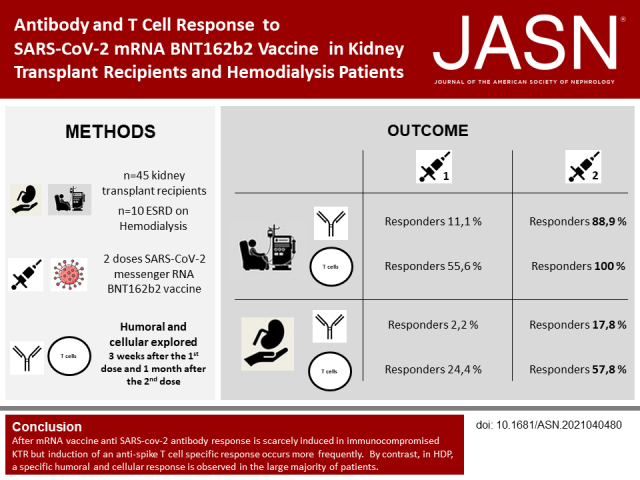
Significance Statement
Antibody and T cell responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines are poorly reported in kidney transplant recipients (KTRs) and patients on hemodialysis (HDPs). The authors investigated the response to BNT162b2 vaccine in 45 KTRs and ten HDPs. After the second dose, 88.9% of HDPs and only 17.8% of KTRs developed anti–SARS-CoV-2 antibodies. A specific T cell response was induced in 100% of HDPs and 57.8% of KTRs. The immune response seemed influenced by the immunosuppressive regimen in KTRs, particularly tacrolimus and belatacept. These results could help to better define the strategy of vaccination in this immunocompromised population.
Keywords: kidney transplantation, hemodialysis, clinical immunology, COVID-19
Visual Abstract
Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with a high rate of mortality in patients with ESKD, and vaccination is hoped to prevent infection.
Methods
Between January 18 and February 24, 2021, 225 kidney transplant recipients (KTRs) and 45 patients on hemodialysis (HDPs) received two injections of mRNA BNT162b2 vaccine. The postvaccinal humoral and cellular response was explored in the first 45 KTRs and ten HDPs.
Results
After the second dose, eight HDPs (88.9%) and eight KTRs (17.8%) developed antispike SARS-CoV-2 antibodies (P<0.001). Median titers of antibodies in responders were 1052 AU/ml (IQR, 515–2689) in HDPs and 671 AU/ml (IQR, 172–1523) in KTRs (P=0.40). Nine HDPs (100%) and 26 KTRs (57.8%) showed a specific T cell response (P=0.06) after the second injection. In responders, median numbers of spike-reactive T cells were 305 SFCs per 106 CD3+ T cells (IQR, 95–947) in HDPs and 212 SFCs per 106 CD3+ T cells (IQR, 61–330) in KTRs (P=0.40). In KTRs, the immune response to BNT162b2 seemed influenced by the immunosuppressive regimen, particularly tacrolimus or belatacept.
Conclusion
Immunization with BNT162b2 seems more efficient in HDPs, indicating that vaccination should be highly recommended in these patients awaiting a transplant. However, the current vaccinal strategy for KTRs may not provide effective protection against COVID-19 and will likely need to be improved.
Pandemic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been particularly deleterious in kidney transplant recipients (KTRs)1–3 and in patients with ESKD4 because of the severity of the disease and the high rate of morbidity and mortality. Moreover, the kidney transplantation activity has been highly affected by the pandemic.5,6 In order to protect these populations, SARS-CoV-2 vaccination is recommended through international guidelines.7,8 However, KTRs and patients on dialysis are considered low responders to vaccines9 and were not included in SARS-CoV-2 preauthorization vaccine clinical trials.
A low immunization after a first of an mRNA coronavirus disease 2019 (COVID-19) vaccine in solid organ transplant recipients has recently been reported.10 Evaluation of the humoral response to a vaccine readily evaluates its efficacy. However, in a population known to have lower seroconversion rates than the general nonimmunosuppressed population, the measurement of the cellular immune response could be particularly helpful and relevant.
We thus aimed in this study to explore the immunogenicity of mRNA BNT162b2 vaccine after the first and second doses not only by measuring vaccine-induced antibodies but also, by evaluating anti–SARS-CoV-2 spike-specific T cell response.
Between January 18 and February 3, 2021, 225 KTRs and 45 patients on hemodialysis received the first injection of the Pfizer SARS-CoV-2 mRNA BNT162b2 vaccine, and 3 weeks later, they received the second injection. Blood samples were collected from the first 45 KTRs (20%) and ten patients on hemodialysis (22.2%), and they were explored for both humoral and cellular immune response on the day of the second injection and 1 month later. Data were retrospectively analyzed. This retrospective study was submitted to the approbation of the Rouen Centre Institutional Review Board.
The anti–SARS-CoV-2 postvaccinal antibody response against the spike protein was assessed using the ARCHITECT IgG II Quant test (Abbott), with titers >50 arbitrary units (AU) per milliliter being considered as positive (detection range: 6.8–40,000 AU/ml; positive agreement, 99.4%; negative agreement, 99.6%).
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation of blood samples and used immediately. PBMCs (in concentrations adjusted to 2 × 105 CD3+ T cells per well) were plated in anti-IFNγ–coated ELISPOT 96-well plates in the presence of overlapping 15-mer peptide pools spanning the sequence of SARS-CoV-2 spike protein S (pool S1 spanning the N-terminal part of the protein including the S1-subunit and pool S2 spanning the C-terminal part) as well as N, M, ORF3A, and ORF7A in order to detect a potential exposition to SARS-CoV-2 (JPT, Strassberg, Germany). Negative and positive control stimulation, respectively, medium only and CEFX (a pool of 176 known peptides from various infectious agents (JPT)) were included in the assay. After an overnight culture, cells were washed, and captured IFNγ was revealed using a colorimetric assay (UCytech, Utrecht, The Netherlands). Spots were counted with an automated ELISPOT reader (AID, Strassberg, Germany). For each stimulation condition, the average spot number observed in wells without antigen was subtracted. Results were expressed as spot-forming cells (SFCs) per 106 CD3+ T cells. For each assay, a specific response was considered positive if the SFC number was superior to three SDs of spot numbers observed in wells without antigens (ranging between nine and 20 SFCs per 106 CD3+ T cells).11
Quantitative data are presented as mean (SD) or median (interquartile range [IQR]). Qualitative data are presented as percentages. The nonparametric Wilcoxon and Mann–Whitney tests were used to compare characteristics between groups with StatView version 5.0 (SAS Institute).
Baseline characteristics of the 45 KTRs are described in Table 1. No patient developed a SARS-CoV-2 infection for up to 1 month after the second injection.
Table 1.
Baseline characteristics of KTRs explored
| Baseline Characteristics | KTRs, n=45 |
|---|---|
| Age, yr | 63.5±16.3 |
| Sex (M/W), n | 23/22 |
| Diabetes mellitus, n (%) | 10 (22.2) |
| Hypertension, n (%) | 36 (80) |
| BMI, kg/m2 | 26.2±4.7 |
| Time from transplantation, yr | |
| Median (range) | 6.9 (0.22–30.2) |
| Immunized KTR, n (%) | 12 (26.7) |
| Median PRA class I | 6 |
| Median PRA class II | 14.5 |
| Previous history of rejection, n (%) | 1 (2.2) |
| eGFR, ml/min per 1.73 m2 | 43.3±15.7 |
| P/C ratio, g/g | 0.26±0.06 |
| Lymphocytes count, per mm3 | |
| CD3+ | 892±476 |
| CD4+ | 447±263 |
| CD8+ | 397±297 |
| Induction therapy for KT, n (%) | |
| ATG | 18 (40) |
| Anti–R-IL2 | 27 (60) |
| IS regimen, n (%) | |
| Tacrolimus | 24 (53.3) |
| Cyclosporin | 8 (17.8) |
| MMF | 37 (82.2) |
| AZA | 4 (8.9) |
| Everolimus | 3 (6.7) |
| Belatacept | 10 (22.2) |
| Steroids | 21 (46.7) |
M, men; W, women; BMI, body mass index; PRA, panel reactive antibodies; P/C, proteinuria/creatininuria; KT, kidney transplantation; ATG, antithymoglobuline; R-IL2, IL-2 receptor; IS, immunosuppressive; MMF, mycophenolate mofetil; AZA, azathioprine.
Regarding patients on hemodialysis, mean age was 71.2±16.4 years. Their median duration of chronic dialysis was 3.14 years (0.6–12.6). One patient on hemodialysis experienced a SARS-CoV-2 infection 3 days after the second injection of vaccine. She was maintained at home until recovery. She was excluded from the analysis after the second dose.
Three weeks after the first injection, only one patient on hemodialysis (11.1%) and one KTR (2.2%) developed anti–SARS-CoV-2 antibodies (P=0.19) (Figure 1A). Antibody titers in responders were 178.9 AU/ml in patients on hemodialysis and 311 AU/ml in KTRs. One month after the second injection, eight patients on hemodialysis (88.9%) and eight KTRs (17.8%) developed anti–SARS-CoV-2 antibodies (P<0.001). Median antibody titers in responders were 1052 AU/ml (IQR, 515–2689) in patients on hemodialysis and 671 AU/ml (IQR, 172–1523) in KTRs (P=0.40).
Figure 1.
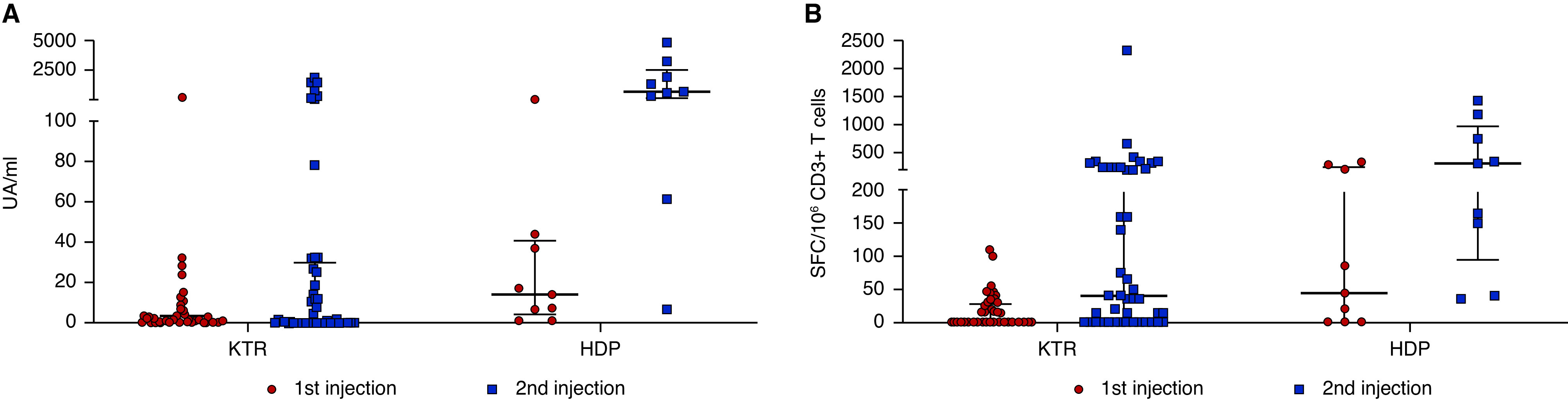
(A) SARS-CoV-2 antispike antibody response and (B) SARS-CoV-2–reactive IFNγ-producing T cells in KTRs and patients on hemodialysis (HDPs) following the first and second injections of the SARS-CoV-2 mRNA BNT162b2 vaccine. Titers of S IgG are shown in the samplings of 45 KTRs and nine HDPs. Medians and IQRs are shown. Numbers of T cells (expressed as SFCs per 106 CD3+ T cells) reactive to overlapping peptide pools spanning SARS-CoV-2 structural protein S (pools S1 and S2) in 45 KTR and nine HDPs are shown. Medians and IQRs are shown. UA, arbitrary units.
The single patient tested positive for SARS-CoV-2, developed after the first significant antibody titer (titer: 161 AU/ml), that dramatically increased after the second injection and COVID-19 (titer: 53,737.6 AU/ml).
Three weeks after the first injection, five patients on hemodialysis (55.6%) and 11 KTRs (24.4%) displayed a significant number of spike-reactive T cells (P=0.06) (Figure 1B). In responders, median numbers of specific T cells were 208 SFCs per 106 CD3+ T cells (IQR, 65–315) in patients on hemodialysis and 45 SFCs per 106 CD3+ T cells (IQR, 35–55) in KTRs (P=0.02). No response to N, M, ORF3A, and ORF7A was evidenced in KTRs and patients on hemodialysis, excluding a potential exposition to SARS-CoV-2.
One month after the second injection, a specific T cell response was detected in nine patients on hemodialysis (100%) and 26 KTRs (57.8%; P=0.06). In responders, median numbers of spike-reactive T cells were 305 SFCs per 106 CD3+ T cells (IQR, 95–947) in patients on hemodialysis and 212 SFCs per 106 CD3+ T cells (IQR, 61–330) in KTRs (P=0.40).
In the patient who tested positive for SARS-CoV-2, spike-specific T cell numbers were 155 SFCs per 106 CD3+ 3 weeks after the first injection and 3245 SFCs per 106 CD3+ after the second injection and SARS-CoV-2 infection. In this case, T cell responses to N, M, ORF3A, and ORF7A were detected.
Baseline characteristics of the 45 KTRs according to the baseline immunosuppressive regimen are described in Table 2.
Table 2.
Baseline characteristics of KTRs explored according to the immunosuppressive regimen
| Baseline Characteristics | Group 1: Tacrolimus-Based IS Regimen, n=24 | P Valuea | Group 2: Belatacept-Based IS Regimen, n=10 | P Valueb | Group 3: Nontacrolimus- and Nonbelatacept-Based IS Regimen, n=11 | P Valuec |
|---|---|---|---|---|---|---|
| Age, yr | 60.2±17.4 | 0.21 | 68.1±13.7 | 0.82 | 66.6±15.5 | 0.29 |
| Sex (M/W), n | 12/12 | 0.59 | 6/4 | 0.51 | 5/6 | |
| Time from transplantation, yr | 6.1±4.9 | 0.92 | 6.3±4.7 | 0.001 | 17.1±7.9 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 45.6±13.7 | 0.005 | 30.4±11.6 | 0.007 | 50.1±17.4 | 0.41 |
| Induction therapy for KT, n (%) | ||||||
| ATG | 11 (45.8) | 0.16 | 2 (20) | 0.21 | 5 (45.5) | |
| Anti–R-IL2 | 13 (54.2) | 8 (80) | 6 (54.5) | |||
| IS regimen, n (%) | ||||||
| Tacrolimus | 24 (100) | <0.001 | 0 | <0.001 | 0 | <0.001 |
| C0 tacrolimus | 6.1±1.8 | 0.007 | — | 0.54 | — | <0.001 |
| Cyclosporin | 0 | 0.78 | 0 | 0.69 | 8 (72.7) | 0.05 |
| C0 cyclosporin | — | 0.14 | — | 0.48 | 67.2±15.3 | 0.26 |
| MMF | 23 (95.8) | 0.12 | 6 (60) | 0.60 | 8 (72.7) | 0.56 |
| MMF median of dosage, mg | 1000 | <0.01 | 625 | <0.001 | 1000 | 0.03 |
| AZA | 1 (4.2) | 0.13 | 2 (20) | 0.12 | 1 (9.1) | 0.77 |
| Everolimus | 0 | 1 10) | 2 (18.2) | |||
| Belatacept | 0 | 10 (100) | 0 | |||
| Steroids | 10 (41.7) | 7 (70) | 4 (36.4) |
IS, immunosuppressive; M, men; W, women; KT, kidney transplantation; ATG, antithymoglobuline; R-IL2, IL-2 receptor; C0, through level; MMF, mycophenolate mofetil; AZA, azathioprine.
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 1 and 3.
One month after the second injection, two KTRs (8.3%) developed anti–SARS-CoV-2 antibodies in group 1, zero KTRs developed anti–SARS-CoV-2 antibodies in group 2, and six KTRs (54.5%) developed anti–SARS-CoV-2 antibodies in group 3 (group 1 versus group 2, P=0.34; group 2 versus group 3, P=0.005; group 1 versus group 3, P=0.002) (Figure 2A).
Figure 2.
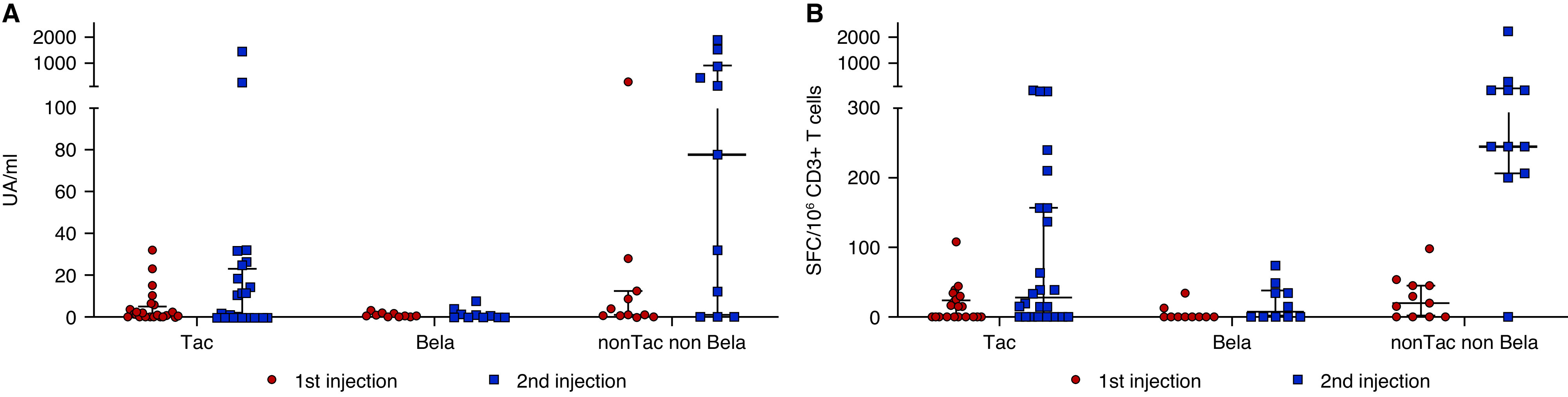
(A) SARS-CoV-2 antispike antibody response and (B) SARS-CoV-2–reactive IFNγ-producing T cells in KTRs following the first and second injections of the SARS-CoV-2 mRNA BNT162b2 vaccine according to the immunosuppressive regimen. Numbers of T cells (expressed as SFCs per 106 CD3+ T cells) reactive to overlapping peptide pools spanning SARS-CoV-2 structural protein S (pools S1and S2) in the samplings of 45 KTRs are shown. Median and IQR are shown. Bela, belatacept; Tac, tacrolimus. UA, arbitrary units.
One month after the second injection, 12 KTRs (50%) in group 1, four KTRs (40%) in group 2, and ten KTRs (90.9%) in group 3 displayed a specific T cell response (group 1 versus group 2, P=0.40; group 2 versus group 3, P=0.01; group 1 versus group 3, P=0.02). (Figure 2B). Median numbers of SARS-CoV-2–reactive T cells, after the second dose, were 160 SFCs per 106 CD3+ T cells (IQR, 46–303) in group 1, 42.5 SFCs per 106 CD3+ T cells (IQR, 35–69) in group 2, and 298 SFCs per 106 CD3+ T cells (IQR, 240–475) in group 3 (group 1 versus group 2, P=0.05; group 2 versus group 3, P=0.005; group 1 versus group 3, P=0.01).
In univariate analysis, predictive factors for a positive antibody response were the duration of kidney transplantation (P=0.003) and a cyclosporin-based immunosuppressive regimen (P<0.001). No factor predictive of a significant T cell response was identified. T cell counts were not associated with the detection or the magnitude of the antibody but were associated with T cell response to the vaccine in univariate analysis (P=0.01 for CD3, P=0.05 for CD4, and P=0.03 for CD8).
We report here the anti–SARS-CoV-2 antibody and T cell responses after two doses of the mRNA vaccine BNT162b2 in a cohort of KTRs and patients on hemodialysis. We demonstrate that an antibody response is scarcely induced in immunocompromised KTRs after a first vaccine dose and in only 17.8% of them after the second dose. Induction of an antispike T cell–specific response occurs more frequently in 51.1% of KTRs after the second injection. By contrast, in patients on hemodialysis, specific humoral and cellular responses are observed in 88.9% and 100%, respectively, of patients after the second dose. These results contrast with the robust and early induced immunity observed during mRNA vaccine trials, showing 100% antispike seroconversion after vaccination with mRNA-127312 or BNT162b2.13 Narasimhan et al.14 observed after the first vaccine dose in a naïve population, using the same serologic assay, median IgG titers of 2217 AU/ml (95% confidence interval, 0 to 44,182) that following the booster dose, dramatically increased 8.2-fold to 18,272 AU/ml (at 98% confidence interval, 11,724 to 21,750; P<0.001). Specific IgG titers found in our patients on hemodialysis and KTRs were significantly lower. Using a similar IFNγ ELISPOT assay, Angyal et al.15(preprint) recently reported (in SARS-CoV-2–naïve health care workers) median numbers of spike-specific T cells of 58 SFUs per 106 PBMCs (IQR, 29–146) after a single dose of BNT162b2 and 165 SFUs per 106 PBMCs (IQR, 101–277) after two doses. These values are in the same range as those observed in our study in KTRs and patients on hemodialysis, especially after the second dose. Thus, postvaccinal T cell immunity in KTRs and patients on hemodialysis seems comparable with that of healthy naïve subjects.
Data regarding vaccination in dialysis are very scarce. Seroconversion rates after influenza vaccine administration in such patients vary in the literature, ranging from 33% to 80%.16–18 Regarding the protection from SARS-CoV-2 of patients on dialysis, among 31 waitlisted patients with ESKD, 87% of them mounted an antibody response after a first vaccine dose (P=0.05).19 Grupper et al.20 reported very recently that most of 56 patients on hemodialysis (96%) developed specific antibodies following full BNT162b2 vaccination but with significantly lower titers than controls. Our data are in line with these results and extend them by showing effective induction of both humoral and T cell responses after two doses of the BNT162b2 vaccine.
In KTRs, vaccinal responses are expected to be impaired, particularly early post-transplantation, after treatment for rejection or rituximab therapy.21–23 KTRs of 65 years and older on ≥2 g daily mycophenolate mofetil generally have reduced humoral responses to influenza vaccines.24 Regarding the efficacy of the SARS-CoV-2 mRNA vaccine in KTRs, Boyarsky et al.10 reported that 82.6% of transplant recipients (n=436) did not mount significant antispike antibody titers after a first dose of mRNA vaccine. In the same vein, Benotmane et al.25 showed that only 10.8% of KTRs had a positive serology 28 days after the first injection of the mRNA-1273 vaccine. The median IgG titer was 224 AU/ml (IQR, 76 − 496 AU/ml). Similarly, Yi et al.19 reported a seroconversion rate of only 6.2% of KTRs after the first mRNA vaccine dose. Our results are thus in line with these studies, showing in addition that after the second vaccine dose, the seroconversion rate is only modestly increased (17.8%). Furthermore, they show that despite a low seroconversion rate, an antispike-specific T cell response is triggered in half of the patients after two vaccine doses. Presence of antispike T cells in absence of specific antibodies could provide some level of protection from SARS-CoV-2 infection by limiting the extent of viral replication, as reported in the context of CMV infection in KTRs.26,27
Our results suggest that the immune response to the BNT262b2 vaccine is essentially influenced by the intensity of the immunosuppressive regimen. Belatacept-treated patients were indeed the worst responders, developing no antibodies and no or only a few specific T cells. Belatacept is in fact suspected to be associated with an increase in the incidence of opportunistic infections28,29 and CMV disease.30 Tacrolimus-treated patients also responded weakly to vaccination, although significant T cell numbers were induced in some of them. It should be noted that the majority of our patients were on mycophenolate mofetil, which may have contributed to impairing postvaccine antibody responses.31
In conclusion, the mRNA BNT162b2 vaccine seems efficient in patients on hemodialysis, indicating that vaccination should be highly recommended in these patients. By contrast, the low seroconversion rate observed in KTRs is worrying. In seronegative patients displaying significant numbers of antispike T cells, a third dose of vaccine might trigger a humoral response. However, in patients failing to generate any response, should we prefer standard adjuvanted vaccines or adenovirus-based vaccines? Alternatively, should immunizing household members and close contacts be the priority? Postvaccination COVID-19 incidence data in KTRs should provide answers to these questions.
Disclosures
J. Plantier reports Research Funding from Abbott, Beckman, and Cepheid. O. Boyer reports Consultancy Agreements with BMS and ARGENX; Research Funding from CSL BEHRING, RA PHARMA, ARGENX, and GRIFOLS; Honoraria from CSL BEHRING, ARGENX, BMS, and ELSEVIER; and Scientific Advisor or Membership via FOCIS board of directors, and the IUIS education committee. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors thank the health care professionals of the University Hospital of Rouen who were involved in the care of the patients and the nurse from the kidney transplant unit.
D. Bertrand, S. Candon, and V. Lemée designed the study; J. Lamulle performed ELISPOT assays; D. Bertrand, S. Candon, and V. Lemée collected data; D. Bertrand, S. Candon, and M. Hamzaoui analyzed the data; D. Bertrand and S. Candon wrote the paper; and all authors provided feedback and critical review.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al.; French SOT COVID Registry : An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int 98: 1549–1558, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaunat O, Legeai C, Anglicheau D, Couzi L, Blancho G, Hazzan M, et al.; French nationwide Registry of Solid Organ Transplant Recipients with COVID-19 : IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int 98: 1568–1577, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al.; French SOT COVID Registry : Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant 21: 1295–1303, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al.: Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98: 1540–1548, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E: COVID-19 and solid organ transplantation: A review article. Transplantation 105: 37–55, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Danziger-Isakov L, Blumberg EA, Manuel O, Sester M: Impact of COVID-19 in solid organ transplant recipients. Am J Transplant 21: 925–937, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention: COVID-19 Vaccination Program Operational Guidance, 2021. Available at: https://www.cdc.gov/vaccines/covid-19/covid19-vaccination-guidance.html. Accessed April 2, 2021
- 8.European Centre for Disease Prevention and Control: COVID-19 vaccination and prioritization strategies in the EU/EEA, 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-vaccination-and-prioritisation-strategies.pdf. Accessed April 2, 2021
- 9.Krueger KM, Ison MG, Ghossein C: Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis 75: 417–425, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al.: Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 325: 1784–1786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candon S, Guerrot D, Drouot L, Lemoine M, Lebourg L, Hanoy M, et al.: T cell and antibody responses to SARS-CoV-2: Experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant 21: 854–863, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al.; mRNA-1273 Study Group : An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 383: 1920–1931, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al.: Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 383: 2439–2450, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, et al.: Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups [published online ahead of print April 7, 2021]. J Clin Microbiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angyal A, Longet S, Moore S, Payne RP, Harding A, Tipton T, et al.; PITCH Consortium : T-cell and antibody responses to first BNT162b2 vaccine dose in previously SARS-CoV-2-infected and infection-naive UK healthcare workers: A multicentre, prospective, observational cohort study. SSRN. 10.2139/ssrn.3812375 (Preprint posted March 25, 2021) [DOI]
- 16.Chang Y-T, Wang J-R, Lin M-T, Wu C-J, Tsai M-S, Wen-Chi CL, et al.: Changes of immunogenic profiles between a single dose and one booster influenza vaccination in hemodialysis patients - an 18-week, open-label trial. Sci Rep 6: 20725, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespo M, Collado S, Mir M, Cao H, Barbosa F, Serra C, et al.: Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol 6: 2208–2214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lertdumrongluk P, Changsirikulchai S, Limkunakul C, Prachukthum P, Punpiput P, Buppanharun R, et al.: Safety and immunogenicity of a 2009 influenza A (H1N1) vaccine in hemodialysis patients. Vaccine 30: 1108–1114, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Yi SG, Knight RJ, Graviss EA, Nguyen DT, Ghobrial RM, Gaber AO, et al.: Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration [published online ahead of print March 19, 2021]. Transplantation [DOI] [PubMed] [Google Scholar]
- 20.Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al.: Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis [published online ahead of print April 6, 2021]. Clin J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice: Vaccination in solid organ transplantation. Am J Transplant 13: 311–317, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Romero P, Bulnes-Ramos A, Torre-Cisneros J, Gavaldá J, Aydillo TA, Moreno A, et al.; Influenza Vaccine in Solid Organ Transplant Recipient Study Group, Spanish Network of Research in Infectious Diseases (REIPI-GESITRA); Influenza Vaccine in Solid Organ Transplant Recipient Study Group Spanish Network of Research in Infectious Diseases REIPI-GESITRA : Influenza vaccination during the first 6 months after solid organ transplantation is efficacious and safe. Clin Microbiol Infect 21: 1040.e11–1040.e18, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T: Serologic vaccination response after solid organ transplantation: A systematic review. PLoS One 8: e56974, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirzel C, Kumar D: Influenza vaccine strategies for solid organ transplant recipients. Curr Opin Infect Dis 31: 309–315, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al.: Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 99: 1487–1489, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litjens NHR, Huang L, Dedeoglu B, Meijers RWJ, Kwekkeboom J, Betjes MGH: Protective cytomegalovirus (CMV)-specific T-cell immunity is frequent in kidney transplant patients without serum anti-CMV antibodies. Front Immunol 8: 1137, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lúcia M, Crespo E, Melilli E, Cruzado JM, Luque S, Llaudó I, et al.: Preformed frequencies of cytomegalovirus (CMV)-specific memory T and B cells identify protected CMV-sensitized individuals among seronegative kidney transplant recipients. Clin Infect Dis 59: 1537–1545, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand D, Chavarot N, Gatault P, Garrouste C, Bouvier N, Grall-Jezequel A, et al.: Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol Dial Transplant 35: 336–345, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Bertrand D, Terrec F, Etienne I, Chavarot N, Sberro R, Gatault P, et al.: Opportunistic infections and efficacy following conversion to belatacept-based therapy after kidney transplantation: A French multicenter cohort. J Clin Med 9: E3479, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavarot N, Divard G, Scemla A, Amrouche L, Aubert O, Leruez-Ville M, et al.: Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept [published online ahead of print December 6, 2020]. Am J Transplant [DOI] [PubMed] [Google Scholar]
- 31.Struijk GH, Minnee RC, Koch SD, Zwinderman AH, van Donselaar-van der Pant KAMI, Idu MM, et al.: Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int 78: 934–940, 2010 [DOI] [PubMed] [Google Scholar]


