Significance Statement
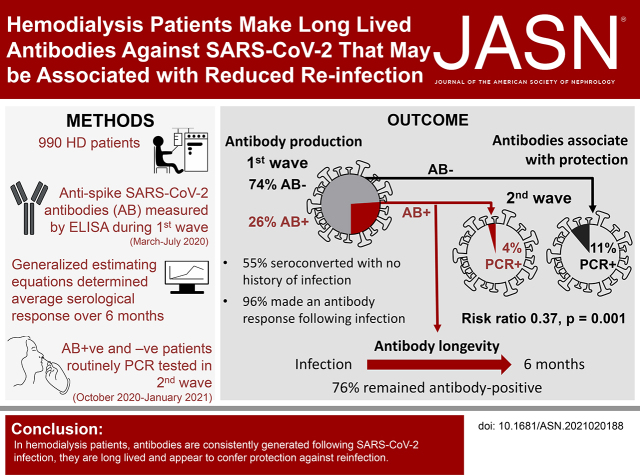
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections have a devastating effect on patients receiving hemodialysis. To what extent infection-induced antibody responses are maintained, or protective, is unknown. This study describes the evolution of antibodies against the SARS-CoV-2 spike protein in a cohort of 990 patients on hemodialysis. During the first wave of the pandemic, 26% of patients had developed antispike SARS-CoV-2 antibodies. Fewer PCR-confirmed second-wave infections were observed in patients with pre-existing antibodies (4.2%) than those without antibodies (11.4%). This study shows that SARS-CoV-2 antibodies in patients on hemodialysis are well maintained and associate with reduced risk of subsequent SARS-CoV-2 infection.
Keywords: hemodialysis, SARS-CoV-2, COVID-19, antibody
Visual Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections have disproportionately affected patients receiving in-center hemodialysis. In England, 11.3% of patients on in-center hemodialysis were reportedly infected by coronavirus disease 2019 (COVID-19), with a 23% fatality rate,1 45 times greater than in nonhemodialysis, age-matched populations.2 Nevertheless, little is known about antibody responses induced by infection and whether these associate with protection.
We report a single-center observational cohort study of 990 patients on hemodialysis, performed between March 10, 2020 and January 9, 2021. We measured the longevity of serological responses to SARS-CoV-2 infection and the risk of reinfection. Participants were recruited from the in-center hemodialysis population at University of Birmingham Hospitals National Health Service (NHS) Foundation Trust. SARS-CoV-2 infection waves were defined as first wave, March to July 2020 and second wave October 2020 to January 2021. Antibodies (combined IgG, IgA, and IgM; IgGAM) against SARS-CoV-2 spike glycoprotein were examined by ELISA in surplus serum from routine clinical samples taken during the first wave.3 We modeled antibody responses longitudinally using generalized estimating equations, allowing for sampling variation between individuals (Supplemental Material). Frequency of PCR-confirmed SARS-CoV-2 infection during the second wave was analyzed according to antibody status.
Clinical data and SARS-CoV-2 infection status were collated from electronic medical records. SARS-CoV-2 infection onset date was defined as the date symptoms started or a positive PCR (PCR+) test, whichever was earlier. In patients testing antibody positive without a history of SARS-CoV-2 infection, the predicted onset date was defined as the date 50% of patients who were symptomatic had developed SARS-CoV-2 within their hemodialysis unit.
Antispike SARS-CoV-2 antibodies were detected in 25.9% (256 out of 990) of patients from the first wave of COVID-19, with 54.7% seroconverting without a history of infection (140 out of 256) (Table 1). In total, 15 patients with PCR-confirmed COVID-19 had no evidence of an antibody response. Six of these 15 patients died after a PCR+ test (median 4 days, range 1–5 days) and six patients had no samples after 14 days after a PCR+ test. Excluding these 12 patients with insufficient samples for analysis, 96% (82 out of 85) of patients who were PCR+ for generated an antibody response.
Table 1.
Comparing outcome, comorbidity, and demographic variables dependent on detection of SARS-CoV-2 antibodies
| Variables | All n=990 | AB− n=734 | AB+ n=256 | P Value |
|---|---|---|---|---|
| Male, n (%) | 579 (58.5) | 447 (60.9) | 132 (51.6) | 0.009 |
| Female, n (%) | 411 (41.5) | 287 (39.1) | 124 (48.4) | |
| Ethnicity, n (%) | 0.006 | |||
| White | 481 (48.6) | 381 (51.9) | 100 (39.1) | |
| Asian | 296 (29.9) | 201 (27.4) | 95 (37.1) | |
| Black | 142 (14.3) | 100 (13.6) | 42 (16.4) | |
| Other | 35 (3.5) | 24 (3.3) | 11 (4.3) | |
| Unknown | 36 (3.6) | 28 (3.8) | 8 (3.1) | |
| Age, yrs, median (IQR) | 65 (54–75) | 64 (54–75) | 67 (55–75) | 0.482 |
| IMD, decile, median (IQR) | 2 (1–5) | 2 (1–5) | 2 (1–4) | 0.096 |
| BMI, kg/m2 median (IQR) | 27 (23–32) | 27 (23–31) | 27 (24–32) | 0.228 |
| CCI, median (IQR) | 7 (5–8) | 7 (5–8) | 7 (6–8) | 0.024 |
| DM, n (%) | 418 (42.2) | 288 (39.2) | 130 (50.8) | 0.001 |
| Immunosuppression medication, n (%) | 155 (15.7) | 120 (16.3) | 35 (13.7) | 0.310 |
| Symptoms reported, n (%) | 227 (22.9) | 111 (15.1) | 116 (45.3) | 0.000 |
| Hospitalized, n (%) | 128 (12.9) | 57 (7.8) | 71 (27.7) | 0.000 |
| Died, n (%) | 93 (9.4) | 66 (9.0) | 27 (10.5) | 0.463 |
| Alive at the start of the second wave | n=700 | n=237 | ||
| PCR+ during second wave, n (%) | 80 (11.4) | 10 (4.2) | 0.001 | |
| Immunosuppression medication, n (%) | 8 (10.0) | 1 (10.0) | 1.000 | |
| Symptoms reported, n (%) | 57 (71.3) | 5 (50.0) | 0.171 | |
| Hospitalized, n (%) | 34 (42.5) | 4 (40.0) | 0.880 | |
| Died, n (%) | 10 (12.5) | 2 (20.0) | 0.511 |
Antibody status determined during the first wave. P values from chi-squared tests for categorical data and Wilcoxon rank-sum tests for continuous data. Current use of immunosuppression medication or intravenous agent within a year of the start of the first wave. Symptoms reported compatible with SARS-CoV-2 infection. Death by January 9, 2021. PCR positivity during the second wave is reported as a percentage of those patients alive at the beginning of the second wave, with associated presence immunosuppression, symptoms, hospitalization, and death reported as a percentage of those who are PCR+. AB−, SARS-CoV-2 antispike IgGAM seronegative; AB+, SARS-CoV-2 antispike IgGAM seropositive; IQR, interquartile range; IMD, Index of Multiple Deprivation 2019; BMI, body mass index; CCI, Charlson Comorbidity Index; DM, diabetes mellitus.
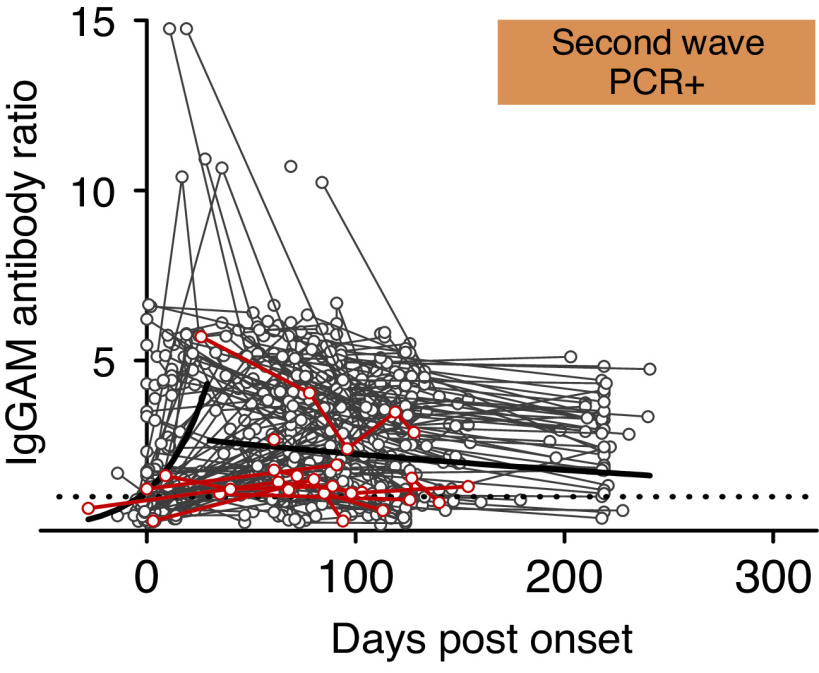
We investigated whether antibodies generated against SARS-CoV-2 persist in patients receiving hemodialysis. In total, 174 patients provided additional samples after testing positive; of these, 132 (75.9%) remained antibody positive at the last sample (median duration 124 days after infection, interquartile range, 95–210). Modeling of our data showed the predicted mean IgGAM antispike response remained positive >200 days after infection but declined over time (Figure 1). Those with symptomatic disease had higher predicted mean IgGAM responses than asymptomatic individuals (P=0.004).
Figure 1.
The serological response to SARS-CoV-2 and the risk of reinfection. Gray and red lines represent individual antibody ratios over time for patients who were antibody positive during the first wave and alive at the start of the second wave. Patients testing PCR+ during the second wave are shown by red lines. Patients who did not test PCR+ during the second wave are shown by gray lines. The predicted mean IgGAM serological response for the cohort is shown by solid black lines. The threshold for antibody positivity is represented by a dotted line.
During the second wave, patients were screened routinely for infection. In total, 90 PCR+ patients were identified out of 937 who were at risk and on hemodialysis—11.4% (80 out of 700) of patients without pre-existing antibodies, but only 4.2% (10 out of 237) of those with pre-existing antibodies (risk ratio, 0.37; 95% confidence interval, 0.19 to 0.70, P=0.001), with no differences in the proportion of patients who were symptomatic, hospitalized, or who died, according to antibody status (Table 1). Eight of the 10 patients with antibodies detected in the first wave, who tested PCR+ during the second wave, had antibody ratios lower than the predicted mean for the cohort (range 65–192 days between last IgGAM and PCR+ tests) (Figure 1).
In this hemodialysis cohort, antibody responses to the SARS-CoV-2 spike protein are maintained, as in other cohorts,4,5 and may be associated with a reduced frequency of reinfection. Those with lower levels of antibodies appear to be at greater risk of reinfection. Our analysis is limited to the quantification of antibody responses; assessment of the neutralizing capacity of these antibodies and associated T cell responses is required to conclude the immune response is protective against reinfection.
We confirmed SARS-CoV-2 infection in 4.2% (10 out of 237) of patients on hemodialysis with pre-existing antibodies, whereas in health care workers only two asymptomatic infections were detected among 1265 antibody-positive participants.6 The different infection rate is, perhaps, unsurprising given the significant immunosuppression associated with hemodialysis,7 differences in testing frequency, the age disparity between the studied groups, and potential differences in symptom expression but this requires further investigation.
This is a large hemodialysis cohort; however, the analysis does have limitations. We were unable to collect samples from most patients who died early in the pandemic, who may not have developed a robust antibody response. Due to our sampling strategy, we were unable to describe the maturation of the immune response comprehensively for an individual from time of infection; however, we used generalized estimating equations to allow for this. Early sampling was biased toward those with symptoms, and we may have missed individuals showing short-lived responses while asymptomatic.
In conclusion, patients on hemodialysis who survive SARS-CoV-2 infection generate an antibody response that is well maintained and appears to be associated with a reduced frequency of reinfection. Given patients with lower responses may be at increased risk of reinfection, the capacity of antivaccine antibody responses to protect patients on hemodialysis should be closely monitored to determine efficacy.
Disclosures
A. Cunningham reports consultancy agreements with Pfizer - vaccine education, Sanofi - vaccine education, and Oxford Immunotec -Immunology education; research funding from GSK - vaccine research; honoraria from Oxford Immunotec; and scientific advisor or membership via BSI, Microbiology Society, and BactiVac. A. Richter reports research funding from The Binding Site - fund joint PhD; and honoraria from CSL- Behring. L. Harper reports research funding from Novartis, Talecris, GSK, Vifor, and Chemocentryx; and honoraria from Novartis, Chemocentryx, and Roche. All remaining authors have nothing to disclose.
Funding
This work was supported by funding for reagents from the Queen Elizabeth Hospital Birmingham Charity grant 16-3-046.
Ethics Approval and Consent to Participate
Ethical approval was granted by the North West-Preston Research Committee (ref 20/NW/0240 IRAS Project ID: 282164) for the NIHR UPH Coronavirus Immunological Analysis study.
Supplementary Material
Acknowledgments
Dr. G. Banham, Prof. A. Cunningham, Prof. L. Harper, and Prof. A. Richter designed the study; Dr. G. Banham and Dr. A. Godlee were involved in data collection and analysis; Dr. S. Faustini and Prof. A. Richter performed the experiments; Dr. G. Banham and Dr. A. Godlee prepared the figure; Dr. G. Banham, Prof. A. Cunningham, Dr. A. Godlee, Prof. L. Harper, and Prof. A. Richter drafted and revised the paper; and all authors approved the final version of the manuscript. We thank Dr. Anna L. Casey, Liz Ratcliffe (Department of Pathology, University Hospitals Birmingham NHS Foundation Trust) for sample collection; Claire Backhouse, Beena Emmanuel, Lynsey A Dunbar (Clinical Immunology Service, University of Birmingham) for processing samples; Dr. Matthew Tabinor and Dr. Megan Fahy (University Hospitals Birmingham NHS Foundation Trust) for assistance with data collection; Dr. Peter Nightingale (University Hospitals Birmingham NHS Foundation Trust) for providing statistical oversight; Prof. Paul Moss (Institute of Immunology and Immunotherapy, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust), the Chief Investigator of the CIA Study, for arranging ethical approval and for his input in study design and Dr. Stephanie Stringer (University Hospitals Birmingham NHS Foundation Trust), the clinical lead for the hemodialysis service, for coordinating the COVID-19 response. None of these individuals received compensation for their contribution. We would like to thank the Department of Pathology, University Hospitals Birmingham NHS Foundation Trust, and the Clinical Immunology Service, University of Birmingham, for processing samples, together with the medical teams and dialysis nurses who cared for these patients.
Footnotes
G.D.B. and A.G. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021020188/-/DCSupplemental.
Supplemental Material. Additional Methods: Serological Testing and Statistical Analysis.
References
- 1.The Renal Association B : UK Renal Registry (2020) COVID-19 surveillance report for renal centres in the UK: A summary of the first wave of the pandemic – March to August 2020. Available at: https://renal.org/sites/renal.org/files/covid_report_first_wave_FINAL_041220.pdf. Accessed January 4, 2021 [Google Scholar]
- 2.Savino M, Casula A, Santhakumaran S, Pitcher D, Wong E, Magadi W, et al.: Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS-CoV-2: A UK Renal Registry data analysis. PLoS One 15: e0241263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook AM, Faustini SE, Williams LJ, Cunningham AF, Drayson MT, Shields AM, et al.: Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients. J Immunol Methods 494: 113046, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al.: Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370: 1227–1230, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al.: Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371: eabf4063, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al.: Antibody status and incidence of SARS-CoV-2 infection in health care workers. NEngl J Med 384: 533–540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al.: Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.