Abstract
Background
Staphylococcus aureus infections are common throughout the lifespan, with recurrent infections occurring in nearly half of infected children. There is no licensed vaccine, underscoring the need to better understand how S. aureus evades protective immunity. Despite much study, the relative contributions of antibodies and T cells to protection against S. aureus infections in humans are not fully understood.
Methods
We prospectively quantified S. aureus-specific antibody levels by ELISA and T-cell responses by ELISpot in S. aureus-infected and healthy children.
Results
S. aureus-specific antibody levels and T-cell responses increased with age in healthy children, suggesting a coordinated development of anti-staphylococcal immunity. Antibody levels against leukotoxin E (LukE) and Panton-Valentine leukocidin (LukS-PV), but not α-hemolysin (Hla), were higher in younger infected children, compared with healthy children; these differences disappeared in older children. We observed a striking impairment of global and S. aureus-specific T-cell function in children with invasive and noninvasive infection, suggesting that S. aureus-specific immune responses are dysregulated during childhood infection regardless of the infection phenotype.
Conclusions
These findings identify a potential mechanism by which S. aureus infection actively evades adaptive immune responses, thereby preventing the development of protective immunity and maintaining susceptibility to recurrent infection.
Keywords: Staphylococcus aureus, MRSA, immunity, T cells, antibody
S. aureus infections are common and frequently recur. In this study, severely impaired T-cell responses were observed during childhood infection. This may be a mechanism by which S. aureus evades protective immunity, thereby maintaining susceptibility to recurrent infection.
Staphylococcus aureus frequently causes infections in community and health care settings in children and adults, ranging from mild, such as skin and soft tissue infections (SSTI), to severe, including osteoarticular infections, pneumonia, and bacteremia [1]. The emergence of virulent methicillin-resistant S. aureus (MRSA) USA300 isolates heralded the onset of an epidemic in the United States [2]. S. aureus infections are a leading cause of hospitalization during childhood [3]. Recurrent infections occur in nearly half of S. aureus-infected children within a year [4]. These observations have prompted an emerging paradigm that S. aureus infections do not reliably elicit protective immunity, highlighting the need for a vaccine [4–6].
Unfortunately, there is no licensed vaccine to prevent S. aureus infections, underscoring the importance of understanding the mechanisms of immune-mediated evasion and protection [6–9]. These efforts are complicated by a nascent understanding of how immunity against S. aureus develops during childhood. Most studies in children have focused on antibody responses against specific staphylococcal antigens, most notably toxins such as leukotoxin ED (LukED), Panton-Valentine leukocidin (PVL, LukSF-PV), and α-hemolysin (α-toxin, Hla), revealing that antibody levels are variable and increase with age in healthy children [10, 11]. Less is understood about T-cell responses to S. aureus and whether they contribute to protection. Rodent models of S. aureus infection demonstrate the importance of antibodies in protection, especially those directed at Hla, while the importance of T cells is more nuanced [12–16]. In contrast, defects in T cell-mediated immunity, but not antibodies, result in human susceptibility to recurrent S. aureus infections [17, 18]. These observations mandate the prioritization of better understanding how T-cell responses develop. Although there have been recent reports of S. aureus-specific T lymphocyte responses in healthy adults and those with S. aureus infection [16, 19, 20], these have not been applied to the pediatric population.
The goals of this study were to quantify T-cell responses in healthy and S. aureus-infected children and to document their relationship to antibody levels and the severity of infection. We observed an age-dependent development of antibodies against LukE and LukS-PV in healthy children, while these antibody levels were already high in the youngest infected children. Despite high antibody levels, effector T-cell responses were markedly impaired in S. aureus-infected children, regardless of whether the infection was superficial or invasive. Together, these findings suggest that S. aureus infection suppresses T-cell responses, potentially precluding the development of protective immunity.
METHODS
Study Design
This single-site prospective observational study was approved by the institutional review board at Nationwide Children’s Hospital. Hospitalized children aged 6 months to 21 years old were identified by positive S. aureus cultures or by clinical criteria with subsequent culture confirmation. Healthy children were enrolled in an outpatient setting. Children who had an infection that was caused by a bacterium other than S. aureus were also included. Exclusion criteria included documented immunodeficiency, receipt of immunosuppressive medications within 2 months, or receipt of an immunoglobulin-based product within 6 months. Following informed consent and assent when applicable, clinical information was entered into a REDCap database. Blood samples were collected within 3 days of culture collection. Blood was separated into mononuclear cell preparation tubes (CPT) and serum separation (SST) tubes (Becton Dickinson). Peripheral blood mononuclear cells (PBMCs) and serum were isolated; PBMCs were frozen in liquid nitrogen and serum at −80°C.
Antibody Quantification
Antibody quantification was modified from our previous study [21]. First, a standardized serum stock was prepared by pooling serum from healthy adults with high levels of S. aureus-specific antibodies. For quantification of antibody levels by enzyme-linked immunosorbent assay (ELISA), 96-well plates (Costar, Corning) were coated with 4 μg/mL of purified Hla, LukE, or LukS-PV. Serial dilutions of the standard were included on each plate to minimize plate-to-plate variability. Serial dilutions of subject sera were added in duplicate. Quantification of immunoglobulin G (IgG) was performed using alkaline phosphatase (AP)-conjugated goat anti-human IgG (1:5000; Kirkegaard and Perry Labs) and AP substrate p-nitrophenyl phosphate (Sigma-Aldrich). Absorbance (OD405) was measured using a GENios spectrophotometer (Tecan). Based on the standards, IgG levels were quantified as arbitrary ELISA units. Results were discarded and repeated if (1) the r2 of the standard curve was <0.995, (2) the coefficient of variance between duplicates was > 20%, (3) the OD405 was <0.5 or >2.5 or outside of the standard range, or (4) blank wells had an OD405 >0.10.
Quantification of Immune Cells
PBMCs were thawed and an aliquot separated for cell counting. Following filtering and washing with phosphate-buffered saline, cells were stained with Live/Dead stain, washed with fluorescence-activated cell sorting (FACS) buffer, and blocked with Human FcR Block. Surface staining was performed with anti-CD3 (UCHT1), anti-CD4 (L200), anti-CD8 (SK1), anti-CD19 (HIB19), anti-CD45 (HI30), anti-CD16 (3G8), anti HLA-DR (L243), anti-CD56 (NCAM1), anti-CD11c (Bu15), and anti-CD14 (M5E2) in Brilliant stain buffer at 4°C for 30 minutes. Except for antibodies for live/dead staining, CD56, and CD4 (Becton Dickinson), all antibodies were purchased from BioLegend. Cells were washed with FACS buffer and fixed in 4% paraformaldehyde overnight at 4°C. The following day, cells were washed and reconstituted with FACS buffer and counting beads added. Flow cytometry was performed on a BD LSRFortessa flow cytometer; data were analyzed using Flowjo.
Quantification of Effector T-Cell Responses
Human interleukin 17 (IL-17) and interferon- γ (IFN-γ) T-cell ELISpot kits (U-Cytech biosciences) were used to quantify T-cell responses. Plates (96-well) were coated with anti-IL-17 or anti-IFN-γ antibody. PBMCs were thawed and added to each well (0.5–4 × 105 cells/well, depending on the conditions). PBMCs were then incubated with phorbal myristate acetate (PMA) and ionomycin, heat-killed S. aureus (5 × 105 colony-forming units/well), or purified antigen (Hla, LukE, or LukS-PV, 20 μg/mL) for 40 hours at 37°C. Following washing, biotin-labeled detection antibodies were added to the wells, followed by avidin-horseradish peroxidase (eBioscience). Spots were counted following addition of the 3-Amino-9-ethylcarbazole (AEC) substrate solution (BD Biosciences) using an Immunospot series 1 analyzer (Cellular Technology).
Statistical Analysis
Differences between cohorts were evaluated with χ 2 or Fisher exact tests, Mann-Whitney tests, or 1-way ANOVA with the Kruskal-Willis post test for multiple comparisons. Multivariable linear regression was used to evaluate differences between groups while accounting for age; in cases where the association between variables differed by age group, we stratified results. To determine appropriate age groupings, the relationship between age and antibody levels was evaluated graphically with a loess curve and statistically with polynomial regression and restricted cubic spline regression. All linear regression analyses were based on log10-transformed antibody levels. The Mann-Whitney test was used to compare immune responses between groups. Differences were considered significant if P < .05. All analyses were performed using R for Statistical Computing or GraphPad Prism.
RESULTS
Study Subjects
Seventy-six hospitalized children with S. aureus infection and 79 healthy children were enrolled between September 2018 and February 2020 at Nationwide Children’s Hospital (Table 1). Infections were classified as invasive (40%) or noninvasive (60%); the most common sites of infection were SSTI (73% of noninvasive infections) and bacteremia (87% of invasive infections). S. aureus-infected children were older compared with healthy children, but there were no differences in sex or race/ethnicity between the groups (Table 1).
Table 1.
Characteristics of the Study Subjects
| Characteristic | Healthy Control | Staphylococcus aureus Infected | P Valuea | P Valueb | ||
|---|---|---|---|---|---|---|
| All | Invasive | Noninvasive | ||||
| Subjects, No. | 79 | 76 | 31 | 45 | ||
| Age, y, median (IQR) | 4.8 (2.4–7.7) | 8.4 (5.5–12.7) | 9.5 (6.4–11.7) | 7.6 (3.2–13.7) | <.001 | .453 |
| Age group, y | <.001 | .186 | ||||
| <5 | 42 (53.2) | 18 (23.7) | 4 (12.9) | 13 (28.9) | ||
| 5–10 | 28 (35.4) | 30 (39.5) | 14 (45.2) | 16 (35.6) | ||
| >10 | 9 (11.4) | 28 (36.8) | 13 (41.9) | 16 (35.6) | ||
| Sex | 1.000 | .985 | ||||
| Male | 45 (56.8) | 43 (56.6) | 17 (54.8) | 26 (57.8) | ||
| Female | 34 (43.2) | 33 (43.4) | 14 (45.2) | 19 (42.2) | ||
| Race/ethnicity | .333 | .672 | ||||
| White | 63 (79.7) | 61 (80.3) | 25 (80.6) | 36 (80.0) | ||
| Black | 5 (6.3) | 8 (10.5) | 3 (9.7) | 5 (11.1) | ||
| Hispanic | 4 (5.1) | 5 (6.6) | 3 (9.7) | 2 (4.44) | ||
| Other | 7 (8.9) | 2 (2.6) | 0 (0.0) | 2 (4.44) | ||
| Complex condition | NA | 21 (28.0) | 11 (35.5) | 10 (22.7) | NA | .342 |
| Prior S. aureus infection | NA | 15 (20.0) | 3 (9.7) | 12 (27.3) | NA | .113 |
| Susceptibility | NA | .261 | ||||
| MRSA | NA | 39 (51.3) | 13 (41.9) | 26 (57.8) | ||
| MSSA | NA | 37 (48.7) | 18 (58.1) | 19 (42.2) | ||
| PVL status | ||||||
| Negative | NA | 23 (30.3) | 15 (48.4) | 8 (17.8) | NA | .013 |
| Positive | NA | 43 (56.6) | 12 (38.7) | 31 (68.9) | ||
| Unknown | NA | 10 (13.2) | 4 (12.9) | 6 (13.3) | ||
| Fever | NA | 44 (60.3) | 25 (83.3) | 19 (44.2) | NA | .002 |
| Site of infectionc | NA | <.001 | ||||
| SSTI | NA | 40 (52.6) | 7 (22.6) | 33 (73.3) | ||
| Blood | NA | 27 (35.5) | 27 (87.0) | 0 (0.0) | ||
| Wound | NA | 15 (19.7) | 0 (0.0) | 15 (33.3) | ||
| Bone/joint | NA | 5 (6.6) | 5 (16.1) | 0 (0.0) | ||
| Respiratory | NA | 3 (3.9) | 3 (9.7) | 0 (0.0) | ||
| Other | NA | 3 (3.9) | 1 (3.2) | 2 (4.4) |
Data are No. (%) except where indicated.
Abbreviations: IQR, interquartile range; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; NA, not applicable; PVL, Panton Valentine leucocidin; SSTI, skin and soft tissue infection.
a P values comparing the differences between healthy controls and S. aureus-infected children.
b P values comparing the differences between S. aureus invasive and noninvasive infection.
cChildren could have more than 1 site of infection.
Antibody Levels in Healthy and S. aureus-Infected Children
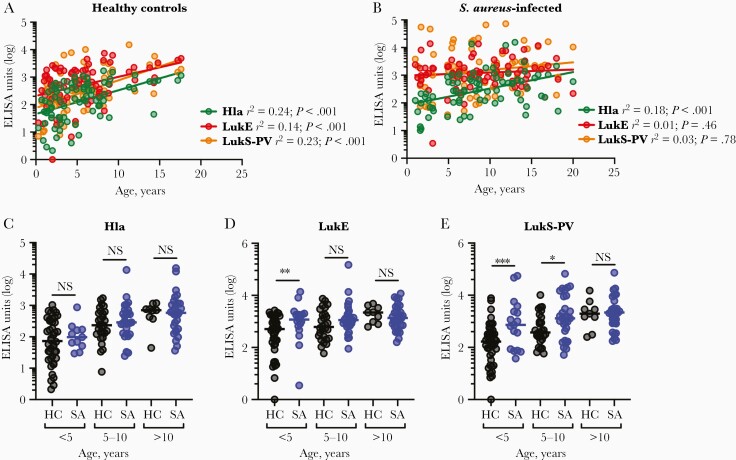
IgG levels against Hla, LukE, and LukS-PV were quantified by ELISA. There was a linear association, albeit weak, between age and the log10-transformed antibody levels against each of the antigens in healthy children, demonstrating an age-dependent increase in antibody levels (Figure 1A). In contrast, only anti-Hla IgG levels correlated with increased age in S. aureus-infected children; there was no association between age and anti-LukE or anti-LukS-PV antibody levels (Figure 1B). This raised the possibility that antibodies against LukE and LukS-PV, but not Hla, increased during infection of younger children.
Figure 1.
Antibody levels in healthy and Staphylococcus aureus-infected children. Antibody levels against Hla, LukE, and LukS-PV were quantified by ELISA in 79 healthy children and 76 children with S. aureus infection. A, Antibody levels against Hla, LukE, and LukS-PV were positively associated with age in healthy children. B, Antibody levels against Hla, but not LukE or LukS-PV, were positively associated with age in children with S. aureus infections. C–E, Antibody levels against Hla, LukE, and LukS-PV in healthy children and children with S. aureus infection aged <5, 5–10, or >10 years. Antibody levels are presented as ELISA units and were log10-transformed for analysis. Correlations were determined by linear regression analysis and antibody levels were compared using the Mann-Whitney test, with lines indicating the median values. *P < .05, ** P < .01, *** P < .001. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HC, healthy children; Hla, α-hemolysin; LukE, leukotoxin E; LukS-PV, Panton-Valentine leucocidin; NS, indicates not significant; SA, S. aureus infection.
To account for differences in age between healthy and infected children, the cohorts were divided into 3 age groups for further analysis (<5, 5–10, >10 years). There were no differences in anti-Hla antibody levels in any group between healthy and infected children (Figure 1C), consistent with Hla-specific IgG increasing with age and being unaffected by infection. In contrast, there were higher levels of anti-LukE (Figure 1D) and anti-LukS-PV (Figure 1E) IgG in younger infected children compared with healthy children, although these differences did not persist in the older children in whom antibody levels were already high. Surprisingly, there were no major differences in antibody levels between children with invasive or noninvasive infection (Supplementary Figure 1A–1C). Antibody levels against LukS-PV, but not Hla or LukE, were higher in children who were infected with PVL-expressing isolates, consistent with the fact that not all S. aureus isolates express PVL (Supplementary Figure 1D–1F). Together, these findings suggest that infection elicited higher levels of LukE- and LukS-PV-specific antibodies only in younger children, but anti-Hla antibodies were not elicited in children of any age. Because antibodies specific for Hla, but not LukE or LukS-PV, are associated with protection [4, 22], this observation underscores the discordance between immunogenicity and protective immunity elicited by S. aureus infection.
Impaired Effector T-Cell Responses in Children With S. aureus Infection
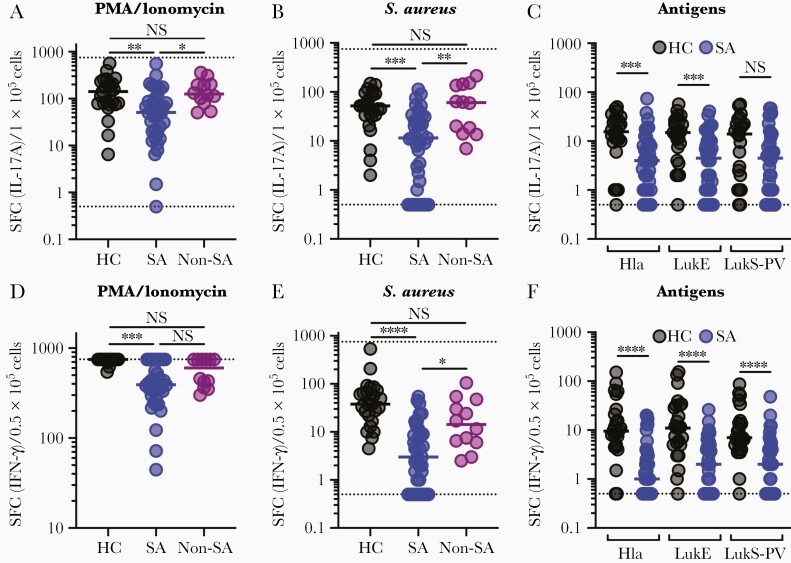
We next quantified functional T-cell responses in an age-matched cohort of 25 healthy children and 39 children with acute S. aureus infection (20 with an invasive infection, 19 with a noninvasive infection) (Supplementary Table 1). First, PBMCs were incubated with PMA and ionomycin to quantify the capacity for T cells to respond to a potent nonspecific stimulus. Following PMA/ionomycin incubation, there were significantly reduced (median 2 to 3-fold) IL-17A and IFN-γ responses among S. aureus-infected children compared with healthy children (Figure 2A and 2D). These findings are consistent with strongly impaired global T-cell function in children with S. aureus infection. To assess whether this impairment was specific to children with S. aureus infection, 12 children with an infection caused by another bacterial pathogen were enrolled (Supplementary Table 1). In contrast to S. aureus-infected children, children with other infections did not have impaired IL-17A or IFN-γ responses compared with healthy children (Figure 2A and 2D). Surprisingly, impairment of T-cell function was independent of the infection phenotype; even children with localized infections had strongly impaired responses (Supplementary Figure 2A and 2D).
Figure 2.
Effector T-cell responses in healthy and Staphylococcus aureus-infected children. Functional effector T-cell responses were quantified by IL-17A (A–C) or IFN-γ (D–F) ELISpot in thawed PBMCs from 25 healthy children, 39 children with S. aureus infection, and 12 children with an infection not caused by S. aureus. Following culture with PMA and ionomycin, there were impaired IL-17A (A) and IFN-γ (D) responses in children with S. aureus infection compared with healthy controls or children with other infections. Following culture with heat-killed S. aureus, there were markedly impaired IL-17A (B) and IFN-γ (E) responses in infected children compared with healthy children or children with other infections. Following culture with purified Hla, LukE, or LukS-PV, there were markedly impaired IL-17A (C) and IFN-γ (F) responses in infected children. Individual data points are plotted with medians overlaid and distributions were compared using 1-way ANOVA with the Kruskal-Willis post test for multiple comparisons (A, B, D, E) or the Mann-Whitney test (C, F). ** P < .01, *** P < .001, **** P < .0001. The dashed lines indicate the upper (750) and lower (0.5) detectable limits of the assay. Abbreviations: ANOVA, analysis of variance; HC, healthy children; Hla, α-hemolysin; IFN-γ, interferon-γ; IL-17A, interleukin 17A; LukE, leukotoxin E; LukS-PV, Panton-Valentine leucocidin; Non-SA, infection not caused by S. aureus; NS, indicates not significant; PBMCs, peripheral blood mononuclear cells; PMA, phorbal myristate acetate; SA, S. aureus infection; SFC, spot-forming colonies.
Next, we compared S. aureus-specific T-cell responses following incubation with heat-killed S. aureus. Strikingly, S. aureus-specific IL-17A responses in infected children were dramatically lower (median 5-fold) compared with healthy children, with a fourth of infected children having responses below the limit of detection (Figure 2B). S. aureus-specific IFN-γ responses were even more strongly impaired in infected children (median 12-fold); a third of infected children had responses that were below the detection limit (Figure 2E). Similar to nonspecific responses, there were no differences in IL-17A and IFN-γ responses in children with invasive or noninvasive infection (Supplementary Figure 2B and 2E), underscoring the potency of even a mild S. aureus infection in impairing S. aureus-specific T-cell function. Again, the impairment of S. aureus-specific T-cell responses was limited to children with S. aureus infection (Figure 2B and 2E). These findings were recapitulated following stimulation with S. aureus toxins, as infected children had significantly decreased Hla- and LukE-specific IL-17A responses (Figure 2C). Similarly, there were markedly reduced IFN-γ responses against all 3 toxins in infected children (Figure 2F). Again, there were no significant differences in antigen-specific IL-17A or IFN-γ responses between children with an invasive or noninvasive infection (Supplementary Figure 2C and 2F). Therefore, as we observed with global T-cell function, S. aureus-specific T-cell responses are strongly impaired during infection, regardless of the infection phenotype.
To test the possibility that T-cell impairment might be due to fewer T cells and/or antigen-presenting cells (APC) in children with S. aureus infection, we quantified immune cell populations in thawed PBMCs by flow cytometry (Supplementary Figure 3A). Although there was a lower percentage of CD3+ T cells in infected children (Supplementary Figure 3B), the differences were modest, and there was a higher percentage of CD4+ T cells in infected children (Supplementary Figure 3C). This decrease in T cells was accompanied by a higher percentage of B cells in infected children (Supplementary Figure 3B), consistent with the higher antibody levels we observed. There were also more monocytes, but fewer dendritic cells (DCs) and natural killer cells in infected children compared with healthy children (Supplementary Figure 3D). These modest differences in lymphocyte and APC populations seem unlikely to explain the severely impaired T-cell responses we observed.
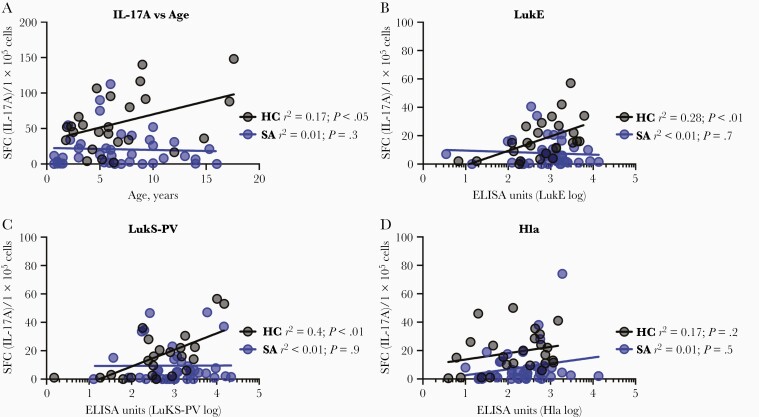
We next tested whether the reduced S. aureus-specific T-cell responses in infected children were dependent on age or antibody levels. As we observed with antibody levels, S. aureus-specific IL-17A responses correlated with age in healthy children, but IL-17A responses were low even in older infected children (Figure 3A). IFN-γ responses were not correlated with age in healthy children and only weakly associated with age in infected children (Supplementary Figure 4A). Similarly, LukE- and LukS-PV–specific IL-17A responses and antibody levels were correlated in healthy, but not infected, children (Figure 3B and 3C). Surprisingly, Hla-specific antibody and IL-17A responses were not correlated for either group (Figure 3D), suggesting a disconnect between these arms of adaptive immunity for Hla-specific responses. While there was a weak association between LukS-PV–specific antibody and IFN-γ responses in healthy children (Supplementary Figure 4C), there were no associations between LukE- and Hla-specific antibody levels and IFN-γ responses in either group (Supplementary Figure 4B and 4D). Together, these findings suggest that antigen-specific antibody and T-cell–mediated immunity develops throughout childhood, but these responses are dysregulated in children with S. aureus infection.
Figure 3.
Correlation of T-cell responses with age and antibody levels. A, Staphylococcus aureus-specific (following incubation with heat-killed S. aureus) IL-17A responses were modestly correlated with age in healthy children but not children with S. aureus infection. B, LukE-specific IL-17A responses were positively associated with log10-transformed LukE-specific antibody levels in healthy children but not children with S. aureus infection. C, LukS-PV–specific IL-17A responses were positively associated with log10-transformed LukS-PV–specific antibody levels in healthy children but not children with S. aureus infection. D, There was no association between Hla-specific IL-17A responses and log10-transformed Hla-specific antibody levels in either group. Associations were determined using linear regression analysis. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HC, healthy children; Hla, α-hemolysin; IL-17A, interleukin 17A; LukE, leukotoxin E; LukS-PV, Panton-Valentine leucocidin; NS, indicates not significant; SA, S. aureus infection.
Discussion
The immune response during S. aureus infection in childhood remains poorly understood. Herein, we found higher antibody levels against the S. aureus toxins LukE and LukS-PV, but not Hla, during infection, compared with healthy children. Despite higher antibody levels, global and S. aureus-specific IL-17A and IFN-γ responses were markedly impaired in infected children, regardless of the severity of infection. These findings suggest a coordinated development of S. aureus-specific antibody and T-cell responses in healthy children, but this is uncoupled in children who have active infection. Our findings additionally suggest a failure to elicit Hla-specific antibodies in children with S. aureus infection. Taken together, our study identifies 2 potential mechanisms, both at the level of T-cell responses and toxin-specific antibody levels, by which S. aureus infection might result in active evasion of the adaptive immune response, providing a potential explanation for sustained susceptibility to recurrent infection.
Emerging clinical evidence and data from mouse models demonstrates roles for both the Th17/IL-17 and Th1/IFN-γ pathways in defense against S. aureus. Th17/IL-17-mediated defense is critical for protection in models of S. aureus bacteremia, SSTI, and pneumonia [14, 15, 23–26], with γδ T cells a major source of IL-17 [15, 27]. Th1/IFN-γ responses protect in some mouse models of S. aureus infection [16, 28], but inhibit protection in others [14, 29]. Human studies are inconclusive; for example, Th17-polarized responses in adults with S. aureus sepsis were associated with higher mortality [30], while adults with S. aureus bacteremia have strongly induced Th1 responses [16] that may be associated with better survival [30]. Together, these studies demonstrate the need to translate findings on the role of T cells in controlling S. aureus infection from preclinical studies to the human population.
The most striking finding of this study is the severely impaired effector T-cell IL-17A and IFN-γ responses in children with active S. aureus infection, independent of the site or extent of infection. Global impairment of T-cell responses was observed, but S. aureus-specific responses were even more profoundly impaired. The impaired T-cell IL-17A and IFN-γ production may reflect a preinfection T-cell phenotype that explains predisposition of individuals to infection. However, nearly 80% of the children in our cohort presented with first-time infection, and infected children had higher levels of antibodies against LukE and LukS-PV and increased percentages of B cells compared with healthy children. Because T cells promote B-cell–mediated antibody responses, these observations argue against baseline immune deficits in these patients. Because we did not quantify lymphocyte subsets prior to cryopreservation of PBMCs, we also cannot exclude the possibility that infected children presented with lymphopenia. An alternative hypothesis is that infection actively suppresses T-cell responses. These findings are consistent with those of Ardura et al, who used a transcriptomic approach to identify decreased T-cell responses in children with active S. aureus infection [31]. Future studies that quantify these responses in children during and following recovery from infection should resolve this question. The suppression of adaptive immune responses may be explained by direct toxicity of staphylococcal toxins to T lymphocytes and DCs, a critical APC population that drives T-cell responses. For example, Hla impairs DC and T-cell responses during murine S. aureus infection [32], and Hla induces apoptosis of T lymphocytes [33]. Similarly, LukED triggers cellular toxicity on T cells and DCs that express its cellular receptor, CCR5 [34]. LukAB also directly kills DCs, impairing their ability to activate CD4+ T cells [35]. Although it remains to be proven, we speculate that expression of one or more of these toxins during infection, together with impaired toxin neutralizing antibody responses, results in suppression of T-cell responses via direct toxicity toward lymphocytes and APCs [32, 36]. Mechanistic studies to test this hypothesis are underway.
Our observation that antibodies against S. aureus toxins increase with age in children are consistent with previous reports [10, 11, 22, 37] and support a conceptual model that exposure to colonizing S. aureus occurs throughout childhood, and the sum of these exposures results in a quantifiable level of immunity. Our findings are also consistent with the notion that the plasticity in S. aureus-specific antibody responses during childhood is lost by adulthood, resulting in the anti-staphylococcal antibody repertoire being fixed by adulthood [38–40]. Nevertheless, the role of antibodies in protection against S. aureus infection in humans remains unclear, despite evidence from mouse models that antibodies, primarily those directed against Hla, are protective [12–14, 41]. Although we found no differences in Hla-specific antibody levels between healthy and infected children during acute infection, an infection-elicited increase in anti-Hla antibodies in children from acute infection to convalescence correlates with lower rates of recurrent infection [4]. Therefore, Hla-specific antibodies may play a more important role in defense against recurrent infection than primary infection. We did not assess the functionality of toxin-specific antibodies. This will be important to assess in the future because healthy children have lower levels of Hla-specific neutralizing antibodies despite similar levels of anti-Hla IgG [37].
A limitation to this study is that immune responses were quantified during acute infection and compared to healthy controls, but we lack baseline responses prior to infection and follow-up studies after the infection has cleared. Nevertheless, this work has several potentially important implications. First, the development of anti-staphylococcal immunity in healthy children follows a predictable trajectory, while children who present with active S. aureus infection appear to have dysregulated responses. Second, impaired T-cell responses may impair clearance of bacteria and prolong resolution of infection. This may also explain why recurrent infections are common in children. Third, a seemingly nonprotective antibody response is elicited at the expense of a protective response. Finally, these findings may have implications for vaccine studies. For example, vaccine efficacy may be optimal during early childhood, prior to the onset of natural nonprotective responses. Additionally, surrogate endpoints for vaccine efficacy might need to include elicited T-cell responses.
In conclusion, we found that S. aureus-specific antibody and T-cell responses develop throughout childhood, but these responses are abnormal in children who develop S. aureus infections. T-cell responses are markedly impaired in children with S. aureus infections, suggesting a mechanism by which the bacterium modulates host immune responses to both impair host defense and prevent the development of protective immunity against subsequent infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to the patients and their families at Nationwide Children’s Hospital for their participation in this study. We acknowledge Jill Popelka, Julie Breuer, and Maggie Flowers for screening and enrolling study subjects.
Financial support. This work was supported by the National Institute for Allergy and Infectious Diseases (grant number AI125489 to C. P. M.); and the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23:616–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goto T, Tsugawa Y, Mansbach JM, CamargoCA, Jr., Hasegawa K. Trends in infectious disease hospitalizations in US children, 2000 to 2012. Pediatr Infect Dis J 2016; 35:e158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fritz SA, Tiemann KM, Hogan PG, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 2015; 13:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev 2020; 44:123–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Proctor RA. Immunity to Staphylococcus aureus: implications for vaccine development. Microbiol Spectr 2019; 7. doi: 10.1128/microbiolspec.GPP3-0037-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verkaik NJ, Lebon A, de Vogel CP, et al. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin Microbiol Infect 2010; 16:1312–7. [DOI] [PubMed] [Google Scholar]
- 11. Dryla A, Prustomersky S, Gelbmann D, et al. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol 2005; 12:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy AD, Bubeck Wardenburg J, Gardner DJ, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010; 202:1050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 2014; 82:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010; 120:1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown AF, Murphy AG, Lalor SJ, et al. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog 2015; 11:e1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan HY, Yang YH, Yu HH, Chien YH, Chiang LL, Chiang BL. Clinical characteristics and outcomes of primary antibody deficiency: a 20-year follow-up study. J Formos Med Assoc 2014; 113:340–8. [DOI] [PubMed] [Google Scholar]
- 18. Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008; 452:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolata JB, Kühbandner I, Link C, et al. The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis 2015; 212:830–8. [DOI] [PubMed] [Google Scholar]
- 20. Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 2012; 484:514–8. [DOI] [PubMed] [Google Scholar]
- 21. Si Y, Zhao F, Beesetty P, et al. Inhibition of protective immunity against Staphylococcus aureus infection by MHC-restricted immunodominance is overcome by vaccination. Sci Adv 2020; 6:eaaw7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermos CR, Yoong P, Pier GB. High levels of antibody to Panton-Valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin Infect Dis 2010; 51:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Microbiol 2009; 55:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 2011; 186:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shibue Y, Kimura S, Kajiwara C, Iwakura Y, Yamaguchi K, Tateda K. Role of interleukin-17 in a murine community-associated methicillin-resistant Staphylococcus aureus pneumonia model. Microbes Infect 2019; 21:33–9. [DOI] [PubMed] [Google Scholar]
- 27. Marchitto MC, Dillen CA, Liu H, et al. Clonal Vγ6+Vδ4+ T cells promote IL-17-mediated immunity against Staphylococcus aureus skin infection. Proc Natl Acad Sci U S A 2019; 116:10917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLoughlin RM, Lee JC, Kasper DL, Tzianabos AO. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J Immunol 2008; 181:1323–32. [DOI] [PubMed] [Google Scholar]
- 29. Karauzum H, Haudenschild CC, Moore IN, Mahmoudieh M, Barber DL, Datta SK. Lethal CD4 T cell responses induced by vaccination against Staphylococcus aureus bacteremia. J Infect Dis 2017; 215:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenberg JA, Hrusch CL, Jaffery MR, et al. Distinct T-helper cell responses to Staphylococcus aureus bacteremia reflect immunologic comorbidities and correlate with mortality. Crit Care 2018; 22:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ardura MI, Banchereau R, Mejias A, et al. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One 2009; 4:e5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee B, Olaniyi R, Kwiecinski JM, Wardenburg JB. Staphylococcus aureus toxin suppresses antigen-specific T cell responses. J Clin Invest 2020; 130:1122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nygaard TK, Pallister KB, DuMont AL, et al. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 2012; 7:e36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alonzo F 3rd, Kozhaya L, Rawlings SA, et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2013; 493:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berends ETM, Zheng X, Zwack EE, et al. Staphylococcus aureus impairs the function of and kills human dendritic cells via the LukAB Toxin. MBio 2019; 10:e01918–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tam K, Lacey KA, Devlin JC, et al. Targeting leukocidin-mediated immune evasion protects mice from Staphylococcus aureus bacteremia. J Exp Med 2020; 217:e20190541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Y, Liu X, Akhgar A, et al. Prevalence of IgG and neutralizing antibodies against Staphylococcus aureus alpha-toxin in healthy human subjects and diverse patient populations. Infect Immun 2018; 86:e00671–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rigat F, Bartolini E, Dalsass M, et al. Retrospective identification of a broad IgG repertoire differentiating patients with s. aureus skin and soft tissue infections from controls. Front Immunol 2019; 10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holtfreter S, Nguyen TT, Wertheim H, et al. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol 2009; 16: 1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelzek AJ, Shopsin B, Radke EE, et al. Human memory B cells targeting Staphylococcus aureus exotoxins are prevalent with skin and soft tissue infection. MBio 2018; 9:e02125–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sampedro GR, DeDent AC, Becker RE, et al. Targeting Staphylococcus aureus α-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis 2014; 210:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.