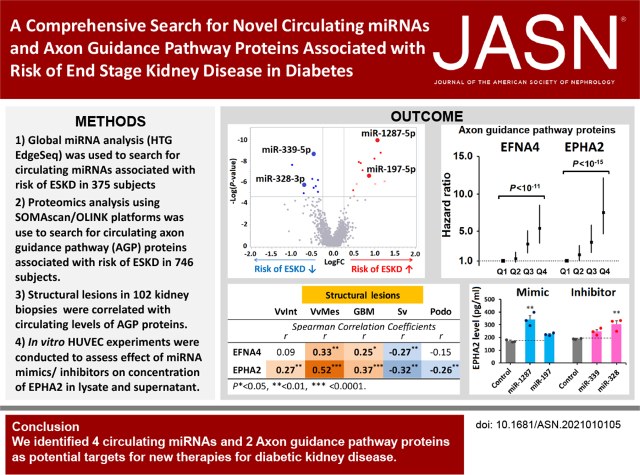
Significance Statement
Mechanisms underlying the progression of diabetic kidney disease to ESKD are not fully understood. Through profiling of circulating microRNAs (miRNAs) and proteins in individuals with type 1 and type 2 diabetes from four independent cohorts, the authors identified a signature of 17 miRNAs and six axon guidance pathway proteins that were robustly associated with severity of early structural lesions in kidney biopsy specimens and with an increased 10-year risk of ESKD. The study reveals novel mechanisms and proteins that govern progression to ESKD and point to the importance of systemic factors in the development of diabetic kidney disease. Some of the circulating miRNAs and axon guidance pathway proteins represent potential targets for new therapies to prevent and treat this condition.
Keywords: chronic kidney disease, diabetic nephropathy, end stage kidney disease, progression of renal failure
Visual Abstract
Abstract
Background
Mechanisms underlying the pro gression of diabetic kidney disease to ESKD are not fully understood.
Methods
We performed global microRNA (miRNA) analysis on plasma from two cohorts consisting of 375 individuals with type 1 and type 2 diabetes with late diabetic kidney disease, and targeted proteomics analysis on plasma from four cohorts consisting of 746 individuals with late and early diabetic kidney disease. We examined structural lesions in kidney biopsy specimens from the 105 individuals with early diabetic kidney disease. Human umbilical vein endothelial cells were used to assess the effects of miRNA mimics or inhibitors on regulation of candidate proteins.
Results
In the late diabetic kidney disease cohorts, we identified 17 circulating miRNAs, represented by four exemplars (miR-1287-5p, miR-197-5p, miR-339-5p, and miR-328-3p), that were strongly associated with 10-year risk of ESKD. These miRNAs targeted proteins in the axon guidance pathway. Circulating levels of six of these proteins—most notably, EFNA4 and EPHA2—were strongly associated with 10-year risk of ESKD in all cohorts. Furthermore, circulating levels of these proteins correlated with severity of structural lesions in kidney biopsy specimens. In contrast, expression levels of genes encoding these proteins had no apparent effects on the lesions. In in vitro experiments, mimics of miR-1287-5p and miR-197-5p and inhibitors of miR-339-5p and miR-328-3p upregulated concentrations of EPHA2 in either cell lysate, supernatant, or both.
Conclusions
This study reveals novel mechanisms involved in progression to ESKD and points to the importance of systemic factors in the development of diabetic kidney disease. Some circulating miRNAs and axon guidance pathway proteins represent potential targets for new therapies to prevent and treat this condition.
Mechanisms of the progression of diabetic kidney disease (DKD) to ESKD are not fully understood. However, circulating microRNAs (miRNAs) and circulating proteins have been implicated in playing a role in this progression, and are studied using high-throughput technologies.
miRNAs are short, endogenous, noncoding RNA molecules that are implicated in many disease processes.1 Over 2600 human miRNAs have been identified, and new ones are continuously being discovered. Up- and downregulation of mature miRNAs results in alterations in translation of many proteins.2–4 Indeed, miRNAs target and regulate approximately 60% of human protein-coding genes.5 In addition to their important intracellular functions, most miRNAs are also found in circulation, where they are protected from degradation and, therefore, can be studied in stored biospecimens. However, the biologic functions of the circulating miRNAs are unclear. A recent report showed that adipose-derived circulating miRNAs regulate gene expression in other tissues,6 and extracellular miRNAs are implicated in crosstalk between tissues and are involved in the pathogenesis of various diseases.7,8 The role of miRNAs in diabetic complications, including DKD, was recently reviewed.9
Using unbiased, global miRNA analysis, we aimed to identify circulating miRNAs associated with progression of DKD to ESKD. A parallel analysis of a targeted proteome was conducted in the same subjects to identify circulating proteins that were regulated by the putative miRNAs. By combining these analyses, we found a specific set of circulating axon guidance pathway (AGP) proteins that is associated with risk of ESKD and with severity of the kidney structural lesions observed in kidney biopsy specimens. We also examined the putative miRNAs for upregulation of the specific AGP protein in in vitro experiments.
Methods
To obtain robust findings that can be generalized to subjects with diabetes, we studied the profiles of circulating miRNAs and AGP proteins in four independent cohorts with type 1 diabetes (T1D) and type 2 diabetes (T2D), who were at various stages of DKD, and were followed for 7–15 years to ascertain progression to ESKD. A subgroup of 105 subjects in the Pima T2D validation cohort had research kidney biopsies in close proximity to the baseline examination. This subgroup was used for studies on the relationships between kidney structural lesions and kidney expression of miRNAs, kidney expression of genes encoding the AGP proteins, and circulating levels of AGP proteins. Subjects for the study cohorts were selected from the Joslin Kidney Study (JKS) and the Pima Indian Kidney Study. More information about the research design and methods is provided in the Supplemental Material.
JKS
The Joslin Diabetes Center Committee on Human Studies approved the informed consent, recruitment, and examination procedures for JKS. JKS is a longitudinal, observational study that investigates the determinants and natural history of kidney function decline in T1D and T2D. Approximately 2000 subjects with T1D and 1500 subjects with T2D were recruited into JKS from the 20,000 subjects attending the Joslin Clinic between 1991 and 2009. The protocols for recruitment, examination, and determination of clinical characteristics for these subjects were previously reported.10–15
Discovery and Replication Cohorts with late DKD
We selected the T1D discovery (n=239) and the T2D replication (n=136) cohorts from JKS participants, with proteinuria and impaired kidney function (eGFR of 20–60 ml/min per 1.73 m2) at baseline, who were enrolled into the study between 1991 and 2009. These subjects were followed for 7–15 years to assessed changes in kidney function and ascertain onset of ESKD. Subjects in these cohorts were previously used in our study on the role of inflammatory circulating proteins in progression to ESKD.12
Validation Cohort with Early DKD
The T1D validation cohort (n=243) included JKS participants with proteinuria and an eGFR of 60–140 ml/min per 1.73 m2 at baseline, who were enrolled into the study between 1991 and 2009. These subjects were followed for 7–15 years to assess changes in kidney function and ascertain onset of ESKD. Subjects in this cohort were previously used in our study on the role of circulating TNF-related proteins in the development of early progressive renal decline.13
Pima Indian Kidney Study
We selected the T2D validation cohort from the subjects participating in the Pima Indian Kidney Study.16–18 This study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. For our T2D validation cohort, we selected 154 of these subjects with a baseline GFR of >60 ml/min. Research kidney biopsies were performed in 105 of these subjects in close proximity to their baseline examinations. Subjects in these cohorts were previously used in our study on the role of inflammatory circulating proteins in progression to ESKD.12
HTG EdgeSeq Assay To Measure miRNAs in Plasma
To determine the plasma concentrations of the majority of currently known miRNAs, we used the HTG Molecular Diagnostics (Tucson, AZ) EdgeSeq miRNA sequence platform.19–21 No RNA extraction was needed for this platform, and whole circulating miRNAs in plasma, including exosomal and protein-bound miRNAs, were measured. Aliquots of plasma (15 μl) from the participants were submitted to HTG Molecular Diagnostics for analysis. In this study, we measured 2083 mature miRNAs.
Analysis of HTG Data
miRNAs with low expression levels were filtered out using the edgeR package (version 3.12.1).22 We defined detectable expression levels as more than one count per million in >90% of the subjects in each cohort. For data analysis, we applied quantile normalization for each miRNA read count with sample weights23 using the limma package (version 3.26.9)24 (see Supplemental Material).
SOMAscan: Aptamer-Based Proteomics Platform
Unique, single-stranded sequences of DNA and RNA, referred to as aptamers, recognize folded protein epitopes with high affinity and specificity.25,26 This property was further advanced by using slow off-rate modified aptamers to develop the SOMAscan platform (SomaLogic, Denver, CO), which we used to assay concentrations of proteins in this study.27–29 All samples in this study were processed at the SomaLogic laboratory in Denver. Results were returned to A.S.K.’s laboratory (see Supplemental Material).
Olink: Proximity Extension Assay–Based Proteomics Platform
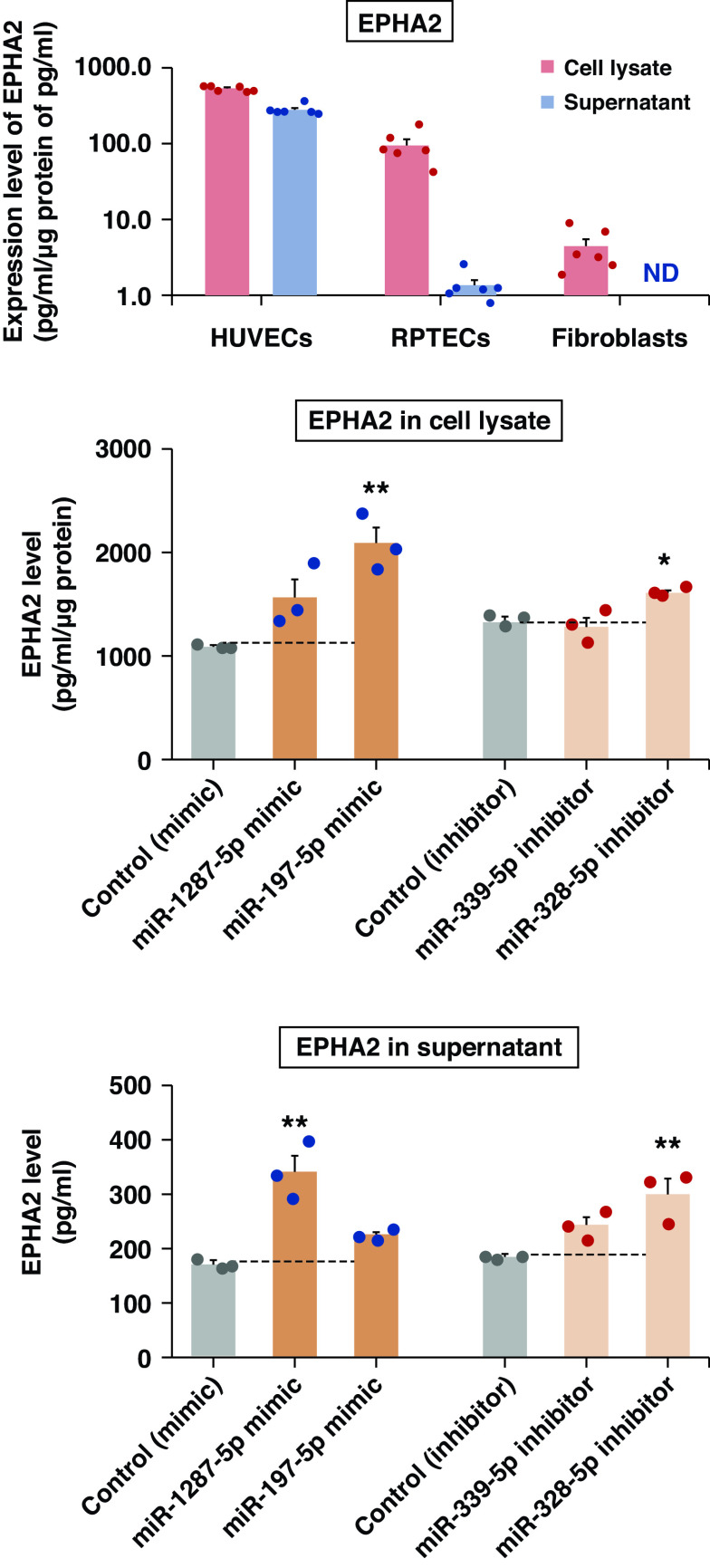
To validate the SOMAscan measurements of AGP proteins, we used the Olink Proteomics platform, which applies the proximity extension assay to measure the concentration of 1100 proteins.30 By comparing the plasma concentration of four AGP proteins measured by the SOMAscan and Olink platforms in 75 subjects from the Pima Indian cohort, we found good agreement between the measurements (Spearman correlation coefficients varied between 0.56 and 0.68; see Supplemental Table 1). Furthermore, hazard ratios (HRs) for time to onset of ESKD, according to baseline concentration of the AGP proteins measured by SOMAscan and Olink platforms, were similar and statistically significant for two proteins (EPHA2 and EPHB6). When measured by the SOMAscan, EFNA4 resulted in a lower and nonsignificant HR compared with the measurement obtained using the Olink platform; measuring UNC5C using the Olink platform resulted in a lower and nonsignificant HR compared with that obtained from SOMAscan (see Supplemental Table 1). These differences were small and insignificant, and were likely due to the small size of the study gr oup. In addition to the validation study, in which commercially available Olink panels were used, we worked with Olink to develop a custom-made assay to measure concentrations of EFNA4 and EPHA2 in the serum from participants from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, and also from the cell lysates and supernatant (medium in which the cells were cultured) in our in vitro studies (see Supplemental Material).
Search for Biologic Pathways Enriched by Genes Targeted by Exemplar miRNAs
To find genes and the encoded proteins targeted by our exemplar miRNAs, we used the bioinformatics approach. Instead of using correlation analysis to search for proteins highly correlated with exemplar miRNAs in our dataset of SOMAscan measurements, we searched for target proteins in a publicly available database to identify the biologic pathways most enriched with such proteins. Next, proteins in the most-enriched pathways were examined for association with ESKD risk during follow-up.
To predict genes/proteins targeted by the exemplar miRNAs, we used the open-access web server miRWALK2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2), which integrates 12 target prediction algorithms.31 To increase prediction accuracy, we required that putative target genes be identified by at least six of the 12 algorithms included in miRWALK2.0. We performed Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis32 to identify overlapped genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/).33 A Fisher exact test P value of <0.01 was used to identify significantly targeted pathways in KEGG and the enriched gene target pathways obtained from these databases.
Measurements of Kidney Structural Lesions
The morphometric techniques were described prev iously.18,34 Briefly, unbiased random samplings of tissue sections from kidney biopsies provided digital images from both electron and light microscopy. We used quantitative morphometric methods to estimate kidney structural parameters by masked observers. The predefined electron microscopy parameters included glomerular basement membrane (GBM) width, mesangial fractional volume (VvMes), glomerular filtration surface area density, podocyte number per glomerulus, podocyte foot process width, percentage of GBM surface with podocyte detachment, and percentage of normally fenestrated glomerular capillary endothelium (%FE). Cortical interstitial fraction volume (VvInt) was measured using light microscopy and, because not all participants had tissue for VvInt, data for this parameter were only available for 102 subjects. Foot process width, percentage of GBM surface with podocyte detachment, and %FE were not measured in one participant. Generally, three glomeruli (or a minimum of two) were examined by electron microscopy per participant, and a median of seven (interquartile range, 3–12) glomeruli were examined by light microscopy for the morphometric measurement s. Morphometric variables for each individual were calculated as the mean of all measurements for that individual.
mRNA Expression of Gene Encoding for AGP Proteins in Kidney Tissue
Of the 105 Pima subjects who had structural measurements, 66 also had gene expression profiling of the glomerular tissue, and 47 had gene expression profiling of the tubulointerstitium for mRNA expression, using a GeneChip Human Genome series U133A and Plus 2.0 Array (Affymetrix, Santa Clara, CA).35–38 mRNAs for five of the six AGP proteins were identified.
Expression of Exemplar miRNAs in Kidney Tissue
Kidney biopsy tissue miRNA expression was determined as previously described.39 Briefly, a small RNA fraction (<200 nt) was isolated from microdissected glomeruli and subjected to barcode adapter ligation and reverse transcription. The cDNA libraries were deep sequenced on an Illumina Genome Analyzer II. Reads were extracted after trimming the adapter sequence, and then aligned to the human genome and small RNA databases. The miRNA sequence reads were normalized by the total reads of each sample.
miRNA In Situ Hybridization Using the miRNAscope Assay
Localization and levels of miRNAs were examined in kidney specimens using miRNAscope Assay in situ hybridization (ISH). For the in silico–designed probes of miR-197-5p, miR-339-5p, and miR-328-3p, we were unable to avoid potential crosshybridization with multiple endogenous human genes. Therefore, probes for miR-1287-5p and miR-658 were used for hybridization (see Supplemental Material).
Immunohistochemical Staining of Kidney Tissue for AGP Proteins
Paraffin sections from two kidney biopsy specimens from the Pima Indian cohort were stained for EFNA4 and EPHA2, using primary antibodies, and the images were obtained using a Leica SP8X confocal microscope (see Supplemental Material).
ACCORD Study
The ACCORD study was a multicenter clinical trial that investigated the effect of treating hyperglycemia, hypertension, and dyslipidemia on cardiovascular event rates. The trial included 10,251 participants with T2D and high cardiovascular risk, who were randomized in a 1:1 ratio to receive intensive (aiming for hemoglobin A1c [HbA1c] <6.0%) or standard (aiming for HbA1c level of 7%–7.9%) glycemic-lowering treatments.40 Our study included 200 of the ACCORD-Lipid (one of the ACCORD study subtrials) participants, who were randomly selected in equal numbers from the four groups defined by the two glycemic treatment arms and the two lipid treatment arms. The resulting sample comprised 100 participants from the intensive glycemic arm and 100 participants from the standard glycemic arm, each including an equal number of subjects from each lipid-treatment arm (fenofibrate or placebo). Using the Olink Proteomics platform described above, EFNA4 and EPHA2 were measured in serum samples obtained at baseline and 12 months after randomization (see Supplemental Material).
Cellular Studies in Renal Proximal Tubule Epithelial Cells, Fibroblasts, and Human Umbilical Vein Endothelial Cells
To determine which cells should be used in our RNA interference (RNAi) experiments with miRNA mimics/inhibitors, we assessed AGP protein expression levels in cell lysates and supernatant in three human cell lines: renal proximal tubule epithelial cells (RPTECs), fibroblasts, and human umbilical vein endothelial cells (HUVECs). RPTECs/TERT1 (CRL-4031) were obtained from American Type Culture Collection (Manassas, VA) and cultured according to the manufacturer’s protocol. Skin fibroblasts from subjects with T1D were provided by M.M. and cultured as previously described.41 HUVECs were isolated from the umbilical cords of newborns and cultured as previously described .42,43 The cell lysates and supernatants from the three cell lines were analyzed using the custom-made Olink assay to measure levels of EPHA2 and EFNA4.
miRNA Mimics and Inhibitors Transfection to HUVECs
HUVECs were transfected with 20 nM of the mimics of hsa-miR-1287-5p and hsa-miR-197-5p, inhibitors of hsa-miR-339-5p and miR-328-3p, and mimic-negative and inhibitor-negative controls (Dharmacon, Lafayette, CO) using Lipofectamine RNAiMax (Invitrogen, Waltham, MA), according to the manufacturer’s protocol. To measure EPHA2 and EFNA4 levels with the custom-made Olink assay, the cell pellets were lysed using M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, Waltham, MA), and the total protein concentration was measured using the Micro BCA Protein Assay Kit (Thermo Scientific). The cell lysate samples were normalized to a concentration of 0.2 mg/ml (see Supplemental Material).
Statistical Analyses
Clinical characteristics and summaries of outcomes were reported as counts and percentages (proportions) for categoric variables, means with SDs for normally distributed continuous variables, and as medians and quartiles for variables with skewed distributions. Differences in miRNA levels between subjects developing ESKD within 10 years and those who did not progress to ESKD were expressed as fold changes between the medians of counts per million and tested with the Wilcoxon rank sum test. Bonferroni correction for n=2083 independent tests (the number of available miRNAs by the study design) yielded a threshold of P<2.4 × 10−5. To examine relationships between the miRNAs significant in the fold-change analysis, we constructed Spearman correlation matrices and performed a hierarchic cluster analysis using an average linkage method, as implemented in the CLUSTER procedure in SAS.
To further examine the association of the miRNAs and AGP proteins with the risk of ESKD, we fitted simple (univariate) and adjusted (multivariable) Cox proportional hazards regression models. In these analyses, to make the results of this study comparable with our recent proteomics publications,12,13,15 the levels of miRNA and AGP proteins at baseline were modeled as one-quartile changes on a continuous scale. In some instances, we presented results with baseline quartiles considered as discrete variables, or compared risk of progression to ESKD in subjects in the fourth quartile versus those in the first quartile for specific miRNAs or AGP proteins. The analyses for AGP proteins were repeated using raw data with changes modeled as one SD. The results of these analyses and distributions of raw data for miRNAs and AGP proteins are presented in the Supplemental Figure 3 and 4.
Two multivariable Cox proportional hazard regression models were used to assess the role of circulating miRNAs and AGP proteins in progression to ESKD. In the models referred to as “etiologic,” the effects of miRNAs and AGP proteins were adjusted for significant clinical exposures and confounders, such as sex, diabetes duration, systolic BP, baseline eGFR (or directly measured GFR, where applicable), HbA1c, and stratification by study cohorts. The urine albumin-creatinine ratio (ACR) was not considered as a covariate in these models because it was an outcome measure and a symptom of the DKD presentation that leads to ESKD. However, in the so-called multivariable “prognostic” models, in which miRNAs and AGP proteins were assessed as predictors of progression to ESKD, clinical predictors such as ACR, HbA1c, and eGFR were also considered. Effects of clinical covariates on progression to ESKD in the two models are shown in Supplemental Table 2.
We used the Kaplan–Meier method to estimate 10-year cumulative risk of ESKD and to plot survivor function estimates according to candidate miRNA levels. To identify the most important miRNAs within each cluster (exemplars), we considered the effect size (HR) and significance level (P value) of the association with ESKD in both univariate and multivariable models. Within each cluster, all miRNAs were highly collinear, and this was reflected by high Spearman correlations and a large variance inflation in Cox regression models. Finally, we analyzed an adjusted Cox regression model that included nonredundant miRNAs that remained nominally significant when included in one model. During model building, we used stepwise variable selection and backward elimination to examine possible combinations of predictors. The fit of the final model was assessed using deviance residuals and by testing interaction with time to check proportionality of hazards.
Enrichment pathway analysis of the miRNA gene/protein targets was performed with a two-sided Fisher exact test against the null hypothesis of no enrichment. The association of the AGP proteins with 10-year risk of ESKD was tested with adjusted Cox regression models. We applied a Bonferroni correction for n=1128 independent tests (the number of proteins measured) performed in the T1D discovery cohort, which yielded a threshold of P<4.4 × 10−5.
Spearman correlation was used to examine association of early structural kidney lesions with kidney expression of miRNAs and AGP genes and circulating AGP proteins.
We examined the performance of two prognostic models using subjects in four study cohorts. Using a Cox proportional hazards model and backward variable elimination, a clinical prognostic model was developed that included clinical covariates such as ACR, HbA1c, and eGFR. The prognostic performance of this model was compared with the prognostic performance of a model that included the three significant clinical covariates and two of the six AGP proteins. Several different methods were used to assess prognostic performances of the two models. The Uno C-statistic was calculated as proportions of pairs for subjects whose observed and predicted outcomes were concordant. The fit of the consecutive nested models was tested using likelihood ratio tests, with the appropriate degrees of freedom and an assumption of chi-squared distribution, and by changes in the Akaike information criterion (AIC) values. To evaluate overall improvement in reclassification, net reclassification improvement (NRI) was used on the basis of several ESKD risk categories (<5%, 5%–9.9%, 10%–19.9%, and ≥20%).
In ACCORD, the effect of intensive glycemic treatment on serum EFNA4 and EPHA2 concentrations was evaluated by linear regression. Due to their right-skewed distributions, both proteins were analyzed after log transformation. For each protein, the linear regression model included the protein concentration at 12 months as the dependent variable, and the glycemic treatment assignment as the independent variable, with the protein concentration at baseline, age, sex, eGFR at baseline and 12 months, and lipid-treatment assignment as covariates. The mean covariate-adjusted ratios between 12 months and baseline protein values were estimated for the two treatment arms by linear regression.
The effect of losartan versus placebo treatment on serum EFNA4 and EPHA2 concentrations by SOMAscan in a subset of the Pima cohort was also evaluated by linear regression. Both proteins were analyzed after log transformation, and 12-month changes were calculated, adjusting for duration of diabetes, sex, systolic BP, baseline GFR, and HbA1c.
All in vitro experiments were performed in triplicate, and the results are expressed as means±SEMs. One-way ANOVA with the Dunnett multiple comparison test was used for statistical analysis. Analyses were performed in SAS version 9.4 and R version 3.2.4.
Results
Clinical Characteristics of Study Cohorts
To obtain robust findings that can be generalized to all subjects with diabetes, several different cohorts were selected for this study. This included two cohorts with impaired kidney function (late DKD), and two cohorts with normal kidney function (early DKD). The cohort with T1D and late DKD was considered a discovery cohort, the cohort with T2D and late DKD was considered a replication cohort, and the two cohorts with early DKD were considered validation cohorts. Clinical characteristics of these cohorts are shown in Table 1. They differed with regard to baseline clinical characteristics, such as age, diabetes duration, HbA1c, ACR, and eGFR. However, a significant proportion of subjects in each cohort developed ESKD during the 7–15 years of follow-up. Progression to ESKD during the first 10 years of follow-up was considered the primary outcome in this study. In the Joslin cohorts, 88% of subjects were of European ancestry, whereas in the Pima cohort all subjects were American Indians.
Table 1.
Clinical characteristics of subjects included in the study cohorts
| Characteristics | Late DKD | Early DKD | |||
|---|---|---|---|---|---|
| Joslin T1D Discovery Cohort (n=239)a | Joslin T2D Replication Cohort (n=136)a | Joslin T1D Validation Cohort (n=243)b | Pima T2D Validation Cohort (n=154)a | Pima T2D Cohort for Auxiliary Study (n=105)c | |
| At baseline | |||||
| Male/female, n | 117/122 | 89/47 | 133/110 | 43/111 | 29/76 |
| Age (yr), mean±SD | 44±10 | 60±6 | 39±9 | 46±10 | 45±10 |
| Duration of DM (yr), mean±SD | 30±9 | 16±9 | 26±9 | 16±7 | 15±6 |
| HbA1c (%), mean±SDd | 8.6±1.6 | 7.5±1.8 | 9.0±1.7 | 9.4±2.3 | 9.2±2.3 |
| SBP (mmHg), mean±SD | 136±20 | 139±19 | 131±19 | 125±14 | 122±13 |
| DBP (mmHg), mean±SD | 77±11 | 75±11 | 78±10 | 77±9 | 77±9 |
| ACR (mg/g), median (IQR) | 793 (274–1855) | 255 (57–1092) | 579 (212–1173) | 55 (13–357) | 40 (12–124) |
| GFR (ml/min), mean±SDe | 42±10 | 49±11 | 97±21 | 150±47 | 151±47 |
| During follow-up | |||||
| New cases of ESKD within 10 yr, n (%) | 127 (53) | 43 (32) | 50 (21) | 37 (24) | 15 (14) |
DM, diabetes mellitus; SBP, systolic BP; DBP, diastolic BP; IQR, interquartile range.
These study cohorts were used to study KRIS proteins, and their clinical characteristics were previously reported.12
This study cohort was used to study TNF-related proteins, and clinical characteristics were previously reported.13
This subgroup from the Pima T2D validation cohort had kidney biopsies and was used to study kidney structural lesions.
Search for Profile of Circulating miRNAs Associated with Risk of ESKD
Figure 1A shows the volcano plot for fold changes in circulating levels of miRNAs in subjects in the T1D discovery cohort who did and did not develop ESKD. The levels of 21 miRNAs were significantly different (P<2.4 × 10−5 after Bonferroni correction) in those who developed ESKD versus those who did not. In the T2D replication cohort, 17 of these 21 miRNAs were statistically significant (P<0.05); of these, eight miRNAs were upregulated and nine were downregulated in subjects progressing to ESKD within 10 years. The fold changes for all 21 miRNAs in the two cohorts are provided in Supplemental Figure 1. Although the discovery and replication cohorts had different types of diabetes and very different baseline clinical characteristics, the fold changes between patients with and without risk of ESKD during the 10-year follow-up were similar, suggesting similar mechanisms underlie the association between these circulating miRNAs and the development of ESKD in T1D and T2D. Consequently, the two cohorts were combined for future analyses.
Figure 1.
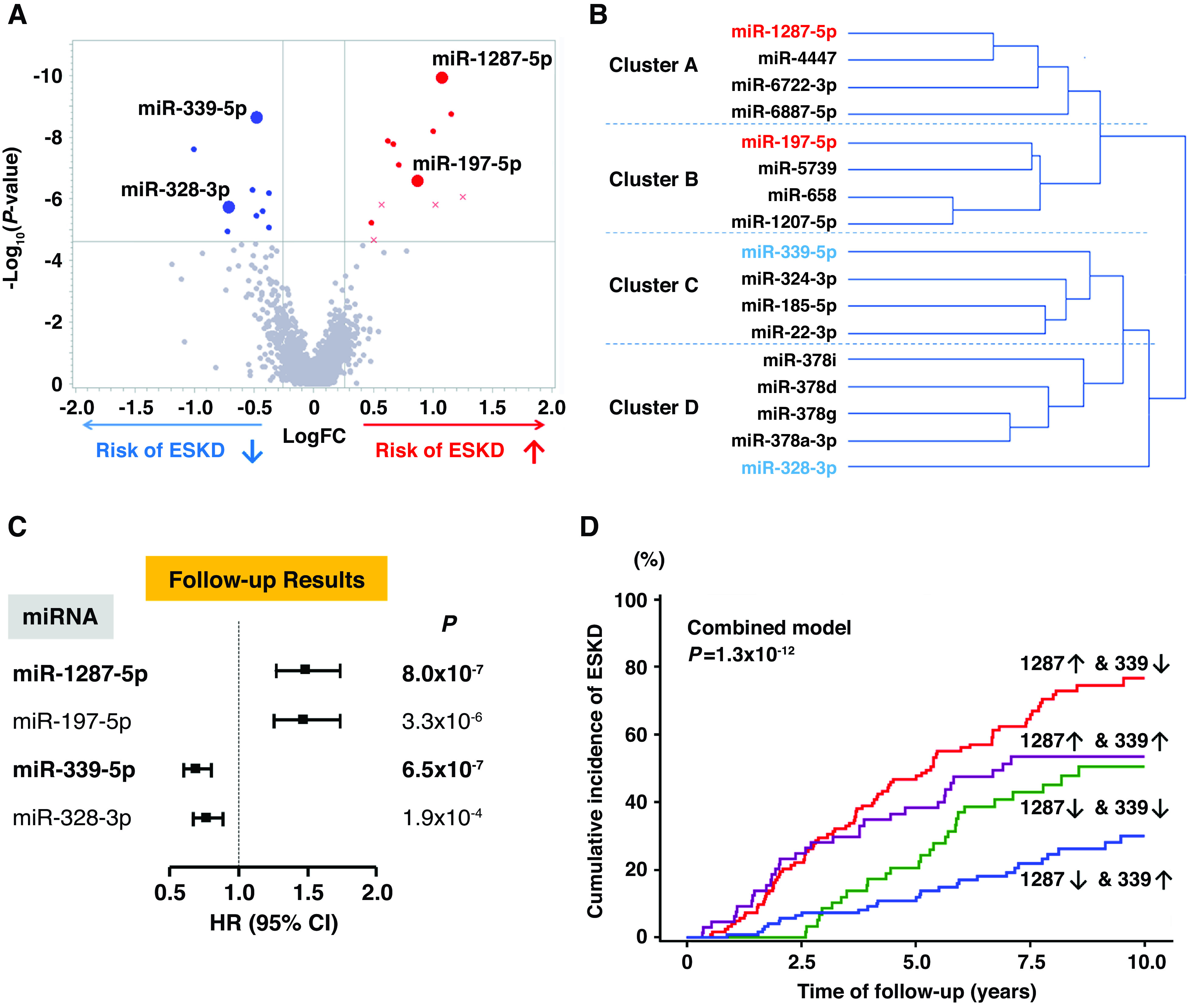
Profile of circulating miRNAs associated with risk of progression to ESKD during 10-year follow-up. (A) Volcano plot of fold changes (FC) and P values for 2083 miRNAs measured in plasma at baseline in 127 subjects with T1D who developed ESKD, compared with 112 subjects with T1D who did not progress to ESKD. A total of 21 miRNAs were associated with risk of ESKD (Bonferroni corrected), and 17 of them were confirmed in the T2D replication cohort (upregulated miRNAs in red, downregulated miRNAs in blue, nonreplicated miRNAs are shown as “x”). Exemplar miRNAs representing different clusters (listed in [B]) are identified in the plot. (B) Cluster analysis of the 17 candidate miRNAs in the combined late DKD cohorts (n=375). The miRNAs in red and blue are exemplar miRNAs that had the strongest association with time to ESKD in Cox models in each cluster (see Supplemental Table 3). (C) HRs with 95% CIs for time to onset of ESKD according to baseline plasma levels of exemplar miRNAs in the combined late DKD cohorts (n=375). Estimates are per one-quartile increase in plasma level of an miRNA after adjusting for clinical covariates important for the etiologic model, i.e., sex, duration of diabetes, systolic BP, baseline HbA1c, and eGFR, with variable stratification by study cohort. For distribution of values of exemplar miRNAs, see Supplemental Figure 3. (D) Cumulative incidence of ESKD according to baseline plasma levels of miR-1287-5p and miR-339-5p in the combined late DKD cohorts. An upward arrow (↑) indicates miRNA level above (inclusive of) the median, and a downward arrow (↓) indicates miRNA level below the median. For statistical analysis, see Supplemental Table 4.
Exemplar miRNAs and Risk of ESKD
The 17 miRNAs associated with development of ESKD were intercorrelated (Supplemental Figure 2) and fell into four clusters: two clusters of upregulated miRNAs (clusters A and B), and two clusters of downregulated miRNAs (cluster C and D) (Figure 1B, Supplemental Figures 3 and 4). To rank the importance of the miRNAs within each cluster, we calculated HRs for risk of ESKD, according to baseline levels of each miRNA, using Cox proportional hazards models (Supplemental Table 3). In each cluster, one exemplar miRNA was selected on the basis of the strongest association with risk of ESKD, as shown in Figure 1B, and their highly statistically significant HRs for time to onset of ESKD in the combined cohorts are shown in Figure 1C.
When the 4 exemplars were analyzed together, the upregulated miR-1287-5p and downregulated miR-339-5p had strong and independent associations with ESKD risk. The other two exemplars also had significant associations, but only in the absence of the first two exemplars, indicating their associations were redundant (collinear) with miR-1287-5p and miR-339-5p. Figure 1D illustrates the cumulative incidence of ESKD during 10-year follow-up according to the combination of different levels of miR-1287-5p and miR-339-5p. The cumulative risk of ESKD was 77% in subjects with high levels (above the median) of miR-1287-5p and low levels (below the median) of miR-339-5p. In contrast, the cumulative ESKD risk was only 30% in subjects with low levels of miR-1287-5p and high levels of miR-339-5p. Subjects with the intermediate miRNA profile had intermediate risks of ESKD. The differences in ESKD risk among these subgroups were highly statistically significant (P=1.3 × 10−12; Supplemental Table 4).
Bioinformatics Search for Genes/Proteins That Were Predicted Targets for Exemplar miRNAs
Many genes were predicted as targets for the exemplar miRNAs and were reported in the KEGG database: 622 target genes for miR-1287-5p, 69 genes for miR-197-5p, 769 genes for miR-339-5p, and 632 genes for miR-328-3p. A total of 23 pathways were enriched by these genes at P<0.01; the highest enrichment was found for the AGP (P=5.6 × 10−11), followed by the Ras signaling pathway (P=2.7 × 10−7; Figure 2A). Both pathways were enriched for the genes targeted by all four exemplar miRNAs. Supplemental Table 5 lists other pathways enriched for genes that are predicted targets for fewer than four exemplar miRNAs. MAPK and HIF-1 signaling pathways were significantly enriched with genes targeted by exemplar miRNAs, and some proteins from these pathways were previously reported to be involved in DKD development.44–46 On the SOMAscan, there were almost 200 proteins representing these pathways; however, only three proteins, EFNA4, EFNA5, and EPHA2 were associated with time to onset of ESKD in the study cohorts (see Supplemental Table 6). Because these proteins are members of the AGP, the results are presented below.
Figure 2.
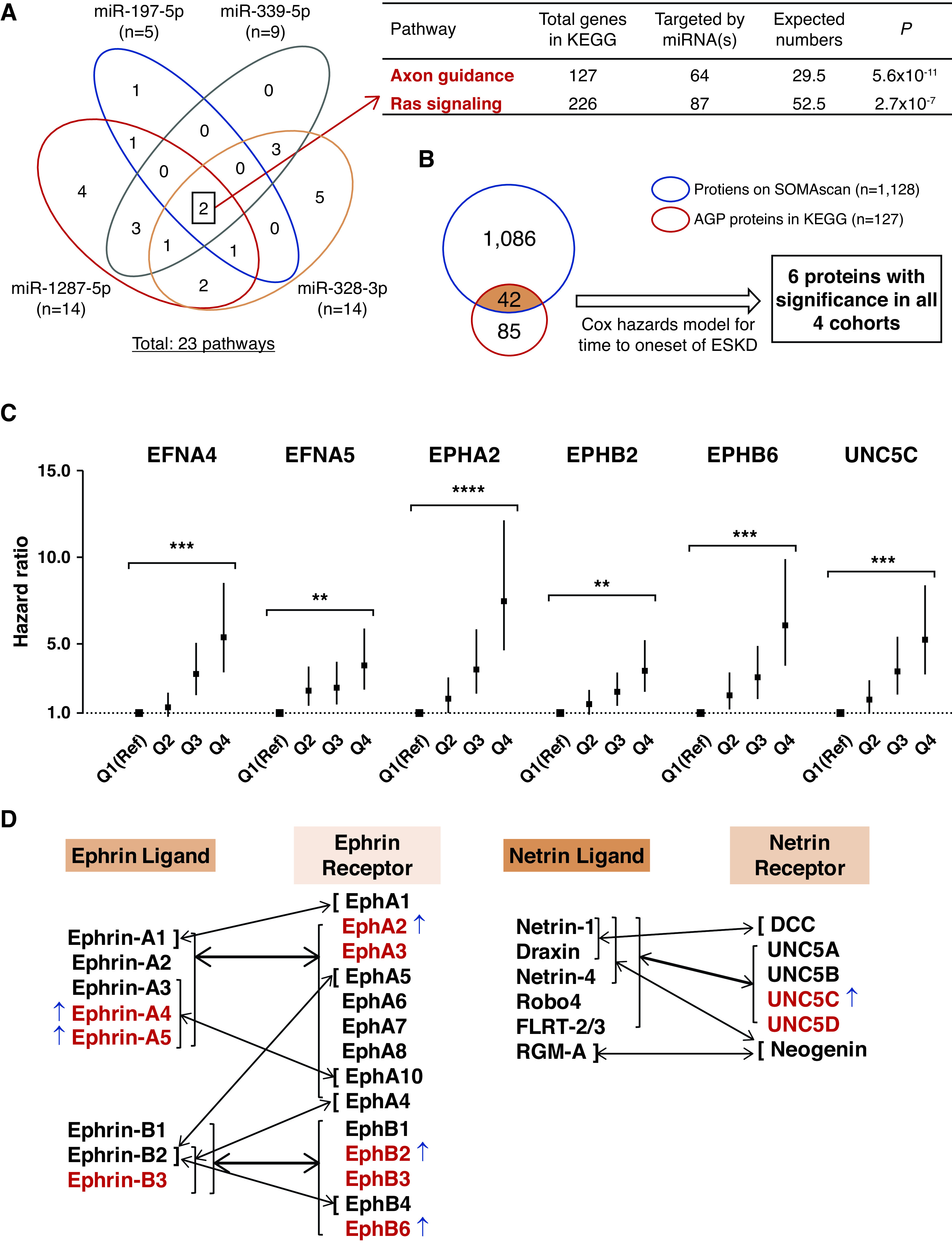
Profile of circulating AGP proteins associated with risk of progression to ESKD during 10-year follow-up. (A) Venn diagram of 23 KEGG pathways enriched by predicted genes targeted by four exemplar miRNAs. There were two overlapping pathways, AGP and Ras signaling pathways, which were enriched by genes targeted by these four miRNAs. (B) Among 127 AGP proteins, 42 were measured by the SOMAscan platform. In Cox regression analysis, the baseline plasma concentration of six AGP proteins were associated with risk of ESKD in each of the four cohorts (see Table 2). (C) HRs with 95% CIs for time to onset of ESKD according to baseline plasma levels of the six AGP proteins in the combined four cohorts (n=745). Estimates are per one-quartile increase (discrete variable) in plasma level of the AGP proteins after adjusting for clinical covariates important for the etiologic model, i.e., sex, duration of diabetes, systolic BP, baseline HbA1c, and eGFR, with variable stratification by study cohort. P values for comparison of quartile 4 (Q4) versus Q1 (Reference [Ref]) are shown (see Table 2). For distribution of values for the six AGP proteins, see Supplemental Figure 4. (D) Binding preferences for Ephrin and Netrin ligands and receptors. Thin arrows indicate selective binding of one ligand with one receptor. Thick arrows represent multiple ligands binding to multiple receptors. Of the 14 AGP proteins available on the SOMAscan platform (in red), six were elevated (indicated with ↑) in subjects at risk of ESKD in the study cohorts. FLRT-2/3, fibronectin and leucine-rich transmembrane protein 2 and 3; RGM-A, repulsive guidance molecule-A. **P<10−7, ***P<10−11, ****P<10−15.
AGP Proteins and Risk of ESKD
AGP was the top candidate pathway identified through bioinformatic analysis. Of the 127 proteins in this pathway listed in KEGG, the concentrations of 42 proteins were measured at baseline in circulation using the SOMAscan proteomics platform (Figure 2B). Of these proteins, 36 did not show association with time to onset of ESKD in Cox regression analyses (Supplemental Tables 7 and 8). However, the concentrations of six of these proteins—EFNA4, EFNA5, EPHA2, EPHB2, EPHB6, and UNC5C—were associated with time to onset to ESKD during 10-year follow-up in each of the different study cohorts, i.e., in cohorts with late and early DKD, T1D and T2D, and in White and Pima Indian subjects. Table 2 and Supplemental Table 7 show the HRs for time to onset of ESKD. Univariate HRs according to quartiles (continuous variable) of the concentrations of six AGP proteins were very similar and statistically significant among the individual study cohorts. When the cohorts were combined and adjusted for clinical covariates relevant for the etiologic model, the HRs became extremely highly statistically significant. The relationship between quartiles (discrete variable) of concentrations of the six AGP proteins and HRs for time to onset of ESKD is shown in Figure 2C and Table 2. In comparison with the first quartile (reference), the HR for time to onset of ESKD in each subsequent quartile increased in a dose-response manner (Figure 2C). The steepest increase in HRs was observed in the quartiles of concentrations of EPHA2. The HRs for time to onset of ESKD between subjects in the first quartile and the fourth quartile were large and highly statistically significant for all six of the proteins. When a similar analysis was performed for the prognostic model that included ACR, the HRs for each of the six AGP proteins diminished; however, they remained large and statistically significant (Table 2).
Table 2.
HRs for time to onse t of ESKD during a 10-year follow-up
| Etiologic Models in Individual Cohorts, with Quartiles as Continuous Variables | Etiologic Models in Combined Cohortsa | Prognostic Modelsb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Late DKD | Early DKD | |||||||||||||
| T1D Discovery Cohort (n=213) | T2D Replication Cohort (n=136) | T1D Validation Cohort (n=243) | T2D Validation Cohort (n=154) | Quartiles as Continuous Values (n=745) | Quartiles as Discrete Values, Comparison of Q4 versus Q1 | Quartiles as Discrete Values, Comparison of Q4 versus Q1 | ||||||||
| Gene | HR | P | HR | P | HR | P | HR | P | HR | P | HR | P | HR | P |
| EFNA4 | 2.10 (1.72-2.57) | 2.4 × 10−13 | 1.61 (1.21-2.14) | 1.0 × 10−3 | 1.98 (1.48-2.65) | 4.1 × 10−6 | 1.65 (1.20-2.25) | 1.9 × 10−3 | 1.84 (1.60-2.12) | 6.4 × 10−18 | 5.36 (3.40-8.47) | 6.0 × 10−13 | 3.35 (2.12-5.31) | 2.5 × 10−7 |
| EFNA5 | 1.70 (1.42-2.05) | 1.8 × 10−8 | 1.64 (1.23-2.18) | 7.6 × 10−4 | 1.40 (1.08-1.81) | 1.1 × 10−2 | 1.89 (1.36-2.62) | 1.6 × 10−4 | 1.45 (1.28-1.65) | 9.7 × 10−9 | 3.75 (2.38-5.89) | 1.1 × 10−8 | 2.21 (1.40-3.51) | 7.2 × 10−4 |
| EPHA2 | 2.15 (1.77-2.61) | 1.4 × 10−14 | 1.89 (1.40-2.55) | 3.0 × 10−5 | 1.68 (1.27-2.21) | 2.5 × 10−4 | 2.00 (1.42-2.80) | 7.3 × 10−5 | 1.99 (1.73-2.29) | 4.5 × 10−22 | 7.48 (4.60-12.15) | 4.8 × 10−16 | 4.24 (2.58-6.99) | 1.4 × 10−8 |
| EPHB2 | 1.45 (1.22-1.72) | 3.2 × 10−5 | 1.45 (1.10-1.91) | 8.6 × 10−3 | 1.29 (1.00-1.67) | 4.6 × 10−2 | 1.62 (1.18-2.22) | 2.8 × 10−3 | 1.51 (1.33-1.71) | 1.7 × 10−10 | 3.41 (2.26-5.15) | 5.6 × 10−9 | 2.03 (1.36-3.04) | 5.9 × 10−4 |
| EPHB6 | 1.74 (1.45-2.10) | 3.7 × 10−9 | 1.88 (1.40-2.52) | 2.3 × 10−5 | 1.54 (1.17-2.02) | 2.0 × 10−3 | 2.12 (1.50-2.98) | 2.2 × 10−5 | 1.78 (1.55-2.05) | 6.1 × 10−16 | 6.07 (3.75-9.82) | 2.1 × 10−13 | 2.86 (1.75-4.65) | 2.5 × 10−5 |
| UNC5C | 1.83 (1.52-2.20) | 1.3 × 10−10 | 1.59 (1.19-2.11) | 1.5 × 10−3 | 1.91 (1.43-2.55) | 1.3 × 10−5 | 1.56 (1.15-2.13) | 4.4 × 10−3 | 1.73 (1.51-1.98) | 3.4 × 10−15 | 5.22 (3.26-8.34) | 5.3 × 10−12 | 2.94 (1.82-4.74) | 1.0 × 10−5 |
HRs were determined according to baseline levels of six AGP proteins and expressed as quartiles, which were considered as either continues or discrete values. Results are presented for individual and combined cohorts for etiologic and prognostic models. Results of analyses according to baseline leve ls of six AGP proteins expressed as one SD change is shown in Supplemental Table 7. HR per one-quartile change when quartiles were considered as continuous variables. Q4, quartile 4; Q1, quartile 1.
Etiologic models were adjusted for sex, duration of diabetes, systolic BP, HbA1c, and eGFR, with variable stratification by study cohort. One subject who had incomplete clinical information was excluded in the combined model, and 745 subjects were used for subsequent analyses.
Prognostic models were adjusted for baseline HbA1c, eGFR, and ACR, with variable stratification by study cohort.
In the analysis in which all six AGP proteins were analyzed together with clinical covariates relevant for the etiologic model, only the EPHA2 receptor and EFNA4 ligand showed independent associations with time to onset of ESKD (HR for EPHA2, 1.67; 95% CI, 1.37 to 2.03; P=3.9 × 10−7; HR for EFNA4, 1.27; 95% CI, 1.04 to 1.55; P=1.7 × 10−2).
The six AGP proteins (EFNA4, EFNA5, EPHA2, EPHB2, EPHB6, and UNC5C) are members of two subfamilies comprising 34 known proteins, i.e., Ephrin ligands and receptors, and Netrin ligands and receptors. Figure 2D lists these proteins and shows known binding preferences.47,48 Of these 34 proteins, 14 (in red) were measured by SOMAscan and six (43%; blue arrows) were strongly associated with the development of ESKD.
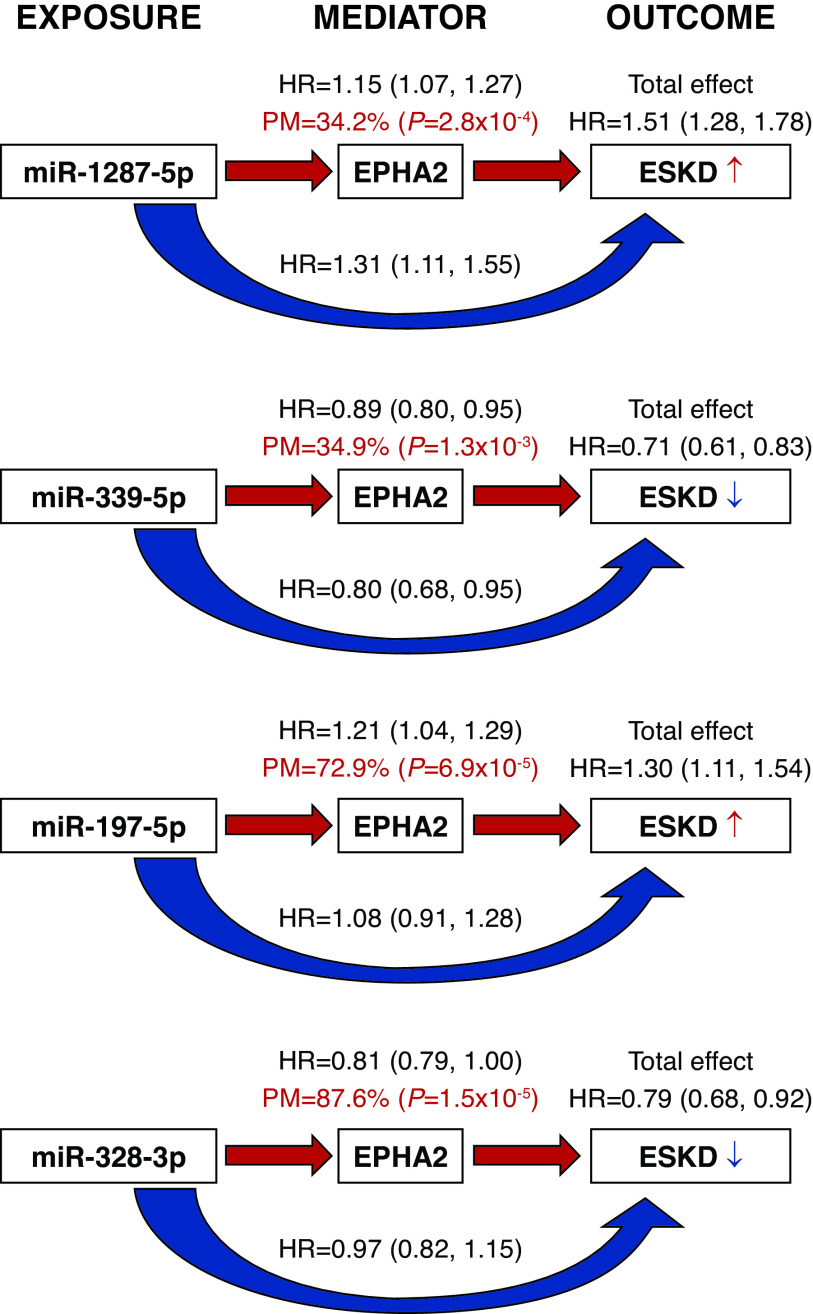
Mediation Analysis: Effect of Exemplar miRNAs on Risk of ESKD Is Mediated through AGP Proteins
To evaluate whether the effects of circulating exemplar miRNAs on risk of ESKD could be mediated through AGP proteins, we performed a mediation analysis. miRNAs were used as exposures because they regulate proteins, and AGP proteins were considered mediators. Using Cox regression models adjusted for relevant covariates, we found that the effects of exemplar miRNAs on time to onset of ESKD were significantly mediated by circulating levels of EPHA2. The proportions of natural indirect effect (percent mediated and indicated by red arrows) were moderate (34%–35%) for miR-1287-5p and miR-339-5p, and high (73%–88%) for miR-197-5p and miR-328-3p (Figure 3). Similar findings were obtained when circulating EFNA4 was considered a mediator (Supplemental Table 9).
Figure 3.
The effects of exemplar miRNAs on time to onset of ESKD were mediated by circulating levels of EPHA2. Shown are the effects of each exemplar miRNA (exposure) from each cluster on risk of ESKD during 10-year follow-up (outcome) mediated by EPHA2 (mediator). Combined T1D discovery and T2D replication cohorts were used in these analyses. The analyses were based on Cox proportional hazards models for time to onset of ESKD, adjusted for sex, duration of diabetes, systolic BP, baseline HbA1c, and eGFR, with variable stratification by study cohort. The effect of an miRNA on the time to ESKD (total effect) is split into a natural indirect effect (red arrow), measured as percent mediated (PM) by EPHA2, which exerts its role on the outcome through modulation of a protein level (mediator) and natural direct effect (blue arrow), which acts on the outcome independently from the mediator. Results of the mediation analysis for EFNA4 are provided in Supplemental Table 9.
Association of Severity of Kidney Structural Lesions with Circulating AGP Proteins but Not with Expression of AGP Genes and Exemplar miRNAs in Kidney Biopsy Specimens
Table 1 shows the clinical characteristics of the 105 Pima Indian subjects who underwent research kidney biopsies and were included in this study.
Kidney Structural Lesions and Circulating Levels of AGP Proteins
Serum AGP protein concentrations correlated positively with VvMes, GBM thickness, and glomerular filtration surface area density (Figure 4A). Among the six AGP proteins, circulating EPHA2 had the strongest correlation with all structural lesions. Circulating EPHB6 and UNC5C levels also correlated significantly with most lesions, whereas the other AGP proteins had much weaker or no association with VvInt, podocyte abnormalities, and %FE.
Figure 4.
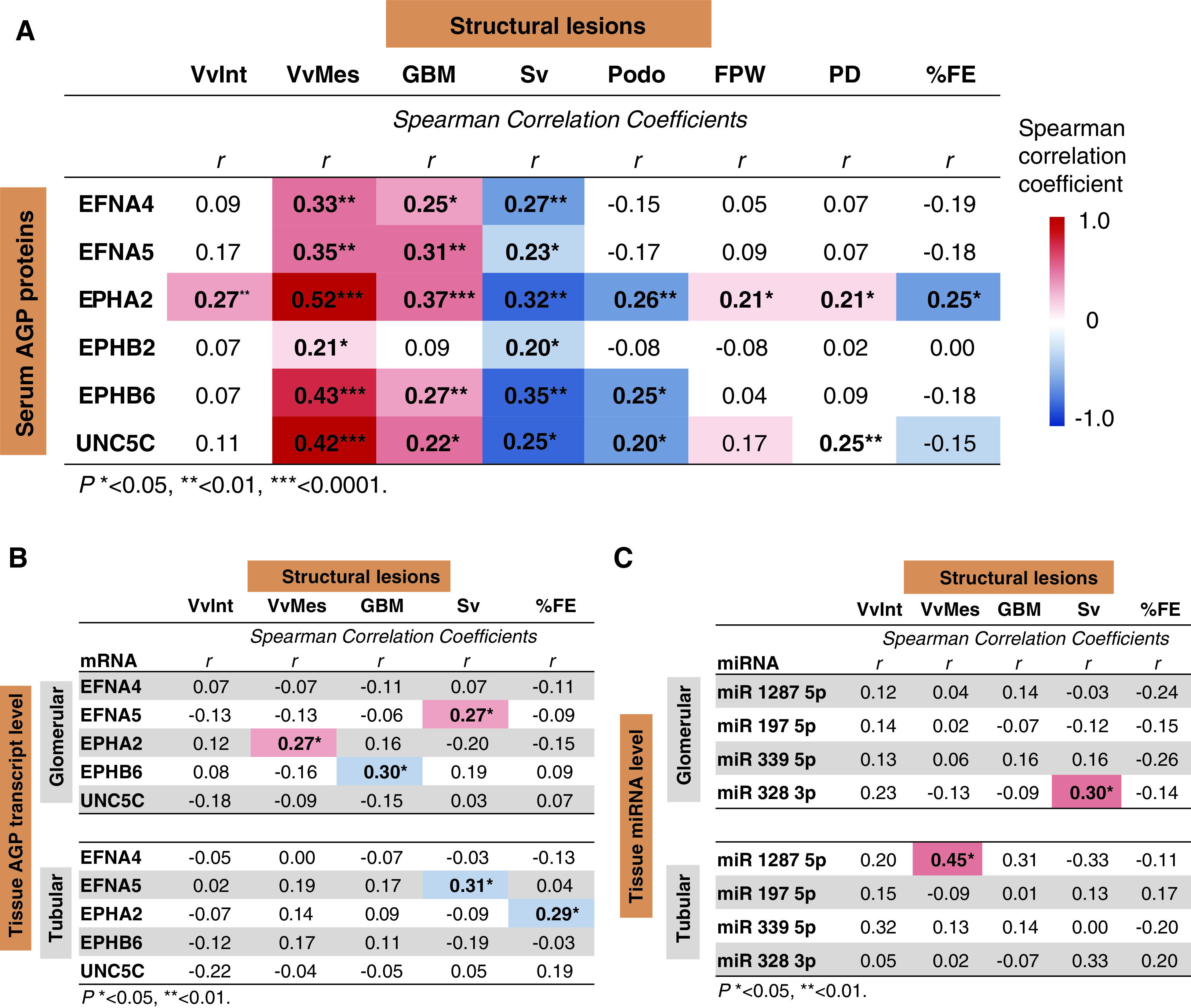
Circulating AGP proteins correlated to structural lesions in kidney biopsy specimens. (A) Correlation between baseline serum concentrations of six AGP proteins and structural lesions observed in kidney biopsy specimens obtained from 105 subjects in the T2D validation cohort. Biopsies were obtained, on average, 1 year later after baseline examination. (B) Correlation between transcript levels of genes encoding for AGP proteins in glomerular (n=66) and tubular (n=47) compartments, and structural lesions in kidney biopsy specimens obtained from the subjects in T2D validation cohort. Transcripts for five of the six AGP proteins were identified. (C) Correlation between expression of exemplar miRNAs in glomerular (n=40) and tubular (n=26) compartments and structural lesions in kidney biopsy specimens obtained from the subjects in T2D validation cohort. Spearman correlation coefficients (r) with statistically significant P values (<0.05) are shown in bold. FPW, podocyte foot process width (nm); PD, percentage of GBM surface with podocyte detachment (%); Podo, mean number of podocytes per glomerulus; Sv, glomerular filtration surface area density. *P<0.05, **P<0.01, ***P<0.0001.
Kidney Structural Lesions and Expression of AGP Genes and Exemplar miRNAs in Kidney Tissue
The mRNA expression levels for the six AGP proteins were measured in glomerular isolates from 66 subjects and in tubular isolates from 47 subjects. Spearman correlations showed little or no correlation between these AGP transcript levels and structural lesions in the same tissue (Figure 4B). Likewise, there was little or no correlation between levels of the four exemplar miRNAs in 40 glomerular and 26 tubular isolates from these biopsy specimens and the structural lesions (Figure 4C), except for a significant correlation between the expression of miR-1287-5p in tubules and VvMes.
In Situ Localization of Exemplar miRNAs in Human Kidney Tissue
Localization and levels of miRNAs were examined in kidney specimens using miRNAscope ISH. miR-1287-5p and miR-658 were detected in tissue affected by DKD, but not in normal kidney tissue (Figure 5). We did not test the probes designed for other miRNAs associated with ESKD that hybridized to multiple endogenous human genes. In the DKD kidney tissue, miR-1287-5p and miR-658 were detected in tubular epithelia (Figure 5, C and D), with lower levels in endothelial and interstitial cells (Figure 5, E and F). Both miRNAs were also detected in cytoplasmic membrane, which may indicate these miRNAs are derived from other cells or tissues. Precursor miRNAs of miR-1287 and miR-658 were also examined, and we observed either no signals or very weak signals in the kidney tissue (data not shown), supporting the hypothesis that these miRNAs originate from outside of the kidney.
Figure 5.
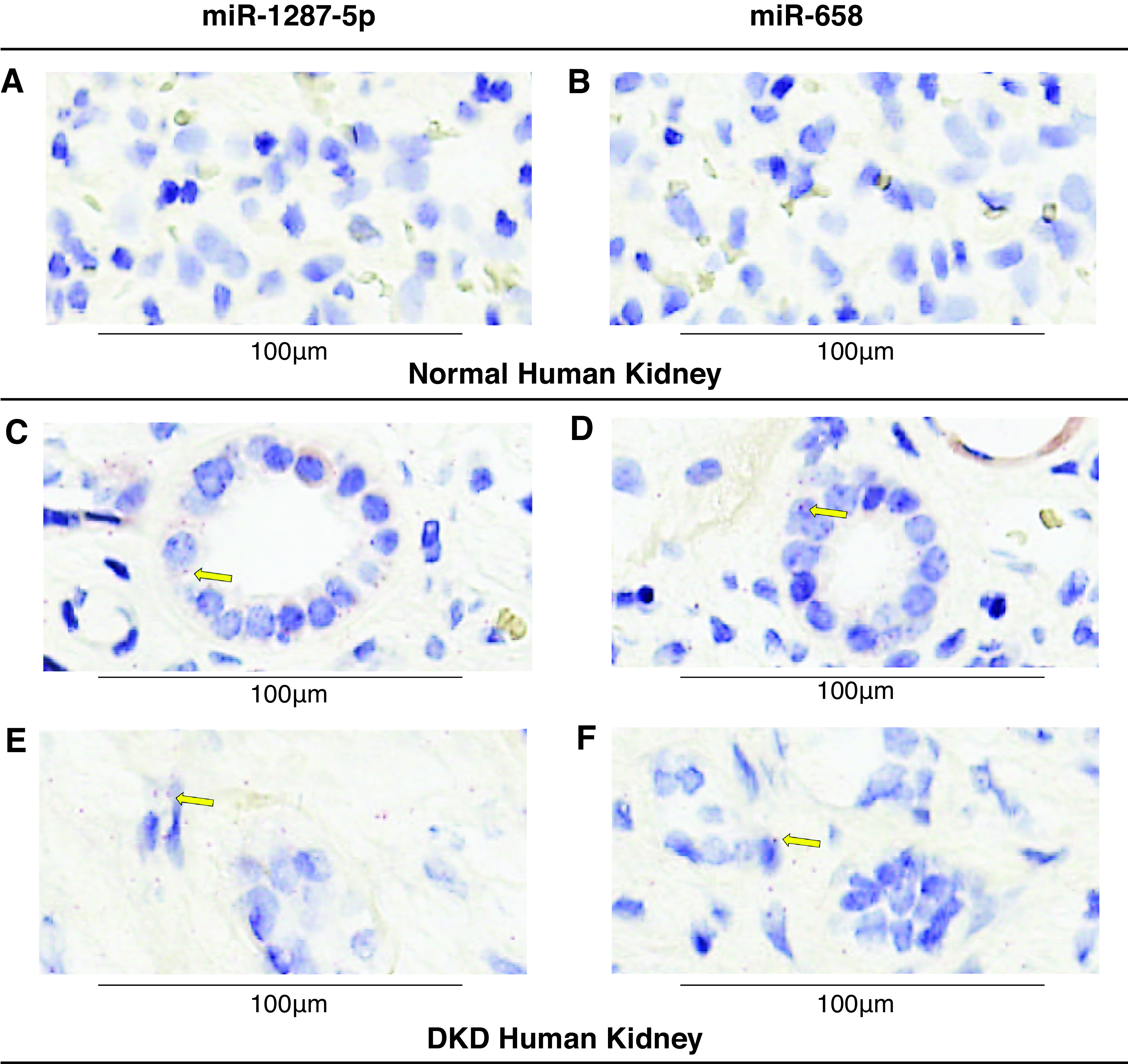
Tissue expression of miR-1287-5p and miR-658 in normal and DKD human kidney samples using miRNAscope ISH assay. (A–B) miR-1287-5p and miR-658 ISH assays in normal human kidney. (C–F) Detection of miR-1287-5p and miR-658 in DKD kidney. Red punctate dots indicate positive staining marked by yellow arrows. Blue hematoxylin counterstains the nuclei. Original magnification, 40×. Scale bar, 100 μm.
Immunofluorescence Staining of AGP Proteins in Kidney Tissue
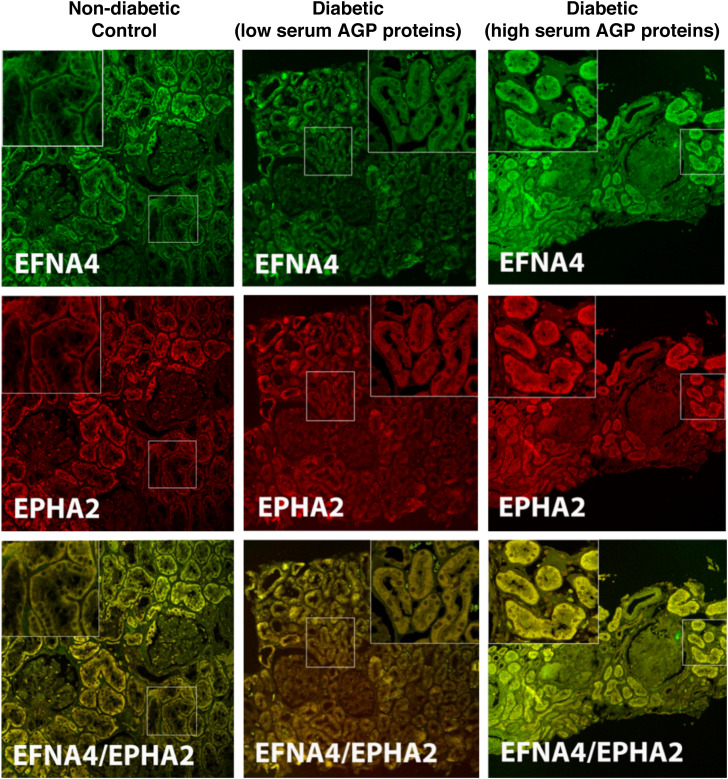
The kidney distribution of AGP proteins was assessed by immunofluorescence microscopy for EPHA2 and EFNA4 (a ligand of EPHA2)49 in two subjects from the Pima cohort with low or high serum AGP proteins and one White subject who was an age-matched, nondiabetic control. Both EPHA2 and EFNA4 were primarily expressed in proximal tubules, with minimal staining in glomeruli, distal tubules, interstitium, and vessels (Figure 6). The proximal tubules from the biopsy specimen of the subject with high serum AGP proteins showed increased staining intensities for EPHA2 and EFNA4 than those from the subject with low serum AGP proteins. The staining intensities in control, nondiabetic tissue were comparable to the biopsy specimen from the subject with diabetes who had low serum AGP proteins. EPHA2 showed almost perfect colocalization with EFNA4. No significant podocyte staining was seen for either protein. Despite the absence of staining for EPHA2 and EFNA4 in glomeruli, both biopsy specimens showed significant mesangial expansion (12% and 27%, respectively), and this lesion was highly correlated with circulating AGP protein levels.
Figure 6.
Immunofluorescence staining for EFNA4, EPHA2, and colocalization of the two AGP proteins in kidney biopsy tissue. A nondiabetic, age-matched control (left column), a Pima subject with T2D (middle column) and low serum AGP proteins (EFNA4, 2150 relative fluorescent units [RFU]; EPHA2, 3453 RFU; and VvMes, 12.3%), and a Pima subject with T2D (right column) and high serum AGP proteins (EFNA4, 2333 RFU; EPHA2, 4462 RFU; and VvMes, 27.2%).
Clinical Relevance of Circulating AGP Proteins
Circulating AGP Proteins and Risk of ESKD during 7, 10, and 15 Years of Follow-Up
The association between circulating AGP proteins and risk of progression to ESKD may vary according to duration of follow-up. To test this, HRs for time to onset of ESKD during 7, 10, and 15 years of follow-up were computed, after adjustment for relevant covariates, according to quartiles of baseline concentration of each of the six AGP proteins. As shown in Table 3, each of the circulating AGP proteins had the strongest association with risk of progression (highest HRs) to ESKD during the first 7 years of follow-up. The HRs declined slightly during longer follow-up. These findings indicate that circulating AGP proteins were associated with fast progression.
Table 3.
Sensitivity analysis
| Proteins | Follow-Up Duration | |||||
|---|---|---|---|---|---|---|
| 7 Years (n=187 ESKD cases) | 10 Years (n=240 ESKD cases) | 15 Years (n=289 ESKD cases) | ||||
| HR | P | HR | P | HR | P | |
| EFNA4 | 1.95 (1.66 - 2.30) |
7.5 × 10−16 | 1.84 (1.60 - 2.12) |
6.4 × 10−18 | 1.72 (1.52 - 1.94) |
1.6 × 10−17 |
| EFNA5 | 1.55 (1.34 - 1.80) |
7.1 × 10−9 | 1.45 (1.28 - 1.65) |
9.7 × 10−9 | 1.42 (1.27 - 1.60) |
1.4 × 10−9 |
| EPHA2 | 2.15 (1.83 - 2.53) |
3.2 × 10−20 | 1.99 (1.73 - 2.29) |
4.5 × 10−22 | 1.86 (1.65 - 2.11) |
1.1 × 10−22 |
| EPHB2 | 1.55 (1.34 - 1.79) |
3.0 × 10−9 | 1.51 (1.33 - 1.71) |
1.7 × 10−10 | 1.42 (1.26 - 1.59) |
2.6 × 10−9 |
| EPHB6 | 1.83 (1.56 - 2.14) |
9.3 × 10−14 | 1.78 (1.55 - 2.05) |
6.1 × 10−16 | 1.68 (1.48 - 1.90) |
9.0 × 10−16 |
| UNC5C | 1.90 (1.62 - 2.22) |
1.8 × 10−15 | 1.73 (1.51 - 1.98) |
3.4 × 10−15 | 1.70 (1.50 - 1.92) |
2.6 × 10−17 |
Comparison of HRs for time to onset of ESKD during 7, 10, and 15 years of follow-up, according to baseline levels of AGP protein, in the four combined cohorts (n=745). HRs are shown for time to onset of ESKD according to a one-quartile increase (continuous variable) in baseline concentration of the candidate AGP proteins. HR were computed for etiologic models adjusted for sex, duration of diabetes, systolic BP, HbA1c, and eGFR, with variable stratification by study cohort.
Circulating AGP Proteins as Predictors of ESKD Risk
A role of the baseline circulating AGP proteins as predictors of progression to ESKD during 10 years of follow-up were evaluated in all four study cohorts by Cox regression. The prognostic performance of two different models was evaluated using C-statistics, AIC, and NRI at 10 years (Table 4). The model with EFNA4, EPHA2, and clinical covariates was characterized by improved fit using the Uno concordance statistic and the AIC, when compared with only the clinical model. Similarly, the categoric NRI for the model with EFNA4 and EPHA2 showed significant improvements compared with the clinical model. These results demonstrated that measuring the levels of EFNA4 and EPHA2 in circulation may have value as predictors of progression to ESKD.
Table 4.
Comparison of performance of a prognostic model that included only clinical covariates versus a prognostic model that included clinical covariates and AGP proteins
| Selected Covariates and Prognostic Performance Tests | Models | |||
|---|---|---|---|---|
| Only Clinical | Clinical and AGP Proteins | |||
| Statistic | P Value | Statistic | P Value | |
| Effect estimates, HR (95% CI)a | ||||
| HbA1c (%) | 1.25 (1.16 to 1.34) | 6.8 × 10−10 | 1.31 (1.22 to 1.41) | 1.2 × 10−13 |
| eGFR per 10 ml/min per 1.73 m2 | 0.85 (0.79 to 0.92) | 2.4 × 10−5 | 0.93 (0.86 to 1.00) | 4.0 × 10−2 |
| log2ACR | 1.37 (1.28 to 1.46) | 1.6 × 10−22 | 1.28 (1.20 to 1.36) | 1.1 × 10−13 |
| EFNA4 per quartile | 1.27 (1.03 to 1.56) | 2.4 × 10−2 | ||
| EPHA2 per quartile | 1.42 (1.16 to 1.75) | 7.9 × 10−4 | ||
| C-statistic±SEMb | 0.7971±0.0122 | — | 0.8162±0.0112 | 4.0 × 10−3 |
| −2 log-likelihood ratio | 2708 | 2650 | ||
| AIC | 2720 | 2666 | ||
| NRI (versus clinical model 1) (95% CI)c | 0.19 (0.13 to 0.25) | 6.5 × 10−10d | ||
Cox regression models were used to evaluate 10-year ESKD risk in the four combined cohorts (n=745). The covariates selected by backward elimination for clinical model included HbA1c, baseline eGFR, ACR, and cohort indicator out of seven examined covariates (see Supplemental Table 2). The covariates selected by backward elimination for combined clinical and AGP proteins included HbA1c, baseline eGFR, ACR, cohort indicator, and six AGP proteins.
The effects are shown as HRs (95% CIs) per one-quartile change of EFNA4 or EPHA2 (continuous variables).
Uno concordance statistics with two-sided P values. Null values for C-statistics are 0.5 and 3013 for AIC, respectively. P value versus model 1.
Risk categories for ESKD are 0%–4.9%, 5.0%–9.9%, 10.0%–19.9%, and ≥20% over 10 years.
P value versus model 1.
Effect of Improved Glycemic Control on Circulating AGP Proteins
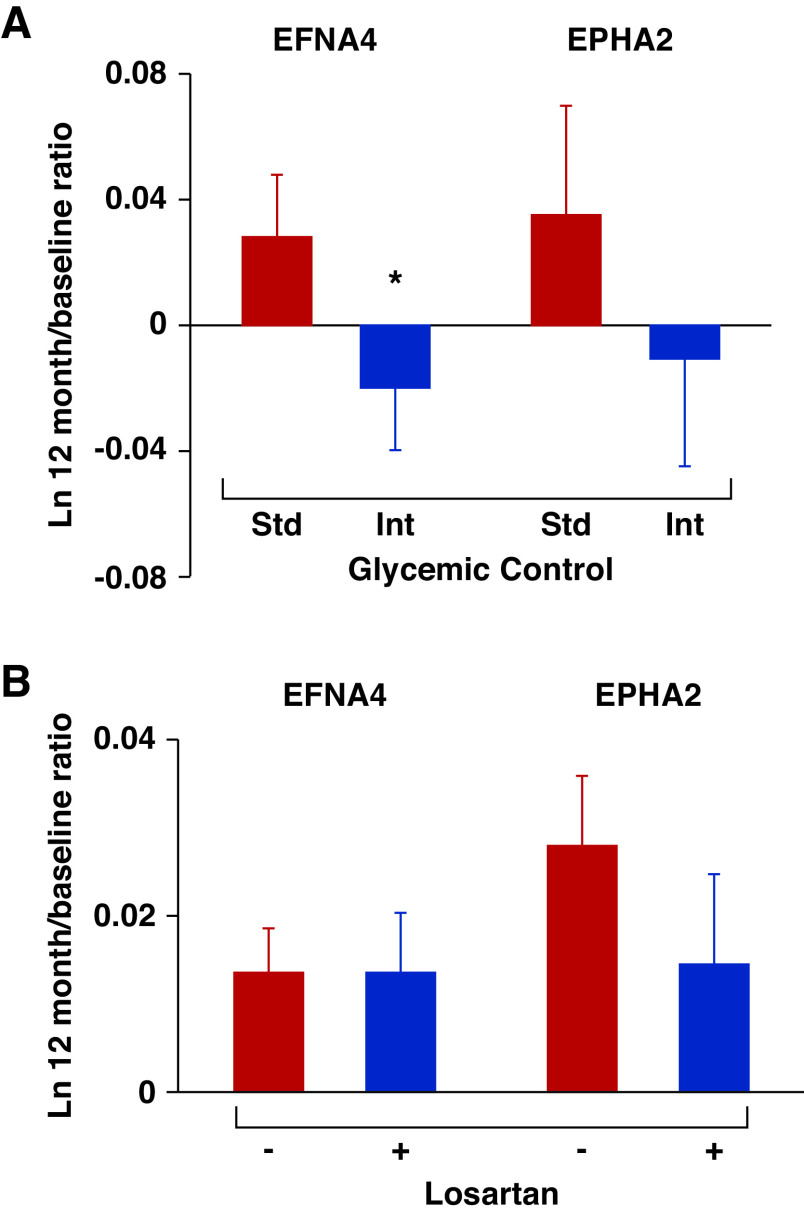
Circulating levels of AGP proteins might be surrogate end points for renoprotective therapies. To test this concept, we measured serum concentrations of EFNA4 and EPHA2 at baseline and at 12 months in a random sample of 200 participants with T2D in the ACCORD study. All baseline clinical characteristics and serum concentrations of EFNA4 and EPHA2 were similar according to glycemic treatment (see Supplemental Table 10). In the standard glycemic control arm, mean HbA1c decreased from 8.2% at baseline to 7.7% at 12 months, whereas it decreased from 8.2% to 6.6% in the intensive glycemic control arm. In parallel with these changes, serum EFNA4 increased, on average, by 2.8% in the standard arm, whereas it decreased by 2.0% in the intensive arm (P=0.04 for the difference in EFNA4 levels between treatment arms at 12 months; Figure 7A). A similar pattern was observed for EPHA2 (3.6% increase in the standard arm, 1.1% decrease in the intensive arm), although the difference between arms did not reach significance with this sample size (P=0.15; Figure 7A).
Figure 7.
Effects of intensive glycemic control and losartan treatment on serum levels of AGP proteins in two clinical trials. (A) Comparison of serum EFNA4 and EPHA2 levels between subjects with standard (n=100) versus intensive glycemic control (n=100) in the ACCORD study. Standard (Std) glycemic control indicated in red; intensive (Int) glycemic control in blue. (B) Comparison of serum EFNA4 and EPHA2 levels between subjects with losartan treatment (blue bars; n=37) versus placebo (red bars; n=47) in T2D Pima cohort. Data are shown as means±SEMs adjusting for sex, duration of diabetes, systolic BP, baseline HbA1c, and GFR. *P<0.05.
Effect of Treatment with Losartan and Circulating AGP Proteins
To examine whether treatment with angiotensin-converting enzyme inhibitors affected circulating AGP proteins, we examined levels of these proteins in 84 of the Pima Indian subjects who participated in a randomized clinical trial of losartan.18 Of these subjects, 47 were in the placebo arm and 37 were in the losartan arm. They received the study drug for at least 6 months before measurement of the AGP proteins. The levels of the six AGP proteins did not differ between the two subgroups (data not shown). Both subgroups had second measurements of their AGP proteins 2–4 years later. Treatment with losartan during this period had no effect on the profile of AGP proteins (Figure 7B). It is noteworthy that losartan treatment did not reduce the risk of kidney function decline in this 6-year trial.50
Effect of Exemplar miRNAs on Regulation of EPHA2 in an In Vitro Study
To study whether the four exemplar miRNAs modify synthesis and excretion of EPHA2, we performed in vitro RNAi experiments using miRNA mimics/inhibitors. Before the RNAi experiment, we assessed expression levels of EPHA2 in three human cell lines: HUVECs, RPTECs, and fibroblasts (Figure 8A). Only HUVECs had high concentrations of EPHA2 in both the lysate and the supernatant, so these cells were used for RNAi experiments. The EPHA2 level increased significantly in the cell lysate after transfection with the miR-197-5p mimic (P<0.01) or miR-328-3p inhibitor (P<0.05) (Figure 8B). The EPHA2 level in the cell lysate also increased after transfection with the miR-1287-5p mimic, but this did not reach statistical significance (P=0.09). A similar increase was observed for EPHA2 supernatant levels after transfection of HUVECs with miRNAs (Figure 8C). EFNA4 levels did not vary with transfection of miRNAs mimics or inhibitors (data not shown) in these experiments.
Figure 8.
Transfection of miRNA mimics and inhibitors into HUVECs increased levels of EPHA2. (A) Expression levels of EPHA2 in three human cell lines. The protein levels in cell lysate (pg/ml per μg protein) and supernatant (medium in which the cells were grown; pg/ml) were measured using the Olink platform (n=6). EPHA2 was not detected in supernatant from fibroblasts. EPHA2 levels in (B) cell lysate and (C) supernatant from HUVECs. HUVECs were transfected with 20 nM of miRNA mimics (blue) or inhibitors (red) (n=3). Cell lysates were normalized to the same protein concentration and measured using the Olink platform. Data are shown as means±SEMs of a representative experiment performed in triplicate. The gray bars indicate controls. ND, not detected. *P<0.05, **P<0.01.
Discussion
Unbiased, global miRNome and targeted proteomic approaches identified novel circulating miRNAs and circulating AGP proteins that were strongly associated with 10-year ESKD risk. Circulating AGP protein levels were also strongly associated with early structural lesions in research kidney biopsy specimens from the Pima cohort, but they were not associated with kidney tissue levels of the gene transcripts encoding for these proteins, or for exemplar miRNAs. The findings were replicated and validated in independent cohorts that included subjects with both diabetes types, in early and late DKD stages, and of different races.
Of the 2083 known circulating miRNAs, 17 were strongly associated with ESKD risk. These significantly intercorrelated miRNAs could be grouped into two upregulated and two downregulated clusters, with each cluster represented by one exemplar miRNA. Indeed, considered together, one exemplar from the upregulated miRNA cluster and one from the downregulated miRNA cluster could account for ESKD risk in the study cohorts. This association pattern suggests these miRNAs have common, yet unknown, mechanisms of dysregulation.
Tissue culture and rodent model studies implicate some of these 17 miRNAs in the development of DKD. These included the miRNAs miR-1207-5p,51 miR-328-3p,52 and miR-378a-3p.53 Multiple circulating miRNAs were reported to be associated with DKD in human studies. Those miRNAs, and their accompanying fold changes obtained in our study, are provided in Supplemental Table 11. Of the 11 circulating miRNAs reported in the literature, only findings for miR-130b-3p were replicated in our study; however, its associated P value did not reach the threshold of Bonferroni adjustment in the T1D cohort. Findings for other miRNAs were not replicated. There are several explanations for these discrepancies. First, findings from all previous studies were uncertain due to small sample size. Second, most of these studies were cross-sectional and could not determine whether abnormalities in circulating miRNAs preceded DKD or were consequences of it. Third, RNA extraction–based methods were used to determine the concentration of miRNAs in all of the previous studies, whereas we used a method that measured total circulating miRNAs in plasma, without extracting RNA, in this study.
Bioinformatic tools were used to predict miRNA-binding sites of known human genes. Pathway analysis of predicted genes targeted by the four exemplar miRNAs revealed enrichment of 23 pathways. Unexpectedly, the strongest enrichment was present for genes encoding for AGP proteins. To validate these results, the SOMAscan proteomics platform was used to measure circulating proteins. Remarkably, although 42 proteins belonging to the AGP were measured, only the increased levels of six AGP proteins, i.e., five ephrin ligands/receptors and one netrin receptor, were strongly associated with the severity of early structural lesions on kidney biopsy specimens and with risk of ESKD during 10-year follow-up.
The AGP is important in the development of the nervous system. AGP proteins—such as ephrins, netrins, Slits, semaphorins, and Wnts—are involved in neurogenesis, the formation of neuronal networks, and regeneration of damaged nerves. Abnormalities of AGP proteins are implicated in neurologic disorders, including Alzheimer disease,54,55 multiple sclerosis,56,57 and amyotrophic lateral sclerosis.58 Importantly, ephrin receptors and ephrin ligands are also recognized to have many functions outside of the central nervous system,47,59–61 including important roles in the development and repair of many cell types in vascularized tissues, and in processes including kidney angiogenesis and blood vessel maturation.62,63
The circulating AGP proteins we identified were strongly correlated with early structural kidney lesions, especially mesangial expansion, i.e., the structural kidney lesion most strongly associated with kidney function loss leading to ESKD in DKD.39,64,65 Surprisingly, kidney tissue levels of the gene transcripts encoding these AGP proteins did not correlate with any early structural lesions. These findings suggest that these early structural DKD lesions are largely determined by circulating factors, such as the miRNAs and AGP proteins we identified, rather than by local mechanisms. Funk et al.66,67 reported that EPHA2 was upregulated in atherosclerotic plaques in humans and mice, and that it regulated both plaque inflammation and progression of advanced atherosclerotic lesions. Our findings challenge the existing kidney-centric model of DKD, in which the major focus is on molecular dysregulation of various kidney compartments, and suggest that factors beyond the kidney should also be considered.
Our findings should stimulate the search for new drug targets to prevent or treat DKD. Such treatments may include miRNA-based therapies to increase circulating levels of downregulated miRNAs, or anti-miRNA sponge systems to neutralize the effects of upregulated miRNAs.68 Further studies are needed to establish whether the exemplars or the other miRNAs, or combinations of these miRNAs, offer the best therapeutic targets to slow or prevent DKD. Likewise, molecules in the Ephrin ligand/receptor pathway for DKD need further study.69,70 Our observation that improved glycemic control reduced circulating EPHA2 and EFNA4 levels is encouraging, but requires confirmation in a larger study.
The miRNAi experiments confirming the effects of miRNAs on regulation of EPHA2 concentration in the cell lysate and supernatant of HUVECs are of great significance for two reasons. First, they validated the bioinformatic findings that AGP genes/proteins were targeted by exemplar miRNAs. Second, they showed that only certain cell lines, i.e., HUVECs but not RPTECs or fibroblasts, secreted AGP proteins. The latter findings point to the complexity involved in regulating the levels of AGP proteins in circulation. At present, the mechanism through which AGP protein levels are regulated by miRNAs is not clear, but direct and indirect effects should be considered. Regarding direct effects, it is assumed that the candidate miRNAs bind to the 3′ untranslated region of target mRNA and inhibit translation. In fact, miR-339-5p and miR-328-3p were predicted to have sequences that target the 3′ untranslated region of EPHA2. On the other hand, in terms of indirect effects, miR-1287-5p was predicted to target CBL in our analysis, and CBL was reported to regulate EPHA2 negatively.71 Therefore, miR-1287-5p may inhibit CBL expression, which results in upregulation of EPHA2 indirectly.
In our study, we evaluated the effects of losartan treatment and improved glycemic control on the levels of circulating AGP proteins. Whereas the losartan did not have any effect, surprisingly, intensive glycemic control reduced circulating levels of AGP proteins. Although there is no explanation for this finding, at this time, it is possible that variation in hyperglycemia might result in variation of circulating exemplar miRNAs, and that this variation affected circulating levels of AGP proteins. Currently, dasatinib is the only available drug showing inhibitory effects on EPHA2 activity.72 Several other AGP protein–targeting therapies are currently being tested in clinical trials for solid tumors (Supplemental Table 12). A novel EPHA2 inhibitor exerted beneficial effects in postinfectious irritable bowel syndrome via the Nrf2 and NF-κB signaling pathways.73 At present, it is unknown whether drugs affecting circulating levels of EPHA2 could modify development of DKD.
The strengths of our study include (1) prospective long-term follow-up in multiple cohorts of different ethnicities and different types of diabetes; (2) use of gold-standard definitions of DKD, i.e., structural lesions visible on research kidney biopsy specimens, as assessed by quantitative morphometry, and time to ESKD onset; (3) reliable and uniform measurements of the concentrations of circulating miRNAs and AGP proteins in all cohorts. There are also some limitations. This study is observational, and causality remains to be established through animal studies and clinical trials; however, mediation analysis and in vitro study support our hypothesis. The study cohorts are relatively small for the evaluation of prognostic models, so further studies with larger cohorts are required to confirm our results.
Disclosures
M. Bitzer reports receiving honoraria from the National Institutes of Health (NIH). A. Doria reports receiving honoraria from the American Diabetes Association (honorarium for serving as associate editor), and serving as associate editor for DIABETES. K. L. Duffin reports being employed by, and receiving research funding from, Eli Lilly and Company; and having ownership interest in Eli Lilly and Pfizer. C. R. Kahn reports serving as scientific advisor for, or member of, with Cellarity Biotech, CohBar, ERX Biotech, and Kaleido Biotech; and having consultancy agreements with, ownership interest in, and honoraria from Cellarity Biotech, CohBar, ERX Biotech, Kaleido Biotech, and Sana Biotech. M. Kretzler reports receiving research funding, via a sponsored research project as principal investigator at the University of Michigan, from amfAR, Angion, AstraZeneca, Boehringer Ingelheim, Certa, Chan Zuckerberg Initiative, Chinook, Elpidera, Gilead, Goldfinch, Ionis, Jansen, JDRF, Lilly, NIH, Novo Nordisk, Regeneron, RenalytixAI, and Travere; having consultancy agreements with Astellas, Boehringer Ingelheim, Certa, Janssen, Novo Nordisk, and Poxel (as an employee of University of Michigan); serving on the editorial boards of JASN, Kidney International, and Kidney Disease; and serving on the advisory board of NephCure Kidney International. R. N. Kulkarni reports receiving research funding from Inversago; having consultancy agreements with Inversago and REDD Pharmaceuticals; and serving as a scientific advisor for, or member of, Novo Nordisk. M. Mauer reports having consultancy agreements with Amicus, Avrobio, Bayer, Boeheringer Ingelheim, Freeline Theraputics, Sangano, and Sanofi/Genzyme; receiving honoraria from Amicus, Freeline Therapeutics, and Sanofi/Genzyme; receiving research funding from Amicus and Sanofi/Genzyme; and serving on the North American Fabry Registry Board. B. Najafian reports having consultancy agreements with Amicus, Avrobio, 4D Molecular Therapeutics, Freeline Therapeutics, Sangamo, and Sanofi; serving as a scientific advisor for, or member of, Amicus, Freeline Therapeutics, and Sanofi; and receiving research funding and honoraria from Amicus and Sanofi. M. A. Niewczas reports receiving honoraria from Indiana Diabetes Research Center (honorarium for the pilot and feasibility grant review), and serving as an editorial board member for Journal of Diabetes Research. M. E. Pavkov reports serving as a member of the Kidney Health Initiative Board of Directors. E. Satake repo rts receiving research funding from Novo Nordisk and Sunstar Foundation. J. M. Wilson reports being employed by Lilly. All remaining authors have nothing to disclose.
Funding
We acknowledge support from the NIH Clinical Center grants DK041526 (to A.S. Krolewski), and DK110350 (to M.G. Pezzolesi), and DK100449 (to M. Bitzer); Novo Nordisk Foundation grant NNF14OC0013659 (PROTON; to A.S. Krolewski); JDRF grants 3-SRA-2015-106-Q-R (to A. S. Krolewski) and 5-CDA-2015-89-A-B (to M. A. Niewczas); the Mary K. Iacocca Fellowship; the Sunstar Foundation; the Hiroo Kaneda Scholarship; the Foundation for Growth Science of Japan (to E. Satake); the Uehara Memorial Foundation (postdoctoral fellowship); and the Japan Society for the Promotion of Science (overseas research fellowship; to H. Kobayashi). This research was also supported by the American Diabetes Association Clinical Science Award 1-08-CR-42 (to R. G. Nelson), by the Centers for Disease Control and Prevention Interagency Agreement 16FED1604631 (to R. G. Nelson), by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program, and by the NIH Clinical Center, Diabetes Endocrinology Research Center grant P30 DK036836 (to Joslin Diabetes Center). This study was also supported by George M. O’Brien Michigan Kidney Translational Core Center, funded by NIH Clinical C enter/NIDDK grant 2P30 DK081943.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the C enters for Disease Control and Prevention.
The authors would like to thank Elsevier Publisher for permitting us to modify our original Figure from ref. 47. The authors would also like to thank Harry Spaulding for his assistance in the preparation of this manuscript.
R. G. Nelson and A. S. Krolewski are the guarantors of this work and, as such, had full access to the data for their cohorts and take responsibility for the integrity of the data and the accuracy of the data analysis.
E. Satake contributed to design of the study, contributed to the miRNome and proteomic data collection in the JKS, performed data analysis, and wrote the manuscript; H. Kobayashi, Z. I. Md Dom, K. Ihara, K. O’Neil, and B. Krolewski contributed to implementation of measurements, generation of data, and analysis of data in the JKS; R. G. Nelson, M. E. Pavkov, P.-J. Saulnier, and H. C. Looker were responsible for design and implementation of the Pima Indian Study, contributed to the proteomic data collection in the Pima Indian Study, performed data analysis, and reviewed and edited the manuscript; M. Kretzler, V. Nair, M. Bitzer, and R. G. Nelson designed the expression study in the Pima Indians, performed data analysis, and reviewed the manuscript; M. Mauer selected and provided the tissue samples for immunostaining and reviewed the manuscript; B. Najafian performed immunostaining experiments, interpreted the results, and reviewed the manuscript; J. M. Wilson and K. L. Duffin developed and implemented experiments to localize miRNAs in kidney tissue, and contributed to interpretation of the results and to the editing of the manuscript; A. Doria was responsible for design and implementation of the analysis of the data from the ACCORD study, and reviewed and edited the manuscript; M. K. Gupta, C. Pipino, B. Krolewski, and R. N. Kulkarni designed and performed the experiments in vitro and reviewed the manuscript; M. A. Niewczas, J. M. Wilson, K. L. Duffin, M. G. Pezzolesi, and C. R. Kahn contributed to interpretation of the results and to the editing of the manuscript; A. S. Krolewski designed the study, supervised the study implementation, and contributed to data analysis, interpretation of findings, and to writing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Predictors of kidney disease progression in diabetes and precision medicine: Something old, something new, and something borrowed,” on pages 2108–2111.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021010105/-/DCSupplemental.
Supplemental Material. Supplementary methods.
Supplemental Table 1. Comparison of AGP proteins measurements performed by SOMAscan platform with measurements of AGP proteins performed by Olink platform.
Supplemental Table 2. Assessment of importance of clinical covariates for time to onset of ESKD in combined 4 cohorts.
Supplemental Figure 1. Ratios of median plasma concentration of each miRNA at baseline in subjects who did and did not develop ESKD during 10-year follow -up.
Supplemental Figure 2. Matrix of Spearman correlations among 17 candidate miRNAs in subjects with late DKD (n=375).
Supplemental Table 3. Association of plasma levels of 17 candidate miRNAs measured at baseline with time to onset of ESKD during 10-year follow-up in subjects with late DKD.
Supplemental Table 4. Statistical data for comparison of cumulative incidence of progression to ESKD during 10-year follow-up according to baseline plasma levels of miR-1287-5p and miR-339-5p grouped into risk categories (discrete variable) in subjects with late DKD.
Supplemental Table 5. List of 23 pathways enriched in genes predicted to be targeted by the 4 exemplar miRNAs.
Supplemental Table 6. Number of proteins in Ras, MAPK and HIF-1 signaling pathways present on SOMAscan platform and their association with risk of ESKD according to study cohorts.
Supplemental Table 7. Hazard ratios per 1-SD increase in AGP proteins levels for time to onset of ESKD during a 10-year follow-up.
Supplemental Table 8. List of 36 AGP proteins present on SOMAscan platform that were not associated with risk of ESKD or not confirmed in study cohorts.
Supplemental Table 9. Effects of exemplar miRNAs on ESKD risk during 10-year follow-up according to plasma levels of AGP proteins (mediators).
Supplemental Table 10. Baseline characteristics of ACCORD participants in whom serum EFNA4 and EPHA2 were measured.
Supplemental Table 11. List of circulating miRNAs previously reported as ones associated with DKD.
Supplemental Table 12. Current and future clinical treatments using ephrin and netrin receptors for any other indications.
Supplemental Figure 3. Distributions of levels of 4 miRNAs in the late DKD cohorts.
Supplemental Figure 4. Distributions of levels of 6 AGP proteins in the 4 cohorts.
References
- 1.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. : The microRNA spectrum in 12 body fluids. Clin Chem 56: 1733–1741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP: Metazoan microRNAs. Cell 173: 20–51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipowicz W, Bhattacharyya SN, Sonenberg N: Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet 9: 102–114, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Kim VN: MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Friedman RC, Farh KKH, Burge CB, Bartel DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. : Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542: 450–455, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR: Extracellular miRNAs: From biomarkers to mediators of physiology and disease. Cell Metab 30: 656–673, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backes C, Meese E, Keller A: Specific miRNA disease biomarkers in blood, serum and plasma: Challenges and prospects. Mol Diagn Ther 20: 509–518, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Natarajan R: Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol 15: 327–345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, et al. : The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 82: 589–597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, et al. : Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. : A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25: 805–813, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihara K, Skupien J, Krolewski B, Md Dom ZI, O’Neil K, Satake E, et al. : A profile of multiple circulating tumor necrosis factor receptors associated with early progressive kidney decline in type 1 diabetes is similar to profiles in autoimmune disorders. Kidney Int 99: 725–736, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skupien J, Warram JH, Smiles A, Galecki A, Stanton RC, Krolewski AS: Improved glycemic control and risk of ESRD in patients with type 1 diabetes and proteinuria. J Am Soc Nephrol 25: 2916–2925, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Md Dom ZI, Satake E, Skupien J, Krolewski B, O’Neil K, Willency JA, et al. : Circulating proteins protect against renal decline and progression to end-stage renal disease in diabetes: Results of global proteomics profiling. Sci Transl Med 13: eabd2699, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson RG, Newman JM, Knowler WC, Sievers ML, Kunzelman CL, Pettitt DJ, et al. : Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 31: 730–736, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al. ; Diabetic Renal Disease Study Group: Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 335: 1636–1642, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, et al. : Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 62: 3224–3231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Songia P, Chiesa M, Valerio V, Moschetta D, Myasoedova VA, D’Alessandra Y, et al. : Direct screening of plasma circulating microRNAs. RNA Biol 15: 1268–1272, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeri A, Courtright A, Danielson K, Hutchins E, Alsop E, Carlson E, et al. : Evaluation of commercially available small RNAseq library preparation kits using low input RNA. BMC Genomics 19: 331, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godoy PM, Barczak AJ, DeHoff P, Srinivasan S, Etheridge A, Galas D, et al. : Comparison of reproducibility, accuracy, sensitivity, and specificity of miRNA quantification platforms. Cell Rep 29: 4212–4222.e5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson MD, McCarthy DJ, Smyth GK: edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolstad BM, Irizarry RA, Astrand M, Speed TP: A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Law CW, Chen Y, Shi W, Smyth GK: voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15: R29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. : Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One 5: e15004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuerk C, Gold L: Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249: 505–510, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, et al. : Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA 315: 2532–2541, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Ngo D, Sinha S, Shen D, Kuhn EW, Keyes MJ, Shi X, et al. : Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation 134: 270–285, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams SA, Murthy AC, DeLisle RK, Hyde C, Malarstig A, Ostroff R, et al. : Improving assessment of drug safety through proteomics: Early detection and mechanistic characterization of the unforeseen harmful effects of torcetrapib. Circulation 137: 999–1010, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, et al. : Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 9: e95192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweep H, Gretz N: miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat Methods 12: 697, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M: KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44[D1]: D457–D462, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. : DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 34.Squarer A, Lemley KV, Ambalavanan S, Kristal B, Deen WM, Sibley R, et al. : Mechanisms of progressive glomerular injury in membranous nephropathy. J Am Soc Nephrol 9: 1389–1398, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Nair V, Komorowsky CV, Weil EJ, Yee B, Hodgin J, Harder JL, et al. : A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney Int 93: 439–449, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, et al. ; European Renal cDNA Bank (ERCB) Consortium: Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55: 2993–3003, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Cohen CD, Frach K, Schlöndorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Martini S, Nair V, Keller BJ, Eichinger F, Hawkins JJ, Randolph A, et al. ; European Renal cDNA Bank; C-PROBE Cohort; CKDGen Consortium: Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 25: 2559–2572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai JY, Luo J, O’Connor C, Jing X, Nair V, Ju W, et al. : MicroRNA-21 in glomerular injury. J Am Soc Nephrol 26: 805–816, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caramori ML, Kim Y, Goldfine AB, Moore JH, Rich SS, Mychaleckyj JC, et al. : Differential gene expression in diabetic nephropathy in individuals with type 1 diabetes. J Clin Endocrinol Metab 100: E876–E882, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Pipino C, Shah H, Prudente S, Di Pietro N, Zeng L, Park K, et al. : Association of the 1q25 diabetes-specific coronary heart disease locus with alterations of the γ-glutamyl cycle and increased methylglyoxal levels in endothelial cells. Diabetes 69: 2206–2216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Tomo P, Canali R, Ciavardelli D, Di Silvestre S, De Marco A, Giardinelli A, et al. : β-Carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol Nutr Food Res 56: 217–227, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Salgado C, Rodríguez-Peña AB, López-Novoa JM: Involvement of small Ras GTPases and their effectors in chronic renal disease. Cell Mol Life Sci 65: 477–492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grynberg K, Ma FY, Nikolic-Paterson DJ: The JNK signaling pathway in renal fibrosis. Front Physiol 8: 829, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase VH: Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, et al. : Eph/Ephrin signaling in injury and inflammation. Am J Pathol 181: 1493–1503, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Larrieu-Lahargue F, Thomas KR, Li DY: Netrin ligands and receptors: Lessons from neurons to the endothelium. Trends Cardiovasc Med 22: 44–47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasquale EB: The Eph family of receptors. Curr Opin Cell Biol 9: 608–615, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Tanamas SK, Saulnier PJ, Fufaa GD, Wheelock KM, Weil EJ, Hanson RL, et al. : Long-term effect of Losartan on kidney disease in American Indians with type 2 diabetes: A follow-up analysis of a randomized clinical trial. Diabetes Care 39: 2004–2010, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez ML, Khosroheidari M, Eddy E, Kiefer J: Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS One 8: e77468, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CH, Cheng CY, Chen YC, Sue YM, Liu CT, Cheng TH, et al. : MicroRNA-328 inhibits renal tubular cell epithelial-to-mesenchymal transition by targeting the CD44 in pressure-induced renal fibrosis. PLoS One 9: e99802, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B, Yao K, Wise AF, Lau R, Shen HH, Tesch GH, et al. : miR-378 reduces mesangial hypertrophy and kidney tubular fibrosis via MAPK signalling. Clin Sci (Lond) 131: 411–423, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Wetzel-Smith MK, Hunkapiller J, Bhangale TR, Srinivasan K, Maloney JA, Atwal JK, et al. ; Alzheimer’s Disease Genetics Consortium: A rare mutation in UNC5C predisposes to late-onset Alzheimer’s disease and increases neuronal cell death. Nat Med 20: 1452–1457, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang B, Li H, Mutlu SA, Bowser DA, Moore MJ, Wang M, et al. : The amyloid precursor protein is a conserved receptor for Slit to mediate axon guidance. eNeuro 4: ENEURO.0185-17.2017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eixarch H, Gutiérrez-Franco A, Montalban X, Espejo C: Semaphorins 3A and 7A: Potential immune and neuroregenerative targets in multiple sclerosis. Trends Mol Med 19: 157–164, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Rezaeepoor M, Shapoori S, Ganjalikhani-Hakemi M, Etemadifar M, Alsahebfosoul F, Eskandari N, et al. : Decreased expression of Sema3A, an immune modulator, in blood sample of multiple sclerosis patients. Gene 610: 59–63, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Tyzack GE, Hall CE, Sibley CR, Cymes T, Forostyak S, Carlino G, et al. : A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat Commun 8: 1164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basile DP, Yoder MC: Getting the “inside” scoop on ephrinB2 signaling in pericytes and the effect on peritubular capillary stability. J Am Soc Nephrol 24: 521–523, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Miao H, Wang B: Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol 41: 762–770, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein E, Schoecklmann H, Daniel TO: Eph family receptors and ligands in vascular cell targeting and assembly. Trends Cardiovasc Med 7: 329–334, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Peuckert C, Aresh B, Holenya P, Adams D, Sreedharan S, Porthin A, et al. : Multimodal Eph/Ephrin signaling controls several phases of urogenital development. Kidney Int 90: 373–388, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Weiss AC, Kispert A: Eph/ephrin signaling in the kidney and lower urinary tract. Pediatr Nephrol 31: 359–371, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Fufaa GD, Weil EJ, Lemley KV, Knowler WC, Brosius FC 3rd, Yee B, et al. : Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 11: 254–261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M; International Diabetic Nephopathy Study Group: The early natural history of nephropathy in type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Funk SD, Yurdagul A Jr, Albert P, Traylor JG Jr, Jin L, Chen J, et al. : EphA2 activation promotes the endothelial cell inflammatory response: A potential role in atherosclerosis. Arterioscler Thromb Vasc Biol 32: 686–695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finney AC, Funk SD, Green JM, Yurdagul A Jr, Rana MA, Pistorius R, et al. : EphA2 expression regulates inflammation and fibroproliferative remodeling in atherosclerosis. Circulation 136: 566–582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebert MS, Neilson JR, Sharp PA: MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4: 721–726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. : Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res 65: 6910–6918, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Saha N, Robev D, Mason EO, Himanen JP, Nikolov DB: Therapeutic potential of targeting the Eph/ephrin signaling complex. Int J Biochem Cell Biol 105: 123–133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Ota S, Kataoka H, Kanamori M, Li Z, Band H, et al. : Negative regulation of EphA2 receptor by Cbl. Biochem Biophys Res Commun 296: 214–220, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Ieguchi K, Maru Y: Roles of EphA1/A2 and ephrin-A1 in cancer. Cancer Sci 110: 841–848, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng L, Li K, Wei H, Hu J, Jiao L, Yu S, et al. : A novel EphA2 inhibitor exerts beneficial effects in PI-IBS in vivo and in vitro models via Nrf2 and NF-κB signaling pathways. Front Pharmacol 9: 272, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.