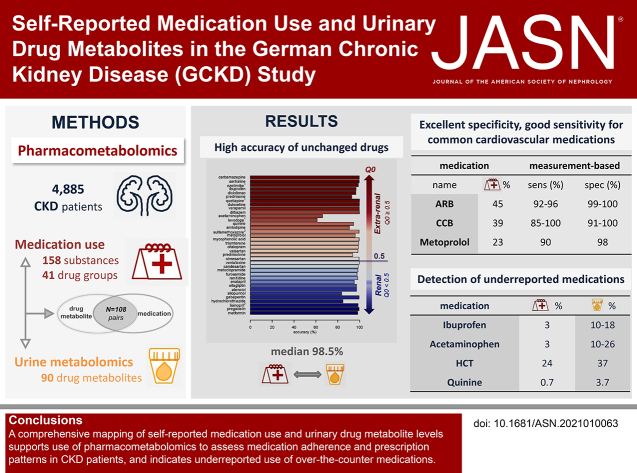
Significance Statement
Medication adherence is a well-recognized problem in the management of patients requiring polypharmacy. Pharmacometabolomics is an emerging approach that may illuminate medication use among persons with CKD. The authors evaluated self-reported use of 158 prescribed substances and 41 medication groups, along with measurements of 90 urinary drug metabolites, among 4885 individuals with CKD participating in a prospective cohort study. Accuracy and specificity were excellent, with high sensitivity for many cardiovascular drugs. Drug metabolites of over-the-counter analgesics were detected at levels higher than the self-reported use of these drugs. Pharmacometabolomics also revealed prescription patterns, including the recommended avoidance of combinations potentially causing serious adverse effects. This study generates a comprehensive resource that maps self-reported medication use and urinary drug metabolite levels, and supports the use of pharmacometabolomics for future research.
Keywords: chronic kidney disease, urine metabolites, pharmacometabolomics, medication use
Visual Abstract
Abstract
Background
Polypharmacy is common among patients with CKD, but little is known about the urinary excretion of many drugs and their metabolites among patients with CKD.
Methods
To evaluate self-reported medication use in relation to urine drug metabolite levels in a large cohort of patients with CKD, the German Chronic Kidney Disease study, we ascertained self-reported use of 158 substances and 41 medication groups, and coded active ingredients according to the Anatomical Therapeutic Chemical Classification System. We used a nontargeted mass spectrometry–based approach to quantify metabolites in urine; calculated specificity, sensitivity, and accuracy of medication use and corresponding metabolite measurements; and used multivariable regression models to evaluate associations and prescription patterns.
Results
Among 4885 participants, there were 108 medication-drug metabolite pairs on the basis of reported medication use and 78 drug metabolites. Accuracy was excellent for measurements of 36 individual substances in which the unchanged drug was measured in urine (median, 98.5%; range, 61.1%–100%). For 66 pairs of substances and their related drug metabolites, median measurement-based specificity and sensitivity were 99.2% (range, 84.0%–100%) and 71.7% (range, 1.2%–100%), respectively. Commonly prescribed medications for hypertension and cardiovascular risk reduction—including angiotensin II receptor blockers, calcium channel blockers, and metoprolol—showed high sensitivity and specificity. Although self-reported use of prescribed analgesics (acetaminophen, ibuprofen) was <3% each, drug metabolite levels indicated higher usage (acetaminophen, 10%–26%; ibuprofen, 10%–18%).
Conclusions
This comprehensive screen of associations between urine drug metabolite levels and self-reported medication use supports the use of pharmacometabolomics to assess medication adherence and prescription patterns in persons with CKD, and indicates under-reported use of medications available over the counter, such as analgesics.
Patients with CKD often develop complications—including anemia, mineral and bone disorder,1 and hypertension—and are at increased risk of cardiovascular and other diseases.2,3 The underlying cause of CKD and multimorbidity lead to the prescription of many medications, defined as polypharmacy when at least five medications per day are used.4 Polypharmacy increases the probability of adverse drug reactions and interactions, which are major causes of hospitalization and death.5–7 Little is known about the urinary excretion of many drugs and their metabolites among patients with CKD.8
A metabolomic approach may provide new insights into these knowledge gaps. Metabolomics experiments can be carried out using easily accessible body fluids (such as serum, plasma, and urine), with most studies so far having concentrated on serum or plasma.9 Urine provides additional valuable information because it captures processes such as metabolite reabsorption or secretion that occur along the nephron. Urine requires fewer sample pretreatments, and its lower complexity and protein content, as compared with blood, make it particularly suitable for metabolomics.10 Several previous studies have shown that metabolite levels in urine and their genetic basis can provide insights into the detoxification capacities of the human body.11,12
The use of pharmacometabolomics is starting to predict the effectiveness and toxicity of individual drugs, and can reveal previously unrecognized, unintentional drug effects.13 For example, additional effects of β-blockers, besides their known effects on the sympathetic nervous system, have been discovered, such as an unexpected increase of several fatty acids and the decrease of serotonin levels.14 In the setting of CKD, pharmacometabolomic studies have received little attention so far, and patients with CKD are frequently under-represented in large medication trials, despite being a high-risk patient group due to polypharmacy. An assessment of the presence of drug metabolites in the urine of patients with CKD and its relation to medication use is a necessary first step for further research in this area. Therefore, using data collected from patients with CKD enrolled in the large German Chronic Kidney Disease (GCKD) study, we aimed to comprehensively study levels of urine drug metabolites in comparison with self-reported medication use and to provide measures of agreement, to assess the statistical associations between drug metabolite levels and reported medication use, and to explore the effect of combinations of medications.
Methods
Study Population
The GCKD study is a prospective cohort study of patients with CKD.15 Details on study design and the study population have been described elsewhere.15,16 In short, the study population comprises 5217 adult patients of European ancestry under regular nephrologic care who provided written informed consent and were recruited between 2010 and 2012. The main inclusion criteria were an eGFR between 30–60 ml/min per 1.73 m2 or an eGFR >60 ml/min per 1.73 m2 in combination with urinary albumin-creatinine ratio (UACR) of ≥300 mg/g or proteinuria ≥500 mg/d. The GCKD study was approved by local ethics committees and registered in the national registry for clinical studies (DRKS 00003971).
Trained and certified personnel followed standardized questionnaires and manuals to systematically collect information, including demographic and clinical data, and a physical examination. The collected data also included information on comorbidities and medication use. Biomaterials—including serum, plasma and urine—were collected in a standardized fashion and transported frozen to a central biobank, where they were stored at −80°C for future analyses.17
An isotope dilution mass spectrometry (MS)–traceable enzymatic assay (Creatinine Plus; Roche) was used to measure serum creatinine, and eGFR (ml/min per 1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.18 UACR (mg/g) was based on urine creatinine, measured with the same assay as in serum, and urine albumin was measured with the ALBU-XS assay. Serum LDL (mg/dl) and triglycerides (mg/dl) were measured with the Cobas (Roche Diagnostics GmbH, Mannheim, Germany) LDL-Cholesterol plus second generation and the Triglyceride Assay, respectively. Blood hemoglobin A1c was measured with the TINA assay (Roche Diagnostics) and reported as a percentage (Diabetes Control and Complications Trial/NGSP units). Systolic and diastolic BPs (mm Hg) were calculated as the mean of three measures taken in an upright sitting position after 5 minutes of rest using a standardized device (M5 Professional Devices; Omron, Mannheim, Germany).
Metabolite Measurements, Quantification, Quality Control, and Data Cleaning
Metabolites were quantified from spot urine specimens that had been collected at the baseline visit, were immediately processed, and frozen at −80°C. Metabolite data were available for 5088 participants. Quantification was performed at Metabolon, Inc. (Durham, NC) using an untargeted MS approach, as described previously in more detail.19 After preparation of the samples,20,21 recovery standards were added for quality control before the first step in the extraction process. Proteins were precipitated with methanol under vigorous shaking for 2 minutes (Glen Mills Genogrinder 2000), followed by centrifugation to remove proteins and to recover chemically diverse metabolites. To ensure injection and chromatographic consistency, vacuum-dried portions of the obtained extract were dissolved in injection solvent containing at least four injection standards (depending on the platform) at predefined concentrations. The resulting extract was divided into different portions and assessed via four ultra high performance liquid chromatography–tandem MS (UPLC-MS/MS) methods: positive ionization chromatographically optimized for hydrophilic compounds, positive ionization chromatographically optimized for hydrophobic compounds, negative ionization–optimized conditions, and negative ionization with hydrophilic interaction chromatography. Chromatography resolves structural isomers. Study samples and three types of control samples (pool of human urine extensively characterized, extracted water samples, and cocktail of standards) were assessed in parallel after randomization across the platform run.22,23 All methods used a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Instruments were tuned and calibrated on a daily basis. The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion. The overall scan range covered 70–1000 m/z. The median relative SD for the 31 standards added to each sample was 5%–7%. The median relative SD of the overall process variability was 7%–9% for all >1000 endogenous metabolites (i.e., noninstrument standards) present in 100% of the pooled human urine samples.
To identify metabolites, an automated comparison of the obtained ion features against a reference library was conducted and curated by visual inspection during quality control.24,25 Metabolon’s spectral library contains >4500 purified chemical standards. Their detectable characteristics have been studied across various UPLC-MS/MS platforms.22 Metabolites reported in this study conformed to the highest confidence level of identification of the Metabolomics Standards Initiative (level 1).26,27 This includes a match to a standard of high purity on the basis of an accurate mass, a narrow retention time window, and a MS/MS fragmentation pattern. As an example, Supplemental Figure 1 shows that the MS/MS fragmentation pattern and peak intensity of hydrochlorothiazide (HCT), a commonly used diuretic in our study, perfectly matched those of its pure reference standard. Only metabolites marked with an asterisk, i.e., the minority reported here, did not yet reach level 1. For these level 2 metabolites, however, Metabolon is highly confident in the identity of the molecule because, for instance, a level 1 identification of a parent molecule (e.g., carboxyibuprofen or ranitidine) was made using a purified standard, and a modified or conjugated metabolite of these molecules (e.g., carboxyibuprofen glucuronide or ranitidine amine-oxide) was inferred on the basis of its accurate mass and MS/MS fragmentation pattern. Level 2 high confidence identifications are facilitated by accurate mass matches, correlations to known drugs/drug metabolites, and an examination of the MS/MS fragmentation pattern. Metabolites were quantified by assessing the area under the curve of respective peaks.25 All measurements were normalized to correct for variation by scaling to the median value for each run day, while preserving intersample variation.
After transfer of the resulting data from Metabolon, we performed data quality control, filtering of metabolites and samples, and correction of measurements to account for differences in urine dilution. In brief, we excluded eight duplicated samples and one sample because it did not cluster with other samples in principal component analysis. No sample showed a high proportion (>50%) of missing data. We excluded 45 metabolites that were only detected in <20 samples. To account for differences in urine dilution, the probabilistic quotient was computed on the basis of endogenous metabolites with <1% missing values.28 None of the remaining metabolites showed low variance (<0.01) or outliers (>5 SD from the mean) in >5% of the samples. The cleaned dataset contained urine levels of 1487 metabolites from 5088 GCKD study participants. The urine dilution-corrected metabolite levels were log2 transformed before statistical analysis.10
Metabolites were assigned to pathway groupings such as “amino acids” and “xenobiotics” by Metabolon. This project investigated 90 drug compounds (superpathway, “xenobiotics”; subpathways, “drug*”) passing quality control and plausibility checks (Supplemental Table 1). Drug compounds were either the parent drug itself or drug metabolites. Throughout this manuscript, the parent drug is called “unmodified drug,” and the metabolized drug compound(s) is/are called “drug metabolite(s).” No imputation of missing values was performed to retain the information when drugs and their metabolites were not detected.
Self-Reported Medication Use
Use of prescribed medications was recorded at the study baseline interview as self-reported by the participants, and validated by trained personnel using the individuals’ current medication schedule from their treating physician.4 Spot urine samples were also collected during this study visit for 98% of the study sample. All individual active ingredients contained in these medications were coded using the Anatomical Therapeutics Chemical (ATC) Classification System of the World Health Organization and are referred to as “substances” herein. Implausibilities, such as inconsistent medication names or discordances between ATC codes and medication names, were reviewed by a committee of trained medical doctors. Medication groups were defined as a set of medications that have similar chemical structures, the same mechanism of action (i.e., bind to the same biologic target), a related mode of action, and/or are used to treat the same disease. They are often reflected by a joint top level of ATC coding. Drug groups were also assigned to substances that have more than one ATC code (e.g., acetylsalicylic acid). In addition to the current use of prescribed medications, participants also answered a question about the use of pain medications (regularly, as needed, never, or unknown).
Substances were analyzed when their use was reported by at least 20 individuals. Medication groups were analyzed when used by at least 100 individuals or, for clinically important medication groups, reported by <100 individuals (methotrexate, fibrates, and contraceptives). This resulted in 158 analyzed substances (Supplemental Table 2) and 41 drug groups (Supplemental Table 3). Published data on drug metabolism and freely available cheminformatics resources (Human Metabolome Database, https://hmdb.ca; PubChem, https://pubchem.ncbi.nlm.nih.gov/; Chemical Entities of Biologic Interest, https://www.ebi.ac.uk/chebi/; DrugBank, https://www.drugbank.ca/) were used for further characterization. The extrarenal excretion (Q0) value when kidney function is normal (percentage) and medication t1/2 (hours) were obtained from the University of Heidelberg (https://dosing.de/), or, when unavailable, from original publications on the particular medication. Except for a few exceptions, all medications had a t1/2 of <24 hours. Throughout this manuscript, substances based on self-reported medications begin with a capital letter (e.g., Prednisolone), whereas measured unmodified drugs in urine start with a lower case letter (e.g., prednisolone).
To compare the proportion of self-reported analgesic use with that based on metabolite measurements, we focused on Acetaminophen (Supplemental Figure 2) and Ibuprofen (Supplemental Figure 3) and their metabolites. Acetaminophen is highly metabolized by the liver and mainly excreted via the kidney as unmodified drug and its respective metabolites (95%–98%) within 24 hours. Ibuprofen is mainly excreted as modified metabolites into the urine. Different metabolites were considered to evaluate over-the-counter (OTC) medication use due to the complexity of their metabolism.29 For Acetaminophen, we used two definitions of metabolomic-based medication use, considering the unmodified drug (4-acetamidophenol) plus its three main drug metabolites (acetaminophenyl glucuronide, acetaminophen sulfate, and acetaminophen-mercapturate): (1) unmodified drug and all three metabolites (conservative approach); and (2) unmodified drug and any two out of three metabolites. For Ibuprofen, only the three main drug metabolites (2-hydroxyibuprofen, carboxyibuprofen, ibuprofen-acyl-glucuronide) were considered to define metabolite-based medication use: (1) all three metabolites (conservative approach); and (2) any two of the three metabolites.
Medication–Drug Metabolite Pair Assignments
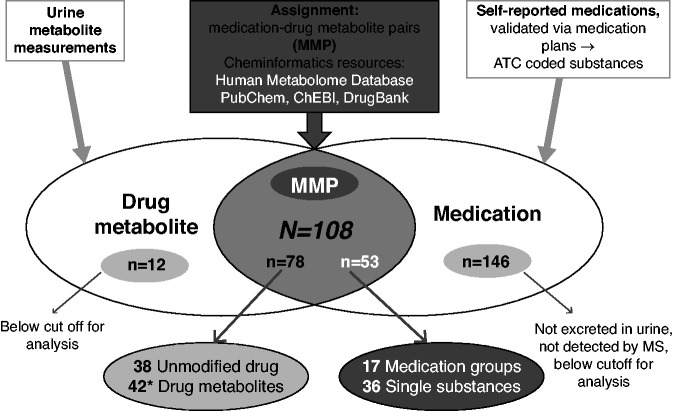
Medications (substances and medication groups) and their corresponding drug metabolites were assigned into medication–drug metabolite pairs (MMPs; Figure 1 and Supplemental Table 1). Among the 90 drug metabolites, 78 were assigned to an MMP, whereas 12 drug metabolites remained without assignment because use of the corresponding medication was not reported by at least 20 persons, the cutoff used for analysis in this project. On the medication side, 53 (36 substances and 17 medication groups) could be assigned to MMPs, whereas 146 could not be assigned to their drug metabolites because of missing or too few measurements. Overall, 108 MMPs were defined. Of these, there were 66 MMPs based on 36 individual substances and any of their metabolites, and 36 MMPs when only the unmodified drug or one individual drug metabolite were considered.
Figure 1.
Overlap of drug metabolites and self-reported medication use in the GCKD study. Overall, 90 drug metabolites and 199 medications (158 substances and 41 medication groups) were available, resulting in 108 MMPs after accounting for multiple assignments of drug metabolites to substances and medication groups and vice versa (gray area). Among 90 drug metabolites, 78 could be assigned to at least one medication (38 unmodified drugs, 42 drug metabolites*). Of the 199 medications, 53 could be assigned to metabolites (36 substances, 17 drug groups). The total of 108 MMPs was composed of 66 MMPs based on substances and 42 MMPs of medication groups. For the remaining 12 metabolites without assignment (left side), the respective medications were reported by <20 users and thus not considered further. The 146 medications not assigned to drug metabolites (right side) had missing or too few measurements. *Because prednisone and prednisolone are interconverted, both metabolites were assigned to both medications (Prednisone, Prednisolone) and thus counted twice, as an unmodified drug and as a drug metabolite. ChEBI, Chemical Entities of Biological Interest.
Statistical Analyses
The analysis was conducted using data from 4885 participants with complete information (Supplemental Figure 4) using R: A Language and Environment for Statistical Computing (version 4.0.1, https://R-project.org). Concordance between self-reported medication use and detection of urine drug metabolites (i.e., nonmissing level) was assessed using: (1) accuracy (percentage), calculated as 100%×(number of patients with concordant information on medication use and metabolite measurement/4885); (2) the robust Cohen κ measure, calculated with the R package irr (R package version 0.84.1; https://CRAN.R-project.org/package=irr; function kappa2); (3) sensitivity and specificity, using self-reported medication use as the reference (“medication-based”) and again using the detection of urine drug metabolites as the reference (“measurement-based”); and (4) via logistic regression analysis to assess the association between a drug metabolite’s detection in urine (outcome) and self-reported medication use (exposure), and to provide a measure of association in terms of odds ratio. This analysis was repeated within strata of eGFR (<45, 45–59, ≥60 ml/min per 1.73 m2), sex (male, female), and age (less than or equal to the median age, or greater than the median age) for MMPs of unmodified drugs, or of one drug metabolite when the unmodified drug was not available, when five or more observations were available in each stratum.
In addition, we conducted multivariable-adjusted linear regression analysis to evaluate the association of urine levels of drug metabolites (log2 transformed, outcome) and self-reported medication use (exposure) in more detail. When not restricting this analysis to MMPs, it allows for detecting associations that might be explained either by treatment indication (e.g., coprescription of medications for the same disease, such as antihypertensive drugs) or by unintended treatment effects. All regression models were adjusted for potential confounders known to be related to CKD status and major comorbidities,30 including demographic variables (age, sex), measures of kidney function and damage (eGFR, log[UACR]), and the following clinical chemistry-based variables related to common CKD comorbidities: systolic BP (for hypertension), log(hemoglobin A1c) (for diabetes), and LDL cholesterol and log(triglycerides) (for dyslipidemia). Because drug metabolites are only detected in some patients, linear regression was only conducted if, for a given pair of outcome and exposure, the number of measurements was >50 and the number of self-reported medication users was more than ten. For any pair not fulfilling both criteria, logistic regression analysis, as described above, was conducted instead. A Bonferroni correction was used to account for the assessment of all pairs of 199 medications (158 substances, 41 medication groups) and 90 drug metabolites (Npairs=17,910). An association was therefore considered significant if the association P value was <2.79 × 10−06 (0.05/17,910).
The interaction of reported medications on drug metabolite levels was evaluated for selected instances of unrelated MMPs with significant associations: For pairs composed of a substance and a drug forming an MMP with another substance, we first calculated residuals from a linear regression of the drug metabolite on adjustment covariates when more than ten patients used both medications and >50 measurements of the metabolite were available. Second, using the residuals as outcome, we fitted another model that contained the two medications as main effects and an interaction term of both. This approach was chosen because of concerns about limited statistical power to identify interactions on a metabolome-wide level. The interaction term was considered significant if Pinteraction<0.05.
Results
General Characteristics of Study Population
Complete baseline information and metabolite measurements were available for 4885 GKCD participants (Table 1, Supplemental Figure 4). The mean±SD age of the study population was 60.1±12.0 years, and 40% were women. The mean±SD eGFR was 49.4±18.2 ml/min per 1.73 m2 and the median (interquartile range) UACR was 50.0 (9.4–380.5) mg/g.
Table 1.
Study sample characteristics of the GCKD study patients
| Characteristic | Statistic |
|---|---|
| Sample size, n | 4885 |
| Age (yr), mean (SD) | 60.1 (12.0) |
| Female sex, n (%) | 1940 (40) |
| Body mass index (kg/m2), mean (SD)a | 29.7 (5.9) |
| Smoking, n (%)a | |
| Nonsmoker | 1985 (41) |
| Exsmoker | 2118 (43) |
| Smoker | 770 (16) |
| Systolic BP (mm Hg), mean (SD) | 139.4 (20.3) |
| eGFR (ml/min per 1.73 m2), mean (SD) | 49.4 (18.2) |
| UACR (mg/g), median (IQR) | 50.0 (9.4–380.5) |
| Serum albumin (g/L), mean (SD)a | 38.4 (4.4) |
| Triglycerides (mg/dl), median (IQR) | 167.9 (117.3–238.6) |
| LDL cholesterol (mg/dl), mean (SD) | 118.3 (43.3) |
| CRP (mg/L), median (IQR) | 2.2 (1.0–5.0) |
| Hemoglobin A1c (%), mean (SD) | 6.3 (1.0) |
| Hypertension, n (%) | 4702 (96) |
| Diabetes mellitus, n (%)a | 1724 (35) |
| CHD, n (%) | 970 (20) |
| Stroke, n (%) | 465 (10) |
| Gout, n (%) | 1181 (24) |
For a definition of hypertension, diabetes, CHD, stroke, and gout, along with the measurement of albumin and CRP, please compare previous publications.16,51 IQR, interquartile range; CRP, C-reactive protein; CHD, coronary heart disease.
Variables with missing values (<1%): body mass index, 46; smoking, 12; serum albumin, 1; diabetes mellitus, 1.
The proportion of urine samples with detectable measurements of the 90 evaluated drug metabolites ranged from 0.3% (3-hydroxyvalproate) to 58% (4-acetaminophen sulfate; Supplemental Table 1). The proportion of self-reported users of 158 substances (Supplemental Table 2) and 41 medication groups (Supplemental Table 3) ranged from 0.4% (Epoetin β, Quetiapine, Mesalazine, Propranolol) to 38% (Simvastatin) for substances, and from 1.1% (methotrexate) to 55% (β-blockers) for medication groups. The most commonly reported medication groups, each reported by >20% of the study population, were those for treating cardiovascular disease (from 24% for thiazides to 55% for β-blockers), antithrombotic agents (34% for platelet aggregation inhibitors), acetylsalicylic acid (33%), vitamin D and analogues (31%), antigout preparations (31%), proton pump inhibitors (27%), and thyroid medications (21%). At the substance level, >30% of study participants reported the use of Simvastatin (38%), Ramipril (32%), and Allopurinol (31%). Of the 158 substances, 29 are mainly excreted unmodified via the kidney (Q0<0.5), whereas 86 are mainly metabolized and/or excreted extrarenally (Q0≥0.5). For 43 substances, Q0 values were not available. t1/2 ranged from 0.4 (Isosorbide dinitrate) to 1440 (Amiodarone) hours.
MMPs To Assess Medication Adherence
MMPs Show High Accuracy
Among 90 drug metabolites, 78 could be assigned to 36 substances and 17 medication groups, thereby forming the MMPs (Figure 1). Due to multiple assignments, this resulted in 108 MMPs, consisting of 66 MMPs with substances (Supplemental Table 4) and 42 MMPs with medication groups (Supplemental Table 5).
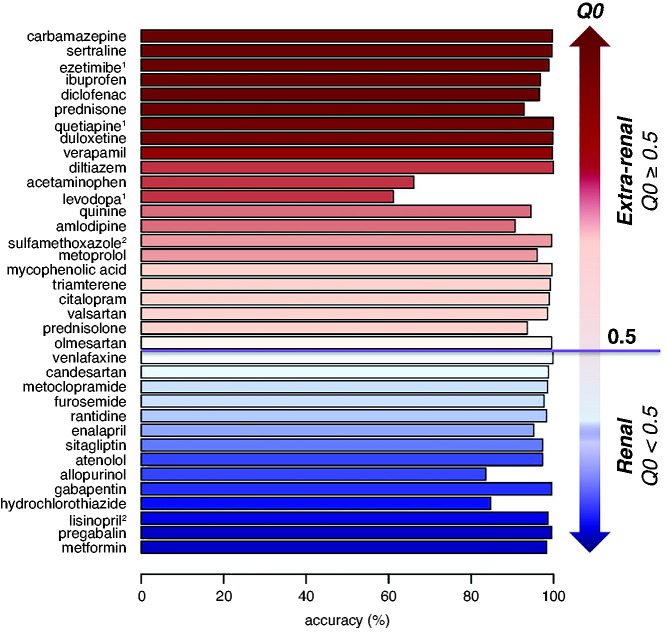
In a first step, we assessed the agreement of 108 MMPs by calculating measures of accuracy, Cohen κ, sensitivity, and specificity (Supplemental Tables 4 and 5). We examined whether accuracy was related to whether the unmodified drug was known to be excreted primarily via the kidney, given our study population of patients with CKD. The median accuracy for 66 MMPs between 36 substances and their metabolites was 97.4% (range, 43.2%–100%). The median accuracy for 36 MMPs, in which the 36 substances were assigned only to their corresponding unmodified drug (n=35, category MMP-0), or to only one drug metabolite (n=3, category MMP-1*) was 98.5% (range, 61.1%–100%). Accuracy was >80% for 34 out of the 36 substances (Figure 2). Among the 14 substances that are mainly excreted unchanged via the kidneys (Q0<0.5), all accuracy measures were >80% (Figure 2), despite potential dose reductions related to the participants’ reduced kidney function. Substances with Q0≥0.5 that are normally mainly excreted via extrarenal routes (metabolization and/or kidney-independent excretion, n=22) were still detected as unmodified drug metabolites (n=19) or modified drug metabolite (n=3) in urine. For unmodified drugs, accuracy was ≥90%, except for acetaminophen (Figure 2). Thus, drug metabolite measurements in urine were generally a highly accurate reflection of the reported use of the respective medication among the GCKD patients, regardless of whether the unmodified drug is normally mainly excreted via the kidneys.
Figure 2.
Accuracy of medication-drug metabolite pairs in relation to the fraction of extrarenal excretion of the unmodified drug. The x axis shows accuracy of 36 MMPs: 33 MMPs based on the unmodified drug (category MMP-0), and 3 MMPs based on a single drug metabolite when the unmodified drug was unavailable (category MMP-1*). Accuracy (%)=100%×(N[++]+N[–])/Ntotal; where ++ represents self-reported medication use with urine measurement, and – represents no self-reported medication use without urine measurement. Q0 values represent the fraction of extrarenal excretion when kidney function is normal (range, 0–1; https://dosing.de); that is, 1-Q0 represents the fraction of a drug that is removed unchanged via the kidneys. Color code: red represents Q0≥0.5, i.e., mainly extrarenal excretion; blue represents Q0<0.5, i.e., mainly renal elimination of unmodified drug. 1Drug metabolites (MMP-1*); 2Q0-values were obtained from a different source than the one described above (Sulfamethoxazole52,53; Lisinopril54).
MMPs Have High Specificity and Variable Sensitivity
In addition, sensitivity and specificity of all MMPs were evaluated (Supplemental Tables 4 and 5), once with respect to drug metabolite measurements and once with reported medication use as the reference. Using metabolite measurements as the reference, the median specificity of the 66 MMPs containing single substances was 99.2% (range, 84.0%–100%; Supplemental Table 4). Median sensitivity was lower (71.7%; range, 1.2%–100%), with 39 MMPs of 20 individual substances showing sensitivity <80% (Figure 3A), including several substances available as OTC medications, such as analgesics or Quinine. Among these 39 MMPs with lower measurement-based sensitivity, indicating their use was not always reported, 24 MMPs had low sensitivity as well when medications were used as the reference (Figure 3). Potential explanations for this observation are discussed below.
Figure 3.
Sensitivity and specificity of MMPs involving substances. A total of 66 MMPs, based on 36 substances and all of their available drug metabolites, were evaluated (A) using measured drug metabolites as the reference (“measurement-based”), and (B) using reported medication use as the reference (“medication-based”). MMPs with <80% sensitivity or specificity are annotated. Tables summarize the results of (A) and (B): both measurement- (meas) and medication-based (med) sensitivity and specificity ≥80% (right table) or both <80% (left table). MMPs with ≥80% specificity for both measurement- and medication-based analysis but <80% sensitivity in either are shown in the middle table. Obs., observation; Sens.: sensitivity; *Metabolon is highly confident in the identity of the metabolite. Metabolites without an asterisk conform to the highest confidence level of identification of the Metabolomics Standards Initiative (Level 1).
Using reported medication use as the reference, median specificity was 98.5% (range, 42.6%–100%), and median sensitivity was 84.7% (range, 13.6%–96.7%; Supplemental Table 4). Eight of the nine MMPs with specificity <80% were related to Ibuprofen or Acetaminophen (Figure 3B), indicating that, among participants who did not report their use, it was still sometimes measured. Similar patterns were observed when evaluating 42 MMPs of medication groups rather than MMPs of substances (Supplemental Table 5). For 23 MMPs, both measurement- and medication-based sensitivity were high (Figure 3).
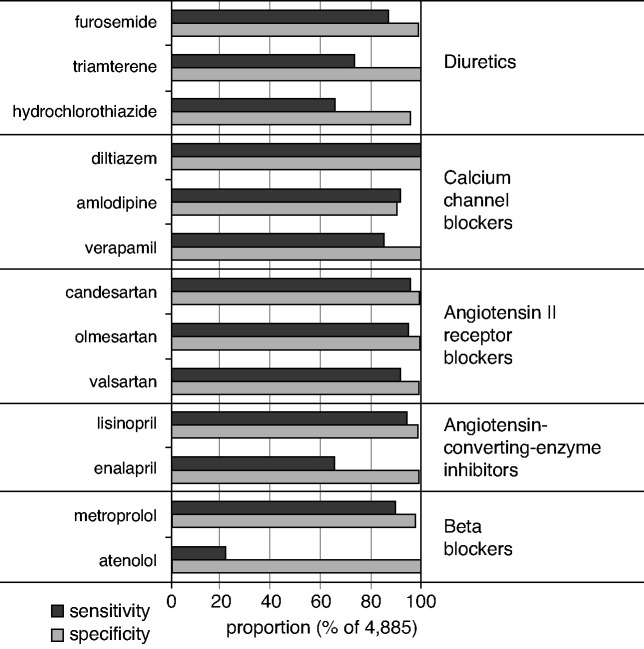
The most commonly used medication group in this CKD population involved medications for treating cardiovascular disease, particularly antihypertensive medications. Across a range of 13 antihypertensive medications with detectable drug metabolites in urine of a sufficient number of study participants, measurement-based sensitivity and specificity were >80% for nine and 13 of 13 medications, respectively (Figure 4). Sensitivity and specificity were especially high for medications commonly prescribed for BP control and cardiovascular risk reduction, including angiotensin II receptor blockers (ranges of 92%–96% and 99%–100%, respectively), calcium channel blockers (ranges of 85%–100% and 91%–100%, respectively), and metoprolol (90% and 98%, respectively). The commonly used diuretic HCT, excreted as unmodified drug in urine, showed only moderate measurement-based sensitivity of 63%. HCT was mostly (72%) prescribed as a combination therapy. The observed overdetection of HCT in urine would be consistent with a proportion of patients newly using HCT combination therapy that was not yet reflected in the prescription lists used to validate self-reported medication use.
Figure 4.
Sensitivity and specificity of cardiovascular medication-drug metabolite pairs. Sensitivity and specificity were calculated in relation to metabolite measurements as the reference. Sensitivity=100%×N(users with metabolite measurements)/N(all patients with metabolite measurements). Specificity=100%×N(nonusers without measurements)/N(all patients without measurements).
Secondary analyses across strata of sex (Supplemental Table 6) and age (Supplemental Table 7) showed similar measures of agreement: mean accuracy was 91.6% for men and 91.5% for women, and 91.7% for those aged ≤63 and 91.4% for those aged >63 years. Measures of agreement were more variable across strata of eGFR (Supplemental Table 8) for some of the MMPs (Supplemental Figure 5). Together, these findings show excellent agreement of reported medication use and drug metabolite measurements among patients with CKD under regular nephrologic care with respect to commonly prescribed medications with detectable metabolites in urine, suggesting good medication adherence.
Metabolites of Acetaminophen and Ibuprofen and Self-Reported Use of OTC Analgesics
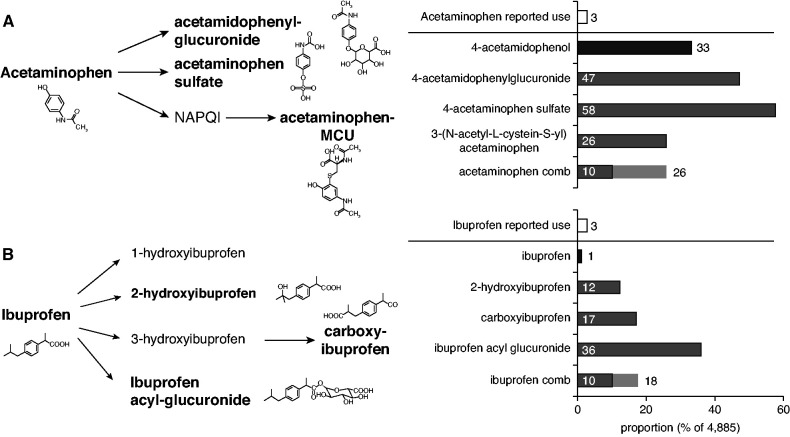
Given that OTC analgesics showed lower measures of agreement and the surprisingly small proportion of participants reporting the use of Acetaminophen (2.6%) and Ibuprofen (2.9%), we evaluated the use of OTC medications more closely. These medications are usually sold without prescription and are, hence, not included in the medication schedules used to verify reported medication use. In addition, study participants may not report the use of OTC medications. In contrast to the low proportion of self-reported use of Acetaminophen and Ibuprofen, the proportions of measured drug metabolite levels were much higher (Figure 5). For Acetaminophen, metabolomic-based medication use was estimated as 10% on the basis of the presence of the unmodified drug and all three main drug metabolites (conservative approach), and as 26% when the unmodified drug and any two of the three main metabolites were present (Figure 5A). For Ibuprofen, the corresponding rates were 10% (all three metabolites) and 18% (two of three metabolites; Figure 5B), consistent with the observed low medication-based specificity (Figure 3B). In support of the use of analgesics available OTC, we find that, among participants without prescribed analgesics but with detectable levels of their metabolites in urine, a much larger group reported that they used pain medication “regularly” or “as needed,” as compared with “never” or “unknown” (Supplemental Table 9).
Figure 5.
Metabolites of (A) Acetaminophen and (B) Ibuprofen and their proportions measured from urine of GCKD participants. Left column: Illustration of initial steps of metabolism.43 Metabolites highlighted in bold have been reported by Loo et al.42 as main drug metabolites in urine, and were used to assess analgesic use based on metabolomics. Right column: Bar plot representing proportions of reported medication use (white) and of urine measurements of unmodified drug metabolites (4-acetamidophenol) and main metabolites (black and gray, respectively). The bottom bar of each bar plot panel represents a summary measure of the measured metabolites, and is used to evaluate the use of the respective medication based on metabolomics. Acetaminophen comb, combination of unmodified drug (4-acetamidophenol) together with at least two of the three main drug metabolites (light gray), or with all three main metabolites (dark gray); Ibuprofen comb, combination of at least two of the three main drug metabolites (light gray), or combination of all three main metabolites (dark gray); MCU, mercapturate (synonym, 3-[N-acetyl-L-cystein-S-yl] acetaminophen); NAPQI, N-acetyl-p-benzoquinone.
Other analgesics were also reported and corresponding metabolites measured. Opioid use was reported by 8% of patients (Supplemental Table 3). The corresponding metabolites included tramadol, morphine, fentanyl, and oxycodone with several drug metabolites. The metabolite measurements ranged from 0.5% (fentanyl) to 3.8% (tramadol) (Supplemental Tables 1 and 9). All of these medications are mainly metabolized extrarenally with Q0≥0.5 (Supplemental Table 5) and may, therefore, not be detected in the urine. Acetylsalicylic acid was only considered as a group and not investigated in more detail because of its complex metabolization and excretion and the different dosing of the two indications as analgesic (ATC code, N02BA01) or antithrombotic (B01AC06).
Unrelated MMPs Reflect Prescription Patterns and Potential Interactions
In addition to the 108 MMPs, we assessed the associations of 17,802 pairs based on 90 drug metabolites and 199 medications that did not form an MMP. That is, the metabolites were not related to the medication of interest, i.e., not the unmodified drug itself or a metabolite thereof. The aim of this analysis was to identify associations that might arise either through coprescription of medications to treat the same disease, or through unintended effects by interactions.
Among 64 pairs with significant associations (P<2.79 × 10−06), 60 concerned a drug metabolite that formed an MMP with another medication (Supplemental Table 10). For example, Trimethoprim was associated with levels of urine N4-acetylsulfamethoxazole (P=2.1 × 10−37), the main metabolite of N4-acetylsulfamethoxazole’s MMP partner Sulfamethoxazole. Sulfamethoxazole is prescribed in a fixed combination with Trimethoprim in Germany. All users of Trimethoprim in the GCKD study indeed also used Sulfamethoxazole (Supplemental Figure 6), highlighting the complete abstraction of single substances for combination medications. In addition to this example of an association due to joint prescription, there were multiple other examples of medication coprescriptions. These included the avoidance of a combination of allopurinol and azathioprine, which can lead to serious adverse reactions; the coprescriptions of laxatives with opioids; and of angiotensin-converting enzyme inhibitors with calcium channel blockers, β-blockers, and diuretics.
When expanding the evaluation to include significant associations with more than ten users of both the reported medication and the MMP partner of a metabolite, two additional pairs were identified: Pantoprazole and Mycophenolic acid each formed a pair with N4-acetylsulfamethoxazole, the main drug metabolite of Sulfamethoxazole, the observed MMP partner. Coadministration of Sulfamethoxazole and Pantoprazole was observed in 31 patients with CKD, among whom the levels of urine N4-acetylsulfamethoxazole were highest (Supplemental Figure 7), as compared with individuals using only one of the medications. The use of Pantoprazole together with Sulfamethoxazole was associated with significantly higher urinary levels of N4-acetylsulfamethoxazole in comparison with use of Sulfamethoxazole alone (Pinteraction=0.02). The interaction analysis for Mycophenolic acid was NS (Pinteraction=0.29).
Discussion
This study represents a comprehensive overview of medication use and corresponding drug metabolite measurements in urine in a well-phenotyped CKD population under regular nephrologic care. A central finding of our study is the high agreement between reported medication use and measurement of the corresponding drug or its main drug metabolites in urine, supporting the use of pharmacometabolomics to assess medication use in patients with reduced kidney function, many of whom use five medications and more.4 Whereas specificity was excellent using either measurements or medication use as the reference, median sensitivity was more variable but still high (70%–80%). Angiotensin II receptor blockers and calcium channel blockers, common drugs used to control BP in patients at high cardiovascular risk, showed excellent sensitivity. This indicates high medication adherence in this study of participants with CKD under regular nephrologic care. Our study also shows that urine metabolite measurements indicate a significantly higher-than-reported use of OTC analgesics from the nonsteroidal anti-inflammatory drug (NSAID) group.
Medication adherence is a well-recognized problem in the management of patients with chronic diseases requiring long-term therapy.31 Nonadherence to multipharmacologic treatment among patients with CKD was found to increase the risk of morbidity, mortality, and hospitalization,32 and thereby also treatment costs.33 Traditional methods for monitoring medication adherence in clinical trials include direct measures (e.g., drug assays of blood or urine, direct observation of the patient receiving the medication) and indirect measures, such as self-reported pill count, electronic monitoring devices, and review of prescription records and claims.34 Because pill counting and electronic monitoring devices are difficult to implement in the setting of an observational study such as ours, we used both a direct measure (urine metabolite levels) and an indirect measure (self-reported, prescription record–validated medication use) to evaluate adherence. High sensitivity for drugs commonly prescribed to reduce cardiovascular risk in patients with CKD, such as several angiotensin II receptor blockers, indicated excellent adherence for these medications.
Our findings are difficult to compare with similarly comprehensive studies, because investigations across the urinary drug metabolome in patients with CKD have not been reported. A systematic review of medication adherence in CKD that included 18 studies across the range of CKD stages reported a pooled medication adherence rate of 67.4%.35 In the Reasons for Geographic and Racial Differences in Stroke study, 23.3% of participants with CKD provided an affirmative answer to at least one question indicating suboptimal adherence based on the Morisky Medication Adherence Scale.36 This study relied on self-reported adherence to prescribed treatments, without quantification of the drugs or their metabolites in biosamples such as in our study. Another review of patients with kidney failure37 reported that adherence to phosphate binders ranged between 22% and 74%, using serum phosphate as the most frequently used adherence indicator rather than drug metabolites. Urine analysis has been used previously to assess adherence to antihypertensive medications, for example in studies of pregnant women38 or patients with resistant hypertension,39 and found variable degrees of adherence. Another study, which used multiple-reaction monitoring on a triple quadrupole mass spectrometer to assess adherence to antihypertensive treatment among 208 patients, reported high total (10.1%) and partial (14.9%) nonadherence rates for 40 antihypertensive medications, defined as complete absence of any drug or of fewer drugs than prescribed, respectively.40 In our study, we reported different measures of agreement (sensitivity, specificity, accuracy), provided information for both individual substances and drug groups, and chose both drug use and metabolite measurements as the reference. The latter analysis provided important novel clues, such as the under-reporting of OTC drug use, which would not have been detected otherwise.
Using drug metabolites as the reference, we observed excellent specificity of reported drug use, with a median of 99.2% for individual substances. Although median sensitivity was good with 71.7%, 39 MMPs of 21 individual substances showed sensitivity of <80%. The lower sensitivity of these substances can be explained because they are not included in the participants’ schedules of permanently used medications and are not reported by the participants. There are several plausible explanations for such instances: firstly, medication schedules of prescribed drugs do not contain information on OTC medications, and patients may not report their use. The fact that many of the substances with evidence of under-reporting (measurement-based sensitivity <80%) were Acetaminophen, Ibuprofen, or Diclofenac and/or their metabolites supports this hypothesis. Interestingly, Quinine also belonged to this group, but an under-reported use of malaria prophylaxis in the GCKD study is unlikely. The antimalarial substance Quinine (M09AA02) is also prescribed in Germany to treat nocturnal muscle cramps, a common problem for patients with CKD.41 At the time of the study baseline visit, quinine was available as an OTC drug to treat leg cramps, thereby providing a plausible explanation. Secondly, medication schedules may not always contain medications that are prescribed for temporary use, and patients may forget to report their use. In support, Prednisone and Prednisolone, Ranitidine, Sulfamethoxazole, and Metoclopramide showed sensitivity of <80%. Third, it may be that a switch from single substances to combination medications is not yet reflected in a medication schedule, as the sensitivity of <80% for substances often part of combination drugs (such as HCT, Triamterene, Atenolol, and Enalapril) may suggest. Lastly, it is conceivable that prescriptions from other specialists may not be known to the treating nephrologists and, hence, may not be included in the medication schedules. We observed this for medications commonly prescribed by neurologists and/or psychiatrists, such as Levodopa, Sertraline, Citalopram, Carbamacepine, and Venlafaxine, which may not be reported by study participants.
For the evaluation of OTC medication use in large epidemiologic studies, direct measures of metabolic profiles are emerging.42 A previous study determined the concordance between self-reported use of NSAIDs and their plasma metabolite levels,43 similar to the accuracy measure we report. This approach proved useful for Acetaminophen (concordance, 80%–100%) but less so for Ibuprofen (concordance, 51%–100%). In another large-scale observational study, the International Study of Macro- and Micro-Nutrients, analgesic use was also evaluated by detection of metabolites in two different 24-hour urine collections.44 The reported rates were 81%–84% for concordance, 15%–17% for under-reporting, and 1% for underdetection. In contrast to the low self-reported use of Acetaminophen and Ibuprofen, with <3% each in our study, we estimated metabolomics-based use of Acetaminophen as 10%–26% and of Ibuprofen as 10%–18%, depending on the number of their metabolites to define metabolomics-based use. These proportions are comparable with findings from other cohorts in the same age group, but without CKD, in Western populations, where the rate of Acetaminophen and Ibuprofen use was reported as >16% (16%–47%).44 Recently, analgesic use in older US adults with CKD was reported as 11%–17%.45 Interestingly, the same study reported frequent opioid use (31%–42%) that increased over time and was associated with CKD progression. The lower use of opioids in our study, both at the metabolomics and reporting level, likely reflects lower opioid prescription rates in a German CKD population, but may also be influenced by differences in t1/2 and pharmacokinetics of individual opioids.46,47
The evaluation of unrelated MMPs in our study supported the accurate abstraction of combination medications and generated insights into physicians’ prescription patterns. N4-acetylsulfamethoxazole is the main drug metabolite of Sulfamethoxazole, one of the most frequently prescribed antibiotics in nephrology.48 In Germany, it is only available in fixed combination with Trimethoprim. The observation that levels of N4-acetylsulfamethoxazole were significantly associated not only with Sulfamethoxazole in an MMP, but also with Trimethoprim in an unrelated MMP, exemplifies a statistical association introduced through coprescription by indication to treat the same disease. At the same time, the fact that high levels of N4-acetylsulfamethoxazole were only detected in patients reporting the use of both substances validates the abstraction process from combination preparations into single substances in the GCKD study. Patterns of coprescriptions include both their avoidance to prevent life-threatening complications, as observed for the significant negative association between levels of the allopurinol metabolites oxypurinol and allopurinol riboside and the reported use of azathioprine, and the intended coprescription of medications, such as of Macrogol and the opioid oxycodone. Lastly, the observation that the combined use of Pantoprazole and Sulfamethoxazole was associated with significantly higher levels of urine N4-acetylsulfamethoxazole has not previously been reported. A potential explanation for such an interaction could be the induction of hepatic Sulfamethoxazole-metabolizing enzymes by Pantoprazole, leading to faster degradation of Sulfamethoxazole to N4-acetylsulfamethoxazole. However, given the relatively small number of individuals using these substances individually and jointly, this observation should be replicated in future studies of the urine drug metabolome in patients with CKD.
Strengths of our study include the large number of participants with CKD under regular nephrologic care, the standardized procedures for biomaterial collection and processing, the extensive quality control of the recorded self-reported use of prescribed medications, and validation of all medication names and composition of combination medications by a committee of trained experts. The urinary drug measurements in this large cohort of patients with CKD using a metabolomics-based approach are very comprehensive. Moreover, our study assessed both reported drug use and drug metabolite measurements as a reference when calculating measures of agreement. This led to important insights, such as the under-reporting of OTC medication use, which would not have been detected when only assessing the presence of prescribed medications in urine. Because NSAIDs, such as ibuprofen, may have several adverse effects on the kidney and further contribute to CKD progression,49 their OTC use is clinically important information for the treating nephrologists.
Potential limitations of our study include the lack of information on drug dosing and the time interval between medication use and urine collection. Because of the design of the GCKD study, our results may not be generalizable to a wider range of eGFR values, and the cohort lacks racial and ethnic diversity. Moreover, prescription patterns in Germany may not reflect those in CKD cohorts situated in other countries. The ability to link self-reported prescribed and OTC medications to comprehensive electronic health record data are not possible in an outpatient setting in Germany, and represents an area for future research in settings that allow for record linkage. Because of differences in genetic polymorphisms that influence drug metabolism by ancestry,50 pharmacometabolomics studies in more diverse study samples are an interesting line of future research. Drug metabolites were measured from spot urine, which may limit the detectability of medications with a short t1/2 when urine collection did not occur within a short time frame of medication use. Metabolite measurements from urine can only be used to evaluate drugs that are at least partially excreted via the kidneys, which limits comparability to previous studies that quantified drug metabolites from blood. In addition, not all commonly prescribed drugs, such as ramipril and bisoprolol, are currently reported by Metabolon. This can be attributed to various reasons, including the MS methods used for metabolite quantification, low abundance in urine, or the current lack of level 1 or 2 confidence to report the metabolite despite its presence in the mass spectra. Finally, some metabolites have known endogenous sources in addition to the reported drugs.
In summary, this study generates a comprehensive resource of the association between urine drug metabolite levels and reported medication use. Although untargeted MS testing to study medication use is currently not available in clinical settings, the presented results are relevant for clinicians because they highlight the substantial under-reporting of the use of OTC medications and of medications that are part of combination therapies. Pharmacometabolomics is a useful approach to assess medication use and prescription patterns in persons with CKD.
Disclosures
M.S. Becker and J. Mielke report being full-time employees of Bayer AG, Division Pharmaceuticals. K.-U. Eckardt reports having consultancy agreements with, and receiving honoraria from, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Genzyme, Otsuka, Travere, and Vifor; receiving research funding from Amgen, AstraZeneca, Bayer, Fresenius, Genzyme, Shire, and Vifor; and serving on the editorial boards of Kidney International and The BMJ. E.D. Karoly reports being a full-time employee of Metabolon, Inc. A. Köttgen reports serving as a scientific advisor for, or member of, American Journal of Kidney Diseases, American Kidney Fund, JASN, Kidney International, and Nature Reviews Nephrology; and receiving honoraria from Sanofi Genzyme. R.P. Mohney reports previously working for Metabolon, Inc., and being employed by, and having ownership interest, in Owlstone Medical Inc. P.J. Oefner reports serving on the editorial board of the oncology section of BioMed Research International, on the editorial board of BioTechniques, as the communicating editor of Human Mutation, previously as the genetic technology and methodology section editor for Investigative Genetics (BioMed Central/Springer), and on the editorial board of Metabolites. All remaining authors have nothing to disclose.
Funding
The work of A. Köttgen and M. Wuttke was funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) project identifier 431984000 – SFB 1453, and A. Köttgen was additionally funded by DFG grant KO 3598/5-1. F. Kotsis was supported by the Else Kröner-Fresenius-Stiftung (NAKSYS), and U.T. Schultheiss and F. Kotsis were supported by the Bundesministerium für Bildung und Forschung (BMBF; German Federal Ministry of Education and Research) within the framework of the e:Med research and funding concept (grant 01ZX1912B). P. Schlosser was supported by the Faculty of Medicine, Albert-Ludwigs-Universität Freiburg EQUIP Program for Medical Scientists. The GCKD study is funded by the BMBF Federal Ministry of Education and Research, grant number 01ER0804, and the KfH Foundation for Preventive Medicine. Metabolomics measurements were supported by Bayer.
Supplementary Material
Acknowledgments
We thank Dr. David Czok for the helpful discussions regarding pharmacologic aspects. We are grateful for the willingness of the patients to participate in the GCKD study. The enormous effort of the study personnel of the various regional centers is highly appreciated. We thank the large number of nephrologists who provide routine care for the patients and collaborate with the GCKD study.
A. Köttgen, P. Sekula, and F. Kotsis designed the study; K.-U. Eckardt, A. Köttgen, P.J. Oefner, U.T. Schultheiss, and F. Kotsis were responsible for recruitment, management, and conduct of the study; R.P. Mohney and E.D. Karoly were responsible for metabolite quantification; P. Sekula, F. Kotsis, and P. Schlosser were responsible for bioinformatics and statistical analysis; F. Kotsis, A. Köttgen, and P. Sekula wrote the manuscript; and all authors edited, critically read, and approved the manuscript.
Footnotes
P.S. and A.K. jointly supervised the project.
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: GCKD Investigators, Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B. Ekici, Susanne Becker, Dinah Becker-Grosspitsch, Ulrike Alberth-Schmidt, Birgit Hausknecht, Anke Weigel, Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Hermann Haller, Jan Menne, Martin Zeier, Claudia Sommerer, Johanna Theilinger, Gunter Wolf, Martin Busch, Rainer Paul, Thomas Sitter, Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021010063/-/DCSupplemental.
Supplemental Table 1. Annotation of drug metabolites and their assignment to self-reported medication use in the GCKD study.
Supplemental Table 2. Annotation of substances.
Supplemental Table 3. Annotation of medication groups.
Supplemental Table 4. Results of medication - drug metabolite pair (MMP) analysis for substances.
Supplemental Table 5. Results of medication - drug metabolite pair (MMP) analysis for medication groups.
Supplemental Table 6. Sex-stratified results of common medication - drug metabolite pair (MMP) analysis for substances.
Supplemental Table 7. Age-stratified results of common medication - drug metabolite pair (MMP) analysis for substances.
Supplemental Table 8. eGFR-stratified results of common medication - drug metabolite pair (MMP) analysis for substances.
Supplemental Table 9. Self-reported use of pain medication among participants without prescribed analgesic medications but with measured metabolites of analgesic drugs in urine.
Supplemental Table 10. Results of unrelated medication - drug metabolite pair analysis.
Supplemental Figure 1. The negative ion fragmentation pattern of hydrochlorothiazide in comparison to a pure reference standard.
Supplemental Figure 2. Map of acetaminophen metabolism and proportion of measured urine metabolites in the GCKD study.
Supplemental Figure 3. Map of ibuprofen metabolism and proportion of measured urine metabolites in the GCKD study.
Supplemental Figure 4. Project-specific selection of the study population.
Supplemental Figure 5. Distribution of drug metabolite levels of common medication - drug metabolite pairs (MMP) across eGFR categories.
Supplemental Figure 6. Distribution of levels of N4-acetylsulfamethoxazole by use of any combination of sulfamethoxazole and trimethoprim.
Supplemental Figure 7. Distribution of levels of N4-acetylsulfamethoxazole by use of any combination of sulfamethoxazole and pantoprazole.
References
- 1.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. ; Kidney Disease Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Drawz P, Rahman M: Chronic kidney disease. Ann Intern Med 162: ITC1–ITC16, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt IM, Hübner S, Nadal J, Titze S, Schmid M, Bärthlein B, et al. : Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: The German Chronic Kidney Disease study. Clin Kidney J 12: 663–672, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarou J, Pomeranz BH, Corey PN: Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279: 1200–1205, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B: Adverse outcomes of polypharmacy in older people: Systematic review of reviews. J Am Med Dir Assoc 21: 181–187, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Niclós G, Olivar T, Rodilla V: A cross-sectional evaluation of the prevalence and detection of predictors of polypharmacy amongst adult in Spain. Int J Pharm Pract 26: 242–249, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM: Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin J Am Soc Nephrol 13: 1085–1095, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wishart DS: Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev 99: 1819–1875, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Zhang, A, Sun, H, Wu, X, Wang, X: Urine metabolomics. Clin Chim Acta 414: 65–69, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, et al. ; CARDIoGRAM: Human metabolic individuality in biomedical and pharmaceutical research. Nature 477: 54–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlosser P, Li Y, Sekula P, Raffler J, Grundner-Culemann F, Pietzner M, et al. ; GCKD Investigators: Genetic studies of urinary metabolites illuminate mechanisms of detoxification and excretion in humans. Nat Genet 52: 167–176, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett JR: Pharmacometabonomics: The prediction of drug effects using metabolic profiling. Handb Exp Pharmacol 260: 263–299, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Altmaier E, Fobo G, Heier M, Thorand B, Meisinger C, Römisch-Margl W, et al. : Metabolomics approach reveals effects of antihypertensives and lipid-lowering drugs on the human metabolism. Eur J Epidemiol 29: 325–336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckardt KU, Bärthlein B, Baid-Agrawal S, Beck A, Busch M, Eitner F, et al. : The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol Dial Transplant 27: 1454–1460, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Titze S, Schmid M, Köttgen A, Busch M, Floege J, Wanner C, et al. ; GCKD study investigators: Disease burden and risk profile in referred patients with moderate chronic kidney disease: Composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 30: 441–451, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Prokosch HU, Mate S, Christoph J, Beck A, Köpcke F, Stephan S, et al. : Designing and implementing a biobanking IT framework for multiple research scenarios. Stud Health Technol Inform 180: 559–563, 2012 [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekula P, Tin A, Schultheiss UT, Baid-Agrawal S, Mohney RP, Steinbrenner I, et al. : Urine 6-bromotryptophan: Associations with genetic variants and incident end-stage kidney disease. Sci Rep 10: 10018, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E: Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81: 6656–6667, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, et al. : High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 4: 132, 2014 [Google Scholar]
- 22.Weiner J 3rd, Parida SK, Maertzdorf J, Black GF, Repsilber D, Telaar A, et al. : Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS One 7: e40221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiltunen TP, Rimpelä JM, Mohney RP, Stirdivant SM, Kontula KK: Effects of four different antihypertensive drugs on plasma metabolomic profiles in patients with essential hypertension. PLoS One 12: e0187729, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehaven CD, Evans AM, Dai H, Lawton KA: Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jump RL, Polinkovsky A, Hurless K, Sitzlar B, Eckart K, Tomas M, et al. : Metabolomics analysis identifies intestinal microbiota-derived biomarkers of colonization resistance in clindamycin-treated mice. PLoS One 9: e101267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, et al. : Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3: 211–221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA: Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom 27: 1897–1905, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieterle F, Ross A, Schlotterbeck G, Senn H: Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78: 4281–4290, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Cohen IV, Cirulli ET, Mitchell MW, Jonsson TJ, Yu J, Shah N, et al. : Acetaminophen (paracetamol) use modifies the sulfation of sex hormones. EBioMedicine 28: 316–323, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonelli M, Wiebe N, Guthrie B, James MT, Quan H, Fortin M, et al. : Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 88: 859–866, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Burnier M: Is there a threshold for medication adherence? Lessons learnt from electronic monitoring of drug adherence. Front Pharmacol 9: 1540, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mechta Nielsen T, Frøjk Juhl M, Feldt-Rasmussen B, Thomsen T: Adherence to medication in patients with chronic kidney disease: A systematic review of qualitative research. Clin Kidney J 11: 513–527, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V: Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 8: e016982, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer KC: Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 21: 1074–1090, discussion 1073, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Seng JJB, Tan JY, Yeam CT, Htay H, Foo WYM: Factors affecting medication adherence among pre-dialysis chronic kidney disease patients: A systematic review and meta-analysis of literature. Int Urol Nephrol 52: 903–916, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Muntner P, Judd SE, Krousel-Wood M, McClellan WM, Safford MM: Low medication adherence and hypertension control among adults with CKD: Data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 56: 447–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karamanidou C, Clatworthy J, Weinman J, Horne R: A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol 9: 2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster LM, Reed K, Myers JE, Burns A, Gupta P, Patel P, et al. : Quantifying adherence to antihypertensive medication for chronic hypertension during pregnancy. Pregnancy Hypertens 17: 12–14, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Pucci M, Martin U: Detecting non-adherence by urine analysis in patients with uncontrolled hypertension: Rates, reasons and reactions. J Hum Hypertens 31: 253–257, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, et al. : High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 100: 855–861, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown SA, Tyrer FC, Clarke AL, Lloyd-Davies LH, Stein AG, Tarrant C, et al. : Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J 10: 788–796, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loo RL, Coen M, Ebbels T, Cloarec O, Maibaum E, Bictash M, et al. ; INTERMAP Research Group: Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. Anal Chem 81: 5119–5129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennis KK, Carter BD, Gapstur SM, Stevens VL: Metabolomics approach for validation of self-reported ibuprofen and acetaminophen use. Metabolites 8: 55, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loo RL, Chan Q, Brown IJ, Robertson CE, Stamler J, Nicholson JK, et al. ; INTERMAP Research Group: A comparison of self-reported analgesic use and detection of urinary ibuprofen and acetaminophen metabolites by means of metabonomics: The INTERMAP Study. Am J Epidemiol 175: 348–358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han Y, Balkrishnan R, Hirth RA, Hutton DW, He K, Steffick DE, et al. : Assessment of prescription analgesic use in older adults with and without chronic kidney disease and outcomes. JAMA Netw Open 3: e2016839, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drugbank Online: Naloxone. Available at: http://www.drugbank.ca/drugs/DB01183. Accessed April 21, 2021
- 47.Drugbank Online: Tramadol. Available at: https://go.drugbank.com/drugs/DB00193. Accessed April 21, 2021
- 48.Delanaye P, Mariat C, Cavalier E, Maillard N, Krzesinski JM, White CA: Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract 119: c187–c193, discussion c193–c194, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Gooch, K, Culleton, BF, Manns, BJ, Zhang, J, Alfonso, H, Tonelli, M, et al. : NSAID use and progression of chronic kidney disease. Am J Med 120: 280.e1–280.e7, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Zanger UM, Schwab M: Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138: 103–141, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Jing J, Kielstein JT, Schultheiss UT, Sitter T, Titze SI, Schaeffner ES, et al. ; GCKD Study Investigators: Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: The German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant 30: 613–621, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N: Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 163: 402–410, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Welling, PG, Craig, WA, Amidon, GL, Kunin, CM: Pharmacokinetics of trimethoprim and sulfamethoxazole in normal subjects and in patients with renal failure. J Infect Dis 128: 556–566, 1973 [DOI] [PubMed] [Google Scholar]
- 54.Ulm EH, Hichens M, Gomez HJ, Till AE, Hand E, Vassil TC, et al. : Enalapril maleate and a lysine analogue (MK-521): Disposition in man. Br J Clin Pharmacol 14: 357–362, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.