo patches were identical to study drug patches" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason withdrawals reported in Figure 1 |
| Selective reporting (reporting bias) | Low risk | No evidenced of selective reporting in the paper. Quote: "The primary outcome variable was NRS [numerical rating scale] score at 1 h", which was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All research personnel, including study subjects and individuals involved in obtaining follow‐up data, were masked to the treatment intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All research personnel, including study subjects and individuals involved in obtaining follow‐up data, were masked to the treatment intervention" |
| Other bias | High risk | Described ITT analysis, withdrawals, co‐interventions were avoided or similar, and timing of outcome assessments were similar. However, baseline groups were not similar, with many more men in the placebo group suggesting a high risk of bias |
Turan 2008.
| Methods | Randomized controlled trial conducted from 1 February 2005 to 1 May 2006 | |
| Participants | Inclusion criteria: women aged > 18 years undergoing abdominal hysterectomy and salpingo‐oophorectomy, within 50% of ideal body Exclusion criteria: known allergy to any of the study medications, contraindications to use of PCA morphine or any anaesthetic drugs, renal insufficiency, peptic ulcer disease, hypertension, pre‐existing pain syndromes, history of CVD, drug abuse |
|
| Interventions | Participants were randomized to nicotine 52.5 mg patch with mean delivery rate of 21 mg per 24 hours or identical placebo patches. Patches were applied 30 minutes before induction of anaesthesia and the same type of patch was placed at 09:00 on the second and third postoperative days (perioperatively) | |
| Outcomes | Primary: pain at 60 minutes 12 and 24 hours post‐surgery, cumulative morphine at 60 minutes post‐surgery, cumulative morphine at 60 minutes, 12 and 24 hours Secondary: nausea, vomiting, patient satisfaction |
|
| Notes | Conducted in Turkey. Additional data provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were prospectively assigned to one of two treatment groups using a computer‐generated random numbers table." |
| Allocation concealment (selection bias) | Unclear risk | Did not specify how patients were selected. Quote: "All patches were identical in appearance, and were placed on the patient’s upper arm and covered with a sterile gauze and tape by an anesthesiology resident not involved in the data collection process" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason withdrawals reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Quote: "Therefore, we tested the hypothesis that transdermal nicotine (TDN) would decrease postoperative pain and opioid analgesic usage, thereby improving the early recovery process after pelvic gynaecological surgery. The secondary objectives of this study were to examine the effect of TDN on recovery of bowel function, resumption of normal activities of daily living, overall quality of recovery, and patient satisfaction with their pain management", and these outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None of the study personnel knew which group the participant was assigned to |
| Other bias | Low risk | Described ITT analysis, withdrawals, baseline groups were similar, co‐interventions were avoided or similar, and timing of outcome assessments were similar |
Yagoubian 2011.
| Methods | Cross‐over randomized controlled trial | |
| Participants | Inclusion criteria: people required third molar extractions aged > 18 years Exclusion criteria: current smokers; people who had smoked within the previous year; and people who had poorly controlled hypertension, CVD, pregnancy, or lactation |
|
| Interventions | Participants were randomized to nicotine 3 mg nasal spray or saline placebo once at the beginning of surgery (preoperatively) | |
| Outcomes | Primary: pain at 24 hours post‐surgery, cumulative morphine at 60 minute Secondary: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not explicitly stated. Quote: "The study drug, nicotine nasal spray (3 mg), or sterile saline placebo was supplied by the research pharmacy according to a block randomization" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to assess the risk of bias arising from allocation concealment. Quote: "The study drug, nicotine nasal spray (3 mg), or sterile saline placebo was supplied by the research pharmacy according to a block randomization and was administered as 3 sprays to each nostril in rapid succession just after injection of local anaesthetic and just before placing the bite block to begin extraction" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completely reported. Number and reason for withdrawals reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Quote: "The primary outcome variable was pain report (NRS [numerical rating scale]). Secondary outcome variables were and hydrocodone 5 mg/acetaminophen 500 mg use during the 5 days after surgery, nausea (NRS), and hemodynamic effects (heart rate and blood pressure) 1 hour after surgery," and these outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if personnel or participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported |
| Other bias | Unclear risk | Described ITT analysis, withdrawals, co‐interventions were avoided or similar, and timing of outcome assessments were similar. However, it was unclear if baseline groups were similar, suggesting an unclear risk of bias |
ASA: American Society of Anesthesiologists;
BMI: body mass index;
CVD: cardiovascular disease;
ITT: intention‐to‐treat;
MI: myocardial infarction;
PCA: patient‐controlled analgesia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benowitz 2008 | Editorial |
| Ionescu 2007 | Controlled trial, but not randomized |
| Souzdalnitski 2009 | Abstract of a review |
Characteristics of studies awaiting assessment [ordered by study ID]
Weingarten 2015.
| Methods | Awaiting classification |
| Participants | Awaiting classification |
| Interventions | Awaiting classification |
| Outcomes | Awaiting classification |
| Notes | Awaiting classification |
Characteristics of ongoing studies [ordered by study ID]
NCT00790829.
| Trial name or title | Preemptive Use of the Nicotine Patch for Postoperative Pain Relief After Open Abdominal Surgery |
| Methods | Allocation: randomized
Endpoint classification: efficacy study
Intervention model: single group assignment
Masking: double blind (participant, carer, investigator)
Primary purpose: prevention If the participant smokes, receives a regional anaesthetic such as an epidural, or is pregnant, then he/she is excluded from the study. There are 2 randomized study groups. Group B receives a 7 mg nicotine transdermal patch and group A receives a placebo patch. Generic 7 mg nicotine patches or identical placebo patches made from band‐aids are glued to a 3 x 4 inch (7.6 x 10 cm) adhesive pad and placed on the person's right upper arm 1 hour before surgery. All participants are given a standardized anaesthetic consisting of a narcotic infusion, propofol, a neuromuscular blocking agent, anaesthetic gas agent, anti‐nausea medication, and a non‐steroidal anti‐inflammatory drug Participants receive postoperative analgesia for 24 hours after surgery with a narcotic or an additional anti‐inflammatory drug. All participants receive intravenous controlled patient‐controlled analgesia (IVPCA) with morphine sulphate 1 mg per 10 minutes, with 40 mg per 4 hours limit. Participants also receive toradol 15 mg for breakthrough pain. The patch is removed from participants 24 hours post IVPCA initiation. The following items are assessed every 4 hours for 24 hours after post anaesthesia care unit discharge: verbal rating of pain, total IVPCA morphine use, nausea occurrence, vomiting occurrence, and sedation score by the nurse |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Drug: transdermal nicotine patch, generic 7 mg nicotine patches for 24 hours Placebo patch for 24 hours |
| Outcomes | n/a |
| Starting date | 13 November 2008 |
| Contact information | Principle investigator: Ursula N Landman, DO; 631‐444‐2975; ulandman@notes.cc.sunnysb.edu |
| Notes |
NCT01194089.
| Trial name or title | Nicotine Administration and Post‐operative Opioid Use With Bariatric Surgery |
| Methods | Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double blind (participant, carer, investigator) Primary purpose: treatment |
| Participants | Life‐long, non‐smoking women aged 18‐60 years undergoing bariatric surgery |
| Interventions | Active comparator: nasal nicotine spray 3 mg of nasal nicotine will be administered postoperatively Placebo comparator: nasal normal saline spray 1 mL of nasal normal saline spray will be administered postoperatively |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 1 September 2010 |
| Contact information | Principle investigator: Toby Weingarten, MD Contact: Laurie Meade; 507‐255‐1829; 1‐866‐265‐9263 |
| Notes |
Differences between protocol and review
In the editorial process, we agreed to delete the outcome variable of cumulative morphine but this did not get deleted from all parts of the published protocol (Matthews 2012). That change has been uniformly made now.
We also examined the following variables, not originally listed in our protocol, in subgroup analyses as potential sources of heterogeneity:
sex (male or female);
nicotine dose per dose (not cumulative) (5 mg or less, between 5 and 10 mg, or 10 mg or greater);
timing of nicotine administration (pre‐ or intraoperatively only or including postoperative administration);
study quality (good, fair, or poor).
We planned to examine the following variables in meta‐regression as potential sources of heterogeneity.
Mean age.
Proportion of males.
Dose of nicotine.
Smoking status (proportion of smokers).
Opioids administered during surgery or postoperatively in morphine equivalents (for outcomes other than postoperative opioid use).
However, because there were fewer than 10 studies, we did not perform meta‐regression, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We also wished to assess publication bias formally using graphical or statistical methods but due to the small numbers of trials available for each analysis were unable to.
Contributions of authors
Conceived the review: Annette M Matthews (AM).
Co‐ordinated the review: AM.
Undertook manual searches: AM.
Screened search results: AM, Tracy Dana (TD), Roger Chou (RC).
Organized retrieval of papers: AM, TD.
Screened retrieved papers against inclusion criteria: AM, TD, RC.
Appraised quality of papers: AM, TD, RC.
Abstracted data from papers: AM, TD, RC.
Wrote to authors of papers for additional information: AM.
Provided additional data about papers: AM.
Obtained and screened data on unpublished studies: AM.
Data management for the review: AM, TD.
Entered data into Review Manager 5 (RevMan 2014): AM, TD.
Performed statistical data analyses using RevMan 2014: AM, TD, RC, Rochell Fu (RF).
Performed other statistical analyses not using Review Manager: RF.
Interpreted data: AM, RC.
Statistical inferences: RC, RF.
Wrote the review: AM, RC.
Secured funding for the review: AM.
Performed previous work that was the foundation of the present study: N/A.
Acted as guarantor for the review: AM.
People responsible for reading and checking review before submission: AM, RC, TD, RF.
Sources of support
Internal sources
-
Oregon Health & Science University Human Investigations Program, USA.
A draft of this protocol was first developed as part of a course on systematic reviews that was sponsored by National Center for Research Resources/National Center for Advancing Translational Sciences (NCRR/NCATS)‐funded Clinical and Translational Science Award (CTSA) grant (UL1RR024140).
External sources
-
Veterans Affairs Clinical Sciences Research and Development (CSR&D) Career Development Award, USA.
Awarded to Annette M. Matthews, MD.
Declarations of interest
Annette M Matthews: received funding from the American Psychiatric Association for: FOCUS Exam Editorial Board Honorarium, reimbursement for Association Business, travel to PRITE Editorial Board meetings, travel to Scientific Program Committee Meetings, to American Psychiatric Association Assembly meetings, and to FOCUS Editorial Board meetings. She received consultancy fees from the Substance Abuse and Mental Health Administration for a Grant review and consultancy fees from the American Psychiatric Association for the Focus Exam Editorial Board. Dr Matthews received from Oregon Psychiatric Association: travel to American Psychiatric Association Assembly meetings. She received from the Oregon Health and Science University, Agency for Healthcare Research and Quality payment for a review of psychiatric medications in the peripartum period. Dr Matthews received from the Veteran Affairs Clinical Sciences Research and Development Program Grant, a career development award. She is employed by Portland VA Medical Center and receives a salary.
Rongwei Fu: none known.
Tracy Dana: none known.
Roger Chou: led a systematic review on postoperative pain management and led the development of a guideline on postoperative pain management for the American Pain Society (nicotine was not included in the guideline).
New
References
References to studies included in this review
Cheng 2008 {published data only}
- Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesthesia and Analgesia 2008;106(1):264‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Czarnetzki 2011 {published data only}
- Czarnetzki C, Schiffer E, Lysakowski C, Haller G, Bertrand D, Tramer MR. Transcutaneous nicotine does not prevent postoperative nausea and vomiting: a randomized controlled trial. British Journal of Clinical Pharmacology 2011;71(3):383‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flood 2004 {published data only}
- Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology 2004;101(6):1417‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Habib 2008 {published data only}
- Habib AS, White WD, Gasim MA, Saleh G, Polascik TJ, Moul JW, et al. Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesthesia and Analgesia 2008;107(3):999‐1004. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hong 2008 {published data only}
- Hong D, Conell‐Price J, Cheng S, Flood P. Transdermal nicotine patch for postoperative pain management: a pilot dose‐ranging study. Anesthesia and Analgesia 2008;107(3):1005‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jankowski 2011 {published data only}
- Jankowski CJ, Weingarten TN, Martin DP, Whalen, FX, Gebhart JB, Liedl LM, et al. Randomised trial of intranasal nicotine and postoperative pain, nausea and vomiting in non‐smoking women. European Journal of Anaesthesiology 2011;28:585‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olson 2009 {published data only}
- Olson LC, Hong D, Conell‐Price JS, Cheng S, Flood P. A transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose‐ranging study. Anesthesia and Analgesia 2009;109(6):1987‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turan 2008 {published data only}
- Turan A, White PF, Koyuncu O, Karamanliodlu B, Kaya G, Apfel CC. Transdermal nicotine patch failed to improve postoperative pain management. Anesthesiology 2008;107(3):1011‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yagoubian 2011 {published data only}
- Yagoubian B, Akkara J, Afzali P, Alfi DM, Olson L, Conell‐Price J, et al. Nicotine nasal spray as an adjuvant analgesic for third molar surgery. Journal of Oral and Maxillofacial Surgery 2011;69(5):1316‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benowitz 2008 {published data only}
- Benowitz NL. Nicotine and postoperative management of pain. Anesthesia and Analgesia 2008;107:739‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ionescu 2007 {published data only}
- Ionescu D, Badescu C, Acalovschi I. Nicotine patch for the prevention of postoperative nausea and vomiting: a prospective randomised trial. Clinical Drug Investigation 2007;27(8):559‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Souzdalnitski 2009 {published data only}
- Souzdalnitski D, Dabu‐Bondoc S, Vadivelu N, Chung K. Nicotine in postoperative pain management. Journal of Pain 2009;10:S34. [Google Scholar]
References to studies awaiting assessment
Weingarten 2015 {published data only}
- Weingarten TN, McGlinch BP, Liedl L, Kendrick ML, Kellogg TA, Schroeder DR, et al. Intranasal nicotine increases postoperative nausea and is Ineffective in reducing pain following laparoscopic bariatric surgery in tobacco‐naive females: a randomized, double blind trial. Obesity Surgery 2015;25(3):506‐13. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00790829 {unpublished data only}
- Preemptive Use of the Nicotine Patch for Postoperative Pain Relief After Open Abdominal Surgery. Ongoing study 13 November 2008.
NCT01194089 {unpublished data only}
- Nicotine Administration and Post‐operative Opioid Use With Bariatric Surgery. Ongoing study 1 September 2010.
Additional references
Apfelbaum 2003
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia and Analgesia 2003;97:534‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brattwall 2010
- Brattwall M, Warren Stomberg M, Rawal N, Segerdahl M, Houltz E, Jakobsson J. Postoperative impact of regular tobacco use, smoking or snuffing, a prospective multi‐center study. Acta Anaesthesiologica Scandinavica 2010;54(3):321‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campbell 2006
- Campbell VC, Taylor RE, Tizabi Y. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Research 2006;1097(1):71‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flood 2002
- Flood P, Sonner JM, Gong D, Coates KM. Isoflurane hyperalgesia is modulated by nicotinic inhibition. Anesthesiology 2002;97(1):192‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girdler 2005
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress‐induced analgesia and pain perception in men and women. Pain 2005;114(3):372‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thomson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kissin 2009
- Kissin I. Patient‐controlled‐analgesia analgesimetry and its problems. Pain Medicine 2009;108:1945‐9. [19448227] [DOI] [PubMed] [Google Scholar]
McQuay 2008
- McQuay HJ, Poon KH, Derry S, Moore RA. Acute pain: combination treatments and how we measure their efficacy. British Journal of Anaesthesia 2008;101(1):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 1962
- Miller WF, Archer RK, Taylor HF, Ossenfort WF. Severe respiratory depression: role of a respiratory stimulant, ethamivan, in the treatment. JAMA 1962;180(1):905‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishriky 2014
- Mishriky BM, Habib AS. Nicotine for postoperative analgesia: a systematic review and meta‐analysis. Anesthesia and Analgesia 2014;119(2):268‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Owen 1990
- Owen H, McMillan V, Rogowski D. Postoperative pain therapy: a survey of patients expectations and their experiences. Pain 1990;41:303‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shi 2010
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology 2010;113(4):977‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Sutton AJ, Loannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;342:d4002. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Svensson 2000
- Svensson I, Sjostrom B, Haljamae H. Assessment of pain experiences after elective surgery. Journal of Pain and Symptom Management 2000;20(3):193‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vadivelu 2010
- Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale Journal of Biology and Medicine 2010;83(1):11‐25. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
van Tudler 2003
- Tudler M, Furlan A, Bombardier C, Bouter L, and the Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28:1290‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vibe Nielsen 2012
- Vibe Nielsen S, Petersen RH, Pachai A. A possible analgesic effect of nicotine on postoperative pain. Ugeskrift for Laeger 2012;23(174):1594‐8. [PubMed] [Google Scholar]
Yan 2009
- Yan S, Dai T‐J, Zeng Y‐M. Nicotinic acetylcholine receptors mediate the hypnotic and analgesic effects of emulsified inhalation anesthetics. Fundamental and Clinical Pharmacology 2009;23(2):235‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Matthews 2012
- Matthews AM, Fu R, Dana T, Chou R. Intranasal or transdermal nicotine for the treatment of postoperative pain. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009634] [DOI] [PMC free article] [PubMed] [Google Scholar]


