Abstract
Small ruminant brucellosis is caused by the Gram negative cocci-bacillus Brucella (B.) melitensis, the most virulent Brucella species for humans. In goats and sheep, middle to late-term gestation abortion, stillbirths and the delivery of weak infected offspring are the characteristic clinical signs of the disease. Vaccination with the currently available Rev. 1 vaccine is the best option to prevent and control the disease, although it is far from ideal. In this study, we investigate the safety of the B. melitensis 16MΔvjbR strain during a 15-month period beginning at vaccination of young goats, impregnation, delivery and lactation. Forty, 4 to 6 months old, healthy female crossbreed goats were randomly divided into four groups (n=10) and immunized subcutaneously with a single vaccine dose containing 1×109 CFU of B. melitensis 16MΔvjbR delivered in alginate microcapsules or non-encapsulated. Controls received empty capsules or the commercially available Rev.1 vaccine. Seven months post-vaccination, when animals were sexually mature, all goats were naturally bred using brucellosis-free males, and allowed to carry pregnancies to term. Blood samples to assess the humoral immune response were collected throughout the study. At two months post-delivery, all dams and their offspring were euthanized and a necropsy was performed to collect samples for bacteriology and histology. Interestingly, none of the animals that received the vaccine candidate regardless of the formulation exhibited any clinical signs associated with vaccination nor shed the vaccine strain through saliva, vagina or the milk. Gross and histopathologic changes in all nannies and offspring were unremarkable with no evidence of tissue colonization or vertical transmission to fetuses. Altogether, these data demonstrate that vaccination with the mutant strain 16MΔvjbR is safe for use in the non-pregnant primary host.
Keywords: Brucella, brucellosis, vaccine, microencapsulation, goats
1. Introduction
Brucellosis is a worldwide chronic infectious, zoonotic disease caused by small aerobic, non-motile, Gram negative coccobacilli of the genus Brucella. Species within the genus are recognized based on preferential host specificity, with goats and sheep being the preferred hosts for Brucella melitensis [1]. Moreover, this species is considered the most virulent among the different Brucella spp. and is capable of causing disease in humans. In small ruminants, middle to late-term gestation abortion, stillbirths and the delivery of weak offspring sometimes followed by the retention of fetal membranes are the characteristic symptoms. In humans, brucellosis is considered a severely debilitating and disabling illness that can result in high morbidity with intermittent fever, chills, sweats, weakness, myalgia, abortion, osteoarticular complications, endocarditis, depression, anorexia, and low mortality [2].
Caprine brucellosis has been controlled in most industrialized countries; however, in low and middle-income nations, it is considered a public health threat as it remains endemic and is associated with an extensive negative impact in flock productivity [3]. Undoubtedly, vaccination is the best option to prevent and control the disease. Far from ideal, the attenuated B. melitensis strain Rev.1 is considered the best vaccine available for prophylaxis of caprine brucellosis [4]. Until today, multiple efforts looking for alternatives to improve the safety of the vaccine strain Rev.1 have proven to be unsuccessful. In previous studies, the mutant strain 16MΔvjbR of B. melitensis has been demonstrated to be a promising vaccine candidate in terms of safety and efficacy in the mouse model [5,6], which has prompted further investigation of the potential use of this vaccine in a natural host. The aim of the present study was to evaluate the vaccine safety in terms of potential undesired side-effects associated with the use of live attenuated vaccines including tissue colonization, concealment, shedding, vertical and horizontal transmission, as well as the induction of humoral responses over a 15-month period that mimics natural conditions including vaccination, impregnation, pregnancy, delivery and lactation.
2. Materials and Methods
2.1. Bacterial strains and vaccine preparation
B. melitensis 16MΔvjbR strain [7], and the commercial vaccine strain B. melitensis Rev.1 (OCUREV®, CZ Vaccines, Pontevedra, Spain) provided by SENASA (Official Veterinary Service of Argentina) were used for this study. The mutant strain was grown from a frozen glycerol stock prepared on tryptose soy agar (TSA) plates. After four days of incubation at 37°C, bacteria were harvested from the surface of the plates into sterile PBS, pH 7.2. The actual number of viable bacteria/ml was retrospectively obtained by serial dilutions in PBS and plated onto TSA for quantification.
Microencapsulation of 16MΔvjbR strain was performed followed a protocol originally published by Abraham et al. (1996) [8], with the modifications suggested by Arenas-Gamboa et al. (2009) [9]. Briefly, a previously determined CFU of 16MΔvjbR strain/ml were resuspended in a 1.5% (w/v) alginate solution in MOPS buffer (10 mM MOPS, 0.85% (w/v) NaCl [pH 7.4]) and loaded into a syringe with a 200-μm nozzle attached. The alginate solution was extruded into a 100mM CaCl2 crosslinking solution under specific voltage and flow rate applied to the syringe by an encapsulator device (Nisco encapsulator, Nisco Engineering AG, Zurich, Switzerland). Following, 15 minutes of continuous stirring, the crosslinking solution was removed and replaced with a 0.1% (w/v) poly-L-lysine solution to permanently crosslink the alginate. To enhance and modify the release profile of the encapsulant, 2.5 mg of vitelline protein B (VpB) was added as previously described [9].
All work with 16MΔvjbR was approved and performed in a biosafety level 2 laboratory at CICVyA-I.N.T.A. (Research Center for Veterinary and Agronomical Sciences- National Institute for Agricultural Technology), per SENASA approved standard operating procedures. Rev.1, approved for field vaccination campaigns in Argentina, was provided by SENASA and manipulated according to the manufacturer’s instructions.
2.2. Animals
Forty, 4 to 6 month old, healthy female crossbreed goats were purchased from a privately owned brucellosis-free flock and housed in outdoor, restricted access, experimental pens at the CICVyA-INTA at Castelar (Bs. As., Argentina). Upon arrival, all animals were confirmed to be negative for brucellosis via buffered plate antigen test (BPAT) and ELISA. All goats were dewormed and vaccinated subcutaneously against Clostridium spp.(Covexin ® 10, MSD, Argentina), weighted, randomly divided in four groups (n=10) and identified by ear tags and implantable LifeChip® Biothermo Identification System chips (Allflex, TX, USA). Animal welfare was determined by daily clinical observation of goats by veterinarians, who evaluated appetite, environmental interaction, body temperature, respiratory frequency and stool consistency. Animals received water ad libitum and were fed hay supplemented with whole corn kernels and alfalfa pellets twice a day. Barnyards were dry-cleaned three times per week. All animal procedures were approved by the Institutional Animal Care and Use Committee (CICUAE) of CICVyA-INTA, under approval number 54/2014. Facilities and procedures involving the use of the genetically modified strain of B. melitensis 16MΔvjbR in goats was approved by CONABIA Argentina (National Advisory Commission on Agricultural Biotechnology) under approval number 3177/2015.
2.3. Experimental design
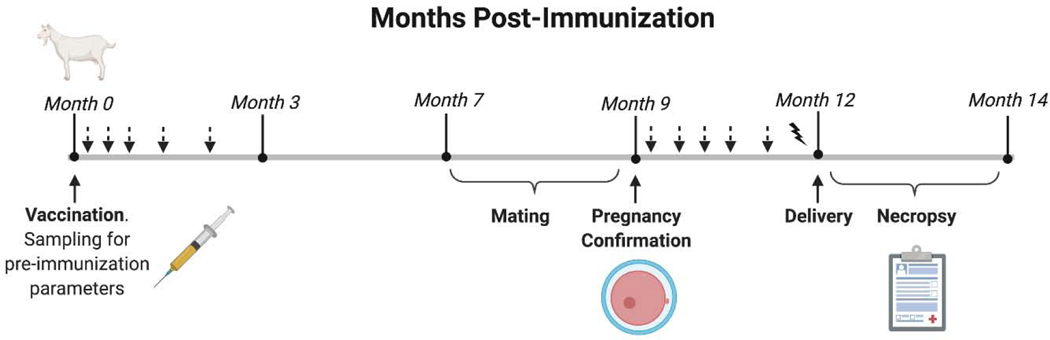
The experimental design is represented in a timeline in Fig. 1. Following a 10 day-acclimation period, goats were immunized with a single dose containing either 1) subcutaneous non-encapsulated 1×109 CFU of B. melitensis 16MΔvjbR, 2) subcutaneous encapsulated 1×109 CFU of 16MΔvjbR, 3) 1×109 intraocular Rev.1, or 4) subcutaneous 1ml of empty capsules suspended in sterile MOPS. Serum samples, oral and vaginal swabs were collected at different time points following immunization. Seven months post-vaccination, all goats (11 to 13 months of age) were bred using brucellosis-free males, and pregnancy was confirmed by ultrasound at 60 days post breeding. Veterinary supervision was increased to twice a week at the time of parturition, which included verification of live-death births, colostrum consumption and offspring-mom interaction. At the time of parturition, samples were collected for bacteriological analysis (blood, vaginal swabs and colostrum) and determination of humoral immune responses. At two months post-partum or immediately following abortion, all dams and their offspring were humanely euthanized by intramuscular application of 0.5–1 ml of xylazine (2%) (Richmond, Bs. As., Argentina) followed by intravenous overdose with sodium pentobarbital (Euthanyle; Brouwer, Bs. As., Argentina), and necropsy was performed to collect samples for further assessment of bacterial colonization as well as histopathological changes. Male goats were euthanized at the end of the breeding season and multiple tissues and serum samples were collected to assess horizontal transmission from the vaccinated females.
Figure 1. Experimental design.
Forty healthy female crossbreed goats of 4 to 6 months old were immunized subcutaneously with 1×109 CFU of non-encapsulated B. melitensis 16MΔvjbR (group A, n=10) or 1×109 CFU of encapsulated 16MΔvjbR (group B, n=10), or by instillation of 1×109 CFU of B. melitensis Rev.1 onto their conjunctiva (group C, n=10), or inoculated subcutaneously with empty capsules suspended in sterile MOPS (group D, n=10). Arrows indicate sampling for immunological assessment (7, 14, 28, 45, 56, 90, 120, 150, 180 and 270 days post vaccination) and detection of shedding in salivary and vaginal fluids (7, 14, 21 and 28 days post-immunization). Solid ray indicates sampling of blood, milk and vaginal secretion as well as placenta (at delivery). Sera from nannies and kids were collected between 30 to 45 days post-delivery (not shown).
2.4. Immune response
2.4.1. Assessment of humoral immune responses
Five ml of blood for serum was collected from the jugular vein of goats at 0, 7, 14, 21, 28, 42, 56, 90, 120, 150, 180 and 270 days post-vaccination, at delivery, 30 to 45 days post-delivery and at necropsy (Fig.1). The presence of Brucella-specific antibodies was assessed in kids (30 to 45 days of age and at necropsy) and in males after the mating period. Buffered Plate Antigen (BPA) and indirect enzyme-linked immunosorbent assays (iELISAs) tests were performed on serum samples to evaluate the presence of Brucella-specific antibodies. For BPA test (CDV, Bs. As., Argentina), positive or negative results were determined by the presence or absence of visible agglutination, respectively. A scale was developed to categorize the degree of agglutination as 1) +++ strong, 2) ++ mild, 3) + weak and 4) – no agglutination. iELISAs were conducted to determine the presence of IgM, total IgG, IgG1 and IgG2 Brucella-specific antibodies. Briefly, ninety-six well polystyrene plates were sensitized overnight at 4°C with 100μl of 1μg/ml B. abortus S1119–3 LPS [10]. The following day, plates were washed five times with PBS-tween (PBST), and blocked with 200μl/well of 10% (w/v) skimmed milk. Following 1 hour of incubation at 37°C, 100μl/well of serum samples (1/100 diluted in blocking buffer for IgM and total IgG, and 1/10 for IgG1 and IgG2), as well as positive and negative control sera, were dispensed in triplicate and incubated for another hour at 37°C. After washing with PBST, 100μl/well of diluted peroxidase-conjugated secondary antibody was added [1/10,000 rabbit-anti-goat IgM polyclonal ab (Bio-Rad; Hercules, CA); 1/3,000 rabbit-anti-goat IgG polyclonal antibody (Sera Care KPL; Milford, MA); 1/100 and 1/250 sheep-anti-bovine IgG1 and IgG2 polyclonal antibody, respectively (Thermo Fisher Sci, Waltham, MA)]. Following 1h incubation and washing, 100μl/well of chromogen 3,3,5,5 ´-tetramethylbenzidine solution (TMB) (Sigma-Aldrich Inc., St. Louis, MI) dissolved in mildly acidic buffer [citrate buffer (pH 5.5), with 30% (v/v) hydrogen peroxide to final concentration of 0.01% acetate] (TMB/H2O2) was added and incubated at room temperature in the dark for 5 min, to visualize the reaction. The colorimetric reaction was stopped by adding 50μl/well of 2N sulfuric acid and an OD was measured at 450 nm in a Multiskan® EX reader (Lab Systems, Bs. As., Argentina). Cut off value was set as the media plus three standard deviations of the values obtained for the negative control.
2.4.2. Detection of Brucella vaccine total IgG antibodies in milk
Milk samples from both mammary glands of all moms were taken at delivery and at necropsy, aliquoted, and stored at −20°C until processing via iELISA. One hundred μl of milk diluted at a 1:1 ratio in blocking buffer, as well as positive and negative controls, were dispensed in triplicate in previously sensitized wells and incubated for one hour at 37°C. After washings with PBST, 100μl/well of secondary antibody (1/3,000) rabbit-anti-goat IgG polyclonal antibody (Sera Care KPL) was added, and the reaction revealed by addition of TMB following 1 h incubation. The OD was measured within 10 minutes at 450 nm wavelength using an ELISA reader.
2.5. Bacteriology
2.5.1. Tissue collection
Tissue colonization was assessed in spleen, liver, mammary gland and milk among other samples for Brucella spp. isolation taken at different time points and from different sources throughout the experiment (Fig. 1 and Table S1). Swabs from the oral (saliva) and vaginal mucosa were collected at multiple time points including pre-immunization (0), and 7, 14, 21 and 28 days post-immunization. Samples from blood, colostrum, vaginal swabs and placenta were taken within 12h post-abortion or delivery. The above mentioned tissues as well as spleen, liver, epididymis, scrotal lymph nodes (LN) and semen from males were collected at necropsy for bacteriology (Table S1).
2.5.2. Tissue processing and culture
One gram of tissue from each organ collected was transferred to a 50 ml conical tube containing 1ml of sterile PBS, macerated with a tissue homogenizer, and 100 μl of the suspension was cultured onto Farrell’s agar media (Oxoid, Hampshire, UK). Swabs were directly streaked onto Farrell’s media as well as 0.1 ml of fluid samples (colostrum, milk, urine, synovial fluid, abomasal content). Plates were incubated at 37°C and cultures monitored daily for 7 to 10 days.
2.6. Histopathology and immunohistochemistry
To assess of histologic changes associated with vaccination, multiple tissues were collected at necropsy from moms, their offspring, and bucks (Table S1). Tissues were fixed in 10% neutral buffered formalin and were then routinely processed and embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Histologic changes between groups were evaluated in a blinded fashion by a boarded veterinary anatomic pathologist. Unstained sections from placenta were adhered to positively charged glass slides for immunohistochemistry. Following deparaffinization and rehydration through a series of xylene and ethanol steps, antigen retrieval and blocking was performed as previously described [11]. Primary incubation was done overnight at 4° C with Brucella polyclonal rabbit antibody (Bioss, Boston, MA) at 1:2,000 ratio. Negative control tissues were incubated with rabbit nonimmune serum diluted in PBS. Vectastain ABC and Betazoid DAB chromogen kits (Biocare Medical, CA) were used following primary incubation according to the manufacturer’s instructions. The slides were counterstained with Meyer’s hematoxylin III.
2.7. Statistical analysis
All analyses were performed using the GraphPad Prism 6.0 software (San Diego, CA, USA) and P values <0.05 were considered significant. Statistical analysis was performed by comparing the mean of the groups using the two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test.
3. Results
3.1. Clinical monitoring
3.1.1. Assessment of body temperature
During the acclimation period, normal body temperature for young female goats was established to be below 40°C, which was in concordance with the literature [12], and remained below this threshold during the first month following immunization regardless of the treatment (Fig S1A). Similarly, no fever was observed following abortion or delivery (Fig S1B).
3.1.2. Adverse side effects
No modifications of physiological or behavioral parameters, like appetite, loss of body weight, trauma, environmental interaction or local inflammation at the site of injection, were observed throughout the course of the experiment.
3.1.3. Pregnancy success
Thirty seven of 40 experimental goats (92.5%) became pregnant after natural service as determined using ultrasound 45 days after the breeding period ended. Specifically, nine of 10 female goats (90%) were pregnant in the control, Rev.1 and encapsulated 16MΔvjbR groups, while all of the goats (n=10) were pregnant in the nonencapsulated 16MΔvjbR immunized group (Table 1).
Table 1.
Pregnancy rates and number of conceptions, abortions, stillborn, perinatal deaths and viable offspring in each experimental group.
| Experimental groups | Pregnancy rate | Number of conceptions | # of abortions1 (% of conceptions) |
# of stillbirths2 (% of conceptions) |
# of perinatal deaths3 (% of conceptions) | # of kids alive (% of conceptions) | Brucella detection 4 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 16MΔvjbR | 100 | 14 | 3 (21.4%) | 2 (14.3%) | 4 (28.6%) | 5 (35.7%) | No |
| 16MΔvjbR-E* | 90 | 10 | 0 | 2 (20%) | 2 (20%) | 6 (60%) | No |
| Rev.1 | 90 | 13 | 0 | 0 | 3 (23.1%) | 10 (76.1%) | No |
| Control | 90 | 11 | 1 (9.1%) | 0 | 1 (9.1%) | (81.8%) | No |
Abortion is defined by the expulsion of dead fetus prior to normal delivery
Stillbirths were classified as delivered of term fetus with no signs of life
Perinatal deaths were those that the kids were born alive but died within 96h after-delivery (by different causes).
This column reflects the outcome of Brucella detection from the assessment of tissues and fluids, culture, and microscopic examination of histological sections and immunohistochemistry of tissues from nannies or kids collected at delivery, abortion or necropsy.
16MΔvjbR-E: 16MΔvjbR encapsulated
Statistical analysis was performed using a One-Way Analysis of Variance (ANOVA) with Holm-Sidak’s multiple comparison test. No significant differences were found between the any of the groups (16MΔvjbR, 16MΔvjbR-E, Rev 1, and the control group) in all variables (pregnancy rates, number of conceptions, abortions, stillbirths, perinatal deaths and viable offspring).
3.1.4. Abortion and deliveries
Three abortions, two stillborn and four perinatal deaths were registered in the group of goats immunized with non-encapsulated 16MΔvjbR strain. One nanny aborted two fetuses, while two delivered one dead and one live full-term kid. Both live kids died within 96h post-delivery. Of the 10 animals vaccinated with the encapsulated mutant strain, there were two stillborn and two perinatal deaths. In this group, one nanny delivered one dead and one weak live kid that died 48h post-partum. In the group of goats immunized with the commercial vaccine B. melitensis Rev.1, three moms delivered three weak offspring, which were found dead within 72h after birth. Unvaccinated controls delivered nine healthy kids, one weak offspring that died 3 days post-delivery, and one nanny aborted one fetus at mid pregnancy (Table 1). Liver, lung, spleen and abomasal content from all aborted, stillbirth and weak offspring as well as their placental tissues were assessed for bacterial growth as well as undergoing histopathological analysis. None of the tissues analyzed revealed any histopathological changes consistent with Brucella infection.
3.2. Evaluation of shedding, tissue colonization and potential transmission of 16MΔvjbR
3.2.1. Vaccine shedding
Oral and vaginal swabs were collected weekly during the first month after vaccination and cultured onto Farrell’s media. Independently of the time point, there was no growth from either oral or vaginal swabs from any of the samples collected (Table 2).
Table 2.
Detection of vaccine shedding in sexually immature female goats through saliva or vaginal samples collected weekly during the first month post-immunization.
| Experimental groups | Anti-vaccine IgG immune response | Vaccine strain excretion | ||
|---|---|---|---|---|
| BPAT | iELISA | Saliva | Vagina | |
|
| ||||
| 16MΔvjbR | ++/+++ | Positive | none | none |
| 16MΔvjbR encapsulated | ++/+++ | Positive | none | none |
| Rev.1 | ++/+++ | Positive | none | none |
| Control | Negative | Negative | none | none |
3.2.2. Vaccine excretion during abortion or parturition
Within 12 h post-abortion or parturition, samples from all nannies including blood, colostrum, vaginal swabs and available placentas (30 of 37) were collected. Brucella isolation was negative in all of the samples collected, independently of the treatment received (Table S2).
3.2.3. Bacterial colonization in nannies
To assess of vaccine persistence in tissues, samples from spleen, liver, lung, brain, uterus, ovaries, mammary gland, mammary and retropharyngeal LNs were collected from all nannies at necropsy, and further processed and cultured (Table S1). No bacteria was isolated from any of the treatment groups including the Rev.1 strain (Table S2) indicating that, like Rev.1, the mutant strain presented a reduced residual virulence in goats.
3.2.4. Assessment of vertical and horizontal transmission of the vaccine candidate strain
No bacteria was isolated from any of the offspring indicating that the vaccine strain does not infect the fetus transplacentally or through the milk, and that the abortions observed were not associated with Brucella infection. In addition, no seroconversion was observed and no Brucella spp. were isolated or detected from tissue samples collected at necropsy from males used to breed the goats (Table S2).
3.3. Immune response
3.3.1. Serological responses in vaccinated animals
3.3.1.1. Anti-Brucella antibody screened by Buffered Plate Antigen Test
All the animals immunized with the non-encapsulated strain exhibited a weak to strong agglutination reaction as soon as 7 days post-immunization, whereas the strongest agglutination was reached at the second week and lasted for 45 days post-immunization. At later time points, agglutination response started to decrease, although six animals remained positive and their response varied from mild to weak agglutination throughout the experiment (Table S3). Although weaker, the dynamic of agglutination response in animals vaccinated with encapsulated strain was similar in comparison with the one observed in the non-encapsulated group. Only one goat (#3) from this group seroconverted at delivery and showed a similar serological response at necropsy (Table S3). Serum samples from control animals remained negative throughout the study, while animals vaccinated with Rev.1 strain exhibited similar agglutination response as compare to goats immunized with the candidate strain (Table S3).
3.3.1.2. Anti-Brucella specific IgM responses in immunized goats
Detection of the anti-Brucella specific IgM antibody following 16MΔvjbR vaccination was performed using an iELISA and results are shown in Figure S2. Immunization with the 16MΔvjbR unencapsulated vaccine candidate elicited an anti-Brucella specific IgM response that was statistically significant (p<0.01) to the control group in the first two weeks post-vaccination. In contrast, anti-Brucella specific IgM response was slightly significant (p<0.05) in the second week post-immunization in animals vaccinated with encapsulated 16MΔvjbRcompare to non-vaccinated animals, while the ELISA optical density (OD) values in serum samples from animals vaccinated with the commercial vaccine Rev.1 strain, was significantly different (p<0.01) to the control animals in the second and third weeks post-inoculation. ELISA OD from control goats’ sera remained below the cut off value for all the time points.
3.3.1.3. Detection of anti-Brucella total IgG in serum of vaccinated goats
The presence of anti-Brucella specific IgG antibodies was evaluated at different time points via iELISA. One week after vaccination, ELISA OD of serum samples from the majority of the B. melitensis 16MΔvjbR vaccinated animals were above of the cut off value, regardless of the formulation. Moreover, serum from all the vaccinated animals (non-encapsulated 16MΔvjbR, encapsulated 16MΔvjbR and Rev.1 vaccinated groups) showed OD values above the threshold at 2 weeks p.v. ( Table 3). Most of the animals vaccinated with the non-encapsulated 16MΔvjbR or Rev.1 strains presented level of antibodies against anti-Brucella LPS above the cut off throughout the study, whereas the number of goats with the level of anti-Brucella LPS specific antibodies above the detection limit decreased in the encapsulated 16MΔvjbR vaccine group at 3 months p.v. As expected, OD values for animals’ sera from the control group remained below the cut off throughout the study.
Table 3.
Anti-Brucella total IgG immune response in serum samples from goats vaccinated with 16MΔvjbR, 16MΔvjbR encapsulated or B. melitensis Rev.1, or inoculated with empty capsules (controls), determined by iELISA before (T0) and after vaccination (1 to 62 weeks post-vaccination).
| Group | Goat # | Weeks Post-Vaccination | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 9 | 13 | 17 | 22 | 26 | 42 | 54 | 57 | 62 | ||
|
| ||||||||||||||||
| 16MΔvjbR | 1 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | − | + | + | + | + | + | + | − | − | + | − | + | + | − | + | |
| 3 | − | + | + | + | + | + | + | + | + | − | − | + | + | + | + | |
| 4 | − | + | + | + | + | + | − | − | − | − | − | − | + | + | + | |
| 5 | − | + | + | + | + | + | + | + | + | + | − | + | + | ND | + | |
| 6 | − | + | + | + | + | + | + | + | + | + | + | + | + | ND | + | |
| 7 | − | − | + | + | − | + | + | + | + | + | + | + | + | ND | + | |
| 8 | − | + | + | + | + | + | + | + | − | + | − | + | + | ND | + | |
| 9 | − | − | + | + | + | + | + | + | + | − | − | + | ND | ND | ND | |
| 10 | − | + | + | + | + | + | + | + | + | + | + | + | ND | ND | ND | |
|
| ||||||||||||||||
| 16MΔvjbR-encapsulated | 1 | − | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| 2 | − | + | + | + | + | + | + | − | − | − | − | − | − | − | − | |
| 3 | − | + | + | + | + | + | + | + | + | − | − | + | − | + | + | |
| 4 | − | + | + | − | + | + | − | − | − | − | − | − | + | − | − | |
| 5 | − | + | + | + | + | + | − | − | − | − | − | − | − | − | + | |
| 6 | − | − | + | − | + | + | + | − | − | − | − | − | + | ND | + | |
| 7 | − | + | + | − | + | + | + | − | − | − | − | − | + | ND | + | |
| 8 | − | + | + | + | + | + | + | + | + | − | − | − | − | ND | − | |
| 9 | − | − | + | + | + | + | + | − | − | − | − | + | ND | ND | ND | |
| 10 | − | + | + | + | + | + | + | − | − | − | − | − | ND | ND | ND | |
|
| ||||||||||||||||
| Rev.1 | 1 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 3 | − | − | + | + | + | + | + | + | + | + | − | + | + | + | + | |
| 4 | − | − | + | + | + | + | + | + | + | + | − | − | + | + | + | |
| 5 | − | − | + | + | + | + | + | + | + | + | + | − | + | + | + | |
| 6 | − | − | + | + | + | + | + | + | + | + | + | − | + | + | + | |
| 7 | − | − | + | + | + | + | + | − | − | + | − | − | + | + | + | |
| 8 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 9 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 10 | − | − | + | + | + | + | + | + | − | + | − | − | ND | ND | + | |
|
| ||||||||||||||||
| Control | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 5 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 6 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 7 | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | |
| 8 | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | ND | ND | − | |
| 10 | − | − | − | − | − | − | − | − | − | − | − | − | ND | ND | − | |
ND = not determined.
3.3.1.4. Evaluation of anti-Brucella IgG1 and IgG2 levels post-immunization
The levels of anti-Brucella IgG isotype 1 and 2 were measured 28 days post-immunization, at the peak of anti-Brucella total IgG response. Our results show that the level of IgG2, but not IgG1, was significantly different in all immunized groups compared with pre-immune levels and the level of this IgG isotype in the control group at 4 weeks post vaccination (Table 4). The OD values of IgG2 were two-fold or higher in animals vaccinated with non-encapsulated 16MΔvjbR, and four-fold or higher in those immunized with Rev.1 in comparison with the level of IgG2 at prevaccination or against the IgG2 level in non-vaccinated animals at the same time point. In contrast, there was no increase in IgG1 levels in vaccinated goats compared to the levels of IgG1 in prevaccinated or naïve goats at the same time point.
Table 4.
Levels of anti-Brucella IgG1 and IgG2 in serum of experimental animals at 4 weeks post vaccination compared with pre-vaccination status determined by iELISA.
| Groups | Goat # | IgG1 | IgG2 | P-value |
|---|---|---|---|---|
|
| ||||
| 16MΔvjbR | 1 | − | + | 0.0309* |
| 3 | − | + | ||
| 5 | − | + | ||
| 8 | − | + | ||
| 9 | − | + | ||
|
| ||||
| 16MΔvjbR encapsulated | 1 | − | + | 0.0195* |
| 2 | − | + | ||
| 3 | − | + | ||
| 7 | − | + | ||
| 8 | − | + | ||
|
| ||||
| Rev.1 | 3 | − | + | 0.0003*** |
| 4 | − | + | ||
| 5 | − | + | ||
| 6 | − | + | ||
| 7 | − | + | ||
|
| ||||
| Co | 1 | − | − | − |
| 2 | − | − | ||
| 3 | − | − | ||
| 4 | − | − | ||
| 5 | − | − | ||
+ = higher level, − = equal level.
Significant (p<0.05),
extremely significant (p<0.001) (One-tailed paired T-test). Five animals were randomly selected from each group.
3.3.2. Detection of anti-Brucella specific IgG antibodies in milk
iELISA was used to evaluate the presence of anti-Brucella IgG antibodies in milk. Colostrum samples from all evaluated dams vaccinated with 16MΔvjbR non-encapsulated and Rev.1 strains and all but one animal (# 10) from the encapsulated 16MΔvjbR vaccinated group, showed ELISA OD above the cut off value (0.37), indicating the presence of IgG antibodies against Brucella in colostrum of immunized animals ( Table 5). Nine weeks post-partum, six (of nine) and five (of seven) milk samples from 16MΔvjbR non-encapsulated and Rev.1 vaccinated animals respectively, still showed absorbance values above the cut off. On the contrary, only one goat vaccinated with 16MΔvjbR encapsulated strain had a detectable level of anti-Brucella IgG antibodies. OD values for animals’ sera from the control group remained below the cut off in both time points. These results indicate a shorter presence of anti-Brucella IgG antibodies in milk of goats vaccinated with non-encapsulated 16MΔvjbR strain.
Table 5.
Presence (+) or absence (−) of anti-Brucella IgG antibodies in milk of goats at parturition and 9 weeks post-partum (p.p.) determined by iELISA.
| Groups | Goats # | Partum | 9 weeks p.p. |
|---|---|---|---|
|
| |||
| 16MΔvjbR | 1 | + | + |
| 2 | + | ND | |
| 3 | + | + | |
| 4 | + | − | |
| 5 | + | − | |
| 6 | + | + | |
| 7 | + | + | |
| 8 | + | + | |
| 9 | + | − | |
| 10 | + | + | |
|
| |||
| 16MΔvjbR-encapsulated | 1 | + | − |
| 2 | + | − | |
| 3 | ND | ND | |
| 4 | ND | ND | |
| 5 | + | + | |
| 6 | + | ND | |
| 7 | + | − | |
| 8 | + | − | |
| 9 | + | − | |
| 10 | − | − | |
|
| |||
| Rev.1 | 1 | ND | ND |
| 2 | + | − | |
| 3 | ND | ND | |
| 4 | + | + | |
| 5 | + | + | |
| 6 | + | + | |
| 7 | + | + | |
| 8 | + | + | |
| 9 | ND | ND | |
| 10 | + | − | |
|
| |||
| Control | 1 | − | − |
| 2 | − | − | |
| 3 | − | − | |
| 4 | − | − | |
| 5 | ND | ND | |
| 6 | ND | − | |
| 7 | ND | ND | |
| 8 | − | − | |
| 9 | − | − | |
| 10 | − | − | |
ND = not determined.
3.3.3. Anti-Brucella antibodies in kids’ serum
Serum was collected from kids at 30–45 days of age and at necropsy. None of the samples showed agglutination to BPAT during the two time points evaluated. Similarly, none of the serum elicited anti-Brucella specific IgM antibodies, except for two individual samples that showed OD above the cut off value, one born from a 16MΔvjbR non-encapsulated group and the other born from a Rev.1 group (Fig S3A). When iELISA was performed to measure the OD of anti-Brucella IgG antibodies in kids’ serum, no statistical differences were found among groups (p>0.05) (Fig S3B). None of the sera from kids born from control goats showed OD values above the cut off.
3.3.4. Lack of anti-Brucella specific antibodies in males following mating
We also evaluated the potential sexual transmission of 16MΔvjbR strain and its consequent seroconversion in bucks after 42 days of co-living with and being bred to immunized goats. All bucks remained serologically negative to BPAT and iELISA (Fig S4).
3.4. Post-mortem examination
3.4.1. Dams’ tissues and placentas
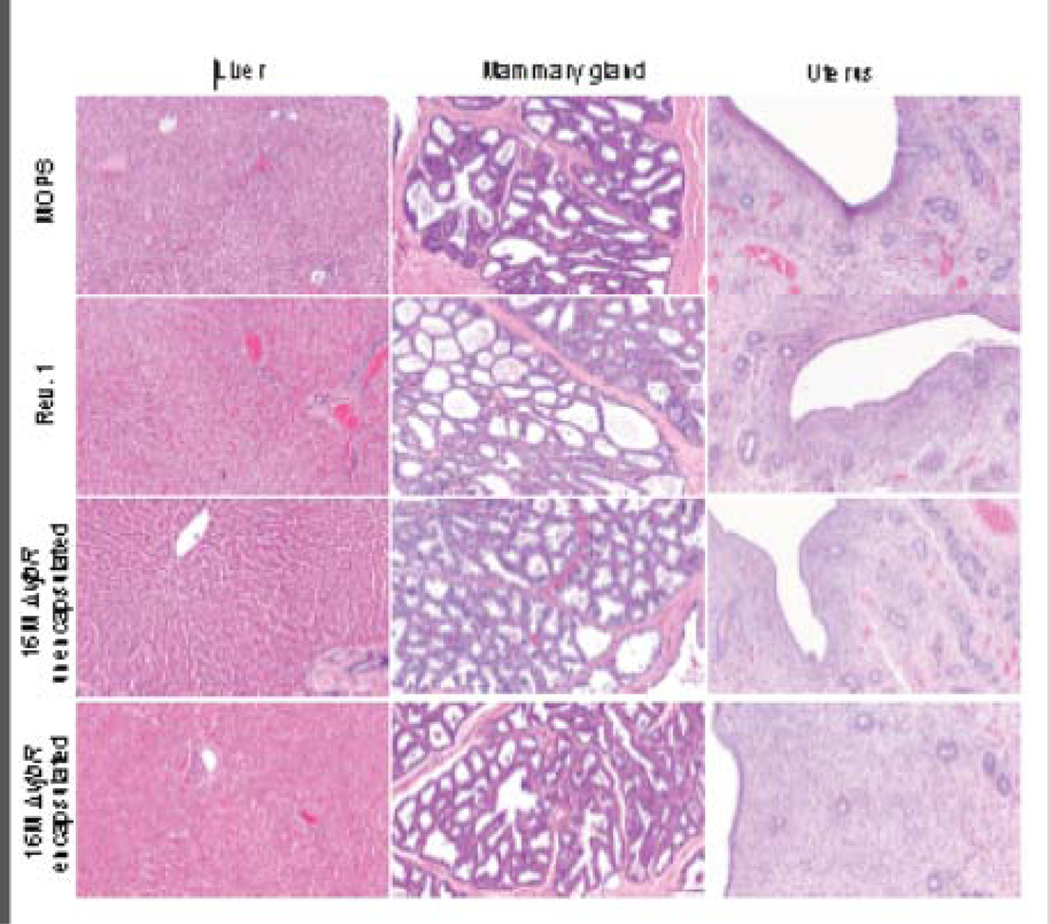
Complete gross evaluation was conducted in all experimental dams with no lesions observed. Tissue samples (n=590) and placentas collected at parturition (30 of 37) were processed and examined for histological lesions associated with the vaccine strain. No microscopic lesions attributable to Brucella spp. infection were noted in any of the tissues examined in any group (Fig. 2). Furthermore, no Brucella antigen was detected by immunohistochemistry in any of the placentas examined (Fig. 3).
Figure 2 . Histological analysis of post-mortem processed tissues.
Samples of liver, mammary gland and uterus from adult goats vaccinated with empty capsules suspended in MOPS (row 1), 1×109 CFU Rev.1 (row 2), 1×109 CFU unencapsulated 16MΔvjbR (row 3) or 1×109 CFU encapsulated 16MΔvjbR (row 4). No significant histopathological abnormalities were noted in any of the tissues. Hematoxylin and eosin (H&E), Magnification = 10×, Bar = 50 μm.
Figure 3 . Histological analysis of placenta.
collected from goats vaccinated with empty capsules suspended in MOPS, 1×109 CFU Rev.1, 1×109 CFU unencapsulated16MΔvjbR, or 1×109 CFU encapsulated 16MΔvjbR. No significant lesions were observed in the placenta regardless of treatment group (H&E. Magnification = 4×. Bar = 100 μm). Additionally, Brucella antigen was not detected by immunohistochemistry (IHC) with an anti-Brucella poly-clonal antibody at 1:2,000 (Inset: Magnification = 20×, Bar = 25 μm).
3.4.2. Offspring
A complete necropsy of aborted fetuses (4), stillbirths (4), perinatal deaths (10) and clinically healthy offspring (30) was performed (Table 1). No gross or histologic lesions compatible with brucellosis were detected in any group despite spontaneous abortions or perinatal deaths occurring in the vaccinated animals (Fig S5).
3.4.3. Assessment of horizontal transmission of the vaccine strain to males
Following 42 days of co-living with immunized goats, bucks were euthanized and tissue samples collected to determine if there was any histologic evidence of Brucella infection secondary to possible sexual transmission of the vaccine strain. All tissues were histologically unremarkable.
4. Discussion
Vaccination is the best option to prevent and control brucellosis in livestock species. The strain Rev.1 of B. melitensis has been employed worldwide for prophylaxis against small ruminant brucellosis since the 1970s. Inoculated in sexually immature females, the vaccine is safe and induces a solid and lifelong protection against B. melitensis infection and abortion [13]. However, it can infect humans, and the vaccination of pregnant animals with strain Rev.1 may cause abortion [14], two reasons why there are many efforts dedicated to the development of an improved vaccine against caprine brucellosis.
In this 15-month trial, we demonstrated that the brucellosis vaccine candidate B. melitensis strain 16MΔvjbR is safe and immunogenic in young goats. When inoculated in sexually immature female goats, no clinical evidence of adverse vaccine reaction was observed following subcutaneous immunization, including fever, local inflammation at the site of inoculation, loss of appetite or lethargy. Previous studies have reported hyperthermia the first days following Rev.1 subcutaneous immunization [15,16], a side-effect not observed in this study with the vaccine candidate strain 16MΔvjbR.
B. melitensis natural infection in non-pregnant female goats is usually asymptomatic, and the pathogen persists in lymphoid tissue and bone marrow [1]. During the breeding season, Brucella disseminates to reach genital organs and causes infertility and abortion [17], reaching 1×1010 colony-forming units (CFU)/ml in allantoic fluid and 1×1013 CFU/g of tissue in cotyledons [18]. Strain 16MΔvjbR, regardless of the formulation, inoculated in young female goats did not affect their fertility as 95% of nannies got pregnant (Table 1). In this study, offspring displayed a variety of birth statuses ranging from live, perinatal death, stillborn and abortion. Independent of birth status, no Brucella were isolated nor any histologic images observed compatible with brucellosis from any aborted offspring, placenta, vaginal swabs or colostrum/milk samples (Table S2). Being Brucella eliminated as etiological agent of abortion, stillbirths and perinatal deaths throughout the assay, no further analysis was performed to find out a possible etiology of these events. However, persistent rain during the calving season and the lack of interest in some dams for their newborn kids could have had a negative impact on kids’ survival.
Lack of shedding is an important parameter to evaluate while developing a Brucella vaccine, since contamination of the environment could potentially pose a risk to other susceptible species residing in the same space [19]. It has been shown that most adult female goats clear the Rev1 strain by approximately 8 weeks after subcutaneous vaccination with 1.5×109 CFU [16]. However, in domestic goats vaccinated conjunctivally, the persistence of Rev.1 in nasal secretions and in oral mucosa has been reported up to two weeks post vaccination [20] [19]. In the present study, vaccine was delivered via a subcutaneous route did not exhibit any shedding, either from saliva or vaginal swabs from any of the collected samples over the first month following vaccination, or at the time of parturition or anytime thereafter. Most importantly, when milk or colostrum was analyzed for bacterial excretion, no Brucella spp. was cultured. These data are in concordance with previous results which have demonstrated that the disruption of vjbR in B. melitensis virulent strains impedes tissue colonization and is cleared from BALB/c mice tissues 4 to 8 weeks post immunization [6,21]. Therefore, these results add support that the vaccine candidate 16MΔvjbR is also highly virulence-attenuated in its natural host.
In parallel with survival and excretion studies, the immunogenic capacity of the strain 16MΔvjbR was monitored and found to be consistent with results in previous publications in which 16MΔvjbR and M5–90ΔvjbR strains elicited humoral and cellular immune responses in BALB/c mice and sheep [6,21]. In the work reported here, nannies immunized with encapsulated 16MΔvjbR strain not only exhibited lower levels of serological IgM and IgG Brucella specific-antibodies, but also became serologically negative earlier than animals immunized with the non-encapsulated 16MΔvjbR strain or Rev.1 strain, as determined by the BPA and iELISA tests. These results are in concordance with those of Zriba et al. (2019) who showed an elevated and prolonged level of anti-Brucella antibodies in sera from pregnant swine vaccinated with unencapsulated S19ΔvjbR strain over those vaccinated with the encapsulated strain [22]. We speculate that the initial amount of Brucella released from microcapsules following vaccination, which is three logs lower than the 109 non-encapsulated Brucella CFU freely available at the time of immunization (either 16MΔvjbR or Rev.1) [6], induced a lower humoral immune response post-vaccination in encapsulated 16MΔvjbR animals than in the other two vaccinated groups. Independently, the long lasting humoral immune response induced by the candidate vaccine (encapsulated or non-encapsulated) was reflected in the increased level of anti-Brucella IgG antibodies in goats’ serum at delivery, which is associated with the physiological phenomenon of an unspecific increase of total IgG concentration in serum a few days ante-partum [23]. Even though there is no agreement regarding the role of humoral immunity in protection against Brucella infection [24], a rapid drop in serum antibodies in encapsulated vaccinated animals would be an advantage to distinguish them from naturally infected goats by current serological diagnostic techniques.
When we investigated the anti-Brucella IgG isotype profile in sera of vaccinated goats, the results demonstrated higher levels of IgG2 than IgG1 at 28 days post immunization. While there was minimal or no increase in the level of the IgG1 isotype over time, the level of IgG2 one month post immunization was two to six fold higher in all immunized groups compared to their pre-immune levels and compared to the level of this IgG isotype in non-vaccinated animals at the same time point (i.e., 4 weeks post immunization). The predominance of Brucella specific-IgG2 over -IgG1 antibodies after 16MΔvjbR immunization would suggest the development of a Type 1 (Th1) immune response, more effective to overcome Brucella invasion [25]. Coincidently with these results, Arenas-Gamboa et al. (2008) reported that the level of IgG2a was higher than the IgG1 in B. melitensis 16MvjbR::Tn5 immunized BALB/c mice [6]. Curiously, Dorneles et al. (2015) reported a predominance of the IgG1 isotype over IgG2 in sera of naïve heifers at 4 weeks post-vaccination with B. abortus S19 or RB51 [26], the two most extensively used and effective vaccines in the control of brucellosis in cattle that have proven to be effective for disease control. Independently, the induction of anti-Brucella IgG isotype of the vaccine candidate strain was similar to the commercially available vaccine Rev.1, well known to induce a solid and lifelong immune response [1].
5. Conclusions
Previous to this investigation, the B. melitensis 16MΔvjbR strain had been studied only in terms of safety and efficacy in the mouse model. In this study, for the first time, the safety and immunogenicity developed by the vaccine strain candidate was demonstrated in the natural host for over a 15 month period. More specifically, these results indicate that female goats immunized with B. melitensis 16MΔvjbR strain showed no evidence of clinical alterations, shedding into the environment, transmission or tissue colonization. Altogether, these favorable results support future studies to demonstrate this vaccine’s efficacy against organ colonization and prevention of abortion against a B. melitensis infection.
Supplementary Material
(A) Body temperature’s average of goats immunized with B. melitensis 16MΔvjbR, B. melitensis 16MΔvjbR encapsulated or B. melitensis vaccine strain Rev.1, or animals inoculated with empty capsules (controls) was monitored at different times during the first month after immunization by a subcutaneously implanted microchip. All temperatures remained below the threshold established for hyperthermia (40°C).
(B) Body temperature of nannies showed no hyperthermia right after abortion or normal delivery independently of the treatment received. Temperature from only 15 animals could be registered after abortion or delivery.
IgM antibody profile was determined in serum by iELISA before (T0) and after vaccination. The results are expressed as the mean of OD values (450nm) with standard error of 10 individual goats. Statistical analysis was performed by comparing the mean of the groups using two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. Cut off value was set at 0.22. Significant differences between vaccine treatment groups and the control group were found at both time point and are indicated by asterisks (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
Serologic response was determined by iELISA and the results were expressed as the mean of OD values (450nm). Statistical analysis was performed by comparing the mean of the groups using two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. (A) The presence of anti-Brucella IgM antibodies was determined in sera of kids at 30–45 days of age (arbitrary unified in week 5 post-partum). No statistical differences between vaccine treatment groups and the control group were found (p>0.05). Cut off = 0.22.
(B) Serologic IgG antibodies in kids born from experimental goats was identified at 30–45 days of age (arbitrary unified in week 5 post-partum) and at necropsy (arbitrary unified in week 9 post-partum). No statistical differences were found among groups (p>0.05). Cut off = 0.39.
The potential seroconversion in bucks as a consequence of sexual transmission of the vaccine strain was evaluated by iELISA. Animals were seronegative at the beginning of the mating season. Anti-Brucella IgG antibodies were not detected immediately after being removing from the females’ pen (T0) nor three weeks afterward (T3).
Tissues from offspring of goats vaccinated with empty capsules suspended in MOPS (row 1), 1×109 CFU Rev.1 (row 2), 1×109 CFU unencapsulated16MΔvjbR (row 3), or 1×109 CFU encapsulated 16MΔvjbR (row 4). No significant histopathological abnormalities were noted in any of the tissues. H&E, Magnification = 10×, Bar = 50 μm.
Acknowledgements
This study was supported by the National Institute of Health (NIH) Grant # K01TW009981 International Research Scientist Development Award (IRSDA/K01) and Instituto Nacional de Tecnología Agropecuaria (INTA), Grant PNSA 1115052. Student stipend support provided by the NIH Institutional Training Grant T32 fellowship (5OD11083-7) (MH) and by CONICET (Argentina) (EM).
The authors want to thank to Mr. Diego Franco and Mr. Claudio Fiorini for their kind help with animal handling. We also thank the veterinarians, Victor Vanzini (EEA-Rafaela-INTA) and Ana Nicola (SENASA Argentina), for their kind donation of the B. abortus 1119-3 LPS and the Rev.1 vaccine, respectively; and Camila Foster (DVM), Dr. Ursula A. Rossi and Mrs. R. Amalia Salvatierra for their technical assistance.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Allison Rice-Ficht, managing partner of NanoRelease Technologies (NRT), LLC Inc., has a 95% equity interest in NRT, a company involved in vaccine delivery platforms. The terms of this arrangement have been reviewed and approved by TXAgriLife Research and Texas A&M University in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alton G. Brucella melitensis. In: Nielsen KH, Duncan J, editor. Anim. Brucell, Boca Raton, Florida, USA: CRC Press, Inc; 1990, p. 383–409. [Google Scholar]
- [2].Adetunji SA, Ramirez G, Foster MJ, Arenas-Gamboa AM. A systematic review and meta-analysis of the prevalence of osteoarticular brucellosis. PLoS Negl Trop Dis 2019;13:e0007112. 10.1371/journal.pntd.0007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rossetti CA, Arenas-Gamboa AM, Maurizio E. Caprine brucellosis: A historically neglected disease with significant impact on public health. PLoS Negl Trop Dis 2017;11:e0005692. 10.1371/journal.pntd.0005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blasco JM. Control and eradication strategies for brucella melitensis infection in sheep and goats. Prilozi 2010;31:145–65. [PubMed] [Google Scholar]
- [5].Arenas-Gamboa AM, Rice-Ficht AC, Fan Y, Kahl-McDonagh MM, Ficht TA. Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1−/− mouse model. Clin Vaccine Immunol 2012;19:249–60. 10.1128/CVI.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect Immun 2008;76:2448–55. 10.1128/IAI.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weeks JN, Galindo CL, Drake KL, Adams GL, Garner HR, Ficht TA. Brucella melitensis VjbR and C12-HSL regulons: contributions of the N-dodecanoyl homoserine lactone signaling molecule and LuxR homologue VjbR to gene expression. BMC Microbiol 2010;10:167. 10.1186/1471-2180-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abraham SM, Vieth RF, Burgess DJ. Novel technology for the preparation of sterile alginate-poly-l-lysine microcapsules in a bioreactor. Pharm Dev Technol 1996;1:63–8. 10.3109/10837459609031419. [DOI] [PubMed] [Google Scholar]
- [9].Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Gomez G, Rice-Ficht AC. The Brucella abortus S19 DeltavjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect Immun 2009;77:877–84. 10.1128/IAI.01017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cherwonogrodzky JW, Nielsen KH. Brucella abortus 1119–3 O-chain polysaccharide to differentiate sera from B. abortus S-19-vaccinated and field-strain-infected cattle by agar gel immunodiffusion. J Clin Microbiol 1988;26:1120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hensel ME, Garcia-Gonzalez DG, Chaki SP, Samuel J, Arenas-Gamboa AM. Characterization of an intratracheal aerosol challenge model of Brucella melitensis in guinea pigs. PLoS One 2019;14:e0212457. 10.1371/journal.pone.0212457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pourjafar M, Badiei K, Chalmeh A, RahmaniShahraki A, Naghib M. Body temperature in horses, cattle, sheep and goats measured by mercury, digital and non-contact infrared thermometers. Online J Vet Res 2012;16:195–203. [Google Scholar]
- [13].Alton GG. Control of Brucella melitensis infection in sheep and goats--a review. Trop Anim Health Prod 1987;19:65–74. 10.1007/bf02297320. [DOI] [PubMed] [Google Scholar]
- [14].Jimenez de Bagues MP, Marin CM, Barberan M, Blasco JM. Responses of ewes to B. melitensis Rev1 vaccine administered by subcutaneous or conjunctival routes at different stages of pregnancy. Ann Rech Vet 1989;20:205–13. [PubMed] [Google Scholar]
- [15].Lantier F, Fensterbank R. Kinetics of Rev.1 infection in sheep. In: Verger JM, Plommet M, editors. Brucella melitensis, Nouzilly, France: Martinus Nijhoff Publishers; 1985, p. 247–52. [Google Scholar]
- [16].Elberg SS, Faunce KJ. Immunization against Brucella infection. VI. Immunity conferred on goats by a nondependent mutant from a streptomycin-dependent mutant strain of Brucella melitensis. J Bacteriol 1957;73:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Diaz Aparicio E. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev Sci Tech 2013;32:53–60. [PubMed] [Google Scholar]
- [18].Alexander B, Schnurrenberger PR, Brown RR. Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet Rec 1981;108:500. 10.1136/vr.108.23.500. [DOI] [PubMed] [Google Scholar]
- [19].Ponsart C, Riou M, Locatelli Y, Jacques I, Fadeau A, Jay M, et al. Brucella melitensis Rev.1 vaccination generates a higher shedding risk of the vaccine strain in Alpine ibex (Capra ibex) compared to the domestic goat (Capra hircus). Vet Res 2019;50:100. 10.1186/s13567-019-0717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zundel E, Verger JM, Grayon M, Michel R. Conjunctival vaccination of pregnant ewes and goats with Brucella melitensis Rev 1 vaccine: safety and serological responses. Ann Rech Vet 1992;23:177–88. [PubMed] [Google Scholar]
- [21].Li Z, Wang S, Zhang H, Xi L, Zhang J, Zhang X, et al. Development and evaluation of in murine model, of an improved live-vaccine candidate against brucellosis from to Brucella melitensis vjbR deletion mutant. Microb Pathog 2018;124:250–7. 10.1016/j.micpath.2018.08.052. [DOI] [PubMed] [Google Scholar]
- [22].Zriba S, Garcia-Gonzalez DG, Khalaf OH, Wheeler L, Chaki SP, Rice-Ficht A, et al. Vaccine safety studies of Brucella abortus S19 and S19DeltavjbR in pregnant swine. Vaccine X 2019;3:100041. 10.1016/j.jvacx.2019.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Micusan V V, Borduas AG. Biological properties of goat immunoglobulins G. Immunology 1977;32:373–81. [PMC free article] [PubMed] [Google Scholar]
- [24].Goenka R, Parent MA, Elzer PH, Baldwin CL. B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis 2011;203:1136–46. 10.1093/infdis/jiq171. [DOI] [PubMed] [Google Scholar]
- [25].Martirosyan A, Moreno E, Gorvel J-P. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev 2011;240:211–34. 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- [26].Dorneles EMS, Lima GK, Teixeira-Carvalho A, Araujo MSS, Martins-Filho OA, Sriranganathan N, et al. Immune Response of Calves Vaccinated with Brucella abortus S19 or RB51 and Revaccinated with RB51. PLoS One 2015;10:e0136696. 10.1371/journal.pone.0136696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Body temperature’s average of goats immunized with B. melitensis 16MΔvjbR, B. melitensis 16MΔvjbR encapsulated or B. melitensis vaccine strain Rev.1, or animals inoculated with empty capsules (controls) was monitored at different times during the first month after immunization by a subcutaneously implanted microchip. All temperatures remained below the threshold established for hyperthermia (40°C).
(B) Body temperature of nannies showed no hyperthermia right after abortion or normal delivery independently of the treatment received. Temperature from only 15 animals could be registered after abortion or delivery.
IgM antibody profile was determined in serum by iELISA before (T0) and after vaccination. The results are expressed as the mean of OD values (450nm) with standard error of 10 individual goats. Statistical analysis was performed by comparing the mean of the groups using two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. Cut off value was set at 0.22. Significant differences between vaccine treatment groups and the control group were found at both time point and are indicated by asterisks (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
Serologic response was determined by iELISA and the results were expressed as the mean of OD values (450nm). Statistical analysis was performed by comparing the mean of the groups using two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. (A) The presence of anti-Brucella IgM antibodies was determined in sera of kids at 30–45 days of age (arbitrary unified in week 5 post-partum). No statistical differences between vaccine treatment groups and the control group were found (p>0.05). Cut off = 0.22.
(B) Serologic IgG antibodies in kids born from experimental goats was identified at 30–45 days of age (arbitrary unified in week 5 post-partum) and at necropsy (arbitrary unified in week 9 post-partum). No statistical differences were found among groups (p>0.05). Cut off = 0.39.
The potential seroconversion in bucks as a consequence of sexual transmission of the vaccine strain was evaluated by iELISA. Animals were seronegative at the beginning of the mating season. Anti-Brucella IgG antibodies were not detected immediately after being removing from the females’ pen (T0) nor three weeks afterward (T3).
Tissues from offspring of goats vaccinated with empty capsules suspended in MOPS (row 1), 1×109 CFU Rev.1 (row 2), 1×109 CFU unencapsulated16MΔvjbR (row 3), or 1×109 CFU encapsulated 16MΔvjbR (row 4). No significant histopathological abnormalities were noted in any of the tissues. H&E, Magnification = 10×, Bar = 50 μm.