To the editor:
In the setting of the rapid spread of the pandemic by the new coronavirus disease 2019 (COVID-19) and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the kidney was recognized as a frequent target.1 With the beginning of vaccination for COVID-19, several cases of postvaccination immunologic manifestations have been described.2 We report herein the first 2 cases of patients who developed biopsy-proven collapsing glomerulopathy (CG) after SARS-CoV-2 adenovirus-vector (AdV)–based vaccination.
Case 1
A 63-year-old afro-descendant woman presented to the hospital complaining of edema of the ankle and foamy urine for 3 months, which began 9 days after receiving the first dose of the Oxford/AstraZeneca (ChAdOx1-S [recombinant] vaccine). She had a previous diagnosis of hypertension, heart failure, and dyslipidemia and reported that 1 month after the onset of symptoms she presented to the emergency department because of dyspnea. On that occasion, the patient underwent a chest X-ray that evidenced a pleural effusion and COVID-19 serology that was negative for IgM and IgG. She was diagnosed with acutely decompensated heart failure, and she was treated with diuretics. Until the current hospital admission, she returned to the emergency department another 2 times, maintaining negative serologies for COVID-19 and pleural effusion. On physical examination at admission, she presented edema (+2/+4) in the lower limbs and a blood pressure of 150/98 mm Hg.
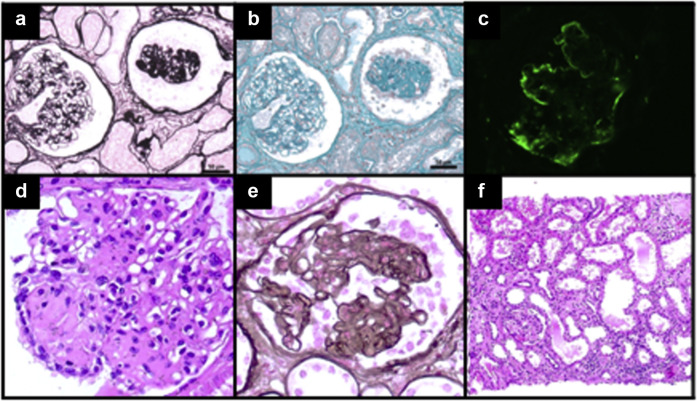
Laboratory tests revealed the following: hemoglobin, 11.8 g/dl; leukocytes, 6.930/mm3; platelets, 240,000/mm3; urea, 19.8 mg/dl; creatinine, 0.88 mg/dl; estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), 70 ml/min per 1.73 m2; urinalysis, proteins (+3) and red cells (+2); and proteinuria, 4131 mg/d; and there was no evidence of electrolyte or acid–base abnormalities. In addition, the following were found: albumin, 2.7 g/dl; total cholesterol, 405 mg/dl; low-density lipoprotein, 294 mg/dl; high-density lipoprotein, 75 mg/dl; and triglycerides, 216 mg/dl. Serologic tests for Chagas, schistosomiasis, HIV, hepatitis B and C, and Venereal Disease Research Laboratory (VDRL) test were negative. Serology for COVID-19 was as follows: IgM, 0.61 arbitrary units (AU)/ml/IgG, 0.14 AU/ml (normal range: cutoff index < 1.0AU/ml). Kidney biopsy revealed at light microscopy glomeruli with synechiae and segmental sclerosis lesions, with some of them showing the collapse of capillary loops in addition to podocyte hyperplasia and hypertrophy (Figure 1 a and b). The tubulointerstitial compartment revealed mild tubular degeneration. Immunofluorescence microscopy highlighted deposits of IgM +2/+3 and C3 +2/+3 (Figure 1c), with a segmental and focal pattern upon the sclerotic sites. Histologic findings were compatible with CG. We performed genotyping for APOL1, which showed the G1/G0 genotype. The patient has started on treatment with oral prednisone, 1 mg/kg/d, and returned to outpatient clinic after 2 months. She has no edema nor dyspnea, and her laboratory tests revealed complete remission of nephrotic syndrome (albumin, 4 g/dl; 24-hour proteinuria, 265 mg; creatinine, 0.88 mg/dl; total cholesterol, 220 mg/dl; low-density lipoprotein, 106 mg/dl; high-density lipoprotein, 71 mg/dl; and triglycerides, 215 mg/dl). Because of the renal injury attributed to the vaccine, the patient decided not to take the second dose of the Oxford-AstraZeneca COVID-19 vaccine, even in the scenario of a negative IgG COVID-19 serology.
Figure 1.
Case 1: (a,b) Kidney biopsy findings depicted glomerulus (right) with global retractions of the capillary tuft and hyperplastic and hypertrophic podocytes surrounding, whereas the glomerulus on the left shows normal aspect (Masson trichrome [a] andperiodicacidsilvermethaminestain[PAMS][b], original magnification ×200). Immunofluorescence microscopy shows global C3 deposits in collapsed glomerulus (original magnification ×400). Case 2: Kidney biopsy revealed at light microscopy (d,e) segmental sclerosis and tuft collapse with overlying podocyte hyperplasia and hypertrophy ([c] hematoxylin-eosin [HE] and [d] PAMS, original magnification ×400), as well as (f) moderate interstitial fibrosis and tubular atrophy, with mild associated inflammation (HE, original magnification ×100). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Case 2
A 58-year-old woman was hospitalized 3 weeks after receiving her first dose of Oxford-AstraZeneca COVID-19 vaccine because of pulmonary congestion, massive edema, and acute-on-chronic kidney disease (CKD). Her medical history included a multiple myeloma, and CKD due to cast nephropathy. In the last 1 year, her serum creatinine and 24-hour proteinuria ranged from 2 to 3 mg/dl and from 0.7 to 1.1 g/d, respectively. In this current hospital stay, an increase in her daily urinary protein excretion to 3.8 g was detected, and she progressed to dialysis. Her urine sediment was bland, and reduced-size kidneys were found on ultrasound. An immunophenotyping of bone marrow excluded a multiple myeloma relapse, and further analyses showed that rheumatoid factor and anti-nuclear antibody were negative, as well as serology tests for hepatitis B and C, HIV, and VDRL. A kidney biopsy revealed 5 of 9 glomeruli were globally sclerotic. The remaining glomeruli presented hypertrophy and hyperplasia of podocyte cells with segmental collapse of the glomerular tuft, and a diagnosis of CG was determined (Figure 1d–f). Parvovirus B19 and COVID-19 reverse transcription–polymerase chain reaction were negative. Interestingly, we found a strong positive IgG serology against SARS-CoV-2. APOL1 genotyping identified high-risk alleles (G2/G2). She remained hemodialysis-dependent and did not receive any specific treatment for her kidney disease, being discharged 1 month later.
CG can be associated with a wide variety of inflammatory conditions (such as systemic lupus erythematosus), glomerular ischemic insult (thrombotic microangiopathy and sickle cell disease), drugs (anthracycline, pamidronate, and interferon [IFN]), and infections (HIV, hepatitis B and C, parvovirus B19, cytomegalovirus, and Epstein-Barr virus).3 , 4
We presented herein the first 2 cases of CG following a SARS-CoV-2 AdV-based vaccine. In the first case, the patient presented only 1 risk allele for APOL1, which, based on current medical literature, is not associated with increased risk of renal disease. In the second case, the patient presented high-risk genotype (HRG)-APOL1, a genetic condition that has previously been associated with an increased risk of CG and CKD. Interestingly, those disorders may manifest following exposure to environmental triggers.S1,S2
Previous medical literature linked vaccines and glomerular lesions, especially influenza vaccines with podocytopathies. Moreover, there are reports of IgA nephropathy flares after receipt of a recombinant zoster vaccine, and flares of minimal change disease after pneumococcal, smallpox, hepatitis B, and tetanus-diphtheria-pertussis vaccines.S3 Although a coincidental occurrence cannot be excluded, the close temporal association between immunization and flares suggests a potential relationship.S4,S5
Vaccines that use mRNA technology and lipid nanoparticle delivery systems or that contain DNA delivered within nonreplicating recombinant AdV systems are being used in large scale to prevent SARS-CoV-2 infections. Both the mRNA and AdV vaccines generate potent neutralizing antibody titers and virus-specific T-cell responses, as measured in blood 2 to 4 weeks after inoculation.S6 In the trials that analyzed the efficacy and safety of the COVID-19 vaccines, renal events are rare. In the phase 1/2 trial of ChAdOx1 nCoV-19/AZD1222 (AstraZeneca)S7 vaccine, there is the report of 1 case of polyuria and 1 case of pollakiuria. An interim analysis of 4 randomized controlled trials using the ChAdOx1 nCoV-19/AZD1222 (AstraZeneca) vaccine in Brazil, South Africa, and the United KingdomS8 detected 1 case of acute kidney injury, 1 case of urinary calculus, and 2 cases of renal colic. The trial of BNT162b2 (Pfizer–BioNTech)S9 revealed a cumulative incidence of acute kidney injury of 0.005%, mRNA-1273 (Moderna)S10 1 of 15,185 cases, and no description of renal events in the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) trial.S11
The proposed susceptibility and triggering mechanisms support a double-hit mechanism for the genesis of CG.S1 The podocyte injury in genetically susceptible individuals with CG may be cytokine-mediated. There is growing evidence linking CG to conditions that increased IFN levels (such as infections and exogenous IFN therapy) in both patients with and without HRG-APOL1.S12
There are several reports of glomerulopathies arising within 3 weeks of SARS-CoV-2 immunization, with most cases arising within the first week,S5 but this is the description of 2 first cases of CG associated with SARS-CoV-2 immunization that symptoms had started 1 to 2 weeks after vaccination. Most reports have been associated with mRNA vaccines, but at least 3 cases were related to SARS-CoV-2 adenovirus-vector based vaccine (SVBV).S4,S5
From the immunologic viewpoint, the development of glomerular diseases shortly after SARS-CoV-2 immunization implies T cells as effectors.S4 Following injection, SVBV targets innate immune cells, like dendritic cells and macrophages, and engages multiple pattern-recognition receptors that bind double-stranded DNA to induce IFN secretion, which can trigger podocytopathies in the susceptible patient or amplify quiescent glomerular diseases.S6 Another potential mechanism for glomerular injury is a molecular mimicry between the SARS-CoV-2 spike protein and self-antigens on the podocytes.S4 Because IFN pathways have an important role in the pathogenesis of CG, notably in patients homozygous for APOL1 high-risk variants, their stimulation by SARS-CoV-2 immunization could be a potential second-hit triggering CG development, especially among genetic susceptible patients.
Finally, we believe that risks of glomerular injury development should not prevent the use of SARS-CoV-2 vaccines. SARS-CoV-2 immunization must not be avoided based on the fear of triggering or worsening a glomerulopathy.
Disclosure
All the authors declared no competing interests.
Ethical Aspects
This case report was approved by the local ethical committee of the Faculty of Medicine of University of São Paulo under the number CAAE 17279219.8.0000.0068. Written informed consent was obtained from the patients.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.de Oliveira P., Cunha K., Neves P., et al. Renal morphology in coronavirus disease: a literature review. Medicina (Kaunas) 2021;57:258. doi: 10.3390/medicina57030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gueguen L., Loheac C., Saidani N., Khatchatourian L. Membranous nephropathy following anti–COVID-19 mRNA vaccination. Kidney Int. 2021;100:1140–1141. doi: 10.1016/j.kint.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales E., Alonso M., Gutiérrez E. Collapsing glomerulopathy: update. Med Clin (Barc) 2019;152:361–367. doi: 10.1016/j.medcli.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Neves P.D., Bridi R.A., Ramalho J.A., et al. Schistosoma mansoni infection as a trigger to collapsing glomerulopathy in a patient with high-risk APOL1 genotype. PloS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.