Abstract
Looking retrospectively at the development of humanity, vaccination is an unprecedented medical landmark that saves lives by harnessing the human immune system. During the ongoing coronavirus disease 2019 (COVID-19) pandemic, vaccination is still the most effective defense modality. The successful clinical application of the lipid nanoparticle-based Pfizer/BioNTech and Moderna mRNA COVID-19 vaccines highlights promising future of nanotechnology in vaccine development. Compared with conventional vaccines, nanovaccines are supposed to have advantages in lymph node accumulation, antigen assembly, and antigen presentation; they also have, unique pathogen biomimicry properties because of well-organized combination of multiple immune factors. Beyond infectious diseases, vaccine nanotechnology also exhibits considerable potential for cancer treatment. The ultimate goal of cancer vaccines is to fully mobilize the potency of the immune system as a living therapeutic to recognize tumor antigens and eliminate tumor cells, and nanotechnologies have the requisite properties to realize this goal. In this review, we summarize the recent advances in vaccine nanotechnology from infectious disease prevention to cancer immunotherapy and highlight the different types of materials, mechanisms, administration methods, as well as future perspectives.
KEY WORDS: Vaccine, Nanotechnology, Infection, Cancer, Nanovaccines, Vaccination, Diseases prevention, Immunotherapy
Graphical abstract
In this review, we summarize the recent advances in the application of vaccine nanotechnology for infectious disease prevention and cancer immunotherapy, involving the contribution of materials, mechanisms, and administration methods.
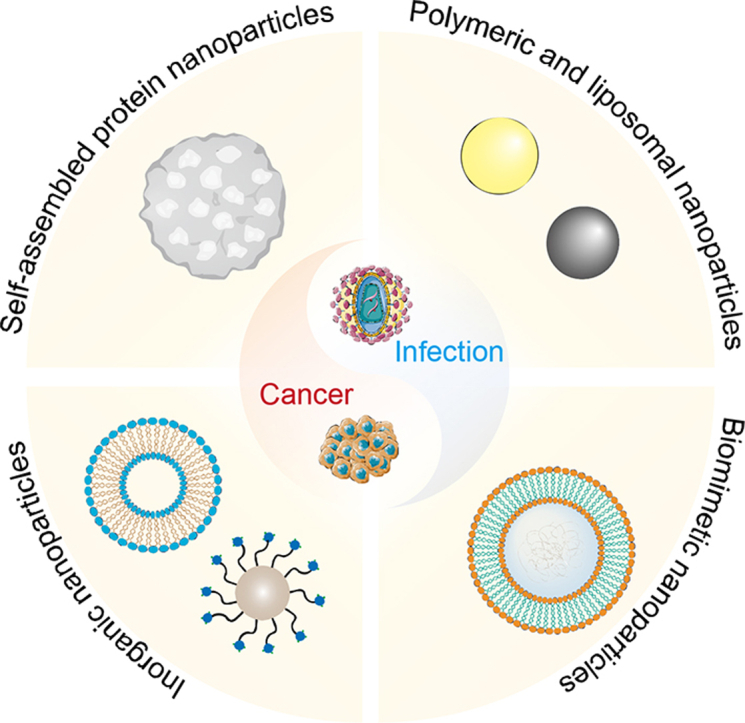
1. Introduction
Without vaccines, infectious diseases cause several disasters for human beings. Vaccines represent an unprecedented milestone in medical history because they successfully harness the human immune system to combat pathogens. Vaccines trace back to “variolation” in the 10th century AD. In 1798, “the father of vaccines,” Dr. Edward Jenner, tested his hypothesis that subjects inoculated with substances from cowpox lesions could receive immunity from smallpox, laying the foundation for modern vaccine's development. Modern vaccines predominately function by two core mechanisms that stimulate active immunity against pathogens and confer passive immunity with pre-existing antibodies or lymphocytes. Throughout human history, vaccination has saved more lives than any other forms of medical intervention, and has played an indispensable role in public health and safety. Vaccination is the most effective and efficient medical modality for combating the coronavirus disease 2019 (COVID-19) pandemic which had a negative impact on the global society and economy.
However, the development of protective vaccines against numerous lethal pathogens lacking effective protective vaccines, such as human immunodeficiency virus (HIV), tuberculosis (TB), and malaria, has proven difficult, contributing to high disease mortality rates in communities with limited resources. The difficulties associated with novel vaccine design are the products of the co-evolutionary history of the pathogen and humans (e.g., virus variability). Moreover, other types of diseases, particularly cancer, benefit from the mobilization of autoimmunity. Therefore, the new era of vaccine development must overcome more challenges, such as the precise combination of humoral and cellular immunity, the ordered activation of immune components, and immune tolerance or low reactivity. The emerging concentrations of material and biomedical science may provide novel technologies for tailorable vaccine design. As one of the most promising and attractive candidates, nanotechnology approaches have a unique position to address the challenges of vaccination and mine more application potentials in cancer treatment. In accordance to their tailored structures, modular compositions, and controlled length scales, vaccine nanotechnologies possess versatile properties, including multivalent target delivery to lymphoid tissues and specific immune cells, multistage stimulating control release of immune components, key immune pathway engagement, and perfect iterative design systems1, 2, 3. It is worth mentioning that the successful application of the Pfizer/BioNTech and Moderna mRNA COVID-19 vaccines have drawn attention to nanotechnology and highlighted its vital role in emerging vaccination development4,5. As an indispensable component of the mRNA vaccine, lipid nanoparticles are vital for protecting mRNA COVID-19 vaccines from nucleases and effectively delivering these vaccines to the right place6.
Cancer immunotherapy, particularly immune checkpoint blockade (ICB) and chimeric antigen receptor T-cell (CAR-T) immunotherapy, has already been validated successfully in the clinic. However, the low patient response rates and off-target adverse events associated with nanotechnologies indicate that there remain many challenges for the successful application of these technologies7,8. During an antitumor immune response, a chain of stepwise events must switch on and expand iteratively, including cancer-associated antigen release, antigen presentation, immune cells activation, T cells infiltration into tumor microenvironment, specific recognition of tumor cells, and the elimination of cancer cells9. Considering the feature of above mentioned antitumor-immunity process, in theory, the absence of any step would result in the final failure of the effective anti-tumor immune response. Therefore, cancer immunotherapy has shifted from focusing on one step to focusing on entire process of the cancer-immunity cycle: the combination of different immune agents that function by different mechanisms, for example, has been promising9. As one of the hopeful cancer immunotherapy candidates with tailorable components and ordered integration, vaccine nanotechnology may support strategies and platforms that realize more effective activation of antitumor immunity.
This review predominately focuses on the nanomaterial vaccines and provides up-to-date progress from infectious diseases prophylaxis to cancer immunotherapy. The rational structural designs of various types of nanovaccines are highlighted, ranging from polymeric nanoparticles, nanogels, inorganic materials, and membrane-coated mimic structures (Fig. 1). Moreover, we investigate the critical roles of immunity activation mechanisms, immune component combination strategies, and different administration strategies in vaccination. Finally, we discuss the perspectives and challenges of vaccine nanotechnology development, especially in clinical translation. The aim of this mini-review, therefore, is to not only summarize the recent advances but also focus on the future application of next-generation vaccine nanotechnology.
Figure 1.
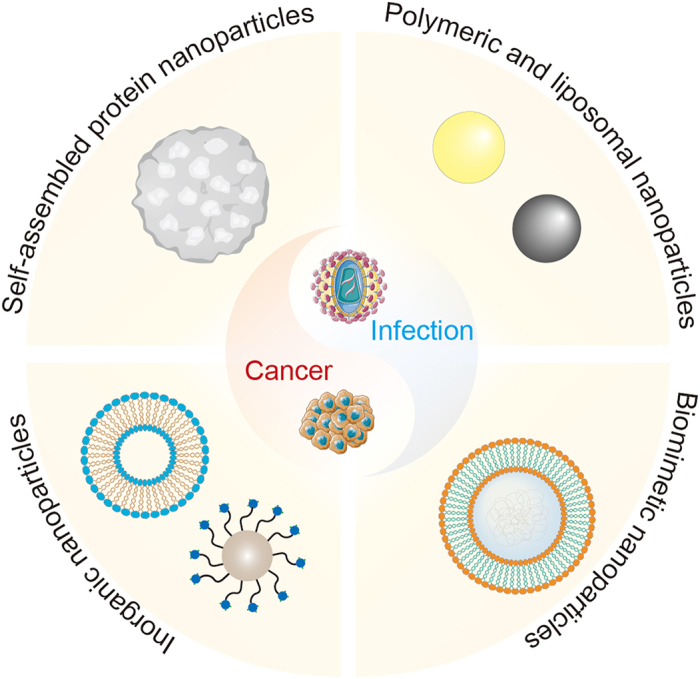
Schematic illustration of emerging vaccine nanotechnology for infectious diseases prevention and cancer immunotherapy. Main types of nanovaccines include self-assembled protein nanoparticles, inorganic nanoparticles, polymeric and liposomal nanoparticles and biomimetic nanoparticles.
2. Representative promising strategies to improve the immune response of nanovaccines
The key principle of vaccination is how to trigger the proper immune response to target antigens. Underlying this process is a complicated network responding to exogenous and endogenous danger stimuli, which are involved in various immune cell types and concerted by innate and adaptive immune responses. The flexible design of nanomaterials endows nanovaccines with improved specific immune responses. The vaccines mainly benefit from the unique drug/antigen delivery properties and nano-enabled immunomodulation of nanomedicine. In this section, we focus on these strategies for evoking the immune response.
2.1. Delivering antigens to key cells and tissues of the immune system
One of the most promising areas in which nanotechnology is applied is drug delivery. As for vaccination, delivering an antigen to the right place in the immune system is also of great importance. Unlike other types of drug delivery to precise cell types, the antigen vaccine delivery process involves spatiotemporal interactions of several cell types, including antigen-presenting cells (APCs), B cells, various T cells, macrophages, and neutrophils. In addition, the above interactions tend to occur in a specific tissue or location, further complicating antigen delivery. Therefore, several promising strategies have been employed to design nanovaccines, such as crossing the biological barrier, lymph node (LN) trafficking, the controlled release of antigen, APCs targeting, cross-presentation, among others10,11.
Researchers widely hypothesized that prolonged persistence of antigen or minimized unnecessary degradation of antigen would enhance immune response. Therefore, nanomedicine researchers were devoted to increasing the persistence of antigens at the injection sites, in lymphoid tissues, and even in APCs by conjugating or encapsulating antigens in nanomaterials12,13. Beyond the precondition of antigen persistence in the internal environment, LN delivery is highly sought after for its design because of the large population of immune cells residing in LNs. Currently, only a small amount of the antigen at the injection site can be delivered to the LNs by APCs; to enhance LN delivery methods, researchers need to further adjust multiple physical properties of nanoparticles, such as their charge, shape, size, and flexibility14, 15, 16, 17. Researchers demonstrated that nanoparticles smaller than 100 nm tend to drain to the LNs and that different nanomaterials may have different optimal sizes for LN delivery18,19. Given the increasing interest in the mucosal immune response, delivery methods that effectively cross the mucosal barrier are also considered highly in vaccine design. Owing to the negatively charged porous mucin glycopolymer structure, mucosal delivery efficacy depends largely on the size and surface charge of nanoparticles20,21. Thus, nanoparticles smaller than the cut-offs of mucosal and cationic nanoparticles (below about 200 nm) can achieve promising mucosal delivery22. Following the delivery of antigens in the appropriate tissues and locations, the internalization, processing, and presentation of antigens by APCs is critical to evoke a strong immune response. Hence, researchers manipulate the physical characteristics of nanoparticles (e.g., their charge, shape, and size) to promote APC uptake of antigens and a consequent strong immune response23,24. For example, 20–200 nm nanoparticles are more easily internalized by a common type of APC, dendritic cells (DCs)25. Targeted nanoparticle delivery to DCs can be achieved by modifying specific ligands for affinity-based targeting DC subsets, such as C-type lectin receptors26. Moreover, it was revealed that multivalent antigen structures can enhance antigen recognition and activation of B cells—another type of APCs27.
2.2. The application of multivalency effect in nanovaccine
Most viruses and bacteria have unique repetitive structures that the immune system can detect. It is revolutionary to consider this repetitive antigen structure in vaccine design. There is evidence to suggest that the multivalency effect elicits a stronger humoral and cellular immune response in self-assembled polypeptide nanoparticles28, multiple antigen conjugated nanoparticles29, and other multivalent assemblies30,31. And most encouragingly, nanotechnology has an absolute advantage in manipulating antigen density and orientation, providing great platforms for investigating the underlying mechanisms of multivalency effect and its optimizing strategies. Puffer and colleagues32 found that the multivalency of hapten induced higher levels of antibodies, correlated with increased Ca2+ signaling in B cells. Moreover, multivalent hapten even confers immunogenicity to low-affinity epitopes33. With more in-depth research, the multivalency of antigens can be programmed to activate immune cells by several pathways, such as complement activation34, during which B cell receptors crosslink through tyrosine-based activation motifs (ITAMs)29. Although the mechanism behind this phenomenon is elusive, virus-like particles (VLPs) with the multivalency effect can enhance B cell activation and downstream immune responses. The studies of nanomaterial-multivalent antigens for combating infectious diseases have exhibited great promise. For example, liposomes with multivalent HIV trimers have been found to increase antibody response breadths against target antigen protein regions, suggesting that multivalency can influence the antibody reservoir35. Further studies suggest that antibody responses can be shaped by programming specific epitopes; the specificity of the vaccine can be improved by burying undesirable epitopes and exposing desirable epitopes, which reduces responses to the immunodominant, non-neutralizing regions of HIV trimers36. Protein-based nanomaterials display alteration was also applied in screening neutralizing region for binding neutralizing antibodies37. Although questions about how antigen orientation influences immune responses remain, nanomaterial platforms are advantageous experimental tools for deeper investigation.
2.3. Delivering nucleic acid for antigen expression in vivo
The successful application of mRNA vaccines to combat the COVID-19 pandemic has demonstrated the limitless potential of nucleic acid-based vaccines. The efficacy of nucleic acid-based vaccines depends predominately on the delivery of DNA or RNA molecules, which upregulate the expression of target encoding antigens and evoke a specific, strong immune response in target immune cells. DNA vaccines were supposed to have great promise in infectious diseases prophylaxis and treatment because they are simple, stable, and inexpensive to mass produce38,39. However, inefficient plasmid DNA (pDNA) delivery in vivo impaired the effectiveness and limited the further preclinical application. For example, traditional DNA vaccines tend to spread rapidly after injection, resulting in a diminished probability of pDNA interacting with APCs. In addition, the inherent risk of traditional viral delivery pushed nonviral vectors, which are relatively safe, into focus. Among the promising nonviral vectors are nanomaterials, which stand out due to their specific delivery advantages; the need to efficiently deliver novel mRNA-based vaccines further advanced the development of nanomedicine in vaccine design. As previously mentioned, nanoparticles can be programmed with specific LN and APC targeting abilities, which may apply to nucleic acid-based vaccines too40,41. Unlike protein/peptide antigen, nucleic acids are more susceptible to degradation by endonucleases. Additionally, the nonspecific immune response to foreign nucleic acids is a nonnegligible hindrance for clinical translation42. Therefore, when designing nucleic acid nano delivery systems, researchers must consider an encapsulating element to protect the nucleic acids from endonuclease enzymes43, 44, 45.
In addition to the double-stranded DNA located in the nucleus, there is single-stranded mRNA, which the ribosomes translate codon-by-codon for protein production in the cytoplasm. Thus, mRNA vaccines can upregulate the expression of antigens in the cytoplasm directly without having to cross the nuclear envelope42,46. Moreover, the undesirable immune response to foreign mRNA can be assuaged by incorporating modified nucleosides, such as pseudouridine and 5-methylcytidine, into the mRNA transcript47,48. Considering these advantages, the mRNA vaccine was supposed to exhibit better antigen expression efficiency and faster clearance, which are conducive to clinical translation. And most encouragingly, this hypothesis was largely confirmed by the approval of the Pfizer/BioNTech and Moderna mRNA COVID-19 vaccines. It is worth mentioning that nanotechnology plays an important role in mRNA COVID-19 vaccines4, 5, 6. The two vaccines are cationic lipid nanoparticles, consisting of a cholesterol, an ionizable cationic lipid, a PEGylated lipid, and a phospholipid distearoylphosphatidylcholine (DSPC) helper lipid6. Cationic lipids, the most commonly employed nanomaterials, are often prepared by prepared by complexing cationic polymers/lipids with negatively charged nucleic acids; this structure, helps protect mRNA from degradation and immunorecognition.
Beyond treating infectious diseases, nucleic acid-based vaccines have long been promising candidates for cancer treatment. However, due to immunosuppression in the tumor microenvironment, vaccine design should involve numerous pathways to activate a sufficient antitumor immune response. It was revealed that nucleic acid molecules also participate in tumor immunomodulation49,50. For example, some nucleic acids can function as immune adjuvants51, and small interfering RNA (siRNA) can inhibit PD-L1 expression for tumor suppression52. Besides, nucleic acids can also be used as vaccine vectors. Liu and co-workers53 developed a DNA nanodevice with a tubular structure that loads molecular adjuvants and antigen peptides, inducing a strong antitumor immune response.
2.4. Trigger tumor antigen release in situ
The existence of tumor heterogeneity and immunosuppression in the tumor microenvironment complicates cancer vaccine design. Among the most difficult challenges is obtaining tumor antigens. Although there are countless types of tumor antigens employed for developing cancer vaccine, such as antigen coding mRNA, model antigens, and neoantigens, it is too difficult to obtain broad-spectrum tumor antigens for clinical vaccination because of tumor heterogeneity. For example, some nanomaterial-based cancer vaccines comprising ovalbumin (OVA) as a model tumor antigen exhibited remarkable antitumor efficacy in OVA-transfected tumor-bearing animal models, but they still stay on theoretical model and would be hard to directly achieve clinical transformation. To escape the cognitive inertia of adopting a single antigen in tumor vaccine design, researchers have studied tumor cell lysate and biomimetic cell membrane-based vaccines54. These strategies are limited by low immunogenicity, and thus, immune adjuvants and other evoking immunity strategies, which are reported below, are indispensable54.
Instead of introducing an antigen via vaccination, it is possible to trigger the release of tumor antigens in vivo. One such mechanism is to trigger immunogenic cell death (ICD), which results in the release of tumor-associated antigens (TAAs), the danger-associated molecular patterns (DAMPs), and proinflammatory factors to evoke adaptive antitumor immunity55. The ICD process can be triggered by a series of antitumor therapies, including certain chemotherapies, phototherapies, radiotherapies, sonodynamic therapies, and local hyperthermia treatments55, 56, 57, 58. The aforementioned therapies are also supposed to reverse “cold tumors” to “hot tumors” behind which the mechanisms could involve ICD induction, promoting immune cells infiltration and macrophage phenotype transition from M2 to M1. Moreover, by harnessing the superior delivery capabilities of nanomedicines, the effect of ICD inducers can be amplified synergistically with other immunotherapeutic agents, such as immune checkpoint inhibitors, indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitors, and stimulator of interferon genes (STING), for combating immunosuppression56. Therefore, besides the classical co-delivery of antigens and immune adjuvants, the co-delivery of ICD inducers and immunotherapeutic agents is a promising design strategy for nanovaccines in solid tumor treatment.
2.5. Immune adjuvants and other immune evoking strategies
Immune adjuvants are an indispensable component of the vaccine and play an auxiliary role in enhancing the immune system's response to the presence of antigens. Recently, immune adjuvant researchers have shifted their attention to natural ligands or synthetic agonist-based adjuvants for targeting pattern recognition receptors (PRRs)51. Although almost all of those adjuvants participate in the activation of APCs, the sciences underlying these mechanisms are diverse. Among those types of adjuvant, toll-like receptor (TLR) agonists have been studied extensively for their application to vaccination51. Bacterial lipopolysaccharides (LPS), for example, are TLR 4 ligands, whose detoxified derivative monophosphoryl lipid A (MPL) serves as a component of the licensed vaccines for HBV and papilloma59. Moreover, synthetic oligodeoxynucleotides (ODN) with optimized CpG motifs (CpG- ODN) are widely used as TLR 9 ligands in nanomaterial-based vaccines, such as antigen-adjuvants vaccines and ICD inducer-adjuvant co-delivery systems51,60. With the help of nanomedicine platforms, researchers can deliver adjuvants more precisely and with improved stability. It is worth mentioning that some nanomaterials exhibit the inherent adjuvant properties of promoting cytokines secretion and activating immune signal pathways61,62. Moreover, nanomaterials possess the properties of phototherapy or ROS generation63, 64, 65, 66, 67, 68, 69, 70, which can also induce ICD effect in cancer immunotherapy63. These self-adjuvant nanomaterials provide more possibilities and potential for the application of nanomedicine in vaccines.
As immunology develops, more immune sensing pathways and immune promoting strategies have been presented. Especially in antitumor immunotherapy, activating CTL functions and combating immunosuppression have received increasing attention. As part of the revolution of tumor immunotherapy, several immune checkpoint inhibitors, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death protein 1 ligand (PD-L1) blocking antibodies, were approved by the US Food and Drug Administration (FDA). Furthermore, vaccines combining multiple immunotherapeutic agents or immune adjuvants have successfully evoked immune response cascades56. Recently, some interesting results involving the enhancement of antitumor immunity have attracted attention, such as intestinal microbiota modulation71. The emerging strategies will yield more novel ideas and practices in combination tumor vaccine design.
3. Administration strategies
Currently, most vaccines employed the parenteral route, which is invasive and has limited compliance, for delivery. The development of nanomedicine provided various options for vaccine routes including postoperative, intradermal/subcutaneous, intranasal, inhalation, and oral administration for both cancer therapy and infectious diseases.
3.1. Postoperative administration
Currently, surgery remains the primary option for solid tumor treatment. However, tumor recurrence remains a challenge as residual tumor cells can be a risk that leads to fast relapse and metastasis. Strategies of nanomedicine are emerging for postoperative tumor drug delivery and immunotherapy72.
To increase the efficiency of postoperative T-cell immunity, a thermo-responsive, curcumin-loaded polymer nanoparticles-assembled hydrogel with antigenic peptide and CpG-ODN was developed. This strategy can induce ICD and consequently enhanced the antitumor immunity73. This immunotherapy strategy promoted the infiltration of CTLs and inhibited local recurrence and pulmonary metastasis. In another study, an implantable 3D porous scaffold was designed to deplete myeloid-derived suppressor cells and present whole tumor lysates with nanogel-based adjuvants for promoting CTLs74. This immune niche strategy modulated the immunosuppressive environment and prevented postoperative tumor recurrence and metastasis. Autologous tumor cells are an excellent source for personalized postoperative nanovaccine development. Li and colleagues75 developed a hydrogel matrix loaded with JQ1, a PD-L1 checkpoint blockade inhibitor, and indocyanine green (ICG). This immunotherapy strategy combined with PDT effectively inhibited tumor relapse and metastasis. Further, they design a PDT-motivated nanovaccine composed of autologous cancer cells and Fmoc-KCRGDK–phenylboronic acid hydrogel. This nanomedicine can be prepared feasible to boost personalized immunotherapy76. Besides, specific autologous cancer cells can be combined with non-specific immune activation such as bacterial-derived membranes77. Postoperative nanovaccines are rising for the treatment of cancer.
3.2. Intradermal/subcutaneous administration
Intradermal/subcutaneous administration is a common route of immunization for DNA vaccines. Both epidermis and dermis layers of the skin contain resident APCs that are targeted for immunization. As the skin is painless, intradermal/subcutaneous administration has been widely applied for prophylactic vaccination.
In recent years, this administration strategy was also explored for anticancer therapy. It has been reported that subcutaneous immunizations using VLPs conjugated with human EGFR 2 epitopes induced elevated HER2-specific antibody titers against the HER2 positive malignancies78. For advanced intradermal administration, versatile microneedle systems have also been explored for tumor and infectious diseases vaccination. A transdermal vaccine may be applied for topical and intratumoral immunotherapy against melanoma. Autonomous active microneedle-mediated propulsive delivery of cowpea mosaic virus nanoparticles and magnesium (Mg) microparticles enhanced the antitumor immunity and greatly suppressed tumor progression79. Microneedles can also be dissolvable for vaccine delivery. Plasmodium falciparum surface protein P47 and CpG were loaded into microneedles and showed potent activation of TLR9 signaling for malaria vaccine80. Recently, a vaccine core and PLGA shell microneedle patch was developed for the long-last and programmed burst release of the vaccine81. This strategy may be used for both prophylactic and therapeutic purposes without repeated vaccination.
3.3. Intranasal administration
Intranasal administration is an important route for respiratory infectious diseases82. Nasal immunization via nanovaccines is promising for preventing diseases through mainly affecting infected respiratory tracts such as TB and for the treatment of cancers.
Chitosan nanoparticles are water-soluble platforms that can be explored for intranasal delivery of antigen for TB vaccination. Thiolated OVA conjugated to N-trimethylaminoethylmethacrylate chitosan showed elevated cellular uptake, deep cervical lymph nodes transport efficiency, and immune responses after intranasal administration83. Recently, inulin acetate, a natural polymer, was developed as an intranasal nanovaccine delivery system for its inherent adjuvant (TLR4 activation) ability84. This nanocarrier has the potential for mucosal vaccination via intranasal administration. For synthetic nanoparticles, a “self-healing microencapsulation” technology has been developed by Bailey and colleagues for the stable loading of antigens in PLGA particles. They used calcium phosphate adjuvant gel as a trapping agent for antigen encapsulation, leading to sustained release of OVA antigen and proliferation of CD8+ T cell via intranasal delivery85 and could be used as a single-dose vaccination platform86. More recently, for the controllable particle size, PLGA nanoparticle was used for intranasal delivery of all trans-Retinoic acid and prolonged the drug release for targeted treatment of TB in the lung87. For intranasal cancer nanovaccine delivery, a recent study developed a self-assembled nanovaccine loaded with multiple OVA peptide antigens. This nanovaccine is safe through nasal administration and prolonged residence time and increased the antigen uptake efficiency, which led to enhanced antigen-specific immune response88.
3.4. Inhalation administration
Inhalation administration is also a promising vaccination route for pulmonary infectious diseases such as TB. Synthetic nanoparticles are useful tools for inhalation formulations.
Polymeric nanocapsules with oily core and polymer shell have been developed for pulmonary delivery of imiquimod, a TLR-7 agonist, and a fusion antigen protein89. Vaccination of this polymeric nanocapsule induced strong immune responses. The development of biomimetic nanotechnology offered strategies for developing nanovaccines by imitating respiratory droplets. In a recent study, a bionic-virus nanovaccine that mimics the structure of SARS-CoV-2 was developed by using liposomes as capsid structure and the receptor binding domains as “spike”90. This inhalable nanovaccine induced strong mucosal immunity and this nanovaccine strategy can also be used for other respiratory infectious diseases.
In addition, inhalation administration can also be applied for cancer nanovaccines such as lung metastasis. It has been reported that inhalation of the VLPs can facilitate the neutrophils infiltration in tumor, and increase cytokines and chemokines production and macrophage inflammatory protein 1α in tumor-bearing mice91. This nanovaccine treatment significantly reduced metastatic tumor burden for various tumor types.
3.5. Oral administration
Oral administration is a noninvasive route with excellent compliance92. Oral vaccines are optimal formulations for administration, immunization, safety, and storage. Despite the existence of lymphatic tissues under the mucous, the intestine forms a barrier for antigens. To develop a vaccine for oral administration, antigens are uptake and transported by epithelial cells and then recognized by immune cells for responses. During the process, antigens may degrade in the gastrointestinal tract resulting in a small number of antigens exposed to the mucosal tissue and limited intestinal uptake.
Several nanocarriers have been developed as TB vaccines that can be orally administrated. Liposome-encapsulated DNA vaccines can induce effective immune responses against TB93. VLP can also be used to carry HIV envelope cDNA with enhanced stability in the gastric environment. This strategy leads to high antigen concentration across intestinal lumen after oral administration94. In another example, polyethyleneimine-coated SPIONs loaded with malarial DNA showed high DNA binding and transfection efficiency even in the acidic environment95.
Oral administration strategy may also be used for cancer vaccines. It has been reported that nanoemulsions have high encapsulation capacity for co-delivery of melanoma antigen, heal shock protein, and staphylococcal toxin A for oral administration. This oral delivery strategy showed comparable immune responses to subcutaneous immunization96.
4. Type of nanomaterial-based vaccines
Recently, various nanomaterials for developing vaccines have been explored, including lipid-based nanoparticles, protein nanoparticles, polymeric nanoparticles, inorganic nanocarriers, and biomimetic nanoparticles. Different types of nanocarriers have distinct physicochemical profiles and behaviors in vivo, that influence vaccination accordingly. Here, we will briefly discuss the different types of nanomaterials for nanovaccines and their features.
4.1. Self-assembled protein nanoparticles
Natural nanomaterials have excellent biocompatibility and biodegradability. Several types of protein nanoparticles made of natural source proteins have been utilized for the delivery of antigens97. Self-assembled protein nanoparticles are promising candidates for nanovaccines. Typical examples of self-assembled protein nanoparticles include ferritin family proteins, pyruvate dehydrogenase (E2), and virus-like particles (VLPs) which have shown potentials in the development of nanovaccines.
VLPs are self-assembled complexes composed of viral proteins, which are supposed to be safe and highly efficient delivery platforms for antigen delivery without genetic components and replication ability98. VLPs have favorable immunological properties as they are self-adjuvants and can be immunologically recognized for the virus size and repetitive surface geometry. VLPs-form polydispersed systems can be efficiently uptaken by APCs and induce immune responses99. Antigens can be chemically coupled or genetically modified to VLPs with high density. VLPs-based vaccines have resulted in successful immunization programs as they are currently available in the market such as Cervarix® and Gardasil® against human papillomavirus virus (HPV) and Sci-B-Vac™ against hepatitis virus.
In contrast to exogenous viral proteins, several endogenous self-assembled proteins can also be explored as nanovaccine platforms, those protein nanoparticles are also called caged protein nanoparticles for their highly organized structures100. Ferritin is a typically caged protein nanoparticle that has widely been used for antigen delivery, drug delivery, imaging, and diagnostic applications101. Classical ferritin is comprised of 24 subunits, forming a central hollow cavity structure (12 nm × 8 nm) that stores iron. Antigen proteins can be genetically modified as subunits to form ferritins or can be incorporated onto ferritins to be efficiently phagocytosed by APCs. It has been reported that ferritin can passively target lymph nodes with a high retention time and induce strong immune responses79.
4.2. Polymeric nanoparticles
Polymeric nanoparticles are colloidal systems with a wide size range (10–1000 nm)102. Polymeric nanoparticles have high immunogenicity and stability for efficient encapsulation and display of antigen, which can be loaded within the core and conjugated to the surface. While polymeric nanoparticles are generally solid, they have controllable size and can be self-adjuvant103. Polymeric nanoparticles can improve the efficiency of antigen uptake by APCs by phagocytosis or endocytosis104.
For the development of nanovaccines, natural polymeric nanomaterials such as chitosan and dextran and synthetic polymeric nanomaterials such as PLA and PLGA are both useful tools. Polymeric nanoparticles from natural sources are highly biocompatible, water-soluble, and cost-saving. For example, chitosan, a typical natural polymer derived from chitin, is a linear cationic polysaccharide that can be used for vaccine delivery. Owing to its cationic charge and bioadhesive properties, chitosan is a competitive candidate for gene delivery and coating other polymeric nanoparticles to improve adherence and immunogenicity105. Moreover, chitosan can be customized depending on purpose by introducing functional groups106.
Compared to natural polymers, synthetic polymeric nanoparticles generally have higher reproducibility and are more controllable for molecular weight compositions and degradation rates107. For example, PLGA nanoparticle is highly biodegradable and its properties can be fine-tuned. PLGA can be coupled with PEG and then self-assembled into a polymeric micelle for hydrophobic peptide antigens delivery with better T cell responses108.
4.3. Lipid-based nanoparticles
Lipid nanoparticles (LNPs) are nanoscale lipid vesicles formed by amphipathic phospholipid molecules through self-assembling. LNPs are promising nanocarriers for nucleic acid delivery with low toxicity, high biocompatibility and controlled release properties109.
LNPs are also vital components for mRNA drugs and vaccines. LNPs have controllable size, shape and charge which are important properties that may affect the efficacy of immune activation. Modification of LNPs can achieve optimal immune responses110. As nanovaccines, LNPs can achieve co-delivery of multiple antigens and adjuvants. Besides, the membrane surface of LNPs can display antigen with enhanced representation of native conformations.
LNPs has shown great potentials for nanovaccine development in a number of preclinical and clinical applications. As mentioned earlier, the lipid nanoparticles play a vital role in protecting mRNA vaccines from nuclease for effective delivery. LNPs have successfully been translated for the delivery of mRNA against COVID-19 recently (mRNA-1273111 and BNT162b2112). There are many other LNP–mRNA formulations that are under ongoing clinical trials for the prevention and treatment of major human health threats including virus infections, cancers and genetic diseases as summarized in a recent review113. Cationic lipids, ionizable lipids and other types of lipids are all suitable components of LNPs. Besides, lipids can be functionalized by modification such as PEGlyation, making LNPs more versatile and powerful for vaccine development114.
4.4. Inorganic nanomaterials
Commonly used inorganic materials in nanomedicine include metals and oxides, non-metal oxides, and inorganic salts. Inorganic materials have low biodegradability but are stable in structure. Many inorganic nanoformulations have inherent adjuvant activity115. However, for nanovaccine application, the physicochemical properties of inorganic nanomaterials need to be modified to improve their biocompatibility. The most widely used inorganic materials for antigen delivery include gold116, iron117, and silica nanoparticles118.
Gold nanoparticles (GNPs) are spherical and positively charged. GNPs have good biocompatibility, low immunogenicity, and high antigen loading capacity. GNPs have size-dependent toxicity119; however, GNPs also have a high affinity to sulfhydryl groups120, which can be utilized for surface engineering to couple with cysteine residues to produce polypeptide antigens with improved safety and pharmacokinetic profiles. In addition, GNPs have intrinsic immunostimulatory effects to induce inflammatory cytokines production121. Therefore, GNPs can be used not only as a transport carrier for antigens but also for stimulating immune responses122. Silica nanoparticles are also potent candidates for nanovaccine carrier materials123. Recent studies have shown that controlling the morphology124 and pore size118,125 of silicon particles can make them have variable porosity, thereby increasing their effective load capacity for different antigens and adjuvants. Their porous structure of silicon particles can fill various active biomolecules or directly wrap on the surface, thereby enhancing the targeting and uptake of the nano-vaccine. Silica nanoparticles have been used to target lymph nodes and accumulate in APCs to deliver antigens and adjuvants126.
4.5. Biomimetic nanomaterials
Biomimetic nanomaterials are emerging for nanovaccine development for their effective and complex biofunctions127,128. Biomimetic nanomaterials are multifunctional and can achieve efficient delivery to the target site or effective interaction with biological systems. Bioinspired nanoparticles have also produced high biocompatibility, extended circulation and unique antigenic properties for the development of effective vaccine formulations.
A simple biomimetic design uses natural ligands or peptides, such as arginylglycylaspartic acid (RGD) and candoxin (CDX) peptides, to modify nanoparticles and enhance binding to improve targeting for efficient delivery129. In addition, molecularly imprinted polymers can also be used to mimic antibodies for developing biomimetic nanoparticles130. The decoration of nanoparticles with an individual natural ligand can endow specific functions, but multiple decorations would be rather difficult and can hardly replicate biological complexity. An emerging biomimetic strategy is to employ biomembranes to fabricate membrane-coated nanoparticles for enhancing biointerfacing131. Cell membrane–coating nanotechnology has been employed widely for improving circulation, targeted delivery, and imaging of nanoparticles132. For vaccine development, camouflaging with cell membrane may help nanoparticles to stay unnoticed in the immune system and target the lesion133. For example, the red blood cell (RBC) membrane can extend circulation and improve the bioavailability of nanoparticles134; platelet membranes can achieve targeting to the damaged vasculature and certain pathogens135; nanoparticles coated with cancer cell membranes have shown autologous targeting to cancer cells136; immune cell membrane-coating can endow the nanoparticle the ability to interaction with tumor tissues137. In addition to cell membranes, intracellular membranes such as outer mitochondrial membrane can also be utilized for specific targeting and detection138. Besides, biomimetic nanoparticles may be exploited as cancer nanovaccines with combined photothermal (PTT) and photodynamic therapy (PDT) against metastasis139,140.
Several other biomimetic strategies are emerging in nanovaccines design for combating infection and cancer. Virosome is a lipidic unilamerllar nanocarriers (60–200 nm) utilized the liposome concept but has structure similar to an enveloped virus with removed nucleocapsid141. Virosome is an emerging biomimetic nanoparticle for the development of nanovaccine against viral infections. Virosome can be developed by different antigen epitope to target host cells of interest and can be modified by polymers for enhanced pharmacokinetic profiles142. Outer membrane vesicles (OMVs) are bacterial-derived nanovesicles carrying various proteins similar to bacterial outer membranes. OMVs are natural antibacterial vaccination for their multi-antigen features143. In addition, OMVs have shown the ability of lymph node entry and can be take up efficiently by APC, making them attractive candidates for antigen delivery and vaccination. Antigenic OMVs can be explored as adjuvant delivery systems to improve vaccine efficacy144.
5. Nanovaccines for diseases prevention and treatments
Nanovaccines have been developed for various diseases. Here, we provide examples of how nanovaccines are being employed against cancers and infectious diseases, including HIV/AIDS, malaria and tuberculosis (TB). The most cutting-edge strategies of developing nanovaccines and their design aspects are included and discussed.
5.1. The prevention and treatment of worldwide infectious disease
HIV/AIDS, malaria, and TB are impacting global health and causing millions of deaths worldwide, highlighting the need for prevention and treatment strategies145, but vaccine strategies can hardly generate protective immunity in the population. HIV has a highly dynamic genome and unclear clear immune protection; malaria is complex for life cycles and there are multiple infection forms (sporozoites, merozoites, and gametocytes); patients with TB infection could be co-infected by HIV and bacteria that are multi-drug resistant. Despite dissimilar pathogens, the development of vaccines for these diseases shares some similarities and antigen delivery remains a key for vaccination146.
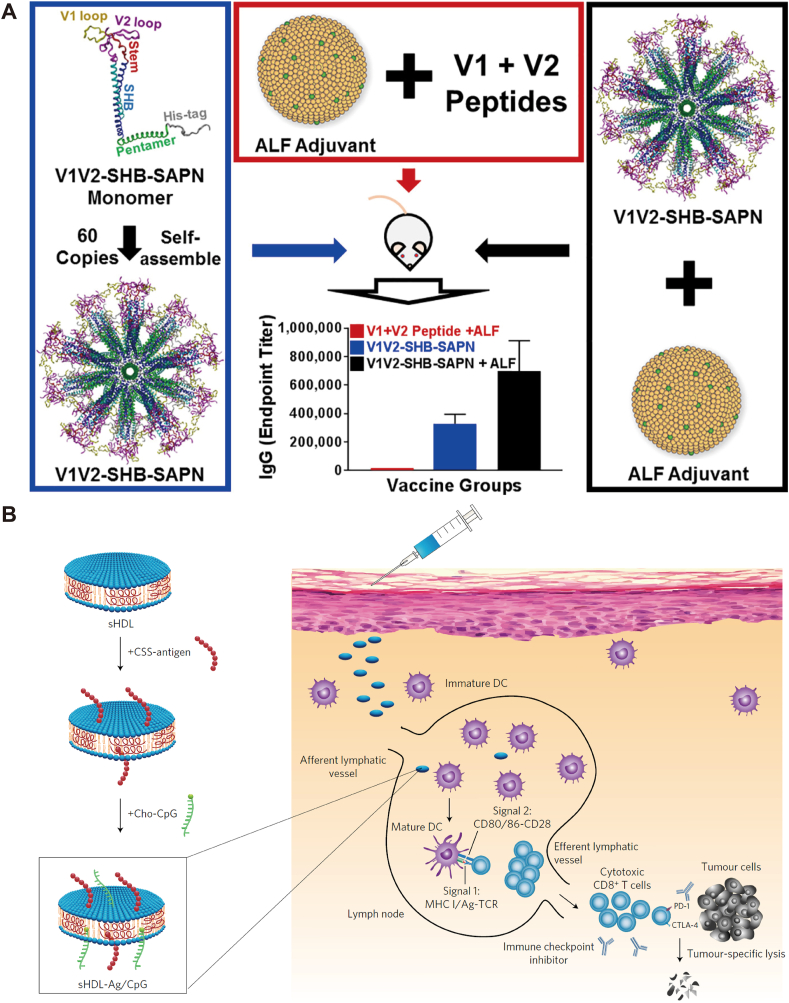
Self-assembled protein nanoparticles are useful platforms for antigen delivery. RTS,S, the first and currently the only malaria vaccine in the market147, uses VLP to deliver antigen. VLP has been tested to display HIV envelope proteins such as V1V2 loop for vaccination and generated specific IgG in mice (Fig. 2A)148. Ferritin nanoparticles have also been employed to display HIV envelope trimers on particle surfaces to increase immunogenicity148,149. Other larger proteins such as dihydrolipoyl acetyltransferase (E2)150 and lumazine synthase151 are also useful for HIV vaccination. A two-component protein nanoparticle152 was recently developed for enhancing the immunogenicity of HIV envelope (SOSIP) trimers by incorporating well-folded trimers into self-assembled nanoparticles. Further studies are exploring how spacing, antigens, and particle (size, shape, and charge) factors are involved in the protective immune responses.
Figure 2.
(A) Self-assembled protein nanoparticles as HIV vaccines. Fully assembled protein nanoparticles display 20 copies of trimers. The protein nanovaccine induces specific IgG in mice and the immune response can be enhanced with army liposome formulation (ALF) as adjuvant. Reprinted with permission from Ref. 148. Copyright © 2019. Elsevier. (B) Design and application of high-density lipoprotein-mimicking nanodisc platform composed of lipids and peptides for co-delivery of Ag and CpG for as cancer vaccine. Subcutaneous injection of the nanovaccine induce DC maturation and elite robust Ag-specific CD8+ T cell responses, killing target cancer cells. This nanovaccine can be used in combination immunotherapy with immune checkpoint blockade. Reprinted with permission from Ref. 167. Copyright © 2017 Springer Nature.
Polymeric nanomaterials have received great interest as vaccine platforms for their synthetic feasibility, low immunogenicity, and high biodegradability. Recently, HIV-1-derived gp140 immunogen with 3M-052 (a TLR-7/8 agonist) were incorporated in PLGA nanoparticles and induced high and persistent frequencies of HIV envelope-specific immune responses in rhesus macaques153. Besides, self-encapsulating PLGA microspheres loaded with calcium phosphate adjuvant gel and ovalbumin (OVA) antigen achieved the sustained release of antigen for more than seven weeks. Administration of OVA-loaded nanoparticles induced strong Th2-type response85.
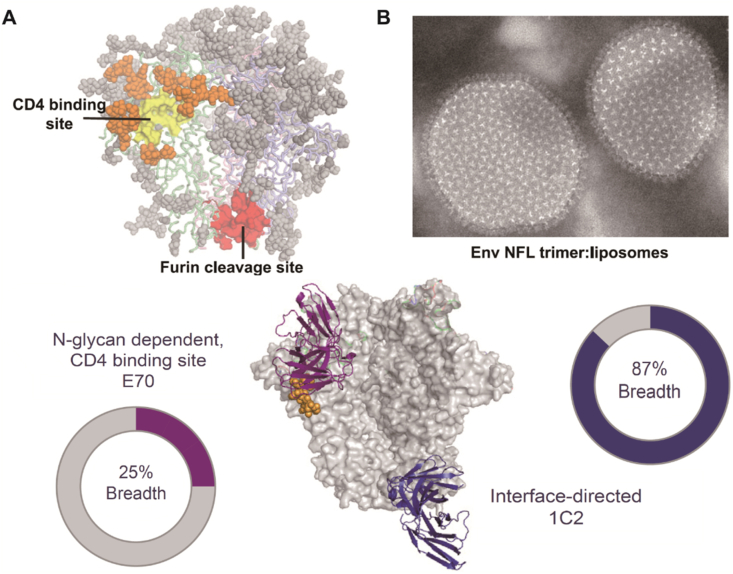
LNPs can stably carry multiple antigens. It has been reported that cobalt porphyrin-phospholide can be loaded into LNP nanovaccine to express antigens coupled to the liposomal surface154. More recently, a heterologous trimer-liposome primer was performed by presenting well-ordered native flexible linked trimers on liposomes with high density and elicited cross-neutralizing antibodies (Fig. 3)155.
Figure 3.
(A) HIV envelope trimer model. Conserved sites of vulnerability were highlighted as CD4bs (yellow) ringed with N-glycans (orange). The interface-directed antibody (1C2) showed significantly higher HIV neutralization breadth (87%) than N-glycan-dependent CD4-binding site E70 (25% HIV neutralization breadth) when vaccinating against a panel of clinical isolates. (B) Morphology of HIV envelope native flexibly linked trimer coupled to liposomes by negative stain electronic microscope. Reprinted with permission from Ref. 155. Copyright © 2019 Cell press.
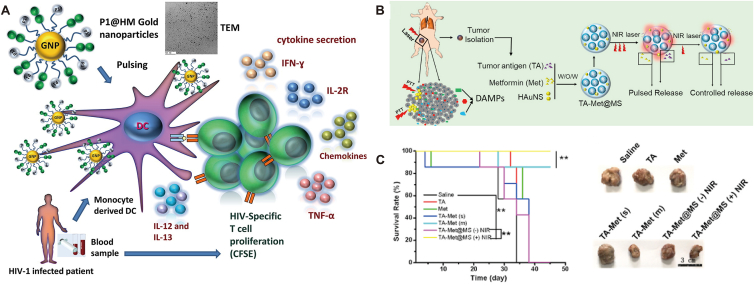
Inorganic nanoparticles are also interesting platforms for developing anti-infection nanovaccines. GNPs-mediated antigen delivery can facilitate the presentation, thus inducing potent immunity. For example, gag p17 of HIV increased proliferation of CD8+ T cells via conjugating onto high-mannoside-modified GNPs (Fig. 4A)156. Fe2O3 nanoparticle containing plasmid DNA TB vaccine induced significant humoral and cellular immune responses157. Enhanced vaccination was recently reported by amine functionalized silica nanoparticles-mediated delivery of antigen in combination with mincle agonist158.
Figure 4.
(A) Gold nanoparticle (GNP) decorated with high-mannoside-type oligosaccharides (P1@HM) and HIV-1-peptides-pulsed DC for HIV-specific T cell immunity. Reprinted with permission from Ref. 156 © Copyright 2018. Elsevier. (B) Fabrication of PLGA nanoparticle loaded with tumor antigen, metformin and hollow gold nanospheres (TA-Met@MS) as tumor vaccines. Photothermal therapy (PTT) stimulate the nanoparticle to release antigen and drug for inducing immunogenicity. (C) TA-Met@MS treatment prolonged the survival of tumor bearing mice and suppressed tumor progression. Reprinted with permission from Ref. 168. Copyright © 2021 Elsevier.
Biomimetic nanoparticles are powerful platforms against infectious diseases. Virosomal vaccine developed by HIV-1 gp41 subunit induced strong mucosal antibodies against HIV challenges159. In another study, Shao et al.160 developed membrane proximal external region (MPER) peptide fragments-bounded liposomes-containing cobalt porphyrin-phospholipid (CoPoP) and a synthetic monophosphoryl lipid A (MPLA) to generate immunogenicity in mice. Immunization with the lipid-presented MPER elicited antibody responses recognizing recombinant HIV gp41 and gp140 proteins. As HIV attack explicitly T cells, Wei et al.161 developed T cell membrane-coated PLGA nanoparticles to neutralize the HIV infection. Membranes of T cells inherit retained antigens of CD4 receptor, C‒C chemokine receptor 5 (CCR5) or C‒X‒C chemokine receptor type 4 (CXCR4) for HIV envelope glycoprotein 120 binding. This biomimetic nanovaccine strategy effectively inhibited viral killing and neutralized HIV infection.
5.2. Suppressing tumor recurrence and metastasis
Cancer remains a leading cause of human death. Developing an anticancer vaccine is a vital step in reaching personalized medicine to treat this prolific disease. Despite tremendous efforts, full elucidation of the cancer pathogenesis is challenging162. Generally, cancerous cells result from the mutation of healthy cells involving multiple environmental and genetic factors. Therefore, unlike infectious diseases, cancer is highly heterologous in cases and the prevention of cancer is extremely difficult163. As small amounts of malignant cells can lead to relapse, the development of vaccines is of significance for the elimination of residual malignant cells and suppression of tumor recurrence and metastasis. Nanomedicine provided opportunities against tumors by using the above-mentioned nanomaterials to enhance either tumor vaccine delivery, in situ tumor antigen exposure, or presentation of the immune activation program.
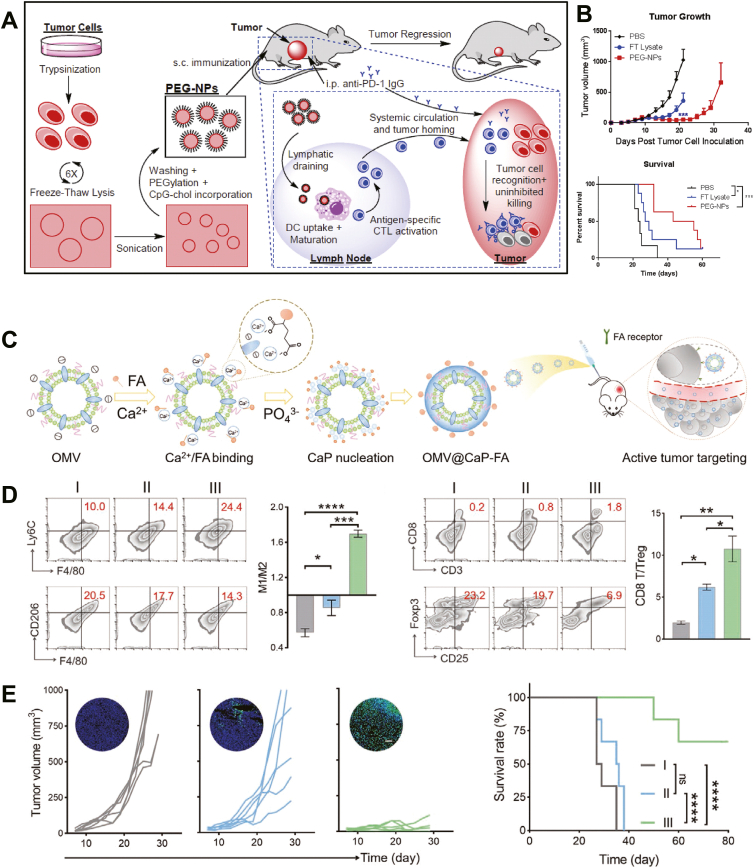
A variety of nanomaterials have been explored as efficient platforms for the delivery of tumor vaccines. VLPs have been used directly for tumor-associated antigens delivery, and vaccination with VLPs could be used in combination with radiation therapy164, chemotherapy165 or immune therapy166. For general stimulation of antitumor immune responses, Kuai and colleagues167 designed nanodiscs mimicking high-density lipoprotein for advanced delivery of antigens and adjuvants to lymphoid organs. The nanodiscs treatment exhibited substantially higher production of neoantigen-specific CTLs control formulations and eliminated tumors in combined immune checkpoint blockade therapy (Fig. 2B)167. Traditional LNPs are also highly effective platforms for tumor vaccine delivery. In a recent study, mRNA encoding tumor antigens were incorporated into cationic C1 LNP, which has adjuvant properties, for efficient delivery and presentation to dendritic cells (Fig. 4B and C)168. The C1 mRNA nanovaccine showed significant prevention and therapeutic effects on tumors.
Membrane-coating technology has been extensively explored for nanovaccine development. RBC-NPs with mannose modification and MPLA as the adjuvant were used to deliver B16F10 melanoma-associated antigen glycoprotein 100 to dendritic cells and inhibited significantly tumor growth169. Cancer cell membranes may be employed as antigens to coat nanoparticles as cancer vaccines. B16F10 cancer cell membrane coating of CpG-loaded emulsion nanoparticles increased significant CTLs levels170. Similarly, the B16-OVA cancer cell membrane modified with mannose coating of polymeric nanoparticles loaded with the TLR7 agonist R837 showed improved APC delivery54. In another study, membrane vesicles from cancer cells was used to coat spermine-modified acetylated dextran that loaded with thermally oxidized porous silicon171. This biomimetic nanoparticle with the immunostimulatory core inhibited the proliferation of autologous cancer cells by inducing potent immunostimulatory response.
In situ exposing tumor antigens is a promising vaccine strategy for preventing recurrence and metastasis. Most in situ tumor vaccinations utilized the ICD172 for activating antitumor immune programs by killing tumor cells and exposing DAMPs to recruit and activate APCs for processing the antigens and activate tumor-specific T cells. Fan and colleagues173 used mitoxantrone to induce ICD and conjugated the dying cells with multilamellar lipid-polymer nanoparticles loaded with CpG. Biomimetic nanoparticles are also useful tools for in situ tumor nanovaccine. Natural killer (NK) cell membrane119 and myeloid-derived suppressor cell membrane174 are useful to coat nanoparticles for enhanced PDT to promote ICD and activate immune responses that suppress significantly both in situ and metastatic tumors. In situ immunotherapy requires multiple steps, to address this issue, a recent study reported a nanomedicine strategy for programmable immune activation driven by the high level of reactive oxygen species induced by supramolecular assembled nanoparticle in the tumor microenvironment175. Release of drug and CpG/PAMAM led to the exposure of tumor antigen and APC activation, and subsequent antitumor immune responses.
As tumor cells are highly heterologous, a single tumor antigen vaccine may have insufficient immune responses to eliminate tumors176. Therefore, the whole tumor cell lysate may be loaded into nanoparticles such as chitosan177 and nanovesicles (Fig. 5A and B)178 for enhanced antigens presentation. Besides, native tumor antigens may have low immunogenicity and induce limited immune responses to combat the tumor. In recent years, artificial antigen-presenting cells (aAPC) technology179 has emerged to stimulate tumor-specific T cells by engineering nanomaterials equipped with peptide epitope and costimulatory molecules, replicating the immune-activation functions of APCs. Nanoscale aAPCs may have core material such as iron oxide180. It has been reported that iron-dextran aAPCs can induce great expansion of T lymphocytes expressing the cognate TCR. Membrane coating has also been used in the development of aAPCs181. The efficacy of aAPCs could be improved by modifying the nanoparticle morphology182, signal coupling183, and fluidity136. Further exploration and establishment of protocols of aAPCs with personalized neoantigens may generate highly effective and personalized tumor vaccines184,185. In a recent study, the immune response of autologous tumor antigen was enhanced by a hybrid membrane delivery strategy77. Bacterial cytoplasmic membrane and tumor cell membranes were used to form a nanoparticle for loading antigen and adjuvant to induce dendritic cell maturation, cytotoxic T cell activation, tumor growth suppression, and recurrence prevention.
Figure 5.
(A) Nanovesicles derived from tumor cell membrane as vaccine platform for cancer therapy. B16F10-OVA cell-derived nanovesicles (PEG-NPs) were obtain by freeze-thawing lysis, sonication, calcium-mediated aggregation, PEGylation, and cholesterol-linked CpG incorporation. Subcutaneous administrated PEG-NPs are taken up by DCs to activate antigen-specific cytotoxic CD8+ T lymphocytes, which recognize and kill cancer cells in synergy with immune checkpoint blockade. (B) PEG-NPs nanovaccines suppressed tumor growth and prolonged survival of tumor bearing mice. Reprinted with permission from Ref. 178. Copyright © 2018 Elsevier. (C) Fabrication of biomineralized OMVs (OMV@CaPs) by Ca2+ binding, CaP nucleation, crystal growth and folic acid modification as targeted tumor vaccines. (D) Flow cytometry analysis showing OMV@CaPs shifted the macrophages polarization from M2 to M1, and increased CD8+ T cell infiltration and decreased Treg in tumors. (E) OMV@CaPs suppressed tumor growth and prolonged survival of tumor bearing mice. Reprinted with permission from Ref. 187. Copyright © 2020 Wiley-VCH.
Non-specific immune activation is another emerging strategy for developing cancer nanovaccines. OMV can induce systemic immune responses and recruit nonspecifically activated immune cells to initiate APC processing and CTLs generation. Intravenous injection of OMVs derived from Escherichia coli into CT26 tumor-bearing mice eradicated tumor cells186; moreover, the growth of re-challenged tumors in mice was suppressed significantly. In a recent study, OMVs were encapsulated into calcium phosphate shells and lead to M2-to-M1 polarization of macrophages in the tumor microenvironment and eliminated in situ tumor, and prevented metastasis with reduced side effects (Fig. 5C‒E)187.
6. Conclusions and perspectives
In the past decades, the rapid development of nanotechnology provided avenues for nanomedicine and vaccine development. Compared to traditional vaccines, nanovaccines utilized a variety of nanoparticles with essential advantages for delivery efficiency, dose regimens, administration route, adjuvants, and vaccination effects. In this review, we summarized major types of nanomaterials for vaccines and discussed cutting-edge examples, highlighting worldwide infectious diseases and cancers. In addition to the nanomaterial design, the development of novel immunogens is of importance toward a desirable prophylactic immune response for infectious diseases; while for cancer nanovaccines development, the safety, targeting ability and efficient whole vaccination cascade is vital for treatment immune response. In regards to the safety of nanovaccine, immunogenicity and toxicity are two major issues. Nanoparticles may activate host immune responses after administration. In addition, nanoparticle-derived products can cause unexpected nonspecific immune responses after biodegradation. Cationic and ionizable nanoparticles may have immunogenicity by increasing proinflammatory cytokine levels188. Cytotoxicity of nanoparticles is closely related to the type of nanomaterials and the dose. Biodegradable components are highly encouraged for developing nanovaccines with improved biocompatibility189.
Focus on the typical clinical approved nanovaccines and vaccine nanotechnology under clinical development, we would get inspirations to find out the direction of next-generation vaccine nanotechnology. As shown in Table 1190, 191, 192, 193, 194, liposomes and lipid nanoparticles have played dominant roles in nanovaccine clinical application, suggesting excellent biocompatibility and biosafety of nanomaterials would still be nonnegligible index in next-generation nanovaccine competition. It is also worth mentioning that the diseases with different underlying immune mechanisms would further push the development of nanovaccine subtypes. Take a panoramic view of current vaccine nanotechnology under clinical development, mRNA-based nanovaccine would have great promise in cancer treatment and infectious disease prevention. Furthermore, many issues, including physicochemical properties, biointerfacing, and quality control remain to be addressed toward successful clinical translation of nanovaccines. The population implementation and the cost-effectiveness of nanovaccines should be considered; while for cancer nanovaccines, the patient-specific antigen is a challenge for personalized vaccination. Taken together, vaccine nanotechnology has shown promising results in experimental studies, and further joint efforts of nanomaterials, immunology, virology, oncology, and pharmaceutical industries will promote the clinical translation and application of nanovaccines for the management of devastating infectious diseases and cancers.
Table 1.
Typical clinical approved vaccines and candidates using nanotechnology for addressing infectious disease or cancer.
| Name | Formulation | Indication | Administration | Trial ID | Ref. |
|---|---|---|---|---|---|
| Mosquirix (RTS,S/AS01E) | Liposomal (AS01) adjuvant and recombinant Plasmodium, Hepatitis B protein | Malaria and hepatitis B | Intramuscular | NCT03162614, NCT02992119 | 190 |
| M72/AS01E | Liposomal (AS01) adjuvant and mycobacterial ‘M72ʼ recombinant fusion antigen | Tuberculosis | Intramuscular | NCT01755598 | 191 |
| HIV-1 gp-41 liposome vaccine | HIV-1 gp-41 MPER peptide 656 liposomes | HIV | Intramuscular | NCT03934541 | NA |
| Lipo-MERIT | RNA formulated with liposomes (RNA-lipoplexes) | Melanoma | Intravenous | NCT02410733 | 192 |
| mRNA-2752 | Lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ | Solid tumors or lymphoma | Intratumoral | NCT03739931 | 193 |
| mRNA-4157 | Lipid nanoparticle encapsulating mRNAs encoding multiple neoantigens | Solid tumors | Intramuscular | NCT03313778 | 194 |
NA, not available.
Acknowledgments
The authors acknowledged the support from the US METAvivor Early Career Investigator Award (No. 2018A020560; to W.T., USA), Harvard Medical School/Brigham and Women’s Hospital Department of Anesthesiology Basic Scientist Grant (No. 2420 BPA075; to W.T., USA) and Center for Neuroscience Research Fund (No. 2019A014810; to W.T., USA). W.T. is a recipient of the Khoury Innovation Award (No. 2020A003219, USA), Gillian Reny Stepping Strong Center for Trauma Innovation Breakthrough Innovator Award (No. 113548, USA), and American Heart Association (AHA) Collaborative Science Award (No. 2018A004190, USA). W.T. also received a start-up package (for 3 years) from the Department of Anesthesiology, Perioperative and Pain Medicine to establish his independent research laboratory at Harvard Medical School and Brigham and Women's Hospital.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Enguo Chen, Email: 3195024@zju.edu.cn.
Wei Tao, Email: wtao@bwh.harvard.edu.
Author contributions
Wei Tao and Chan Feng conceived the manuscript. Chan Feng, Yongjiang Li, Bijan Emiliano Ferdows, Dylan Neal Patel, Jiang Ouyang, Zhongmin Tang, and Na Kong co-wrote the draft. Wei Tao and Enguo Chen refined the draft. All authors discussed and edited the manuscript at all stages. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Eppler H.B., Jewell C.M. Biomaterials as tools to decode immunity. Adv Mater. 2020;32:1903367. doi: 10.1002/adma.201903367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields Iv C.W., Wang L.L.-W., Evans M.A., Mitragotri S. Materials for immunotherapy. Adv Mater. 2020;32:1901633. doi: 10.1002/adma.201901633. [DOI] [PubMed] [Google Scholar]
- 3.Tang Z.M., Xiao Y.F., Kong N., Liu C., Chen W., Huang X.G., et al. Nano-bio interfaces effect of two-dimensional nanomaterials and their applications in cancer immunotherapy. Acta Pharm Sin B. 2021;11:3447–3464. doi: 10.1016/j.apsb.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Z.M., Zhang X.C., Shu Y.Q., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36:101019. doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z.M., Kong N., Zhang X.C., Liu Y., Hu P., Mou S., et al. A materials-science perspective on tackling COVID-19. Nat Rev Mater. 2020;5:847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana A., Allawadhi P., Khurana I., Allwadhi S., Weiskirchen R., Banothu A.K., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingwell K. Uncoupling resistance to cancer immunotherapy. Nat Rev Drug Discov. 2019;18:171. doi: 10.1038/d41573-019-00025-8. [DOI] [PubMed] [Google Scholar]
- 8.McLeod H.L., Mariam A., Schveder K.A., Rotroff D.M. Assessment of adverse events and their ability to discriminate response to anti-PD-1/PD-L1 antibody immunotherapy. J Clin Oncol. 2019;38:103–104. doi: 10.1200/JCO.19.01712. [DOI] [PubMed] [Google Scholar]
- 9.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Chen F.M., Wang Y.J., Gao J., Saeed M., Li T.L., Wang W.Q., et al. Nanobiomaterial-based vaccination immunotherapy of cancer. Biomaterials. 2021;270:120709. doi: 10.1016/j.biomaterials.2021.120709. [DOI] [PubMed] [Google Scholar]
- 11.Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat Nanotechnol. 2021;16:16–24. doi: 10.1038/s41565-020-00790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demento S.L., Cui W.G., Criscione J.M., Stern E., Tulipan J., Kaech S.M., et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon J.J., Suh H., Li A.V., Ockenhouse C.F., Yadava A., Irvine D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109:1080. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schudel A., Chapman A.P., Yau M.K., Higginson C.J., Francis D.M., Manspeaker M.P., et al. Programmable multistage drug delivery to lymph nodes. Nat Nanotechnol. 2020;15:491–499. doi: 10.1038/s41565-020-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P., et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 16.Sixt M., Kanazawa N., Selg M., Samson T., Roos G., Reinhardt D.P., et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Rohner N.A., Thomas S.N. Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater Sci Eng. 2017;3:153–159. doi: 10.1021/acsbiomaterials.6b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole J.B., Pamela T., Eric M.B., Kristy M.A. Drug delivery for cancer immunotherapy and vaccines. Pharm Nanotechnol. 2018;6:232–244. doi: 10.2174/2211738506666180918122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H.P., Moynihan K.D., Zheng Y.R., Szeto G.L., Li A.V., Huang B., et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster B.S., Suk J.S., Woodworth G.F., Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34:3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B., Maharjan S., Cho K.H., Cui L.H., Park I.K., Choi Y.J., et al. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int J Biol Macromol. 2018;110:54–64. doi: 10.1016/j.ijbiomac.2017.10.101. [DOI] [PubMed] [Google Scholar]
- 23.Champion J.A., Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S.S., Lau C.M., Thomas S.N., Jerome W.G., Maron D.J., Dickerson J.H., et al. Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. Int J Nanomed. 2012;7:799–813. doi: 10.2147/IJN.S28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang S.D., Scholzen A., Minigo G., David C., Apostolopoulos V., Mottram P.L., et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Figdor C.G., van Kooyk Y., Adema G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.M., Pan J.Y., Korbel G.A., Peperzak V., Boes M., Ploegh H.L. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci U S A. 2006;103:3327–3332. doi: 10.1073/pnas.0511315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babapoor S., Neef T., Mittelholzer C., Girshick T., Garmendia A., Shang H., et al. A Novel Vaccine Using Nanoparticle Platform to Present Immunogenic M2e against Avian Influenza Infection. Influenza Res Treat. 2011;2011:126794. doi: 10.1155/2011/126794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista F.D., Neuberger M.S. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 2000;19:513–520. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T., et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett J.C., Ulery B.D., Trent A., Liang S., David N.A., Tirrell M.V. Modular peptide amphiphile micelles improving an antibody-mediated immune response to group A streptococcus. ACS Biomater Sci Eng. 2017;3:144–152. doi: 10.1021/acsbiomaterials.6b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puffer E.B., Pontrello J.K., Hollenbeck J.J., Kink J.A., Kiessling L.L. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 33.Mora-Solano C., Wen Y., Han H.F., Chen J.J., Chong A.S., Miller M.L., et al. Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials. 2017;149:1–11. doi: 10.1016/j.biomaterials.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokatlian T., Read B.J., Jones C.A., Kulp D.W., Menis S., Chang J.Y.H., et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pejawar-Gaddy S., Kovacs J.M., Barouch D.H., Chen B., Irvine D.J. Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in HIV vaccination. Bioconjugate Chem. 2014;25:1470–1478. doi: 10.1021/bc5002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulp D.W., Steichen J.M., Pauthner M., Hu X., Schiffner T., Liguori A., et al. Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nat Commun. 2017;8:1655. doi: 10.1038/s41467-017-01549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazzill J.D., Ochyl L.J., Giang E., Castillo S., Law M., Moon J.J. Interrogation of antigen display on individual vaccine nanoparticles for achieving neutralizing antibody responses against hepatitis C virus. Nano Lett. 2018;18:7832–7838. doi: 10.1021/acs.nanolett.8b03601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z., Wise M.C., Chokkalingam N., Walker S., Tello-Ruiz E., Elliott S.T.C., et al. In vivo assembly of nanoparticles achieved through synergy of structure-based protein engineering and synthetic DNA generates enhanced adaptive immunity. Adv Sci. 2020;7:1902802. doi: 10.1002/advs.201902802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes A., Vandermeulen G., Preat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38:146. doi: 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng L., Mohan T., Chang T.Z., Gonzalez G.X., Wang Y., Kwon Y.M., et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun. 2018;9:359. doi: 10.1038/s41467-017-02725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamp H.D., Swanson K.A., Wei R.R., Dhal P.K., Dharanipragada R., Kern A., et al. Design of a broadly reactive Lyme disease vaccine. NPJ Vaccines. 2020;5:33. doi: 10.1038/s41541-020-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Islam M.A., Xu Y., Tao W., Ubellacker J.M., Lim M., Aum D., et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Eng. 2018;2:850–864. doi: 10.1038/s41551-018-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong N., Tao W., Ling X., Wang J.Q., Xiao Y.L., Shi S.J., et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao W., Yurdagul A., Kong N., Li W., Wang X., Doran A.C., et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam M.A., Rice J., Reesor E., Zope H., Tao W., Lim M., et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266:120431. doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson B.R., Muramatsu H., Nallagatla S.R., Bevilacqua P.C., Sansing L.H., Weissman D., et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010;38:5884–5892. doi: 10.1093/nar/gkq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 49.McNamara M.A., Nair S.K., Holl E.K. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linares-Fernandez S., Lacroix C., Exposito J.Y., Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng D.W., Gao F., Cheng Q., Bao P., Dong X., Fan J.X., et al. A vaccine-based nanosystem for initiating innate immunity and improving tumor immunotherapy. Nat Commun. 2020;11:1985. doi: 10.1038/s41467-020-15927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S.L., Jiang Q., Zhao X., Zhao R.F., Wang Y.N., Wang Y.M., et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2021;20:421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 54.Yang R., Xu J., Xu L.G., Sun X.Q., Chen Q., Zhao Y.H., et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 55.Duan X.P., Chan C., Lin W.B. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou L., Zhang P.C., Wang H., Wang D., Li Y.P. Smart Nanosized drug delivery systems inducing immunogenic cell death for combination with cancer immunotherapy. Acc Chem Res. 2020;53:1761–1772. doi: 10.1021/acs.accounts.0c00254. [DOI] [PubMed] [Google Scholar]
- 57.Huang W., He L.Z., Ouyang J., Chen Q., Liu C., Tao W., et al. Triangle-shaped tellurium nanostars potentiate radiotherapy by boosting checkpoint blockade immunotherapy. Matter. 2020;3:1725–1753. [Google Scholar]
- 58.Ouyang J., Tang Z.M., Farokhzad N., Kong N., Kim N.Y., Feng C., et al. Ultrasound mediated therapy: recent progress and challenges in nanoscience. Nano Today. 2020;35:100949. [Google Scholar]
- 59.Casella C.R., Mitchell T.C. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall J.D., Higgins D., Abbate C., Yee P., Teshima G., Ott G., et al. Polymyxin B enhances ISS-mediated immune responses across multiple species. Cell Immunol. 2004;229:93–105. doi: 10.1016/j.cellimm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Chen Q., Xu L.G., Liang C., Wang C., Peng R., Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong X.F., Zhang Y.T., Tan L., Zheng T., Hou Y.Y., Hong X.Y., et al. An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J Control Release. 2019;300:81–92. doi: 10.1016/j.jconrel.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 63.Li W., Yang J., Luo L.H., Jiang M.S., Qin B., Yin H., et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C., Sun S., Feng Q., Wu G.W., Wu Y.T., Kong N., et al. Arsenene nanodots with selective killing effects and their low-dose combination with ß-Elemene for cancer therapy. Adv Mater. 2021;33:2102054. doi: 10.1002/adma.202102054. [DOI] [PubMed] [Google Scholar]
- 65.Kong N., Zhang H.J., Feng C., Liu C., Xiao Y.F., Zhang X.C., et al. Arsenene-mediated multiple independently targeted reactive oxygen species burst for cancer therapy. Nat Commun. 2021;12:4777. doi: 10.1038/s41467-021-24961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu C., Shin J., Son S., Choe Y., Farokhzad N., Tang Z.M., et al. Pnictogens in medicinal chemistry: evolution from erstwhile drugs to emerging layered photonic nanomedicine. Chem Soc Rev. 2021;50:2260–2279. doi: 10.1039/d0cs01175d. [DOI] [PubMed] [Google Scholar]
- 67.Ouyang J., Ji X.Y., Zhang X.C., Feng C., Tang Z.M., Kong N., et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc Natl Acad Sci U S A. 2020;117:28667. doi: 10.1073/pnas.2016268117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C., Kim H.S., Won M., Jung E., Kim J.S. Navigating 2D monoelemental materials (Xenes) for cancer nanomedicine. Matter. 2020;3:12–13. [Google Scholar]
- 69.Kong N., Ji X.Y., Wang J.Q., Sun X.N., Chen G.Q., Fan T.J., et al. ROS-mediated selective killing effect of black phosphorus: mechanistic understanding and its guidance for safe biomedical applications. Nano Lett. 2020;20:3943–3955. doi: 10.1021/acs.nanolett.0c01098. [DOI] [PubMed] [Google Scholar]
- 70.Ji X.Y., Kang Y., Ouyang J., Chen Y.H., Artzi D., Zeng X.B., et al. Synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy. Adv Sci. 2019;6:1901211. doi: 10.1002/advs.201901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong X., Pan P., Zheng D.W., Bao P., Zeng X., Zhang X.Z. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y.W., Jiang C. Postoperative cancer treatments: in-situ delivery system designed on demand. J Control Release. 2021;330:554–564. doi: 10.1016/j.jconrel.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 73.Liu X., Feng Z.J., Wang C.R., Su Q., Song H.J., Zhang C.N., et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials. 2020;230:119649. doi: 10.1016/j.biomaterials.2019.119649. [DOI] [PubMed] [Google Scholar]
- 74.Phuengkham H., Song C., Um S.H., Lim Y.T. Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: postoperative immunotherapy. Adv Mater. 2018;30 doi: 10.1002/adma.201706719. [DOI] [PubMed] [Google Scholar]
- 75.Li X.M., Hu Z.P., Ma J.L., Wang X.Y., Zhang Y.P., Wang W., et al. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf, B. 2018;167:260–266. doi: 10.1016/j.colsurfb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Fang L., Zhao Z.T., Wang J., Zhang P.C., Ding Y.P., Jiang Y.Y., et al. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L., Qin H., Zhao R.F., Zhao X., Lin L.R., Chen Y., et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abc2816. [DOI] [PubMed] [Google Scholar]
- 78.Shukla S., Myers J.T., Woods S.E., Gong X., Czapar A.E., Commandeur U., et al. Plant viral nanoparticles-based HER2 vaccine: immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017;121:15–27. doi: 10.1016/j.biomaterials.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 79.Boone C.E., Wang C., Lopez-Ramirez M.A., Beiss V., Shukla S., Chariou P.L., et al. Active microneedle administration of plant virus nanoparticles for cancer in situ vaccination improves immunotherapeutic efficacy. ACS Appl Nano Mater. 2020;3:8037–8051. doi: 10.1021/acsanm.0c01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yenkoidiok-Douti L., Barillas-Mury C., Jewell C.M. Design of Dissolvable microneedles for delivery of a Pfs47-based malaria transmission-blocking vaccine. ACS Biomater Sci Eng. 2021;7:1854–1862. doi: 10.1021/acsbiomaterials.0c01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tran K.T.M., Gavitt T.D., Farrell N.J., Curry E.J., Mara A.B., Patel A., et al. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat Biomed Eng. 2021;5:998–1007. doi: 10.1038/s41551-020-00650-4. [DOI] [PubMed] [Google Scholar]
- 82.Bernocchi B., Carpentier R., Betbeder D. Nasal nanovaccines. Int J Pharm. 2017;530:128–138. doi: 10.1016/j.ijpharm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 83.Liu Q.F., Zhang C., Zheng X.Y., Shao X.Y., Zhang X., Zhang Q.Z., et al. Preparation and evaluation of antigen/N-trimethylaminoethylmethacrylate chitosan conjugates for nasal immunization. Vaccine. 2014;32:2582–2590. doi: 10.1016/j.vaccine.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 84.Bakkari M.A., Valiveti C.K., Kaushik R.S., Tummala H. Toll-like receptor-4 (TLR4) agonist-based intranasal nanovaccine delivery system for inducing systemic and mucosal immunity. Mol Pharm. 2021;18:2233–2241. doi: 10.1021/acs.molpharmaceut.0c01256. [DOI] [PubMed] [Google Scholar]
- 85.Bailey B.A., Desai K.H., Ochyl L.J., Ciotti S.M., Moon J.J., Schwendeman S.P. Self-encapsulating poly(lactic-co-glycolic acid) (PLGA) microspheres for intranasal vaccine delivery. Mol Pharm. 2017;14:3228–3237. doi: 10.1021/acs.molpharmaceut.7b00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey B.A., Ochyl L.J., Schwendeman S.P., Moon J.J. Toward a single-dose vaccination strategy with self-encapsulating plga microspheres. Adv Healthc Mater. 2017;6:1601418. doi: 10.1002/adhm.201601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Connor G., Krishnan N., Fagan-Murphy A., Cassidy J., O'Leary S., Robertson B.D., et al. Inhalable poly(lactic-co-glycolic acid) (PLGA) microparticles encapsulating all-trans-Retinoic acid (ATRA) as a host-directed, adjunctive treatment for Mycobacterium tuberculosis infection. Eur J Pharm Biopharm. 2019;134:153–165. doi: 10.1016/j.ejpb.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y., Ge S., Song Z., Zhao A., Zhao L.Q., Hu Z.M., et al. A novel self-assembled epitope peptide nanoemulsion vaccine targeting nasal mucosal epithelial cell for reinvigorating CD8+ T cell immune activity and inhibiting tumor progression. Int J Biol Macromol. 2021;183:1891–1902. doi: 10.1016/j.ijbiomac.2021.05.158. [DOI] [PubMed] [Google Scholar]
- 89.Diego-Gonzalez L., Crecente-Campo J., Paul M.J., Singh M., Reljic R., Alonso M.J., et al. Design of polymeric nanocapsules for intranasal vaccination against mycobacterium tuberculosis: influence of the polymeric shell and antigen positioning. Pharmaceutics. 2020;12:489. doi: 10.3390/pharmaceutics12060489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng B., Peng W.C., Guo M.M., Huang M.Q., Gu Y.X., Wang T., et al. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem Eng J. 2021;418:129392. doi: 10.1016/j.cej.2021.129392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lizotte P.H., Wen A.M., Sheen M.R., Fields J., Rojanasopondist P., Steinmetz N.F., et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao Y.F., Tang Z.M., Wang J.Q., Liu C., Kong N., Farokhzad O.C., et al. Oral insulin delivery platforms: strategies to address the biological barriers. Angew Chem Int Ed Engl. 2020;59:19787–19795. doi: 10.1002/anie.202008879. [DOI] [PubMed] [Google Scholar]
- 93.Wang D., Xu J., Feng Y.H., Liu Y., McHenga S.S., Shan F.P., et al. Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response. Vaccine. 2010;28:3134–3142. doi: 10.1016/j.vaccine.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 94.Takamura S., Niikura M., Li T.C., Takeda N., Kusagawa S., Takebe Y., et al. DNA vaccine-encapsulated virus-like particles derived from an orally transmissible virus stimulate mucosal and systemic immune responses by oral administration. Gene Ther. 2004;11:628–635. doi: 10.1038/sj.gt.3302193. [DOI] [PubMed] [Google Scholar]
- 95.Al-Deen F.N., Ho J., Selomulya C., Ma C., Coppel R. Superparamagnetic nanoparticles for effective delivery of malaria DNA vaccine. Langmuir. 2011;27:3703–3712. doi: 10.1021/la104479c. [DOI] [PubMed] [Google Scholar]
- 96.Ge W., Li Y., Li Z.S., Zhang S.H., Sun Y.J., Hu P.Z., et al. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following peroral administration route. Cancer Immunol Immunother. 2009;58:201–208. doi: 10.1007/s00262-008-0539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Neill C.L., Shrimali P.C., Clapacs Z.P., Files M.A., Rudra J.S. Peptide-based supramolecular vaccine systems. Acta Biomater. 2021;133:153–167. doi: 10.1016/j.actbio.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frietze K.M., Peabody D.S., Chackerian B. Engineering virus-like particles as vaccine platforms. Curr Opin Virol. 2016;18:44–49. doi: 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mohsen M.O., Zha L., Cabral-Miranda G., Bachmann M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Neek M., Kim T.I., Wang S.W. Protein-based nanoparticles in cancer vaccine development. Nanomedicine. 2019;15:164–174. doi: 10.1016/j.nano.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tesarova B., Musilek K., Rex S., Heger Z. Taking advantage of cellular uptake of ferritin nanocages for targeted drug delivery. J Control Release. 2020;325:176–190. doi: 10.1016/j.jconrel.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 102.Kang H., Rho S., Stiles W.R., Hu S., Baek Y., Hwang D.W., et al. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simon-Vazquez R., Peleteiro M., Gonzalez-Fernandez A. Polymeric nanostructure vaccines: applications and challenges. Expert Opin Drug Del. 2020;17:1007–1023. doi: 10.1080/17425247.2020.1776259. [DOI] [PubMed] [Google Scholar]
- 104.Das A., Ali N. Nanovaccine: an emerging strategy. Expert Rev Vaccines. 2021;20:1273–1290. doi: 10.1080/14760584.2021.1984890. [DOI] [PubMed] [Google Scholar]
- 105.Wu D.J., Zhu L.X., Li Y., Zhang X.L., Xu S.M., Yang G.S., et al. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: a review. Carbohydr Polym. 2020;238:116126. doi: 10.1016/j.carbpol.2020.116126. [DOI] [PubMed] [Google Scholar]
- 106.Butkovich N., Li E., Ramirez A., Burkhardt A.M., Wang S.W. Advancements in protein nanoparticle vaccine platforms to combat infectious disease. Wiley interdiscip. 2021;13 doi: 10.1002/wnan.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karlsson J., Vaughan H.J., Green J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu Rev Cell Biol. 2018;9:105–127. doi: 10.1146/annurev-chembioeng-060817-084055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rietscher R., Schröder M., Janke J., Czaplewska J., Gottschaldt M., Scherließ R., et al. Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity. Eur J Pharm Biopharm. 2016;102:20–31. doi: 10.1016/j.ejpb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev. 2020;154:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 111.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2:17056. [Google Scholar]
- 115.Hess K.L., Medintz I.L., Jewell C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi: 10.1016/j.nantod.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dykman L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev Vaccines. 2020;19:465–477. doi: 10.1080/14760584.2020.1758070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y., Zhao X.T., Cheng Y., Guo X.S., Yuan W.E. Iron oxide nanoparticles-based vaccine delivery for cancer treatment. Mol Pharmaceut. 2018;15:1791–1799. doi: 10.1021/acs.molpharmaceut.7b01103. [DOI] [PubMed] [Google Scholar]
- 118.Hong X.Y., Zhong X.F., Du G.S., Hou Y.Y., Zhang Y.T., Zhang Z.R., et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci Adv. 2020;6:eaaz4462. doi: 10.1126/sciadv.aaz4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deng G.J., Sun Z.H., Li S.P., Peng X.H., Li W.J., Zhou L.H., et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 120.Sharma M., Salisbury R.L., Maurer E.I., Hussain S.M., Sulentic C.E. Gold nanoparticles induce transcriptional activity of NF-kappaB in a B-lymphocyte cell line. Nanoscale. 2013;5:3747–3756. doi: 10.1039/c3nr30071d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzalez-Ballesteros N., Diego-Gonzalez L., Lastra-Valdor M., Rodriguez-Arguelles M.C., Grimaldi M., Cavazza A., et al. Immunostimulant and biocompatible gold and silver nanoparticles synthesized using the Ulva intestinalis L. aqueous extract. J Mater Chem B. 2019;7:4677–4691. doi: 10.1039/c9tb00215d. [DOI] [PubMed] [Google Scholar]
- 122.Ferrando R.M., Lay L., Polito L. Gold nanoparticle-based platforms for vaccine development. Drug Discov Today Technol. 2020;38:57–67. doi: 10.1016/j.ddtec.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Mody K.T., Popat A., Mahony D., Cavallaro A.S., Yu C., Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167–5179. doi: 10.1039/c3nr00357d. [DOI] [PubMed] [Google Scholar]
- 124.Lee J.Y., Kim M.K., Nguyen T.L., Kim J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl Mater Interfaces. 2020;12:34658–34666. doi: 10.1021/acsami.0c09484. [DOI] [PubMed] [Google Scholar]
- 125.Maria de Souza Morais S., Ferreira Rodigues N., Ingrid Oliveira da Silva N., Aparecido Salvador E., Rodrigues Franco I., Augusto Pires de Souza G., et al. Serum albumin nanoparticles vaccine provides protection against a lethal Pseudomonas aeruginosa challenge. Vaccine. 2018;36:6408–6415. doi: 10.1016/j.vaccine.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 126.An M., Li M., Xi J., Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Interfaces. 2017;9:23466–23475. doi: 10.1021/acsami.7b06024. [DOI] [PubMed] [Google Scholar]
- 127.Hu H.Z., Yang C., Zhang F., Li M.Q., Tu Z.X., Mu L.Z., et al. A versatile and robust platform for the scalable manufacture of biomimetic nanovaccines. Adv Sci. 2021:2002020. doi: 10.1002/advs.202002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang J.L., Zhang X.C., Liu C., Wang Z., Deng L.F., Feng C., et al. Biologically modified nanoparticles as theranostic bionanomaterials. Prog Mater Sci. 2021;118:100768. [Google Scholar]
- 129.Dehaini D., Fang R.H., Zhang L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med. 2016;1:30–46. doi: 10.1002/btm2.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu S.X., Wang L.S., Liu Z. Molecularly imprinted polymer nanoparticles: an emerging versatile platform for cancer therapy. Angew Chem Int Ed. 2021;60:3858–3869. doi: 10.1002/anie.202005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang J., Zhu M.T., Nie G.J. Biomembrane-based nanostructures for cancer targeting and therapy: from synthetic liposomes to natural biomembranes and membrane-vesicles. Adv Drug Deliv Rev. 2021;178:113974. doi: 10.1016/j.addr.2021.113974. [DOI] [PubMed] [Google Scholar]
- 132.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30 doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou J.R., Kroll A.V., Holay M., Fang R.H., Zhang L.F. Biomimetic nanotechnology toward personalized vaccines. Adv Mater. 2020;32 doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rao L., Bu L.L., Xu J.H., Cai B., Yu G.T., Yu X., et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11:6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 135.Ho W., Gao M.Z., Li F.Q., Li Z.Y., Zhang X.Q., Xu X.Y. Next-generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao L., Yu G.T., Meng Q.F., Bu L.L., Tian R., Lin L.S., et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv Funct Mater. 2019;29:1905671. [Google Scholar]
- 137.Oroojalian F., Beygi M., Baradaran B., Mokhtarzadeh A., Shahbazi M.-A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17:2006484. doi: 10.1002/smll.202006484. [DOI] [PubMed] [Google Scholar]
- 138.Gong H., Zhang Q., Komarla A., Wang S., Duan Y., Zhou Z., et al. Nanomaterial biointerfacing via mitochondrial membrane coating for targeted detoxification and molecular detection. Nano Lett. 2021;21:2603–2609. doi: 10.1021/acs.nanolett.1c00238. [DOI] [PubMed] [Google Scholar]
- 139.Ouyang X., Wang X., Kraatz H.-B., Ahmadi S., Gao J., Lv Y., et al. A Trojan horse biomimetic delivery strategy using mesenchymal stem cells for PDT/PTT therapy against lung melanoma metastasis. Biomater Sci. 2020;8:1160–1170. doi: 10.1039/c9bm01401b. [DOI] [PubMed] [Google Scholar]
- 140.Shi S.R., Wang Y., Wang B.B., Chen Q., Wan G.Y., Yang X.Y., et al. Homologous-targeting biomimetic nanoparticles for photothermal therapy and Nrf2-siRNA amplified photodynamic therapy against oral tongue squamous cell carcinoma. Chem Eng J. 2020;388:124268. [Google Scholar]
- 141.Asadi K., Gholami A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: a review. Int J Biol Macromol. 2021;182:648–658. doi: 10.1016/j.ijbiomac.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Felnerova D., Viret J.-F., Glück R., Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518–529. doi: 10.1016/j.copbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 143.Gnopo Y.M.D., Watkins H.C., Stevenson T.C., DeLisa M.P., Putnam D. Designer outer membrane vesicles as immunomodulatory systems—reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev. 2017;114:132–142. doi: 10.1016/j.addr.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 144.Irene C., Fantappie L., Caproni E., Zerbini F., Anesi A., Tomasi M., et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc Natl Acad Sci U S A. 2019;116:21780–21788. doi: 10.1073/pnas.1905112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kirtane A.R., Verma M., Karandikar P., Furin J., Langer R., Traverso G. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol. 2021;16:369–384. doi: 10.1038/s41565-021-00866-8. [DOI] [PubMed] [Google Scholar]
- 146.Fries C.N., Curvino E.J., Chen J.-L., Permar S.R., Fouda G.G., Collier J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat Nanotechnol. 2021;16:1–14. doi: 10.1038/s41565-020-0739-9. [DOI] [PubMed] [Google Scholar]
- 147.Adepoju P. RTS, S malaria vaccine pilots in three African countries. Lancet. 2019;393:1685. doi: 10.1016/S0140-6736(19)30937-7. [DOI] [PubMed] [Google Scholar]
- 148.Karch C.P., Bai H., Torres O.B., Tucker C.A., Michael N.L., Matyas G.R., et al. Design and characterization of a self-assembling protein nanoparticle displaying HIV-1 Env V1V2 loop in a native-like trimeric conformation as vaccine antigen. Nanomedicine: NBM. 2019;16:206–216. doi: 10.1016/j.nano.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 149.Sliepen K., Ozorowski G., Burger J.A., van Montfort T., Stunnenberg M., LaBranche C., et al. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. 2015;12:1–5. doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Molino N.M., Neek M., Tucker J.A., Nelson E.L., Wang S.W. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;86:83–91. doi: 10.1016/j.biomaterials.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jardine J., Julien J.-P., Menis S., Ota T., Kalyuzhniy O., McGuire A., et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brouwer P.J., Antanasijevic A., Berndsen Z., Yasmeen A., Fiala B., Bijl T.P., et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat Commun. 2019;10:1–17. doi: 10.1038/s41467-019-12080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kasturi S.P., Rasheed M.A.U., Havenar-Daughton C., Pham M., Legere T., Sher Z.J., et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang W.C., Deng B., Lin C., Carter K.A., Geng J., Razi A., et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat Nanotechnol. 2018;13:1174–1181. doi: 10.1038/s41565-018-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dubrovskaya V., Tran K., Ozorowski G., Guenaga J., Wilson R., Bale S., et al. Vaccination with glycan-modified HIV NFL envelope trimer-liposomes elicits broadly neutralizing antibodies to multiple sites of vulnerability. Immunity. 2019;51:915–929. doi: 10.1016/j.immuni.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Climent N., García I., Marradi M., Chiodo F., Miralles L., Maleno M.J., et al. Loading dendritic cells with gold nanoparticles (GNPs) bearing HIV-peptides and mannosides enhance HIV-specific T cell responses. Nanomedicine: NBM. 2018;14:339–351. doi: 10.1016/j.nano.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 157.Yu F.L., Wang J., Dou J., Yang H.T., He X.F., Xu W.G., et al. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomedicine: NBM. 2012;8:1337–1344. doi: 10.1016/j.nano.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 158.Abdelwahab W.M., Riffey A., Buhl C., Johnson C., Ryter K., Evans J.T., et al. Co-adsorption of synthetic Mincle agonists and antigen to silica nanoparticles for enhanced vaccine activity: a formulation approach to co-delivery. Int J Pharmaceut. 2021;593:120119. doi: 10.1016/j.ijpharm.2020.120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bomsel M., Tudor D., Drillet A.S., Alfsen A., Ganor Y., Roger M.G., et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 160.Shao S., Huang W.C., Lin C., Hicar M.D., LaBranche C.C., Montefiori D.C., et al. An Engineered biomimetic mper peptide vaccine induces weakly hiv neutralizing antibodies in mice. Ann Biomed Eng. 2020;48:1991–2001. doi: 10.1007/s10439-019-02398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei X.L., Zhang G., Ran D., Krishnan N., Fang R.H., Gao W.W., et al. T-Cell-Mimicking nanoparticles can neutralize hiv infectivity. Adv mater. 2018;30 doi: 10.1002/adma.201802233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blass E., Ott P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Turajlic S., Sottoriva A., Graham T., Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet. 2019;20:404–416. doi: 10.1038/s41576-019-0114-6. [DOI] [PubMed] [Google Scholar]
- 164.Patel R., Czapar A.E., Fiering S., Oleinick N.L., Steinmetz N.F. Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression. ACS Omega. 2018;3:3702–3707. doi: 10.1021/acsomega.8b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee K.L., Murray A.A., Le D.H.T., Sheen M.R., Shukla S., Commandeur U., et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 2017;17:4019–4028. doi: 10.1021/acs.nanolett.7b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lebel M.E., Chartrand K., Tarrab E., Savard P., Leclerc D., Lamarre A. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Lett. 2016;16:1826–1832. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- 167.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luo L.H., Li X., Zhang J.L., Zhu C.Q., Jiang M.S., Luo Z.Y., et al. Enhanced immune memory through a constant photothermal-metabolism regulation for cancer prevention and treatment. Biomaterials. 2021;270:120678. doi: 10.1016/j.biomaterials.2021.120678. [DOI] [PubMed] [Google Scholar]
- 169.Guo Y.Y., Wang D., Song Q.L., Wu T.T., Zhuang X.T., Bao Y.L., et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 170.Kroll A.V., Fang R.H., Jiang Y., Zhou J., Wei X., Yu C.L., et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29:1703969. doi: 10.1002/adma.201703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fontana F., Shahbazi M.A., Liu D., Zhang H., Makila E., Salonen J., et al. Multistaged nanovaccines based on porous silicon@acetalated dextran@cancer cell membrane for cancer immunotherapy. Adv Mater. 2017;29:201603239. doi: 10.1002/adma.201603239. [DOI] [PubMed] [Google Scholar]
- 172.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 173.Fan Y., Kuai R., Xu Y., Ochyl L.J., Irvine D.J., Moon J.J. Immunogenic cell death amplified by co-localized adjuvant delivery for cancer immunotherapy. Nano Lett. 2017;17:7387–7393. doi: 10.1021/acs.nanolett.7b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu G.T., Rao L., Wu H., Yang L.L., Bu L.L., Deng W.W., et al. Myeloid-derived suppressor cell membrane-coated magnetic nanoparticles for cancer theranostics by inducing macrophage polarization and synergizing immunogenic cell death. Adv Funct Mater. 2018;28:1801389. [Google Scholar]
- 175.Zhang Y., Ma S., Liu X.M., Xu Y.D., Zhao J.Y., Si X.H., et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv Mater. 2021;33 doi: 10.1002/adma.202007293. [DOI] [PubMed] [Google Scholar]
- 176.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 177.Shi G.N., Zhang C.N., Xu R., Niu J.F., Song H.J., Zhang X.Y., et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 178.Ochyl L.J., Bazzill J.D., Park C., Xu Y., Kuai R., Moon J.J. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials. 2018;182:157–166. doi: 10.1016/j.biomaterials.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eggermont L.J., Paulis L.E., Tel J., Figdor C.G. Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends Biotechnol. 2014;32:456–465. doi: 10.1016/j.tibtech.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Perica K., De Leon Medero A., Durai M., Chiu Y.L., Bieler J.G., Sibener L., et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine. 2014;10:119–129. doi: 10.1016/j.nano.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Q.M., Wei W., Wang P.L., Zuo L.P., Li F., Xu J., et al. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy. ACS Nano. 2017;11:10724–10732. doi: 10.1021/acsnano.7b04955. [DOI] [PubMed] [Google Scholar]
- 182.Meyer R.A., Sunshine J.C., Perica K., Kosmides A.K., Aje K., Schneck J.P., et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–1525. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kosmides A.K., Necochea K., Hickey J.W., Schneck J.P. Separating T cell targeting components onto magnetically clustered nanoparticles boosts activation. Nano Lett. 2018;18:1916–1924. doi: 10.1021/acs.nanolett.7b05284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang D.K.Y., Cheung A.S., Mooney D.J. Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds. Nat Protoc. 2020;15:773–798. doi: 10.1038/s41596-019-0249-0. [DOI] [PubMed] [Google Scholar]
- 185.Ichikawa J., Yoshida T., Isser A., Laino A.S., Vassallo M., Woods D., et al. Rapid Expansion of highly functional antigen-specific T cells from patients with melanoma by nanoscale artificial antigen-presenting cells. Clin Cancer Res. 2020;26:3384–3396. doi: 10.1158/1078-0432.CCR-19-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim O.Y., Park H.T., Dinh N.T.H., Choi S.J., Lee J., Kim J.H., et al. Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response. Nat Commun. 2017;8:626. doi: 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qing S., Lyu C.L., Zhu L., Pan C., Wang S., Li F., et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32 doi: 10.1002/adma.202002085. [DOI] [PubMed] [Google Scholar]
- 188.Sedic M., Senn J.J., Lynn A., Laska M., Smith M., Platz S.J., et al. Safety evaluation of lipid nanoparticle–formulated modified mrna in the sprague-dawley rat and cynomolgus monkey. Vet Pathol. 2017;55:341–354. doi: 10.1177/0300985817738095. [DOI] [PubMed] [Google Scholar]
- 189.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Laurens M.B. RTS,S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccines Immunother. 2020;16:480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tait D.R., Hatherill M., Van Der Meeren O., Ginsberg A.M., Van Brakel E., Salaun B., et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 192.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 193.Patel M.R., Bauer T.M., Jimeno A., Wang D., LoRusso P., Do K.T., et al. A phase I study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral (iTu) injection alone and in combination with durvalumab. J Clin Oncol. 2020;38:3092. [Google Scholar]
- 194.Burris H.A., Patel M.R., Cho D.C., Clarke J.M., Gutierrez M., Zaks T.Z., et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J Clin Oncol. 2019;37:2523. [Google Scholar]