Abstract
Chronic stress contributes to the development of psychiatric disorders including anxiety and depression. Several inflammatory-related effects of stress are associated with increased interleukin-1 (IL-1) signaling within the central nervous system and are mediated by IL-1 receptor 1 (IL-1R1) on several distinct cell types. Neuronal IL-1R1 is prominently expressed on the neurons of the dentate gyrus, but its role in mediating behavioral responses to stress is unknown. We hypothesize that IL-1 acts on this subset of hippocampal neurons to influence cognitive and mood alterations with stress. Here, mice subjected to psychosocial stress showed reduced social interaction and impaired working memory, and these deficits were prevented by global IL-1R1 knockout. Stress-induced monocyte trafficking to the brain was also blocked by IL-1R1 knockout. Selective deletion of IL-1R1 in glutamatergic neurons (nIL-1R1−/−) abrogated the stress-induced deficits in social interaction and working memory. In addition, viral-mediated selective IL-1R1 deletion in hippocampal neurons confirmed that IL-1 receptor in the hippocampus was critical for stress-induced behavioral deficits. Furthermore, selective restoration of IL-1R1 on glutamatergic neurons was sufficient to reestablish the impairments of social interaction and working memory after stress. RNA-sequencing of the hippocampus revealed that stress increased several canonical pathways (TREM1, NF-κB, complement, IL-6 signaling) and upstream regulators (INFγ, IL-1β, NF-κB, MYD88) associated with inflammation. The inductions of TREM1 signaling, complement, and leukocyte extravasation with stress were reversed by nIL-1R1−/−. Collectively, stress-dependent IL-1R1 signaling in hippocampal neurons represents a novel mechanism by which inflammation is perpetuated and social interactivity and working memory are modulated.
Keywords: Social Stress, Interleukin-1 beta, IL-1 Receptor, Neurons
Introduction
Psychosocial stress is a major contributing factor to anxiety and depression1, 2. There is evidence that stress is associated with increased inflammation3–6. For example, increases in inflammatory cytokines, circulating monocytes, and microglial activation are detected in patients with anxiety and mood disorders7, 8. This intersection between stress, inflammation, and anxiety has been recapitulated in rodent models of stress including repeated social defeat (RSD)9–11, repeated social defeat stress12–15, and paired fighting16, 17. We have previously detailed how psychosocial stress results in peripheral immune activation, increased levels of circulating monocytes, and robust neuroimmunological responses in the brain18–20. These responses include increases in interleukin-1 (IL-1) beta and other cytokines/chemokines, microglial activation, astrogliosis, monocyte trafficking to the brain, and enhanced neuronal activity21, 22.
Several inflammatory-related effects of stress are associated with increased IL-1 signaling within the central nervous system (CNS). Our previous work using RSD shows that stress-induced anxiety-like behavior is augmented by inflammatory, IL-1β-producing monocytes which are actively recruited to the brain vasculature by microglia11. Moreover, expression of the IL-1 receptor (IL-1R1) on endothelial cells is critical in mediating monocyte communication to the brain endothelium during stress23. Additionally, endothelial IL-1R1 contributes to microglial activation via release of soluble factors24, 25. Notably, if IL-1 can act directly on neurons to cause behavioral alterations has not been determined. This is relevant because direct IL-1 action on neurons might translate neuroinflammatory signals to behavioral outcomes.
We have created and validated a unique genetic model to restrict IL-1R1 expression in different cell types24–26. We have since defined how cell type-specific IL-1R1 regulates distinct neuroimmune activities in the brain24, 25. For example, endothelial IL-1R1 mediates IL-1β-induced sickness behavior via cyclooxygenase-II (COX-2) signaling, and both endothelial and ependymal IL-1R1 are involved in recruiting peripheral myeloid cells to the brain. While we discovered that IL-1R1 is prominently expressed on glutamatergic neurons of the dentate gyrus, its role in mediating responses to stress is unknown. To define the IL-1R1-mediated neuronal response, we used novel and comprehensive IL-1R1 transgenic/reporter lines in which one can selectively delete IL-1R1 or restore IL-1R1 on specific cell types26, including glutamatergic neurons. We also used viral vectors to determine the specific role of hippocampal neurons on the stress response. The purpose of this study was to determine the degree to which IL-1 acts directly on hippocampal neurons to influence cognitive and mood changes with stress. Here we show novel data that social defeat-dependent IL-1R1 signaling in hippocampal neurons perpetuated inflammation and promoted deficits in social interaction and working memory.
Methods
Mice:
Male C57BL/6 mice were housed in cohorts of two per cage, while CD-1 aggressors were singly housed. Transgenic mouse lines were bred in-house and randomly distributed to groups. All procedures were in accordance with the NIH Guidelines and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Social stress paradigm:
Mice were subjected to social stress as described in Supplementary Materials. In brief, experimental mice were placed individually into the cages of CD-1 aggressor mice for one hour (between 16:00 to 18:00) per day for six consecutive days. During each cycle, submissive behaviors were observed to ensure that the experimental mice showed subordinate behavior.
Behavioral testing:
Distance traveled in the open field was determined as a measure of baseline activity, working memory was determined via the Y-maze, and social exploratory behavior was measured as described in Supplementary Materials.
Isolation of cells from blood and brain:
Tissues were collected immediately following CO2 asphyxiation. Cells from blood and brain were isolated as previously described27.
RNA-sequencing of hippocampal tissue:
Bulk tissue RNA-sequencing was performed as described in Supplementary Materials.
Statistical analysis:
Data were analyzed as described in Supplementary Materials. All data are expressed as mean ± SEM.
Results
Stress-induced social withdrawal and working memory deficits are dependent on IL-1R1.
This study aimed to augment our understanding of IL-1R1 signaling in physiological and behavioral responses to stress. Here, a modified social stress paradigm was implemented using aggressive CD-1 intruders (Fig.1A), and IL-1R1+/+ (wild-type IL-1R1 expression with tdTomato reporter) and IL-1R1−/− mice (IL-1R1 knockout) were generated (Fig.1B–C). First, IL-1R1 expression in the brain was assessed by tdTomato. Stress increased endothelial tdTomato expression in IL-1R1+/+ mice throughout the brain, with robust neuronal expression within the granule cell layer of the dentate gyrus (Fig.1D–E).
Figure 1. Stress-induced social withdrawal and working memory deficits are dependent on IL-1R1.
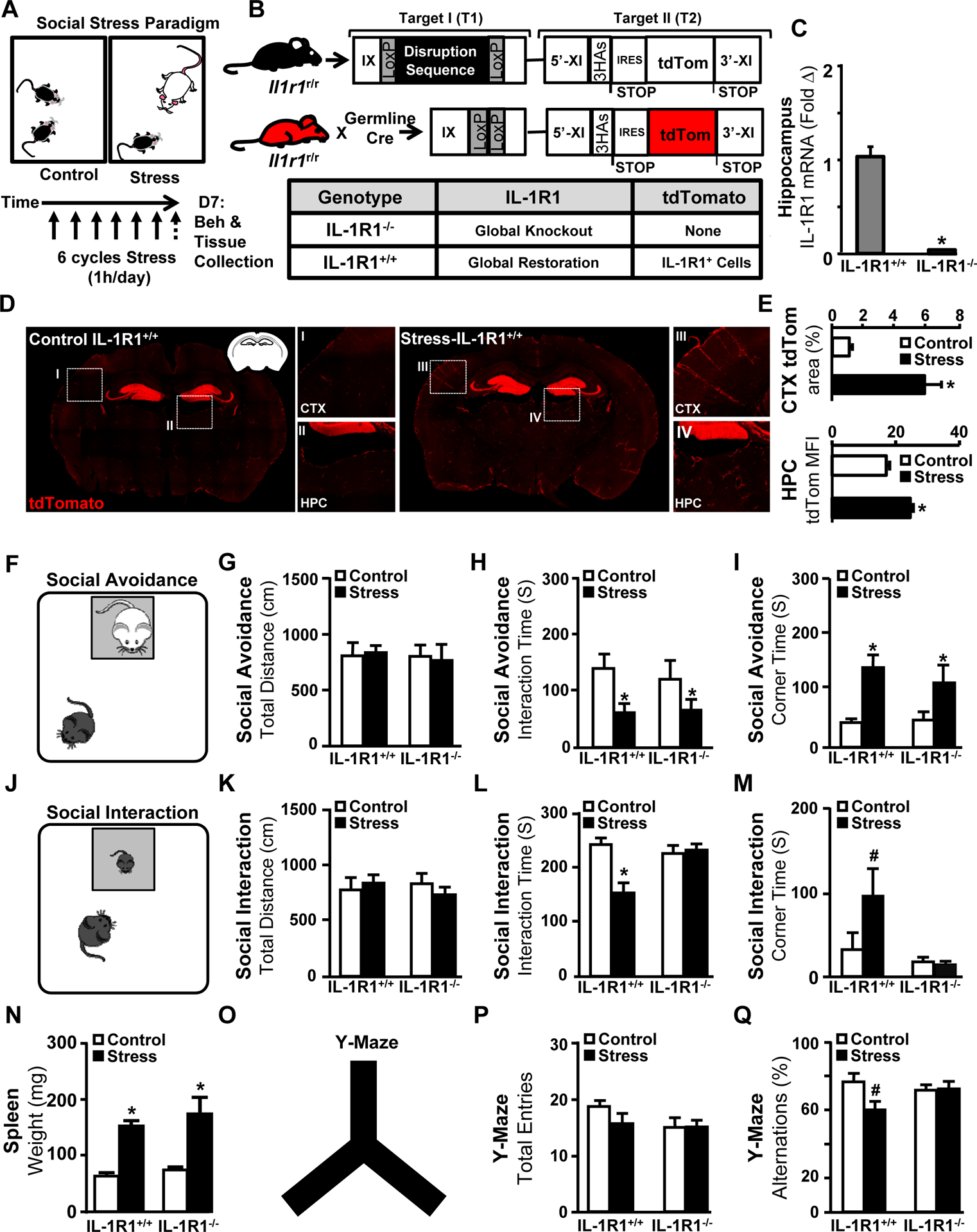
A) Male IL-1R1+/+ and IL-1R1−/− mice were left undisturbed in their home cage (Control) or exposed individually to a male CD-1 aggressor mouse for 1 h per day for six consecutive days (Stress). 14 h after Stress, behavior was determined and then the brain was collected for tdTomato analysis. B) Illustration and denotation of the IL-1R1 transgenic construct in C57BL/6 mice. IL-1R1+/+ mice are homozygous and express germline Cre, resulting in global restoration of wild-type-like genotype in which all IL-1R1 mRNA is tagged with tdTomato. IL-1R1−/− mice are functional knockouts of IL-1R1 in all cell types. C) Validation by qPCR for IL-1R1−/− mice (P < 0.001, Student’s t-test). D) Representative images of tdTomato levels in IL-1R1+/+ mice exposed to Control or Stress in the cortex (CTX) and hippocampus (HPC). E) Quantification via percent area in CTX (P = 0.006; HPC), and via mean fluorescence intensity (MFI) in the HPC neurons (P < 0.001) (n = 6). F) Social avoidance of a CD-1 aggressor mouse was assessed (n = 6, two replicates) with G) Total distance travelled (n.s.), H) Interaction time with a CD-1 mouse (main effect of Stress; F(1,29) = 5.6, P = 0.01) and I) time spent in corner (main effect of Stress; F(1,29) = 5.2, P = 0.01). J) In a separate experiment, mice were subjected to stress as above and social interaction with novel C57BL/6 juvenile mice was determined (n = 11, three replicates). K) Total distance traveled (n.s.), L) Interaction time with the C57BL/6 juvenile (Stress x Genotype; F(2,47) = 12.6, P = 0.0001), and M) time spent in corner (Stress x Genotype; F(2,47)=3.8, P = 0.1). N) Spleen weight (main effect of Stress, F(1,26) = 17.8, P < 0.001). O) In the same mice, working memory was determined in the Y-maze. P) Total entries (n.s.) and Q) percentage of spontaneous alternations (Stress x Genotype; F(2,32) = 3.4, P = 0.10). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from the corresponding controls (P< 0.05), and means with (#) tended to be different from control (P < 0.1), according to F-protected post hoc analysis.
Next, IL-1R1+/+ and IL-1R1−/− mice were exposed to social stress and several behaviors were determined. In assessments of social avoidance of an aggressor, mice were exposed to a caged male CD-1 mouse (Fig.1F). Baseline activity was unaffected (Fig.1G), but stress caused avoidance of the CD-1 aggressor with reduced interaction time (Fig.1H) and increased corner time (Fig.1I) regardless of genotype. To investigate “sociability”, a social interaction test with a juvenile conspecific was performed (Fig.1J). Baseline activity was again unaffected by stress (Fig.1K). Stress reduced interaction time with the juvenile (Fig.1L) and increased time spent in the corner in IL-1R1+/+ mice, but not in IL-1R1−/− mice (Fig.1M). Stress increased spleen weight, a biomarker for psychosocial stress18, 28, independent of IL-1R1 expression (Fig.1N).
Stress-induced working memory deficits in IL-1R1+/+ and IL-1R1−/− mice were assessed by spontaneous alternations in the Y-maze29, 30 (Fig.1O). Overall mobility in the Y-maze was unaffected (Fig.1P), but there were IL-1R1-dependent reduced spontaneous alternations in stressed mice (Fig.1Q). These stress effects were recapitulated in wild-type mice (Supplementary Fig.S1). Thus, social defeat-induced reductions in generalized sociability and working memory were IL-1-dependent.
IL-1R1 is associated with increased neuronal activity, microglial restructuring, endothelial reactivity, and monocyte accumulation in the brain with social stress.
We investigated if stress-induced neuronal activity and long-term alteration (ΔFosB)31, microglial restructuring (Iba-1)11, and endothelial reactivity (VCAM)32 were dependent on IL-1R1 signaling (Fig.2A). Stress increased ΔFosB expression in the hippocampus in both IL-1R1+/+ and IL-1R1−/− mice (Fig.2B). Notably, there was less ΔFosB expression in IL-1R1−/− mice compared to IL-1R1+/+ mice. Microglial labeling (Iba-1) and endothelial reactivity (VCAM) were increased by stress in IL-1R1+/+ mice (Fig.2C–D). These stress effects were attenuated in IL-1R1−/− mice. Stress-induced VCAM expression was highly correlated with IL-1R1 expression on endothelial cells (Fig.2E–F).
Figure 2. IL-1R1 is required for vascular adhesion and monocyte accumulation with social stress.
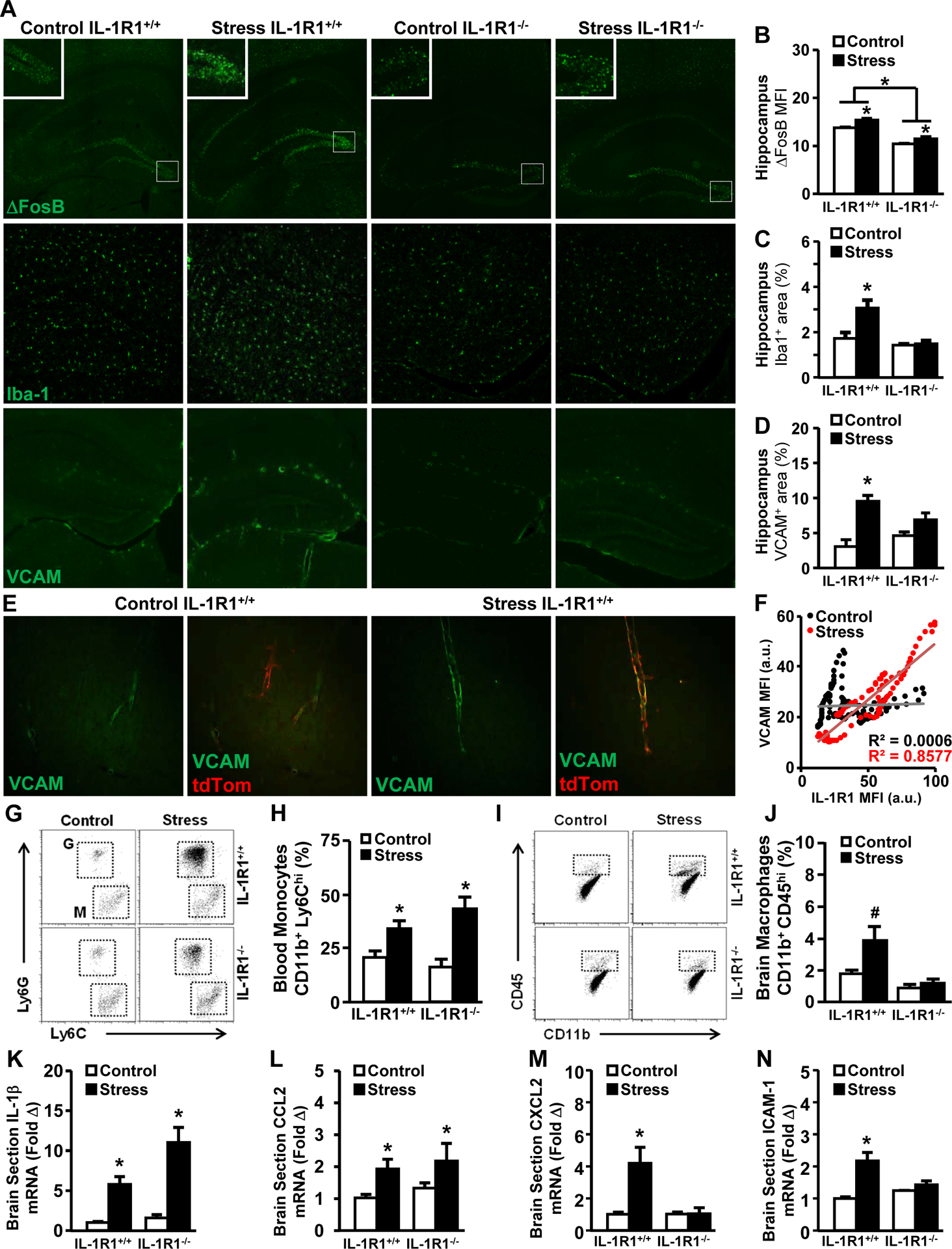
Male IL-1R1+/+ and IL-1R1−/− mice were left undisturbed in their home cage (Control) or exposed individually to a male CD-1 aggressor mouse for 1 h per day for six consecutive days (Stress). 14 h after stress, mice were sacrificed, brains were PFA fixed and the hippocampal sections were labeled (ΔFosB, Iba-1 or VCAM-1). A) Representative images are shown for each experimental group (n = 5, two replicates). Boxes indicate the areas used in insets. B) Mean fluorescence intensity of neuronal ΔFosB labeling in the dentate gyrus 14 h after stress (main effect of Stress; F(1,17) = 8.3, P = 0.02; main effect of Genotype; F(1,7) = 28.9, P = 0.001). C) Percent area of Iba-1 microglial labeling in the hippocampus after stress (Stress x Genotype; F(2,18) = 7.9, P = 0.02). D) Percent area of VCAM labeling in the hippocampus after stress (Stress x Genotype; F(2,23) = 10.1, P = 0.004). E) Representative labeling of VCAM and expression of tdTomato in the cortex of control and stress mice. F) Quantification and scatterplot of VCAM/IL-1R1 colocalization of Control (R2 = 0.0006, P = 0.78) and Stress (R2 = 0.8577, P < 0.001) cortical blood vessels. In a separate experiment, IL-1R1+/+ and IL-1R1−/− mice were subjected to social stress as above and the percentage of monocytes were determined in the blood and brain (n = 6, two replicates). G) Representative bivariate dot blots of Ly6C+ and Ly6G+ cells in circulation. H) Percentage of Ly6Chi monocytes in circulation (main effect of Stress; F(1,19) = 11.1, P = 0.01). I) Representative bivariate dot blots of CD45+ and CD11b+ cells in the brain. J) Percentage of CD45hi macrophages in the brain (Stress x Genotype; F(2,25) = 3.01, P = 0.09). In a separate experiment (n = 4, two replicates), IL-1R1+/+ and IL-1R1−/− mice were subjected to social stress as above; mice were transcardially perfused with PBS 14h after the final cycle of stress and a 1mm coronal section through the hippocampus was used to determine the mRNA levels of K) IL-1β (main effect of Stress; F(1,13) = 11.4, P = 0.004), L) CCL2 (main effect of Stress; F(1,13) = 4.7, P = 0.05), M) CXCL2 (Stress x Genotype; F(2,13) = 8.8, P = 0.01), and N) ICAM-1 (Stress x Genotype; F(2,14) = 4.1, P = 0.002). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different (P < 0.05), and means with (#) tended to be different (P < 0.1) from the corresponding control mice, according to F-protected post hoc analysis.
Next, monocyte mobilization in the blood, accumulation in the brain, and neuroinflammation were assessed. Stress increased circulating CD11b+/Ly6Chi monocytes in both IL-1R1+/+ and IL-1R1−/− mice (Fig.2G–H). There was increased monocyte accumulation in the brain of stressed IL-1R1+/+ mice, but not in IL-1R1−/− mice (Fig.2I–J). Thus, stress-induced increases in circulating monocytes were IL-1R1-independent, while monocyte trafficking to the CNS was IL-1R1-dependent. In the same study, mRNA expression levels of several cytokines, chemokines, and adhesion molecules were determined in a 1mm coronal brain section. IL-1β (Fig.2K) and CCL2 (Fig.2L) mRNA levels were increased by stress, but were unaffected by IL-1R1 knockout. CXCL2 (Fig.2M) and ICAM-1 (Fig.2N) mRNA levels were also increased by stress and these inductions were absent in IL-1R1−/− mice. Collectively, knockout of IL-1R1 attenuated stress-induced activation of neurons, microglia, and endothelial cells and reduced monocyte accumulation in the brain.
IL-1R1 on glutamatergic neurons is sufficient for stress-induced social withdrawal and working memory deficits.
Previous data using RSD show that there are cell type-specific IL-1R1 responses to stress, including monocyte-to-endothelium communication32. Based on the robust hippocampal expression of IL-1R1 (Fig.1D) and evidence of cytokine-induced neuronal activity33, we next investigated neuronal-specific responses to IL-1β with social stress. To address this, glutamatergic neuron-specific Vglut2-Cre-driven deletion of IL-1R1 (nIL-1R1−/−) and restoration of IL-1R1 in knockout mice (nIL-1R+/+) were generated (Fig.3A–B). These transgenic lines were compared to IL-1R1+/+ mice after stress.
Figure 3. IL-1R1 on glutamatergic neurons is sufficient for stress-induced social withdrawal and working memory deficits.
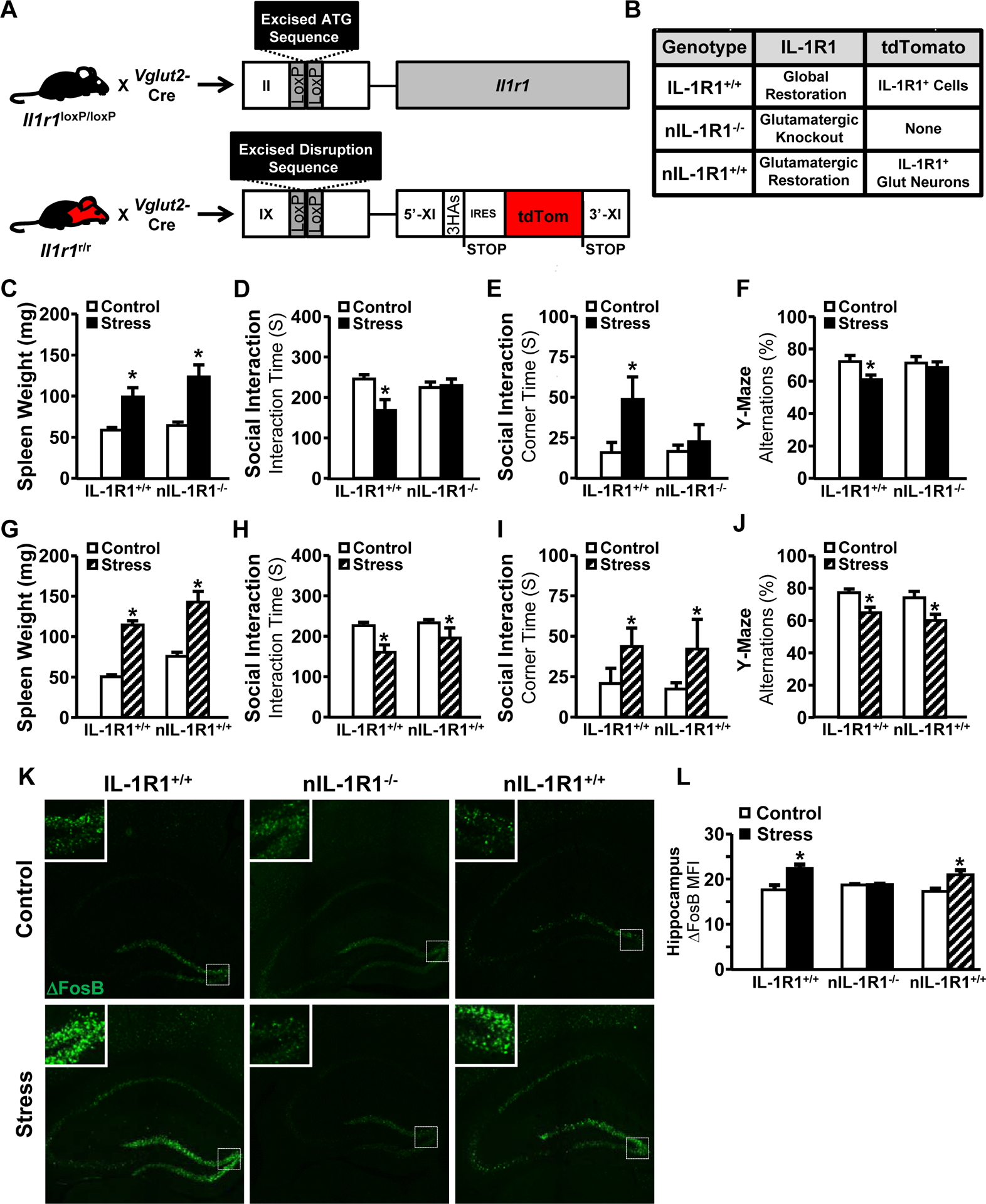
Male transgenic mice were left undisturbed in their home cage (Control) or exposed individually to a male CD-1 aggressor mouse for 1 h per day for six consecutive days (Stress). 14 h after stress, IL-1R1+/+ and nIL-1R1−/− mice were subjected to the social interaction and spontaneous alternations tests (n = 12, three replicates). A) Illustration and denotation of the IL-1R1 transgenic construct in C57/BL6 mice. IL-1R1+/+ mice are homozygous and express Cre in all cell types resulting in a global restoration of wild type genotype in which all IL-1R1 mRNA is tagged with tdTomato. nIL-1R1−/− mice are functional knockouts of IL-1R1 in Vglut2+ neurons, and nIL-1R1+/+ mice have restoration of IL-1R1 in Vglut2+ neurons. B) Chart summarizing transgenic lines. C) Spleen weight (main effect of Stress; F(1,31) = 9.8, P < 0.01), D) time spent interacting with novel caged C57BL/6 juvenile mice (Stress x Genotype; F(2,31) = 6.6, P = 0.01), E) time spent in either far corner of the arena (Stress x Genotype; F(2,31) = 7.01, P = 0.01), and F) percentage of spontaneous alternations among the arms of the Y-maze (Stress x Genotype; F(2,31) = 6.01, P = 0.02). Measures are displayed in IL-1R1+/+ and nIL-1R1+/+ mice for G) spleen weight (main effect of stress; F(1,37) = 34.4, P < 0.0001), H) time spent interacting with novel caged C57BL/6 juvenile mice (main effect of Stress; F(1,37) = 8.02, P = 0.007), I) time spent in either far corner of the arena (main effect of Stress; F(1,37) = 4.03, P = 0.05), and J) percentage of spontaneous alternations in the Y-maze (main effect of Stress; F(1,37) = 6.9, P = 0.01). K) Representative images within the hippocampus of control (top) and stressed (bottom) mice that were labeled for ΔFosB (n = 5, two replicates). Boxes indicate the areas used in insets. L) ΔFosB mean fluorescence intensity quantified within HPC (Stress x Genotype; F(2,19) = 2.7, P = 0.02). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from the corresponding control mice (P < 0.05), and means with (#) tended to be different from control mice (P < 0.1), according to F-protected post hoc analysis.
Stress increased spleen weight independent of nIL-1R1 (Fig.3C). When exposed to a juvenile, stress reduced sociability in IL-1R1+/+ mice but nIL-1R1−/− mice had neither reduced social interactivity (Fig.3D) nor increased corner time (Fig.3E). Stress-induced spontaneous alternation deficits in the Y-maze were also abrogated in nIL-1R1−/− mice (Fig.3F). The same behavioral assessments were completed using nIL-1R1+/+ mice. In these mice, only Vglut2+ glutamatergic neurons have been restored to functional IL-1R1 expression. Stressed nIL-1R1+/+ mice had reduced social investigation of a juvenile mouse (Fig.3G–H) and increased corner time (Fig.3I). Additionally, stress-induced decreases in spontaneous alternations were evident in nIL-1R1+/+ mice (Fig. 3J). Behaviorally, nIL-1R1−/− mice resembled IL-1R1−/− mice, but expression of nIL-1R1 was sufficient to recapitulate stress-induced behavioral deficits evident in IL-1R1+/+ mice.
We assessed if nIL-1R1 was involved in neuronal activity (ΔFosB) and microglial restructuring (Iba-1) in the dentate gyrus following social stress. ΔFosB labeling was unchanged in stressed nIL-1R1−/− mice, while nIL-1R1+/+ mice had increased ΔFosB after stress that was consistent with IL-1R1+/+ mice (Fig.3K–L). The stress-induced microglial Iba-1 labeling increases, however, were independent of nIL-1R1 (Supplementary Fig.S2). These data indicate that nIL-1R1 was crucial for stress-induced neuronal activity in the hippocampus.
Hippocampal-specific knockout of nIL-1R1 prevents stress-induced behavioral deficits.
We show a central role for the hippocampus in stress-induced nIL-1R1-mediated behaviors. Therefore, we selectively knocked out hippocampal IL-1R1 by bilaterally injecting AAV2-CreGFP (HPC-IL-1R1−/−) into the dentate gyrus of Il1r1loxP/loxP mice (Fig.4A). AAV2-GFP injections were used as controls (Sham-IL-1R1+/+). This virus specifically targeted neurons as validated by GFP (Fig.4B), and induced regionally-specific IL-1R1 knockout within the hippocampus. As expected, stress increased spleen weight independent of viral manipulation (Fig.4C). Additionally, there were no differences in baseline activity (Fig.4D). The stress-induced reduction in social interaction with a juvenile (Fig.4E) was not evident in the HPC-IL-1R1−/− mice. Corner time also appeared to be increased with stress, but these results were not significant (Fig.4F). In the Y-Maze, reduction in the spontaneous alternations in stressed Sham-IL-1R1+/+ mice were not evident in HPC-IL-1R1−/− mice (Fig.4G–H). Notably, a hippocampal-selective restoration approach was also used and the restoration of IL-1R1 in hippocampal neurons was less effective for restoring the social interaction effects of stress (Supplementary Fig.S3C–D) compared to Vglut2-driven restoration (Fig.3H–I). Collectively, our data indicate that IL-1R1 signaling in hippocampal neurons was critical for social withdrawal and cognitive deficits after stress.
Figure 4. Hippocampal-specific knockout of the neuronal IL-1 receptor is sufficient to eliminate stress-induced behavioral changes.
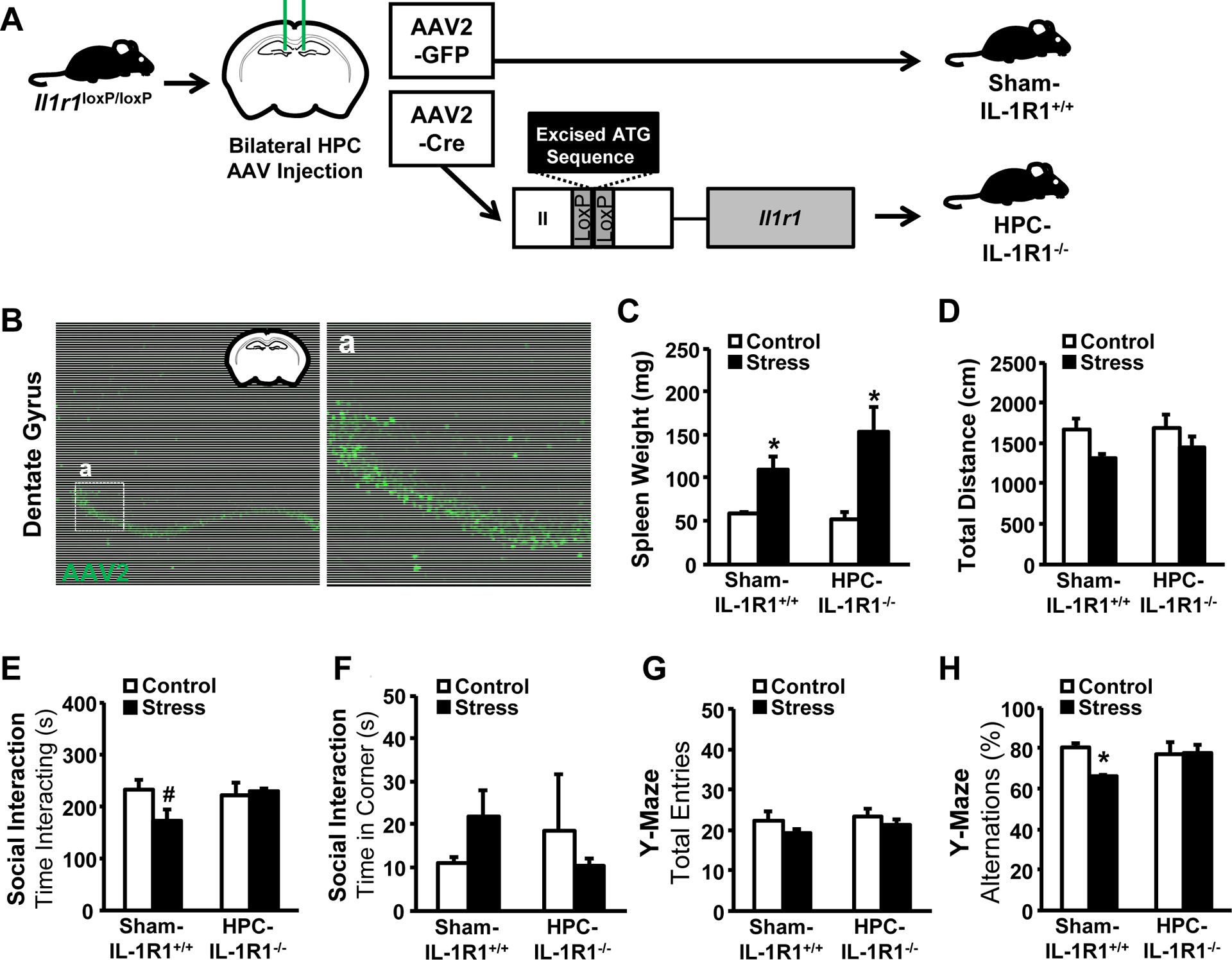
Male Il1r1loxP/loxP mice were injected bilaterally with either AAV2-GFP (Sham-IL-1R1+/+) or with AAV2-CreGFP (HPC-IL-1R1−/−) into the dentate gyrus (coordinates AP −1.82, DV 2.26, ML ±0.75). After two weeks of recovery, mice were either left undisturbed in their home cage (Control) or exposed individually to a male CD-1 aggressor mouse for 1 h per day for six consecutive days (Stress). A) Experimental design is shown. 14 h after stress, behavior was determined and brains were fixed with 4% PFA. B) Representative images of hippocampal sections that were examined for verification of viral injection into the dentate gyrus (n = 8, two replicates). Inset (a) shown to the right. Measures are displayed for C) spleen weight (main effect of Stress; F(2,23) = 15.06, P = 0.0007), D) total distance traveled (n.s.), E) time spent interacting with a novel caged C57BL/6 juvenile mouse (Stress x Condition; F(2,23) = 3.56, P = 0.10), F) time spent in the corner of the social interaction arena (n.s.), G) total entries into the arms of the Y-Maze (n.s.), and H) percentage of spontaneous alternations in the Y-Maze (Stress x Condition; F(2,23) = 4.15, P = 0.05). Means with asterisk (*) are significantly different from the corresponding control mice (P < 0.05), and means with (#) tended to be different from control mice (P < 0.1), according to F-protected post hoc analysis.
Stress-induced hippocampal gene expression is mediated by IL-1R1 on glutamatergic neurons.
Our findings indicate a central role for nIL-1R1 in stress-induced behavioral alterations. The prevention of behavioral effects in nIL-1R1−/− mice led us to hypothesize that there were neuronal processes initiated by nIL-1R1 driving these behaviors. In order to determine the nIL-1R1-specific gene-expression effects of stress, we performed RNA-sequencing on microdissected hippocampi from IL-1R1+/+ and nIL-1R1−/− mice.
There were 101 genes that were differentially expressed in the hippocampus after stress in IL-1R1+/+ mice (Fig.5A). Stress increased expression of 97 genes and decreased expression of 4 genes. Of these, 27 genes (25 increased, 2 decreased) were reversed by nIL-1R1−/− and 15 were partially reversed. A heatmap of increased and decreased genes is shown (Fig.5B), and all genes significantly increased and decreased in expression by stress are provided (Supplementary Fig.S4). Ingenuity Pathway Analysis (IPA) was used to determine canonical pathways (Fig.5C) and upstream regulators (Fig.5D) that were affected by stress. Here, pathways of interest were selected from the larger list of pathways that were altered by stress (Supplementary Fig.S5). Next, we analyzed the effect of nIL-1R1 knockout. Previously-increased canonical pathways (Fig.5E) and upstream regulators (Fig.5F) were reversed in stressed nIL-1R1−/− mice compared to stressed IL-1R1+/+ mice. TREM1 signaling, leukocyte extravasation, pattern recognition receptor signaling, and complement system were all decreased in stressed nIL-1R1−/− mice compared to stressed IL-1R1+/+ mice. Collectively, stress-induced neuronal IL-1R1 signaling augmented inflammatory and immune signaling profiles within the hippocampus.
Figure 5. Stress-induced hippocampal gene expression is mediated by IL-1R1 on glutamatergic neurons.
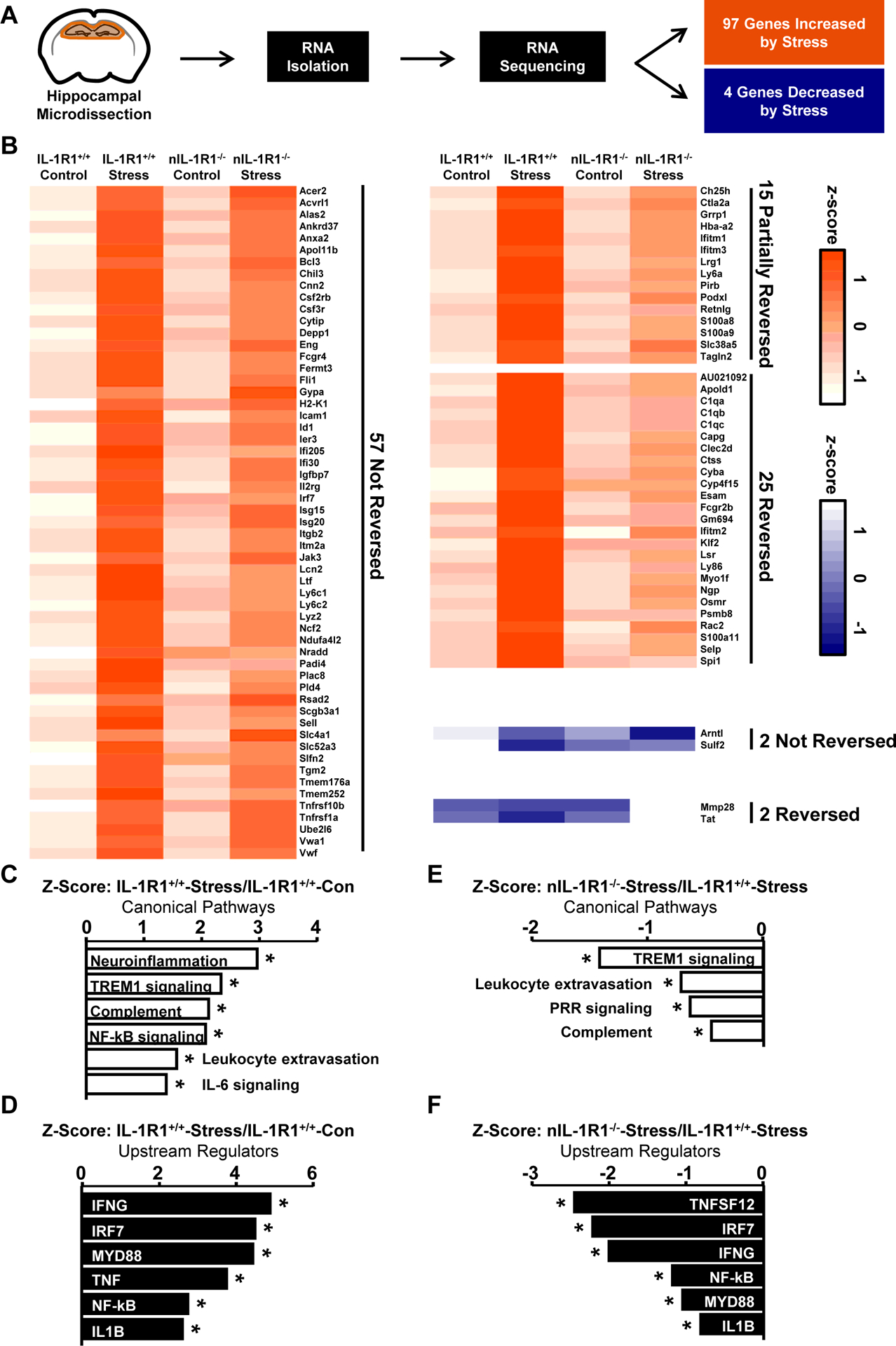
Male IL-1R1+/+ and nIL-1R1−/− mice were left undisturbed in their home cage (Control) or exposed individually to a male CD-1 aggressor mouse for 1 h per day for six consecutive days (Stress). A) 14 h after stress, mice were sacrificed, brains were harvested, and hippocampi were microdissected for RNA-sequencing (n = 6, two replicates). B) Heat map shows mean expression of 101 genes significantly increased or decreased by stress (IL-1R1+/+ Control vs. IL-1R1+/+ Stress: P-adj < 0.05 and fold change > 1.5). These are divided into categories based on patterns of expression following neuronal IL-1R1 knockout: Partially Reversed (nIL-1R1−/− Stress vs. IL-1R1+/+ Stress: P < 0.05; nIL-1R1−/− Stress vs. IL-1R1+/+ Control: P < 0.05), or Reversed (nIL-1R1−/− Stress vs. IL-1R1+/+ Stress: P < 0.05; nIL-1R1−/− Stress vs. IL-1R1+/+ Control: P ≥ 0.05). Heat maps are normalized by row. Ingenuity Pathway Analysis (IPA) was used to determine significantly altered C) canonical pathways and D) upstream regulators of differentially expressed genes in the IL-1R1+/+ Stress versus IL-1R1+/+ Control comparison. Bars represent pathway z-score and (*) denotes P < 0.05. IPA determined significantly altered E) canonical pathways and F) upstream regulators of differentially expressed genes in the nIL-1R1−/− Stress versus IL-1R1+/+ Stress comparison. Detailed gene lists and mRNA counts can be found in Supplementary Data.
Discussion
Previous work shows that social stress-induced behavioral deficits are augmented by inflammatory IL-1β-producing monocytes, which are actively recruited to the brain vasculature by microglia11. Expression of inflammatory receptors on endothelia and blood-brain barrier integrity are critical in mediating cytokine communication with the brain after stress23, 34–37. IL-1R1 is prominently expressed on glutamatergic neurons within the hippocampus (Fig.1D), but its role in modulating stress responses is unclear. Thus, we aimed to determine if IL-1 acts on neurons to influence cognitive and behavioral impairments with stress. To address this, we used a comprehensive IL-1R1 transgenic/reporter line in which one can selectively knockout or restore IL-1R1 on specific cell types26. Consistent with other findings, IL-1R1 knockout prevented social defeat-induced neuronal and microglial activation, social withdrawal, and working memory deficits, while social avoidance of an aggressor mouse was IL-1R1-independent. Selective knockout of IL-1R1 in glutamatergic neurons (nIL-1R1−/−) abrogated stress-induced deficits in social interaction and working memory. In addition, selective restoration of nIL-1R1 was sufficient to reestablish changes in social interaction and working memory with stress. These data were corroborated by a hippocampus-specific knockout of neuronal IL-1R1. Hippocampal RNA-sequencing data suggested that IL-1R1-mediated neuronal activation with stress corresponds with enhanced inflammatory gene expression. Overall, stress-dependent IL-1R1 signaling in hippocampal neurons represents a novel mechanism by which specific behaviors are modulated in association with neuron-dependent expression of inflammatory genes.
We used a modified paradigm of repeated social defeat (RSD) in the current study. Paired fighting social stress was used so that aggressive interactions would be abbreviated and individually monitored. Similar to previous studies using RSD10, 11, 38, stressed mice showed social withdrawal, cognitive impairment, and monocyte accumulation in the brain. For manipulation of IL-1R1 expression, we used previously-validated transgenic/reporter mouse lines in which one can selectively delete or express IL-1R1 on specific cell types24, 26. This construct is an improvement over previous knockout models because it prevents expression of IL-1R1 and its truncated isotype IL-1R339, and carries a tdTomato reporter that tracks IL-1R1 mRNA expression. Consistent with previous studies23, stress-induced increases in IL-1R1 were prominent within the blood vessels of regions associated with fear and threat appraisal, including the frontal cortex and hippocampus. It was also highly correlated with stress-induced VCAM expression on endothelial cells. The hippocampus is important because it is a key region of neuronal plasticity and memory integration40, 41. The robust expression of hippocampal IL-1R1 at baseline suggests that the hippocampus is modulated by IL-1 signaling.
There were unique differences in working memory and degrees of sociability with social stress. Stress reduced Y-Maze spontaneous alternations in IL-1R1+/+ mice, but had no effect in IL-1R1−/− mice. Spontaneous alternations represent a measure of hippocampal working memory, and a reduced percentage of alternations indicates a deficit with spatial reasoning and memory30. We interpret these results to indicate that stress-induced memory impairment is IL-1R1-mediated, consistent with previous literature42, 43 and with studies using RSD38 and other stress models44–46. It is important to discuss that one aspect of sociability, social interaction with a juvenile, is dependent on IL-1R1 but social avoidance of an aggressive mouse is not. Consistent with our previous studies38, the IL-1R1-independent avoidance of the aggressor CD-1 mouse indicates that this type of fear learning is neuronally-driven via fear circuitry. This suggests that evolutionarily-conserved fear circuitry and learned fear responses are IL-1R1-independent, and that stress-induced withdrawal from novel juveniles is a behavior augmented by IL-1 signaling. Taken together, reduced social interaction with a juvenile and increased corner time are aspects of anxiety-like or depressive-like behavior that are IL-1R1-dependent.
It is important to highlight other results from social stress that were either IL-1R1-dependent or -independent. Neurons (ΔFosB), microglia (Iba-1), and endothelia (VCAM) in the hippocampus had increased activity after stress, and all of these increases were dependent on IL-1R1. Monocyte trafficking to the brain with social stress was eliminated by IL-1R1 knockout while learned fear (discussed above), splenomegaly, and monocyte increases in circulation occurred independently from IL-1R1. Some aspects of inflammation detected in a coronal brain section through the hippocampus were also IL-1R1-dependent. Note that coronal brain section mRNA levels showed no difference from microdissected hippocampal mRNA levels, likely due to the relatively large increase in mRNA levels after stress and the amount of the coronal brain section that was comprised of hippocampal tissue (Supplementary Fig.S6). Stress increased expression of CXCL2 and ICAM-1 mRNA, and this induction was prevented in the IL-1R1−/− mice. While mRNA levels of IL-1β and CCL2 were increased by stress, these increases were not prevented by IL-1R1 knockout. This finding is surprising because their induction by RSD is important for increased microglial labeling and monocyte accumulation9, 11, both of which were attenuated by IL-1R1 knockout. It is possible that this IL-1β is being transported into the brain47 or that IL-1R1 knockout leads to a compensatory increase in IL-1β mRNA, while the increased CCL2 was unable to recruit monocytes due to the prevention of ICAM-1 induction. Furthermore, increased microglial Iba-1 labeling does not fully align with cytokine production/release48, and there are functional aspects of microglia activation that still occur after IL-1R1 knockout. These data indicate that IL-1R1−/− mice still interpreted the stress and sent sympathetic signals to the immune system to release monocytes. Therefore, IL-1R1-mediated behavioral deficits are mediated within the CNS rather than in the periphery.
A key advancement here is that there are selective behaviors induced by social stress that are dependent on glutamatergic neuron expression of IL-1 receptor (nIL-1R1). Since glutamate is the primary neurotransmitter in the dentate gyrus49, we used Vglut2-Cre-driven manipulations to study aspects of nIL-1R1 signaling. Previous studies have linked psychosocial stress norepinephrine in the locus coeruleus50–52, neurons in the prefrontal cortex53, 54 or undergoing dendritic atrophy55, while other studies show central roles for oxidative stress56 or BDNF57–59. There are indeed other brain regions and substrates important for regulating anxiety and mood disorders, but our focus on the hippocampus is relevant for its major role in limbic pathways and mood regulation60–62. Here, we used a nIL-1R1 knockout (nIL-1R1−/−) and a selective restoration of nIL-1R1 (nIL-1R1+/+) to target the glutamatergic neurons throughout the brain, including the dense population of Vglut2+ neurons within the hippocampus. The stress-induced reduction of juvenile social interaction was prevented in nIL-1R1−/− mice, while the same social interaction effect was restored in nIL-1R1+/+ mice. A similar dependence on nIL-1R1 was evident with stress-induced working memory deficits. For instance, nIL-1R1 knockout prevented Y-Maze deficits after stress, and these working memory deficits were restored in nIL-1R1+/+ mice. Thus, nIL-1R1−/− mice recapitulated the stress-resistant behavioral phenotype observed in global IL-1R1 knockout mice. Furthermore, selective restoration of nIL-1R1 was sufficient to restore the stress-induced deficits in social interaction and working memory. These behavioral deficits were paralleled by differences in stress-induced cumulative neuronal activity in the hippocampus, as ΔFosB was increased by stress in IL-1R1+/+ and nIL-1R1+/+ mice, but not nIL-1R1−/− mice. Taken together, nIL-1R1 is both necessary and sufficient to mediate deficits in social interaction and working memory after social stress.
To further investigate the role of the hippocampus-specific neurons in responding to IL-1 and driving behavioral alterations, we used an approach to knockout IL-1R1 in hippocampal neurons with AAV2-CreGFP63, 64. The prevention of stress-induced behavioral deficits in HPC-IL-1R1−/− mice was indicative of the central role for hippocampal neurons in the IL-1-driven behaviors we have discussed. Notably, the restoration of IL-1R1 in hippocampal neurons was less effective in restoring the social interaction effects of stress (Supplementary Fig.S3). In addition, the hippocampal-selective restoration approach did not affect cognition in the Y-Maze. Again, nIL-1R1 restoration using Vglut2-Cre was effective at recapitulating stress-induced social withdrawal and cognitive deficits. Thus, we interpret these data to indicate that Vglut2+ neurons inside and outside the hippocampus were involved in these effects, and a region-specific restoration of IL-1R1 may be more nuanced than the related knockout approach. Overall, we conclude that hippocampal neuronal IL-1R1 signaling was critical for the social withdrawal and cognitive deficits after psychosocial stress.
Our novel data have shown that social stress increased neuronal activation in the hippocampus through an IL-1R1-dependent mechanism. Next, we investigated if nIL-1R1 knockout prevented stress-altered neuronal transcription. RNA-sequencing of the hippocampus revealed that genes altered by social stress in IL-1R1+/+ mice were associated with increases in neuroinflammatory pathways. These pathways share genes of interest from previous stress work11, 22. General neuroinflammation, TREM1 signaling, leukocyte extravasation, NF-κB signaling, and complement system pathways were increased after stress in IL-1R1+/+ mice. Notable stress-dependent genes reversed by nIL-1R1−/− include those involved in complement signaling (C1qa, C1qb, C1qc), interferon signaling (Ifitm1, Ifitm2, Ifitm3), and the integrin pathway (Cyba, Esam, Myo1f, Rac2). Stress-induced upstream regulators of interest include interferon-gamma (IFNγ), interferon regulatory factor 7 (IRF7), MYD88, and NF-κB. The RNA-sequencing results suggest that these upstream regulators and associated inflammatory pathways that were induced by stress in the hippocampus were attenuated in nIL-1R1−/− mice. These pathways were associated with increased microglia activation. There were still increases in Iba-1 labeling with stress in the nIL-1R1−/− mice, indicating that stress-induced microglia activation was reliant on both nIL-1R1-dependent and nIL-1R1-independent pathways. This coincides with previous studies showing a role for endothelia and endothelial IL-1R1 in microglial reactivity23, 24. It is unclear from RNA-sequencing which IL-1R1-dependent neuronal signal can activate microglia, so follow-up studies are needed. Taken together, IL-1-mediated activation of neurons in the hippocampus was paralleled by an enhanced inflammatory profile and corresponded with deficits in social interaction and working memory.
In conclusion, IL-1R1 has a critical, cell-specific role in interpreting stress responses. Here, the activity of hippocampal neurons was directly tied to the behavioral consequences of psychosocial stress, and these neurons were activated by IL-1R1 (summarized in Supplementary Fig.S7). We do not exclude the role of other brain regions and neuron subtypes in mediating stress effects, but by combining these novel data with previous studies about peripheral monocyte trafficking and endothelial reactivity we provide a more complete picture of the brain after stress. Additionally, we show IL-1R1 as a target for future pharmaceuticals, as nIL-1R1 and HPC-IL-1R1 knockout prevented the manifestation of stress-induced social and cognitive deficits.
Supplementary Material
Acknowledgements
This research was supported by NIMH R01-MH-109165 and R21-MH-099482 (to NQ), NIMH R01-MH-119670 and NIMH R01-MH-116670 (to JPG and JFS), and NIA R01-AG-051902 (to JPG). DJD, DPN, and SO were supported by a National Institute of Dental and Craniofacial Research Training Grant T32-DE014320 (to JFS). KGW was supported by the OSU Presidential Fellowship. Our RNA-sequencing was made possible by an allotment of resources from the Ohio Supercomputing Center. The authors declare no competing financial interests.
Footnotes
Authors report no conflict of interest.
References
- 1.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of general psychiatry 2003; 60(8): 789–796. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. The Journal of nervous and mental disease 1998; 186(11): 661–669. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012; 37(1): 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. Journal of leukocyte biology 2012; 92(5): 959–975. [DOI] [PubMed] [Google Scholar]
- 6.Cytokine Dantzer R., sickness behavior, and depression. Immunology and allergy clinics of North America 2009; 29(2): 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsson J, Bjerke M, Sahebi S, Isgren A, Ekman CJ, Sellgren C et al. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. Journal of psychiatry & neuroscience : JPN 2015; 40(4): 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences of the United States of America 2011; 108(7): 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 2013; 33(34): 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in neuroscience 2014; 8: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry 2018; 23(6): 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfau ML, Menard C, Cathomas F, Desland F, Kana V, Chan KL et al. Role of Monocyte-Derived MicroRNA106b approximately 25 in Resilience to Social Stress. Biological psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols 2011; 6(8): 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AM, Roy S, Wong K, Gaikwad S, Chung KM, Kalueff AV. Cytokine and endocrine parameters in mouse chronic social defeat: implications for translational ‘cross-domain’ modeling of stress-related brain disorders. Behav Brain Res 2015; 276: 84–91. [DOI] [PubMed] [Google Scholar]
- 15.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 2014; 111(45): 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol 2002; 132(1–2): 66–71. [DOI] [PubMed] [Google Scholar]
- 17.Avitsur R, Stark JL, Dhabhar FS, Kramer KA, Sheridan JF. Social experience alters the response to social stress in mice. Brain Behav Immun 2003; 17(6): 426–437. [DOI] [PubMed] [Google Scholar]
- 18.McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF. Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell reports 2018; 25(9): 2552–2562 e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez K, Fornaguera-Trias J, Sheridan JF. Stress-Induced Microglia Activation and Monocyte Trafficking to the Brain Underlie the Development of Anxiety and Depression. Current topics in behavioral neurosciences 2017; 31: 155–172. [DOI] [PubMed] [Google Scholar]
- 20.Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015; 289: 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niraula A, Sheridan JF, Godbout JP. Microglia Priming with Aging and Stress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2017; 42(1): 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niraula A, Witcher KG, Sheridan JF, Godbout JP. Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biological psychiatry 2019; 85(8): 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014; 34(7): 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O et al. Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity 2019; 50(3): 764–766. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Liu X, Nemeth DP, DiSabato DJ, Witcher KG, McKim DB et al. Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav Immun 2019; 81: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Yamashita T, Chen Q, Belevych N, McKim DB, Tarr AJ et al. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci 2015; 35(7): 2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry 2016; 79(10): 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biological psychiatry 2014; 75(12): 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 2004; 28(5): 497–505. [DOI] [PubMed] [Google Scholar]
- 30.Miedel CJ, Patton JM, Miedel AN, Miedel ES, Levenson JM. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-beta and Tau Neuropathology. J Vis Exp 2017; (123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niraula A, Wang Y, Godbout JP, Sheridan JF. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. The Journal of neuroscience : the official journal of the Society for Neuroscience 2018; 38(9): 2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawicki CM, McKim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM et al. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience 2015; 302: 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson BDS, Kahoud RJ, McCarthy CB, Howe CL. Inflammatory cytokine-induced changes in neural network activity measured by waveform analysis of high-content calcium imaging in murine cortical neurons. Scientific reports 2017; 7(1): 9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 2017; 20(12): 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambree O, Ruland C, Scheu S, Arolt V, Alferink J. Alterations of the Innate Immune System in Susceptibility and Resilience After Social Defeat Stress. Front Behav Neurosci 2018; 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stankiewicz AM, Goscik J, Swiergiel AH, Majewska A, Wieczorek M, Juszczak GR et al. Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci 2014; 15: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci 2007; 27(35): 9301–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016; 36(9): 2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F et al. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proceedings of the National Academy of Sciences of the United States of America 2012; 109(30): 12189–12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. European psychiatry : the journal of the Association of European Psychiatrists 2002; 17 Suppl 3: 300–305. [DOI] [PubMed] [Google Scholar]
- 41.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65(1): 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 2003; 13(7): 826–834. [DOI] [PubMed] [Google Scholar]
- 43.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25(2): 181–213. [DOI] [PubMed] [Google Scholar]
- 44.Gaikwad S, Stewart A, Hart P, Wong K, Piet V, Cachat J et al. Acute stress disrupts performance of zebrafish in the cued and spatial memory tests: the utility of fish models to study stress-memory interplay. Behavioural processes 2011; 87(2): 224–230. [DOI] [PubMed] [Google Scholar]
- 45.Yun J, Koike H, Ibi D, Toth E, Mizoguchi H, Nitta A et al. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: possible involvement of a brain-specific transcription factor Npas4. Journal of neurochemistry 2010; 114(6): 1840–1851. [DOI] [PubMed] [Google Scholar]
- 46.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011; 60(3): 307–317. [DOI] [PubMed] [Google Scholar]
- 47.Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 2009; 156(7): 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura Y, Si QS, Kataoka K. Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 1999; 35(2): 95–100. [DOI] [PubMed] [Google Scholar]
- 49.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 2007; 163: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P et al. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav Immun 2017; 59: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS et al. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One 2017; 12(2): e0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K et al. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol 2004; 156(1–2): 153–162. [DOI] [PubMed] [Google Scholar]
- 53.Reznikov R, Bambico FR, Diwan M, Raymond RJ, Nashed MG, Nobrega JN et al. Prefrontal Cortex Deep Brain Stimulation Improves Fear and Anxiety-Like Behavior and Reduces Basolateral Amygdala Activity in a Preclinical Model of Posttraumatic Stress Disorder. Neuropsychopharmacology 2018; 43(5): 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ et al. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis 2020; 134: 104669. [DOI] [PubMed] [Google Scholar]
- 55.Francis TC, Chandra R, Gaynor A, Konkalmatt P, Metzbower SR, Evans B et al. Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry 2017; 22(11): 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015; 144(3): 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu WX, Wang J, Xie ZM, Xu N, Zhang GF, Jia M et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology (Berl) 2016; 233(3): 405–415. [DOI] [PubMed] [Google Scholar]
- 58.Boulle F, Pawluski JL, Homberg JR, Machiels B, Kroeze Y, Kumar N et al. Prenatal stress and early-life exposure to fluoxetine have enduring effects on anxiety and hippocampal BDNF gene expression in adult male offspring. Dev Psychobiol 2016; 58(4): 427–438. [DOI] [PubMed] [Google Scholar]
- 59.Licznerski P, Jonas EA. BDNF signaling: Harnessing stress to battle mood disorder. Proc Natl Acad Sci U S A 2018; 115(15): 3742–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Femenia T, Gomez-Galan M, Lindskog M, Magara S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain research 2012; 1476: 58–70. [DOI] [PubMed] [Google Scholar]
- 61.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of psychiatry & neuroscience : JPN 2004; 29(6): 417–426. [PMC free article] [PubMed] [Google Scholar]
- 62.Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR et al. An Amygdala-Hippocampus Subnetwork that Encodes Variation in Human Mood. Cell 2018; 175(6): 1688–1700 e1614. [DOI] [PubMed] [Google Scholar]
- 63.Hammond SL, Leek AN, Richman EH, Tjalkens RB. Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection. PLoS One 2017; 12(12): e0188830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connor B, Sun Y, von Hieber D, Tang SK, Jones KS, Maucksch C. AAV1/2-mediated BDNF gene therapy in a transgenic rat model of Huntington’s disease. Gene Ther 2016; 23(3): 283–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


