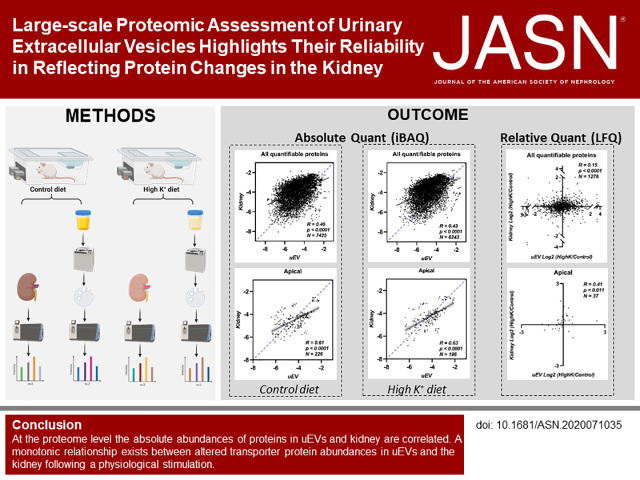
Significance Statement
Measurement of urinary extracellular vesicle (uEV) protein abundances is frequently used to reflect ongoing (patho)physiologic processes in the kidney. However, whether protein abundances in uEVs and the kidney directly correlate, or whether (patho)physiologic alterations in protein levels in the kidney can be determined by assessing protein changes in uEVs, has never been comprehensively determined. Here, quantitative proteomic data indicate protein abundances in uEVs and kidney in rats are correlated, with a monotonic relationship between altered transporter protein abundance in uEVs and the kidney after a physiologic stimulation. Therefore, it is valid to draw conclusions from altered protein levels in uEVs for particular protein classes and relate them to changes in the kidney.
Keywords: urinary extracellular vesicle, kidney, proteomics, label-free, exosome, biomarker, transgenic mouse
Visual Abstract
Abstract
Background
Urinary extracellular vesicles (uEVs) are secreted into urine by cells from the kidneys and urinary tract. Although changes in uEV proteins are used for quantitative assessment of protein levels in the kidney or biomarker discovery, whether they faithfully reflect (patho)physiologic changes in the kidney is a matter of debate.
Methods
Mass spectrometry was used to compare in an unbiased manner the correlations between protein levels in uEVs and kidney tissue from the same animal. Studies were performed on rats fed a normal or high K+ diet.
Results
Absolute quantification determined a positive correlation (Pearson R=0.46 or 0.45, control or high K+ respectively, P<0.0001) between the approximately 1000 proteins identified in uEVs and corresponding kidney tissue. Transmembrane proteins had greater positive correlations relative to cytoplasmic proteins. Proteins with high correlations (R>0.9), included exosome markers Tsg101 and Alix. Relative quantification highlighted a monotonic relationship between altered transporter/channel abundances in uEVs and the kidney after dietary K+ manipulation. Analysis of genetic mouse models also revealed correlations between uEVs and kidney.
Conclusion
This large-scale unbiased analysis identifies uEV proteins that track the abundance of the parent proteins in the kidney. The data form a novel resource for the kidney community and support the reliability of using uEV protein changes to monitor specific physiologic responses and disease mechanisms.
Analysis of urine is attractive in kidney disease biomarker discovery, due to the noninvasive simplicity of collection in large and repeated quantities.1–6 However, a challenge in this field is that the urinary proteome is composed of proteins/peptides from plasma, secreted proteins, urinary extracellular vesicles (uEVs), and whole cells originating from the complete genitourinary tract.7
uEVs are nanosized vesicles released from epithelial cells within the kidney, the urinary tract, and the male reproductive tract. Although a heterogeneous population, microvesicles and exosomes (of different cellular origins) are the two major subtypes found in the urine.8 uEVs can be readily isolated from urine and are a relatively stable source of proteins and other molecular cargo (e.g., RNA, microRNA).9–15 Thus, uEVs are an attractive source for biomarker discovery and assessment of protein levels in uEVs is frequently used as a surrogate marker for physiologic and pathophysiologic changes occurring within the kidney.16–21 For example, uEVs have been used to assess the abundance or role of various proteins in the setting of patients with familial hyperkalemic hypertension,22 Bartter syndrome, Gitelman syndrome,23 primary aldosteronism,24,25 polycystic kidney disease,26 and nephrogenic diabetes insipidus.27
A longstanding assumption in the renal field is that changes in uEV protein content faithfully reflect changes within kidney tissue. This assumption was challenged recently, because no correlation was detected in the abundance of nine proteins in kidneys and uEVs samples isolated from patients undergoing nephrectomy.28 It is unclear if these observations on a limited number of proteins are applicable to other proteins in the kidney. Therefore, this study was designed to test the following hypotheses: (1) protein abundance in uEVs correlates with the abundance of proteins in the kidney; (2) some classes of proteins have higher correlations than other classes; (3) physiologic changes in proteins within the kidney can be detected in uEVs; and (4) relevant correlations between uEVs and kidney can be detected in animal models of disease. To test the first three hypotheses in a large-scale unbiased manner, we assessed protein abundance in uEVs and kidney tissue isolated from rats fed a normal or a high potassium (K+) diet, using liquid chromatography with tandem mass spectrometry (LC-MS/MS) shotgun proteomics. For animal models of disease, we assessed uEV protein abundance using immunoblotting in two mouse models with genetic alterations in the WNK-SPAK-NCC signaling pathway and electrolyte balance abnormalities.29–32 Our proteome-wide data indicate the abundance of proteins in uEVs and kidney are correlated, and a monotonic relationship exists between altered transporter/channel protein abundance in uEVs and the kidney after a physiologic stimulation. Furthermore, changes in key proteins in the kidney due to physiologic manipulation or genetic changes mimicking disease can also be detected by analyzing uEVs. However, our study also highlights that caution must be exercised in drawing direct comparisons of protein levels in uEVs and the kidney for certain cytosolic protein classes.
Materials and Methods
Rat Studies
All animal protocols comply with the European community guidelines for the use of experimental animals and were approved and performed under a license issued by the Danish Ministry of Justice (Dyreforsøgstilsynet). In study 1, 12 male Wistar rats, approximately 180 g bodyweight, were initially housed under a 12:12 hour light:dark cycle in standard rodent cages with free access to standard rodent chow (1324 pellets, Altromin) and tap water. Rats were acclimatized to metabolic cages for 2 days then randomly assigned (six animals per group) to receive either a control diet (Research Diets, D16021401, 0.4% sodium [Na+], 0.8% K+) or a high K+ diet (D1602143, 0.4% Na+, 5% K+ as K+ citrate) for 4 days. Study 2 was performed similarly to study 1, but rats were randomly assigned (4–5 animals per group) to receive either a control diet (0.3% Na+, 1.05% K+, Teklad Diet: TD.190005) or a high K+ citrate (0.3% Na+, 5.37% K+) diet for 2 or 4 days. The high K+ citrate diet was prepared by adding K+ citrate (Sigma-Aldrich) to the control diet. In both studies, urine was collected over a 24-hour period into tubes containing protease and phosphatase inhibitors (Roche) (tablets were dissolved in a small volume of PBS and put in the tube before the collection, one protease inhibitor tablet and one phosphatase inhibitor tablet per 10 ml of estimated 24-hour urine volume). Urine was spun at low speed (1000 g) for 30 minutes to remove whole cells and any food debris before storage at -80°C before further processing. Rats were anesthetized using isoflurane and blood samples collected from the heart, before euthanization using cervical dislocation. Kidneys were dissected and frozen immediately on liquid N2 before storage at −80°C before further processing. Plasma or urine aldosterone concentrations were determined using an enzyme immunoassay kit (EIA-5298; range: 20–1,000 pg/ml; QC: standards, DRG International, Springfield, NJ). Urine Na+, chloride (Cl−), and K+ concentrations and plasma Na+ and Cl− concentrations were analyzed by the Clinical Pathology Laboratory at the Medical Research Council (Harwell, Oxfordshire, United Kingdom).
Mouse Studies
Mice with renal tubule-specific deletion of Kcnj10 (KS-kcnj10−/−)29 or a constitutively active form of the Stk39 encoded kinase SPAK (CA-SPAK) in the distal convoluted tubule (DCT)30 were housed in metabolic cages, with free access to standard rodent chow and tap water. For KS-kcnj10−/− mice, urine was collected over two 24-hour periods and processed independently. For CA-SPAK studies, urine was collected over two 24-hour periods, and the two urine collections were combined before processing. For both models, urine was collected as detailed in “rat studies” and uEVs isolated as described below. Studies were performed in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Maryland School of Medicine (CA-SPAK studies) or the Oregon Health and Science University Animal Care and Use Committees (KS-kcnj10−/− studies).
uEVs
These were purified as previously described.9 Briefly, urine was thawed and vortexed vigorously several times. Urine was centrifuged twice at 17,000 g for 30 min at 4°C to remove urinary sediment including whole cells, large membrane fragments, and other debris. The supernatant was further centrifuged twice at 200,000 g (Beckman 70Ti) for 1 hour at 4°C to pellet the uEVs. Supernatant was removed and the pellet was resuspended in 200 µl of PBS containing 1% SDS and Complete Protease Inhibitor Tablets and PhosSTOP phosphatase inhibitor tablets (Roche Diagnostics). Samples were further processed on ice using a probe-sonicator for five cycles (one cycle was comprised of 10 seconds on and 10 seconds off) before centrifugation at 17,000 g for 30 minutes at 4°C, to remove any possible remaining cell debris and larger particles that may inhibit downstream processing steps. Protein concentration measurements were made using a standard bicinchoninic acid assay.
Kidney Samples for Mass Spectrometry
Whole kidneys were homogenized in ice-cold dissection buffer (0.3 M sucrose, 25 mM imidazole, and 1 mM EDTA, pH 7.2) containing Complete Protease Inhibitor Tablets and PhosSTOP phosphatase inhibitor tablets (Roche Diagnostics). Homogenate containing 100 µg of protein was mixed with PBS solution with 1% SDS, and samples further processed as described for uEVs.
Filter-aided Sample Preparation and Peptide Fractionation
Filter-aided sample preparation was performed as previously described.33 Briefly, protein samples (60 for uEV samples and 100 µg for kidney samples) were loaded on filtration units (Vivacon 500, 30 KDa cutoff) and washed multiple times with UA buffer (8 M urea in 100 mM tetraethylammonium bromide) to remove SDS. Subsequently, reduction was done by adding 100 µl of 50 mM dithiothreitol (DTT) in UA buffer, and incubating at 56°C for 1 hour, followed by centrifugation to remove excess DTT. Then alkylation was performed by adding 100 µl of 50 mM iodoacetamide in UA buffer, and incubating at room temperature for 10 minutes in the dark. After one more wash in UA buffer, 40 µl of Lys-C in UA buffer was added at an enzyme to protein ratio of 1:100, and the spin unit was incubated at 37°C for 3 hours, followed by addition of 400 µl of trypsin in 100 mM tetraethylammonium bromide at an enzyme to protein ratio of 1:50 and incubation at 37°C overnight. Resultant peptides were released from the membrane by centrifugation and vacuum dried before high pH fractionation using a commercial kit following the manufacturer’s guidelines (high pH fractionation kit, Thermo Fisher Scientific). Eight fractions from each sample were collected and vacuum dried before LC-MS/MS analysis.
LC-MS/MS Analysis
One third of every peptide fraction was subjected to LC-MS/MS analysis as a single run, with each sample run twice. In study 1, where we analyzed the uEV and kidney samples from rats under control diet and high K+ diet for 4 days, LC-MS/MS was performed using a QExactive mass spectrometer (Thermo Fisher Scientific) coupled to an easy nLC-1000 (Thermo Fisher Scientific). A 60-minute gradient with a 40-minute separation window (5%–35% acetonitrile in 0.1% formic acid) was used to separate peptides. Data-dependent acquisition with top 10 precursors was performed, with precursor scan range 300–1800, a resolution of 70,000, an automatic gain control (AGC) of 1e6, and a maximum injection time of 20 ms. Fragment scan was done at a resolution of 17,500, AGC of 5e5, and a maximum injection time of 100 ms. High collision dissociation energy was set at 30%. Dynamic exclusion was enabled with an exclusion window of 30 seconds. Single charged, >8 charged, and unknown charged ions were excluded from fragmentation. In study 2 (2 day high K+ diet), LC-MS/MS was performed using a Fusion mass spectrometer (Thermo Fisher Scientific) coupled to an easy nLC-1200 (Thermo Fisher Scientific). A 60-minute gradient with a 42-minute separation window (5%–35% acetonitrile in 0.1% formic acid) was used to separate peptides. Data-dependent acquisition with a cycle time of 2 seconds was performed, with precursor scan range 375–1500, a resolution of 60,000, an AGC of 4e5, and a maximum injection time of 50 ms. The fragment scan was done at a resolution of 15,000, an isolation window of 1.6 Da, AGC of 5e4, and a maximum injection time of 22 ms. High collision dissociation energy was set at 32%. Dynamic exclusion was enabled with an exclusion window of 30 seconds. Single charged, >6 charged, and unknown charged ions were excluded from fragmentation.
Database Search
iBAQ.
All raw files from one sample were combined and searched in MaxQuant (v 1.6.3.4) with the iBAQ algorithm enabled. The database was rat uniprot database downloaded on September 11, 2020. Carbamidomethylation of cysteine was set as a fixed modification, whereas acetylation of protein N-term, carbamylation of lysine and peptide N-terminus, oxidation of methionine, and phosphorylation of serine, threonine, and tyrosine were set as variable modifications. Peptide and protein false discovery rates were both set at 0.01.
Label-free Relative Quantification.
The 48 raw files from rats on the control diet (6 rats×8 fractions) and 48 raw files from rats fed a high K+ diet were combined into one MaxQuant (v 1.6.3.4) search, with 48 designated as fractions in control experiment and the other 48 as fractions in high K+ experiment. uEV and kidney samples were searched separately. All search parameters were the same as described in the iBAQ section, with the following additional label-free relative quantification (LFQ) settings: LFQ, fast LFQ, stabilize large LFQ ratios enabled, and LFQ minimum ratio count set at 1.
Data Analysis
iBAQ.
iBAQ values were converted to percentages by dividing any single iBAQ value with the sum of all iBAQ values from a sample. These percentages were logarithmically transformed, then the results from six uEV and six kidney samples under one physiologic condition were compiled. They were then normalized to the largest number of protein IDs in these 12 samples in the following manner to account for the differences in the number of identified proteins:
LFQ.
LFQ intensities from control and high K+ experiments reported by MaxQuant were used to calculate the fold-change. Only proteins with valid LFQ intensities in both control and high K+ conditions were taken into consideration. Fold-changes were logarithmically transformed to calculate the log2 ratio.
Bioinformatics
Databases.
The number of transmembrane helices in individual proteins were predicted using the TMHMM server v2.034 (http://www.cbs.dtu.dk/services/TMHMM/). Transporter/channels, protein kinases, and transcription factor databases used were retrieved from https://helixweb.nih.gov/ESBL/Database/NephronRNAseq/.35 The E3 ligase database was retrieved from https://hpcwebapps.cit.nih.gov/ESBL/Database/E3-ligases/.36 The DUB database was retrieved from https://hpcwebapps.cit.nih.gov/ESBL/Database/DUBs/.
Segment-restricted or Apical/Basolateral Proteins.
“Segment-restricted proteins” were calculated using an in-house Java script generated using rat renal tubule transcriptomic and proteome data.35,37 The source code and example files can be accessed at https://github.com/qiwu-au/SlidingWindowTrue. The renal tubule was divided into 14 continuous segments. Any genes exhibiting at least 10-fold higher expression values in any continuous region (including one segment and 2–6 adjacent segments, an intrasegment variation below 10-fold, minimal absolute expression value above 10) than all other segments were considered as highly expressed only in a certain continuous region of the renal tubule, and thus defined as “segment-restricted” proteins. Apical and basolateral distribution of transporter/channel proteins were determined using a combined approach of the cellular component in Quick GO (https://www.ebi.ac.uk/QuickGO/) and manual literature mining. Proximal and distal proteins were defined using the proteomic ruler value in the segment-specific proteome database. We defined the proximal tubule as the combination of S1, S2, and S3, and distal tubule as the combination of the DCT, the connecting tubule (CNT), the cortical, outer medullary, and inner medullary collecting ducts (CCD, OMCD, and IMCD, respectively). We further defined the proximal proteins as proteins fulfilling the following criteria: average proteomic ruler expression of (S1, S2, S3) >10× average proteomic ruler expression of (DCT, CNT, CCD, OMCD, IMCD), and vice versa for distal proteins: average proteomic ruler expression of (DCT, CNT, CCD, OMCD, IMCD) >10× average proteomic ruler expression of (S1, S2, S3).
Gene Ontology.
Statistical overrepresentation tests were performed on datasets from study 1 using PANTHER to: (1) look for enriched Gene Ontology (GO) terms of uEV proteins in rats on a control diet. Input was the combination of uEV enriched proteins (iBAQ count in both uEV and kidney ≥4, false discovey rate <1%, Log2 [uEV/kidney] >0) and uEV unique proteins (at least one valid iBAQ value in uEV, none in kidney), background was all identified proteins in kidney (defined by at least one iBAQ value in kidney, regardless of their appearances in uEV). (2) To look for enriched GO terms of uEV proteins in rats on a high K+ diet. Input and background were selected as for the control diet, but the data source was high K+ data. PANTHER version was 16.0 (released January 12, 2020), with GO-slim Biologic Process, Molecular Function, and Cellular Component in use. GO terms with enrichment fold >1, passing the false discovery rate 5% threshold were retained. If there were more than ten GO terms, only the top ten with the highest enrichment folds were plotted. GO functional analysis was performed by ClueGO on proteins that exhibited large changes in uEV, but small changes in kidney after the high K+ intervention, on the basis of the following criteria: log2 fold-change in uEV >1 or less than −1, and log2 fold change in kidney is between −0.5 and 0.5. ClueGO (v2.5.7) was run under the Cytoscape framework (v3.8.2), with GO Biologic Process, Molecular Function, Cellular Component (all annotations updated on February 11, 2021), and KEGG (annotation updated on February 8, 2021) in use. Evidence level was set at “All_without_IEA”; “use GO Term fusion” function was enabled; and only pathways with P<0.05 were shown.
Correlation Analysis
iBAQ.
Logarithmically transformed and normalized iBAQ percentages of the same protein identified in uEV and in kidney were plotted. For averaged correlations, the values of the same protein within uEV or kidney were averaged and plotted. Simple linear regression was performed with a 95% confidence interval. Pearson and Spearman correlation coefficients were calculated. All plots and correlation analysis were performed by GraphPad Prism (v 8.4.1).
LFQ.
Log2 ratios (high K+/control) of the same proteins in uEV and in kidney were plotted. Spearman correlation coefficient was calculated. All plots and correlation analysis were performed by GraphPad Prism (v 8.4.1).
Data Availability.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository,38 with the dataset identifier PXD018363.
Immunoblotting of Mouse and Rat uEVs.
Protein concentrations of uEVs and kidneys were determined using a standard bicinchoninic acid assay (Thermo Fisher Scientific) and gel samples of equal concentration made in Laemmli sample buffer containing DTT (15 mg/ml). Equal protein amounts were loaded on SDS-PAGE gels and Coomassie stained to adjust for equal protein loading, with maximal deviations in total protein loading between samples on individual blots +/−10%. Immunoblotting was performed using standard protocols and the following antibodies: rabbit anti-NCC (SPC-402D; StressMarq Biosciences Inc., Victoria, BC, Canada; dilution 1:1000), rabbit anti–pT58-NCC,39 rabbit anti-NKCC2,40 rabbit α-ENaC antibody,41 rabbit anti-Kir4.1 antibody (Alomone), rabbit anti-SPAK/OSR1 antibody,42 rabbit anti-phosphoSPAK/OSR1 (pS373/S325) antibody (07–2273, Millipore), mouse anti-Calbindin 28kD antibody (10R-C106A, Fitzgerald), mouse anti-parvalbumin (PV235, Swant), mouse anti-histone H2A (#2578, Cell Signaling), mouse anti-RhoA (sc-418, Santa Cruz), mouse anti–CRHSP-24 (sc-137072, Santa Cruz), mouse ant-PEPCK (sc-271029, Santa Cruz), mouse anti-Nedd8 (sc-373741, Santa Cruz), rabbit anti-Histone H3 (14269, Cell Signaling), rabbit anti-TSG101 (ab125011, Abcam), rabbit anti-NHERF1 (PA1–090, Invitrogen), mouse anti-Glut5 (sc-271055, Santa Cruz), rabbit anti-NHE3,43 rabbit anti-NaPi2a,44 rabbit anti-NaKATPase α-1 (06–520, EMD Millipore), and rabbit anti–β-actin (SAB5600204, Sigma). Specificity of the commercial antibodies has been validated previously by specific labeling using immunohistochemistry, and for immunoblotting was on the basis of that they either gave a single unique band corresponding to the target proteins predicted molecular weight, or the most prominent band on the immunoblot was at the target proteins predicted molecular weight (with no other bands of similar size). Pairwise comparisons of data meeting the statistical assumptions of normality and variance homogeneity were performed using a two-sided t test.
Results
Correlations of Absolute Protein Abundances between uEVs and Kidney
Rats were randomly assigned (six per group) to be fed either a control diet or a high K+ diet for 4 days. Measured physiologic parameters of the rats are in Supplemental Figure 1. The 24-hour urine output, urine concentrations of Na+, Cl−, and K+ and urine excretion of K+ were significantly different after a high K+ diet. There was also a small, but significant, decrease in plasma Cl− concentrations in rats given the high K+ citrate diet. Aldosterone levels significantly increased from 105.7±9 pg/ml in controls to 393±126 in the high K+ group. uEVs and the kidneys from the same animals were prepared and processed for shotgun proteomics (see Methods). Approximately 2000 and 5000 unique proteins were identified in each uEV and kidney sample, respectively. The iBAQ algorithm45 was enabled within the MaxQuant search framework,46 to estimate the absolute amount of each protein in every sample. The iBAQ percentages were logarithmically transformed and normalized to account for the variations in the number of identified proteins from each sample. Subsequently, simple linear regression45 was used to correlate the abundance of proteins in the kidney and their corresponding levels in uEVs from the same animal.
In samples collected from rats on control diet, GO term overrepresentation analysis on uEV abundant proteins (see Methods) confirmed the successful enrichment of uEVs and some of their potential biologic processes and activities (Supplemental Figure 2). For example, uEVs were enriched for cytokines, in line with their importance for immune responses.47,48 Comparison of all proteins identified by mass spectrometry in uEVs and kidney (Supplemental Table 1), highlighted a positive correlation between their absolute protein abundance (Pearson correlation coefficient, R=0.46, P<0.0001; Spearman correlation coefficient=0.47, P<0.0001) (Figure 1A). Where possible, proteins were subclassified into quantifiable groups, including transporters/channels (from here on referred to as “transporters”) (Figure 1B), apical membrane transporters (Figure 1C), basolateral membrane transporters (Figure 1D), protein kinases (Figure 1E), transcription factors (TFs) (Figure 1F), E3 ubiquitin ligases (Figure 1G), deubiquitylases (Figure 1H), and proteins that have at least one predicted transmembrane helix34 (“PredHel > 0”) (Figure 1I), and proteins that are within the list of the 100 most common EV proteins (http://microvesicles.org/extracellular_vesicle_markers) (Figure 1J). Furthermore, using transcriptomics35 and proteomics data37 from microdissected rat renal tubules, proteins were stratified to a “segment-restricted” category (see Methods) if they were expressed only in a narrow continuous region along the renal tubule on the basis of mRNA expression (Figure 1K) or protein abundance values (Figure 1L). Using the same segment-specific proteome database,37 proteins were further defined as being highly abundant in the proximal (Figure 1M) or distal tubule (Figure 1N) (see Methods), and crossreferenced with the “segment-restricted (proteome)” proteins (Figure 1, O and P). The strongest correlation was observed for apical membrane transporters, which fits well with the pool of analyzed uEVs being enriched for exosomes. Furthermore, transporter/channel proteins in general, PredHel >0 proteins and segment-restricted proteins all had good positive overall correlations between abundances in uEVs and kidney (Pearson correlation coefficients >0.5, P<0.0001). Their relationship is shown in a Venn diagram (Supplemental Figure 3). In contrast, cytosolic proteins such as E3 ubiquitin ligases, protein kinases, and TFs had very low or negative correlations between uEVs and kidney abundances. A greater number of identified proteins were classified as proximal relative to distal, but both proximal and distal proteins (also when crossreferenced with segment-restricted proteins) had correlations similar to “all quantifiable proteins”. Although deubiquitylases also had a positive correlation (R=0.59, P<0.0001), their abundance in the kidney is approximately 100-fold higher than in uEVs.
Figure 1.
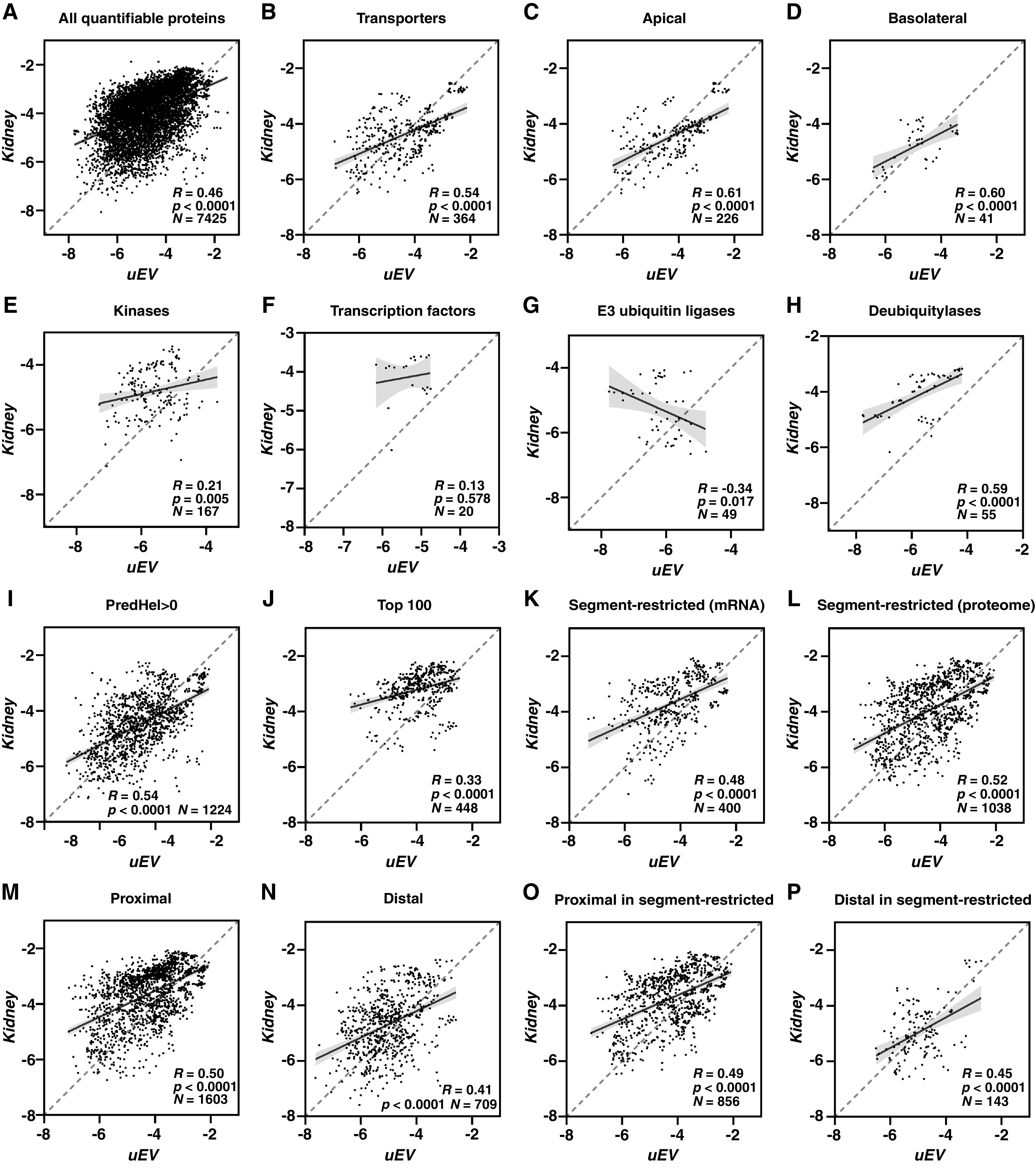
Correlations of absolute protein abundances between uEV and kidney on a standard diet showed higher correlations for transporter, PredHel >0, and segment-restricted proteins. The x axis denotes normalized log10 iBAQ percentages for uEV, whereas the y axis denotes the same for kidney. As the iBAQ percentage is a value between 0 and 1, logarithmic transformation produces a negative value (but they are indicative of the absolute abundances of the proteins). R denotes Pearson’s correlation coefficient, P denotes the significance of Pearson’s correlation, and N denotes the number of data points in the figure. The dashed diagonal line denotes perfect positive correlation, the black line inside the cluster of dots denotes the actual linear regression line, and the gray area denotes the 95% confidence interval. (A) all quantifiable proteins identified; (B) proteins defined as transporter/channel proteins (see Methods); (C) transporter/channel proteins defined as apically located (see Methods); (D) transporter/channel proteins defined as basolaterally located (see Methods); (E) protein kinases (see Methods); (F) transcription factors (see Methods); (G) ubiquitin E3 ligases (see Methods); (H) deubiquitylases (see Methods); (I) proteins that have at least one predicted transmembrane domain (see Methods); (J) most commonly identified proteins from other extracellular vesicle studies (see Methods); (K) proteins with restricted localization along the kidney tubule based on mRNA expression data (see Methods); (L) proteins with restricted localization along the kidney tubule based on proteomic data (see Methods); (M) proteins defined as being located in the proximal region of the kidney tubule (see Methods); (N) proteins defined as being located in the distal region of the kidney tubule(see Methods); (O) proximally located proteins that are expressed in a restricted region based on proteomic data (see Methods); (P) distally located proteins that are expressed in a restricted region based on proteomic data (see Methods).
GO term overrepresentation analysis on uEV abundant proteins from rats on a high K+ diet further confirmed the successful enrichment of uEVs (Supplemental Figure 4). For the majority of categories, correlation analysis between proteins identified in uEVs and kidney showed similar results to rats on control diet (Figure 2, Supplemental Table 2), suggesting the abundance correlation between uEVs and kidney for most protein classes is not hugely influenced by physiologic status. To investigate this further, a similar study was performed in rats fed a high K+ diet for only 2 days (physiologic parameters are in Supplemental Figure 5), where steady-state conditions due to the dietary challenge were unlikely to have been reached. Samples from this study were analyzed on an alternative mass spectrometer to rule out technical influence on the results. Correlation analysis on these samples (Supplemental Figure 6, Supplemental Table 3) showed similar trends to rats on control diet or rats on high K+ diet for 4 days.
Figure 2.
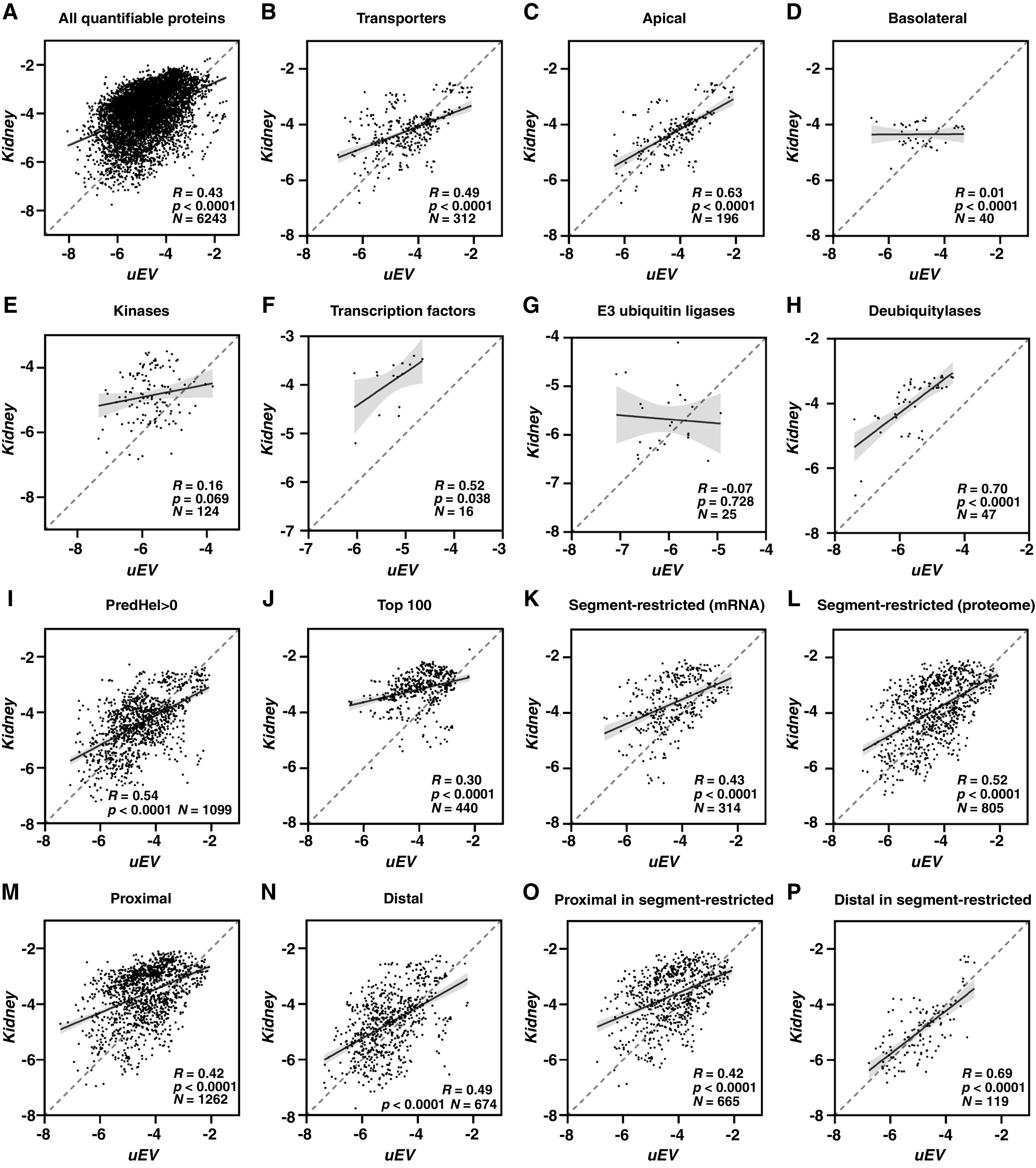
Correlations of absolute protein abundances between uEVs and kidney on a high K+ diet showed highly similar trends to standard diet. The x axis denotes normalized Log10 iBAQ percentages for uEV, whereas the y axis denotes the same for kidney. R denotes Pearson’s correlation coefficient, P denotes the significance of Pearson’s correlation, and N denotes the number of data points in the figure. The dashed diagonal line denotes perfect positive correlation, the black line inside the cluster of dots denotes the actual linear regression line, and the gray area denotes the 95% confidence interval. See Figure 1 for detailed explanations of figure panels A-P.
The large datasets from the control-fed rats and the 4-day high K+ diet were used to investigate which proteins had the best absolute abundance correlation between uEVs and the kidney with minimal variation between subjects. To assess this, correlation analysis was performed on proteins that only fulfilled the following criteria: (1) the protein was identified at least four times in both sample types; (2) the relative standard deviation of the protein abundances within uEVs and kidney was below 10%; and (3) the average fold difference between absolute protein abundance in uEVs and the kidney was less than 5-fold (Supplemental Tables 1 and 2). Out of the 1124 and 917 proteins that had at least four iBAQ values in both uEVs and kidney under the control and high K+ diets, respectively, 360 and 270 proteins passed these criteria. This corresponds to approximately 30% of all consistently identified proteins. Proteins that passed these criteria demonstrated an almost perfect linear regression (R=0.92, P<0.0001) (Figure 3, A and F). Although there were some differences within the biologic replicates (Figure 3, B–E and G–J), four well-established marker proteins for uEVs, Alix, Tsg101, Cd63, and Cd81 were within a group of around 200 proteins that fitted the criteria under both dietary conditions (Supplemental Table 4). In addition, well-studied proteins such as Aqp1, Aqp2, the sodium/phosphate cotransporter NaPi-2a, and cubilin also demonstrated very high absolute correlations.
Figure 3.
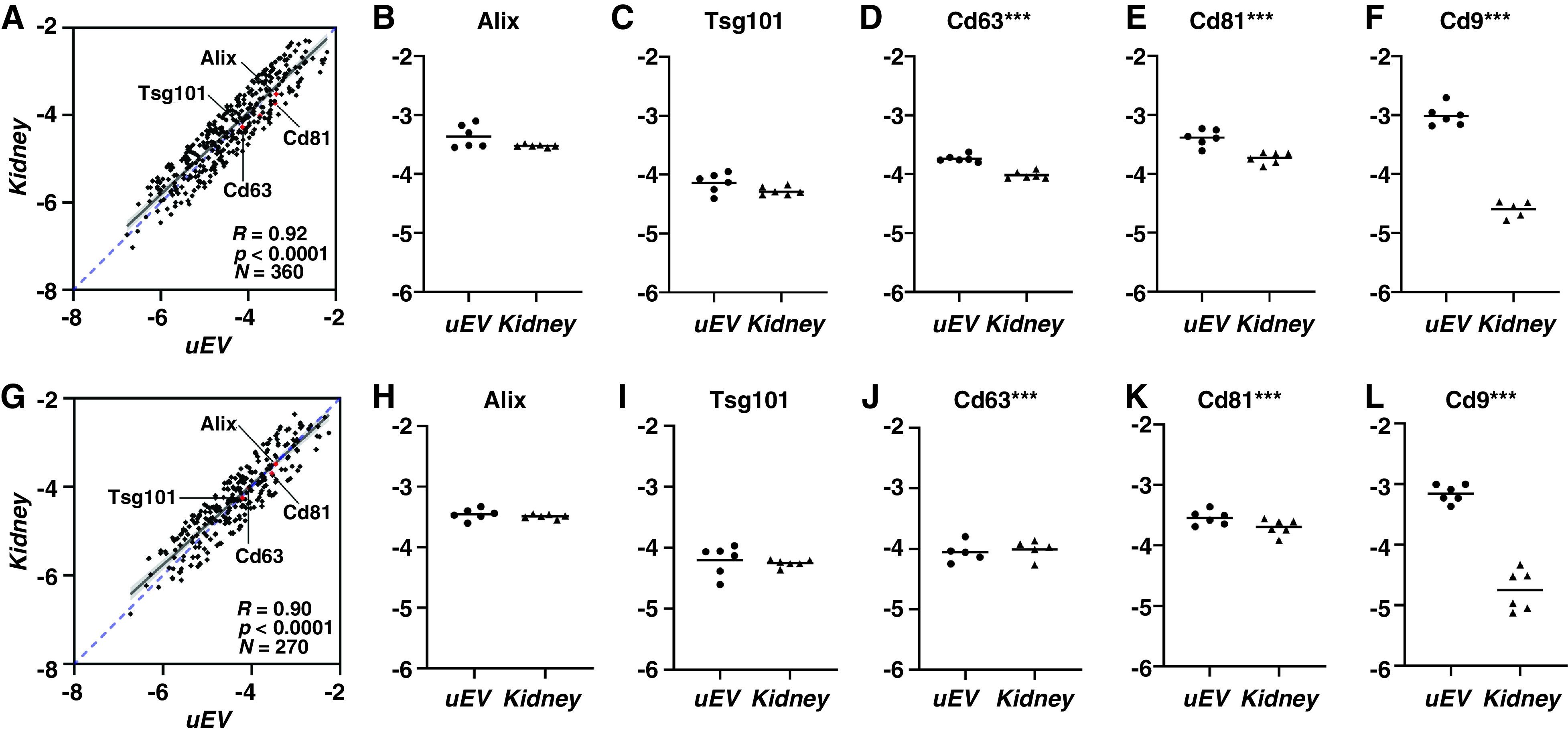
Highly correlated proteins and individual absolute protein levels for four uEV marker proteins in individual rats showed almost perfect correlations. (A–F): control diet; (G –L): high K+ diet. R denotes Pearson’s correlation coefficient, the blue dashed diagonal denotes perfect positive correlation, a single asterisk (*) denotes 0.01<P<0.05; a double asterisk (**) denotes 0.001<P<0.01; a triple asterisk (***) denotes 0.0001<P<0.001; and a quadruple asterisk (****) denotes P<0.0001. Although some proteins showed P<0.05, they still fulfilled the stringent criteria we set (except Cd9, see text). All axes denote normalized log10 iBAQ percentages for uEV or kidney. Cd9, an established marker protein for uEVs, had greater (100-fold) protein abundance in uEVs than in kidney.
Relative Quantification of uEV and Kidney After Dietary Intervention
LFQ and correlation analysis was used to determine if, after dietary manipulation, the alterations in protein abundances in the kidney were reflected by a similar alteration in the same protein in uEVs. The relationship between the fold-change in kidney (high K+ abundance versus control) and uEVs were plotted for quantifiable proteins after dietary manipulation. Because in this scenario we were most interested in knowing whether a change in protein abundance in the uEV pool reflected a changed abundance in the kidney (in the same direction), Spearman’s correlation was used to assess the strength of a monotonic relationship between the two groups. As shown in Figure 4A, there is a significant, but weak, correlation (R=0.14, P<0.0001) between all quantifiable proteins in uEVs and kidney (Supplemental Table 5). Proteins that change in the same direction (in uEV and kidney) and both to a reasonable extent (log2 fold-change >0.5 or less than −0.5) were highlighted in column “Both >0.5 or less than −0.5” (Supplemental Table 5). Quantifiable proteins were again subclassified into transporters (Figure 4B), apical membrane transporters (Figure 4C), basolateral membrane transporters (Figure 4D), kinases (Figure 4E), TFs (Figure 4F), E3 ubiquitin ligases (Figure 4G), deubiquitylases (Figure 4H), “PredHel >0” (Figure 4I), top 100 (Figure 4J), “segment-restricted (mRNA)” (Figure 4K), “segment-restricted (proteome)” (Figure 4L), proximal (Figure 4M), distal (Figure 4N), proximal in segment-restricted (proteome) (Figure 4O), and distal in segment-restricted (proteome) (Figure 4P). Transporters (especially apically located), PredHel >0 proteins and segment-restricted proteins showed reasonable overall significant correlations between the groups. Protein kinases, deubiquitylases, TFs, and E3 ubiquitin ligases again showed no significant correlation, but this must be interpretated with caution due to the small number of proteins in each of these classes. Confirmatory immunoblotting of kidney samples from control or 4-day high K+ diet fed rats for 13 randomly selected proteins showed a very good correlation (R=0.74) with the mass spectrometry data (Supplemental Figure 7).
Figure 4.
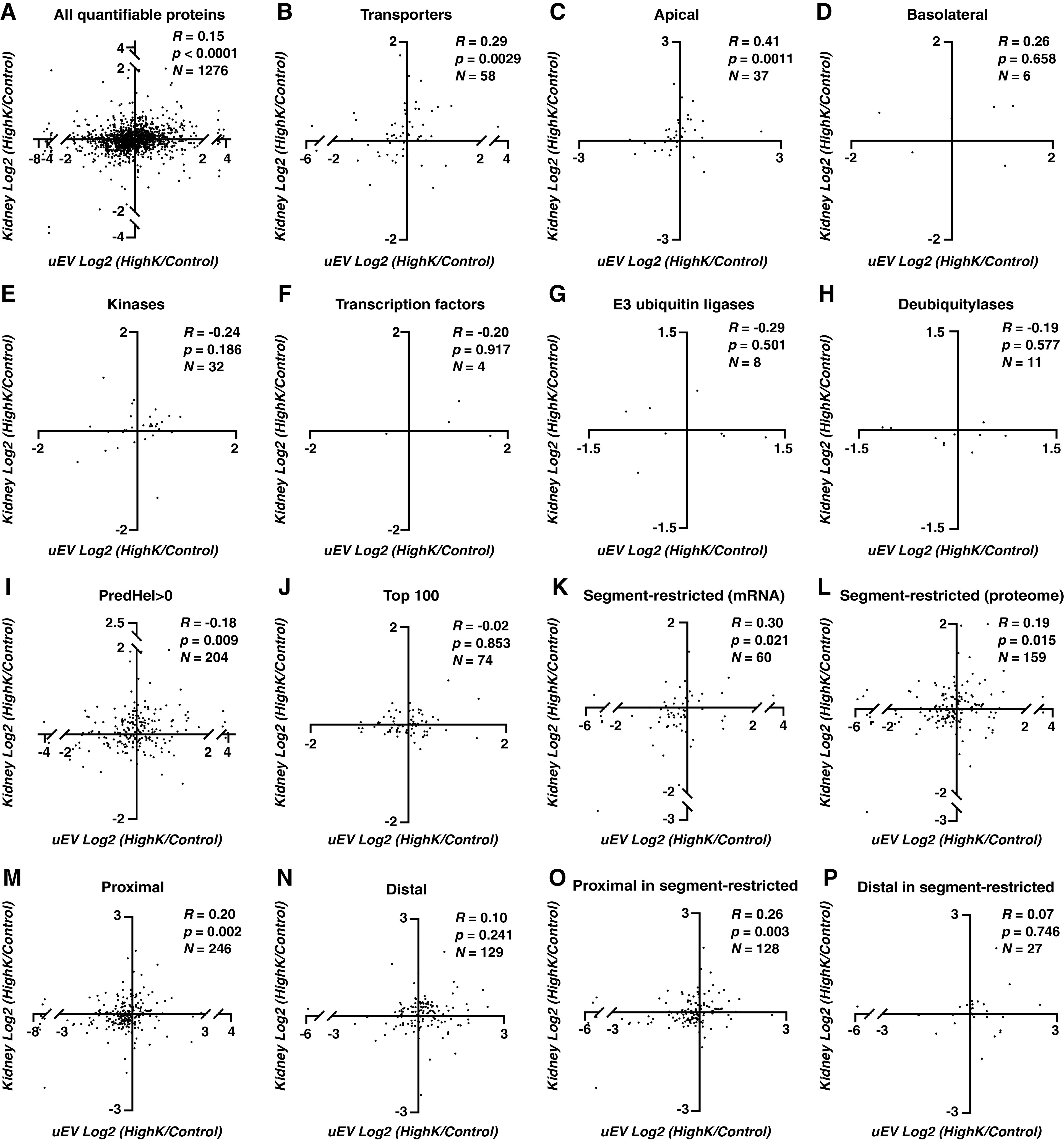
Relative correlations of fold changes induced by diet (high K+ versus control) of the same protein between uEV and kidney showed higher correlations for transporter and segment-restricted proteins. R denotes Spearman’s correlation coefficient, P denotes the significance of Spearman’s correlation, N denotes the number of proteins in the figure. See Figure 1 for detailed explanations of figure panels A-P.
GO functional analysis was performed on the proteins that had large changes in uEVs but small changes in the kidney after 4 days of high K+ intervention in an attempt to uncover novel biologic functions of uEVs during the dietary manipulation (Supplemental Figure 8). Although the significantly enriched processes in the uEVs are varied, they include positive regulation of potassium ion transport and vesicle trafficking to the plasma membrane, and an enrichment for ESCRT complex proteins in the uEVs.
The abundances of the epithelial sodium channel ENaC, the sodium chloride cotransporter NCC (and its active phosphorylated form, pT58-NCC), the Na-K-ATPase and the sodium/phosphate cotransporter NaPi-2a change in the kidney after dietary K+ manipulation.49,50 To see if similar responses can be observed in uEVs, immunoblotting for these proteins was performed on uEVs and corresponding kidney samples from rats fed control or high K+ diet for 2 or 4 days. The dietary-induced changes observed in the kidney were mirrored by changes in uEVs, with a good correlation between the altered protein abundance in uEVs and kidney from the same rat (Supplemental Figure 9). This strengthens the idea that uEV proteins of certain classes track reliably with the abundance of the parent proteins in kidney.
Correlations between uEVs and Kidney in Animal Models of Disease
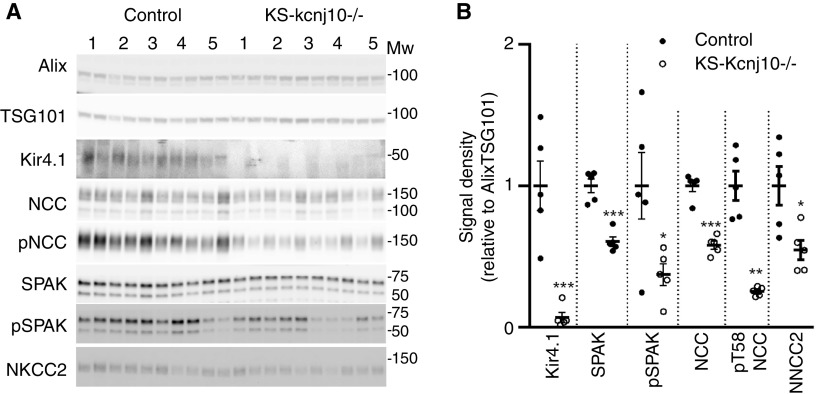
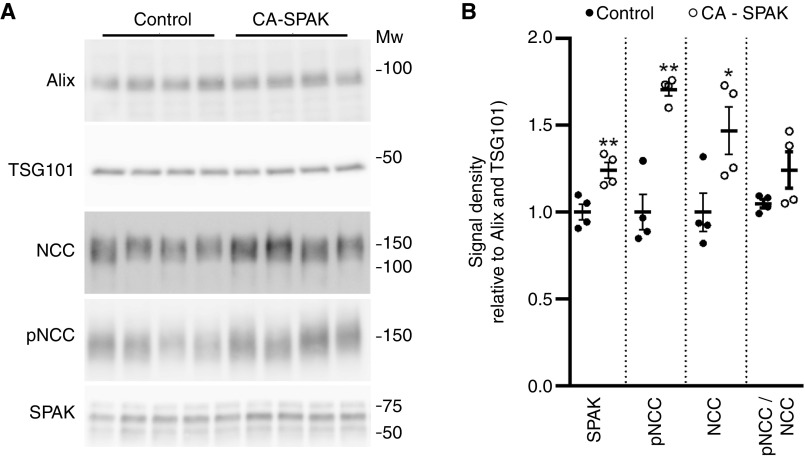
In disease settings, prolonged changes in protein abundance within the kidney may also be reflected in uEV protein abundances.51 To assess this, two genetic mouse models used to study the role of the WNK-SPAK-NCC signaling pathway for maintenance of blood pressure and electrolyte balance were used: (1) mice with renal tubule-specific deletion of Kcnj10 encoding the potassium channel Kir4.1 (KS-kcnj10−/−)29,32; (2) mice with DCT-specific expression of a constitutively active form of the Stk39 encoded kinase SPAK (CA-SPAK).30 As the focus here was on a small number of proteins known to be affected by disease states, we used immunoblotting. Kir4.1 protein was virtually undetectable in uEVs isolated from KS-kcnj10−/− mice relative to controls (Figure 5). The uEV protein abundances of SPAK, phosphorylated SPAK, NCC, phosphorylated NCC (pT58-NCC), and NKCC2 were significantly lower in uEVs from KS-kcnj10−/− mice (Figure 5). In uEVs isolated from CA-SPAK mice, protein levels of SPAK, NCC, and pT58-NCC were significantly higher relative to controls (Figure 6). In both models, these observed differences in protein abundances in uEVs correlate very well with previous observations of their abundances in the kidney29–31 (see Discussion).
Figure 5.
Altered protein abundances in uEVs isolated from KS-kcnj10−/− are similar to those reported in the kidney. (A) uEVs were isolated from 24-hour urine and assessed by immunoblotting. Each duplet represents uEVs isolated from two sequential 24-hour collection periods from the same mouse. (B) Summary data of relative abundances of uEV proteins in control and KS-kcnj10−/− mice. Each data point represents the average abundance of the protein from the two 24-hour collection periods. Data correlate well with relative abundances reported in the literature (see text). A single asterisk (*) denotes 0.01<P<0.05; a double asterisk (**) denotes 0.001<P<0.01; and a triple asterisk (***) denotes 0.0001<P<0.001, relative to control.
Figure 6.
Altered protein abundances in uEVs isolated from CA-SPAK mice are similar to those reported in the kidney. (A) uEVs were isolated from 24-hour urine and assessed by immunoblotting. Each lane represents uEVs isolated from a 24-hour collection period from the same mouse. (B) Summary data of relative abundances of uEV proteins in control and CA-SPAK mice. Data correlate well with relative abundances reported in the literature (see text). A single asterisk (*) denotes 0.01<P<0.05; a double asterisk (**) denotes 0.001<P<0.01, relative to control.
Discussion
uEVs are extensively studied as a source of biomarkers for renal and nonrenal disorders.1,52–54 Furthermore, and especially in clinical settings, measurement of uEV protein abundance is frequently used to reflect ongoing (patho)physiologic processes in the kidney.1,2,55 However, whether protein abundances in uEVs and the kidney directly correlate, or whether (patho)physiologic alterations in protein levels in the kidney can be determined by assessing protein changes in uEVs has never been comprehensively determined. To fill this major knowledge void, this study set out to answer four questions: (1) On a proteome scale can absolute protein abundances in uEVs be an indicator of protein abundances in the kidney? (2) What is the degree of correlation between proteins in uEVs and the kidney, and are there protein classes that have better correlations? (3) During a physiologic manipulation, can assessment of uEVs proteins be relied on to reflect similar changes in the kidney? (4) In genetic models of disease, do proteins in uEVs track with their abundance in the kidney?
To answer the first three questions, we used LC-MS/MS–based shotgun proteomics to quantify thousands of proteins in uEVs and kidney tissue isolated from three different dietary cohorts in an unbiased manner. To maximize the number of identified proteins from the two sample types (biofluid and tissue), we used label-free absolute quantification (iBAQ). This allowed us to assess different total protein quantities by LC-MS/MS (total protein quantities in uEVs much lower than kidney tissue) and preserve the information of protein abundance of every identified protein within one sample. An important finding was that, at a proteome level, independent of dietary conditions (Figures 1 and 2), there is a significant positive correlation between absolute protein abundances in uEVs and their abundance in the kidney. This is reinforced by the good agreement (Figure 3) of the abundances of established uEV marker proteins (Alix, Tsg101, Cd63, and Cd81)56 in our uEV pool and the kidney. The strongest significant correlation under both dietary conditions was for proteins classified as apical membrane transporters/channels. Furthermore, for transmembrane proteins, including transporters/channels, and proteins that had a restricted location within the renal tubule, both the absolute protein abundances and the relative change in abundance between the kidney and uEVs after a high K+ diet correlated relatively well. This is highlighted by the good correlations in abundances of ENaC, NCC, pT58-NCC, the Na-K-ATPase, and NaPi-2a in uEVs and kidney from the same rats after 2 or 4 days of dietary K+ manipulation. Together, this indicates uEV proteins of these classes track reliably with the abundance of the parent proteins in kidney.
For numerous protein kinases, TFs and E3 ubiquitin ligases there were poor correlations between their absolute abundance in uEVs and in the kidney. These data alone are not surprising—we had no reason to believe the absolute abundance of every protein in a uEV pool would be equal to its abundance in the kidney. However, changes in the kidney abundance of these protein classes after a dietary manipulation also did not show a similar change in uEVs (Figure 4), highlighting a potential limitation of uEV assessment for several of these classes of proteins in a physiologic setting. At a proteome-wide level, what is the basis for the lack of correlation for some proteins? One possibility is that it could reflect the more generalized expression of these protein classes in multiple cell types, with changes in their quantities within uEVs derived from a particular cell type “masked” in the general analysis of kidney tissue. Another possibility is that the lack of correlation relates to the origin of uEVs. Our uEV pool was enriched for exosomes (of plasma membrane and/or endosomal origin) and possibly some small microvesicles (fragments of the plasma membrane) by ultracentrifugation. Therefore, some of the small soluble proteins detected in uEVs may simply be contaminants that are randomly observed in our preparation. Alternatively, proteins of nontubular origin, including endothelial cells, connective tissue cells (including blood), immune cells, and nerve cells, contribute to the kidney but not to the uEV proteome, and may bias the analysis. Although this limitation could potentially be avoided using cell-enrichment techniques (microdissection, FACS), our overall aim here was to see how good the correlation was between protein abundances in uEV and whole kidney, which is better suited to a clinical or diagnostic setting.
Another question that we addressed was whether in genetically altered mouse disease models, protein abundance in uEVs reflected the changes in kidney tissue. To address this, we performed targeted studies using immunoblotting of uEV proteins isolated from two different genetic mouse models. Relative to controls, mice with renal tubule–specific deletion of Kir4.1, phenotypically mimicking Gitelman/EAST syndromes, had reduced uEV protein abundances of Kir4.1 (7%±4%), SPAK (61±4%), phosphorylated SPAK (37±7%), NCC (58±3%), phosphorylated NCC (25±3%), and NKCC2 (55±7%). In previous reports,29–31 relative to controls KS-kcnj10−/− mice had kidney abundances of Kir4.1 (4% ± 2%), SPAK (35±6%), phosphorylated SPAK (49±6%), NCC (47±8%), pT53-NCC (31±8%), and NKCC2 (52±5%). Relative to controls, CA-SPAK mice, which phenotypically mimic familial hyperkalemic hypertension,30 had increased NCC (147%±12%), pT58-NCC (170%±4%), and pT58-NCC/NCC ratios (120%±9%). Again, this matches well with relative kidney abundances previously reported; NCC (200%±8%), pT58-NCC (302%±11%), and pT58-NCC/NCC ratios (142%±9%).30 Thus, taken together, the data indicate that in mouse models of disease, protein abundances in uEVs reflected the changes in kidney tissue. A wider implication of this result is that analysis of proteins in uEVs may be a reliable way to measure protein changes in the kidney of patients with certain monogenetic diseases and fixed changes in genes affecting kidney function.
This study is not without limitations. One thing that was apparent is that even in well-defined conditions with genetically identical rats, there was greater variation in the absolute abundance levels of proteins within uEVs relative to the kidney samples. This may be due to the intrinsic property of uEVs, with greater variations in their proteome due to fluctuations of, for example, circadian rhythm, fluid status etc.,28 or it may be a result of technical variations introduced by the procedure for isolation of uEVs. This variation is especially important to consider when assessing changes in a particular protein on an individual-subject level, rather than assessment of average changes in a cohort of subjects. For example, the average absolute abundances of four well-established uEV markers Alix, Tsg101, Cd63, and Cd81 agreed very well with absolute kidney abundances with very little variation across six samples (Figure 3). However, if examined at an individual level, the correlations of these proteins in uEV and the kidney are much weaker (Supplemental Figure 10). Similar weak correlations on an individual level were observed in a study examining nine different proteins in human uEVs and kidney tissue using western blotting.28 Another point to stress is that the abundance of a given protein in uEVs may change without corresponding changes in expression in the kidney. This may particularly apply to proteins that undergo “stimulus-induced trafficking” to the apical plasma membrane, such as AQP2,57 which could increase their abundance only in uEVs via their endocytic retrieval to multivesicular bodies and subsequent targeting to exosomes. Furthermore, we cannot exclude that secretion rates of uEVs and their respective cargo are altered under certain (patho)physiologic conditions, whereas their abundance in the kidney is not.
In conclusion, we provide a unique resource and guide that facilitates the use of uEVs as a surrogate to study kidney function. Our data suggest that at the proteome-wide level, the absolute abundances of proteins in uEVs and kidney are correlated. Transmembrane proteins and proteins with a restricted localization in the renal tubule have the greatest positive correlations, whereas cytosolic proteins have a weak correlation. Furthermore, a monotonic relationship exists between altered transporter and channel protein abundances in uEVs and the kidney after a physiologic stimulation. This suggests that drawing conclusions from altered protein levels in uEVs for these particular protein classes and relating it to changes in the kidney is valid.
Disclosures
D.H. Ellison reports being a scientific advisor or member as an Author for UpToDate, Consulting Editor for Hypertension, Editorial Board American Journal Physiology-Renal Physiology; and reports other interests/relationships as a member of Leadership Council for American Society of Nephrology. E. Delpire reports receiving honoraria from University of Toronto (Speaker honorarium); reports being a scientific advisor or member of the American Journal of Physiology Cell Physiology, and the European Journal of Physiology. P. Welling reports receiving honoraria from American Physiological Society; reports being a scientific advisor or member of the American Journal of Physiology Renal Editorial Board, Chair of Finance Committee for American Physiological Society, and Chair of Kidney Molecular Biology and Development for the National Institutes of Health. R.A. Fenton reports being a scientific advisor or member as Associate Editor for American Journal of Physiology Renal, Editorial board member of Journal of the American Society of Nephrology (2008–), Editorial board member of PLOS One (2011–), and Editorial board member of Nature Scientific Reports (2016–). Q. Wu reports being an Associate Editor for Frontiers in Molecular Biosciences (section Protein Chemistry and Enzymology, 2021-) and Editorial board member of PLOS One (2020-). All remaining authors have nothing to disclose.
Funding
This work is supported by a grant from the Leducq Foundation (Potassium in Hypertension Network). Funding to R.A. Fenton is provided by the Novo Nordisk Foundation, the Lundbeck Foundation, and the Danish Medical Research Council. Q. Wu was supported by European Union Horizon 2020 Marie Skłodowska-Curie Individual Fellowship (Project 705682). The work with the CA-SPAK mouse was funded by National Institutes of Health grant DK93501 (to E. Delpire and P. Welling).
Supplementary Material
Acknowledgments
R.A. Fenton conceptualized the study; R.A. Fenton, P. Grimm, S.K. Murali, S.B. Poulsen, X.-T. Su, and Q. Wu performed experiments; E. Delpire provided novel mouse model; D.H. Ellison, P. Welling, and Q. Wu analyzed and interpreted data; R.A. Fenton and Q. Wu drafted the manuscript; and all authors approved the final manuscript and agreed to be accountable for all aspects of the work.
We would like to acknowledge the technical assistance of Ahmed Abduljabar, Golshah Ayoubi, and Helle Høyer.
Footnotes
S.B.P and S.K.M contributed equally to this study.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071035/-/DCSupplemental.
Supplemental Figure 1. Measured physiological parameters of rats fed either a control diet (0.4% Na+, 0.8% K+) or a high K+ diet (0.4% Na+, 5% K+ as potassium citrate) for 4 days.
Supplemental Figure 2. Gene Ontology overrepresentation analysis on uEV abundant proteins under control diet.
Supplemental Figure 3. Venn diagram of four categories under control diet: transporter proteins, segment-restricted proteins screened by transcriptomics (mRNA) and proteomics (proteome) databases, and predicted transmembrane proteins (PredHel >0).
Supplemental Figure 4. Gene Ontology overrepresentation analysis on uEV abundant proteins under high K+ diet.
Supplemental Figure 5. Measured physiological parameters of rats fed either a control (0.3% Na+, 1.05% K+) or a high potassium citrate (0.3% Na+, 5.25% K+) diet for 2 or 4 days.
Supplemental Figure 6. Correlations of absolute protein abundances between uEV and kidney on a short-term high K+ diet (2 days) showed similar trend to control diet and high K+ diet (4 days).
Supplemental Figure 7. Immunoblotting of 13 randomly selected proteins in kidney samples from rats fed a control or high K+ diet for 4 days.
Supplemental Figure 8. Gene Ontology functional analysis on proteins that have large changes in uEVs but small changes in kidney.
Supplemental Figure 9. Correlation of protein abundances in uEV and kidney after dietary K+ manipulation.
Supplemental Figure 10. Correlations of the individual absolute protein levels for five well-established uEV marker proteins in six rats.
Supplemental Table 1. All identified and quantified proteins from uEV and kidney under control diet.
Supplemental Table 2. All identified and quantified proteins from uEV and kidney under high K+ diet.
Supplemental Table 3. All identified and quantified proteins from uEV and kidney under high K+ diet (short-term, 2 day).
Supplemental Table 4. Protein list that passed the criteria of abundance RSD<10% (in both uEV and kidney) plus fold-change smaller than 5 (between uEV and kidney) under both control and high K+ diets.
Supplemental Table 5. All LFQ quantifiable proteins in uEVs and kidney.
References
- 1.Merchant ML, Rood IM, Deegens JKJ, Klein JB: Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat Rev Nephrol 13: 731–749, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karpman D, Ståhl AL, Arvidsson I: Extracellular vesicles in renal disease. Nat Rev Nephrol 13: 545–562, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Thomas S, Hao L, Ricke WA, Li L: Biomarker discovery in mass spectrometry-based urinary proteomics. Proteomics Clin Appl 10: 358–370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siwy J, Mullen W, Golovko I, Franke J, Zürbig P: Human urinary peptide database for multiple disease biomarker discovery. Proteomics Clin Appl 5: 367–374, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Zürbig P, Jerums G, Hovind P, Macisaac RJ, Mischak H, Nielsen SE, et al.: Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 61: 3304–3313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullen W, Delles C, Mischak H; EuroKUP COST action: Urinary proteomics in the assessment of chronic kidney disease. Curr Opin Nephrol Hypertens 20: 654–661, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Fenton RA: Proteomic approaches in kidney disease biomarker discovery. Am J Physiol Renal Physiol 315: F1817–F1821, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Svenningsen P, Sabaratnam R, Jensen BL: Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol (Oxf) 228: e13346, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal M: Exosomes: Revisiting their role as “garbage bags”. Traffic 20: 815–828, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al.: Reassessment of exosome composition. Cell 177: 428–445.e18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessvik NP, Llorente A: Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193–208, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig N, Whiteside TL, Reichert TE: Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci 20: 4684, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayala-Mar S, Donoso-Quezada J, Gallo-Villanueva RC, Perez-Gonzalez VH, González-Valdez J: Recent advances and challenges in the recovery and purification of cellular exosomes. Electrophoresis 40: 3036–3049, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolarz M, Pietrowska M, Matysiak N, Mielańczyk Ł, Widłak P: Proteome profiling of exosomes purified from a small amount of human serum: The problem of co-purified serum components. Proteomes 7: 18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE: Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat Rev Urol 10: 206–218, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK: Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 82: 1024–1032, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Huebner AR, Somparn P, Benjachat T, Leelahavanichkul A, Avihingsanon Y, Fenton RA, et al.: Exosomes in urine biomarker discovery. Adv Exp Med Biol 845: 43–58, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS: Urine exosomes: An emerging trove of biomarkers. Adv Clin Chem 78: 103–122, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z: Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311: F844–F851, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayan H, Attar-Herzberg D, Shaharabany M, Holtzman EJ, Farfel Z: Increased urinary Na-Cl cotransporter protein in familial hyperkalaemia and hypertension. Nephrol Dial Transplant 23: 492–496, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Corbetta S, Raimondo F, Tedeschi S, Syrèn ML, Rebora P, Savoia A, et al.: Urinary exosomes in the diagnosis of Gitelman and Bartter syndromes. Nephrol Dial Transplant 30: 621–630, 2015 [DOI] [PubMed] [Google Scholar]
- 24.van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AH, et al.: The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 60: 741–748, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Wolley MJ, Wu A, Xu S, Gordon RD, Fenton RA, Stowasser M: In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol 28: 56–63, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salih M, Demmers JA, Bezstarosti K, Leonhard WN, Losekoot M, van Kooten C, et al.; DIPAK Consortium : Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J Am Soc Nephrol 27: 3079–3092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinrichs GR, Hansen LH, Nielsen MR, Fagerberg C, Dieperink H, Rittig S, et al.: A novel mutation affecting the arginine-137 residue of AVPR2 in dizygous twins leads to nephrogenic diabetes insipidus and attenuated urine exosome aquaporin-2. Physiol Rep 4: e12764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabaratnam R, Geertsen L, Skjødt K, Højlund K, Dimke H, Lund L, et al.: In human nephrectomy specimens, the kidney level of tubular transport proteins does not correlate with their abundance in urinary extracellular vesicles. Am J Physiol Renal Physiol 317: F560–F571, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, et al.: Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, et al.: SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, et al.: Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Moeller HB, Stevens DA, Sanchez-Hodge R, Childers G, Kortenoeven MLA, et al.: CHIP regulates Aquaporin-2 quality control and body water homeostasis. J Am Soc Nephrol 29: 936–948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krogh A, Larsson B, von Heijne G, Sonnhammer EL: Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol 305: 567–580, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medvar B, Raghuram V, Pisitkun T, Sarkar A, Knepper MA: Comprehensive database of human E3 ubiquitin ligases: Application to aquaporin-2 regulation. Physiol Genomics 48: 502–512, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limbutara K, Chou CL, Knepper MA: Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol 31: 1255–1266, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al.: The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47[D1]: D442–D450, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA: Localization and regulation of the rat renal Na(+)-K(+)-2Cl− cotransporter, BSC-1. Am J Physiol 271: F619–F628, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, et al.: WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Kim GH, Ecelbarger C, Knepper MA, Packer RK: Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935–942, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O, et al.: Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol 301: C850–C861, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al.: Global quantification of mammalian gene expression control. Nature 473: 337–342, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Tyanova S, Temu T, Cox J: The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11: 2301–2319, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Lv LL, Feng Y, Tang TT, Liu BC: New insight into the role of extracellular vesicles in kidney disease. J Cell Mol Med 23: 731–739, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiemstra TF, Charles PD, Gracia T, Hester SS, Gatto L, Al-Lamki R, et al.: Human urinary exosomes as innate immune effectors. J Am Soc Nephrol 25: 2017–2027, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA: Regulation of the Renal NaCl Cotransporter and Its Role in Potassium Homeostasis. Physiol Rev 100: 321–356, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, et al.: Mechanisms of phosphate transport. Nat Rev Nephrol 15: 482–500, 2019 [DOI] [PubMed] [Google Scholar]
- 51.Blijdorp CJ, Hoorn EJ: Urinary extracellular vesicles: the mothership connection. Am J Physiol Renal Physiol 317: F648–F649, 2019 [DOI] [PubMed] [Google Scholar]
- 52.Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE, et al.: Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol 12: 346–357, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Szabo G, Momen-Heravi F: Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol 14: 455–466, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsuda T, Kosaka N, Ochiya T: The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics 14: 412–425, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Erdbrügger U, Le TH: Extracellular vesicles in renal diseases: More than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, et al.: Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6: 22519, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenton RA, Murali SK, Moeller HB: Advances in Aquaporin-2 trafficking mechanisms and their implications for treatment of water balance disorders [published online ahead of print May 20, 2202]. Am J Physiol Cell Physiol 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository,38 with the dataset identifier PXD018363.