Diabetes mellitus is the leading cause of ESKD worldwide. The last few years have seen an explosion of evidence for the efficacy of sodium-glucose transporter type 2 inhibitors (SGLT2i), glucagon-like peptide-1 receptor agonists, and mineralocorticoid receptor antagonists to slow progression of diabetic kidney disease (DKD). However, timely guideline-based treatment of DKD at treatable stages before irreversible fibrosis is rampant is woefully inadequate. In fact, a recent study demonstrated that <10% of patients with diabetes and CKD are treated with SGLT2i, despite the plethora of data that show their renal and cardiovascular protection in this population.1 Can better ascertainment of various pathophysiologic processes in DKD—a complex syndrome with varying degrees of cellular stress, metabolic imbalance, and tubulointerstitial fibrosis—ultimately enable better identification of patients that will experience progression of DKD and allow for more efficient and effective clinical care?
Traditional clinical models for DKD progression have included clinical predictors such as age, HbA1c, systolic blood pressure, urine albumin-creatinine ratio, and eGFR (baseline and slope), obtaining modest performance.2 In 2010, the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases sponsored the CKD Biomarker consortium with the aim of identifying biomarkers in prognostication models that could better serve to risk-stratify kidney disease progression. During the first phase of the CKD Biomarker consortium (2010–2015), biomarkers that reflected kidney tubule injury were largely assessed in several large cohorts, and revealed that, although there were unadjusted associations with progression by levels of urinary injury marker, those associations were lost after adjusting for baseline covariates, such as eGFR and urine albumin-creatinine ratio.3 During phase 2 (2015–2020), the portfolio of biomarkers was expanded to include several plasma and urinary biomarkers reflective of inflammation and fibrosis, in addition to solely tubular injury. The proposals for biomarker measurements built upon, among others, the seminal work of the Joslin Kidney Study group that first demonstrated the marked strength of TNF receptors (TNFR) to prognosticate future progression of kidney disease in both type 1 and type 2 diabetes mellitus.4,5 In addition, TNFR have consistently been found to be prognostic in other cohorts,6,7 including the follow-up work by the Joslin Kidney Study Group that found that out of 194 circulating inflammatory proteins in subjects with diabetes, 17 novel proteins were highly enriched for TNFR superfamily members, which associated with a 10-year risk of ESKD.8 Similarly, other biomarkers such as plasma kidney injury molecule-1, soluble urokinase plasma activator receptor, monocyte chemoattractant protein-1, and chitinase-3 like-protein-1 have been shown to be independently associated with risk of progression of DKD.7
In this issue of JASN, Satake and colleagues9 elegantly describe an unbiased global miRNA analysis that uncovered 17 novel circulating miRNAs that were strongly associated with future ESKD in both type 1 and type 2 diabetes. Using bioinformatics approaches, the investigators found that 23 pathways were enriched by four exemplar miRNAs, of which the axon guidance pathway (AGP) was the top candidate pathway. To validate these results, the investigators used the SOMAscan aptamer-based platform to measure 42 AGP proteins. Six of the 42 aptamers (five ephrin ligands/receptors and one netrin receptor) were strongly associated with the severity of early structural kidney biopsy lesions and with the risk of ESKD during the 10-year follow-up. Importantly, the authors found a discrepancy between kidney tissue levels of genes transcripts encoding these AGP proteins and early structural DKD lesions, suggesting that circulating miRNAs (presumably from an extrarenal source) regulated the expression of AGP proteins. Interestingly, AGP proteins have been previously described to participate in rearranging the local cytoskeleton and plasma membrane in growth cones and axons in the Alzheimer’s disease literature. However, an increasing body of evidence shows that AGP proteins are also involved in gene expression control via local translation and transcription.10 Notably, ephrin receptors and ephrin ligands have several functions outside of the central nervous system, including the development and repair of vascularized tissues, kidney angiogenesis, and blood vessel maturation, which could arguably take part in the pathophysiology of DKD.
These new findings on AGP proteins expand the realm of the biomarker landscape in DKD beyond the well-established importance of inflammatory and kidney injury biomarkers. In fact, AGP may represent the fourth important pathophysiologic domain that biomarkers can ascertain in a prognostic manner for DKD. The third pathway, more closely related to inflammation and injury, is fibrosis. In this regard, the Joslin investigators recently examined circulating WAP four-disulfide core domain protein 2 and matrix metalloproteinase 7—surrogate markers for renal fibrosis—and their independent association with DKD among patients with normal eGFR, with or without albuminuria.11 In that analysis, the risk of fast early renal function decline during the next 6–12 years was greater among those within the highest quartiles of WAP four-disulfide core domain protein 2 and matrix metalloproteinase 7 concentrations. The fibrosis markers were independently associated with fast kidney function decline, as were the markers of kidney tubular injury such as plasma kidney injury molecule-1 and urinary EGF/ monocyte chemoattractant protein-1.
What are the next steps for using these AGP proteins as biomarkers in DKD? First, the associations of these AGP biomarkers need to be tested in cohorts outside of the Joslin patient population to replicate and externally validate the findings. Second, a parsimonious group of biomarkers providing potentially orthogonal signals representative of different pathophysiologic domains described above—inflammation, kidney tubular injury/health, fibrosis, and the heretofore unrecognized AGP—needs to be consolidated to forecast kidney disease progression (Figure 1). Finally, the fitness for purpose of this integrative approach needs to be tested prospectively in the real world and through clinical trials. In this manner, the final parsimonious set of markers could be used to risk stratify patients in clinical practice and assist with clinical decisions including prompt guideline-based medication initiation and referrals to specialists. Moreover, the final panel of markers could be used to enrich clinical trials of new agents for DKD to reduce sample size or the length of follow-up needed to have sufficient statistical power. In addition, the array of markers could be used to assess response to novel clinical treatments. Finally, although much work is still needed to prove this approach, multi-dimensional markers could be used to subphenotype patients with DKD and guide more precise treatment. For example, patients with high inflammatory markers could need additional anti-inflammatory treatment with mineralocorticoid receptor antagonists, whereas those with high injury markers may benefit the most from treatments such as SGLT2i or glucagon-like peptide-1 receptor agonists. We are reaching the cusp for “precision medicine” in DKD, and integration of known disease pathways with these new “nontraditional” markers, such as the novel AGP proteins, could be the final lynchpin for true prognostication and individualization of treatments in this field.
Figure 1.
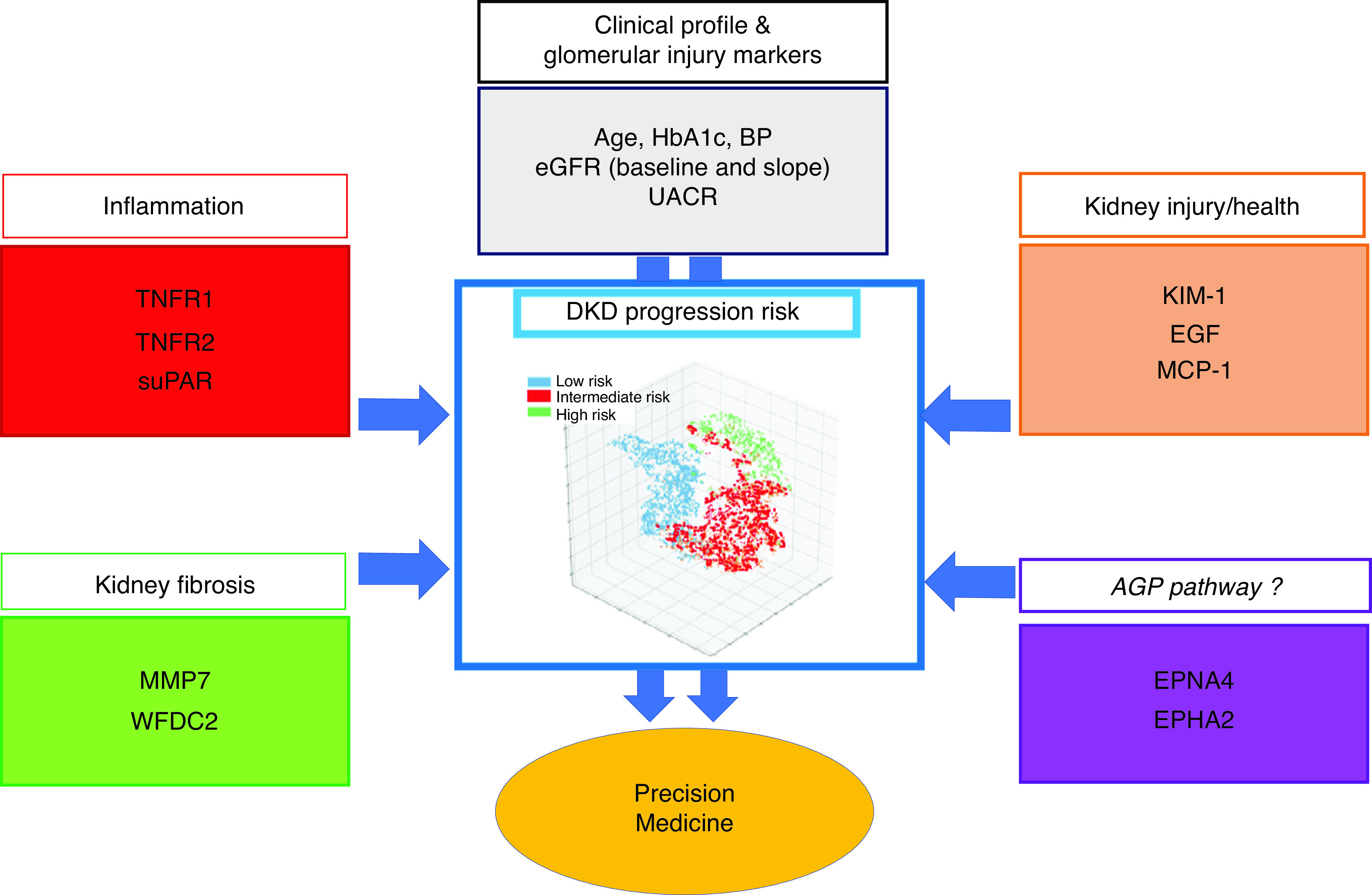
Schematic review of different dimensions of kidney disease and health on the basis of biomarker profiles in DKD progression integrated into a unified multidimensional predictive model which ascertains patients at low risk, intermediate risk, and high risk. suPAR, soluble urokinase plasma activator receptor; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; MMP-7, matrix metallopeptidase 7; WFDC2, WAP four-disulfide core domain protein; UACR, urine albumin-creatinine ratio; AGP, axon guidance proteins; EFNA4, Ephrin ligand 4; EPHA2, Ephrin receptor 2.
Disclosures
In the past 3 years, S.G. Coca reports receiving fees for advisory boards or steering committee roles for Akebia, Bayer, Boehringer-Ingelheim, CHF Solutions, ProKidney, Quark, Reprieve Cardiovascular, Renalytix, Takeda, and Vifor; reports owning equity in pulseData and Renalytix; reports having patents and inventions with Renalytix; reports receiving salary and research support from the NIH (grants U01DK106962, R01DK115562, R01HL85757, R01DK112258, U01OH011326, and R01DK126477), ProKidney, Renalytix, the Renal Research Institute, and XORTX; and reports being listed as coinventor for KidneyIntelX (patent pending). The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
G. Vasquez-Rios and S.G. Coca drafted the manuscript, made significant intellectual contributions, and reviewed the literature. All authors accepted the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related original article, “Comprehensive search for novel circulating miRNAs and Axon guidance pathway proteins associated with risk of ESKD in diabetes,” on pages 2331–2351.
References
- 1.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, et al. : Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 4: e216139, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunkler D, Gao P, Lee SF, Heinze G, Clase CM, Tobe S, et al. ; ONTARGET and ORIGIN Investigators: Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol 10: 1371–1379, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CY, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, et al. ; CRIC Study Investigators; CKD Biomarkers Consortium: Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 91: 196–203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al. : Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al. ; CKD Biomarkers Consortium and the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol 32: 115–126, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. : A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25: 805–813, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satake E, Saulnier PJ, Kobayashi H, Gupta M, Looker H, Wilson J, et al. : Comprehensive search for novel circulating miRNAs and Axon guidance pathway proteins associated with risk of ESKD in diabetes. J Am Soc Nephrol 32: 2331–2351, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell SA, Bashaw GJ: Axon guidance pathways and the control of gene expression. Dev Dyn 247: 571–580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihara K, Skupien J, Kobayashi H, Md Dom ZI, Wilson JM, O’Neil K, et al. : Profibrotic circulating proteins and risk of early progressive renal decline in patients with type 2 diabetes with and without albuminuria. Diabetes Care 43: 2760–2767, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


