Abstract
β-Catenin is an oncogenic protein involved in regulation of cell-cell adhesion and gene expression. Accumulation of cellular β-catenin occurs in many types of human cancers. Four mechanisms are known to cause increases in β-catenin: mutations of β-catenin, adenomatous polyposis coli, or axin genes and activation of Wnt signaling. We report a new cause of β-catenin accumulation involving oncogenic mutants of RON and MET receptor tyrosine kinases (RTKs). Cells transfected with oncogenic RON or MET were characterized by β-catenin tyrosine phosphorylation and accumulation; constitutive activation of a Tcf transcriptional factor; and increased levels of β-catenin/Tcf target oncogene proteins c-myc and cyclin D1. Interference with the β-catenin pathway reduced the transforming potential of mutated RON and MET. Activation of β-catenin by oncogenic RON and MET constitutes a new pathway, which might lead to cell transformation by these and other mutant growth factor RTKs.
β-Catenin (Drosophila homologue Armadillo) is a multifunctional protein discovered as a component of adherens junctions (77). β-Catenin associated with E-cadherin and α-catenin at the plasma membrane regulates cell adhesion, whereas cytoplasmic β-catenin is involved in signal transduction and activation of gene expression (12). The amount of uncomplexed cytoplasmic β-catenin is tightly regulated by a multiprotein complex containing axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3β (GSK3β). Physical interaction between these proteins promotes β-catenin phosphorylation on serine residues by GSK3β, an event leading to β-catenin ubiquitination and proteasomal degradation (8).
Increased cellular β-catenin due to mutations in APC tumor suppressor or β-catenin genes occurs in many human cancers, including those of colon and skin (14, 57, 68, 92). Mutations in axin leading to β-catenin accumulation have been found in hepatocellular carcinomas (16, 96). All these mutations result in reduced degradation of β-catenin, which is believed to promote tumor formation by constitutive activation of β-catenin targets (68, 84). Another pathway leading to β-catenin stabilization is activation of the Wnt signaling, the vertebrate homologue of Drosophila Wingless (75, 82, 102). Wnt genes are tumorigenic in mice (75) and may also be implicated in human cancer (84).
The MET receptor tyrosine kinase (RTK) family contains three members: MET (17, 34, 81), RON (89), and c-Sea (42) (Sea may be a chicken RON orthologue). MET is the receptor for hepatocyte growth factor (HGF) (10). MET is expressed in a number of cell types, including epithelial cells (23, 105), endothelial cells (13, 35), myoblasts (2), spinal motor neurons (27), and hematopoietic cells (30, 74). Interaction of HGF with MET activates multiple intracellular signaling pathways involved in muscle and liver formation (32, 62, 97), cell proliferation (64, 73, 91), morphogenesis (66), and motility (105, 106). In addition to regulation of normal cell functions, MET is implicated in development and progression of a number of tumors. Increased MET expression has been found in papillary carcinomas of the thyroid gland; carcinomas of colon, pancreas, and ovary; and osteogenic sarcomas (23–25, 28, 33, 50). Point mutations in the MET kinase domain have been identified in hereditary and sporadic papillary renal carcinomas (98, 99).
RON, the receptor for macrophage-stimulating protein (MSP, also known as HGF-like protein) (115), is another member of the MET RTK family sharing many common features with MET (89). RON is expressed in different cell types, including macrophages (47), epithelial cells (113, 114), osteoclasts (59), and hematopoietic cells, such as erythroid and myeloid progenitor cells (48) and bone marrow megakaryocytes (3). Ligand-stimulated RON activates the pathways regulating cell adhesion and motility, growth, and survival (20, 21). Recent investigations have shown that activated RON is expressed in human primary breast carcinomas (61) and in a number of cancer cell lines that are removed from the host tissue environment (15, 31). Truncated RON confers susceptibility to Friend virus-induced erythroleukemia in mice (83), and the avian oncogene v-sea, which is highly homologous to RON, causes erythroblastosis in chickens (104). Although RON has not yet been implicated as a cause of any human neoplasm, the above data indicate its oncogenic potential.
Activating mutations in growth factor RTKs may result in oncogenic cell transformation and tumor development. A number of human neoplastic syndromes are associated with activating point mutations in a highly conserved region of the tyrosine kinase domains of the KIT, RET, and MET receptors (98). Mutation of aspartic acid at position 816 (D816V) in the KIT receptor causes mast cell leukemia and mastocytosis (71, 110). Substitution of a threonine for methionine in RET (M918T) and in MET (M1268T) receptors is associated with multiple endocrine neoplasia (93) and renal papillary carcinoma (98), respectively. Experimental mutations in the conserved region of the RON kinase domain (D1232V, KIT type; M1254T, RET/MET type) result in a tumorigenic phenotype (95, 117).
In the present work we show that expression of tumorigenic RON (M1254T and D1232V) or MET (M1268T) mutants causes cellular accumulation of β-catenin. The increase in cellular β-catenin is accompanied by constitutive activation of the Tcf-4 transcriptional factor and Tcf-dependent accumulation of c-myc and cyclin D1 oncogenes. Biochemical data suggest that the initiating step may be β-catenin tyrosine phosphorylation by mutated RON and MET. Inhibition of the β-catenin pathway resulted in reduction of RON and MET mutant transforming ability. In light of numerous data reporting increased cellular β-catenin in cancer (7, 68, 84), this pathway may contribute to cell transformation by mutated growth factor receptors of the MET family.
MATERIALS AND METHODS
Antibodies.
RON receptor was immunoprecipitated from cell lysates by using mouse monoclonal anti-RON antibodies (clone ID2; a gift from F. Montero-Julian) (65). Rabbit anti-RON antibodies (C-20; Santa Cruz, Santa Cruz, Calif.) were used for Western blotting. Protein tyrosine phosphorylation was detected by Western blotting using antiphosphotyrosine (anti-PY) antibodies (clone 4G10; UBI, Lake Placid, N.Y.). Rabbit anti-C-terminal β-catenin (H-102, Santa Cruz) and anti-N-terminal β-catenin (UBI) antibodies were used for β-catenin immunoprecipitation and Western blotting, respectively. Antiaxin (Zymed, South San Francisco, Calif.) and mouse monoclonal anti-GSK3 (0011-A; Santa Cruz) antibodies were used for axin and GSK3 immunoprecipitation and Western blotting. Anti-c-myc (monoclonal, C-8), anti-cyclin D1 (monoclonal, HD11), anti-human and -mouse MET (rabbit, C-28 and SP260), antiubiquitin (monoclonal, P1A6), and anti-JNK1 (rabbit, FL) antibodies were from Santa Cruz. Mouse monoclonal anti-pMAPK antibodies were from New England BioLabs, Beverly, Mass. Mouse monoclonal anti-mitogen-activated protein kinase (anti-MAPK) antibody was from Transduction Laboratories (Lexington, Ky.). Rabbit anti-phospho-JNK antibodies were from Promega (Madison, Wis.). Mouse monoclonal anti-β-actin antibody (clone AC-15) was from Sigma (St. Louis, Mo.).
Site-directed mutagenesis of RON receptor.
M1254T and D1232V RON mutants were generated using the GeneEditor (Promega) mutagenesis kit with mutagenesis oligonucleotides described earlier (95).
Cells and transfections.
MDCK and NIH 3T3 cells (purchased from the American Type Culture Collection (Manassas, Va.) were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS). For stable expression of RON, cells in 10-cm-diameter dishes were transfected with 10 μg of RON cDNA by Superfect reagent (Qiagen, Santa Clarita, Calif.) and were placed in medium with 500 μg of Geneticin (Life Technologies, Inc.). RON expression was measured by Western blotting with anti-RON antibodies (Santa Cruz) in total cell lysates as well as in RON immunoprecipitates (IPs). NIH 3T3 cells expressing MET WT or M1268T mutant used in this work have been previously described (51, 72).
Cell stimulation.
Cells were incubated overnight in medium without serum and were then collected from dishes and stimulated with 5 nM MSP for 15 min. Cells incubated in medium alone served as a control. For some experiments cells were pretreated with 1 mM sodium pervanadate (PV) for 15 min, after which PV was removed by changing culture medium after cell centrifugation. The PV solution was prepared as previously described (85). PV-pretreated cells were used for subsequent stimulation. To inhibit proteasomal protein degradation, cells were incubated with 25 μM N-acetyl-Leu-Leu-norleucinal (ALLN) for 6 h.
Cell lysis, immunoprecipitation, and Western blotting.
After stimulation, cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, 1% Triton X-100, 10 μg of leupeptin/ml, 10 U of aprotinin/ml, and 1 mM phenylmethylsulfonyl fluoride). Cell lysates were immunoprecipitated or were used directly for detection of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
RON kinase assay in vitro.
RON was immunoprecipitated from cell lysates by RON antibodies. IPs were washed twice in HNTG buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, and 10% glycerol) and twice in kinase buffer (20 mM HEPES, pH 7.4, 10% glycerol, 10 mM MgCl2, 10 mM MnCl2, and 150 mM NaCl). To initiate kinase reactions, 15 μCi of [γ32-P]ATP (3,000 Ci/mmol; 10 μCi/ml) was added, and IPs were incubated 30 min at room temperature in 15 μl of total volume. The exogeneous substrate, myelin basic protein, was added to the kinase reaction mixture at a concentration of 0.5 μg/reaction tube. Reactions were stopped with 5 μl of 4× sample buffer. Phosphorylated RON or its substrate, myelin basic protein, was visualized after SDS-PAGE by autoradiography.
RON phosphorylation of β-catenin in a kinase assay in vitro.
After stimulation with MSP (5 nM) for 15 min, cells were lysed and RON was immunoprecipitated. For the kinase assay, immunoprecipitated RON wild type (WT) or M1254T mutant from stimulated or unstimulated cells was mixed with immunoprecipitated β-catenin from unstimulated MDCK cells. The kinase assay was performed as described above. Phosphorylated RON or β-catenin was visualized after SDS-PAGE and transfer onto a nitrocellulose membrane by autoradiography. The amount of RON or β-catenin in each sample was determined by probing the same membrane with appropriate antibodies.
Pulse-chase analysis of β-catenin.
MDCK or NIH 3T3 cells expressing RON WT or mutants were labeled with [35S]methionine and [35S]cysteine (100 μCi/ml) for 60 min at 37°C in methionine- and cysteine-free DMEM containing 8% dialyzed FCS. Labeled cells were rinsed twice in normal DMEM containing methionine and cysteine and were incubated in this medium for the indicated times, after which β-catenin was immunoprecipitated from the lysed cells. After SDS–8% PAGE of the IPs, proteins were transferred to a nitrocellulose membrane for radioautography and quantification of the labeled proteins by scanning and integration with National Institutes of Health Image software. Bands corresponding to β-catenin were identified by immunoblotting.
Luciferase assay.
The reporter plasmids 3× WT Tcf-binding site (TOPFLASH) and 3× mutated Tcf-binding site (FOPFLASH) were from Promega. Adenoviral constructs containing β-galactosidase (β-Gal) or dominant-negative (DN) human Tcf-4 were prepared as previously described (38). Cells expressing RON or MET WT or mutants in 10-cm-diameter dishes were infected with adenovirus encoding β-Gal or DN Tcf-4 as described previously (37). Twenty-four hours later, these cells were transfected with luciferase reporter plasmids (10 μg/dish; using Superfect reagent as described above) containing WT (TOPFLASH) or mutated (FOPFLASH) Tcf promoter. After an additional 24 h, Tcf activity was determined by luciferase assay according to the standard Promega protocol. Luciferase activity was measured in cell lysates by using a chemiluminometer and was normalized to total protein.
Cell infection with adenoviral constructs and detection of cyclin D1 and c-myc expression.
NIH 3T3 cells expressing MET WT or M1268T mutant or MDCK cells expressing RON WT or M1254T mutant were infected with adenovirus encoding β-Gal or DN Tcf-4. After 48 h the amount of c-myc and cyclin D1 was determined in total lysates from cells by Western blotting with anti-c-myc or anti-cyclin D1 antibody.
Focus-forming assay.
A focus-forming assay was performed as described (29). Briefly, NIH 3T3 cells stably expressing RON WT or RON M1254T or MET WT or MET M1268T were transfected by Superfect reagent (Qiagen) with 2 μg of pMSCVpuro vector (Clontech, Palo Alto, Calif.) carrying enhanced green fluorescent protein (EGFP) as a control or the kinase-dead RON (RONkd) gene in a six-well tissue culture plate. RONkd (K1114 M) was generated using the GeneEditor (Promega) mutagenesis kit as described previously (22). Two days later cells were split and placed in DMEM containing 10% FCS, 500 μg of Geneticin (Life Technologies, Inc.), and 1 μg of puromycin (Clontech)/ml. Transfected cells were used for the focus-forming assay and for Western blotting 3 weeks after transfection.
RESULTS
RON mutants induce constitutive phosphorylation of β-catenin.
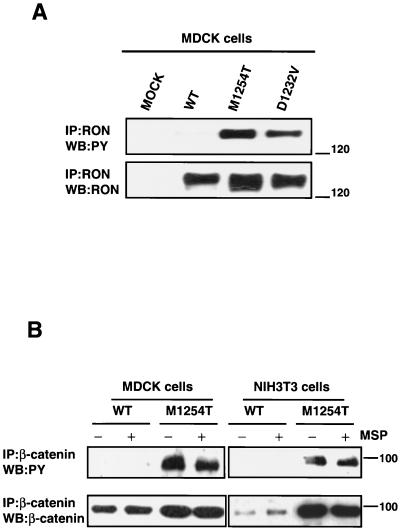
Single amino acid substitutions (D1232V or M1254T) in the RON tyrosine kinase domain that mimic the oncogenic mutations found in KIT (D814V) in human mastocytosis (110) and RET (M918T) in MEN2B (93) convert normal RON into an oncogenic receptor (95, 117). To investigate signaling pathways activated by RON M1254T and D1232V mutants that might contribute to oncogenic cell transformation, we established MDCK and NIH 3T3 cell lines expressing the WT, M1254T, or D1232V RON receptor. To avoid artifacts caused by clonal selection, we used in all experiments a polyclonal population of stable transfectants. We observed ligand-independent tyrosine phosphorylation of RON M1254 and D1232V receptor mutants in both MDCK (Fig. 1A) and NIH 3T3 cell lines (data not shown). Tyrosine phosphorylation of mutant RON receptors correlated with enhanced kinase activity of the receptors (data not shown). Both RON mutants displayed transforming potential, as determined by an in vitro focus-forming assay (data not shown). Our results are in agreement with published data on the constitutive activity and tumorigenicity of these mutants (95).
FIG. 1.
(A) Constitutive ligand-independent tyrosine phosphorylation of RON M1254T and D1232V receptor mutants. MDCK cells expressing RON WT or M1254T or D1232V mutants were lysed, and RON receptor was immunoprecipitated from lysates by anti-RON antibodies. RON tyrosine phosphorylation was detected by Western blotting (WB) with anti-PY antibodies (upper panel). The lower panel represents reblotting with anti-RON antibodies to obtain the amount of RON in precipitates. Positions of molecular-weight markers are indicated on the right in kilodaltons. MOCK, empty vector. (B) Mutant RON M1254T induces constitutive tyrosine phosphorylation of β-catenin. MDCK or NIH 3T3 cells expressing RON WT or M1254T mutant were stimulated with 5 nM MSP for 15 min. After stimulation cells were lysed, and β-catenin was immunoprecipitated with anti-β-catenin antibodies. Tyrosine phosphorylation of β-catenin (WB:PY) was detected by Western blotting with anti-PY antibodies (upper panel). The amount of β-catenin in precipitates was determined with anti-β-catenin antibodies (WB:β-catenin). Positions of molecular-weight markers are indicated on the right in kilodaltons.
A search for intracellular molecules that are constitutively tyrosine phosphorylated in cells expressing mutant RON revealed β-catenin (Fig. 1B, upper panel). In the absence of MSP stimulation, β-catenin was tyrosine phosphorylated in cells with RON M1254T (Fig. 1B, upper panel) and with RON D1232V (data not shown) but not in cells with RON WT (Fig. 1B, upper panel). Moreover, treatment of cells expressing RON WT with MSP did not cause β-catenin tyrosine phosphorylation (Fig. 1B, upper panel). These data suggest that activating mutations M1254T and D1232V may lead to a switch of RON kinase substrate specificity: β-catenin is a substrate for mutant RON but not for RON WT.
Association of β-catenin with RON receptor.
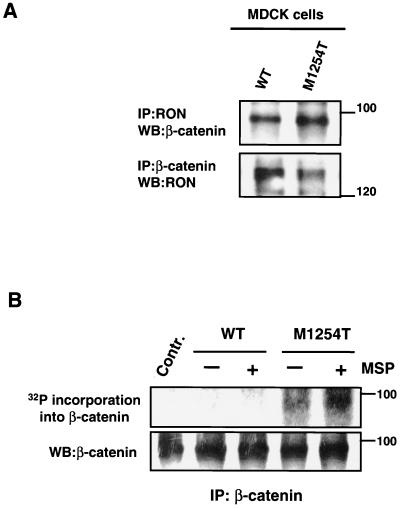
To investigate the potential association of RON with β-catenin in intact cells, MDCK-RON-expressing cell lines were lysed and immunoprecipitated with antibodies either against RON or β-catenin. Western blotting showed that RON and β-catenin were reciprocally coprecipitated from cell lysates (Fig. 2A). The association was constitutive (Fig. 2A) and was not affected by ligand stimulation (data not shown). β-Catenin coprecipitated with either immunoprecipitated RON WT or M1254T mutant (Fig. 2A, upper blot), and both receptors coprecipitated with immunoprecipitated β-catenin (Fig. 2A, lower blot). These data indicate that RON and β-catenin are spatially associated. We believe that β-catenin-RON association is not due to RON overexpression. RON expression in established MDCK and NIH 3T3 cell lines is no greater than endogenous RON expression in a HaCat cell line (data not shown).
FIG. 2.
(A) RON association with β-catenin. MDCK cells expressing RON WT or M1254T mutant were lysed. The cells from which these lysates were made were not stimulated with MSP. RON and β-catenin were immunoprecipitated from cell lysates with anti-RON and anti-β-catenin antibodies, respectively. β-Catenin in RON precipitates was detected by Western blotting (WB) with anti-β-catenin antibodies (upper panel). RON in β-catenin precipitates was detected with anti-RON antibodies (lower panel). Positions of molecular-weight markers are indicated on the right. (B) RON M1254T mutant but not RON WT can phosphorylate β-catenin in vitro. Nonstimulated or MSP-stimulated MDCK cells expressing RON WT or M1254T mutant were lysed, and RON was immunoprecipitated from cell lysates. After that beads containing immunoprecipitated RON were mixed with beads containing β-catenin immunoprecipitated from nonstimulated, starved MDCK parental cells (without detectable RON expression). Mixed, immunoprecipitated RON and β-catenin were incubated in kinase buffer with radioactive ATP at 30°C for 30 min. Proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Incorporation of 32P into β-catenin was detected by autoradiography (upper panel). The amount of β-catenin in each sample was detected by probing of the same membrane with anti-β-catenin antibodies (lower panel). Control, all components except for immunoprecipitated RON. Positions of molecular-weight markers are indicated on the right.
Mutated RON tyrosine kinase can phosphorylate β-catenin in vitro.
The association of RON with β-catenin suggests that the observed tyrosine phosphorylation of β-catenin in cells expressing M1254T mutant RON might be mediated by RON kinase. To investigate whether β-catenin is a potential substrate for RON kinase, we assayed the capacity of RON to phosphorylate β-catenin in vitro. RON was immunoprecipitated from nonstimulated or MSP-stimulated MDCK cells expressing either RON WT or M1254T mutant. β-Catenin was immunoprecipitated from nonstimulated, starved, parental MDCK cells, which do not express detectable RON. After that, beads carrying precipitated RON receptor (WT or M1254T mutant) were mixed with beads carrying precipitated β-catenin and were incubated in kinase buffer containing radioactive ATP. We were thus able to detect kinase-mediated incorporation of 32P into β-catenin. β-Catenin was phosphorylated when mixed with precipitated RON M1254T mutant but not with RON WT (Fig. 2B, upper panel). RON WT did not phosphorylate β-catenin (Fig. 2B, upper panel), even after receptor activation by the ligand (Fig. 2B, upper panel). β-Catenin phosphorylation by mutant RON was independent of ligand stimulation (Fig. 2B, upper panel). The kinase assay data (Fig. 2B) correlate with the results obtained in vivo (Fig. 1B, upper panel). Only RON M1254T mutant caused phosphorylation of β-catenin in vivo (Fig. 1B, upper panel) or in vitro (Fig. 2B, upper panel). The in vitro results show that mutant RON, or a kinase that coprecipitates with mutant RON, can phosphorylate β-catenin.
β-Catenin tyrosine phosphorylation correlates with its metabolic stability.
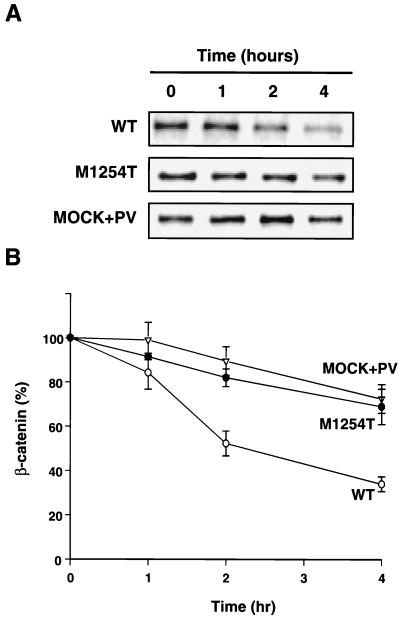
Because of the high amounts of β-catenin in cells expressing RON M1254T (Fig. 1B, lower panel), we postulated that β-catenin catabolism might be decreased in these cells. A pulse-chase assay was performed to determine the effect of RON M1254T on β-catenin catabolism. The β-catenin degradation rate in cells expressing RON M1254T was significantly lower than the rate in cells with RON WT (Fig. 3). To answer whether tyrosine phosphorylation of β-catenin affects its stability, we pretreated parental MDCK cells without detectable RON expression that had been transfected with the empty vector (MOCK) (control cell line) with PV, an inhibitor of tyrosine-specific phosphatases (53, 107). Exposure of cells to PV for 15 to 20 min resulted in tyrosine phosphorylation of a number of cellular proteins, including β-catenin. The level of β-catenin tyrosine phosphorylation was stable and comparable at all experimental time points (data not shown). PV also decreased the degradation rate of β-catenin (Fig. 3). From the data in Fig. 3, the estimated half-life of β-catenin was approximately 2 h in cells expressing RON WT and longer than 4 h in cells expressing RON M1254T or in MDCK-MOCK cells pretreated with PV. Since the delayed degradation of β-catenin in both of these experimental models is associated with tyrosine phosphorylation of β-catenin, phosphorylation may mediate β-catenin metabolic stability.
FIG. 3.
Expression of RON M1254T or cell treatment with PV increases metabolic stability of β-catenin. MDCK cells expressing RON WT, M1254T mutant, or MOCK (“empty vector”-expressing cells) pretreated with PV for 15 min were pulse labeled with [35S]methionine and/or cysteine for 1 h. Radioactive medium was then replaced by unlabeled medium, and cells were lysed at the indicated times. β-Catenin was immunoprecipitated from cell lysates, and the incorporation of 35S into β-catenin was detected by radioautography (A). The level of 35S incorporation into β-catenin was calculated by densitometry of the labeled β-catenin bands on the radioautographs and was expressed as the percentage of the value at time zero. Graph data are means ± standard errors of three independent experiments (B).
RON mutants inhibit β-catenin-axin/GSK3β complex formation.
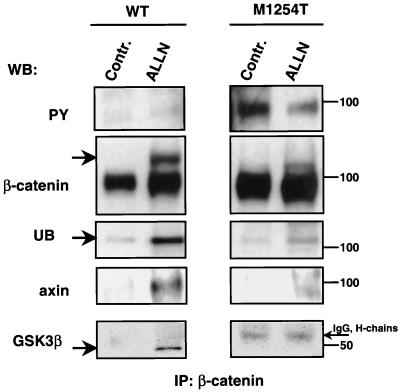
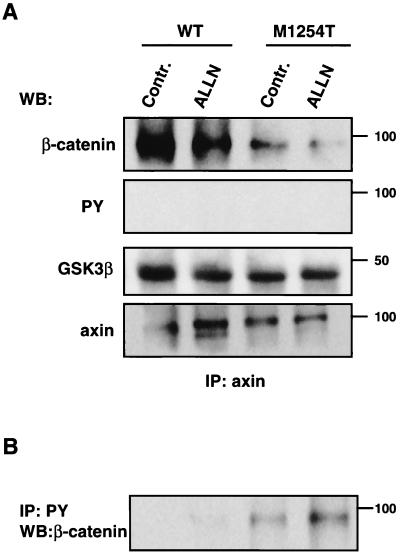
Four mechanisms are known to cause increases in cellular levels of β-catenin: (i) mutations in the β-catenin gene, (ii) mutations in the APC gene, (iii) mutations in the axin gene, and (iv) activation of Wnt signaling. All four result in the stabilization of β-catenin (84). The amount of β-catenin in cells is regulated by ubiquitin-dependent proteolysis (1). Pretreatment of cells with ALLN inhibits proteasome-mediated proteolysis of ubiquitinated proteins, which leads to accumulation of proteins degraded by this pathway (18). This approach allows detection of ubiquitinated proteins, including β-catenin (1). Pretreatment of cells expressing RON WT with ALLN led to the appearance of a high-molecular-weight β-catenin, which was consistent with β-catenin coupled to ubiquitin (Fig. 4, β-catenin, arrow). By probing the same membrane with antiubiquitin antibodies, we showed that the high-molecular-weight β-catenin band contained ubiquitin (Fig. 4, UB). In contrast to cells expressing RON WT, β-catenin IPs from cells expressing RON M1254T, with or without ALLN treatment, were tyrosine phosphorylated (Fig. 4, PY) and were not ubiquitinated (Fig. 4, UB). Our results suggest that β-catenin tyrosine phosphorylation occurring constitutively in cells with RON M1254T protects this molecule from ubiquitination and proteasome-induced degradation.
FIG. 4.
Expression of RON M1254T mutant prevents association of β-catenin with the axin/GSK3β kinase complex and consequent ubiquitination. WB, Western blotting; Contr., control. MDCK cells expressing RON WT or M1254T mutant were cultured in the presence of 25 μM proteasome inhibitor ALLN for 6 h. After that, cells were lysed and β-catenin was immunoprecipitated. Tyrosine phosphorylation of β-catenin was detected by Western blotting with anti-PY antibodies (PY). The β-catenin row shows results of reprobing with anti-β-catenin antibodies. High-molecular-weight β-catenin is labeled by the arrow on the left. Ubiquitinated β-catenin was detected by reprobing with antiubiquitin (UB). Its location corresponded to the position of high-molecular-weight β-catenin. Axin in β-catenin precipitates was detected by Western blotting with antiaxin antibodies (axin). GSK3β kinase in β-catenin precipitates was detected by anti-GSK3β antibodies (GSK3β). The GSK3β band is labeled by the arrow on the left. The immunoglobulin G (IgG) heavy chains corresponding to nonspecific bands are labeled by the arrow on the right. Positions of molecular-weight markers are indicated on the right.
Regulation of β-catenin degradation is a complex process involving a number of intracellular proteins. β-Catenin ubiquitination is preceded by phosphorylation of its N-terminal serine residues by GSK3β (44), which occurs only in a multimolecular complex formed between β-catenin, axin or axil (related scaffold protein [5, 44, 56]), GSK3β, and APC protein (5, 8, 43, 84). Expression of RON M1254T or treatment of cells expressing RON WT with PV blocked association of β-catenin with axin and GSK3β (Fig. 4, axin and GSK3β). The amounts of axin or GSK3β in cell lysates were comparable for the experimental conditions illustrated in Fig. 4 (data not shown). We conclude from these experiments that mutant RON stabilizes β-catenin by inhibiting complex formation between β-catenin and axin, which prevents β-catenin serine phosphorylation by GSK3β and its subsequent ubiquitination and degradation. Because expression of RON M1254T or pretreatment of RON WT cells with PV each causes β-catenin tyrosine phosphorylation and increased stability, we suggest that inhibition of complex formation between β-catenin and axin might be caused by tyrosine phosphorylation of β-catenin.
Tyrosine-phosphorylated β-catenin does not interact with axin.
To evaluate the possibility that β-catenin tyrosine phosphorylation inhibits its association with axin, we estimated the amount of β-catenin in axin IPs by Western blotting. The amount of β-catenin coprecipitated with axin was decreased in cells expressing RON M1254T mutant (Fig. 5A, β-catenin), despite the increased total amount of β-catenin in these cells (Fig. 1B, lower panel). Reprobing of the same membrane with anti-PY antibodies gave a negative result: there is no tyrosine-phosphorylated β-catenin in the complex with axin. Conversely, we were able to detect uncomplexed, tyrosine-phosphorylated β-catenin in cell lysate supernatants collected after immunoprecipitation of axin (Fig. 5B). We also investigated the association between axin and GSK3β kinase. Expression of RON M1254T mutant did not inhibit axin and GSK3β association (Fig. 5A, GSK3β). Thus our results indicate that RON M1254T mutant-induced β-catenin tyrosine phosphorylation prevents complex formation between β-catenin and axin, an essential event for β-catenin proteasomal degradation.
FIG. 5.
(A) Tyrosine-phosphorylated β-catenin is not associated with axin. WB, Western blotting; Contr., control. MDCK cells expressing RON WT or M1254T mutant were cultured in the presence of 25 μM proteasome inhibitor ALLN for 6 h. After that, cells were lysed and axin was immunoprecipitated and prepared for Western blotting by SDS-PAGE and transfer to a nitrocellulose membrane. Tyrosine-phosphorylated proteins (PY), β-catenin , GSK3β, and axin itself in axin precipitates were detected by anti-PY, anti-β-catenin, anti-GSK3, and antiaxin antibodies, respectively. Positions of molecular-weight markers are indicated on the right. (B) Tyrosine-phosphorylated β-catenin is detected in axin-uncomplexed fraction. After removal of axin from cell lysates by immunoprecipitation (described for panel A), tyrosine-phosphorylated proteins were immunoprecipitated by anti-PY antibodies and separated by SDS-PAGE. One of the phosphorylated proteins was β-catenin, detected by Western blotting with anti-β-catenin antibodies. Positions of molecular-weight markers are indicated on the right.
RON mutants cause constitutive transcriptional activation of Tcf-4 and increase c-myc and cyclin D1 expression by the Tcf-dependent mechanism.
Increased β-catenin occurs in many human cancers (68, 84). It is believed that an increase in β-catenin promotes tumor formation through activation of its downstream targets. β-Catenin can form a complex with Tcf/Lef transcriptional factors (57, 69). Tcf-4 transactivates transcription only when it is associated with β-catenin (57). c-myc and cyclin D1 genes contain a Tcf/Lef binding site in their promoter regions (37, 108). These genes are potential targets for transcriptional activation by β-catenin/Tcf (37, 101, 108) and have been implicated in oncogenic cell transformation and tumor development (9, 49, 58, 60, 86).
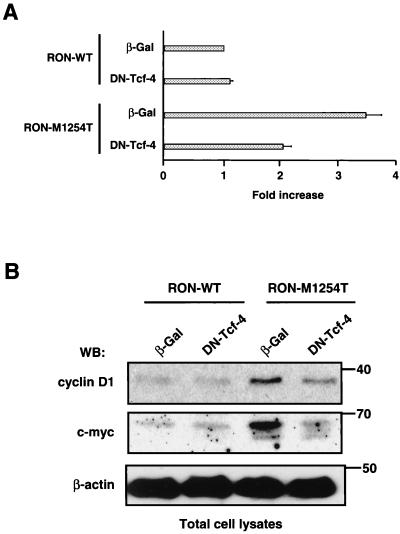
To investigate whether increased β-catenin in cells expressing RON mutants results in transcriptional activation of Tcf-4, we transfected luciferase reporter plasmids containing three copies of the Tcf-binding site (TOPFLASH) or three copies of mutated Tcf-binding site (FOPFLASH, used as a negative control, data not shown). Twenty-four hours before the transfection, these cells were infected with adenoviral constructs containing the β-Gal gene (a control construct) or DN Tcf-4. Results show that expression of RON M1254T mutant leads to constitutive transcriptional activation of Tcf as determined by the luciferase assay (Fig. 6A, third bar from top). In RON M1254T cells carrying the TOPFLASH and β-Gal-containing constructs, we observed an approximately fourfold increase of luciferase activity. Similar results were obtained when cells were transfected with only the TOPFLASH plasmid (data not shown). Expression of DN Tcf-4 in RON M1254T cells inhibited activity of endogenous Tcf, as shown by a decrease of luciferase activity in these cells (Fig. 6A, fourth bar from top).
FIG. 6.
(A) RON M1254T receptor mutant causes constitutive transactivation of Tcf consensus sequence (TOPFLASH)-driven transcription in MDCK cells. MDCK cells expressing RON WT or M1254T mutant were infected with adenovirus encoding β-Gal or DN Tcf-4, and 24 h later these cells were transfected with luciferase reporter plasmids containing WT (TOPFLASH) or mutated (FOPFLASH) Tcf promoter. Tcf activity was determined by a luciferase assay as described in Materials and Methods. Data were normalized for total protein concentration. Data for a negative control FOPFLASH are not shown. Luciferase activity was calculated in fold increase, where luciferase activity in cells expressing RON WT and β-Gal was taken for 1. Bar graph data are means ± standard errors of three independent experiments. (B) The increased level of c-myc and D1 expression in cells with mutated RON is mediated by Tcf. MDCK cells expressing RON WT or M1254T mutant were infected with adenovirus encoding β-Gal or DN Tcf-4. After 48 h the amount of c-myc and cyclin D1 was determined in total lysates from cells by Western blotting (WB) with anti-c-myc and anti-cyclin D1 antibodies. The β-actin panel serves as a control showing an equal amount of protein in each sample. Positions of molecular-weight markers are indicated on the right.
Because c-myc and cyclin D1 are potential targets of β-catenin/Tcf, we studied expression of these molecules in cells with WT or M1254T RON. We detected c-myc and cyclin D1 in lysates from MDCK cells expressing RON M1254T (Fig. 6B). In all instances, expression levels were higher than those from lysates of cells expressing RON WT. Expression of DN Tcf-4 decreased the level of both c-myc and cyclin D1 proteins in cells with RON M1254T mutant (Fig. 6B). Our results indicate that expression of RON M1254T leads to constitutive transcriptional activation of Tcf and to a consequent increase in c-myc and cyclin D1 by the Tcf-dependent mechanism.
Tumorigenic MET mutant M1268T also activates β-catenin pathway.
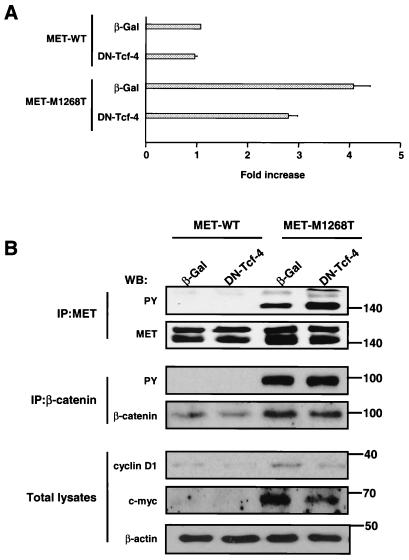
The finding of germ line and somatic mutations in the MET proto-oncogene in papillary renal carcimonas directly implicated the MET gene in the pathogenesis of this disease (98, 99). The oncogenic mutation M1268T in the MET kinase domain is at the position that is homologous to the M1254T mutation in RON. The fact that the RON M1254T mutant causes activation of the β-catenin pathway suggested that MET M1268T could also induce activation of the same pathway. To test this hypothesis, we used a polyclonal population of NIH 3T3 cell lines stably expressing MET M1268T or MET WT (as a control) (72). In agreement with previous data (51, 72), we observed constitutive tyrosine phosphorylation of the M1268T MET mutant (Fig. 7B). Results also demonstrated that expression of MET M1268T causes constitutive activation of Tcf transcriptional activity (Fig. 7A) and that increased c-myc and cyclin D1 are dependent on Tcf activity (Fig. 7B). Thus, both mutated RON and MET induce β-catenin accumulation and its activation.
FIG. 7.
(A) MET M1268T receptor mutant causes constitutive transactivation of Tcf consensus sequence (TOPFLASH)-driven transcription in NIH 3T3 cells. Activity of Tcf-4 in NIH 3T3 cells expressing MET WT or M1268T was determined by luciferase assay as described in the legend to Fig. 6A. Luciferase activity in cells expressing MET WT and β-Gal was set equal to 1. Bar graph data are means ± standard errors of three independent experiments. (B) The increased level of c-myc and D1 expression in cells with mutated MET is mediated by Tcf. NIH 3T3 cells expressing MET WT or M1268T were infected with an adenovirus encoding β-Gal or DN Tcf-4. After 48 h the amount of c-myc and cyclin D1 was determined in total lysates from cells by Western blotting with anti-c-myc and anti-cyclin D1 antibodies. MET tyrosine phosphorylation was determined by anti-PY antibodies in MET IPs. To estimate the amount of the MET in precipitates, the blot was probed with anti-MET antibodies (upper band, immature MET [170 kDa]; lower band, mature MET [140 kDa]). Tyrosine phosphorylation of β-catenin was detected by Western blotting (WB) with anti-PY antibodies. The amount of β-catenin in precipitates was determined with anti-β-catenin antibodies. The β-actin panel serves as a control showing an equal amount of protein in each sample. Positions of molecular-weight markers are indicated on the right.
RONkd inhibits activation of β-catenin pathway and decreases transforming ability of MET M1268T mutant.
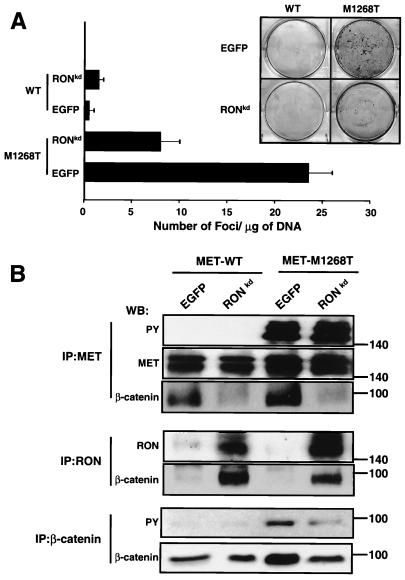
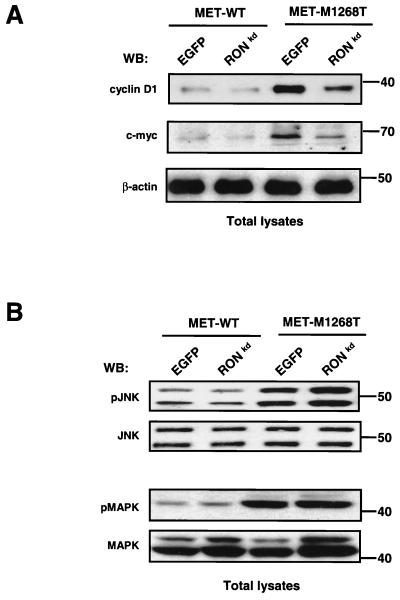
It was shown previously that coexpression of RONkd (lysine at position 1114 in the ATP-binding pocket of the RON kinase domain was replaced by alanine) with oncogenic MET mutants reduced the number of foci induced by the MET mutants (29). Thus, MET transforming ability was impaired by RONkd; however, the molecular mechanism underlying the effect of RONkd was not elucidated. We hypothesized that RONkd may inhibit activation of the β-catenin pathway mediated by the oncogenic MET mutants. To test this hypothesis, RONkd (in our case, Lys1114 was replaced by Met) was expressed in NIH 3T3 cells carrying M1268T or WT MET (as a control). The effect of RONkd on mutant MET-mediated transformation was studied in a focus-forming assay. Results show that RONkd significantly inhibited the number of foci formed by NIH 3T3 cells expressing the MET mutant (Fig. 8A). We found that RONkd inhibited association between MET M1268T and β-catenin (Fig. 8B). β-catenin is coimmunoprecipitated with both MET WT and M1268T from cells transfected with a control plasmid (containing an EGFP gene), whereas β-catenin is not coprecipitated with MET from cells expressing RONkd (Fig. 8B, IP:MET, WB:β-catenin). β-Catenin appeared in RON precipitates (Fig. 8B, IP:RON, WB: β-catenin). Relocation of β-catenin from MET to RON precipitates is accompanied by reduction of both the intracellular level of β-catenin and its tyrosine phosphorylation level (Fig. 8B, IP:β-catenin), but it does not affect M1268T MET tyrosine phosphorylation (Fig. 8B, IP:MET, WB:PY). Decreased β- catenin in cells coexpressing M1268T MET and RONkd correlates with the decreased level of β-catenin downstream targets c-myc and cyclin D1 (Fig. 9A), but there is no reduction in activation of JNK and MAPK pathways induced by the M1268T MET mutant (Fig. 9B). Similar results were obtained when RONkd was expressed in cells carrying the M1254T RON mutant (data not shown).
FIG. 8.
(A) Expression of RONkd reduces the transforming ability of the oncogenic M1268T MET mutant. RONkd or EGFP (a control) was coexpressed in NIH 3T3 cells carrying M1268T or WT (as a control) MET. MET transforming activity was determined by a focus-forming assay 3 weeks after transfection and culture in the presence of selective antibiotics. To count foci, cells were subjected to Giemsa staining. The bar graph (data are means ± standard errors of three independent experiments) represents the results. The inset shows representative plates. (B) Expression of RONkd interferes with MET/β-catenin association, leading to reduction of the β-catenin level and inhibition of its tyrosine phosphorylation. NIH 3T3 cells expressing WT or M1268T MET were transfected by RONkd or EGFP (a control) cDNA, and 3 weeks later cell lysates were analyzed by Western blotting (WB). MET tyrosine phosphorylation was determined by anti-PY antibodies in MET IPs. To estimate the amount of MET or β-catenin in precipitates, the blot was probed with anti-MET (upper band, immature MET [170 kDa]; lower band, mature MET [140 kDa]) or anti-β-catenin antibody, respectively. The amount of RON and β-catenin in RON precipitates was determined with anti-RON and anti-β-catenin antibodies, respectively. Tyrosine phosphorylation of β-catenin was detected by Western blotting with anti-PY antibodies. The amount of β-catenin in precipitates was determined with anti-β-catenin antibodies. Positions of molecular-weight markers are indicated on the right.
FIG. 9.
Expression of RONkd reduces the level of c-myc and cyclin D1 in cells with M1268T MET but does not affect M1268T MET-induced activation of JNK and MAPK. NIH 3T3 cells with WT or M1268T mutant were transfected with RONkd or EGFP cDNAs, and 3 weeks later cell lysates were analyzed by Western blotting (WB). (A) The amount of c-myc and cyclin D1 was determined in total lysates from cells by Western blotting with anti-c-myc and anti-cyclin D1 antibodies, respectively. The β-actin panel serves as a control showing an equal amount of protein in each sample. Positions of molecular-weight markers are indicated on the right. (B) Activation of JNK and MAPK in cell lysates was determined by anti-phospho-JNK and anti-phospho-MAPK antibodies, respectively. The amount of JNK (the two bands represent p46 JNK1 and p54 JNK2) and of MAPK (the two bands represent p42 and p44 ERK1/ERK2) was determined by reprobing of the membrane with JNK and anti-MAPK antibodies, respectively. Positions of molecular-weight markers are indicated on the right.
DISCUSSION
Experimental mutations in the conserved region of the RON kinase domain (D1232V, KIT type; M1254T, RET/MET type) cause the RON receptor to become oncogenic (95, 117). To investigate signaling pathways contributing to RON mutant-mediated oncogenic cell transformation, we established MDCK and NIH 3T3 cell lines expressing WT or M1254T or D1232V RON receptors. Because RON is an RTK which became constitutively active as a result of the M1254T or D1232V mutations, we looked for tyrosine-phosphorylated intracellular proteins that might be substrates for RON mutants.
The investigation showed that β-catenin is constitutively tyrosine phosphorylated in cells expressing mutated but not WT RON even after its activation by the ligand (Fig. 1B, upper panels). These data suggest that in addition to constitutive ligand-independent kinase activation, M1254T and D1232V mutations in RON might cause a switch of RON kinase substrate specificity or inhibition of catalytic activity of tyrosine-specific phosphatases responsible for β-catenin dephosphorylation. Previously observed effects on cell signaling caused by the same RON mutants (95) or by oncogenic RET and MET receptor mutants (4, 51, 78, 93) favor the possibility that the kinase substrate specificity of the mutant receptor differs from that of the WT (54).
What kinases are responsible for growth factor RTK-induced β-catenin tyrosine phosphorylation? We obtained evidence that oncogenic RON mutants can phosphorylate β-catenin. RON and β-catenin were reciprocally coprecipitated (Fig. 2A), and in an in vitro kinase assay, nonphosphorylated β-catenin was phosphorylated when it was mixed with RON mutant IPs (Fig. 2B, upper panel). This indicates that β-catenin is a substrate for mutated RON or for a kinase that associates with and is activated by mutated RON. In other systems, β-catenin has been found associated with the epidermal growth factor (EGF), c-Erb-2, or MET receptor (38, 41, 54) and β-catenin is tyrosine phosphorylated in cells stimulated by ligands for these receptors (40, 41, 45, 80). The capacity to directly phosphorylate β-catenin has been shown only for the EGF receptor (41). Another possibility is that growth factor-induced β-catenin tyrosine phosphorylation is mediated by c-Src or c-Src-related kinases. Cell stimulation with growth factors causes activation of c-Src in many cases (67, 112), and active c-Src can phosphorylate β-catenin as well other proteins of adherens junctions (11, 36, 87, 111).
RON mutant-induced β-catenin tyrosine phosphorylation is associated with increased cellular β-catenin (Fig. 1B, lower panel) and a decreased degradation rate (Fig. 3). Likewise, treatment of cells with PV, a tyrosine phosphatase inhibitor, causes both tyrosine phosphorylation of β-catenin and its metabolic stability (Fig. 3). These data suggest that β-catenin tyrosine phosphorylation may play a role in its stabilization. Consistent with this idea is a positive correlation between an EGF-induced increase in the cellular pool of β-catenin and the level of β-catenin phosphorylation (70).
A search for the mechanism underlying RON mutant-induced β-catenin accumulation revealed that expression of RON mutants inhibited complex formation between β-catenin and axin (Fig. 4), a step required for ubiquitination and proteasomal degradation of β-catenin (26, 44). Analysis of axin-coupled and uncoupled fractions of β-catenin showed no phosphorylated tyrosine residues in the β-catenin coupled to axin (Fig. 5A), whereas axin-free β-catenin was tyrosine phosphorylated (Fig. 5B). These results suggest that tyrosine phosphorylation of β-catenin prevents its association with axin. A similar protein stabilization mechanism has been described for IκB. In resting cells, NF-κB is sequestered in an inactive state by association with IκB. Release from this sequestration is caused by phosphorylation of IκB at a consensus phosphorylation motif that includes serines 32 and 36. This leads to ubiquitination and proteasome-mediated degradation of IκB. Serine phosphorylation of IκB and its resulting degradation can be prevented by phosphorylation of tyrosine 42 (46, 103). Thus, tyrosine phosphorylation might be a general mechanism for protecting proteins from ubiquitin-dependent proteolysis.
Three possible mechanisms of β-catenin dissociation from axin have been proposed: (i) Wnt-dependent dephosphorylation of axin leading to its rapid degradation, (ii) Wnt-induced dephosphorylation of axin that decreases its affinity for β-catenin and GSK3β, and (iii) involvement of FRAT1, a protein which binds to GSK3β and inhibits GSK-dependent phosphorylation (55, 109, 116, 118). Our data suggest the existence of an additional mechanism involving tyrosine phosphorylation of β-catenin. Because RON mutants did not block axin interaction with GSK3β (Fig. 5A) and because we did not detect changes in axin and GSK3β expression levels or inhibition of GSK3β kinase activity (data not shown), the simplest explanation is that tyrosine phosphorylation of β-catenin causes conformational changes that decrease its affinity for axin. The data showing that tyrosine-phosphorylated β-catenin undergoes conformational changes during mouse oocyte development (76) support this view. On the other hand, our data cannot exclude that tyrosine-phosphorylated β-catenin and axin are located in spatially different subcellular pools, which prevents complex formation between these proteins.
Tyrosine phosphorylation of β-catenin has been observed in a number of apparently unrelated conditions: in the response to growth factors and cytokines (39–41, 45, 54, 70, 100), in Src-transformed cells (36, 90, 111), and in mouse oocyte development (76). What is the physiological role of β-catenin tyrosine phosphorylation? Two distinct roles for β-catenin have been established, one in the maintenance of cell-cell adhesion and the other in transcription of genes involved in growth and development. It is believed that tyrosine phosphorylation of β-catenin causes loss of intercellular adhesion mediated by E-cadherins (6, 19, 63, 70). In addition to effects on E-cadherin-associated β-catenin, tyrosine phosphorylation is involved in regulation of the free cytoplasmic pool of β-catenin, the pool that transduces signaling and activates gene expression (68, 79, 84).
We have shown that expression of RON RTK oncogenic mutants leads to increases in β-catenin and its potential downstream oncogene target products, c-myc and cyclin D1. The increased levels of c-myc and cyclin D1 proteins in cells expressing M1254T or D1232V RON receptor mutants is due to constitutive transcriptional activation of Tcf-4 (Fig. 6), which is known to be in the β-catenin pathway. It is believed that the increase in β-catenin which occurs in many human cancers (14, 16, 57, 69, 92, 96) promotes tumor formation through activation of these downstream targets. c-myc and cyclin D1 genes have been implicated in oncogenic cell transformation and tumor development (9, 49, 58, 60, 86).
We also found that activation of the β-catenin pathway by constitutively active mutated RTK is not unique for RON. Expression of the oncogenic MET M1268T mutant affects β-catenin in a similar way. In cells with the MET mutant we detected accumulation of β-catenin, constitutive activation of Tcf, and a Tcf-dependent increase in c-myc and cyclin D1 expression (Fig. 7).
To show a role of the β-catenin pathway in cellular transformation induced by mutated RON and MET, RONkd was coexpressed in NIH 3T3 cells carrying mutated RON or MET. Our results (Fig. 8A) correlate with previously published data that the expression of RONkd reduces the number of foci induced by the active MET mutants (29). We found that RON kd competes with activating MET (Fig. 8B) and RON mutants for β-catenin association. It can make β-catenin unavailable as a substrate for constitutively active MET and RON kinases, leading to reduced β-catenin tyrosine phosphorylation and decrease in β-catenin, c-myc, and cyclin D1 (Fig. 8B and 9A). At the same time, expression of RONkd does not affect activation of JNK and MAPK pathways (Fig. 9B) caused by mutated MET. It was shown previously that activation of JNK and MAPK pathways is essential for cell transformation by MET (4, 52, 88). Inhibition of either pathway resulted in significant but partial reduction of MET transforming potential. It has been shown that expression of the stabilized β-catenin mutant which activates Tcf is not sufficient to cause cell transformation, whereas Wnt-1-induced β-catenin accumulation and Tcf activation correlate with cell transformation supports (119). These data together with our results suggest that several pathways can contribute to MET-induced transformation; cooperation between these pathways might be important.
Using RONkd, we selectively interfered with activation of the β-catenin pathway, leading to inhibition of MET and RON mutant transforming potential. Thus, the mechanism of β-catenin accumulation described in this paper is involved in oncogenic cell transformation induced by tumor-causing RON and MET mutants.
ACKNOWLEDGMENTS
We thank B. Vogelstein (Johns Hopkins University, Oncology Center, Baltimore, Md.) for β-Gal- and DN Tcf-4-containing adenoviral constructs, R. Breathnach (INSERM U211, Nantes, France) for human RON WT cDNA, F.A. Montero-Julian (Immunotech, Nantes, France) for mouse monoclonal anti-RON antibodies, and P. Dashner (Carcinogenesis and Cellular Defense Section, Basic Research Laboratory, SAIC, Frederick, Md.) for his help in luciferase assays.
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banu N, Price D J, London R, Deng B, Mark M, Godowski P J, Avraham H. Modulation of megakaryocytopoiesis by human macrophage-stimulating protein, the ligand for the RON receptor. J Immunol. 1996;156:2933–2940. [PubMed] [Google Scholar]
- 4.Bardelli A, Longati P, Gramaglia D, Basilico C, Tamagnone L, Giordano S, Ballinari D, Michieli P, Comoglio P M. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc Natl Acad Sci USA. 1998;95:14379–14383. doi: 10.1073/pnas.95.24.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens J, Jerchow B A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel M M, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Ze'ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- 8.Bienz M. APC: the plot thickens. Curr Opin Genet Dev. 1999;9:595–603. doi: 10.1016/s0959-437x(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 9.Bodrug S E, Warner B J, Bath M L, Lindeman G J, Harris A W, Adams J M. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottaro D P, Rubin J S, Faletto D L, Chan A M, Kmiecik T E, Vande Woude G, Aaronson S A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogen product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 11.Brown M T, Cooper J A. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Bullions L C, Levine A J. The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol. 1998;10:81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Bussolino F, Di Renzo M F, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio P M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan E F, Gat U, McNiff J M, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Seol D W, Carr B, Zarnegar R. Expression of c-Myc in response to colony-stimulating factor-1 requires mitogen-activated protein kinase kinase-1. J Biol Chem. 1999;274:6553–6558. doi: 10.1074/jbc.274.10.6553. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Axin and hepatocellular carcinomas. Nat Genet. 2000;24:206–208. doi: 10.1038/73396. [DOI] [PubMed] [Google Scholar]
- 17.Cooper C S, Park M, Blair D G, Tainsky M A, Huebner K, Croce C M, Vande Woude G. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 18.Coux O, Tanaka K, Goldberg A L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 19.Daniel J H, Reynolds A B. Tyrosine phosphorylation and cadherin/catenin function. BioEssays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- 20.Danilkovitch A, Donley S, Skeel A, Leonard E J. Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells. Mol Cell Biol. 2000;20:2218–2227. doi: 10.1128/mcb.20.6.2218-2227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danilkovitch A, Leonard E J. Kinases involved in MSP/RON signaling. J Leukoc Biol. 1999;65:345–348. doi: 10.1002/jlb.65.3.345. [DOI] [PubMed] [Google Scholar]
- 22.Danilkovitch-Miagkova A, Angeloni D, Skeel A, Donley S, Lerman M, Leonard E J. Integrin-mediated RON growth factor receptor phosphorylation requires tyrosine kinase activity of both the receptor and c-Src. J Biol Chem. 2000;275:14783–14786. doi: 10.1074/jbc.C000028200. [DOI] [PubMed] [Google Scholar]
- 23.Di Renzo M F, Narsimhan R P, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio P M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997–2003. [PubMed] [Google Scholar]
- 24.Di Renzo M F, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti M A. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 1992;7:2549–2553. [PubMed] [Google Scholar]
- 25.Di Renzo M F, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- 26.Easwaran V, Song V, Polakis P, Byers S. The ubiquitin-proteasome pathway and serine kinase activity modulate adenomatous polyposis coli protein-mediated regulation of beta-catenin-lymphocyte enhancer-binding factor signaling. J Biol Chem. 1999;274:16641–16645. doi: 10.1074/jbc.274.23.16641. [DOI] [PubMed] [Google Scholar]
- 27.Ebens A, Brose K, Leonardo E D, Hanson M G J, Bladt F, Birchmeier C, Barres B A, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 28.Ferracini R, Di Renzo M F, Scotlandi K, Baldini N, Olivero M, Lollini P, Cremona O, Campanacci M, Comoglio P M. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene. 1995;10:739–749. [PubMed] [Google Scholar]
- 29.Follenzi A, Bakovic S, Gual P, Stella M C, Longatti P, Comoglio P M. Cross-talk between the proto-oncogenes MET and RON. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 30.Galimi F, Bagnara G P, Bonsi L, Cottone E, Follenzi A, Simeone A, Comoglio P M. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol. 1994;127:1743–1754. doi: 10.1083/jcb.127.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo K A, Godowski P J, Comoglio P M. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherardi E, Stoker M. Hepatocyte growth factor—scatter factor: mitogen, motogen, and met. Cancer Cells. 1991;3:227–232. [PubMed] [Google Scholar]
- 33.Giordano S, Di Renzo M F, Olivero M, Mondino A, Zhen Z, Medico E, Comoglio P M. The c-met/HGF receptor in human tumours. Eur J Cancer Prev. 1992;1(Suppl. 3):45–49. doi: 10.1097/00008469-199210003-00007. [DOI] [PubMed] [Google Scholar]
- 34.Giordano S, Ponzetto C, Di Renzo M F, Cooper C S, Comoglio P M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- 35.Grant D S, Kleinman H K, Goldberg I D, Bhargava M M, Nickoloff B J, Kinsella J L, Polverini P, Rosen E M. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 38.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiscox S, Jiang W G. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun. 1999;261:406–411. doi: 10.1006/bbrc.1999.1002. [DOI] [PubMed] [Google Scholar]
- 40.Hiscox S, Jiang W G. Hepatocyte growth factor/scatter factor disrupts epithelial tumour cell-cell adhesion: involvement of beta-catenin. Anticancer Res. 1999;19:509–517. [PubMed] [Google Scholar]
- 41.Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huff J L, Jelinek M A, Borgman C A, Lansing T J, Parsons J T. The protooncogene c-sea encodes a transmembrane protein-tyrosine kinase related to the Met/hepatocyte growth factor/scatter factor receptor. Proc Natl Acad Sci USA. 1993;90:6140–6144. doi: 10.1073/pnas.90.13.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilan N, Mahooti S, Rimm D L, Madri J A. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci. 1999;112:3005–3014. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- 46.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J F. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 47.Iwama A, Wang M H, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard E J. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–3403. [PubMed] [Google Scholar]
- 48.Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15:5866–5875. [PMC free article] [PubMed] [Google Scholar]
- 49.Jamerson M H, Johnson M D, Dickson R B. Dual regulation of proliferation and apoptosis: c-myc in bitransgenic murine mammary tumor models. Oncogene. 2000;19:1065–1071. doi: 10.1038/sj.onc.1203268. [DOI] [PubMed] [Google Scholar]
- 50.Jeffers M, Rong S, Vande Woude G F. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med. 1996;74:505–513. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- 51.Jeffers M, Schmidt L, Nakaigawa N, Webb C P, Weirich G, Kishida T, Zbar B, Vande Woude G F. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffers M, Fiscella M, Webb C P, Anver M, Koochekpour S, Vande Woude G F. The mutationally activated MET receptor mediates motility and metastasis. Proc Natl Acad Sci USA. 1998;95:14417–14422. doi: 10.1073/pnas.95.24.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadota S, Fantus I G, Deragon G, Guyda H J, Hersh B, Posner B I. Peroxide(s) of vanadium: a novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem Biophys Res Commun. 1987;147:259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- 54.Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimoto S, Hirohashi S. c-erbB-2 gene product directly associates with beta-catenin and plakoglobin. Biochem Biophys Res Commun. 1995;208:1067–1072. doi: 10.1006/bbrc.1995.1443. [DOI] [PubMed] [Google Scholar]
- 55.Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- 56.Kikuchi A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999;10:255–265. doi: 10.1016/s1359-6101(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 57.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 58.Kourea H P, Cordon-Cardo C, Dudas M, Leung D, Woodruff J M. Expression of p27(kip) and other cell cycle regulators in malignant peripheral nerve sheath tumors and neurofibromas: the emerging role of p27(kip) in malignant transformation of neurofibromas. Am J Pathol. 1999;155:1885–1891. doi: 10.1016/S0002-9440(10)65508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurihara N, Iwama A, Tatsumi J, Ikeda K, Suda T. Macrophage-stimulating protein activates STK receptor tyrosine kinase on osteoclasts and facilitates bone resorption by osteoclast-like cells. Blood. 1996;87:3704–3710. [PubMed] [Google Scholar]
- 60.Lin S Y, Xia W, Wang J C, Kwong K Y, Spohn B, Wen Y, Pestell R G, Hung M C. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maggiora P, Marchio S, Stella M C, Giai M, Belfiore A, De Bortoli M, Di Renzo M F, Costantino A, Sismondi P, Comoglio P M. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- 62.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 63.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michalopoulos G K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176–187. [PubMed] [Google Scholar]
- 65.Montero-Julian F A, Dauny I, Flavetta S, Ronsin C, Andre F, Xerri L, Wang M H, Marvaldi J, Breathnach R, Brailly H. Characterization of two monoclonal antibodies against the RON tyrosine kinase receptor. Hybridoma. 1998;17:541–551. doi: 10.1089/hyb.1998.17.541. [DOI] [PubMed] [Google Scholar]
- 66.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 67.Mori S, Ronnstrand L, Yokote K, Engstrom A, Courtneidge S A, Claesson-Welsh L, Heldin C H. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993;12:2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morin P J. Beta-catenin signaling and cancer. BioEssays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 69.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 70.Muller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 71.Nagata H, Worobec A S, Oh C K, Chowdhury B A, Tannenbaum S, Suzuki Y, Metcalfe D D. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakaigawa N, Weirich G, Schmidt L, Zbar B. Tumorigenesis mediated by MET mutant M1268T is inhibited by dominant-negative Src. Oncogene. 2000;19:2996–3002. doi: 10.1038/sj.onc.1203628. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishino T, Hisha H, Nishino N, Adachi M, Ikehara S. Hepatocyte growth factor as a hematopoietic regulator. Blood. 1995;85:3093–3100. [PubMed] [Google Scholar]
- 75.Nusse R, Varmus H E. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 76.Ohsugi M, Butz S, Kemler R. Beta-catenin is a major tyrosine-phosphorylated protein during mouse oocyte maturation and preimplantation development. Dev Dyn. 1999;216:168–176. doi: 10.1002/(SICI)1097-0177(199910)216:2<168::AID-DVDY7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 77.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandit S D, Donis-Keller H, Iwamoto T, Tomich J M, Pike L J. The multiple endocrine neoplasia type 2B point mutation alters long-term regulation and enhances the transforming capacity of the epidermal growth factor receptor. J Biol Chem. 1996;271:5850–5858. doi: 10.1074/jbc.271.10.5850. [DOI] [PubMed] [Google Scholar]
- 79.Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- 80.Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun. 1998;247:851–858. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- 81.Park M, Dean M, Kaul K, Braun M J, Gonda M A, Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 83.Persons D A, Paulson R F, Loyd M R, Herley M T, Bodner S M, Bernstein A, Correll P H, Ney P A. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 84.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 85.Pumiglia K M, Lau L F, Huang C K, Burroughs S, Feinstein M B. Activation of signal transduction in platelets by the tyrosine phosphatase inhibitor pervanadate (vanadyl hydroperoxide) Biochem J. 1992;286:441–449. doi: 10.1042/bj2860441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao R N, Stamm N B, Otto K, Kovacevic S, Watkins S A, Rutherford P, Lemke S, Cocke K, Beckmann R P, Houck K, Johnson D, Skidmore B J. Conditional transformation of rat embryo fibroblast cells by a cyclin D1-cdk4 fusion gene. Oncogene. 1999;18:6343–6356. doi: 10.1038/sj.onc.1203009. [DOI] [PubMed] [Google Scholar]
- 87.Reynolds A B, Herbert L, Cleveland J L, Berg S T, Gaut J R. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–2445. [PubMed] [Google Scholar]
- 88.Rodrigues G A, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ronsin C, Muscatelli F, Mattei M G, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 90.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 91.Rubin J S, Chan A M, Bottaro D P, Burgess W H, Taylor W G, Cech A C, Hirschfield D W, Wong J, Miki T, Finch P W. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rubinfeld B, Robbins P, El Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 93.Santoro M, Carlomagno F, Romano A, Bottaro D P, Dathan N A, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus M H. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 94.Santoro M, Collesi C, Grisendi S, Gaudino G, Comoglio P M. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol Cell Biol. 1996;16:7072–7083. doi: 10.1128/mcb.16.12.7072. . (Erratum, 17:1758, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santoro M, Penengo L, Minetto M, Orecchia S, Cilli M, Gaudino G. Point mutations in the tyrosine kinase domain release the oncogenic and metastatic potential of the Ron receptor. Oncogene. 1998;17:741–749. doi: 10.1038/sj.onc.1201994. [DOI] [PubMed] [Google Scholar]
- 96.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt L, Duh F M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J, Walther M M, Tsui L C, Geil L, Orcutt M L, Stackhouse T, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, Lubensky I, Neumann H P, Brauch H, Decker J, Vocke C, Brown J A, Jenkins R, Richard S, Bergerheim U, Gerrard B, Dean M, Linehan W M, Zbar B. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 100.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- 101.Shtutman H, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siegfried E, Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. BioEssays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- 103.Singh S, Darnay B G, Aggarwal B B. Site-specific tyrosine phosphorylation of IkappaBalpha negatively regulates its inducible phosphorylation and degradation. J Biol Chem. 1996;271:31049–31054. doi: 10.1074/jbc.271.49.31049. [DOI] [PubMed] [Google Scholar]
- 104.Smith D R, Vogt P K, Hayman M J. The v-sea oncogene of avian erythroblastosis retrovirus S13: another member of the protein-tyrosine kinase gene family. Proc Natl Acad Sci USA. 1989;86:5291–5295. doi: 10.1073/pnas.86.14.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 106.Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- 107.Swarup G, Cohen S, Garbers D L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- 108.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 109.Thomas G M, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- 110.Tsujimura T. Role of c-kit receptor tyrosine kinase in the development, survival and neoplastic transformation of mast cells. Pathol Int. 1996;46:933–938. doi: 10.1111/j.1440-1827.1996.tb03571.x. [DOI] [PubMed] [Google Scholar]
- 111.Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Twamley-Stein G M, Pepperkok R, Ansorge W, Courtneidge S A. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci USA. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang M H, Dlugosz A A, Sun Y, Suda T, Skeel A, Leonard E J. Macrophage-stimulating protein induces proliferation and migration of murine keratinocytes. Exp Cell Res. 1996;226:39–46. doi: 10.1006/excr.1996.0200. [DOI] [PubMed] [Google Scholar]
- 114.Wang M H, Montero-Julian F A, Dauny I, Leonard E J. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene. 1996;13:2167–2175. [PubMed] [Google Scholar]
- 115.Wang M H, Ronsin C, Gesnel M C, Coupey L, Skeel A, Leonard E J, Breathnach R. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994;266:117–119. doi: 10.1126/science.7939629. [DOI] [PubMed] [Google Scholar]
- 116.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams T A, Longati P, Pugliese L, Gual P, Bardelli A, Michieli P. METprc mutations in the Ron receptor result in upregulation of tyrosine kinase activity and acqisition of oncogenic potential. J Cell Physiol. 1999;181:507–514. doi: 10.1002/(SICI)1097-4652(199912)181:3<507::AID-JCP15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 119.Young C S, Kitamura M, Hardy S, Kitajewski J. Wnt-1 induces growth, cytosolic β-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]