Abstract
BACKGROUND
Hypertension (HTN) has the greatest population-attributable risk for aortic dissection and is highly prevalent among patients with thoracic aortic aneurysms (TAAs). Although HTN is diagnosed based on brachial blood pressure (bBP), central HTN (central systolic blood pressure [cSBP] ≥130 mm Hg) is of interest as it better reflects blood pressure (BP) in the aorta. We aimed to (i) evaluate the prevalence of central HTN among TAA patients without a diagnosis of HTN, and (ii) assess associations of bBP vs. central blood pressure (cBP) with aneurysm size and growth.
METHODS
One hundred and five unoperated subjects with TAAs were recruited. With validated methodology, cBP was assessed with applanation tonometry. Aneurysm size was assessed at baseline and follow-up using imaging modalities. Aneurysm growth rate was calculated in mm/year. Multivariable linear regression adjusted for potential confounders assessed associations of bBP and cBP with aneurysm size and growth.
RESULTS
Seventy-seven percent of participants were men and 49% carried a diagnosis of HTN. Among participants without diagnosis of HTN, 15% had central HTN despite normal bBP (“occult central HTN”). In these patients, higher central systolic BP (cSBP) and central pulse pressure (cPP) were independently associated with larger aneurysm size (β ± SE = 0.28 ± 0.11, P = 0.014 and cPP = 0.30 ± 0.11, P = 0.010, respectively) and future aneurysm growth (β ± SE = 0.022 ± 0.008, P = 0.013 and 0.024 ± 0.009, P = 0.008, respectively) while bBP was not (P > 0.05).
CONCLUSIONS
In patients with TAAs without a diagnosis of HTN, central HTN is prevalent, and higher cBP is associated with larger aneurysms and faster aneurysm growth.
Keywords: blood pressure, central hypertension, hypertension, thoracic aortic aneurysm
Graphical Abstract
Graphical Abstract.
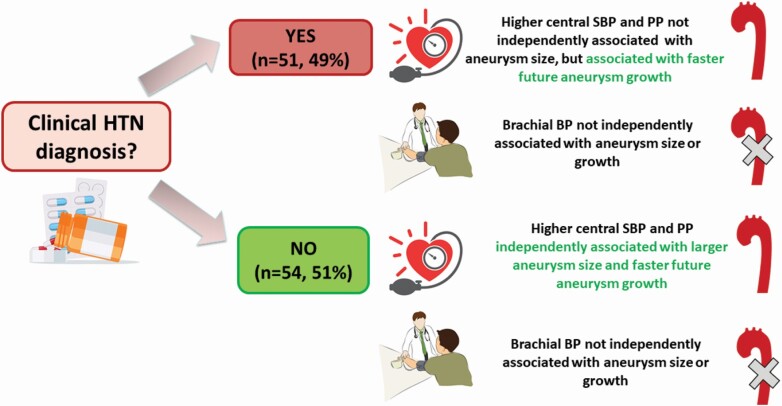
Association of central hypertension to TAA growth. Higher central systolic and pulse pressures were associated with faster aneurysm growth while brachial blood pressure was not, among patients with an established diagnosis of hypertension. Among those without a diagnosis of hypertension, higher central systolic and pulse pressures were associated with larger aneurysms at baseline, and with faster aneurysm growth during follow-up. Abbreviations: SBP, systolic blood pressure; TAA, thoracic aortic aneurysm.
Arterial hypertension (HTN) is the greatest population-attributable risk factor leading to the development of thoracic aortic aneurysms (TAAs).1–3 Being highly prevalent among TAA patients, HTN is a predictor of acute aortic syndromes in these patients and negatively influences their overall survival.1,2,4–6
The rate of aortic dilatation is a precursor to aortic rupture and dissection, both of which are potentially fatal events.4 However, at present, there are no reliable risk stratification tools to predict accelerated TAA growth. The size of the TAA, which is the standard of care for decision-making, has been shown to be an imperfect predictor of TAA-related risk,7 highlighting a critical need to identify additional factors contributing to aneurysm growth. In this context, we must turn our attention to aortic function and aortic pressure as a potential means to understand TAA disease activity. The aorta is an elastic artery that is uniquely designed to withstand biomechanical forces due to pulsatile blood flow from the heart,8 serving as our main pressure buffer. More recently, it has been hypothesized that the architecture of the smooth muscle cell contractile-elastin unit protects the aorta from mechanical forces.8 However, risk factors for TAAs, such as HTN, increase hemodynamic forces on the ascending aorta.8 In these cases, the aortic smooth muscle cell’s mechanosensing is stressed by the increased forces on the wall, leading to misperceptions of high stress as low stress and resulting in maladaptive remodeling.8,9 Importantly, wall stress increases almost linearly with systolic blood pressure (BP).10,11 Thus, controlling HTN is particularly important in TAA in order to reduce aortic wall stress10 and, in turn, target disease prevention.9,12
Understanding the importance of HTN in TAA, we must acknowledge that HTN is typically diagnosed based on brachial BP (bBP), which does not perfectly reflect central BP (cBP)—shown to better predict cardiovascular events.13 Central HTN, defined as central systolic blood pressure (cSBP) ≥130 mm Hg14 is of particular interest in TAA as it better reflects HTN in the aorta, where the aneurysm is located. However, the role of central HTN in TAA has not been prospectively evaluated, especially among individuals who do not carry a clinical diagnosis of HTN and may otherwise be considered “lower risk.” Thus, in this study we aimed to (i) evaluate the prevalence of central HTN in TAA patients without a clinical diagnosis of HTN, and (ii) assess the associations of bBP vs. cBP with TAA size and growth.
METHODS
Study participants
Study participants were recruited from the University of Ottawa Heart Institute’s Aorta Clinic and included unoperated adults with TAA. Participants were eligible if they had a documented TAA (aortic root and/or ascending and/or descending aorta ≥40 mm) due to any etiology. Exclusion criteria included a previous history of aortic dissection, rupture, intramural hematoma, or surgery; worse than mild aortic stenosis, worse than moderate aortic regurgitation, or history of coarctation of the aorta. Participants gave informed consent and the study was approved by University of Ottawa Heart Institute’s Research Ethics Board. Details about the determination of baseline characteristics are outlined in Supplementary Material online.
bBP and cBP estimation
bBP was assessed at baseline using a digital sphygmomanometer (3 times, 2 minutes apart, average used for analyses). Mean arterial pressure was calculated as 1/3 of the systolic BP +2/3 of the diastolic BP. Brachial pulse pressure (bPP) was calculated as brachial systolic − diastolic BP. We estimated cBP with arterial applanation tonometry of the carotid artery as described in our previous studies,15–17 using validated equipment (Non-Invasive Hemodynamics, Cardiovascular Engineering, Norwood, MA). In summary, participants were asked to fast, refrain from alcohol, tobacco, and caffeine use, and withhold antihypertensive medications for 12 hours prior to study. Study was performed with patients in a supine position, in a darkened room with controlled temperature by trained cardiac sonographers. Immediately following bBP measurement as above, applanation arterial tonometry was used to obtain arterial waveforms from the brachial and carotid arteries, in this order and in rapid succession. Approximately 15 beats were obtained from each tonometry site, and signal-averaged final brachial and carotid artery waveforms were obtained. bBP was used to calibrate the peak and trough of the signal-averaged brachial artery tonometry waveform. We then used the diastolic and integrated mean brachial pressures to calibrate the carotid artery waveform, which was then used as a surrogate of cBP. This allowed us to estimate central systolic (cSBP), diastolic (cDBP), and pulse pressure (cPP, calculated as cSBP − cDBP). Central HTN was defined as cSBP ≥130 mm Hg.14 This methodology allows estimation of cBP in a manner that is free of assumptions, reproducible (coefficient of variation: 2.3%)18 and highly correlated with invasive aortic BP (r = 0.96, P < 0.001 for cBP).18
Assessment of aneurysm size and growth rates
All imaging studies were done at our Institution and were available for assessment. Imaging modalities assessed included transthoracic echocardiography, computed tomography, and magnetic resonance imaging. Imaging protocols are outlined in Supplementary Material online. Whenever possible, the same imaging modality was used to compute aneurysm growth (“concordant imaging modality”). When the original modality was not subsequently repeated, we then used a different imaging modality to calculate aneurysm expansion (“discordant imaging modality”). Maximum thoracic aorta size at baseline (time of study enrollment) and follow-up was measured systematically by an imaging cardiologist (T.C.), in diastole, according to published guidelines,19 blinded to BP, and indexed to height. Our group has previously shown excellent reproducibility in TAA measurements across different imaging modalities.20 Aneurysm growth rate was calculated as the indexed growth of the aneurysm at its maximal location divided by the total follow-up time [(mm/m)/year].
Statistical analyses
Continuous variables were reported as mean ± SD and compared between those with and without diagnosis of HTN using a t-test for normally distributed variables, and Wilcoxon rank-sum test for skewed variables. Nominal variables were reported as total number and percentages and compared between those with and without diagnosis of HTN using a chi-square test. We also compared baseline characteristics among groups defined based on a combination of bBP and cBP at the time of the baseline hemodynamic study. Age- and sex-adjusted linear regression assessed associations of bBP and cBP components with baseline TAA size and future TAA expansion in individuals with and without diagnosis of HTN. This was followed by multivariable linear regression additionally adjusted for BSA, diabetes, smoking history, type of imaging modality, aneurysm location, and etiology to assess associations of bBP and cBP components, using separate models for those with and without a diagnosis of HTN. Similar models were performed to assess associations of bBP and cBP components with future TAA expansion, this time also adjusting for baseline aneurysm size, follow-up time, and the concordant/discordant nature of the imaging modalities at baseline and follow-up. Given the role of aging on central hemodynamics, we included interaction terms for age* each of brachial SBP, brachial DBP, bPP, cSBP, cDBP, and cPP in the models evaluating TAA size and TAA growth. If an interaction term was significant, we repeated the respective analysis while stratifying the sample for age ≤ or >50 years. All analyses were performed using JMP version 13 (SAS Institute, Cary, NC). P values ≤0.05 were considered statistically significant.
RESULTS
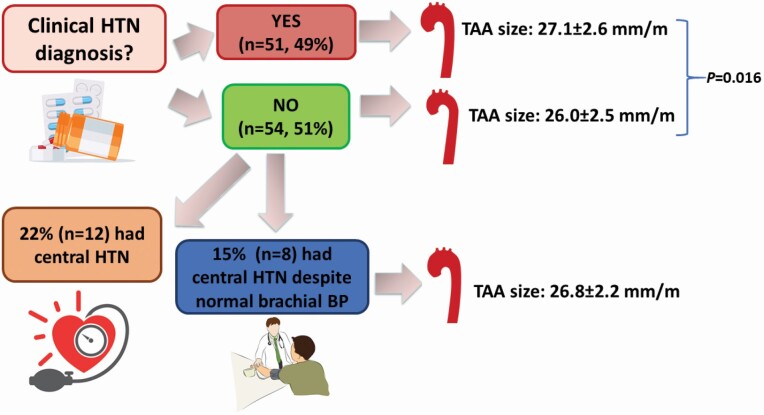
Participant characteristics are summarized in Table 1. Forty-nine percent of participants carried a clinical diagnosis of HTN based on bBP. All participants carrying a history of HTN were taking antihypertensive medications. Only 6 participants without a diagnosis of HTN were taking antihypertensive medications (4 on beta-blockers, 2 on angiotensin-receptor blockers) due to the heritable nature of their aortopathy (Marfan’s or Loeys–Dietz syndromes). Among participants without a clinical diagnosis of HTN, 15% had central HTN despite nonhypertensive bBP. Those with established HTN had larger aneurysms than those without. However, participants without a diagnosis of HTN but who had central HTN had aneurysm sizes comparable to those with established HTN (Figure 1). Supplementary Table S1 online illustrates the characteristics of participants based on bBP and cBP on the day of the study. Compared with those with normal bBP and cBP, those with isolated central HTN were older and had larger aneurysms at baseline. TAA growth was fastest among those with both elevated bBP and cBP, followed by those with isolated central HTN. Five individuals had normal cBP but elevated bBP. The clinical characteristics of these 5 individuals were not different than the rest of the cohort, but it was apparent that their cBP was much lower, and their TAA growth rate was much slower. Among these 5 participants, we found that they had a 27% lower aortic characteristic impedance and twice as high proximal aortic compliance than the rest of the group (analyses not shown), which led to their cBP being lower. Thus, it is possible that these 5 individuals had very favorable aortic elastic properties, and the greater impedance gradient between the very elastic proximal aorta and stiffer brachial artery may have contributed to greater peripheral pulse pressure amplification.
Table 1.
Baseline characteristics of the study participants
| Variable | All (n = 105) | With previous HTN diagnosis (n= 51) | Without previous HTN diagnosis (n = 54) | P value |
|---|---|---|---|---|
| Age, years | 62.77 ± 11.32 | 66.62 ± 10.08 | 58.98 ± 11.46 | 0.0004 |
| Men, n (%) | 81 (77%) | 41 (82%) | 41 (75%) | 0.354 |
| Height, cm | 175 ± 8.75 | 174.66 ± 9.55 | 175.04 ± 8.06 | 0.025 |
| Weight, kg | 87.99 ± 17.54 | 91.41 ± 19.56 | 84.65 ± 14.84 | 0.025 |
| Body surface area, m2 | 2.03 ± 0.22 | 2.06 ± 0.24 | 2.00 ± 0.19 | 0.081 |
| Body mass index, kg/m2 | 28.78 ± 5.01 | 29.86 ± 5.65 | 27.55 ± 3.93 | 0.009 |
| Brachial SBP, mm Hg | 127 ± 15 | 129 ± 16 | 126 ± 16 | 0.215 |
| Brachial DBP, mm Hg | 67 ± 10 | 67 ± 10 | 67 ± 11 | 0.417 |
| Mean arterial pressure, mm Hg | 90 ± 10 | 90 ± 10 | 90 ± 10 | 0.283 |
| Brachial PP, mm Hg | 60 ± 16 | 61± 17 | 59 ± 17 | 0.269 |
| Central SBP, mm Hg | 126 ± 19 | 134 ± 19 | 119 ± 16 | <0.0001 |
| Central DBP, mm Hg | 69 ± 11 | 69 ± 10 | 67± 12 | 0.182 |
| Central PP, mm Hg | 58 ± 20 | 65 ± 21 | 51 ± 16 | 0.0002 |
| Brachial systolic HTN >140 mm Hg on the day of the study, n (%) | 22 (21%) | 13 (27%) | 9 (16%) | 0.205 |
| Central systolic HTN >130 mm Hg on the day of the study, n (%)a | 40 (38%) | 27 (55%) | 12 (22%) | 0.0004 |
| Diabetes, n (%) | 4 (3.8%) | 4 (8%) | 0 | 0.013 |
| Smoking, n (%) | 58 (55%) | 31 (62%) | 27 (49%) | 0.183 |
| Maximal aneurysm location (root/ascending aorta) | 29/76 | 40/10 | 36/19 | 0.094 |
| Degenerative/heritable TAA | 61/44 | 39/11 | 22/33 | <0.0001 |
| Baseline aneurysm size, mm | 46.24 ± 3.84 | 47.17 ± 3.79 | 45.40 ± 3.72 | 0.009 |
| Indexed baseline TAA size, mm/m | 26.51 ± 2.59 | 27.08 ± 2.55 | 25.99 ± 2.54 | 0.016 |
| Imaging modality at baseline (TTE/CT/MRI) | 39/23/43 | 19/11/20 | 20/12/23 | 0.980 |
| Follow-up time, years | 2.92 ± 1.01 | 2.88 ± 0.99 | 2.96 ± 1.04 | 0.681 |
| Concordant/discordant nature of imaging modalities at baseline and follow-up | 63/42 | 35/15 | 42/13 | 0.462 |
| TAA size at follow-up, mm | 47.45 ± 4.17 | 48.55 ± 4.16 | 46.45 ± 3.95 | 0.010 |
| Indexed TAA size at follow-up, mm/m | 27.21 ± 2.84 | 27.88 ± 2.91 | 26.59 ± 2.66 | 0.021 |
| TAA growth rate, mm/year | 0.39 ± 0.40 | 0.50 ± 0.38 | 0.36 ± 0.34 | 0.047 |
| Indexed TAA growth rate, (mm/m)/year | 0.25 ± 0.22 | 0.29 ± 0.24 | 0.21 ± 0.20 | 0.048 |
Abbreviations: CT, computed tomography; DBP, diastolic blood pressure; HTN, hypertension; MAP, mean arterial pressure; MRI, magnetic resonance imaging; PP, pulse pressure; SBP, systolic blood pressure; TAA, thoracic aortic aneurysm; TTE, transthoracic echocardiogram.
aOn day of the study.
Figure 1.
Distribution of central hypertension and relationship to TAA size. Abbreviations: HTN, hypertension; SBP, systolic blood pressure; TAA, thoracic aortic aneurysm.
The results of age- and sex-adjusted models are summarized in Table 2. We found that, among participants without known HTN, cSBP and cPP were independently associated with larger TAA size at baseline and faster TAA growth, while bBP components were not. Among participants with established HTN, neither cBP nor bBP components were associated with aneurysm size at baseline, but cSBP and cPP were associated with faster TAA growth. Inferences remained unchanged after adjustment for additional confounders in the multivariable models (Tables 3 and 4, Graphical Abstract), confirming the independent role of cSBP and cPP on TAA size and growth. Of note, antihypertensive medication use was not associated with TAA size or growth (analyses not shown).
Table 2.
Summary of age- and sex-adjusted linear regression models evaluating aneurysm size and expansion
| Variable | Aneurysm size, mm/m | Aneurysm growth, (mm/m)/year | ||
|---|---|---|---|---|
| With hypertension diagnosis (β ± SE) | Without hypertension diagnosis (β ± SE) | With hypertension diagnosis (β ± SE) | Without hypertension diagnosis (β ± SE) | |
| Brachial SBP, 5 mm Hg | 0.12 ± 0.11; P = 0.283 | −0.025 ± 0.11; P = 0.820 | 0.019 ± 0.0082; P = 0.023 | 0.0081 ± 0.0079; P = 0.313 |
| Brachial DBP, 5 mm Hg | −0.069 ± 0.19; P = 0.718 | −0.17 ± 0.16; P = 0.287 | 0.0057 ± 0.014; P = 0.693 | −0.0037 ± 0.012; P = 0.761 |
| MAP, 5 mm Hg | 0.027 ± 0.18; P = 0.883 | −0.12 ± 0.170; P = 0.472 | 0.024 ± 0.013; P = 0.077 | 0.010 ± 0.013; P = 0.435 |
| Brachial PP, 5 mm Hg | 0.14 ± 0.11; P = 0.210 | 0.045 ± 0.10; P = 0.651 | 0.016 ± 0.0080; P = 0.051 | 0.0086 ± 0.0074; P = 0.255 |
| Central SBP, 5 mm Hg | 0.18 ± 0.094; P = 0.061 | 0.29 ± 0.11; P = 0.010 | 0.021 ± 0.068; P = 0.003 | 0.027 ± 0.0079; P = 0.001 |
| Central DBP, 5 mm Hg | −0.015 ± 0.20; P = 0.939 | 0.0019 ± 0.15; P = 0.990 | −0.013 ± 0.015; P = 0.398 | −0.0015 ± 0.011; P = 0.891 |
| Central PP, 5 mm Hg | 0.16 ± 0.089; P = 0.072 | 0.30 ± 0.11; P = 0.009 | 0.021 ± 0.0063; P = 0.001 | 0.029 ± 0.0079; P = 0.001 |
Abbreviations as in Table 1.
Table 3.
Summary of fully adjusted multivariable linear regression models evaluating baseline aneurysm size (mm/m)
| Variable | With hypertension diagnosis (β ± SE) | Without hypertension diagnosis (β ± SE) |
|---|---|---|
| Brachial SBP, 5 mm Hg | 0.084 ± 0.12; P = 0.484 | −0.029 ± 0.11; P = 0.794 |
| Brachial DBP, 5 mm Hg | −0.13 ± 0.20; P = 0.502 | −0.20 ± 0.16; P = 0.216 |
| MAP, 5 mm Hg | −0.043 ± 0.19; P = 0.824 | −0.14 ± 0.17; P = 0.404 |
| Brachial PP, 5 mm Hg | 0.12 ± 0.11; P = 0.296 | 0.055 ± 0.10; P = 0.591 |
| Central SBP, 5 mm Hg | 0.17 ± 0.10; P = 0.099 | 0.28 ± 0.11; P = 0.014 |
| Central DBP, 5 mm Hg | −0.014 ± 0.20; P = 0.945 | −0.021 ± 0.15; P = 0.887 |
| Central PP, 5 mm Hg | 0.15 ± 0.097; P = 0.116 | 0.30 ± 0.11; P = 0.010 |
Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Table 4.
Summary of fully adjusted multivariable linear regression models evaluating aneurysm growth [(mm/m)/year]
| Variable | With hypertension diagnosis (β ± SE) | Without hypertension diagnosis (β ± SE) |
|---|---|---|
| Brachial SBP, 5 mm Hg | 0.019 ± 0.008; P = 0.026 | 0.010 ± 0.008; P = 0.192 |
| Brachial DBP, 5 mm Hg | 0.0084 ± 0.014; P = 0.550 | 0.003 ± 0.012; P = 0.788 |
| MAP, 5 mm Hg | 0.026 ± 0.013; P = 0.052 | 0.016 ± 0.012; P = 0.213 |
| Brachial PP, 5 mm Hg | 0.017 ± 0.009; P = 0.078 | 0.008 ± 0.007; P = 0.298 |
| Central SBP, 5 mm Hg | 0.019 ± 0.008; P = 0.021 | 0.022 ± 0.008; P = 0.013 |
| Central DBP, 5 mm Hg | −0.011 ± 0.015; P = 0.458 | −0.001 ± 0.011; P = 0.916 |
| Central PP, 5 mm Hg | 0.019 ± 0.007; P = 0.012 | 0.024 ± 0.009; P = 0.008 |
Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Interaction term analyses demonstrated that the only significant interactions were for age × cSBP (P = 0.045) and age × cPP (P = 0.020) in the evaluation of TAA growth among participants without a diagnosis of HTN. Thus, we stratified the sample of participants without diagnosis of HTN based on age and demonstrated that cSBP (β ± SE: 0.093 ± 0.036, P = 0.015) and cPP (β ± SE: 0.012 ± 0.003, P = 0.0009) were independently associated with future TAA growth among those older than 50 years (n = 41), but not among individuals younger than 50 (n = 14, β ± SE: 0.003 ± 0.004, P = 0.496 and 0.001 ± 0.006, P = 0.876 for cSBP and cPP, respectively).
DISCUSSION
We examined the prevalence of central HTN among people with TAA without a clinical history of brachial HTN and assessed associations of bBP vs. cBP with TAA size and growth. In this context, we found that while participants with a history of HTN had larger aneurysms than those without this diagnosis, those patients with “occult central HTN” (i.e., central HTN despite normal bBP, in the absence of HTN diagnosis) had aneurysm sizes that were nearly identical to those of established hypertensive patients. Furthermore, higher central systolic and pulse pressures were associated with larger aneurysms and with faster aneurysm expansion among those without a diagnosis of HTN, and associated with faster aneurysm growth among known hypertensives. Among individuals without a diagnosis of HTN, age appears to be an effect modifier of the associations of cBP with aneurysm growth, as these associations were only observed among those older than 50 years. To our knowledge, this is the first study to explore the implications of central HTN in patients with TAA considered to be normotensive on a clinical basis, and to compare the potential effects of cBP vs. bBP among TAA patients, adding novel information regarding the importance of cBP assessment in these individuals.
According to current practice guidelines, it is recommended that patients with TAA undergo yearly surveillance to monitor aneurysm size.21 However, although aortic diameter is a predictor of acute aortic syndromes,22,23 studies suggest that it does not fully account for the risk of adverse outcomes.5,24 Thus, it is important to identify additional physiologic measures contributing to aneurysm growth, playing a potential role in risk stratification and disease progression. These gaps in clinical care motivated us to pursue alternative parameters for assessing TAA disease activity, focusing on cBP as a correlate of aortic health and function and, as such, a marker of disease activity.
In our study, 22% of patients without a clinical diagnosis of HTN had central HTN on the day of the study, and 15% had central HTN despite normal bBP. Although bBP is routinely measured in the clinical setting for evaluation and management of HTN and known to have a strong relationship with cardiovascular outcomes,25 the target organs are directly exposed to cBP.26 Furthermore, bBP is a surrogate of cBP and, because of peripheral pulse pressure augmentation, does not always accurately reflect the true cBP. Recent studies have suggested that cBP may be a more accurate predictor of cardiovascular outcomes compared with bBP, with cBP being an independent risk factor for end-organ damage, cardiovascular events, and mortality.13,27 Relevant to this study, cBP is also potentially more applicable to TAA, since it may better reflect the BP and hemodynamic forces in the aorta. As a result, our study adds to existing literature by supporting the estimation of cBP to identify centrally hypertensive individuals who may be at risk of TAA-related adverse outcomes.
Central vs. brachial HTN: effects on aneurysm size and expansion
It has been previously reported that aortic wall stress increases with higher systolic bBP.10 At the same level of BP, aortic wall stress is greater in larger compared with smaller aneurysms.10 Thus, this stress on the aortic wall drives further TAA expansion. In the present study, we found that participants with established HTN had larger aneurysms than those without. It has been demonstrated that when systolic bBP is reduced from 140 to 110 mm Hg, there is a 21% reduction in aortic wall stress.10 This implicates the importance of controlling BP in potentially reducing adverse aortic events in patients with TAA.
However, bBP does not fully account for the risk of adverse outcomes. cSBP is an important hemodynamic parameter,28 as cardiovascular events decrease with lower cSBP levels.27,29–33 Further, in a study of treated hypertensives without TAA, 62% of patients were unable to attain the target for treated cSBP whereas bBP targets were achieved by all patients.34 This highlights the importance of considering cBP when trying to mitigate the adverse effects of HTN. Notably, in our study 15% of participants had central HTN despite normal bBP and no clinical diagnosis of HTN, representing a subgroup of hypertensive patients who would otherwise go clinically unnoticed.
Importantly, we found that among TAA patients without clinical diagnosis of HTN (which is made based on bBP), cSBP and cPP, both reflecting the aorta’s health and overall pressure-buffering function, were independently associated with larger aneurysms. Further, higher cSBP and cPP were independently associated with faster future aneurysm growth among those with and without clinical diagnosis of HTN. Conversely, bBP measures displayed no independent relationship with aneurysm size or growth rate, confirming the notion that assessing cBP may be more relevant for TAA risk stratification than bBP. Our findings are in agreement with previously reported studies demonstrating that higher cBP is a risk factor for faster abdominal aortic aneurysm expansion35 and can be used as a marker of risk in patients with a known aortic aneurysm and well-controlled bBP.36 Our findings are also in agreement with a previous study that concluded cPP was a determinant of ascending aorta diameter in patients with Marfan syndrome, whereas bPP was not.37 Based on our findings, cBP can be used to: (i) identify TAA patients with occult central HTN despite normal bBP and (ii) predict TAA expansion regardless of clinical HTN diagnosis. Since cBP can be simply, cheaply, and reliably estimated with noninvasive techniques, our findings highlight the clinical potential of assessing cBP in the evaluation of TAA disease activity and its associated risk.
Limitations
Our study is not without limitations. We relied on a clinical diagnosis of HTN and/or use of antihypertensive medications to categorize participants as hypertensives, and it is possible that some patients could have been clinically missed. However, as participants were recruited from an Aorta Clinic, which operates based on referrals from Primary Care, Emergency and Cardiovascular Physicians, the likelihood that participants had not had their BP checked recently is low. Further, we did not have data on ambulatory 24-hour BP monitoring, which could have identified masked HTN if present. In addition, the diagnostic criteria for brachial HTN currently varies: in Canada, HTN is diagnosed with automatic bBP ≥135/85 mm Hg or nonautomatic bBP ≥140/90 mm Hg.38 Similarly, in Europe, HTN is diagnosed as bBP ≥140/90 mm Hg.39 In the United States, bBP >130/80 mm Hg is considered hypertensive since 2017.40 Given our location and timing of commencement of the research study (Canada, 2014), Canadian/European guidelines were used to define HTN for the purpose of this study. Lastly, we demonstrated the effect of age as an effect modifier of associations of cBP with TAA growth among individuals without a diagnosis of HTN but cannot exclude the possibility of underpowering as the absolute number of normotensive individuals younger than age 50 was low (n = 14).
CONCLUSIONS
Central HTN is prevalent among patients with TAA without a clinical diagnosis of HTN, and higher central systolic and pulse pressures are associated with larger aneurysms among normotensives, and faster future TAA expansion among all TAA patients. Conversely, bBP bears no independent relationship to TAA size or growth. Our findings highlight the potential clinical role of central HTN in assessing TAA disease activity. Future studies are warranted to: (i) determine the role of cBP on predicting acute aortic syndromes; (ii) define the clinical utility of including cBP as part of a personalized TAA risk algorithm; and (iii) understand the role of cBP as a therapeutic target to curb TAA expansion and improve outcomes.
FUNDING
This research was funded by an Early Research Leaders Initiative grant from the Canadian Vascular Network and the Canadian Institutes of Health Research, and a Grant-in-Aid from the Heart and Stroke Foundation of Canada.
DISCLOSURE
Dr Coutinho is supported by a Clinician Scientist Stage 1 Award from the Heart and Stroke Foundation of Ontario.
Supplementary Material
REFERENCES
- 1. Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat Clin Pract Cardiovasc Med 2007; 4:418–427. [DOI] [PubMed] [Google Scholar]
- 2. LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol 2011; 8:103–113. [DOI] [PubMed] [Google Scholar]
- 3. Rabkin SW, Mathewson AL, Tate RB. Predicting risk of ischemic heart disease and cerebrovascular disease from systolic and diastolic blood pressures. Ann Intern Med 1978; 88:342–345. [DOI] [PubMed] [Google Scholar]
- 4. Chan KK, Rabkin SW. Increasing prevalence of hypertension among patients with thoracic aorta dissection: trends over eight decades—a structured meta-analysis. Am J Hypertens 2014; 27:907–917. [DOI] [PubMed] [Google Scholar]
- 5. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators . Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116:1120–1127. [DOI] [PubMed] [Google Scholar]
- 6. Joyce JW, Fairbairn JF II, Kincaid OW, Juergen JL. Aneurysms of the thoracic aorta. A clinical study with special reference to prognosis. Circulation 1964; 29:176–181. [PubMed] [Google Scholar]
- 7. Mansour AM, Peterss S, Zafar MA, Rizzo JA, Fang H, Charilaou P, Ziganshin BA, Darr UM, Elefteriades JA. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology 2018; 139:139–146. [DOI] [PubMed] [Google Scholar]
- 8. Milewicz DM, Prakash SK, Ramirez F. Therapeutics targeting drivers of thoracic aortic aneurysms and acute aortic dissections: insights from predisposing genes and mouse models. Annu Rev Med 2017; 68:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology. Dysfunctional mechanosensing in aneurysms. Science 2014; 344:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabkin SW, Janusz MT. Aortic wall stress in hypertension and ascending thoracic aortic aneurysms: implications for antihypertensive therapy. High Blood Press Cardiovasc Prev 2013; 20:265–271. [DOI] [PubMed] [Google Scholar]
- 11. Okamoto RJ, Xu H, Kouchoukos NT, Moon MR, Sundt TM III. The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. J Thorac Cardiovasc Surg 2003; 126:842–850. [DOI] [PubMed] [Google Scholar]
- 12. Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res 2015; 116:1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension 2007; 50:197–203. [DOI] [PubMed] [Google Scholar]
- 14. Cheng HM, Chuang SY, Sung SH, Yu WC, Pearson A, Lakatta EG, Pan WH, Chen CH. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol 2013; 62:1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coutinho T, Goel K, Corrêa de Sá D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol 2013; 61:553–560. [DOI] [PubMed] [Google Scholar]
- 16. Coutinho T, Pellikka PA, Bailey KR, Turner ST, Kullo IJ. Sex differences in the associations of hemodynamic load with left ventricular hypertrophy and concentric remodeling. Am J Hypertens 2016; 29:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boczar KE, Cheung K, Boodhwani M, Beauchesne L, Dennie C, Nagpal S, Chan K, Coutinho T. Sex differences in thoracic aortic aneurysm growth. Hypertension 2019; 73:190–196. [DOI] [PubMed] [Google Scholar]
- 18. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol 1992; 20:952–963. [DOI] [PubMed] [Google Scholar]
- 19. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuéllar-Calàbria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Taylor AJ, Weinsaft JW, Zamorano JL, Dietz H, Eagle K, Elefteriades J, Jondeau G, Rousseau H, Schepens M. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015; 28:119–182. [DOI] [PubMed] [Google Scholar]
- 20. Cheung K, Boodhwani M, Chan K, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc 2017; 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, Therrien J, Siu SC; Canadian Cardiovascular Society . Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol 2014; 30:577–589. [DOI] [PubMed] [Google Scholar]
- 22. Shimada I, Rooney SJ, Pagano D, Farneti PA, Davies P, Guest PJ, Bonser RS. Prediction of thoracic aortic aneurysm expansion: validation of formulae describing growth. Ann Thorac Surg 1999; 67:1968–1970; discussion 1979. [DOI] [PubMed] [Google Scholar]
- 23. Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, Scognamiglio G, D’Oria V, De Feo M. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging 2013; 6:1301–1310. [DOI] [PubMed] [Google Scholar]
- 24. Kim EK, Choi SH, Sung K, Kim WS, Choe YH, Oh JK, Kim DK. Aortic diameter predicts acute type A aortic dissection in patients with Marfan syndrome but not in patients without Marfan syndrome. J Thorac Cardiovasc Surg 2014; 147:1505–1510. [DOI] [PubMed] [Google Scholar]
- 25. Ryuzaki M, Morimoto S, Niiyama M, Seki Y, Yoshida N, Oshima Y, Mizuguchi Y, Watanabe D, Ando T, Ichihara A. The relationships between the differences in the central blood pressure and brachial blood pressure and other factors in patients with essential hypertension. Intern Med 2017; 56:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chi C, Yu X, Auckle R, Lu Y, Fan X, Yu S, Xiong J, Bai B, Teliewubai J, Zhou Y, Ji H, Li J, Zhang Y, Xu Y. Hypertensive target organ damage is better associated with central than brachial blood pressure: the Northern Shanghai Study. J Clin Hypertens (Greenwich) 2017; 19:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CAFE Investigators A-SCOTI, CAFE Steering Committee and Writing Committee, Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation 2006; 113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 28. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 29. Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 2007; 50:154–160. [DOI] [PubMed] [Google Scholar]
- 30. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens 2011; 29:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trudeau L. Central blood pressure as an index of antihypertensive control: determinants and potential value. Can J Cardiol 2014; 30:S23–S28. [DOI] [PubMed] [Google Scholar]
- 33. Palatini P, Casiglia E, Gąsowski J, Głuszek J, Jankowski P, Narkiewicz K, Saladini F, Stolarz-Skrzypek K, Tikhonoff V, Van Bortel L, Wojciechowska W, Kawecka-Jaszcz K. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag 2011; 7:725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bulas J, Potočárová M, Murín J, Kozlíková K, Luha J, Čaprnda M. Central systolic hypertension in patients with well-controlled hypertension. Biomed Res Int 2017; 2017:8158974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruegg G, Mason RH, Hardinge M, Perkins J, Husmann M, Russi EW, Bloch KE, Stradling JR, Kohler M. Augmentation index and central aortic blood pressure in patients with abdominal aortic aneurysms. J Hypertens 2010; 28:2252–2257. [DOI] [PubMed] [Google Scholar]
- 36. Moon J, Lee SH, Ko YG, Jang Y, Shim WH, Choi DH. Central aortic pressure in aortic aneurysm and aortic dissection: a novel prognostic marker. Acta Cardiol 2010; 65:303–308. [DOI] [PubMed] [Google Scholar]
- 37. Jondeau G, Boutouyrie P, Lacolley P, Laloux B, Dubourg O, Bourdarias JP, Laurent S. Central pulse pressure is a major determinant of ascending aorta dilation in Marfan syndrome. Circulation 1999; 99:2677–2681. [DOI] [PubMed] [Google Scholar]
- 38. Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Padwal RS, Tran KC, Grover S, Rabkin SW, Moe GW, Howlett JG, Lindsay P, Hill MD, Sharma M, Field T, Wein TH, Shoamanesh A, Dresser GK, Hamet P, Herman RJ, Burgess E, Gryn SE, Grégoire JC, Lewanczuk R, Poirier L, Campbell TS, Feldman RD, Lavoie KL, Tsuyuki RT, Honos G, Prebtani APH, Kline G, Schiffrin EL, Don-Wauchope A, Tobe SW, Gilbert RE, Leiter LA, Jones C, Woo V, Hegele RA, Selby P, Pipe A, McFarlane PA, Oh P, Gupta M, Bacon SL, Kaczorowski J, Trudeau L, Campbell NRC, Hiremath S, Roerecke M, Arcand J, Ruzicka M, Prasad GVR, Vallée M, Edwards C, Sivapalan P, Penner SB, Fournier A, Benoit G, Feber J, Dionne J, Magee LA, Logan AG, Côté AM, Rey E, Firoz T, Kuyper LM, Gabor JY, Townsend RR, Rabi DM, Daskalopoulou SS; Hypertension Canada . Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34:506–525. [DOI] [PubMed] [Google Scholar]
- 39. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 40. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.