Significance Statement
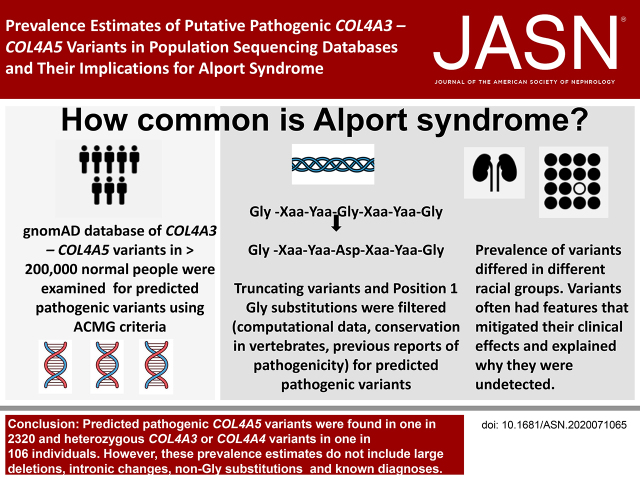
The population frequencies of Alport syndrome vary greatly in different reports. This study examined a population sequencing database of individuals not known to have kidney disease using filtering steps corresponding to the American College of Medical Genetics and Genomics/Association for Molecular Pathology criteria for “predicted pathogenic” variants in COL4A3–COL4A5, which considered collagen chain position 1 Gly residues “critical domains.” Predicted pathogenic COL4A5 variants occurred in at least one in 2320 individuals. Heterozygous COL4A3 or COL4A4 variants affected one in 106; compound heterozygous COL4A3 or COL4A4 variants affected one in 88,866. The actual prevalences are even greater because they also include already diagnosed disease and other variants not examined here. The high frequency of predicted pathogenic COL4A3–COL4A5 variants suggests that other genetic and environmental factors mitigate the corresponding clinical manifestations of disease.
Keywords: Alport syndrome, thin basement membrane nephropathy, hematuria, COL4A5, COL4A3, COL4A4, collagen IV, genetic renal disease, glomerular basement membrane, Gly substitutions
Visual Abstract
Abstract
Background
The reported prevalence of Alport syndrome varies from one in 5000 to one in 53,000 individuals. This study estimated the frequencies of predicted pathogenic COL4A3–COL4A5 variants in sequencing databases of populations without known kidney disease.
Methods
Predicted pathogenic variants were identified using filtering steps based on the ACMG/AMP criteria, which considered collagen IV α3–α5 position 1 Gly to be critical domains. The population frequencies of predicted pathogenic COL4A3–COL4A5 variants were then determined per mean number of sequenced alleles. Population frequencies for compound heterozygous and digenic combinations were calculated from the results for heterozygous variants.
Results
COL4A3–COL4A5 variants resulting in position 1 Gly substitutions were confirmed to be associated with hematuria (for each, P<0.001). Predicted pathogenic COL4A5 variants were found in at least one in 2320 individuals. p.(Gly624Asp) represented nearly half (16 of 33, 48%) of the variants in Europeans. Most COL4A5 variants (54 of 59, 92%) had a biochemical feature that potentially mitigated the clinical effect. The predicted pathogenic heterozygous COL4A3 and COL4A4 variants affected one in 106 of the population, consistent with the finding of thin basement membrane nephropathy in normal donor kidney biopsy specimens. Predicted pathogenic compound heterozygous variants occurred in one in 88,866 individuals, and digenic variants in at least one in 44,793.
Conclusions
The population frequencies for Alport syndrome are suggested by the frequencies of predicted pathogenic COL4A3–COL4A5 variants, but must be adjusted for the disease penetrance of individual variants and for the likelihood of already diagnosed disease and non-Gly substitutions. Disease penetrance may depend on other genetic and environmental factors
Alport syndrome is an inherited renal disease characterized by progressive kidney failure, hearing loss, lenticonus, and fleck retinopathy.1 X-linked (XL) inheritance results from pathogenic variants in COL4A5,2 and autosomal recessive (AR) disease results from two pathogenic variants in COL4A3 or COL4A4 in trans.3 The COL4A3–COL4A5 genes code for the collagen IV α3, α4, and α5 chains that undergo trimerization to form a network that represents the major component of the basement membranes of the glomerulus, cochlea, lens, and retina.
Individuals with heterozygous pathogenic COL4A3 or COL4A4 variants are carriers for AR Alport syndrome and are sometimes diagnosed with thin basement membrane nephropathy,4,5 even without a renal biopsy being performed. The term “autosomal dominant” (AD) Alport syndrome is also used, but affected individuals typically have persistent hematuria and not the hearing loss, ocular abnormalities, or typical glomerular basement membrane (GBM) lamellation.6 In contrast to XL and AR disease, the risk of renal failure for heterozygous pathogenic COL4A3 or COL4A4 variants is uncertain because most reported series comprise only hospital-based patients.7 Digenic Alport syndrome occurs with heterozygous pathogenic variants in two different COL4A3–COL4A5 genes,8 and clinical features may be more severe than with only a heterozygous COL4A3 or COL4A4 variant.8 In addition, any variant or combination of variants in COL4A3–COL4A5 may result in FSGS, probably secondary to a defective GBM and podocyte loss.9,10
Alport syndrome has been considered rare because it affects fewer than one in 2000 individuals,11 and the United States has <200,000 patients with Alport syndrome.7 Its exact prevalence is not known.12 Population-based studies have suggested frequencies of one in 5000 in Utah,13 one in 17,000 in southern Sweden,14 and one in 53,000 in Finland.15 However, persistent hematuria and GBM thinning, which correspond to heterozygous COL4A3 or COL4A4 variants, occur in 1%–2.5% of children and adults on population screening,16,17 and, depending on the definition of thinning, in 4%–7% of normal donor kidney transplant biopsy specimens.18
Alport syndrome is suspected when persistent hematuria occurs together with a family history of hematuria or renal impairment, or an abnormal GBM appearance or collagen IV chain composition.19 Hematuria is highly penetrant,20,21 but many of those affected are undiagnosed. These individuals are often men with XL disease and late-onset kidney failure, or others who have no family history or their kidney biopsy specimen is too scarred to demonstrate the characteristic features. Although women with XL disease are affected twice as often as men, their diagnosis is often even more problematic22 because they typically have only hematuria and a thinned, rather than lamellated, GBM.22
The most sensitive method for the diagnosis of Alport syndrome is genetic testing19,23 using massively parallel sequencing, which detects causative variants in up to 90% of individuals.23 Identifying likely pathogenic variants in the COL4A3–COL4A5 genes in datasets of individuals not known to have kidney disease—using databases such as the Genome Aggregation Database (gnomAD), gnomAD controls, Exome Variant Server (EVS), or TOPMed—indicates more accurately the population frequencies of undiagnosed COL4A5, heterozygous COL4A3 or COL4A4 variants, and, hence, compound heterozygous and digenic disease. However, these databases share some datasets and include no clinical information. Conversely, the Genomics England 100,000 Genomes Project is an independent dataset that includes clinical information, but its recruitment of individuals with inherited kidney disease precludes its use in determining population frequencies.
Genomic variants are usually assessed for pathogenicity using the American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) criteria.24 Nonsense variants, canonic splice site variants, and frameshift changes in the COL4A3–COL4A5 genes are likely to be pathogenic because loss of function is a recognized disease mechanism for these genes.24 However, the most common variants are missense (databases.lovd.nl/shared/genes/). The interpretation of missense variants from a population not known to have kidney disease is complicated by the absence of clinical, segregation, and functional data (Supplemental Table 1), and this is compounded by the lack of expert panel assertions for most variants (https://clinvarminer.genetics.utah.edu/).
However, affecting a “critical domain” also represents one of the ACMG/AMP criteria for pathogenicity (PM1),24 and, in osteogenesis imperfecta, position 1 glycine (Gly) residues in the collagen I α1 chain Gly-Xaa-Yaa repeats are considered critical because of their disruptive effect on the molecule.25 Gly is the smallest amino acid, position 1 Gly residues are found within the interior of the collagen triple helix, and their replacement by any larger residues disturbs the helix formation. The most common reported missense variants in COL4A3–COL4A5 result in position 1 Gly substitutions in the intermediate domain of the collagen IV α3, α4, and α5 chains,12,25 and the recent Chandos House meeting of the Alport Variant Consortium recommended that these are generally considered pathogenic.26
There are, nevertheless, also important differences in the biochemistry of collagen IV and I. The collagen IV chains retain their noncollagenous amino and carboxy termini, and include multiple noncollagenous interruptions, in the Gly-Xaa-Yaa repeats of the intermediate domains (Supplemental Table 2).27–29 Gly residues in the amino and carboxy termini, the interruptions, and positions 2 and 3 in the Gly-Xaa-Yaa repeats29 are not considered critical.25,30
This study used the United Kingdom’s 100,000 Genomes Project to confirm that predicted pathogenic position 1 Gly substitutions in the collagen IV α3, α4, and α5 chains were associated with hematuria. We identified predicted pathogenic COL4A5 variants in the large, unbiased gnomAD version 2.1.1 dataset, and confirmed the strategy using the gnomAD control, EVS, and TOPMed cohorts. The amount of overlap between the datasets was determined from the number of shared variants. A similar approach has been used previously to determine the population frequency of polycystic kidney and liver disease.31
We then identified predicted pathogenic COL4A3 and COL4A4 variants in gnomAD, and used these to calculate the frequencies of compound heterozygous and digenic variants. These population frequencies provide an approximate guide to the prevalence of XL, AR (compound heterozygous), and digenic Alport syndrome and thin basement membrane nephropathy. However, the precise prevalence must also take into account the penetrance of individual variants and the occurrence of already diagnosed disease and missense variants due to non-Gly substitutions.
Methods
Datasets
gnomAD (version 2.1.1; www.gnomAD.broadinstitute.org)32 is a large dataset that includes COL4A3–5 alleles from unrelated adults, with equal numbers of males and females, who have undergone exomic (in about 90%) or genomic sequencing in disease and population studies. It includes individuals with adult-onset diseases—such as diabetes, cardiac disease, and psychiatric conditions—and excludes those with severe pediatric diseases and their close family members. Participants were not known to have inherited kidney disease, and individuals with any severe disease were expected less frequently than in the general population. gnomAD indicates variants that are homozygous, but variants are not otherwise linked to an individual and it is not possible to directly identify a second different variant in COL4A3–COL4A5 and, hence, compound heterozygous or digenic combinations. Ancestries are available, but the clinical phenotypes are not recorded.
Three other datasets were also examined for predicted pathogenic variants in COL4A5. These were the “gnomAD controls” (version 2.1.1; www.gnomAD.broadinstitute.org), which comprises alleles from the normal individuals recruited as controls for gnomAD studies; EVS (www.evs.gs.washington.edu/EVS/), which includes alleles from exomic sequencing of individuals recruited for mainly cardiac and pulmonary studies; and TOPMed (www.bravo.sph.umich.edu/freeze8/hg38/), with alleles from genomic sequencing of individuals recruited for heart, lung, blood, and sleep studies. Although participants in these cohorts were not known to have inherited kidney disease, they had not been specifically phenotyped for these conditions, and it was uncertain how many had underlying renal disease or impaired kidney function. The gnomAD dataset includes all of the gnomAD control cohort, and some of the cohorts from other studies. The amount of overlap was assumed to include the COL4A3–COL4A5 variants randomly, and was quantitated from the number of shared variants with the same sex.
In contrast, the Genomics England 100,000 Genomes Project database (www.genomicsengland.co.uk/)33 includes whole-genome sequencing data from individuals with rare diseases, including inherited kidney disease, and their unaffected relatives. This dataset also includes clinical phenotypes, including hematuria, noted in the hospital records. This database was used to examine whether substitutions affecting position 1 Gly substitutions in the collagen IV α3, α4, and α5 chains were associated with hematuria.
Ethics approval was obtained at the time of recruitment into each of the constituent studies. The databases are freely accessible online and were examined between December 24, 2019 and February 1, 2021.
COL4A3–5 Variants
COL4A5 variants were described using the GRCh37/hg19 sequence with 53 exons and the reference sequence for the collagen IV α5 chain (NM_033380.2, LRG_232t2). COL4A3 and COL4A4 variants were described using the GRCh37/hg19 sequence and the reference sequences for collagen IV α3 (NM 000091.4, LRG_230t1) and α4 chains (NM 000092.4, LRG_231t1), respectively. The locations of the noncollagenous termini and interruptions in the collagenous domains of each chain were noted (Supplemental Table 2).27–29
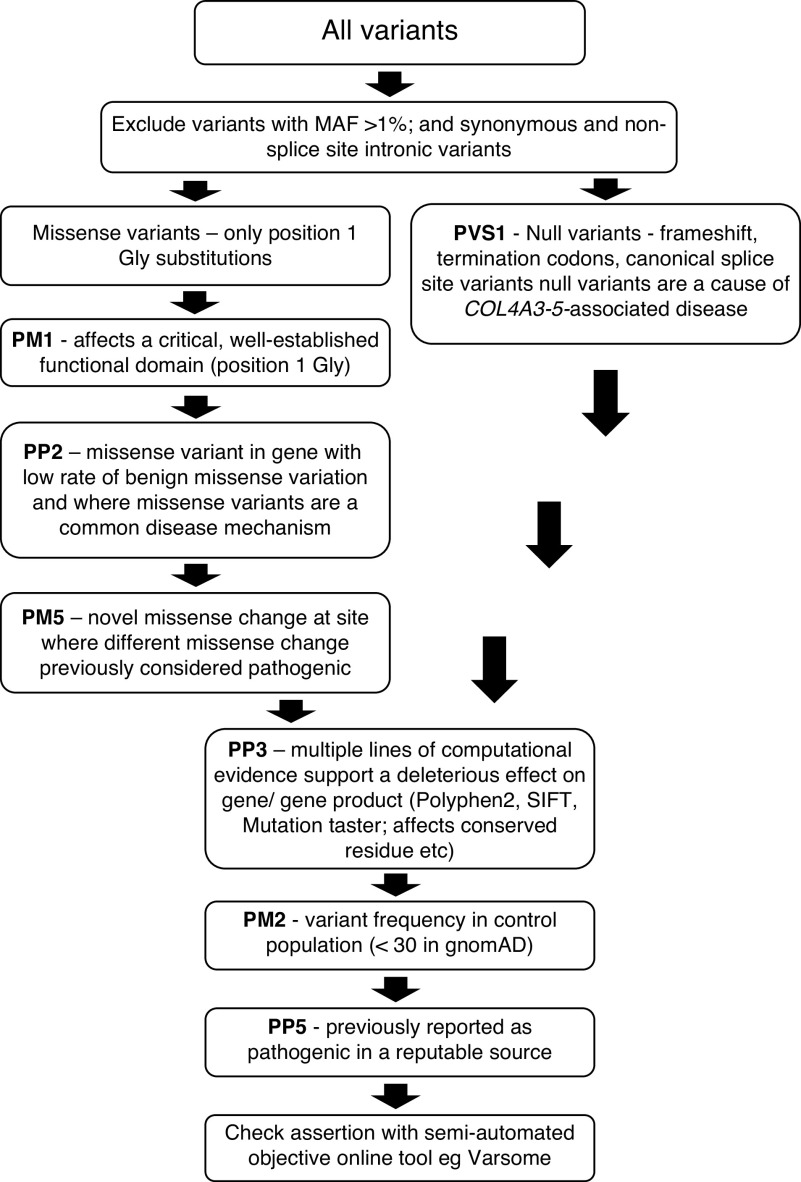
Filtering Steps To Determine Predicted Pathogenic Variants on the Basis of the ACMG/AMP Criteria
Variants were filtered to exclude those that failed any step to be confident of the corresponding population frequencies. Variants were filtered according to their effect on the canonic transcripts using the following steps and ACMG/AMP criteria (Figure 1, Supplemental Table 1).34 The term “predicted pathogenic” was used for variants that were not excluded, to differentiate them from the “pathogenic” and “likely pathogenic” classes derived from the ACMG/AMP classification in individuals with clinical features of disease.
Synonymous variants and intronic variants other than those affecting a +/− 1 or 2 splice site were excluded.
Variants that resulted in a protein-truncating change, frameshift, or canonic splice site change were graded PVS1, because loss of function is a known disease mechanism for disease associated with COL4A3–COL4A5.24
Missense variants resulting in position 1 Gly substitutions within the intermediate collagenous domains of the collagen IV α3−α5 chains were graded as PP2, because missense variants are a common mechanism for disease associated with COL4A3–COL4A5, with a low rate of benign missense variation . Variants that resulted in a position 1 Gly substitution were considered to affect a critical and well-established functional domain and were graded PM1.
Missense variants were examined with individual computational tools: PolyPhen-2 (sc ore >0.8; www.genetics.bwh.harvard.edu/pph2/), Sorting Intolerant From Tolerant (for classification of deleterious or polymorphism; www.sift.bii.a-star.edu.sg/), and MutationTaster (for classification as disease causing or polymorphism; www.mutationtaster.org/). Missense variants were also examined for conservation among vertebrates (www.asia.ensembl.org/) (PP3).
Variants that occurred in gnomAD at an arbitrary cutoff of <30 times were graded PM2.34 The formula used to determine the cutoff is normally based on the disease prevalence and contribution of the gene, which were not known.35 Although the cutoff varies for different genes, this level has been used for other heterogeneous genes resulting in AD kidney disease.
Variants were assessed for previous reports of being likely pathogenic or pathogenic in a reputable source, such as ClinVar (www.ncbi.nlm.nih.gov/clinvar/) or LOVD (www.databases.lovd.nl/shared/genes/COL4A3-5) (PP5), despite this practice being controversial.36,37 Variants in ClinVar reported as benign, likely benign, variant of uncertain significance, or conflicting were excluded. Variants in LOVD reported as benign or likely benign were excluded. Variants that resulted in the same amino acid change as a previously established pathogenic variant, regardless of nucleotide change, in ClinVar or LOVD were noted (PS1). In these cases, the submitters’ evaluations were accepted because their assessments included the associated clinical data, which we were not able to access.
Variants were examined to determine if they resulted in a novel missense change at an amino acid where a different change was previously reported to be pathogenic (PM5). (
Figure 1.
Filtering steps used to determine pathogenic and likely pathogenic variants using the ACMG/AMP criteria. PVS1 represents very strong evidence of pathogenicity; PM1, PM2, and PM5 represent moderate evidence of pathogenicity; PP2, PP3, and PP5 represent supporting evidence of pathogenicity.24 MAF, minor allele frequency; SIFT, Sorting Tolerant from Intolerant.
All COL4A3–COL4A5 variants were also assessed by VarSome (www.varsome.com/) using the ACMG/AMP criteria,38 and COL4A5 variants in gnomAD were assessed in Alamut (www.interactive-biosoftware.com/alamut-visual/). These semiautomated online tools were chosen because they are objective, not subject to individual laboratory practice, and are widely available. They used slightly different criteria from our filtering steps, for example, they used numerous computational tools, and GERP scores for conservation. Variants assessed as benign, likely benign, or a variant of uncertain significance by VarSome or Alamut were excluded.
Predicted Pathogenic COL4A3–COL4A5 Variants Resulting in Position 1 Gly Substitutions in the 100,000 Genomes Project Database and Hematuria
Individuals in the 100,000 Genomes Project database with hematuria were identified from hematuria-related Human Phenotype Ontology terms after excluding those with cancer of the kidney or bladder, or those with kidney stones. Predicted pathogenic variants resulting in a position 1 Gly substitution in COL4A3–COL4A5 were identified from all Gly substitutions in these genes using our filtering strategy, and the total number of individuals with a predicted pathogenic variant were compared in the cohorts with hematuria and without (chi square with Yates correction, two tailed, GraphPad).
Prevalence of Predicted Pathogenic Variants in COL4A5, COL4A3, and COL4A4
The population frequencies of predicted pathogenic variants were calculated on the basis of the mean number of alleles examined. For COL4A5, which is located on the X chromosome, the corresponding number of individuals was determined from the number of alleles after correcting for the number of males, because they have only one allele for COL4A5. The total number of individuals recruited in the whole cohort was greater than the number calculated from the number of alleles examined because some regions of a gene were not sequenced or failed quality testing.
Prevalence of Variants in Different Ancestries and Founder Variants
The frequencies of predicted pathogenic COL4A5, COL4A3, and COL4A4 variants were noted in people of different ancestries (African, Latino, Ashkenazi Jew, East Asian, European Finnish, European non-Finnish, South Asians, and “Others” who did not unambiguously cluster with any of the major populations by principal component analysis). Any founder variants (arbitrarily set at ≥18 alleles) were noted.
Biochemical Features Likely to Mitigate Clinical Phenotypes for Predicted Pathogenic COL4A5 Variants
The cohort with predicted pathogenic COL4A5 variants were then assessed for any biochemical features associated with clinically milder phenotypes that might explain the lack of previous diagnosis. Mitigating features included female sex22; location within the 20 amino-terminal exons including the amino noncollagenous domain (204 residues)39; position adjacent to a noncollagenous domain or interruption30; and Gly substitutions with a less disruptive residue, such as alanine (Ala), serine (Ser), or cysteine (Cys).40,41
Calculation of Population Frequencies for Heterozygous, Compound Heterozygous, and Digenic COL4A3 and COL4A4 Variants
The population frequencies of predicted pathogenic heterozygous COL4A3 and COL4A4 variants were derived from the sum of the allele frequencies for each variant, assuming they were inherited independently and that no or very few individuals had inherited two variants.
The population frequency of AR Alport syndrome represents the sum of the frequencies of predicted pathogenic homozygous and compound heterozygous variants in trans in COL4A3 plus COL4A4. Previous reports suggest homozygous inheritance represents 30% of all individuals with AR Alport syndrome.42 Homozygous variants are indicated in gnomAD and usually result from relationships that are consanguineous or consanguineous by descent. However, in Alport syndrome, homozygous predicted pathogenic variants result in early-onset kidney failure, which would have precluded recruitment into gnomAD. The likelihood of individual homozygous COL4A3 or COL4A4 variants occurring by chance was very small and not calculated here.
It was not possible to identify compound heterozygous and digenic variants from the databases because variants were not linked to any individual. In addition, compound heterozygous and digenic variants were likely to be under-represented because they too are associated with early-onset kidney failure. Thus, the population frequencies of predicted pathogenic compound heterozygous variants in COL4A3 or COL4A4 were calculated simply from the likelihood of the occurrence of each allele occurring independently.
Digenic variants were calculated from two predicted pathogenic variants in COL4A3 and COL4A4 in cis or trans, and from a predicted pathogenic variant in COL4A5 and either COL4A3 or COL4A4.
Results
Predicted Pathogenic COL4A3–COL4A5 Variants Resulting in Position 1 Gly Substitutions in the 100,000 Genomes Project Database and Hematuria
This cohort comprised 2221 individuals with documented hematuria and 37,200 with none. It included 19 predicted pathogenic COL4A5 variants that affected a position 1 Gly and were associated with hematuria, and nine that were not, consistent with an association with hematuria (chi square, 95.83; P<0.001). COL4A3 and COL4A4 variants resulting in predicted pathogenic position 1 Gly substitutions were also associated with hematuria (P<0.0001 for each; Table 1, Supplemental Tables 3–5).
Table 1.
Association of predicted pathogenic COL4A3–COL4A5 variants resulting in position 1 Gly substitutions with hematuria in 100,000 Genomes Project database
| Gene | Number of Variants Resulting in Position 1 Gly Substitutions in 100,000 Genomes Project, n | Number of Variants Resulting in Position 1 Gly Substitutions Excluded after Filtering, n (%) | Number of Individuals with a Predicted Pathogenic Position 1 Gly Substitution | Chi Square (with Yates correction) | Two-Tailed P Value | |
|---|---|---|---|---|---|---|
| In Total with Hematuria (n=2221) | In Total without Hematuria (n=37,200) | |||||
| COL4A5 | 20 | 1 (5) | 12 | 9 | 95.83 | <0.001 |
| COL4A3 | 87 | 14 (16) | 21 | 85 | 37.54 | <0.001 |
| COL4A4 | 84 | 13 (15) | 19 | 95 | 24.14 | <0.001 |
COL4A5
Predicted Pathogenic COL4A5 Variants in gnomAD
A total of 54 variants in COL4A5 (frameshift, nonsense, and canonic splice site variants, position 1 Gly substitutions) were identified for further assessment (Table 2). These included one deletion resulting in a termination codon and six canonic splice site variants, all predicted pathogenic, and found in eight individuals. There were also 47 position 1 Gly substitutions, but 13 were excluded on filtering (including p.[Gly953Val], Supplemental Table 3), resulting in 34 Gly substitutions in 51 individuals (Table 2).
Table 2.
Assessment of COL4A5 variants in the gnomAD database and gnomAD control subset
| Total gnomAD Database | gnomAD Control Subset | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position hg19 and Protein consequence | Transcript | Affected Individuals’ Sex, Total Alleles | PolyPhen-2 | SIFT | MT | Conserved | Previously Reported; Alternative Pathogenic Residue (LOVD) | ClinVar | Pathogenicity (VarSome) | Pathogenicity (Alamut) | Evidence Supports Pathogenicity | Affected Individuals’ Sex, Total Alleles |
| 107783018 p.(Gly42S er) |
c.124G>A | 1M, 21,780 | 0a | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Noa | |
| 107783019 p.(Gly42Ala) |
c.125G>C | 1F, 182,802 |
0.003a | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Noa | 1F, 80,278a |
| 107783019 p. (Gly42Asp) |
c.125G>A | 1F, 182,802 |
0.739a | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Noa | |
| 107802357 p.(Gly69Arg) |
c.205G>C | 1F, 183,072 |
1.00b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1F)b | |
| 107807131 p.(Gly84Glu) |
c.251G>A | 1F, 182,367 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Strongb | Yes (1F)b | |
| 107811896 p.(Gly105Ala) |
c.314G>C | 1M, 183,404 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Moderateb | Yes (1M)b | 1M, 80,333b |
| 107812008 p.(Gly114Ala) |
c.341G>C | 1F, 183,513 |
1b | Delb | DCb | Glyb | No, but p.(Gly114Arg) reported in LOVDb | Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Moderateb | Yes (1F)b | 1F, 80,345b |
| 107812044 p.(Gly126Glu) |
c.377G>A | 1F, 21,871 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Moderateb | Yes (1F)b | 1F, 7600b |
| 107814670 p.(Gly138Ser) |
c.412G>A | 1F, 183,271 |
1b | Tola | DCb | Glyb | Yes, Fallerini et al.58; and p(Gly138Asp) in LOVDb | Pathogenic (no assertion criteria)b | Likely pathogenic, PM1, PM2, PM5, PP2, PP3, PP5b | Weak | Noa | |
| 107819195 p.(Gly201Ala) |
c.602G>C | 1M, 168,800 |
0.999b | Delb | DCb | Glyb | No, but p(Gly201Val), Hertz et al.61,b | Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Moderateb | Yes (1M)b | |
| 107823808 p.(Gly276Ser) |
c.826G>A | 1F, 183,230 |
1b | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | 1F, 80,319a |
|
| 107823912 p.(Gly279Arg) |
c.835G>C | 1M, 183,017 |
1b | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Moderateb | Noa | |
| 107823913 p.(Gly279Ala) |
c.836G>C | 1M, 183,034 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1M)b | 1M, 80,323b |
| p.(Gly283Val) | c.848G>T | 1F, 183,010 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1F)b | |
| p.(Gly286Cys) | c.856G>T | 1F, 182,986 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1F)b | 1F, 80,324b |
| 107829879 p.(Gly356Ala) |
c.1067G>C | 1F, 21,220 |
0.997b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1F)b | |
| 107829879 p.(Gly356Glu) |
c.1067G>A | 1M, 183,308 |
0.99b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1M)b | |
| p.(Gly512Glu) | c.1535G>A | 1M, 145,435 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1M)b | |
| 107840625 p.(Gly536Ser) |
c.1606G>A | 1F, 183,226 |
1b | Delb | DCb | Glyb | No, but p(Gly536Asp) p(Gly536Val), in LOVDb |
Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Strongb | Yes (1F)b | |
| 107844640 p.(Gly624Asp) |
c.1871G>A | 4M, 12F, 182,998 | 1b | Delb | DCb | Glyb | Yes, Slajpah et al.42,b | Pathogenic (2 stars)b | Likely pathogenic PM1, PP2, PP3, and PP5b | Strongb | Yes (4M,12F)b | 2M, 6F, 80,268b |
| 107842028 p.(Gly626Ser) |
c.1876G>A | 2M,5F, 204,674 |
1b | Delb | DCb | Glyb | Yes, Hertz et al.61,b | Likely benign (one star)a | Likely pathogenic, PM1, PM2, PM5, PP2, PP3, BP6b | Strongb | Nob | 1M, 4F, 87,809b |
| 107844640 p.(Gly656Ser) |
c.1966G>A | 1M, 181,478 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1M)b | |
| 107845177 p.(Gly702Ser) |
c.2104G>A | 1F, 129,179 |
1b | Delb | DCb | Glyb | No, but p(Gly702Val), in LOVD (VUS)b | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (1F)b | 1M, 55,168b |
| 107846274 p.(Gly743Ser) |
c.2227G>A | 2F, 148,007 |
1b | Delb | DCb | Glyb | No, but p.(Gly743Asp), Plant et al.62,b | Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Weak | Noa | 2F, 63,637a |
| 107849982 p.(Gly752Val) |
c.2255G>T | 2M, 183,227 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Strongb | Yes (2M)b | |
| 107850087 p.(Gly787Ala) |
c.2360G>C | 1F, 183,169 |
1b | Delb | DCb | Glyb | No, but p(Gly787Val), King et al.63,b |
Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Strongb | Yes (1F)b | |
| 107858210 p.(Gly822Glu) |
c.2465G>A | 1M, 182,957 |
1b | Delb | DCb | Glyb | No, but p(Gly822Arg), Cruz-Robles et al.64,b |
Not found | Likely pathogenic, PM1, PM2, PM5, PP2, PP3b | Strongb | Yes (1M)b | |
| 107865033 p.(Gly893Val) |
c.2678G>T | 1F, 180,196 |
1b | Delb | DCb | Glyb | Yes, Bekheirnia et al.,65 p(Gly893Arg), Mohammad et al.66,b | Pathogenic (no assertion criteria)b | Likely pathogenic, PM1, PM2, PM5, PP2, PP3, PP5b | Weak | Yes (1F)b | |
| p.(Gly953Val) | c.2858G>T | 249M, 442F, 204,819 |
1b | Delb | Pola | Glyb | Yes, Knebelmann et al.67,b | Conflicting interpretation, B, LB, VUSa | Benign, PM1, PP2, PP3, BS1, BS2a | Benigna | Noa | 125M, 230F, 87,876a |
| 107866020 p.(Gly961Val) |
c.2882G>T | 2M,3F, 182,593 |
0.001a | Delb | Pola | Glyb | No | Not found | VUS, PM1, PP2, BP4a | Weak | Noa | 1M, 1F, 80,233a |
| 107866037 p.(Gly967Arg) |
c.2899G>A | 1F, 181,344 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Moderateb | Yes (1F)b | |
| 107869494 p(Gly1054Asp) |
c.3161G>A | 1M, 183,204 |
1b | Delb | DCb | Glyb | LOVDb | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Moderateb | Yes (1M)b | |
| 107869553 p(Gly1074Arg) |
c.3220G>C | 1M, 183,246 |
0.073a | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Noa | |
| 107869553 p.(Gly1074Ser) |
c.3220G>A | 4M, 3F, 204,841 |
0.129a | Delb | DCb | Glyb | No | Not found | VUS, PM1, PP2, PP3, BS2a | Weak | Noa | 1M, 2F, 80,317a |
| p.(Gly1134Cys) | c.3400G>T | 1F, 180,336 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | 1F, 79,040b |
| p.(Gly1170Ser) | c.3508G>A | 1F, 174,685 |
1b | Delb | DCb | Glyb | Yesb, Inoue et al.68 | Pathogenic (no assertion criteria)b | Pathogenic, PS1, PM1, PM2, PM5, PP2, PP3, PP5b | Weak | Yes (1F)b | 1F, 76,459b |
| 107909824 p.(Gly1185Ser) |
c.3553G>A | 1F, 159,615 |
0.99b | Delb | DCb | Glyb | No | Not found | Pathogenic, PVS1, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | |
| 107911689 p.(Gly1249Arg) |
c.3745G>A | 1F, 174,771 |
0.99b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | |
| 107920766 p.(Gly1282Glu) |
c.3845G>A | 1M, 183,125 |
1b | Delb | DCb | Glyb | No, but p(Gly1282Val), in LOVDb | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1M)b | |
| 107920774 p.(Gly1285Ser) |
c.3853G>A | 1F, 183,121 |
0.026a | Tola | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Noa | 1F, 80,288a |
| 107920820 p.(Gly1300Ala) |
c.3899G>C | 1F, 182,971 |
0.999b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | |
| 107920829 p.(Gly1303Ala) |
c.3908G>C | 1M, 182,951 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1M)b | |
| 107923928 p.(Gly1321Val) |
c.3962G>T | 1F, 182,976 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | |
| 107923937 p.(Gly1324Glu) |
c.3971G>A | 1F, 182,990 |
1b | Delb | DCb | Glyb | No | Not found | Likely pathogenic, PM1, PM2, PP2, PP3b | Weak | Yes (1F)b | 1F, 80,251b |
| p.(Gly1333Cys) | c.3997G>T | 1M, 182,997 |
1b | Delb | DCb | Glyb | No, but p(Gly1333Ser), Plant et al.62,b | Not found | Pathogenic, PVS1, PM1, PM2, PM5, PP2, PP3b | Weak | Yes (1M)b | 1M, 80,243b |
| 107925082 p.(Gly1394Cys) |
c.4180G>T | 2F, 182,474 |
1b | Delb | DCb | Glyb | No | Not found | Pathogenic and predicted to result in termination codon, PP3b | Moderateb | Yes (2F)b | |
| 107929314 p.(Gly1424Ser) |
c.4270G>A | 1F, 182,592 |
1b | Delb | DCb | Glyb | Yes, LOVD, p(Gly1424Glu), Zhang et al.69,b | Not found | Likely pathogenic, PM2, PM3, PM5, PP2, PP3b | Weak | Yes (1F)b | |
| 107802385 | c.231 + 2T>C | 1M, 182,806 |
No | Not found | Pathogenic, PVS1, PM2, PP3b | Exon 3 skip likely, likely abolition of donor site (3 of 4 tools)b | Yes (1M)b | 1F, 80,152b |
||||
| 107821614 | c.780 + 1G>T | 1F, 183,138 |
No | Not found | Pathogenic, PVS1, PM2, PP3b | Exon 13 skip likely, likely abolition of donor site (4 of 4 tools)b | Yes (1F)b | 1M, 80,312b |
||||
| 107863487 | c.1340–1G>A | 1F, 183,216 |
No | Likely pathogenic (one star)b | Pathogenic, PVS1, PM2, PP3, PP5b | Exon21 skip likely, likely abolition of acceptor site (4 of 4 tools)b | Yes (1F)b | 1M, 80,317b |
||||
| 107863487 | c.2510–2A>G | 1F, 181,241 |
No | Pathogenic (no assertion criteria)b | Pathogenic, PVS1, PM2, PP3, PP5b | Exon 31 skip likely, likely abolition of acceptor site (3 of 4 tools)b | Yes (1F)b | 1M, 80,268b |
||||
| 107917984 | c.3808 + 1G>C | 1F, 169,376 |
No | Not found | Pathogenic, PVS1, PM2, PP3, PP5b | Exon 43 skip likely, likely abolition of donor site (3 of 4 tools)b | Yes (1F)b | |||||
| 107929360 | c.4315 + 1G>A | 2F, 200,378 |
No | Pathogenic (one star)b | Pathogenic, PVS1, PM2, PP3, PP5b | Exon 48 skip likely, likely abolition of donor site (3 of 4 tools)b | Yes (2F)b | |||||
| 107898559 p.(Gly1083Ter) |
c.3247_3248 delGG | 1F, 182,859 |
No | Not found | Pathogenic, PVS1, PM2, PP3b | Likely abolition of acceptor site (4 of 4 tools) interrupts reading frame prematurelyb | Yes (1F)b | 1F, 80,169b |
||||
| 54 variants assessed | 88 individuals potentially affected (29M, 59F) | 47 variants pathogenic | 47 variants pathogenic | 52 variants pathogenic | 45 missense variants conserved | 16 previous reports of pathogenicity | 9 decisions; 7 pathogenic |
51 variants predicted pathogenic in VarSome | 30 variants moderately or strongly pathogenic | 9 decisions; 7 pathogenic | 23 predicted pathogenic alleles (9M, 14F) in mean 73,849 alleles examined | |
Polyphen-2, SIFT (Sorting Intolerant from Tolerant), and MutationTaster are computational tools to assess pathogenicity, and scores of >0.80, Del, or DC are consistent with pathogenicity, respectively. Conservation of Gly among vertebrates was examined ). ClinVar uses the terms Pathogenic and Likely Pathogenic, Variant of Uncertain Significance, Benign, Likely Benign, Conflicting, and and a star system (one to four stars) for the quality of assertion. VarSome uses ACMG/AMP grading of pathogenic, LP, P, VUS, LB and B; and criteria of PVS (pathogenic very strong), PM (pathogenic moderate), PP (pathogenic supporting), BS (benign strong), etc. Although individual tools may be consistent with a pathogenic or benign classification, these are not individually sufficient to assert variant pathogenicity. The tools used here have been principally to exclude variants where the evidence is not totally supportive of pathogenicity. M, male; F, female; Tol, tolerated; DC, disease causing; and Del, deleterious, Pol polymorphism.
Evidence against pathogenicity.
Evidence consistent with pathogenicity.
Thus, gnomAD included 59 individuals with a predicted pathogenic COL4A5 variant in a mean allele number of 170,190 (113,460 individuals) or one in 1923 individuals. If the population frequency were calculated from the total gnomAD cohort of 136,920 individuals at the time of examination, then the population frequency was one in 2320.
Two of the predicted pathogenic variants found in gnomAD (p.[Gly624Asp] and p.[Gly752Val]) were also present in the 100,000 Genomes Project database, but none of the four individuals with these variants had hematuria.
Population Frequencies of Predicted Pathogenic COL4A5 Variants in Different Ancestries in gnomAD
The population frequencies of predicted pathogenic COL4A5 variants differed in people of different ancestries (Table 3). Predicted pathogenic COL4A5 variants were more common in people of European (one in 1800), African (one in 1733), or East Asian (one in 2310) ancestry. In general, COL4A5 variants were less common in Ashkenazim (one in 4961) and not found in Finns.
Table 3.
Prevalence of predicted pathogenic variants in COL4A5 in people of different ancestries in gnomAD
| Ethnicity | Number of Individuals Tested | Number of Predicted Pathogenic Variants | Prevalence of Predicted Pathogenic Variants in COL4A5 |
|---|---|---|---|
| African | 12,132 | 7 | One in 1733 |
| Ashkenazi Jewish | 4961 | 1 | One in 4961 |
| East Asian | 9243 | 4 | One in 2310 |
| European | 59,512 | 33 | One in 1800 |
| Finnish | 12,242 | 0 | None |
| Latino | 18,105 | 6 | One in 3018 |
| South Asian | 12,948 | 4 | One in 3237 |
| Other | 3140 | 4 | One in 785 |
| Total | 136,920 | 59 | One in 2320 |
The most common predicted pathogenic variant, p.(Gly624Asp), was considered pathogenic by ClinVar with two stars and has been reported pathogenic many times.43 It was present 16 times in 54,463 individuals European ancestry, corresponding to a prevalence of one in 3403. Nearly half (16 of 33) of all Europeans with a predicted pathogenic COL4A5 variant had p.(Gly624Asp), and it was not present in other ancestries. This variant is associated with late-onset kidney failure in men,44,45 and a 50% cumulative probability of end stage kidney failure by the age of 54, which is nearly 30 years later than with other variants. The substitution is located immediately adjacent to an interruption in the collagenous sequence, and was found in 12 females and only four males, which help explain its association with a milder phenotype and why it had not been detected previously in individuals recruited into gnomAD.
Founder COL4A5 Variants in gnomAD
Most COL4A5 variants resulting in a Gly substitution were found only once, but five were found more often, usually in a single ancestral group: p.(Gly624Asp) (n=16) and p.(Gly626Ser) (n=7) in Europeans, p.(Gly961Val) (n=5) and p.(Gly1074Ser) (n=7) in Latinos, and p.(Gly953Val) in South and East Asians (n=691). All of these variants are located adjacent to a noncollagenous interruption, and are less likely to be disruptive than other position 1 Gly substitutions (Supplemental Table 2).
Predicted Pathogenic COL4A5 Variants in Other Cohorts
In the normal control subset of gnomAD, comprising a mean of 87,876 alleles or 58,584 individuals, 24 individuals had 17 predicted pathogenic variants, occurring in one in 2441 individuals (Table 2). All of these variants were also found in the whole gnomAD cohort, as expected.
In the EVS dataset, comprising a mean of 10,563 alleles or 7042 individuals, three individuals had predicted pathogenic variants, corresponding to a population frequency of one in 2347 (Supplemental Table 6). Five of the eight COL4A5 variants found in the EVS dataset were also present with a consistent sex in gnomAD, suggesting, at most, an overlap of five out of eight (63%) variants.
In the TOPMed cohort of a mean of 125,568 alleles or 83,713 individuals, 40 individuals had predicted pathogenic variants, corresponding to a population frequency of one in 2093 (Supplemental Table 7). Only two of the 45 COL4A5 variants found in the TOPMed dataset (p.[Gly524Asp] and p.[Gly1424Ser]) were also present with a consistent sex in gnomAD, suggesting very little, if any, overlap in these datasets. The population frequency of predicted pathogenic COL4A5 variants from TOPMed might, therefore, be considered independent and confirmatory of the gnomAD results.
Thus, the population frequencies of predicted pathogenic COL4A5 variants varied from one in 2320 (on the basis of the total participants recruited) or one in 1923 (on the basis of the alleles examined) in gnomAD, one in 2441 in the gnomAD normal controls, one in 2347 in EVS, and one in 2093 in TOPMed.
Mitigating Features for Phenotypes of Predicted Pathogenic COL4A5 Variants in gnomAD
Features that might mitigate the clinical features (for example, result in late-onset kidney failure) were found for 54 of the 59 COL4A5 variants (92%). These included being identified in a woman (41 of 59, 69%) and, for Gly substitutions, being located in the 20 amino terminal exons (11 of 51, 22%) or adjacent to a noncollagenous interruption (23 of 51, 45%), or a Gly substitution with a less disruptive residue (19 of 51, 37%) (Supplemental Table 8).
COL4A3
Predicted Pathogenic COL4A3 Variants in gnomAD
A total of 574 variants (2.6%) were identified in COL4A3 in gnomAD for further assessment. Fifteen position 1 Gly substitutions were excluded because evidence was not all consistent with pathogenicity (Supplemental Table 9). Overall, there were 559 predicted pathogenic variants in a mean of 245,889 (0.23%) alleles, corresponding to a population frequency of 0.45%. These variants were position 1 Gly substitutions (n=380, in 68% of variants, 0.16% of the population), frameshift (n=110, 20% of the variants, 0.04%), nonsense (n=33, 6% of the variants, 0.01%), or splicing variants (n=36, 6% of the variants, 0.01%). No predicted pathogenic COL4A3 variant was found in the homozygous form.
In the total cohort of 136,930 individuals, the population frequency of a predicted pathogenic COL4A3 variant was 0.41%.
Population Frequencies of Predicted Pathogenic COL4A3 Variants in Different Ancestries
Most predicted pathogenic COL4A3 variants were present in a single ancestry.
Predicted pathogenic heterozygous COL4A3 variants were most common in people of Latino (0.80%) or East Asian (0.64%) ancestries, and least common in Askenazim (0.14%) and Finns (0.14%) (Table 4).
Table 4.
Prevalence of predicted pathogenic variants in COL4A3 and COL4A4 in people of different ancestries in gnomAD
| Variant | Probably Pathogenic Variants/Mean Total Alleles Examined | Prevalence (%) | African | Latino | Ashkenazim | East Asian | European Finnish | European Non-Finnish | Other | South Asians |
|---|---|---|---|---|---|---|---|---|---|---|
| COL4A3 | ||||||||||
| Position 1 Gly substitutions | 380/234,398 | 0.16 | 32/15,280 | 96/32,139 | 5/9400 | 46/16,842 | 7/20,376 | 140/106,288 | 8/5739 | 46/28,333 |
| Frameshift variants | 110/280,838 | 0.04 | 3/15,431 | 26/35,368 | 0/10,360 | 2/17,966 | 6/25,004 | 48/128,652 | 5/7148 | 18/30,602 |
| Nonsense variants | 33/245,590 | 0.01 | 8/18,361 | 4/32,413 | 2/9487 | 2/17,393 | 1/21,567 | 12/111,937 | 0/6117 | 4/28,315 |
| Splicing variants | 36/264,528 | 0.01 | 4/14,588 | 5/30,169 | 0/8840 | 4/15,827 | 1/19,207 | 13/99,841 | 2/5421 | 7/26,560 |
| Predicted pathogenic alleles/mean total alleles | 559/245,889 | 0.23 | 47/15,755 (0.30%) | 131/32,713 (0.40%) | 7/9425 (0.07%) | 54/16,829 (0.32%) | 15/22,228 (0.07%) | 213/111,253 (0.19%) | 15/6166 (0.24%) | 75/28,711 (0.26%) |
| Percentage of individuals with COL4A3 variant | 0.45 | 0.60 | 0.80 | 0.14 | 0.64 | 0.14 | 0.38 | 0.48 | 0.52 | |
| COL4A4 | ||||||||||
| Position 1 Gly substitutions | 458/234,586 | 0.20 | 44/15,805 | 114/31,850 | 6/9292 | 52/16,793 | 9/20,406 | 174/106,590 | 7/5759 | 52/28,090 |
| Frameshift variants | 42/223,504 | 0.02 | 5/14,880 | 2/30,446 | 1/8878 | 3/16,014 | 1/19,435 | 24/101,495 | 0/5482 | 6/26,870 |
| Nonsense variants | 48/228,799 | 0.02 | 2/15,211 | 2/31,170 | 1/9098 | 2/16,386 | 0/19,838 | 29/104,040 | 3/5609 | 4/27,444 |
| Splicing variants | 29/246,884 | 0.01 | 2/15,273 | 5/34,351 | 0/10,009 | 3/17,838 | 0/21,428 | 14/111,706 | 0/6006 | 5/30,271 |
| Predicted pathogenic alleles/mean total alleles | 577/233,916 | 0.25 | 53/15,675 (0.34%) | 123/31,917 (0.39%) | 8/9216 (0.09%) | 60/16,758 (0.35%) | 10/20,309 (0.05%) | 241/106,073 (0.23%) | 10/5714 (0.18%) | 67/28,104 (0.24%) |
| Percentage of individuals with COL4A4 variant | 0.49 | 0.68 | 0.78 | 0.18 | 0.70 | 0.10 | 0.46 | 0.36 | 0.48 | |
| Percentage with COL4A3 or COL4A4 variant | 0.94 | 1.28 | 1.58 | 0.32 | 1.34 | 0.24 | 0.84 | 0.84 | 1.00 | |
These population frequencies are determined on the basis of the mean total alleles examined for each variant and not on the total number of individuals examined.
COL4A4
Predicted Pathogenic COL4A4 Variants in gnomAD
A total of 599 variants were identified in COL4A4 in gnomAD for further assessment. Of these, 22 position 1 Gly substitutions were excluded because evidence was not all consistent with pathogenicity (Supplemental Table 9). The variants p.(Gly545Ala) and p.(Gly999Glu) were considered benign, and are discussed in the footnote to Supplemental Table 9.
In COL4A4, there were 577 predicted pathogenic variants in a mean of 233,916 alleles (0.25% alleles), corresponding to a population frequency of 0.49%. Variants were position 1 Gly substitutions (n=458, 82% of all variants, in 0.20% of the population), frameshift (n=42, 0.02%), nonsense (n=48, 0.02%), or splicing variants (n=29, 0.01%). No homozygous predicted pathogenic COL4A4 variant was found.
In the total cohort of 136,930 individuals, the population frequency of a predicted pathogenic COL4A4 variant was 0.42%.
Population Frequencies of Predicted Pathogenic COL4A4 Variants in Different Ancestries
Heterozygous predicted pathogenic COL4A4 variants were most common in people of Latino (0.78%) and East Asian (0.70%) ancestries, and least common in Finns (0.10%) and Ashkenazim (0.18%) (Table 4).
Most founder variants were found only once in any ancestral group, but there were also seven more common COL4A4 founder variants, which were all position 1 Gly substitutions (Supplemental Table 10). These were mainly found in one population each, including Africans (p.[Gly445Ala]), Latinos (p.[Gly481Ser]), East Asians (p.[Gly816Glu]), non-Finnish Europeans (p.[Gly1178Ser] and p.[Ser969Ter]), and Others (p.[Gly774Arg]). No founder variants were found in Ashkenazim or Finns. p.(Ser969Ter) was common in Europeans, occurring in 18 alleles of the 280,956 examined, or one in 7804 individuals, but otherwise no founder variants were frameshift or splicing variants.
Population Frequencies of Heterozygous Predicted Pathogenic COL4A3 and COL4A4 Variants
The population frequency of heterozygous predicted pathogenic variants in COL4A3 or COL4A4 was 0.94% for all individuals (Supplemental Table 11).
Heterozygous predicted pathogenic COL4A3 and COL4A4 variants were most common in people of Latino (in 1.58%), East Asian (1.34%), or African (1.28%) ancestries, and least common in Finns (0.24%) and Ashkenazim (0.32%).
Association of Heterozygous Predicted Pathogenic COL4A3 and COL4A4 Variants in gnomAD with Hematuria in the 100,000 Genomes Project Dataset
Seventeen COL4A3 and 27 COL4A4 predicted pathogenic Gly substitutions found in gnomAD were also present in the 100,000 Genomes database. Of the 2221 individuals with hematuria, 14 had one of these variants, and 78 of the 37,200 individuals without hematuria also had one (chi squared, 14.17; P=0.0002), which indicated that these variants are associated with hematuria. This confirmed that the heterozygous predicted pathogenic COL4A3 and COL4A4 variants resulting in Gly substitutions found in gnomAD were associated with hematuria.
Calculated Population Frequencies of Compound Heterozygous and Digenic COL4A3 and COL4A4 Variants
The population frequency of 0.94% for heterozygous predicted pathogenic COL4A3 or COL4A4 variants corresponded to about 0.9% of the population, or one in 106 people.
The population frequency of two compound heterozygous predicted pathogenic variants in COL4A3 or COL4A4 was 1.13 × 10−5 (or 0.001%, or one in 88,866) when the variants occurred independently.
The population frequency of a digenic predicted pathogenic COL4A3 and a COL4A4 variant was 2.23 × 10−5 (or 0.002%, or one in 44,793) if each variant were in cis or trans, and the variants occurred independently. Digenic predicted pathogenic variants in COL4A5 plus COL4A3 or COL4A4 were much less common, occurring in about one in 2320 (for COL4A5) x one in 106 (for COL4A3 or COL4A4), equal to one in 245,920 of the population.
Discussion
Previous estimates of the population frequency of XL Alport syndrome have differed,13–15 but examination of a large variant database from people without known kidney disease predicted pathogenic COL4A5 variants in at least one in 2320 individuals, and heterozygous pathogenic COL4A3 or COL4A4 variants in one in 106 individuals. The accuracy of population frequencies for these variants is suggested by the consistency of predicted pathogenic COL4A5 variants in a control subset, an overlapping cohort, and an independent dataset.
However, the population frequencies of the corresponding diseases, being directly related to the prevalence of the predicted pathogenic COL4A3–COL4A5 variants, must be corrected for the penetrance of clinical features for individual variants. Penetrance probably depends on genetic and other factors, and we demonstrated, for most COL4A5 variants, that there were mitigating features that explained why variants had not been detected previously.
The population frequency of one in 106 for heterozygous predicted pathogenic variants in COL4A3 and COL4A4 corresponded to a population frequency for compound heterozygous COL4A3 or COL4A4 variants of one in 88,866, and of digenic variants in each of COL4A3 and COL4A4 of one in 44,793, with a lesser contribution from COL4A5 and COL4A3 or COL4A4. The calculated frequency for compound heterozygous variants was less than expected for AR Alport syndrome,7 which previous reports have suggested affects one in six Alport families,46 or one in 40,000 of the population.7,47 Recessive Alport syndrome results from compound heterozygous variants that occur by chance, and from homozygous variants due to consanguinity or consanguinity by descent, which account for a further 30% of patients.42 Thus, the overall population frequency for AR Alport syndrome must also take into account a further 30% from homozygous variants. Digenic COL4A3–COL4A5 variants occur together by chance, and the calculated frequencies are more accurate.
These population frequencies of predicted pathogenic variants are still underestimates because they do not include individuals with already diagnosed disease, with the large deletions or deep intronic splicing variants that are not detected with whole exome sequencing,23 or with pathogenic non-Gly substitutions, which were not evaluated.12,48
So what are the implications of these results for the prevalence of different modes of inheritance of Alport syndrome? In general, pathogenic COL4A5 variants are highly penetrant for hematuria and renal failure in males,20 and for hematuria in females.21 This study only examined high-risk predicted pathogenic variants, that is, nonsense variants and position 1 Gly substitutions, but, even so, many had biochemical features that potentially mitigated the clinical phenotype.
Interestingly, the population frequency of heterozygous predicted pathogenic COL4A3 and COL4A4 variants approximated the 1% estimated from the prevalence of persistent hematuria16,17 and thin membrane nephropathy in normal donor kidney biopsy specimens.18
However, the penetrance of persistent hematuria with COL4A3 and COL4A4 variants is about 70%,7 and the penetrance of a thinned GBM, FSGS, and renal impairment are not known. This means that the population frequencies of thin basement membrane nephropathy or AD Alport syndrome deduced from the prevalence of heterozygous predicted pathogenic COL4A3 or COL4A4 variants must be adjusted for the incomplete penetrance associated with these variants. The prevalence of AR Alport syndrome due to compound heterozygous variants and of digenic Alport syndrome is probably affected less because hematuria and renal impairment are more likely with two COL4A3–COL4A5 variants.8,49
Genetic testing is the most sensitive method for the detection of COL4A3–5 variants and, hence, for detection of the different forms of Alport syndrome.19 Examination of data from the United Kingdom’s 100,000 Genomes Project confirmed that heterozygous predicted pathogenic position 1 Gly substitutions in COL4A5, COL4A3, or COL4A4 were each associated with hematuria, and that these Gly residues were critical domains. However, some position 1 Gly substitutions in COL4A3–COL4A5 were not considered pathogenic, and assertions from collagen I variants in osteogenesis imperfecta suggest that nearby amino acids determine whether chain flexibility from a non-Gly substitution is tolerated.50
The gnomAD and TOPMed datasets represented cohorts of individuals not known to have kidney disease and were used to determine the population frequencies of predicted pathogenic COL4A3–COL4A5 variants. Sequencing was not available for all study participants at each residue, and, in general, the population frequencies were calculated from the mean number of alleles examined and the deduced number of individuals. However, we have cited the more conservative value of COL4A5 variants in the whole cohort. There were also limitations in applying the ACMG/AMP criteria24 to variants where clinical data were not available, but although our strategy may have excluded some variants that were pathogenic, these small numbers were unlikely to have significantly affected the overall frequencies.
The large number of undetected COL4A5 variants identified in gnomAD may be explained by mitigating factors, for instance, the associated clinical features were mild, with hematuria or late-onset kidney failure but without extrarenal features. Thus, predicted pathogenic variants were found twice as often in women, which was consistent with XL inheritance, but different from the female representation in most previous reports.12–14 Affected women often have only hematuria, but identifying affected women is still important because of their own risk of end stage kidney failure, because half of their sons develop kidney failure,22 and because treatment is effective.51 Most other COL4A5 variants identified in gnomAD were associated with a clinically mitigating feature, such as location in the amino terminus or adjacent to an interruption, or a Gly substitution with Ala, Ser, or Cys.
This study also demonstrated that the population frequencies of predicted pathogenic COL4A3–COL4A5 variants varied in people of different ancestries. Predicted pathogenic variants in COL4A5 were more common in people from a European or African background, present at a low level in Ashkenazim, and absent from Finns. This was consistent with the previously reported low population frequency in Finns.15 In addition, heterozygous predicted pathogenic COL4A3 and COL4A4 variants affected >1% of people of Latino, African, and East Asian ancestries, which was at least four times more common than in Ashkenazim and Finns. It may not be that COL4A3–COL4A5 variants are more common in some ancestries, but rather that they are uncommon in others due to geographic and cultural isolation.52
In conclusion, predicted pathogenic COL4A5 variants occur in at least one in 2320 of the population, but are often unrecognized.53–57,59,60 Heterozygous predicted pathogenic COL4A3 or COL4A4 variants occur in at least one in 106 of the population. The true prevalence of potentially pathogenic COL4A3–COL4A5 variants is even greater because they must also include already diagnosed disease, variants that were not detected with exomic sequencing, and non-Gly substitutions. However, the prevalence of the different forms of inheritance of Alport syndrome depends on the penetrance of hematuria and renal impairment with these variants. Future studies should address penetrance of these clinical features.
Disclosures
L. Burnett reports serving as a nonexecutive director of Australasian Association for Clinical Biochemistry and Laboratory Medicine Services Pty Ltd., member of Clinical Immunogenomics Research Consortium Australasia, and member of the KidGen Renal Genetics Flagship Study of Australian Genomics (these roles are honorary); serving as a member of the Community Genetics New South Wales (NSW) Advisory Committee, a member of the genetics services executive committee of the NSW Health Genetics Network, and a member of the genetics advisory committee of Royal College of Pathologists of Australasia (these roles are honorary); and receiving an investigator-initiated grant for genomic screening of Gaucher disease from Takeda (Shire). D. Gale repo rts receiving honoraria from Alexion and Otsuka, and serving as a trustee for Alport UK. J. Savige reports having other interests in/relationships with Alport Foundation Australia and the PKD Australia Scientific Board. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors would like to thank gnomAD and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about. The authors would like to thank the National Heart, Lung, and Blood Institute Gene Ontology (GO) Exome Sequencing Project and the following ongoing studies that produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the Women’s Health Initiative Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926), and the Heart GO Sequencing Project (HL-103010). Finally, the authors would also like to thank the hosts, contributors, and administrators of the many websites used to evaluate variants for predicted pathogenicity—VarSome in particular, and the LOVD database.
This research was also made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and National Health Service (NHS) England. The Wellcome Trust, Cancer Research UK, and the Medical Research council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the NHS as part of their care and support.
J. Gibson undertook this study while an undergraduate student at the University of Melbourne.
J. Gibson undertook the initial analysis and made major contributions to the initial draft; R. Fieldhouse, L. Burnett, V. Izzi, A.V. Persikov, and H. Storey devised the list of interruptions, provided associated disease phenotypes, and helped in the assessments of predicted pathogenicity; M.M.Y. Chan, O. Sadeghi-Alavijeh, and D.P. Gale helped with the acquisition of data, analysis and interpretation of data, and with revisions; V. Izzi, A.V. Persikov, and H. Storey also contributed to the acquisition of data, analysis, and interpretation of data; J. Savige designed the study, undertook some of the analysis, and drafted and revised the manuscript; and all authors contributed to the writing, have approved the final submission, and agree to be accountable for the data.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071065/-/DCSupplemental.
Supplemental Table 1. Applicability of ACMG/AMP criteria for COL4A3–COL4A5 variants in population frequency databases 24.
Supplemental Table 2. Non-collagenous domains and interruptions in collagen IV α5, α3 and α4 chains 28, 26, 27.
Supplemental Table 3. Assessment of COL4A5 variants in the 100,000 Genomes Project database and correlation with haematuria.
Supplemental Table 4. Assessment of COL4A3 variants in the 100,000 Genomes Project database and correlation with haematuria.
Supplemental Table 5. Assessment of COL4A4 variants in the 100,000 Genomes Project database and correlation with haematuria.
Supplemental Table 6. Predicted pathogenic variants in COL4A5 in the Exome Variant Server database.
Supplemental Table 7. COL4A5 predicted pathogenic variants in the TOPMed database.
Supplemental Table 8. Mitigating features for the clinical effects of COL4A5 predicted pathogenic variants resulting in position 1 Gly substitutions in gnomAD: Associations with gender, location, and replacement residues.
Supplemental Table 9. Variants in COL4A3 and COL4A4 in gnomAD with inconsistent assessments subsequently excluded from population frequency studies.
Supplemental Table 10. Predicted pathogenic founder variants in COL4A3 and COL4A4 in different ancestries in gnomAD.
Supplemental Table 11. Estimated population frequencies for predicted pathogenic heterozygous, compound heterozygous, and digenic variants.
References
- 1.Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, et al. : Alport’s syndrome. A report of 58 cases and a review of the literature. Am J Med 70: 493–505, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, et al. : Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, et al. : Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Buzza M, Wilson D, Savige J: Segregation of hematuria in thin basement membrane disease with haplotypes at the loci for Alport syndrome. Kidney Int 59: 1670–1676, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Buzza M, Dagher H, Wang YY, Wilson D, Babon JJ, Cotton RG, et al. : Mutations in the COL4A4 gene in thin basement membrane disease. Kidney Int 63: 447–453, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Savige J: Should we diagnose autosomal dominant Alport syndrome when there is a pathogenic heterozygous COL4A3 or COL4A4 variant? Kidney Int Rep 3: 1239–1241, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY: Thin basement membrane nephropathy. Kidney Int 64: 1169–1178, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Mencarelli MA, Heidet L, Storey H, van Geel M, Knebelmann B, Fallerini C, et al. : Evidence of digenic inheritance in Alport syndrome. J Med Genet 52: 163–174, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Voskarides K, Damianou L, Neocleous V, Zouvani I, Christodoulidou S, Hadjiconstantinou V, et al. : COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol 18: 3004–3016, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Wickman L, Hodgin JB, Wang SQ, Afshinnia F, Kershaw D, Wiggins RC: Podocyte depletion in thin GBM and Alport syndrome. PLoS One 11: e0155255, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldovino S, Moliner AM, Taruscio D, Daina E, Roccatello D: Rare diseases in Europe: from a wide to a local perspective. Isr Med Assoc J 18: 359–363, 2016 [PubMed] [Google Scholar]
- 12.Hertz JM, Thomassen M, Storey H, Flinter F: Clinical utility gene card for: Alport syndrome – update 2014. Eur J Hum Genet 23: 1269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasstedt SJ, Atkin CL: X-linked inheritance of Alport syndrome: Family P revisited. Am J Hum Genet 35: 1241–1251, 1983 [PMC free article] [PubMed] [Google Scholar]
- 14.Persson U, Hertz JM, Wieslander J, Segelmark M: Alport syndrome in southern Sweden. Clin Nephrol 64: 85–90, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Pajari H, Kääriäinen H, Muhonen T, Koskimies O: Alport’s syndrome in 78 patients: Epidemiological and clinical study. Acta Paediatr 85: 1300–1306, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Dodge WF, West EF, Smith EH, Bruce Harvey 3rd: Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediatr 88: 327–347, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Ritchie CD, Bevan EA, Collier SJ: Importance of occult haematuria found at screening. Br Med J (Clin Res Ed) 292: 681–683, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dische FE, Anderson VE, Keane SJ, Taube D, Bewick M, Parsons V: Incidence of thin membrane nephropathy: Morphometric investigation of a population sample. J Clin Pathol 43: 457–460, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F: Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol 24: 364–375, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Jais JP, Knebelmann B, Giatras I, Marchi M, Rizzoni G, Renieri A, et al. : X-linked Alport syndrome: Natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, et al. : X-linked Alport syndrome: Natural history and genotype-phenotype correlations in girls and women belonging to 195 families: A “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14: 2603–2610, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Savige J, Colville D, Rheault M, Gear S, Lennon R, Lagas S, et al. : Alport syndrome in women and girls. Clin J Am Soc Nephrol 11: 1713–1720, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savige J, Ariani F, Mari F, Bruttini M, Renieri A, Gross O, et al. : Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatr Nephrol 34: 1175–1189, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. ; ACMG Laboratory Quality Assurance Committee: Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myllyharju J, Kivirikko KI: Collagens and collagen-related diseases. Ann Med 33: 7–21, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Savige J, Storey H, Watson E, Hertz JM, Deltas C, Renieri A, et al. : Consensus statement on standards and guidelines for the molecular diagnostics of Alport syndrome: Refining the ACMG criteria [published online ahead of print April 15, 2021]. Eur J Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariyama M, Leinonen A, Mochizuki T, Tryggvason K, Reeders ST: Complete primary structure of the human alpha 3(IV) collagen chain. Coexpression of the alpha 3(IV) and alpha 4(IV) collagen chains in human tissues. J Biol Chem 269: 23013–23017, 1994 [PubMed] [Google Scholar]
- 28.Leinonen A, Mariyama M, Mochizuki T, Tryggvason K, Reeders ST: Complete primary structure of the human type IV collagen alpha 4(IV) chain. Comparison with structure and expression of the other alpha (IV) chains. J Biol Chem 269: 26172–26177, 1994 [PubMed] [Google Scholar]
- 29.Zhou J, Hertz JM, Leinonen A, Tryggvason K: Complete amino acid sequence of the human alpha 5 (IV) collagen chain and identification of a single-base mutation in exon 23 converting glycine 521 in the collagenous domain to cysteine in an Alport syndrome patient. J Biol Chem 267: 12475–12481, 1992 [PubMed] [Google Scholar]
- 30.Demosthenous P, Voskarides K, Stylianou K, Hadjigavriel M, Arsali M, Patsias C, et al. ; Hellenic Nephrogenetics Research Consortium: X-linked Alport syndrome in Hellenic families: Phenotypic heterogeneity and mutations near interruptions of the collagen domain in COL4A5. Clin Genet 81: 240–248, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Lanktree MB, Haghighi A, Guiard E, Iliuta IA, Song X, Harris PC, et al. : Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 29: 2593–2600, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. ; Exome Aggregation Consortium: Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genomics England: The National Genomics Research and Healthcare Knowledgebase v5, 2019. https://www.genomicsengland.co.uk/wp-content/uploads/2019/08/The-National-Genomics-Research-and-Healthcare-Knowledgebase-v5-1.pdf
- 34.van der Ven AT, Connaughton DM, Ityel H, Mann N, Nakayama M, Chen J, et al. : Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 29: 2348–2361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly MA, Caleshu C, Morales A, Buchan J, Wolf Z, Harrison SM, et al. : Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: Recommendations by ClinGen’s Inherited Cardiomyopathy Expert Panel. Genet Med 20: 351–359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biesecker LG, Harrison SM; ClinGen Sequence Variant Interpretation Working Group: The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet Med 20: 1687–1688, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards CS, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. ; ACMG/AMP Interpretation of Sequence Variants Work Group 2015: Response to Biesecker and Harrison. Genet Med 20: 1689–1690, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, et al. ; ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI): Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat 39: 1517–1524, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimura Y, Nozu K, Kaito H, Nakanishi K, Fu XJ, Ohtsubo H, et al. : Milder clinical aspects of X-linked Alport syndrome in men positive for the collagen IV α5 chain. Kidney Int 85: 1208–1213, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Savige J, Storey H, Il Cheong H, Gyung Kang H, Park E, Hilbert P, et al. : X-linked and autosomal recessive Alport syndrome: Pathogenic variant features and further genotype-phenotype correlations. PLoS One 11: e0161802, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persikov AV, Pillitteri RJ, Amin P, Schwarze U, Byers PH, Brodsky B: Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum Mutat 24: 330–337, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA: COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol 24: 1945–1954, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slajpah M, Gorinsek B, Berginc G, Vizjak A, Ferluga D, Hvala A, et al. : Sixteen novel mutations identified in COL4A3, COL4A4, and COL4A5 genes in Slovenian families with Alport syndrome and benign familial hematuria. Kidney Int 71: 1287–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Macheroux EP, Braunisch MC, Pucci Pegler S, Satanovskij R, Riedhammer KM, Günthner R, et al. : The hypomorphic variant p.(Gly624Asp) in COL4A5 as a possible cause for an unexpected severe phenotype in a family with X-linked Alport syndrome. Front Pediatr 7: 485, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Żurowska AM, Bielska O, Daca-Roszak P, Jankowski M, Szczepańska M, Roszkowska-Bjanid D, et al. : Mild X-linked Alport syndrome due to the COL4A5 G624D variant originating in the Middle Ages is predominant in Central/East Europe and causes kidney failure in midlife. Kidney Int 99: 1451–1458, 2021 [DOI] [PubMed] [Google Scholar]
- 46.Feingold J, Bois E, Chompret A, Broyer M, Gubler MC, Grünfeld JP: Genetic heterogeneity of Alport syndrome. Kidney Int 27: 672–677, 1985 [DOI] [PubMed] [Google Scholar]
- 47.Lemmink HH, Nillesen WN, Mochizuki T, Schröder CH, Brunner HG, van Oost BA, et al. : Benign familial hematuria due to mutation of the type IV collagen alpha 4 gene. J Clin Invest 98: 1114–1118, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B: Amino acid propensities for the collagen triple-helix. Biochemistry 39: 14960–14967, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Fallerini C, Baldassarri M, Trevisson E, Morbidoni V, La Manna A, Lazzarin R, et al. : Alport syndrome: impact of digenic inheritance in patients management. Clin Genet 92: 34–44, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Makareeva E, Mertz EL, Kuznetsova NV, Sutter MB, DeRidder AM, Cabral WA, et al. : Structural heterogeneity of type I collagen triple helix and its role in osteogenesis imperfecta. J Biol Chem 283: 4787–4798, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, et al. ; Study Group Members of the Gesellschaft für Pädiatrische Nephrologie: Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Kääriäinen H, Muilu J, Perola M, Kristiansson K: Genetics in an isolated population like Finland: A different basis for genomic medicine? J Community Genet 8: 319–326, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashtan CE: Alport Syndrome. Gene Reviews. In: GeneReviews, edited by Adam MP, Ardinger HH, Pagon RA, Wallace SE, Seattle, WA, University of Washington, Seattle, 2001, pp 1993–2020 [Google Scholar]
- 54.Pierides A, Voskarides K, Athanasiou Y, Ioannou K, Damianou L, Arsali M, et al. : Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/ COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant 24: 2721–2729, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Matthaiou A, Poulli T, Deltas C: Prevalence of clinical, pathological and molecular features of glomerular basement membrane nephropathy caused by COL4A3 or COL4A4 mutations: A systematic review. Clin Kidney J 13: 1025–1036, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillion V, Dahan K, Cosyns JP, Hilbert P, Jadoul M, Goffin E, et al. : Genotype and outcome after kidney transplantation in Alport syndrome. Kidney Int Rep 3: 652–660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, et al. : Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 380: 142–151, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fallerini C, Dosa L, Tita R, Del Prete D, Feriozzi S, Gai G, et al. : Unbiased next generation sequencing analysis confirms the existence of autosomal dominant Alport syndrome in a relevant fraction of cases. Clin Genet 86: 252–257, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, et al. : A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int 94: 363–371, 2018 [DOI] [PubMed] [Google Scholar]
- 60.Gross O, Tönshoff B, Weber LT, Pape L, Latta K, Fehrenbach H, et al. ; German Pediatric Nephrology (GPN) Study Group and EARLY PRO-TECT Alport Investigators: A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport’s syndrome. Kidney Int 97: 1275–1286, 2020 [DOI] [PubMed] [Google Scholar]
- 61.Hertz JM, Juncker I, Persson U, Matthijs G, Schmidtke J, Petersen MB, et al. : Detection of mutations in the COL4A5 gene by SSCP in X-linked Alport syndrome. Hum Mutat 18: 141–148, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Plant KE, Green PM, Vetrie D, Flinter FA: Detection of mutations in COL4A5 in patients with Alport syndrome. Hum Mutat 13: 124–132, 1999 [DOI] [PubMed] [Google Scholar]
- 63.King K, Flinter FA, Green PM: A two-tier approach to mutation detection in the COL4A5 gene for Alport syndrome. Hum Mutat 27: 1061, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Cruz-Robles D, García-Torres R, Antignac C, Forestier L, de la Puente SG, Correa-Rotter R, et al. : Three novel mutations in the COL4A5 gene in Mexican Alport syndrome patients. Clin Genet 56: 242–243, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Bekheirnia MR, Reed B, Gregory MC, McFann K, Shamshirsaz AA, Masoumi A, et al. : Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol 21: 876–883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammad M, Nanra R, Colville D, Trevillian P, Wang Y, Storey H, et al. : A female with X-linked Alport syndrome and compound heterozygous COL4A5 mutations. Pediatr Nephrol 29: 481–485, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Knebelmann B, Breillat C, Forestier L, Arrondel C, Jacassier D, Giatras I, et al. : Spectrum of mutations in the COL4A5 collagen gene in X-linked Alport syndrome. Am J Hum Genet 59: 1221–1232, 1996 [PMC free article] [PubMed] [Google Scholar]
- 68.Inoue Y, Nishio H, Shirakawa T, Nakanishi K, Nakamura H, Sumino K, et al. : Detection of mutations in the COL4A5 gene in over 90% of male patients with X-linked Alport’s syndrome by RT-PCR and direct sequencing. Am J Kidney Dis 34: 854–862, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, Ding J, Wang F, Zhao D: Mutation detection of COL4An gene based on mRNA of peripheral blood lymphocytes and prenatal diagnosis of Alport syndrome in China. Nephrology (Carlton) 16: 377–380, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.