Abstract
O-methylation of small molecules is a common modification widely present in most organisms. Type III polyketides undergo O-methylation at hydroxyl end to play a wide spectrum of roles in bacteria, plants, algae, and fungi. Mycobacterium marinum harbours a distinctive genomic cluster with a type III pks gene and genes for several polyketide modifiers including a methyltransferase gene, mmar_2193. This study reports functional analyses of MMAR_2193 and reveals multi-methylating potential of the protein. Comparative sequence analyses revealed conservation of catalytically important motifs in MMAR_2193 protein. Homology-based structure-function and molecular docking studies suggested type III polyketide cores as possible substrates for MMAR_2193 catalysis. In vitro enzymatic characterization revealed the capability of MMAR_2193 protein to utilize diverse polyphenolic substrates to methylate several hydroxyl positions on a single substrate molecule. High-resolution mass spectrometric analyses identified multi-methylations of type III polyketides in cell-free reconstitution assays. Notably, our metabolomics analyses identified some of these methylated molecules in biofilms of wild type Mycobacterium marinum. This study characterizes a novel mycobacterial O-methyltransferase protein with multi-methylating enzymatic ability that could be exploited to generate a palette of structurally distinct bioactive molecules.
Introduction
Methylation is an important biological activity with implications in several cellular processes including DNA repair, signal transduction, regulation of hormones and neurotransmitters, and secondary metabolites biosynthesis. Methyltransferases (MTases) in an enzymatic mechanism catalyze nucleophilic substitution reaction by the transfer of methyl group from universal donor S-Adenosyl L-methionine (SAM) to a nucleophile containing carbon (C), sulfur (S), nitrogen (N) and oxygen (O) [1]. Methylation in secondary metabolic pathways occurs on several biological molecules affecting the natural properties of the final products [2]. Based on the nucleophiles targeted for methylation, MTases are termed as C-methyltransferases (CMTs), S-methyltransferases (SMTs), N-methyltransferases (NMTs) and the most abundantly found, O-methyltransferases (OMTs). OMTs methylate the electron-rich O-position of the acceptor molecule and are present in diverse organisms, including plants, animals, bacteria, and fungi conferring a wide range of biological functions [3]. Two classes of OMTs are reported based on protein sequence and phylogenetic analysis [4]. Class I proteins with a size of 23–28 kDa require divalent cation Mg2+ as a cofactor [5] to organize the substrate-binding site [6]. It includes alfalfa (Medicago sativa) caffeoyl coenzyme A 3-OMT (CCoAOMT) [7], rat (Rattus norvegicus) catechol OMT (COMT) [8], different variants of repair enzyme protein of humans [9], L-isoaspartyl (D-aspartyl) OMTs from Pyrococcus furiosus [10] and Vibrio cholerae [11], and Streptomyces clavuligerus Cmcl [12]. Among the OMTs, catechol O-methyltransferases (COMTs) are most widely studied [8]. COMTs methylate hydroxyl moiety of catechol, neurotransmitters, and xenobiotics and have a role in inactivating the catechol-type compounds such as L-Dopamine [13]. Plant OMTs biosynthesize specialized metabolites like lignin from methylated caffeoyl-CoA, flavonoids, phenylpropanoid conjugate from methylation of phenolic compounds [14]. Class II MTases with larger subunit sizes (38-43kDa) involves a catalytic histidine residue in place of Mg2+ for methylation. This class includes alfalfa chalcone OMT [15] that carries out single methylation at 2’-position of isoliquiritigenin (4, 2’, 4’-trihydroxychalcone) giving 4, 4’-dihydroxy-2’-methoxychalcone, a signalling molecule and an inducer of nodulation in soil rhizobia [16, 17]. Class II OMTs also include isoflavone OMT [15] and caffeic acid OMT [18] from alfalfa, and isoflavonoid OMT from Medicago truncatula [19]. The domain analysis of Class II methyltransferases shows C-terminal end with a role in catalysis and N-terminal for dimerization of protein [20].
Class I AdoMet-dependent OMTs are also present in bacterial species like Leptospira interrogans (LiOMTs) [21] and Bacillus cereus (BcOMT2) [20]. Methyltransferase protein CheR1 along with flagella mediated chemotaxis is shown to be essential for biofilm generation and maintenance in Pseudomonas aeruginosa [22]. Methyltransferase proteins encoded by Rv2952 and Rv2959c are also implicated in methylation of pathogenicity determinants like phenolglycolipids (PGL), dimycocerosate of phthiocerol (DIM) and related p-hydroxybenzoic acid derivatives (p-HBAD). These molecules upon methylation increase the virulence of pathogenic M. tuberculosis [23].
Bacteria produce an array of biologically potent aromatic and polyphenolic products from important class of enzymes called type III polyketide synthases (PKSs). These proteins catalyze condensation of an acyl-CoA starter substrate, iteratively with malonyl-CoA and/or methylmalonyl-CoA extender units. The type III polyketide products get variously modified for biological roles. In a recent study, M. smegmatis is shown to harbour an alkyl benzoquinone biosynthetic cluster comprising of a type III PKS (MSMEG_0808), methyltransferase (MSMEG_0809) and oxidoreductase (MSMEG_0809) [24]. Proteins from the cluster biosynthesize methylated polyketide quinones (PKQs) that aid anaerobic respiration in mycobacterial biofilms.
In this study, we have biochemically characterized a methyltransferase, mmar_2193, from a type III pks gene cluster in Mycobacterium marinum (Mmar). MMAR_2193 protein works as an O-methyltransferase, and high-resolution mass spectrometry revealed its capability to methylate hydroxyl positions in polyketide core molecules. MMAR_2193 could methylate multiple hydroxyl positions on a single substrate molecule to produce multimethylated products. Our metabolomics analyses identified some of these methylated products in wild type Mycobacterium marinum biofilms. This study thus characterizes a novel methyltransferase from Mmar with multi-methylation potential that could be utilized to generate unusual biologically active compounds.
Materials and methods
Bacterial strains and materials
We used Escherichia coli XL-1 blue and BL21 (DE3) as bacterial hosts for cloning and expression, respectively. Wild-type M. marinum (strain ATCC BAA-535/M) kindly provided by Prof. Y. Singh (IGIB, India) was grown on Middlebrook 7H9, Middlebrook 7H11 media. The strain was further used for genomic DNA isolation and for biofilm growth on Sauton’s Fluid Media Base. The MtbPKS18 expression clone was kindly gifted by R. S. Gokhale (NII, India) and was used for substrate biosynthesis. S-adenosyl L-methionine (SAM), acyl-CoA starter and extender substrates were procured from Sigma. Escherichia coli strains were grown on LB medium. Restriction endonucleases and PCR master mixture procured from New England Biolabs. PCR cleanup kit and Ni2+-NTA agarose were purchased from Qiagen. UPLC and MS grade solvents were purchased from Merck and Sigma.
Genomic DNA isolation
Genomic DNA was isolated from M.marinum culture. The bacterial culture was streaked onto 7H11 Agar Medium with ADC supplement. Genomic DNA was isolated using the boiling method. Colonies of bacterial cells were scrapped and resuspended in 100μl water in microcentrifuge (MCT) tube. To the mixture equal volume of chloroform was added, vortexed and boiled for 10-15mins. Chloroform was again added, and the mixture was heated to evaporate the solvent followed by the addition of 100μl autoclaved water. The mixture was centrifuged to pellet down cell debris, proteins and lipids, and the supernatant containing genomic DNA was collected.
Cloning of gene and sequence analysis
Bacterial genomic DNA was used as a template to amplify methyltransferase gene using a set of gene specific forward primer (5’TTCATATGGATTTTGATGCG CTG3’) containing NdeI restriction enzyme site and reverse primer (5’TTAAGCTTGTTTTGCCGCCGCGC3’) containing HindIII restriction site. The mmar_2193 gene was amplified and cloned into pBLuescriptSK(+) cloning vector (Stratagene). Identity of the clone was confirmed by restriction digestion and automated nucleotide sequencing. The mmar_2193 gene was further sub-cloned into pET 21c (Novagen) expression vector, for protein purification.
Expression and purification of protein
The MMAR_2193 protein was expressed as a C-terminal hexa-histidine tagged protein in the BL21/ (DE-3) strain of E.coli. The recombinant E.coli BL21 (DE3) cells harbouring expression plasmid was grown in Luria Bertini broth incubated at 30°C until the optical density at 600 nm reached 0.5 units. It is followed by the induction of culture using the 1mM final concentration of isopropyl-1-thio-β-D-thiogalactopyranoside (IPTG) and incubated at 22°C. After 16h, the culture was harvested to pellet down the cells, and the bacterial pellet was re-suspended in lysis buffer (50mM Tris, pH 8.0 with 10% glycerol, 0.15M NaCl). The recombinant protein was released by sonication (30s cycle, 10s rest, 15 cycles at 30%amplitude) and the lysate formed was centrifuged at 17000rpm for 40 min at 4˚C to remove cell debris. The supernatant was collected and used for binding with 0.5ml Ni-NTA slurry. Unbound protein was washed with wash buffer (50mM Tris pH 8.0, 10% glycerol). The Ni-NTA bound protein was eluted through the imidazole gradient using gravity flow from 5mM to 150mM. SDS-PAGE was used to check the purified protein elution (see Fig 3D). For the coenzyme assay, MtbPKS18 was purified using Ni-NTA based affinity purification.
Fig 3. Cavity volume comparison of ligand/ products binding cavities of MMAR_2193 and fractionation of methylated products using Ultra-Fast Liquid Chromatograph (UFLC).
Homology model of MMAR_2193 was used to study cavity volume near SAH binding cavity for fitting of methylated products Rm’ and Rm” (A) of resorcinol, methylated products Om’ and Om” (B) of olivetol, and methylated products Pm’, Pm” and Pm”‘ (C) of phloroglucinol. (D) shows the purified protein with approximately 25kDa size. (E), (F) and (G) display the fractionation of methylated polyketide products with respect to the controls RCt, PCt, and OCt. (E) shows the fractionation of mono- and di-methylated (Rm’ and Rm”) resorcinol together in 4 min. (F) shows the fractionation of mono- and di-methylated (Pm’ and Pm”) phloroglucinol from 23.1 to 23.4 min and fractionation of tri-methylated (Pm”‘) phloroglucinol at 24.1 min. (G) shows the fractionation of mono- and di-methylated (Om’ and Om”) olivetol from 22.25 to 23.25 min.
Generation of methylated products and polyketide assay
Enzyme activity assay for methyltransferase was carried out using reaction mixture consisting of three probable phenolic products (resorcinol, phloroglucinol, and olivetol) of type III PKS as a substrate based on the result of cDocker and and cDocker interaction energy. The probable substrates and products are docked on to the model of MMAR_2193 with S-adenosyl-L-homocysteine after the protein and ligand preparation to predict the substrate for in vitro work.
The in-vitro reaction was performed for MtbPKS18, a type III PKS together with MMAR_2193, a methyltransferase. C-chain starters: C16-CoA with an extender malonyl (C2)-CoA were used for the first reaction with MtbPKS18 to get type III polyketide products. The enzymatic reaction was carried out using 100μM each starter molecule (C16-CoA) and 100μM malonyl-CoA as extender molecule. The enzymatic reactions were carried using 50μg of purified Mtbpks18 at 30˚C for 120 min. The reaction was quenched using 5% acetic acid, and the products were extracted using 2×300μl of ethyl acetate. The extracts were dried using a vacuum and were resuspended and dissolved using 10% ethanol in 50mM HEPES buffer. The products obtained from the first reaction were used as a starter substrate for the second sequential reaction getting O-methylated products.
Methyltransferase assay was done using a combination of 50mM extracted products from first reaction or commercially available standard resorcinol, olivetol and phloroglucinol as starter with 400μM S-adenosyl L-methionine (SAM) as methyl donor in a reaction buffer (50mM HEPES, 10mM MgSo4.7H2O, 0.1% BSA, pH 7.4), and 0.9495 mg/ml of the protein. Control reactions were kept without enzyme. Reactions were incubated at 30°C for 8h. Products were quenched by adding 5% of acetic acid. Products were extracted with the 2×equal volume of ethyl acetate was added to the reaction mixture and dried under vacuum. The extracts from the assay were resuspended using 50μl methanol. The methylated polyketide products from standard commercially available resorcinol, olivetol and phloroglucinol was further fractionated in UFLC, and that from the sequential reaction was directly characterized using SCIEX Triple TOF 5600 high-resolution mass spectrometry (HRMS)
UFLC analysis: Reaction products were resolved in reverse phase column (ES Industries, Sonama C5, 5μ 100°A; 25cm×4.6mm). A shallow gradient of 5% CH3CN in water (each containing 1% acetic acid) to 30% CH3CN over 5 min, 60% CH3CN in 15 min, 80% CH3CN in 25 min, 90% CH3CN in 35 min, and 100% CH3CN in 45min was used for separation of the products from the assay. Methylated products were characterized using SCIEX Triple TOF 5600 HRM.
Metabolomic analyses of mycobacterial biofilms
M. marinum wild-type biofilm pellicles were grown from 1% inoculum of primary culture in triplicate using (150 mm ×25 mm) sterile polystyrene coated cell culture dish (SIGMA) with 70 ml Sauton’s fluid medium base supplemented with glucose (2%) and glycerol (2%) and was incubated for 14 days. The pellicle from biofilm was scraped out and resuspended in 100 mM Tris, pH 8.0 and further acidified to pH 4.0. The metabolites were extracted with the 2×equal volume of ethyl acetate from the acid-based hydrolysed mixture and dried under vacuum. The extracts from the assay were resuspended using 200μl methanol. Methylated products were characterized using SCIEX Triple TOF 5600 HRMS.
In silico studies
Cluster analysis of M. marinum, M complete genome (accession number: CP000854) for type III PKS cluster was done using AntiSMASH [25]. Multiple Sequence alignments of different SAM-dependent methyltransferase were done by using JalView Software Waterhouse et al [26] and are given in supplementary figure (with an illustration of N-terminal and C-terminal domain). The NCBI (RefSeq or GenBank) accession number of protein sequences used for sequence comparison were as follows:NodS-like (sam-dependent methyltransferase, M. liflandii), AGC62714; NodS-like (sam-dependent methyltransferase, M. psudohottsii), GAQ31737; NodS-like (SAM-dependent methyltransferase, M. marinum), ACC40642; class I SAM-dependent (methyltransferase, M. ulcerans), WP_096371067; tansferase (M.tuberculosis), CKU58587; class I SAM-dependent (methyltransferase, M. tuberculosis), WP_070890772,; class I SAM-dependent (methyltransferase, M. shinjukuense), WP_083048499; ChainA (Echinomycin biosynthesis),4NEC_A; ChainA (Sam-dependent methyltransferase, Metahnosarcina mazei), 3SM3_A; Chain A (sam-dependent metyltransferase, Pyrococcus horikoshii Ot3), 1WZN_A.
The FASTA format of MMAR_2193 gene sequence (ACC40642) was used as the target sequence to search for the template based on the sequence alignment with Protein Databank (PDB) database. Homology modelling of MMAR_2193 model was performed based on the crystal structure of 4NEC_A downloaded from RCSB protein databank using Biovia Discovery Studio. The model was superimposed with its template. The structure of the ligand library was generated using ChemDraw Professional 17.0. Protein and ligand were prepared for in silico docking. Different docked poses of protein with different ligands were generated using Biovia Discovery Studio CDOCKER tool. CHARMm-based energy forcefields were used to generate the docked score and predict the correct poses [27].
Results
Homology based functional analyses of MMAR_2193
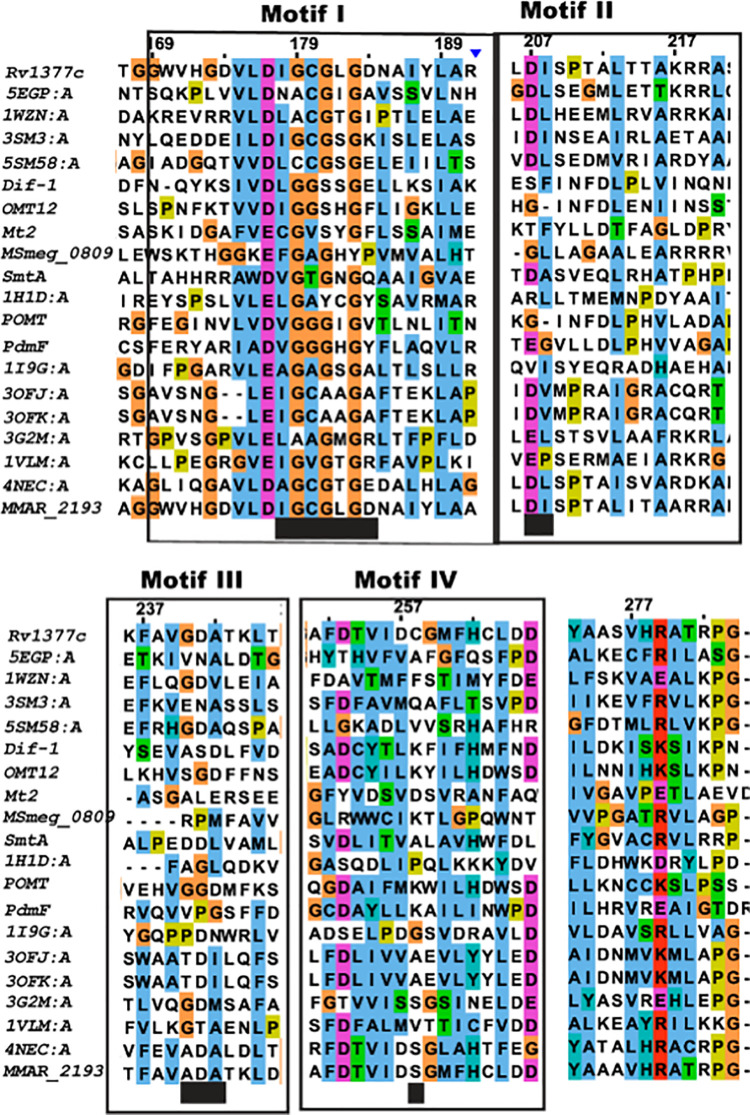
Comparative sequence analysis has revealed a plethora of genomic clusters specific to pathogenic mycobacterial strains. Mmar shows a type III pks gene cluster with mmar_2189 (a putative desaturase) mmar_2190 (a putative type III pks), mmar_2191 and mmar_2192 (two probable sulfotransferase genes), followed by mmar_2193 (a putative methyltransferase gene) as shown in Fig 1 in S1 Text. Our sequence-based homology comparisons predicted mmar_2193 to belong to Nod-like SAM-dependent methyltransferase family. A comparison with RCSB protein databank entries revealed MMAR_2193 (GI: ACC40642.1) to be 37% identical (with 98% query cover) to 4NEC_A, an ADOMET methyltransferase superfamily protein that catalyzes conversion of disulphide bond into thioacetal group during echinomycin biosynthesis. Multiple sequence alignment (MSA) was carried out for MMAR_2193 with similar-length NodS–like SAM-dependent methyltransferases(AGC62714, GAQ31737), class I SAM-dependent methyltransferases (WP_096371067, WP_070890772), a transferase sequence (WP_09637106) and few structurally characterized transferases (4NEC_A, 3SM3_A, 1WZN_A). As presented in Fig 1, the MSA revealed conservation of functionally important domains in MMAR_2193 protein. The S-Adenosyl-l-methionine binding site at the N-terminal region and substrate binding site on C-terminal region of MMAR_2193 are similar to other SAM-dependent methyltransferases. The probable SAM-binding site in MMAR_2193 was identified to be conserved across the sequence in four amino acid motif positions, 49IGCGLGD55, 71DI72, 96ADA98, and 114S.
Fig 1. Multiple sequence alignment (MSA) of MMAR_2193.
The MSA was generated to find N-terminal and C-terminal conserved amino acid for all bacterial and a few O-methylated representative plant O-methyltransferases (OMTs). Four conserved motifs were analyzed for MMAR_2193 generally conserved in OMTs.
In an attempt to predict the probable substrates and possible products of the protein, we carried out computational homology modeling of MMAR_2193. The model was made using ligand-bound 4NEC_A as the structural template. As depicted in Fig 2A and 2B, the residues interacting with SAH in 4NEC_A protein (03F07Y24W48D50GC, 71DI72, 96ADAT99, and 114S) are similar to the motifs interacting with the docked methyl donor, SAM, in MMAR_2193 model.
Fig 2. Homology model of MMAR_2193 with interacting S-adenosyl homocysteine (SAH) and S-adenosyl L-methionine (SAM).
Template structure of 4NEC_A from Protein Data Bank (PDB) was used to generate homology model using Biovia Discovery study version 4.5. The interaction between protein and methyl donor, SAM (B) and reduced SAH (A) was derived using CHARM based force field. Methyl group of SAM is shown in black color.
With SAM identified as the methyl donating substrate, we went ahead to identify possible acceptor substrates for the mycobacterial protein. mmar_2193 clusters with a type III pks gene in the genome. Microbial type III PKSs are known to biosynthesize several polyphenolic lipids with aliphatic extensions on cores of resorcinol/ phloroglucinol/ α-pyrone scaffolds [28–31]. To acertain the possibility of MMAR_2193 being a polyketide modifier, we carried out molecular docking experiments using resorcinol, phloroglucinol, 5-pentyl-resorcinol (olivetol), and triketide- and tetraketide-α-hexanoylpyrones as the acceptor substrates. Notably, MMAR_2193 protein model could flexibly accommodate the polyketide cores as well as methyl-substituted probable products as shown in Fig 3A–3C. These methylated products fitted best in the cavity volume of the protein model near SAH binding site. Table 1 in S1 Text enlists the docking energies and residues interacting with each probable methylated product of all the acceptor substrates analyzed. Our computational studies thus provided clues to polyketide modifying capability of MMAR_2193 protein.
Multiple O-methylations by MMAR_2193
Our in silico studies predicted MMAR_2193 protein to catalyze O-methylation of varied substrates. For functional characterization, mmar_2193 gene was amplified from Mmar genomic DNA and cloned into E. coli expression vector system. MMAR_2193 was expressed as a hexa-histidine tagged protein and purified using Ni2+-nitrilotriacetic acid affinity chromatography as a single protein band of ~25 kDa as determined on SDS-PAGE (Fig 3D). Comparative structural modeling and docking studies with MMAR_2193 predicted SAM-dependent catalysis for methylation of different polyketide cores. Purified recombinant MMAR_2193 was used to perform in vitro enzymatic assays using SAM as a methyl donor and resorcinol/phloroglucinol/olivetol as the acceptor substrates. Extracted reaction products resolved using ultra-fast liquid chromatography (UFLC) and subjected to high-resolution mass spectrometry (HRMS) corroborated in silico predictions. The UFLC peaks in Fig 3E–3G revealed methylated products from resorcinol, phloroglucinol and olivetol, respectively, in our HRMS analyses. UFLC profile in Fig 3E revealed ions with [M-H]- at m/z 122.9031 and m/z 136.9603 from resorcinol primed reaction. Reaction with phloroglucinol led to product ions with [M-H]- at m/z 138.9629, m/z 152.9535 and m/z 166.9535 in UFLC peaks shown in Fig 3F. Olivetol primed reactions formed product ions with [M-H]- at m/z 191.1410 and m/z 207.0473 in profile observed in Fig 3G.
Tandem MS/MS analyses as shown in Fig 4A–4G, confirmed these product ions as mono-methylated resorcinol (Rm’, [M-H]- at m/z 122.9031: fragments at m/z 95.0132, 93.0363, 68.9971 and 53.0019); di-methylated resorcinol (Rm”, [M-H]- at m/z 136.9603: fragments at m/z 120.9648, 93.0545 and 76.9827); mono-methylated phloroglucinol (Pm’, [M-H]- at m/z 138.9629: fragments at m/z 122.9633, 109.0423, 96.9766, 94.9446 and 80.9300); di-methylated phloroglucinol (Pm”, [M-H]- at m/z 152.9535: fragments at m/z 120.9789, 108.9605 and 96.9756); tri-methylated phloroglucinol (Pm”‘, [M-H]- at m/z 166.9535: fragments at m/z 151.0087, 134.9532); mono-methylated olivetol (Om’, [M-H]- at m/z 191.1410: fragments at m/z 177.0893, 165.1183, 149.0827 and 135.0674) and di-methylated olivetol (Om”, [M-H]- at m/z 207.0473: fragments at m/z 191.1410, 144.9777, 143.0722 and 117.0314). Table 2 in S1 Text provides details of mass spectrometric characterization of the methylated molecules. Our functional characterization of MMAR_2193 as summarized in Fig 4H provided evidence for the O-methylation potential of the mycobacterial protein. Notably, MMAR_2193 displayed a potential to perform multiple O-methylations on a single substrate molecule.
Fig 4. Tandem mass spectrometry for different methylated products formed from resorcinol, phloroglucinol and olivetol and overall reaction illustrating summary of methylated products formed from standard polyketide compounds.
The identity of the mono- and di-methylated resorcinol (Rm’, Rm”); mono-, di- and tri-methylated phloroglucinol (Pm’, Pm” and Pm”‘) with [M-H]- of m/z 122.9031 (A) and 136.9603 (B), 138.9629 (C), 152.9535 (D) and 166.9535 (E) was established by the distinctive product profile of each molecule. Tandem mass spectrometry for mono- and di-methylated olivetol (Om’ and Om”) with [M-H]- of m/z 195.0995 (F) and 209.603 (G). (H) summarizes biofunctional assays of MMAR_2193 with resorcinol, phloroglucinol and olivetol using SAM as methyl donor. Methyl group is shown in red.
O-methylation of α-alkyl pyrones from type III polyketide synthase
Type III polyketide synthases catalyze formation of products with diverse scaffolds. The most commonly biosynthesized products include triketide-and tetraketide-α-alkyl pyrones that are many times co-produced with alkyl-resorcinols or acyl-phloroglucinols. Our biochemical studies with resorcinol, phloroglucinol and olivetol provided evidence for the O-methylation potential of MMAR_2193. We further set out to examine MMAR_2193 mediated possible methylation of α-alkyl pyrones. MtbPKS18, a type III PKS from Mycobacterium tuberculosis has been previously characterized to catalyze biosynthesis of long-chain triketide- and tetraketide-α-alkyl pyrones in cell-free assays [29]. In a similar in vitro reaction, we incubated MtbPKS18 protein with palmitoyl-CoA (C16-CoA) and malonyl-CoA to biosynthesize triketide- and tetraketide-α-palmitoylpyrones, 16A and 16B, respectively. These metabolites were extracted from the quenched assays and used as substrates for a sequential reaction with SAM and MMAR_2193 protein. A HRMS analysis of the methyltransferase reaction products identified ions of [M-H]- at m/z 335.1987 and m/z 377.2351. Tandem MS/MS of these precursor ions confirmed the identity of these molecules as methylated α-alkyl pyrones, 16A’ and 16B’, respectively (Fig 5A and 5B). Our studies displayed the ability of MMAR_2193 to catalyze O-methylations on α-alkyl pyrone polyketides.
Fig 5. Tandem mass spectrometry of methylated α-triketidepyrones (16A’), α-tetraketidepyrone (16B’) from coupled assay and wild-type M. marinum biofilm extracts.
Sequential assay using products of Mtbpks18 as substrates for methyltransferase shows formation of methylated α-triketidepyrone 16A’ (A) and α-tetraketidepyrone 16B’ (B) formed from C16-acyl CoA as a starter and malonyl-CoA as an extender. The metabolomics profiling of extracts from wild-type M. marinum also shows presence of α-triketidepyrone (12A’ and 16A’) as shown in (C) and (D). (E) shows the fragmentation pattern for the respective methylated triketide and tetraketide pyrones.
O-methylated polyketides from mycobacterial biofilms
Mmar harbours several genomic clusters with polyketide biosynthetic genes including the MMAR_2193 cluster that is exclusively identified in pathogenic species. Biofilms have lately been associated with several pathogenic diseases [32–35] and are a natural form of existence in mycobacteria. We probed the possibility of presence of O-methylated polyketides in wild-type Mmar biofilms. Biofilm pellicles were developed for Mmar cells (Fig 2 in S1 Text) and extracted for HRMS metabolomics analyses. A multiple-reaction-monitoring (MRM) based metabolomics approach identified two ions of [M-H]- at m/z 279.2257 and m/z 335.1987. Tandem MS/MS confirmed identities of O-methylated triketide-α-lauroylpyrone (12A’) and triketide-α-palmitoylpyrone (16A’) corresponding to the two identified ions, respectively (Fig 5C–5E). Our results revealed presence of O-methylated polyketides in wild-type Mmar biofilms.
Discussion
Pathogenic mycobacterial genomes reveal several genomic clusters dedicated to virulent lipid biosynthesis. Polyketide synthases (PKSs) work in conjunction with fatty acid synthases to biosynthesize these molecules. Post-synthesis modification of polyketide cores is crucial for biological activity of these metabolites. Mycobacterial genomes reveal a plethora of genes homologous to methyltransferases that are important polyketide modifiers. In a distinct organization, mmar_2193, a probable methyltransferase was identified to be clustered with a type III pks and other modifying genes in pathogenic genomes. Our homology-based sequence/structure analyses predicted a SAM-dependent catalysis for MMAR_2193 protein. Structural modeling and docking studies predicted polyketide cores as probable substrates for methylation.
Interestingly, our docking studies proposed a multi-methylating potential of MMAR_2193 protein. Our biochemical studies using polyketide core molecules as substrates corroborated the in silico analyses. High-resolution mass spectrometry confirmed methylated polyketide products from MMAR_2193 catalyzed reactions. It was interesting to note that this mycobacterial protein utilized SAM as a donor to biosynthesize variably methylated products. Notably, MMAR_2193 exhibited the potential to methylate several hydroxyl positions on a single polyphenolic substrate molecule generating a palette of variably methylated products. Polyphenolic compounds in differing methylated states could play diverse physiological roles. MMAR_2193 utilized phloroglucinol to produce methylated products, including tri-methylated phloroglucinol or tri-methoxy benzene (TMB). TMB is the key volatile molecule that imparts typical floral scent to the Chinese rose, Rosa chinensis. However, the plant requires two separate classes of O-methyltransferases to achieve complete methylation of phloroglucinol precursor and production of TMB [36, 37]. Plant O-methytransferases involved in secondary metabolism generally display strict substrate specificity and through methylation direct small molecules into various metabolic pathways [15, 18, 19, 38–41]. Attempts to generate chimeric O-methytransferase proteins have been shown to change substrate specificity though with limited region-selectivity [42–44]. Recently, a catechol O-methytransferase from Mycobacterium tuberculosis was reported to display promiscuous substrate specificity and relaxed region-selectivity [45]. The protein however, could generate only mono-methylated products.
O-methyltransferases occur in several secondary metabolite generating genomic clusters. A sequencial assay of MMAR_2193 with reaction products of MtbPKS18 generated methylated α-pyrones in vitro. Based on the genomic placement of mmar_2193 in a type III pks gene cluster and further the capability of the protein to methylate polyketide cores and products, it is tempting to speculate that MMAR_2193 could play crucial roles in modifying type III polyketides in M. marinum. Interestingly, O-methylated triketide α-pyrones could be identified in M. marinum biofilms, suggesting roles of these molecules in mycobacterial physiology. This study provides functional analyses of an unusual O-methyltransferase from M. marinum. The distinctive ability of MMAR_2193 to perform multi-methylations on varied substrates could be utilized to generate a palette of novel methylated bioactive metabolites.
Conclusion
O-methyltransferase, mmar_2193 belongs to a part of type III pks cluster found exclusively in pathogenic bacterial strains. Efficient O-methylation of hydroxyl groups seems to be essential to produce varied methylated type III PKS products. Our study reveals multiple O-methylating potential of MMAR_2193 to methylate all hydroxyl positions on a given substrate. O-methylation is reported to determine bacterial pathogenicity and survival in adverse conditions. The presence of O-methylated products in biofilm culture of wildtype M. marinum suggest the importance of O-methylation. Further, the enzyme can be utilized for generation of novel methylated scaffolds.
Supporting information
(DOCX)
(PDF)
Acknowledgments
We are thankful to Dr. Rajesh S. Gokhale (NII, India) for the kind gift of plasmid for expression of MtbPKS18. We acknowledge Prof Y. Singh (IGIB, India) for providing M. marinum culture (strain ATCC BAA-535/M) for genomic DNA isolation and biofilm culture based study. We are thankful to Sciex Gurugram for Mass Spectrometry facility and data collection. G.R.G. acknowledges Prof. Lakshmaiah Sreerama (Qatar University) for helpful discussions on mass spectrometry data analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support for the study was provided to PS by Innovative Young Biotechnologist Award (IYBA), Department of Biotechnology (DBT) (https://dbtindia.gov.in), Ministry of Science and Technology, Government of India, Core Research Grant (CRG/2018/002229), Science and Engineering Research Board (SERB) (http://www.serb.gov.in/home.php), Department of Science and Technology (DST), Government of India and Startup Grant, South Asian University (SAU) (http://www.sau.int/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klimasauskas S, Weinhold E. A new tool for biotechnology: AdoMet-dependent methyltransferases. Trends in biotechnology. 2007;25(3):99–104. doi: 10.1016/j.tibtech.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Law BJC, Struck AW, Bennett MR, Wilkinson B, Micklefield J. Site-specific bioalkylation of rapamycin by the RapM 16-O-methyltransferase. Chemical science. 2015;6(5):2885–92. doi: 10.1039/c5sc00164a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim RK, Bruneau A, Bantignies B. Plant O-methyltransferases: molecular analysis, common signature and classification. Plant Molecular Biology. 1998;36(1):1–10. doi: 10.1023/a:1005939803300 [DOI] [PubMed] [Google Scholar]
- 4.Joshi CP, Chiang VL. Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltransferases. Plant Molecular Biology. 1998;37(4):663–74. doi: 10.1023/a:1006035210889 [DOI] [PubMed] [Google Scholar]
- 5.Ibdah M, Zhang X-H, Schmidt J, Vogt T. A Novel Mg2+-dependent O-Methyltransferase in the Phenylpropanoid Metabolism of Mesembryanthemum crystallinum. Journal of Biological Chemistry. 2003;278(45):43961–72. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y-J, Bruice TC. A Theoretical Examination of the Factors Controlling the Catalytic Efficiency of a Transmethylation Enzyme: Catechol O-Methyltransferase. Journal of the American Chemical Society. 1997;119(35):8137–45. [Google Scholar]
- 7.Ferrer JL, Zubieta C, Dixon RA, Noel JP. Crystal structures of alfalfa caffeoyl coenzyme A 3-O-methyltransferase. Plant physiology. 2005;137(3):1009–17. doi: 10.1104/pp.104.048751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidgren J, Svensson LA, Liljas A. Crystal structure of catechol O-methyltransferase. Nature. 1994;368(6469):354–8. doi: 10.1038/368354a0 [DOI] [PubMed] [Google Scholar]
- 9.Smith CD, Carson M, Friedman AM, Skinner MM, Delucas L, Chantalat L, et al. Crystal structure of human L-isoaspartyl-O-methyl-transferase with S-adenosyl homocysteine at 1.6-Å resolution and modeling of an isoaspartyl-containing peptide at the active site. Protein Science. 2002;11(3):625–35. doi: 10.1110/ps.37802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith SC, Sawaya MR, Boutz DR, Thapar N, Katz JE, Clarke S, et al. Crystal structure of a protein repair methyltransferase from Pyrococcus furiosus with its l-isoaspartyl peptide substrate11Edited by I. A. Wilson. Journal of Molecular Biology. 2001;313(5):1103–16. doi: 10.1006/jmbi.2001.5095 [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee T, Mukherjee D, Banerjee M, Chatterjee BK, Chakrabarti P. Crystal structure and activity of protein L-isoaspartyl-O-methyltransferase from Vibrio cholerae, and the effect of AdoHcy binding. Archives of biochemistry and biophysics. 2015;583:140–9. doi: 10.1016/j.abb.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 12.Öster LM, Lester DR, Terwisscha van Scheltinga A, Svenda M, van Lun M, Généreux C, et al. Insights into Cephamycin Biosynthesis: the Crystal Structure of CmcI from Streptomyces clavuligerus. Journal of Molecular Biology. 2006;358(2):546–58. doi: 10.1016/j.jmb.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 13.Bonifacio MJ, Archer M, Rodrigues ML, Matias PM, Learmonth DA, Carrondo MA, et al. Kinetics and crystal structure of catechol-o-methyltransferase complex with co-substrate and a novel inhibitor with potential therapeutic application. Molecular pharmacology. 2002;62(4):795–805. doi: 10.1124/mol.62.4.795 [DOI] [PubMed] [Google Scholar]
- 14.Noel JP, Dixon RA, Pichersky E, Zubieta C, Ferrer J-L. Chapter two Structural, functional, and evolutionary basis for methylation of plant small molecules. In: Romeo JT, editor. Recent Advances in Phytochemistry. 37: Elsevier; 2003. p. 37–58. [Google Scholar]
- 15.Zubieta C, He XZ, Dixon RA, Noel JP. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nature structural biology. 2001;8(3):271–9. doi: 10.1038/85029 [DOI] [PubMed] [Google Scholar]
- 16.Maxwell CA, Harrison MJ, Dixon RA. Molecular characterization and expression of alfalfa isoliquiritigenin 2’-O-methyltransferase, an enzyme specifically involved in the biosynthesis of an inducer of Rhizobium meliloti nodulation genes. The Plant journal: for cell and molecular biology. 1993;4(6):971–81. doi: 10.1046/j.1365-313x.1993.04060971.x [DOI] [PubMed] [Google Scholar]
- 17.Maxwell CA, Edwards R, Dixon RA. Identification, purification, and characterization of S-adenosyl-L-methionine: isoliquiritigenin 2’-O-methyltransferase from alfalfa (Medicago sativa L.). Archives of biochemistry and biophysics. 1992;293(1):158–66. doi: 10.1016/0003-9861(92)90379-b [DOI] [PubMed] [Google Scholar]
- 18.Zubieta C, Kota P, Ferrer JL, Dixon RA, Noel JP. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. The Plant cell. 2002;14(6):1265–77. doi: 10.1105/tpc.001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CJ, Deavours BE, Richard SB, Ferrer JL, Blount JW, Huhman D, et al. Structural basis for dual functionality of isoflavonoid O-methyltransferases in the evolution of plant defense responses. The Plant cell. 2006;18(12):3656–69. doi: 10.1105/tpc.106.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho JH, Park Y, Ahn JH, Lim Y, Rhee S. Structural and functional insights into O-methyltransferase from Bacillus cereus. J Mol Biol. 2008;382(4):987–97. doi: 10.1016/j.jmb.2008.07.080 [DOI] [PubMed] [Google Scholar]
- 21.Hou X, Wang Y, Zhou Z, Bao S, Lin Y, Gong W. Crystal structure of SAM-dependent O-methyltransferase from pathogenic bacterium Leptospira interrogans. Journal of Structural Biology. 2007;159(3):523–8. doi: 10.1016/j.jsb.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt J, Musken M, Becker T, Magnowska Z, Bertinetti D, Moller S, et al. The Pseudomonas aeruginosa chemotaxis methyltransferase CheR1 impacts on bacterial surface sampling. PloS one. 2011;6(3):e18184. doi: 10.1371/journal.pone.0018184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez E, Constant P, Laval F, Lemassu A, Laneelle MA, Daffe M, et al. Molecular dissection of the role of two methyltransferases in the biosynthesis of phenolglycolipids and phthiocerol dimycoserosate in the Mycobacterium tuberculosis complex. The Journal of biological chemistry. 2004;279(41):42584–92. doi: 10.1074/jbc.M406134200 [DOI] [PubMed] [Google Scholar]
- 24.Anand A, Verma P, Singh AK, Kaushik S, Pandey R, Shi C, et al. Polyketide Quinones Are Alternate Intermediate Electron Carriers during Mycobacterial Respiration in Oxygen-Deficient Niches. Molecular cell. 2015;60(4):637–50. doi: 10.1016/j.molcel.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic acids research. 2015;43(W1):W237–43. doi: 10.1093/nar/gkv437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–91. doi: 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu G, Robertson DH, Brooks CL, 3rd, Vieth M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. Journal of computational chemistry. 2003;24(13):1549–62. doi: 10.1002/jcc.10306 [DOI] [PubMed] [Google Scholar]
- 28.Parvez A, Giri S, Giri GR, Kumari M, Bisht R, Saxena P. Novel Type III Polyketide Synthases Biosynthesize Methylated Polyketides in Mycobacterium marinum. Scientific reports. 2018;8(1):6529. doi: 10.1038/s41598-018-24980-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena P, Yadav G, Mohanty D, Gokhale RS. A new family of type III polyketide synthases in Mycobacterium tuberculosis. The Journal of biological chemistry. 2003;278(45):44780–90. doi: 10.1074/jbc.M306714200 [DOI] [PubMed] [Google Scholar]
- 30.Funa N, Ohnishi Y, Ebizuka Y, Horinouchi S. Properties and substrate specificity of RppA, a chalcone synthase-related polyketide synthase in Streptomyces griseus. The Journal of biological chemistry. 2002;277(7):4628–35. doi: 10.1074/jbc.M110357200 [DOI] [PubMed] [Google Scholar]
- 31.Funabashi M, Funa N, Horinouchi S. Phenolic lipids synthesized by type III polyketide synthase confer penicillin resistance on Streptomyces griseus. The Journal of biological chemistry. 2008;283(20):13983–91. doi: 10.1074/jbc.M710461200 [DOI] [PubMed] [Google Scholar]
- 32.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews Microbiology. 2004;2(2):95–108. doi: 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 33.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annual review of microbiology. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720 [DOI] [PubMed] [Google Scholar]
- 34.Davies D. Understanding biofilm resistance to antibacterial agents. Nature reviews Drug discovery. 2003;2(2):114–22. doi: 10.1038/nrd1008 [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty P, Kumar A. The extracellular matrix of mycobacterial biofilms: could we shorten the treatment of mycobacterial infections? Microbial cell. 2019;6(2):105–22. doi: 10.15698/mic2019.02.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Watanabe N, Mita S, Ueda Y, Shibuya M, Ebizuka Y. Two O-methyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. Journal of bioscience and bioengineering. 2003;96(2):119–28. [PubMed] [Google Scholar]
- 37.Scalliet G, Piola F, Douady CJ, Rety S, Raymond O, Baudino S, et al. Scent evolution in Chinese roses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5927–32. doi: 10.1073/pnas.0711551105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petronikolou N, Hollatz AJ, Schuler MA, Nair SK. Loganic Acid Methyltransferase: Insights into the Specificity of Methylation on an Iridoid Glycoside. Chembiochem: a European journal of chemical biology. 2018;19(8):784–8. doi: 10.1002/cbic.201700679 [DOI] [PubMed] [Google Scholar]
- 39.Coiner H, Schroder G, Wehinger E, Liu CJ, Noel JP, Schwab W, et al. Methylation of sulfhydryl groups: a new function for a family of small molecule plant O-methyltransferases. The Plant journal: for cell and molecular biology. 2006;46(2):193–205. doi: 10.1111/j.1365-313X.2006.02680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiens B, De Luca V. Molecular and biochemical characterization of a benzenoid/phenylpropanoid meta/para-O-methyltransferase from Rauwolfia serpentina roots. Phytochemistry. 2016;132:5–15. doi: 10.1016/j.phytochem.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 41.Ma QH, Xu Y. Characterization of a caffeic acid 3-O-methyltransferase from wheat and its function in lignin biosynthesis. Biochimie. 2008;90(3):515–24. doi: 10.1016/j.biochi.2007.09.016 [DOI] [PubMed] [Google Scholar]
- 42.Lebe KE, Cox RJ. O-Methylation steps during strobilurin and bolineol biosynthesis. RSC Advances. 2019;9(54):31527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grocholski T, Dinis P, Niiranen L, Niemi J, Metsa-Ketela M. Divergent evolution of an atypical S-adenosyl-l-methionine-dependent monooxygenase involved in anthracycline biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(32):9866–71. doi: 10.1073/pnas.1501765112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morishige T, Tamakoshi M, Takemura T, Sato F. Molecular characterization of O-methyltransferases involved in isoquinoline alkaloid biosynthesis in Coptis japonica. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2010;86(7):757–68. doi: 10.2183/pjab.86.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Kang J, Kim J. Structural and biochemical characterization of Rv0187, an O-methyltransferase from Mycobacterium tuberculosis. Scientific reports. 2019;9(1):8059. doi: 10.1038/s41598-019-44592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.