Abstract
STUDY QUESTION
Is there an association between maternal occupational exposure to endocrine-disrupting chemicals (EDCs) early in pregnancy and subgroups of congenital anomalies of kidney and urinary tract (CAKUT), and hypospadias?
SUMMARY ANSWER
Exposure to specific EDCs can increase the risk of CAKUT and no association with hypospadias was observed.
WHAT IS KNOWN ALREADY
Previous studies showed an association between maternal occupational exposure to EDCs and hypospadias. However, little is known about the effect of these chemicals on the development of CAKUT, especially subgroups of urinary tract anomalies.
STUDY DESIGN, SIZE, DURATION
For this case–control study, cases with urogenital anomalies from the European Concerted Action on Congenital Anomalies and Twins Northern Netherlands (Eurocat NNL) registry and non-malformed controls from the Lifelines children cohort (living in the same catchment region as Eurocat NNL) born between 1997 and 2013 were selected. This study included 530 cases with CAKUT, 364 cases with hypospadias, 7 cases with both a urinary tract anomaly and hypospadias and 5602 non-malformed controls. Cases with a genetic or chromosomal anomaly were excluded, and to avoid genetic correlation, we also excluded cases in which a sibling with the same defect was included.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Information on maternal occupation held early in pregnancy was collected via self-administered questionnaires. Job titles were translated into occupational exposure to EDCs using a job-exposure matrix (JEM). Adjusted odds ratios (aORs) and 95% CIs were estimated to assess the association between maternal occupational exposure to EDCs (and to specific types of EDCs) and CAKUT and hypospadias.
MAIN RESULTS AND THE ROLE OF CHANCE
For CAKUT and hypospadias, 23.1% and 22.9% of the cases were exposed to EDCs, respectively, whereas 19.8% of the controls were exposed. We found an association between maternal occupational exposure to organic solvents/alkylphenolic compounds and CAKUT (aOR 1.41, 95% CI 1.01–1.97) that became stronger when combinations of urinary tract anomalies co-occurred with other defects (aOR 7.51, 95% CI 2.41–23.43). An association was also observed for exposure to phthalates/benzophenones/parabens/siloxanes and CAKUT (aOR 1.56, 95% CI 1.06–2.29), specifically urinary collecting system anomalies (aOR 1.62, 95% CI 1.03–2.54) and combinations of urinary tract anomalies (aOR 2.90, 95% CI 1.09–7.71). We observed no association between EDC exposure and hypospadias.
LIMITATIONS, REASONS FOR CAUTION
The different study designs of Eurocat NNL and Lifelines could have introduced differential information bias. Also, exposure misclassification could be an issue: it is possible that the actual exposure differed from the exposure estimated by the JEM. In addition, women could also have been exposed to other exposures not included in the analysis, which could have resulted in residual confounding by co-exposures.
WIDER IMPLICATIONS OF THE FINDINGS
Women, their healthcare providers, and their employers need to be aware that occupational exposure to specific EDCs early in pregnancy may be associated with CAKUT in their offspring. An occupational hygienist should be consulted in order to take exposure to those specific EDCs into consideration when risk assessments are carried out at the workplace.
STUDY FUNDING/COMPETING INTEREST(S)
N.S. was paid by the Graduate School of Medical Sciences (MD/PhD programme), University Medical Center Groningen (UMCG), Groningen, the Netherlands. Eurocat Northern Netherlands is funded by the Dutch Ministry of Health, Welfare and Sports. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University Groningen and the Northern Provinces of the Netherlands. The authors report no conflict of interest.
TRIAL REGISTRATION NO
N/A.
Keywords: congenital anomalies of kidney and urinary tract, hypospadias, periconceptional, occupation, exposure, endocrine-disrupting chemicals
Introduction
Urogenital anomalies are congenital anomalies representing any defect in the organs and tissues responsible for the formation and excretion of urine. The total prevalence of congenital anomalies of kidney and urinary tract (CAKUT) is 31 per 10 000 births in Europe (EUROCAT, 2017). These anomalies comprise a broad range of disorders that result from abnormal embryonic renal development, such as renal parenchymal malformations, abnormalities in renal migration or abnormalities of the urinary collecting system. The severity of urinary anomalies can differ. Total absence of the kidneys will cause neonatal death, whereas milder kidney and urinary collecting system anomalies (e.g. vesico-ureteral-renal reflux—the retrograde passage of urine from the bladder into the upper urinary tract) can lead to chronic renal failure if untreated (Salmasi et al., 2010).
Hypospadias is the most common genital anomaly, with a total prevalence of 14 per 10 000 births in Europe (EUROCAT, 2017). Hypospadias is present only in males and is characterised by an abnormal position of the urethral opening that ranges from the urethral opening being near the tip of the penis to further down the shaft of the penis, scrotum or in the perineum. Most types of hypospadias need surgical correction after birth.
Foetal development occurs under the influence of hormones. It has been proposed that hypospadias develops through disruption of the androgenic stimulation required for the development of the normal male external genitalia. Both genetic and environmental factors can negatively affect androgenic stimulation (Baskin, 2019). For CAKUT, the pathogenesis is largely unknown, but both genetic and environmental factors are thought to be involved (Rosenblum, 2017). Exposure to certain chemicals can influence hormonal activity and adversely affect foetal development of the urogenital tract (Yiee and Baskin, 2010). These potentially endocrine-disrupting chemicals (EDCs) can be man-made or naturally occurring. Sources of EDC exposure in the general population are diet, personal care products, cosmetics, plastics, textiles and construction materials, among others (Brouwers et al., 2009; World Health Organisation and United Nations Environment Program, (UNEP), 2012). However, a relatively high level of exposure may occur in specific occupations as EDCs are present in a large variety of materials and products used in the workplace, such as pesticides, phthalates and organic solvents, or can be by-products formed during manufacturing, for example dioxins (Brouwers et al., 2009; World Health Organisation and United Nations Environment Program, (UNEP), 2012).
Several studies have found an association between maternal occupational exposures to EDCs and hypospadias (Ormond et al., 2009; Haraux et al., 2016), while a few other studies have found associations between specific EDCs, such as pesticides (Rocheleau et al., 2009) and solvents (Kalfa et al. 2015; Warembourg et al., 2018), and hypospadias. However, other studies have found no association between maternal occupational exposure to pesticides or metals and increased risk of hypospadias (Zhu et al., 2006; Spinder et al., 2019).
Studies regarding maternal occupational exposure early in pregnancy in relation to CAKUT are limited. One study found an association between maternal occupational exposure to solvents and urinary tract malformations (Garlantézec et al., 2009). However, only 13 cases were included in this prospective study. Another study found no association between solvent exposure and urinary tract malformations (Cordier et al., 1997). Neither of these studies differentiated between specific subcategories of CAKUT. The aim of our study was thus to examine the association between maternal occupational exposure to EDCs early in pregnancy and subgroups of CAKUT and hypospadias in the offspring.
Materials and methods
Study design
This is a case–control study. Cases were selected from the European Concerted Action on Congenital Anomalies and Twins Northern Netherlands (Eurocat NNL) registry. Since 1981, this registry has been collecting data on children born with congenital anomalies in the northern Dutch provinces of Groningen, Friesland and Drenthe. Eurocat NNL registers live births (up to 10 years of age at notification), stillbirths, miscarriages and pregnancies terminated because of a congenital anomaly. After the parents have given informed consent, they are asked to complete a questionnaire that includes items on pregnancy characteristics, parental medical history, demographic and occupational characteristics, pre-pregnancy weight and height, and smoking, alcohol and medication use during the periconceptional period (3 months before pregnancy to the end of the first trimester).
Controls for this study were selected from the Lifelines cohort. Lifelines is a multi-disciplinary prospective population-based cohort study examining in a three-generation design the health and health-related behaviours of 167 729 persons living in the same catchment region as Eurocat NNL. Participants were recruited through general practitioners in the study area. Children were invited to participate if one parent was already a participant in Lifelines. Children were included from 6 months of age until their 18th birthday after parents or the child has given informed consent. Parents of participating children received a questionnaire with questions on pregnancy, childbirth and health of the child in the first 6 months of life. Detailed information about the Lifelines cohort study has been published previously (Scholtens et al., 2015).
Ethical approval
Eurocat NNL and Lifelines operate within the scope of the Dutch data protection act according to the principles of the Declaration of Helsinki, and in accordance with the Code of Good conduct. Eurocat NNL did not require ethics committee approval to collect and store data according to the medical ethical committee of the University Medical Center Groningen, in line with the Dutch act Medical Research involving Human Subjects. Lifelines is approved by the medical ethical committee of the University Medical Center Groningen. All parents and participants of Eurocat NNL and Lifelines give informed consent before participation.
Case and control definition
Children, foetuses and terminated pregnancies affected by a major anomaly of the urogenital tract born between 1997 and 2013 were selected from Eurocat NNL. Coding and classification of congenital anomalies were performed according to EUROCAT guidelines (Greenlees et al., 2011; EUROCAT, 2013). Since there are many different types of CAKUT, we classified cases into four groups of urinary tract anomalies: one, malformations of the renal parenchyma; two, anomalies of the urinary collecting system; three, abnormal embryonic migration of kidneys and other urinary tract anomalies; and four, combinations of urinary anomalies (Rosenblum, 2017; Bakker et al., 2018). The anomalies included in these categories are listed in Table I. The primary urinary tract anomaly was used for categorisation. For example, if a child developed renal dysplasia as a consequence of an ureteropelvic junction stenosis (UPJ stenosis), the anomaly was classified as UPJ stenosis and categorised under ‘anomalies of the urinary collecting system’. The only genital tract anomaly included in this study was hypospadias (Table I). Infants with both hypospadias and CAKUT were counted in both main categories (n = 7). Cases with a genetic or chromosomal anomaly were excluded. To avoid genetic correlation, we also excluded cases in which a sibling with the same defect was included.
Table I.
Classes of urogenital anomalies.
| Anomaly | Classes | Included urinary tract anomalies |
|---|---|---|
| Urinary anomalies | I Malformations of the renal parenchyma | Renal agenesis, renal hypoplasia, multicystic dysplastic kidneys, cystic kidney and renal dysplasia |
| II Anomalies of the urinary collecting system | Hypdronephrosis (end stage of obstructive anomalies), ureteropelvic junction stenosis, megaloureter, hydroureter, duplication of ureter, vesico-uretero-renal reflux, epispadias, exstrophy of urinary bladder, OEIS complex, (posterior) urethral valves, stenosis, atresia of urethra and bladder neck, and absence of bladder and urethra | |
| III Abnormal embryonic migration of kidneys and other urinary tract anomalies | Pelvic kidney, horseshoe kidney, other malformations of the urinary system | |
| IV Combinations of urinary anomalies | Presence of at least two types of urinary tract anomalies, both considered to be primary anomalies and belonging to at least two categories | |
| Genital anomalies | Hypospadias | Glandular,* coronal, penile, penoscrotal and perineal hypospadias |
Included from birth year 2005 onwards according to EUROCAT guidelines (Greenlees et al., 2011; EUROCAT, 2013).
EUROCAT, the European Concerted Action on Congenital Anomalies and Twins Northern Netherlands registry; OEIS complex, omphalocele-exstrophy-imperforate anus-spinal defects.
Controls were defined as children without congenital anomalies born between 1997 and 2013. We only selected infants whose biological mother participated in Lifelines. Parents were asked if their child was born with a congenital anomaly. Since linkage with Eurocat NNL was not possible and parental descriptions of the congenital anomaly were poor, we could not include these infants as cases. Lifelines is a three-generation study, which aimed at including multiple family members, therefore, one infant per mother was included to avoid genetic correlation.
Exposure assessment
Mothers were asked to report their occupation, including industry of employment, held during early pregnancy (first trimester). By restricting our analyses to employed women, we controlled for confounding by employment status and related factors. This is important because employment status is related to sociodemographic and (reproductive) health characteristics that are generally recognised risk factors for adverse pregnancy outcomes (Rocheleau et al., 2017; Johnson et al., 2019). The maternal job descriptions of children included from the Eurocat registry and Lifelines cohort were coded by two authors (N.S. and H.K.) into the International Standard Classification of Occupational 1988 (ISCO88; International Labor Office, 1988), without knowledge of case or study details. To translate the ISCO88 codes into occupational exposure, we used a job-exposure matrix (JEM) developed by van Tongeren et al. (2002). The original JEM was developed to study the risk of hypospadias after maternal exposure to EDCs (van Tongeren et al., 2002) and later updated to improve its performance (Brouwers et al., 2009). Chemicals incorporated in this JEM were identified from the literature and classified into nine chemical groups: polycyclic aromatic hydrocarbons (PAHs), polychlorinated organic compounds, pesticides, phthalates, organic solvents, bisphenol A, alkylphenolic compounds, brominated flame retardants and a miscellaneous group (benzophenones, parabens and siloxanes). Three occupational experts scored the exposure to each chemical group as ‘unlikely, possible or probable’ using job titles from the Standard Occupation Classification (SOC). Two authors (N.S. and H.K.) translated SOC codes into the ISCO88 classification to make the JEM applicable to the data in this study. Owing to sparse data (<5 exposed cases), exposures to polychloride organic compounds, bisphenol A and flame retardants were not analysed. Detailed information on the JEM has been published elsewhere (Brouwers et al., 2009).
Statistical analyses
The selection of covariates was based on associations observed in prior literature. Infant and maternal characteristics for cases and controls were tabulated. Variables registered in both Eurocat NNL and the Lifelines cohort were: child sex, birth year, maternal age at delivery, maternal body BMI—the self-reported pre-pregnancy weight and height for Eurocat NNL cases or objective measurement at baseline visit for Lifelines controls (underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2))—, maternal education level (low (primary school, lower vocational education, pre-vocational education), middle (secondary vocational education, general secondary education or pre-university education) or high (higher professional education or academic education)), maternal smoking (no, yes/some period during pregnancy), maternal alcohol use during pregnancy (no, yes/some period during pregnancy), folic acid use (no/wrong period, yes/sometime during periconceptional period) and fertility problems (no, yes (self-reported fertility problems or fertility treatment)) (Pierik et al., 2004; Ormond et al., 2009; Brouwers et al., 2010; Nicolaou et al., 2015).
The correlation between EDC subgroups was explored in mothers of controls to determine the best modelling strategy. Owing to high correlation, organic solvents and alkylphenolic compounds were combined into a single exposure group (exposure to at least one exposure in this group), as were phthalates, benzophenones, parabens and siloxanes.
The association between maternal occupational exposures was assessed separately for the four classes of CAKUT and hypospadias. Because hypospadias is only present in boys, only boys were selected as controls for the hypospadias analyses. The associations between any occupational exposures (possible/probable) and CAKUT and hypospadias were assessed using univariate and multivariate logistic regression to estimate crude odds ratios (OR) and adjusted odds ratios (aORs), respectively. Cases and controls with no occupational exposure to EDCs included in the analysis were used as the reference category. Multivariate logistic regression analyses were adjusted for birth year, maternal age at delivery, maternal BMI, and smoking and alcohol use during pregnancy, based on χ2 tests (Supplementary Table SI). Multivariate logistic regressions regarding CAKUT were additionally adjusted for child sex, folic acid use and fertility problems. Stratified analyses were conducted for isolated urogenital defects and for urogenital defects co-occurring with congenital anomalies because these may differ in aetiology. To account for the likelihood of exposure, subgroup analyses were performed only including mothers who had a job ‘probably’ involving exposure to EDCs, meaning that exposure was likely to occur in more than 10% of workers with this job. We also performed analyses using ‘no exposure’ to any occupational EDCs as the reference category. Also, stratified analyses were performed for males and females. The Statistical Package for the Social Sciences version 23 (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA) was used to perform all analyses.
Results
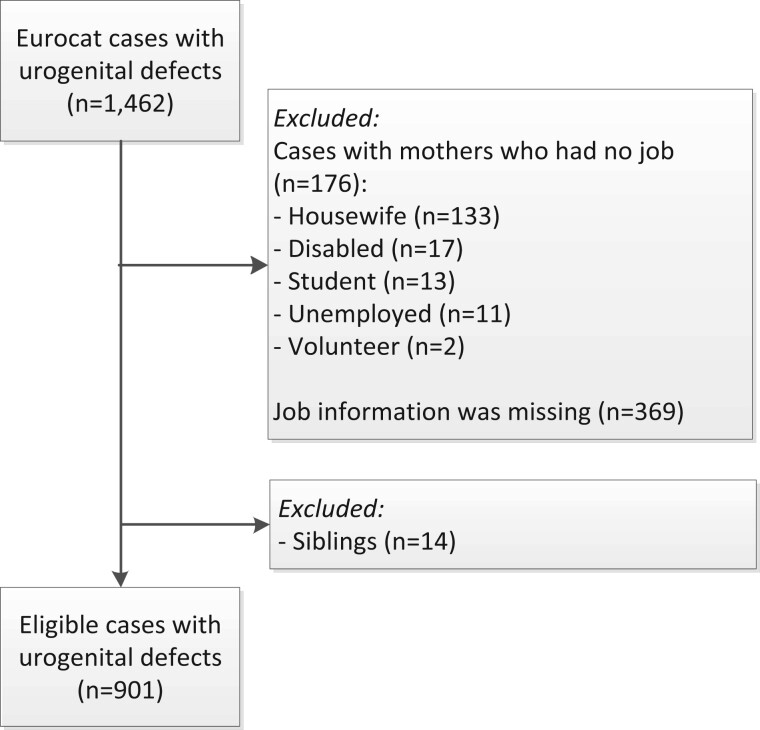
We selected 1462 cases with urogenital defects from Eurocat NNL (Fig. 1). Cases were excluded because mothers had no job early in pregnancy (n = 176) or because job information was missing (n = 369). Fourteen cases were excluded because siblings with the same defect were included. We included 530 cases with CAKUT, 364 cases with hypospadias and 7 cases with both a urinary tract anomaly and hypospadias. For CAKUT cases, 12% were miscarriages, stillbirths or terminated pregnancies because of a congenital anomaly. All hypospadias cases were live births.
Figure 1.
Flowchart for case selection from the European Concerted Action on Congenital Anomalies and Twins Northern Netherlands registry, 1997–2013.
The Lifelines children’s cohort consisted of 12 494 potentially eligible infants born from 1997 to 2013 (Fig. 2). From these, we excluded 163 non-biological infants. A further 814 children were excluded because their parents reported one or more congenital anomalies or it was unknown if the child was born with a congenital anomaly. Another 3029 children were excluded because their mother did not work early in pregnancy or job information was missing. One child per parent was selected, which resulted in the exclusion of another 2886 children. In total, 5602 children without congenital anomalies were selected as the control group.
Figure 2.
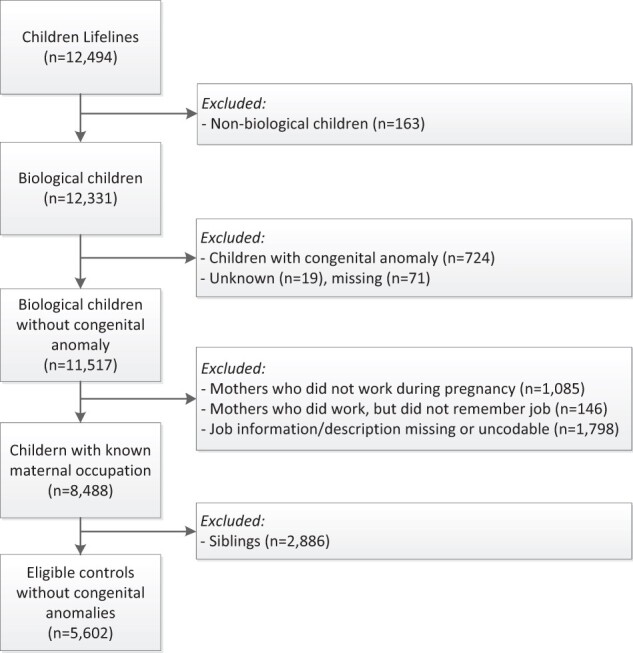
Flowchart for control selection from the Lifelines population-based cohort study Northern Netherlands, 1997–2013.
Children affected by a urinary tract anomaly were more often boys (70.2% of Eurocat NNL cases were male versus 48.8% of the Lifelines cohort, Supplementary Table SI). For both CAKUT and hypospadias cases, the mothers appeared to be younger at delivery and had a lower BMI compared to controls. Mothers of cases were also more often smokers and more often used alcohol during pregnancy compared to controls. The mothers of CAKUT cases used folic acid less often and more often had fertility problems.
Congenital anomalies of kidney and urinary tract
The percentage of women exposed to EDCs and the ORs for CAKUT are shown in Table II. For CAKUT, 23.1% of the cases and 19.8% of the controls were exposed to ‘any’ EDCs. After adjustment, an OR of 1.21 (95% CI 0.96–1.53) was observed between exposure to ‘any’ EDCs and CAKUT when comparing to non-exposed infants. When we looked into specific types of EDCs, we observed associations for exposure to organic solvents/alkylphenolic compounds (aOR 1.41, 95% CI 1.01–1.97) and exposure to phthalates/benzophenones/parabens/siloxanes (aOR 1.56, 95% CI 1.06–2.29). No associations were observed for exposure to PAHs or pesticides when comparing to non-exposed infants (aOR 1.06, 95% CI 0.73–1.53 and aOR 0.53, 95% CI 0.22–1.25, respectively).
Table II.
Prevalence, crude and adjusted odds ratios of maternal occupational exposure to endocrine-disrupting chemicals, and the risk of urinary anomalies in the offspring (Eurocat) compared to non-malformed controls (Lifelines), Northern Netherlands, 1997–2013.
Organic solvents/alkylphenolic compoundsc
| Occupational exposure | Total | Unexposed |
Exposeda |
Unadjusted |
Adjustedb |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | n | (%) | n | (%) | OR | 95% CI | OR | 95% CI | |
| Any EDC | |||||||||
| Controls | 5602 | 4491 | (80.2) | 1111 | (19.8) | 1.00 | 1.00 | ||
| Urinary anomalies | 537 | 413 | (76.9) | 124 | (23.1) | 1.21 | (0.98–1.50) | 1.21 | (0.96–1.53) |
| Malformations of the renal parenchyma | 109 | 85 | (78.0) | 24 | (22.0) | 1.14 | (0.72–1.80) | 1.04 | (0.64–1.69) |
| Anomalies of the urinary collecting system | 360 | 274 | (76.1) | 86 | (23.9) | 1.27 | (0.99–1.63) | 1.29 | (0.98–1.69) |
| Abnormal embryonic migration of kidneys | 25 | 20 | (80.0) | 5 | (20.0) | 1.01 | (0.38–2.67) | 0.84 | (0.28–2.52) |
| Combination of urinary tract anomalies | 43 | 34 | (79.1) | 9 | (20.9) | 1.07 | (0.51–2.24) | 1.10 | (0.51–2.35) |
| PAHs | |||||||||
| Controls | 5602 | 5251 | (93.7) | 351 | (6.3) | 1.00 | 1.00 | ||
| Urinary total | 537 | 498 | (92.7) | 39 | (7.3) | 1.17 | (0.83–1.65) | 1.06 | (0.73–1.53) |
| Malformations of the renal parenchyma | 109 | 104 | (95.4) | 5 | (4.6) | 0.72 | (0.29–1.78) | 0.51 | (0.18–1.41) |
| Anomalies of the urinary collecting system | 360 | 330 | (91.7) | 30 | (8.3) | 1.36 | (0.92–2.01) | 1.28 | (0.84–1.94) |
| Abnormal embryonic migration of kidneys | 25 | <5 | NC | NC | |||||
| Combination of urinary tract anomalies | 43 | <5 | NC | NC | |||||
| Pesticides | |||||||||
| Controls | 5602 | 5486 | (97.9) | 116 | (2.1) | 1.00 | 1.00 | ||
| Urinary total | 537 | 531 | (98.8) | 6 | (1.1) | 0.53 | (0.23–1.22) | 0.53 | (0.22–1.25) |
| Malformations of the renal parenchyma | 109 | <5 | NC | NC | |||||
| Anomalies of the urinary collecting system | 360 | 355 | (98.6) | 5 | (1.4) | 0.67 | (0.27–1.64) | 0.65 | (0.25–1.66) |
| Abnormal embryonic migration of kidneys | 25 | <5 | NC | NC | |||||
| Combination of urinary tract anomalies | 43 | <5 | NC | NC | |||||
| Organic Solvents/Alkylphenolic compoundsc | |||||||||
| Controls | 5602 | 5220 | (93.2) | 382 | (6.8) | 1.00 | 1.00 | ||
| Urinary total | 537 | 486 | (90.5) | 51 | (9.5) | 1.43 | (1.06–1.95) | 1.41 | (1.01–1.97) |
| Malformations of the renal parenchyma | 109 | 100 | (91.7) | 9 | (8.3) | 1.23 | (0.62–2.45) | 1.17 | (0.58–2.38) |
| Anomalies of the urinary collecting system | 360 | 326 | (90.6) | 34 | (9.4) | 1.43 | (0.99–2.06) | 1.39 | (0.93–2.06) |
| Abnormal embryonic migration of kidneys | 25 | <5 | NC | NC | |||||
| Combination of urinary tract anomalies | 43 | 37 | (86.0) | 6 | (14) | 2.22 | (0.93–5.28) | 2.16 | (0.88–5.30) |
| Phthalates/benzophenones/parabens/siloxanesc | |||||||||
| Controls | 5602 | 5348 | (95.5) | 254 | (4.5) | 1.00 | 1.00 | ||
| Urinary total | 537 | 500 | (93.1) | 37 | (6.9) | 1.56 | (1.09–2.23) | 1.56 | (1.06–2.29) |
| Malformations of the renal parenchyma | 109 | 104 | (95.4) | 5 | (4.6) | 1.01 | (0.41–2.51) | 0.92 | (0.36–2.32) |
| Anomalies of the urinary collecting system | 360 | 334 | (92.8) | 26 | (7.2) | 1.64 | (1.08–2.49) | 1.62 | (1.03–2.54) |
| Abnormal embryonic migration of kidneys | 25 | <5 | NC | NC | |||||
| Combination of urinary tract anomalies | 43 | 38 | (88.4) | 5 | (11.6) | 2.77 | (1.08–7.10) | 2.90 | (1.09–7.71) |
Possible or probable exposure.
Adjusted for child sex, birth year, maternal age and BMI, smoking, alcohol, and folic acid use during pregnancy and fertility problems.
Exposure to at least one exposure in this group.
OR, odds ratio; EDCs, endocrine-disrupting chemicals; PAHs, polycyclic aromatic hydrocarbons; NC, not calculated owing to sparse data (<5 exposed cases).
Subgroup analyses for specific classes of CAKUT were performed (Table II). The aOR for anomalies of the urinary collecting system was higher compared to all urinary tract anomalies (aOR 1.29, 95% CI 0.98–1.69). The aORs for other subgroups anomalies were lower and ranged from 0.84 (95% CI 0.28–2.52) for abnormal embryonic migration of the kidneys to 1.10 (95% CI 0.51–2.35) for combinations of urinary tract anomalies. An association was found between occupational exposure to phthalates/benzophenones/parabens/siloxanes and anomalies of the urinary collecting system (aOR 1.62, 95% CI 1.03–2.54) and combinations of CAKUT (aOR 2.90, 95% CI 1.09–7.71). We found no associations with anomalies of the renal parenchyma and abnormal embryonic migration of the kidney.
When we performed a stratified analysis for isolated CAKUT (n = 420, Supplementary Table SII) and CAKUT co-occurring with other congenital anomalies (n = 117, Supplementary Table SIII), the association between occupational exposure to phthalates/benzophenones/parabens/siloxanes was stronger for isolated cases (aOR 1.63, 95% CI 1.07–2.49) than for CAKUT co-occurring with congenital anomalies (aOR 1.20, 95% CI 0.51–2.80), specifically for anomalies of the urinary collecting system (aOR 1.76, 95% CI 1.11–2.79). The association between exposure to organic solvents/alkylphenolic compounds and combinations of urinary tract anomalies became stronger when restricting the analysis to CAKUT co-occurring with congenital anomalies (aOR 7.51, 95% CI 2.41–23.43).
Stratified analyses for males and females affected by CAKUT were performed (n = 377 and n = 160, respectively, Supplementary Tables SIV and SV). The association between exposure to phthalates/benzophenones/parabens/siloxanes and anomalies of the urinary collecting system was only present in male offspring (aOR 1.85, 95% CI 1.06–3.23), whereas the association between organic solvents/alkylphenolic compounds and CAKUT was only present in females (aOR 1.87, 95% CI 1.11–3.14).
Subgroup analyses performed for mothers with ‘probable’ exposure to EDCs according to the JEM and for women not exposed to any EDC (the reference category) did not materially change the results (Supplementary Tables SVI and SVII).
Hypospadias
The exposure rates and ORs for hypospadias are shown in Table III. For hypospadias, 22.9% of the cases and 18.9% of the controls were exposed to ‘any’ EDCs. We observed no association between EDCs in general and hypospadias in offspring (aOR 1.26, 95% CI 0.95–1.65), nor did we observe an association between subcategories of EDCs and hypospadias.
Table III.
Prevalence, crude and adjusted OR of maternal occupational exposure to EDCs and the risk of hypospadias in the offspring (Eurocat) compared to non-malformed controls (Lifelines), Northern Netherlands, 1997–2013.
| Occupational exposure | Total | Unexposed |
Exposeda |
Unadjusted |
Adjustedb |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | n | (%) | n | (%) | OR | 95% CI | OR | 95% CI | |
| Any EDC | |||||||||
| Controlsc | 2731 | 2214 | (81.1) | 517 | (18.9) | 1.00 | 1.00 | ||
| Hypospadias | 371 | 286 | (77.1) | 85 | (22.9) | 1.27 | (0.98–1.65) | 1.26 | (0.95–1.65) |
| PAHs | |||||||||
| Controlsc | 2731 | 2560 | (93.7) | 171 | (6.3) | 1.00 | 1.00 | ||
| Hypospadias | 371 | 340 | (91.6) | 31 | (8.4) | 1.37 | (0.92–2.03) | 1.37 | (0.91–2.07) |
| Pesticides | |||||||||
| Controlsc | 2731 | 2685 | (98.3) | 46 | (1.7) | 1.00 | 1.00 | ||
| Hypospadias | 371 | 366 | (98.7) | 5 | (1.3) | 0.80 | (0.32–2.02) | 0.76 | (0.30–1.98) |
| Organic solvents/alkylphenolic compoundsd | |||||||||
| Controlsc | 2731 | 2560 | (96.7) | 171 | (3.3) | 1.00 | 1.00 | ||
| Hypospadias | 371 | 347 | (93.5) | 24 | (6.5) | 1.02 | (0.66–1.59) | 0.94 | (0.59–1.48) |
| Phthalates/benzophenones/parabens/siloxanesd | |||||||||
| Controlsc | 2731 | 2620 | (95.9) | 111 | (4.1) | 1.00 | 1.00 | ||
| Hypospadias | 371 | 351 | (95.6) | 20 | (5.4) | 1.35 | (0.83–2.19) | 1.21 | (0.73–2.01) |
Possible or probable exposure.
Adjusted for birth year, maternal age and BMI smoking and alcohol use during pregnancy.
Only boys are selected as controls.
Exposure to at least one exposure in this group.
EDC, endocrine-disrupting chemical; PAHs, polycyclic aromatic hydrocarbons.
We performed stratified analyses of isolated hypospadias (n = 341) and hypospadias co-occurring with other congenital anomalies (n = 30; Supplementary Table SVIII). An increased aOR was found for ‘any’ EDCs exposure and hypospadias that co-occurred with another congenital anomalies (aOR 1.46, 95% CI 0.63–3.39) compared to isolated hypospadias (aOR 1.23, 95% CI 0.92–1.64). Subgroup analyses for mothers with ‘probable’ exposure to EDCs or using women not exposed to any EDC as reference category did not materially change the results (Supplementary Tables SIX and SX).
Discussion
In this study, we found an association between maternal occupational exposure to organic solvents/alkylphenolic compounds and phthalates/benzophenones/parabens/siloxanes and CAKUT, specifically for anomalies of the urinary collecting system when more than one urinary tract anomaly was present. Women exposed to organic solvents/alkylphenolic compounds were working in the agricultural sector or as life-science technicians. Women exposed to phthalates/benzophenones/parabens/siloxanes worked mainly as cleaners, hairdressers or beauticians.
Strengths and weaknesses of the study
For this case–control study, we used case data from the high-quality population-based Eurocat NNL registry. Detailed medical information was available for each case, and all cases were coded by trained registry staff according to international coding guidelines (EUROCAT, 2013). These factors made it possible to distinguish between classes of urinary tract anomalies and between isolated defects and urinary tract defects that co-occurred with other anomalies. A major strength of this study is that we used non-malformed controls. We selected controls from Lifelines, a large population-based cohort that recruited its participants from the same region as Eurocat. The Lifelines cohort is representative for the population in the Northern Netherlands (Klijs et al., 2015). Another strength is that we used a JEM for occupational exposure assessment. When compared to self-reported exposures, the use of a JEM limits the effect of recall bias on exposure estimates as well as the differential misclassification of exposure compared to self-reported exposure (Kromhout and Vermeulen, 2001; Mannetje and Kromhout, 2003).
The different study designs of Eurocat and Lifelines could have introduced differential information bias. Eurocat aimed to conduct research specifically to identify risk factors for congenital anomalies, whereas Lifelines aimed to collect data that could be used to examine diseases in general. Eurocat questionnaires therefore focused specifically on risk factors for congenital anomalies, whereas the Lifelines questionnaire included a wide variety of risk factors for neonatal and childhood diseases such as low birthweight, asthma, allergies and congenital anomalies. This is of particular importance when assessing our confounder variables because questions were asked with different intentions. An example that illustrates the difference in study design is BMI. In Eurocat, weight was self-reported, whereas in Lifelines, participants’ weight was measured at the study clinic. It is likely that self-reported weight is underestimated for overweight women, which could have an impact on our results. Questions regarding smoking and drinking alcohol were very similar in Eurocat and Lifelines (self-reported), limiting the introduction of differential information bias. However, recall bias could have been an issue. There was also a lack of detailed information for several covariates. For example, if women smoked during the first trimester, there was no detailed information on how many cigarettes women were smoking at that time. Furthermore, not all the data on potential covariates were available. For example, there were no data on maternal medication use for infants from Lifelines. Previous literature showed that maternal medication use, such as hormones for fertility treatment, increases the risk of hypospadias (Raghavan et al., 2018). In addition, supplementation with multivitamins was not studied and, although a previous study showed that intake of multivitamins can decrease the risk of CAKUT (Groen In 't Woud et al., 2016), it is possible that residual confounding was introduced.
Bias was unlikely for our main risk factor of interest: maternal occupational exposure early in pregnancy. In both questionnaires, the mothers were asked to report their job during pregnancy. In addition, recall bias could have been introduced, as the time between birth and notification was 16 months for Eurocat cases and 7.5 years for Lifelines controls. An underestimation of the effect between occupational exposure and urogenital anomalies owing to recall bias is expected to be small, because exposure is assigned based on job description/industry of employment, and recall bias for self-reported job description has been reported to be limited (Teschke et al., 2002). A limitation of JEMs in general is that they assign exposure at job level, which under normal conditions will result in a Berkson type error resulting in unbiased, but less precise, risk estimates. Another limitation is that the JEM estimates exposure as possible exposed (involves <10% of workers with this job title) and probably exposed (exposure was likely to occur in more than 10% of workers with this job; Brouwers et al., 2009). No studies have evaluated how the exposure assigned by the JEM relates to the actual exposures of the females involved. Non-differential misclassification could be introduced in three different ways. First, the JEM does not account for the years in which the job was performed, and previous studies have shown that occupational exposure can vary over time (Burstyn and Kromhout, 2002; Peters et al., 2011). Second, circumstances at the workplace are often unpredictable and can vary within a job and between companies (Teschke et al., 2002). Finally, the JEM does not account for the possibility that women avoided certain exposures because of their pregnancy, which could have led to bias towards the null. We have no information regarding the number of hours per week mothers worked early in pregnancy or other characteristics regarding employment. Therefore, it is possible that the actual exposure is lower or absent than the exposure estimated by the JEM. Besides the variability of exposures assessed by the JEM, women could also have been exposed to other exposures not included in the JEM. This could have resulted in residual confounding by co-exposures. One other limitation is that the JEM assigns a probability of exposure rather than a level of exposure, which precludes performing dose–response analyses.
Interpretation
Studies regarding occupational exposure and urinary anomalies are scarce and only performed for maternal occupational exposure to solvents. One earlier study also found an association between solvent exposure and urinary tract anomalies (Garlantézec et al., 2009), but the results of two other studies were not in line with our finding (Cordier et al., 1997, 2001). However, one of these studies did find an association between glycol ethers (a specific group of organic solvents) and multiple congenital anomalies (Cordier et al., 1997), which is in line with the association we found in our subgroup analysis for cases with a combination of urinary tract anomalies co-occurring with congenital anomalies. We did not observe a specific pattern of co-occurring congenital anomalies in the five exposed cases that could explain the association between solvents and multiple anomalies. The number of included CAKUT cases in those previous studies (Cordier et al., 1997, 2001; Garlantézec et al., 2009) was low compared to the present study (n = 12–76 versus n = 537), and therefore no subgroup analyses were performed.
It is known that EDCs can interfere with hormone levels during foetal development, and hormones such as gonadotrophins, oestrogen and androgens play an important role in the development of many tissues during foetal growth. Therefore, it seems possible that maternal occupational exposure to solvents increases the risk of multiple, co-occurring congenital anomalies. Additionally, it has been suggested that exposure to EDCs is associated with inappropriate modulation of hormone receptors and can, therefore, interfere with the development of the (male) reproductive tract (Yoon et al., 2014). The development of the urinary tract system is closely related to that of the reproductive tract.
However, despite the hypothesis that EDCs can interfere with the development of the reproductive tract, we did not find an association between maternal occupational exposure to EDCs and hypospadias, nor did we find one with specific groups of EDCs. Previous studies have reported contradictory results. Four studies found an association between maternal occupational exposure to EDCs in general and hypospadias (Giordano et al., 2010; Morales-Suárez-Varela et al., 2011; Kalfa et al., 2015; Haraux et al., 2016), but several other studies and the present study found no association (Vrijheid et al., 2003; Pierik et al., 2004; Carbone et al., 2007; Nassar et al., 2010). A few studies performed subgroup analysis for specific EDCs (Vrijheid et al., 2003; Pierik et al., 2004; Ormond et al., 2009; Giordano et al., 2010; Nassar et al., 2010; Morales-Suárez-Varela et al., 2011). One study that found an effect for EDC exposure in general, found that specifically phthalates and other compounds (e.g. parabens) had an effect (Morales-Suárez-Varela et al., 2011). However, another study that found an association, did not find an effect for specific EDC subgroups (Giordano et al., 2010). Two studies that found no association between EDC exposure and hypospadias, also found no specific subgroup effects (Pierik et al., 2004; Carbone et al., 2007). Only one study found an association between maternal occupational exposure to metals and mild hypospadias (Nassar et al., 2010). Two studies showed an association between maternal occupational exposure to phthalates and hypospadias (Vrijheid et al., 2003; Ormond et al., 2009). Two studies showing an association between EDC exposure and hypospadias included a small number of cases (n = 57–80), which could have resulted in imprecise estimates (Giordano et al., 2010; Haraux et al., 2016). As studies were conducted in different countries, it is possible that exposure levels varied over time and by country owing to (pregnancy) policy differences. The difference in results could not be explained by exposure assessment methods as all studies used the same JEM produced by van Tongeren et al. (2002).
The association between maternal occupational exposure to pesticides and hypospadias has been examined in several studies. This study did not show an association between maternal occupational exposure to pesticides and hypospadias. This is in line with two published reviews, including previous studies with comparable exposure assessment methods to this study (Rocheleau et al., 2009; Spinder et al., 2019).
Conclusion
This study showed an association between maternal occupational exposure to organic solvents/alkylphenolic compounds and phthalates/benzophenones/parabens/siloxanes early in pregnancy and CAKUT in offspring. We also found some indication that exposure to those specific EDCs is associated with a combination of urinary tract anomalies that co-occur with other congenital anomalies. In the Netherlands, since 1997, employers have been obliged by law to identify occupational risks for pregnant employees by carrying out risk assessments at the workplace (Wetboek, 1997). Women, their healthcare providers and their employers need to be aware that occupational exposure to specific EDCs early in pregnancy may be associated with CAKUT in offspring. An occupational hygienist should be consulted to take exposure to those specific EDCs into consideration when risk assessments are carried out at the workplace.
Data availability
The Eurocat Northern Netherlands data underlying this article will be shared on reasonable request to the corresponding author. The Lifelines data underlying this article were provided by Lifelines Biobank under licence.
Supplementary Material
Acknowledgements
The authors thank all those who are involved in providing and processing information to Eurocat NNL, including the affected families, clinicians, health professionals and registry staff. They wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines and all the study participants. They thank Kate McIntyre of the University of Groningen, University Medical Center Groningen for editorial assistance.
Authors’ roles
N.S.: study design, congenital anomaly classification, job coding, data analysis and manuscript drafting. J.E.H.B.: study design, Eurocat NNL data collection and congenital anomaly classification. M.v.T.: job-exposure matrix designer. H.M.B.: study design and statistical expertise. H.K.: job coding, study design and statistical expertise. H.E.K.d.W.: study design and Eurocat NNL data collection. All authors contributed to the interpretation of the results, revision of the manuscript and approved the final version of the manuscript.
Funding
N.S. was paid by the Graduate School of Medical Sciences (MD/PhD programme), University Medical Center Groningen (UMCG), Groningen, the Netherlands. Eurocat Northern Netherlands is funded by the Dutch Ministry of Health, Welfare and Sports. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University Groningen and the Northern Provinces of the Netherlands.
Conflict of interest
The authors report no conflict of interest.
References
- Bakker MK, Bergman JEH, Fleurke-Rozema H, Streefland E, Gracchi V, Bilardo CM, De Walle HEK.. Prenatal diagnosis of urinary tract anomalies, a cohort study in the Northern Netherlands. Prenat Diagn 2018;38:130–134. [DOI] [PubMed] [Google Scholar]
- Baskin LS. Hypospadias: pathogenesis, diagnosis, and evaluation. 2019. https://www.uptodate.com/contents/hypospadias-pathogenesis-diagnosis-and-evaluation (31 August 2021, date last accessed).
- Brouwers MM, van der Zanden LF, de Gier RP, Barten EJ, Zielhuis GA, Feitz WF, Roeleveld N.. Hypospadias: risk factor patterns and different phenotypes. BJU Int 2010;105:254–262. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, van Tongeren M, Hirst AA, Bretveld RW, Roeleveld N.. Occupational exposure to potential endocrine disruptors: further development of a job exposure matrix. Occup Environ Med 2009;66:607–614. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Kromhout H.. Trends in inhalation exposure to hydrocarbons among commercial painters in the Netherlands. Scand J Work Environ Health 2002;28:429–438. [DOI] [PubMed] [Google Scholar]
- Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, Figa-Talamanca I.. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: a population-based case-control study in rural Sicily. Int J Androl 2007;30:3–13. [DOI] [PubMed] [Google Scholar]
- Cordier S, Bergeret A, Goujard J, Ha M, Aymé S, Bianchi F, Calzolari E, De Walle HEK, Knill-Jones R, Candela S. et al. Congenital Malformations and Maternal Occupational Exposure to Glycol Ethers. Occupational Exposure and Congenital Malformations Working Group. Epidemiology 1997;4:355–363. [DOI] [PubMed] [Google Scholar]
- Cordier S, Szabova E, Fevotte J, Bergeret A, Plackova S, Mandereau L.. Congenital malformations and maternal exposure to glycol ethers in the Slovak Republic. Epidemiology 2001;5:592–593. [DOI] [PubMed] [Google Scholar]
- EUROCAT. EUROCAT Guide 1.4: instruction for the registration of congenital anomalies. 2013.
- EUROCAT. Prevalence charts and tables. 2017. January 15, 2020.
- Garlantézec R, Monfort C, Rouget F, Cordier S.. Maternal occupational exposure to solvents and congenital malformations: a prospective study in the general population. Occup Environ Med 2009;66:456–463. [DOI] [PubMed] [Google Scholar]
- Giordano F, Abballe A, De Felip E, Di Domenico A, Ferro F, Grammatico P, Ingelido AM, Marra V, Marrocco G, Vallasciani S. et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol 2010;88:241–250. [DOI] [PubMed] [Google Scholar]
- Greenlees R, Neville A, Addor MC, Amar E, Arriola L, Bakker M, Barisic I, Boyd PA, Calzolari E, Doray B. et al. Paper 6: EUROCAT member registries: organization and activities. Birth Defects Res A Clin Mol Teratol 2011;91(Suppl 1):S51–S100. [DOI] [PubMed] [Google Scholar]
- Groen In 't Woud S, Renkema KY, Schreuder MF, Wijers CH, van der Zanden LF, Knoers NV, Feitz WF, Bongers EM, Roeleveld N, van Rooij IA.. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: a case-control study. Birth Defects Res A Clin Mol Teratol 2016;106:596–603. [DOI] [PubMed] [Google Scholar]
- Haraux E, Braun K, Buisson P, Stephan-Blanchard E, Devauchelle C, Ricard J, Boudailliez B, Tourneux P, Gouron R, Chardon K.. Maternal exposure to domestic hair cosmetics and occupational endocrine disruptors is associated with a higher risk of hypospadias in the offspring. Int J Environ Res Public Health 2016;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Labor Office. International Standard Classification of Occupations. 1988.
- Johnson CY, Rocheleau CM, Grajewski B, Howards PP.. Structure and control of healthy worker effects in studies of pregnancy outcomes. Am J Epidemiol 2019;188:562–569. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Paris F, Philibert P, Orsini M, Broussous S, Fauconnet-Servant N, Audran F, Gaspari L, Lehors H, Haddad M. et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur Urol 2015;68:1023–1030. [DOI] [PubMed] [Google Scholar]
- Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N.. Representativeness of the LifeLines Cohort Study. PLoS One 2015;10:e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout H, Vermeulen R.. Application of job-exposure matrices in studies of the general population: some clues to their performance. Eur Respirtory Rev 2001;11:80–90. [Google Scholar]
- Mannetje A, Kromhout H.. The use of occupation and industry classifications in general population studies. Int J Epidemiol 2003;32:419–428. [DOI] [PubMed] [Google Scholar]
- Morales-Suárez-Varela MM, Toft GV, Jensen MS, Ramlau-Hansen C, Kaerlev L, Thulstrup A-M, Llopis-González A, Olsen J, Bonde JP.. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish national birth cohort study. Environ Health Global Health 2011;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Abeywardana P, Barker A, Bower C.. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med 2010;67:585–589. [DOI] [PubMed] [Google Scholar]
- Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV.. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 2015;11:720–731. [DOI] [PubMed] [Google Scholar]
- Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, Elliott P.. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: Case-control study. Environ Health Perspect 2009;117:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, Savary B, Lavoué J, Cavallo D, Cattaneo A. et al. Modelling of occupational respirable crystalline silica exposure for quantitative exposure assessment in community-based case-control studies. J Environ Monit 2011;13:3262–3268. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA.. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect 2004;15:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R, Romano ME, Karagas MR, Penna FJ.. Pharmacologic and environmental endocrine disruptors in the pathogenesis of hypospadias: a review. Curr Environ Health Rep 2018;5:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CM, Bertke SJ, Lawson CC, Romitti PA, Desrosiers TA, Agopian AJ, Bell E, Gilboa SM; National Birth Defects Prevention Study. Factors associated with employment status before and during pregnancy: implications for studies of pregnancy outcomes. Am J Ind Med 2017;60:329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, Dennis LK.. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol 2009;5:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum ND. Overview of congenital anomalies of the kidney and urinary tract (CAKUT). 2017. [DOI] [PubMed]
- Salmasi G, Grady R, Jones J, McDonald SD; On behalf of the Knowledge Synthesis Group. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 2010;89:423–441. [DOI] [PubMed] [Google Scholar]
- Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, van Dijk F, van Zon SK, Wijmenga C, Wolffenbuttel BH. et al. Cohort profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 2015;44:1172–1180. [DOI] [PubMed] [Google Scholar]
- Spinder N, Prins JR, Bergman JEH, Smidt N, Kromhout H, Boezen HM, de Walle HEK.. Congenital anomalies in the offspring of occupationally exposed mothers: a systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum Reprod 2019;34:903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke K, Olshan AF, Daniels JL, De Roos AJ, Parks CG, Schulz M, Vaughan TL.. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 2002;59:575–593; discussion 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tongeren M, Nieuwenhuijsen MJ, Gardiner K, Armstrong B, Vrijheid M, Dolk H, Botting B.. A job-exposure matrix for potential endocrine-disrupting chemicals developed for a study into the association between maternal occupational exposure and hypospadias. Ann Occup Hyg 2002;46:465–477. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Armstrong B, Dolk H, van Tongeren M, Botting B.. Risk of hypospadias in relation to maternal occupational exposure to potential endocrine disrupting chemicals. Occup Environ Med 2003;60:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg C, Botton J, Lelong N, Rouget F, Khoshnood B, Le Gleau F, Monfort C, Labat L, Pierre F, Heude B. et al. Prenatal exposure to glycol ethers and cryptorchidism and hypospadias: a nested case-control study. Occup Environ Med 2018;75:59–65. [DOI] [PubMed] [Google Scholar]
- Wetboek B. Arbeidsomstandighedenbesluit—Afdeling 9. Zwangere werknemers en werknemers tijdens de lactatie. 1997.
- World Health Organisation (WHO), United Nations Environment Program (UNEP). State of the Science of Endocrine Disrupting Chemicals 2012;1/296. [Google Scholar]
- Yiee JH, Baskin LS.. Environmental factors in genitourinary development. J Urol 2010;184:34–41. [DOI] [PubMed] [Google Scholar]
- Yoon K, Kwack SJ, Kim HS, Lee BM.. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev 2014;17:127–174. [DOI] [PubMed] [Google Scholar]
- Zhu JL, Hjollund NH, Andersen A-N, Olsen J.. Occupational exposure to pesticides and pregnancy outcomes in gardeners and farmers: a study within the Danish national birth cohort. J Occup Environ Med 2006;48:347–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Eurocat Northern Netherlands data underlying this article will be shared on reasonable request to the corresponding author. The Lifelines data underlying this article were provided by Lifelines Biobank under licence.