Abstract
Boron dipyrromethene, commonly known as BODIPY, based metal-organic macrocycles (MOCs) and metal-organic frameworks (MOFs) represent an interesting part of materials due to their versatile tunability of structure and functionality as well as significant physicochemical properties, thus broadening their applications in various scientific domains, especially in biomedical sciences. With increasing concern over the efficacy of cancer drugs versus quality of patient’s life dilemma, scientists have been trying to fabricate novel comprehensive therapeutic strategies along with the discovery of novel safer drugs where research with BODIPY metal complexes has shown vital advancements. In this review, we have exclusively examined the articles involving studies related to light harvesting and photophysical properties of BODIPY based MOCs and MOFs, synthesized through self-assembly process, with a special focus on biomolecular interaction and its importance in anti-cancer drug research. In the end, we also emphasized the possible practical challenges involved during the synthetic process, based on our experience on dealing with BODIPY molecules and steps to overcome them along with their future potentials. This review will significantly help our fellow research groups, especially the budding researchers, to quickly and comprehensively get the near to wholesome picture of BODIPY based MOCs and MOFs and their present status in anti-cancer drug discovery.
Keywords: BODIPY, Metal Organic Macrocycles, Metal Organic Frameworks, Therapeutic, Fluorescence
Graphical Abstract
This review highlights the recent development of BODIPY-based metal-organic macrocycles and metal-organic frameworks and their importance as therapeutic agents. Their current challenges and potential opportunities are also highlighted.
1. Introduction
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (also known as BODIPY), where BF2 is chelated to dipyrromethene, the half form of porphyrin, has received much attention from researchers for decades [1]. Due to their large molar extinction coefficients, high fluorescence quantum yields, relatively long excited-state lifetimes, and remarkable photo-stability, BODIPY has been adopted by diverse fields such as bioimaging, photodynamic therapy (PDT), and artificial photosynthetic systems. This chromophore mainly exhibits intense absorption and emission spectra with small Stokes shifts in the green region (490–570 nm). The fact that various moieties can be functionalized at eight positions via nucleophilic or electrophilic aromatic substitution, C-H activations and transition metal-catalyzed cross-coupling reactions in the BODIPY core allows many chemists to finely tune the characteristics of absorption and emission properties of BODIPY [2,3]. This opened many novel ideas related to labeling biomolecules and photodynamic therapy with BODIPY derivatives [4,5]. It may be noted that apart from their photophysical versatility, BODIPY derivatives are blighted with some prominent limitations and challenges as well [6]. Among several challenges, the low water solubility of BODIPY species is the most troublesome one [6–8]. This actually creates a lot of difficulties while designing the treatment dosage for biological experiments as the efficacy of any drug candidate largely depends on its water solubility, especially while conducting mechanistic studies. In addition, low solubility can lead to severe aggregation of BODIPY chromophores, resulting in self-quenching of the fluorescence [6]. Another weakness is the low intersystem crossing efficiency of BODIPY resulting from the weak spin-orbit coupling. This shortcoming is fatal in the field of PDT, where energy is transferred from the triplet state of the photosensitizer to triplet oxygen to produce singlet reactive oxygen.
Fluorescent transition metal complexes have attracted a fair deal of interest for decades due to their intriguing photophysical properties, which make them capable of interacting as well as modifying biomolecules as ameliorating agents against cancer [9–11]. The use of fluorescent metal complexes as candidate drugs against cancer has been receiving considerable interest in medicinal chemistry lately [12–15]. The advantage of using such compounds is not just the synergistic bioactivity of the metal core and the linkers but also the increased selectivity in killing cancer cells [8,13,14]. As an additional advantage, visualizing the localization of compound deposition within the treated cell is possible by virtue of the fluorescent moiety. Information regarding affected organelles, nuclear membrane breach, and interaction with the genetic materials can be obtained by observing the treated cells by a confocal laser scanning microscope [13,14,16,17]. Judicious selection of transition metals and organic ligands can lead to fine-tuning for photophysical properties of fluorescent transition metal complexes, and strong spin-orbit coupling due to heavy transition metal results in highly intense phosphorescent emission with prolonged lifetimes. Using BODIPY as a ligand of choice in the fluorescent transition metal complexes is justified by results showing improved photophysical properties of the later while alleviating certain drawbacks associated with BODIPY itself. The combination of BODIPY and metal enables control of BODIPY emission properties by heavy atom effect. Incidentally, the presence of metal ions may improve the solubility of BODIPY-based transition metal complexes in water, which is advantageous for biological applications.
As an extended form of transition metal complexes, metal-organic macrocycles (MOCs) and metal-organic frameworks (MOFs) are two important versatile platforms in the field of material science. Eventually, these two materials have matured and grown into various topics, including design methodology, post-self-assembly modification, and applications. Both are constructed by coordination-driven self-assembly and directional bonding between metal centers and ligands [18–21]. MOCs and MOFs have a common advantage that structural diversification is straightforward through the combination of various metal ion selection and ligand symmetry control, and essential functionality can be easily introduced through the pre- and post-self-assembly modifications [22]. On the other side, these two materials are conspicuously different classes of complexes; MOCs are well-defined structures with confined cavities that are mostly soluble in common organic solvents (including water in many cases), whereas MOFs are formed as repeating, infinite networks having certain inner porosity and a channel, formed as insoluble crystalline products. The common and distinct characteristics of these materials have led to their active application in the field of biomedicine. When the BODIPY participates in the structure of the well-ordered MOCs or MOFs as a ligand, it brings the following advantages.
Spatially well-separated BODIPY in MOCs and MOFs can avoid their fluorescence self-quenching resulting from chromophore aggregation.
The directional transition dipole moment of BODIPY in rigid structures of MOCs and MOFs enables intriguing photophysical properties compared to pristine BODIPY itself having a random orientation of transition dipole moments.
The guest molecules that can enter the space within the MOCs and MOFs can interact electronically with the BODIPY ligand, thus affecting BODIPY ‘s photophysical properties.
Because the regularity of chromophores observed in natural photosynthetic systems can be rendered in the ordered framework of MOCs and MOFs, the efficient energy transfer phenomenon in photosynthesis can also be in the BODIPY chromophore incorporating MOCs and MOFs.
The excellent fluorescent properties of BODIPY chromophores present in MOCs and MOFs architectures can be used as a tracking tool to understand the fate of metallodrug candidates undergoing in vitro and in vivo studies. This helps not only in localizing the drug candidates within the treated cells but also helps while understanding their mechanism of action.
The unsubstituted BODIPY chromophores (BODIPY core) are usually not suitable as efficient photosensitizers. However, slight modification or introducing heavy atoms, such as iodine, at the 2- and 6-positions can undergo intersystem crossing, enhancing the properties of photodynamic therapy. Briefly, BODIPY ligands used to design MOCs and MOFs systems can be easily modified to fine-tune their absorption/emission properties for PDT studies.
Therefore, various BODIPY-based MOCs and MOFs were synthesized and widely used in applications such as photocatalysis, bioimaging, photodynamic therapy, theranostics, and light-harvesting (Fig. 1). In this review, we will be discussing the evolution and recent advances in the synthesis of BODIPY based MOCs and MOFs and their current uses in anticancer research.
Fig. 1.
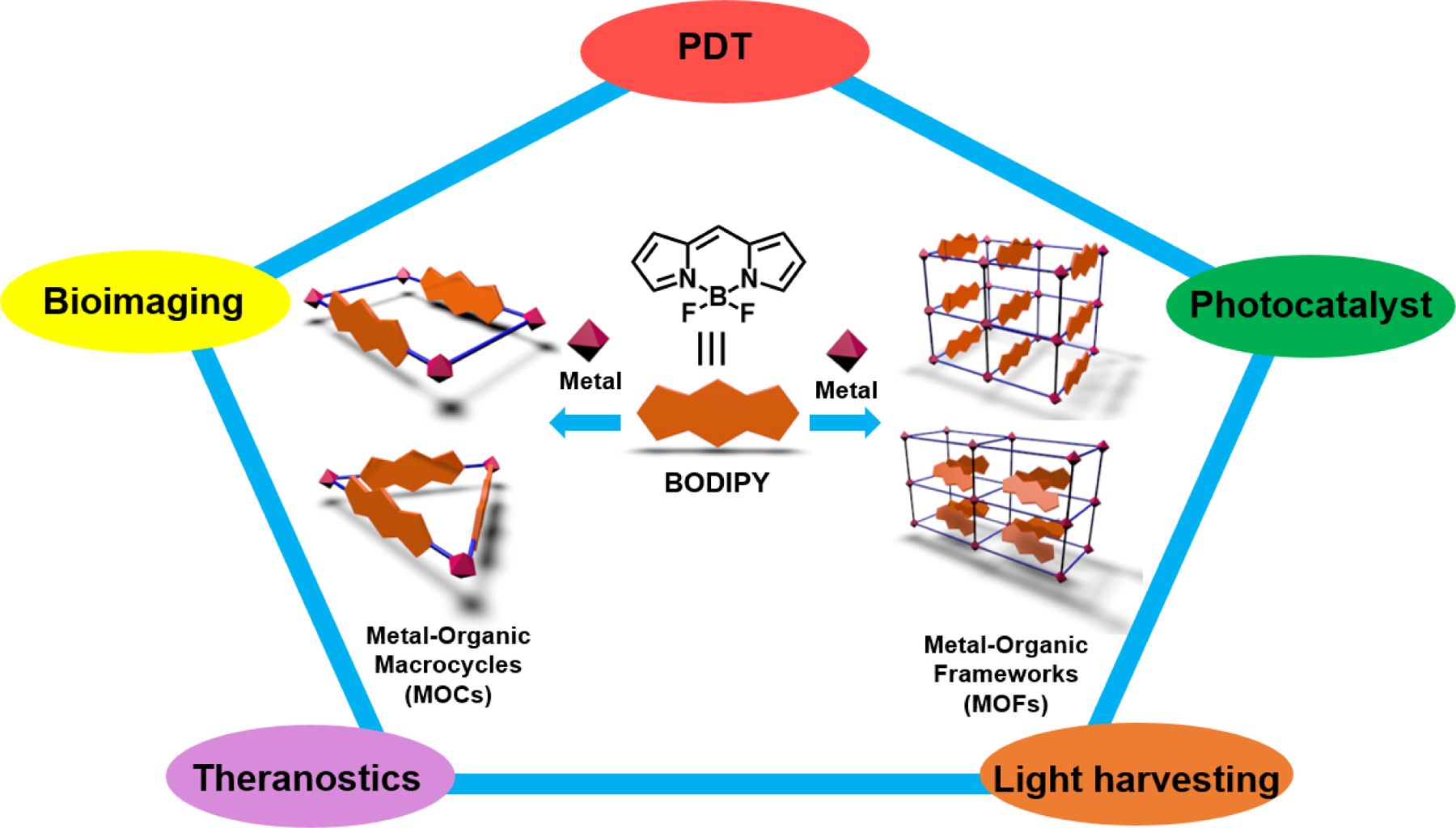
Representation of different molecular geometry and applications of MOCs and MOFs designed with BODIPY chromophore.
2. Metal-Organic Macrocycles
The pioneering work to develop metal macrocycles by the self-assembly process was first developed by Verkade in 1983. He synthesized several 20-membered tetranuclear rings by reacting a diphosphine bridging ligand with metal or mixed metal carbonyl precursors (Fig. 2, left) [23]. Then, with the idea of inclusion of organic guest into an inorganic host solution, Fujita developed the first palladium metal-based molecular square in 1990, where a Pd (II) complex was bridged with a bipyridine ligand resulting in a square-planar structure (Fig. 2, middle) [24]. The amine caped palladium square was obtained by reacting ethanoic solution of the 4,4’-bipyridine ligand with (en)Pd(NO3)2 in a mixed solvent of methanol and water. The formation of the water-soluble molecular square was achieved in 10 min of stirring at room temperature, and the structure was confirmed by elemental analysis and NMR spectroscopy studied in D2O solvent. Following a similar strategy, a platinum-based molecular square was also developed by Stang (Fig. 2, right) [25]. In this case, the platinum source of 1,3-bis(diphenylphosphino) propane was used, which on reaction with 4,4’-bipyridine in CH2Cl2 produced the molecular box in high yield.
Fig. 2.
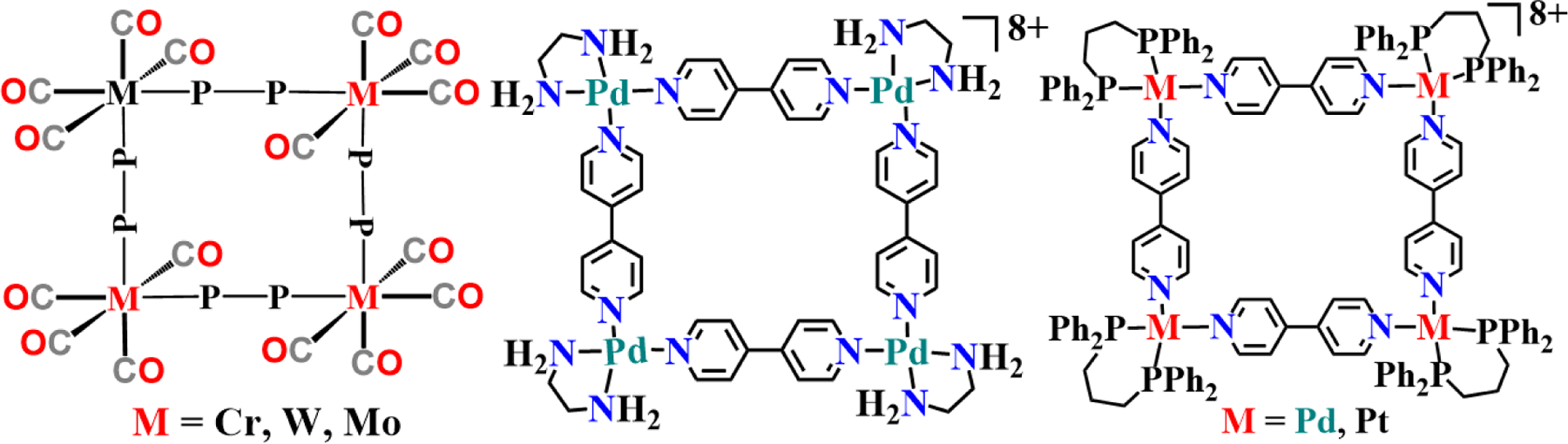
The first metal macrocycles developed by Verkade (L), and the first palladium- and platinum-based macrocycles developed by Fujita (M) and Stang (R).
Since then, the design and study of self-assembled MOCs have received considerable interest in various fields of chemistry [26–37]. Dynamic formation of self-assemblies into sophisticated supramolecular architectures allows us to understand certain characteristics and functions not normally observed with typical single molecules [38]. The popular metals of choice are platinum, palladium, ruthenium, iridium, iron, zinc, and silver among others. The chemistry of self-assembled supramolecular complexes has been a flourishing area of research due to their potential applications in various important fields of chemistry, material science, and especially in biochemistry. Many such supramolecules are being found to selectively kill cancer cells of various origins or hinder their proliferation rate in vitro [39].
2.1. BODIPY-based Metal-Organic Macrocycles
The BODIPY ligand for the design of a fluorescent rhomboid cavitand using a platinum precursor was initiated by Pistolis and co-workers in 2010 [40]. Various metal sources and BODIPY ligands used for designing MOCs are shown in Chart 1 and 2. They designed a highly symmetrical and rigid 3D molecular platform where the BODIPY core was linked with a 4-ethynylpyridine molecule to obtain the L1 ligand. Then L1 was reacted with Pt1 in CHCl3 or CH2Cl2 solution to produce a brilliantly fluorescent rhomboidal shaped cavitand MOC 1. The monoclinic space group (C2/c) of the cavitand was confirmed by single-crystal X-ray diffraction (SCXRD) (Fig. 3, MOC 1). The crystal structure displays the formation of a cavitand where the pyridyl groups of the two BODIPY ligands are bonded to two platinum molecules via the nitrogen atoms. A distortion of around 5º of the N-Pt-N angle was observed relative to the ideal 90º. However, interestingly no change was observed in the sp3 hybridized boron corners. The triflate counter anions were located outside the cavity space. It was also quite intriguing to notice that the overall photophysical properties of the BODIPY chromophore, such as high fluorescence quantum yield (ɸ = 0.86), lifetime (τ =6.9 ns), and high anisotropy (r = 0.37), were preserved even after the formation of the cavitand. Controlled reactions were performed to understand the stability, rigidity, and photophysical properties of the synthesized cavitand. For instance, when the 8-position (meso position) of the BODIPY core was replaced by a pyridine moiety and reacted similarly with triflate salt of Pt1 in CH2Cl2, resulting in the formation of a product that has completely different physical properties with much lower quantum yield (ɸ = 0.06). Unlike the cavitand MOC 1, the pyridyl BODIPY supramolecule complex was found to be less stable under the ESI-MS condition. This result suggests that the overall photophysical properties of the BODIPY chromophore are preserved and enhanced only in the self-assembled cavitand molecule MOC 1 and not in the pyridyl supramolecule. The interesting features of MOC 1 type metal macrocycles could be significant in several applications, including host-guest interaction studies, which were further demonstrated in their future reports.
Chart 1.
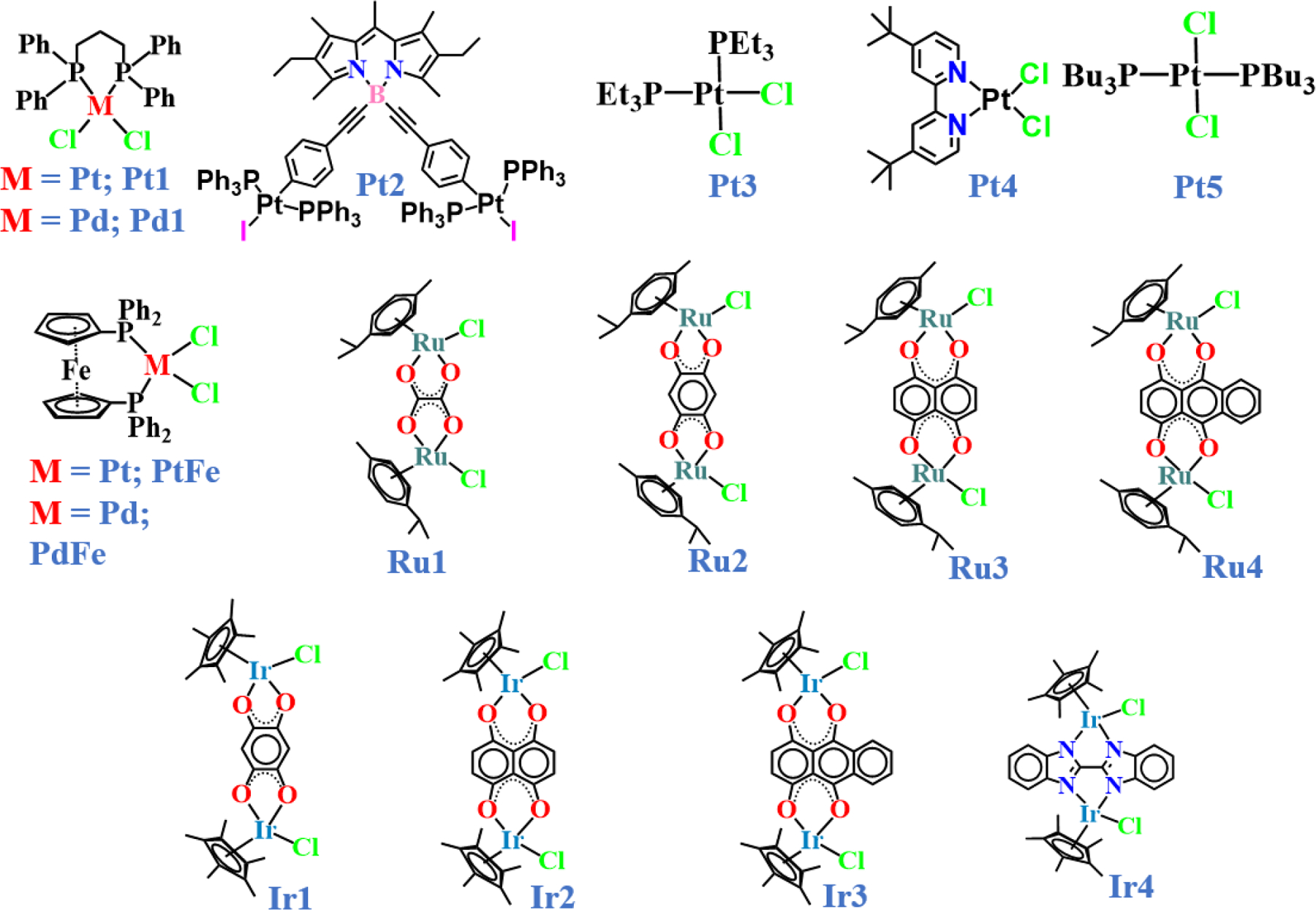
Structural representation of various metal clips used in the self-assembly process of metal organic macrocycles.
Chart 2.
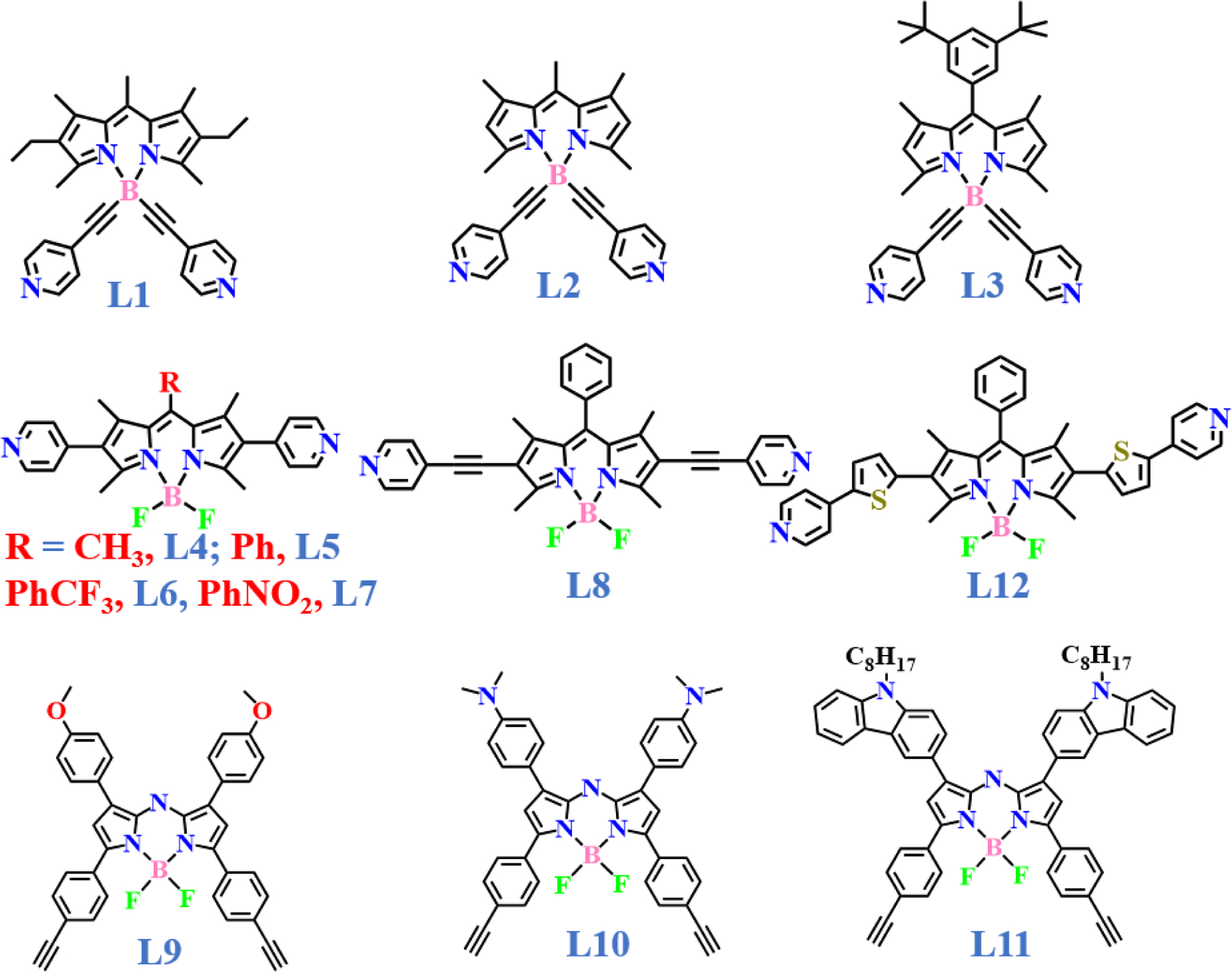
Different BODIPY ligands used in the synthesis of metal organic macrocycles.
Fig. 3.
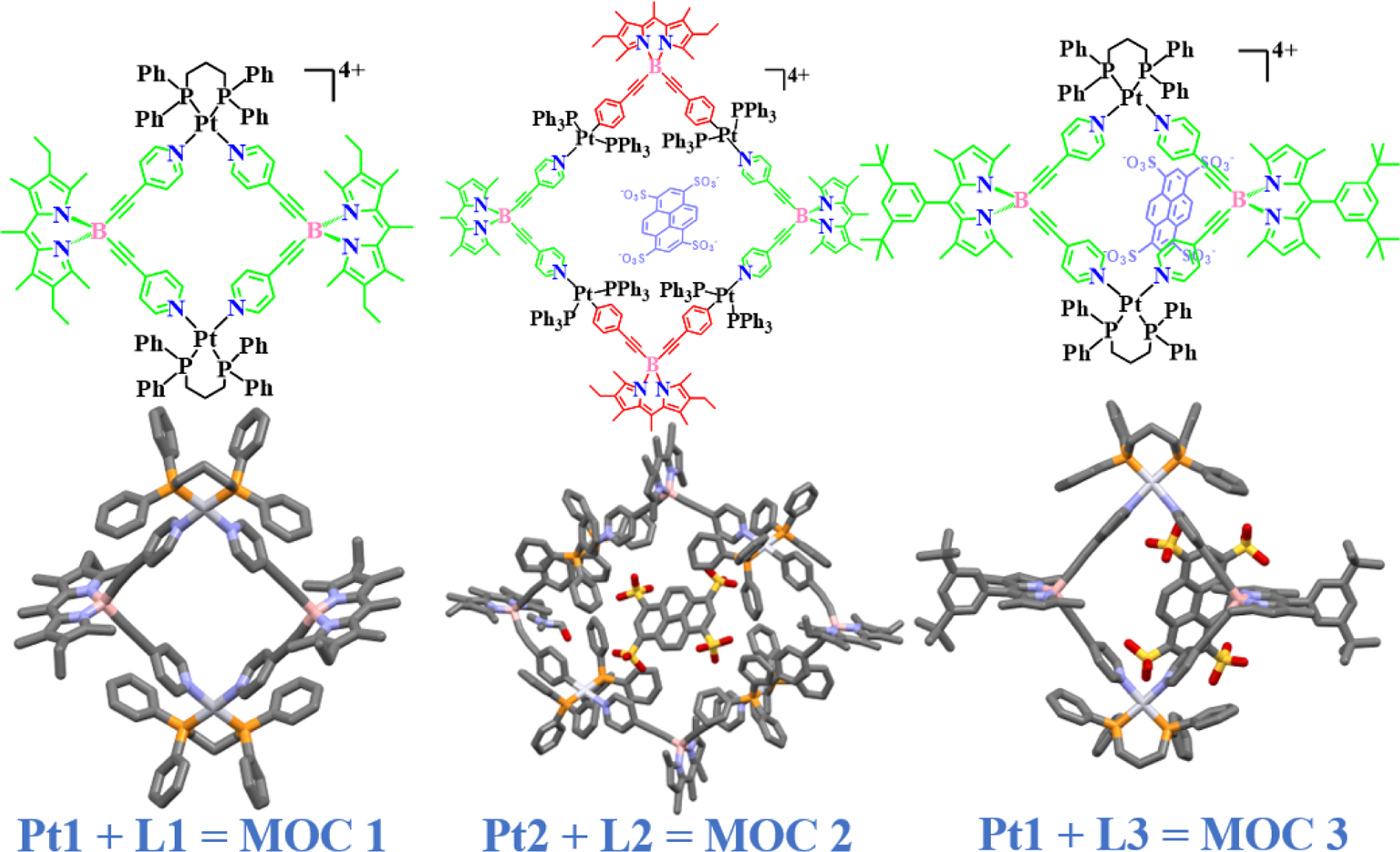
Molecular structures of platinum based BODIPY macrocycles.
Following the successful synthesis of MOC 1, Pistolis and co-workers next described the construction of a platinum-based highly fluorescent hexagonal supramolecule with light-harvesting properties [41]. The reaction of Pt2 with 4,4ʹ-bipyridine gives a unique hexagonal macrocycle MOC 4 (Fig. 4). The hexagonal product consists of 12 platinum units with 6 BODIPY chromophores connected with 6 bipyridine units in a hexagonal fashion with 12 triflate counter anions. The mutually parallel orientation of the BODIPY chromophores fixed within the hexagonal macrocycle allowed effective energy transfer between the BODIPY subunits. The excellent light-harvesting properties shown by MOC 4 open the potential for solar harvesting and photonics research on such fluorophore-based metal macrocycles.
Fig. 4.
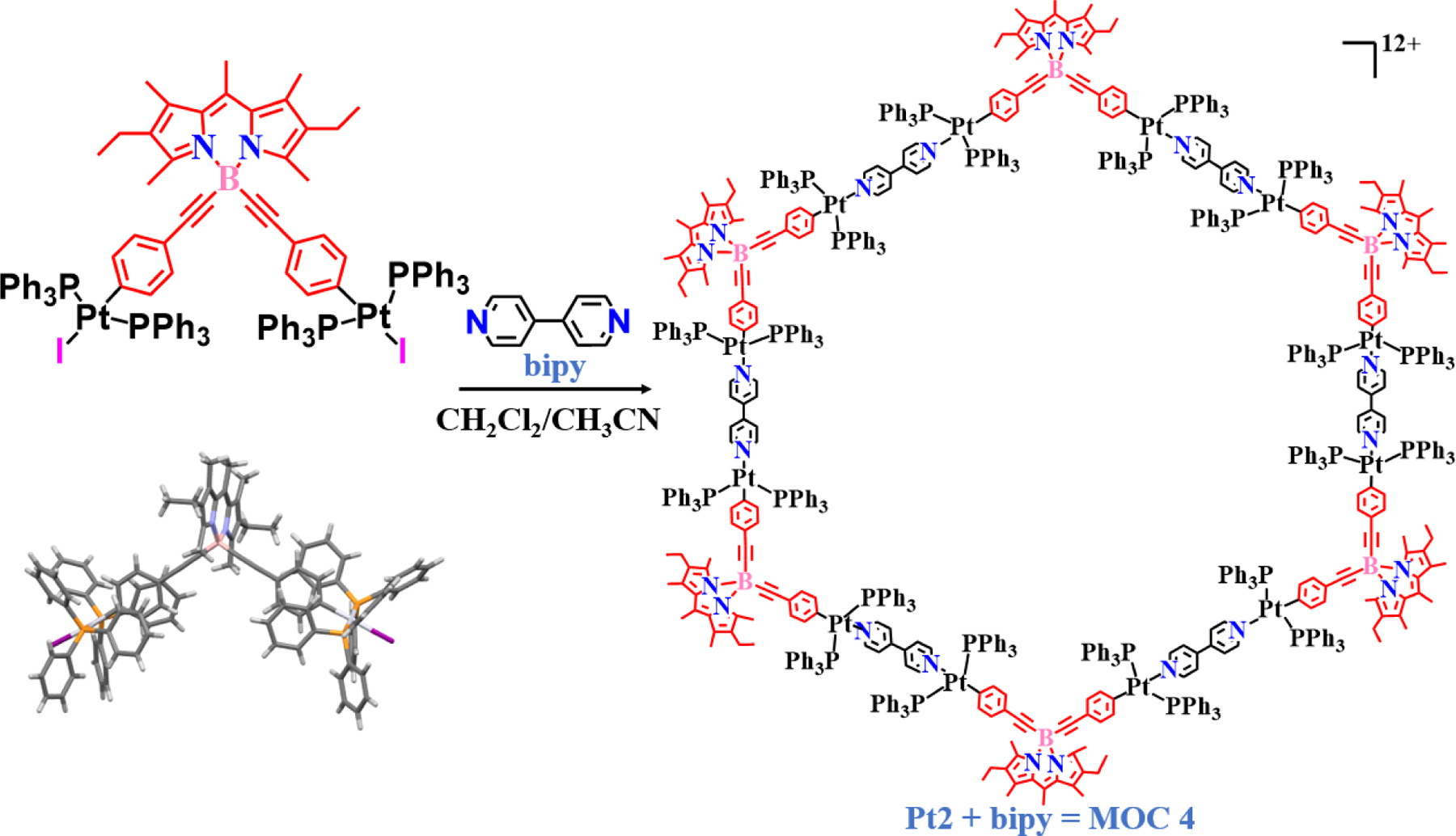
Synthetic scheme showing the formation of the platinum-based hexagonal macrocycle.
A follow-up study described the synthesis and physical studies of a large macrocycle MOC 2, which can accommodate the guest 1,3,6,8-tetrasulfopyrene in its cavity or can interact with MOC 3 (Fig. 3) [42,43]. The molecular structure obtained from the single crystal XRD confirms the formation of the [2+2] rhomboid cavitand where the molecular planes of the two BODIPY (Pt2 and L2) ligands are near perpendicular to the least-square plane of the rhomboid. The Pt-Pt distances were 17.8 and 18.2 Å, which were also the dimensions of the cavitand and large enough to tightly encapsulate the guest molecule 1,3,6,8-tetrasulfopyrene within its cavity. This is interesting since both the donor as well as the acceptor ligands are derived from the BODIPY chromophore. The supramolecule MOC 2 showed highly efficient and unidirectional intra-host (BODIPY-BODIPY) and guest-to-host (pyrene-BODIPY) energy transfer between the host and the guest molecules, which was confirmed by femtosecond fluorescence spectroscopy. Furthermore, they also reported a highly-ordered multichromophoric network, constructed by electrostatic interaction between the rhomboidal cavitand and the 1,3,6,8-tetrasulfonate pyrene (4SPy4-). In this ordered network, effective energy transfer and polarization switching between the pyrene and BODIPY subunits were observed.
3. BODIPY-based Metal-Organic Macrocycles with Therapeutic properties
3.1. Palladium and Platinum MOCs
DNA is possibly the most important intracellular molecular target for organometallic drug candidates in cancer therapy [44,45]. However, in addition to the double helix architecture of DNA, alternative secondary structures like G-quadruplexes are also found in the telomeric region of a chromosome due to the folding of the single stranded guanine-rich 3’ end [13,46], which inhibits the telomerase enzyme resulting in tumor suppression [47]. Platinum square complexes have been found to share structural compatibility with G-quadruplexes. In 2008, Sleiman and co-workers had demonstrated through in silico approaches that self-assembled platinum squares can be efficient G-quadruplex binders and inhibitors of the enzyme telomerase [48]. They found that the interaction between a Pt-square and a G-quadruplex 22-mer is most favorable when the electrostatic interactions, as well as the distance between Pt and the phosphates (from DNA backbone), are consistent, along with strong H-bonding of the amino and aromatic groups of the complex with the phosphate oxygens and guanine bases, respectively. Considering the ease of designing supramolecules, the rapid optimization of such Pt-squares can be adapted for the efficient binding with DNA secondary structures resulting in targeted anticancer therapy.
Lee and co-workers developed the first BODIPY based Pd macrocycles, MOC 5 - MOC 8, with triangular and square architectures which existed in equilibrium in solution (Fig. 5) [49]. The triangle/square equilibrium was observed only in chlorinated solvents, but in highly polar solvents the formation of only the triangle was observed. Such transformation of macrocycles to a single stable form by the change of solvent was interesting. Furthermore, the biological activity of these complexes was found to be highly active against brain cancer cells with IC50 values similar or lower than the standard cisplatin. In general, the ideal nature of toxicity expected from a metal macrocyclic compound for being an efficient anticancer agent is quite tricky. They are expected to impart lethal damage in a higher percentage of cancer cells while sparing non-malignant cells to a greater extent. This leads to the concept of selective cancer cell toxicity. Observing this trait forms the basis of similar studies in higher models and/or future clinical studies involving real human subjects, in case a drug candidate proves to be a potential anticancer agent in preliminary mechanistic studies. Lee and co-workers found significant selectivity of MOC 5 – MOC 8 in killing glioblastoma cells over non-malignant lung fibroblast cells, in vitro. For example, MOC 5 showed more than 3 times higher toxicity against U87 cells (IC50 = 4.96 ± 0.17 µM) as compared to normal WI 38 cells (16.71 ± 0.33 µM). Microscopic studies indicated cytoplasmic routes of action for their high bioactivity. The formation of nanoaggregates due to the likely intrinsic hydrophobicity of the aromatic group was observed. They showed a prominent tendency to deposit near the perinuclear area, thereby indicating the affinities of the complexes towards nuclear membrane-bound proteins, receptors or other biomolecules. The affinities of the complexes towards intracellular bio-molecules were also proven by their dose-dependent affinities to bind with the BSA protein as well as the genomic DNA.
Fig. 5.
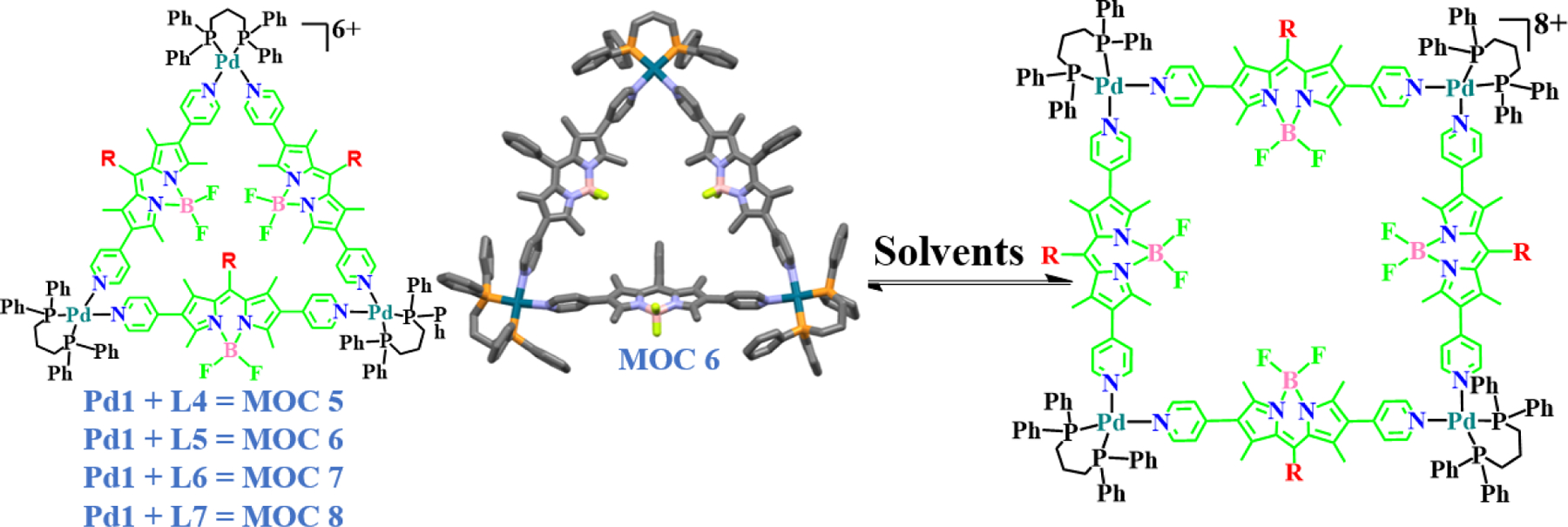
Synthetic scheme and single crystal XRD structure of the first palladium based BODIPY macrocycles with triangle-square architecture.
Huang and Cook, and co-workers reported the self-assembly of two highly emissive platinum(II) centered [3+3] macrocycles MOC 9 and MOC 10, having triangular architecture containing an L8 fluorophore which was further employed for photodynamic therapy studies [50]. The triangular architectures were synthesized by the reaction of Pt(II) acceptors (Pt3 and Pt4) with L8 in a combination of CH2Cl2/DMA solvent at 60 ºC for 24 h (Fig. 6). CLSM studies confirmed the intracellular uptake of the complexes by HeLa cells. This cellular uptake of the triangles was favored due to the latter’s cationic nature and the negative potential of the cell membrane, which through inductively coupled plasma optical emission spectrometry (ICP-OES) studies was also found to increase over time. Moreover, this internalization rate was considerably higher than that of the free Pt(II) acceptor and the BODIPY ligand, a common phenomenon observed with almost all BODIPY based macrocycles. Both triangles showed low IC50 values against proliferating HeLa cells, which were comparable to that of the Pt(II) ligand. Even though the complexes were nearly 2–6 times less toxic than the standard drug cisplatin, the former had the advantage of being fluorescent, which would act as an upper hand when supramolecules like these go for further intensive anticancer drug testing. The level of synergism in the activities of more than one molecule in the assemblage is measured and quantified using the drug combination index. The combination indices of the complexes were found to be 0.56 and 0.48, confirming their synergistic activity against cervical cancer cells in vitro. In another important finding, cisplatin-resistant A2780cis cells showed increased mortality when treated with MOC 9 and MOC 10, in combination with photodynamic therapy. Photodynamic therapy was also found to gradually enhance the generation of singlet oxygen, more in the case of the triangles rather than the BODIPY ligand per se.
Fig. 6.
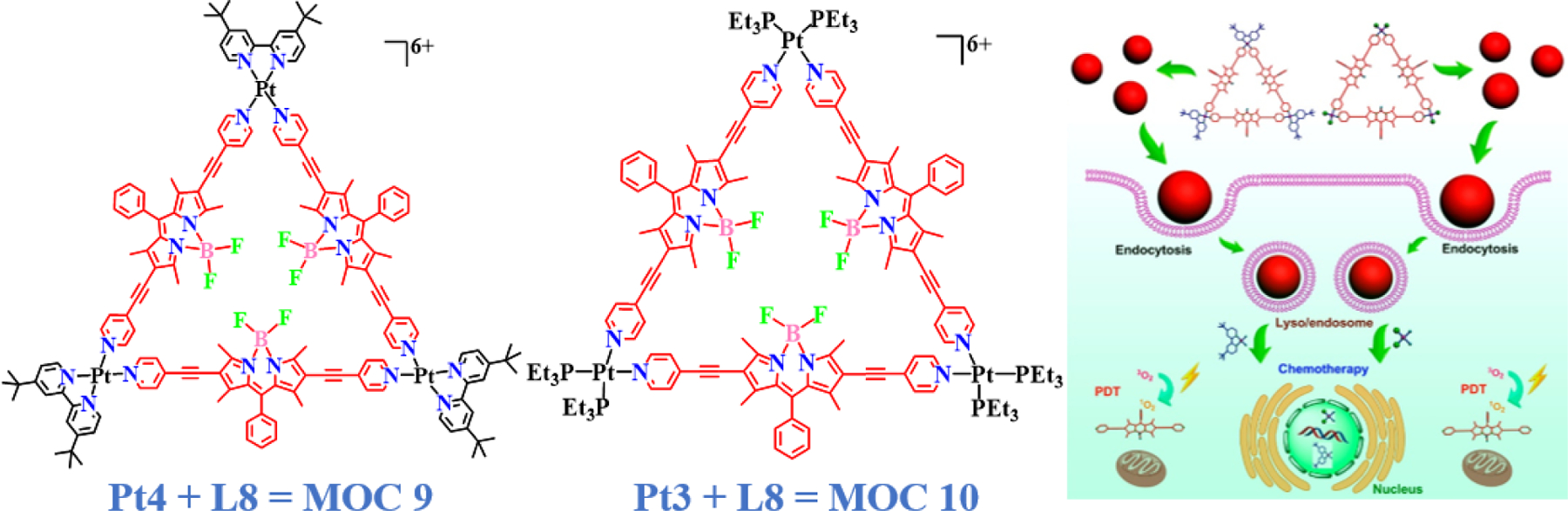
Molecular structures of the first platinum-based BODIPY macrocycles with triangular architecture along with cartoon representation of their cellular uptake. Reproduced with permission from ref. [50]. Copyright 2018 American Chemical Society.
The availability of photosensitizer (PS) agents showing strong absorption in the NIR range significantly contributes to the overall bioactivity of the complexes and BODIPY derivatives [50,51]. Traits like higher stability and strong absorption in the NIR region facilitate penetration and heat generation within tissues, thereby enhancing the therapeutic efficacy of the complex under consideration [52–54]. Approaches like PDT try to exploit the concept of phototoxicity, which basically leads to the generation of highly reactive free radicals/reactive oxygen species (ROS) within the cells. BODIPY linkers act like ideal photosensitizers (PS), making the complexes interact with molecular oxygen in the presence of light of designated wavelength to primarily produce singlet oxygen (1O2). This highly cytotoxic ROS is generated mostly in the illuminated locations where the PS accumulates, giving PDT an edge over chemotherapy and radiotherapy in regard of causing less normal cell toxicity. Other ROS like the hydroxyl radical (HO•) and hydrogen peroxide (H2O2) are also generated which alongside 1O2 are capable for causing indirect damage to DNA by breaking base pairing in the double helix, causing carbonylation in vital regulatory proteins as well as lipid peroxidation. BODIPY as well as porphyrin-based derivatives, have been verified to be efficient PSs due to their high extinction coefficient along with low dark cytotoxicities in this minimally invasive mode of cancer therapy. Most recently, Zhao and co-workers have reported three highly stable metallacycles having intense NIR absorption [55]. Three metallacycles MOC 11 – MOC 13 were synthesized using Pt5 and aza-BODIPY L9 – L11 (Fig. 7). These metallacycles showed excellent photophysical properties in chloroform with NIR absorption peaks between 735–760 nm and emission peaks above 808 nm. The relative quantum yields were 0.0457, 0.0889, and 0.0118 for MOC 11, MOC 12, and MOC 13, respectively, which were quenched as compared to the starting BODIPY L9 – L11 due to the heavy atom effect of platinum. For further biological applications, and to have high stability in a biological medium, the metallacycles MOC 11 – MOC 13 were encapsulated into DSPE-mPEG5000 NPs producing MOC 11-NPs, MOC 12-NPs and MOC 13-NPs, respectively. MOC 13-NPs otherwise having low dark toxicities imparted significant cell death in HeLa cells, when irradiated with lasers of 730, 660 and 785 nm, thus providing evidence of irradiation-dependent generation of singlet oxygen and hyperthermia. To understand the difference between the effect of ROS damage and PTT per se, the potent singlet oxygen scavenger vitamin C was used. The net damage on cells was found to be higher in the absence of vitamin C, indicating a synergistic cytotoxicity which included singlet oxygen damage as well as thermal injury, resulting in greater efficiency of PDT/PTT as compared to PTT only. This synergistic effect was further substantiated by checking the intracellular singlet oxygen via DCFH-DA staining and type of cell mortality using calcein AM and propidium iodide (PI) dual staining. Similarly, dual staining experiments with FITC and PI in a flow cytometer, showed maximum mortality in cells under late apoptosis due to the PDT/PTT synergism, where the presence of vitamin C inhibited the cells from dying. They also conducted in vivo experiments with HeLa tumor bearing nude mice, where MOC 13-NPs were injected intratumorally followed by infrared thermal imaging. Twenty days after the first intratumoral injection and irradiation, tumor sizes were found to reduce considerably and were completely eliminated after the second treatment. Additionally, pathomorphological analyses of mice organs revealed no significant tissue damage in the heart, lung, spleen, liver and kidney that could have occurred due to the dosage, indicating a high safety index of the MOC 13-NPs in vivo. The strong affinity to absorb in the NIR, the higher photothermal conversion potency, along with excellent photostability confirmed the ability of BODIPY based MOC 13-NPs as potential phototherapy agents in cancer treatment.
Fig. 7.
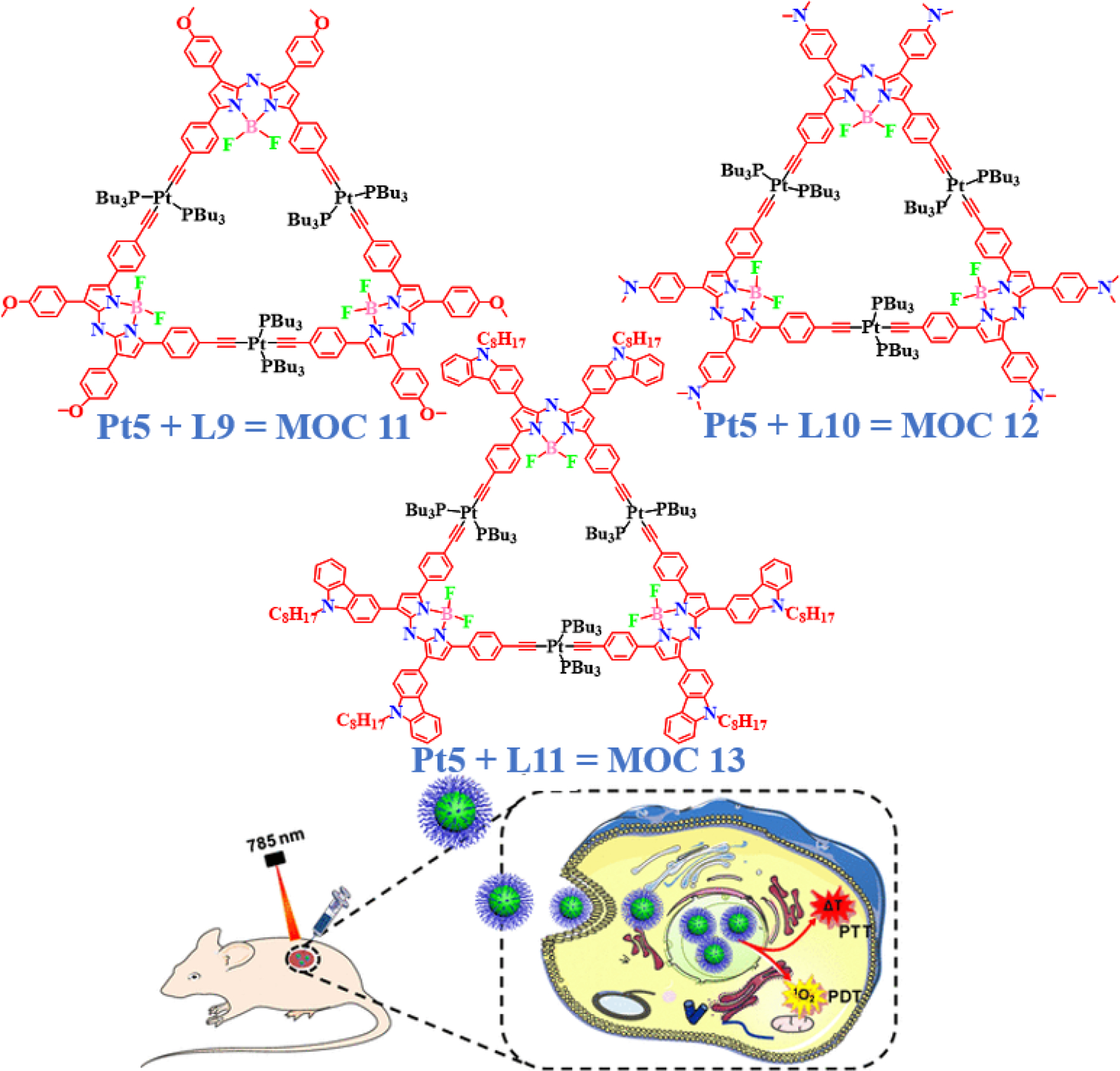
Molecular structures of NIR-absorbing platinum macrocycles for PDT application. Reproduced with permission from ref. [55]. Copyright 2020 American Chemical Society.
Ferrocene-based palladium and platinum precursors were also extensively used for the development of self-assembled macrocycles for various applications [56–58]. However, biological applications of such macrocycles were not studied until Chi and co-workers described the syntheses of two new tetracationic hetero-bimetallacycles [59]. Biological studies also showed them to be more effective than cisplatin against a number of cancer cell lines.
Following a similar strategy, Lee and co-workers utilized the BODIPY ligands (L5 and L8) to synthesize new octanuclear heterometallic systems MOC 14 – MOC 17 [60]. The complexes were highly fluorescent, and their molecular structures are shown in Fig. 8. Owing to selective killing of cancer cells, these macrocycles were able to efficiently kill PC-3, MCF-7, and A549 cells and non-malignant Human Kidney Fibroblast cells; at times at multifold lower IC50 values than cisplatin. Moreover, cisplatin was found to be highly toxic against normal kidney fibroblast cells, owing to nephrotoxicity as one of its side effect during chemotherapy. One of the Pd-rectangles MOC 15 showed lower IC50 values than cisplatin against PC-3 and A549 cells. The mechanism of cell death was further studied using a flowcytometry. However, using fluorescent dyes for dual staining, like FITC (Fluorescein isothiocyanate) and Propidium Iodide (PI), it becomes difficult to distinguish between their respective fluorescence and the net fluorescence of the complexes. So after deducting the fluorescence readings from the cells treated with complexes and without any dual staining, the net results showed macrocycles MOC 14 and MOC 15 resulting in apoptosis, while the other two macrocycles causing necrosis in the treated cells. This shows that in addition to the metal core, it is the type of BODIPY ligand used that not only aids to the net activity of the entire macrocycle, but also makes a difference in their respective mechanisms of action. It is because the L5 ligand was used in MOC 14 and MOC 15, while MOC 16 and MOC 17 had the comparatively larger L8 ligand.
Fig. 8.
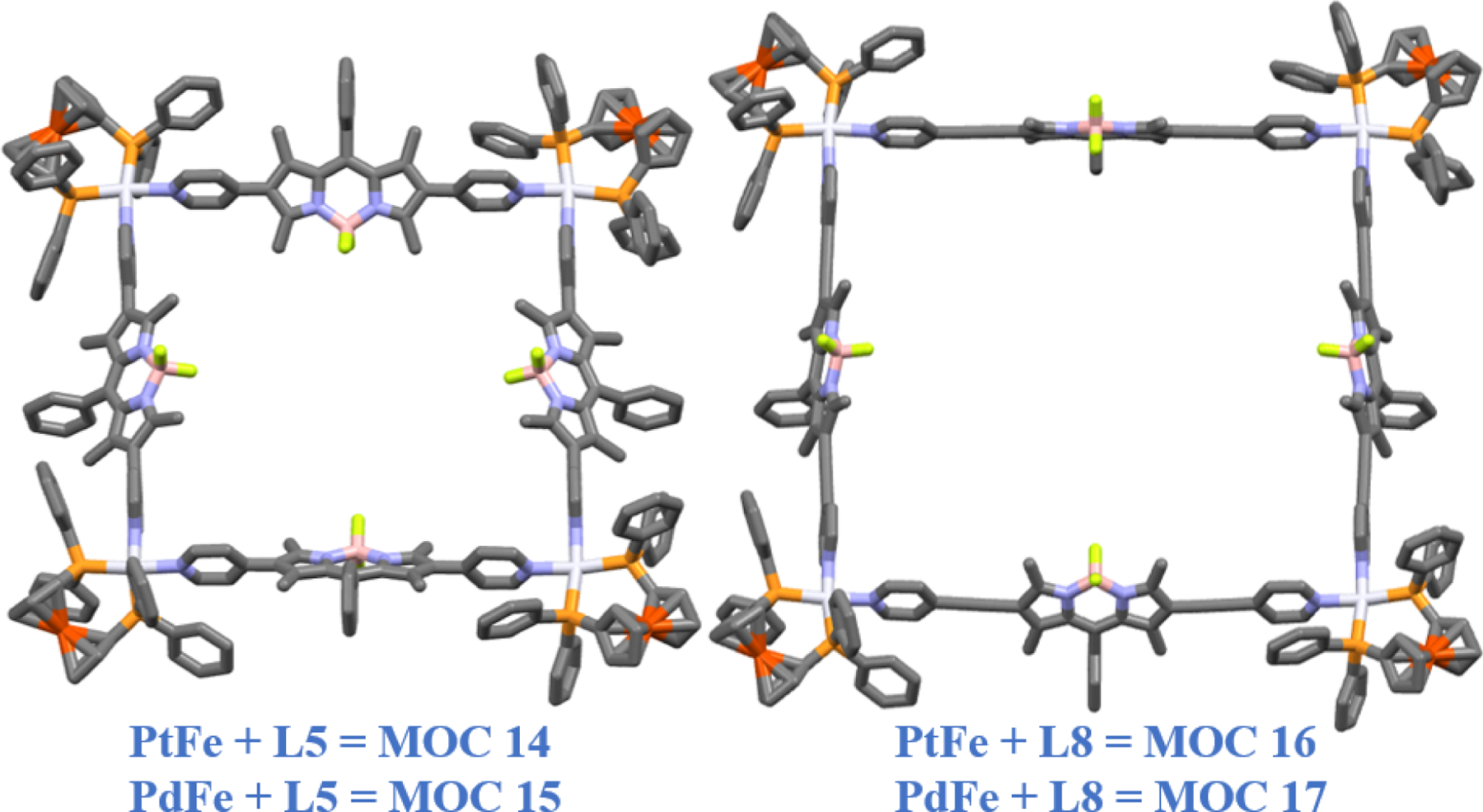
Molecular structures of octanuclear heterometallic PdFe and PtFe macrocycles.
3.2. Ruthenium and Iridium MOCs
Coordination-driven self-assembly of arene-based ruthenium and pentamethylcyclopentadienyl iridium and rhodium complexes are well known in the design and synthesis of supramolecular architecture. In 1997, Süss-Fink and co-workers pioneered the self-assembly process to develop arene-based metalla- rectangles by utilizing p-cymene ruthenium-based metal clips with pyridyl donor ligands (Fig. 9) (L) [61]. The reaction of triflate salt of Ru1 with 4,4’-bipyridine ligand in methanol gave the (2+2) rectangle, which was confirmed by a single crystal structure. Thereafter, Therrien and co-workers utilized such metal clips (Chart 1) to develop new ruthenium-based trigonal prism showing good biological properties (Fig. 9 (R)) [62]. The prism was self-assembled into a trigonal structure by reacting two equivalents of tritopic tripyridyl ligand with three equivalents of dinuclear ruthenium molecular clips. Upon further studies, it was shown that the prism was capable of encapsulating a molecule of Pt/Pd acetylacetonato complex into its cavity. These supramolecules, when tested against the proliferation of A2780 human ovarian cancer cells, showed that the Pt/Pd host/guest system was more effective than the free host or the guest molecules alone. This property indicated the strategy of selective cancer cell killing. Since then, several pyridyl based bidentate, tridentate, and tetradentate ligands with different functionalization were utilized by several groups to develop a library of rectangles, triangles/prisms and cages; as well as Borromean Rings, solomon rings, ring in ring structures, and catenated structures, among others [63–67].
Fig. 9.

Molecular structures of the first arene ruthenium-based rectangle developed by Süss-Fink (L) and the first arene ruthenium-based prism used as host-guest system developed by Therrien (R).
In recent years, several interesting reviews on arene and Cp* based Ru(II), Ir(III) and Rh(III) macrocycles have been published by different research groups. For example, Chi and co-workers have described the use of different pyridyl ligands that were used for the synthesis of self-assembled metallacycles and metallacages along with their potential biological applications [39]. Several complexes like these have shown good to excellent anticancer properties. They were also found to be stable under physiological conditions and bind strongly with DNA and protein. Following a similar strategy, Lee and co-workers recently introduced a highly fluorescent BODIPY based dipyridyl ligand L5 to develop (2+2) ruthenium and iridium rectangles MOC 18 - MOC 21 using the self-assembly approach (Fig. 10) [13]. The inclusion of BODIPY facilitates cellular labeling experiments for the detection and localization of their associating complexes within the target cells in living organisms [68,69]. These ruthenium and iridium rectangles were synthesized by reacting metal clips (Ru2, Ru3, Ir1 and Ir2) with L5. The molecular structure (crystal grown from methanol/DEE) of the ruthenium rectangle MOC 19 was found to be twisted by up to 99º (Fig. 10, SCXRD MOC 19-twisted) as compared to the iridium analogue (MOC 21). Such a twist in ruthenium rectangles is unusual. The distance between the two BODIPY units was around 4.2 Å, which hinders any π-π interaction between them. In order to understand if solvent plays any role in the formation of such a twisted structure, single crystals using another combination of solvents (CH2Cl2/DEE) were grown. Interestingly, in this case, the expected linear structure similar to that of the ruthenium rectangle was obtained (Fig. 10, SCXRD MOC 19). Thus, it was concluded that solvent played an important role in the structural arrangement of the rectangles, which has also led to the reduction of the distance between the boron molecules by up to 1.72 Å.
Fig. 10.
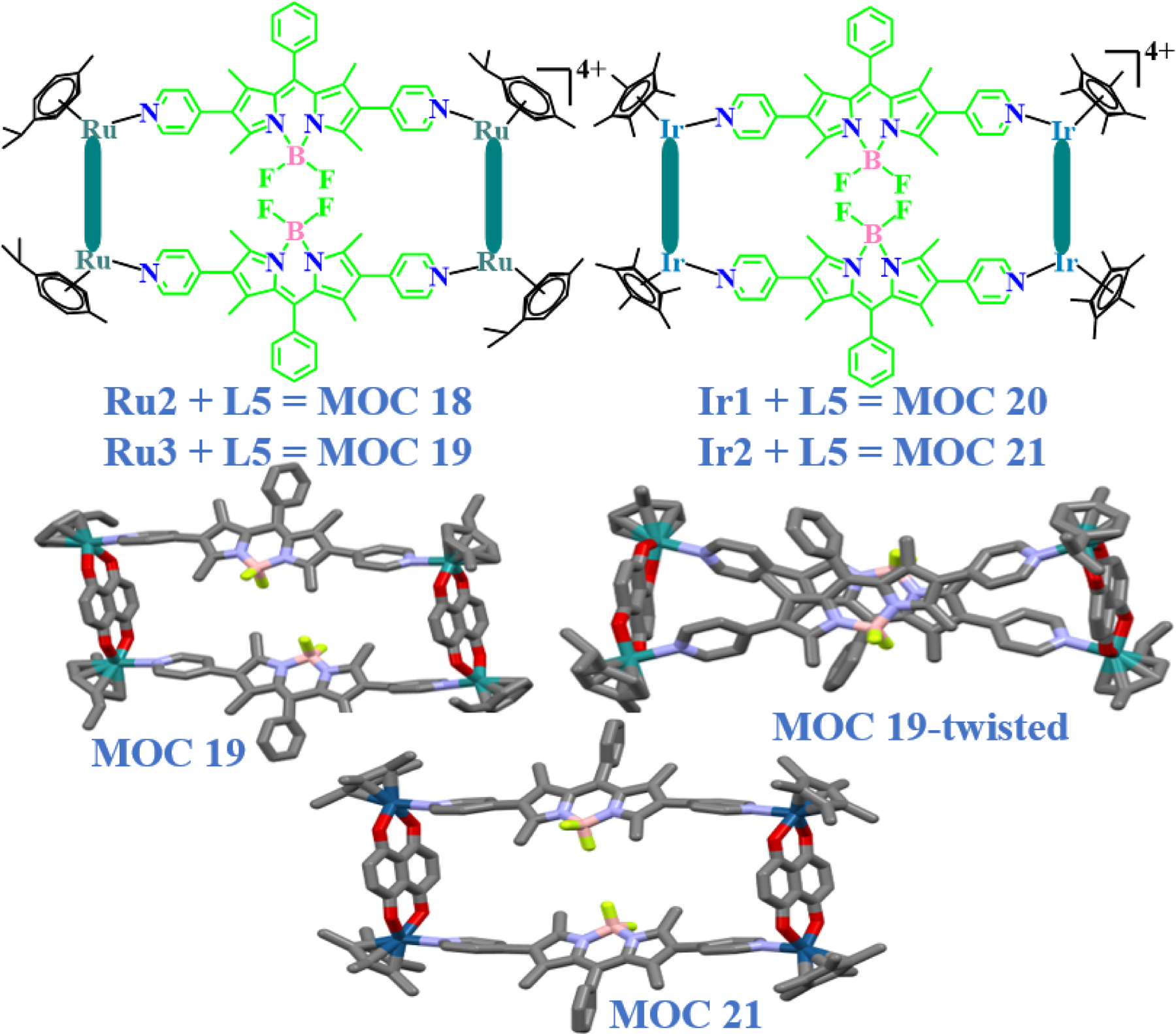
Molecular structures of first BODIPY based Ru(II) and Ir(III) rectangles obtained from clips Ru2, Ru3, Ir1 and Ir2 with ligand L5. Single-crystal XRD structures of the twisted and normal form of the rectangles.
To investigate the respective efficacies of these new fluorescent rectangles against malignant cell growth, cancer cell lines of four different types (A549, MCF-7, HeLa and U87) were studied. To check for the possibility of selective cancer cell killing, the extent of toxicity induced by each compound was compared with those obtained against the non-malignant WI-38 cell line. In the case of lung carcinoma, none of the complexes showed any selectivity, although all displayed a better cytotoxic potential as compared to the standard drug cisplatin. Some compounds indicated selective killing of the other cancer cell lines, and a few were even better than cisplatin. It was also observed that, irrespective of the selectivity and the cell line used, Ir-based rectangles (IC50 range = 0.24 – 4.31 µM) were several times more toxic than the Ru-based rectangles (IC50 range = 3.79 – 83.63 µM). BODIPY groups as such are known to impart low phototoxicity along with high biocompatibilities in vitro [20]; likewise the ligand used in these four complexes also showed minimum toxicity in all the studied cell lines (IC50 = 148.39 – 428.48 µM).
Additionally, the BODIPY ligand gave maximum fluorescence within the 450–550 nm range (maxima at 488 nm) of the visible spectra, hence it was investigated whether the compounds within the treated cells show any sort of fluorescence, when observed under a confocal microscope. The CLSM images demonstrated intracellular localization of the compounds as nanoaggregates. The green fluorescence of the BODIPY ligand in these rectangles was thus used as a tool to check whether the localization of the compounds is intra- or extra-nuclear, or did any of the compounds failed to even enter the cells. Moreover, the treated cells were found to lose cellular integrity, undergo shrinkage and pyknosis, indicating the onset of programmed cell death. The authors concluded that, the biological activities against cancer cell lines involved different intracellular molecular targets, and further in-depth study at transcriptional and translational levels was required. Given this biological activity of BODIPY based ruthenium and iridium rectangles and to further enhance the anticancer activities of such metalla-rectangles, the same group further designed new thiophene functionalized BODIPY L12 [14]. Thiophene-based drugs are well-known due to their wide range of pharmacological potency, including anticancer properties [70–72]. Four arene-ruthenium precursors, Ru1-Ru4, were reacted with ligand L12 yielding MOC 22 - MOC 25 (Fig. 11).
Fig. 11.
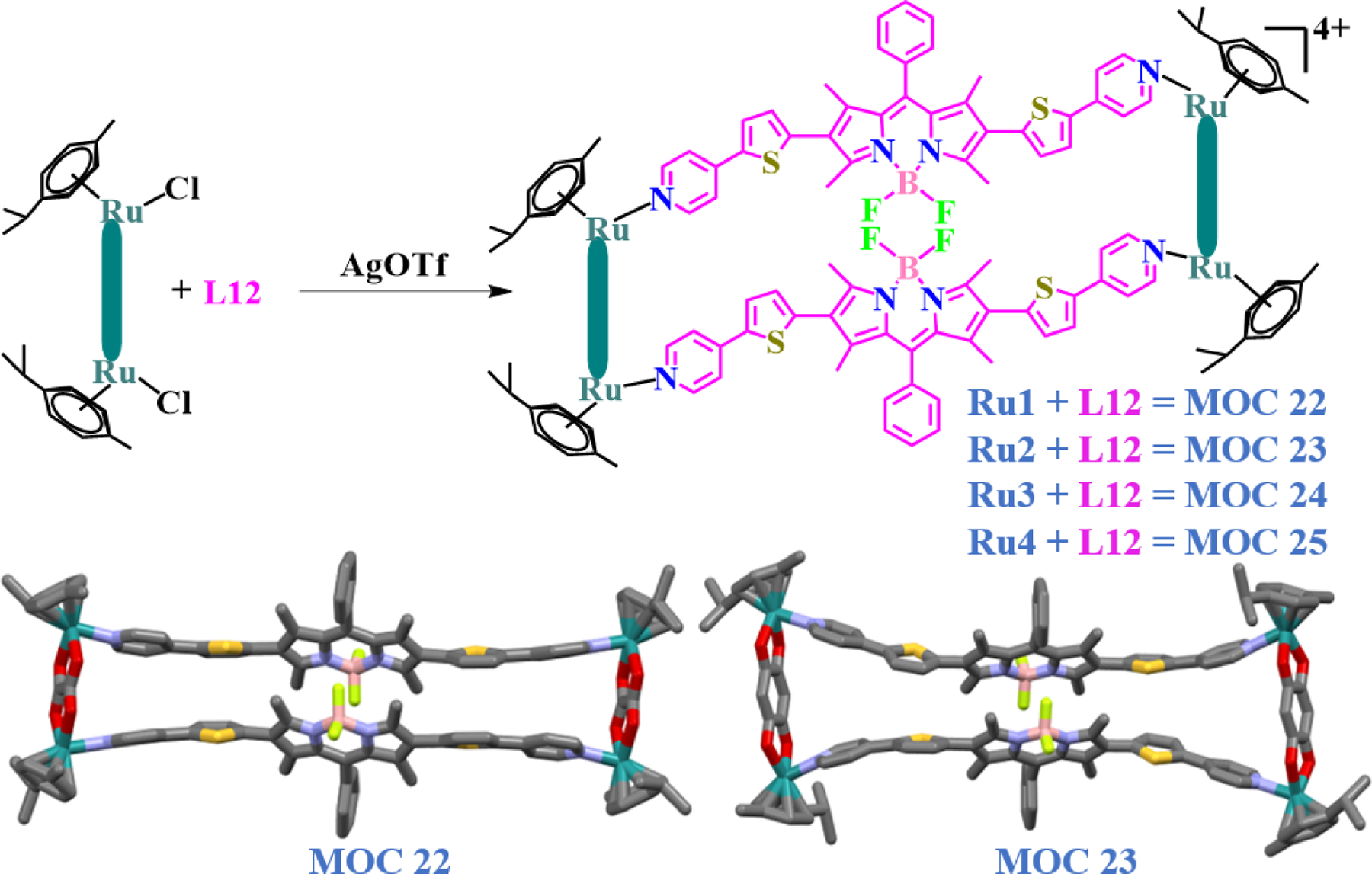
Molecular structures of thiophene-based BODIPY ruthenium rectangles derived from clips Ru1 – Ru4 and ligand L12.
The complexes were tested against proliferating MCF-7, HeLa & U87 cancer cells and the non-malignant WI38 cells. Even though, these complexes showed comparatively higher IC50 values than the Ru rectangles previously discussed [13], their selectivities in killing cancer cells increased quite significantly. All 4 compounds were highly as well as selectively toxic against breast cancer cells (IC50 range = 10.54 – 40.21 µM); with complexes MOC 22 and MOC 23 showing IC50 values as high as 936 and >2000 µM, respectively against normal lung fibroblast cells. It is understood that thiophene compounds having potent antioxidant activities are activated primarily by cytochrome P450-mediated oxidation. Cancer cells are already under oxidative stress because of the high concentration of intracellular ROS owing to anomalous mitochondrial function. Therefore, upon treatment with such complexes, the redox balance tends to disrupt more effectively as compared to normal cells, thereby causing selective cancer cytotoxicity. Nuclear disintegration was largely observed in MCF-7 and HeLa cells (Fig. 12). In addition, many pyknotic cells were observed in breast and brain cancer cells. The above observation further supported the substantial rise in sub-G1 peak from the cell-cycle studies. Previously reported BODIPY rectangles showed both nuclear and cytoplasmic localizations [13], however in this case, the rectangles were predominantly localized in the cytoplasmic region both uniformly as well as in the form of nano-aggregates. Further, binding studies by the spectroscopic method showed the ability of these rectangles to interact strongly with DNA and protein. This indicates that the incorporation of thiophene groups in the BODIPY ligand augment the overall efficacy of a Ru- supramolecule, especially its selectivity towards killing cancer cells in vitro.
Fig. 12.
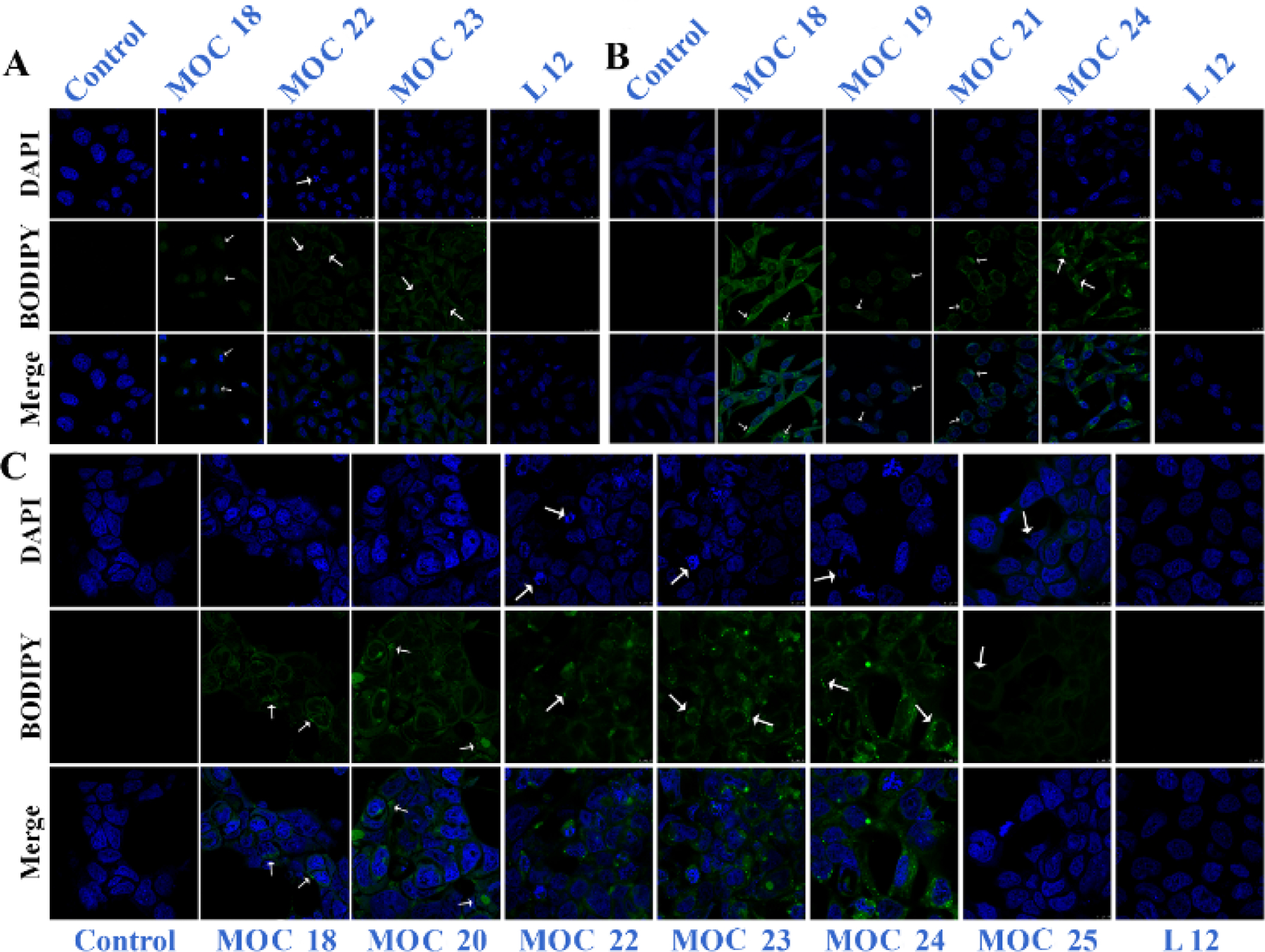
Confocal microscopic images of macrocycles MOC 18-25. Reproduced with permission from ref. [13]. Copyright 2016 Royal Society of Chemistry. And ref. [14]. Copyright 2017 Wiley-VCH.
Iridium complexes have previously displayed potent activities against proliferating cancer cell lines [73–77]. Compared to the ruthenium supramolecules, their biologically active iridium counterparts are usually found to be more toxic against dividing cancer cells in vitro [13]. Later in some analogous studies, Lee and co-workers also developed rectangles MOC 26 – MOC 27 [78], with an ethynyl functional group introduced in between the BODIPY core and the pyridyl moiety (L8, Fig. 13). The rectangles and the free BODIPY ligand were investigated against proliferating cervical carcinoma and glioblastoma cell lines. The biologically active complex (MOC 27) was found to be toxic for not only the cancer cells but also for normal cells. However, the complex was found to be 4 times more toxic in killing HeLa cells and twice as toxic against U87 cells, compared to cisplatin. Furthermore, the complex did not seem to break the nuclear membrane even after 48 h of incubation, indicating a cytoplasmic route of action for its toxicity and showed excellent dose-dependent interactions with biomolecules.
Fig. 13.
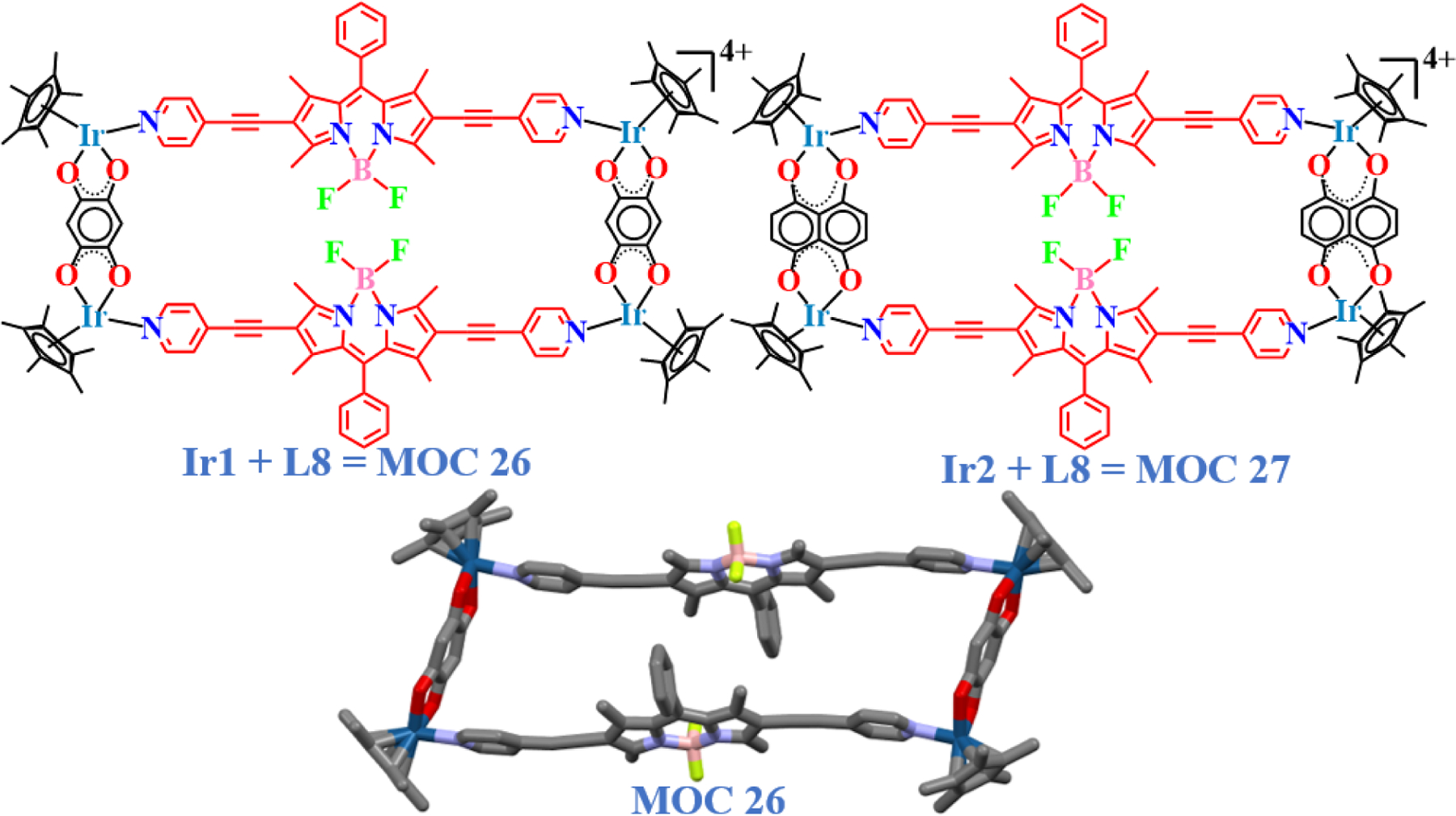
Molecular structures of BODIPY based iridium rectangles derived from clips Ir1 and Ir2 and ligand L8 and single-crystal XRD structure of MOC 26.
Selective toxicity, which was an issue with this work, was addressed later when the same group further extended their work by replacing the O,O-bridging clips with the N,N-bridged bis-benzimidazole clip Ir4 [79]. Several bis-benzimidazole derivatives are known to possess a wide range of pharmacological activities due to the presence of two benzimidazole nuclei [80,81]. Chi and co-workers reported that the bis-benzimidazole based ruthenium rectangle and prism could act as potent anticancer agents [82]. Following a similar protocol MOC 28 – MOC 29 were synthesized (Fig. 14) [79], where MOC 29 was found to be at least 10 times more effective in reducing the viability of both HeLa and U87 cells to 50% than the normal WI38 cells. The degree of selectivity was comparable to that of cisplatin, however it was at least 7 times safer, compared to normal cell (WI38) exposure. Both complexes followed a cytoplasmic route of action for their toxicities against U87 cells. Moreover, they showed significant propensities to localize around the perinuclear region of the cells, indicating their possible affinities towards membrane receptors and the presence of suitable binding sites, which cause their biological activities.
Fig. 14.
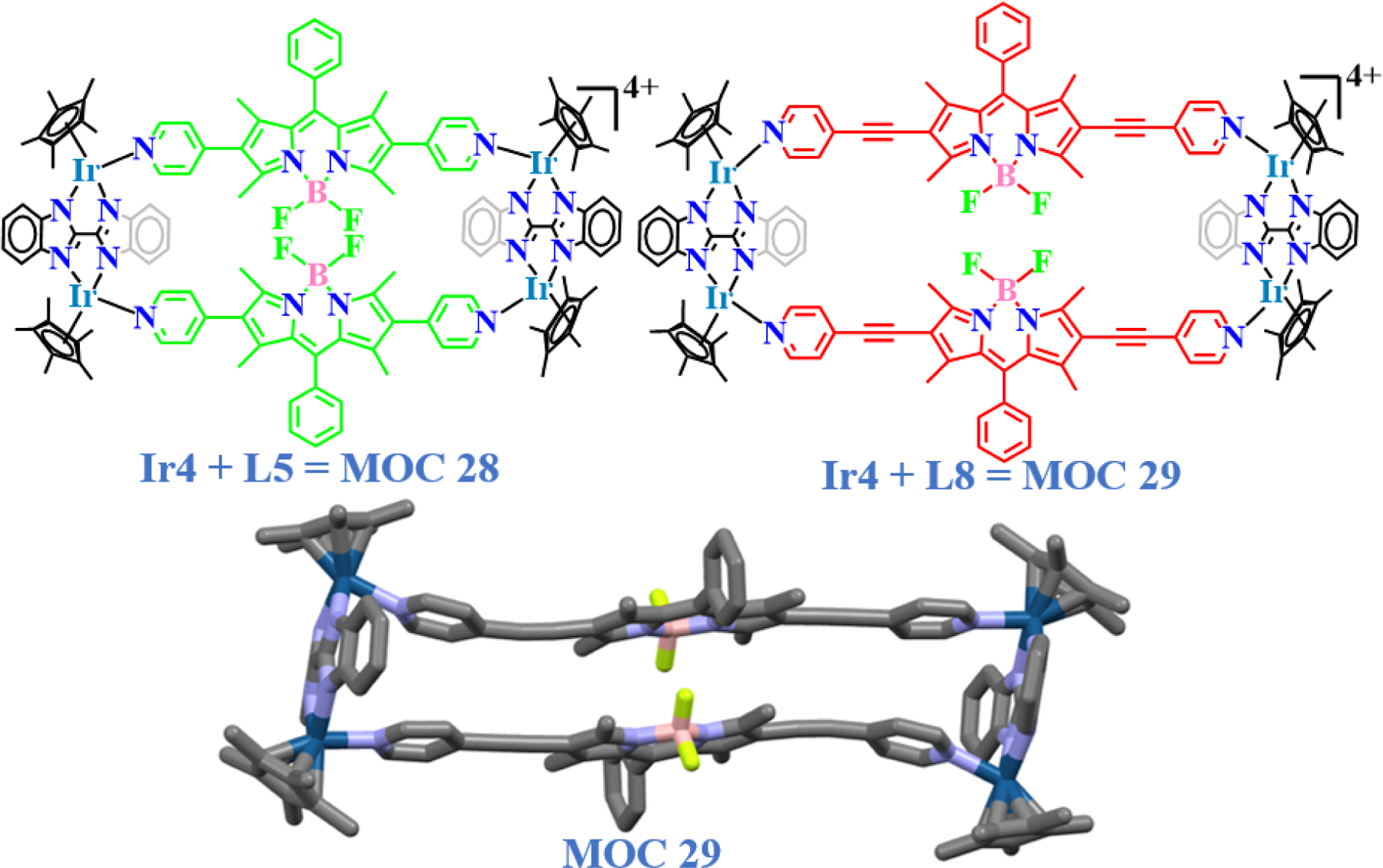
Molecular structures of bisbenzimidazole based BODIPY iridium rectangles derived from clip Ir4 and ligand L5 and L8 and single-crystal XRD structure of MOC 29.
The highly fluorescent BODIPY molecule present in all the series of complexes studied can be used as an important tool for intracellular localization by confocal microscopy studies in biological applications. It was among one of the significant outcomes from work done by Lee and co-workers. It was also proved that the fluorescence of the BODIPY ligands at the designated wavelength(s) resulted only when in their respective supramolecular forms and not in their free forms. The presence of polar functional groups within a supramolecular structure usually improves its water solubility; however, some amount of intrinsic hydrophobicities caused by the aromatic groups led to the formation of nanoaggregates within the cell [13,79,83].
In their most recent work, Lee and co-workers tried to look further into the biomolecular strategies behind the anticancer activities of another series of Ru & Ir supramolecules against malignant cells [16]. Macrocycles MOC 30 – MOC 35 were synthesized by reacting clips Ru4 and Ir3 with BODIPY ligands L4, L5, and L6 (Fig. 15). All six supramolecules were found to be effective in selectively killing breast and brain cancer cells. Additionally, all complexes showed higher cytotoxic activities than cisplatin against the proliferating MCF-7 cells, while the BODIPY ligands remained ineffective when used alone. The results were compared with those from a previous study [13], where it was found that the Ir-based complexes showed improved toxicities as compared to their previous counterparts. Flowcytometric results showed that a 10 µM dose of MOC 33 killed nearly 50% of breast cancer cells, but MOC 32 was found to be the most efficient killer. It was quite encouraging to see that the respective bioactivities of both these complexes were parallel with the IC50 results obtained. In the case of HeLa and U87 cells, MOC 34 and MOC 35 resulted in a rise in the cell death percentage at the highest doses, along with a decrease in the number of cells in G1 and S phases when checked with a flow cytometer. This phenomenon may have caused due to the failure of the already aberrant DNA repair mechanism after a certain load of compound treatment, resulting in a high percentage of synchronized cell death. CLSM studies revealed that some complexes were not only able to enter the nuclear membrane, but also initiate early signs of apoptosis in MCF-7 cells. By virtue of dual staining, DAPI can demarcate the release of chromatin and irreversible disintegration of the nuclear matter in some treated cells; whereas the presence of BODIPY in the complexes made it possible to see the site of compound localization and action. These results indicated that the complexes might prefer more than one paths of action to instigate programmed cell death. Moreover, MOC 32 and MOC 35 with the highest toxicities against all cell lines also showed maximum interaction with DNA as well as with the protein backbone and aromatic amino acids. Complexes having significant interaction with single and double stranded DNA [13,16,49], indicate towards their capability to manipulate the genetic arrangement within a cancer cell leading to disruption of cell cycle kinetics.
Fig. 15.
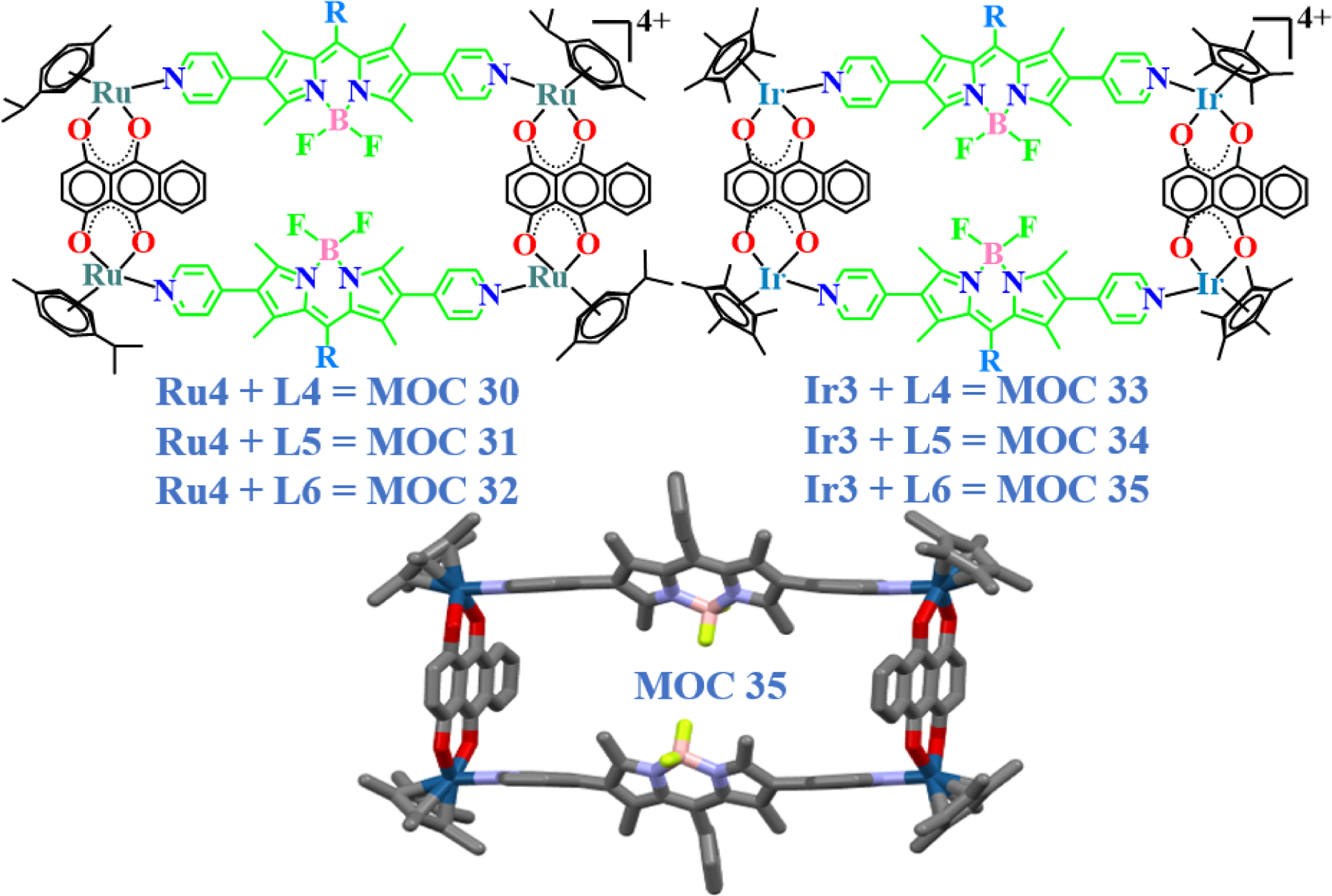
Molecular structures of quinone-based BODIPY ruthenium and iridium rectangles and single-crystal XRD structure of MOC 35.
It was also desirable to explore the molecular mechanisms by which these compounds manipulate the aberrant cytokinetics of cancer cells. Signaling pathways in programmed cell death involve a complicated cascade of successive up-regulation and down-regulation of several anti- and pro-apoptotic proteins. Western Blotting studies revealed that MOC 33 initiated an increase in the Bax/Bcl-2 protein ratio in all three cell types. The treatment also resulted in time-dependent activation of caspase-9 and caspase-3 as well as enhanced levels Apaf-1 followed by proteolytic degradation of PARP, thereby confirming the intrinsic pathway of apoptosis. Caspase-8 has the ability to trigger the downstream executioner caspase-3, which in return initiates the extrinsic pathway of apoptosis [84]; on the other hand, it can also activate the intrinsic pathway of apoptosis by causing the cleavage of Bid. Caspase-8 is therefore considered to play a vital role in connecting both the pathways of apoptosis [85,86], if both caspase-3 and t-Bid are expressed significantly at the translational level. It was found that MOC 33 also initiated time-dependent cleavage of procaspase-8 to caspase-8 and Bid to t-Bid in all the cell lines, signifying evidence of the extrinsic pathway. p53 is believed to be the most important tumor suppressor protein, and over 50% of cancers are caused by its mutation. In this study, a significant time-dependent augmentation in its levels was observed, which meant that the mechanism of programmed cell death here was also p53 dependent. A representation of the mechanism that might be involved in this process is described in Fig. 16.
Fig. 16.
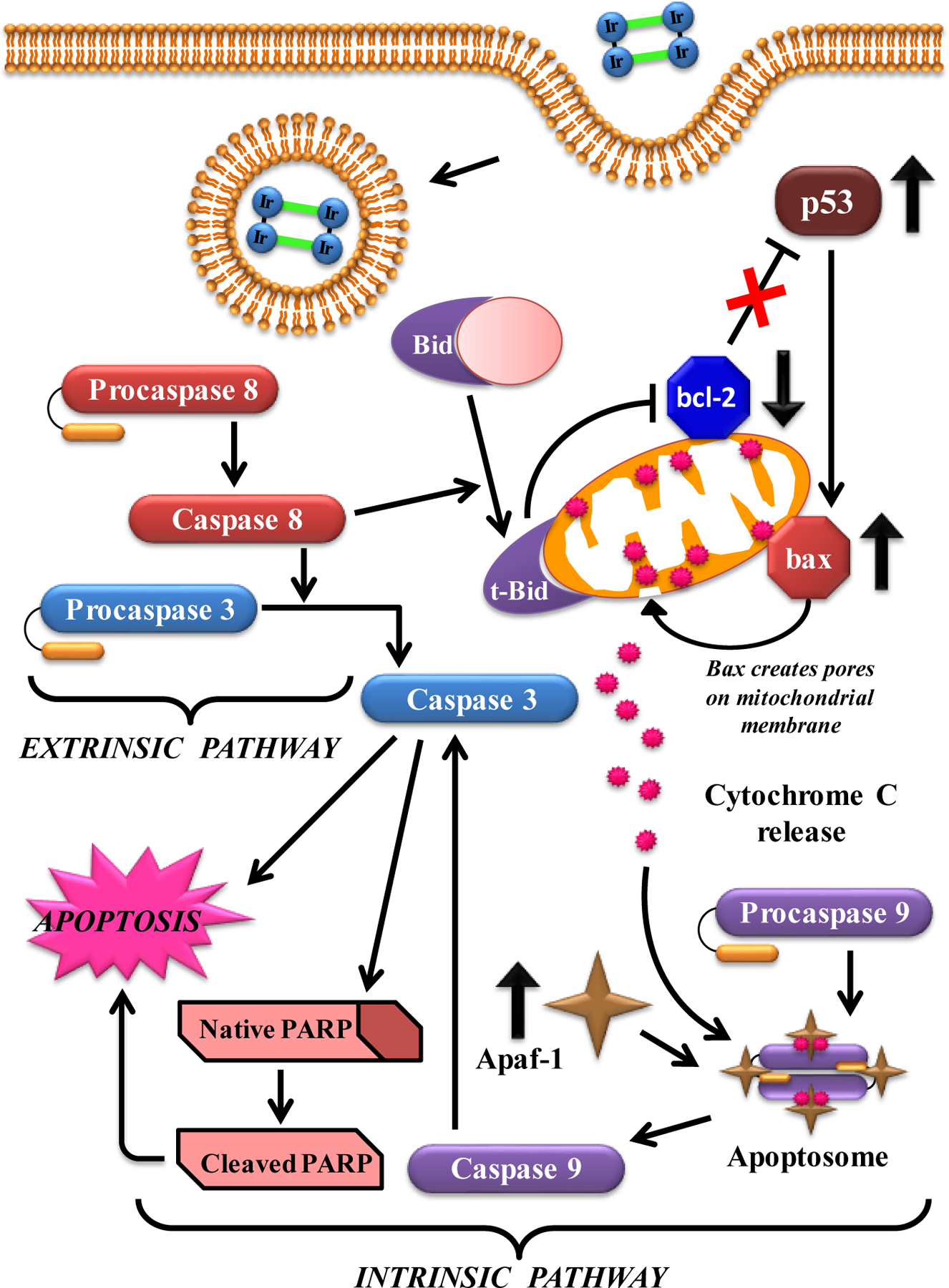
Cartoon representation showing the mechanism involved with MOC 33.
4. Metal-Organic Frameworks
Coordination polymers or metal organic frameworks, simply known as MOFs, are crystalline materials with extremely high porosity and chemical stability [87]. They are synthesized by reacting organic building blocks, otherwise known as ligands, with a metal source by solvothermal reaction with high boiling solvents [88–90]. The product is precipitated as crystals usually insoluble in common organic solvents. The formation and morphology of the MOFs are dependent on the type of metal source and ligands used in the reaction process. The most commonly used metals are zinc (II) and copper (II) salts, however, other transition metal ions are also extensively used; whereas the organic ligands are either O- and N-donor containing molecules [88,91,92].
In 1995 Yaghi and co-workers developed a novel class of crystalline materials with permanent porosity and high surface area termed as metal-organic frameworks [93–95]. The striking difference of these materials from that of zeolites is that they are manmade synthesized in the laboratory, whereas zeolites are usually natural or synthetic materials. They are highly porous and thermally stable at high temperatures and various chemical conditions [87,96]. These materials offer a wider range of applications due to their versatile physical and chemical properties and have emerged as an alternative to zeolites as well as activated carbons. To date, they are the most porous materials known to mankind with surface areas of as high as 7140 m2/g, however further structural modifications of such materials can show a higher value of above 9,000 m2/g [97]. They are one of the most studied materials with over 100,000 crystal structures deposited at the Cambridge Crystallographic Data Center (CCDC), and the number is rapidly increasing [97]. Besides several important applications of MOFs such as gas storage and separation [98], sensor [99], catalysis [100], light-harvesting [101], magnetic and electronic devices [102], etc., MOFs have also shown significant success in biomedical applications [103,104]. Various MOFs, along with their modified version, were found to have stability in the biological medium, making them suitable candidates for anticancer and photodynamic therapy studies.
The structural advantage of MOFs allows BODIPY chromophores to be easily introduced into MOFs. First, BODIPY containing pyridine or carboxylic acid is designed so that it can be directly involved as a ligand during MOF synthesis. Second, since MOFs can provide a confined space suitable for appropriately sized molecule encapsulation, BODIPY can be loaded in the MOFs as a guest. Third, the possibility of functionalization and exchange of ligands and the capability of additional coordination to metal nodes in the MOFs suggest that BODIPY can be incorporated into the MOFs via post-synthetic modifications (PSMs) (Fig. 17).
Fig. 17.
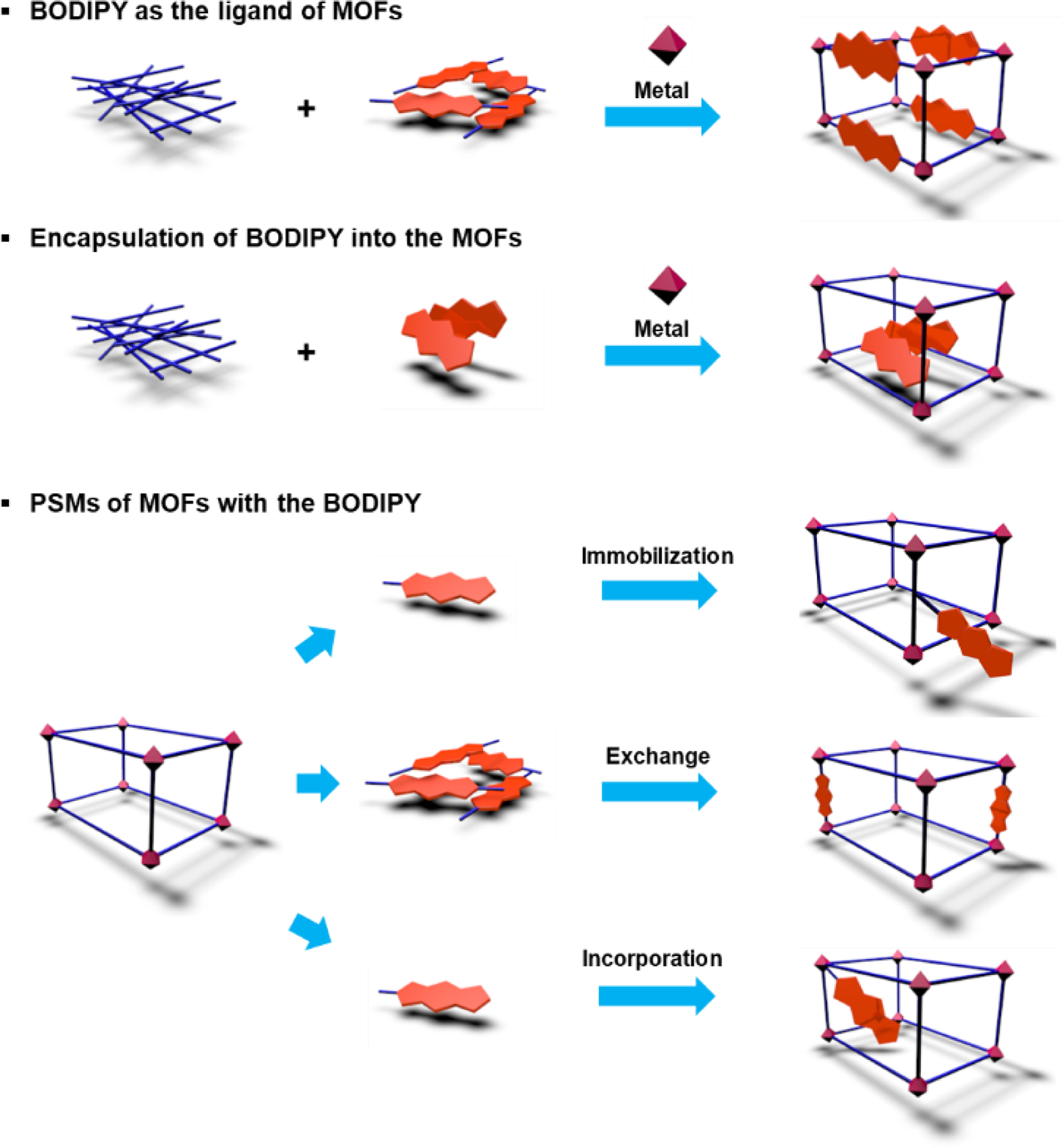
Common strategies representing the formation and functionalization of MOFs.
4.1. BODIPY-based MOFs (BMOFs): incorporation of BODIPY as the ligand
The first BODIPY-based ligand (L5) for constructing metal-organic frameworks was designed and synthesized by Hupp and co-workers [105]. The BMOF-1 and BMOF-2 were synthesized in high yield using linkers L13 (for BMOF-1) and L14 (for BMOF-2) with dipyridyl BODIPY L5 and Zn(NO3)2 ‧ 6H2O (For linkers, see Chart 3). The single-crystal structures (Fig. 18) showed a similar type of 2D non-interpenetrated system formed by the coordination of paddlewheel nodes of Zn(II) ions to tetracarboxylates which were further pillared by dipyridyl BODIPY ligands having permanent porosity. The MOF featuring BODIPY and porphyrin ligands showed excellent light-harvesting properties throughout the entire visible spectrum. Energy transfer from the BODIPY chromophore to the Zn(porphyrin) unit was the result of this interesting antenna behavior. Effective energy transfer occurs only when the chromophore is inserted into a well-ordered MOF structure. This result provides structural insights necessary when mimicking photosynthesis in nature.
Chart 3.
Presentation of different ligands used to generate MOFs.
Fig. 18.

View of the molecular structures of MOFs BMOF-1 and BMOF-2 and the confocal laser scanning microscopy images of the crystals. Black crystals indicate no emission, whereas color crystals denote emission. Reproduced with permission from ref. [105]. Copyright 2011 American Chemical Society.
Following the above report described by Hupp and co-workers, other groups utilized the pyridyl-based BODIPY ligand for the synthesis of MOFs. However, only the synthesis and photophysical characterization were studied for the reported MOFs [106]. During this time, copper and silver metal coordination polymers based on pyridyl BODIPY ligands were also described [107–110]. In almost all the reported BODIPY based MOFs, the pyridyl BODIPY ligand was used as a pillar to form the described 2D or 3D architectures. Fan and co-workers designed the BODIPY ligand L15 where carboxylic groups were directly introduced into the BODIPY core in its 2 and 6 positions, which acted as a linker to bind with auxiliary bipyridine co-ligands 1,3-bi(4-pyridyl)propane (bpp) and 1,2-bi-(4-pyridyl)ethane (bpe) to give the 2D (BMOF-5, BMOF-6) or 3D (BMOF-3, BMOF-4, BMOF-7) coordination polymers [111]. Interestingly, the photophysical properties differ depending upon how the BODIPY chromophores are oriented within the rigid frameworks. In BMOF-4, the BODIPY moieties show a characteristic J-dimer absorption band at λmax = 705 nm because they are located close to each other with the coplanar displacement of θ = 37.9 °. On the other hand, in BMOF-5, such J-dimer transition disappears, and intact monomer-like BODIPY fluorescence is observed because the BODIPY moieties have a parallel arrangement of transition dipoles with sufficient distance and a θ = 84 ° slip angle (Fig. 19).
Fig. 19.

Single-crystal X-ray structures of BMOF-3~7 and partial packing diagrams of BODIPY chromophore in BMOF-4 and BMOF-5. Reproduced with permission from ref. [111]. Copyright 2015 American Chemical Society.
Peng and co-workers reported a 3-fold interpenetrated pillared-paddlewheel type MOF (BMOF-8) constructed by dipyridyl BODIPY L5, 4,4’-biphenyldicarboxylic acid (H2BPDC), and Zn (II) ion [112]. Subsequently, BMOF-8 served as a support for platinum nanoparticles to obtain photocatalyst (Pt/BMOF-8) for H2 production from water (Fig. 20). The fluorescence intensity of BODIPY moiety in the BMOF-8 decreased when a Pt nanoparticle was incorporated in BMOF-8, and the average fluorescence lifetime of Pt/BMOF-8 (τav = 1.84 ns) is shorter than that of BMOF-8 (τav = 2.09 ns). Overall the fluorescence properties suggest that the Pt nanoparticles endow BMOF-8 with an efficient charge separation via trapping of BODIPY’s excited electron. Pt/BMOF-8 had an H2 production rate (4680 μmol g−1h−1) and remarkable quantum efficiency (9.06% at 420 nm) compared to the comparable catalyst in an aqueous medium. This photocatalytic performance results from an inherent narrow bandgap and suitable band positions of BMOF-8 as well as suppression of the recombination of the photoinduced e--h+ charge separation state by the Pt nanoparticles and the metal node in BMOF-8.
Fig. 20.
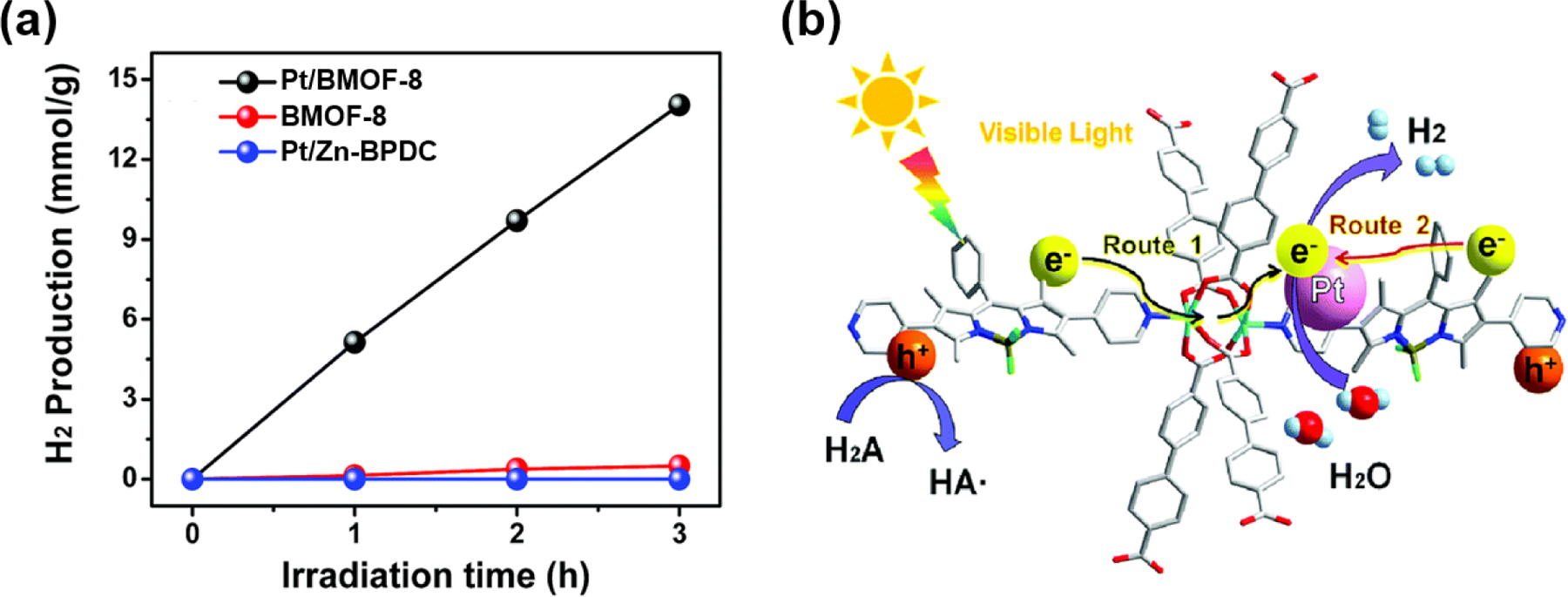
(a) Photocatalytic H2 production over different samples. (b) Proposed mechanism of photocatalytic H2 production over Pt/BMOF-8 under visible light irradiation. Reproduced with permission from ref. [112]. Copyright 2019 Royal Society of Chemistry.
Recently, an anticancer drug-loaded BODIPY-based MOF was synthesized by Meng and co-workers, and it was applied to photodynamic and chemotherapeutic agents (Fig. 21) [113]. Self-assembly of dipyridyl BODIPY L16, H2BPDC, and Cd(NO3)2 afforded two-fold interpenetration pillar-layered BMOF-9. The strong photoluminescent emission peak corresponding to the BODIPY moiety in BMOF-9 was observed at 643 nm upon excitation at 470 nm. This BODIPY linker-based emission peak was red-shifted compared with that of BODIPY L16 (λem= 603 nm), suggesting a π-π overlap between the BODIPY chromophores through reverse offset face-to-face stacking of BODIPY in restricted BMOF-9 frameworks. The singlet oxygen quencher, 1,3-diphenylisobenzofuran (DPBF) was used to verify reactive oxygen generation ability by BMOF-9. As displayed in Fig. 21b, BMOF-9 showed excellent singlet oxygen generation abilities, demonstrating its potential as a PDT photosensitizer. The representative anticancer drug, doxorubicin (DOX) was successfully loaded in BMOF-9 by a simple impregnation method. Due to the luminescence properties of the DOX and BODIPY moieties, cellular localization of DOX-loaded BMOF-9 and gradual release of DOX were visually confirmed by fluorescence microscopy images. The most efficient anticancer activity was achieved in DOX-loaded BMOF-9 in the presence of 660 nm light irradiation, indicating a synergistic effect of photodynamics and chemotherapy.
Fig. 21.
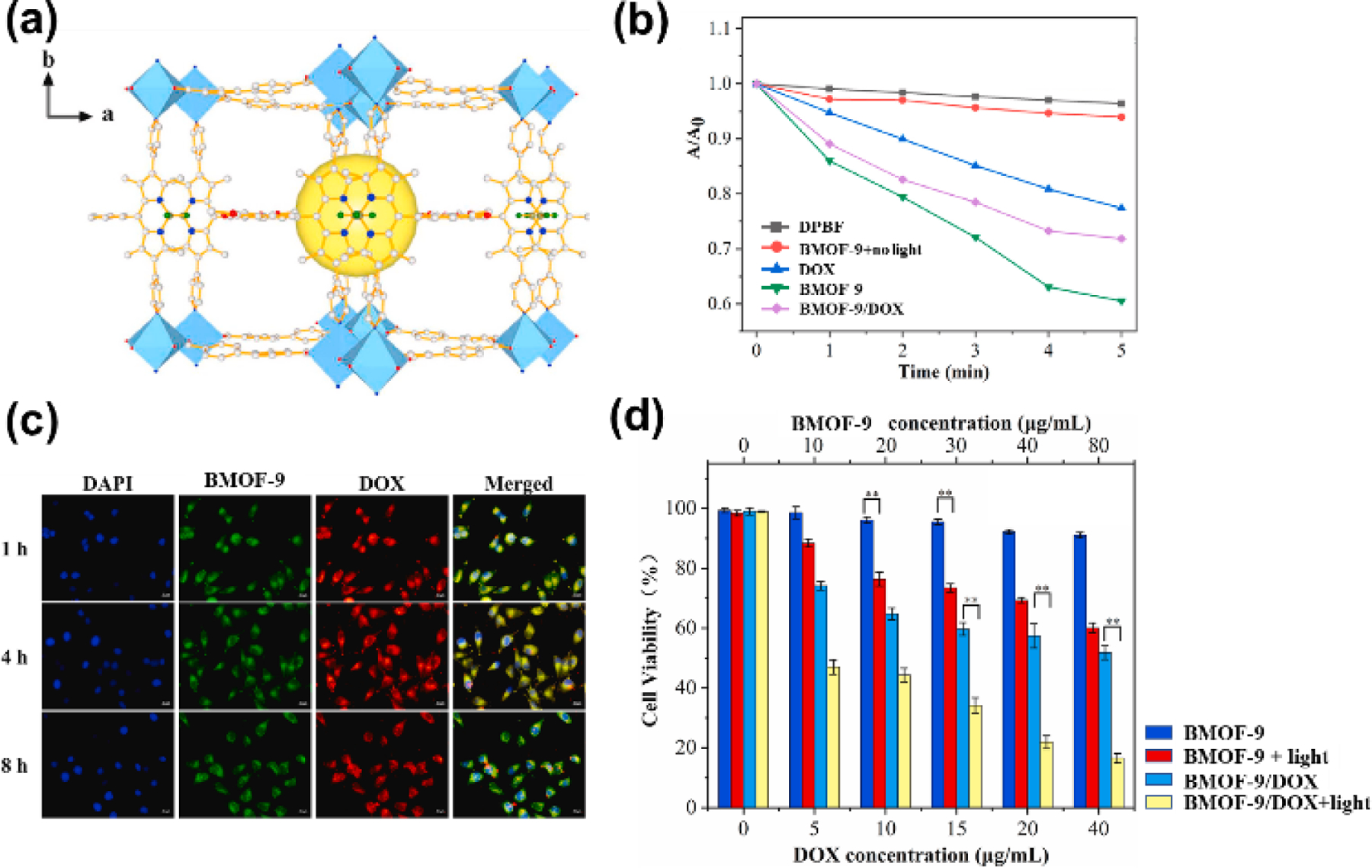
(a) X-ray crystal structure of BMOF-9. (b) Singlet oxygen (1O2) generation ability as monitored by the change in absorption signal (λab=416 nm) of DPBF. Reproduced with permission from ref. [113]. Copyright 2021 Elsevier.
4.2. BODIPY-based MOFs (BMOFs): encapsulation of BODIPY into the MOFs
An important contribution of such an encapsulation system, where a BODIPY chromophore was trapped into a MOF, leading to excellent photophysical properties, was described by Glembockyte and co-workers [114]. In this approach, BODIPY fluorophore L17 was entrapped within a ZIF-8 framework forming L17@ZIF8 by solvent-free ion- and liquid-assisted grinding (ILAG) or an accelerated aging (AA) method (Fig. 22). Because the molecular dimension of L17 is larger than the pore aperture size of ZIF-8, a simple impregnating of L17 into pre-synthesized ZIF-8 is excluded and BODIPY is encapsulated through the in situ entrapment method. L17 does not show fluorescence due to nonemissive H-aggregate formation in the solid-state, whereas L17 encapsulated in ZIF-8 exhibits strong fluorescence resulting from a spatially separated dye dispersal in the framework. Interestingly, the emission properties of L17@ZIF-8 is dependent of the L17 loading amount. High loading of the BODIPY dye in ZIF-8 formed a double pore occupancy resulting in non-emissive or weakly emissive H-aggregates. Time-resolved fluorescence spectroscopies revealed a gradual shortening of fluorescence lifetimes with increasing L17 loading (1.5 wt% or higher), implying that the through-pore energy transfer process between the L17 fluorophores in different ZIF-8 pores occurred at high BODIPY loading. The entrapped BODIPY chromophore resulted in a 10- fold enhancement of its photostability by showing lower photobleaching after 100 minutes of irradiation.
Fig. 22.
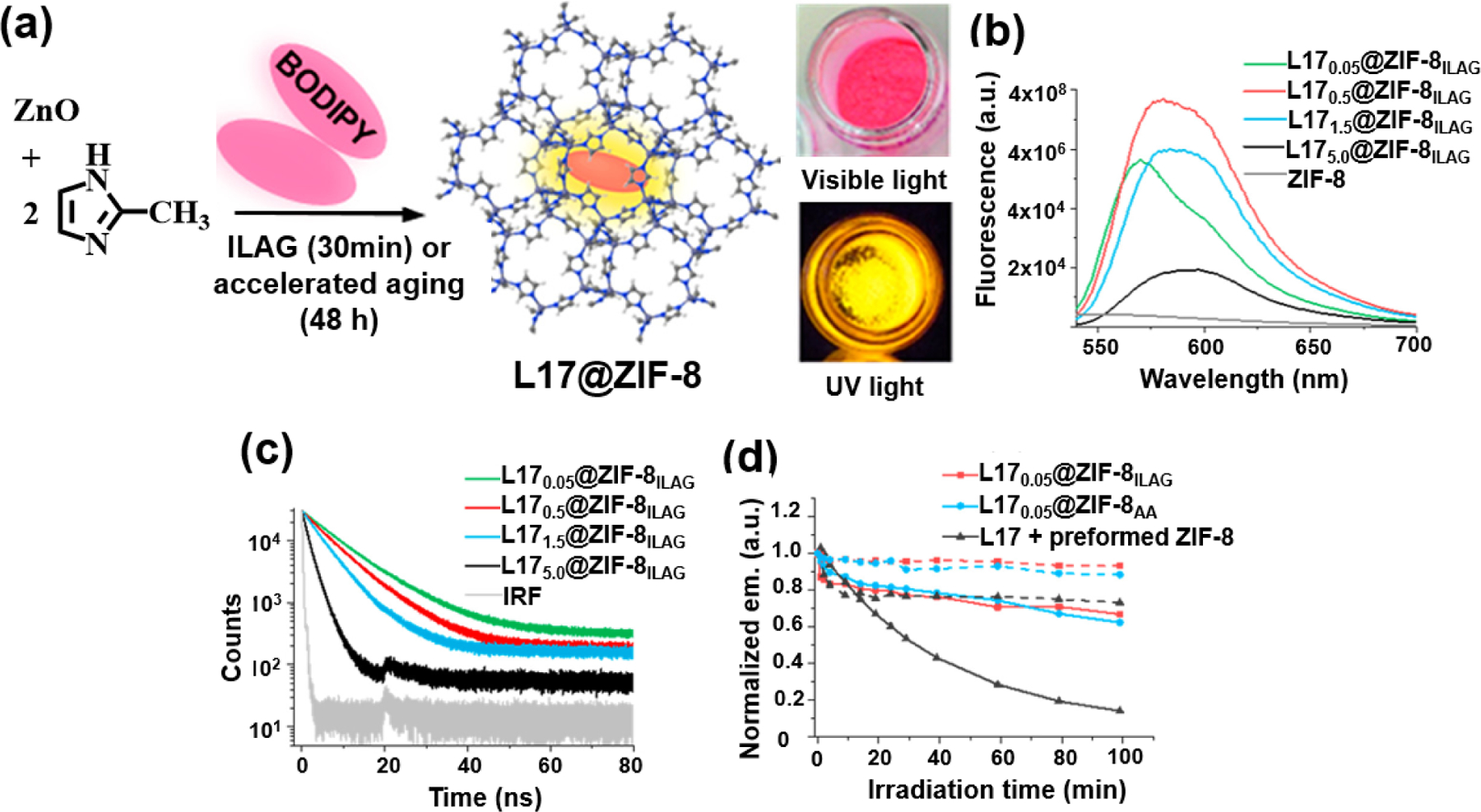
(a) Solid-state mechanochemical or accelerated aging synthesis of L17@ZIF-8 and L17@ZIF-8 sample viewed under room light and ultraviolet light (b) Fluorescence emission spectra for L17n@ZIF-8 (λex=520 nm, n is the wt % of loaded dye) (c) Time-resolved fluorescence decay profiles of L17n@ZIF-8 (λex=467 nm and monitored at 590 nm) (d) Normalized integrated emission of L170.05@ZIF-8ILAG and L170.05@ZIF-8AA samples and L17 + preformed ZIF-8 containing acetonitrile monitored after different irradiation times (solid lines). The extent of L17 degradation resulting from photobleaching can be determined by comparing the fluorescence intensity obtained to that of dark control samples (dashed lines). Reproduced with permission from ref. [114]. Copyright 2018 American Chemical Society.
Burrows and co-workers reported another BODIPY-encapsulated MOF [115]. The tripodal ligand, 2,4,6-tris(4-pyridyl)-1,3,5-triazine (L18) and Zn metal ion were assembled to obtain a crystalline sponge, [(ZnI2)3(tpt)2]·x(solvent), which acted as the host for incubating the BODIPY chromophore. After activation of the MOF, it was soaked in a BODIPY solution for one week to afford BODIPY-encapsulated MOF. The solid-state fluorescence emission of BODIPY was red-shifted with a concomitant quenching in emission intensity upon being hosted in the MOF. The authors presented various reasons for this behavior, including emission filtering by the MOF framework, exciplex formation between BODIPY and the electron-deficient framework, and collisional quenching between the host and guest.
BODIPY was applied as a luminescent probe molecule by Hupp and co-workers to investigate the process and mechanism of molecular transport within the solvent-filled metal-organic framework (Fig. 23) [116]. The diffusion of the guest molecule BODIPY along the 1D channel in individual zirconium-based MOF NU-1008 crystals was monitored by in situ confocal fluorescence microscopy due to the highly fluorescent BODIPY dye. The BODIPY movement in the crystal can be explained by the one-dimensional diffusion along the channel, and the result was inversely proportional to the diffusivity of the dye to the viscosity of the solvent, qualitatively consistent with the expected result from the Stokes−Einstein equation for molecular diffusion. Quantitatively, the confirmed diffusion coefficient of BODIPY through the channel was smaller by 2-orders of magnitude than the value in the solution due to the channel-confinement effect.
Fig. 23.
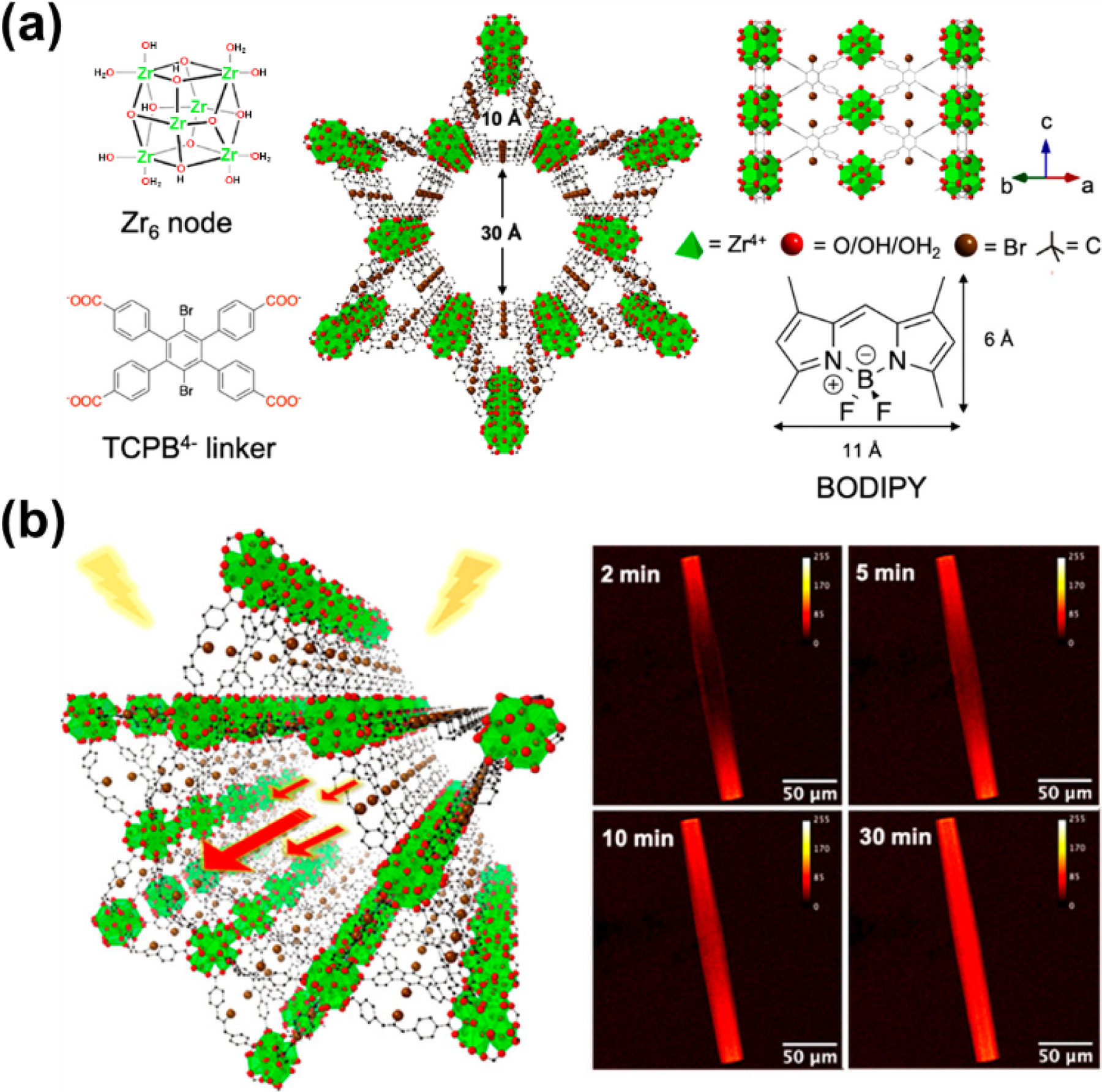
(a) Structural information of single-crystal NU-1008 (SC-NU-1008) and the structure of the guest molecule BODIPY. (b) In situ images of transport of BODIPY within SC-NU-1008 (at the center of z stack) filled with methanol. Reproduced with permission from ref. [116]. Copyright 2020 American Chemical Society.
Another example of BODIPY encapsulated MOFs, 2I-BodipyPh-NO2@ZIF-90, was developed by Dong and co-workers [8]. Diiodo-BODIPY, 2I-BodipyPh-NO2, was encapsulated into ZIF-90 by a facile one-pot in situ self-assembly approach (Fig. 24). ZIF-90 acts as a pH-triggered charge reversed material that converts the zeta potential from negative to positive when the pH decreases. Therfore, positive charge conversion was induced in ZIF-90 in the environment of cancer cells with low pH, and its strong affinity for negatively charged cell membranes made it possible for ZIF-90 to selectively uptaken by cancer cells.
Fig. 24.
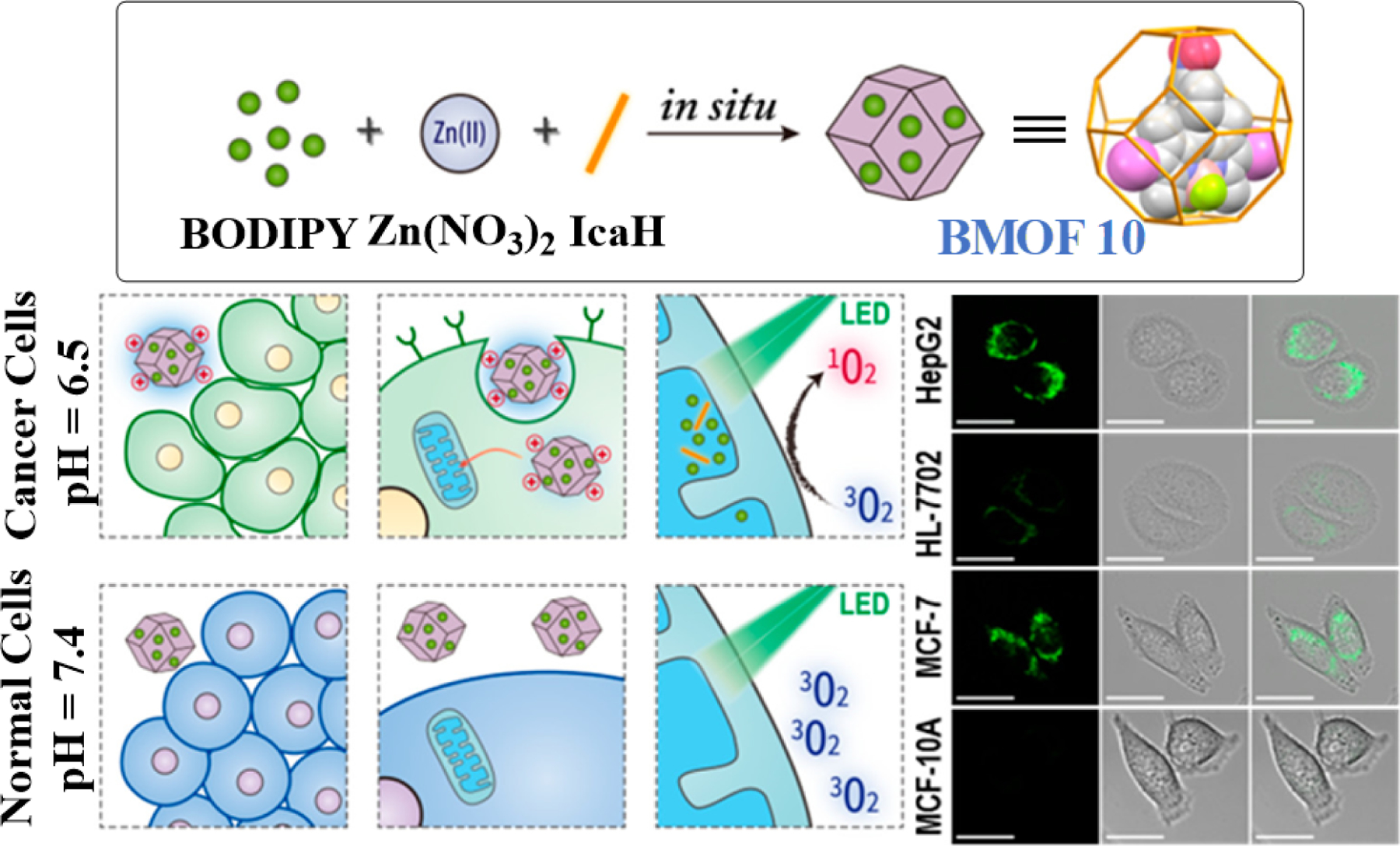
Schematic diagram showing the formation of 2I-BodipyPh-NO2@ZIF-90 (BMOF 10) from L24, Zn(NO3)2 and imidazolate-2-carboxyaldehyde (IcaH). The cartoon presentation (L) of its pH-driven selective uptake toward cancer and normal cells and mitochondria targeting under PDT conditions and the in vitro CLSM images (R) of different cells incubated with 2I-BodipyPh-NO2@ZIF-90 in PBS solutions. Reproduced with permission from ref. [8]. Copyright 2018 American Chemical Society.
2I-BodipyPh-NO2@ZIF-90 was found to be photostable under 540 nm green illumination, unlike the free 2I-BodipyPhNO2 molecules, which means 2I-BodipyPh-NO2@ZIF-90 will remain structurally stable at near physiological pH within the circulatory systems in a patient’s body, unless it comes in contact with any tumor microenvironment, which is when the acidic pH driven site-specific PS release would be triggered. This selective phenomenon was also observed with cell lines where a much higher selective uptake and retention of the green fluorescence was seen in breast cancer cells than their non-malignant counterparts. As previously stated, any nano species with a net positive charge after amassing within an acidic cancer microenvironment would have a higher affinity to pass through the selectively permeable negatively charged cell membrane. Further CLSM studies revealed that upon internalization, the nanocrystals BMOF 10 had an excellent affinity to target and localize within the mitochondria of MCF-7 and HepG2 cells. This was ascribed to the positively charged ZIF-90 shell showing a pronounced inclination to accumulate within the negatively charged inner membrane of mitochondria. Using lysosomal and mitochondrial tracking fluorescent dyes a gradual decline in the levels of BMOF 10 within the lysosomes of a hepatic cancer cell was observed with time, whereas in mitochondria a time-dependent increase in retention was seen. This is because the internalization of any particle within a cell starts through endosomes leading up to the lysosomes. In this case, the endosomal membrane is assumed to be weakened due to the imidazole protonation of BMOF 10, while the acidic pH in lysosomes facilitated the gradual decomposition of the ZIF-90 shell. It means BMOF 10 in its partially decomposed status protects the PS from enzymatic degradation while escaping from the lysosomes and then targeting the mitochondria. The authors also showed increased generation of singlet oxygen by BMOF 10 under acidic conditions as compared to that obtained at physiological pH through spectrophotometric studies and intracellularly in cancer and normal cell lines by using a Singlet Oxygen Sensor Green (SOSG) probe. Moreover, the authors also provided evidence through flow cytometric studies that the phototoxicity caused by BMOF 10 is due to induction of programmed cell death or apoptosis.
Chen and co-workers reported a photo-driven smart “Domino” agent for antibacterial activity with BODIPY derivatives encapsulated ZIF-8 (Fig. 25) [117]. They prepared BCNBA@ZIF by encapsulating BC, which is coupled with the chemical antibacterial phenethyl caffeate (CAFE) and the photodynamic antibacterial BODIPY in ZIF-8 together with 2-nitro-benzaldehyde (o-NBA) acting as a photoacid. In the presence of light, the encapsulated o-NBA releases protons and lowers the pH, resulting in the decomposition of BCNBA@ZIF, which releases Zn2+ and BC. These interesting photoresponsive properties led to the biological application of BCNBA@ZIF. The cytotoxicity of BCNBA@ZIF to BGC-823 cells was insignificant in the absence of light, but showed clear cytotoxicity with an IC50 value of 312.3 μg mL−1 under blue LED illumination. A study on the antibacterial activity of BCNBA@ZIF was also conducted. Under blue LED light illumination, BCNBA@ZIF showed an antibiotic effect equivalent to that of the commercial drug Ampicillin, but exhibited low activity in the absence of light. The high anti-bacterial effect of BCNBA@ZIF may be due to the proton produced by light irradiation causing destruction of the ZIF structure and releasing BC to achieve the antibacterial activity.
Fig. 25.
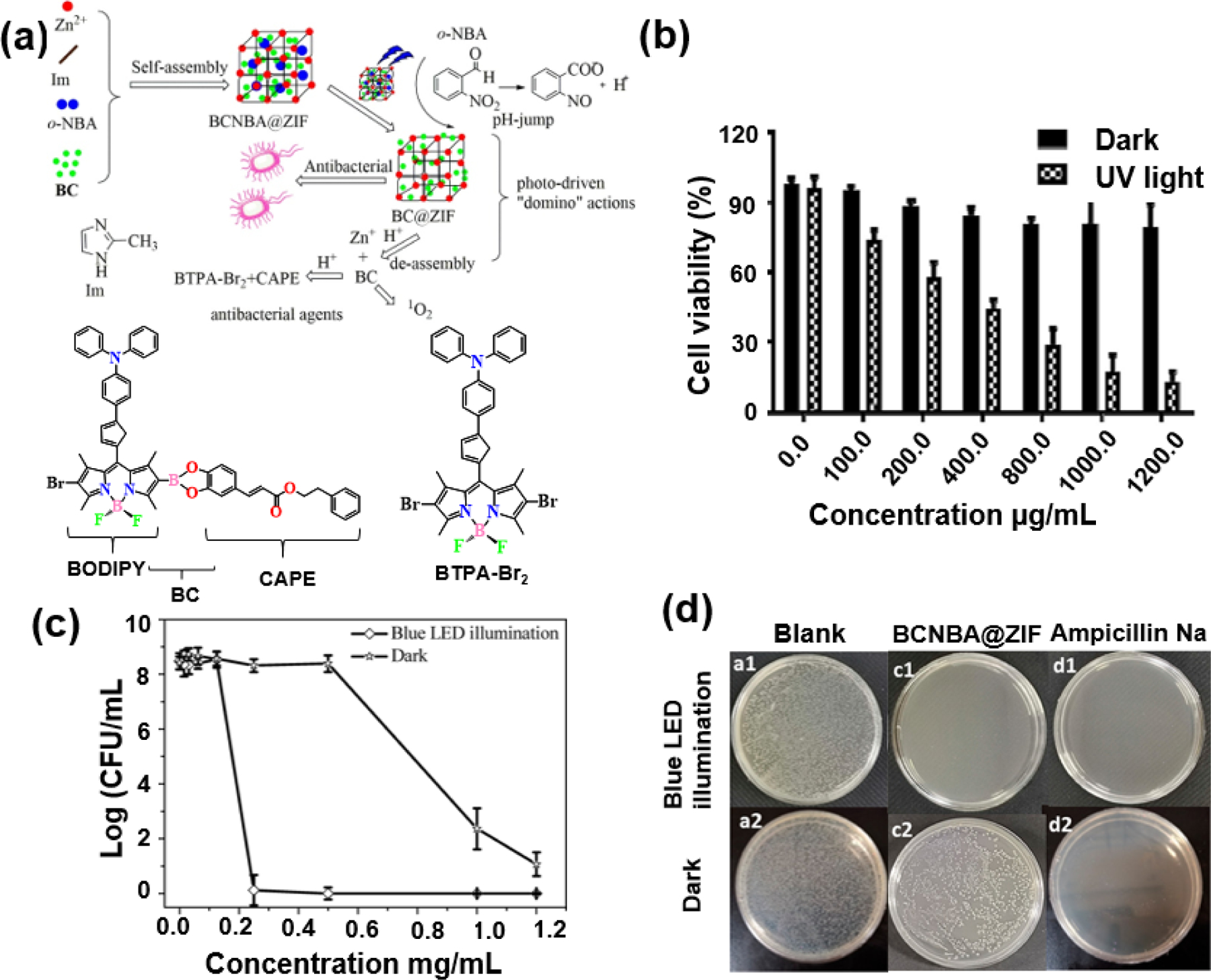
(a) Schematic Illustration of BTPA-Br, BC, the Synthesis and Mechanism of BC@ZIF, and BCNBA@ZIF Nanoparticles. (b) Cytotoxicity of different concentrations of BCNBA@ZIF to BGC-823 cells in dark conditions and blue LED light for 120 min. (c) Concentration-dependent resistance study for BCNBA@ ZIF in dark and blue LED illumination condition against E. coli ([E. coli] = 1.2 × 108 CFU mL−1). The results are represented as mean ± SD (n = 3). (d) Image of E. coli ([E. coli] = 1.2 × 104 CFU mL−1). (a1) E. coli with 30 min LED light irradiation. (a2) Only E. coli. (c1) E. coli + 1 mg mL−1 BCNBA@ZIF+ 30 min LED light irradiation. (c2) E. coli + 1 mg mL−1 BCNBA@ZIF. (d1) E. coli + 500 μgmL−1 Ampicillin Na+ 30 min LED light irradiation. (d2) E. coli + 500 μgmL−1 Ampicillin Na. Reproduced with permission from ref. [117]. Copyright 2020 American Chemical Society.
4.3. BODIPY-based MOFs (BMOFs): Post-synthetic modification (PSM) of MOFs with BODIPY
The insertion of BODIPY into the MOFs through the post-synthetic modification process reported so far can be summarized in three ways. The first is an immobilization method that post-processes BODIPY, which has a functional group capable of reacting with the ligand’s reaction site. Second, an exchange method in which a BODIPY derivative having a functional group such as a carboxylic acid or a pyridyl group is treated afterward to replace the ligand of the existing MOFs structure. The third is the incorporation method in which the BODIPY with a carboxyl group displaces the terminal aqua or hydroxo group on the Zr6 node of the zirconium-based MOFs.
4.3.1. Immobilization
Lin and co-workers reported the first immobilization method of BODIPY in 2009 (Fig. 26) [118]. An amino group functionalized MIL-101(Fe) was synthesized by coordinating a ligand mixture of terephthalic acid (1,4-bezenedicarboxylic acid, BDC) and 2-amino terephthalic acid to iron(III). The introduction of amino groups into frameworks enabled the covalent attachment of biologically relevant compounds through the PSM method. In this work, 1,3,5,7-tetramethyl-4,4-difluoro-8-bromomethyl-4-bora-3a,4a-diaza-s-indacene (Br-BODIPY) was immobilized by a nucleophilic substitution reaction between the alkyl bromide of BODIPY and the amino group of MIL-101(Fe)’s ligand. The BODIPY-loaded MIL-101(Fe) (1b) was decomposed in 8mM PBS buffer at 37°C to release the BODIPY dye in the form of BDC-NH-BODIPY. Due to the instability of MIL-101(Fe) in the PBS buffer, an additional silica protective layer was coated onto it to slow down the release of BODIPY. Silica-coated 1b (1b@SiO2) was used as an optical contrast agent due to the inherent fluorescence characteristics of BODIPY. As a result of treatment with 1b@SiO2 on HT-29 human colon adenocarcinoma cells, strong BODIPY fluorescence in the cell was confirmed by laser scanning confocal microscopy, whereas no fluorescence was observed in the cell without 1b@SiO2. When only the comparative test compound (BDC-NH-BODIPY) was treated in the cell, fluorescence did not appear, probably because it did not pass through the cell membrane.
Fig. 26.
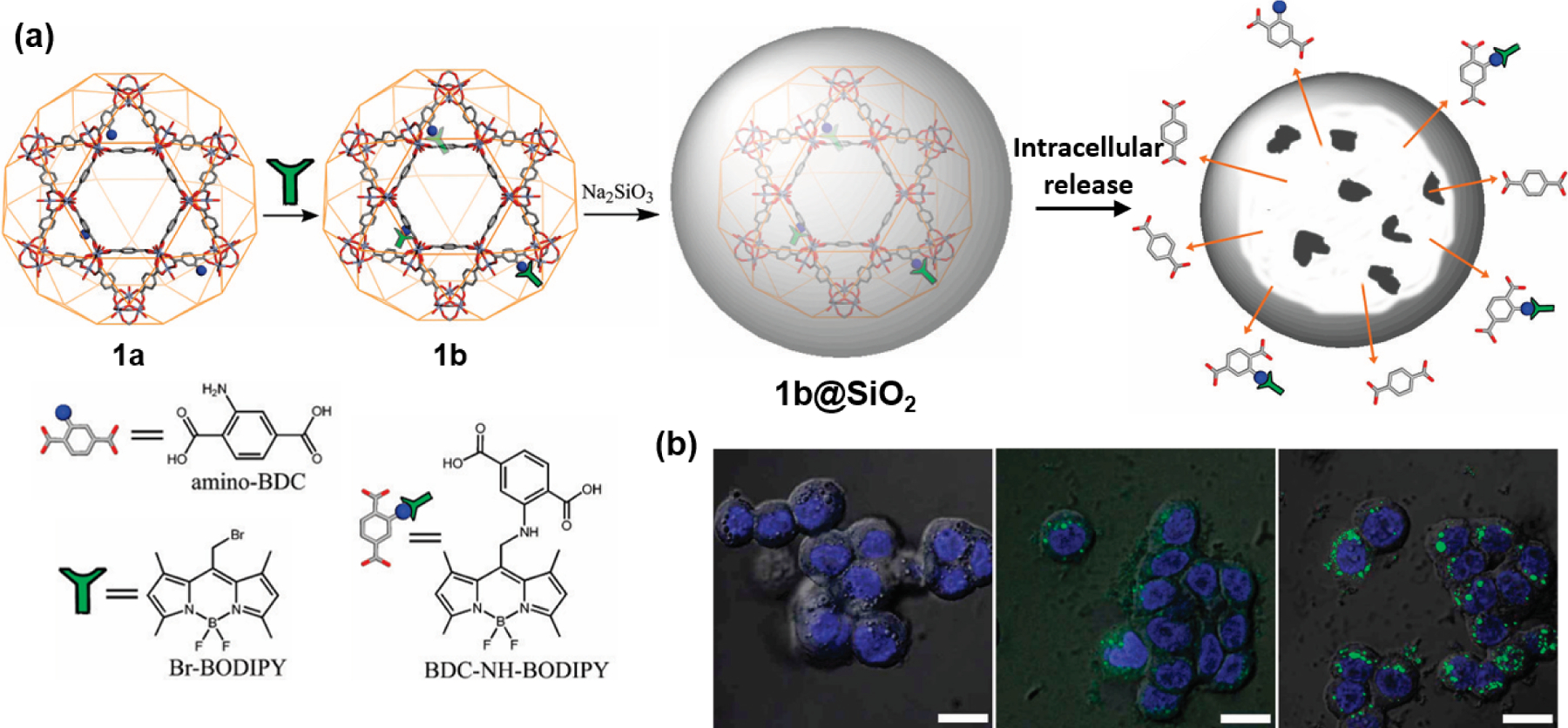
(a) Schematic diagram showing the formation of BODIPY (L17, L19-L21) loaded MOFs 1b and 1b@SiO2. (b) Overlayed DIC and confocal fluorescence images of the DRAQ5 channel (blue, nuclear stain) and the BDC-NH-BODIPY channel (green) of HT-29 cells incubated with no particles (left), 0.19 mg/mL of 1b@SiO2 particles (equivalent to 17 μM BODIPY) (middle), and 0.38 mg/mL of 1b@SiO2 particles (equivalent to 34 μM BODIPY) (right). The bars represent 25 μm. Reproduced with permission from ref. [118]. Copyright 2009 American Chemical Society.
Similarly, Wang and co-workers covalently immobilized diiodo-BODIPY to amine-tagged Zr-MOF UiO-68-NH2 [119]. Covalent immobilization of diiodo-BODIPY was completed by the reaction between acyl chloride moiety on BODIPY and the amino group of the bridging ligand in UiO-68-NH2. The resulting deeply red solid UiO-68-BP was tested as a photoredox catalyst for the aerobic cross dehydrogenative coupling (CDC) reaction of tetrahydroisoquinoline with nitromethane in the presence of air. The desired cross-coupling product was obtained by photocatalyst UiO-68-BP with high yield under the irradiation of green LEDs (λmax = 520–530 nm) at room temperature. Electron spin resonance (ESR) measurements were performed to identify the active species for the photocatalytic reaction. They suggested that a single electron transfer (SET) from tetrahydroisoquinoline to excited diiodo-BODIPY (PS*) occurred, generating radical anion PS−•, followed by transfer of electrons to oxygen molecules to produce superoxide radical anion O2−•. The superoxide radical anion acts as a crucial intermediate in cross dehydrogenative coupling. UiO-68-BP was also tested as a photocatalyst for a series of oxidation–[3 + 2] cycloaddition-aromatization tandem reaction and pyrrolo[2,1-a]isoquinoline products were obtained in good yield.
4.3.2. Exchange
The first ligand exchange method for BODIPY was performed by Xie and co-workers [120]. Carboxyl-functionalized diiodo-substituted BODIPY (I2BDP, L23) was inserted into nano-sized UiO-66 MOFs by solvent-assisted ligand exchange (SALE) to afford the final product of UiO-PDT (Fig. 27). The intracellular uptake of UiO-PDT in B16F10 murine melanoma cells was visualized under a confocal microscope. A time-dependent increase in the characteristic red fluorescence of I2BDP was seen in both cases, where the respective treatments sustainably accumulated in the cytoplasm. Moreover, UiO-PDT was also able to pass through the cell membrane more efficiently than I2BDP alone, possessed enhanced photo-stability, and was an effective generator of singlet oxygen. The UiO-PDT nanocrystals showed good biocompatibility with low dark toxicities when tested against proliferating B16F10, C26, and CT26 cell lines. On the other hand, upon irradiation, a significant rise in toxicity was observed, both in UiO-PDT and I2BDP against every cell line.
Fig. 27.

(a) Schematic diagram showing the synthesis of UiO-PDT from UiO-66 NMOF for PDT application. (b) and (c) In vitro CLSM images of B16F10 cells treated with free BODIPY (I2BDP) and UiO-PDT, respectively. (d) In vitro cytotoxicities of free I2BDP and UiO-PDT nanocrystals against B16F10 cells before and after being irradiated with visible light at a power density of 80 mWcm−2 for 10 minutes. (e) Half maximal inhibitory concentration (IC50) of I2BDP and UiO-PDT with and without light irradiation. Reproduced with permission from ref. [120]. Copyright 2016 Royal Society of Chemistry.
Owing to the presence of iodinated aromatic groups in UiO-PDT, Xie and co-workers further tried to exploit them in the field of clinical diagnosis by investigating their ability as contrasting agents in computerized tomography (CT) (Fig. 28) [121]. Heavy elements like iodine, barium and bismuth have strong X-ray attenuation ability, which makes them ideal choices for developing prospective contrasting agents for CT [122]. Apart from the aforementioned advantages, the ability of NMOFs to carry high metal/ligand concentrations per nanocrystal unit within the framework makes them competent contrasting agents [121]. As anticipated, nano-crystals of UiO-PDT did show a concentration-dependent ability to attenuate X-rays when scanned with a micro CT at 80 kVp, along with a parallel increase in the intensity of the CT signal resulting in brighter images. UiO-PDT was previously found to be ineffective against three proliferating cell lines in vitro. Further testing against HepG2 cells also resulted in less than 20% mortality, attesting to their biocompatibility and low cytotoxic potential. Following this, post intravenous administration of UiO-PDT in male Kunming mice caused neither a change in body weight nor acute toxicity. Additionally, histopathological studies of mice organs confirmed that the tissues were unharmed by the treatment. Similarly, serum biochemistry, haematological tests, liver function markers, gallbladder, and vital kidney function indicators also indicated the non-toxic nature of the NMOFs.
Fig. 28.
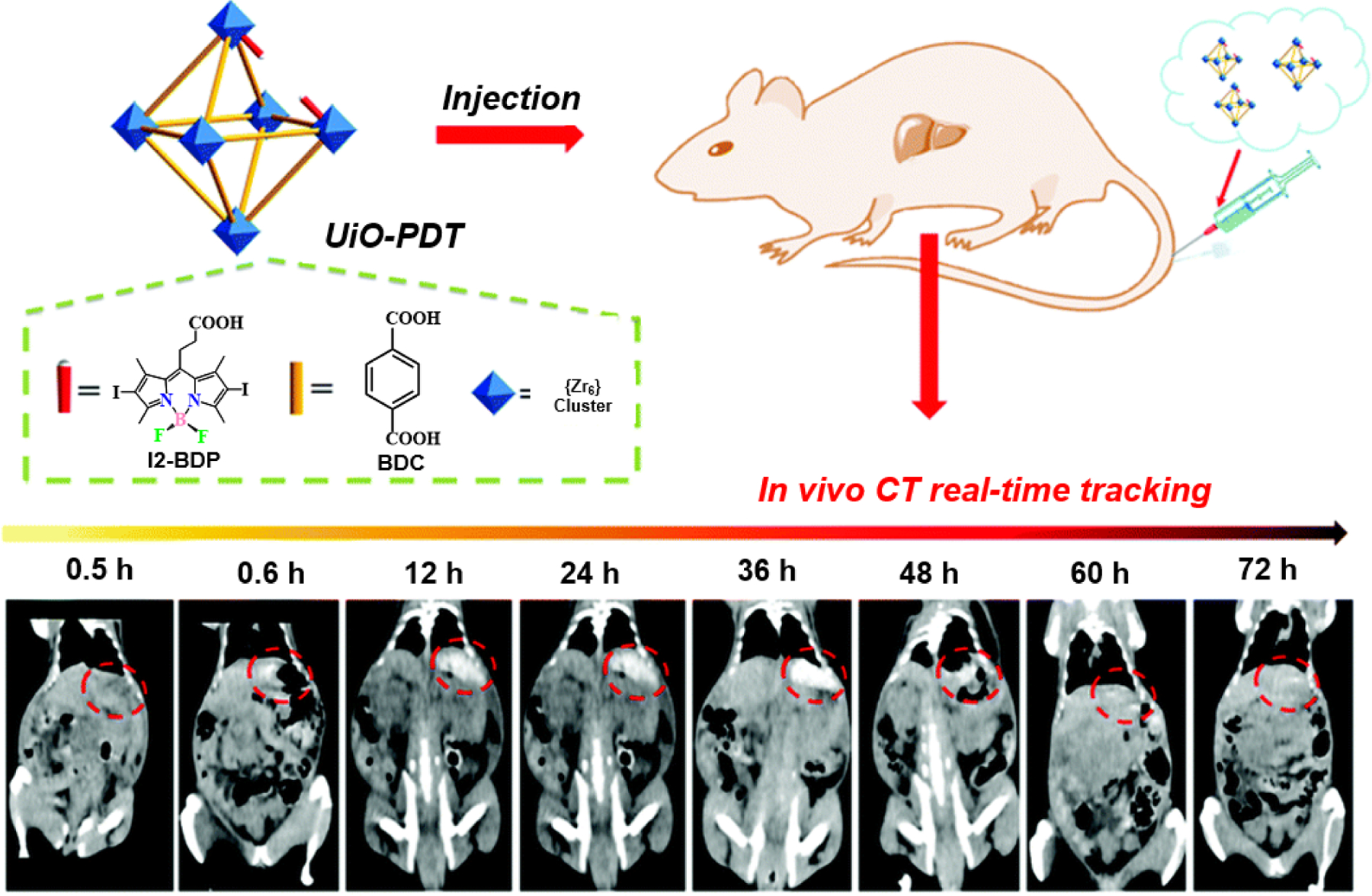
Schematic illustration of the synthesis of UiO-PDT nanocrystals and their application for in vivo X-ray CT imaging and biological studies. Reproduced with permission from ref. [121]. Copyright 2017 Royal Society of Chemistry.
Before conducting conclusive in vivo biological experiments with any nanomaterial, understanding its hemocompatibility is very critical as upon intravenous administration those molecules travel within the subject’s body through blood circulation. UiO-PDT neither caused the significant release of hemoglobin (an indicator of hemolysis) nor did it interfere with any plasma coagulation factors in rabbit blood, thus establishing its high hemocompatibility. The major advantages of using nanoparticles as CT contrast agents over other molecular CT contrast agents are their longer circulation time, higher stability, biocompatibility, lesser side effects, and good contrast effect at lower concentrations. The authors also tested its efficacy in real-time on hepatic tumor-bearing Sprague-Dawley rats, where results showed significant improvement in the reconstructed image resolution with better visibility of tumor growth.
4.3.3. Incorporation
BODIPY’s incorporation in MOFs can be achieved by the solvent-assisted ligand incorporation (SALI) process. In the SALI process, 8 terminal -OH groups in the octahedral Zr6 node of an eight connected Zr-based MOF are replaced by carboxylic acid-containing functional groups. Farah and coworkers introduced BODIPY derivatives containing Br atom (Br-BDP, L26) to the zirconium-based MOF, NU-1000, through the SALI process for the first time (Fig. 29). BODIPY incorporated NU-1000 (Br-BDP@NU-1000) was tested as a heterogeneous photocatalyst to produce singlet oxygen under green LED irradiation [123]. Photooxidation of toxic sulfur mustard simulant 2-chloroethyl ethyl sulfide (CEES) to a less toxic sulfoxide product 2-chloroethyl ethyl sulfoxide (CEESO) was carried out by the Br-BDP@NU-1000. Due to the superior singlet oxygen generation activity of the heavy atom-containing BODIPY photosensitizer, Br-BDP@NU-1000 exhibited fast conversion comparable to that of the homogeneous Br-BDP system. Moreover, Br-BDP@NU-1000 catalyst showed a faster photooxidation rate of 2 to 5 times compared to other MOF-based sensitizers previously studied. The catalyst retained its crystallinity well after the catalytic reaction and maintained its activity in four consecutive catalyst cycles.
Fig. 29.
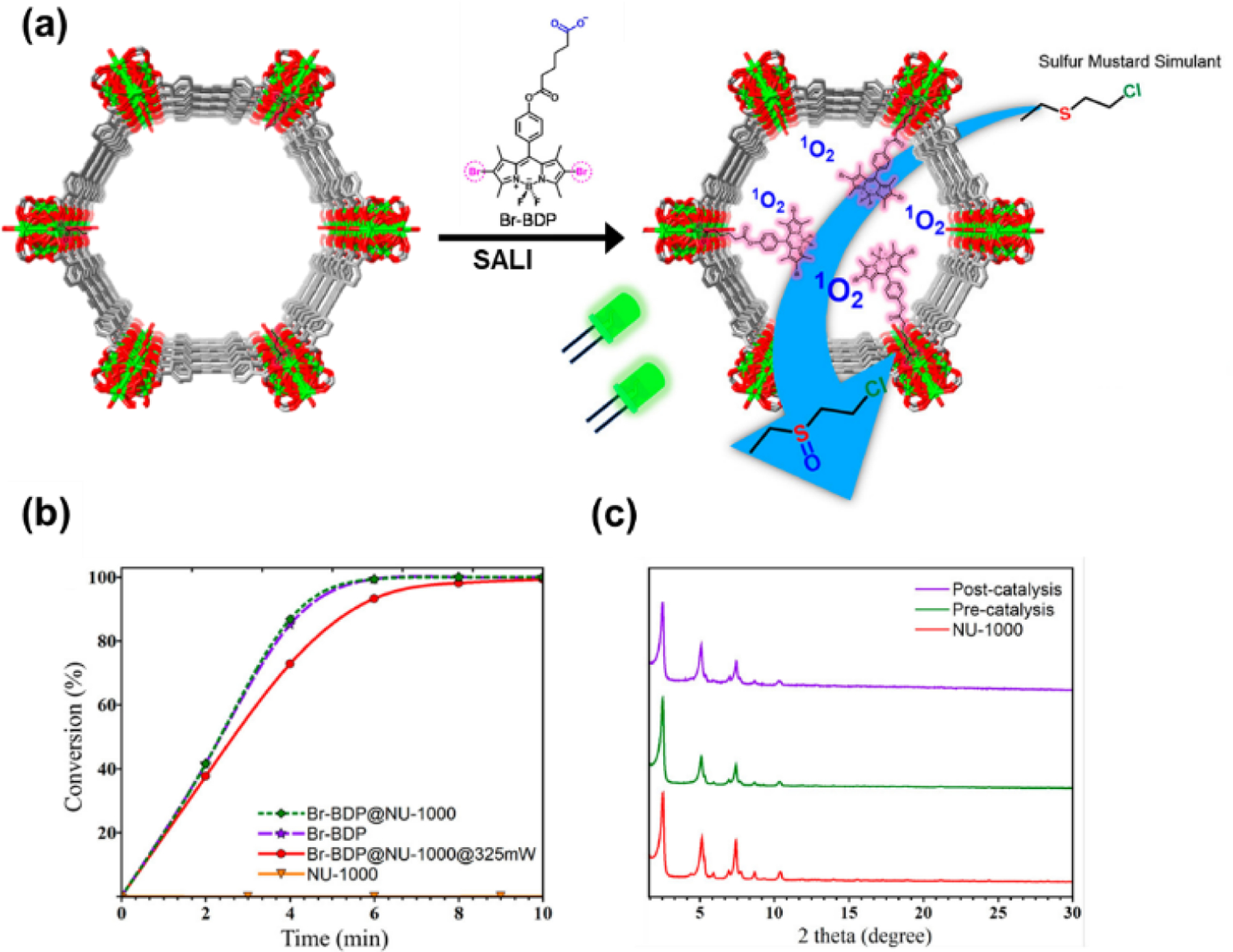
(a) Schematic representation of solvent-assisted ligand incorporation of Br-BDP in NU-1000 and schematic diagram showing the detoxification of sulfur mustard chemical warfare agent CEES to CEESO with BODIPY functionalized NU-1000. (b) Comparison of oxidation of CEES with Br-BDP in the homo/heterogeneous phase and NU-1000 under 450 mW cm−2 green light. Comparison of catalytic efficiency of Br-BDP@NU- 1000 under 325 mW and 450 mW light power. (c) powder X-ray diffraction patterns of NU-1000, Br-BDP@NU-1000, and Br-BDP@NU-1000 after catalysis. Reproduced with permission from ref. [123]. Copyright 2017 American Chemical Society.
BODIPY and iodine modified BODIPY, I2BODIPY were incorporated into nano-sized porphyrinic MOFs, nPCN-222 by Kim and coworkers via the SALI process [124]. The resonance energy transfer from the donor (BODIPY/I2BODIPY) to the acceptor (porphyrin in nPCN-222) within the MOF’s framework resulted in significantly enhancing the fluorescence of nPCN-222. The energy transfer phenomena were visually manifested by the time-resolved and space-resolved fluorescence imaging of the nano-scaled MOFs. Nanoscale grains of nPCN-222 displays no fluorescence below 600 nm due to the absence of BODIPY in confocal fluorescence maps and representative spectra (Figure 30 a and b). When BODIPY was incorporated into nPCN-222 to afford nPCN-BDP, MOFs grains showed less intense emission than the background emission in the BODIPY emission region (Figure 30 c and d). Considering that the emission from the background is derived from the uninserted free BODIPY residue, the observed quenching suggests that the excited energy of the BODIPY in the nPCN-BDP has been transferred to another moiety. On the other hand, the emission from porphyrin in the nPCN-BDP was enhanced compared to that of nPCN-222, indicating energy transfer from the excited BODIPY to porphyrin (Figure 30 h). The I2BODIPY incorporated nPCN-222 (nPCN-I2BDP) exhibited a somewhat weak enhancement in emission intensity of porphyrin compared to nPCN-BDP due to the presence of additional pathways for non-radiative decay of the I2BODIPY emission through inter-system crossing via the heavy-atom effect (Fig. 30 c, d and i).
Fig. 30.
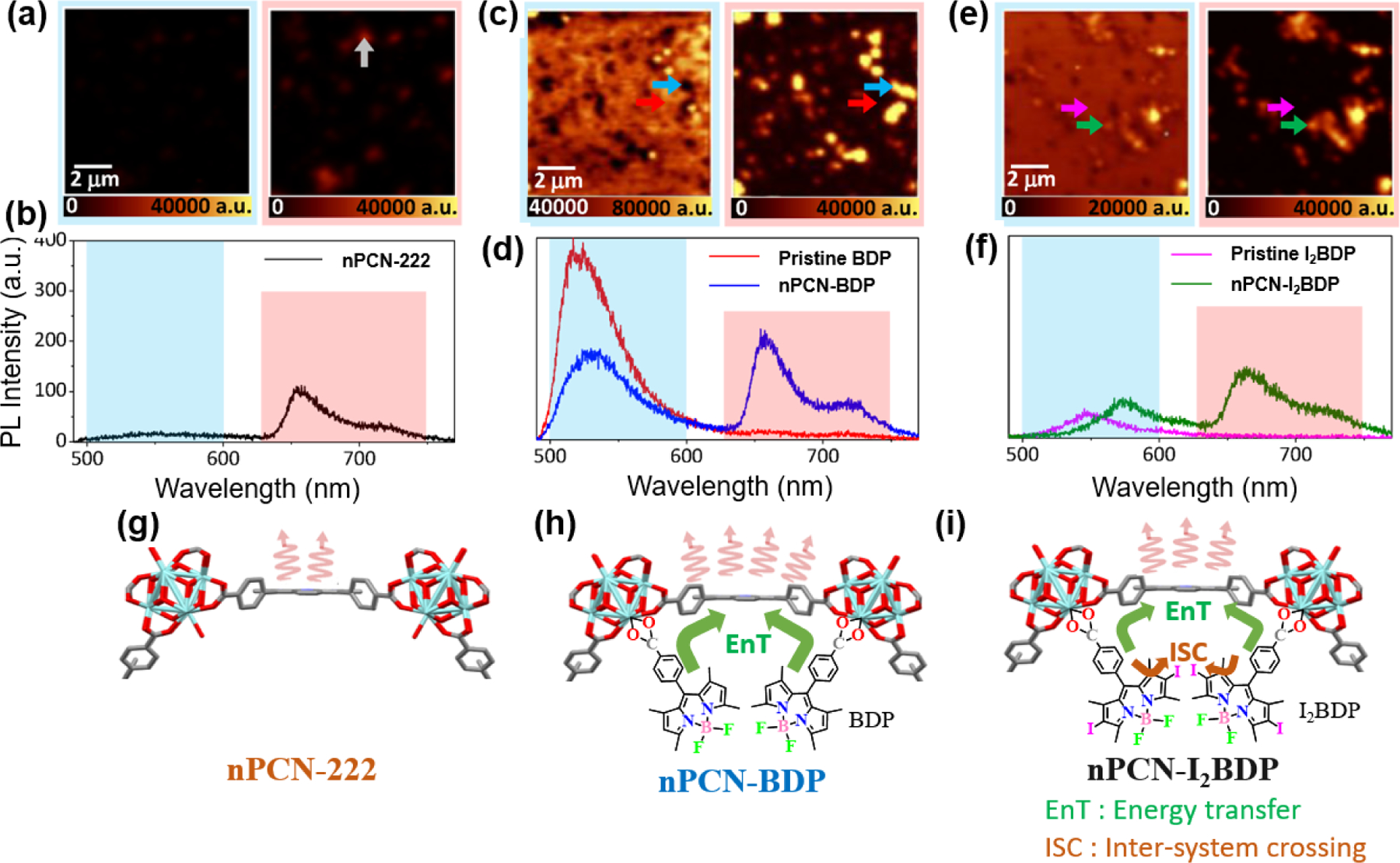
(a) Fluorescence maps and representative local fluorescence spectra of (a,b) nPCN-222, (c,d) nPCN-BDP, and (e,f) nPCN-I2BDP samples. The fluorescence maps were obtained by integrating the donor (BODIPY/I2BODIPY) emission range of 500–600 nm (blue box) and acceptor (nPCN-222) emission range of 630–750 nm (red box). In each map, local representative fluorescence spectra were obtained from the location marked by the arrows. (g-i) Expectation of transition pathways of the incorporated MOFs system. (g) Fluorescence emission of nPCN-222. (h) Enhanced fluorescence emission of nPCN-222 (acceptor) induced by resonance energy transfer from BODIPY (donor) in nPCN-BDP. (i) Energy transfer and inter-system crossing can occur in nPCN-I2BDP. Reproduced with permission from ref. [124]. Copyright 2020 MDPI.
Lee and coworkers tested nPCN-I2BDP as a photosensitizer for PDT (Fig. 31) [125]. nPCN-222 is known to be a highly stable NMOF having a large surface area with optimum sized pores for efficient oxygen diffusion [126]. nPCN-I2BDP was found to be more efficient than PCN-222 in generating singlet oxygen due to the heavy atom effect and improved light properties of I2BDP (L27) that is attached to the former. Both nPCN-I2BDP and nPCN-222 were tested against proliferating breast adenocarcinoma (MCF-7) and murine melanoma (B16F10) cells. While otherwise having very low dark toxicities, upon inclusion of a 4 mW/cm2 light irradiation step both molecules imparted exponential death rates on the cells. The effectiveness of PDT was increased by about 10,000 times in B16F10 cells, where the IC50 of nPCN-I2BDP was brought down from 79640 ± 2.88 nM to 9.197 ± 0.229 nM whereas in case MCF-7 cells it was about 2000 times. When compared with the toxicities induced by nPCN-222, the IC50 values of nPCN-I2BDP were found to be at least 10 times better than the former. The reason behind such augmented cytotoxicity of nPCN-I2BDP may be attributed to its ability to generate more singlet oxygen (quantum yield of 78%) than nPCN-222 (quantum yield of 49%). This activity was further justified through a DCF-DA assay, where nPCN-I2BDP treated cells showed higher green fluorescence than nPCN-222 within their intracellular organelles. This indicated that inclusion of BODIPY species exponentially improved the PDT efficiency of nPCN-222, in vitro. Confocal microscopy studies revealed that the mechanism of action of both these NMOFs was mainly cytoplasmic as none of them were able to break and enter through the nuclear barrier.
Fig. 31.
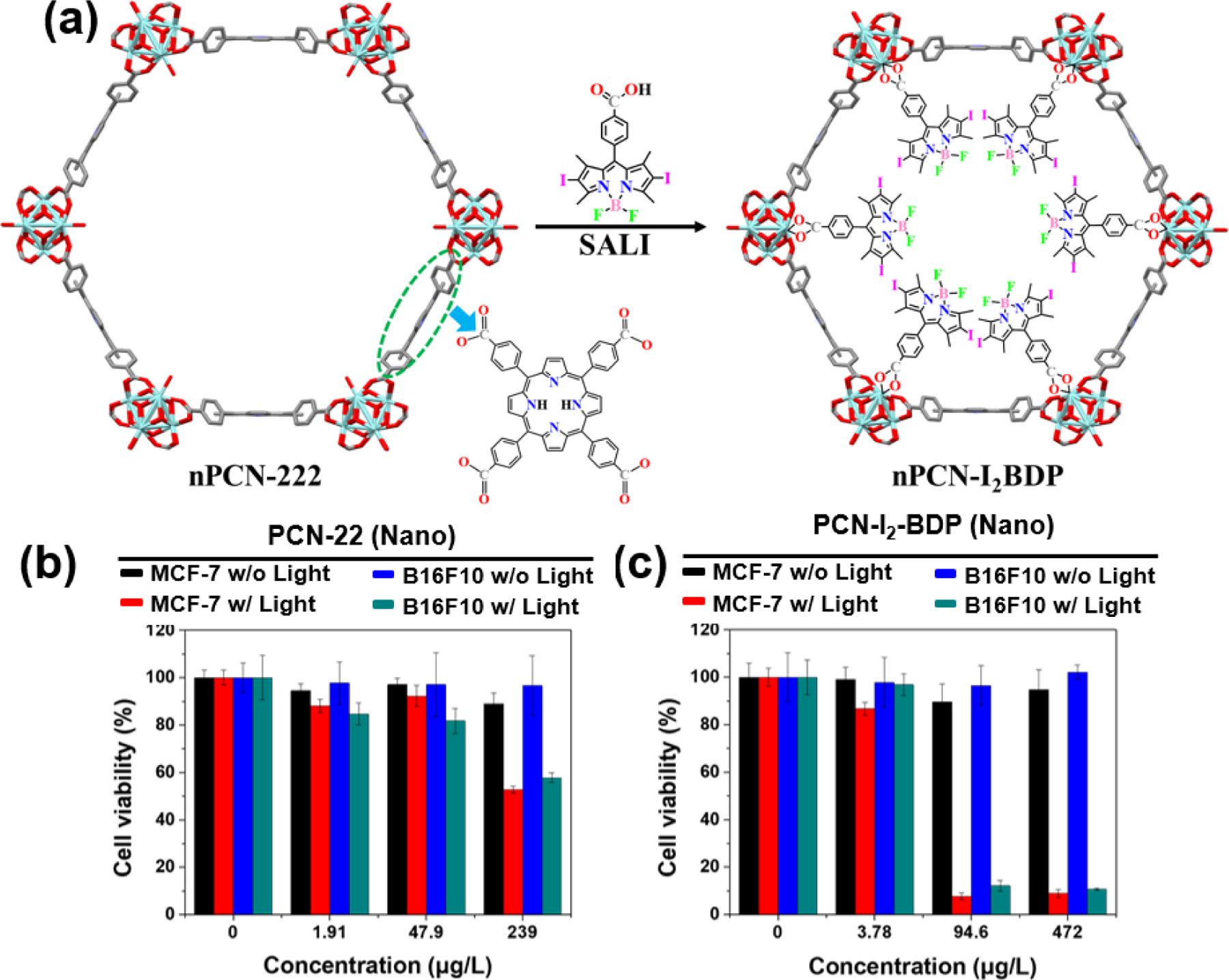
(a) Schematic representation of the formation of nPCN-I2BDP from PCN-222 (b- c) Cytotoxicities of nPCN-222 and nPCN–I2BDP in MCF-7 cells and B16F10 cells. Reproduced with permission from ref. [125]. Copyright 2019 Elsevier.
5. Summary and outlooks
In this review, we have brought together the recent developments of the highly fluorescent BODIPY based metal organic macrocycles and metal-organic frameworks designed by the self-assembly process. This review accentuates the evidence where the inclusion of BODIPY has made metal complexes not just more toxic against cancer cells but also has improved their selectivity in killing cancer cells per se. An ideal anti-cancer drug candidate is expected to induce apoptosis in the already aberrant cell cycle machinery of a malignant cell. BODIPY-based MOCs have also been found to induce programmed cell death in quite an efficient manner. The mechanism of action of such complexes has also been studied, an understanding of which would indeed help future researchers for tailoring novel complexes as better and safer drug candidates. We have discussed the importance of PDT and the role of BODIPY as a photosensitizer to improve its stance as an effective anticancer strategy. It was demonstrated that BODIPY based NMOFs are capable of instigating charge conversion within the cancer microenvironment under the characteristic acidic conditions. This in return caused selective assimilation of more and more PS agents near and within the conglomeration of malignant cells, thereby substantially augmenting the efficiency of PDT. For BODIPY incorporation researchers have either used well-known NMOFs with some known biological activities or have synthesized new ones. Owing to the innate fluorescence of these complexes, it becomes quite easy to locate the intracellular areas where the complexes can enter or deposit.
While considering the improvement in bio-efficiency, there are also some limiting factors of BODIPY-based complexes which need attention: (a) Their moderate to low solubility in the aqueous or biological medium is one of the main limiting factors which might hinder the detailed biological studies, especially the mechanistic experiments. (b) BODIPY based MOCs and MOFs are usually synthesized in milligram levels. The synthetic process of BODIPY ligands used to construct MOCs and MOFs is time-consuming and expensive. More convenient, easy, and economical procedures need to be developed for efficient large-scale development of such materials. (c) Using BODIPY-based MOCs and MOFs as a tracking tool to understand the intracellular mechanism and site(s) of action is a bonus; although this forte is laced with limitations because a lot of research still needs to be done to properly establish their real-time monitoring capabilities. (d) Toxicity of metal macrocycles depends on several important factors, such as metals, ligands, overall charge, solubility, particle morphology, etc. So based on limited studies, we cannot come to a conclusion about its toxicity and need detailed studies to make scientifically reliable conclusions. Understanding the mechanism of action of a drug candidate becomes more comprehensive if the malignant and non-malignant cells considered for the study share the same tissue origin. It is important to study the pattern of killing a particular cell type more than others by complexes with a particular metal or type of BODIPY linker. This cultivates a substantial understanding of possible modifications that could be made while designing future complexes which might effectively amplify the overall potency of the drug candidate.
As far as works on BODIPY macrocycles are considered, only bidentate bipyridyl functionalized BODIPY ligands were used. We believe tridentate and tetradentate pyridyl ligands can also be designed using the BODIPY core. So far, it is quite clear that research with BODIPY based metal complexes has many challenges, but these have not kept us from new opportunities. Exploiting those opportunities while handling the limitations and challenges will not just expand our understanding in photodynamics, novel ways of biomolecular interaction with fluorescent moieties, or in tailoring novel biocompatible and stable complexes, but also help us in the search for better and safer drugs against cancer.
Highlights.
The field of BODIPY-based inorganic materials is growing fast with diverse applications.
BODIPY-based Metal Organic Macrocycles and Metal Organic Frameworks are highlighted.
Their applications in cancer therapy is discussed in details.
Advantages, challenges and potential suggestions of BODIPY and BODIPY-based materials are also described.
Acknowledgements
CYL acknowledges funding from the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2021R1A2C1011731). PJS thanks the NIH (R01-CA215157) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedication. In memory of Prof. Nripendranath Mandal
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Treibs A, Kreuzer F-H, Difluorboryl-Komplexe von Di- und Tripyrrylmethenen, Justus Liebigs Ann. Chem 718 (1968) 208–223. 10.1002/jlac.19687180119. [DOI] [Google Scholar]
- [2].Boens N, Verbelen B, Dehaen W, Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy, European J. Org. Chem 2015 (2015) 6577–6595. 10.1002/ejoc.201500682. [DOI] [Google Scholar]
- [3].Turksoy A, Yildiz D, Akkaya EU, Photosensitization and controlled photosensitization with BODIPY dyes, Coord. Chem. Rev 379 (2019) 47–64. 10.1016/j.ccr.2017.09.029. [DOI] [Google Scholar]
- [4].Loudet A, Burgess K, BODIPY dyes and their derivatives: Syntheses and spectroscopic properties, Chem. Rev 107 (2007) 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- [5].Karolin J, Johansson LBA, Strandberg L, Ny T, Fluorescence and Absorption Spectroscopic Properties of Dipyrrometheneboron Difluoride (BODIPY) Derivatives in Liquids, Lipid Membranes, and Proteins, J. Am. Chem. Soc 116 (1994) 7801–7806. 10.1021/ja00096a042. [DOI] [Google Scholar]
- [6].Quan L, Liu S, Sun T, Guan X, Lin W, Xie Z, Huang Y, Wang Y, Jing X, Near-infrared emitting fluorescent BODIPY nanovesicles for in vivo molecular imaging and drug delivery, ACS Appl. Mater. Interfaces 6 (2014) 16166–16173. 10.1021/am5042115. [DOI] [PubMed] [Google Scholar]
- [7].Zhu S, Zhang J, Vegesna G, Luo FT, Green SA, Liu H, Highly water-soluble neutral BODIPY dyes with controllable fluorescence quantum yields, Org. Lett 13 (2011) 438–441. 10.1021/ol102758z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guan Q, Le Zhou L, Li YA, Bin Dong Y, Diiodo-Bodipy-Encapsulated Nanoscale Metal-Organic Framework for pH-Driven Selective and Mitochondria Targeted Photodynamic Therapy, Inorg. Chem 57 (2018) 10137–10145. 10.1021/acs.inorgchem.8b01316. [DOI] [PubMed] [Google Scholar]
- [9].Mauro M, Aliprandi A, Septiadi D, Kehr NS, De Cola L, When self-assembly meets biology: Luminescent platinum complexes for imaging applications, Chem. Soc. Rev 43 (2014) 4144–4166. 10.1039/c3cs60453e. [DOI] [PubMed] [Google Scholar]
- [10].Zhao Q, Huang H, Li F, Phosphorescent heavy-metal complexes for bioimaging, Chem. Soc. Rev 40 (2011) 2508–2524. 10.1039/c0cs00114g. [DOI] [PubMed] [Google Scholar]
- [11].Fernández-Moreira V, Thorp-Greenwood FL, Coogan MP, Application of d6 transition metal complexes in fluorescence cell imaging, Chem. Commun 46 (2010) 186–202. 10.1039/b917757d. [DOI] [PubMed] [Google Scholar]
- [12].Gupta G, Kumari P, Ryu JY, Lee J, Mobin SM, Lee CY, Mitochondrial localization of highly fluorescent and photostable BODIPY-based ruthenium(II), rhodium(III), and iridium(III) metal complexes, Inorg. Chem 58 (2019) 8587–8595. 10.1021/acs.inorgchem.9b00898. [DOI] [PubMed] [Google Scholar]
- [13].Gupta G, Das A, Ghate NB, Kim T, Ryu JY, Lee J, Mandal N, Lee CY, Novel BODIPY-based Ru(II) and Ir(III) metalla-rectangles: Cellular localization of compounds and their antiproliferative activities, Chem. Commun 52 (2016) 4274–4277. 10.1039/c6cc00046k. [DOI] [PubMed] [Google Scholar]
- [14].Gupta G, Das A, Panja S, Ryu JY, Lee J, Mandal N, Lee CY, Self-Assembly of Novel Thiophene-Based BODIPY RuII Rectangles: Potential Antiproliferative Agents Selective Against Cancer Cells, Chem. - A Eur. J 23 (2017) 17199–17203. 10.1002/chem.201704368. [DOI] [PubMed] [Google Scholar]
- [15].Yu G, Yu S, Saha ML, Zhou J, Cook TR, Yung BC, Chen J, Mao Z, Zhang F, Zhou Z, Liu Y, Shao L, Wang S, Gao C, Huang F, Stang PJ, Chen X, A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy, Nat. Commun 9 (2018). 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta G, Das A, Panja S, Ryu JY, Lee J, Mandal N, Lee CY, Selective cytotoxicity of self-assembled BODIPY metalla-rectangles: Evidence of p53-Dependent apoptosis via both intrinsic and extrinsic pathways, Dye. Pigment 180 (2020) 108478. 10.1016/j.dyepig.2020.108478. [DOI] [Google Scholar]
- [17].Qin Y, Liu X, Jia PP, Xu L, Yang HB, BODIPY-based macrocycles, Chem. Soc. Rev 49 (2020) 5678–5703. 10.1039/c9cs00797k. [DOI] [PubMed] [Google Scholar]
- [18].Cook TR, Zheng Y-R, Stang PJ, Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials, Chem. Rev 113 (2013) 734–777. 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li FZ, Yin JF, Kuang GC, BODIPY-based supramolecules: Construction, properties and functions, Coord. Chem. Rev 448 (2021) 214157. 10.1016/j.ccr.2021.214157. [DOI] [Google Scholar]
- [20].Singh PK, Majumdar P, Singh SP, Advances in BODIPY photocleavable protecting groups, Coord. Chem. Rev 449 (2021) 214193. 10.1016/j.ccr.2021.214193. [DOI] [Google Scholar]
- [21].Xia Q, Zhang J, Chen X, Cheng C, Chu D, Tang X, Li H, Cui Y, Synthesis, structure and property of boron-based metal–organic materials, Coord. Chem. Rev 435 (2021) 213783. 10.1016/j.ccr.2021.213783. [DOI] [Google Scholar]
- [22].Prakash MJ, Lah MS, Metal-organic macrocycles, metal-organic polyhedra and metal-organic frameworks, Chem. Commun (2009) 3326–3341. 10.1039/b902988e. [DOI] [PubMed]
- [23].Stricklen PM, Volcko EJ, Verkade JG, Novel Homo- and Heterometallic Coordination Macrocycles, J. Am. Chem. Soc 105 (1983) 2494–2495. 10.1021/ja00346a076. [DOI] [Google Scholar]
- [24].Fujita M, Yazaki J, Ogura K, Preparation of a Macrocyclic Polynuclear Complex, [(en)Pd(4,4′-bpy)]4(NO3)8, 1 Which Recognizes an Organic Molecule in Aqueous Media, J. Am. Chem. Soc 112 (1990) 5645–5647. 10.1021/ja00170a042. [DOI] [Google Scholar]
- [25].Stang PJ, Cao DH, Transition Metal Based Cationic Molecular Boxes. Self-Assembly of Macrocyclic Platinum(II) and Palladium(II) Tetranuclear Complexes, J. Am. Chem. Soc 116 (1994) 4981–4982. 10.1021/ja00090a051. [DOI] [Google Scholar]
- [26].Saha ML, Yan X, Stang PJ, Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and its Applications, Acc. Chem. Res 49 (2016) 2527–2539. 10.1021/acs.accounts.6b00416. [DOI] [PubMed] [Google Scholar]
- [27].Brown CJ, Toste FD, Bergman RG, Raymond KN, Supramolecular Catalysis in Metal-Ligand Cluster Hosts, Chem. Rev 115 (2015) 3012–3035. 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]
- [28].Sun Y, Chen C, Wang X, Zhang F, Lu S, Li X, Suo X, Lin Z, Self-Assembly of Metallacages into Centimeter Films with Tunable Size and Emissions, J. Am. Chem. Soc 142 (2020) 17933–17937. 10.1021/jacs.0c09781. [DOI] [PubMed] [Google Scholar]
- [29].Hong T, Zhang Z, Sun Y, Tao JJ, Tang JD, Xie C, Wang M, Chen F, Xie SS, Li S, Stang PJ, Chiral Metallacycles as Catalysts for Asymmetric Conjugate Addition of Styrylboronic Acids to α,β-Enones, J. Am. Chem. Soc 142 (2020) 10244–10249. 10.1021/jacs.0c01563. [DOI] [PubMed] [Google Scholar]
- [30].McConnell AJ, Wood CS, Neelakandan PP, Nitschke JR, Stimuli-Responsive Metal-Ligand Assemblies, Chem. Rev 115 (2015) 7729–7793. 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]
- [31].Sepehrpour H, Fu W, Sun Y, Stang PJ, Biomedically Relevant Self-Assembled Metallacycles and Metallacages, J. Am. Chem. Soc 141 (2019) 14005–14020. 10.1021/jacs.9b06222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun Y, Chen C, Liu J, Stang PJ, Recent developments in the construction and applications of platinum-based metallacycles and metallacages: Via coordination, Chem. Soc. Rev 49 (2020) 3889–3919. 10.1039/d0cs00038h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang M, Yin S, Zhang J, Zhou Z, Saha ML, Lu C, Stanga PJ, Metallacycle-cored supramolecular assemblies with tunable fluorescence including white-light emission, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 3044–3049. 10.1073/pnas.1702510114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zheng YR, Lan WJ, Wang M, Cook TR, Stang PJ, Designed post-self-assembly structural and functional modifications of a truncated tetrahedron, J. Am. Chem. Soc 133 (2011) 17045–17055. 10.1021/ja207217t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ghosh K, Yang HB, Northrop BH, Lyndon MM, Zheng YR, Muddiman DC, Stang PJ, Coordination-driven self-assembly of cavity-cored multiple crown ether derivatives and poly[2]pseudorotaxanes, J. Am. Chem. Soc 130 (2008) 5320–5334. 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]
- [36].Sun Y, Stang PJ, Metallacycles, metallacages, and their aggregate/optical behavior, Aggregate (2021) 1–21. 10.1002/agt2.94. [DOI]
- [37].Sun Y, Chen C, Liu J, Liu L, Tuo W, Zhu H, Lu S, Li X, Stang PJ, Self-Assembly of Porphyrin-Based Metallacages into Octahedra, J. Am. Chem. Soc 142 (2020) 17903–17907. 10.1021/jacs.0c08058. [DOI] [PubMed] [Google Scholar]
- [38].Matsuoka R, Nabeshima T, Functional supramolecular architectures of dipyrrin complexes, Front. Chem 6 (2018) 1–13. 10.3389/fchem.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cook TR, Vajpayee V, Lee MH, Stang PJ, Chi KW, Biomedical and biochemical applications of self-assembled metallacycles and metallacages, Acc. Chem. Res 46 (2013) 2464–2474. 10.1021/ar400010v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kaloudi-Chantzea A, Karakostas N, Raptopoulou CP, Psycharis V, Saridakis E, Griebel J, Hermann R, Pistolis G, Coordination-driven self assembly of a brilliantly fluorescent rhomboid cavitand composed of bodipy-dye subunits, J. Am. Chem. Soc 132 (2010) 16327–16329. 10.1021/ja1064679. [DOI] [PubMed] [Google Scholar]
- [41].Kaloudi-Chantzea A, Karakostas N, Pitterl F, Raptopoulou CP, Glezos N, Pistolis G, Efficient supramolecular synthesis of a robust circular light-harvesting Bodipy-dye based array, Chem. Commun 48 (2012) 12213–12215. 10.1039/c2cc36825k. [DOI] [PubMed] [Google Scholar]
- [42].Karakostas N, Mavridis IM, Seintis K, Fakis M, Koini EN, Petsalakis ID, Pistolis G, Highly efficient and unidirectional energy transfer within a tightly self-assembled host-guest multichromophoric array, Chem. Commun 50 (2014) 1362–1365. 10.1039/c3cc48076c. [DOI] [PubMed] [Google Scholar]
- [43].Kaloudi-Chantzea A, Martinou E, Seintis K, Karakostas N, Giastas P, Pitterl F, Oberacher H, Fakis M, Pistolis G, Formation of a highly-ordered rigid multichromophoric 3D supramolecular network by combining ionic and coordination-driven self-assembly, Chem. Commun 52 (2016) 3388–3391. 10.1039/c5cc10335e. [DOI] [PubMed] [Google Scholar]
- [44].Komor AC, Barton JK, The path for metal complexes to a DNA target, Chem. Commun 49 (2013) 3617–3630. 10.1039/c3cc00177f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang CX, Lippard SJ, New metal complexes as potential therapeutics, Curr. Opin. Chem. Biol 7 (2003) 481–489. 10.1016/S1367-5931(03)00081-4. [DOI] [PubMed] [Google Scholar]
- [46].Spiegel J, Adhikari S, Balasubramanian S, The Structure and Function of DNA G-Quadruplexes, Trends Chem 2 (2020) 123–136. 10.1016/j.trechm.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, Coviello GM, Wright WE, Weinrich SL, Shay JW, Specific association of human telomerase activity with immortal cells and cancer, Science (80-. ) 266 (1994) 2011–2015. 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- [48].Kieltyka R, Englebienne P, Fakhoury J, Autexier C, Moitessier N, Sleiman HF, A platinum supramolecular square as an effective G-quadruplex binder and telomerase inhibitor, J. Am. Chem. Soc 130 (2008) 10040–10041. 10.1021/ja8014023. [DOI] [PubMed] [Google Scholar]
- [49].Gupta G, Das A, Park KC, Tron A, Kim H, Mun J, Mandal N, Chi KW, Lee CY, Self-Assembled Novel BODIPY-Based Palladium Supramolecules and Their Cellular Localization, Inorg. Chem 56 (2017) 4615–4621. 10.1021/acs.inorgchem.7b00260. [DOI] [PubMed] [Google Scholar]
- [50].Zhou J, Zhang Y, Yu G, Crawley MR, Fulong CRP, Friedman AE, Sengupta S, Sun J, Li Q, Huang F, Cook TR, Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles, J. Am. Chem. Soc 140 (2018) 7730–7736. 10.1021/jacs.8b04929. [DOI] [PubMed] [Google Scholar]
- [51].Zhou Z, Liu J, Rees TW, Wang H, Li X, Chao H, Stang PJ, Heterometallic Ru–Pt metallacycle for two-photon photodynamic therapy, Proc. Natl. Acad. Sci. U. S. A 115 (2018) 5664–5669. 10.1073/pnas.1802012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lu H, MacK J, Yang Y, Shen Z, Structural modification strategies for the rational design of red/NIR region BODIPYs, Chem. Soc. Rev 43 (2014) 4778–4823. 10.1039/c4cs00030g. [DOI] [PubMed] [Google Scholar]
- [53].Knox HJ, Hedhli J, Kim TW, Khalili K, Dobrucki LW, Chan J, A bioreducible N-oxide-based probe for photoacoustic imaging of hypoxia, Nat. Commun 8 (2017). 10.1038/s41467-017-01951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang L, Li Z, Zhao Y, Zhang Y, Wu S, Zhao J, Han G, Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy, J. Am. Chem. Soc 138 (2016) 14586–14591. 10.1021/jacs.6b05390. [DOI] [PubMed] [Google Scholar]
- [55].Li G, Zhang X, Zhao W, Zhao W, Li F, Xiao K, Yu Q, Liu S, Zhao Q, Stable and Well-Organized Near-Infrared Platinum(II)-Acetylide-Based Metallacycles-Mediated Cancer Phototherapy, ACS Appl. Mater. Interfaces 12 (2020) 20180–20190. 10.1021/acsami.0c01695. [DOI] [PubMed] [Google Scholar]
- [56].Singh N, Singh J, Kim D, Kim DH, Kim EH, Lah MS, Chi KW, Coordination-Driven Self-Assembly of Heterotrimetallic Barrel and Bimetallic Cages Using a Cobalt Sandwich-Based Tetratopic Donor, Inorg. Chem 57 (2018) 3521–3528. 10.1021/acs.inorgchem.7b02653. [DOI] [PubMed] [Google Scholar]
- [57].Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS, Self-assembly of a nanoscopic Pt12Fe12 heterometallic open molecular box containing six porphyrin walls, Angew. Chemie - Int. Ed 47 (2008) 8455–8459. 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]
- [58].Xu L, Wang YX, Chen LJ, Yang HB, Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions, Chem. Soc. Rev 44 (2015) 2148–2167. 10.1039/c5cs00022j. [DOI] [PubMed] [Google Scholar]
- [59].Mishra A, Lee SC, Kaushik N, Cook TR, Choi EH, Kaushik NK, Stang PJ, Chi KW, Self-assembled supramolecular hetero-bimetallacycles for anticancer potency by intracellular release, Chem. - A Eur. J 20 (2014) 14410–14420. 10.1002/chem.201403372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gupta G, You Y, Hadiputra R, Jung J, Kang DK, Lee CY, Heterometallic bodipy-based molecular squares obtained by self-assembly: synthesis and biological activities, ACS Omega 4 (2019) 13200–13208. 10.1021/acsomega.9b01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yan H, Süss-fink G, Neels A, Stoeckli-evans H, Mono-, di- and tetra-nuclear, 6 (2000) 4345–4350. [Google Scholar]
- [62].Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ, The “complex-in-a-complex” cations [(acac)2M⊂ Ru6-(p-iPrC6H4Me)6(tpt) 2(dhbq)3]6+: A trojan horse for cancer cells, Angew. Chemie - Int. Ed 47 (2008) 3773–3776. 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]
- [63].Therrien B, Biologically relevant arene ruthenium metalla-assemblies, CrystEngComm 17 (2015) 484–491. 10.1039/c4ce02146k. [DOI] [Google Scholar]
- [64].Therrien B, Transporting and shielding photosensitisers by using water-soluble organometallic cages: A new strategy in drug delivery and photodynamic therapy, Chem. - A Eur. J 19 (2013) 8378–8386. 10.1002/chem.201301348. [DOI] [PubMed] [Google Scholar]
- [65].Han YF, Jin GX, Half-sandwich iridium- and rhodium-based organometallic architectures: Rational design, synthesis, characterization, and applications, Acc. Chem. Res 47 (2014) 3571–3579. 10.1021/ar500335a. [DOI] [PubMed] [Google Scholar]
- [66].Lu Y, Zhang HN, Jin GX, Molecular Borromean Rings Based on Half-Sandwich Organometallic Rectangles, Acc. Chem. Res 51 (2018) 2148–2158. 10.1021/acs.accounts.8b00220. [DOI] [PubMed] [Google Scholar]
- [67].Kim T, Singh N, Oh J, Kim EH, Jung J, Kim H, Chi KW, Selective Synthesis of Molecular Borromean Rings: Engineering of Supramolecular Topology via Coordination-Driven Self-Assembly, J. Am. Chem. Soc 138 (2016) 8368–8371. 10.1021/jacs.6b04545. [DOI] [PubMed] [Google Scholar]
- [68].Boens N, Leen V, Dehaen W, Fluorescent indicats based on BODIPY, Chem. Soc. Rev 41 (2012) 1130–1172. 10.1039/c1cs15132k. [DOI] [PubMed] [Google Scholar]
- [69].Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K, BODIPY dyes in photodynamic therapy, Chem. Soc. Rev 42 (2013) 77–88. 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O’Donnell JP, Biotransformation reactions of five-membered aromatic heterocyclic rings, Chem. Res. Toxicol 15 (2002) 269–299. 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]
- [71].Le Dang N, Hughes TB, Miller GP, Swamidass SJ, Computational Approach to Structural Alerts: Furans, Phenols, Nitroaromatics, and Thiophenes, Chem. Res. Toxicol 30 (2017) 1046–1059. 10.1021/acs.chemrestox.6b00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Petti F, Thelemann A, Kahler J, McCormack S, Castaldo L, Hunt T, Nuwaysir L, Zeike L, Haack H, Sullivan L, Garton A, Haley JD, Temporal quantitation of mutant Kit tyrosine kinase signaling attenuated by a novel thiophene kinase inhibitor OSI-930, Mol. Cancer Ther 4 (2005) 1186–1197. 10.1158/1535-7163.MCT-05-0114. [DOI] [PubMed] [Google Scholar]
- [73].Gupta G, Kumar JM, Garci A, Nagesh N, Therrien B, Exploiting natural products to build metalla-assemblies: The anticancer activity of embelin-derived Rh(III) and Ir(III) metalla-rectangles, Molecules 19 (2014) 6031–6046. 10.3390/molecules19056031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gupta G, Oggu GS, Nagesh N, Bokara KK, Therrien B, Anticancer activity of large metalla-assemblies built from half-sandwich complexes, CrystEngComm 18 (2016) 4952–4957. 10.1039/c6ce00139d. [DOI] [Google Scholar]
- [75].Gupta G, Denoyelle-Di-Muro E, Mbakidi JP, Leroy-Lhez S, Sol V, Therrien B, Delivery of porphin to cancer cells by organometallic Rh(III) and Ir(III) metalla-cages, J. Organomet. Chem 787 (2015) 44–50. 10.1016/j.jorganchem.2015.03.035. [DOI] [Google Scholar]
- [76].Zhang P, Sadler PJ, Advances in the design of organometallic anticancer complexes, J. Organomet. Chem 839 (2017) 5–14. 10.1016/j.jorganchem.2017.03.038. [DOI] [Google Scholar]
- [77].Liu Z, Habtemariam A, Pizarro AM, Fletcher SA, Kisova A, Vrana O, Salassa L, Bruijnincx PCA, Clarkson GJ, Brabec V, Sadler PJ, Organometallic half-sandwich iridium anticancer complexes, J. Med. Chem 54 (2011) 3011–3026. 10.1021/jm2000932. [DOI] [PubMed] [Google Scholar]
- [78].Gupta G, Das A, Lee J, Mandal N, Lee CY, Self-Assembled BODIPY-Based Iridium Metallarectangles: Cytotoxicity and Propensity to Bind Biomolecules, Chempluschem 83 (2018) 339–347. 10.1002/cplu.201800035. [DOI] [PubMed] [Google Scholar]
- [79].Gupta G, Das A, Lee SW, Ryu JY, Lee J, Nagesh N, Mandal N, Lee CY, BODIPY-based Ir(III) rectangles containing bis-benzimidazole ligands with highly selective toxicity obtained through self-assembly, J. Organomet. Chem 868 (2018) 86–94. 10.1016/j.jorganchem.2018.04.034. [DOI] [Google Scholar]
- [80].Özil M, Emirik M, Beldüz A, Ülker S, Molecular docking studies and synthesis of novel bisbenzimidazole derivatives as inhibitors of α-glucosidase, Bioorganic Med. Chem 24 (2016) 5103–5114. 10.1016/j.bmc.2016.08.024. [DOI] [PubMed] [Google Scholar]
- [81].Elumalai P, Jeong YJ, Park DW, Kim DH, Kim H, Kang SC, Chi KW, Antitumor and biological investigation of doubly cyclometalated ruthenium(ii) organometallics derived from benzimidazolyl derivatives, Dalt. Trans 45 (2016) 6667–6673. 10.1039/c5dt04400f. [DOI] [PubMed] [Google Scholar]
- [82].Vajpayee V, Lee SM, Park JW, Dubey A, Kim H, Cook TR, Stang PJ, Chi KW, Growth inhibitory activity of a bis-benzimidazole-bridged arene ruthenium metalla-rectangle and -prism, Organometallics 32 (2013) 1563–1566. 10.1021/om301174s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev 115 (2015) 11718–11940. 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- [84].Zou H, Li Y, Liu X, Wang X, An APAf-1 · cytochrome C multimeric complex is a functional apoptosome that activates procaspase-9, J. Biol. Chem 274 (1999) 11549–11556. 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- [85].Li H, Zhu H, Xu CJ, Yuan J, Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis, Cell 94 (1998) 491–501. 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- [86].Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB, Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl, Carcinogenesis 23 (2002) 143–150. 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- [87].Howarth AJ, Liu Y, Li P, Li Z, Wang TC, Hupp JT, Farha OK, Chemical, thermal and mechanical stabilities of metal-organic frameworks, Nat. Rev. Mater 1 (2016) 1–15. 10.1038/natrevmats.2015.18. [DOI] [Google Scholar]
- [88].Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM, The chemistry and applications of metal-organic frameworks, Science (80-. ) 341 (2013). 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- [89].Lu W, Wei Z, Gu ZY, Liu TF, Park J, Park J, Tian J, Zhang M, Zhang Q, Gentle T, Bosch M, Zhou HC, Tuning the structure and function of metal-organic frameworks via linker design, Chem. Soc. Rev 43 (2014) 5561–5593. 10.1039/c4cs00003j. [DOI] [PubMed] [Google Scholar]
- [90].Li M, Li D, O’Keeffe M, Yaghi OM, Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle, Chem. Rev 114 (2014) 1343–1370. 10.1021/cr400392k. [DOI] [PubMed] [Google Scholar]
- [91].Lin ZJ, Lü J, Hong M, Cao R, Metal-organic frameworks based on flexible ligands (FL-MOFs): Structures and applications, Chem. Soc. Rev 43 (2014) 5867–5895. 10.1039/c3cs60483g. [DOI] [PubMed] [Google Scholar]
- [92].Silva P, Vilela SMF, Tomé JPC, Almeida Paz FA, Multifunctional metal-organic frameworks: From academia to industrial applications, Chem. Soc. Rev 44 (2015) 6774–6803. 10.1039/c5cs00307e. [DOI] [PubMed] [Google Scholar]
- [93].F. Containing, L. Rectangular, Yaghi_1995_Jacs, (2001) 1–2. papers2://publication/uuid/314D644C-7F5A-44FD-BE7E-2C092BF548BA.
- [94].Yaghi OM, Li G, Li Hailian, Yaghi-Selective binding and removal of guests in a imcroporous metal-organic framework-Nature 1985, Nature 378 (1995) 703–706. [Google Scholar]
- [95].Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J, Reticular synthesis and the design of new materials, Nature 423 (2003) 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- [96].Burtch NC, Jasuja H, Walton KS, Water stability and adsorption in metal-organic frameworks, Chem. Rev 114 (2014) 10575–10612. 10.1021/cr5002589. [DOI] [PubMed] [Google Scholar]
- [97].Zhang X, Chen Z, Liu X, Hanna SL, Wang X, Taheri-Ledari R, Maleki A, Li P, Farha OK, A historical overview of the activation and porosity of metal-organic frameworks, Chem. Soc. Rev 49 (2020) 7406–7427. 10.1039/d0cs00997k. [DOI] [PubMed] [Google Scholar]
- [98].Barea E, Montoro C, Navarro JAR, Toxic gas removal-metal-organic frameworks for the capture and degradation of toxic gases and vapours, Chem. Soc. Rev 43 (2014) 5419–5430. 10.1039/c3cs60475f. [DOI] [PubMed] [Google Scholar]
- [99].Lustig WP, Mukherjee S, Rudd ND, Desai AV, Li J, Ghosh SK, Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications, Chem. Soc. Rev 46 (2017) 3242–3285. 10.1039/c6cs00930a. [DOI] [PubMed] [Google Scholar]
- [100].Mahmood A, Guo W, Tabassum H, Zou R, Metal-Organic Framework-Based Nanomaterials for Electrocatalysis, Adv. Energy Mater 6 (2016) 1–26. 10.1002/aenm.201600423. [DOI] [Google Scholar]
- [101].So MC, Wiederrecht GP, Mondloch JE, Hupp JT, Farha OK, Metal-organic framework materials for light-harvesting and energy transfer, Chem. Commun 51 (2015) 3501–3510. 10.1039/c4cc09596k. [DOI] [PubMed] [Google Scholar]
- [102].Mínguez Espallargas G, Coronado E, Magnetic functionalities in MOFs: From the framework to the pore, Chem. Soc. Rev 47 (2018) 533–557. 10.1039/c7cs00653e. [DOI] [PubMed] [Google Scholar]
- [103].Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Férey G, Morris RE, Serre C, Metal-organic frameworks in biomedicine, Chem. Rev 112 (2012) 1232–1268. 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- [104].McKinlay AC, Morris RE, Horcajada P, Férey G, Gref R, Couvreur P, Serre C, BioMOFs: Metal-organic frameworks for biological and medical applications, Angew. Chemie - Int. Ed 49 (2010) 6260–6266. 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]
- [105].Lee CY, Farha OK, Hong BJ, Sarjeant AA, Nguyen ST, Hupp JT, Light-harvesting metal-organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs, J. Am. Chem. Soc 133 (2011) 15858–15861. 10.1021/ja206029a. [DOI] [PubMed] [Google Scholar]
- [106].Zhou L, Xue YS, Xu Y, Zhang J, Bin Du H, Two photoluminescent metal-organic frameworks based on a BODIPY-derived bipyridine ligand, CrystEngComm 15 (2013) 7315–7320. 10.1039/c3ce41002a. [DOI] [Google Scholar]
- [107].Zhang F, Baudron SA, Hosseini MW, Solvent and anion effects on the organization of a luminescent [2 + 2] BODIPY/Ag(i) metallamacrocycle in the crystalline state, CrystEngComm 19 (2017) 4393–4400. 10.1039/c7ce00997f. [DOI] [Google Scholar]
- [108].Mazel A, Baudron SA, Hosseini MW, AzaBODIPY based coordination polymers, CrystEngComm 19 (2017) 897–900. 10.1039/c6ce02582j. [DOI] [Google Scholar]
- [109].Hassanain H, Davies ES, Lewis W, Kays DL, Champness NR, Structural characterization and optical properties of two copper(i)-iodide BODIPY coordination polymers, CrystEngComm 21 (2019) 4551–4556. 10.1039/c9ce00845d. [DOI] [Google Scholar]
- [110].Baudron SA, Luminescent metal–organic frameworks based on dipyrromethene metal complexes and BODIPYs, CrystEngComm 18 (2016) 4671–4680. 10.1039/C6CE00450D. [DOI] [Google Scholar]
- [111].Li M, Yao Y, Ding J, Liu L, Qin J, Zhao Y, Hou H, Fan Y, Spectroscopic and crystallographic investigations of novel Bodipy-derived metal-organic frameworks, Inorg. Chem 54 (2015) 1346–1353. 10.1021/ic502219y. [DOI] [PubMed] [Google Scholar]
- [112].Yang H, Wang J, Ma J, Yang H, Zhang J, Lv K, Wen L, Peng T, A novel BODIPY-based MOF photocatalyst for efficient visible-light-driven hydrogen evolution, J. Mater. Chem. A 7 (2019) 10439–10445. 10.1039/c9ta02357g. [DOI] [Google Scholar]
- [113].Meng Y, Du Y, Lin Y, Su Y, Li R, Feng Y, Meng S, A two-fold interpenetration pillar-layered metal-organic frameworks based on BODIPY for chemo-photodynamic therapy, Dye. Pigment 188 (2021). 10.1016/j.dyepig.2021.109174. [DOI] [Google Scholar]
- [114].Glembockyte V, Frenette M, Mottillo C, Durantini AM, Gostick J, Štrukil V, Friščić T, Cosa G, Highly Photostable and Fluorescent Microporous Solids Prepared via Solid-State Entrapment of Boron Dipyrromethene Dyes in a Nascent Metal-Organic Framework, J. Am. Chem. Soc 140 (2018) 16882–16887. 10.1021/jacs.8b09608. [DOI] [PubMed] [Google Scholar]
- [115].Gee WJ, Shepherd HJ, Dawson DM, Ashbrook SE, Raithby PR, Burrows AD, Solid-state host-guest influences on a BODIPY dye hosted within a crystalline sponge, New J. Chem 44 (2020) 14108–14115. 10.1039/d0nj02969f. [DOI] [Google Scholar]
- [116].Wang R, Bukowski BC, Duan J, Sheridan TR, Atilgan A, Zhang K, Snurr RQ, Hupp JT, Investigating the Process and Mechanism of Molecular Transport within a Representative Solvent-Filled Metal-Organic Framework, Langmuir 36 (2020) 10853–10859. 10.1021/acs.langmuir.0c01999. [DOI] [PubMed] [Google Scholar]
- [117].Lu JY, Zhang PL, Chen QY, A Nano-BODIPY Encapsulated Zeolitic Imidazolate Framework As Photoresponsive Integrating Antibacterial Agent, ACS Appl. Bio Mater 3 (2020) 458–465. 10.1021/acsabm.9b00905. [DOI] [PubMed] [Google Scholar]
- [118].Taylor-Pashow KML, Della Rocca J, Xie Z, Tran S, Lin W, Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery, J. Am. Chem. Soc 131 (2009) 14261–14263. 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Quan Y, Li QY, Zhang Q, Zhang WQ, Lu H, Yu JH, Chen J, Zhao X, Wang XJ, A diiodo-BODIPY postmodified metal-organic framework for efficient heterogeneous organo-photocatalysis, RSC Adv 6 (2016) 23995–23999. 10.1039/c6ra03516g. [DOI] [Google Scholar]
- [120].Wang W, Wang L, Li Z, Xie Z, BODIPY-containing nanoscale metal-organic frameworks for photodynamic therapy, Chem. Commun 52 (2016) 5402–5405. 10.1039/c6cc01048b. [DOI] [PubMed] [Google Scholar]
- [121].Zhang T, Wang L, Ma C, Wang W, Ding J, Liu S, Zhang X, Xie Z, BODIPY-containing nanoscale metal-organic frameworks as contrast agents for computed tomography, J. Mater. Chem. B 5 (2017) 2330–2336. 10.1039/c7tb00392g. [DOI] [PubMed] [Google Scholar]
- [122].Lusic H, Grinstaff MW, X-ray-computed tomography contrast agents, Chem. Rev 113 (2013) 1641–1666. 10.1021/cr200358s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Atilgan A, Islamoglu T, Howarth AJ, Hupp JT, Farha OK, Detoxification of a Sulfur Mustard Simulant Using a BODIPY-Functionalized Zirconium-Based Metal-Organic Framework, ACS Appl. Mater. Interfaces 9 (2017) 24555–24560. 10.1021/acsami.7b05494. [DOI] [PubMed] [Google Scholar]
- [124].Seo C, Kim M, Lee J, Lee CY, Kim J, Spectroscopic evidence of energy transfer in bodipy-incorporated nano-porphyrinic metal-organic frameworks, Nanomaterials 10 (2020) 1–13. 10.3390/nano10101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Oh JS, You Y, Park KC, Gupta G, Kang DK, Lee CY, Toward an efficient photosensitizer for photodynamic therapy: Incorporating BODIPY into porphyrinic nanoscale MOFs through the solvent-assisted ligand incorporation, Dye. Pigment 170 (2019) 107576. 10.1016/j.dyepig.2019.107576. [DOI] [Google Scholar]
- [126].Feng D, Gu ZY, Li JR, Jiang HL, Wei Z, Zhou HC, Zirconium-metalloporphyrin PCN-222: Mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts, Angew. Chemie - Int. Ed 51 (2012) 10307–10310. 10.1002/anie.201204475. [DOI] [PubMed] [Google Scholar]


