Abstract
STUDY QUESTION
Is cesarean delivery associated with earlier offspring pubertal development?
SUMMARY ANSWER
We identified that boys born by cesarean delivery developed puberty earlier, evidenced by an earlier age at peak height velocity and earlier attainment of puberty score > 1, than boys born by vaginal delivery.
WHAT IS KNOWN ALREADY
Cesarean delivery is posited to have long-term effects on health outcomes. However, few studies have examined whether mode of delivery is related to pubertal development.
STUDY DESIGN, SIZE, DURATION
Prospective pre-birth cohort study consisting of 1485 mother–child pairs enrolled during pregnancy from obstetric practices and followed up until early adolescence (median age 12.9 years). Participant inclusion required data on mode of delivery and at least one measure of pubertal development.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants are children from the Project Viva study. We abstracted information on delivery mode from electronic medical records from children followed since birth (1999–2002) and examined the following markers of pubertal development: age at peak height velocity (APHV); age at menarche (girls only); parent-reported pubertal development score; and child-reported pictograph Tanner pubic hair staging. We used multivariable regression models to examine associations of delivery mode with these four pubertal indices, adjusting for the following confounders: demographic and socioeconomic factors; maternal height, pre-pregnancy BMI, total gestational weight gain, pregnancy conditions, parity, and maternal age at menarche; paternal height and BMI; gestational age at delivery and birthweight-for-gestational-age z-score.
MAIN RESULTS AND THE ROLE OF CHANCE
In this study, 23.2% of children were born by cesarean delivery. Girls had an earlier APHV, had a higher pubertal score throughout childhood and in early adolescence, and were more likely to attain puberty score >1 and Tanner pubic hair Stage >1 earlier compared to boys. Mean (SD) age at menarche in girls was 12.4 (1.0) years. Boys born by cesarean delivery had significantly earlier APHV (β −0.23 years; 95% CI −0.40, −0.05) and higher risk of earlier attainment of puberty score > 1 (hazard ratio 1.09; 95% CI 1.01, 1.19) than boys born by vaginal delivery, after adjusting for confounders. These associations were not mediated by pre-pubertal BMI and were similar for planned (no labor) and unplanned (labor) cesarean delivery. No associations were observed between delivery mode and time to attain Tanner pubic hair Stage > 1 in boys. In girls, mode of delivery was not associated with any of the measured pubertal development markers.
LIMITATIONS, REASONS FOR CAUTION
This study used, as secondary outcomes, parent- and child-reported measures of pubertal development, which may be more prone to error and misclassification than information collected by trained observers or physicians during clinical examinations. The findings may also not be generalizable to populations from different settings, because all participants lived in one geographic area, were well educated, and had health care.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings provide support for cesarean delivery as a potential indicator of identifying children who are likely to experience earlier pubertal development; however, more studies are needed to confirm or refute these observations.
STUDY FUNDING/COMPETING INTEREST(S)
The project was funded by grants from the National Institutes of Health. The authors have no financial relationships or competing interests to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: cesarean delivery, pubertal development, lifecourse epidemiology, developmental origins of disease, cohort study
Introduction
Cesarean delivery is one of the most common surgical procedures in both developed and developing countries (Betran et al., 2007, 2016). Globally, more than 18.5 million cesareans are performed each year (Gibbons et al., 2010), and the rate of cesarean delivery has doubled in the last 15 years to 21% and is increasing annually by 4% (Boerma et al., 2018). When medically indicated, cesareans reduce the risk of morbidity to mother and fetus and can be a life-saving intervention (Gregory et al., 2012). Despite these benefits, concerns have been raised about possible associations between cesarean delivery and several adverse childhood health outcomes. For example, prior studies have reported that children born by cesarean delivery may have increased rates of respiratory illness in the first year of life and beyond (Thavagnanam et al., 2008; Geller et al., 2010; Souza et al., 2010), child overweight and obesity (Huh et al., 2012; Kuhle et al., 2015; Martin-Calvo et al., 2020), and diabetes (Cardwell et al., 2008; Chavarro et al., 2020). Although the association between cesarean delivery and child obesity is not entirely consistent (Rifas-Shiman et al., 2018, 2021), recent experimental data in rodents suggests this link is causal (Martinez et al., 2017). Moreover, the increasing evidence that the elevated risk of obesity may persist through adolescence (Yuan et al., 2016; Mueller et al., 2021) raises the possibility that the adverse long-term offspring health effects of cesarean birth may extend to other obesity-related conditions, such as an earlier pubertal development (Chen et al., 2019; Reinehr and Roth, 2019; Aris et al., 2019a,b; Brix et al., 2020; Deardorff et al., 2021).
Puberty is a time of psychological and biological transition from childhood to adulthood, which normally starts at ages 9–14 years in boys and at ages 8–13 years in girls (Sun et al., 2002). While excessive childhood adiposity is a well-known risk factor for earlier onset of puberty (Reinehr and Roth, 2019), less attention has been paid to the role of other risk factors beyond adiposity, such as cesarean delivery. Clarifying the extent to which cesarean delivery may contribute to earlier pubertal development in offspring will be of paramount importance, given the high frequency of cesarean delivery worldwide (Gibbons et al., 2010; Boerma et al., 2018) and that an earlier pubertal onset has been associated with an increased chronic disease risk later in life, including type 2 diabetes, cardiovascular disease, and cancer (Widen et al., 2012; Berentzen et al., 2017; Cheng et al., 2020).
To address these research gaps, we used data from a pre-birth cohort in eastern MA, USA, to examine associations of cesarean delivery with offspring pubertal developmental markers. We hypothesized that birth by cesarean delivery would be associated with earlier pubertal development in both boys and girls.
Materials and methods
Study population
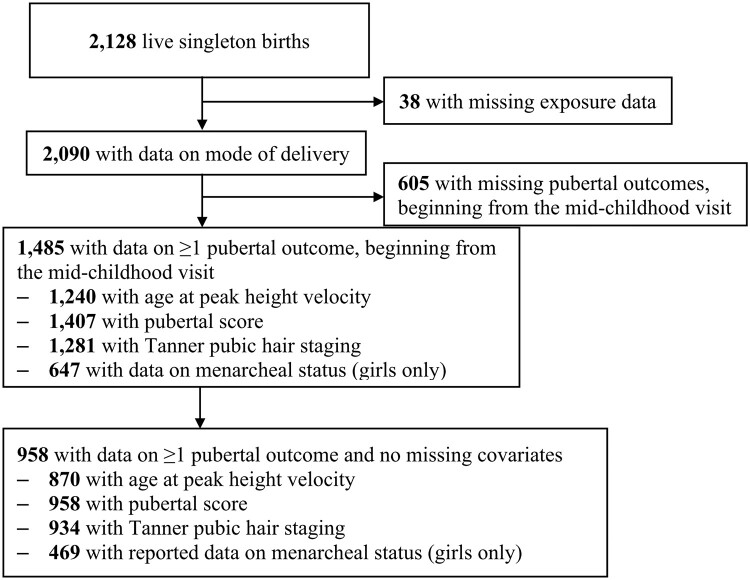
Project Viva is an ongoing study of pre- and perinatal influences on maternal, fetal and child health (Oken et al., 2015). Briefly, we approached 4102 women for participation and recruited 2670 pregnant women during their first prenatal appointment between April 1999 and November 2002 from obstetric practices at Atrius Harvard Vanguard Medical Associates in eastern MA. After recruitment we found 329 women to be ineligible (i.e. 115 moved out of the study area, 19 had multiple gestations, and 195 had stillbirths or miscarriages), leaving 2341 eligible enrollees, of whom 2128 were still enrolled at the time of delivery and had live births. Mothers provided written informed consent at enrollment and follow-up visits, and children provided verbal assent at the mid-childhood and early adolescent visits. The Institutional Review Board of Harvard Pilgrim Health Care approved the project in line with ethical standards established by the Declaration of Helsinki. Of 2128 live singleton births, 2090 (98%) had data on mode of delivery. We further limited this analysis to 1485 (70%) children with at least one measure of pubertal development (Fig. 1).
Figure 1.
Flowchart of sample in a study of association of mode of delivery with offspring pubertal development.
Exposure: mode of delivery
We obtained information about mode of delivery from electronic hospital records. For each participant who had a cesarean delivery recorded on electronic birth logs, we reviewed the operative report to confirm cesarean delivery and to abstract the primary indication for operative delivery and the timing of cesarean delivery in relation to onset of labor. Using this information, we divided cesarean deliveries into cesarean delivery preceded by spontaneous labor, cesarean delivery preceded by induced labor, and cesarean deliveries not preceded by labor. We also defined an unplanned cesarean delivery as a delivery in which the operative report described a failed induction of labor, prolonged latent phase, prolonged active phase, arrest of dilation, ‘failure to progress’, arrest of descent in the second stage or failed operative vaginal delivery, ‘non-reassuring fetal heart rate tracing’, non-reassuring testing prompting immediate cesarean delivery, cord prolapse or abruption (Huh et al., 2012). We defined planned cesarean deliveries as those for which participants did not undergo a trial of labor (elective repeat cesarean without trial of labor, malpresentation, placenta previa, suspected macrosomia, maternal request, or other indication precluding trial of labor).
Primary outcome: age at peak height velocity
At in-person research visits during early childhood (median 3.2 years; range 2.8–6.0), mid-childhood (7.8 years; 6.6–10.9), and early adolescence (12.9 years; 11.9–16.7), trained research assistants measured standing height to the nearest 0.1 cm using a wooden stadiometer (Shorr Productions, Olney, MD, USA). We also obtained additional data on height from medical records, where pediatricians recorded height at routine clinic visits (i.e. well-child visits at which healthcare providers would monitor growth and development and make recommendations to parents for optimal child health (Committee on Practice and Ambulatory Medicine and Bright Futures Periodicity Schedule Workgroup, 2015)). Supplementary Fig. S1 describes the distribution of the number of height measurements according to the method of ascertainment (i.e. research visits (n = 2811 measurements) versus medical records (n = 12 259 measurements)).
Linear growth typically accelerates during puberty due to activation of the hypothalamic–pituitary axis (Breehl and Caban, 2021) and the timing of the pubertal growth spurt (i.e. age at peak height velocity [APHV]) can be used as an objective marker of pubertal timing. We estimated APHV using longitudinal height data from both research visits and medical records [mean (range) = 12 (3–45) height measurements per child; age range = 2.5–18.3 years]. We fit subject-specific height growth curves using the SITAR (SuperImposition by Translation And Rotation) growth model (Cole et al., 2010). Briefly, SITAR uses a shape-invariant natural cubic spline curve and a nonlinear random-effects model to estimate a population average height growth curve for the entire sample and each subject’s deviation from the population average curve as random effects. We identified the optimal model using the Bayesian information criterion. We estimated APHV for each child by differentiating the individually predicted height curves and locating the maximum inflection point during adolescence for each individually differentiated curve, where the derivative equals to zero. We fitted these models with the SITAR package in R v.4.0.2 (R Core Team, Vienna, Austria).
Secondary outcomes: child- and parent-reported pubertal development markers
Pubic hair staging
Between the ages 10 and 16 years, as well as at the early adolescent visit (median 12.9 years; interquartile range 12.5–13.8), we obtained self-reported pubic hair staging in boys and girls through questionnaires using Tanner’s criteria for stage of maturation (Stages 1–5) with descriptions accompanied by pictographs (Marshall and Tanner, 1969, 1970). Supplementary Fig. S2 provides a spaghetti plot visualization of Tanner pubic hair staging across all visits according to mode of delivery. We considered Tanner Stage >1 (i.e. Stage 2 or higher) as pubertal onset in accordance with previous studies (Kubo et al., 2018; Aghaee et al., 2019).
Puberty score
We evaluated pubertal development via a widely used and validated written pubertal development scale (PDS) (Petersen et al., 1988) filled out by parents at the mid-childhood visit (median 7.8 years; range 6.6–10.9) and the early adolescent visit (median 12.9 years; range 11.9–16.7), and from questionnaires distributed between ages 8 and 16 years. Prior studies (Mensah et al., 2013) have utilized the PDS at a similar early age (∼8 years), thus validating its use during mid-childhood in our study. A recent study (Koopman-Verhoeff et al., 2020) showed moderate to high agreement between self- and parent-reported PDS (Kendall’s Tau 0.67–0.76). Moreover, the PDS has been moderately correlated with physician breast Tanner staging in girls (Brooks-Gunn et al., 1987) and pubic hair staging in boys (Schmitz et al., 2004). PDS questions for boys include four items: voice deepening, facial and body hair growth, acne, and growth spurt. PDS questions for girls include five items: breast development, body hair growth, acne, growth spurt and menarche. The response options for each item (except for menarche) were: ‘not yet started’ (1 point), ‘barely started’ (2 points), ‘definitely started’ (3 points), ‘seems complete’ (4 points) and ‘I don’t know’ (coded as missing). A ‘yes’ answer on the menarche question receives 4 points, while a ‘no’ answer receives 1 point. Supplementary Tables SI and SII describe the distribution of responses for each of these items across study visits. We derived a continuous puberty score ranging from 1 to 4 for each participant, by summing the point values and averaging across all items. Supplementary Fig. S3 provides a spaghetti plot visualization of the puberty score across all visits according to mode of delivery. We then dichotomized the variable as pre-pubertal (puberty score = 1) versus pubertal (puberty score > 1) in accordance with a previous study (Perng et al., 2018).
Age at menarche
We obtained data on age at menarche from a series of parent-reported questionnaires that were distributed approximately annually between the ages of 9 and 16 years, and at the early adolescent visit (median 12.9 years; interquartile range 12.5–13.8). Briefly, mothers indicated whether their daughters had attained their first menstrual period (yes or no) and the month and year of its occurrence.
Covariates
Mothers reported their age, pre-pregnancy weight, height, race/ethnicity, smoking history, age at first menstrual period, highest education level, household income, and father’s weight and height via questionnaires and interviews at recruitment in early pregnancy. Trained research assistants asked mothers the question: ‘Which of the following best describes your race or ethnicity?’. Mothers had a choice of one or more of the following racial/ethnic groups: Hispanic or Latina; white or Caucasian; Black or African American; Asian or Pacific Islander; American Indian or Alaskan Native; and other (please specify). For participants who chose ‘other’ race/ethnicity, we compared the specified responses to the US census definition for the other five race and ethnicities and reclassified them where appropriate. If a participant chose more than one racial/ethnic group, we classified them in the ‘other’ category. Owing to the small sample size, we combined mothers whose race/ethnicity were Other or more than one race/ethnicity into a single category. We categorized education as having obtained a college degree (yes or no), household income as ≤$70 000/year or >$70 000/year, and smoking history as never smoked, smoked before pregnancy, or smoked during pregnancy. We calculated pre-pregnancy BMI as self-reported pre-pregnancy weight divided by height squared. We used the last prenatal weight (within 4 weeks of delivery) recorded in prenatal care records and self-reported pre-pregnancy weight to calculate total gestational weight gain. We obtained results of a 2-stage clinical glycemic screening and used them to categorize women as having gestational diabetes (GDM) based on criteria previously detailed (Gingras et al., 2018). We also extracted data on parity (categorized as nulliparous versus multiparous) as well as vital signs, laboratory results, and diagnoses of hypertensive disorders of pregnancy from outpatient and hospital medical records to identify women as having pre-eclampsia.
We extracted data on infant sex and birthweight from hospital medical records. We calculated length of gestation in days by subtracting the date of the last menstrual period (LMP) from the date of delivery. We used the result from the second trimester ultrasound estimate of gestational duration if it differed from the LMP estimate by more than 10 days. We calculated birthweight-for-gestational-age z-scores from a US national reference (Oken et al., 2003). We used weight and height data from research visits during mid-childhood to calculate offspring BMI and derived age- and sex-specific BMI z-scores using the World Health Organization Child Growth Standards (de Onis et al., 2007).
Statistical analysis
We decided a priori to conduct all analyses separately in boys and girls, given the known sex differences in pubertal development (Fechner, 2002). In our primary analyses, we used mode of delivery as a two-category variable: cesarean or vaginal delivery. In secondary analyses, we used data on timing and type of labor in relation to delivery and on planning of cesarean delivery to define mode of delivery as a three-category variable (planned cesarean without trial of labor; unplanned cesarean following spontaneous or induced labor; and vaginal delivery).
We used multivariable linear regression models to estimate mean differences in APHV by delivery mode. As we had collected Tanner pubic hair stage, puberty score and menarche data on an annual basis, we used parametric maximum-likelihood survival models to estimate associations of delivery mode with time to attain pubic hair Stage >1, puberty score > 1 and menarche, respectively. However, we do not know the exact age at which a child attained Stage >1 of pubic hair development or puberty score > 1. Rather, we know what pubic hair stage or puberty score was reported at the time each questionnaire was completed, leading to interval censoring (Christensen et al., 2010; Brix et al., 2019a,b,c). Thus, we used parametric survival analysis for interval-censored data to estimate associations of delivery mode with time to attain Tanner pubic hair Stage >1 and time to attain puberty score > 1. We considered the data left-censored if the event (i.e. Tanner pubic hair Stage >1 or puberty score > 1) was attained by the first questionnaire, interval-censored if the event was attained sometime between two questionnaires and right-censored if the event was not attained by the early adolescent study visit.
For all analyses, we applied the following models: Model 1 adjusted for gestational age at delivery and birthweight-for-gestational-age z-score; Model 2 adjusted for the same covariates as Model 1 as well as mother’s age at enrollment, educational level, household income, parity, race/ethnicity, smoking history, pregnancy conditions (i.e. GDM and pre-eclampsia), total gestational weight gain, height, and pre-pregnancy BMI; Model 3 adjusted for the same covariates as Model 2 as well as maternal age at menarche; Model 4 adjusted for the same covariates as Model 3 as well as paternal height and BMI. To examine whether these associations could be explained by differences in pre-pubertal weight, we further adjusted our analyses for BMI z-score in mid-childhood. We used chained equation multiple imputation to impute values for missing covariates. We generated 50 imputed datasets for all 2128 Project Viva children. The imputation model included all exposures, outcomes, and covariates under study. We combined imputed datasets using MI ESTIMATE in Stata after excluding those who did not satisfy the inclusion criteria for this study, i.e. children without any pubertal outcomes (n = 605). We conducted a series of sensitivity analyses to assess the robustness of our study findings by: repeating all analyses in subjects without missing exposure or covariate data (n = 958; Fig. 1); conducting inverse probability of censoring weighting analyses to control for selection bias (Hernan et al., 2004) owing to loss to follow-up, i.e. children without pubertal outcome data beginning from the mid-childhood visit; repeating all analyses in children without macrosomia as well as children whose mothers did not experience GDM or pre-eclampsia (n = 737). We performed all analyses using Stata 16.1 software (StataCorp LP, TX, USA).
Results
In this study, 23.2% of children were born by cesarean delivery, similar to that in MA as a whole (23.8%) and USA (22.9%) for the year 2000 (Osterman and Martin, 2014). Women who delivered by cesarean section (all cesarean deliveries, planned or unplanned cesarean) were shorter, had higher pre-pregnancy BMI, and had an earlier age at menarche. Children born by cesarean delivery (all cesarean deliveries, planned or unplanned cesarean) had higher BMI z-score in mid-childhood than those born by vaginal delivery (Table I and Supplementary Table SIII). No other maternal, paternal, or child characteristics substantially differed by mode of delivery.
Table I.
Characteristics of study sample according to mode of delivery.
|
Vaginal
n = 736 (76.8%) |
Cesarean
n = 222 (23.2%) |
P-valuea | |
|---|---|---|---|
| Maternal characteristics | Mean (SD) or % | ||
| Age at enrollment (years) | 32.6 (4.7) | 33.2 (4.4) | 0.12 |
| Race/ethnicity | 0.07 | ||
| White | 76.1 | 67.6 | |
| Black | 10.6 | 14.4 | |
| Hispanic | 5.6 | 7.7 | |
| Asian | 5.0 | 4.9 | |
| Other/More than 1 race or ethnicity | 2.7 | 5.4 | |
| College graduate | 75.3 | 76.6 | 0.69 |
| Household income >$70 000/year | 66.4 | 60.8 | 0.12 |
| Smoking history | 0.89 | ||
| Never | 70.5 | 72.1 | |
| Before pregnancy | 20.8 | 19.4 | |
| During pregnancy | 8.7 | 8.6 | |
| Height (m) | 1.66 (0.07) | 1.64 (0.07) | 0.002 |
| Pre-pregnancy BMI (kg/m2) | 24.4 (4.8) | 26.0 (6.2) | <0.001 |
| Total gestational weight gain (kg) | 15.6 (5.1) | 15.8 (5.7) | 0.51 |
| Nulliparous | 47.8 | 52.7 | 0.20 |
| Gestational diabetes | 4.6 | 5.4 | 0.63 |
| Pre-eclampsia | 2.7 | 5.0 | 0.10 |
| Age at menarche (years) | 12.8 (1.5) | 12.6 (1.5) | 0.03 |
| Paternal characteristics | |||
| Height (m) | 1.79 (0.08) | 1.80 (0.08) | 0.14 |
| BMI (kg/m2) | 26.3 (3.9) | 26.8 (4.0) | 0.07 |
| Child characteristics | |||
| Male sex | 49.7 | 54.9 | 0.17 |
| Gestational age at delivery (weeks) | 39.6 (1.5) | 39.6 (1.6) | 0.84 |
| Birthweight-for-gestational-age z-score (SD units) | 0.18 (0.91) | 0.31 (1.0) | 0.08 |
| Mid-childhood BMI z-score (SD units) | 0.49 (1.10) | 0.79 (1.18) | 0.002 |
| Child pubertal development markers | |||
| Number of visits/questionnaires from which information on puberty was collected | 5 (2) | 6 (2) | 0.44 |
| Age at peak height velocity (years) | 12.2 (1.3) | 12.1 (1.3) | 0.20 |
| No. of serial height measures obtained | 13.4 (6.2) | 13.4 (6.9) | 0.87 |
| Puberty score at the mid-childhood visit (units) | 1.1 (0.4) | 1.1 (0.4) | 0.99 |
| Puberty score at 8 years (units) | 1.3 (0.4) | 1.3 (0.4) | 0.41 |
| Puberty score at 9 years (units) | 1.3 (0.4) | 1.4 (0.4) | 0.23 |
| Puberty score at 10 years (units) | 1.5 (0.5) | 1.6 (0.5) | 0.05 |
| Puberty score at 11 years (units) | 1.7 (0.6) | 1.8 (0.7) | 0.02 |
| Puberty score at the early adolescent visit (units) | 2.6 (0.8) | 2.6 (0.8) | 0.51 |
| Puberty score at 14 years (units) | 3.0 (0.6) | 3.1 (0.6) | 0.35 |
| Puberty score at 15 years (units) | 3.3 (0.5) | 3.3 (0.5) | 0.59 |
| Puberty score at 16 years (units) | 3.5 (0.4) | 3.5 (0.4) | 0.89 |
| Pubic hair stage > 1 at 10 years | 44.7 | 53.2 | 0.05 |
| Pubic hair stage > 1 at 11 years | 74.1 | 77.3 | 0.46 |
| Pubic hair stage > 1 at the early adolescent visit | 96.7 | 96.4 | 0.85 |
| Pubic hair stage > 1 at 14 years | 99.2 | 98.7 | 0.67 |
| Pubic hair stage > 1 at 15 years | 99.7 | 100.0 | 0.60 |
| Pubic hair stage > 1 at 16 years | 100.0 | 100.0 | – |
| Age at menarche—girls only (years) | 12.4 (0.9) | 12.3 (0.8) | 0.13 |
P-value calculated using one-way ANOVA (for continuous variables) and chi-square tests (for categorical variables).
As expected, girls had an earlier APHV (11.2 versus 13.1 years), had higher pubertal score throughout childhood and in early adolescence, and were more likely to attain puberty score >1 and Tanner pubic hair Stage >1 earlier compared to boys (Supplementary Tables SI and SII). Mean (SD) age at menarche was 12.4 (1.0) years among girls who achieved menarche by their last menarcheal status report (n = 558; 86.2%). As expected, in both boys and girls, APHV showed moderate negative correlations with puberty score and Tanner pubic hair stage, while puberty score showed moderate positive correlations with Tanner pubic hair stage (Supplementary Figs S4 and S5). In girls, age at menarche was positively correlated with APHV and negatively correlated with puberty score and Tanner pubic hair stage (Supplementary Fig. S5). At ages 10–16 years, parent-reported body hair growth was moderately and positively correlated with child-reported Tanner pubic hair stage in boys (10 y: r = 0.46; 11 y: r = 0.55; 12 y: r = 0.69; 14 y: r = 0.40; 15 y: r = 0.22; 16 y: r = 0.37, Supplementary Fig. S6). Similar observations were noted in girls (10 y: r = 0.61; 11 y: r = 0.76; 12 y: r = 0.74; 14 y: r = 0.65; 15 y: r = 0.30; 16 y: r = 0.34, Supplementary Fig. S7). In both boys and girls, APHV was negatively correlated with parent-reported growth spurt (Supplementary Figs S8 and S9).
Associations of delivery mode with pubertal development in boys
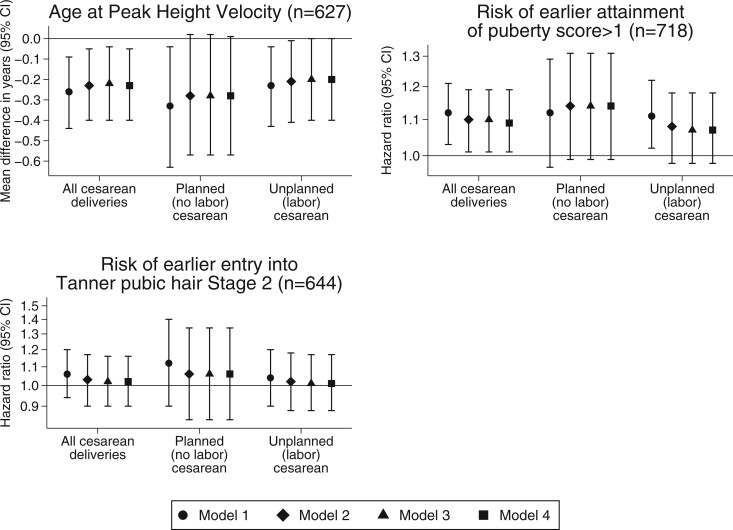
After adjusting for maternal, paternal, and child confounders, boys born by cesarean delivery had significantly earlier APHV (β −0.23 years; 95% CI −0.40, −0.05) and higher risk of earlier attainment of puberty score > 1 (hazard ratio 1.09; 95% CI 1.01, 1.19) than boys born by vaginal delivery. Similarly, planned (no labor) cesarean delivery and unplanned (labor) cesarean delivery were both associated with earlier APHV and higher risk of earlier attainment of puberty score > 1 (Fig. 2, Model 2). These associations remained significant after additionally adjusting for maternal age at menarche (Fig. 2, Model 3), and paternal height and BMI (Fig. 2, Model 4). The associations of cesarean delivery with APHV (β −0.18 years; 95% CI −0.35, 0.00) and time to attain puberty score > 1 (hazard ratio 1.09; 95% CI 1.00, 1.18) were attenuated slightly after adjusting for BMI z-score in mid-childhood. No associations were observed between delivery mode and time to attain Tanner pubic hair Stage >1 (Fig. 2).
Figure 2.
Multiple imputation analyses for the association of mode of delivery with pubertal outcomes in boys. Model 1: adjusted for gestational age at delivery and birthweight-for-gestational-age z-score. Model 2: Model 1 + mother’s age at enrollment, educational level, household income, race/ethnicity, smoking history, parity, gestational diabetes, pre-eclampsia, total gestational weight gain, height, and pre-pregnancy BMI. Model 3: Model 2 + maternal age at menarche. Model 4: Model 3 + paternal height and BMI.
Associations of delivery mode with pubertal development in girls
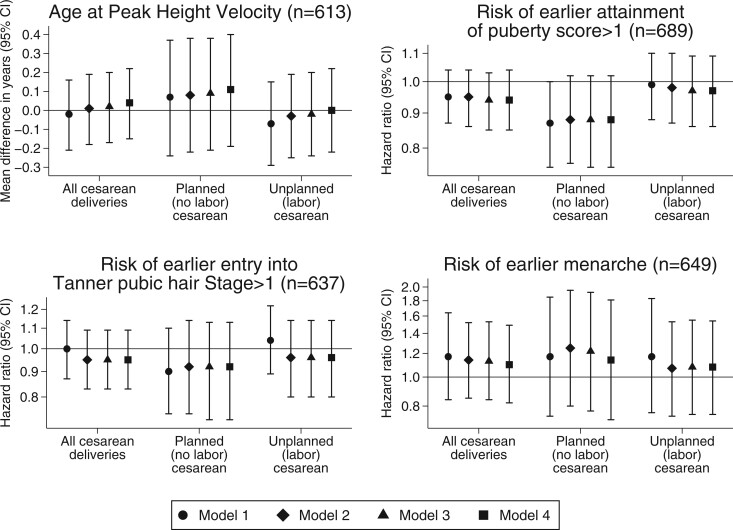
After adjusting for maternal, paternal and child confounders, we observed no significant association of cesarean versus vaginal delivery with APHV (β 0.04 years; 95% CI −0.15, 0.22), time to attain puberty score > 1 (hazard ratio 0.94; 95% CI 0.85, 1.04), time to attain Tanner pubic hair stage >1 (hazard ratio 0.94; 95% CI 0.82, 1.08) and time to menarche (hazard ratio 1.10; 95% CI 0.82, 1.49). We also observed no associations with pubertal outcomes in those born by planned (no labor) or unplanned (labor) cesarean delivery (Fig. 3).
Figure 3.
Multiple imputation analyses for the association of mode of delivery with pubertal outcomes in girls. Model 1: adjusted for gestational age at delivery and birthweight-for-gestational-age z-score. Model 2: Model 1 + mother’s age at enrollment, educational level, household income, race/ethnicity, smoking history, parity, gestational diabetes, pre-eclampsia, total gestational weight gain, height, and pre-pregnancy BMI. Model 3: Model 2 + maternal age at menarche. Model 4: Model 3 + paternal height and BMI.
Sensitivity analyses
In boys, our covariate complete case analyses, inverse probability of censoring weighting analyses, as well as analyses excluding those with macrosomia, GDM, or pre-eclampsia, showed no appreciable changes from our main analyses for the outcomes of APHV, time to attain pubic hair Stage >1, and time to attain puberty score > 1. However, our covariate complete case analyses and exclusion analyses showed that girls born by cesarean delivery had a higher risk of earlier menarche (Supplementary Tables SIV and SV).
Discussion
In this study, we observed sex-specific associations of mode of delivery with offspring pubertal development. Compared with boys born by vaginal delivery, those born by cesarean delivery had earlier pubertal development evidenced by an earlier APHV in early adolescence and higher risk of earlier attainment of puberty score > 1. These associations, however, were not seen in the main analyses in girls. We also observed similar associations in boys born by planned (no labor) cesarean delivery or unplanned (labor) cesarean delivery. Although we have no clear biological explanation for this observation, the findings are consistent with our previous report that showed the association of cesarean delivery with offspring BMI z-score persisted regardless of the type of labor (Mueller et al., 2021).
Increasing evidence has shown that children born by cesarean may experience higher rates of adverse health outcomes later in life, including type 1 (Cardwell et al., 2008) and type 2 diabetes (Chavarro et al., 2020), allergies and asthma (Thavagnanam et al., 2008; Abrahamsson et al., 2014), multiple sclerosis (Nielsen et al., 2013), chronic immune disorders (Sevelsted et al., 2015), and obesity (Huh et al., 2012; Yuan et al., 2016; Martin-Calvo et al., 2020; Mueller et al., 2021). To date, it remains unknown whether cesarean delivery is predictive of offspring pubertal development, an important question given that puberty is a period of significant maturation of reproductive functions, and that an earlier pubertal onset is linked with increased chronic disease risk in adulthood, including type 2 diabetes, cardiovascular disease, and cancer (Widen et al., 2012; Berentzen et al., 2017; Cheng et al., 2020). Recent studies have identified other early life risk factors for earlier pubertal development such as maternal pre-pregnancy obesity (Aris et al., 2019a; Brix et al., 2019a), maternal smoking during pregnancy (Hakonsen et al., 2013; Brix et al., 2019,c), gestational diabetes (Kubo et al., 2018), hypertensive disorders of pregnancy (Lunddorf et al., 2020), lower placental weight z-score (Ernst et al., 2021), and small-for-gestational-age (Hvidt et al., 2019). Our study thus makes an important contribution to the extant literature by providing the first evidence of a prospective relationship between cesarean delivery and earlier pubertal development, independently of the aforementioned risk factors. The clinical relevance of an altered APHV or earlier attainment of puberty score > 1 should be explored further. Past studies have shown that a 1-year change in APHV is linked to hormonal changes associated with cancer risk (Sandhu et al., 2006) and cardiovascular disease mortality in adulthood (Imai et al., 2013). Our observed differences in APHV of ∼0.3 years when born by cesarean delivery may potentially have similar, albeit smaller, health consequences.
We have previously shown that cesarean delivery is associated with higher childhood BMI z-score (Huh et al., 2012; Mueller et al., 2021) and that excess pre-pubertal child weight is a strong risk factor for earlier pubertal development (Aris et al., 2019b). We further explored pathways from cesarean delivery to offspring pubertal development in boys through pre-pubertal BMI, and found evidence of a direct effect that is not largely mediated by pre-pubertal BMI. This finding is consistent with our previous work showing that maternal pre-pregnancy obesity—a strong risk factor for cesarean delivery (Chu et al., 2007)—was associated with earlier pubertal development only in boys, independently of pre-pubertal BMI (Aris et al., 2019a). We speculate that the observed adiposity-independent effects of cesarean delivery on pubertal development in boys might be due to the fact the relation of adiposity with timing of puberty is stronger and more consistent for girls than for boys (Li et al., 2017; Aris et al., 2019b; Brix et al., 2020).
Taken together, our findings provide support for cesarean delivery as a potential indicator of identifying children who are likely to experience earlier pubertal development in adolescence. We further speculate that cesarean delivery may exert ‘programming’ effects on pubertal development in male offspring, perhaps through exposure to sex hormone alterations. Pubertal onset is typically driven by an increase in the pulsatile secretion of GnRH which stimulates the release of FSH and LH and in turn, triggering production of testosterone (in boys) and estradiol (in girls) (Bordini and Rosenfield, 2011). It is possible that testosterone production from the testis in boys is more sensitive to early life exposures, such as cesarean delivery, as compared with sex steroid production in girls that are adrenal or ovarian in origin (Buck Louis et al., 2008). It is also possible that cesarean delivery may exert programming effects on pubertal development through epigenetic alterations and/or gut microbiota changes. Cesarean delivery has been shown to be associated with an accelerated epigenetic age (Khouja et al., 2018), which in turn, has been independently linked with earlier pubertal development (Suarez et al., 2018). A recent animal experiment has also shown that cesarean-born mice not only gained more body mass after weaning, but also lacked the dynamic developmental gut microbiota changes observed in control mice (Martinez et al., 2017). Further studies are clearly warranted to elucidate these mechanisms. Moreover, since to our knowledge, this is the first report of an association between cesarean delivery and timing of puberty, it is also especially important that this relation is examined in independent study populations.
Strengths of our study include its prospective study design, long-term follow-up, and wide range of covariates (including mothers’ age at menarche) obtained by highly trained staff using standardized protocols. Further, most of the existing research on pubertal development has only focused on age at menarche in girls, leaving important gaps in the understanding of the determinants of pubertal development in boys, and on the development and progression of secondary sexual characteristics (such as pubic hair development) in both boys and girls. Our study directly addresses this research gap by utilizing multiple indicators of pubertal development in both boys and girls. Our study also benefits from the use of repeated height measures from early childhood to late adolescence to estimate APHV as a measure of pubertal timing in boys and girls, which has been shown to be a reliable and objective indicator of pubertal development (Granados et al., 2015).
Our study has several limitations. First, we used child-reported measures of pubic hair staging and parent-reported measures of pubertal score, which may be more prone to error and misclassification than information collected by trained observers or physicians during clinical examinations. Although clinical assessment of pubertal development is considered the gold standard, it is still dependent on observer training and experience (Chavarro et al., 2017). Further, a recent review of pubertal assessment methods suggested low acceptability of clinical examination in a research setting (Walker et al., 2020). By using parent- and child-reported information on pubertal development, we were able to conduct a larger population-based study examining the relationship between cesarean delivery and pubertal development, albeit at the cost of some measurement error. Moreover, in large population-based studies, self-assessment of secondary sexual characteristics (including pubic hair development) performs similarly well to assessments by trained physicians when both are compared against objective biomarkers of pubertal development (Chavarro et al., 2017), suggesting that the cost in terms of measurement error may be minor. Additionally, our findings were consistent across different pubertal indicators (e.g. APHV and puberty score in boys) further suggesting the reliability of the reported measures. Second, we had distributed the first questionnaire for self-reported Tanner pubic hair stage at a relatively late age (∼10 years), resulting in a moderate proportion of children (∼43% boys and ∼50% girls) who had already attained Tanner stage >1. Third, it is possible that our findings may be the result of events leading to cesarean delivery and not cesarean delivery itself (i.e. confounding by indication). However, we do not think that there is extensive confounding by indication in our study, as the most common indications for cesarean delivery in the USA are failure to progress and non-reassuring fetal heart tracing (Boyle et al., 2013), neither of which are known risk factors for pubertal development. We also observed no substantial difference in pubertal development outcomes for children born by planned or unplanned cesarean delivery, further supporting the argument against confounding by indication. Moreover, our analyses have adjusted for several maternal characteristics (e.g. GDM, pre-eclampsia, maternal pre-pregnancy BMI) which are established risk factors for pregnancy complications that, in turn, are relative indications for cesarean delivery. Further, our sensitivity analyses excluding children with these characteristics showed no appreciable changes from the main findings. Fourth, we excluded 30% of children from the original cohort without data on mode of delivery or pubertal development markers. Differences between those included and lost to follow-up might have led to some selection bias, but we adjusted for this to a certain degree by conducting inverse probability of censoring weighting analyses, which showed no appreciable changes from our main findings. Lastly, our findings may also not be generalizable to populations from different settings, because all participants lived in Eastern MA, USA, were well educated, and had health care coverage.
Conclusion
Our findings suggest that cesarean delivery is significantly associated with earlier pubertal development in boys. More importantly, these observations provide support for cesarean delivery as a potential indicator of identifying children who are likely to experience earlier pubertal development in adolescence. While these findings are intriguing, it is imperative that this question is addressed in independent studies to confirm or refute these observations, given the scarcity of data on this topic and its potential public health impact.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
I.M.A. and J.E.C. conceptualized and designed the study and drafted the manuscript. S.L.R.S., M.F.H. and E.O. acquired the data. I.M.A. and S.L.R.S. analyzed the data. All authors helped interpret the data, reviewed the article critically for the intellectual content, approved the final version to be published and agrees to be accountable for all aspects of the work.
Funding
The project was funded by grants from the National Institutes of Health (R01HD093761, R01HD034568, R01ES024765 and UH3 OD023286).
Conflict of interest
The authors have no financial relationships or competing interests to disclose.
Supplementary Material
References
- Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC.. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 2014;44:842–850. [DOI] [PubMed] [Google Scholar]
- Aghaee S, Deardorff J, Greenspan LC, Quesenberry CP Jr, Kushi LH, Kubo A.. Breastfeeding and timing of pubertal onset in girls: a multiethnic population-based prospective cohort study. BMC Pediatr 2019;19:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris IM, Rifas-Shiman SL, Li LJ, Fleisch AF, Hivert MF, Kramer MS, Oken E.. Parental obesity and offspring pubertal development: project viva. J Pediatr 2019a;215:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris IM, Rifas-Shiman SL, Zhang X, Yang S, Switkowski K, Fleisch AF, Hivert MF, Martin RM, Kramer MS, Oken E.. Association of BMI with linear growth and pubertal development. Obesity (Silver Spring) 2019b;27:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berentzen NE, Wijga AH, van Rossem L, Postma DS, Gehring U, Smit HA.. Pubertal timing and cardiometabolic markers at age 16 years. J Pediatr 2017;187:158–164. [DOI] [PubMed] [Google Scholar]
- Betran AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, Wagner M.. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol 2007;21:98–113. [DOI] [PubMed] [Google Scholar]
- Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR.. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, Moller AB, Say L, Hosseinpoor AR, Yi M. et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018;392:1341–1348. [DOI] [PubMed] [Google Scholar]
- Bordini B, Rosenfield RL.. Normal pubertal development: Part I: the endocrine basis of puberty. Pediatr Rev 2011;32:223–229. [DOI] [PubMed] [Google Scholar]
- Boyle A, Reddy UM, Landy HJ, Huang CC, Driggers RW, Laughon SK.. Primary cesarean delivery in the United States. Obstet Gynecol 2013;122:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breehl L, Caban O.. Physiology, Puberty. Treasure Island (FL): StatPearls, 2021. [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Arah OA, Nohr EA, Olsen J, Henriksen TB, Ramlau-Hansen CH.. Maternal pre-pregnancy obesity and timing of puberty in sons and daughters: a population-based cohort study. Int J Epidemiol 2019a;48:1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Parner E, Stovring H, Olsen J, Henriksen TB, Ramlau-Hansen CH.. Timing of puberty in boys and girls: a population-based study. Paediatr Perinat Epidemiol 2019b;33:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, Henriksen TB, Ramlau-Hansen CH.. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol 2020;49:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Parner ET, Olsen J, Henriksen TB, Ramlau-Hansen CH.. Maternal smoking during pregnancy and timing of puberty in sons and daughters: a population-based cohort study. Am J Epidemiol 2019c;188:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J.. Validity of self-report measures of girls’ pubertal status. Child Dev 1987;58:829–841. [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, Sippell W, Abbott DH, Soto A, Tyl RW. et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics 2008;121 Suppl 3:S192–S207. [DOI] [PubMed] [Google Scholar]
- Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Parslow RC, Pozzilli P, Brigis G, Stoyanov D. et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51:726–735. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Martin-Calvo N, Yuan C, Arvizu M, Rich-Edwards JW, Michels KB, Sun Q.. Association of birth by cesarean delivery with obesity and type 2 diabetes among adult women. JAMA Netw Open 2020;3:e202605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Watkins DJ, Afeiche MC, Zhang Z, Sanchez BN, Cantonwine D, Mercado-Garcia A, Blank-Goldenberg C, Meeker JD, Tellez-Rojo MM. et al. Validity of self-assessed sexual maturation against physician assessments and hormone levels. J Pediatr 2017;186:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Fan HY, Yang C, Hsieh RH, Pan WH, Lee YL.. Assessing causality between childhood adiposity and early puberty: a bidirectional Mendelian randomization and longitudinal study. Metabolism 2019;100:153961. [DOI] [PubMed] [Google Scholar]
- Cheng TS, Day FR, Lakshman R, Ong KK.. Association of puberty timing with type 2 diabetes: a systematic review and meta-analysis. PLoS Med 2020;17:e1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, Ness A, Drews-Botsch C, Dominguez C, McGeehin MA. et al. Progression through puberty in girls enrolled in a contemporary British cohort. J Adolesc Health 2010;47:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM.. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev 2007;8:385–394. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Donaldson MD, Ben-Shlomo Y.. SITAR – a useful instrument for growth curve analysis. Int J Epidemiol 2010;39:1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Practice and Ambulatory Medicine and Bright Futures Periodicity Schedule Workgroup. 2015 Recommendations for Preventive Pediatric Health Care Committee on Practice and Ambulatory Medicine and Bright Futures Periodicity Schedule Workgroup. Pediatrics 2015;136:e727. [DOI] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J.. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff J, Reeves JW, Hyland C, Tilles S, Rauch S, Kogut K, Greenspan LC, Shirtcliff E, Lustig RH, Eskenazi B. et al. Childhood overweight and obesity and pubertal onset among Mexican American boys and girls in the CHAMACOS longitudinal study. Am J Epidemiol 2021; doi: 10.1093/aje/kwab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lunddorf LLH, Olsen J, Ramlau-Hansen CH.. Placental weight Z-score and pubertal timing: a population-based cohort study. Paediatr Perinat Epidemiol 2021;35:206–216. [DOI] [PubMed] [Google Scholar]
- Fechner PY. Gender differences in puberty. J Adolesc Health 2002;30:44–48. [DOI] [PubMed] [Google Scholar]
- Geller EJ, Wu JM, Jannelli ML, Nguyen TV, Visco AG.. Neonatal outcomes associated with planned vaginal versus planned primary cesarean delivery. J Perinatol 2010;30:258–264. [DOI] [PubMed] [Google Scholar]
- Gibbons L, Belizán JM, Lauer JA, Betrán AP, Merialdi M, Althabe F.. The global numbers and costs of additionally needed and unnecessary caesarean sections performed per year: overuse as a barrier to universal coverage. World Health Report 2010;30:1–31. [Google Scholar]
- Gingras V, Rifas-Shiman SL, Derks IPM, Aris IM, Oken E, Hivert MF.. Associations of gestational glucose tolerance with offspring body composition and estimated insulin resistance in early adolescence. Diabetes Care 2018;41:e164–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados A, Gebremariam A, Lee JM.. Relationship between timing of peak height velocity and pubertal staging in boys and girls. J Clin Res Pediatr Endocrinol 2015;7:235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KD, Jackson S, Korst L, Fridman M.. Cesarean versus vaginal delivery: whose risks? Whose benefits? Am J Perinatol 2012;29:7–18. [DOI] [PubMed] [Google Scholar]
- Hakonsen LB, Olsen J, Stovring H, Ernst A, Thulstrup AM, Zhu JL, Shrestha A, Ramlau-Hansen CH.. Maternal cigarette smoking during pregnancy and pubertal development in sons. A follow-up study of a birth cohort. Andrology 2013;1:348–355. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JA.. Structural approach to selection bias. Epidemiology 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- Huh SY, Rifas-Shiman SL, Zera CA, Edwards JW, Oken E, Weiss ST, Gillman MW.. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child 2012;97:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidt JJ, Brix N, Ernst A, Lauridsen LLB, Ramlau-Hansen CH.. Size at birth, infant growth, and age at pubertal development in boys and girls. Clin Epidemiol 2019;11:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai CM, Gunnarsdottir I, Gudnason V, Aspelund T, Birgisdottir BE, Thorsdottir I, Halldorsson TI.. Early peak height velocity and cardiovascular disease mortality among Icelandic women. Ann Med 2013;45:545–550. [DOI] [PubMed] [Google Scholar]
- Khouja JN, Simpkin AJ, O’Keeffe LM, Wade KH, Houtepen LC, Relton CL, Suderman M, Howe LD.. Epigenetic gestational age acceleration: a prospective cohort study investigating associations with familial, sociodemographic and birth characteristics. Clin Epigenet 2018;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman-Verhoeff ME, Gredvig-Ardito C, Barker DH, Saletin JM, Carskadon MA.. Classifying pubertal development using child and parent report: comparing the pubertal development scales to tanner staging. J Adolesc Health 2020;66:597–602. [DOI] [PubMed] [Google Scholar]
- Kubo A, Deardorff J, Laurent CA, Ferrara A, Greenspan LC, Quesenberry CP, Kushi LH.. Associations between maternal obesity and pregnancy hyperglycemia and timing of puberty onset in adolescent girls: a population-based study. Am J Epidemiol 2018;187:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle S, Tong OS, Woolcott CG.. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev 2015;16:295–303. [DOI] [PubMed] [Google Scholar]
- Li W, Liu Q, Deng X, Chen Y, Liu S, Story M.. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health 2017;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunddorf LLH, Brix N, Ernst A, Arendt LH, Stovring H, Clemmensen PJ, Olsen J, Ramlau-Hansen CH.. Hypertensive disorders in pregnancy and timing of pubertal development in daughters and sons. Hum Reprod 2020;35:2124–2133. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM.. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM.. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Calvo N, Martinez-Gonzalez MA, Segura G, Chavarro JE, Carlos S, Gea A.. Caesarean delivery is associated with higher risk of overweight in the offspring: within-family analysis in the SUN cohort. J Epidemiol Commun Health 2020;74:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KA, Devlin JC, Lacher CR, Yin Y, Cai Y, Wang J, Dominguez-Bello MG.. Increased weight gain by C-section: functional significance of the primordial microbiome. Sci Adv 2017;3:eaao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah FK, Bayer JK, Wake M, Carlin JB, Allen NB, Patton GC.. Early puberty and childhood social and behavioral adjustment. J Adolesc Health 2013;53:118–124. [DOI] [PubMed] [Google Scholar]
- Mueller NT, Zhang M, Rifas-Shiman SL, Oken E, Hivert MF, Chavarro J.. Mode of delivery, type of labor, and measures of adiposity from childhood to teenage: Project Viva. Int J Obes 2021;45:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NM, Bager P, Stenager E, Pedersen BV, Koch-Henriksen N, Hjalgrim H, Frisch M.. Cesarean section and offspring’s risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler 2013;19:1473–1477. [DOI] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM. et al. Cohort profile: project viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW.. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman MJ, Martin JA.. Trends in low-risk cesarean delivery in the United States, 1990-2013. Natl Vital Stat Rep 2014;63:1–16. [PubMed] [Google Scholar]
- Perng W, Rifas-Shiman SL, Hivert MF, Chavarro JE, Oken E.. Branched chain amino acids, androgen hormones, and metabolic risk across early adolescence: a prospective study in Project Viva. Obesity (Silver Spring) 2018;26:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A.. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988;17:117–133. [DOI] [PubMed] [Google Scholar]
- Reinehr T, Roth CL.. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health 2019;3:44–54. [DOI] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Gillman MW, Hawkins SS, Oken E, Taveras EM, Kleinman KP.. Association of cesarean delivery with body mass index z score at age 5 years. JAMA Pediatr 2018;172:777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Huh SY, Martin RM, Kramer M, Patel R, Bogdanovich N, Vilchuck K, Thompson J, Oken E.. Delivery by caesarean section and offspring adiposity and cardio-metabolic health at ages 6.5, 11.5 and 16 years: results from the PROBIT cohort in Belarus. Pediatr Obes 2021;16:e12783. [DOI] [PubMed] [Google Scholar]
- Sandhu J, Davey Smith G, Holly J, Cole TJ, Ben-Shlomo Y.. Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab 2006;91:3150–3157. [DOI] [PubMed] [Google Scholar]
- Schmitz KE, Hovell MF, Nichols JF, Irvin VL, Keating K, Simon GM, Gehrman C, Jones KL.. A validation study of early adolescents’ pubertal self-assessments. J Early Adolesc 2004;24:357–384. [Google Scholar]
- Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H.. Cesarean section and chronic immune disorders. Pediatrics 2015;135:e92–e98. [DOI] [PubMed] [Google Scholar]
- Souza JP, Gülmezoglu A, Lumbiganon P, Laopaiboon M, Carroli G, Fawole B, Ruyan P; WHO Global Survey on Maternal and Perinatal Health Research Group. Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: the 2004-2008 WHO Global Survey on Maternal and Perinatal Health. BMC Med 2010;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Girchenko P, Andersson S, Strandberg TE, Reynolds RM, Kajantie E, Binder EB.. The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clin Epigenet 2018;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS.. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 2002;110:911–919. [DOI] [PubMed] [Google Scholar]
- Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR.. A meta-analysis of the association between caesarean section and childhood asthma. Clin Exp Allergy 2008;38:629–633. [DOI] [PubMed] [Google Scholar]
- Walker IV, Smith CR, Davies JH, Inskip HM, Baird J.. Methods for determining pubertal status in research studies: literature review and opinions of experts and adolescents. J Dev Orig Health Dis 2020;11:168–187. [DOI] [PubMed] [Google Scholar]
- Widen E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen A-L, Laitinen J, Pouta A, Kaprio J, Jarvelin M-R. et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care 2012;35:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, Field AE, Chavarro JE.. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr 2016;170:e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.