Abstract
Background
Prior studies have documented lower cardiovascular disease (CVD) risk among people with a higher adherence to a plant-based dietary pattern. Non-Hispanic black Americans are an understudied group with high burden of CVD, yet studies of plant-based diets have been limited in this population.
Methods and findings
We conducted an analysis of prospectively collected data from a community-based cohort of African American adults (n = 3,635) in the Jackson Heart Study (JHS) aged 21–95 years, living in the Jackson, Mississippi, metropolitan area, US, who were followed from 2000 to 2018. Using self-reported dietary data, we assigned scores to participants’ adherence to 3 plant-based dietary patterns: an overall plant-based diet index (PDI), a healthy PDI (hPDI), and an unhealthy PDI (uPDI). Cox proportional hazards models were used to estimate associations between plant-based diet scores and CVD incidence and all-cause mortality. Over a median follow-up of 13 and 15 years, there were 293 incident CVD cases and 597 deaths, respectively. After adjusting for sociodemographic characteristics (age, sex, and education) and health behaviors (smoking, alcohol intake, margarine intake, physical activity, and total energy intake), no significant association was observed between plant-based diets and incident CVD for overall PDI (hazard ratio [HR] 1.06, 95% CI 0.78–1.42, p-trend = 0.72), hPDI (HR 1.07, 95% CI 0.80–1.42, p-trend = 0.67), and uPDI (HR 0.95, 95% CI 0.71–1.28, p-trend = 0.76). Corresponding HRs (95% CIs) for all-cause mortality risk with overall PDI, hPDI, and uPDI were 0.96 (0.78–1.18), 0.94 (0.76–1.16), and 1.06 (0.86–1.30), respectively. Corresponding HRs (95% CIs) for incident coronary heart disease with overall PDI, hPDI, and uPDI were 1.09 (0.74–1.61), 1.11 (0.76–1.61), and 0.79 (0.52–1.18), respectively. For incident total stroke, HRs (95% CIs) for overall PDI, hPDI, and uPDI were 1.00 (0.66–1.52), 0.91 (0.61–1.36), and 1.26 (0.84–1.89) (p-trend for all tests > 0.05). Limitations of the study include use of self-reported dietary intake, residual confounding, potential for reverse causation, and that the study did not capture those who exclusively consume plant-derived foods.
Conclusions
In this study of black Americans, we observed that, unlike in prior studies, greater adherence to a plant-based diet was not associated with CVD or all-cause mortality.
In a cohort study, Leah J. Weston and colleagues investigate the associations between consumption of plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans.
Author summary
Why was this study done?
Plant-based diets have been linked, largely through studies of “vegetarian” diets, with health benefits, including lower risk of heart disease; however, studies of these associations among more general populations have produced mixed results.
Investigating plant-based dietary patterns has allowed researchers to study how levels of adherence to plant-based dietary patterns and the healthfulness of plant-based diets correlate with cardiovascular disease (CVD) risk.
This study was conducted to expand the generalizability of conclusions about plant-based diets and CVD risk in African American men and women in the US who were following a southern dietary pattern.
What did the researchers do and find?
We used data on 3,635 African American adults from the Jackson Heart Study with a mean follow-up of 13 years to assess the association of 3 plant-based dietary patterns (overall, healthy, and unhealthy) with CVD incidence and all-cause mortality.
Overall diet quality was low for all participants, and participants with the most plant-rich diets still regularly included animal-based foods.
Incidence of CVD and all-cause mortality was the same among participants whose diets were most similar to a plant-based dietary pattern and among those whose diets were least plant-based.
Among individual food groups, legumes were associated with a lower risk for CVD, while vegetable oils were associated with higher risk for CVD, and whole grains and sugar-sweetened beverages were associated with higher all-cause mortality.
What do these findings mean?
Plant-based diet index scores have gained popularity among researchers for their ability to characterize overall dietary patterns; however, their use may be limited in populations with minimal gradations in the plant richness of participants’ diets.
Health benefits linked to plant-based diets may depend on the overall healthfulness of an individual’s diet and might require higher levels of adherence to a plant-based diet than what is typically practiced among those with the most plant-rich diets in this cohort of African Americans residing in the southern region of the US.
Introduction
Plant-based diets are gaining attention as more studies suggest both health and environmental sustainability benefits of dietary patterns characterized by lower meat consumption and higher consumption of fruit, vegetables, legumes, whole grains, nuts, and seeds [1]. Although observational studies have consistently found that vegetarians and vegans tend to have lower cardiometabolic risk factors and lower risk of heart disease, diabetes, kidney disease, and some cancers, there have been mixed findings among prospective studies investigating the association of plant-based diets with cardiovascular disease (CVD) and CVD risk factors [2–5]. These conflicting findings may be related to the attributes of the populations studied and variability in the healthiness of the vegetarian or vegan diets studied. Many cohorts have specifically recruited vegetarians, vegans, and health-conscious controls [5]. These groups tend to differ from the general population in several factors, including sociodemographics and health behaviors, which may limit the comparability and generalizability of these studies to the general US population [5,6].
To better address the possibility that these contrasting findings may be due to variability in the underlying healthfulness of participants’ diets, more recent studies have investigated plant-based diets in populations with wider generalizability [7–9]. In addition, rather than studying diets based on complete exclusion of food groups (i.e., vegetarian or vegan), there has been a trend toward characterizing diets based on relative adherence to a plant-based diet and to consider both unhealthy and healthy plant-based diet patterns. Diet indices reduce variability, contextualize the meaning of study findings, and allow for replication of the same scoring system in different study populations. However, not all of these large cohort studies are consistent in the magnitude or significance of their findings with respect to CVD incidence and mortality [7–9].
One limitation of existing research on plant-based diets is that this research may not adequately capture the dietary patterns of all Americans, particularly African Americans, who remain an understudied population with regard to plant-based dietary patterns. In a subgroup analysis of 592 black Americans (75% African Americans and 25% West Indians) in the Adventist Health Study published in 2015, vegetarians had lower odds of cardiometabolic risk factors compared with nonvegetarians, similar to in the overall Adventist Health Study cohort [10]. It has been reported that the prevalence of CVD is lower among black Americans in the Adventist Health Study compared to the overall US black American population. Also, the Adventist Health Study has limited generalizability, given differences in other influential health and lifestyle factors [10].
As one of the largest community-based cohorts of African American adults in the US, the Jackson Heart Study (JHS) provides a unique opportunity to investigate the association of plant-based diets with CVD morbidity and mortality [11]. The aim of this study is to evaluate whether 3 plant-based dietary patterns—an overall plant-based diet, a healthy plant-based diet, and an unhealthy plant-based diet—are associated with the risk of incident CVD or all-cause mortality in a southern African American population. Studying this cohort will allow us to increase the certainty and generalizability of conclusions on plant-based diets and CVD in African Americans, and expand our understanding of plant-based diets.
Methods
Study design
We conducted an analysis of prospectively collected data from the JHS, a longitudinal cohort study investigating CVD risk in African American individuals, aged 21–95 years, in Jackson, Mississippi [11]. Details of the study design, recruitment procedures, and measures have been published elsewhere [11–13]. The institutional review boards at Jackson State University, Tougaloo College, and the University of Mississippi Medical Center reviewed the protocol, and participants provided written informed consent. Participants underwent baseline assessments between 2000 and 2004 during which researchers conducted physical examinations and laboratory studies and collected data on medical history, medications, sociodemographic factors, and behavioral risk factors. This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (S1 STROBE Checklist).
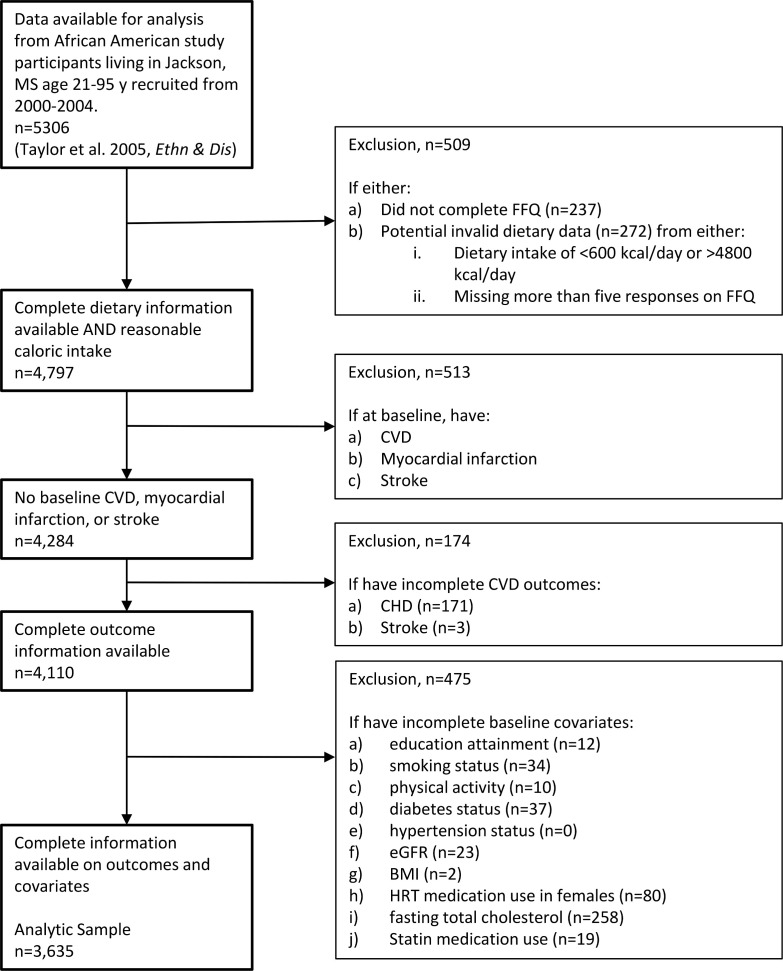
The study enrolled 5,306 participants at baseline. We excluded 509 participants who did not complete the food frequency questionnaire (FFQ) (n = 237) or who had invalid or unavailable dietary data (n = 272) (defined as extremely low or high energy intake [<600 or >4,800 kcal/day] or missing more than 5 responses on the FFQ), leaving 4,797 participants with valid dietary assessment (Fig 1). Participants were further excluded if they had CVD, myocardial infarction, or stroke at baseline (n = 513) or if they had incomplete outcome information (missing coronary heart disease [CHD] or stroke, n = 174). We also excluded participants if they had missing data on covariates (education attainment, smoking status, physical activity, alcohol intake, margarine intake, fasting total cholesterol, body mass index [BMI], hypertension, diabetes, estimated glomerular filtration rate [eGFR], hormone replacement therapy [HRT] in women, and statin use; n = 475), leaving a final analytic sample of 3,635 participants.
Fig 1. Participant selection flowchart.
There were no missing data for age, sex, and total energy intake. Abbreviations: BMI, body mass index; CVD; cardiovascular disease; eGFR, estimated glomerular filtration rate; FFQ, food frequency questionnaire; HRT, hormone replacement therapy; MS, Mississippi.
Dietary assessment
Dietary intake was assessed using an interviewer-administered, culturally appropriate, and validated FFQ developed for the study population, administered at baseline [14,15]. The JHS FFQ was based on the Delta NIRI FFQ, developed from 24-hour recalls in Mississippi, US [14,16]. Participants were asked to self-report the frequency and portion size of 158 food items consumed over the previous year. The reproducibility and validity of the FFQ used in the JHS was studied in a subset of 499 JHS participants, comparing the FFQ to 24-hour dietary recall data that were collected at the initial clinic visit and at 4 subsequent monthly administrations beginning 1 month after the initial clinic visit [15]. Average daily intakes of foods in servings per day were calculated using University of Minnesota Nutrition Data System for Research (NDSR) software (version 5.0–35, 2004; Nutrition Coordinating Center, University of Minnesota, Minneapolis).
Plant-based diet scores
We used established plant-based diet indices (PDIs) for the present study, generating an overall PDI, a healthy PDI (hPDI), and an unhealthy PDI (uPDI) [7,9,17]. In previous studies, another plant-based diet score was developed in a Mediterranean population, i.e., a provegetarian diet index [18,19]. Given that the present study was conducted in participants from the US, we conducted our analysis using the overall PDI, hPDI, and uPDI. All food items were derived using the NDSR software based on participants’ responses on the FFQ. Then, the food categories from the NDSR were sorted into 1 of 18 food groups (S1 Table). These 18 food groups were further categorized into broader categories of animal food groups (animal fats, dairy, eggs, meat, and fish and seafood), healthy plant food groups (whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea and coffee), and less healthy plant food groups (refined grains, potatoes, fruit juices, sugar-sweetened beverages [SSBs], sweets and desserts, and miscellaneous unhealthy plant-based foods). To account for existing dietary patterns of our study population, we modified the original indices by adding a “miscellaneous unhealthy plant-based foods” category and excluding the “mixed animal-based foods” category. Miscellaneous unhealthy plant-based foods included fried fruits, fried vegetables, and vegetable-based savory snacks. We did not include a “mixed animal-based foods” category in this index because such foods were already categorized into a primary food group by the NDSR software (pizza was categorized as cheese, beef-based tomato sauce as beef, etc.). Healthful and unhealthful foods were categorized based on their reported associations in the literature with chronic conditions, including type 2 diabetes, CVD, obesity, and hypertension [7,9,17,18]. Notably, the relative healthfulness of different food groups is not accounted for in the indices because all food groups are given equal weight in the diet index scores. The trans-fat content of margarine has changed in recent years [20]. Therefore, we did not include margarine in the index, but instead controlled for margarine intake in multivariable models, consistent with the approach from prior publications [7, 9,17,18]. We also did not include alcohol in our index, and instead controlled for it in our multivariable models, similar to previous studies [7, 9,17,18].
Indices were calculated by computing energy-adjusted consumption of each of the 18 food groups using the residual method [21,22] and dividing the energy-adjusted values into quintiles, assigned a score from 1 to 5. For the overall PDI, quintiles with the greatest relative consumption of healthy and less healthy plant foods were assigned a score of 5, and quintiles with the least relative consumption of healthy and less healthy plant foods were assigned a score of 1, with middle quintiles assigned a score of 2, 3, or 4. Participants with the highest relative consumption of animal foods were given reverse scores, such that the highest relative animal food consumption quintile received a score of 1 and the lowest relative animal food consumption quintile received a score of 5.
For hPDI, only healthy plant foods received positive scores: Participants in the highest quintile of healthy plant food consumption received a score of 5 (positive score), while participants in the highest quintile of unhealthy plant food consumption and the highest quintile of animal food consumption received a score of 1 (reverse score). For the uPDI, only the less healthy plant foods received positive scores, such that participants in the highest quintile of unhealthy plant food consumption received a score of 5 (positive score), while participants in the highest quintiles of healthy plant food consumption and animal food consumption received a score of 1 (reverse score). Indices had a theoretical range of 18 to 90, where 18 represents the least possible adherence to the particular index and 90 represents the greatest possible adherence to the diet index. All PDIs were divided into tertiles for analysis.
Life’s Simple 7 total score and Life’s Simple 7 healthy diet score
In addition to PDIs, we calculated Life’s Simple 7 total score to examine the baseline characteristics of participants and Life’s Simple 7 healthy diet score to examine the nutritional characteristics of the plant-based diet scores. Life’s Simple 7 total score is a composite score describing cardiovascular health ranging from 0 to 14 that sums American Heart Association poor (0), intermediate (1), or ideal (2) health scores for smoking, diet, physical activity level, BMI, blood pressure, total cholesterol, and fasting plasma glucose [23]. Life’s Simple 7 healthy diet score is a measure of adherence to 5 healthy diet factors with the score ranging from 0 (least healthy) to 5 (most healthy) [24]. Healthy diet score components are as follows: fruits and vegetables, ≥4.5 cups/day; fish, ≥2 3.5-ounce servings/week; fiber-rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate), ≥3 1-ounce servings per day; sodium, ≤1,500 mg/day; and SSBs, <36 fluid ounces/week (≤450 kcal/week). Dietary recommendations are scaled according to a 2,000-kcal/day diet.
Outcome assessment
Surveillance for CVD events and deaths began on September 26, 2000, and continued until May 31, 2018. Details of the identification and classification of CVD events and deaths has been described elsewhere [25]. Briefly, CVD illnesses and deaths were identified through a combination of standardized annual telephone follow-up interviews and surveillance of hospitalizations and death certificates with adjudication by trained medical professionals. Every year, participants’ contact information was verified to help maintain contact in the following year. All-cause mortality was defined as deaths attributable to any cause. Incident CVD was defined as any new CHD event (including fatal CHD, myocardial infarction, or cardiac procedure) or stroke event that occurred during the follow-up window in an individual without prior history of CVD, myocardial infarction, or stroke. We did not include incident heart failure in our measure of CVD because monitoring for heart failure hospitalization did not begin until 2005. For all outcomes, participants were censored at loss to follow-up or end of study, and for CVD incidence analysis, participants were additionally censored at death.
Covariate assessment
Participants’ sociodemographic information (age, sex, and education), health behaviors (cigarette smoking, physical activity, total energy intake, alcohol use, and margarine intake), medical history (hypertension status, diabetes status, HRT use, and statin medication use), BMI, and laboratory information (total cholesterol and eGFR) were collected at baseline [11]. Trained staff measured participants’ height to the nearest centimeter and weight to the nearest 0.1 kilogram, which were used to calculate BMI (kg/m2). Physical activity level was measured using the JHS Physical Activity Cohort Survey [26]. Alcohol use, margarine intake, and total energy intake were estimated using data reported in the FFQ. Hypertension was defined as having blood pressure ≥ 140/90 mm Hg or use of blood-pressure-lowering medication within 2 weeks prior to the clinic visit. Diabetes was defined as having fasting glucose ≥ 7.0 mmol/L, having hemoglobin A1c (HbA1c) ≥ 6.5%, or use of diabetic medication within 2 weeks prior to the clinic visit. eGFR was assessed using the 4-variable Chronic Kidney Disease Epidemiology Collaboration equation [27].
Statistical analyses
Differences in baseline characteristics and nutritional characteristics, according to tertiles of PDI scores (overall PDI, hPDI, and uPDI) were evaluated by the chi-squared test for categorical variables and ANOVA for continuous variables. We examined macro- and micro-nutrient intake and Life’s Simple 7 healthy diet score (a measure of adherence to 5 healthy dietary goals including higher intake of fruits and vegetables, fish, and whole grains, and lower intake of sodium and SSBs) to describe nutritional characteristics of each of the PDIs [24].
For the primary analysis, we used Cox proportional hazards regression models to describe associations between PDIs and incident CVD and all-cause mortality. Length of follow-up (time since baseline) was used as the time metric. We adjusted the analysis for potential confounders in 3 progressively adjusted models. In model 1, we adjusted for age, sex, and total energy intake (kcal/day). Model 2 was adjusted for all variables in model 1 and further for educational attainment (less than high school, high school or General Educational Development [GED], greater than high school), smoking status (current, former, never), physical activity (continuous), alcohol intake (g/day), and margarine intake (servings/day). Model 3 was adjusted for all variables in model 2 and further for diabetes (yes/no), hypertension (yes/no), total cholesterol (continuous), eGFR (continuous), BMI (continuous), HRT medication use (yes/no), and statin medication use (yes/no). We calculated p-trend to assess the linear trend of hazard ratios (HRs) in Cox proportional hazards regression models, using the median value of each diet score tertile. HRs and 95% CIs were calculated by tertile. Finally, we conducted stratified analyses to determine whether associations differed by sex, BMI category (18.5 to <25 kg/m2, 25 to <30 kg/m2, ≥30 kg/m2), hypertension status, or diabetes status.
As secondary analyses, we modeled (1) components within the PDIs (healthy plant foods, unhealthy plant foods, and animal foods) and (2) 18 individual food groups within the indices together, instead of the scores, to test if a specific component or food group was associated with CVD incidence and all-cause mortality. All analyses were conducted using Stata statistical software, version 16.1 (StataCorp, College Station, TX), and significance was defined as a 2-sided p-value < 0.05.
As post hoc analyses, we (1) analyzed plant-based diets as continuous variables (HR per 1 standard deviation higher), (2) analyzed CVD separately as CHD (n = 173) and stroke (n = 148), (3) analyzed stroke separately as ischemic (n = 135) and hemorrhagic stroke (n = 12), (4) examined if there were departures from linearity by formally testing for linear association and modeling plant-based diet scores using restricted cubic splines with 4 knots at the 5th, 35th, 65th, and 95th percentiles, (5) simultaneously adjusted for hPDI and uPDI, (6) divided plant-based diet scores into quintiles instead of tertiles, (7) compared the baseline characteristics of the participants in our analytic sample (n = 3,635) and the total eligible study population including those with missing covariates (n = 4,110), and (8) used multiple imputation by chained equations to impute missing covariates (educational attainment, smoking status, alcohol intake, margarine intake, physical activity, BMI, total cholesterol, diabetes, eGFR, HRT medication use, and statin medication use) to assess the robustness of our findings [28]. For the analysis of stroke subtypes, we excluded 1 participant with missing stroke subtype information. For hemorrhagic stroke, we examined plant-based diets only as a continuous variable, due to the small number of hemorrhagic stroke cases (n = 12).
Results
Baseline characteristics
The overall PDI ranged from 30 to 76, while hPDI ranged from 34 to 82 and the uPDI ranged from 30 to 76. Participants with higher overall PDI and hPDI were more likely to be older, female, more educated, and more physically active, and to have lower eGFR and higher Life’s Simple 7 total score. Participants with higher overall PDI were more likely to have lower total energy intake, to have lower alcohol intake, to be nonsmokers, to have higher fasting total cholesterol, to have lower BMI, and to use statin medication (Table 1). Participants with higher hPDI were more likely to have higher intake of total energy, alcohol, and margarine (S2 Table). Conversely, participants with higher uPDI were more likely to be younger and to be male, and to have higher intake of total energy and alcohol, lower educational attainment, lower physical activity, and lower overall Life’s Simple 7 total score (S3 Table). Those with higher uPDI were also less likely to have hypertension or diabetes, had lower BMI, and lower HbA1c (p < 0.05 for all comparisons). Baseline characteristics were similar for the participants included in our analyses and the total eligible study population including those with missing data (S4 Table). Imputing missing covariates did not substantially change the results (S5 Table).
Table 1. Selected baseline demographic, socioeconomic, and health characteristics by tertiles of plant-based diet index in the Jackson Heart Study.
| Characteristic | Overall plant-based diet index | p-Value | ||
|---|---|---|---|---|
| Tertile 1 n = 1,237 (34.0%) |
Tertile 2 n = 1,258 (34.6%) |
Tertile 3 n = 1,140 (31.4%) |
||
| Median index score (range) | 48 (30–51) | 55 (52–57) | 61 (58–76) | |
| Age, years | 51.9 (12.8) | 54.2 (12.6) | 55.5 (12.1) | <0.001 |
| Female | 52.5 | 67.8 | 72.5 | <0.001 |
| Total energy intake, kcal/day | 2,525 (899) | 2,142 (916) | 2,103 (836) | <0.001 |
| Educational attainment | 0.021 | |||
| Less than high school/GED | 16.2 | 15.1 | 14.2 | |
| High school or GED completion | 21.1 | 20.4 | 16.8 | |
| Attended college or trade school | 62.7 | 64.5 | 68.9 | |
| Smoking status | <0.001 | |||
| Current smoker, percent | 14.4 | 10.6 | 8.4 | |
| Former smoker, percent | 20.0 | 15.8 | 17.1 | |
| Physical activity index* | 2.1 (0.8) | 2.1 (0.8) | 2.2 (0.8) | 0.01 |
| Alcohol intake, g/day | 6.4 (18.0) | 3.1 (9.2) | 1.9 (6.3) | <0.001 |
| Margarine intake, servings/day | 1.4 (1.7) | 1.2 (1.8) | 1.2 (1.7) | 0.052 |
| Fasting total cholesterol, mmol/L | 5.1 (1.0) | 5.1 (1.0) | 5.3 (1.0) | 0.001 |
| Hypertension† | 49.7 | 53.3 | 54.3 | 0.059 |
| Diabetes‡ | 20.9 | 18.2 | 17.1 | 0.053 |
| eGFR, ml/min/1.73 m2§ | 98.3 (20.5) | 95.5 (20.5) | 94.5 (19.7) | <0.001 |
| BMI, kg/m2 | 32.2 (7.4) | 31.9 (7.3) | 31.1 (6.8) | <0.001 |
| HRT medication use, percent of females | 14.4 | 19.9 | 19.5 | 0.507 |
| Statin medication use | 9.1 | 11.1 | 13.2 | 0.006 |
| Systolic blood pressure, mm Hg | 127 (16) | 127 (17) | 127 (17) | 0.613 |
| HbA1c, mmol/mol | 41 (13.1) | 40 (12.0) | 40 (12.0) | 0.141 |
| Life’s Simple 7 total score|| | 6.9 (2.1) | 7.2 (2.0) | 7.3 (2.1) | <0.001 |
Values are mean (standard deviation) for continuous variables and percent for categorical variables, unless otherwise noted. Statistical differences by tertiles of plant-based diet index were tested using analysis of variance for continuous variables and chi-squared tests for categorical variables, with p < 0.05 denoting statistical significance.
*Physical activity index is a measure from 0 (low) to 5 (high) of activity in daily living.
†Hypertension was defined as having blood pressure ≥ 140/90 mm Hg or use of blood-pressure-lowering medication within 2 weeks prior to the clinic visit.
‡Diabetes was defined as having fasting glucose ≥ 7.0 mmol/L, having HbA1c ≥ 6.5%, or use of diabetic medication within 2 weeks prior to the clinic visit.
§eGFR was assessed using the 4-variable Chronic Kidney Disease Epidemiology Collaboration equation.
||Life’s Simple 7 total score is a composite score describing cardiovascular health ranging from 0 to 14 that sums American Heart Association poor (0), intermediate (1), or ideal (2) health scores for smoking, diet, physical activity level, BMI, blood pressure, total cholesterol, and fasting plasma glucose.
BMI, body mass index; eGFR, estimated glomerular filtration rate; GED, General Educational Development; HRT, hormone replacement therapy; HbA1c, hemoglobin A1c.
Nutritional characteristics
Nutritional characteristics of the diet differed significantly across tertiles of plant-based diet scores (Tables 2, S6, and S7). Participants in the highest tertiles of overall PDI, hPDI, and uPDI, respectively, met an average of 1.5, 1.6, and 0.8 of the 5 diet metrics in the Life’s Simple 7 healthy diet score (p-values for all tests < 0.001). Participants in the highest tertile of overall PDI had slightly lower total energy intake than those in the lowest tertile of overall PDI, whereas participants in the highest tertile of hPDI had slightly higher total energy intake than those in the lowest tertile of hPDI (p-values for all tests < 0.001). There was no linear trend in total energy intake across uPDI tertiles.
Table 2. Selected nutritional characteristics by tertiles of plant-based diet index in the Jackson Heart Study.
| Characteristic | Overall plant-based diet index | p-Value | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Life’s Simple 7 healthy diet score* | 1.1 (1.0) | 1.3 (0.9) | 1.5 (0.9) | <0.001 |
| Total energy intake, kcal/day | 2,525 (899) | 2,142 (916) | 2,103 (836) | <0.001 |
| Total fat, g/day | 106.9 (42.9) | 86.2 (40.9) | 81.4 (38.1) | <0.001 |
| Protein, g/day | 98.2 (39.4) | 76.6 (35.1) | 69.2 (30.7) | <0.001 |
| Alcohol, g/day | 6.4 (17.6) | 3.2 (8.6) | 2.1 (6.3) | <0.001 |
| Saturated fatty acid, g/day | 35.5 (14.9) | 26.6 (13.0) | 23.2 (11.5) | <0.001 |
| Carbohydrates, g/day | 290 (117) | 269 (123) | 283 (115) | <0.001 |
| Dietary fiber, g/day | 21.1 (9.6) | 21.4 (10.3) | 24.7 (11.1) | <0.001 |
| Fruit, servings/day | 2.5 (2.7) | 2.8 (2.9) | 3.7 (3.3) | <0.001 |
| Vegetables, servings/day | 3.9 (2.4) | 3.8 (2.4) | 4.4 (2.7) | <0.001 |
| Whole grains, servings/day | 0.8 (0.8) | 1.0 (0.9) | 1.3 (1.0) | <0.001 |
| Nuts, g/day | 7.3 (11.5) | 7.6 (10.8) | 9.0 (11.7) | <0.001 |
| Fish, g/day | 24.5 (36.4) | 19.7 (26.9) | 18.9 (23.0) | <0.001 |
| Processed meat, g/day | 28.0 (31.1) | 18.3 (20.5) | 13.7 (15.5) | <0.001 |
| Beverages, g/day | 359 (369) | 314 (330) | 301 (327) | <0.001 |
| Sweetened beverages, servings/week | 12.9 (17.7) | 11.0 (15.0) | 9.9 (12.2) | <0.001 |
| Animal protein, g/day | 70.0 (32.9) | 47.6 (25.7) | 36.8 (21.3) | <0.001 |
| Vegetable protein, g/day | 23.5 (9.5) | 22.0 (9.7) | 23.3 (9.5) | <0.001 |
| Cholesterol, mg/day | 459 (218) | 297 (163) | 221 (127) | <0.001 |
| Monounsaturated fatty acids, g/day | 38.1 (16.3) | 29.6 (15.5) | 27.0 (14.1) | <0.001 |
| Polyunsaturated fatty acids, g/day | 22.0 (9.9) | 18.9 (10.0) | 18.8 (9.7) | <0.001 |
| Sodium, mg/day | 4,001 (1,533) | 3,283 (1,461) | 3,077 (1,343) | <0.001 |
| Potassium, mg/day | 2,781 (1,122) | 2,448 (1,084) | 2,421 (1,049) | <0.001 |
| Phosphorus, mg/day | 1,449 (570) | 1,131 (508) | 1,026 (454) | <0.001 |
| Calcium, mg/day | 894 (413) | 717 (347) | 647 (291) | <0.001 |
| Magnesium, mg/day | 281 (100) | 250 (99) | 257 (99) | <0.001 |
| Iron, mg/day | 14.9 (6.2) | 12.8 (5.9) | 12.7 (5.5) | <0.001 |
| Vitamin A, mg/day | 7,181 (3,268) | 6,745 (3,396) | 7,002 (3,047) | 0.003 |
| Vitamin C, mg/day | 113 (83) | 110 (79) | 118 (75) | 0.063 |
| Folate, mg/day | 296 (123) | 266 (115) | 277 (113) | <0.001 |
| Vitamin B12, μg/day | 6.7 (4.2) | 4.9 (3.4) | 4.0 (2.6) | <0.001 |
| Zinc, mg/day | 12.7 (5.6) | 10.0 (4.8) | 9.1 (4.3) | <0.001 |
Values are mean (standard deviation). Statistical differences were tested using analysis of variance for continuous variables with p < 0.05 denoting statistical significance. Dietary data were self-reported.
*Life’s Simple 7 healthy diet score is a measure of adherence to 5 healthy dietary goals with score ranging from 0 (least healthy) to 5 (most healthy). Healthy diet score components are as follows: fruits and vegetables, ≥4.5 cups/day; fish, ≥2 3.5-ounce servings/week; fiber-rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate), ≥3 1-ounce servings per day; sodium, ≤1,500 mg/day; and sugar-sweetened beverages, <36 fluid ounces/week (≤450 kcal/week). Dietary recommendations are scaled according to a 2,000-kcal/day diet.
Participants in the highest versus lowest tertile of overall PDI and hPDI reported higher consumption of fruits and vegetables, whereas those in the highest versus lowest uPDI tertile reported lower consumption of fruits and vegetables (Tables 2, S6, and S7). Those in the highest versus lowest tertile of overall PDI consumed less animal protein, processed meat, saturated fatty acids, and SSBs (Table 2). Those in the highest versus lowest tertile of hPDI consumed less processed meat, and similar amounts of animal protein and SSBs (S6 Table). Those in the highest versus lowest tertile of uPDI consumed less animal protein, more SSBs, and similar amounts of processed meat (S7 Table).
Plant-based diets and CVD incidence and all-cause mortality
During a median follow-up of 13 years, there were 293 observed cases of incident CVD. During a median follow-up time of 15 years, there were 597 observed deaths. Incidence rates for CVD and all-cause mortality did not differ by PDI score (overall PDI, hPDI, or uPDI) (S8 Table). In multivariable regression models, there were no significant differences in risk of incident CVD or all-cause mortality by tertiles of plant-based diet (Table 3). HRs and 95% CIs for CVD incidence in the highest versus lowest tertile of overall PDI, hPDI, and uPDI were 1.06 (95% CI 0.78–1.42, p-trend = 0.72), 1.07 (95% CI 0.80–1.42, p-trend = 0.67), and 0.95 (95% CI 0.71–1.28, p-trend = 0.76), respectively, after adjusting for age, sex, energy intake, education, smoking, physical activity, and alcohol and margarine intake (model 2). A standard deviation increase in overall PDI (HR 1.05, 95% CI 0.93–1.20), hPDI (HR 1.07, 95% CI 0.95–1.20), and uPDI (HR 1.01, 95% CI 0.89–1.14) was not associated with incident CVD. HRs for all-cause mortality risk for the highest versus lowest tertile of overall PDI, hPDI, and uPDI were 0.96 (95% CI 0.78–1.18, p-trend = 0.67), 0.94 (95% CI 0.76–1.16, p-trend = 0.63), and 1.06 (95% CI 0.86–1.30, p-trend = 0.59), respectively. In a model adjusting for age, sex, and total energy intake (model 1), a standard deviation increase in hPDI was inversely associated with all-cause mortality (HR 0.89, 95% CI 0.82–0.97), whereas uPDI was positively associated with all-cause mortality (HR 1.09, 95% CI 1.00–1.19). However, these associations were attenuated in the most adjusted model (model 3) (hPDI: HR 0.93, 95% CI 0.85–1.02; uPDI: HR 1.09, 95% CI 1.00–1.19).
Table 3. Hazard ratios (95% confidence intervals) of incident cardiovascular disease and all-cause mortality among dietary patterns for progressively adjusted models.
| Dietary index | Measure | Incident cardiovascular disease | All-cause mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (ref) | Tertile 2 | Tertile 3 | p-Trend | Per SD higher score | p-Value | Tertile 1 (ref) | Tertile 2 | Tertile 3 | p-Trend | Per SD higher score | p-Value | ||
| Overall plant-based diet index | Median score | 48 | 55 | 61 | 48 | 55 | 61 | ||||||
| Cases/N | 90/1,237 | 103/1,258 | 100/1,140 | 194/1,237 | 203/1,258 | 200/1,140 | |||||||
| Person-years | 14,507 | 14,787 | 13,470 | 17,998 | 18,412 | 16,659 | |||||||
| HR (95% CI) | |||||||||||||
| Model 1 | 1 | 1.02 (0.76–1.36) | 1.06 (0.79–1.43) | 0.69 | 1.05 (0.93, 1.19) | 0.40 | 1 | 0.87 (0.71–1.06) | 0.93 (0.76–1.14) | 0.49 | 0.97 (0.89, 1.05) | 0.44 | |
| Model 2 | 1 | 0.98 (0.73–1.31) | 1.06 (0.78–1.42) | 0.72 | 1.05 (0.93, 1.20) | 0.41 | 1 | 0.86 (0.70–1.05) | 0.96 (0.78–1.18) | 0.67 | 0.98 (0.89, 1.08) | 0.72 | |
| Model 3 | 1 | 1.02 (0.75–1.37) | 1.09 (0.80–1.47) | 0.60 | 1.07 (0.94, 1.22) | 0.30 | 1 | 0.93 (0.76–1.15) | 1.07 (0.87–1.33) | 0.52 | 1.03 (0.95, 1.14) | 0.45 | |
| Healthy plant-based diet index | Median score | 48 | 54 | 60 | 48 | 54 | 60 | ||||||
| Cases/N | 101/1,295 | 91/1,155 | 101/1,185 | 201/1,295 | 226/1,155 | 170/1,185 | |||||||
| Person-years | 15,179 | 13,531 | 14,054 | 18,906 | 16,644 | 17,518 | |||||||
| HR (95% CI) | |||||||||||||
| Model 1 | 1 | 0.97 (0.73–1.29) | 1.06 (0.80–1.41) | 0.68 | 1.06 (0.94, 1.19) | 0.36 | 1 | 1.24 (1.02–1.50) | 0.87 (0.70–1.07) | 0.21 | 0.89 (0.82, 0.97) | 0.008 | |
| Model 2 | 1 | 0.97 (0.73–1.30) | 1.07 (0.80–1.42) | 0.67 | 1.07 (0.95, 1.20) | 0.27 | 1 | 1.27 (1.05–1.55) | 0.94 (0.76–1.16) | 0.63 | 0.93 (0.85, 1.01) | 0.095 | |
| Model 3 | 1 | 0.97 (0.72–1.29) | 1.02 (0.76–1.36) | 0.92 | 1.04 (0.92, 1.18) | 0.52 | 1 | 1.29 (1.06–1.57) | 0.94 (0.76–1.17) | 0.65 | 0.93 (0.85, 1.02) | 0.11 | |
| Unhealthy plant-based diet index | Median score | 48 | 54 | 61 | 48 | 54 | 61 | ||||||
| Cases/N | 103/1,289 | 109/1,247 | 81/1,099 | 195/1,289 | 221/1,247 | 181/1,099 | |||||||
| Person-years | 15,183 | 14,536 | 13,045 | 18,869 | 18,103 | 16,097 | |||||||
| HR (95% CI) | |||||||||||||
| Model 1 | 1 | 1.08 (0.82–1.41) | 0.97 (0.72–1.30) | 0.85 | 1.02 (0.90, 1.15) | 0.76 | 1 | 1.17 (0.96–1.42) | 1.14 (0.93–1.40) | 0.21 | 1.09 (1.00, 1.19) | 0.045 | |
| Model 2 | 1 | 1.06 (0.81–1.40) | 0.95 (0.71–1.28) | 0.76 | 1.01 (0.89, 1.14) | 0.93 | 1 | 1.10 (0.90–1.34) | 1.06 (0.86–1.30) | 0.59 | 1.04 (0.96, 1.14) | 0.31 | |
| Model 3 | 1 | 1.01 (0.83–1.45) | 1.03 (0.76–1.40) | 0.82 | 1.05 (0.93, 1.19) | 0.42 | 1 | 1.19 (0.98–1.46) | 1.15 (0.93–1.42) | 0.20 | 1.09 (1.00, 1.19) | 0.047 | |
Dietary data were self-reported. Incident cardiovascular disease is a composite of coronary heart disease and/or stroke events. Model 1 was adjusted for age, sex, and total energy intake. Model 2 was adjusted for all the covariates in model 1 and was further adjusted for educational attainment, smoking status, alcohol intake, margarine intake, and physical activity. Model 3 was adjusted for all the covariates in model 2 and was further adjusted for body mass index, total cholesterol, hypertension, diabetes, estimated glomerular filtration rate, hormone replacement therapy medication use, and statin medication use. Standard deviation (SD) for the overall plant-based diet index was 6.7, SD for the healthy plant-based diet index was 6.0, and SD for the unhealthy plant-based diet index was 6.7.
When CVD was analyzed separately, we found no association between any of the PDIs and incident CHD (p-trend for all tests > 0.05) (S9 Table), hPDI was inversely associated with ischemic stroke (HR 0.86, 95% CI 0.56–1.32), and uPDI was positively associated with ischemic stroke (HR 1.17, 95% CI 0.77–1.79), but none of these associations were statistically significant (p-trend for all tests > 0.05) (S10 Table). No significant association was observed for plant-based diet scores and hemorrhagic stroke (p-values for all tests > 0.05). We did not find departures from linearity when we tested for nonlinear associations for CVD and all-cause mortality (p for nonlinear association > 0.05 for all indices) or when we examined the shape of the association using restricted cubic splines (S1–S6 Figs). Simultaneously adjusting for hPDI and uPDI (range of HRs for hPDI and uPDI 0.96–1.09, p-trend for all tests > 0.05) or using quintiles instead of tertiles did not change the results for incident CVD or all-cause mortality (S11 Table).
Results for population subgroups, by sex, BMI, hypertension status, and diabetes status were similar to the main results, and there was no difference in association by subgroups (p-interaction > 0.05 for all tests) (Table 4).
Table 4. Adjusted hazard ratios (95% CIs) for incident cardiovascular disease and all-cause mortality for highest versus lowest tertile of plant-based diet scores according to sex and baseline diabetes status, hypertension status, and body mass index (BMI).
| Dietary index | Subgroup | Incident cardiovascular disease | All-cause mortality | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-Interaction | Hazard ratio (95% CI) | p-Interaction | ||
| Overall plant-based diet index | Overall | 1.09 (0.80–1.47) | 0.60 | 1.07 (0.87–1.33) | 0.52 |
| Females | 1.02 (0.99–1.05) | 0.13 | 1.02 (0.99–1.04) | 0.20 | |
| Males | 0.99 (0.95–1.03) | 0.99 (0.96–1.02) | |||
| BMI 18.5 to <25 kg/m2 | 0.96 (0.89–1.03) | 0.29 | 1.04 (0.99–1.09) | 0.30 | |
| BMI 25 to <30 kg/m2 | 0.99 (0.95–1.03) | 0.99 (0.96–1.03) | |||
| BMI ≥ 30 kg/m2 | 1.03 (1.00–1.06) | 1.01 (0.98–1.03) | |||
| Diabetes | 1.04 (1.00–1.09) | 0.18 | 1.02 (0.99–1.05) | 0.38 | |
| No diabetes | 0.99 (0.96–1.02) | 1.00 (0.98–1.02) | |||
| Hypertension | 1.01 (0.99–1.04) | 0.77 | 1.00 (0.98–1.02) | 0.52 | |
| No hypertension | 0.99 (0.93–1.04) | 1.01 (0.98–1.05) | |||
| Healthy plant-based diet index | Overall | 1.02 (0.76–1.36) | 0.92 | 0.94 (0.76–1.17) | 0.65 |
| Females | 1.01 (0.98–1.04) | 0.47 | 1.00 (0.98–1.03) | 0.08 | |
| Males | 0.99 (0.95–1.03) | 0.98 (0.95–1.01) | |||
| BMI 18.5 to <25 kg/m2 | 0.99 (0.92–1.07) | 0.18 | 1.04 (0.99–1.09) | 0.14 | |
| BMI 25 to <30 kg/m2 | 0.97 (0.93–1.02) | 1.00 (0.97–1.03) | |||
| BMI ≥ 30 kg/m2 | 1.02 (0.99–1.06) | 0.98 (0.96–1.01) | |||
| Diabetes | 1.01 (0.96–1.05) | 0.94 | 0.99 (0.96–1.02) | 0.92 | |
| No diabetes | 1.00 (0.97–1.03) | 1.00 (0.98–1.02) | |||
| Hypertension | 1.01 (0.99–1.04) | 0.09 | 0.99 (0.97–1.01) | 0.50 | |
| No hypertension | 0.95 (0.89–1.00) | 1.01 (0.97–1.05) | |||
| Unhealthy plant-based diet index | Overall | 0.97 (0.72–1.30) | 0.85 | 1.15 (0.93–1.42) | 0.20 |
| Females | 1.00 (0.97–1.03) | 0.80 | 1.01 (0.99–1.03) | 0.99 | |
| Males | 1.00 (0.96–1.04) | 1.01 (0.98–1.04) | |||
| BMI 18.5 to <25 kg/m2 | 0.98 (0.92–1.05) | 0.87 | 0.99 (0.95–1.03) | 0.36 | |
| BMI 25 to <30 kg/m2 | 1.01 (0.97–1.05) | 1.00 (0.97–1.03) | |||
| BMI ≥ 30 kg/m2 | 1.00 (0.96–1.03) | 1.02 (1.00–1.04) | |||
| Diabetes | 1.00 (0.96–1.04) | 0.78 | 1.03 (1.00–1.06) | 0.27 | |
| No diabetes | 1.00 (0.98–1.03) | 1.00 (0.99–1.02) | |||
| Hypertension | 0.99 (0.97–1.02) | 0.09 | 1.01 (1.00–1.03) | 0.46 | |
| No hypertension | 1.04 (0.99–1.10) | 1.00 (0.96–1.03) | |||
Dietary data were self-reported. Models adjusted for age, sex, total energy intake, educational attainment, smoking status, alcohol intake, margarine intake, physical activity, body mass index (BMI), total cholesterol, hypertension, diabetes, estimated glomerular filtration rate, hormone replacement therapy medication use, and statin medication use.
Analyses on score components and individual food groups
We found no significant association between score components (healthy plant-based foods, unhealthy plant-based foods, and animal-based foods) and incident CVD or all-cause mortality when controlling for all covariates and other score components (S12 Table). In the analysis of individual food groups, we observed significant associations, per 1-serving increase, of whole grain consumption with all-cause mortality (HR 1.13, 95% CI 1.02–1.25), SSB consumption with all-cause mortality (HR 1.07, 95% CI 1.00–1.14), legume consumption with lower CVD risk (HR 0.59, 95% CI 0.35–0.99), and healthy oil consumption with higher CVD risk (HR 1.10, 95% CI 1.01–1.20) after adjusting for covariates and all other individual food groups (Table 5).
Table 5. Adjusted hazard ratios (95% CIs) for incident cardiovascular disease and all-cause mortality per 1-serving increase in individual food groups.
| Food group | Incident cardiovascular disease | All-cause mortality | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-Trend | Hazard ratio (95% CI) | p-Trend | |
| Healthy plant foods | ||||
| Whole grains | 1.03 (0.89–1.19) | 0.71 | 1.13 (1.02–1.25) | 0.019 |
| Vegetables | 1.17 (0.96–1.41) | 0.12 | 1.00 (0.86–1.16) | 0.99 |
| Fruits | 0.87 (0.72–1.06) | 0.16 | 1.03 (0.97–1.09) | 0.34 |
| Nuts | 0.88 (0.71–1.08) | 0.23 | 0.92 (0.79–1.06) | 0.24 |
| Legumes | 0.59 (0.35–0.99) | 0.047 | 0.71 (0.50–1.00) | 0.053 |
| Healthy vegetable oils | 1.10 (1.01–1.20) | 0.019 | 1.03 (0.96–1.10) | 0.40 |
| Unsweetened coffee and tea | 1.02 (0.95–1.08) | 0.62 | 1.02 (0.97–1.07) | 0.53 |
| Unhealthy plant foods | ||||
| Refined grains | 1.05 (0.94–1.16) | 0.39 | 1.05 (0.97–1.13) | 0.21 |
| Potatoes | 1.19 (0.85–1.68) | 0.30 | 1.11 (0.86–1.44) | 0.41 |
| Fruit juice | 1.01 (0.92–1.12) | 0.77 | 1.05 (0.98–1.12) | 0.15 |
| Sugar-sweetened beverages | 1.06 (0.97–1.16) | 0.21 | 1.07 (1.00–1.14) | 0.045 |
| Sweets and desserts | 0.98 (0.94–1.02) | 0.32 | 0.99 (0.96–1.02) | 0.43 |
| Miscellaneous unhealthy plant-based foods | 1.07 (0.77–1.48) | 0.69 | 1.21 (0.97–1.51) | 0.10 |
| Animal-based foods | ||||
| Animal fat | 0.95 (0.85–1.07) | 0.39 | 1.05 (0.97–1.12) | 0.22 |
| Dairy | 1.03 (0.88–1.21) | 0.74 | 1.05 (0.94–1.17) | 0.42 |
| Eggs | 1.02 (0.80–1.31) | 0.86 | 1.12 (0.95–1.32) | 0.17 |
| Fish and seafood | 0.99 (0.91–1.08) | 0.84 | 0.98 (0.92–1.04) | 0.50 |
| Meat | 1.02 (0.94–1.11) | 0.59 | 1.02 (0.96–1.08) | 0.53 |
Dietary data were self-reported. Models adjusted for all other individual food groups in addition to age, sex, total energy intake, educational attainment, smoking status, alcohol intake, margarine intake, physical activity, body mass index, total cholesterol, hypertension, diabetes, estimated glomerular filtration rate, hormone replacement therapy medication use, and statin medication use.
Discussion
In our analysis of 3,635 African American participants in the JHS, there was no significant association between plant-based dietary patterns and CVD incidence, all-cause mortality, or CVDs analyzed separately (CHD, total stroke, ischemic stroke, and hemorrhagic stroke). This lack of an association persisted when stratifying by sex, BMI, hypertension status, and diabetes status and was observed for the overall PDI as well as hPDI and uPDI. Despite this lack of association for the dietary indices, several individual food groups were associated with CVD or mortality risk. Specifically, each additional serving of legumes was associated with a 41% reduction in CVD risk, while an additional serving of healthy oils was associated with a 10% increase in CVD risk. Additional daily servings of whole grains and SSBs were associated with a 13% and 7% increased risk for all-cause mortality, respectively.
Our results are not uniform and show a number of similarities to and differences from previous studies. In contrast to observational studies on vegetarians and vegans that have consistently found a lower risk for CVD and all-cause mortality [3,10,29,30], we did not observe this association when using PDIs to describe dietary patterns. Stratifying CVD by type, we did not observe any elevation in incident stroke risk (total, ischemic, or hemorrhagic) among participants with higher PDI scores, whereas a vegetarian diet has been previously associated with higher risk for stroke, particularly hemorrhagic stroke [31].
We modeled our 3 PDIs after those used in several other studies of American populations, including the National Health and Nutrition Examination Survey (NHANES) and the Atherosclerosis Risk in Communities (ARIC) study [7,8,32]. In ARIC participants, those with the highest versus lowest adherence to an overall plant-based dietary pattern had 8%–25% lower risk of CHD or CVD risk. Importantly, in the Nurses’ Health Study and Health Professionals Follow-Up Study, nearly all participants were white, and had lower baseline rates of hypertension and diabetes and lower BMI compared with participants in the JHS. Among NHANES participants, there was no association between CVD mortality and overall PDI, hPDI, or uPDI scores [8]. An inverse association was found only among participants with hPDI score above the median, where a 10-point higher hPDI score was associated with a 5% reduction in all-cause mortality risk. This finding suggests that health benefits related to plant-based diets may only be evident once a minimum level of plant-based eating is achieved. This observation may help to explain our results.
The quality and variability of overall diet among JHS participants are important considerations in interpreting our findings. While overall diet quality can be difficult to infer from FFQs and ranked scores like our PDIs, the Life’s Simple 7 healthy diet score is an absolute measure of dietary quality in that it uses absolute thresholds to classify participants according to their intake of specific foods and nutrients. As such, the Life’s Simple 7 healthy diet score is a useful metric for overall dietary quality that can be compared across populations. In the Life’s Simple 7 healthy diet score, a score of <2 indicates poor diet quality, 2–3 indicates intermediate diet quality, and 4–5 indicates ideal diet quality [24]. In prior investigations of the JHS cohort, 57.4% of participants were found to have poor diet quality by this metric, whereas only 0.9% met the criteria for an ideal diet [33,34]. In our study, those in the lowest overall PDI tertile met, on average, only 1.1 of the 5 Life’s Simple 7 criteria for an ideal diet. Moreover, those in the highest tertile of overall PDI met on average only 1.5 criteria, and those in the highest tertile of hPDI still met on average only 1.6 of the 5 criteria. These findings suggest poor overall diet and low variability in the diet quality of JHS participants.
The Dietary Approaches to Stop Hypertension (DASH) diet score can also be considered as a measure of overall healthfulness of participants’ diets. The DASH scores of JHS participants are also low overall. Tyson et al. investigated DASH diet adherence in the JHS and observed a median DASH score of 1.0 among participants, with 75% of participants scoring ≤1.5 on an 8-point scale [35]. By contrast, the mean DASH score observed in studies of NHANES participants was approximately 2.9 on a similar 9-point scale (which also included sodium intake scores), suggesting that NHANES participants likely have healthier diets than JHS participants [36,37]. In an urban community-based cohort, the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study, the median DASH score was 1.5 [38]. In each of these studies, black race was associated with lower DASH scores [36–38].
Our findings illuminate several important considerations when using the PDI approach to study the impact of plant-based diets on health outcomes. The use of sample-based scoring methods for scoring plant-based diets may, in part, explain the lack of association observed in our study. If there is a threshold for the effect of diet healthfulness on CVD risk, as the NHANES PDI study suggests [8], it may be that we were unable to observe such a relationship in this study by comparing intake between participants because diet quality was low on average for the entire study population. Furthermore, the PDI scores, although designed to rank participants according to degree of adherence to plant-based dietary patterns, did not capture those who exclusively consume plant-derived foods. For example, participants in the highest tertile of overall PDI still consumed, on average, 37 g of animal protein, 14 g of processed meat, 19 g of fish, and only 25 g of fiber per day. As such, future diet indices with an absolute scoring system may better represent the health impacts of a plant-based diet.
Additionally, the use of FFQs may make the diet scores more challenging to interpret because preparation methods and other dietary behaviors and preferences may not have been adequately captured in our study population. For example, it is not possible to discern whether there was high consumption of fried foods (either animal- or plant-based) in this population. In the dietary data, cooking oils were not separated from other oils in participants’ oil consumption but rather grouped under a general vegetable oils category. Prior PDIs have categorized plant-based oils as “healthy oils,” and we also used this categorization in our indices. If frying foods was a large contributor to this “healthy oils” category, it may have tempered the beneficial impacts of other plant food categories. The observation of a 10% higher CVD risk associated with each additional serving of healthy oils is consistent with this possibility that cooking and preparation methods impact the overall association of the PDIs with CVD.
The positive association between whole grain intake and mortality was also unexpected, but may be related to limited variability in the whole grain intake of the population or may suggest reverse causality. A prior analysis of dietary patterns in the JHS found that, among Life’s Simple 7 criteria for a healthy diet, whole grain intake had the lowest adherence, with only 4.1% of the JHS cohort meeting the recommendation of 3 or more 1-ounce servings per day [33]. Although we implemented measures to reduce reverse causation, we cannot exclude the possibility of reverse causation influencing our results, particularly for whole grains. A prior study investigating rates of hypertension and DASH diet scores also found an unexpected, and difficult to explain, positive association between DASH diet score and hypertension in the JHS cohort [35].
Our observed statistically significant inverse association between legumes and CVD risk, as well as the positive association between SSBs and CVD risk, is consistent with prior knowledge. Legumes are a rich source of fiber, are low in fat, and contain a variety of bioactive phytochemicals (e.g., phytate, polyphenols, and flavonoids), which can reduce blood pressure, inflammation, and risk of CVD [39]. The American Heart Association recommends consumption of plant-based sources of protein as part of an overall healthy dietary pattern for CVD prevention [40]. The added sugar and calories from added sugar in SSBs can result in weight gain [41]. Obesity is an established risk factor for the development of CVD [42].
The findings of our study should be interpreted within the context of the study strengths and limitations. One limitation of this study is the use of self-reported dietary intake, which may result in measurement error. However, the FFQ used in the JHS was developed specifically for assessing diet in American individuals residing in the southern region of the US, and calibration and validation studies in a subset of JHS participants found that it had reasonable validity and performed similarly for most nutrients when compared to both 24-hour recalls and a longer version of the Delta NIRI FFQ [14,15]. This tailoring of the FFQ and dietary index to our population’s dietary patterns is a marked strength of our study and may reduce misclassification bias [43].
While our analysis adjusted for many sociodemographic and behavioral factors and relevant medical history, this study may still be limited by residual confounding. Reverse causation, as described earlier, may also be a potential concern if participants at higher risk for CVD had intentionally adopted a more plant-based dietary pattern. Notably, prevalences of diabetes and hypertension in JHS participants at baseline were about double those in the ARIC study [9], and average BMI among JHS participants was about 3 kg/m2 higher [44]. However, our models adjusted for risk factors for CVD, and the consistency of results in analyses stratified by hypertension and diabetes status adds to the validity of our findings. In addition, the prospective analysis (i.e., dietary assessment preceded outcome ascertainment) and the exclusion of participants with CVD, myocardial infarction, or stroke at baseline minimizes the potential for reverse causation. Additionally, the number of incident CVD cases was relatively small (293 CVD cases out of 3,536 participants) in our study; thus, we may not have detected a statistically significant association due to low power.
This study has a number of important strengths, including a relatively large sample size comprised exclusively of African American adults, long duration of follow-up, and rigorous ascertainment of outcomes. It adds to a growing body of research to understand the association between plant-based dietary patterns and disease risk in populations reflective of the general American public. The black American population is particularly underrepresented in research, yet experiences a disproportionate burden of CVD risk factors and outcomes [45–48]. Moreover, eating patterns have cultural and regional determinants [49–51], and more research is needed to understand the role of these differing dietary patterns, to address health disparities. It is also unclear whether the components of a plant-based dietary pattern differ meaningfully among racial groups or regions, and whether specific patterns within a plant-based diet mediate the potential associations of plant-based diets with health and disease prevention. This study can begin to contextualize such questions.
Conclusion
In summary, our results found no association of an overall, healthy, or unhealthy plant-based dietary pattern with CVD incidence or all-cause mortality in a community-based population of African American individuals in the southern region of the US who consumed a range of both plant-derived and animal-derived foods.
Supporting information
(DOCX)
The histogram shows the distribution of the overall plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the healthy plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the unhealthy plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the overall plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the healthy plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the unhealthy plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors wish to thank the staff and participants of the Jackson Heart Study.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- eGFR
estimated glomerular filtration rate
- FFQ
food frequency questionnaire
- hPDI
healthy plant-based diet index
- HR
hazard ratio
- JHS
Jackson Heart Study
- HRT
hormone replacement therapy
- NDSR
Nutrition Data System for Research
- NHANES
National Health and Nutrition Examination Survey
- PDI
plant-based diet index
- SSB
sugar-sweetened beverage
- uPDI
unhealthy plant-based diet index
Data Availability
The data underlying the results presented in the study are available from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/jhs/).
Funding Statement
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. CMR is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782, R03 DK128386) and grants from the National Heart, Lung, and Blood Institute (R21 HL143089, R56 HL153178). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. doi: 10.1016/S0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 2.Appleby PN, Key TJ. The long-term health of vegetarians and vegans. Proc Nutr Soc. 2016;75(3):287–93. doi: 10.1017/S0029665115004334 [DOI] [PubMed] [Google Scholar]
- 3.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. doi: 10.1080/10408398.2016.1138447 [DOI] [PubMed] [Google Scholar]
- 4.Glenn AJ, Viguiliouk E, Seider M, Boucher BA, Khan TA, Blanco Mejia S, et al. Relation of vegetarian dietary patterns with major cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. Front Nutr. 2019;6:80. doi: 10.3389/fnut.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benatar JR, Stewart RAH. Cardiometabolic risk factors in vegans; a meta-analysis of observational studies. PLoS ONE. 2018;13(12):e0209086. doi: 10.1371/journal.pone.0209086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alles B, Baudry J, Mejean C, Touvier M, Peneau S, Hercberg S, et al. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the NutriNet-Sante Study. Nutrients. 2017;9(9):1023. doi: 10.3390/nu9091023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. doi: 10.1016/j.jacc.2017.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Caulfield LE, Rebholz CM. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr. 2018;148(4):624–31. doi: 10.1093/jn/nxy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8(16):e012865. doi: 10.1161/JAHA.119.012865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser G, Katuli S, Anousheh R, Knutsen S, Herring P, Fan J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015;18(3):537–45. doi: 10.1017/S1368980014000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–4–17. [PubMed] [Google Scholar]
- 12.Wyatt SB, Diekelmann N, Henderson F, Andrew ME, Billingsley G, Felder SH, et al. A community-driven model of research participation: the Jackson Heart Study Participant Recruitment and Retention Study. Ethn Dis. 2003;13(4):438–55. [PubMed] [Google Scholar]
- 13.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethnic Dis. 2005;15(4):S18–29. [PubMed] [Google Scholar]
- 14.Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, et al. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–49–55. [PubMed] [Google Scholar]
- 15.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, et al. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109(7):1184–93. doi: 10.1016/j.jada.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, et al. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr. 2005;8(1):87–96. [PubMed] [Google Scholar]
- 17.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. doi: 10.1371/journal.pmed.1002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Gonzalez MA, Sanchez-Tainta A, Corella D, Salas-Salvado J, Ros E, Aros F, et al. A provegetarian food pattern and reduction in total mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr. 2014;100(Suppl 1):320S–8S. doi: 10.3945/ajcn.113.071431 [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Donoso C, Martinez-Gonzalez MA, Martinez JA, Gea A, Sanz-Serrano J, Perez-Cueto FJA, et al. A provegetarian food pattern emphasizing preference for healthy plant-derived foods reduces the risk of overweight/obesity in the SUN cohort. Nutrients. 2019;11(7):1553. doi: 10.3390/nu11071553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garsetti M, Balentine DA, Zock PL, Blom WA, Wanders AJ. Fat composition of vegetable oil spreads and margarines in the USA in 2013: a national marketplace analysis. Int J Food Sci Nutr. 2016;67(4):372–82. doi: 10.3109/09637486.2016.1161012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med. 2011;12(4):255–7. doi: 10.2459/JCM.0b013e328343e986 [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 25.Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15(4 Suppl 6):S6–62–70. [PubMed] [Google Scholar]
- 26.Smitherman TA, Dubbert PM, Grothe KB, Sung JH, Kendzor DE, Reis JP, et al. Validation of the Jackson Heart Study Physical Activity Survey in African Americans. J Phys Act Health. 2009;6(Suppl 1):S124–32. doi: 10.1123/jpah.6.s1.s124 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 29.Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr. 2013;97(3):597–603. doi: 10.3945/ajcn.112.044073 [DOI] [PubMed] [Google Scholar]
- 30.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr. 2009;89(5):1613S–9S. doi: 10.3945/ajcn.2009.26736L [DOI] [PubMed] [Google Scholar]
- 31.Tong TYN, Appleby PN, Bradbury KE, Perez-Cornago A, Travis RC, Clarke R, et al. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford study. BMJ. 2019;366:l4897. doi: 10.1136/bmj.l4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Rebholz CM, Garcia-Larsen V, Steffen LM, Coresh J, Caulfield LE. Operational differences in plant-based diet indices affect the ability to detect associations with incident hypertension in middle-aged US adults. J Nutr. 2020;150(4):842–50. doi: 10.1093/jn/nxz275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djousse L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, et al. Prevalence and changes over time of ideal cardiovascular health metrics among African-Americans: the Jackson Heart Study. Prev Med. 2015;74:111–6. doi: 10.1016/j.ypmed.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer LC, Redmond N, Slusser JP, Scott CG, Chamberlain AM, Djousse L, et al. Stress and achievement of cardiovascular health metrics: the American Heart Association Life’s Simple 7 in blacks of the Jackson Heart Study. J Amer Heart Assoc. 2018;7(11):e008855. doi: 10.1161/JAHA.118.008855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyson CC, Davenport CA, Lin PH, Scialla JJ, Hall R, Diamantidis CJ, et al. DASH diet and blood pressure among black Americans with and without CKD: the Jackson Heart Study. Am J Hypertens. 2019;32(10):975–82. doi: 10.1093/ajh/hpz090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168(3):308–14. doi: 10.1001/archinternmed.2007.119 [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Andrade FC. Diagnostic status of hypertension on the adherence to the Dietary Approaches to Stop Hypertension (DASH) diet. Prev Med Rep. 2016;4:525–31. doi: 10.1016/j.pmedr.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crews DC, Kuczmarski MF, Miller ER 3rd, Zonderman AB, Evans MK, Powe NR. Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr. 2015;25(2):103–10. doi: 10.1053/j.jrn.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mudryj AN, Yu N, Aukema HM. Nutritional and health benefits of pulses. Appl Physiol Nutr Metab. 2014;39(11):1197–204. doi: 10.1139/apnm-2013-0557 [DOI] [PubMed] [Google Scholar]
- 40.American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158 [DOI] [PubMed] [Google Scholar]
- 41.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–102. doi: 10.3945/ajcn.113.058362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik VS. Sugar sweetened beverages and cardiometabolic health. Curr Opin Cardiol. 2017;32(5):572–9. doi: 10.1097/HCO.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 43.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. doi: 10.1093/aje/154.12.1089 [DOI] [PubMed] [Google Scholar]
- 44.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Folsom AR, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2(1):18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown AF, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168(8):541–9. doi: 10.7326/M17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6(9):e006027. doi: 10.1161/JAHA.117.006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnett E, Halverson J. Disparities in premature coronary heart disease mortality by region and urbanicity among black and white adults ages 35–64, 1985–1995. Public Health Rep. 2000;115(1):52–64. [PMC free article] [PubMed] [Google Scholar]
- 48.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329(9):600–6. doi: 10.1056/NEJM199308263290902 [DOI] [PubMed] [Google Scholar]
- 49.Champagne CM, Bogle ML, McGee BB, Yadrick K, Allen HR, Kramer TR, et al. Dietary intake in the lower Mississippi delta region: results from the Foods of our Delta Study. J Am Diet Assoc. 2004;104(2):199–207. doi: 10.1016/j.jada.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 50.Block G, Rosenberger WF, Patterson BH. Calories, fat and cholesterol: intake patterns in the US population by race, sex and age. Am J Public Health. 1988;78(9):1150–5. doi: 10.2105/ajph.78.9.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr. 2014;1:29. doi: 10.3389/fnut.2014.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The histogram shows the distribution of the overall plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the healthy plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the unhealthy plant-based diet score. The solid line represents hazard ratios for incident CVD, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the overall plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the healthy plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
The histogram shows the distribution of the unhealthy plant-based diet score. The solid line represents hazard ratios for all-cause mortality, adjusting for age, sex, total energy intake, educational attainment, smoking status, physical activity, alcohol intake, margarine intake, diabetes, hypertension, total cholesterol, estimated glomerular filtration rate, body mass index, hormone replacement therapy medication use, and statin medication use. The dashed lines represent 95% confidence intervals.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/jhs/).