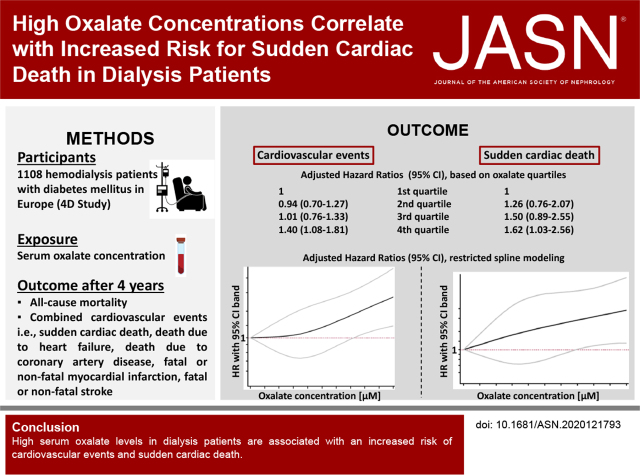
Significance Statement
Oxalate is a toxic end product of metabolism that is highly elevated in patients with kidney failure requiring chronic dialysis. Basic research has demonstrated that oxalate is a potential trigger of systemic inflammation, progression of CKD, and cardiovascular complications. The authors conducted a post-hoc analysis of the randomized German Diabetes Dialysis Study of 1255 European patients with diabetes on hemodialysis. In their analysis of 1108 patients, they found those with higher serum oxalate concentrations were more likely than those with lower levels of serum oxalate to experience cardiovascular mortality, particularly sudden cardiac death. These findings suggest oxalate-lowering therapeutic strategies might have potential for decreasing cardiovascular mortality in patients with kidney disease.
Keywords: uremic toxins, oxalate, sudden cardiac death, cardiovascular disease, chronic hemodialysis, chronic kidney failure
Visual Abstract
Abstract
Background
The clinical significance of accumulating toxic terminal metabolites such as oxalate in patients with kidney failure is not well understood.
Methods
To evaluate serum oxalate concentrations and risk of all-cause mortality and cardiovascular events in a cohort of patients with kidney failure requiring chronic dialysis, we performed a post-hoc analysis of the randomized German Diabetes Dialysis (4D) Study; this study included 1255 European patients on hemodialysis with diabetes followed-up for a median of 4 years. The results obtained via Cox proportional hazards models were confirmed by competing risk regression and restricted cubic spline modeling in the 4D Study cohort and validated in a separate cohort of 104 US patients on dialysis after a median follow-up of 2.5 years.
Results
A total of 1108 patients had baseline oxalate measurements, with a median oxalate concentration of 42.4 µM. During follow-up, 548 patients died, including 139 (25.4%) from sudden cardiac death. A total of 413 patients reached the primary composite cardiovascular end point (cardiac death, nonfatal myocardial infarction, and fatal or nonfatal stroke). Patients in the highest oxalate quartile (≥59.7 µM) had a 40% increased risk for cardiovascular events (adjusted hazard ratio [aHR], 1.40; 95% confidence interval [95% CI], 1.08 to 1.81) and a 62% increased risk of sudden cardiac death (aHR, 1.62; 95% CI, 1.03 to 2.56), compared with those in the lowest quartile (≤29.6 µM). The associations remained when accounting for competing risks and with oxalate as a continuous variable.
Conclusions
Elevated serum oxalate is a novel risk factor for cardiovascular events and sudden cardiac death in patients on dialysis. Further studies are warranted to test whether oxalate-lowering strategies improve cardiovascular mortality in patients on dialysis.
Despite the improvement of survival rates in patients with kidney failure over the last decades, excess mortality in these patients is still unacceptably high compared with the general population.1 Thus, for patients who are dialysis dependent and aged <50 years, an average loss of approximately 30 life-years has been reported.2,3 Although many traditional cardiovascular risk factors contribute to mortality in patients with kidney failure,4,5 these do not conclusively explain the pathophysiology of premature death.6,7 Nontraditional risk factors, such as uremic toxins, inflammation, and oxidative stress, directly damage the heart and blood vessels and lead to endothelial dysfunction and arterial calcification.8,9 At least 150 uremic retention solutes have been identified. However, for most of these putative toxins, biologic effects and mortality associations have not been investigated.8,9
Oxalate is a terminal metabolite and potential trigger of systemic inflammation10 and cardiovascular complications,11,12 and contributes to the progression of CKD and kidney allograft failure.13,14 Moreover, oxalate is among the 20 uremic toxins with the highest relative increase in uremia.15 Although healthy adults maintain plasma oxalate concentrations around 1–3 µM,16 concentrations in patients with kidney failure may exceed the suggested supersaturation threshold for calcium oxalate in plasma of 30 µM.17 In primary and enteric hyperoxaluria, extreme oxalate concentrations of 80–125 µM lead to systemic oxalosis, progressive CKD, and premature death.17,18 For patients with other causes of kidney failure, clinical determinants of oxalate concentrations and their influence on mortality remain imperfectly defined.19–21
We hypothesized that increased oxalate concentrations may be associated with excess mortality in patients on chronic dialysis. We tested this hypothesis by examining the relationship between oxalate concentrations and mortality and cardiovascular events in 1108 participants with kidney failure and type 2 diabetes mellitus from the German Diabetes and Dialysis (4D) Study.
Methods
Study Design and Participants
Design and methods of the 4D Study have been previously reported.22 In brief, the 4D Study was a prospective randomized controlled trial including 1255 patients with type 2 diabetes mellitus, aged 18–80 years, who had begun hemodialysis (HD) within the previous 2 years. No patient was enrolled with a known history of primary hyperoxaluria; a history of nephrolithiasis was not available. Between March 1998 and October 2002, patients were recruited in 178 dialysis centers in Europe and randomly assigned to double-blind treatment with 20 mg atorvastatin (n=619) or placebo (n=636) once daily. At each follow-up every 6 months until March 2004, blood samples were taken and clinical information was recorded, including any adverse events and an electrocardiogram. The study conforms with the principles outlined in the Declaration of Helsinki. It was approved by the medical ethics committee of the University of Würzburg, Germany, and all patients gave their written informed consent before inclusion.
Study design and methods of the cross-sectional US cohort used to investigate determinants of plasma oxalate and replicate the results of this study are detailed in the Supplemental Cohort.
Data Collection
Demographic and clinical information was obtained through patient interviews and reports from the treating nephrologists. Coronary artery disease was defined by a history of myocardial infarction, coronary artery bypass grafting surgery, percutaneous coronary intervention, or the presence of typical vascular findings by coronary angiography.
Outcome Assessment
We analyzed risk associations for different end points of the 4D Study. The primary end point of the 4D Study was a composite of cardiac death, nonfatal myocardial infarction, and fatal or nonfatal stroke, whichever occurred first (composite cardiovascular end point). Death from cardiac causes comprised sudden cardiac death, death due to congestive heart failure, fatal myocardial infarction, death due to coronary disease during or within 28 days after an intervention, and all other deaths attributable to coronary artery disease. Sudden cardiac death was defined as death verified by terminal rhythm disorders in an electrocardiogram, death observed by witnesses within 1 hour after the onset of cardiac symptoms, sudden cardiac death confirmed by autopsy, or unexpected death presumably or possibly of cardiac origin, and in the absence of a potassium level ≥7.5 mmol/L before the start of the three most recent HD sessions. Death due to heart failure was on the basis of the adjudication of detailed documentation, including hospital discharge letters, death certificates, and, in a few patients, autopsy findings by independent cardiologists and nephrologists. Myocardial infarction was diagnosed when two of the following three criteria were met: typical symptoms, elevated levels of cardiac enzymes (i.e., a level of creatine kinase type muscle-brain >5% of the total level of creatine kinase, a level of lactic dehydrogenase 1.5 times the upper limit of normal, or a level of troponin T >2 ng/ml), or diagnostic changes on the electrocardiogram. Stroke was defined as a neurologic deficit lasting >24 hours. Computed tomographic or magnetic resonance imaging was available in all but 16 patients. The 4D Study end points were centrally adjudicated and categorized by three members of the end point adjudication committee blinded to study treatment and according to predefined criteria.22 For the present analysis, all-cause mortality, composite cardiovascular end point, sudden cardiac death, death due to congestive heart failure, myocardial infarction (fatal and nonfatal), and stroke (fatal and nonfatal), were chosen as separate outcome measures, and thus include the core cardiovascular outcome measures as suggested by the Standardized Outcomes in Nephrology-Hemodialysis initiative.23
Sample Handling and Oxalate Measurement
Oxalate concentrations were measured in baseline serum samples taken 1 week before randomization, before the start of dialysis sessions and administration of drugs, and stored at −80°C. Frozen serum samples were slowly thawed, vigorously vortexed, filtered, and the filtrate was acidified with 4 μl 1N hydrochloric acid per 100 μl of serum, and measured as previously described.24 In the acidified filtrate, oxalate was measured enzymatically using oxalate oxidase (Trinity Biotech; Bray, County Wicklow, Ireland).25,26 The lower limit of detection of our assay was 2 µM.
The median coefficient of variation for repeated measurements in the stored eluate of 36 randomly selected samples was 4.1% (samples were remeasured within 2 months of the first measurement). Sodium oxalate solutions (10 and 50 µM) and two pooled patient samples (15 and 30 µM) were used as quality controls.
Statistical Analyses
We calculated means (SDs) or medians (interquartile ranges) for continuous and frequency tables for categorical variables. We compared characteristics between groups by Student’s t tests, ANOVA, Mann–Whitney U tests, or chi-squared tests where appropriate. Patient characteristics are presented in subgroups defined by quartiles of oxalate concentrations at baseline, with the following cutoff points: ≤29.6 µM, >29.6 to ≤42.3 µM, >42.3 to ≤59.6 µM, and >59.6 µM. The risk of all-cause mortality, reaching the composite cardiovascular end point, sudden cardiac death, death due to congestive heart failure, myocardial infarction, and stroke were assessed by Cox regression models considering any competing fatal events as censored observations. As sensitivity analysis to explore selection bias due to competing events, we fitted a competing risk regression model.27 Oxalate concentration was coded as restricted cubic spline model aimed to visualize the increase of risk as a function of continuous oxalate exposure. In addition, oxalate was coded as quartiles (with the first quartile being the reference group) and as a continuous variable (per doubling the concentrations and per standard deviation). First, we fitted a model including the main confounding variables, namely, age, sex, use of atorvastatin, time on HD, and use of diuretics (model 1). The variable “use of atorvastatin” addresses the original interventional study design of 4D. Diuretic prescription was used as a surrogate for residual kidney function, which was not assessed in 4D. Both dialysis vintage and residual kidney function were identified as relevant confounders for oxalate concentration in patients on dialysis in a separate cohort of 104 patients with kidney failure in the United States (see Supplemental Cohort, Supplemental Tables 1–3, and Supplemental Figure 1), and thus chosen for model 1. Second, we fitted a model additionally adjusting for other risk factors of mortality in kidney failure as recently identified by a meta-analysis,28 including C-reactive protein, body mass index, hemoglobin, albumin, and previous coronary artery disease, representing the basic markers of the inflammatory, nutritional, and cardiovascular status of patients with kidney failure (model 2—core model). To explore potential mediating pathways, we calculated another model adding NT-proBNP (model 3—mediating model). Supplemental Table 4 summarizes all adjustment models. All models were fit as complete patient analyses, so the 147 patients out of the original 1255 patients of the 4D Study in which oxalate measurement was not available were not imputed. P values are two sided. Statistical analyses were conducted using STATA (StataCorp 2017, Stata Statistical Software: Release 15, College Station, TX: StataCorp LLC).
Results
Oxalate Concentrations and Clinical Outcomes in the 4D Study
A total of 1108 patients had baseline oxalate measurements. The baseline patient characteristics are shown in Table 1. The median (IQR) oxalate concentration at baseline was 42.4 µM (30), with no significant difference between the atorvastatin and placebo group. Patients with oxalate concentrations in the highest quartile were younger, more likely to be female, had higher serum albumin, higher HDL cholesterol, higher serum phosphate, higher leukocyte counts, and higher serum creatinine (Table 1). In addition, as observed in the US cohort (see Supplemental Cohort), higher oxalate concentrations were positively associated with longer time on HD (P<0.001). Furthermore, there was a strong correlation between serum oxalate concentration and NT-proBNP (P=0.001) concentrations. Table 2 shows the total number of events per quartile at the end of the 4D Study after a median follow-up of 4.0 years in the atorvastatin arm and 4.1 years in the placebo arm. During follow-up, 548 patients died, including 139 (25.4%) who died from sudden cardiac death and 38 (6.9%) who died due to congestive heart failure. A total of 413 patients reached the primary composite cardiovascular end point with myocardial infarction (fatal or nonfatal) and stroke (fatal or nonfatal) occurring in 177 (32.3%) and 90 (16.4%) patients, respectively.
Table 1.
Characteristics of 1108 patients requiring chronic HD stratified by quartiles of oxalate concentration at baseline (4D Study)
| Characteristics | Quartile 1 ≤29.6 µM n=274 |
Quartile 2 29.7–42.3 µM n=279 |
Quartile 3 42.4–59.6 µM n=274 |
Quartile 4 ≥59.7 µM n=281 |
P valuea |
|---|---|---|---|---|---|
| Age, years | 67.6 (8.1) | 66.1 (8.3) | 66.1 (8.1) | 65.6 (8.5) | 0.03 |
| Male, n (%) | 159 (58) | 153 (55) | 148 (54) | 143 (51) | 0.41 |
| Atorvastatin treatment, n (%) | 132 (48) | 140 (50) | 137 (50) | 135 (48) | 0.95 |
| Smoking, n (%) | 30 (11) | 22 (8) | 22 (8) | 25 (9) | 0.43 |
| Systolic blood pressure, mmHg | 143 (23) | 146 (21) | 145 (21) | 147 (23) | 0.08 |
| Diastolic blood pressure, mmHg | 76 (11) | 75 (10) | 77 (11) | 76 (12) | 0.54 |
| Body mass index, kg/m2 | 27.6 (4.4) | 27.8 (5.2) | 27.0 (4.3) | 27.2 (5.0) | 0.20 |
| Duration of diabetes, yrs | 18.3 (9.1) | 17.9 (8.3) | 17.8 (8.5) | 18.4 (8.4) | 0.81 |
| Time on HD, mo | 6.4 (6.0) | 7.4 (6.2) | 9.1 (7.4) | 9.8 (7.2) | <0.001 |
| Cholesterol total, mg/dl | 215 (41) | 217 (44) | 217 (40) | 217 (36) | 0.91 |
| LDL cholesterol, mg/dl | 125 (28) | 127 (33) | 125 (29) | 126 (27) | 0.95 |
| HDL cholesterol, mg/dl | 35 (11) | 37 (14) | 37 (14) | 39 (14) | 0.004 |
| Triglycerides, mg/dl | 249 (142) | 237 (115) | 241 (124) | 228 (113) | 0.241 |
| Hemoglobin, g/dl | 10.8 (1.3) | 10.8 (1.4) | 11.0 (1.3) | 11.0 (1.3) | 0.46 |
| Albumin,g/dl | 3.77 (0.3) | 3.81 (0.3) | 3.84 (0.3) | 3.90 (0.3) | 0.001 |
| CRP, µg/ml, median (IQR) | 5.2 (8.5) | 6.1 (8.8) | 6.5 (8.3) | 7.6 (8.6) | 0.47 |
| Phosphate, mg/dl | 5.3 (1.3) | 6.1 (1.6) | 6.2 (1.5) | 6.5 (1.7) | <0.001 |
| Leukocytes, x 103/µl | 7.8 (2.3) | 8.1 (2.5) | 8.3 (2.5) | 8.2 (2.5) | 0.04 |
| HbA1c, % | 6.8 (1.2) | 6.7 (1.2) | 6.7 (1.3) | 6.7 (1.3) | 0.40 |
| ADMA, µmol/L | 0.86 (0.2) | 0.86 (0.15) | 0.87 (0.15) | 0.88 (0.17) | 0.43 |
| Creatinine, mg/dl | 5.5 (1.9) | 6.6 (2.1) | 7.5 (2.2) | 8.1 (2.1) | <0.001 |
| NT-proBNP, pg/ml | 5771.6 | 7760.5 | 9218.4 | 17745.0 | 0.001 |
| Aldosteron, ng/dl | 66.60 (120.6) | 83.00 (165.2) | 108.5 (197.6) | 123.2 (297.9) | 0.007 |
| TNF, pg/ml | 0.86 (0.2) | 0.86 (0.15) | 0.87 (0.15) | 0.88 (0.17) | 0.454 |
| Use of diuretics, n (%) | 227 (83) | 223 (80) | 206 (75) | 211 (75) | 0.60 |
| Hypertension, n (%) | 244 (89) | 243 (87) | 247 (90) | 250 (89) | 0.73 |
| Coronary artery disease, n (%) | 85 (31) | 75 (27) | 85 (31) | 79 (28) | 0.64 |
| Chronic heart failure, n (%) | 107 (39) | 98 (35) | 96 (35) | 87 (31) | 0.26 |
| Peripheral vascular disease, n (%) | 44 (16) | 46 (16) | 48 (18) | 43 (15) | 0.653 |
Continuous variables are expressed as mean (SD) or median (IQR) where appropriate, categorical variables as n (%). CRP, C-reactive protein; IQR, interquartile range; ADMA, Asymmetric dimethylarginine.
P value of ANOVA F statistic (for continuous outcomes) or Pearson chi square statistic (for categorical outcomes).
Table 2.
Number of events at the end of follow-up of the 4D Study stratified by quartiles of oxalate concentration at baselinea
| Events | Quartile 1 ≤29.6 µM n=274 |
Quartile 2 29.7–42.3 µM n=279 |
Quartile 3 42.4–59.6 µM n=274 |
Quartile 4 ≥59.7 µM n=281 |
|---|---|---|---|---|
| All-cause mortality (n=548) | 121 | 142 | 134 | 151 |
| Cardiovascular eventsb (n=413) | 93 | 97 | 91 | 132 |
| Sudden cardiac death (n=139) | 26 | 35 | 37 | 41 |
| Death due to heart failure (n=38) | 7 | 8 | 9 | 14 |
| Myocardial infarction (n=177) | 39 | 44 | 37 | 57 |
| Stroke (n=90) | 25 | 16 | 17 | 32 |
The median follow-up was 4.0 years in the atorvastatin arm and 4.1 years in the placebo arm.
Combined cardiovascular events were defined as a composite of death from cardiac causes, fatal or nonfatal stroke, and nonfatal myocardial infarction, whichever occurred first. Death from cardiac causes comprised death due to congestive heart failure, sudden cardiac death, fatal myocardial infarction, death due to coronary artery disease during or within 28 days after an intervention, and all other deaths attributable to coronary artery disease.
Oxalate and Mortality Risk
In a model examining oxalate as quartiles in the 4D Study, higher oxalate concentrations were associated with a tendency toward an increased risk of all-cause death. Patients in the highest oxalate quartile had a 23% increased risk of death compared with the lowest quartile; however, the lower bound of the 95% confidence interval (95% CI) did cross below 1 (hazard ratio [HR], 1.23; 95% CI, 0.97 to 1.56, P=0.08; Figure 1).
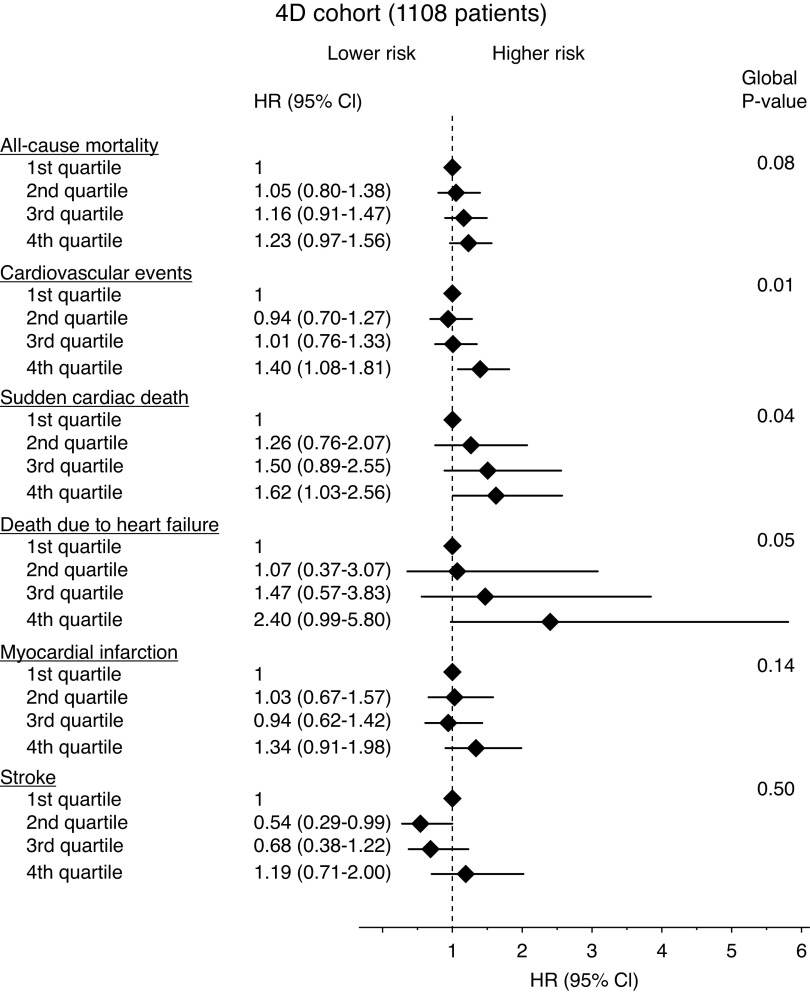
Figure 1.
Oxalate concentrations and the risk of mortality/cardiovascular events in 1108 patients (4D Study cohort) with kidney failure requiring chronic HD (Forest plot). Multivariable-adjusted HRs and 95% CIs (error bars) for all-cause mortality, combined cardiovascular events, sudden cardiac death, death due to heart failure, myocardial infarction, and stroke in the 4D Study cohort stratified by oxalate concentrations at baseline (quartiles). Models are adjusted for age, sex, use of atorvastatin, time on HD, use of diuretics, C-reactive protein, body mass index, hemoglobin, albumin, and previous coronary artery disease (core model).
Combined Cardiovascular Events and Sudden Cardiac Death
The incidence of combined cardiovascular events, the primary composite outcome of the 4D Study, was markedly higher at higher oxalate concentrations (Figure 1). Patients in the highest oxalate quartile had a 40% increased risk to reach the combined end point after adjustment for the main confounders for oxalate concentration in patients on dialysis, and markers of the inflammatory, nutritional, and cardiovascular status (model 2, see Methods and Supplemental Table 4) (adjusted HR, 1.40; 95% CI, 1.08 to 1.81, P=0.01; Figure 1 and Supplemental Table 5).
The risk of sudden cardiac death as a separate outcome measure was found to increase incrementally and significantly with higher oxalate concentrations (Figure 1). Patients in the highest quartile exhibited a 62% higher risk of sudden death (adjusted HR, 1.62; 95% CI, 1.03 to 2.56, P=0.04) compared with those in the lowest quartile (Figure 1 and Supplemental Table 5). To investigate potential intermediate pathways, we additionally adjusted our analyses for NT-proBNP, and observed a mild attenuation of the association (adjusted HR, 1.51; 95% CI, 0.94 to 2.43) for the highest compared with the lowest quartile (model 3).
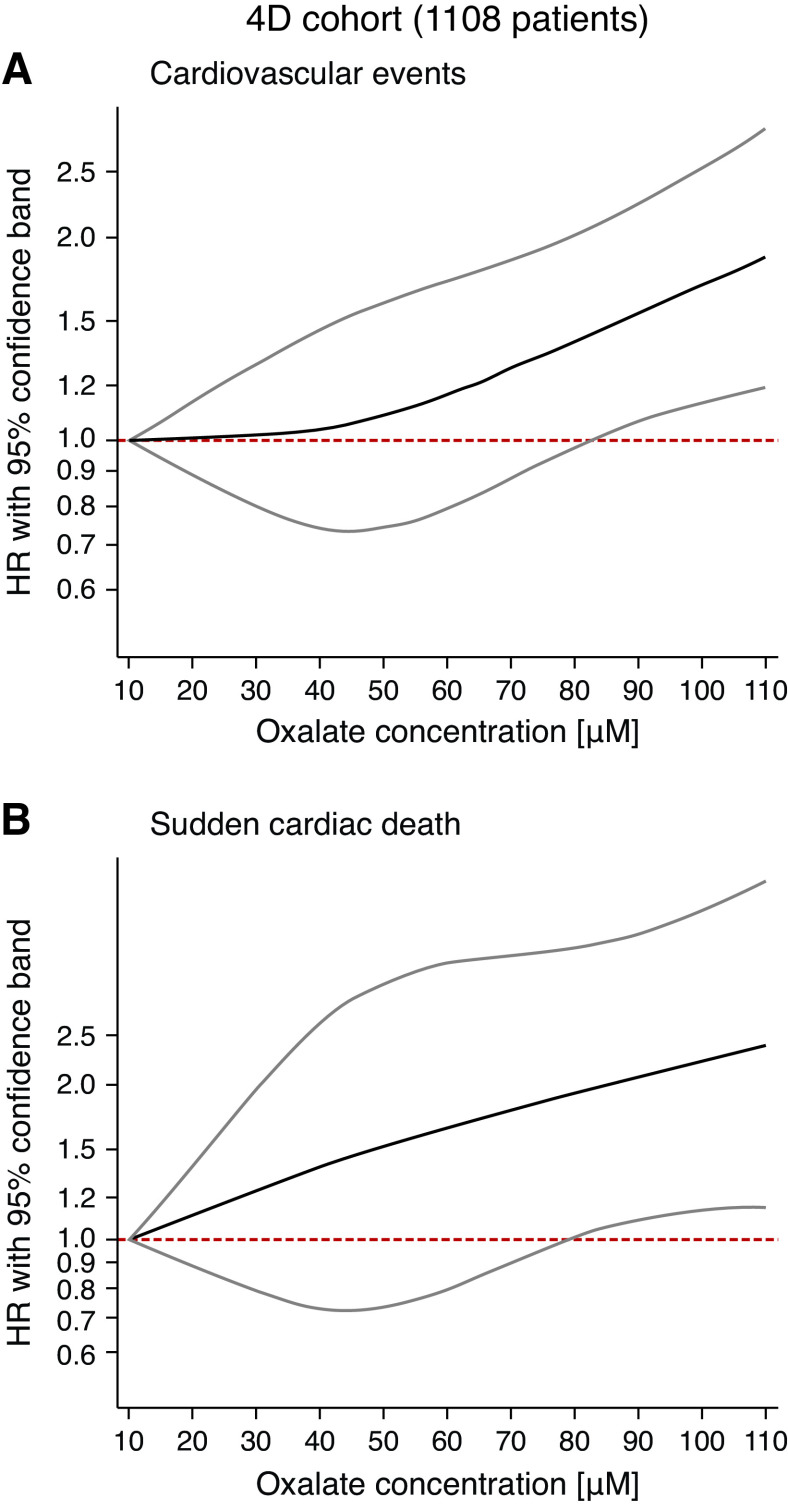
Restricted cubic splines modeling oxalate as a continuous variable confirmed our finding of incrementally increasing risk for cardiovascular events and sudden cardiac death with higher oxalate concentrations (Figure 2, A and B). The risk for sudden cardiac death increased almost linearly (adjusted HR, 1.22; 95% CI, 0.98 to 1.51, per doubling concentrations, and HR, 1.12; 95% CI, 1.00 to 1.26, per SD increase, respectively, Figure 2B). Figure 2A demonstrates that the risk for combined cardiovascular events remained relatively stable for lower oxalate concentrations and started to rise constantly above a cutoff of about 40 µM.
Figure 2.
Risk of (A) combined cardiovascular events and (B) sudden cardiac death as a function of continuous oxalate exposure in the 4D Study (restricted splines). Risk was assessed using restricted cubic spline modeling, and adjusted for age, sex, use of atorvastation, time on dialysis, use of diuretics, C-reactive protein, body mass index, hemoglobin, albumin, and previous coronary artery disease (n=1108). The black line represents the HR, the grey lines indicate the upper and lower 95% CIs. The pink dashed line marks an HR of 1.0.
Death Due to Heart Failure, Myocardial Infarction, and Stroke
Higher baseline oxalate concentrations were also associated with a tendency toward an incrementally higher risk of death due to congestive heart failure, suggesting a more than two-fold increased risk of death due to heart failure for patients in the highest quartile compared with patients in the lowest quartile (adjusted HR, 2.40; 95% CI, 0.99 to 5.80, P=0.05; Figure 1 and Supplemental Table 5).
Associations between serum oxalate and myocardial infarction individually were consistent with the overall findings for the composite events and sudden cardiac death, but the associations were weaker and were not statistically significant alone; no consistent signal was observed for stroke as a separate outcome (Figure 1 and Supplemental Table 5).
Corresponding restricted cubic splines showing the risk for death due to heart failure, myocardial infarction, and stroke as a function of continuous oxalate exposure are shown in the Supplemental Figure 2.
Competing Risk Analysis
Competing risk models fitted for all chosen 4D Study outcomes separately and for a new combined composite outcome (sudden cardiac death or death by cardiac heart failure because we assume both events share a common risk) showed the same direction and magnitude of association with the quantile-based oxalate cutoff points (Supplemental Table 6). This indicates that bias induced by neglected competing risk pathways is unlikely. For stroke as a separate outcome, competing risk models did not show any evidence for an effect on this end point (Supplemental Table 6), suggesting the reduced HRs in oxalate quartiles 2 and 3 in the cause-specific hazards models were likely due to a competing risk effect (Figure 1 and Supplemental Table 5).
Validation in the Supplemental US Cohort
To validate whether our findings could also be replicated in a different cohort, we analyzed mortality in the separate US-based cohort, which we had used to investigate determinants of oxalate concentration in patients on dialysis. As shown in Supplemental Table 1, 15 of 104 patients on dialysis died after a follow-up of 2.5 years. Patients with oxalate concentrations in the highest quartile had a trend to higher mortality rates compared with patients in quartiles 1–3 (HR, 2.23; 95% CI, 0.79 to 6.26, P=0.13, Supplemental Figure 3A). This trend became even more robust, when concentrating on patients with even higher oxalate concentrations: patients with oxalate concentrations in the highest quintile had a more than three-fold increased mortality compared with patients with oxalate concentrations in quintiles 1–4 (HR, 3.06; 95% CI, 1.09 to 8.61, P=0.03, Supplemental Figure 3B).
Discussion
In a well-established cohort of patients with kidney failure who were dialysis dependent, we found that high serum oxalate concentrations were associated with an increased risk of experiencing a cardiovascular event and dying of sudden cardiac death. These associations were robust after adjusting for a comprehensive panel of covariates, including treatment with statins as the interventional measure in the 4D Study, and accounting for competing risks in a sensitivity analysis.
Sudden cardiac death is the single largest cause of death on dialysis and accounts for 22%–26% of all-cause mortality in kidney failure.4,22,29 Reduced left ventricular ejection fraction due to ischemic cardiomyopathy and proarrhythmic triggers, such as rapid electrolyte shifts during dialysis sessions, are associated with sudden cardiac death.7,30 In contrast, coronary artery disease and heart failure do not correlate with the rate of sudden death in patients with CKD.31,32 Consequently, in kidney failure, alternative mechanisms, such as myocardial fibrosis or microvessel disease, are believed to play a major pathophysiological role.7,33 Our results suggest oxalate concentrations might be mechanistically involved, which is in agreement with several recent studies that report an association between oxalate and inflammation, fibrosis, and altered hemodynamic parameters.10–12,34 The oxalate concentrations in our patients on dialysis with excess mortality risk exceeded the suggested threshold of 30 µM for supersaturation of calcium oxalate, although the absolute values of oxalate concentration in the 4D Study are presumably higher than expected as a result of the preanalytical conditions (see below).35 Thus, one might hypothesize that formation and deposition of calcium-oxalate microcrystals can lead to cardiovascular stiffening, endothelial dysfunction, and particularly cardiac conduction abnormalities, providing a possible pathophysiological link between oxalate, compromised cardiac function, and sudden death.11,36–38 This is in line with recent studies suggesting that bradyarrhythmias leading to sudden death are more common in prevalent patients on dialysis than tachyarrhythmias.39 Despite this, our analysis only provides a statistical correlation between oxalate and sudden cardiac death, and does not allow any biologic or causal conclusions.
Dialysis vintage and residual kidney function were identified as two independent and relevant confounders of oxalate concentration in a supplemental cohort. Because residual kidney function is an important predictor of overall survival in patients with kidney failure and decreases with time on dialysis,40,41 one might argue that the association of elevated oxalate concentrations and death simply reflects the loss of residual kidney function over time. One might even suggest oxalate might solely be a marker of loss of kidney function as a result of gradual loss of cardiac function. However, all subjects of the 4D Study had been receiving maintenance HD for <2 years (mean duration of 8 months) at the time of inclusion.22 Hence, the proportion of patients with residual kidney function, reflected by the variable ‘use of diuretics’, was high (>75%). As shown in Table 1, it did not differ between oxalate quartiles. Similarly, congestive heart failure as a pre-existing condition was also equally distributed among oxalate quartiles. These considerations contradict the assumption that serum oxalate concentration may solely represent a marker for residual kidney function or poor cardiac function in the 4D cohort.
After adjusting for these two critical confounders, time on dialysis and “use of diuretics” as a surrogate marker of kidney function, the observed associations remained. Results were further adjusted for treatment with atorvastatin, to address the original interventional study design, and for the main risk factors of mortality in patients with kidney failure according to a recent meta-analysis, representing the basic markers of the inflammatory, nutritional, and cardiovascular status of patients with kidney failure.28 Our multivariate models indicated evidence for a dose-response relationship: for the two end points that were consistently related to oxalate exposure, namely cardiovascular events and sudden cardiac death, our multivariate models showed a trend of increasing risk both over continuously increasing and over quartiles of exposure. By adjusting all potential confounding variables and accounting for competing risks, this exposure-risk pattern became even more robust. The decrease of the effect estimates after adjustment to NT-proBNP suggests a role for this marker as a potential mediator of oxalate action.
The results of our primary model suggest a trend of increased risk of death from heart failure, but the number of total events was too small (n=38) to provide reliable statistical power. When conducting competing risk regression, we added a new composite end point of sudden cardiac death and heart failure, because both share some commonalities in terms of risk profile. Associations of oxalate concentrations with the new composite end point were consistent with the association observed for both outcomes individually. However, the difficulties of adjudicating heart failure in patients with kidney failure, the nature of our study, and the complex relationship between oxalate, kidney function, and heart failure42 do not allow for conclusions regarding causality and/or directionality and deserve more distinct scrutiny in separate studies.
In the 4D Study, oxalate concentrations were higher than expected from previous studies25 or from the data obtained in our separate US cohort. This finding can be explained by the preanalytical handling of the 4D Study samples: oxalate concentrations were measured in serum samples that had been stored at −80°C for almost 20 years. Spontaneous generation of oxalate might have occurred before freezing, which may cause a tendency to higher oxalate results, compared with immediate processing.24 Our results on the association of serum oxalate with cardiovascular mortality should not be biased, assuming the relative rank order remained intact. In addition, all patients in the 4D Study cohort had type 2 diabetes mellitus. Several studies have reported an association between diabetes mellitus and increased urinary oxalate excretion, or calcium oxalate deposition in kidney allografts.13,14,43 This is highly suggestive of alterations in oxalate homeostasis in patients who are diabetic and, hence, might also contribute to the observation of higher oxalate concentrations in the 4D Study.
In the 4D Study cohort, no patient was enrolled with a known history of primary hyperoxaluria. Although it cannot be excluded that some patients may have suffered from this rare genetic disorder and have remained undiagnosed, the mean age of 64 years and known duration of type 2 diabetes mellitus of 18 years in this study make this unlikely.22 Similarly, a history of primary or secondary hyperoxaluria was an exclusion criterion for the US study. Information in the history of nephrolithiasis was not available in the 4D Study data. In the US cohort, only three patients reported previous nephrolithiasis, and only one of them was in the group with the highest oxalate concentration.
Validating the identified associations in a well-established cohort and a supplemental cohort with varying dialysis patterns, practices, and ethnicities is a critical strength of our study. Results remained robust implementing different statistical methods. Nonetheless, several limitations should be considered in interpreting our findings. Our study in the 4D Study cohort was a post-hoc analysis of an interventional trial within a selected cohort of patients who were diabetic and residing in Europe. Hence, results might not be generalizable. The number of adjustments was only modest but deliberately chosen on the basis of the determinants of oxalate concentrations established in the supplemental US patient cohort. In the 4D Study, all patients were diabetic, which is itself a strong risk factor for mortality in patients on dialysis and might have an effect on oxalate homeostasis, as discussed above.28 Thus, an extended analysis in a more general population of patients who are diabetic and nondiabetic might help to define the role of oxalate in kidney failure and/or diabetes mellitus more specifically. Because residual kidney function was not assessed in the 4D Study, we cannot exclude that oxalate may solely represent a surrogate marker for residual kidney function. Finally, the small patient number in our supplemental validation cohort places constraints on the complexity and power of statistical models.
Randomized prospective interventional trials are necessary to determine if active reduction of oxalate levels efficiently lowers the risk for sudden cardiac death and other cardiovascular events in patients on dialysis. Two common parameters of nutritional status, creatinine and albumin,44 were positively correlated with higher oxalate concentrations in the 4D Study cohort. This may point to novel strategies including avoidance of foods high in oxalate content to lower serum oxalate concentration and cardiovascular mortality in patients on dialysis. Further potential treatment options might involve new pharmacological advances as administration of orally available oxalate decarboxylase enzyme, and even silencing RNA technology to reduce liver production of oxalate.45,46
In summary, this analysis of a well-established cohort of patients who were dialysis dependent shows elevated serum oxalate concentrations are associated with adverse clinical events in kidney failure. Our findings are consistent with a large body of clinical and preclinical evidence suggesting oxalate is a uremic toxin. Oxalate lowering strategies in patients on dialysis are warranted to test whether our findings are causal in nature and could represent a novel treatment paradigm.
Disclosures
A. Pfau reports receiving personal fees from Alnylam Pharmaceuticals, outside the submitted work; reports being an employee of the nonprofit institute Charité-Universitätsmedizin Berlin, which recently filed a patent application for oxalate-lowering agents in patients on dialysis, with A. Pfau listed as one of two inventors; the patent has not yet been granted, A. Pfau has not received any inventor's compensation to date, and exploitation activities are still pending. C. Drechsler reports receiving research funding from Genzyme. C. Wanner reports receiving personal fees from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Eli-Lilly, Fresenius Medical Care, GlaxoSmithKline, MSD, Mundipharma, Reata, Shire-Takeda, and Tricida, and grants from Boehringer Ingelheim, Idorsia, and Sanofi-Genzyme, outside the submitted work; reports having consultancy agreements with Gilead and Vifor; reports receiving honoraria from Astellas; and reports other interests/relationships with European Renal Association-European Dialysis and Transplant Association. F. Finkelstein reports employment with Metabolism Associates; and reports receiving research funding from Fresenius Medical Care. F. Knauf reports receiving personal fees from Advicenne, Allena Pharmaceuticals, Alnylam Pharmaceuticals, Fresenius Medical Care, Oxthera Pharmaceuticals, and Sanofi Pharmaceuticals outside the submitted work; reports having consultancy agreements with Chinook Pharmaceuticals USA; reports receiving honoraria from ECoR1 and Medice; reports having patents and inventions with PocketDoktor Medical Books; and reports being a scientific advisor or member of the Scientific Advisory Board of Oxalosis and Hyperoxaluria Foundation in New York. K.-U. Eckardt reports receiving personal fees from Akebia, Johnson&Johnson, and Retrophin; reports receiving grants from Amgen, AstraZeneca, Fresenius, and Shire; and reports receiving grants and personal fees from Bayer, Genzyme, and Vifor during the conduct of the study; reports being a scientific advisor or member of the Editorial Boards of Kidney International and BMJ and the Scientific Advisory Boards of Swiss National Science Foundation, German Research Foundation, and Kidney Disease: Improving Global Outcomes (KDIGO) (member of the Board of Directors). M. Tio reports receiving research funding from the American Kidney Fund. P. Aronson reports receiving honoraria from various universities and academic societies for seminars and lectures; reports patents and inventions via royalties for antitransporter monoclonal antibodies used in research; and reports being a scientific advisor or member with Editorial Board of the American Journal of Physiology, and Editorial Board of Function. S.G. Coca reports receiving personal fees from 3ive, Bayer, Boehringer-Ingelheim, CHF Solutions, inRegen, Quark, Relypsa, Takeda, and grants, personal fees, and other from RenalytixAI, outside the submitted work; reports consultancy agreements with Akebia; reports having an ownership interest in pulseData; reports patents and inventions with RenalytixAI; reports being a scientific advisor or member of RenalytixAI; and other interests/relationships as Associate Editor for Kidney360, Editorial Board CJASN, JASN, and Kidney International. S. Waikar reports receiving personal fees from Allena Pharmaceuticals, Bain, Barron and Budd (vs Fresenius), Biomarin, Bunch and James, Cerus, CVS, GE Health Care, GlaxoSmithKline, Harvard Clinical Research Institute (aka Baim), JNJ, Kantum Pharma, Mallinckrodt, Mass Medical International, Metro Biotechnology, Oxidien, Pfizer, Public Health Advocacy Institute, Regeneron, Roth Capital Partners, Sironax, Strataca/3ive, Takeda, Venbio, and Wolters Kluewer outside the submitted work; reports having consultancy agreements with Allena; reports receiving research funding from Vertex; reports being a scientific advisor or member of Kantum (scientific advisory board); and reports other interests/relationships as an expert witness for litigation involving cisplatin toxicity, and litigation related to DaVita lab testing, Fresenius product Granuflo, Gilead product tenofovir, and GE product Omniscan. W. März reports current employment with Synlab Holding Deutschland; reports consultancy agreements with Abbott Diagnostics, Akzea Therapeutics, Amgen, Amryt Pharmaceuticals, Novartis, and Sanofi; reports receiving research funding from Abbott Diagnostics, Akzea Therapeutics, Amgen, Amryt, BASF, Bayer Vital, bestbion dx, Boehringer Ingelheim, Daiichi Sankyo, Immundiagnostik, Sanofi, Siemens Healthineers, and Novartis; reports receiving honoraria from Abbott Diagnostics, Akzea Therapeutics, Amgen, Amryt Pharmaceuticals, Novartis Pharma, Sanofi, and Vifor Pharma; reports being a scientific advisor or member with European Heart Journal; reports speakers bureau with Abbott Diagnostics, Akzea Therapeutics, Amgen, Amryt Pharmaceuticals, Novartis Pharma, Sanofi, and Vifor Pharma; reports being employed by Synlab Holding Deutschland during the conduct of the study; reports receiving grants from Siemens Healthineers, grants and personal fees from Abbott Diagnostics, Aegerion Pharmaceuticals, Akzea Therapeutics, Amgen, Amryt Pharmaceuticals, AstraZeneca, BASF, Bayer Vital, Berlin-Chemie, bestbion dx, Boehringer Ingelheim Pharma, Immundiagnostik, Merck Chemicals, Numares, Novartis Pharma, Olink Proteomics, Sanofi, and Vifor Pharmaoutside the submitted work. All remaining authors have nothing to disclose.
Funding
This study was supported by Deutsche Forschungsgemeinschaft grants 394046635—SFB 1365, and the Oxalosis and Hyperoxaluria Foundation (to F. Knauf and K.-U. Eckardt) and National Institutes of Health grant DK33793 (to P. Aronson). The US study received Renal Research Institute grant support.
Supplementary Material
Acknowledgments
The authors thank all patients who participated in the US study and in the German Diabetes and Dialysis Study. We would like to thank Prof. Fred Luft for careful review and suggestions to improve our manuscript. We also thank all investigators, physicians, study nurses, and collaborators involved in patient care, recruitment, sample, and data handling, and the laboratory staff at the Universities of Freiburg, Würzburg, Erlangen, and Berlin (Charité). We thank A. Schäfer for their support in setting up the enzymatic oxalate assay and the Metabolism Associates team in New Haven, in particular M. Cimini, for their extensive administrative assistance. Dr. T. Ermer was recipient of a TRENAL medical student scholarship (a thematic network grant) from the Deutscher Akademischer Austauschdienst. Dr. M. Tiós work is supported by the American Kidney Fund’s Clinical Scientist in Nephrology Fellowship, which is funded with thanks in part to Amgen and the Hearst Foundation. The funders had no role in study design, collection, analysis, and interpretation of data, writing the report, and the decision to submit the report for publication. S.G. Coca, F. Knauf, and A. Pfau designed the study; T. Ermer and A. Pfau performed the measurements; C. Drechsler, T. Ermer, A. Pfau, and C. Wanner collected and provided data; B. Genser and A. Pfau performed statistical analyses. P. Aronson, K.-U. Eckardt, F. Finkelstein, and F. Knauf supervised experiments; S.G. Coca, C. Drechsler, B. Genser, F. Knauf, A. Pfau, M. Tio, and S. Waikar interpreted the data; C. Drechsler, T. Ermer, and A. Pfau wrote the paper; P. Aronson, F. Knauf, and S. Waikar revised the paper; all authors approved the manuscript. A. Pfau and F. Knauf declare that elements of this publication are also contents of a European patent application that has been filed recently.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020121793/-/DCSupplemental.
Supplemental Cohort (US cohort). Study design and participants, data collection, sample handling and oxalate measurement, statistical analyses.
Supplemental Table 1. Characteristics of 104 US patients with kidney failure requiring chronic dialysis (US cohort).
Supplemental Table 2. Correlation between oxalate concentration and clinical parameters in 104 US patients with kidney failure requiring chronic dialysis (US cohort).
Supplemental Table 3. Multivariable regression of oxalate concentration, age, BMI, Kt/V, time on dialysis, and residual kidney function in 104 US patients with kidney failure requiring chronic dialysis (US cohort).
Supplemental Table 4. Adjustment models used in the 4D Study.
Supplemental Table 5. Risk of all-cause mortality, combined cardiovascular events, sudden cardiac death, death due to heart failure, myocardial infarction, and stroke, using cause-specific hazards models, stratified by quartiles of oxalate concentration at baseline (4D Study, n=1108).
Supplemental Table 6. Risk of combined cardiovascular events, sudden cardiac death, death due to heart failure, and the composite outcome of both, myocardial infarction, and stroke, using subdistribution hazard models, stratified by quartiles of oxalate concentration at baseline (4D Study, n=1108) (Fine–Gray competing risk regression).
Supplemental Figure 1. Oxalate concentration and its correlation with residual kidney function in 104 US patients with kidney failure requiring chronic dialysis (US cohort).
Supplemental Figure 2. Risk of (A) all-cause mortality, (B) death due to heart failure, (C) myocardial infarction, and (D) stroke as a function of continuous oxalate exposure in the 4D Study (restricted splines).
Supplemental Figure 3. Kaplan–Meier curves for overall survival in 104 US patients with kidney failure requiring chronic dialysis (US cohort) comparing (A) the highest oxalate quartile versus quartiles 1–3 and (B) the highest oxalate quintile versus quintiles 1–4.
References
- 1.Foster BJ, Mitsnefes MM, Dahhou M, Zhang X, Laskin BL: Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin J Am Soc Nephrol 13: 91–99, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Walraven C, Manuel DG, Knoll G: Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 63: 491–499, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. : US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 75[Suppl 1]: A6–A7, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. ; HEMO Study Group: Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, et al. ; EVOLVE Trial Investigators: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 6.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. : Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Makar MS, Pun PH: Sudden cardiac death among hemodialysis patients. Am J Kidney Dis 69: 684–695, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanholder R, Fouque D, Glorieux G, Heine GH, Kanbay M, Mallamaci F, et al. ; European Renal Association European Dialysis; Transplant Association (ERA-EDTA) European Renal; Cardiovascular Medicine (EURECA-m) working group: Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Endocrinol 4: 360–373, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Lisowska-Myjak B: Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract 128: 303–311, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, et al. : NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulhan B, Turkmen K, Aydin M, Gunay M, Cıkman A, Kara M: The relationship between serum oxalic acid, central hemodynamic parameters and colonization by oxalobacter formigenes in hemodialysis patients. Cardiorenal Med 5: 164–174, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulay SR, Eberhard JN, Pfann V, Marschner JA, Darisipudi MN, Daniel C, et al. : Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am J Physiol Renal Physiol 310: F785–F795, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palsson R, Chandraker AK, Curhan GC, Rennke HG, McMahon GM, Waikar SS: The association of calcium oxalate deposition in kidney allografts with graft and patient survival. Nephrol Dial Transplant 35: 888–894, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waikar SS, Srivastava A, Palsson R, Shafi T, Hsu CY, Sharma K, et al. ; Chronic Renal Insufficiency Cohort study investigators: Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern Med 179: 542–551, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, et al. ; European Uremic Toxin Work Group: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DM, Liedtke RR: Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference: Reference values and sample handling procedures. Clin Chem 37: 1229–1235, 1991 [PubMed] [Google Scholar]
- 17.Ogawa Y, Machida N, Ogawa T, Oda M, Hokama S, Chinen Y, et al. : Calcium oxalate saturation in dialysis patients with and without primary hyperoxaluria. Urol Res 34: 12–16, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Cochat P, Rumsby G: Primary hyperoxaluria. N Engl J Med 369: 649–658, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Salyer WR, Keren D: Oxalosis as a complication of chronic renal failure. Kidney Int 4: 61–66, 1973 [DOI] [PubMed] [Google Scholar]
- 20.Tomson CR, Channon SM, Ward MK, Laker MF: Plasma oxalate concentration, oxalate clearance and cardiac function in patients receiving haemodialysis. Nephrol Dial Transplant 4: 792–799, 1989 [PubMed] [Google Scholar]
- 21.Liu Y, Weisberg LS, Langman CB, Logan A, Hunter K, Prasad D, et al. : Plasma oxalate levels in prevalent hemodialysis patients and potential implications for ascorbic acid supplementation. Clin Biochem 49: 1133–1139, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, et al. ; German Diabetes and Dialysis Study Investigators: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 23.O’Lone E, Viecelli AK, Craig JC, Tong A, Sautenet B, Herrington WG, et al. ; SONG-HD CVD Consensus Workshop Investigators: Establishing core cardiovascular outcome measures for trials in hemodialysis: Report of an international consensus workshop. Am J Kidney Dis 76: 109–120, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Pfau A, Wytopil M, Chauhan K, Reichel M, Coca SG, Aronson PS, et al. : Assessment of plasma oxalate concentration in patients with CKD. Kidney Int Rep 5: 2013–2020, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ermer T, Kopp C, Asplin JR, Granja I, Perazella MA, Reichel M, et al. : Impact of regular or extended hemodialysis and hemodialfiltration on plasma oxalate concentrations in patients with end-stage renal disease. Kidney Int Rep 2: 1050–1058, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladwig PM, Liedtke RR, Larson TS, Lieske JC: Sensitive spectrophotometric assay for plasma oxalate. Clin Chem 51: 2377–2380, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 28.Ma L, Zhao S: Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int J Cardiol 238: 151–158, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Wheeler DC, London GM, Parfrey PS, Block GA, Correa-Rotter R, Dehmel B, et al. ; EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) Trial Investigators: Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: The EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc 3: e001363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, et al. : Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 69: 266–277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP: Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 76: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joki N, Tokumoto M, Takahashi N, Nishimura M: Current perspectives on sudden cardiac death in hemodialysis patients. Contrib Nephrol 196: 5–12, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Jonassen JA, Cao LC, Honeyman T, Scheid CR: Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr 13: 55–72, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Worcester EM, Nakagawa Y, Bushinsky DA, Coe FL: Evidence that serum calcium oxalate supersaturation is a consequence of oxalate retention in patients with chronic renal failure. J Clin Invest 77: 1888–1896, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recht PA, Tepedino GJ, Siecke NW, Buckley MT, Mandeville JT, Maxfield FR, et al. : Oxalic acid alters intracellular calcium in endothelial cells. Atherosclerosis 173: 321–328, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Mookadam F, Smith T, Jiamsripong P, Moustafa SE, Monico CG, Lieske JC, et al. : Cardiac abnormalities in primary hyperoxaluria. Circulation 74: 2403–2409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito T, Ikeda M, Asai K, Shimizu W: Crystalline cardiomyopathy due to secondary oxalosis after short-bowel syndrome and end-stage renal failure. Clin Res Cardiol 105: 714–716, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Kalra PA, Green D, Poulikakos D: Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 93: 781–783, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Kong J, Davies M, Mount P: The importance of residual kidney function in haemodialysis patients. Nephrology (Carlton) 23: 1073–1080, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K: Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 24: 2502–2510, 2009 [DOI] [PubMed] [Google Scholar]
- 42.House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. ; Conference Participants: Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 95: 1304–1317, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Efe O, Verma A, Waikar SS: Urinary oxalate as a potential mediator of kidney disease in diabetes mellitus and obesity. Curr Opin Nephrol Hypertens 28: 316–320, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K: A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif 49: 202–211, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Lingeman JE, Pareek G, Easter L, Pease R, Grujic D, Brettman L, et al. : ALLN-177, oral enzyme therapy for hyperoxaluria. Int Urol Nephrol 51: 601–608, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weigert A, Martin-Higueras C, Hoppe B: Novel therapeutic approaches in primary hyperoxaluria. Expert Opin Emerg Drugs 23: 349–357, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.