Abstract
Magnesium is an essential cofactor in many cellular processes, and aberrations in magnesium homeostasis can have life-threatening consequences. The kidney plays a central role in maintaining serum magnesium within a narrow range (0.70–1.10 mmol/L). Along the proximal tubule and thick ascending limb, magnesium reabsorption occurs via paracellular pathways. Members of the claudin family form the magnesium pores in these segments, and also regulate magnesium reabsorption by adjusting the transepithelial voltage that drives it. Along the distal convoluted tubule transcellular reabsorption via heteromeric TRPM6/7 channels predominates, although paracellular reabsorption may also occur. In this segment, the NaCl cotransporter plays a critical role in determining transcellular magnesium reabsorption. Although the general machinery involved in renal magnesium reabsorption has been identified by studying genetic forms of magnesium imbalance, the mechanisms regulating it are poorly understood. This review discusses pathways of renal magnesium reabsorption by different segments of the nephron, emphasizing newer findings that provide insight into regulatory process, and outlining critical unanswered questions.
Keywords: cell and transport physiology, magnesium, claudin, ion transport, Gitelman syndrome
Introduction
Magnesium (Mg2+) is the fourth most abundant cation in the body and the second most abundant intracellularly after potassium (K+). The concentrations of free cytosolic and extracellular Mg2+ are similar (0.70–1.10 mmol/L), but total intracellular concentrations are high (5–20 mmol/L) because it is 90% bound and acts as a cofactor for >300 enzymes involved in essential processes including maintenance of ionic gradients; ATP production by the mitochondria; and DNA, RNA, and protein synthesis.1 Given its diverse roles, disturbances of Mg2+ homeostasis can cause systemic defects, some of which are potentially life-threatening. Hypermagnesemia (serum [Mg2+]>1.10 mmol/L) is primarily observed in the setting of renal failure because the kidney is the key regulator of serum [Mg2+], or when a large Mg2+ load is given (e.g., to lower neuromuscular excitability in preeclampsia). In severe cases, severe respiratory paralysis or cardiac arrest can occur. Hypomagnesemia (serum [Mg2+]<0.70 mmol/L) is common in hospitalized patients (up to 12%), and has a prevalence as high as 65% in ICU patients.1 It has many causes including uncontrolled type 2 diabetes, alcoholism, medications, and several genetic diseases. Symptoms reflect neuromuscular hyperexcitability and include tremor, muscle fasciculations, and muscle cramps; in more severe cases, tetany, and generalized seizures.2 Hypomagnesemia is frequently associated with hypokalemia and/or hypocalcemia, which can result in cardiac arrhythmia and sudden death.2 With the notable exception of impaired intestinal uptake seen with proton pump inhibitor usage, dysregulation of renal Mg2+ handling plays a central role in most cases of hypomagnesemia. In total, 70%–80% of serum Mg2+ is in ionized or complexed forms with anions and is ultrafilterable by the kidney. Compared with other cations, renal handling of filtered Mg2+ is unusual because it is primarily reabsorbed along the thick ascending limb (TAL) (65%–70% of filtered load),3–6 rather than the proximal tubule (PT), which only reabsorbs 10%–25% of the filtered load.3,4,6 The distal convoluted tubule (DCT) only reabsorbs 3%–7% of the filtered load,6–8 but plays an important role in fine-tuning Mg2+ homeostasis. About 4% of the filtered Mg2+ is excreted in the urine to maintain whole body balance. In this review we will summarize mechanisms of Mg2+ reabsorption along the nephron, and discuss new findings that expand our understanding of this process. Along the TAL, careful characterization of claudin expression suggests that paracellular pathways for Na+ and Mg2+ may be distinct, raising the possibility of complex regulatory mechanisms. New data also suggest regulation of paracellular Mg2+ reabsorption by membrane trafficking of claudin-16, and claudin-14–mediated regulation of claudin-16/19. Along the DCT, recent data suggest that in addition to regulation by EGF, Mg2+ reabsorption via the channel transient receptor potential channel subfamily M, member 6 (TRPM6) may also undergo regulation by uromodulin (UMOD), also known as Tamm–Horsfall protein, and fluid flow.
Proximal Tubule
The PT displays high permeability for small ions such as Na+, K+, Ca2+, and Cl−, and for water, reabsorbing around two-thirds of the filtered loads for each by the end of the segment. In contrast, permeability to Mg2+ is relatively low, with only about one-third reabsorbed by the late PT.5,9 Mg2+ reabsorption along the PT is considered to occur by a passive paracellular mechanism that is likely to be largely unregulated because it remains linear and unsaturable over a wide range of luminal [Mg2+].3,10 Thiazide diuretics likely cause hypocalciuria, in part, by provoking increased Ca2+ reabsorption as a result of increased transepithelial electrochemical gradients linked to enhanced sodium reabsorption along the PT; PT Mg2+ reabsorption does not parallel Na+ reabsorption, which may explain why thiazides do not reduce urinary Mg2+ excretion. The molecular pathways of PT Mg2+ reabsorption have not been identified, but two members of the claudin family, which act as gatekeepers of tight junction movement, are expressed at significant levels along the adult PT. Claudin-2 is expressed at high levels along the PT.11,12 Claudin-2 knockout mice display renal calcium (Ca2+) wasting,13 but fractional excretion of Mg2+ (FEMg2+) is unaffected.14 Increased distal compensatory reabsorption may occur to maintain Mg2+ homeostasis; micropuncture studies using claudin-2 knockout mice would resolve this issue. Claudin-10a is also expressed at high levels in mouse PT,15 but its anion selectivity argues against a role in the Mg2+ reabsorptive pathway. Given that the TAL expresses claudins permeable to Na+, Mg2+, and Ca2+, the physiologic reason for the PT not doing so, and shifting Mg2+ permeability distally, is unknown.
Thick Ascending Limb
The main site of Mg2+ reabsorption along the nephron is the TAL, which reabsorbs 65%–70% of the filtered load.16 Reabsorption along the TAL is highly regulated, as demonstrated by a decrease in reabsorption as plasma [Mg2+] increases with a concomitant increase in delivery of filtered Mg2+ to this segment.3 However, as will be discussed, the precise nature of the reabsorptive pathway(s) and regulatory mechanisms remain poorly defined. The TAL also reabsorbs 20%–30% of the filtered Na+ load primarily via the apical Na+-K+-2Cl− cotransporter (NKCC2). The importance of the TAL in Mg2+ reabsorption and homeostasis is demonstrated by the development of renal Mg2+ wasting and hypomagnesemia in patients chronically administered loop-diuretics that block NKCC217,18 (Figure 1A). The resulting TAL dysfunction impairs the generation of the lumen-positive transepithelial voltage necessary for paracellular Mg2+ reabsorption.
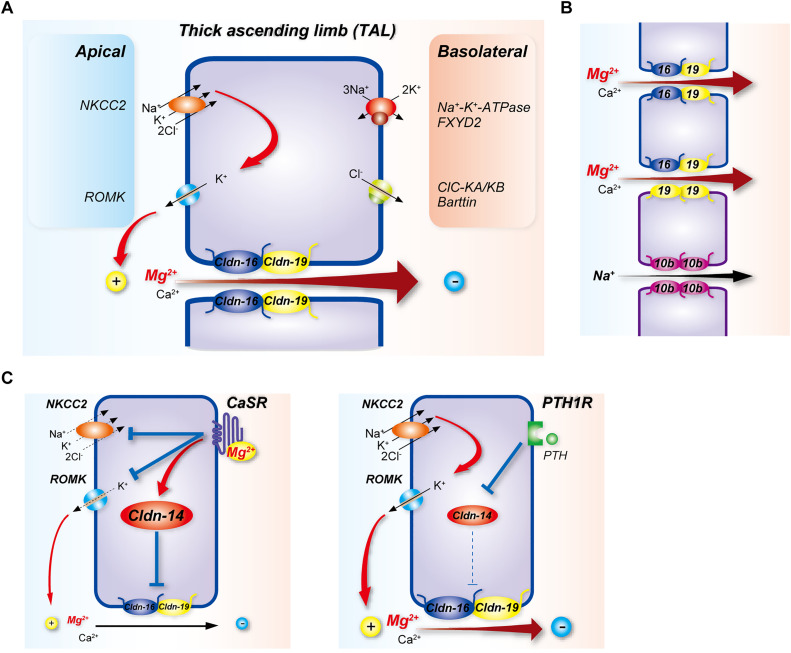
Figure 1.
Mg2+ reabsorption along the TAL. (A) Mg2+ pathways and regulation. The basolateral Na+-K+-ATPase with its regulatory γ-subunit FXYD2 provides the drive for transcellular entry of Na+, K+, and Cl− through the NKCC2. Recycling of K+ through ROMK contributes to the generation of the transepithelial voltage that provides the drive for paracellular movement of Mg2+ and Ca2+ through the claudin-16/19 complex (Cldn-16/19). Claudin-14 (Cldn-14) inhibits movement through Cldn-16/19, and its expression is stimulated by Mg2+ activation of CaSR or repressed by activation of the PTH1 receptor (PTH1R), through mechanisms that involve microRNAs. Chloride exits at the basolateral membrane via CLC-K channels. (B) Recent data suggest TAL expression of claudins displays a mosaic pattern, suggesting greater complexity than shown in (A). Proposed scheme for pore composition is on the basis of immunolocalization37 and single-cell RNA sequencing. Cells outlined in blue express claudins 16 (blue) and 19 (yellow), whereas those outlined in purple express claudins 10b (purple) and 19 but not claudin-16. Claudins form dimers within the same cell (cis interactions) and interact with dimers in adjacent cells to form ion pores (trans interactions). Adjacent cells expressing claudin 16 and 19 heterodimers form a Ca2+/Mg2+ pore. Cells expressing claudin-10b homodimers form an Na+ pore, which may regulate paracellular Ca2+/Mg2+ flux by modulating the transepithelial voltage. In claudin-10b–expressing cells, claudin-19 homodimers may form a Ca2+/Mg2+ pore with claudin-16/19 heterodimers in an adjacent cell. (C) Claudin-14 inhibits Ca2+/Mg2+ movement through Cldn-16/19, and its expression is stimulated by Mg2+ activation of CaSR (left), or inhibited by activation of PTH1R (right). CaSR also exerts inhibitory effects on NKCC2 and ROMK (left).
Claudins are critical for paracellular Mg2+ movement along the TAL. Mutations in the CLDN16 gene, encoding claudin-16, cause the rare autosomal recessive disease familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), due to renal Mg2+ and Ca2+ wasting.19 The median onset of FHHNC is 1–8 years of age, and the disease progresses to CKD. Furosemide or MgCl2 infusion, which both inhibit Mg2+ and Ca2+ reabsorption by the TAL of normal subjects, do not do so in in patients with FHHNC, supporting a primary defect in this segment.20 Management includes administration of thiazide diuretics to limit Ca2+ excretion and the progression of nephrocalcinosis combined with Mg2+ supplementation. Whether claudin-16 forms the paracellular Mg2+ pore is controversial and remains to be resolved.21 In support, in MDCK cells expressing wild type claudin-16, Mg2+ permeability was significantly higher compared with mock-transfected cells, whereas Na+ and Ca2+ permeabilities did not differ.22 Furthermore, TAL isolated from claudin-16 knockout mice (which phenocopy FHHNC) displays lower Mg2+ permeability, but unchanged Na+ permeability.23 Contrasting these findings, Hou and colleagues found no difference in Na+/Li+ or Na+/Mg2+ permeabilities in TAL from mice with transgenic siRNA-mediated knockdown of claudin-16.24 Because these mice displayed significantly higher FEMg2+, how might a nonselective pore affect Mg2+ movement? NaCl reabsorption along the water-impermeable TAL results in a dilute luminal fluid. “Backleak” of Na+ through claudin-16 may then generate the luminal potential that permits paracellular Mg2+ movement.25 Interactions with claudin-19 may play an important role in determining selectivity (see below). Phosphorylation of claudin-16 mediates its localization to the paracellular tight junction.26 Marunaka and colleagues recently identified a novel regulator of claudin-16 membrane localization,27 PDZRN3, a protein containing a RING-finger domain (involved in ubiquitination) and a PDZ domain (involved in membrane anchoring). Immunolocalization confirmed PDZRN3 expression in TAL. In MDCK cells expressing claudin-16, inhibition of protein kinase A with H-89 increased the association between PDZRN3 and claudin-16, and increased claudin-16 monoubiquitination. Lysine 275 is the major ubiquitination site, and dephosphorylation at serine 217 mediates its association with PDZRN3. H-89 decreased Mg2+ permeability in cells expressing wild type claudin-16, but this effect was reversed by knockdown of PDZRN3 with siRNA. These data suggest that PDZRN3 mediates internalization of dephosphorylated claudin-16, which may then decrease TAL Mg2+ permeability. Future in vivo studies are needed to determine whether changing dietary Mg2+ levels alters PDZRN3 trafficking or expression levels, and whether any changes correlate with claudin-16 localization.
Mutations in CLDN19, encoding claudin-19, cause a syndrome similar to FHHNC, with the addition of severe ocular manifestations that lead to visual impairment.28 Knockdown of claudin-19 by siRNA in mice results in an FHHNC phenotype very similar to that seen with claudin-16 disruption.23,24,29 Claudin-16 and claudin-19 directly interact with each other, and disease-causing mutations can disrupt these interactions.29,30 Subsequently it was shown that claudin-19 can interact with all other claudins expressed along the TAL, but claudin-16 can only interact with claudin-19.29 In FHHNC mouse models, lower claudin-16 or claudin-19 abundance disrupts the localization of the other claudin at the tight junction, suggesting the interaction is functionally important. Some data suggest that claudin-16/19 interactions affect Na+ permeability and are an important determinant of the transepithelial voltage driving Mg2+ reabsorption, rather than claudin-16/19 acting as the Mg2+ pore itself.30 However, studies exploring claudin-10b function and its relationship to claudin-16/19 suggest otherwise. Mutations in CLDN10, which is also highly expressed along the TAL,31 cause hypohidrosis, electrolyte imbalance, lacrimal gland dysfunction, ichthyosis, and xerostomia (HELIX syndrome), with manifestations including include hypokalemia, metabolic alkalosis, salt-wasting, hypocalciuria, and hypermagnesemia.32–35 Electrolyte imbalances observed in HELIX are recapitulated in renal tubule–specific claudin-10 knockout mice.36 In TAL isolated from these mice, Na+ permeability is lower, whereas Mg2+/Na+ and Ca2+/Na+ permeabilities are higher than in wild type. A study examining spatial separation of claudin-10b and claudin-16/19 expression along TAL showed that in TAL cortical and inner stripe of the outer medulla tight junctions, claudin-10b is expressed at tight junctions in cells distinct form those expressing claudins 3, 16, and 19 at junctions.37 Milatz and colleagues reported that claudin-10b junctions are preferentially permeable to Na+, whereas those containing claudins 3, 16, and 19 are more permeable to Mg2+.37 This mosaic distribution along TAL may allow independent regulation of the two pathways but refinement of the model is needed. Indeed, a recent single-cell RNA-sequencing study strongly suggests that although claudin-19 is expressed in all TAL cells, cells expressing claudin-16 are distinct from those expressing claudin-10b.38 To reconcile the findings of Milatz and these new data, we propose the model shown in Figure 1B. In this scheme, when claudin-10b– and claudin-16–expressing cells are in contact, claudin-16/19 heterodimers form in cells expressing claudin-16, whereas only claudin-19/19 homodimers form in claudin-10b–expressing cells. A consequence of differential transinteractions between cells (homodimer/homodimer or homodimer/heterodimer) might be the formation of pores with different properties, e.g., permeability.
Finally, claudin-14 is also highly expressed along the TAL,39,40 and its expression in mice is directly proportional to dietary Mg2+ intake.40 Claudin-14 knockout mice have lower FEMg2+,39 whereas transgenic mice overexpressing it along TAL display increased FEMg2+.41 It may act to modulate paracellular Mg2+ reabsorption by directly blocking claudin-16/19 permeability39 (Figure 1C). A genome-wide association study identified a single-nucleotide polymorphism (rs172639) within an intergenic region downstream of the 3′ untranslated region of the claudin-14 mRNA. This variant is associated with lower urinary Mg2+/Ca2+, which may result from lower expression of claudin-14,40 possibly by mechanisms similar to Ca2+-mediated claudin-14 regulation via microRNAs.41,42 This finding also raises the possibility that claudin-14 differentially modulates claudin-16/19 Mg2+ and Ca2+ permeabilities.
Recently, the transcription factor hepatocyte nuclear factor–1β (HNF1β) was reported to upregulate calcium-sensing receptor (CaSR) expression both in vitro and in vivo, through an effect on the CaSR gene promoter.43 This would be predicted to promote urinary Mg2+ excretion through stimulatory effects on claudin-14 (and hence claudin-16/19) and inhibitory effects on NKCC2 and the renal outer medullary potassium channel (ROMK)44 (Figure 1C). However, inactivating mutations in HNF1β cause autosomal dominant tubulointerstitial kidney disease (ADTKD-HNF1β), which features renal cysts; maturity-onset diabetes of the young; and electrolyte disturbances including hypokalemia, hypocalciuria, and hypomagnesemia, rather than hypermagnesemia.45 Effects of HNF1β on DCT function (see below) may therefore be more important in the disease. In opposition to CaSR activation are the effects of parathyroid hormone (PTH) which doubled Mg2+ transport in isolated rat TAL.46 Disruption of the Parathyroid hormone 1 (PTH1) receptor in mice increased claudin-14 abundance and led to hypercalciuria47; effects on Mg2+ handling were not determined but increased urinary Mg2+ excretion might be expected due to loss of claudin-14 inhibition (Figure 1C).
Distal Convoluted Tubule
The DCT reabsorbs about 5% of the filtered Mg2+ load with no evidence of further reabsorption beyond this segment. The DCT is defined by expression of the NaCl cotransporter (NCC), but it is heterogeneous and can be divided into early (DCT1) and late portions (DCT2) (Figure 2A). The basis for this division is expression of parvalbumin with NCC along DCT1, and expression of the epithelial sodium channel (ENaC) with NCC along DCT2.38 Mg2+ reabsorption is likely to occur along DCT1 and DCT2, given the presence of Mg2+ transport–related transcripts in both.38 In contrast to the PT and TAL, Mg2+ is transported via an active transcellular pathway. Entry at the apical membrane is through a channel, TRPM6.48,49 Interaction with TRPM7 is required for maximal surface expression of TRPM6, and disruption of TRPM7 ablates TRPM6-dependent Mg2+ entry in trophoblast stem cells.50 This interaction generates a TRPM6/7 heterodimeric channel with high activity regardless of physiologic and intracellular Mg2+-ATP concentrations, permitting continuous Mg2+ reabsorption.51 Inactivating mutations in TRPM6 cause hypomagnesemia with secondary hypocalcemia in humans.48,49,52 An extensive study of 21 families revealed multiple causative mutations including stop mutations, frame-shift mutations, splice-site mutations, and exon deletions.52 Hypomagnesemia with secondary hypocalcemia was typically diagnosed within the first few months of life with most patients displaying serum [Mg2+]<0.3 mmol/L. Generalized seizures due to either hypomagnesemia or hypocalciuria occurred in most patients, and in some cases brain development was severely impaired. Importantly, acute intravenous Mg2+ infusion followed by lifelong oral supplementation alleviated symptoms. Despite these severe effects on humans, neither kidney-specific Trpm650 nor Trpm753 knockout mice display frank Mg2+ wasting, and are normomagnesemic. One possibility for this apparent discrepancy is that reabsorption along the TAL may be sufficiently enhanced in mice but not in humans. A larger sample number and determination of FEMg2+, as is done clinically, might uncover disruption subtle phenotype in knockout mice. Activation of the EGF receptor (EGFR) induces TRPM6 expression and activity in vitro54,55 (Figure 2B). In humans, mutations in the gene encoding EGF cause isolated, recessive hypomagnesemia due to renal Mg2+ wasting.56 The high frequency of the development of hypomagnesemia in patients with colorectal cancer administered cetuximab or panitumumab, monoclonal antibodies targeting the EGFR,56,57 underscores the importance of EGFR signaling in TRPM6 regulation. The membrane potential of −70 mV that drives Mg2+ entry through TRPM6/7 is believed to be determined by the voltage-gated potassium channel subtype 1.1 (Kv1.1) because patients with mutations in KCNA1 encoding it fail to lower fractional renal Mg2+ excretion in the presence of hypomagnesemia.58 However, the physiologic contribution of Kv1.1 to renal Mg2+ handling has not been assessed in experimental animal models.
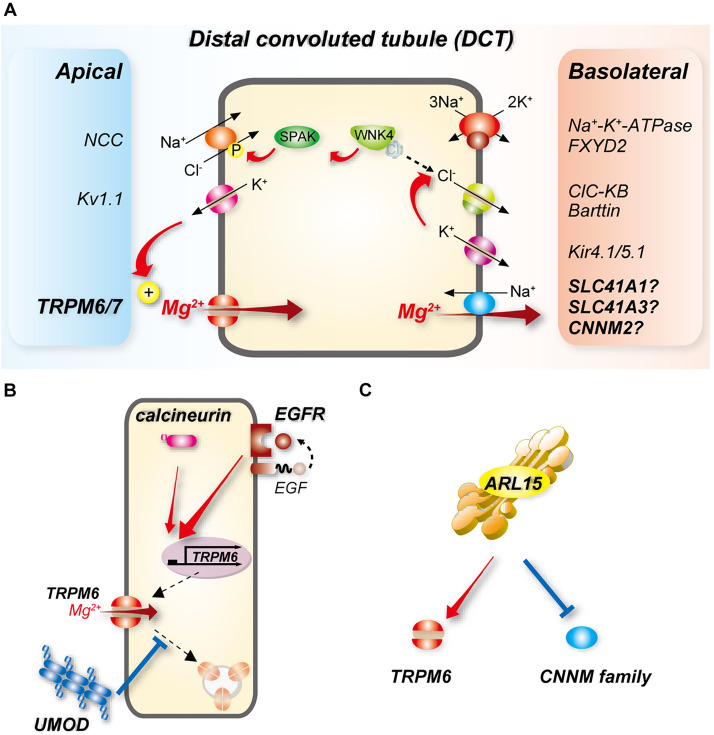
Figure 2.
Transcellular Mg2+ reabsorption along the DCT. (A) Mg2+ enters cells along the early (DCT1) and late DCT (DCT2) through TRPM6, which requires interaction with TRPM7 for maximum activity. The basolateral exit pathway remains to be identified, but candidates are shown. Because transport through NCC is electroneutral, the potassium channel Kv1.1 is believed to generate the drive for Mg2+ entry at the apical membrane. NCC is activated by phosphorylation via the WNK-SPAK kinase pathway. WNK kinases are inhibited by direct binding of Cl−; coupling of K+ and Cl− efflux via Kir4.1/5.1 and CLC-KB as a result of increased plasma [K+] lowers intracellular [Cl−] leading to WNK kinase activation. Energy consumption by the basolateral Na+-K+-ATPase drives all of these processes (see Figure 3). (B) Activation of EGFR induces TRPM6 mRNA expression and activity, and calcineurin may promote this. UMOD may stimulate TRPM6 activity by inhibiting its endocytosis. (C) ARL15, a GTP-binding protein, may activate TRPM6 but inhibit cyclin and CNNMs.
UMOD is highly expressed along the TAL. After cleavage from the apical membrane by the luminal protease hepsin,59 UMOD is excreted at high levels in the urine.60 UMOD enhances membrane expression of NKCC2 and ROMK, which may increase the drive for paracellular Mg2+ reabsorption. Consistent with this, UMOD knockout mice exhibit renal Mg2+ wasting with compensatory upregulation of mRNA expression of Trpm6, Hnf1b, and Egf.61 UMOD may also directly affect DCT Mg2+ reabsorption. UMOD acting on the DCT may be derived from TAL cleavage, but lower expression along DCT1 has also been demonstrated.62 Despite increasing Trpm6 mRNA levels, UMOD deletion paradoxically decreased apical TRPM6 protein abundance.61 In cotransfection experiments in HEK293 cells, UMOD increased TRPM6 membrane abundance and activity; administration of purified UMOD to TRPM6-expressing cells had the same effects.61 The mechanism may involve decreased TRPM6 endocytosis (Figure 2B). Several key regulators of UMOD trafficking and release expressed along the TAL are also expressed along the DCT: hepsin,59 CaSR,63,64 and ROMK,65,66 raising the possibility that there is DCT-centered regulation of TRPM6 by UMOD. Segment-specific knockout mice would help determine the roles of each of these factors in TRPM6 regulation. The relative contributions of TAL- versus DCT-derived UMOD in renal Mg2+ handling also remain to be determined.
Changes in fluid flow rate cause shear stress that is detected by primary cilia or mechanosensing proteins located at the apical membrane of the renal epithelia. Verschuren and colleagues used an in vitro microfluidic system to determine the effects of fluid shear stress (FSS) on Mg2+ uptake by mDCT15 cells.67 Mg2+ uptake was stimulated, but the pathways or regulatory mechanisms are unclear. CRISPR/Cas9 disruption of intraflagellar transport 140 homolog (IFT140), which is required for primary cilium function, affected static uptake but had no effect on FSS-induced uptake. This suggests Mg2+ uptake is independent of the primary cilia. Pharmacologic inhibition of TRPM7 or CRISPR/Cas9 disruption of TRPM6 also had no effect on uptake. Abundances of mRNAs encoding proteins implicated in DCT Mg2+ reabsorption including pro-EGF, Kv1.1, TRPM6, and TRPM7 were also unchanged by FSS. Polycystin-1 (PC1) directly acts as a mechanosensor, and mutations in PKD1, which encodes it, cause autosomal dominant polycystic kidney disease. A recent study determined the effects of PC1 disruption before cyst development using an inducible kidney-specific knockout mouse model.68 Precystic knockout mice displayed urinary Mg2+ wasting and lower plasma [Mg2+]. This was associated with lower expression of mRNAs encoding claudin-16, ROMK, NKCC2, TRPM6, UMOD, and NCC (see below), as well as suggesting a broad effect on TAL and DCT Mg2+ reabsorption. Together, these studies suggest a potential role for flow in renal Mg2+ handling along the DCT, but more studies are needed to confirm this and the mechanisms involved.
Corre and colleagues identified an association of intronic variant of ADP-ribosylation factor–like protein 15 (ARL15), which encodes a GTP-binding protein, with lower 24-hour urinary Mg2+ excretion.69 In mouse, ARL15 expression was high along TAL and DCT, stimulated TRPM6 activity in transfected HEK293 cells, and affected Mg2+ homeostasis when disrupted in zebrafish69 (Figure 2C). A recent preprint70(preprint) also suggests that ARL15 may negatively regulate members of the cyclin and CBS domain divalent metal cation transport mediator (CNNM) family, implicated in basolateral Mg2+ exit at the basolateral membrane of the DCT (see below), by promoting their N-glycosylation (Figure 2C). This may also affect Mg2+ reabsorption by the DCT. Whether ARL15 modulates Mg2+ entry through effects on claudins along the TAL has not been determined. Along the DCT, binding of Ca2+ to a carrier protein (calbindin-D28K) is necessary to avoid fluctuations in cytosolic [Ca2+] during its transcellular movement. However, there is no strong evidence for the existence of a carrier protein for Mg2+. Calbindin-D28K knockout mice display unchanged serum [Mg2+] and urinary Mg2+ excretion.71 Furthermore, mice lacking the divalent cation-binding protein parvalbumin, which is expressed at high levels along the DCT1, display only mild urinary Mg2+ wasting.72
The basolateral Mg2+ extrusion mechanism remains unclarified and the subject of controversy (for a detailed review see reference).73 One candidate is solute carrier family 41 member A1 (SLC41A1), an Na+-Mg2+ exchanger, which is expressed along the DCT.74 However, an inactivating mutation that causes nephronophthisis-related disorders is not associated with abnormalities in serum or urine Mg2+.74 Another candidate is SLC41A3, believed to be an Na+-Mg2+ exchanger. Although Slc41a3 mRNA is enriched in DCT and Slc41a3 knockout mice display hypomagnesemia, it may act at the mitochondria rather than the basolateral membrane.75 In Slc41a3 knockout mice, reduced efflux of Mg2+ might affect Mg2+ reabsorption along the DCT by dysregulating metabolism, or limiting Mg2+ available for the basolateral Na+-K+-ATPase which is needed to drive basolateral exit of Mg2+ against the electrochemical gradient (−70 mV). Immunolocalization of SLC41A3 within DCT cells and generation of renal tubule–specific knockout mice might clarify its roles. Finally, mutations in cyclin and CBS domain divalent metal cation transport mediator–2 (CNNM2), which is present at the basolateral membrane of the human DCT, cause renal Mg2+ wasting and hypomagnesemia.76 Dietary Mg2+ restriction potently increases Cnnm2 mRNA abundance,77 and tubule-specific Cnnm2 knockout mice display lower serum [Mg2+], but its role in basolateral Mg2+ efflux is highly debated.78,79 Although some studies suggest it directly transports Mg2+,80 others suggest it only acts to regulate transmembrane Mg2+ movement.81,82 Furthermore, tubule-specific Cnnm2 knockout mice display reduced blood pressure,83 raising the possibility of effects on NCC activity, although these would be indirect given the apical expression of NCC.
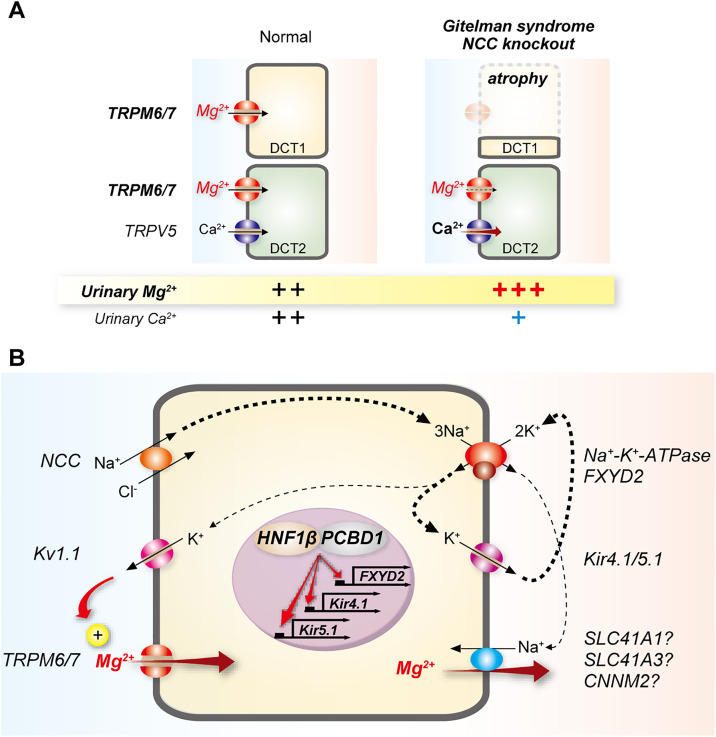
Activity of NCC is an important determinant of DCT Mg2+ reabsorption. Disruption of NCC activity by thiazide diuretics and genetic mutations in SLC12A3 (encoding NCC) in the disease Gitelman syndrome, or in Slc12a3 knockout mice, are all associated with renal Mg2+ wasting and hypomagnesemia. Furthermore, two related mechanisms are likely to play a role (for a detailed recent review see reference).84 First, lower NCC or absent activity causes atrophy of the DCT,85–87 which may lead to lower total TRPM6 expression and transport capacity86 (Figure 3A). Some recent data suggest that effects on TRPM6 expression may be direct, because TRPM6 expression is reduced before DCT atrophy in newborn Slc12a3 knockout mice.88 Similarly, DCT-specific disruption of the transcription factor Prox-1 in mice causes mild hypomagnesemia associated with lower abundances of NCC and TRPM6 despite unchanged DCT volume, suggesting lower NCC activity might somehow affect TRPM6 independently of DCT atrophy.89 Second, reduced Na+ entry through NCC may lower activity of the basolateral Na+-K+-ATPase, which would lower the drive for apical Mg2+ entry through TRPM6/7 and basolateral efflux (Figure 3B). DCT1-specific disruption of the transcription factor KCTD1 results in Mg2+ wasting and hypomagnesemia, and this was associated with reduced abundance of NCC, but not of TRPM6, supporting a role for NCC activity, although this was not measured using a thiazide-response test. Mutations in the Na+-K+-ATPase γ-subunit (FXYD2) cause renal Mg2+ wasting in humans,90 and de novo germline mutations in the α-1 subunit (ATPA1A) not only cause a severe neurologic phenotype but also severe hypomagnesemia and renal Mg2+ wasting.91 Other factors that regulate activities of both NCC and the Na+-K+-ATPase contribute to DCT Mg2+ reabsorption. In addition to direct effects on TRPM6 activity, UMOD may affect TRPM6 indirectly by altering NCC activity. In UMOD knockout mice abundance of the phosphorylated active form of NCC is lower in DCT1, and UMOD facilitates NCC activation in HEK293 cells.62 The basolateral Kir4.1/Kir5.1 potassium channel may affect apical Mg2+ entry through effects on both NCC and the Na+-K+-ATPase. Mutations in KCNJ10, which encodes Kir4.1, cause epilepsy, ataxia, sensorineural deafness, and tubulopathy (EAST syndrome, also called SeSAME syndrome) with renal manifestations similar to Gitelman syndrome, including hypomagnesemia.92,93 Kcnj10 knockout mice display a dramatic reduction in NCC expression and phosphorylation, DCT atrophy, and urinary Mg2+ wasting.94 Na+-K+-ATPase activity depends on K+ recycling via Kir4.1/Kir5.1, so inhibition may contribute to lower NCC activity indirectly (Figure 3B), but a growing body of data support the idea that Kir4.1/Kir5.1 is a component of a chloride-dependent NCC regulatory pathway.95 In this scheme, lower extracellular [K+] promotes K+ efflux from DCT cells through Kir4.1/5.1 which then provides the drive for basolateral Cl− exit through the channel CLC-KB (Figure 2A). This alleviates inhibition of with-no-lysine[K] (WNK) kinases by Cl−, which they directly bind, and ultimately phosphorylation-dependent activation of NCC by SPAK, a kinase downstream of WNK kinases. Importantly, expression of both CLK-Kb96 and its essential β subunit barttin along the DCT likely explains the presence of hypomagnesemia in Bartter syndrome due to mutations in the genes encoding CLC-KB (type 3 Bartter syndrome) or barttin (type 4 Bartter syndrome). Reduced capacity for Cl− efflux along the DCT leads to increased WNK inhibition and thus lower NCC activity. Forms of Bartter syndrome that affect only the TAL do not present with hypomagnesemia. Dysregulation of Kir4.1/Kir5.1 activation by c-Src in Cav1 (caveolin-1) knockout mice also leads to renal Mg2+ wasting.97 The transcription factor HNF1β mutated in ADTKD-HNF1β, which features hypomagnesemia, regulates expression of both FXYD298 and Kir4.1/Kir5.199 (Figure 3B). Mutations in PCBD1, a dimerization cofactor for HNF1β, also cause hypomagnesemia and renal Mg2+ wasting by reducing FXYD2 transcription98 (Figure 3B), but whether they also affect Kir4.1/Kir5.1 has not been demonstrated.
Figure 3.
Links between activities of NCC and the Na+-K+-ATPase and DCT Mg2+ reabsorption. (A) DCT1 atrophy occurs with disruption of NCC activity, and is seen in Gitelman syndrome (GS, NCC mutation) and in NCC knockout mice; and in epilepsy, ataxia, sensorineural deafness, and tubulopathy (EAST) syndrome (Kir4.1 mutation); Bartter syndrome types 3 and 4 (CLC-KB and barttin mutations); and Kir4.1 knockout mice (not shown). This reduces the capacity for Mg2+ reabsorption in DCT1, leading to hypomagnesemia. The DCT2 does not compensate for this effect, and Mg2+ reabsorption may also be lower in this segment due to NCC inhibition. Calcium (Ca2+) is not reabsorbed along the DCT1, but along the DCT2 and connecting segment (not shown). In GS, Ca2+ reabsorption is paradoxically enhanced leading to hypocalciuria; several mechanisms have been proposed, including enhanced entry along the DCT2, although the evidence better supports increased Ca2+ PT reabsorption.111 (B) Generation of the driving force for Mg2+ reabsorption requires activity of the basolateral Na+-K+-ATPase. Consistent with this, mutations in its γ-subunit (FXYD2, shown in brown) cause renal Mg2+ wasting. The Na+-K+-ATPase maintains intracellular K+; apical efflux of K+ through Kv1.1 may determine the membrane potential for apical Mg2+ entry. Na+-K+-ATPase–mediated generation of an Na+ gradient at the basolateral membrane may provide the driving force for Mg2+ exit through an Na+-Mg2+ exchanger. Apical entry of Na+ via the NCC also contributes to maintenance of Na+-K+-ATPase activity. Thicker arrows represent established pathways for ion movement, whereas thinner arrows present proposed pathways. The transcription factor HNF1β and its dimerization cofactor PCBD1 both regulate expression of FXYD2, and when mutated cause hypomagnesemia. HNF1β has also been shown to regulate expression of Kir4.1 and Kir5.1.
The relationship between NCC hyperactivation and Mg2+ handling is more complex. Hypermagnesemia is not observed in the disease familial hyperkalemic hypertension (FHHt, also called Gordon syndrome or pseudohypoaldosteronism type II) in which NCC is hyperactivated.100 It is not clear why this is the case, but Trpm6 mRNA was unchanged in a mouse model that mimics FHHt by expressing constitutively active SPAK.101 These mice also display increased DCT mass, so compensatory TRPM6 downregulation (less TRPM6 per unit DCT mass) might contribute. Tacrolimus is a calcineurin inhibitor used to prevent rejection after solid-organ transplantation and to treat autoimmune disease. Tacrolimus use is often accompanied by hypomagnesemia and hypertension through effects on the kidney, with the hypertension partly mediated by impaired dephosphorylation-mediated inactivation of NCC.102 Thus, renal Mg2+ wasting occurs in the context of NCC hyperactivation. One mechanism may involve downregulation of Trpm6 mRNA and protein levels.103 Tacrolimus must bind to the 12-kDa FK506-binding protein (FKBP12) to inhibit calcineurin. Tacrolimus-induced downregulation of Trpm6 mRNA and hypomagnesemia are abolished in kidney-specific Fkbp12 knockout mice.104 The mechanism may involve an effect of calcineurin on EGFR signaling, which induces TRPM6 expression105 (Figure 2B). The precise mechanisms permitting differential effects of tacrolimus on NCC and TRPM6 remain to be determined. Note that calcineurin inhibitor cyclosporine use can also cause hypomagnesemia, but this may involve effects on both TRPM6 and claudin-16 expression,106 whereas tacrolimus does not change abundances of claudin-16 or caludin-19 in mice.104
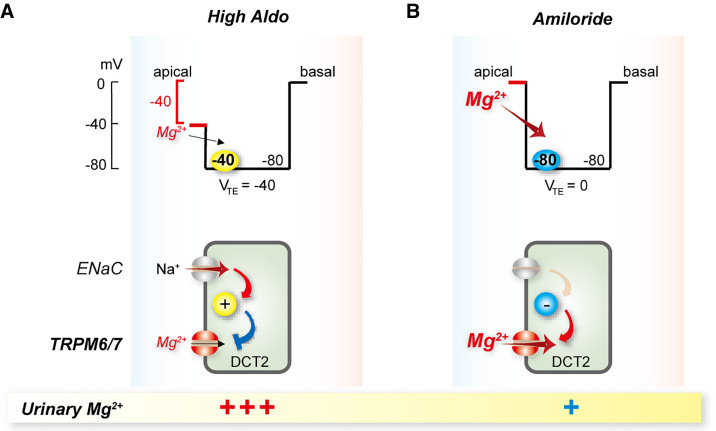
In patients with hypomagnesemia resulting from thiazide or loop diuretic therapy, Gitelman or Bartter syndromes, or cisplatin nephrotoxicity, addition of the ENaC blocker amiloride can increase plasma [Mg2+].107–110 In states of high aldosterone as might occur in Gitelman syndrome, apical membrane depolarization due to Na+ entry through ENaC (expressed along DCT2) would be predicted to reduce the electrochemical gradient favoring Mg2+ entry through TRPM6/7 (Figure 4). ENaC blockade with amiloride would repolarize the membrane and promote Mg2+ entry via TRPM6 along DCT2, but not DCT1 (which lacks ENaC).
Figure 4.
Proposed mechanism of amiloride inhibition of Mg2+ wasting along the late distal convoluted tubule (DCT2). (A) In states of high aldosterone (High Aldo) the apical membrane is depolarized as a result of sodium entry via the ENaC (top panel shows voltage trace). This means that the transepithelial voltage is oriented with the lumen negative, relative to the basal side. The depolarized apical membrane voltage drives little Mg2+ into the cell through TRPM6/7, as the membrane voltage across the apical membrane is the primary driving force. The bottom panel shows this schematically. (B) Amiloride treatment inhibits sodium entry through ENaC, eliminating the negative transepithelial potential and hyperpolarizing the apical membrane (top panel shows voltage trace), leading to a net increase in the driving force for Mg2+ to enter the cell across the apical membrane through TRPM6/7. The bottom panel shows this schematically. VTE, transepithelial voltage.
Conclusions
Genetic diseases featuring defective renal Mg2+ handling have facilitated identification of the pathways involved in Mg2+ reabsorption. Questions still remain regarding the composition of the paracellular pore along the PT, but claudin-2 is the strongest candidate. Along the TAL, claudin-16/19 is likely to form the pore, with claudins 14 and 10b playing regulatory roles. Na+ reabsorption via claudin-10b appears to be spatially distinct from Mg2+ movement, increasing the possibility for regulation. Along the DCT, UMOD has been identified as a novel regulator of TRPM6. Flow may also contribute to Mg2+ handling by this segment, but further studies are needed to confirm this. Finally, details regarding the link between NCC activity and Mg2+ handling remain to be elucidated. Major gaps for future studies include regulatory mechanisms along the TAL, and the nature of the basolateral efflux pathway along the DCT.
Disclosures
D.H. Ellison reports Scientific Advisor or Membership as an Author for UpToDate, Consulting Editor for Hypertension, and Editorial Board member of American Journal of Physiology: Renal Physiology; reports receiving research funding from the National Institutes of Health and the Veterans Affairs Health Services Research & Development, and the Leducq Foundation; and Other Interests/Relationships with the Leadership Council of the American Society of Nephrology. Y. Maeoka reports receiving research funding from the Uehara Foundation. J. McCormick reports Scientific Advisor or Membership via the Editorial boards for Frontiers in Physiology: Renal and Epithelial Physiology, American Journal of Physiology: Renal Physiology, and Kidney360; and reports receiving funding from the National Institutes of Health.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jahnen-Dechent W, Ketteler M: Magnesium basics. Clin Kidney J 5: i3–i14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Baaij JH, Hoenderop JG, Bindels RJ: Magnesium in man: implications for health and disease. Physiol Rev 95: 1–46, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Wong NL, Dirks JH, Quamme GA: Tubular reabsorptive capacity for magnesium in the dog kidney. Am J Physiol 244: F78–F83, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Wong NL, Whiting SJ, Mizgala CL, Quamme GA: Electrolyte handling by the superficial nephron of the rabbit. Am J Physiol 250: F590–F595, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Brunette MG, Vigneault N, Carriere S: Micropuncture study of magnesium transport along the nephron in the young rat. Am J Physiol 227: 891–896, 1974 [DOI] [PubMed] [Google Scholar]
- 6.de Rouffignac C, Quamme G: Renal magnesium handling and its hormonal control. Physiol Rev 74: 305–322, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Quamme GA: Effect of furosemide on calcium and magnesium transport in the rat nephron. Am J Physiol 241: F340–F347, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Quamme GA, Dirks JH: Intraluminal and contraluminal magnesium on magnesium and calcium transfer in the rat nephron. Am J Physiol 238: F187–F198, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Quamme GA, Wong NL, Dirks JH, Roinel N, De Rouffignac C, Morel F: Magnesium handling in the dog kidney: a micropuncture study. Pflugers Arch 377: 95–99, 1978 [DOI] [PubMed] [Google Scholar]
- 10.Carney SL, Wong NL, Quamme GA, Dirks JH: Effect of magnesium deficiency on renal magnesium and calcium transport in the rat. J Clin Invest 65: 180–188, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enck AH, Berger UV, Yu AS: Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 281: F966–F974, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S: Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Curry JN, Saurette M, Askari M, Pei L, Filla MB, Beggs MR, et al.: Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J Clin Invest 130: 1948–1960, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, et al.: Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 107: 8011–8016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM: Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Poujeol P, Chabardes D, Roinel N, De Rouffignac C: Influence of extracellular fluid volume expansion on magnesium, calcium and phosphate handling along the rat nephron. Pflugers Arch 365: 203–211, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Bashir Y, Sneddon JF, Staunton HA, Haywood GA, Simpson IA, McKenna WJ, et al.: Effects of long-term oral magnesium chloride replacement in congestive heart failure secondary to coronary artery disease. Am J Cardiol 72: 1156–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Cohen N, Almoznino-Sarafian D, Zaidenstein R, Alon I, Gorelik O, Shteinshnaider M, et al.: Serum magnesium aberrations in furosemide (frusemide) treated patients with congestive heart failure: pathophysiological correlates and prognostic evaluation. Heart 89: 411–416, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al.: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, et al.: Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59: 2206–2215, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Curry JN, Yu ASL: Magnesium handling in the kidney. Adv Chronic Kidney Dis 25: 236–243, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Günzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, Hunziker W, et al.: Claudin-16 affects transcellular Cl− secretion in MDCK cells. J Physiol 587: 3777–3793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, et al.: Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol 298: F1152–F1161, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, et al.: Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem 282: 17114–17122, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hou J, Goodenough DA: Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens 19: 483–488, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, et al.: Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci 119: 1781–1789, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Marunaka K, Furukawa C, Fujii N, Kimura T, Furuta T, Matsunaga T, et al.: The RING finger- and PDZ domain-containing protein PDZRN3 controls localization of the Mg2+ regulator claudin-16 in renal tube epithelial cells. J Biol Chem 292: 13034–13044, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al.: Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, et al.: Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, et al.: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, et al.: Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122: 1507–1517, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Bongers EMHF, Shelton LM, Milatz S, Verkaart S, Bech AP, Schoots J, et al.: A novel hypokalemic-alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J Am Soc Nephrol 28: 3118–3128, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, et al.: Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet 13: e1006897, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadj-Rabia S, Brideau G, Al-Sarraj Y, Maroun RC, Figueres ML, Leclerc-Mercier S, et al.: Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet Med 20: 190–201, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Meyers N, Nelson-Williams C, Malaga-Dieguez L, Kaufmann H, Loring E, Knight J, et al.: Hypokalemia associated with a claudin 10 mutation: a case report. Am J Kidney Dis 73: 425–428, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, et al.: Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A 109: 14241–14246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, et al.: Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci U S A 114: E219–E227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Chou CL, Knepper MA: Targeted single-cell RNA-seq identifies minority cell types of kidney distal nephron. J Am Soc Nephrol 32: 886–896, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, et al.: Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corre T, Olinger E, Harris SE, Traglia M, Ulivi S, Lenarduzzi S, et al.: Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine. Pflugers Arch 469: 91–103, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Gong Y, Hou J: Claudin-14 underlies Ca++-sensing receptor-mediated Ca++ metabolism via NFAT-microRNA-based mechanisms. J Am Soc Nephrol 25: 745–760, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong Y, Himmerkus N, Plain A, Bleich M, Hou J: Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J Am Soc Nephrol 26: 663–676, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kompatscher A, de Baaij JHF, Aboudehen K, Farahani S, van Son LHJ, Milatz S, et al.: Transcription factor HNF1β regulates expression of the calcium-sensing receptor in the thick ascending limb of the kidney. Am J Physiol Renal Physiol 315: F27–F35, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Gamba G, Friedman PA: Thick ascending limb: the Na+:K+:2Cl− co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch 458: 61–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, et al.: HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20: 1123–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shareghi GR, Agus ZS: Magnesium transport in the cortical thick ascending limb of Henle’s loop of the rabbit. J Clin Invest 69: 759–769, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T, Courbebaisse M, Ide N, Fan Y, Hanai JI, Kaludjerovic J, et al.: Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14. Proc Natl Acad Sci U S A 114: E3344–E3353, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, et al.: Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, et al.: Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31: 171–174, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Chubanov V, Ferioli S, Wisnowsky A, Simmons DG, Leitzinger C, Einer C, et al.: Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. eLife 5: e20914, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferioli S, Zierler S, Zaißerer J, Schredelseker J, Gudermann T, Chubanov V: TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci Rep 7: 8806, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlingmann KP, Sassen MC, Weber S, Pechmann U, Kusch K, Pelken L, et al.: Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J Am Soc Nephrol 16: 3061–3069, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Mittermeier L, Demirkhanyan L, Stadlbauer B, Breit A, Recordati C, Hilgendorff A, et al.: TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc Natl Acad Sci U S A 116: 4706–4715, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ: EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol 20: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikari A, Sanada A, Okude C, Sawada H, Yamazaki Y, Sugatani J, et al.: Up-regulation of TRPM6 transcriptional activity by AP-1 in renal epithelial cells. J Cell Physiol 222: 481–487, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Groenestege WM, Thébault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, et al.: Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest 117: 2260–2267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maliakal P, Ledford A: Electrolyte and protein imbalance following anti-EGFR therapy in cancer patients: a comparative study. Exp Ther Med 1: 307–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaudemans B, van der Wijst J, Scola RH, Lorenzoni PJ, Heister A, van der Kemp AW, et al.: A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest 119: 936–942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, et al.: The serine protease hepsin mediates urinary secretion and polymerisation of zona pellucida domain protein uromodulin. eLife 4: e08887, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf MTF, Zhang J, Nie M: Uromodulin in mineral metabolism. Curr Opin Nephrol Hypertens 28: 481–489, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nie M, Bal MS, Liu J, Yang Z, Rivera C, Wu XR, et al.: Uromodulin regulates renal magnesium homeostasis through the ion channel transient receptor potential melastatin 6 (TRPM6). J Biol Chem 293: 16488–16502, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tokonami N, Takata T, Beyeler J, Ehrbar I, Yoshifuji A, Christensen EI, et al.: Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int 94: 701–715, 2018 [DOI] [PubMed] [Google Scholar]
- 63.Graca JA, Schepelmann M, Brennan SC, Reens J, Chang W, Yan P, et al.: Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am J Physiol Renal Physiol 310: F518–F533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokonami N, Olinger E, Debaix H, Houillier P, Devuyst O: The excretion of uromodulin is modulated by the calcium-sensing receptor. Kidney Int 94: 882–886, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, et al.: Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiano G, Glaudemans B, Olinger E, Goelz N, Müller M, Loffing-Cueni D, et al.: The urinary excretion of uromodulin is regulated by the potassium channel ROMK. Sci Rep 9: 19517, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verschuren EHJ, Hoenderop JGJ, Peters DJM, Arjona FJ, Bindels RJM: Tubular flow activates magnesium transport in the distal convoluted tubule. FASEB J 33: 5034–5044, 2019 [DOI] [PubMed] [Google Scholar]
- 68.Verschuren EHJ, Mohammed SG, Leonhard WN, Overmars-Bos C, Veraar K, Hoenderop JGJ, et al.: Polycystin-1 dysfunction impairs electrolyte and water handling in a renal precystic mouse model for ADPKD. Am J Physiol Renal Physiol 315: F537–F546, 2018 [DOI] [PubMed] [Google Scholar]
- 69.Corre T, Arjona FJ, Hayward C, Youhanna S, de Baaij JHF, Belge H, et al.: Genome-wide meta-analysis unravels interactions between magnesium homeostasis and metabolic phenotypes. J Am Soc Nephrol 29: 335–348, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zolotarov Y, Ma C, González-Recio I, Hardy S, Franken G, Uetani N, et al.: ARL15 modulates magnesium homeostasis through N-glycosylation of CNNMs. bioRxiv 2020.09.09.289835 (Preprint posted September 10, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CT, Ng HY, Lee YT, Lai LW, Lien YH: The role of calbindin-D28k on renal calcium and magnesium handling during treatment with loop and thiazide diuretics. Am J Physiol Renal Physiol 310: F230–F236, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olinger E, Schwaller B, Loffing J, Gailly P, Devuyst O: Parvalbumin: calcium and magnesium buffering in the distal nephron. Nephrol Dial Transplant 27: 3988–3994, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Schäffers OJM, Hoenderop JGJ, Bindels RJM, de Baaij JHF: The rise and fall of novel renal magnesium transporters. Am J Physiol Renal Physiol 314: F1027–F1033, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Hurd TW, Otto EA, Mishima E, Gee HY, Inoue H, Inazu M, et al.: Mutation of the Mg2+ transporter SLC41A1 results in a nephronophthisis-like phenotype. J Am Soc Nephrol 24: 967–977, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mastrototaro L, Smorodchenko A, Aschenbach JR, Kolisek M, Sponder G: Solute carrier 41A3 encodes for a mitochondrial Mg2+ efflux system. Sci Rep 6: 27999, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stuiver M, Lainez S, Will C, Terryn S, Günzel D, Debaix H, et al.: CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 88: 333–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Baaij JH, Groot Koerkamp MJ, Lavrijsen M, van Zeeland F, Meijer H, Holstege FC, et al.: Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg2+ handling. Am J Physiol Renal Physiol 305: F1563–F1573, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Arjona FJ, de Baaij JHF: CrossTalk opposing view: CNNM proteins are not Na+/Mg2+ exchangers but Mg2+ transport regulators playing a central role in transepithelial Mg2+ (re)absorption. J Physiol 596: 747–750, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Funato Y, Furutani K, Kurachi Y, Miki H: CrossTalk proposal: CNNM proteins are Na+/Mg2+ exchangers playing a central role in transepithelial Mg2+ (re)absorption. J Physiol 596: 743–746, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamazaki D, Funato Y, Miura J, Sato S, Toyosawa S, Furutani K, et al.: Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet 9: e1003983, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sponder G, Mastrototaro L, Kurth K, Merolle L, Zhang Z, Abdulhanan N, et al.: Human CNNM2 is not a Mg2+ transporter per se. Pflugers Arch 468: 1223–1240, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Arjona FJ, de Baaij JH, Schlingmann KP, Lameris AL, van Wijk E, Flik G, et al.: CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet 10: e1004267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Funato Y, Yamazaki D, Miki H: Renal function of cyclin M2 Mg2+ transporter maintains blood pressure. J Hypertens 35: 585–592, 2017 [DOI] [PubMed] [Google Scholar]
- 84.Maeoka Y, McCormick JA: NaCl cotransporter activity and Mg2+ handling by the distal convoluted tubule. Am J Physiol Renal Physiol 319: F1043–F1053, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, et al.: Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, et al.: SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schnoz C, Carrel M, Loffing J: Loss of sodium chloride co-transporter impairs the outgrowth of the renal distal convoluted tubule during renal development. Nephrol Dial Transplant 35: 411–432, 2020 [DOI] [PubMed] [Google Scholar]
- 89.Schnoz C, Moser S, Kratschmar DV, Odermatt A, Loffing-Cueni D, Loffing J: Deletion of the transcription factor Prox-1 specifically in the renal distal convoluted tubule causes hypomagnesemia via reduced expression of TRPM6 and NCC. Pflugers Arch 473: 79–93, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meij IC, Koenderink JB, van Bokhoven H, Assink KF, Groenestege WT, de Pont JJ, et al.: Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase gamma-subunit. Nat Genet 26: 265–266, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Schlingmann KP, Bandulik S, Mammen C, Tarailo-Graovac M, Holm R, Baumann M, et al.: Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet 103: 808–816, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, et al.: Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al.: Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, et al.: Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su XT, Yang CL, Ellison DH: Kidney is essential for blood pressure modulation by dietary potassium. Curr Cardiol Rep 22: 124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, et al.: The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28: 209–217, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Zhang C, Su X, Lin DH, Wang W: Caveolin-1 deficiency inhibits the basolateral K+ channels in the distal Convoluted tubule and impairs renal K+ and Mg2+ transport. J Am Soc Nephrol 26: 2678–2690, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrè S, de Baaij JH, Ferreira P, Germann R, de Klerk JB, Lavrijsen M, et al.: Mutations in PCBD1 cause hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 25: 574–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kompatscher A, de Baaij JHF, Aboudehen K, Hoefnagels APWM, Igarashi P, Bindels RJM, et al.: Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1β drives autosomal dominant tubulointerstitial kidney disease. Kidney Int 92: 1145–1156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z: Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87: 3248–3254, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, et al.: The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nijenhuis T, Hoenderop JG, Bindels RJ: Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Gratreak BDK, Swanson EA, Lazelle RA, Jelen SK, Hoenderop J, Bindels RJ, et al.: Tacrolimus-induced hypomagnesemia and hypercalciuria requires FKBP12 suggesting a role for calcineurin. Physiol Rep 8: e14316, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ikari A, Okude C, Sawada H, Takahashi T, Sugatani J, Miwa M: Down-regulation of TRPM6-mediated magnesium influx by cyclosporin A. Naunyn Schmiedebergs Arch Pharmacol 377: 333–343, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Chang CT, Hung CC, Tian YC, Yang CW, Wu MS: Ciclosporin reduces paracellin-1 expression and magnesium transport in thick ascending limb cells. Nephrol Dial Transplant 22: 1033–1040, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Ryan MP: Magnesium- and potassium-sparing effects of amiloride. Review and recent findings. Magnesium 3: 274–288, 1984 [PubMed] [Google Scholar]
- 108.Murdoch DL, Forrest G, Davies DL, McInnes GT: A comparison of the potassium and magnesium-sparing properties of amiloride and spironolactone in diuretic-treated normal subjects. Br J Clin Pharmacol 35: 373–378, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Devane J, Ryan MP: Dose-dependent reduction in renal magnesium clearance by amiloride during frusemide-induced diuresis in rats. Br J Pharmacol 80: 421–428, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dyckner T, Wester PO, Widman L: Amiloride prevents thiazide-induced intracellular potassium and magnesium losses. Acta Med Scand 224: 25–30, 1988 [DOI] [PubMed] [Google Scholar]
- 111.Reilly RF, Huang CL: The mechanism of hypocalciuria with NaCl cotransporter inhibition. Nat Rev Nephrol 7: 669–674, 2011 [DOI] [PubMed] [Google Scholar]