Abstract
Late-life depression (LLD) is associated with an increased risk of all-cause dementia and may involve Alzheimer's disease pathology. Twenty-one LLD patients who met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for a current major depressive episode and 21 healthy controls underwent clinical and neuropsychological assessments, magnetic resonance imaging to measure gray matter volumes, and high-resolution positron emission tomography to measure beta-amyloid (Aβ) deposition. Clinical and neuropsychological assessments were repeated after 10–12 weeks of Citalopram or Sertraline treatment (LLD patients only). LLD patients did not differ from healthy controls in baseline neuropsychological function, although patients improved in both depressive symptoms and visual-spatial memory during treatment. Greater Aβ in the left parietal cortex was observed in LLD patients compared with controls. Greater Aβ was correlated with greater depressive symptoms and poorer visual-spatial memory, but not with improvement with treatment. The study of LLD patients with prospective measurements of mood and cognitive responses to antidepressant treatment is an opportunity to understand early neurobiological mechanisms underlying the association between depression and subsequent cognitive decline.
Keywords: Beta-amyloid, Positron emission tomography, Late-Life, Depression, Aging, Selective serotonin reuptake inhibitors, Citalopram, Sertraline
1. Introduction
A considerable body of evidence has shown that depression in late life is associated with cognitive deficits and an increased risk of development of all-cause dementia, including Alzheimer's disease (AD) and vascular dementia (Byers and Yaffe, 2011; Diniz et al., 2013). Approximately 60% of late-life depressed (LLD) patients (aged ≥60 years, who meet Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-V], criteria for major depressive disorder) that do not meet clinical criteria for dementia have deficits in one or more cognitive domains (Butters et al., 2004a,b). The cognitive domains affected in late-life depression (LLD) include executive function, verbal and visual-spatial memory, and processing speed (Butters et al., 2004a; Lockwood et al., 2000, 2000). Cognitive impairment commonly persists, even after remission of depressive symptoms with antidepressant treatment (Alexopoulos et al., 1993; Bhalla et al., 2006). Persistent cognitive impairment has been hypothesized to be associated with a greater risk of dementia and an underlying neuropathological process (Butters et al., 2004a,b, 2008a,b). Understanding the cognitive domains that respond or fail to respond to antidepressant treatment and their underlying neurobiological mechanisms may help to identify patients with incipient dementia and to inform the development of more effective treatments (Meyers and Bruce, 1998). Thus, studying LLD patients during the course of antidepressant treatment with serial mood and neuropsychological assessments and molecular imaging is an important opportunity to obtain such information. Importantly, eliminating depression has been estimated to reduce the incidence of dementia to a greater extent than other risk factors, such as obesity, diabetes, hypertension, and physical inactivity (4% vs. 1%–3%; Livingston et al., 2017; Dafsari and Jessen, 2020).
Previous positron emission tomography (PET) studies have identified functional neuroanatomic changes associated with LLD and response to antidepressant treatment. Increased cerebral glucose metabolism in LLD patients compared with controls was observed in cortical regions, including superior and medial frontal gyrus, middle temporal gyrus, precuneus, and inferior parietal lobule (Smith et al., 2009). Citalopram treatment decreased glucose metabolism in a subcortical-limbic-frontal network that was associated with improvement in affect (depression and anxiety), as well as in a medial temporal-parietal-frontal network that was associated with improvement in cognition (verbal memory and verbal fluency; Diaconescu et al., 2011). The regions that comprise the “cognitive network” include regions that demonstrate increased cerebral glucose metabolism as well as AD neuropathology, including Tau and beta-amyloid deposition (Aβ; Arnold et al., 1991; Smith et al., 1992). In contrast, lower cortical and limbic gray matter (GM) volumes measured with magnetic resonance imaging (MRI) have not been reported consistently in LLD patients compared with controls, particularly in patients without substantial cognitive impairment (Colloby et al., 2011; Jamieson et al., 2019). Having identified the neural circuitry of LLD and treatment response, the logical next step is to investigate underlying neurobiological mechanisms with selective PET radiotracers.
The development of radiotracers to image Aβ deposition has been a major advance in understanding the earliest neuropathological changes associated with cognitive decline (Mathis et al., 2003; as reviewed by Villemagne, 2014). [11C]-PiB is the best characterized and validated of Aβ radiotracers with high affinity and specificity to Aβ in AD brain (Mathis et al., 2003; Klunk et al., 2004; Maeda et al., 2007; Ikonomovic et al., 2008). Its regional distribution in vivo in AD patients corresponds to that observed in postmortem AD brain tissue (Ziolko et al., 2006). The regional distribution of Aβ overlaps with regions that comprise the “default mode network” (Buckner et al., 2005). Increased activity of the “default mode network” over the lifespan has been suggested to predispose these brain regions to a neurodegenerative process. [11C]-PiB binding is associated with subtle cognitive impairment and may predict cognitive decline in individuals who are cognitively normal and those with mild cognitive impairment (MCI; Mormino et al., 2009; Pike et al., 2007; Sojkova et al., 2008). Recent advances in radiochemistry have led to the development of Tau radiotracers (as reviewed by Lois et al., 2019). Molecular imaging studies support a model of preclinical AD in which cortical Aβ accumulation begins before symptom onset, whereas cortical Tau is more temporally linked to, neuronal dysfunction, and cognitive deficits (Dubois et al., 2016; Hanseeuw et al., 2019). Pathologic Tau causes morphologic changes in neurites and synapses and impairs cellular trafficking and synaptic function (Menkes-Caspi et al., 2015). Tau spreads from transentorhinal cortex to mesial and inferior temporal lobe, posterior cingulate, and isocortical brain areas in a transynaptic manner along functional networks and disrupts network function, causing neurodegeneration (Arnold et al., 1991; Braak et al., 2006). Cerebrospinal fluid (CSF) biomarkers, including lower Aβ42 and Aβ42:Aβ40 ratio and higher total Tau (t-tau) and phosphorylated Tau (p-tau, especially p-tau181), are associated with the development of dementia in normal aging and MCI and have shown more reliable findings than blood biomarkers for Aβ and Tau, thus far (as reviewed by Blennow and Zetterberg, 2018; Hameed et al., 2020; Hampel et al., 2018; Mattsson-Carlgren et al., 2020).
To understand neurobiological mechanisms that underlie the association between LLD and cognitive decline, fluid and molecular imaging biomarkers of preclinical AD have been studied in cognitively normal individuals with depressive symptoms and LLD patients who meet the criteria for major depressive disorder. Many, but not all, molecular imaging studies in cognitively normal individuals have shown associations between greater Aβ and Tau and depressive symptoms cross-sectionally (Gatchel et al., 2019, 2017; Lavretsky et al., 2009). Some studies have shown an association between Aβ measured with PET and higher CSF tau:Aβ42 ratios and development of depressive symptoms after 1- to 1.5-year follow-up (Babulal et al., 2016; Donovan et al., 2018). In LLD patients, CSF and plasma measures of Aβ biomarkers have shown mixed results. A meta-analysis showed that LLD patients had a higher plasma Aβ40:Aβ42 ratio compared with nondepressed participants and a marginally significant reduction of CSF Aβ42 levels (Nascimento et al., 2015). PET studies of Aβ in LLD patients have shown either no significant difference or less or increased Aβ deposition relative to controls in a more limited neuroanatomical distribution (temporal and parietal cortices) compared with LLD patients with MCI or individuals with either MCI or AD (De Winter et al., 2017; Kumar et al., 2011; Li et al., 2017; Mackin et al., 2020; Butters. et al., 2008a; Wu et al., 2014). Greater Aβ was associated with more frequently documented episodes of treatment resistance in LLD patients (Li et al., 2017). In a recent PET study of cognitively normal individuals, participants with elevated Tau, particularly those who were also taking antidepressants, were more likely to be depressed, whereas Aβ did not show the same association (Babulal et al., 2020). Factors that may contribute to differences between studies in LLD patients include age at onset and history of depressive episodes (chronic, early onset vs. late onset), presence or absence of neuropsychological impairment, and history of antidepressant treatment response.
The purpose of this study was to measure Aβ using PET in LLD patients compared with demographically matched, healthy controls. LLD patients were enrolled with limited or no previous antidepressant treatment. The controls and LLD patients were matched as a group for apolipoprotein E4 (ApoE4) genotype status. After completing clinical and neuropsychological assessments, MRI and Aβ PET scans, LLD patients began treatment with the selective serotonin reuptake inhibitor (SSRI) Citalopram or Sertraline. Citalopram (later, Sertraline) was used because when the study began, both medications were regarded as safe and effective with less possibility of drug-drug interactions than other antidepressants used in the treatment of LLD (Solai et al., 2001). The clinical and neuropsychological assessments were repeated after reaching a stable change in depressive symptoms by 8–10 weeks of treatment. Correlations between regional Aβ and baseline depressive symptoms or neuropsychological function, as well as change in these outcomes after antidepressant treatment, were performed. The hypotheses were tested that (1) LLD patients compared with controls would show greater Aβ deposition in frontal, temporal, and parietal association cortices and little or no cortical atrophy and (2) greater Aβ would be correlated with greater depressive symptoms and greater neuropsychological deficits at baseline and with less improvement in these domains after treatment. This study differed from previous Aβ studies in LLD patients, as depressive symptoms and multidomain neuropsychological function were evaluated before and after antidepressant treatment, and these measures were correlated with the regional distribution of Aβ.
2. Materials and methods
2.1. Participant screening and selection
Potential participants were recruited from advertisements in the community or from Johns Hopkins University Alzheimer's Disease Research Center (2P50AG005146). All potential LLD patients and controls underwent screening procedures that included physical and neurologic examination, laboratory testing and toxicology screening, and psychiatric and neuropsychological evaluations. The evaluations included the Structured Clinical Interview for DSM-V by a clinical psychologist (N.G.), clinical dementia rating (CDR) scale and Mini-Mental State Examination (First et al., 1995; Folstein et al., 1975; Morris, 1993). The history of antidepresant treatment was determined by the administration of the SCID and the antidepressant treatment history form (Sackeim, 2001). LLD patients were enrolled who were over age 60 years and who had a DSM-V diagnosis of a current major depressive episode (non-bipolar and non-psychotic) and a Hamilton Depression Rating Scale score of 17 or higher (24-item scale; Hamilton, 1960). The exclusionary criteria were (1) a history of or active neurologic or Axis I psychiatric disorders, except for a diagnosis of current major depressive episode in LLD patients; (2) medical instability or chronic medical conditions that were not well controlled (i.e., hypertension and/or diabetes); (3) a positive toxicology screening or use of psychotropic drugs or medications with central nervous system effects (e.g., antihistamines and cold medications) that persisted for 2 weeks before enrollment; and (4) contraindications for MRI scanning (e.g., pacemaker, metal implants, and aneurism clamps). The study protocol and consent forms were approved by the Institutional Review Board and the Radiation Research Committee of Johns Hopkins University School of Medicine. Participants received transcribed and verbal descriptions of the study, and written informed consent was obtained.
2.2. Antidepressant treatment
After baseline study procedures were completed, LLD patients began treatment with a 10 mg/d oral dose of Citalopram (Celexa) for 1 week. If significant clinical improvement was not observed at the 10 mg dose, based on a rating of ≥3 on the Clinical Global Impression (CGI) scale (Guy, 1976), the dose was increased to 20 mg/d and maintained for 4 weeks. If significant clinical improvement was not observed at the 20 mg dose, the dose was increased to 30 mg and then to 40 mg/d, depending on CGI ratings. All patients were followed weekly. Clinical ratings for depressive symptoms were acquired, including Hamilton Depression Rating Scale-24 (HDRS) item (Hamilton, 1960), Beck Depression Inventory (BDI) (Beck and Steer, 1993), and CGI. During the course of the study, a “black box” warning was issued for Citalopram (Vieweg et al., 2012). LLD patients enrolled after this time were treated with Sertraline (Zoloft). LLD patients started at a 25 mg/d oral dose for 2 weeks, and then, the dose was increased by 25 mg weekly to a maximum dose of 150 mg, using the same CGI threshold for dosage increase as Citalopram.
2.3. Neuropsychological testing
A multidomain neuropsychological test battery was administered to LLD patients at baseline and after 10–12 weeks of treatment, based on domains impaired in LLD patients and improved with antidepressant treatment (Butters et al., 2004a; Lockwood et al., 2000, 2002). Sixteen elderly controls who overlapped with controls enrolled in the present study were tested twice during the same time interval as the LLD patients. Delis-Kaplan Executive Function System Letter and Category Fluency*, Trail Making Test,* Color-Word Interference Test* and Sorting Test* were used to measure executive function (Delis et al., 2001). California Verbal Learning Test (CVLT)* and Wechsler Memory Scale*, Logical Memory (Delis et al., 1987; Wechsler, 1997) were used to measure auditory-verbal memory. The visual-spatial memory tests used were Brief Visual Memory Test-Revised (BVMT-R)* and Rey Complex Figure Test (Benedict et al., 1996; Rey, 1941). Symbol Digit Modalities Test was used to measure attention (Smith, 1968). Iowa Gambling Test was used to measure decision-making (Bechara, 2007). Alternate forms of the tests were used where available (*).
2.4. Genotyping
ApoE genotyping was performed (in the laboratory of DA) using polymerase chain reaction amplification of genomic DNA digestion with Hhal restriction enzyme and gel electrophoresis, as previously described (Avramopoulos et al., 1996; Wenham et al., 1991).
2.5. MRI procedures
MRI brain scans were acquired before the PET scan at the F. M. Kirby Research Center for Functional Brain Imaging of the Kennedy Krieger Institute, as described previously (Smith et al., 2017). A Phillips 3.0 T Achieva MRI instrument was used with an 8-channel head coil (Philips Medical Systems, Best, the Netherlands). The magnetization-prepared rapid acquisition with gradient-echo pulse sequence (TE = 4, TR = 8.9, flip angle = 8°, NSA = 1, 0.7 mm isotropic voxel size) was used for volumetric analyses and PET image processing.
2.6. PET imaging procedures
PET scans were performed at the PET Center of the Russell H. Morgan Department of Radiology, Johns Hopkins University School of Medicine. The scanner used was a second-generation High-Resolution Research Tomograph scanner (Siemens Healthcare, Knoxville, TN, USA), a cerium-doped lutetium oxyorthosilicate (Lu25i05[Ce] or LSO) detector-based, dedicated brain PET scanner. Each subject was fitted with a thermoplastic mask modeled to their face to reduce head motion during the PET study. Attenuation maps were generated from a 6-minute transmission scan performed with a [137Cs] point source before the emission scans.
N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole ([11C]-PiB) was synthesized as previously described (Klunk et al., 2004). Dynamic scanning began immediately upon a 15 mCi ±10% radiotracer injection and lasted for 90 minutes. Data were acquired in list mode. Images were reconstructed using iterative ordered subset expectation maximization (OSEM) algorithm (with 6 iterations and 16 subsets), with correction for radioactive decay, dead time, attenuation, scatter, and randoms (Rahmim et al., 2005) and re-binned into 30 frames (four 15 seconds, four 30 seconds, three 1 minute, two 2 minutes, five 4 minutes and twelve 5 minutes frames). Reconstructed image space consisted of 256 (left-to-right) by 256 (nasion-to-inion) by 207 (neck-to-cranium) cubic voxels, each 1.22 mm in dimension. Final spatial resolution is less than 2.5 mm full width at half-maximum (FWHM) in 3 directions (Sossi et al., 2005).
2.7. MRI processing and analysis
Magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) images were submitted to FreeSurfer (version 6.0) (Fischl et al., 2002); for automated parcellation of brain regions that consisted of 82 left and right cortical and subcortical volumes of interest (VOIs) and whole cerebellum GM (Desikan et al., 2006). VOIs were transferred from MRI to PET space using parameters that were obtained using the coregistration module in SPM12 (Institute of Neurology, London) running on MATLAB 7.10 (MathWorks, Natick, MA, USA).
FreeSurfer-derived gray and white matter (GM and WM, respectively) masks of individual participants were submitted to the “Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra” (DARTEL) algorithm (Ashburner, 2007) to generate a binary GM template in Montreal Neurological Institute (MNI) space that preserved features of FreeSurfer-derived masks. Then, DARTEL-derived spatial normalization parameters were applied to individual GM and WM masks with or without the ‘reserve’ option to generate spatially normalized GM and WM masks for voxel-based morphometry (VBM) analysis and to generate probabilistic maps, respectively. Instead of using the 6-layer templates supplied by SPM12, 6-layer templates were generated from the FreeSurfer-derived GM and WM masks that were averaged across subjects. GM masks for VBM analyses were smoothed by a Gaussian kernel of 8 mm FWHM. VBM using SPM12 was performed to evaluate between-group differences in GM volumes.
2.8. PET tracer kinetic modeling and image processing
Regional distribution volume ratio (DVR) values of [11C]-PiB were obtained by multilinear reference tissue method with 2 parameters (MRTM2) with cerebellar GM (excluding vermis) as reference region (Ichise et al., 2002; Klunk et al., 2004). Briefly, the assumed t* (the time when the free-plasma ratio approaches time-invariant) was set to 25 minutes. Brain-to-blood clearance rate constant (k2R) of the cerebellum was set to 0.149 per minute, which corresponded to a population mean value of k2R from the initial MRTM2 analyses of the dataset. To reduce the number of outliers (voxels with negative values or exceeding 5 times a subject's maximal regional value), dynamic PET frames were smoothed with a 6 mm Gaussian kernel before generating DVR maps.
Preprocessing and voxel-wise statistical analyses of parametric [11C]-PiB DVR images were performed with SPM12. First, as mentioned, MRIs were spatially normalized to MNI space using the FreeSurfer-derived, 6-layer template from DARTEL in place of the SPM12-supplied template. Second, [11C]-PiB DVR images were transferred to MNI space by combining spatial normalization and PET-to-MRI coregistration parameters. DVR images in MNI space were smoothed with a 5 mm (FWHM) Gaussian kernel. The combination of smoothing of dynamic PET frames with smoothing of DVR images is equivalent to smoothing native PET images with an 8 mm (FWHM) Gaussian kernel. A binarized FreeSurfer-derived GM + WM mask, after smoothing with a 10 mm (FWHM) Gaussian kernel (height threshold = 0.2) limited the search area (explicit mask).
2.9. Statistical analysis
One-way and repeated measures analyses of variance tested for differences in demographic, clinical, and neuropsychological variables between and within groups, respectively. In SPM12, 2 sample t-tests tested for differences in Aβ and GM volumes between LLD patients and controls. Multiple regression analyses correlated Aβ with baseline depressive symptoms and neuropsychological variables in the combined group of LLD patients and controls. Multiple regression analyses were performed also to correlate Aβ with change in depressive symptoms and neuropsychological variables from before to during SSRI treatment in LLD patients. Significance criteria used for reporting SPM results were a cluster-level, family-wise error corrected threshold of p ≤ 0.05 and a peak voxel uncorrected threshold of p ≤ 0.001: Height threshold p = 0.01 and extent threshold (k) = 50 voxels. Peak voxels within anatomic regions belonging to the same cluster are represented on different rows on each of the tables. Brain locations are reported as x, y, z coordinates in MNI space with approximate Brodmann areas (BA) identified by mathematical transformation into Talairach space with a nonlinear mapping approach (Lacadie et al., 2008). To determine the rates of Aβ positivity, mean cortical values were calculated, and cutoffs were applied using a method validated against postmortem data (Villeneuve et al., 2015). A weighted mean of the following Freesurfer VOIs (right and left hemispheres) was calculated: frontal pole, rostral middle frontal, superior frontal lobe, caudal middle frontal, frontal operculum, orbital operculum, lateral orbital gyrus, medial orbital gyrus, rostral anterior cingulate, caudal anterior cingulate, superior temporal lobe, middle temporal lobe, inferior temporal lobe, isthmus/cingulate, posterior cingulate, precuneus, superior parietal lobe, inferior parietal lobe, supramarginal gyrus, and lingual gyrus. Two cutoffs were used that were less (DVR ≥1.08) and more conservative (DVR ≥1.20) to divide mean cortical Aβ values into negative and positive Aβ groups . Using the lower Aβ cutoff (DVR ≥1.08) is supported by evidence that cognitively normal individuals with levels of Aβ in this range, in combination with depressive symptoms, show evidence of subsequent cognitive decline (Gatchel et al., 2019). Analysis of variance was performed to determine whether within each group (control/LLD patients), Aβ status (positive/negative) was associated with differences in mood and cognitive variables. The hypotheses were tested that Aβ positivity would be associated with greater depressive symptoms and worse performance on neuropsychological measures in controls and LLD patients and that Aβ-positive LLD patients would show less change in mood and cognitive variables with antidepressant treatment.
3. Results
Twenty-one LLD patients and 21 healthy controls were enrolled in the study. Means and standard deviations for demographic and clinical characteristics are shown in Table 1. Groups did not differ significantly in age and sex distribution and years of education or the baseline neuropsychological measures (including MMSE, CVLT, BVMT-R, Rey Complex Figure, Category, and Letter Fluency). One of the controls and no LLD patients were left handed. All controls received a CDR score of 0 (normal). All but one LLD patient received a CDR score of 0. This patient received a score of 0.5 (MCI: sum of boxes = 1.5 for memory, orientation, judgment, and problem-solving subscales). One patient received a CDR of 0 and a sum of boxes = 0.5 (judgment and problem-solving subscale). In addition, groups were matched on numbers of ApoE4 alleles (1 subject = 4, 4; 3 subjects = 3, 4 in each group).
Table 1.
Demographic and clinical characteristics for late-life depressed (LLD) patients and controls
| Demographic variable | Healthy older controls (n = 21) | LLD patients (n = 21) at baseline | LLD patients (n = 20) during SSRI treatment |
|---|---|---|---|
| Age | 66 ± 6 | 67 ± 7 | |
| Sex (F/M) | 11/10 | 11/10 | |
| Education (in years) | 15 ± 3 | 15 ± 2 | |
| Race (number of participants who are African American/White) | 1/20 | 2/19 | 2/18 |
| MMSE | 29 ± 1 | 29 ± 1 | 29 ± 1 |
| HDRS | 1 ± 1 | 17 ± 2a | 5 ± 3b |
| BDI | 6 ± 12 | 24 ± 8c | 9 ± 7d |
| CVLT total recall (sum of first 5 trials) | 59 ± 11 | 56 ± 10 | 58 ± 9 |
| CVLT delayed free recall | 12 ± 2 | 12 ± 3 | 13 ± 3 |
| DKEFS™ Category fluency | 41 ± 8 | 41 ± 8 | 42 ± 2 |
| DKEFS™ Letter fluency | 45 ± 12 | 40 ± 11 | 45 ± 12e |
| BVMT-R total recall (sum of first 3 trials) | 19 ± 7 | 20 ± 8 | 24 ± 6f |
| BVMT-R delayed recall | 8 ± 3 | 7 ± 3 | 9 ± 3g,h |
| Rey complex figure test-copy | 32 ± 3 | 31 ± 4 | 32 ± 3 |
| Rey complex figure test-immediate recall | 17 ± 6 | 16 ± 7 | 22 ± 7i |
| Rey complex figure test-delayed recall | 16 ± 5 | 15 ± 6 | 22 ± 7j |
Key: BDI, Beck Depression Inventory; BVMT-R, Brief Visual Memory Test-Revised; CVLT, California Verbal Learning Test; D-KEFS, Delis-Kaplan Executive Function System; HDRS, Hamilton Depression Rating Scale; MMSE, modified Mini-Mental State Examination; SSRI, Selective Serotonin Reuptake Inhibitor.
Significant difference between controls and LLD patients (F(1,40) = 900.42, p < 0.001).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 229.31, p < 0.001).
Significant difference between controls and LLD patients (F(1,40) = 35.51, p < 0.001).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 42.52, p < 0.001).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 4.96, p < 0.05).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 8.77, p < 0.01).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 16.00, p < 0.001).
Significant change in performance between controls and LLD patient groups (F(1,19) = 8.59, p < 0.01).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 38.30, p < 0.001).
Significant difference in the LLD patients before and during SSRI treatment (F(1,19) = 65.07, p < 0.001).
As expected, LLD patients showed significantly higher HDRS and BDI scores than controls at baseline (F(1,40) = 900.42; p < 0.001); F(1,40) = 35.51, p < 0.001, respectively), within a range of moderate to moderate-to-severe depressive symptoms, respectively. Three LLD patients were previously treated with SSRIs in late-life, although not within 2 years of enrollment. There were no significant differences between control and LLD patient groups in baseline neuropsychological measures. All but one LLD patient completed follow-up clinical and neuropsychological assessments. When the follow-up clinical and neuropsychological assessments were performed, LLD patients were taking a mean Citalopram dose of 21 mg ± 9 mg (range 10–40 mg) or a mean Sertraline dose of 92 mg ± 52 mg (range 50–150 mg). None of the LLD patients reported any side effects from treatment. All but one of 21 LLD patients met the criteria for treatment response (HDRS score of 10 or below on HDRS) and remission (HDRS score of 10 or below for 2 consecutive weeks; Dew et al., 1997; Sheffrin et al., 2009). Significant improvement in depressive symptoms (HDRS: F(1,19) = 229.31, p < 0.001; BDI: F(1,19) = 42.52, p < 0.001) was observed. During SSRI treatment, significant improvement in Verbal (letter) fluency was observed (F(1,19) = 4.96; p < 0.05). The 2 visual-spatial memory tests also showed significant improvement in immediate and delayed memory components. BVMT-R sum of first 3 trials and delayed recall measures showed significant improvement with SSRI treatment (F(1,19) = 8.77, p < 0.01; F(1,19) = 16.00; p < 0.01). Rey Complex Figure immediate and delayed recall but not copy condition showed significant improvement (F(1,19) = 38.30, p < 0.001; F(1,19) = 65.07, p < 0.05; F(1,19) = 1.21 p > 0.05). There was no statistically significant change in other neuropsychological measures with SSRI treatment. As mentioned, healthy control data were available for 16 subjects tested over a 10- to 12-week interval, some of whom were enrolled in the present study. A comparison of change over time between groups (testing session 2—testing session one for controls and LLD patients) revealed that improvement in performance was greater for LLD patients than controls for verbal (letter) fluency (controls 2% ± 17%; LLD patients 13% ± 30), BVMT-R total recall (controls 16% ± 12%; LLD patients 36% ± 71), and BVMT-R delayed recall (control 2% ± 11%; LLD patient 36% ± 62), but not Rey Complex Figure Test (immediate recall: control 51% ± 70%; LLD patient ± 53% ± 47; delayed recall: control 82% ± 172; LLD patient ±62% ± 53). Improvement in performance was significantly greater for the LLD patients compared to controls only for BVMT-R delayed recall (F(1,19) = 8.59, p < 0.01; Table 1).
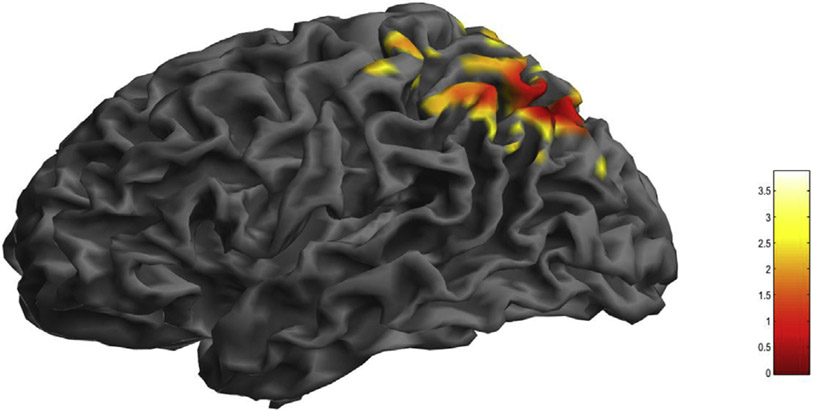
Regions of greater Aβ in LLD patients compared with controls are shown in Table 2 and Fig. 1. Aβ was greater n LLD patients than controls in left superior parietal lobule (BA 7) and left inferior parietal lobule (BA 39). There were no significant differences in GM volumes between groups. For correlation analyses, mood outcomes were used , HDRS and BDI, represent core depressive/vegetative and cognitive aspects of depression, respectively. Cognitive outcomes included CVLT (total number of words recalled without perseverations and intrusions over the first 5 trials) and BVMT-R (number of shapes, and their location recalled over 3 learning trials) to represent auditory-verbal and visual-spatial memory, respectively. No covariates were used in the analysis, as groups were well-matched for age, sex, and ApoE4 status. Greater Aβ was correlated with greater severity of depressive symptoms (HDRS) in left superior parietal lobule (BA 7), left inferior parietal lobule (BA 7, 39) and left supramarginal gyrus (BA 39; Table 3). Greater Aβ was correlated with worse visual-spatial memory performance (BVMT-R) in left precentral gyrus (BA 6), left superior temporal gyrus (BA 22), and left middle temporal gyrus (BA 21; Table 4). Significant correlations were not observed between Aβ and change in these measures during SSRI treatment in LLD patients. However, correlations were observed between greater Aβ and less change in BVMT in left temporal and parietal cortical regions that were significant at the voxel (p ≤ 0.001), not cluster level (p ≥ 0.05).
Table 2.
Greater beta-amyloid (Aβ) deposition in late-life depressed (LLD) patients compared with controls
| Left hemisphere |
Structure | ||
|---|---|---|---|
| MNI coordinates |
Talairach coordinates |
Z score | |
| X Y Z (mm) | X Y Z (mm) | ||
| −26 −74 54 | −26 −69 49 | 3.36 | Superior parietal lobule (BA 7) |
| −40 −72 46 | −40 −68 43 | 3.53 | Inferior parietal lobule (BA 39) |
| −28 −72 44 | −28 −68 41 | 3.33 | Inferior parietal lobule (BA 39) |
The results are reported at a significance level of cluster-level, family-wise error (FWE) corrected threshold of p ≤ 0.05 and a peak voxel uncorrected threshold of p ≤ 0.001: Height threshold p = 0.01 and extent threshold (k) = 50 voxels. The cluster size (kE) is 2280. Note: No effects in the right hemisphere were observed.
Key: BA, Brodmann area; MNI, Montreal Neurological Institute (MNI).
Fig. 1.
Greater beta-amyloid (Aβ) deposition in late-life depressed (LLD) patients compared with healthy controls. Statistically significant voxel-wise results (T-maps generated in SPM12) are displayed on a three-dimensional MRI rendering.
Table 3.
Positive correlations between greater beta-amyloid (Aβ) deposition and greater depressive symptoms (Hamilton Depression Rating Scale) in late-life depressed (LLD) patients and healthy controls
| Left hemisphere |
Structure | ||
|---|---|---|---|
| MNI coordinates |
Talairach coordinates |
Z-score | |
| X Y Z (mm) | X Y Z (mm) | ||
| −50 −52 54 | −50 −49 50 | 3.07 | Supramarginal gyrus (BA 39) |
| −24 −74 56 | −24 −69 51 | 3.50 | Superior parietal lobule (BA 7) |
| −26 −72 42 | −26 −68 39 | 3.41 | Inferior parietal lobule (BA 7) |
| −38 −76 46 | −38 −72 43 | 3.30 | Inferior parietal lobule (BA 39) |
The results are reported at a significance level of cluster-level, family-wise error (FWE) corrected threshold of p ≤ 0.05 and a peak voxel uncorrected threshold of p ≤ 0.001: Height threshold p = 0.01 and extent threshold (k) = 50 voxels. The cluster size (kE) is 2276. Note: No effects in the right hemisphere were observed.
Key: BA, Brodmann area; MNI, Montreal Neurological Institute (MNI).
Table 4.
Negative correlations between greater Beta-(Aβ) deposition and poorer visual-spatial memory performance (Brief Visual Memory Test-Reviseda) in late-life depressed (LLD) patients and healthy controls
| Left hemisphere |
Structure | ||
|---|---|---|---|
| MNI coordinates |
Talairach coordinates |
Z score | |
| X Y Z (mm) | X Y Z (mm) | ||
| −62 −2 14 | −59 −3 15 | 3.34 | Precentral gyrus (BA 6) |
| −54 −4 −10 | −51 −7 −5 | 3.31 | Superior temporal gyrus (BA 22) |
| −68 −14 −20 | −64 −17 −14 | 3.43 | Middle temporal gyrus (BA 21) |
The results are reported at a significance level of cluster-level, family-wise error (FWE) corrected threshold of p ≤ 0.05 and a peak voxel uncorrected threshold of p ≤ 0.001: Height threshold p = 0.01 and extent threshold (k) = 50 voxels. The cluster size (kE) is 2580.
Key: BA, Brodmann area; MNI, Montreal Neurological Institute (MNI). Note: No effects in the right hemisphere were observed.
Total number of shapes recalled on the first 3 trials.
Using a cutoff of DVR ≥1.08, 17 of 21 controls (81%) and 14 of 21 LLD patients (76%) were Aβ positive. Using a cutoff of DVR ≥1.2, 4 of 21 controls (19%) and 6 of 21 LLD patients (29%) were Aβ positive. Using cutoffs of DVR ≥1.08 or DVR ≥1.20, there was no significant difference in any of mood or neuropsychological measures in comparing Aβ-positive to Aβ-negative participants within control or LLD patient groups (Supplemental Table 1a and b). For both cutoffs, Aβ positive compared with Aβ negative in both groups showed lower scores on neuropsychological measures (Supplemental Table 1a and b). There were no significant differences between magnitude change from treatment to baseline for measures of depressive symptoms or neuropsychological function for Aβ-positive compared with Aβ-negative LLD patients (Supplemental Table 2a and b). Although Aβ-positive LLD patients showed improvement with treatment, scores on some of the tests were lower when comparing testing during treatment for 2 groups, especially for verbal fluency, CVLT, and Rey Complex Figure Test.
4. Discussion
Greater left parietal cortical Aβ in LLD patients compared with controls was observed using PET and the radiotracer [11C]-PiB. Previous studies that show greater Aβ in LLD patients failing to meet criteria for MCI or dementia observe Aβ in parietal cortex, with some studies also showing Aβ in frontal and temporal cortices (Kumar et al., 2011a,b; Li et al., 2017; Wu et al., 2014). The percentage of Aβ-positive LLD patients was 76% using a cutoff that was comparable to most studies (DVR ≥1.08) and 29% using a more conservative criterion (DVR ≥1.20), relative to 81% and 19% of controls, respectively. Other studies have reported lower Aβ positivity rates, 19% of LLD patients and 30% of controls using a relatively less restrictive threshold (SUVR = 1.11; Mackin et al., 2020) and comparable rates with a more stringent threshold, 21% of LLD patients and 23% of controls (SUVR = 1.37; De Winter et al., 2017). As lower levels of Aβ (DVR ≥1.06) in combination with depressive symptoms may be associated with longitudinal cognitive decline (Gatchel et al., 2019), the continuum of Aβ and the relationship to depressive symptoms and cognitive decline in LLD patients should be evaluated. In the present study, poorer baseline cognitive functioning in both LLD patients and controls was observed in Aβ positive compared to Aβ negative- participants, but the magnitude of improvement with treatment in LLD patients did not differ based on Aβ status, consistent with results of voxel-wise analyses that did not show a correlation between and change in mood and neuropsychological outcomes . For both Aβ cutoffs, Aβ-positive LLD patients showed lower scores in most baseline measures compared with Aβ-negative LLD patients. Using more conservative criteria, despite Aβ-positive LLD patients showing improvement with treatment, their scores during treatment remained lower compared to scores of Aβ-negative LLD patients for measures of verbal fluency, auditory-verbal, and visual-spatial memory. In individuals who meet criteria for major depressive disorder compared with individuals with depressive symptoms, similarities and differences in regional distributions of Aβ and their clinical correlates may be observed. Thus, a voxel-wise analysis of Aβ was used in the present study. GM volumes were not significantly different between LLD patients and healthy controls. Lower GM volumes in LLD patients compared with controls have not been consistently reported (Jamieson et al., 2019). Thus, it is possible that Aβ deposition may occur in advance of significant cerebral atrophy in LLD patients.
Although this study is limited in sample size, the focus on (1) LLD in a homogeneous group of patients with limited to no prior antidepressant treatment history and (2) prospective assessment of both depressive symptoms and multi-domain neuropsychological function before and during antidepressant treatment are unique aspects. The treatment duration of 3 months may be considered to be relatively short. However, a 3-month antidepressant trial is consistent with previous research studies that have repeatedly shown mood, neuropsychological, and neurobiological effects of antidepressant treatment in LLD after 3 months of treatment (e.g., Barch et al., 2012; Smith et al., 2011). Another study limitation is the possibility of practice effects in the repeated neuropsychological measures. A comparison of change in neuropsychological assessments performed before and during treatment in patients was compared with a healthy control sample tested during a similar interval. A significantly greater increase in visual-spatial memory only (BVMT-R, delayed recall) was observed in LLD patients relative to controls. The use of alternate forms may not completely address the impact of practice effects on measuring relatively short-term outcomes of antidepressant treatment.
Previous molecular imaging studies of Aβ in LLD have shown variable results. LLD patient heterogeneity and differences in PET data analysis strategies (region of interest or voxel-wise analyses or using an Aβ-positive/Aβ-negative dichotomy) may have contributed to differences between studies. The initial Aβ imaging study in LLD patients reported greater Aβ relative to controls, mainly in LLD patients who also met criteria for amnestic MCI, in contrast to those meeting criteria for nonamnestic MCI or those who were cognitively normal (Butters et al., 2008a). Using the radiotracer [18F]-FDDNP that detects AD pathology including beta-Aβ and tau, higher global binding, as well as higher binding in lateral temporal and posterior cingulate cortices, was observed in LLD patients compared with controls (Kumar et al., 2011). Several subsequent studies using selective radiotracers for Aβ did not show higher Aβ in LLD patients, including LLD patients with cognitive deficits (Diniz et al., 2015; Morin et al., 2019; Youn et al., 2019). In a sample of mainly early onset LLD patients, higher Aβ relative to controls was not observed and such depression history variables such as greater number of lifetime episodes and longer duration of SSRI treatment exposure were not associated with greater Aβ (Mackin et al., 2020). The investigators suggested that greater risk of cognitive decline may be observed in late-onset depressed patients. Higher Aβ was correlated with greater antidepressant treatment resistance determined retrospectively in LLD patients (Li et al., 2017). Associations between greater AD neuropathology and depressive and anxiety symptoms have been more consistently shown in cognitively normal subjects using the radiotracer [18F]-FDDNP, as well as selective radiotracers for Aβ and Tau (Babudal et al., 2020; Gatchel et al., 2019, 2017; Lavretsky et al., 2009). Furthermore, greater Aβ has been associated with an increase in depressive symptoms over time in cognitively normal individuals (Babulal et al., 2016; Donovan et al., 2018). These results suggest that depressive symptoms with a late-life onset compared with early onset may be more likely to reflect an underlying AD or other neuropathological process associated with increased risk for cognitive decline.
The present study also revealed that greater left parietal cortical Aβ correlated with greater depressive symptoms, as reported in some previous studies in cognitively normal individuals (Yasuno et al., 2016). Greater Aβ deposition in motor and temporal cortices correlated with greater deficits in visual-spatial memory. Previous studies have shown correlations between higher global Aβ and worse visual-spatial memory performance (Konijnenberg et al., 2019). Correlations observed in temporal cortex may be expected, given the role of temporal cortical pathology in memory and because Aβ deposition in temporal cortex has been observed in some studies in LLD patients with memory deficits (Butters et al., 2008a). Significant associations in pre-central gyrus are unexpected, as Aβ is not typically observed in primary sensory and motor regions until later in the course of AD (Butters et al., 2008a; Ziolko et al., 2006). However, increased cerebral glucose metabolism in LLD patients compared with controls has been observed in precentral gyrus, as well as temporal and parietal cortices (Smith et al., 2009). Higher glucose metabolism was correlated with Aβ deposition in a study in MCI (a negative correlation was observed in AD patients) (Cohen et al., 2009). Higher glucose metabolism in LLD patients may represent a compensatory response to an underlying neurodegenerative process. Associations were not observed between Aβ and improvement in depressive symptoms or cognition with SSRI treatment. LLD patients enrolled in this study had no or only limited prior history of antidepressant treatment. Furthermore, the majority of LLD patients were antidepressant responders, and as a group, they did not have significant cognitive impairment. Limited variability in treatment response and in improvement in cognition may have affected the ability to detect the association between Aβ and treatment response.
Similar to molecular imaging studies, postmortem studies have shown that AD pathology is observed mainly in LLD patients meeting criteria for MCI or dementia (Sweet et al., 2004; Tsopelas et al., 2011). In patients with depressive symptoms or major depression, AD pathology was not associated with incident dementia (Wilson et al., 2016). A population-based sample of LLD patients not meeting criteria for dementia before death did not find greater AD pathology or cerebrovascular disease (strokes, lacunar infarctions, or small vessel disease) relative to controls (Tsopelas et al., 2011). In contrast to the lack of AD pathology, other findings are observed in LLD patients not meeting criteria for dementia compared with controls, including greater cell loss in hippocampus, nucleus basalis, substantia nigra and raphe nucleus, and subcortical Lewy bodies in brainstem monoaminergic neurons (locus coeruleus, substantia nigra, and dorsal raphe nucleus; Tsopelas et al., 2011; Wilson et al., 2013). Heterogeneity observed in neuroimaging and neuropathological studies of LLD is consistent with hypotheses regarding the multi-factorial nature of LLD that includes vascular and neurodegenerative mechanisms, for example (Butters et al., 2004b, 2008b), and calls for studies that precisely control such factors that otherwise impede interpretation of findings.
5. Conclusion
In summary, greater Aβ in left parietal cortex was observed in LLD patients relative to controls in LLD patients with limited or no previous antidepressant exposure. Higher cortical Aβ deposition correlated with greater depressive symptoms and worse visual-spatial memory performance before treatment. Although LLD patients improved with antidepressant treatment in depressive symptoms, verbal (letter) fluency, and visual-spatial memory, magnitude of improvement was not correlated with Aβ pathology. Neurobiological mechanisms associated with antidepressant treatment response may involve neurochemical or other neurodegeneration processes (e.g., monoamine degeneration, Tau, and alpha-synuclein). Proteomic studies have identified additional neurobiological mechanisms (e.g., inflammation, neurotrophic responses) that should be investigated further in relation to treatment response and cognitive outcomes (Diniz et al., 2015). Further molecular imaging studies in LLD patients with a history of poor mood and cognitive responses to antidepressant treatment may further elucidate neurodegenerative processes underlying vulnerability to cognitive decline (Li et al., 2017). Integration of serial assessment of depressive symptoms and neuropsychological outcomes and molecular imaging into treatment studies will provide critical mechanistic information that could inform the development of more effective intervention and prevention strategies.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Karen Edmonds, Bineyam Gebrewold, Michael Hans, Jose Leon, and David J. Clough for their invaluable contribution to the acquisition of PET data and Terri Brawner, Ivana Kusevic, and Kathy Kahl for their invaluable contribution to the acquisition of MRI data.
This study was supported by the National Institute of Health (USA): National Institute of Mental Health [MH064823 (GSS) and MH086881 (GSS),} and National Institute on Aging [AG038893 (GSS), AG041633 (GSS) and AG059390 (GSS)} and National Center for Advancing Translational Sciences [UL1 TR003098 (DEF)].
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2021.01.002.
Conflict of Interest: The authors have no competing interests to declare.
References
- Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T, 1993. The course of geriatric depression with “reversible dementia”: a controlled study. Am. J. Psychiatry 150, 1693–1699. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW, 1991. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1, 103–116. [DOI] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Avramopoulos D, Mikkelsen M, Vassilopoulos D, Grigoriadou M, Petersen MB, 1996. Apolipoprotein E allele distribution in parents of Down’s syndrome children. Lancet. [DOI] [PubMed] [Google Scholar]
- Babulal GM, Ghoshal N, Head D, Vernon EK, Holtzman DM, Benzinger TLS, Fagan AM, Morris JC, Roe CM, 2016. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am. J. Geriatr. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, Morris JC, Ances BM, 2020. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J. Alzheimers Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, Garcia KS, Gersing K, Doraiswamy PM, Sheline YI, 2012. Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am. J. Geriatr. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, 2007. Iowa gambling task professional Manual. Psychol. Assess. Res [Google Scholar]
- Beck AT, Steer RA, 1993. Manual for the Beck Depression Inventory. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Benedict RHB, Groninger L, Schretlen D, Dobraski M, Shpritz B, 1996. Revision of the brief visuospatial memory test: studies of normal performance, reliability and, validity. Psychol. Assess [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, Becker JT, 2006. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am. J. Geriatr. Psychiatry [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, 2018. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J. Int. Med [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Tredici K, 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA, 2005. Molecular, structural and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid and memory. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Mulsant BH, Mazumdar S, Houck PR, Begley AE, Dew MA, Pollock BG, Nebes RD, Becker JT, Reynolds CF, 2004a. Executive functioning, illness course and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am. J. Geriatr. Psychiatry [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, Becker JT, 2004b. The nature and determinants of neuropsychological functioning in late-life depression. Arch. Gen. Psychiatry [DOI] [PubMed] [Google Scholar]
- Butters MA, Klunk WE, Mathis CA, Price JC, Ziolko SK, Hoge JA, Tsopelas ND, Lopresti BJ, Reynolds CF, DeKosky ST, Meltzer CC, 2008a. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh compound-B. Alzheimer Dis. Assoc. Disord [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, DeKosky ST, Becker JT, 2008b. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, 2011. Depression and risk of developing dementia. Nat. Rev. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, Nebes RD, Saxton JA, Snitz BE, Aizenstein HA, Wolk DA, Dekosky ST, Mathis CA, Klunk WE, 2009. Basal cerebral metabolism may modulate the cogniti [J Neurosci. 2009] - PubMed result. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloby SJ, Firbank MJ, Vasudev A, Parry SW, Thomas AJ, O’Brien JT, 2011. Cortical thickness and VBM-DARTEL in late-life depression. J. Affective Disord 133, 158–164. [DOI] [PubMed] [Google Scholar]
- Dafsari FS, Jessen F, 2020. Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter FL, Emsell L, Bouckaert F, Claes L, Jain S, Farrar G, Billiet T, Evers S, Van Den Stock J, Sienaert P, Obbels J, Sunaert S, Adamczuk K, Vandenberghe R, Van Laere K, Vandenbulcke M, 2017. No association of lower Hippocampal volume with Alzheimer’s disease pathology in late-life depression. Am. J. Psychiatry 174, 237–245. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, 2001. The Delis-Kaplan Executive Function System. Examiners Manual, San Antonio. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B, 1987. California Verbal Learning Test (CVLT) Manual. The Psychological Corporation, San Antonio. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ, 1997. Temporal profiles of the course of depression during treatment: predictors of pathways toward recovery in the elderly. Arch. Gen. Psychiatry [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, Eidelberg D, Mcintosh AR, Smith GS, 2011. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Human Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF, 2013. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, Becker JT, Lopez OL, Lotze MT, Klunk WE, Reynolds CF, Butters MA, 2015. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol. Psychiatry 20, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, Johnson KA, Sperling RA, 2018. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am. J. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Jack CR, 2016. Preclinical Alzheimer’s disease: definition, natural history and diagnostic criteria. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams J, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, 1995. The Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. Biomet. Res. Department [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. Mini-mental state. J. Psychiatr. Res [DOI] [PubMed] [Google Scholar]
- Gatchel JR, Donovan NJ, Locascio JJ, Schultz AP, Becker JA, Chhatwal J, Papp KV, Amariglio RE, Rentz DM, Blacker D, Sperling RA, Johnson KA, Marshall GA, 2017. Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. J. Alzheimers Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel JR, Rabin JS, Buckley RF, Locascio JJ, Quiroz YT, Yang HS, Vannini P, Amariglio RE, Rentz DM, Properzi M, Donovan NJ, Blacker D, Johnson KA, Sperling RA, Marshall GA, 2019. Longitudinal association of depression symptoms with cognition and cortical amyloid among community-dwelling older adults. JAMA Netw. Open [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W, 1976. Clinical global impressions. In: ECDEU Assess. Man. Psychopharmacology—Revised. [Google Scholar]
- Hameed S, Fuh J-L, Senanarong V, Ebenezer EGM, Looi I, Dominguez JC, Park KW, Karanam AK, Simon O, 2020. Role of fluid biomarkers and PET imaging in early diagnosis and its clinical implication in the management of Alzheimer’s disease. J. Alzheimer’s Dis. Rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurgery and Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, O’Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, Kiddle SJ, Batrla R, Blennow K, 2018. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat. Rev. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K, 2019. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Toyama H, Innis RB, Carson RE, 2002. Strategies to improve neuro-receptor parameter estimation by linear regression analysis. J. Cereb. Blood Flow Metab [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST, 2008. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A, Goodwill AM, Termine M, Campbell S, Szoeke C, 2019. Depression related cerebral pathology and its relationship with cognitive functioning: a systematic review. J. Affective Disord 250, 410–418. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Långström B, 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann. Neurol [DOI] [PubMed] [Google Scholar]
- Konijnenberg E, den Braber A, ten Kate M, Tomassen J, Mulder SD, Yaqub M, Teunissen CE, Lammertsma AA, van Berckel BNM, Scheltens P, Boomsma DI, Visser PJ, 2019. Association of amyloid pathology with memory performance and cognitive complaints in cognitively normal older adults: a monozygotic twin study. Neurobiol. Aging [DOI] [PubMed] [Google Scholar]
- Kumar A, Kepe V, Barrio JR, Siddarth P, Manoukian V, Elderkin-Thompson V, Small GW, 2011a. Protein binding in patients with late-life depression. Arch. Gen. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kepe V, Barrio JR, Siddarth P, Manoukian V, Elderkin-Thompson V, Small GW, 2011b. Protein binding in patients with late-life depression. Arch. Gen. Psychiatry 68, 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X, 2008. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, Bookheimer SY, Huang SC, Barrio JR, Small GW, 2009. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am. J. Geriatr. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Hsiao IT, Liu CY, Chen CH, Huang SY, Yen TC, Wu KY, Lin KJ, 2017. Beta-amyloid deposition in patients with major depressive disorder with differing levels of treatment resistance: a pilot study. EJNMMI Res. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Mukadam N, 2017. Dementia prevention, intervention and care. Lancet. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG, 2000. Subtypes of cognitive impairment in depressed older adults. Am. J. Geriatr. Psychiatry 8, 201–208. [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Van Gorp WG, 2002. Executive dysfunction in geriatric depression. Am. J. Psychiatry [DOI] [PubMed] [Google Scholar]
- Lois C, Gonzalez I, Johnson KA, Price JC, 2019. PET imaging of tau protein targets: a methodology perspective. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RS, Insel P, Landau S, Bickford D, Morin R, Rhodes E, Tosun D, Rosen H, Butters M, Aisen P, Raman R, Saykin A, Toga A, Jack C, Koeppe R, Weiner M, Nelson C, 2020. Late life depression is associated with reduced cortical amyloid burden: findings from the ADNI depression project. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Ji B, Irie T, Tomiyama T, Maruyama M, Okauchi T, Staufenbiel M, Iwata N, Ono M, Saido TC, Suzuki K, Mori H, Higuchi M, Suhara T, 2007. Longitudinal, quantitative assessment of amyloid, neuroinflammation and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE, 2003. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem [DOI] [PubMed] [Google Scholar]
- Mattsson-Carlgren N, Palmqvist S, Blennow K, Hansson O, 2020. Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nat. Commun 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA, 2015. Pathological tau disrupts ongoing network activity. Neuron. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Bruce ML, 1998. The depression-dementia conundrum: integrating clinical and epidemiological perspectives. Arch. Gen. Psychiatry [DOI] [PubMed] [Google Scholar]
- Morin RT, Insel P, Nelson C, Butters M, Bickford D, Landau S, Saykin A, Weiner M, Mackin RS, 2019. Latent classes of cognitive functioning among depressed older adults without dementia. J. Int. Neuropsychological Soc 25, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ, 2009. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, 1993. The clinical dementia rating (cdr): current version and scoring rules. Neurology. [DOI] [PubMed] [Google Scholar]
- do Nascimento KKF, Silva KP, Malloy-Diniz LF, Butters MA, Diniz BS, 2015. Plasma and cerebrospinal fluid amyloid-β levels in late-life depression: a systematic review and meta-analysis. J. Psychiatr. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC, 2007. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. [DOI] [PubMed] [Google Scholar]
- Rahmim A, Cheng JC, Blinder S, Camborde ML, Sossi V, 2005. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Phys. Med. Biol [DOI] [PubMed] [Google Scholar]
- Rey A, 1941. L’examen psychologique dans les cas d’encephalopathie traumatique (Psychological examination of brain damage cases). Archives de Psychologie. [Google Scholar]
- Sackeim HA, 2001. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry [PubMed] [Google Scholar]
- Sheffrin M, Driscoll HC, Lenze EJ, Mulsant BH, Pollock BG, Miller MD, Butters MA, Dew MA, Reynolds CF, 2009. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J. Clin. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, 1968. The Symbol-Digit Modalities Test: a neuropsychologic test for economic screening of learning and other cerebral disorders. Learn. Disord [Google Scholar]
- Smith GS, Leon MJ, George AE, Kluger A, Volkow ND, Mcrae T, Golomb J, Ferris SH, Reisberg B, Ciaravino J, Regina ME, 1992. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease: pathophysiologic implications. Arch. Neurol 49. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D, 2009. The functional neuroanatomy of geriatric depression. Int. J. Geriatr. Psychiatry 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Kahn A, Sacher J, Rusjan P, Van Eimeren T, Flint A, Wilson AA, 2011. Serotonin transporter occupancy and the functional neuroanatomic effects of citalopram in geriatric depression. Am. J. Geriatr. Psychiatry 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Barrett FS, Joo JH, Nassery N, Savonenko A, Sodums DJ, Marano CM, Munro CA, Brandt J, Kraut MA, Zhou Y, Wong DF, Workman CI, 2017. Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol. Dis 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojkova J, Beason-Held L, Zhou Y, An Y, Kraut MA, Ye W, Ferrucci L, Mathis CA, Klunk WE, Wong DF, Resnick SM, 2008. Longitudinal cerebral blood flow and amyloid deposition: an emerging pattern? J. Nucl. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solai LKK, Mulsant BH, Pollock BG, 2001. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. [DOI] [PubMed] [Google Scholar]
- Sossi V, De Jong HWAM, Barker WC, Bloomfield P, Burbar Z, Camborde ML, Comtat C, Eriksson LA, Houle S, Keator D, Knöß C, Krais R, Lammertsma AA, Rahmim A, Sibomana M, Teräs M, Thompson CJ, Trébossen R, Votaw J, Wong DF, 2005. The second generation HRRT - a multi-centre scanner performance investigation. IEEE Nucl. Sci. Symp. Conf. Rec. [Google Scholar]
- Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Lopez OL, DeKosky ST, Reynolds CF, 2004. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology 29, 2242–2250. [DOI] [PubMed] [Google Scholar]
- Tsopelas C, Stewart R, Savva GM, Brayne C, Ince P, Thomas A, Matthews FE, 2011. Neuropathological correlates of late-life depression in older people. Br. J. Psychiatry 198, 109–114. [DOI] [PubMed] [Google Scholar]
- Vieweg WVR, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, Pandurangi AK, 2012. Citalopram, QTc interval prolongationand torsade de pointes. How should we apply the recent FDA ruling? Am. J. Med [DOI] [PubMed] [Google Scholar]
- Villemagne VL, 2014. New developments in molecular imaging of the pathology of neurodegeneration. Neurobiol. Aging [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, La Joie R, Arthur-Bentil SK, Vogel JW, Marks SM, Lehmann M, Rosen HJ, Reed B, Olichney J, Boxer AL, Miller BL, Borys E, Jin LW, Huang EJ, Jagust W, 2015. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Memory Scale - Third Edition Administration and Scoring Manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wenham PR, Price WH, Blundell G, 1991. Apolipoprotein E genotyping by one-stage PCR. Lancet. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Shah RC, Schneide JA, Arnold SE, Bennett DA, 2013. Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Capuano AW, Shah RC, Hoganson GM, Nag S, Bennett DA, 2016. Late-life depression is not associated with dementia-related pathology. Neuropsychology 30, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hsiao IT, Chen CS, Chen CH, Hsieh CJ, Wai YY, Chang CJ, Tseng HJ, Yen TC, Liu CY, Lin KJ, 2014. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging [DOI] [PubMed] [Google Scholar]
- Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, Yamamoto A, Matsuoka K, Kosaka J, Kudo T, Iida H, Kishimoto T, Nagatsuka K, 2016. High amyloid-β deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int. J. Geriatr. Psychiatry [DOI] [PubMed] [Google Scholar]
- Youn HC, Lee S, Han C, Kim SH, Jeong HG, 2019. Association between brain amyloid accumulation and neuropsychological characteristics in elders with depression and mild cognitive impairment. Int. J. Geriatr. Psychiatry 34, 1907–1915. [DOI] [PubMed] [Google Scholar]
- Ziolko SK, Weissfeld LA, Klunk WE, Mathis CA, Hoge JA, Lopresti BJ, DeKosky ST, Price JC, 2006. Evaluation of voxel-based methods for the statistical analysis of PIB PET amyloid imaging studies in Alzheimer’s disease. Neuroimage. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.