ABSTRACT
Objective
The objective of this study was to investigate in a real-life context the effectiveness of long-term opioid therapy for reducing pain intensity and interference and improving health-related quality of life (QOL) in patients with chronic noncancer pain.
Methods
Participants were 893 patients (age = 52.4 ± 14.1, female = 62.4%) enrolled in the Quebec Pain Registry (2008–2011) who completed questionnaires before their first visit at one of three multidisciplinary pain management clinics and 6 and 12 months thereafter. Based on their opioid use profile (OUP), patients were categorized as nonusers, non-lasting users, or lasting users. Data were analyzed using generalized estimating equations.
Results
More than 60% of patients newly initiated on opioid therapy stopped their medication mainly because of adverse effects and/or lack of pain relief. OUP significantly predicted pain intensity and interference and physical QOL (pQOL; P values < 0.001). Lasting users of opioids reported higher levels of pain intensity and interference and poorer pQOL than nonusers and/or non-lasting users over the 12-month follow-up (P values < 0.001). However, all effect sizes were small, thus questioning the clinical significance of these group differences. Among lasting users, more than 20% of patients experienced a meaningful amelioration in pain intensity and interference as well as mental QOL (mQOL), whereas only 8% exhibited improved pQOL.
Discussion
A significant subgroup of patients may benefit from long-term opioid therapy in terms of pain severity and mQOL but the majority do not. The challenge facing clinicians is how to identify who the responders will be.
KEYWORDS: opioids, chronic pain, Quebec Pain Registry, multidisciplinary pain treatment facility, treatment effectiveness
RÉSUMÉ
Objectif: L'objectif de cette étude était d'investiguer dans un contexte de vraie vie l'efficacité à long terme des opioïdes pour réduire l'intensité et l'interférence de la douleur et améliorer la qualité de vie reliée à la santé (QDV) des patients souffrant de douleur chronique non cancéreuse.
Méthodes: Les participants à cette étude étaient 893 patients (âge = 52.4 ± 14.1, femmes = 62,4%) enrôlés dans le Registre Québec Douleur (2008–2011) et qui avaient complété des questionnaires avant leur premiére visite dans l'un des trois centres multidisciplinaires de gestion de la douleur ainsi qu’à 6 et 12 mois plus tard. Selon leur profil d'utilisation d'opioïdes (PUO), les patients ont été classés en non-utilisateurs, utilisateurs non continus, ou utilisateurs continus. Les données ont été analysées à l'aide de modéles d'équation d'estimation généralisée.
Résultats: Plus de 60% des patients nouvellement initiés à une thérapie à base d’opioïdes en avaient cessé la prise principalement à cause des effets indésirables et/ou d’un manque de soulagement de leur douleur. Le PUO prédisait d’une maniére significative l'intensité de la douleur et son interférence ainsi que la QDV physique (QDVp; valeurs p < 0.001). Comparés aux non-utilisateurs et/ou utilisateurs non continus, les utilisateurs continus rapportaient des niveaux plus élevés d'intensité et d’interférence de la douleur ainsi qu’une moins bonne QDVp au cours des 12 mois de suivi (valeurs p < 0.001). Cependant, la magnitude de ces effets était de petite taille, remettant ainsi en question la signification clinique des différences observées entre ces groupes. Parmi les utilisateurs continus, plus de 20% d'entre eux montraient une amélioration significative de leur condition douloureuse en termes d'intensité et d’interférence ainsi que de la QDV mentale (QDVm), alors que seulement 8% présentaient une amélioration de leur QDVp.
Discussion: Un sous-groupe important de patients peut bénéficier d’un traitement à long terme à base d’opioïdes en termes de sévérité de la douleur et de QDVm, mais ce n’est pas le cas pour la majorité des patients. Le défi auquel les cliniciens doivent faire face est de déterminer les patients les plus susceptibles de bénéficier de ce type de traitement.
Introduction
In Canada and the United states, opioids (e.g., morphine, oxycodone, fentanyl) are among the most widely used drugs to treat chronic noncancer pain (CNCP) along with acetaminophen and nonsteroidal anti-inflammatory drugs.1–3 Opioids are potent analgesics, but their use is associated with several side effects such as respiratory depression, nausea, or constipation.4 Over the past 2 decades and until 2010, the use of opioids for CNCP has dramatically increased,5,6 as have associated serious damages such as overdose, abuse, or addiction.7–12 However, since 2011, the general use of opioids and rates of death related to their prescription tend to decrease,6 whereas the death rates due to illicit use of opioids have increased.13
There is a lack of evidence in the literature supporting the effectiveness of long-term opioid therapy among CNCP patients. A literature review published in 2009 by the American Pain Society in collaboration with the American Academy of Pain Medicine found that very few studies have investigated the long-term benefits of opioids (≥6 months) in CNCP,14 among which two high-quality systematic reviews found that opioids were discontinued by a high proportion of patients due to adverse events or insufficient pain relief.15,16 Noble et al. concluded that only weak evidence supports the fact that patients who are able to continue opioids on a long-term basis experience clinically significant pain relief, though the evidence for improvement in health-related quality of life and physical functioning is inconclusive.16 The most recent literature review published in 2015 came to similar conclusions.12 To date, research on pharmacological agents for chronic pain management has been limited mainly to clinical trials, which are often of short duration and have stringent selection criteria.17–19 Furlan et al. reported that 74% of randomized clinical trials on opioid therapy had a duration of less than 6 weeks.20 Some cross-sectional studies have evaluated the effectiveness of long-term opioid therapy for improving pain severity and/or quality of life among patients with CNCP. Two studies found that patients with CNCP using long-term opioid therapy reported more pain and greater disability than those not using opioids.21,22 Two other studies, conducted among long-term opioid users only, found that patients reported relatively high pain intensity and interference,23,24 with an important proportion of patients having a deteriorated quality of life.23
Recently, Moulin et al. conducted a long-term national study in patients suffering from neuropathic pain and found that those who were on high dose of opioids at baseline and at follow-up exhibited poorer outcomes at 12-month follow-up.25 These authors therefore concluded that opioid therapy may not be beneficial in the majority of patients with chronic neuropathic pain.25 A subsequent subanalysis of this study, conducted by Bostick et al. involving 537 patients with chronic neuropathic pain, found that physical functioning and disability did not improve in patients who were prescribed opioids compared with those who were not.26 In contrast, Watson et al. surveyed 84 patients using opioids on a regular basis and found that the majority reported at least 50% or greater pain relief and a moderate improvement in disability.27 However, this study involved highly selected patients and the authors acknowledge that their results may not be generalizable to all CNCP patients in whom opioids are being initiated. Brooks et al. conducted a qualitative study focusing on the lived experience of nine patients using opioids to manage their CNCP came to the conclusion that positive effects of opioids outweighed the negative for most participants.28 However, further observational studies involving large samples of patients suffering from a variety of CNCP syndromes are needed in this field as the results can complement those of randomized clinical trials for evidence-based guidance of treatment decisions.
The objective of the present longitudinal study was to investigate in a real-life context the effectiveness of long-term opioid therapy for reducing pain severity (intensity and interference) and improving health-related quality of life (physical and mental) in patients with CNCP over a 1-year period.
Materials and methods
Participants
Participants were recruited from the Quebec Pain Registry (QPR), which is a vast database of patients referred to university-affiliated multidisciplinary pain treatment clinics in the province of Quebec, Canada.29 Patients were enrolled in the QPR between October 2008 and November 2014 if they were (1) scheduled for a first visit at the pain clinic for multidisciplinary treatment considerations, (2) aged 18 years or more, (3) fluent in spoken and written French and/or English, and (4) physically and cognitively able to complete questionnaires. Biopsychosocial data were collected prior to the initial visit at the pain clinic (baseline) and 6 months later. Up to March 2012, additional follow-up data were gathered at 12 and 24 months but only in those patients who had not been discharged from the pain clinic in the meantime, mainly because they do not have a family physician. Patients suffering from CNCP (≥3 months) were therefore selected for the present study among those enrolled in the QPR between October 2008 and April 2011. Only patients who provided written consent for their QPR data to be used for research purposes (more than 90% of patients29) and who were not currently taking opioids at the time of their first visit at the pain clinic were included in the study. Given small sample size at 24-month follow-up due to patients’ discharge in the meantime, only data collected up to 12 months were taken into account in the present study.
Procedure
The Research Ethics Boards of the Centre hospitalier de l’Université de Montréal, McGill University Health Center, and Centre hospitalier de l’Université de Sherbrooke approved the QPR project. Data at baseline and follow-up (e.g., pain intensity and interference, health-related quality of life) were collected with a self-report questionnaire (patient self-administered questionnaire) and medical/clinical data (e.g., pain duration, pain diagnosis, analgesic medication, etc.) were gathered by the QPR nurses using a structured interview protocol (nurse-administered questionnaire).
Questionnaires
All of the questionnaires were structured so that patients who reported multiple pain sites were asked to focus on the most painful one to complete the questionnaires.
Patient self-report questionnaires
Numeric Rating Scale for pain intensity
The Numeric Rating Scale (NRS)30 is a widely used scale to measure pain intensity.31 It consists of an 11-point scale (0 = no pain; 10 = worst possible pain) on which the participants are asked to select the number corresponding to the intensity of their pain. The NRS has good reliability, validity, and sensitivity.30,31 Participants were asked to rate on the NRS their average and worst pain intensity over the past 7 days at each time point (baseline and follow-ups).
Pain interference items of the Brief Pain Inventory
The Brief Pain Inventory (BPI-10)32 consists of ten items (as opposed to seven items in the original version of BPI33,34) assessing the extent to which pain impacts on various aspects of daily living. Participants are asked to rate on a scale from 0 (does not interfere) to 10 (completely interferes) the extent to which pain has interfered in the past 7 days with general activity, mood, mobility, normal work, relationships with others, sleep, enjoyment of life, self-care, recreational activities, and social activities.32 A global interference score can be derived by adding the ratings on all items, with higher scores indicating greater pain interference. The psychometric qualities of the BPI are well documented32,33 and it has been shown to have good validity and sensitivity to change in chronic pain patients attending a multidisciplinary pain treatment clinic.35 The BPI has been translated into French using a forward–backward translation method.36
Short-Form-12 Health Survey Version 2
The Short-Form-12 Health Survey Version 2 (SF-12v2)37 is a valid and reliable 12-item scale that assesses health-related quality of life (QOL). For each item, patients are asked to choose the answer that best describes their condition. The SF-12v2 generates norm-based scores for eight different domains as well as two composite scores representing physical and mental health–related QOL. Higher scores indicate better QOL.
Nurse-administered questionnaires
Pain history information and medication
Patients were asked information on their pain history (e.g., pain duration and frequency) and type(s) of medication currently used and used in the past 6 months to treat their pain.
Douleur Neuropathique 4
The Douleur Neuropathique 4 (DN4)38 is a well-validated screening tool that assesses the presence of neuropathic pain through self-report and physical examination. Each of the ten items is answered yes (score 1) or no (score 0). The total score is calculated as the sum of the ten items and the cutoff value for the presence of neuropathic pain is a total score of four out of ten.
Pain diagnosis
A summary of the data collected with the patient and nurse questionnaires was given to the pain physician at the patient’s initial visit. Once the visit was over, the physician was provided with a standardized form on which she or he was invited to record the patient’s diagnostic code(s) using the QPR pain diagnostic grid.39
Opioid use profile and type of pain classification
The opioid use profile (OUP) variable was created by classifying patients into one of three categories based on current and past medication reported at each time point (baseline, 6- and 12-month follow-up). All patients included in the study were opioid naïve at baseline. Patients who reported not taking any opioids during the course of the study (at baseline, 6 and 12 months) were categorized as nonusers. Patients who started using opioids within the first 6 months but stopped taking them thereafter (at 6 or 12 month) were categorized as non-lasting users. Patients who were put on opioids within the first 6 months and continued taking them at each follow-up period (6 and 12 months) were categorized as lasting users.
A type of pain variable was also created and was composed of the three following categories: patients who were diagnosed with neuropathic pain by the physician of the pain clinic and had a DN4 score ≥ 4 were classified into the neuropathic pain category. Patients with a neuropathic pain diagnosis and DN4 score < 4 as well as those with a nonneuropathic pain diagnosis and DN4 score ≥ 4 were categorized as having mixed evidence of neuropathic pain. Last, patients with a pain diagnosis other than a neuropathic origin and a DN4 score < 4 were categorized as having nonneuropathic pain.
Data analysis
Independent Student’s t tests and Pearson’s chi-square tests were employed to compare the baseline characteristics of patients who did and did not complete all questionnaires at each time point (baseline, 6 and 12 months). Mean and standard deviations along with frequency tables were used to describe participants’ characteristics. All patients included in this study were not using opioids at baseline.
Generalized estimating equations (GEEs) adjusting for sex, age, pain duration, and frequency were used to examine whether OUP and type of pain were associated over the 1-year follow-up period (time effect) with reported average and worst pain intensities, pain interference global scores, physical health–related quality of life (pQOL), and mental health–related quality of life (mQOL). In our five GEE models, the time effect encompassed measures collected at baseline, 6 months, and 12 months, thereby allowing group comparisons over time and at each time point in the event of a significant interaction. Pairwise comparisons were then used to assess the statistical significance of the group differences.
Effect sizes of group differences (Cohen’s d40 for continuous variables, the phi (φ) statistic for binary categorical variables, and Cramér’s v for discrete variables with more than two categories41) were also examined given that significant testing in studies involving large sample sizes like the present one can be misleading because even small differences can reach statistical significance, whereas clinically, they can be viewed as trivial and not meaningful.42–44 Only differences reaching a Cohen’s d value equal to or greater than ±0.5 or a φ or a Cramér’s v value equal to or greater than ±0.3 were considered meaningful and clinically important.42–44
Frequency tables were used to determine the proportion of lasting opioid users whose pain condition and quality of life improved, remained stable, or deteriorated. Based on the IMMPACT recommendations, a change of 20% or more in pain intensity and interference was considered as meaningful.45 For SF-12v2 quality of life scores, we considered a change of at least one SD of the mean norm-based scores of the general population as a meaningful change.
Results
A total of 2650 patients were enrolled in the QPR during the selected study period and consented for their information to be used for research purposes. As shown in Figure 1, a total of 893 opioid-naïve patients completed all of the nurse-administered questionnaires at each time point (baseline, 6 months, 12 months), so it was possible to categorize them according to their OUP. More than half of them (51.5%) were classified as non-opioid users, 30.6% as non-lasting users, and 17.9% as lasting users. Results of the statistical analysis comparing participants who did and did not complete all of the questionnaires at each time point (baseline, 6 and 12 months) in terms of age, sex, pain duration, type of pain, average and worst pain intensity, pain interference, pQOL, or mQOL at baseline revealed some statistically significant differences, but all of the effect sizes were small (Cohen’s d < 0.5; φ or Cramér’s v value < 0.3), suggesting that these differences were not clinically meaningful.
Figure 1.
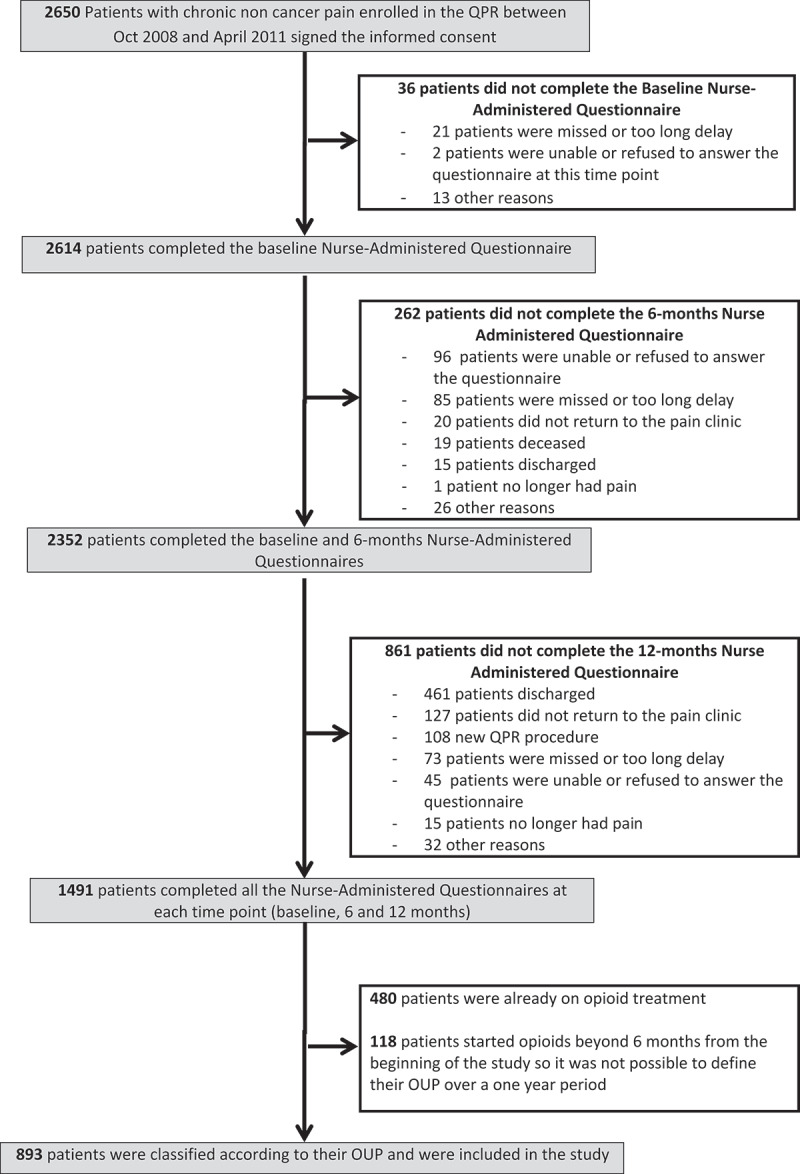
Study flow diagram.
Descriptive statistics
Table 1 shows the demographic and pain characteristics of the entire sample of participants and for each patient’s OUP category. Participants’ ages ranged from 18 and 92 years with a median of 51 years, and 62% were female (n = 554). Patients suffered from CNCP from 3 months to 60 years, the median being 3 years. More than 75% of the participants were classified as having neuropathic pain or mixed evidence of neuropathic pain. At baseline, pain intensity on the average in the past 7 days was 6.75 (SD = 1.9) on the 0–10 NRS.
Table 1.
Patient demographics and pain characteristics at baseline according to the opioid use profile and for the total sample.
| Nonusers | Non-lasting users | Lasting users | Total | |
| |
N (%) |
N (%) |
N (%) |
N (%) |
| N | 460 (51,5%) | 273 (30,6%) | 160 (17,9%) | 893 |
| Sex | ||||
| Females | 284 (61,7%) | 176 (64,5%) | 94 (58,8%) | 554 (62%) |
| Males | 176 (38,3%) | 97 (35,5%) | 66 (41,2%) | 339 (38%) |
| Age | ||||
| Age≤40 | 88 (19,6%) | 46 (17,0%) | 24 (15,1%) | 158 (18%) |
| 40<Age<60 | 217 (48,2%) | 151 (55,9%) | 95 (59,7%) | 463 (52,7%) |
| Age≥60 | 145 (32,2%) | 73 (27,1%) | 40 (25,2%) | 258 (29,4%) |
| Pain duration (in yrs) | ||||
| 1 yr or less | 74 (16,1%) | 44 (16,2%) | 24 (15,0%) | 142 (15,9%) |
| more than 1 up to 5 yrs | 220 (47,8%) | 151 (55,5%) | 67 (41,9%) | 438 (49,1%) |
| more than 5 yrs | 166 (36,1%) | 77 (28,3%) | 69 (43,1%) | 312 (35%) |
| Pain frequency in past 7 days | ||||
| Always present | 383 (83,3%) | 245 (90,1%) | 147 (91,9%) | 775 (86,9%) |
| Occasionally | 77 (16,7%) | 27 (9,9%) | 13 (8,1%) | 117 (13,1%) |
| Type of pain | ||||
| Non neuropathic | 99 (24,3%) | 48 (19,4%) | 43 (29,23%) | 190 (23,7%) |
| Mixed evidence | 189 (46,3%) | 104 (42,1%) | 65 (44,2%) | 358 (44,6%) |
| Neuropathic | 120 (29,4%) | 95 (38,5%) | 39 (26,5%) | 254 (31,7%) |
| Means ± SD | ||||
| Average pain in past 7 days | 6,52 ± 2,0 | 6,94 ± 1,9 | 7,07 ± 1,6 | 6,75 ± 1,9 |
| Worst pain in past 7 days | 7,92 ± 1,9 | 8,39 ± 1,6 | 8,67 ± 1,3 | 8,20 ± 1,7 |
| Pain interference (BPI-10) | 52,74 ± 22,0 | 59,61 ± 22,2 | 61,19 ± 19,5 | 56,38 ± 21,9 |
| Physical quality of life (SF-12v2 score) | 30,72 ± 9,4 | 28,41 ± 8,1 | 28,32 ± 7,8 | 29,58 ± 8,8 |
| Mental health–related quality of life (SF-12v2 score) | 41,52 ± 11,8 | 39,87 ± 11,7 | 38,87 ± 11,7 | 40,47 ± 11,8 |
BPI-10 = Brief Pain Inventory-10; SF-12v2 = Short-Form-12 Health Survey Version 2.
Among the users of opioids, close to two thirds (63.1%) stopped taking them during the follow-up period, mainly because of side effects (36.2%) and/or lack of efficacy (20.5%).
GEE analyses
Results of the GEE, which are summarized in Table 2, showed that after adjusting for age, sex, pain duration, and frequency, the patient’s OUP was a statistically significant predictor of average pain intensity, χ2 (df = 2) = 20.41, P < 0.001, worst pain intensity, χ2 (df = 2) = 54.13, P < 0.001, pain interference, χ2 (df = 2) = 20.89, P < 0.001, and pQOL, χ2 (df = 2) = 28.29, P < 0.001, over time (OUP × time, all P > 0.05). This was true irrespective of the type of pain the patients were suffering from (neuropathic, nonneuropathic, or mixed evidence of neuropathic pain; OUP × type of pain, all P > 0.05). As shown in Figure 2, pairwise comparisons revealed that lasting users reported higher pain intensity (average pain, P < 0.001, Cohen’s d = 0.27, and worst pain, P < 0.001, Cohen’s d = 0.46), greater pain interference (P < 0.001; Cohen’s d = 0.38), as well as poorer pQOL (P < 0.001, Cohen’s d = 0.35) over time than the nonusers. Compared to nonusers, non-lasting users reported higher pain intensity (average pain, P < 0.05, Cohen’s d = 0.14, and worst pain, P = 0.001, Cohen’s d = 0.23), greater pain interference (P < 0.05; Cohen’s d = 0.26), and poorer pQOL (P < 0.001; Cohen’s d = 0.30). Comparison between non-lasting users and lasting users revealed that the latter group reported higher pain intensity (average pain, P < 0.05, Cohen’s d = 0.11, and worst pain, P < 0.001; Cohen’s d = 0.21) during the follow-up period. All of the above Cohen’s d values were less than ±0.5, thereby questioning the clinical significance of the observed differences.
Table 2.
Results of the generalized estimating equation analyses.
| Predictive variable |
χ2 |
df |
P value |
| Average pain intensity | |||
| Opioid use profile | 20.408 | 2 | <0.001 |
| Type of pain | 6.981 | 2 | 0.05 |
| Time | 84.732 | 1 | <0.001 |
| Sex | 0.316 | 1 | 0.574 |
| Age | 7.402 | 1 | 0.007 |
| Pain duration | 28.957 | 2 | <0.001 |
| Pain frequency | 26.073 | 1 | <0.001 |
| Worst pain intensity | |||
| Opioid use profile | 54.127 | 2 | <0.001 |
| Type of pain | 15.384 | 2 | <0.001 |
| Time | 88.432 | 1 | <0.001 |
| Sex | 0.877 | 1 | 0.349 |
| Age | 0.305 | 1 | 0.581 |
| Pain duration | 37.005 | 2 | <0.001 |
| Pain frequency | 19.775 | 1 | <0.001 |
| Pain interference | |||
| Opioid use profile | 20.890 | 2 | <0.001 |
| Type of pain | 8.479 | 2 | 0.14 |
| Time | 67.182 | 1 | <0.001 |
| Sex | 1.082 | 1 | 0.298 |
| Age | 1.646 | 1 | 0.199 |
| Pain duration | 42.196 | 2 | <0.001 |
| Pain frequency | 38.336 | 1 | <0.001 |
| Physical quality of life | |||
| Opioid use profile | 28.287 | 2 | <0.001 |
| Type of pain | 1.955 | 2 | 0.376 |
| Time | 43.747 | 1 | <0.001 |
| Sex | 0.536 | 1 | 0.464 |
| Age | 15.432 | 1 | <0.001 |
| Pain duration | 6.032 | 2 | <0.05 |
| Pain frequency | 17.027 | 1 | <0.001 |
| Mental health–related quality of life | |||
| Opioid use profile | 5.153 | 2 | 0.076 |
| Type of pain | 4.548 | 2 | 0.103 |
| Time | 17.672 | 1 | <0.001 |
| Sex | 0.680 | 1 | 0.410 |
| Age | 14.813 | 1 | <0.001 |
| Pain duration | 9.414 | 2 | <0.05 |
| Pain frequency | 9.285 | 1 | <0.005 |
Figure 2.
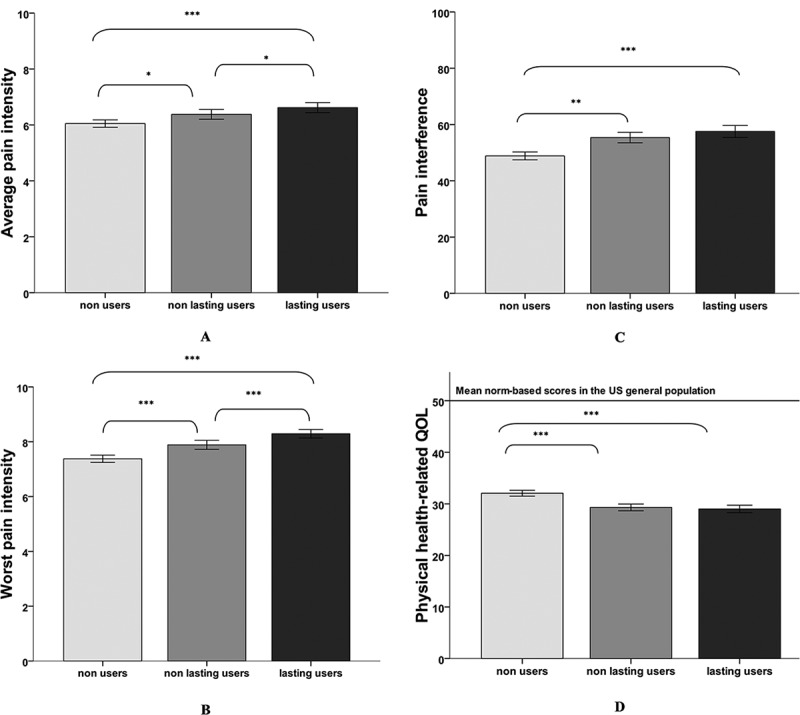
Results of the generalized estimating equations analyses. Error bars: 95% confidence interval. *P < 0.05. **P < 0.01. ***P < 0.001.
Examination of the types of response among the lasting users of opioids revealed that at 12-month follow-up, close to one in four lasting users experienced a significant improvement (≥20%) in their pain intensity and interference. The same was true for their mQOL. In contrast, pQOL improved from baseline to 12 months among only 8% of the lasting users (see Table 3).
Table 3.
Types of responses among lasting users of opioids over time (12 months).
| Outcome |
Changea |
% of patients |
| Improved | 27.7 | |
| Average pain intensity | Remained stable | 61.1 |
| Deteriorated | 11.2 | |
| Improved | 21.6 | |
| Worst pain intensity | Remained stable | 72.4 |
| Deteriorated | 6 | |
| Improved | 25 | |
| Pain interference | Remained stable | 68.1 |
| Deteriorated | 6.9 | |
| Improved | 7.9 | |
| Physical quality of life | Remained stable | 85.1 |
| Deteriorated | 7 | |
| Improved | 20.2 | |
| Mental quality of life | Remained stable | 69.3 |
| Deteriorated | 10.5 |
aA change of at least 20% (for pain intensity and interference scores) or at least one SD (for quality of life scores) was considered meaningful (improvement or deterioration). Patients with a change below 20% (or below one SD for quality of life scores) were considered stable.
Discussion
To our knowledge, this multicenter study is the first to have examined the effectiveness of long-term opioid therapy for reducing pain severity and improving health-related QOL in a large heterogeneous sample of patients with CNCP newly initiated on this type of treatment and followed in a real-life context. Our study revealed statistically significant differences in the patients’ outcomes according to their OUP. However, in studies involving large sample sizes like the present one, examination of effect sizes may prove to be more informative than statistical significance testing.42 In this article, none of the group differences reached a medium to large size effect (all Cohen’s d < 0.5). Although Cohen’s criteria are guides rather than absolutes, the clinical significance of the mean group differences can be viewed as questionable. In other words, although a significant subgroup among those who took opioids over a 1-year period did experience improvement in pain severity (intensity and interference) and mQOL, the majority did not and continued to report high pain severity scores as well as poor health-related quality of life (mental and physical).
Use of opioids for CNCP
Results of our study showed that nearly half of patients referred to specialized pain clinics and who were not discharged before the end of the 1-year follow-up period were on opioids at one time or another during the course of their treatment, a finding that is consistent with Moulin et al.’s results.25 Studies carried out in primary care settings showed much lower prevalence rates of opioid use ranging from 7.1% to 19.9%.21,46–48 The high rate of opioid use among our sample may be explained by the fact that these patients were recruited in tertiary care centers and have typically tried many other alternative therapies without significant success.49 However, as important, if not more important, to emphasize is the high percentage of patients (63.1%) who interrupted the use of opioids during the follow-up period, because this can be viewed as an important treatment failure rate. These results are consistent with those of the literature reviews of Noble et al.,50 Furlan et al.,20 and Abdel Shaheed et al.,51 who reported high rates of opioid discontinuation across studies mainly due to adverse events and/or insufficient pain relief.
Opioid effectiveness for reducing pain severity
The lasting opioid users continued to report high mean pain intensity scores (average and worst pain) during the follow-up period. This result is consistent with those of two studies that found that long-term opioid therapy did not contribute to lower pain intensity on average among patients with CNCP.21,22 In one of these studies, persistent opioid use was defined based on dispensed opioid volume and number of prescriptions during 365 days,22 whereas in the other, patients were considered long-term opioid therapy users if they reported using this type of medication on a regular basis.21
Our study also showed the same pattern of results with regard to the effects of long-term opioid use on pain interference in various aspects of daily living. In his systematic review, Ballantyne reported that function has been investigated in a limited number of trials, and these studies vary considerably in both their design and principal findings.52 Since then, several other studies have assessed functional outcomes among patients with CNCP using long-term opioid therapy and found that opioids did not improve physical functioning and disability.23–26
Relatively little scientific evidence exists on the reasons why long-term opioid treatment may fail to improve patients’ pain severity. It is commonly believed that sustained administration of this type of medication can be accompanied by a tolerance phenomenon leading to a progressive loss of the drug effects with a decrease in the apparent analgesic efficacy.4 Another possible reason for the lack of long-term opioid effectiveness may be related to the fact that this type of medication can also induce hyperalgesia, a phenomenon called “opioid-induced hyperalgesia” (OIH) and defined as a state of nociceptive sensitization caused by exposure to opioids and characterized by a paradoxical response whereby a patient receiving opioids for the treatment of pain may actually become more sensitive to certain painful stimuli.53–55 However, the precise mechanism of OIH is not yet understood, and this phenomenon has been mainly studied in the context of a short-time exposure rather than a long-time exposure to opioids.54 That patients with CNCP on long-term opioid therapy may develop OIH cannot be excluded, and this issue certainly merits further investigation.
Opioid effectiveness for improving health-related quality of life
In our sample, lasting users of opioids continued to report poor QOL on the average. These results are consistent with Eriksen et al.’s21 and Campbell et al.’s23 observations suggesting that long-term use of opioids would not be really helpful for improving health-related quality of life. This is problematic in view of the fact that the amelioration of patients’ quality of life commonly constitutes the primary goal of CNCP management, a goal that is often set as important as reducing the pain itself.56–58 In an earlier study, Choinière et al found that health-related quality of life was remarkably low in patients with CNCP on waitlists of multidisciplinary pain treatment.59 Similar results were obtained in the present study, where the patients’ norm-based scores on the physical scale of the SF-12v2 were much lower than those in the general population, and this was true whether or not they were on opioid treatment. These findings further highlight the deteriorated quality of life of this group of patients.
Response to treatment among the lasting users of opioids
Results from this study showed that though the majority of lasting users remained stable over time, about one in five patients experienced a significant improvement in their pain condition and mental health–related quality of life. This is similar to the findings of Moulin el al.’s study where close to one fifth of patients with neuropathic pain treated with opioids in tertiary care pain centers showed clinically significant improvement in their pain and function 12 months later.25 This is not negligible, because these results suggest that long-term use of opioids may be effective for a subgroup of patients with CNCP. Further research is clearly needed to identify the characteristics of patients who are most likely (and least likely) to benefit from long-term opioid treatment. Some earlier reports60–63 suggest that factors such as sex, depression, anxiety, and treatment expectations may play a role, but additional studies involving large sample sizes are needed to address this important issue.
Study limitations
Like any other studies, the present one has limitations that merit comments. First, our sample did not include 476 patients who were discharged from the pain clinic within the 12-month follow-up and therefore did completed all follow-up questionnaires. Thus, we may have missed some patients who continued to use opioids and were doing well or patients who did not respond to opioids and were discharged from the clinic because no other treatments could be offered. Second, in our sample, the follow-up period was limited to 12 months, though it may take more than a year to really be able to assess the effectiveness of long-term opioid therapy. Third, this study is limited to patients with CNCP attending tertiary care pain clinics. Most of them had chronic pain for years and had tried several approaches to treating their condition. In addition, this study does not capture patients who are doing well on opioids in the community and who are not referred to pain clinics. This may impact the generalizability of the findings. However, one can suspect that opioids are more commonly prescribed at the tertiary care level because many family physicians may not feel comfortable using this type of medication due to the risk of substance abuse.64 Furthermore, it is important to mention that access to tertiary care clinics in the province of Quebec requires a physician referral; access to these clinics is free but limited due to relatively long waiting lists, as is the case in other Canadian provinces.65 As a result, it is unclear how the data obtained in the present study compare to what would be obtained in other health care systems (self-referrals or other systems of access to the specialized pain clinics). In addition, patients were classified into different OUP categories based on their self-reported current medication use at the time of the first visit at the pain clinic and at follow-up interviews at 6 and 12 months; it is possible that patients interrupted their opioid treatment for a while within each 6-month period separating the follow-up interviews, and this was not taken into account in the OUP classification. Finally, because our study was not a randomized controlled trial, it is unknown whether or not persistent opioid users’ conditions would have worsened over time if they had not taken this type of medication.
Conclusions
Close to one quarter of patients who were lasting users of opioids experienced a significant improvement in terms of pain intensity, pain interference, and mental health–related quality of life. The majority of opioid-naïve patients who were followed at the pain clinic for a 1-year period did not benefit from opioid therapy, and a great proportion of them discontinued opioids due to lack of effect or presence of side effects. Results are in line with the literature suggesting that though most patients do not benefit from long-term opioid therapy, there is a significant subgroup of patients who do benefit from this therapy. The challenge facing clinicians is how to identify who the responders will be.
Acknowledgments
The authors thank all of the nurses and assistants for their dedicated work during the development/implementation of the QPR and the data collection process at the multidisciplinary pain treatment clinics of the CHUM, MUHC, CHUS, CHUQ, and HDL. Special thanks are due to Hélène Lanctôt, the QPR nurse coordinator, and to her assistant, Lucie Germain. The authors also thank the clinicians working in each participating site and to the patients who gave consent for their QPR data to be used for research purposes. John Padoba and his team from Dacima Software Inc. also deserve thanks for their work developing the first version of the electronic web-based software for inputting QPR data. The authors thank Benoit Duchaine and his team from Typhon Solutions Inc. who developed the updated electronic CRFs and database of the QPR. Finally, thanks are due to Marc Dorais (StatSciences Inc.), who conducted the statistical analyses carried out in the early phases of the QPR project.
Disclosure Statement
Dr. Manon Choinière received funding from the Research Center of the Centre Hospitalier de l’Université de Montréal to carry out this study. The Quebec Pain Registry (QPR) Project, led by Drs. Manon Choinière and Mark Ware, was supported by the Quebec Pain Research Network (QPRN), which was itself funded by a governmental grant from the Fonds de recherche du Québec–Santé (FRQS). The QPRN was also supported by the Quebec Health Ministry, Pfizer Canada Inc., Astra Zeneca Inc., and, to a lesser extent, by Janssen Inc., whose contributions were all channeled through the FRQS via an official financial partnership. All authors of the present article certify that they have no conflicts of interest with any financial organisation regarding the material presented and discussed in this article.
References
- 1.International Narcotics Control Board . Availability of internationally controlled drugs: ensuring adequate access for medical and scientific purposes. New York (NY): United Nations; 2016. [Google Scholar]
- 2.International Narcotics Control Board . Narcotic drugs: estimated world requirements for 2004, statistics for 2002. New York (NY): United Nations; 2004. [Google Scholar]
- 3.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell CI, Merrill JO, Silverberg MJ, Banta-Green C, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman LS, Brunton LL, Chabner B, Knollmann BC.. Goodman & Gilman’s the pharmacological basis of therapeutics.New York (NY): McGraw-Hill Medical; 2011. [Google Scholar]
- 5.Dart RC, Severtson SG, Bucher-Bartelson B.. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(16):1573–1574. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 6.Statistics Canada . Canadian Tobacco, Alcohol and Drugs Survey (CTADS). 2013 [accessed 2016 Sep 28]. http://www23.statcan.gc.ca/.
- 7.Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Center for Behavioral Health Statistics and Quality (U.S.). National admissions to substance abuse treatment services. Rockville (MD): Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2011. [accessed 2015 Oct 13]. http://wwwdasis.samhsa.gov/teds09/TEDS2k9NWeb.pdf. [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Center for Behavioral Health Statistics and Quality (U.S.). The DAWN report: highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Rockville (MD): Office of Applied Studies Center for Behavioral Health Statistics and Quality; 2012. [Google Scholar]
- 9.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ.. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–720. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS Data Brief. 2011;81:1–8. [PubMed] [Google Scholar]
- 11.Rolita L, Spegman A, Tang X, Cronstein BN. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61(3):335–340. doi: 10.1111/jgs.2013.61.issue-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 13.Ontario’s opioid related death rates quadruple over the past 25 years . Emerging trends in opioid-related deaths in Ontario: 1991 to 2015. Ontario Drug Policy Research Network, Toronto; 2017. [Google Scholar]
- 14.American Pain Society and American Academy of Pain Medicine . Guideline for the use of chronic opioid therapy in chronic noncancer pain: evidence review. Glenview, IL: American Pain Society; 2009.
- 15.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage. 2008;35(2):214–228. doi: 10.1016/j.jpainsymman.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Reid MC, Bennett DA, Chen WG, Eldadah BA, Farrar JT, Ferrell B, Gallagher RM, Hanlon JT, Herr K, Horn SD, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12(9):1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellows BK, Kuo KL, Biltaji E, Singhal M, Jiao T, Cheng Y, McAdam-Marx C. Real-world evidence in pain research: a review of data sources. J Pain Palliat Care Pharmacother. 2014;28(3):294–304. doi: 10.3109/15360288.2014.941131. [DOI] [PubMed] [Google Scholar]
- 19.McQuay H, Moore A. Utility of clinical trial results for clinical practice. Eur J Pain. 2007;11(2):123–124. doi: 10.1016/j.ejpain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Furlan A, Chaparro LE, Irvin E, Mailis-Gagnon A. A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag. 2011;16(5):337–351. doi: 10.1155/2011/465281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1–2):172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Fredheim OM, Mahic M, Skurtveit S, Dale O, Romundstad P, Borchgrevink PC. Chronic pain and use of opioids: a population-based pharmacoepidemiological study from the Norwegian prescription database and the Nord–Trondelag health study. Pain. 2014;155:1213–21. doi: 10.1016/j.pain.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Campbell G, Nielsen S, Bruno R, Lintzeris N, Cohen M, Hall W, Larance B, Mattick RP, Degenhardt L. The Pain and Opioids IN Treatment Study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain. 2015;156(2):231–242. doi: 10.1097/01.j.pain.0000460303.63948.8e. [DOI] [PubMed] [Google Scholar]
- 24.LeResche L, Saunders K, Dublin S, Thielke S, Merrill JO, Shortreed SM, Campbell C, Von Korff MR. Sex and age differences in global pain status among patients using opioids long term for chronic noncancer pain. J Womens Health (Larchmt). 2015;24(8):629–635. doi: 10.1089/jwh.2015.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulin DE, Clark AJ, Gordon A, Lynch M, Morley-Forster PK, Nathan H, Smyth C, et al. Long-term outcome of the management of chronic neuropathic pain: a prospective observational study. J Pain. 2015;16(9):852–861. doi: 10.1016/j.jpain.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Bostick GP, Toth C, Carr EC, Stitt LW, Morley-Forster P, Clark AJ, Lynch M, Gordon A, Nathan H, Smyth C, et al. Physical functioning and opioid use in patients with neuropathic pain. Pain Med. 2015;16(7):1361–1368. doi: 10.1111/pme.12702. [DOI] [PubMed] [Google Scholar]
- 27.Watson CP, Watt-Watson J, Chipman M. The long-term safety and efficacy of opioids: a survey of 84 selected patients with intractable chronic noncancer pain. Pain Res Manag. 2010;15(4):213–217. doi: 10.1155/2010/867201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks EA, Unruh A, Lynch ME. Exploring the lived experience of adults using prescription opioids to manage chronic noncancer pain. Pain Res Manag. 2015;20(1):15–22. doi: 10.1155/2015/314184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choiniere M, Ware MA, Page MG, Lacasse A, Lanctot H, Beaudet N, Boulanger A, Bourgault P, Cloutier C, Coupal L, et al. Development and implementation of a registry of patients attending multidisciplinary pain treatment clinics: the Quebec Pain Registry. Pain Res Manag. 2017;2017:8123812. doi: 10.1155/2017/8123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 2nd ed. New York (NY): Guilford Press; 2001. p. 15–34. [Google Scholar]
- 31.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Tyler EJ, Jensen MP, Engel JM, Schwartz L. The reliability and validity of pain interference measures in persons with cerebral palsy. Arch Phys Med Rehabil. 2002;83(2):236–239. doi: 10.1053/apmr.2002.27466. [DOI] [PubMed] [Google Scholar]
- 33.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 34.Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67(2–3):267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 35.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Larue F, Carlier AM, Brasseur L, Colleau SM, Cleeland CS, editors. Assessing the prevalence and severity of cancer pain in France: the French Brief Pain Inventory. Paper presented at: American Pain Society 10th Annual Scientific Meeting; 1991. Nov 7–10; New Orleans, LA. [Google Scholar]
- 37.Ware J Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Choinière M, Ware M, Pagé M, Lacasse A, Lanctôt H, Beaudet N, Boulanger A, Bourgault P, Cloutier C, Coupal L, et al. Development and Implementation of a Registry of Patients Attending Multidisciplinary Pain Treatment Clinics: The Quebec Pain Registry. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2017;2017:8123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J. Statistical power analysis for behavioral sciences. Hillsdale, NJ: Laurence Erlbaum; 1988. [Google Scholar]
- 41.Pearson K. On the theory of contingency and its relation to association and normal correlation. London (UK): Delay & Co.; 1904. [Google Scholar]
- 42.Wilkinson L, Statistical methods in psychology journals: Guidelines and explanations. Stat Methods Psychol J Am Psychol. 1999;54:594–604. [Google Scholar]
- 43.Kline RB. Beyond significance testing. Washington (DC): American Psychological Association; 2004. [Google Scholar]
- 44.Gliklich R, Dreyer N, Leavy M, editors. Registries for evaluating patient outcomes: a user’s guide. 3rd ed. Vol. 2. Rockville (MD: ): Agency for Healtcare Research and Quality; 2014. June 22, 2017; AHRQ Publication No. 13 (14)-EHC111. http://www.effectivehealthcare.ahrq.gov/registries-guide-3.cfm. [PubMed] [Google Scholar]
- 45.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD, Banta-Green CJ, Merrill JO, Silverberg MJ, Boudreau D, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine. 2004;29(8):884–890; discussion 891. doi: 10.1097/00007632-200404150-00012. [DOI] [PubMed] [Google Scholar]
- 48.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in U.S.: 1980 vs. 2000. Pain. 2004;109(3):514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Guerriere DN, Choiniere M, Dion D, Peng P, Stafford-Coyte E, Zagorski B, Banner R, Barton PM, Boulanger A, Clark AJ, et al. The Canadian STOP-PAIN project—part 2: what is the cost of pain for patients on waitlists of multidisciplinary pain treatment facilities? Can J Anaesth. 2010;57(6):549–558. doi: 10.1007/s12630-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 50.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel Shaheed C, Cg M, Ka W, Day R, Aj M. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958–968. doi: 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- 52.Ballantyne JC. Opioids for chronic nonterminal pain. South Med J. 2006;99(11):1245–1255. doi: 10.1097/01.smj.0000223946.19256.17. [DOI] [PubMed] [Google Scholar]
- 53.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 54.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 55.Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–684. [PubMed] [Google Scholar]
- 56.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 57.Kalso E, Allan L, Dellemijn PL, Faura CC, Ilias WK, Jensen TS, Perrot S, Plaghki LH, Zenz M. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7(5):381–386. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- 58.Kalso E. Opioids for persistent non-cancer pain. BMJ. 2005;330(7484):156–157. doi: 10.1136/bmj.330.7484.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choiniere M, Dion D, Peng P, Banner R, Barton PM, Boulanger A, Clark AJ, Gordon AS, Guerriere DN, Guertin MC, et al. The Canadian STOP-PAIN project—part 1: who are the patients on the waitlists of multidisciplinary pain treatment facilities? Can J Anaesth. 2010;57(6):539–548. doi: 10.1007/s12630-010-9305-5. [DOI] [PubMed] [Google Scholar]
- 60.Riley JL III, Hastie BA. Individual differences in opioid efficacy for chronic noncancer pain. Clin J Pain. 2008;24(6):509–520. doi: 10.1097/AJP.0b013e31816c6654. [DOI] [PubMed] [Google Scholar]
- 61.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 62.Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, Sarton E. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151(1):61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav. 2010;58(1):72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Lynch ME, Katz J. “One size fits all” doesn’t fit when it comes to long-term opioid use for people with chronic pain. Can J Pain. 2017;1:2–7. doi: 10.1080/24740527.2017.1319733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng P, Choiniere M, Dion D, Intrater H, Lefort S, Lynch M, Ong M, Rashiq S, Tkachuk G, Veillette Y, et al. Challenges in accessing multidisciplinary pain treatment facilities in Canada. Can J Anaesth. 2007;54(12):977–984. doi: 10.1007/BF03016631. [DOI] [PubMed] [Google Scholar]


