Abstract
Patients with cancer are more likely to have impaired immune responses to SARS-CoV-2 vaccines. We study the breadth of responses against SARS-CoV-2 variants after primary vaccination in 178 patients with a variety of tumor types and after booster doses in a subset. Neutralization of alpha, beta, gamma, and delta SARS-CoV-2 variants is impaired relative to wildtype, regardless of vaccine type. Regardless of viral variant, mRNA1273 is the most immunogenic, followed by BNT162b2, and then Ad26.COV2.S. Neutralization of more variants (breadth) is associated with a greater magnitude of wildtype neutralization, and increases with time since vaccination; advancing age associates with a lower breadth. The concentrations of anti-spike protein antibody are a good surrogate for breadth (positive predictive value of =90% at >1,000 U/mL). Booster SARS-CoV-2 vaccines confer enhanced breadth. These data suggest that achieving a high antibody titer is desirable to achieve broad neutralization; a single booster dose with the current vaccines increases the breadth of responses against variants.
Keywords: SARS-CoV-2, mRNA1273, BNT162b2, Ad26.COV2.S, booster dose, breadth, neutralization, variants, cancer
Graphical abstract
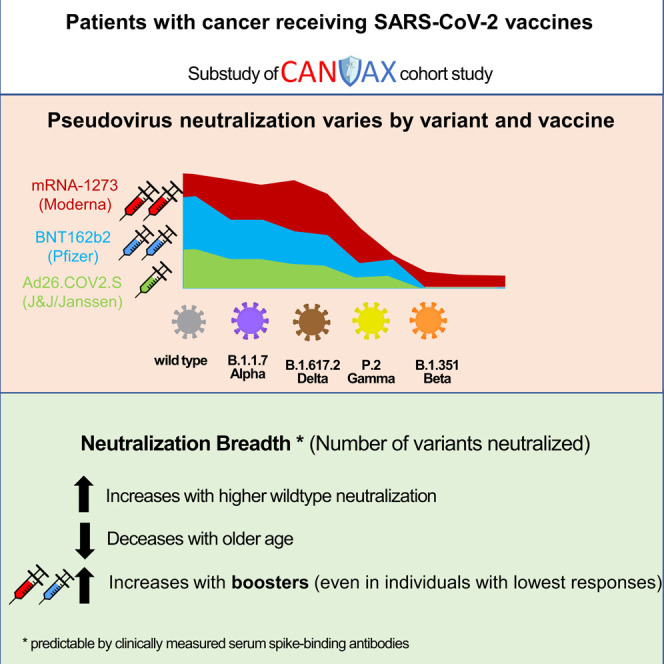
Naranbhai et al. examine how well current wildtype-based SARS-CoV-2 vaccines perform in patients with cancer in neutralizing viral variants. The findings support preferring the most immunogenic wildtype vaccines (i.e., mRNA1273 or BNT162b2) for patients at high risk to generate breadth against variants.
Patients with cancer are at an increased risk of severe disease and/or death from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Bakouny et al., 2020; Kuderer et al., 2020) and vaccination against SARS-CoV-2 is a cornerstone of prevention. The magnitude of anti-SARS-CoV-2 spike protein antibodies, receptor-binding domain (RBD) antibodies and neutralization titer against wildtype SARS-CoV-2 are robust correlates of vaccine-mediated protection (Earle et al., 2021; Khoury et al., 2021), but are not sufficiently validated for use clinically. In the Cancer, COVID and Vaccination study (CANVAX) of more than 750 patients with cancer, we observed lower humoral immune responses than in controls without cancer (Naranbhai et al., 2021a), consistent with findings from other cohorts (Addeo et al., 2021; Bird et al., 2021; Van Oekelen et al., 2021; Thakkar et al., 2021). In both controls without cancer and patients with cancer, the magnitude of response was strongly associated with prior SARS-CoV-2 infection and vaccine type: mRNA1273 was the most immunogenic followed by BNT162b2. Both mRNA vaccines were markedly more immunogenic than Ad26.COV2.S. Finally, booster vaccines were able to overcome poor responses in CANVAX (Naranbhai et al., 2021a) and other studies (Greenberger et al., 2021; Shapiro et al., 2021).
The evolution of the SARS-CoV-2 variants with mutations that confer greater transmissibility or evasion of immune responses pose an ongoing threat, as exemplified by the rapid rise of the delta variant to global dominance during 2021. We, and others, have previously observed marked variation of in vitro neutralization of viral variants in healthy individuals (Garcia-Beltran et al., 2021a; Tada et al., 2021). There are few robust data regarding the degree of protection against each variant after different vaccines in immunocompromised patients, but the frequency of breakthrough infection resulting in hospitalization seems to be markedly higher for immunocompromised patients than in the general population, highlighting the impact of lower immunogenicity and higher risk of severe disease (Hippisley-Cox et al., 2021). Based on these and other data, additional booster vaccine doses have been recommended for immunocompromised patients in many developed countries. Although these vaccine increase the magnitude of response (Greenberger et al., 2021; Shapiro et al., 2021), whether homologous (i.e. wildtype strain based) booster doses enhance the breadth of protection against variants is uncertain (Cho et al., 2021).
Here, we examine the magnitude and breadth of neutralization of SARS-CoV-2 variants after the primary series, and after booster doses of vaccination in patients with cancer who received one of the SARS-CoV-2 US Food Drug Administration (FDA) Emergency Use Authorization (EUA) vaccines in the United States.
Results
The CANVAX study is an ongoing prospective cohort study of SARS-CoV-2 vaccines in patients with cancer. For this report, we selected 178 participants of CANVAX without prior SARS-CoV-2 infection who were sampled 14 or more days after vaccination stratifying by vaccine type: 58 mRNA1273 (Moderna), 60 BNT162b2 (Pfizer/BioNTech), and 60 Ad26.COV2S (J&J/Janssen). The baseline participant characteristics known to affect immunogenicity are shown according to vaccine type in Table 1 , and recapitulate those of the overall CANVAX study: Ad26.COV2.S recipients were slightly older but the sex, cancer type, and therapy types were similar between groups.
Table 1.
Baseline characteristics of participants in this study
| Characteristic | Overall (n = 178) | mRNA1273 (n = 58) | BNT162b2 (n = 60) | Ad26.CoV2.S (n = 60) | p Value |
|---|---|---|---|---|---|
| Age, years (IQR) | 68 (61–72) | 66 (61–71) | 64 (57–71) | 69 (67–73) | .014 |
| Sex | |||||
| Female | 109 (61%) | 34 (59%) | 38 (63%) | 37 (62%) | .9 |
| Male | 69 (39%) | 24 (41%) | 22 (37%) | 23 (38%) | |
| Chemotherapya | 59 (33%) | 21 (36%) | 19 (32%) | 19 (32%) | .8 |
| Immunotherapya | 29 (16%) | 8 (14%) | 11 (18%) | 10 (17%) | .8 |
| Time after first dose, days (IQR) | 68 (55–93) | 67 (62–86) | 74 (51–111) | 65 (42–86) | .054 |
| Cancer type | |||||
| Solid | 141 (79%) | 41 (71%) | 50 (83%) | 50 (83%) | .13 |
| BMT | 23 (13%) | 13 (22%) | 6 (10%) | 4 (6.7%) | |
| Hematologic | 14 (7.9%) | 4 (6.9%) | 4 (6.7%) | 6 (10%) | |
Within 12 months of vaccination.
Neutralization of the alpha, beta, gamma, and delta SARS-CoV-2 variants is impaired in vaccine recipients
We assessed in vitro neutralization of the wildtype SARS-CoV-2 (ancestral strain) and 4 viral variants (alpha, beta, delta and gamma strains) using an extensively validated high-throughput pseudovirus neutralization assay (Garcia-Beltran et al., 2021a, 2021b). These variants represent recent waves of the pandemic, and harbor both shared and distinct mutations (Table S1) that are targeted by immune responses induced by vaccination with current vaccines, which all encode wildtype SARS-CoV-2 spike protein. We quantified the serum pseudovirus neutralization titer (pNT50) associated with 50% decrease in viral entry into angiotensin-converting enzyme 2 (ACE2)-expressing 293T cells.
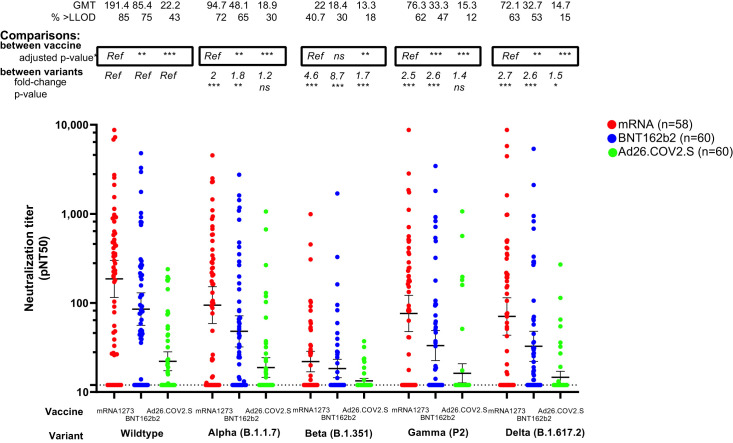
Consistent with the overall CANVAX population and other studies (Naranbhai et al., 2021b; Tada et al., 2021), neutralization of wildtype SARS-CoV-2 was highest for mRNA1273 recipients, followed by BNT162b2, and lowest among patients receiving Ad26.COV2.S. Adjusting for covariates, neutralization was lower among BNT162b2 recipients than mRNA1273 for the alpha, gamma, and delta variants (Figure 1 and Table S2).
Figure 1.
Neutralization of SARS-CoV-2 variants after vaccination with mRNA1273 (n = 58), BNT162b2 (n = 60), or Ad26.COV2.S (n = 60) in patients with cancer
The y axis shows pseudovirus neutralization titer 50 (pNT50, defined as the titer at which the serum achieves 50% neutralization of SARS-CoV-2 wildtype pseudovirus entry into ACE2-expressing 293T cells).. Briefly, lentiviral particles encoding both luciferase and ZsGreen reporter genes were pseudotyped with the SARS-CoV-2 spike protein from the strain indicated (see Table S1 for sequences) and produced in 293T cells, titered using ZsGreen expression by flow cytometry and used in an automated neutralization assay with 50–250 infectious units of pseudovirus co-incubated with 3-fold serial dilutions of serum for 1 h. Neutralization was determined on 293T-ACE2 cells. A horizontal dotted line is shown at a pNT50 titer of 12, which is the lower limit of detection of this assay; a pNT50 titer of 20 corresponds with the clinical threshold for positivity defined previously (Garcia-Beltran et al., 2021a). The geometric mean titer, proportion positive (at a threshold of 1:12). Statistical comparison of neutralization titers against each strain between recipients of different vaccines is details in Table S2 and denoted by a ∗ on the graph where p value are adjusted for covariates previously shown to be associated with wildtype virus neutralization namely age, chemotherapy, immunotherapy, timing after vaccination, and cancer type. Comparison of neutralization titers for recipients of each vaccine type, against different strains is shown as the fold change in neutralization, and corresponding p value (based on a Dunnet's test conducted in GraphPad Prism v9.0). Horizontal lines denote geometric mean titers, whiskers extend to 2.5th and 97.5th centiles to encompass the 95% CI.
We observed marked and significant neutralization of the alpha, beta, delta, and gamma variants for most vaccine groups (Figure 1). The differences were most striking for the beta variant, where fewer than one-half of all evaluated donors (41% of mRNA1273, 30% of BNT162b, and 18% of Ad26.COV2.S recipients) had neutralization measurable above the assay limit of detection. Few patients with cancer in this study had measurable neutralization against wildtype SARS-CoV-2 and the 4 variants tested after receipt of Ad26.COV2.S were 43% against wildtype, 30% against alpha, 18% against beta, 12% against gamma, and 15% against delta.
Neutralization breadth is associated with wildtype neutralization titer and time since vaccination, and is reduced with age
Next, we sought to identify correlates of vaccine breadth. In a multivariate regression analysis, the magnitude of neutralization of wildtype SARS-CoV-2 was the strongest correlate of breadth (effect estimate 1.4 additional variants neutralized per log10 increase in neutralization titer, 95% confidence interval [CI], 1.1–1.6; adjusted p < 0.001) (Table 2 ). Vaccine type did not independently associate with breadth of neutralization (p > 0.1 for each vaccine). Older age was associated with narrower breadth of response (effect estimate per 5-year increase in age −0.09; 95% CI, –0.17 to –0.01; adjusted p = 0.029). The breadth of the response tended to increase with time after vaccination, even adjusting for the expected initial increase and later waning in the magnitude of response (effect estimate per week after vaccination, 0.04; 95% CI, 0–0.08; adjusted p = 0.061).
Table 2.
Multivariate regression analysis to identify correlates of breadth of neutralization
| Effect estimatea | 95% CI | p Value | |
|---|---|---|---|
| Neutralization titer against wildtype SARS-CoV-2 | 1.4 | 1.1 to 1.6 | <.001 |
| Age (per 5-year increase) | −0.09 | −0.17 to −0.01 | .029 |
| Vaccine type | |||
| mRNA1273 | Ref | ||
| BNT162b2 | −0.16 | −0.57 to 0.25 | .4 |
| Ad26.COV2.S | −0.29 | −0.77 to 0.18 | .2 |
| Chemotherapy | −0.14 | −0.50 to 0.22 | .4 |
| Immunotherapy | 0.16 | −0.30 to 0.62 | .5 |
| Time after first dose (per week) | 0.04 | 0.00 to 0.08 | .061 |
| Cancer type | |||
| Solid | Ref | ||
| Bone marrow transplanted | −0.04 | −0.55 to 0.47 | .9 |
| Hematologic | 0.2 | −0.42 to 0.81 | .5 |
Effect estimates shown are per additional variant neutralized at measurable levels (above the lower limit of detection).
Anti-spike binding antibody concentrations are a surrogate for breadth
We measured total binding antibodies against SARS-CoV-2 spike protein (combined IgA/M/G) with Roche Elecsys assay (FDA and EUA approved) and specific isotypes (IgA, IgG, IgM, and combined IgA/M/G) of antibodies binding the RBD with a validated assay we previously developed (Garcia-Beltran et al., 2021a). Anti-RBD responses were dominated by IgG isotype responses, and responses varied by vaccine as previously reported (Figure S1). Wildtype neutralization was more robustly correlated with anti-RBD concentrations (Pearson R = 0.63; 95% CI, 0.53–0.71; p < 0.001) than overall anti-spike responses (Pearson R = 0.53; 95% CI, 0.41–0.63; p < 0.001), but both correlations were modest (Figure S2). A response composed of more isotypes was not associated with greater breadth after adjusting for neutralization titer against wildtype virus as having a more isotype diverse response was associated with neutralization titer (p < 0.001), as in other studies (Noval et al., 2021).
Anti-spike IgA/M/G total antibodies are easily measured in clinical practice, whereas neutralization is not. An anti-spike IgA/G/M titer of more than 1,000 U/mL on the Roche Elecsys FDA EUA assay was predictive of neutralization breadth (the positive predictive value for neutralization of >2 variants at a titer of >20 was 90%; negative predictive value of 88%; overall sensitivity of 95%; overall specificity of 78%) (Figure S3).
Additional booster SARS-CoV-2 vaccines confer enhanced variant neutralization breadth
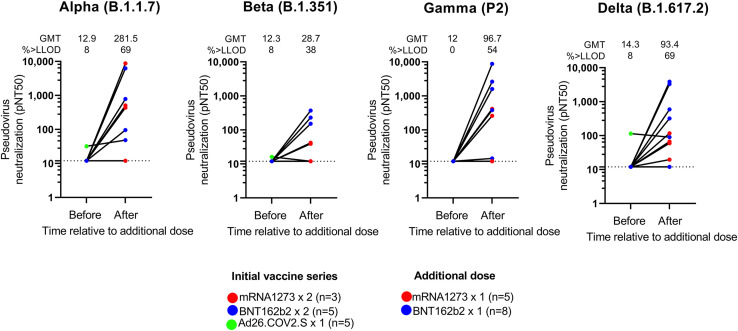
Since the magnitude of wildtype response associated with breadth, and booster doses increase the magnitude of wildtype response, we hypothesized that an additional homologous vaccine dose (or booster) would elicit enhanced heterologous breadth. The safety of additional doses in this cohort was comparable with the primary series (Naranbhai et al., 2021a). In 13 participants with a low baseline response (Table S3), booster doses enhanced the neutralization of the alpha, beta, gamma, and delta variants (Figure 2 ). Notwithstanding that these participants had low pre-booster titers (only 1 had measurable neutralization of any strain), the magnitude of neutralization of the alpha, beta, gamma, and delta variants was numerically higher after the booster doses than the overall evaluated population who had received the full vaccine series. In this subset of participants with poor pre-booster responses, neutralization breadth increased from median 0 (interquartile range [IQR], 0–0) variants neutralized before the booster to a median of 2 (IQR, 1–4) variants neutralized after the booster.
Figure 2.
Effect of booster doses on neutralization of SARS-CoV-2 viral variants in patients with cancer (n = 13)
The color of each dot indicates the initial vaccine series and additional vaccine as indicated in legend. The geometric mean titer (GMT) and proportion above the lower limit of detection (lower limit of detection = 12) is shown.
Discussion
SARS-CoV-2 vaccination induces lower antibody responses in patients with cancer. Here we studied the breadth of response against SARS-CoV-2 variants as these represent the leading threat to vaccinated individuals. As in patients without cancer, vaccination with SARS-CoV-2 vaccines induces lower neutralization of variants, particularly beta, than wildtype. The vaccine types varied in magnitude of response, but crucially, the magnitude of wildtype neutralization response was the primary correlate of breadth of neutralization. As predicted and concordant with another small study of 4 individuals (Iketani et al., 2021), booster doses even with wildtype vaccines increase breadth against viral variants.
These data have several potential clinical implications. First, to achieve the greatest neutralizing breadth, the most immunogenic vaccine may be preferred as the primary series for patients at high risk, where feasible. Second, the hierarchy of effectiveness of the 3 FDA EUA vaccines in preventing breakthrough infection by variants, where mRNA1273 provides the highest protection, followed by BNT162b2 and then Ad26.COV2.S (Naranbhai et al., 2021b) are likely accounted for by differences in immunogenicity against wildtype SARS-CoV-2. Finally, booster doses with wildtype vaccines would be expected to increase protection against variants in patients with cancer, even as we await the next generation of vaccines. This observation is likely because of boosting of polyclonal responses capable of binding conserved sites in current and predicted future variants. The magnitude of increase in variant naturalization seems to be robust, even when responses to initial vaccination are poor, suggesting that a single booster dose may be adequate for most patients to overcome the apparent reduced priming of responses. Whether these patients may require additional doses owing to waning responses remains unclear.
There are several additional noteworthy observations. Advancing age was associated with a reduced breadth of neutralization (independent of magnitude of response), consistent with impaired immune functions such as decrease in somatic hypermutation with age (Troutaud et al., 1999). Interestingly, we observed a trend toward an enhanced breadth over time, but this is likely small and likely not at the magnitude seen in individuals with natural infection who continue to accrue increased breadth over time, potentially because of antigen persistence (Cho et al., 2021). Interestingly, an anti-spike antibody titer of higher than 1000 U/mL on the Roche Elecsys assay—an assay that is widely available in clinical practice—was a good surrogate for breadth. This may be a helpful threshold in counseling patients regarding need for boosters, notwithstanding the limitations of inferring clinical protection from immunologic measures.
A key limitation of this study is the focus on in vitro immunogenicity. The effectiveness of vaccines against variants is likely to be more complex, involving differences in viral infectivity, exposure rates, transmissibility, and possibly virulence in combination with largely humoral immune responses and cellular responses. This is illustrated by the exceptional success of the delta variant in transmission, but its relatively modest escape of neutralization. The number of individuals evaluated after booster doses is modest, but these represent the extra tail of the curve of patients who failed to make adequate responses after initial vaccination.
In conclusion, while current wildtype-based SARS-CoV-2 vaccines induce a lower magnitude responses in patients with cancer that show impaired neutralization of viral variants, boosting these responses can safely restore breadth against current viral variants.
Consortia
Ryan J Sullivan, Aditya Bardia, Laura M. Spring, Steven J. Isakoff, Jocelyn R. Farmer, Leyre Zubiri, Gabriella S. Hobbs, Joan How, Andrew M. Brunner, Amir T. Fathi, Clarie A. Pernat, A. Gavralidis, Jennifer L. Peterson, Mustafa Sakhi, Grace Hambelton, Elyssa N. Denault, Lindsey J. Mortensen, Lailoo A. Periello, Marissa N. Bruno, Bruno MN, Brittany Y. Betraux, Alyssa R. Lawless Melissa A. Jackson, Elizabeth Niehoff, Claire Barabell, Christain N Nambu, Erika Nakajima, Thomas Reinecke, Cyndi Bowes, Grace E. Kirkpatrick, Julia C. Thierauf, Kerri Reynolds, Henning Willers, Anand S. Dighe, Rebecca Saff, Kimberley Blumenthal.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CR3022-IgG1 | Obtained from the lab of Dr. Aaron Schmidt | IEDB Cat# CR3022, RRID:AB_2848080 |
| Anti-human IgG+IgM+IgA (HL) HRP | Bethyl | Cat# A80-152P |
| Polyclonal human sera | This study | N/A |
| Bacterial and virus strains | ||
| SARS-CoV-2 ΔC18 (wild type) pseudotyped pHAGE-CMV-Luc2-IRES-ZsGreen-W lentivirus | Garcia-Beltran et al. (2021b) | N/A |
| SARS-CoV-2 ΔC18 B.1.1.7 pseudotyped pHAGE-CMV-Luc2-IRES-ZsGreen-W lentivirus | Garcia-Beltran et al. (2021b) | N/A |
| SARS-CoV-2 ΔC18 B.1.351 v1 pseudotyped pHAGE-CMV-Luc2-IRES-ZsGreen-W lentivirus | Garcia-Beltran et al. (2021b) | N/A |
| SARS-CoV-2 ΔC18 P2 pseudotyped pHAGE-CMV-Luc2-IRES-ZsGreen-W lentivirus | Garcia-Beltran et al. (2021b) | N/A |
| SARS-CoV-2 ΔC18 B.1.617.2 v1 pseudotyped pHAGE-CMV-Luc2-IRES-ZsGreen-W lentivirus | This study | NA |
| Chemicals, Peptides, and Recombinant Proteins | ||
| SARS-CoV-2 receptor binding domain protein | Obtained from the lab of Dr. Aaron Schmidt | N/A |
| Experimental Models: Cell Lines | ||
| 293T/ACE2.MF | Obtained from the lab of Dr. Michael Farzan | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, A. John Iafrate (aiafrate@partners.org).
Materials availability
The study did not generate new unique reagents.
Data and code availability
-
•
Code for computing and analyzing neutralization breadth measures is available in the supplementary appendix
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request
Experimental model and subject details
Patients
The CANVAX study enrolled consenting adult patients at the Massachusetts General Hospital Cancer Center between April 21 and July 21, 2021. Recruitment and enrolment procedures have been previously described. For this analysis, we randomly selected participants without prior SARS-CoV-2 infection (confirmed by anti-nucleocapsid antibody testing) who had received each of the 3 FDA EUA vaccines (by allocating random numbers to each participant and selecting the first 60). Participants were samples 14 or more days after their final dose of vaccine. This study was approved by the Mass General Brigham Human Research Committee (2021P000746). Adult participants provided written (or in exceptional cases verbal) informed consent to participation in this study.
Method details
Neutralization assays
We used pseudovirus neutralization assay that we have previously described in detail elsewhere. In brief, pseudovirus neutralization titer 50 was calculated by taking the inverse of the serum concentration that achieved 50% neutralization of SARS-CoV-2 pseudotyped lentivirus particles entry into ACE2-expressing 293T cells. We introduced mutations corresponding to the SARS-CoV-2 variants of concern shown in Table S1 by site-directed mutagenesis and confirmed clones by sequencing.
Binding antibody assays
We measured antibodies against the spike protein with the Roche Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics), at the Massachusetts General Hospital Core Clinical laboratory, a Clinical Laboratory Improvement Amendments laboratory. Anti-receptor binding domain antibodies were measured with an enzyme-linked immunosorbent assay (ELISA) (Garcia-Beltran et al., 2021a, 2021b). Briefly, we used an indirect ELISA with a standard consisting of anti-SARS-CoV and -CoV-2 monoclonal antibody (CR3022) (IgG1 isotype). The 96-well ELISA plates were coated with purified wildtype SARS-CoV-2 RBD. Plates were blocked with bovine serum albumin and washed. A 7-point standard curve was created using CR3022-IgG1 starting at 2 μg/mL by performing 1:3 serial dilutions with dilution buffer, and serum samples were diluted 1:100 with dilution buffer. Diluted samples and standards were added to corresponding wells and incubated for 1 h at 37°C, followed by washing. Total antibodies were detected with anti-human IgG + IgA + IgM (H+L)-HRP (Bethyl) diluted 1:25,000 for a 30-min incubation at room temperature. After washing, TMB substrate (Inova) was added to each well and incubated for 5 to 15 min before stopping with 1 M H2SO4. The optical density (O.D.) was measured at 450 nm with subtraction of the O.D. at 570 nm as a reference wavelength on a SpectraMax ABS microplate reader. Anti-RBD antibody levels were calculated by interpolating onto the standard curve and correcting for sample dilution; 1 U/mL was defined as the equivalent reactivity seen by 1 μg/mL of CR3022.
Quantification and statistical analysis
Analyses were performed in R (v4.05) using the gtsummary packages and lm() functions. Details are provided in the figure legends and below. We modelled log10 transformed pseudovirus neutralization titers as the dependent variable. We selected covariates found to associated with neutralization of wildtype SARS-CoV-2 in the overall CANVAX study including more than 600 individuals; these were age, days after vaccination, vaccine type, cancer type (categorized into solid, bone marrow transplant, or hematologic), receipt of chemotherapy in the prior year, and receipt of immunotherapy in the prior year as independent variables. The statistical comparisons in Figure 1 are shown relative to either mRNA1273 for each comparison of neutralization response between vaccine groups for each variant, or relative to wildtype virus for each comparison of neutralization response between variants for each vaccine group. The distribution of the data after log10 transformation was visually assessed in R to confirm suitability for linear regression. Comparisons between vaccine groups were performed by linear regression adjusting for all the above covariates in R (as detailed in Table S2). Comparisons between variants were performed by Dunnet’s test in GraphPad Prism v9.0. Figures were made in GraphPad Prism.
Acknowledgments
We thank the Peter and Ann Lambertus Family Foundation and Donald Glazer for support of the CANVAX study. We thank Andrea Nixon and the MGH Core laboratory for assistance in performing assays. We thank Michael Farzan, PhD, for providing ACE2-expressing 293T cells. A.J.I. and this study were supported by the Peter and Ann Lambertus Family Foundation. A.B.B. was supported by the National Institutes for Drug Abuse (NIDA) Avenir New Innovator Award DP2DA040254, the MGH Transformative Scholars Program as well as funding from the Charles H. Hood Foundation.
Research support: Peter and Ann Lambertus Family Foundation; Donald Glazer Fund
Author contributions
Conceptualization, V.N., A.J.I.
Methodology, V.N., J.F.G., A.B.B, A.J.I., W.F.G.B.,
Formal Analysis, V.N., K.J.S.D, E.C.L.
Investigation, V.N. K.J.S.D, E.C.L, O.O., W.F.G.B., C.B., A.B.B.
Data Curation, V.N.
Writing—Original Draft, V.N.
Writing—Review and Editing, V.N., K.J.S.D, E.C.L, O.O., W.F.G.B., C.B., A.B., J.F.G., A.B.I., A.J.I.
Visualization, V.N.
Supervision, J.F.G., A.B.B, A.J.I.
Funding Acquisition, J.F.G., A.B.B, A.J.I.
Declaration of interests
J.F.G. has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech, Ariad/Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Moderna, AstraZeneca, Pfizer, Novartis, Merck, and GlydeBio; research support from Novartis, Genentech/Roche, and Ariad/Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. A.J.I. has served as a compensated consultant for Oncoclinicas Brasil, Kinnate, Repare, and Paige.ai.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: January 10, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ccell.2021.12.002.
Contributor Information
CANVAX team:
Ryan J. Sullivan, Aditya Bardia, Laura M. Spring, Steven J. Isakoff, Jocelyn R. Farmer, Leyre Zubiri, Gabriella S. Hobbs, Joan How, Andrew M. Brunner, Amir T. Fathi, Clarie A. Pernat, A. Gavralidis, Jennifer L. Peterson, Mustafa Sakhi, Grace Hambelton, Elyssa N. Denault, Lindsey J. Mortensen, Lailoo A. Periello, Marissa N. Bruno, M.N. Bruno, Brittany Y. Betraux, Alyssa R. Lawless, Melissa A. Jackson, Elizabeth Niehoff, Claire Barabell, Christain N. Nambu, Erika Nakajima, Thomas Reinecke, Cyndi Bowes, Grace E. Kirkpatrick, Julia C. Thierauf, Kerri Reynolds, Henning Willers, Anand S. Dighe, Rebecca Saff, and Kimberley Blumenthal
Supplemental information
References
- Addeo A., Shah P.K., Bordry N., Hudson R.D., Albracht B., Di Marco M., Kaklamani V., Dietrich P.-Y., Taylor B.S., Simand P.-F., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098-e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouny Z., Hawley J.E., Choueiri T.K., Peters S., Rini B.I., Warner J.L., Painter C.A. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S., Panopoulou A., Shea R.L., Tsui M., Saso R., Sud A., West S., Smith K., Barwood J., Kaczmarek E., et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A., Muecksch F., Schaefer-Babajew D., Wang Z., Finkin S., Gaebler C., Ramos V., Cipolla M., Mendoza P., Agudelo M., et al. Anti-SARS-CoV-2 receptor binding domain antibody evolution after mRNA vaccination. Nature. 2021;600:517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., Hauser B.M., Caradonna T.M., Clayton K.L., Nitido A.D., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., St. Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–1299. doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C.A., Mehta N., Keogh R.H., Diaz-Ordaz K., Khunti K., Lyons R.A., Kee F., Sheikh A., Rahman S., et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:2244. doi: 10.1136/bmj.n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S., Liu L., Nair M.S., Mohri H., Wang M., Huang Y., Ho D.D. A third COVID-19 vaccine shot markedly boosts neutralizing antibody potency and breadth. MedRxiv. 2021 2021.08.11.21261670. [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., Lopes G.de L., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V., Pernat C.A., Gavralidis A., St Denis K.J., Lam E.C., Spring L.M., Isakoff S.J., Farmer J.R., Zubiri L., Hobbs G.S., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J. Clin. Oncol. 2021 doi: 10.1200/JCO.21.01891. JCO2101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V., Garcia-Beltran W.F., Chang C.C., Mairena C.B., Thierauf J.C., Kirkpatrick G., Onozato M.L., Cheng J., Denis K.J. St., Lam E.C., et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J Infect Dis. jiab593. 2021 doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval M.G., Kaczmarek M.E., Koide A., Rodriguez-Rodriguez B.A., Louie P., Tada T., Hattori T., Panchenko T., Romero L.A., Teng K.W., et al. Antibody isotype diversity against SARS-CoV-2 is associated with differential serum neutralization capacities. Sci. Rep. 2021 doi: 10.1038/s41598-021-84913-3. 2021 111 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oekelen O., Gleason C.R., Agte S., Srivastava K., Beach K.F., Aleman A., Kappes K., Banu R., Bermúdez-González M.C., Chernet R.L., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L.C., Thakkar A., Campbell S.T., Forest S.K., Pradhan K., Gonzalez-Lugo J.D., Quinn R., Bhagat T.D., Choudhary G.S., McCort M., et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Zhou H., Samanovic M.I., Dcosta B.M., Cornelius A., Mulligan M.J., Landau N.R. Comparison of neutralizing antibody titers elicited by mRNA and adenoviral vector vaccine against SARS-CoV-2 variants. BioRxiv. 2021 2021.07.19.452771. [Google Scholar]
- Thakkar A., Gonzalez-Lugo J.D., Goradia N., Gali R., Shapiro L.C., Pradhan K., Rahman S., Kim S.Y., Ko B., Sica R.A., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutaud D., Drouet M., Decourt C., Morvan C. Le, Cogné M. Age-related alterations of somatic hypermutation and CDR3 lengths in human Vκ4-expressing B lymphocytes. Immunology. 1999;97:197. doi: 10.1046/j.1365-2567.1999.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Code for computing and analyzing neutralization breadth measures is available in the supplementary appendix
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request