Abstract
Chiari malformation Type I (CMI) is characterized by herniation of the cerebellar tonsils through the foramen magnum. The pathophysiology of CMI is not well elucidated; however, the prevailing theory focuses on the underdevelopment of the posterior cranial fossa which results in tonsillar herniation. Symptoms are believed to be due to the herniation causing resistance to the natural flow of cerebrospinal fluid (CSF) and exerting a mass effect on nearby neural tissue. However, asymptomatic cases vastly outnumber symptomatic ones and it is not known why some people become symptomatic. Recently, it has been proposed that CMI symptoms are primarily due to instability of either the atlanto-axial (AA) or the atlanto-occipital (AO) joint and the cerebellar tonsils herniate to prevent mechanical pinching. However, only a small percentage of patients exhibit clinical instability and these theories do not account for asymptomatic herniations. We propose that the pathophysiology of adult CMI involves a combination of craniocervical abnormalities which leads to tonsillar herniation and reduced compliance of the cervical spinal canal. Specifically, abnormal AO and/or AA joint morphology leads to chronic cervical instability, often subclinical, in a large portion of CMI patients. This in turn causes overwork of the suboccipital muscles as they try to compensate for the instability. Over time, the repeated, involuntary activation of these muscles leads to mechanical overload of the myodural bridge complex, altering the mechanical properties of the dura it merges with. As a result, the dura becomes stiffer, reducing the overall compliance of the cervical region. This lower compliance, combined with CSF resistance at the same level, leads to intracranial pressure peaks during the cardiac cycle (pulse pressure) that are amplified during activities such as coughing, sneezing, and physical exertion. This increase in pulse pressure reduces the compliance of the cervical subarachnoid space which increases the CSF wave speed in the spinal canal, and further increases pulse pressure in a feedback loop. Finally, the abnormal pressure environment induces greater neural tissue motion and strain, causing microstructural damage to the cerebellum, brainstem, and cervical spinal cord, and leading to symptoms. This hypothesis explains how the combination of craniocervical bony abnormalities, anatomic CSF restriction, and reduced compliance leads to symptoms in adult CMI.
Keywords: Chiari malformation Type I, Pathophysiology, Compliance, Myodural bridge complex, Hydrodynamics
Introduction
Chiari malformation type I
Chiari malformation Type I (CMI) is a serious neurological disorder characterized by herniation of the cerebellar tonsils through the foramen magnum at the cranio-vertebral junction (CVJ) as first described by Hans Chiari more than 100 years ago [1]. Common symptoms in adults include cough or Valsalva-induced occipital headaches, neck/shoulder/upper back pain, balance issues, brainstem/cranial nerve issues, and cognitive dysfunction [2]. In some patients, the herniation can lead to the formation of a syrinx, or fluid-filled cyst in the spinal cord tissue, causing paresthesia, muscle weakness, and paralysis [1]. Symptom onset can occur at any age, but in adults symptoms often emerge in the late 20’s or early 30’s [3]. Symptoms are thought to be due to disruption of the natural flow of cerebrospinal fluid (CSF) across the CVJ and the herniation exerting a mass effect on nearby neural tissue. Still, the precise mechanism underlying most symptoms is poorly understood [4].
Diagnostically, CMI is traditionally defined as a herniation of 5 mm or more measured at the mid-sagittal plane [1]. However, this definition has proven to be problematic as up to 1% of the adult population meets this radiographic criterion, but the vast majority of people will never experience any symptoms [5]. This is true even for CSF related symptoms such as cough associated headache and the presence of a syrinx [3,6]. This has led many surgeons to reject the 5 mm herniation criterion as a diagnostic rule and has left the medical community searching for improved diagnostic criteria [4]. For the purposes of this paper, CMI will be used to refer to symptomatic herniation of the cerebellar tonsils.
Symptomatic CMI theories
Small posterior cranial fossa (PCF)
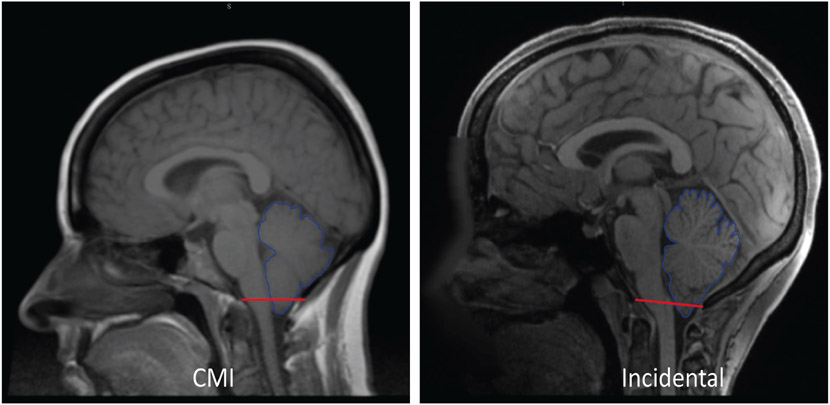
The pathophysiology of CMI is not well elucidated; however, the prevailing theory focuses on the underdevelopment of the PCF, which does not provide enough space for the cerebellum resulting in herniation of the tonsils [3]. This theory is supported by morphometric analyses of the PCF, which have shown abnormal PCF structures and reduced PCF space in CMI patients compared to controls [3,7-8]. For example, our group compared 229 adult female CMI patients to age, race, and BMI matched controls and found that the PCF of the CMI group was significantly different as characterized by the reduced height of PCF structures and a flattening of the clivus angulation [7]. A separate study using many of the same subjects found reduced CSF spaces, or crowding, around the cerebellar tonsils [9]. Despite this supporting evidence, the small PCF theory does not address the issue of asymptomatic cases of tonsillar herniation being far more prevalent than symptomatic ones. In fact, from a mid-sagittal view, it can be difficult to distinguish any difference between symptomatic CMI and asymptomatic tonsillar herniation. This is illustrated in Fig. 1, which shows a symptomatic CMI adult on the left taken from the Chiari 1000 Patient Database, and an asymptomatic adult on the right obtained from the Human Connectome Project. The small PCF theory also fails to adequately address the link between herniation and symptoms. If symptoms were completely a result of the herniation blocking CSF and exerting a mass effect, one would expect the amount of herniation to correlate with the severity of symptoms, but this is not the case [3,6]. Rather it appears that some people are able to accommodate the restricted CSF flow by some other means.
Fig. 1.
Sagittal view of the cerebellum (outlined in blue) for a CMI subject showing tonsillar descent below the foramen magnum (left) and an incidental (right). The red line indicates the McRae line.
Tethered Cord
While the small PCF theory is generally accepted in the neurosurgical community, other theories have emerged over the years. In 1997 Royo-Salvador proposed that CMI (and other conditions) are due to a tight filum terminale essentially pulling down on the spinal cord. The evidence to support this theory is limited primarily to his own results in treating CMI patients by sectioning of the filum [10]. However, a cadaver study that used weights to simulate this tension found that the traction force exerted on the spinal cord is quickly dispersed and does not reach the level of the cerebellar tonsils [11].
Atlanto-axial (AA) Joint Instability
More recently, Goel et al. proposed that CMI symptoms mainly arise from AA joint instability and that the cerebellar tonsils herniate as a protective measure against mechanical pinching [12]. They present favorable clinical results by treating a series of patients with C1-C2 stabilization surgery (as opposed to the widely accepted decompression procedure). Still, this theory does not address the issue of asymptomatic cases or the significant morphometric evidence of PCF variations. In addition, clinically identified cervical instability in CMI is not overly common and is usually associated with Ehlers-Danlos Syndrome [13]. A survey of 1,315 adult female CMI patients found that only 10% had been diagnosed with cervical instability. Similarly, a survey of 699 adult female surgical CMI patients found that only 13% had undergone any type of fusion or stabilization procedure [14].
Atlanto-occipital (AO) Joint Instability
In a morphometric study of the AO joint, Wan et al. used high-resolution computerized tomography (CT) to show that symptomatic CMI patients have smaller than normal occipital condyles and shallower facets on the atlas [15-16]. This finding, combined with a study that showed the stabilizing transverse and alar ligaments are also abnormal in CMI patients [17], led Wan et al. to postulate that AO joint instability plays a role in CMI. While Wan et al. also posit that this instability could cause the actual tonsillar herniation, they do acknowledge that it could instead play a role in the development of symptoms. However, they do not propose what this role might be or speculate on the physiological link between the two.
Physical Trauma/Stress
There is substantial evidence that physical trauma and activities play a role in symptom onset for at least a subset of CMI patients [3,14]. Both patient surveys and clinical reports indicate that up to one-third of patients cite a specific event that triggered their symptoms. For example, a survey of 460 adult CMI patients undertaken by our group found that the most common triggering events were related to traumas such as car accidents (16%), falls (11%), head trauma (10%), and sports injuries (5%). However, pregnancy (13% of female respondents) and physical exertion (9%) were also commonly cited [14]. Clinically, there have been case reports of CMI symptoms being sparked by car accidents, head trauma, and skydiving [18-20]. Currently, no satisfying explanation has been proposed for the role that physical trauma plays in symptom onset.
Hypothesis
We propose that symptomatic CMI originates from a combination of craniocervical bony and ligamentous abnormalities of the PCF, the AO joint, and the AA joint, which leads to both tonsillar herniation and cranio-cervical instability (which is often not identified during standard clinical and radiographic evaluation for CMI), respectively. This, in turn, leads to a combined hydrodynamic effect of CSF restriction due to the herniation and reduced spinal canal and dural compliance (dV/dP) at the same level due to repeated activation of the suboccipital muscles causing mechanical failure of the myodural bridge complex (MDBC). This combination disrupts the pressure equilibrium between the cranial and spinal compartment during the cardiac cycle as CSF flows back and forth across the CVJ. Specifically, an elevated CSF pulse pressure environment results, especially during activities that naturally increase pressure, such as coughing, sneezing, and physical exertion. The elevated pulse pressure amplitude, in turn, causes increased motion and strain of the cerebellum, brainstem, and upper spinal cord leading to many of the common CMI symptoms. Fig. 2 illustrates the cascading sequence of events and factors that culminate in CMI symptoms.
Fig. 2.
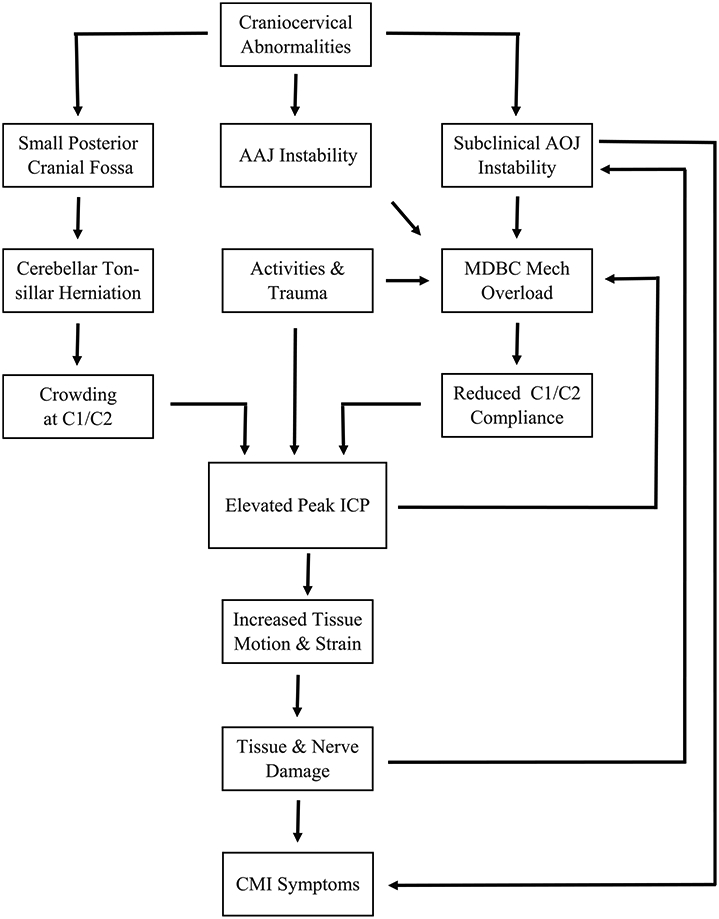
Flow chart depicting the factors that culminate in adult CMI symptoms.
Subclinical AO Joint Instability
While the PCF and AA joint in CMI patients have been studied extensively, the AO joint has not received as much attention. The AO joint is normally cup-shaped with the convex occipital condyles situated in the convex articulating surfaces of the atlas [16]. Stability is provided by a combination of the joint morphology and surrounding ligaments. Recent morphometric studies of the AO joint have identified the potential for instability in CMI patients [16]. Specifically, Wan et al. used thin sliced CT to calculate the depth to length ratio of the convex surfaces of the atlas in 47 adult CMI patients as compared to age and gender-matched controls and found that the ratio was significantly reduced in the CMI group. This flattening is similar to morphometric studies in Down syndrome patients with clinically identified AO joint instability [15]. Wan et al. also used thin CT slices to examine the occipital condyles of 73 adult CMI patients compared to matched controls and found the average length, width, and height of the condyles in the CMI group were significantly smaller [16]. Additionally, an MRI study of the ligaments of the CVJ found that both the transverse and alar ligaments were significantly shorter in adult CMI patients compared to controls [17]. Based on this, we propose that a large portion of adult CMI patients experience subclinical AO joint instability, which causes the suboccipital muscles to work harder in an attempt to compensate. Eventually, this leads to mechanical overload and failure of the MDBC. For the purposes of this paper, subclinical refers to a level of instability not routinely identified during clinical and standard radiographic evaluation for CMI.
Myodural Bridge Complex
A fibrous connection between the rectus capitis posterior minor muscle and the cervical dura was first identified by Hack in 1995 and labeled a myodural bridge [21-22]. Since that time, similar connections have been identified linking additional suboccipital muscles (rectus capitis posterior major and obliquus capitis inferior) to the cervical dura. In combination, these connections have been termed the MDBC and are believed to stabilize the spinal cord and regulate the tension of the dura to prevent infolding [21-22]. Examination of the MDBC by scanning electron microscope found that the fibers of the MDBC penetrate and merge into the dura [23]. From their analysis, Jiang et al. state, “The fibers of the myodural bridge merged into the spinal dura mater and gradually became a superficial layer of the spinal dural mater” supporting the concept that the MDBC is capable of passing tension from the suboccipital muscles to the dura.
The MDBC has also been theorized to promote CSF movement and in essence act as a pump to aid in pushing CSF back and forth across the CVJ [21]. Xu et al. used phase-contrast magnetic resonance imaging (PCMR) on healthy controls to demonstrate that head movement significantly influenced CSF flow at the CVJ by increasing CSF stroke volume, and proposed the MDBC played a key role in this effect [23]. The idea that the MDBC controls the tension of the dura and influences CSF dynamics is further supported by Ma et al., who used a dog study to show that both passive head movement and electrical stimulation of suboccipital muscles significantly increased CSF pressure. When the MDBC connection was severed, the CSF pressure remained steady when the muscles were stimulated [24]. However, the role of the MDBC in CMI has not previously been established.
The suboccipital muscles respond reflexively to involuntary head and neck movements [22]. We propose that subclinical AO joint instability in CMI patients leads to repeated involuntary contractions of the suboccipital muscles, especially during physical activities that amplify the inherent instability. Over time, these repeated contractions lead to mechanical overload of the MDBC, which ceases to function properly. Eventually, the mechanical properties of the dura - which the failed MDBC connects to - are altered, becoming stiffer and less compliant. This is supported by the finding of Klinge et al. that the surgically removed MDBCs of adult CMI patients showed signs of fibril pathology suggestive of mechanical overload [25]. Since the MDBC merges with the dura rather than simply connecting to it, it is reasonable to conclude that this pathology will extend to the dura as well. It is worth noting that this reduction in compliance can also occur in cases of clinically significant instability of the AO joint, the AA joint, or both. In fact, any mechanism, such as a whiplash trauma, that can cause mechanical overload of the MDBC, either suddenly or over time, has the potential to reduce the cervical, dural compliance in CMI.
Reduced Cervical Spinal Caned Compliance
Our group has developed preliminary data using PCMR showing that adult CMI patients have abnormal stiffness and reduced spinal canal compliance in the cervical region. The institutional review board (IRB) at Emory University approved this study, and all subjects provided written informed consent (IRB#8711). All subjects were scanned at the Center for Systems Imaging at the Emory University School of Medicine. Specifically, the CSF flow rate was measured at the C2 and T2 levels on 4 adult CMI patients and three healthy controls. From this, the CSF flow volumes at the input (C2) and output (T2) were calculated by integrating the flow rate over the duration of the systole. Next, the change in CSF volume was calculated as the difference between volume in and volume out. Finally, volumetric expansion was calculated as a percentage based on the change in volume divided by the volume in. The average volumetric expansion was 62% for the control group, but for the CMI group, this was reduced to just 26%.
This indicates that the control group showed high levels of compliance in the cervical region, meaning that as CSF was pushed out of the brain and across the CVJ in response to systole, the subarachnoid space expanded in the cervical region to accommodate the influx of CSF. However, this was not the case in the CMI group, where increased stiffness limited the natural expansion of the subarachnoid space and altered the pressure environment, similar to how stiff arteries increase systolic blood pressure [26].
Elevated Pulse Pressure
We believe that the combination of CSF restriction due to the herniated tonsils and the reduced compliance via the MDBC leads to abnormal pressure peaks during the cardiac cycle as more pressure is required to move CSF across the CVJ. In a healthy person, during systole CSF can flow freely across the CVJ, and a compliant dura expands in response to the pulsatile increase of CSF volume in the cervical region, thus maintaining pressure equilibrium (Fig. 3). However, in a CMI patient, there is both blockage of CSF flow due to herniated tonsils and a stiff, less compliant dura, the combination of which leads to elevated pulse pressure. Clinical pressure monitoring of CMI patients supports this theory. Fric et al. simultaneously monitored both static and pulse intracranial pressure (ICP) and lumbar CSF pressure in 26 adult CMI patients [27]. They defined abnormal pulse pressure as mean ICP wave amplitude (MWA) > 4 mmHg combined with a MWA > 5 mmHg in more than 10 % of the recording time and borderline as average MWA of 4–5 mmHg. Abnormal pressure gradient was defined as a difference of more than 2 mmHg between the MWA ICP and the MWA lumbar. Using these criteria, they found that 66.5% had abnormal pulse pressure, 7.7% had borderline pulse pressure, and 71% showed an abnormal pressure gradient. The two measures were significantly correlated with each other (R = 0.72, p < 0.001), but neither was correlated with tonsillar position, implying a reduction in compliance was the cause. Similarly, Dyson et al. used continuous ICP monitoring to compare pre-surgical CMI patients, failed surgical CMI patients, and a control group [28]. While the median ICP did not differ significantly between the groups, the pulse amplitude did. Specifically, both the ICP pulse amplitude of the pre-surgical and failed surgical groups was significantly higher than the control group.
Fig. 3.
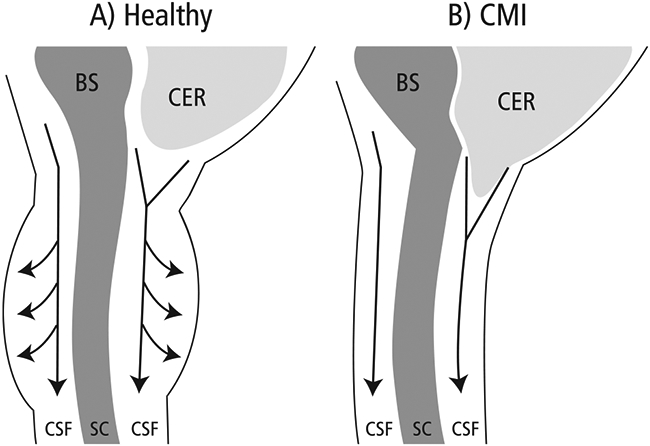
SC = spinal cord; BS = brainstem; CER = cerebellum; CSF = cerebrospinal fluid In a healthy person, CSF flows freely across the CVJ during systole and a compliant dura in the cervical region expands to accommodate the influx of CSF volume. Thus pressure equilibrium is maintained between the brain and spinal compartments. In a CMI patient, herniated tonsils cause resistance to CSF flow and increase the pressure required to push the CSF across the junction. In addition, a stiff, less compliant dura can’t accommodate the extra CSF volume in the cervical region, which also increases the pressure (similar to how stiff arteries increase systolic blood pressure). The combination of increased resistance and reduced compliance creates an elevated pulse pressure environment.
It is important to note the ICP monitoring in these studies was done while the subjects were at rest. The ICP peaks in CMI patients are likely magnified even more during activities such as coughing, sneezing, postural change, and physical exertion, which naturally involve elevated pressure. This aligns with patient reports of activities that aggravate symptoms [14].
An increase in the pressure pulse amplitude can create a feedback loop that further increases pulse pressure. Since the resistance to CSF flow is elevated due to a reduction in the cross-section area of subarachnoid space (herniated cerebellar tonsils), greater pulse pressure is required to move CSF into and out of the spinal canal across the CVJ. This puts the outer wall of the spinal canal, or the dura, under greater tension during these elevated peak pressures. This increased tension leads to spinal canal stiffness and increases wave speed propagation which, in turn, leads to a further increase in pulse pressure through wave reflection similar to that described for the arterial tree [29]. This increases the pulse pressure during each heartbeat resulting in a feedback loop of increasing pulse pressure and tension.
Microstructural Damage
We propose this increased pulse pressure is the cause of an observed increase in motion and strain of the cerebellum, brainstem, and upper spinal cord. Our group used displacement encoding with stimulated echoes (DENSE) MRI to capture the displacement of seven different brain regions in 43 adult CMI patients and 25 controls [30]. Mean compression and extension strain were then calculated for each brain region based on the displacement values. Displacement of the cerebellum and brainstem were found to be 106% and 64% higher, respectively, in the CMI group. In addition, the CMI group had a 52% increase in the mean compression strain in the cerebellum and a 50% and 41% increase in the mean extension strain in the cerebellum and brainstem, respectively. In addition, Lawrence et al. used cardiac gated PCMR to measure the tissue motion of the upper spinal cord of 20 CMI adults, both pre and post-surgery, plus 15 controls [31]. The pre-surgical CMI group showed significantly increased motion compared to the control group, and this motion decreased significantly post-surgery.
We propose that over time this strain damages the cerebellum, brainstem, and upper spinal cord leading to many of the most common CMI symptoms. For example, our group used diffusion tensor imaging (DTI) to examine the white matter microstructure in a group of 18 adult CMI patients and 18 age and education-matched controls [32]. Abnormal fractional anisotropy (FA) and diffusivity were found in the cerebellum of the CMI group as compared to the control group. Similarly, Krishna used DTI to examine the lower brainstem of adult CMI patients versus healthy controls. The CMI group showed abnormal FA of the anterior brainstem before surgery, but this tended to normalize after surgery except for some patients with syringomyelia [33]. Finally, a third DTI study found abnormal diffusivity in the cervical spinal cord of CMI patients versus a control group and abnormal FA in the pontobulbar region [34].
Discussion
This theory explains, with substantial supporting evidence, several previously confusing aspects of adult CMI. First and foremost, it offers an explanation for why there are many more cases of asymptomatic tonsillar herniation than symptomatic CMI. Namely, symptoms are not due to tonsillar position alone, but rather a combination of tonsillar position and reduced cervical compliance. Under this theory, people with tonsillar herniation but without symptoms have adequate compliance in the cervical spinal canal to compensate for the anatomically caused CSF restriction and therefore don’t experience the damaging increase in pulse pressure. Since most asymptomatic cases do not become symptomatic [35], it is likely that these cases do not have the abnormal AO or AA joint morphology found in symptomatic CMI cases, even though they may have the abnormal PCF morphology leading to tonsillar herniation.
Similarly, this theory accounts for why tonsillar position is not correlated with symptom severity. In symptomatic cases, the severity of symptoms is related to the amplitude and frequency of the pulse pressure peaks, which is a function of CSF restriction due to tonsillar herniation and reduced cervical compliance via the MDBC, and physical stress factors such as Valsalva type activities and minor traumas. In practical terms, this explains why someone with a small herniation – even less than 5 mm – can experience severe symptoms because of extreme stiffness and low compliance, while a person with a large herniation – well above 5 mm – may be symptom-free due to maintaining high compliance. If patient-specific compliance were able to be measured, this theory predicts that symptom severity would be correlated to the sum of geometric space around the tonsils for CSF flow and the overall compliance at the same level.
This theory also details the underlying mechanism for many of the most common CMI symptoms such as cough/Valsalva occipital headache, neck pain, dizziness and cerebellum/brainstem/spinal cord issues. Currently, it is believed that in general irritation of the C1/C2 nerve roots, nociceptors in the dura and/or small blood vessels in the skull base can lead to occipital headaches [36-37]. In addition, abnormal CSF flow around the herniated tonsils has been implicated as a causative mechanism of Chiari related headahces. McGirt et al. used PCMR to show elevated CSF velocities just below the tonsils in CMI patients as compared to both controls and other CMI patients with frontal or generalized headaches [38]. Our group used computational fluid dynamics to simulate patient-specific CSF flow and calculate the integrated longitudinal impedance (ILI) of 51 symptomatic adult CMI patients [39]. ILI is a commonly used technique to obtain the resistance within a conduit to unsteady fluid motion. We found that the ILI was significantly higher in patients with cough-associated headaches than in those without such headaches. Further, based on receiver operator characteristic curves, ILI was more predictive of cough associated headaches than tonsillar position [39]. In addition, our group has recently shown that a smaller anterior CSF space was related to an increase in pain [40]. From this, it is reasonable to conclude that the Chiari headache is due at least in part to the altered pulse pressure environment irritating nerve roots, pain receptors in the dura, and/or the blood vessels of the skull base.
We believe that much of the neck pain common with CMI originates from subclinical AO/AA joint instability. The pain can arise both directly from overworked muscles trying to compensate and indirectly from the muscle tension causing the MDBC to fail, inducing an abnormal pressure environment which then affects cervical nerves. In addition, since the suboccipital muscles are innervated from the dorsal ramus of the C1 spinal nerve, it is possible that a feedback loop is created whereby damage to the C1 nerve causes spasticity of the suboccipital muscles, which then transmits even more tension to the cervical dura through the MDBC and increases the pulse pressure correspondingly. It is interesting to note that decompression surgery is reported to improve neck pain only 52–66% of the time [41-42], perhaps indicating residual instability or permanent nerve damage.
It is likely that a mass effect from the tonsillar herniation still plays a role in some symptoms, causing a situation that can lead to both pressure on the adjacent C1 and C11 nerves (equivalent to a vascular compression syndrome) and compression of the posterior inferior cerebellar artery with vascular steal phenomenon. For example, from author observations during surgery (PK) we believe dizziness and syncope in CMI may be due to compression of the posterior inferior cerebellar artery.
The increased tissue motion and strain due to the elevated pulse pressure environment is the causative mechanism for many of the common CMI symptoms that involve the cerebellum, brainstem, cranial nerves, and upper spinal cord, as evidenced by the microstructural changes found on DTI.
This theory may also offer an explanation for how trauma can suddenly trigger symptoms. For example, a whiplash injury from a car accident could lead to a sudden failure of the MDBC and alteration of the cervical compliance due to inflammation. The effects of such an injury are likely amplified in someone with underlying AO or AA joint instability. Any type of traumatic injury which triggers an inflammatory response may play a similar role in increasing cervical stiffness, lowering compliance, and triggering CMI type symptoms. This theory may also account for the emergence of symptoms in the third and fourth decade of life in some patients. Namely, the mechanical failure of the MDBC occurs due to an accumulation of stresses over a long period of time, similar to a bridge or other man-made structure.
Finally, this theory may account for the presence of arachnoid adhesions which are commonly found during CMI surgery when the dura is fully opened [43]. Specifically, the adhesions could be an extension of the change in tissue structure in response to the mechanical failure of the MDBC. It is also possible that the adhesions are linked to the altered CSF dynamics, as surgical observations (PK) have noted the alignment of such adhesions is different in the area of disrupted CSF flow. Further investigation is required to quantify the impact (if any) the adhesions have on CSF resistance and compliance in the cervical region.
While this theory has the potential to explain certain aspects of CMI, it is not all-encompassing. There are indications that CMI involves several subgroups with different etiologies [44-46]. This theory is most applicable to what is sometimes referred to as “classic” Chiari; i.e., patients with a small PCF and CSF-related symptoms such as occipital headaches and syringomyelia. It is not clear how it applies, for example, to patients with concomitant Ehlers-Danlos Syndrome (EDS), who tend to have smaller tonsillar herniations but clinically recognized C1/C2 instability and systemic connective tissue issues [46]. It is possible that many of the symptoms for these patients arise from the C1/C2 instability directly and systemic connective tissue issues.. However, it is also possible that reduced compliance still plays a role in these cases as the C1/C2 instability is likely to have an effect on the suboccipital muscles or that the MDBC itself is congenitally altered and not functioning properly. Similarly, it is difficult to untangle the origin of symptoms for patients with intracranial hypertension and CMI, as there is extensive overlap in the symptomatology of the two conditions.
This theory also does not cover the psychological impact of CMI. Garcia et al. found high rates of moderate-severe depression and anxiety among a large sample of adult CMI women which persisted even after decompression surgery [47]. It is likely that factors beyond what is discussed in this paper play a role in the psychological impact of CMI on patients. Despite these limitations, if proven correct, this theory has significant implications not only for advancing the understanding of adult CMI, but for diagnosis and treatment as well. Current diagnostic criteria are insufficient for identifying symptomatic CMI cases that will respond to treatment. With this theory, diagnostic imaging and analysis could quickly expand to include examining the AO joint, the MDBC, and indications of reduced compliance such as lack of volumetric expansion. Over time a new quantitative measure could be developed which incorporates both the geometry around the herniated tonsils and the cervical compliance and obviates the 5 mm herniation rule.
For treatment, the relative contributions of the tonsillar herniation and cervical compliance may influence decisions on surgical technique, such as the use of duraplasty and tonsillar reduction. For cases with minimal CSF blockage due to herniation, it may be possible to achieve symptom relief from severing the MDBC connection only, if the dural stiffness has not yet been permanently altered.
The confirmed presence of abnormal AO or AA joint morphology may lead to more frequent stabilization in addition to the standard decompression. It is even possible to envision new treatment approaches focused on reducing tension and inflammation in the suboccipital muscles and restoring cervical compliance without the trauma and morbidity risk of surgery.
Testing the Hypothesis
Different aspects of this theory can be evaluated using a number of existing experimental techniques, such as imaging, cadaver studies, and finite element modeling (FEM). The AO joint morphology of a large set of CMI patients could easily be quantified using CT and compared to matched controls. Similarly, MRI can be used to evaluate the structure, composition, and function of the MDBC and associated muscles [48]. Or a comprehensive imaging study could be undertaken, which includes those modalities plus PCMR to capture cervical volumetric expansion; DENSE to capture the motion of the cerebellum, brainstem, cervical spine; and DTI to assess microstructural damage to the same regions for different groups of CMI patients and healthy controls.
Cadaver studies have previously been used to quantify both normal AO joint functionality and AO joint instability [49,50]. It may be possible to refashion a cadaveric AO joint to match the flattening found in CMI patients and examine the impact on stability and the supporting muscles and ligaments. FEM has also been used to study the AO joint under different conditions [51]. It may be informative to use this approach to study how CMI type AO joint morphology responds to everyday motions and traumas.
Conclusion
We propose that symptoms in adult CMI are due to a combination of CSF restriction from herniated tonsils and reduced cervical compliance. Abnormal AO and/or AA joint morphology causes instability, often subclinical, leading to compensatory overactivation of the suboccipital muscles and mechanical overload and failure of the MDBC. Over time, this alters the mechanical properties of the dura the MDBC merges with, causing it to become stiffer and reducing the overall cervical compliance. The combination of CSF restriction and reduced compliance leads to an elevated pulse pressure environment, causing strain on the cerebellum, brainstem, and upper spinal cord. Over time, this results in microstructural damage and leads to symptoms such as cough headaches, neck pain, balance issues, abnormal sensations, and brainstem/cranial nerve issues. This theory is supported by a significant number of published studies along with pilot data from our group. It accounts for several previously unexplained aspects of adult CMI, such as why asymptomatic cases far outnumber symptomatic individuals, why the tonsillar position is not correlated with symptom severity, and how environmental factors such as physical activity and trauma influence symptoms. Finally, the theory is testable through imaging, cadaver studies, and other means. If proven correct, it will not only further the understanding of adult CMI but will impact both diagnosis and treatment as well.
Acknowledgements
The authors would like to thank Conquer Chiari (Grant No. CC-18-1459 and CC-20-1667) and the National Institutes of Health, (Grant No. 1R15NS109957-01A1 and 1R15NS071455-01) for providing funding for this research work.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Labuda R, Loth F, Slavin K. National Institutes of Health Chiari Research Conference: state of the research and new directions. Neurological research. 2011;33(3):227–3 Epub 2011/04/26. 10.1179/016164111x12962202723689. PubMed PMID: 21513642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mueller DM, Oro JJ. Prospective analysis of presenting symptoms among 265 patients with radiographic evidence of Chiari malformation type I with or without syringomyelia. Journal of the American Academy of Nurse Practitioners. 2004;16(3):134–8. Epub 2004/05/08. 10.1111/j.1745-7599.2004.tb00384.x. PubMed PMID: 15130068. [DOI] [PubMed] [Google Scholar]
- [3].Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44(5):1005–17. PubMed PMID: 10232534. [DOI] [PubMed] [Google Scholar]
- [4].Bolognese PA, Brodbelt A, Bloom AB, Chiari KRW, Malformation I. Opinions on Diagnostic Trends and Controversies from a Panel of 63 International Experts. World Neurosurg 2019;130:e9–16. 10.1016/j.wneu.2019.05.098. PubMed PMID: 31121369, Epub 2019/05/24. [DOI] [PubMed] [Google Scholar]
- [5].Smith BW, Strahle J, Bapuraj JR, Muraszko KM, Garton HJ, Maher CO. Distribution of cerebellar tonsil position: implications for understanding Chiari malformation. Journal of neurosurgery. 2013;119(3):812–9. Epub 2013/06/19. 10.3171/2013.5.Jns12182 PubMed PMID: 23767890. [DOI] [PubMed] [Google Scholar]
- [6].Nwotchouang BST, Eppelheimer MS, Ibrahimy A, Houston JR, Biswas D, Labuda R, Bapuraj JR, Allen PA, Frim D, Loth F. Clivus length distinguishes between asymptomatic healthy controls and symptomatic adult women with Chiari malformation type I. Neuroradiology. 2020;62(11):1389–400. Epub 2020/05/18. 10.1007/s00234-020-02453-5. PubMed PMID: 32418026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Houston JR, Eppelheimer MS, Pahlavian SH, Biswas D, Urbizu A, Martin BA, et al. A morphometric assessment of type I Chiari malformation above the McRae line: A retrospective case-control study in 302 adult female subjects. J Neuroradiol 2018;45(1):23–31. 10.1016/j.neurad.2017.06.006. PubMed PMID: 28826656. [DOI] [PubMed] [Google Scholar]
- [8].Noudel R, Gomis P, Sotoares G, Bazin A, Pierot L, Pruvo JP, Bordet R, Roche PH. Posterior fossa volume increase after surgery for Chiari malformation Type I: a quantitative assessment using magnetic resonance imaging and correlations with the treatment response. Journal of neurosurgery. 2011;115(3):647–5 Epub 2011/02/010.3171/2010.11.Jns10214PubMed PMID: 21294619. [DOI] [PubMed] [Google Scholar]
- [9].Biswas D, Eppelheimer MS, Houston JR, Ibrahimy A, Bapuraj JR, Labuda R, et al. Quantification of Cerebellar Crowding in Type I Chiari Malformation. Ann Biomed Eng 2019;47(3):731–43. 10.1007/s10439-018-02175-z. [DOI] [PubMed] [Google Scholar]
- [10].Royo-Salvador MB. A new surgical treatment for syringomyelia, scoliosis, Arnold-Chiari malformation, kinking of the brainstem, odontoid recess, idiopathic basilar impression and platybasia. Revista de neurologia 1997;25(140):523–30. Epub 1997/04/01 PubMed PMID: 9172910. [PubMed] [Google Scholar]
- [11].Tubbs RS, Loukas M, Shoja MM, Oakes WJ. Observations at the craniocervical junction with simultaneous caudal traction of the spinal cord. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(4):367–9. Epub 2007/01/05. 10.1007/s00381-006-0286-1. PubMed PMID: 17203326. [DOI] [PubMed] [Google Scholar]
- [12].Goel A, Jadhav D, Shah A, Rai S, Dandpat S, Vutha R, Dhar A, Prasad A. Chiari 1 Formation Redefined-Clinical and Radiographic Observations in 388 Surgically Treated Patients. World Neurosurg. 2020;141:e921–e34. Epub 2020/06/21. 10.1016/j.wneu.2020.06.076. PubMed PMID: 32562905. [DOI] [PubMed] [Google Scholar]
- [13].Henderson FC Sr., Austin C, Benzel E, Bolognese P, Ellenbogen R, Francomano CA, Ireton C, Klinge P, Koby M, Long D, Patel S, Singman EL, Voermans NC. Neurological and spinal manifestations of the Ehlers-Danlos syndromes. American journal of medical genetics Part C, Seminars in medical genetics. 2017;175(1): 195–211. Epub 2017/02/22. 10.1002/ajmg.c.31549. PubMed PMID: 28220607. [DOI] [PubMed] [Google Scholar]
- [14].Chiari C. Chiari 1000 2021. [cited 2021 02/9/2021]. Available from: http://chiari1000results.info/.
- [15].Wan M, Zong R, Tong HY, Zhang ZZ, Zhao B, Yu XG. A morphometric study of the atlanto-occipital joint in adult patients with Chiari malformation type I. British journal of neurosurgery. 2020:1–4. Epub 2020/09/25. 10.1080/02688697.2020.1823940. PubMed PMID: 32969751. [DOI] [PubMed] [Google Scholar]
- [16].Wan M, Zong R, Xu HL, Qiao GY, Tong HY, Shang AJ, et al. Feasibility of occipital condyle screw placement in patients with Chiari malformation type I: a computed tomography-based morphometric study. Acta Neurochir (Wien) 2021. 10.1007/s00701-021-04714-5. PubMed PMID: 33462712, Epub 2021/01/20. [DOI] [PubMed] [Google Scholar]
- [17].Karaaslan B, Börcek A, Uçar M, Aykol Ş. Can the Etiopathogenesis of Chiari Malformation Be Craniocervical Junction Stabilization Difference? Morphometric Analysis of Craniocervical Junction Ligaments. World Neurosurg 2019;128:e1096–101. 10.1016/j.wneu.2019.05.072. PubMed PMID: 31103770, Epub 2019/05/20. [DOI] [PubMed] [Google Scholar]
- [18].Wrobel CJ, Taubman K. Syringomyelia in skydivers. The New England journal of medicine. 2003;349(3):309–10. Epub 2003/07/10.1056/nejm200307173490325. PubMed PMID: 12867621. [DOI] [PubMed] [Google Scholar]
- [19].Wan MJ, Nomura H, Tator CH. Conversion to symptomatic Chiari I malformation after minor head or neck trauma. Neurosurgery. 2008;63(4):748–53; discussion 53. Epub 2008/11/05. 10.1227/01.Neu.0000325498.04975.C0. PubMed PMID: 18981886. [DOI] [PubMed] [Google Scholar]
- [20].Bunc G, Vorsic M. Presentation of a previously asymptomatic Chiari I malformation by a flexion injury to the neck. Journal of neurotrauma. 2001;18(6):645–8. Epub 2001/07/05. 10.1089/089771501750291882. PubMed PMID: 11437087. [DOI] [PubMed] [Google Scholar]
- [21].McElroy A, Rashmir A, Manfredi J, Sledge D, Carr E, Stopa E, Klinge P. Evaluation of the Structure of Myodural Bridges in an Equine Model of Ehlers-Danlos Syndromes. Scientific reports. 2019;9(1):9978. Epub 2019/07/12. 10.1038/s41598-019-46444-w. PubMed PMID: 31292490; PMCID: PMC6620297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Enix DE, Scali F, Pontell ME. The cervical myodural bridge, a review of literature and clinical implications. The Journal of the Canadian Chiropractic Association. 2014;58(2):184–92. Epub 2014/06/17. PubMed PMID: 24932022; PMCID: PMC4025088. [PMC free article] [PubMed] [Google Scholar]
- [23].Xu Q, Yu SB, Zheng N, Yuan XY, Chi YY, Liu C, Wang XM, Lin XT, Sui HJ. Head movement, an important contributor to human cerebrospinal fluid circulation. Scientific reports. 2016;6:31787. Epub 2016/08/20. 10.1038/srep31787. PubMed PMID: 27538827; PMCID: PMC4990938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ma Y, Tang W, Gong DZ, Li XY, Zhang JH, Sun JH, Wang B, Zhang Y, Chen YX, Zhang ZH, Zheng N, Okoye CS, Chi YY, Wu CW, Yu SB, Sui HJ. The morphology, biomechanics, and physiological function of the suboccipital myodural connections. Scientific reports. 2021;11(1):8064. Epub 2021/04/15. 10.1038/s41598-021-86934-4. PubMed PMID: 33850172; PMCID: PMC8044117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Klinge P, McElroy A, Donahue J, Brinker T, Gokaslan Z, Beland M. Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. JNS Spine. 2021;Accepted. [DOI] [PubMed] [Google Scholar]
- [26].Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–9. Epub 2003/06/11. 10.1161/01.Cir.0000069826.36125.B4. PubMed PMID: 12796414. [DOI] [PubMed] [Google Scholar]
- [27].Frič R, Lindstrøm EK, Ringstad GA, Mardal KA, Eide PK. The association between the pulse pressure gradient at the cranio-cervical junction derived from phase-contrast magnetic resonance imaging and invasively measured pulsatile intracranial pressure in symptomatic patients with Chiari malformation type 1. Acta Neurochir (Wien). 2016;158(12):2295–304. Epub 2016/10/16. 10.1007/s00701-016-2979-x. PubMed PMID: 27743249. [DOI] [PubMed] [Google Scholar]
- [28].Dyson EW, Chari A, Toma AK, Thorne LW, Watkins LD. Failed Foramen Magnum Decompression in Chiari I Malformation Is Associated With Failure to Restore Normal Intracranial Compliance: An Observational Cohort Study. Neurosurgery. 2020;86(6):E552–e7. Epub 2020/04/14. 10.1093/neuros/nyaa079. PubMed PMID: 32282048. [DOI] [PubMed] [Google Scholar]
- [29].O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. British journal of clinical pharmacology. 2001;51(6):507–22. Epub 2001/06/26. 10.1046/j.0306-5251.2001.01400.x. PubMed PMID: 11422010; PMCID: PMC2014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nwotchouang BST, Eppelheimer MS, Pahlavian SH, Barrow JW, Barrow DL, Qiu D, et al. Regional Brain Tissue Displacement and Strain is Elevated in Subjects with Chiari Malformation Type I Compared to Healthy Controls: A Study Using DENSE MRI. Ann Biomed Eng 2021. 10.1007/s10439-020-02695-7. PubMed PMID: 33398617, Epub 2021/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lawrence BJ, Luciano M, Tew J, Ellenbogen RG, Oshinski JN, Loth F, Culley AP, Martin BA. Cardiac-Related Spinal Cord Tissue Motion at the Foramen Magnum is Increased in Patients with Type I Chiari Malformation and Decreases Postdecompression Surgery. World Neurosurg. 2018;116:e298–e307. Epub 2018/05/08. 10.1016/j.wneu.2018.04.191. PubMed PMID: 29733988; PMCID: PMC6063776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Houston JR, Hughes ML, Bennett IJ, Allen PA, Rogers JM, Lien MC, Stoltz H, Sakaie K, Loth F, Maleki J, Vorster SJ, Luciano MG. Evidence of Neural Microstructure Abnormalities in Type I Chiari Malformation: Associations Among Fiber Tract Integrity, Pain, and Cognitive Dysfunction. Pain medicine (Malden, Mass). 2020;21(10):2323–35. Epub 2020/05/11. 10.1093/pm/pnaa094. PubMed PMID: 32388548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krishna V, Sammartino F, Yee P, Mikulis D, Walker M, Elias G, Hodaie M. Diffusion tensor imaging assessment of microstructural brainstem integrity in Chiari malformation Type I. Journal of neurosurgery. 2016;125(5):1112–9. Epub 2016/11/02. 10.3171/2015.9.Jns151196. PubMed PMID: 26848913. [DOI] [PubMed] [Google Scholar]
- [34].Gok H, Naderi S. Prognostic Value of Craniovertebral Junction Diffusion Tensor Imaging in Patients with Chiari Type 1 Malformation. Turkish neurosurgery. 2020;30(3):400–6. Epub 2020/02/25. 10.5137/1019-5149.Jtn.27144-19.2. PubMed PMID: 32091118. [DOI] [PubMed] [Google Scholar]
- [35].Dantas FLR, Dantas F, Caires AC, Botelho RV. Natural History and Conservative Treatment Options in Chiari Malformation Type I in Adults: A Literature Update. Cureus. 2020;12(12):e12050. Epub 2021/01/16. 10.7759/cureus.12050. PubMed PMID: 33447479; PMCID: PMC7802397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fontaine D, Almairac F, Santucci S, Fernandez C, Dallel R, Pallud J, Lanteri-Minet M. Dural and pial pain-sensitive structures in humans: new inputs from awake craniotomies. Brain : a journal of neurology. 2018;141(4):1040–8. Epub 2018/02/02. 10.1093/brain/awy005. PubMed PMID: 29390108. [DOI] [PubMed] [Google Scholar]
- [37].Noseda R, Melo-Carrillo A, Nir RR, Strassman AM, Burstein R. Non-Trigeminal Nociceptive Innervation of the Posterior Dura: Implications to Occipital Headache. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(10):1867–80. Epub 2019/01/10. 10.1523/jneurosci.2153-18.2018. PubMed PMID: 30622169; PMCID: PMC6407291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McGirt MJ, Nimjee SM, Floyd J, Bulsara KR, George TM. Correlation of cerebrospinal fluid flow dynamics and headache in Chiari I malformation. Neurosurgery. 2005;56(4):716–21; discussion -21. Epub 2005/03/29. 10.1227/01.neu.0000156203.20659.14. PubMed PMID: 15792510. [DOI] [PubMed] [Google Scholar]
- [39].Ibrahimy A, Huang CC, Bezuidenhout AF, Allen PA, Bhadelia RA, Loth F. Association Between Resistance to Cerebrospinal Fluid Flow Near the Foramen Magnum and Cough-Associated Headache in Adult Chiari Malformation Type I. J Biomech Eng 2021. 10.1115/1.4049788. PubMed PMID: 33454731, Epub 2021/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].García M, Eppelheimer MS, Houston JR, Houston ML, Nwotchouang BST, Kaut KP, et al. Adult Age Differences in Self-Reported Pain and Anterior CSF Space in Chiari Malformation. The Cerebellum 2021. 10.1007/s12311-021-01289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Giammattei L, Messerer M, Daniel RT, Aghakhani N, Parker F. Long-term outcome of surgical treatment of Chiari malformation without syringomyelia. Journal of neurosurgical sciences. 2020;64(4):364–8. Epub 2017/07/06. 10.23736/s0390-5616.17.04063-2. PubMed PMID: 28677937. [DOI] [PubMed] [Google Scholar]
- [42].De Vlieger J, Dejaegher J, Van Calenbergh F. Multidimensional, patient-reported outcome after posterior fossa decompression in 79 patients with Chiari malformation type I. Surgical neurology international. 2019;10:2 Epub 2020/01/02. 10.25259/sni_377_2019. PubMed PMID: 31893143; PMCID: PMC6935946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ellenbogen RG, Armonda RA, Shaw DW, Winn HR. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurgical focus. 2000;8(3):E6. Epub 2006/05/09. 10.3171/foc.2000.8.3.6. PubMed PMID: 16676929. [DOI] [PubMed] [Google Scholar]
- [44].Eppelheimer MS, Houston JR, Bapuraj JR, Labuda R, Loth DM, Braun AM, Allen NJ, Heidari Pahlavian S, Biswas D, Urbizu A, Martin BA, Maher CO, Allen PA, Loth F. A Retrospective 2D Morphometric Analysis of Adult Female Chiari Type I Patients with Commonly Reported and Related Conditions. Frontiers in neuroanatomy. 2018;12:2. Epub 2018/02/07. 10.3389/fnana.2018.00002. PubMed PMID: 29403363; PMCID: PMC5785719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mukherjee S, Kalra N, Warren D, Sivakumar G, Goodden JR, Tyagi AK, Chumas PD. Chiari I malformation and altered cerebrospinal fluid dynamics-the highs and the lows. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2019;35(10):1711–7. Epub 2019/06/17. 10.1007/s00381-019-04233-w. PubMed PMID: 31203396. [DOI] [PubMed] [Google Scholar]
- [46].Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien). 2010;152(7):1117–27. Epub 2010/05/05. 10.1007/s00701-010-0636-3. PubMed PMID: 20440631; PMCID: PMC2887504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garcia MA, Allen PA, Li X, Houston JR, Loth F, Labuda R, Delahanty DL. An examination of pain, disability, and the psychological correlates of Chiari Malformation pre- and post-surgical correction. Disability and health journal. 2019;12(4):649–56. Epub 2019/05/31. 10.1016/j.dhjo.2019.05.004. PubMed PMID: 31147250. [DOI] [PubMed] [Google Scholar]
- [48].Sun MY, Sui HJ, Eteer K, Yu SB, Hu JN. Utilization of MR imaging in myodural bridge complex with relevant muscles: current status and future perspectives. Journal of musculoskeletal & neuronal interactions. 2020;20(3):382–9. Epub 2020/09/04. PubMed PMID: 32877974; PMCID: PMC7493449. [PMC free article] [PubMed] [Google Scholar]
- [49].Siccardi D, Buzzatti L, Marini M, Cattrysse E. Analysis of three-dimensional facet joint displacement during two passive upper cervical mobilizations. Musculoskeletal science & practice. 2020;50:102218. Epub 2020/09/04. 10.1016/j.msksp.2020.102218. PubMed PMID: 32882623. [DOI] [PubMed] [Google Scholar]
- [50].Panjabi M, Dvorak J, Duranceau J, Yamamoto I, Gerber M, Rauschning W, Bueff HU. Three-dimensional movements of the upper cervical spine. Spine. 1988;13(7):726–30. Epub 1988/07/01. 10.1097/00007632-198807000-00003. PubMed PMID: 3194778. [DOI] [PubMed] [Google Scholar]
- [51].Zhang H, Bai J. Development and validation of a finite element model of the occipito-atlantoaxial complex under physiologic loads. Spine. 2007;32(9):968–74. Epub 2007/04/24. 10.1097/01.brs.0000261036.04919.91. PubMed PMID: 17450071. [DOI] [PubMed] [Google Scholar]