Abstract
Background
The virulent and unpredictable nature of COVID-19 combined with a change in reimbursement mechanisms both forced and enabled the rapid adoption of telemedicine around the world. Thus, it is important to now assess the effects of this rapid adoption and to determine whether the barriers to such adoption are the same today as they were under prepandemic conditions.
Objective
The objective of this systematic literature review was to examine the research literature published during the COVID-19 pandemic to identify facilitators, barriers, and associated medical outcomes as a result of adopting telemedicine, and to determine if changes have occurred in the industry during this time.
Methods
The systematic review was performed in accordance with the Kruse protocol and the results are reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We analyzed 46 research articles from five continents published during the first year of the COVID-19 pandemic that were retrieved from searches in four research databases: PubMed (MEDLINE), CINAHL, Science Direct, and Web of Science.
Results
Reviewers identified 25 facilitator themes and observations, 12 barrier themes and observations, and 14 results (compared to a control group) themes and observations. Overall, 22% of the articles analyzed reported strong satisfaction or satisfaction (zero reported a decline in satisfaction), 27% reported an improvement in administrative or efficiency results (as compared with a control group), 14% reported no statistically significant difference from the control group, and 40% and 10% reported an improvement or no statistically significant difference in medical outcomes using the telemedicine modality over the control group, respectively.
Conclusions
The pandemic encouraged rapid adoption of telemedicine, which also encouraged practices to adopt the modality regardless of the challenges identified in previous research. Several barriers remain for health policymakers to address; however, health care administrators can feel confident in the modality as the evidence largely shows that it is safe, effective, and widely accepted.
Keywords: telemedicine, pandemic, technology acceptance, COVID-19, digital health, telehealth, health policy, health care
Introduction
Rationale
The virulent nature of COVID-19 forced social distancing and a decrease of in-person visits to clinics around the world. Telemedicine presented health care providers with solutions that enabled a social-distancing window into the clinical environment and a continuation of the doctor-patient relationship.
Telemedicine is defined by the World Health Organization as healing from a distance through information communications technologies by all health care professionals for the “exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation” [1]. Telemedicine is not a perfect means of patient care; however, it offers great advantages to overcome geographical barriers to improve health outcomes [1]. Validated and peer-reviewed international statistics are elusive on adoption figures, but a recent question-and-answer session indicates overall low adoption of telemedicine internationally [2]. In the United States, prior to the pandemic, telemedicine had only been adopted by 8% of providers [3]. Providers have recognized wide acceptance of telemedicine by patients; however, prior to the desperate circumstances of COVID-19, they had not been willing to adopt telemedicine on a wide scale [4]. The largest challenges to the adoption of telemedicine were identified as technically challenged staff, resistance to change, cost, reimbursement, and education level of the patient [5]. Telemedicine saves patients time, consultation fees, and travel expenses [6]. However, telemedicine requires users at both ends to possess certain levels of technological skills such as those required to enable video teleconferencing [7]. Fortunately, some countries enacted legislation to expand the adoption of telemedicine. For example, in the United States, telemedicine was not easily reimbursed by federal programs until the Coronavirus Aid, Relief, and Economic Security (CARES) Act legislation [8], which greatly increased reimbursement mechanisms for the telemedicine modality. This change in reimbursement structure should not be ignored, and it most likely provided a significant catalyst to the adoption of telemedicine.
A large number of articles were published in the first 12 months of the pandemic (February 2020 to February 2021) on the rapid implementation efforts of telemedicine to enable clinics and hospitals to continue to see patients and care for their needs [9,10]. However, providers acknowledge some of the shortfalls inherent to this modality, such as lack of technical infrastructure, cost, lack of technical staff, computer literacy of both staff and patients, and a negative impact on the patient-to-provider relationship [4,11-13]. A systematic review performed in 2020 on telemedicine and COVID-19 evaluated 44 articles along four service lines and identified 10 themes of efficiency [14]. However, the authors did not evaluate facilitators and barriers to adoption or health outcomes. Another systematic review [5] was performed in 2016 on the barriers to the adoption of telemedicine worldwide, which evaluated 30 articles across all service lines in all countries; however, it also did not evaluate facilitators or health outcomes.
Although analyses have been published that highlight the advantages to the adoption of telemedicine, with an 8% adoption rate in the United States, the conclusions of these previous studies may not be as robust as possible. The circumstances presented by the pandemic have encouraged wider adoption of this modality of care. Therefore, with proper systematic review techniques, reviewer observations this far into the pandemic will undoubtedly be more robust and widely applicable to medicine.
Objectives
The purpose of this systematic review was to evaluate the facilitators and barriers to the adoption of telemedicine worldwide, including an analysis of health outcomes and patient satisfaction. A brief comparison of the results of this review with those of reviews performed prior to COVID-19 was further performed to identify changes in these factors in light of the pandemic.
Methods
Protocol and Registration
The Kruse protocol for writing a systematic review was followed, and the findings are reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [15,16]. This systematic review was registered in PROSPERO on August 2, 2021 (ID CRD42021235933).
Eligibility Criteria
The search parameters were established to find articles published in 2020 and 2021 concerning telemedicine in all aspects of care and for all ages of patients, published in peer-reviewed journals, using any method of study (mixed method, quantitative, and qualitative). Other systematic reviews were excluded because we wanted to compare our results to these previous reviews without confounding the findings. The Johns Hopkins Nursing Evidence-Based Practice Rating Scale (JHNEBP) was used to assess the quality of all articles analyzed [17]. Any studies below level IV C were discarded due to poor quality.
Information Sources
Four research databases were searched: PubMed (MEDLINE), CINAHL (excluding MEDLINE), Web of Science, and Science Direct. We also performed a journal-specific search of the Journal of Medical Internet Research.
Search Strategy
Google Scholar was used to determine the general trends of publication on this topic previously and to collect key terms from published articles. These key terms were entered into the US Library of Medicine’s Medical Subject Headings (MeSH) to create an exhaustive search string using Boolean terms. The actual search string used was: (telemedicine OR telehealth OR “mobile health” OR mhealth OR ehealth) AND (COVID-19 OR coronavirus). The same search string was used in all databases. Similar filters were used in each database (not all filters are the same between databases).
Study Selection Process
Once the search string was entered into each database, we filtered the results and screened abstracts for applicability. Although filters for the four research databases differ, we generally filtered for the date range (2020-2021), scholarly journals (no theses or opinions), and “full text” to ensure that we would have access to the entire article. Articles were rejected for a variety of reasons: protocol (no results to analyze); opinion (no data); reviews; did not use telemedicine; or did not contribute to our objective statement of identifying facilitators, barriers, or effects on patient satisfaction. The κ statistic was calculated to identify the level of agreement between reviewers [18].
Data Collection Process
An Excel spreadsheet was used as a data-extraction tool to collect data for reporting and analysis. This spreadsheet was standardized according to the Kruse protocol [15]. We held three consensus meetings to screen abstracts, analyze articles, and discuss possible themes. After the second consensus meeting, we performed a narrative analysis to identify themes in the articles analyzed [19]. Because there were only two authors on this project, both authors analyzed all articles (n=46).
Data Items
In accordance with the Kruse protocol, PRISMA standard, and JHNEBP, the following fields were collected: database source; date of publication; journal; authors; study title; PICOS (participants, intervention, results, outcomes, study design); sample size; bias within study; effect size; country of origin; statistics used; quality metrics from the JHNEBP scale; and reviewer observations as they relate specifically to the objective statement in areas of patient satisfaction, and facilitators and barriers to adoption [15,17,20]. All data items were independently collected and discussed in subsequent consensus meetings.
Risk of Bias Within and Across Studies
The JHNEBP rating scale was used for assessment of bias within and across studies. Observations of bias and methodological weaknesses were noted [17]. The JHNEBP ratings also provided insight into bias because poor-quality results can limit the external validity of the experiment.
Summary Measures
Because we included mixed methods and qualitative studies, we were unable to standardize summary measures as would be performed in a meta-analysis.
Additional Analyses
We performed a narrative, or thematic, analysis of the observations to convert them into themes (common threads between articles) [19]. We calculated the frequency of occurrence of both themes and individual observations and report these in a series of affinity matrices (tables). This technique was used to identify the statistical probability for identifying each theme, which does not identify a level of importance but rather identifies a frequency of mention of these themes in the literature during the period of observation.
Results
Study Selection
The database search and study selection process are illustrated in Figure 1. The κ statistic was 0.95, indicating almost perfect agreement between reviewers [18,21]. Several studies made it through all filters, but were still eliminated because they were protocols (no results), opinions, out of the date range, or other systematic reviews.
Figure 1.
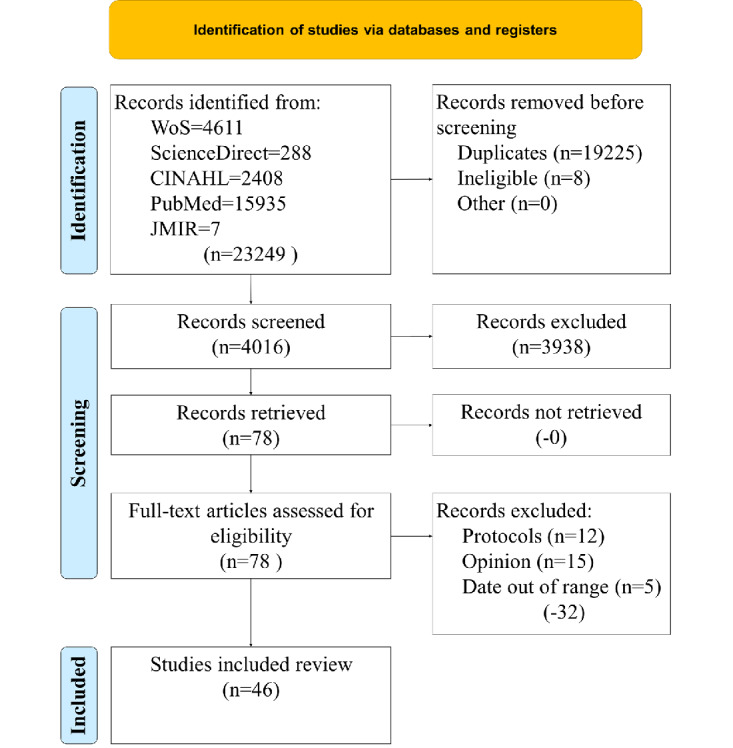
Article search and selection process. WoS: Web of Science.
Study Characteristics
Reviewers collected study characteristics identified by the PRISMA standard such as PICOS (see Table 1). Of the 46 six studies analyzed over the 15-month period, 2 (4%) involved adolescents, 6 (13%) involved adults >60 years, and 38 (83%) involved adults >18 years as participants. Most participants were current or former patients who agreed to participate in studies. More than half the interventions were mobile health (mHealth), telephone/televideo, or eHealth (26/46, 56%). The rest were interventions involving telemonitoring, patient portals, telecoaching, web chat, and social media, which could be cross-platform. In these 46 studies, 18 resulted in a positive outcome over a control group (23%), 12 of which involved medically measured outcomes (21%) as opposed to clinical and administrative outcomes. Only 9 of the 46 (20%) studies resulted in no statistically significant difference between the intervention and control groups, which means that positive results could be obtained through telemedicine commensurate with those obtained using traditional means of care. Four articles analyzed were published in 2021 [22-25], with the remaining 42 articles published in 2020 [26-67]. Further explanation of the results and medical outcomes can be found the Additional Analysis subsection.
Table 1.
Characteristics of the included studies according to the PICOS (Participants, Intervention, Results, Outcomes, and Study Design) structure.
| Study | Participant | Intervention | Results (compared to the control group or other studies) | Medical outcomes | Design |
| Ben-Arye et al [22] | Adult patients (>18 years) undergoing adjuvant, neoadjuvant, or palliative treatment for solid tumors | eHealth | Improved compliance/adherence | Not reported | Prospective, controlled, and nonrandomized study |
| Yu et al [25] | Older adult patients (50% >60 years, 60% women, 68% one-time telehealth users) and 45 physicians | Telephone or televideo | Improved patient satisfaction | Not reported | Cross-sectional |
| Richards et al [24] | Adult respondents from a neurosurgical outpatient clinic (mean age 63 years, 50.3% men) | Telephone or televideo | Improved patient satisfaction | Not reported | Qualitative |
| Kurihara et al [23] | Adult patients with Parkinson disease (61% women, mean age 67 years) at Fukuoka University Hospital | Telemedicine self-testing | No control group (nonexperimental) | Not reported | Cross-sectional |
| Alkire et al [26] | Adults (Gen X, Millennial) | Patient portals | No control group (nonexperimental) | Not reported | Nonexperimental |
| Ballin et al [27] | Older adults, 70-year-old men, and women with central obesity | Supervised and web-based | No significant difference; decreased fat mass | Improved in at least one area: decreased fat mass | Randomized controlled trial |
| Banbury et al [28] | Adults >50 years with at least one chronic condition | Telemonitoring | Telemedicine improved results compared to control: companionship, emotional support, health literacy, self-management | Not reported | Mixed methods, quasiexperimental, nonrandomized trial |
| Barnett et al [29] | Adults (22-27 years; 10 men, 10 women), clients of an alcohol and drug counseling service across Australia, and 8 counselors | Webchat | No control group (nonexperimental) | Not reported | Qualitative study, nonexperimental |
| Batalik et al [30] | Adult cardiac rehabilitation patients | Home-based telerehab | No statistically significant difference | No statistically significant difference | Randomized controlled trial |
| Beller et al [31] | Adult patients scheduled for video visits through the University of Virginal urology departments | Televideo | No control group (nonexperimental) | Not reported | Cohort |
| Bernabe-Ortiz et al [32] | Adult participants from a randomized clinical trial on a 1-year mHealtha intervention on blood pressure and body weight 4 years postcompletion | mHealth | Telemedicine improved results compared to control: decreased fat mass | Improved in at least one area; decreased body weight | Retrospective study of a randomized clinical trial |
| Bilgrami et al [33] | Adults with inflammatory bowel disease | Telemedicine self-testing | No statistically significant difference | No statistically significant difference | Randomized controlled trial |
| Broers et al [34] | Adult patients with cardiovascular disease | eHealth | No statistically significant difference; increased quality of life | Not reported | Randomized controlled trial |
| Cho et al [35] | Adult participants (30-59 years) with at least 2 conditions defined by the Third Report of the National Cholesterol Education Program expert panel (abdominal obesity, high blood pressure, high triglycerides, low high-density lipoprotein cholesterol, and high fasting glucose level) | mHealth | Telemedicine improved results compared to control (decreased fat mass) | Improved in at least one area: decreased fat mass, decreased body weight | Randomized controlled trial |
| Claes et al [36] | Adult patients with cardiovascular disease from 3 European hospitals | eHealth | Improved health behaviors | Not reported | Randomized controlled trial |
| Coorey et al [37] | Adults who had completed 12 months of follow-up from the Consumer Navigation of Electronic Cardiovascular Tools trial | eHealth | No control group (nonexperimental): improved self-management, improved health literacy | Not reported | Qualitative analysis of a randomized controlled study |
| Ding et al [38] | Adults (mean age 70.1 years) with chronic heart failure | Telemonitoring | Telemedicine improved results compared to controls: improved compliance/adherence | Not reported | Randomized controlled trial |
| Geramita et al [39] | Adult lung transplant recipients | mHealth | No statistically significant difference | Not reported | Randomized controlled follow-up study |
| Gong et al [40] | Adult hypertension | mHealth | Telemedicine improved results compared to controls: improved compliance/adherence | Improved in at least one area: reductions in blood pressure | Randomized controlled trial |
| Han et al [41] | Adults (<55 years) prepandemic (S1) and 273 follow-up surveys (S2); university-affiliated, and physicians | eHealth | No control group (nonexperimental): telemedicine improved results compared to controls, improved compliance/adherence | Not reported | Qualitative |
| Harding et al [42] | Adult caregivers with 837 patient assessment outcomes | mHealth | No control group (nonexperimental) | Not reported | Qualitative (pilot study) |
| Hsia et al [43] | Pediatric patients with asthma | mHealth | Telemedicine improved results compared to controls: improved self-management, improved patient satisfaction | Improved self-management, decreased medication use, increase in controlled asthma | Prospective study |
| Hsieh et al [44] | Insured adults (>20 years) | Patient portals | No control group (nonexperimental) | Not reported | Qualitative |
| Hutchesson et al [45] | Adult Australian women with a recent history of preeclampsia | mHealth | No statistically significant difference | No statistically significant difference | Pilot randomized controlled trial |
| Jiménez-Marrero et al [46] | Adult patients with chronic heart failure | Televideo | Telemedicine improved results compared to controls, decreased cost | Improved in at least one area: decreased incidence of heart failure | Randomized controlled trial |
| Katt et al [47] | 180 patients with upper-extremity condition and 302 physicians | Telephone or televideo | Improved patient satisfaction | Not reported | Qualitative |
| Kobe et al [48] | Adult patients (52% men, mean age 62 years, 55.5% African American) of Duke University Health System with type 2 diabetes, poorly controlled hypertension, and on prescription hypertension and diabetes medication | Telephone or televideo | Telemedicine improved results compared to control | Improved in at least one area, improved annual rate eGFRb decline | Secondary analysis of randomized controlled trial |
| Lai et al [49] | Adults with Parkinson disease (telehealth mean age 63 years, control mean age 70 years; 70% men, predominantly White) | Telemonitoring | Telemedicine improved results compared to control: improved compliance/adherence, health behaviors, and patient satisfaction | Not reported | Mixed methods |
| Lemelin et al [50] | Adult women (mean age 32 years) with gestational diabetes mellitus | Telecoaching | Improved patient satisfaction: telemedicine improved results compared to control | Identified other areas for intervention | Prospective and controlled clinical trial |
| Manning et al [51] | Adults from families with toddlers | Televideo | No statistically significant difference | Not reported | Mixed method quasiexperimental and longitudinal design |
| Marques et al [52] | Adult Valladolid University students (74% women, 67.5% aged 18-23 years) | mHealth | No control group (nonexperimental) | Not reported | Qualitative |
| Martins et al [53] | Adult patients (mean age 62 years, 50% women) with suspected acute strokes at a Brazil university hospital | mHealth | Telemedicine improved results compared to control | Improved in at least one area: decreased mortality, decreased intracranial hemorrhage | Prospective observational |
| McGillicuddy et al [54] | Adults (mean 51.5-52.1 years) with kidney transplants (majority men, African American) | mHealth | Telemedicine improved results compared to control | Improved in at least one area: reduction in mean tacrolimus trough coefficient of variation | Randomized controlled clinical trial |
| Mo et al [55] | Adult patients (51.7-53.5 years) with chronic heart failure (approximately 66% men) | Telephone or televideo | Telemedicine improved results compared to control: improved emotional support | Improved in at least one area: mental health inventory, quality of life | Open-label interventional study |
| Mustonen et al [56] | Adult patients (>45 years; mean age 65 years) with type 2 diabetes and coronary artery disease (approximately 40% women) | Telecoaching | No statistically significant difference | Not reported | Posttrial analysis of a randomized controlled trial |
| O’Shea et al [57] | Adults (77% men, mean age 61 years) | eHealth | Not reported | Not reported | Posttrial analysis of an acceptability and feasibility trial |
| Perri et al [58] | Adults (mean 55.4 years) from 14 counties in Florida (83% women, 73.9% White) | Telephone or televideo | Telemedicine improved results compared to control: decreased fat mass, improved self-management | Improved in at least one area: decreased body weight | Randomized clinical trial |
| Piera-Jiménez et al [59] | Adults (majority 50-70 years and men) from Spain, the Netherlands, and Taiwan | Telemonitoring | Telemedicine improved results compared to control | Improved in at least one area, improved quality of life | Financial randomized controlled trial |
| Press et al [60] | Adults (mean 54.5 years) with asthma or chronic obstructive pulmonary disease (majority Black women) | mHealth | Telemedicine improved results compared to control: improved self-management health behaviors | Increase in controlled asthma | Randomized controlled trial |
| Ramirez-Correa et al [61] | Adults (mean 39.9 years, 56% men) | Telemedicine self-testing | No control group (nonexperimental) | Not reported | Cross-sectional |
| Ronan et al [62] | Adults with cystic fibrosis involved in a study on an online Tai Chi intervention | Televideo | No statistically significant difference, improved health behaviors | Not reported | Qualitative analysis of a mixed methods randomized controlled feasibility study |
| Sacco et al [63] | Older adults (mean age 88.2 years), 59.8% women | Telephone or video | Improved patient satisfaction, improved emotion support | Not reported | Cross-sectional survey |
| Scheerman et al [64] | Adolescents (12-17 years) and mothers | Social media | Telemedicine improved results compared to control, improved health behaviors | Not reported | Cluster randomized controlled trial |
| Schrauben et al [65] | Adult Chronic Renal Insufficiency Cohort (CRIC) Study participants (mean age 68 years, eGFR 54 mL/min/1.73, 59% men) | mHealth | No control group (nonexperimental) | Not reported | Cross-sectional survey |
| Shareef et al [66] | Elderly and disabled people (average age 74.5 years, 59% women) in retirement homes and rehabilitation centers | Robotics or artificial intelligence | Improved companionship | Not reported | Experiment and follow-up survey |
| van Dijk et al [67] | Adult women (mean age 30 years), either less than 13 weeks pregnant or trying to become pregnant, and 36 men | mHealth | Improved compliance/adherence, improved health behaviors | Improved in at least one area, improved self-management | Randomized controlled trial |
amHealth: mobile health.
beGFR: estimated glomerular filtration rate.
Risk of Bias Within and Across Studies
Table 2 summarizes the quality indicators assessed for each article with the JHNEBP tool. The strength of evidence most frequently observed was level III followed by level I and level II. Nearly half of the articles reported strong-evidence studies that included both a control group and randomization; the next most common study type was nonexperimental (no control group) or qualitative, with the least frequent type being quasiexperimental (included a control group but no randomization). The quality of evidence most frequently observed was A (high quality), followed by B (good quality). The most common combination of strength and quality was III B, followed closely by I A, which speaks to both the strength and quality of evidence evaluated by this review. The III B combination highlights the number of qualitative studies with smaller samples or selection bias.
Table 2.
Summary of quality assessments (N=46).
| Evidence | Occurrence, n (%) | |
| Strength | ||
|
|
I (Experimental study or randomized controlled trial) | 22 (48) |
|
|
III (Nonexperimental, qualitative) | 17 (37) |
|
|
II (quasiexperimental) | 7 (15) |
| Quality | ||
|
|
A (High quality) | 27 (59) |
|
|
B (Good quality) | 17 (37) |
|
|
C (Low quality) | 2 (4) |
Many studies used geographically localized samples, which may limit the external validity of the results. Some studies focused only on one gender or race, speaking to the convenience sample or volunteer-basis of their design. Asking for volunteers in a technology-oriented experiment invites bias because the self-selection allows for those who are already technology-oriented or comfortable with technology to participate. This group as the intervention can skew the results because those already comfortable with technology will not experience the frustration experienced by those who are not comfortable with technology. This selection bias also limits the external validity of the results. A comprehensive list of bias, country of origin, sample size, strength, and quality of evidence identified for each study can be found in Multimedia Appendix 1.
Thematic Analysis Based on Results of Individual Studies
During the analysis phase of the systematic review process, the reviewers recorded observations to identify instances of patient satisfaction, as well as both facilitators and barriers to the adoption of telemedicine. A thematic analysis was then performed to make sense of the observations [19]. Multiple instances of the same observation become a theme. A translation of observations to themes is provided in Multimedia Appendix 2. The summary of analysis is provided in Table 3, which lists the themes/observations from reviewers that correspond with the objective statement and sorts articles from the most recent to the oldest.
Table 3.
Summary of thematic analysis for individual studies.
| Authors | Patient satisfaction | Facilitators | Barriers |
| Ben-Arye et al [22] | Not reported | Technical literacy, availability of technology, past experience with technology | Availability of technology, confidentiality/security |
| Yu et al [25] | Strong satisfaction | Concerns adequately addressed, improved health behaviors, pandemic created acceptance of technology | Some patients prefer in-person consultations, decrease in patient-provider communication, technical literacy |
| Richards et al [24] | Strong satisfaction | Convenience of telemedicine, increased patient-provider communication, concerns adequately addressed, increased access | Not reported |
| Kurihara et al [23] | Not reported | Pandemic created acceptance of technology, past experience with technology | Some patients prefer in-person consultations, technical literacy |
| Alkirie et al [26] | Not reported | Technical literacy, past experience with technology, perceived usefulness, increased patient-provider communication, perceived ease of use | Technology needs further development, technical literacy |
| Ballin et al [27] | Not reported | Increased connectedness, self-management, flexibility, and access | Technology needs further development |
| Banbury et al [28] | Not reported | Enabled social interaction; decreased anxiety; increased connectedness, technical literacy, and access; televideo enables reading of body language; education; convenience of telemedicine | Health literacy, availability of technology, technical literacy |
| Barnett et al [29] | Not reported | Increased efficiency, access, and patient-provider communication, and improved standard of care | Technology needs further development, decrease in patient-provider communication, technical literacy, confidentiality/security |
| Batalik et al [30] | Not reported | Technical literacy, increased self-management, increased access, increased flexibility | Discomfort for wearable monitors, technical literacy, technology needs further development |
| Beller et al [31] | Not reported | Pandemic created acceptance of technology, availability of technology, fewer miles driven to appointment, convenience of telemedicine, faster initiation of treatment, decreased costs | Limits of reimbursement for telemedicine, some patients prefer in-person consultations, connectivity, technical literacy |
| Bernabe-Ortiz et al [32] | Not reported | Increased connectedness, increased adherence, improved health behaviors | Perceived lack of usefulness, lack of personal desire to get better, some patients prefer in-person consultations |
| Bilgrami et al [33] | Not reported | Pandemic created acceptance of technology | Not reported |
| Broers et al [34] | Strong satisfaction | Perceived usefulness, perceived ease of use, increased adherence | Decrease in quality of life after intervention |
| Cho et al [35] | Not reported | Increased adherence, increased self-management, increased weight loss, technical literacy | Technical literacy, availability of technology |
| Claes et al [36] | Not reported | Technical literacy, perceived ease of use | Technology needs further development |
| Coorey et al [37] | Not reported | Increased adherence, increased self-management | Lack of personal desire to get better, technology needs further development, technical literacy |
| Ding et al [38] | Not reported | Increased adherence, increased self-management | Technology needs further development, cost |
| Geramita et al [39] | Not reported | Long-term use may not be required to develop good habits | Cost, confidentiality/security, technology needs further development |
| Gong et al [40] | Not reported | Increased adherence, increased self-management | Not reported |
| Han et al [41] | Not reported | Pandemic created acceptance of technology, increased efficiency, increased self-management, increased access, availability of technology | Cost, technical literacy, interoperability, availability of technology |
| Harding et al [42] | Not reported | Not reported | Connectivity, confidentiality/security, technical literacy |
| Hsia et al [43] | Strong satisfaction | Increased quality of life, decreased emergency room visits, increased adherence, availability of technology, pandemic created acceptance of technology, perceived ease of use, convenience of telemedicine | Connectivity, technical literacy, cost, availability of technology |
| Hsieh et al [44] | Not reported | Health literacy, perceived usefulness, perceived ease of use | Some patients prefer in-person consultations, technical literacy, cost |
| Hutchesson et al [45] | Strong satisfaction | Increased self-management, perceived usefulness, perceived ease of use | Technology needs further development, perceived lack of usefulness |
| Jiménez-Marrero et al [46] | Not reported | Decreased costs, increased adherence, increased self-management | Cost |
| Katt et al [47] | Strong satisfaction | Convenience of telemedicine, pandemic created acceptance of technology, faster initiation of treatment, perceived ease of use | Some patients prefer in-person consultations, workflow issues for providers |
| Kobe et al [48] | Not reported | Not reported | Some patients prefer in-person consultations |
| Lai et al [49] | Strong satisfaction | Convenience of telemedicine, increased social support, increased self-management | Technology needs further development, connectivity, decrease in patient-provider communication, technical literacy |
| Lemelin et al [50] | Strong satisfaction | Education, increased social support | Not reported |
| Manning et al [51] | Not reported | Pandemic created acceptance of technology | Connectivity, availability of technology |
| Marquez et al [52] | Not reported | Past experience with technology, decreased costs, pandemic created acceptance of technology, faster initiation of treatment, increased access | Some patients prefer in-person consultations |
| Martins et al [53] | Not reported | Faster initiation of treatment, availability of technology, increased access | Lack of infrastructure, limits of reimbursement for telemedicine, connectivity, confidentiality/security |
| McGillicuddy et al [54] | Not reported | Increased social support, health literacy | Not reported |
| Mo et al [55] | Not reported | Increased quality of life, increased social support | Not reported |
| Mustonen et al [56] | Not reported | Decreased costs | Not reported |
| O’Shea et al [57] | Satisfaction | Increased self-management | Technical literacy, perceived lack of usefulness, technology needs further development |
| Perri et al [58] | Not reported | Increased weight loss, increased adherence, increased self-management | Not reported |
| Piera-Jiménez et al [59] | Not reported | Decreased costs, no significant difference in cost care | Cost |
| Press et al [60] | Not reported | Decreased costs, education, increased access | Availability of technology, technical literacy |
| Ramirez-Correa et al [61] | Not reported | Increased patient-provider communication, education, pandemic created acceptance of technology | Connectivity |
| Ronan et al [62] | Not reported | Convenience of telemedicine, pandemic created acceptance of technology, increased social support | Technical literacy, technology needs further development, availability of technology |
| Sacco et al [63] | Strong satisfaction | Increased social support, increased connectedness | Not reported |
| Scheerman et al [64] | Not reported | Increased social support, improved standard of care | Not reported |
| Schrauben et al [65] | Not reported | Health literacy, education | Technical literacy, health literacy, confidentiality/security |
| Shareef et al [66] | Not reported | Enabled social interaction, increased social support | Confidentiality/security, technical literacy, perceived lack of usefulness |
| van Dijk et al [67] | Not reported | Improved health behaviors, increased adherence | Not reported |
Patient satisfaction was reported as “strong satisfaction” or “satisfaction” in 9 (20%) and 1 (2%) of the 46 studies, respectively, and 36 studies did not report any measure of patient satisfaction. No studies reported a decline in patient satisfaction as a result of using telemedicine as the intervention.
Twenty-five facilitator themes and seven individual observations were identified in the literature by the two reviewers. Only two studies did not identify facilitators. Facilitator themes are listed in Table 4.
Table 4.
Facilitator themes and individual observations (N=132).
| Themes/observations | References | Occurrence, n (%) |
| Increased self-management | [27,30,35,37,38,40,41,45,46,49,57,58] | 12 (9.1) |
| Pandemic created acceptance of technology | [23,25,31,33,41,43,47,51,52,61,62] | 11 (8.3) |
| Increased adherence | [32,34,35,37,38,40,43,46,58,67] | 10 (7.6) |
| Increased access | [24,27-30,41,52,53,60] | 9 (6.8) |
| Increased social support | [49,50,54,55,62-64,66] | 8 (6.1) |
| Convenience of telemedicine | [24,28,31,43,47,49,62] | 7 (5.3) |
| Perceived ease of use | [26,34,36,43-45,47] | 7 (5.3) |
| Decreased costs | [31,46,52,56,59,60] | 6 (4.5) |
| Education | [28,50,60,61,65] | 5 (3.8) |
| Technical literacy | [22,26,30,35,36] | 5 (3.8) |
| Availability of technology | [22,31,41,43,53] | 5 (3.8) |
| Increased patient-provider communication | [24,26,29,61] | 4 (3.0) |
| Faster initiation of treatment | [31,47,52,53] | 4 (3.0) |
| Increased connectedness | [27,28,32,63] | 4 (3.0) |
| Perceived usefulness | [26,34,44,45] | 4 (3.0) |
| Past experience with technology | [22,23,26,52] | 4 (3.0) |
| Health literacy | [44,54,65] | 3 (2.3) |
| Improved health behaviors | [25,32,67] | 3 (2.3) |
| Increased efficiency | [29,41] | 2 (1.5) |
| Concerns adequately addressed | [24,25] | 2 (1.5) |
| Enabled social interaction | [28,66] | 2 (1.5) |
| Increased quality of life | [43,55] | 2 (1.5) |
| Improved standard of care | [29,64] | 2 (1.5) |
| Increased flexibility | [27,30] | 2 (1.5) |
| Increased weight loss | [35,58] | 2 (1.5) |
| Decreased anxiety | [28] | 1 (0.8) |
| Increased technical literacy | [28] | 1 (0.8) |
| Televideo enables reading of body language | [28] | 1 (0.8) |
| Fewer miles driven to appointment | [31] | 1 (0.8) |
| Long-term use may not be required to develop good habits | [39] | 1 (0.8) |
| Decreased emergency room visits | [43] | 1 (0.8) |
| No significant difference in cost of care | [59] | 1 (0.8) |
| Not reported | [42,48] | 2 (N/Aa) |
aN/A: not applicable.
The most commonly identified themes were increased self-management, acceptance of the technology from the pandemic, adherence to treatment protocols, access, and social support. For the 46 articles, these themes represent 38% of all 132 occurrences. Other themes included convenience of telemedicine and perceived ease of use, decreased cost, opportunity for education, technical literacy, availability of technology, an increase in patient-provider communication, faster initiation of treatment, increased connectedness, perceived usefulness, and past experience with technology. Health literacy and improved health behaviors were identified less frequently, and increased office efficiencies, medical concerns adequately addressed, enabled social interaction, increased quality of life, improved standard of care, increased flexibility, and increased weight loss were the least frequent themes identified. The following seven individual observations accounted for 5% of the total observations: decreased anxiety, increased technical literacy, televideo enabled reading of body language, fewer miles driven to appointment, long-term use may not be required to develop good habits, decreased emergency room visits, and no significant difference in cost of care.
Twelve themes and five individual observations were identified as barriers from the literature by the reviewers; 11 studies did not identify barriers (11%). Table 5 lists the themes and individual observations.
Table 5.
Barrier themes and individual observations (N=86).
| Themes/observations | References | Occurrence, n (%) |
| Technical literacy | [23,25,26,28-31,35,37,41-44,49,57,60,62,65,66] | 19 (22) |
| Technology needs further development | [26,27,29,30,36-39,45,49,57,62] | 12 (14) |
| Availability of technology | [22,28,35,41,43,51,60,62] | 8 (9) |
| Cost | [38,39,41,43,44,46,59] | 7 (8) |
| Connectivity | [31,42,43,49,51,53,61] | 7 (8) |
| Confidentiality/security | [22,29,39,42,53,65,66] | 7 (8) |
| Some patients prefer in-person consultations | [23,25,31,32,44,47,48,52] | 8 (9) |
| Perceived lack of usefulness | [32,45,57,66] | 4 (5) |
| Decrease in patient-provider communication | [25,29,49] | 3 (3) |
| Health literacy | [28,65] | 2 (2) |
| Limits of reimbursement for telemedicine | [31,53] | 2 (2) |
| Lack of personal desire to get better | [32,37] | 2 (2) |
| Decrease in quality of life after intervention | [34] | 1 (1) |
| Discomfort for wearable monitors | [30] | 1 (1) |
| Workflow issues for providers | [47] | 1 (1) |
| Lack of infrastructure | [53] | 1 (1) |
| Interoperability | [41] | 1 (1) |
| Not reported | [24,33,40,50,54-56,58,63,64,67] | 11 (N/Aa) |
aN/A: not applicable.
The most commonly listed barriers were technical literacy, technology needs further development, availability of technology, and patient preference, accounting for 55% of the total 86 occurrences. Cost, connectivity, and confidentiality/security were also identified, as well as health literacy, limits of reimbursement for telemedicine, and lack of personal desire to get better with less frequent occurrences (2 each). The remaining five observations made up a total of 6% of the total occurrences: decrease in quality of life after intervention, discomfort for wearing monitors, workflow issues for providers, lack of data infrastructure, and interoperability.
Additional Analyses
Distribution of Publications by Country
Eighteen of the 46 studies (39%) were performed in North America, 11 (24%) were performed in Europe, 7 (15%) were performed in Asia, 5 (11%) were performed in Australia, 3 (7%) were performed in South America, and 2 (4%) were performed in multiple countries and continents.
Comparisons to a Control Group
Table 6 summarizes the themes and observations recorded for results as compared to the control group identified by the two reviewers. There is some overlap between this set of observations and medical outcomes; the latter represent clinical observations only, whereas the former are both clinical and administrative in nature. Ten themes and four individual observations were identified by the reviewers for a total of 66 occurrences in the literature. Eleven studies were nonexperimental in nature, which had no control group.
Table 6.
Themes and individual observations for studies with a control group comparison (N=66).
| Themes/observations | References | Occurrence, n (%) |
| Telemedicine improved results compared to control | [28,32,35,38,40,41,43,46,48-50,53-55,58-60,64] | 18 (27) |
| No statistically significant difference | [27,30,33,34,39,45,51,56,62] | 9 (14) |
| Improved patient satisfaction | [24,25,43,47,49,50,63] | 7 (11) |
| Improved health behaviors | [36,49,60,62,64,67] | 6 (9) |
| Improved compliance/adherence | [22,38,40,41,49,67] | 6 (9) |
| Improved self-management | [28,37,43,58,60] | 5 (8) |
| Decreased fat mass | [27,32,35,58] | 4 (6) |
| Improved emotional support | [28,55,63] | 3 (5) |
| Improved companionship | [28,66] | 2 (3) |
| Improved health literacy | [28,37] | 2 (3) |
| Improved informational support | [28] | 1 (2) |
| Decreased cost | [46] | 1 (2) |
| Increased quality of life | [34] | 1 (2) |
| Not reported | [57] | 1 (2) |
| No control group (nonexperimental) | [23,26,29,31,37,41,42,44,52,61,65] | 11 (N/Aa) |
aN/A: not applicable.
Eighteen of the studies demonstrated either a clinical or administrative improvement compared to the control group, whereas nine reported no statistically significant results from the control group. Both of these themes demonstrate the efficacy of the telemedicine modality. The remainder of the list in Table 6 demonstrates the specific improvements that occurred (multiple improvements occurred in multiple articles), including improved patient satisfaction, improved behaviors, improved compliance/adherence to treatment protocol, improved self-management of condition or disease, decreased fat mass, improved emotional support, improved companionship, and improved health literacy. The remainder were individual observations that combined accounted for 5% of the total observations: improved informational support, decreased cost, and increased quality of life. Only one article did not report a result as compared to the control group because it was a posttrial analysis and it did not address the control group.
Medical Outcomes Commensurate With an Intervention
Table 7 summarizes the medical outcomes observed. Seven themes and nine individual observations were recorded commensurate with the adoption of telemedicine for a total of 30 occurrences. Twenty-eight studies did not report clinical outcomes.
Table 7.
Medical outcome themes and individual observations commensurate with adoption of the intervention/technology (N=30).
| Themes/observations | References | Occurrence, n (%) |
| Improved in at least one area | [27,32,35,40,46,48,53-55,58,59,67] | 12 (40) |
| No statistically significant difference | [30,33,45] | 3 (10) |
| Decreased body weight | [32,35,58] | 3 (10) |
| Decreased fat mass | [27,35] | 2 (7) |
| Improved self-management | [43,67] | 2 (7) |
| Increase in controlled asthma | [43,60] | 2 (7) |
| Improved quality of life | [55,59] | 2 (7) |
| Reductions in blood pressure | [40] | 1 (3) |
| Reduction in mean tacrolimus trough coefficient of variation | [54] | 1 (3) |
| Improved annual rate of eGFRa decline | [48] | 1 (3) |
| Decreased medication use | [43] | 1 (3) |
| Decreased incidence of heart failure | [46] | 1 (3) |
| Identified other areas for intervention | [50] | 1 (3) |
| Decreased mortality | [53] | 1 (3) |
| Improved mental health inventory | [55] | 1 (3) |
| Decreased intracranial hemorrhage | [53] | 1 (3) |
| Not reported | [22-26,28,29,31,34,36-39,41,42,44,47,49,51,52,56,57,61-66] | 28 (N/Ab) |
aeGFR: estimated glomerular filtration rate.
bN/A: not applicable.
Twelve studies reported 12 statistically significant improvements in clinical outcomes and three reported no statistically significant difference between modalities of care. Both of these themes demonstrated the efficacy of the telemedicine modality. The most commonly observed theme for medical outcomes was decreased body weight, followed by decreased fat mass, improved self-management, increase in controlled asthma, and increased quality of life. The following individual observations contributed to 30% of the total observations: reduction in blood pressure, reduction in mean tacrolimus trough coefficient of variation, improved annual rate of estimated glomerular filtration rate (eGFR) decline, decrease in medication use, decrease incidence of heart failure, decreased mortality, improved mental health inventory, decreased intracranial hemorrhage, and telemedicine identified other areas for intervention.
Interactions Between Observations
Interventions of mHealth resulted in seven occurrences of a result (clinical and administrative outcomes) and six occurrences of an improvement in at least one clinical outcome. The interventions with telephone or televideo resulted in four instances of improved patient satisfaction and a decrease in eGFR and weight loss. The interventions of eHealth resulted in very few instances of either clinical or administrative outcomes other than improved compliance and health behaviors.
Discussion
Principal Findings
Telemedicine is examined in countries worldwide, and it is clear that the COVID-19 pandemic caused a rapid adoption of this modality of medicine to ensure the viability of practices. A key issue for discussion is the differences in findings between this systematic review and another recent similar review [14]. This systematic review identified key facilitators and barriers, and further analyzed health outcomes. The other similar review identified themes of effectiveness but failed to meet the expectations for a systematic review in terms of medical outcomes [68]. Common themes between the two reviews were: rapid telemedicine expansion, education, improved access, convenience, and patient satisfaction.
Summary of Evidence
This systematic review exercised a set Boolean search string in four common research databases to analyze 46 articles originating from five continents for themes of facilitators, barriers, and medical outcomes. Nearly 50% of the articles demonstrated the strongest evidence and nearly 60% demonstrated the highest quality of evidence. Various forms of telemedicine were examined: eHealth, mHealth, audio only, telemonitoring, telecoaching, telerehab, robotics or artificial intelligence, and televideo. Twenty-five facilitator themes and individual observations, 12 results themes and observations, and 7 medical outcome themes and observations were recorded and analyzed. Forty-one percent of barrier themes recorded either an improvement or no statistically significant improvement in results compared to the control group. Forty percent of the observations recorded an improvement in at least one medical outcome.
Health care administrators can focus on the findings demonstrating that implementation of telemedicine will increase self-management [27,30,35,37,38,40,41,45,46,49,57,58], adherence [32,34,35,37,38,40,43,46,58,67], access [24,27-30, 41,52,53,60], and social support [49,50,54,55,62-64,66]. Telemedicine is shown to be an effective modality of treatment [28,32,35,38,40,41,43,46,48-50,53-55,58-60,64] at a decreased cost [31,46,52,56,59,60]. Patients perceive the modality to be convenient and easy to use [26,34,36,43-45,47], and its implementation increases patient satisfaction [24,25,43, 47,49,50,63].
Health policymakers should focus on several barriers to increase the adoption of telemedicine. Because technical literacy, availability of technology, and connectivity are listed as the most often cited barriers, public programs should be offered to assist those with these difficulties. Technical literacy is often associated along age or socioeconomic lines, and researchers acknowledge the dearth of research in the area of how to overcome this obstacle [69]. However, community centers that provide access to computers, classes on computers, and a dedicated broadband connection can all contribute to solutions to these barriers.
A key similarity between the 2020 systematic review [14] and this review is the rapid expansion of telemedicine. Eleven articles analyzed in this review used a phrase similar to “the pandemic created an acceptance of telemedicine technology” [23,25,31,33,41,43,47,51,52,61,62]. A systematic review published in 2018 cited cost as the chief barrier to adoption, whereas this review only found cost as a barrier in 8% of all observations [5]. The COVID-19 pandemic forced acceptance of the technology and enabled providers to not focus so intently on the cost of its implementation.
Limitations
This systematic review selected 46 articles for analysis from four commonly available research databases. A larger group for analysis could have yielded richer results. This review also only utilized two researchers to analyze the data; additional researchers could have identified additional themes. Selection bias was controlled through independent analysis of all articles by both reviewers followed by consensus meetings. Publication bias is the largest limitation because we were unable to query and analyze unpublished articles.
Conclusion
The COVID-19 pandemic caused huge problems to deliver medicine traditionally. However, these problems created an environment that limited face-to-face medical encounters and fostered legislation to reimburse the telemedicine modality for broad and rapid adoption of telemedicine to expand the access of care beyond the physical walls of the clinic. Physicians should feel confident that the telemedicine modality will be reimbursed and will have very little effect on patient satisfaction. Health care administrators who have not already adopted telemedicine should feel confident in the technology; however, they should ensure that sufficient confidentiality and security measures are in place. Policymakers should enact legislation to remove or mitigate barriers such as availability of technology, technical literacy, and connectivity, as these are commonly referred to in the literature.
Abbreviations
- CARES
Coronavirus Aid, Relief, and Economic Security
- eGFR
estimated glomerular filtration rate
- JHNEBP
Johns Hopkins Nursing Evidence-Based Practice Rating Scale
- MeSH
Medical Subject Headings
- mHealth
mobile health
- PICOS
participants, intervention, results, outcomes, study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Sample size, bias, effect size, country of origin, and statistics used.
Observation-to-theme conversion for patient satisfaction as well as facilitators and barriers to adoption.
Footnotes
Authors' Contributions: KH and CK contributed equally to this project. Both authors were involved in each step of the research and publication process.
Conflicts of Interest: None declared.
References
- 1.World Health Organization . Telemedicine: opportunities and developments in member states: report on the second global survey on eHealth 2009. Geneva, Swizerland: World Health Organization; 2010. [Google Scholar]
- 2.FitzGerald M, Williams R. Commonwealth Fund. 2020. Nov 20, [2021-02-15]. https://www.commonwealthfund.org/blog/2020/variations-telemedicine-across-world-qa-tiago-cravo-oliveira-hashiguchi .
- 3.Bokolo AJ. Exploring the adoption of telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic. Ir J Med Sci. 2021 Feb;190(1):1–10. doi: 10.1007/s11845-020-02299-z. http://europepmc.org/abstract/MED/32642981 .10.1007/s11845-020-02299-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fieux M, Duret S, Bawazeer N, Denoix L, Zaouche S, Tringali S. Telemedicine for ENT: Effect on quality of care during Covid-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020 Sep;137(4):257–261. doi: 10.1016/j.anorl.2020.06.014. https://linkinghub.elsevier.com/retrieve/pii/S1879-7296(20)30153-8 .S1879-7296(20)30153-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruse SC, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J Telemed Telecare. 2016 Oct 16;24(1):4–12. doi: 10.1177/1357633x16674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda N. Decision making on telemedicine for patients with epilepsy during the coronavirus disease 2019 (COVID-19) crisis. Front Neurol. 2020;11:722. doi: 10.3389/fneur.2020.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Pazi H, Beni-Adani L, Lamdan R. Accelerating telemedicine for cerebral palsy during the COVID-19 pandemic and beyond. Front Neurol. 2020;11:746. doi: 10.3389/fneur.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royce TJ, Sanoff HK, Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020 Nov 01;6(11):1698–1699. doi: 10.1001/jamaoncol.2020.2684.2768022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Z, Li N, Li D, Li J, Li B, Xiong W, Lu L, Li W, Zhou D. Telemedicine during the COVID-19 pandemic: experiences from Western China. J Med Internet Res. 2020 May 08;22(5):e19577. doi: 10.2196/19577. https://www.jmir.org/2020/5/e19577/ v22i5e19577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojdehbakhsh RP, Rose S, Peterson M, Rice L, Spencer R. A quality improvement pathway to rapidly increase telemedicine services in a gynecologic oncology clinic during the COVID-19 pandemic with patient satisfaction scores and environmental impact. Gynecol Oncol Rep. 2021 May;36:100708. doi: 10.1016/j.gore.2021.100708. https://linkinghub.elsevier.com/retrieve/pii/S2352-5789(21)00013-8 .S2352-5789(21)00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang PJ, Jay GM, Kalpakjian C, Andrews C, Smith S. Patient and provider-reported satisfaction of cancer rehabilitation telemedicine visits during the COVID-19 pandemic. PM R. 2021 Dec;13(12):1362–1368. doi: 10.1002/pmrj.12552. http://europepmc.org/abstract/MED/33455066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte A, Gouveia E Melo R, Lopes A, Rato JP, Valente J, Pedro LM. Lessons learned from the impact of the COVID-19 pandemic in a vascular surgery department and preparation for future outbreaks. Ann Vasc Surg. 2021 May;73:97–106. doi: 10.1016/j.avsg.2021.01.060. http://europepmc.org/abstract/MED/33493593 .S0890-5096(21)00060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regelmann MO, Conroy R, Gourgari E, Gupta A, Guttmann-Bauman I, Heksch R, Kamboj MK, Krishnan S, Lahoti A, Matlock K, PES Drug and Therapeutics Committee and Practice Management Committee Pediatric endocrinology in the time of COVID-19: considerations for the rapid implementation of telemedicine and management of pediatric endocrine conditions. Horm Res Paediatr. 2020;93(6):343–350. doi: 10.1159/000513060. https://www.karger.com?DOI=10.1159/000513060 .000513060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betancourt JA, Rosenberg MA, Zevallos A, Brown JR, Mileski M. The impact of COVID-19 on telemedicine utilization across multiple service lines in the United States. Healthcare (Basel) 2020 Oct 01;8(4):380. doi: 10.3390/healthcare8040380. https://www.mdpi.com/resolver?pii=healthcare8040380 .healthcare8040380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse CS. Writing a systematic review for publication in a health-related degree program. JMIR Res Protoc. 2019 Oct 14;8(10):e15490. doi: 10.2196/15490. https://www.researchprotocols.org/2019/10/e15490/ v8i10e15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33782057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newhouse R, Dearholt S, Poe S, Pugh L, White K. The Johns Hopkins nursing evidence-based practice rating scale. Johns Hopkins University School of Nursing. 2005. [2021-02-15]. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd =&ved=2ahUKEwjuheqN3-30AhXummoFHa7zBSsQFnoECAMQAQ&url=http%3A%2F%2Fevidencebasednurse.weebly.com%2Fuploads%2F4%2F2%2F0%2F8%2F42081989%2Fjhnedp_evidence _rating_scale.pdf&usg=AOvVaw0TqXuL2_eg1w4Da9XXoKNu .
- 18.Light RJ. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol Bull. 1971;76(5):365–377. doi: 10.1037/h0031643. [DOI] [Google Scholar]
- 19.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006 Jan;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://dx.plos.org/10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282. http://www.biochemia-medica.com/2012/22/276 . [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Arye E, Gressel O, Ben-Arye E, Samuels N. Feasibility of an online integrative oncology treatment program during COVID-19. J Pain Symptom Manage. 2021 Feb;61(2):e1–e3. doi: 10.1016/j.jpainsymman.2020.11.009. http://europepmc.org/abstract/MED/33189855 .S0885-3924(20)30874-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurihara K, Nagaki K, Inoue K, Yamamoto S, Mishima T, Fujioka S, Ouma S, Tsuboi Y. Attitudes toward telemedicine of patients with Parkinson’s disease during the COVID‐19 pandemic. Neurol Clin Neurosci. 2020 Nov 18;9(1):77–82. doi: 10.1111/ncn3.12465. [DOI] [Google Scholar]
- 24.Richards AE, Curley K, Christel L, Zhang N, Kouloumberis P, Kalani MA, Lyons MK, Neal MT. Patient satisfaction with telehealth in neurosurgery outpatient clinic during COVID-19 pandemic. Interdisciplin Neurosurg. 2021 Mar;23:101017. doi: 10.1016/j.inat.2020.101017. [DOI] [Google Scholar]
- 25.Yu J, Afridi SM, Cozart AC, Isea L, Guan J. Evaluation and feedback for telehealth from patients and physicians during the early stage of COVID-19 pandemic period. Cureus. 2021 Jan 11;13(1):e12633. doi: 10.7759/cureus.12633. http://europepmc.org/abstract/MED/33585121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkire (née Nasr) L, O'Connor GE, Myrden S, Köcher S. Patient experience in the digital age: An investigation into the effect of generational cohorts. J Retail Consum Serv. 2020 Nov;57:102221. doi: 10.1016/j.jretconser.2020.102221. [DOI] [Google Scholar]
- 27.Ballin M, Hult A, Björk S, Lundberg E, Nordström P, Nordström A. Web-based exercise versus supervised exercise for decreasing visceral adipose tissue in older adults with central obesity: a randomized controlled trial. BMC Geriatr. 2020 May 12;20(1):173. doi: 10.1186/s12877-020-01577-w. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-020-01577-w .10.1186/s12877-020-01577-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banbury A, Nancarrow S, Dart J, Gray L, Dodson S, Osborne R, Parkinson L. Adding value to remote monitoring: Co-design of a health literacy intervention for older people with chronic disease delivered by telehealth - The telehealth literacy project. Patient Educ Couns. 2020 Mar;103(3):597–606. doi: 10.1016/j.pec.2019.10.005.S0738-3991(19)30441-0 [DOI] [PubMed] [Google Scholar]
- 29.Barnett A, Savic M, Pienaar K, Carter A, Warren N, Sandral E, Manning V, Lubman DI. Enacting 'more-than-human' care: Clients' and counsellors' views on the multiple affordances of chatbots in alcohol and other drug counselling. Int J Drug Policy. 2021 Aug;94:102910. doi: 10.1016/j.drugpo.2020.102910. http://europepmc.org/abstract/MED/33059955 .S0955-3959(20)30249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batalik L, Dosbaba F, Hartman M, Batalikova K, Spinar J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: A randomized controlled trial. Medicine (Baltimore) 2020 Mar;99(11):e19556. doi: 10.1097/MD.0000000000019556.00005792-202003130-00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beller HL, Rapp DE, Noona SW, Winkelman AJ, Zillioux JM, Smith RP, Sims T, Corbett ST, Krupski TL, Greene KL, Schenkman NS. Tele-urology during COVID-19: rapid implementation of remote video visits. Urol Pract. 2020 Nov;7(6):442–447. doi: 10.1097/upj.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 32.Bernabe-Ortiz A, Pauschardt J, Diez-Canseco F, Miranda JJ. Sustainability of mHealth effects on cardiometabolic risk factors: five-year results of a randomized clinical trial. J Med Internet Res. 2020 Apr 21;22(4):e14595. doi: 10.2196/14595. https://www.jmir.org/2020/4/e14595/ v22i4e14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilgrami Z, Abutaleb A, Chudy-Onwugaje K, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Ghazi L, Patil SA, Quezada SM, Russman KM, Quinn CC, Jambaulikar G, Beaulieu DB, Horst S, Cross RK. Effect of TELEmedicine for inflammatory bowel disease on patient activation and self-efficacy. Dig Dis Sci. 2020 Jan;65(1):96–103. doi: 10.1007/s10620-018-5433-5. http://europepmc.org/abstract/MED/30604373 .10.1007/s10620-018-5433-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broers ER, Kop WJ, Denollet J, Widdershoven J, Wetzels M, Ayoola I, Piera-Jimenez J, Habibovic M. A personalized eHealth intervention for lifestyle changes in patients with cardiovascular disease: randomized controlled trial. J Med Internet Res. 2020 May 22;22(5):e14570. doi: 10.2196/14570. https://www.jmir.org/2020/5/e14570/ v22i5e14570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho SMJ, Lee JH, Shim J, Yeom H, Lee SJ, Jeon YW, Kim HC. Effect of smartphone-based lifestyle coaching app on community-dwelling population with moderate metabolic abnormalities: randomized controlled trial. J Med Internet Res. 2020 Oct 09;22(10):e17435. doi: 10.2196/17435. https://www.jmir.org/2020/10/e17435/ v22i10e17435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claes J, Cornelissen V, McDermott C, Moyna N, Pattyn N, Cornelis N, Gallagher A, McCormack C, Newton H, Gillain A, Budts W, Goetschalckx K, Woods C, Moran K, Buys R. Feasibility, acceptability, and clinical effectiveness of a technology-enabled cardiac rehabilitation platform (Physical Activity Toward Health-I): randomized controlled trial. J Med Internet Res. 2020 Feb 04;22(2):e14221. doi: 10.2196/14221. https://www.jmir.org/2020/2/e14221/ v22i2e14221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coorey G, Peiris D, Neubeck L, Redfern J. A realist evaluation approach to explaining the role of context in the impact of a complex eHealth intervention for improving prevention of cardiovascular disease. BMC Health Serv Res. 2020 Aug 18;20(1):764. doi: 10.1186/s12913-020-05597-5. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-020-05597-5 .10.1186/s12913-020-05597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding H, Jayasena R, Chen SH, Maiorana A, Dowling A, Layland J, Good N, Karunanithi M, Edwards I. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020 Jul 08;22(7):e17559. doi: 10.2196/17559. https://www.jmir.org/2020/7/e17559/ v22i7e17559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geramita E, DeVito Dabbs AJ, DiMartini A, Pilewski J, Switzer G, Posluszny D, Myaskovsky L, Dew MA. Impact of a mobile health intervention on long-term nonadherence after lung transplantation: follow-up after a randomized controlled trial. Transplantation. 2020 Mar;104(3):640–651. doi: 10.1097/TP.0000000000002872. http://europepmc.org/abstract/MED/31335759 .00007890-202003000-00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong K, Yan Y, Li Y, Du J, Wang J, Han Y, Zou Y, Zou XY, Huang H, She Q, APP Study Group et al Mobile health applications for the management of primary hypertension: A multicenter, randomized, controlled trial. Medicine (Baltimore) 2020 Apr;99(16):e19715. doi: 10.1097/MD.0000000000019715.00005792-202004170-00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han JK, Al-Khatib SM, Albert CM. Changes in the digital health landscape in cardiac electrophysiology: a pre-and peri-pandemic COVID-19 era survey. Cardiovasc Dig Health J. 2021 Feb;2(1):55–62. doi: 10.1016/j.cvdhj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding R, Carrasco JM, Serrano-Pons J, Lemaire J, Namisango E, Luyirika E, Immanuel T, Paleri AK, Mathews L, Chifamba D, Mupaza L, Martínez CL, Zirimenya L, Bouësseau MC, Krakauer EL. Design and evaluation of a novel mobile phone application to improve palliative home-care in resource-limited settings. J Pain Symptom Manage. 2021 Jul;62(1):1–9. doi: 10.1016/j.jpainsymman.2020.09.045.S0885-3924(20)30893-9 [DOI] [PubMed] [Google Scholar]
- 43.Hsia BC, Singh AK, Njeze O, Cosar E, Mowrey WB, Feldman J, Reznik M, Jariwala SP. Developing and evaluating ASTHMAXcel adventures: a novel gamified mobile application for pediatric patients with asthma. Ann Allergy Asthma Immunol. 2020 Nov;125(5):581–588. doi: 10.1016/j.anai.2020.07.018. http://europepmc.org/abstract/MED/32711031 .S1081-1206(20)30495-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh P, Lai H. Exploring people's intentions to use the health passbook in self-management: An extension of the technology acceptance and health behavior theoretical perspectives in health literacy. Technol Forecast Soc Change. 2020 Dec;161:120328. doi: 10.1016/j.techfore.2020.120328. [DOI] [Google Scholar]
- 45.Hutchesson MJ, Taylor R, Shrewsbury VA, Vincze L, Campbell LE, Callister R, Park F, Schumacher TL, Collins CE. Be Health for Your Heart: a pilot randomized controlled trial evaluating a web-based behavioral intervention to improve the cardiovascular health of women with a history of preeclampsia. Int J Environ Res Public Health. 2020 Aug 10;17(16):5779. doi: 10.3390/ijerph17165779. https://www.mdpi.com/resolver?pii=ijerph17165779 .ijerph17165779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiménez-Marrero S, Yun S, Cainzos-Achirica M, Enjuanes C, Garay A, Farre N, Verdú JM, Linas A, Ruiz P, Hidalgo E, Calero E, Comín-Colet J. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: A sub-analysis of the iCOR randomized trial. J Telemed Telecare. 2018 Sep 07;26(1-2):64–72. doi: 10.1177/1357633x18796439. [DOI] [PubMed] [Google Scholar]
- 47.Katt BM, Imbergamo C, Fletcher D, Aita D, Nakashian M, Kwok M, Beredjiklian PK. Telehealth for upper extremity conditions: perceptions of the patient and provider. JAAOS Glob Res Rev. 2020 Sep 10;4(9):e20.00127–13. doi: 10.5435/jaaosglobal-d-20-00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobe E, Diamantidis C, Bosworth H, Davenport C, Oakes M, Alexopoulos AS, Pendergast J, Patel UD, Crowley MJ. Racial differences in the effectiveness of a multifactorial telehealth intervention to slow diabetic kidney disease. Med Care. 2020 Nov;58(11):968–973. doi: 10.1097/MLR.0000000000001387. http://europepmc.org/abstract/MED/32833935 .00005650-202011000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai B, Bond K, Kim Y, Barstow B, Jovanov E, Bickel CS. Exploring the uptake and implementation of tele-monitored home-exercise programmes in adults with Parkinson’s disease: A mixed-methods pilot study. J Telemed Telecare. 2018 Aug 22;26(1-2):53–63. doi: 10.1177/1357633x18794315. [DOI] [PubMed] [Google Scholar]
- 50.Lemelin A, Paré G, Bernard S, Godbout A. Demonstrated cost-effectiveness of a telehomecare program for gestational diabetes mellitus management. Diabetes Technol Ther. 2020 Mar;22(3):195–202. doi: 10.1089/dia.2019.0259. [DOI] [PubMed] [Google Scholar]
- 51.Manning B, Harpole A, Harriott E, Postolowicz K, Norton E. Taking language samples home: feasibility, reliability, and validity of child language samples conducted remotely with video chat versus in-person. J Speech Lang Hear Res. 2020 Dec 14;63(12):3982–3990. doi: 10.1044/2020_JSLHR-20-00202. http://europepmc.org/abstract/MED/33186507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marques G, Drissi N, Díez de la Torre I, de Abajo BS, Ouhbi S. Impact of COVID-19 on the psychological health of university students in Spain and their attitudes toward Mobile mental health solutions. Int J Med Inform. 2021 Mar;147:104369. doi: 10.1016/j.ijmedinf.2020.104369.S1386-5056(20)31905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martins SC, Weiss G, Almeida AG, Brondani R, Carbonera LA, de Souza AC, Martins MCO, Nasi G, Nasi LA, Batista C, Sousa FB, Rockenbach MA, Gonçalves FM, Vedolin LM, Nogueira RG. Validation of a smartphone application in the evaluation and treatment of acute stroke in a comprehensive stroke center. Stroke. 2020 Jan;51(1):240–246. doi: 10.1161/strokeaha.119.026727. [DOI] [PubMed] [Google Scholar]
- 54.McGillicuddy JW, Chandler JL, Sox LR, Taber DJ. Exploratory analysis of the impact of an mHealth medication adherence intervention on tacrolimus trough concentration variability: post hoc results of a randomized controlled trial. Ann Pharmacother. 2020 Dec;54(12):1185–1193. doi: 10.1177/1060028020931806. http://europepmc.org/abstract/MED/32506922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo Y, Wang H, Huang G, Chu M. Effectiveness of nurse-led program on mental health status and quality of life in patients with chronic heart failure. Medicine (Baltimore) 2020 Aug 14;99(33):e21746. doi: 10.1097/MD.0000000000021746.00005792-202008140-00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mustonen E, Hörhammer I, Absetz P, Patja K, Lammintakanen J, Talja M, Kuronen R, Linna M. Eight-year post-trial follow-up of health care and long-term care costs of tele-based health coaching. Health Serv Res. 2020 Apr;55(2):211–217. doi: 10.1111/1475-6773.13251. http://europepmc.org/abstract/MED/31884682 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Shea O, Woods C, McDermott L, Buys R, Cornelis N, Claes J, Cornelissen V, Gallagher A, Newton H, Moyna N, McCaffrey N, Susta D, McDermott C, McCormack C, Budts W, Moran K. A qualitative exploration of cardiovascular disease patients' views and experiences with an eHealth cardiac rehabilitation intervention: The PATHway Project. PLoS One. 2020;15(7):e0235274. doi: 10.1371/journal.pone.0235274. https://dx.plos.org/10.1371/journal.pone.0235274 .PONE-D-20-00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perri MG, Shankar MN, Daniels MJ, Durning PE, Ross KM, Limacher MC, Janicke DM, Martin AD, Dhara K, Bobroff LB, Radcliff TA, Befort CA. Effect of telehealth extended care for maintenance of weight loss in rural US communities: a randomized clinical trial. JAMA Netw Open. 2020 Jun 01;3(6):e206764. doi: 10.1001/jamanetworkopen.2020.6764. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2020.6764 .2767136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piera-Jiménez J, Winters M, Broers E, Valero-Bover D, Habibovic M, Widdershoven JWMG, Folkvord F, Lupiáñez-Villanueva F. Changing the health behavior of patients with cardiovascular disease through an electronic health intervention in three different countries: cost-effectiveness study in the Do Cardiac Health: advanced new generation ecosystem (Do CHANGE) 2 randomized controlled trial. J Med Internet Res. 2020 Jul 28;22(7):e17351. doi: 10.2196/17351. https://www.jmir.org/2020/7/e17351/ v22i7e17351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Press VG, Arora VM, Kelly CA, Carey KA, White SR, Wan W. Effectiveness of virtual vs in-person inhaler education for hospitalized patients with obstructive lung disease: a randomized clinical trial. JAMA Netw Open. 2020 Jan 03;3(1):e1918205. doi: 10.1001/jamanetworkopen.2019.18205. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.18205 .2758209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramírez-Correa P, Ramírez-Rivas C, Alfaro-Pérez J, Melo-Mariano A. Telemedicine acceptance during the COVID-19 pandemic: an empirical example of robust consistent partial least squares path modeling. Symmetry. 2020 Sep 25;12(10):1593. doi: 10.3390/sym12101593. [DOI] [Google Scholar]
- 62.Ronan P, Mian A, Carr SB, Madge SL, Lorenc A, Robinson N. Learning to breathe with Tai Chi online - qualitative data from a randomized controlled feasibility study of patients with cystic fibrosis. Eur J Integr Med. 2020 Dec;40:101229. doi: 10.1016/j.eujim.2020.101229. http://europepmc.org/abstract/MED/33106755 .S1876-3820(20)31410-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacco G, Lléonart S, Simon R, Noublanche F, Annweiler C, TOVID Study Group Communication technology preferences of hospitalized and institutionalized frail older adults during COVID-19 confinement: cross-sectional survey study. JMIR Mhealth Uhealth. 2020 Sep 18;8(9):e21845. doi: 10.2196/21845. https://mhealth.jmir.org/2020/9/e21845/ v8i9e21845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheerman JFM, Hamilton K, Sharif MO, Lindmark U, Pakpour AH. A theory-based intervention delivered by an online social media platform to promote oral health among Iranian adolescents: a cluster randomized controlled trial. Psychol Health. 2020 Apr;35(4):449–466. doi: 10.1080/08870446.2019.1673895. [DOI] [PubMed] [Google Scholar]
- 65.Schrauben S, Appel L, Rivera E, Lora C, Lash J, Chen J, Hamm LL, Fink JC, Go AS, Townsend RR, Deo R, Dember LM, Feldman HI, Diamantidis CJ, CRIC Study Investigators Mobile health (mHealth) technology: assessment of availability, acceptability, and use in CKD. Am J Kidney Dis. 2021 Jun;77(6):941–950.e1. doi: 10.1053/j.ajkd.2020.10.013.S0272-6386(20)31139-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shareef MA, Kumar V, Dwivedi YK, Kumar U, Akram MS, Raman R. A new health care system enabled by machine intelligence: Elderly people's trust or losing self control. Technol Forecast Soc Change. 2021 Jan;162:120334. doi: 10.1016/j.techfore.2020.120334. [DOI] [Google Scholar]
- 67.van Dijk MR, Koster MPH, Oostingh EC, Willemsen SP, Steegers EAP, Steegers-Theunissen RPM. A mobile app lifestyle intervention to improve healthy nutrition in women before and during early pregnancy: single-center randomized controlled trial. J Med Internet Res. 2020 May 15;22(5):e15773. doi: 10.2196/15773. https://www.jmir.org/2020/5/e15773/ v22i5e15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018 Nov 19;18(1):143. doi: 10.1186/s12874-018-0611-x. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-018-0611-x .10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumeyer X, Santos SC, Morris MH. Overcoming barriers to technology adoption when fostering entrepreneurship among the poor: the role of technology and digital literacy. IEEE Trans Eng Manage. 2021 Dec;68(6):1605–1618. doi: 10.1109/TEM.2020.2989740. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample size, bias, effect size, country of origin, and statistics used.
Observation-to-theme conversion for patient satisfaction as well as facilitators and barriers to adoption.


