ABSTRACT
Background: Chronic postsurgical pain is a highly prevalent public health problem associated with substantial emotional, social, and economic costs.
Aims: (1) To review the major risk factors for chronic postsurgical pain (CPSP); (2) to describe the implementation of the Transitional Pain Service (TPS) at the Toronto General Hospital, a multiprofessional, multimodal preventive approach to CPSP involving intensive, perioperative psychological, physical, and pharmacological management aimed at preventing and treating the factors that increase the risk of CPSP and related disability; and (3) to present recent empirical evidence for the efficacy of the TPS.
Methods: The Toronto General Hospital TPS was specifically developed to target patients at high risk of developing CPSP. The major known risk factors for CPSP are perioperative pain, opioid use, and negative affect, including depression, anxiety, pain catastrophizing, and posttraumatic stress disorder–like symptoms. At-risk patients are identified early and provided comprehensive care by a multidisciplinary team consisting of pain physicians, advanced practice nurses, psychologists, and physical therapists.
Results: Preliminary results from two nonrandomized, clinical practice–based trials indicate that TPS treatment is associated with improvements in pain, pain interference, pain catastrophizing, symptoms of anxiety and depression, and opioid use. Almost half of opioid-naïve patients and one in four opioid-experienced patients were opioid free by the 6-month point.
Conclusions: These promising results suggest that the TPS benefits patients at risk of CPSP. A multicenter randomized controlled trial of the TPS in several Ontario hospitals is currently underway.
KEYWORDS: Transitional Pain Service, acceptance and commitment therapy, chronic postsurgical pain, risk factors, opioid use, opioid weaning, pain, pain interference
Abstract
Contexte: La douleur chronique postopératoire est un problème de santé publique très prévalent, associé à des coûts émotionnels, sociaux et économiques considérables.
Buts: (1) Étudier les facteurs de risque importants pour la douleur chronique postopératoire; (2) décrire la mise en œuvre du Service de la douleur transitoire à l’Hôpital général de Toronto, une approche préventive multiprofessionnelle, multimodale à la douleur chronique postopératoire fondée sur une prise en charge intensive, périopératoire, psychologique, physique et pharmacologique pour prévenir et traiter les facteurs qui augmentent le risque de douleur chronique postopératoire et l’incapacité qui s’y rattache; et (3) présenter des données probantes empiriques récentes en ce qui concerne l’efficacité du Service de douleur transitoire.
Méthodes: Le Service de la douleur transitoire de l’Hôpital général de Toronto a été expressément mis sur pied pour cibler les patients ayant un risque élevé de souffrir de douleur chronique postopératoire. Les principaux facteurs de risque connus pour la douleur chronique postopératoire sont la douleur périopératoire, la consommation d’opiacés et un affect négatif, y compris la dépression, l’anxiété, la catastrophisation de la douleur et les symptômes apparentés au syndrome de stress post-traumatique. Les patients à risque sont repérés de manière précoce et des soins complets leurs sont prodigués par une équipe multidisciplinaire composée de médecins spécialistes de la douleur, d’infirmières en pratique avancée, de psychologues et de physiothérapeutes.
Résultats: Les résultats préliminaires de deux essais cliniques non randomisés fondés sur la pratique indiquent que le traitement prodigué par le Service de la douleur transitoire est associé à des améliorations en ce qui concerne la douleur, l’interférence de la douleur, la catastrophisation de la douleur, les symptômes d’anxiété et de dépression, et la consommation d’opiacés. Près de la moitié des patients n’ayant jamais consommé d’opioïdes et un patient sur quatre parmi ceux qui avaient déjà consommé des opioïdes n’en consommaient plus après six mois.
Conclusions: Ces résultats prometteurs suggèrent que le Service de la douleur transitoire profite aux patients à risque de douleur chronique postopératoire. Un essai multicentre randomisé et contrôlé du Service de la douleur transitoire est actuellement en cours dans plusieurs hôpitaux ontariens.
Chronic pain is the silent epidemic of our times.1 It causes enormous human suffering and drains the Canadian economy and health care system of valuable resources. Health-related quality of life of Ontarians with chronic pain is lower than that reported by people with most other chronic diseases, including heart disease, diabetes, and chronic obstructive pulmonary disease.2 The annual incremental medical cost to manage chronic pain in Ontario is estimated at $1742 per person in 2014 Canadian dollars,3 which translates to a ~$10 billion burden annually to the Canadian health care system, not including direct out-of-pocket costs incurred by the person with pain or indirect costs such as lost income. Chronic postsurgical pain (CPSP) is a significant driver of this cost4 given the high rates of CPSP. The magnitude of the problem is evident when one jointly considers that 312 million major surgeries are performed annually worldwide5 and the one-year incidence of moderate-to-severe CPSP is ~12% and ~22% for adults6 and children,7 respectively. It is not surprising, then, that more than 20% of adults8 and 17% of children9 attending specialized chronic pain centers have been referred for CPSP. Innovative research designs10–12 and novel solutions13,14 are needed to halt—and potentially reverse—the transition of acute pain to chronic pain, improve quality of life, and reduce personal/system costs related to unnecessary hospitalizations, medications, disability, and unemployment.
The aims of the present article to are (1) review the major known risk factors for CPSP; (2) describe the implementation of the Transitional Pain Service (TPS) at the Toronto General Hospital, a multiprofessional, multimodal preventive approach to CPSP involving intensive, perioperative psychological, physical, and pharmacological management aimed at preventing and treating the factors that increase the risk of CPSP and related disability; and (3) present recent empirical evidence for the efficacy of the TPS.
Chronic postsurgical pain—Definition
Substantial variability exists among surgical procedures, including the anatomic structures and physiologic processes affected as well as the time required to heal and recover. Nevertheless, the following 6-point definition appears to capture the most important aspects of CPSP.15,16 The pain (1) developed after a surgical procedure, (2) is at least 2 months in duration, (3) interferes significantly with health-related quality of life, (4) is a continuation of acute postsurgical pain or develops after an asymptomatic period, (5) is localized to the surgical field and/or projected to territory or dermatome innervated by a nerve in the surgical field and (6) is not caused by other factors (e.g., preoperative pain, recurrence after surgery for cancer, chronic infection have been ruled out).
Risk factors for chronic postsurgical pain
Considerable progress has been made in identifying risk and protective factors for CPSP,7,17–26 although the question of causality remains unanswered.19,27–29 There are many risk factors for CPSP. The following are those most consistently reported in the literature. Type of surgery has long been recognized as a major risk factor for CPSP, with surgeries that involve deliberate or inadvertent damage to nerves showing the highest prevalence,17,19 though not all CPSP is neuropathic.30
The most robust risk factor for CPSP is pain itself.19,31 The data clearly show that the presence32 and intensity of preoperative chronic pain,6,33–35 the intensity of acute postoperative pain,6,23,32,33,36 the time spent in severe pain after surgery,6 pain intensity in the weeks after surgery,35,37,38 and pain in other body parts32,34,39 reliably predict CPSP across a range of surgical procedures. Higher consumption of postoperative analgesics, typically a proxy for intense postoperative pain, is associated with more intense CPSP.38,40,41 Negative cognitive–affective states including perioperative depression,42,43 anxiety,25,32,35,43,44 pain catastrophizing,18,44 and posttraumatic stress symptoms45 also predict the development of CPSP. Finally, preoperative opioid use, with a prevalence of ~25%,46 is a risk factor for CPSP,32,47 in part due to opioid-induced hyperalgesia.48,49
Negative affect and pain catastrophizing are also risk factors for intense, acute postoperative pain and excessive opioid use.50–52 Inadequately controlled acute pain and excessive opioid use delay recovery and hospital discharge after many surgeries.53–56 Notably, many of the above risk factors for CPSP are also associated with persistent opioid use after surgery, including mood disorders,57 anxiety,57 pain catastrophizing,58 preoperative neuropathic and nonneuropathic pain,57 and greater pain intensity on the day of surgery.58
Management of these known risks before and after surgery is hypothesized not only to reduce pain, suffering, and opioid misuse but also to benefit the health care system by facilitating earlier discharge and reducing costs.59–61 The following sections describe the Toronto General Hospital TPS and preliminary clinical outcomes.
Toronto General Hospital Transitional Pain Service—Development and description
The Toronto General Hospital TPS59–61 was established in 2014 to address the problem of CPSP with a seamless approach to perioperative pain and opioid use using multidisciplinary, integrated care. Patients are assessed and managed as early as the preoperative visit, treatment is extended into the in-hospital setting after surgery, and it is maintained for up to 6 months across the post–hospital discharge period once patients have returned home. The primary aim of the TPS is to offer timely and effective treatment to patients at high risk of developing chronic postsurgical pain and persistent opioid use after undergoing a variety of surgical procedures, including those for cancer (e.g., thoracic, breast, gastrointestinal, head and neck), cardiac disease (e.g., coronary artery bypass graft, heart valve repair), and organ transplants (e.g., kidney, lung, liver, heart, pancreas). The three major goals of the TPS are to (1) provide comprehensive pre- and postoperative pain management for patients who are at high risk of developing chronic postsurgical pain and pain disability, (2) manage opioid medication while in hospital and after discharge, and (3) improve coping and functioning in the immediate and long term to provide as high a quality of life as possible. At present, clinical services at the TPS include multimodal medication optimization by anesthesiologists, postsurgical physical therapy and acupuncture, and a pain psychology intervention consisting of pain education, mindfulness training, brief hypnosis, and a form of cognitive–behavioral treatment called acceptance and commitment therapy (ACT). The service also includes an administrative assistant and a patient care coordinator. In 2016, the TPS began a partnership with ManagingLife, whose mobile platform and app, Manage My Pain, allows TPS patients to quickly and easily track their pain on a daily basis using an Apple iPhone, Android smartphone, or a responsively designed web app through their mobile or desktop browser.62
The TPS provides proactive, timely support in a multidisciplinary setting to inpatients and outpatients with complex postsurgical pain for up to 6 months after surgery. Patients are screened for physical and mental health problems and flagged for known risk factors (Table 1) if they have a history of anxiety, depression, high levels of pain catastrophizing, chronic opioid use, and/or preexisting chronic pain. Intensive intervention is provided to patients who are at the highest risk of developing CPSP and persistent, high-dose opioid use.
Table 1.
Referral criteria for admission to the Transitional Pain Service.a
| “Pain alert” patients |
| Presurgical chronic pain |
| History of drug abuse |
| Currently on opioid, methadone, or buprenorphine maintenance therapy |
| Severe postsurgical pain |
| Prolonged Acute Pain Service stay |
| Surgical patients with repeat Acute Pain Service consultation |
| Medically stable postsurgical patients with complex pain problems that prevent discharge |
| High postsurgical opioid consumption |
| Consumption of > 90 MME/day |
| Methadone or buprenorphine patients without a community pain specialist |
| Patients discharged with a prescription for a long-acting opioid |
| Interventional postsurgical procedures (e.g., stump catheters postamputation) |
| Emotional distress |
| Depression |
| Anxiety |
| Pain catastrophizing |
| Other psychosocial concern(s) identified by questionnaires or Acute Pain Service/Transitional Pain Service member |
aAdapted with permission from Katz et al.59
MME = morphine milligram equivalents.
Toronto General Hospital Transitional Pain Service—Implementation
Psychology at the TPS
There is growing understanding of the important role that psychological interventions can play in reducing pain perception, negative affect, and avoidance behaviors after surgery.29 The main goal of the TPS ACT intervention is to teach patients a mindful approach to their postsurgical pain that allows them to live a fuller life.29,63 The ACT intervention teaches them how to stop the negative cycle of intense postoperative pain, emotional distress, behavioral avoidance, and escalating opioid use that inhibits functioning and degrades quality of life. Patients learn to expand their capacity to experience pain—including the negative thoughts and feelings that inevitably arise when pain is present. Pain sensations—as well as the patients’ reactions to them—are observed neutrally and nonreactively, while focusing on enhancing motivation and commitment to engage in personally meaningful, achievable, goal-oriented activities. They are taught to do so without engaging in problematic avoidance behaviors that typically make the pain worse and limit functioning. Ultimately, patients become more psychologically flexible; they learn to adapt their behavior in a way that enables them to live a rich and meaningful life while open to their internal psychological and emotional experiences, including pain.
Other Disciplines at the TPS
In addition to our psychology team, the nursing, physiotherapy/acupuncture specialists, and recently our yoga specialist are all integral to the TPS. The Acute Pain Service nurse practitioners are the backbone of the TPS. They identify patients immediately after surgery who are not recovering as they should and then refer to our inpatient coordinator, who then arranges an in-hospital visit and the scheduling of the outpatient clinic visit. The outpatient clinic has also recently expanded to include a pain and opioid misuse (addiction) specialist nurse practitioner who has been a critical addition to the team and a significant resource to the hospital. The nurse practitioner’s role is to manage complex pain patients with opioid and other use disorders within the institution (expanding beyond perioperative care). Our acupuncturist manages patients who are amenable to treatment in the outpatient setting and we are planning to evaluate its effectiveness in the immediate postoperative time period. Finally, we have a certified hatha yoga instructor who has developed a postoperative yoga pathway that we are currently evaluating for efficacy.
Challenges in the implementation of the TPS
We encountered several barriers to implementation of the TPS. Some have been overcome but we continue to grapple with others. First, institutional acceptance of the program took time, and acceptance by our surgical colleagues regarding the added value of this novel care pathway was initially lukewarm. Today, the referrals overwhelm the team and currently a major limitation is the inability to keep up with the increased capacity demands within the institution. Discharging patients after they have stabilized has also proven to be a challenge. This is partly due the patients’ positive connection to the interdisciplinary team and their reluctance to move back to a single-provider model of care. The lack of primary care providers for some patients has also proven to be a factor limiting our ability to discharge TPS patients within the 3- to 6-month postoperative time frame. The added burden of the opioid crisis has also delayed our ability to discharge stable TPS patients who were unable to wean off their opioid medications because many primary care providers simply refuse to inherit opioid prescriptions not initiated by them or refuse outright to prescribe opioids at all. We also encountered initial challenges integrating the Manage My Pain (MMP) app into the daily clinical service of the TPS. One of the biggest hurdles in the implementation of the MMP platform at Toronto General Hospital was safe stewardship of patient health information. Issues involved data security, sharing patient data with the care team, authenticating the clinical user, and access to patient data after TPS treatment has ended. These concerns regarding the safeguarding of patients’ electronic private health information have been resolved to satisfaction of the TPS, MMP, and patients.62
Preliminary evaluation of the TPS
At present, the best evidence we have for the efficacy of the TPS comes from two clinical practice–based cohort studies64,65 and a detailed case report63 showing promising psychosocial outcomes and opioid weaning rates. The two clinical practice–based studies are reviewed in detail below. A multicenter randomized controlled trial of the TPS in several Ontario hospitals is currently underway, funded by the Ontario Ministry of Health and Long-Term Care.
TPS ACT-based psychological outcomes
In the first study, patients receiving TPS psychological services (ACT group) were compared to those not receiving psychological services (no ACT group) on measures of pain, pain interference, key psychological constructs, and opioid use for the duration of TPS treatment.64
A total of 382 TPS patients participated in the study. Ninety-one received ACT and 252 did not (no ACT). Pain intensity, pain interference, sensitivity to pain traumatization,66 pain catastrophizing,67 symptoms of anxiety and depression,68 and opioid use were compared between the two groups and across time, beginning with the first TPS visit and ending with the last. Patients referred to the ACT group were significantly more likely to report a mental health condition preoperatively, had significantly higher opioid use at the first postsurgical visit, and, at both time points, reported significantly higher sensitivity to pain traumatization and anxiety scores than the no ACT group. These pretreatment differences are not surprising given that patients who are referred to TPS psychology services typically are the most distressed and are having the most difficulty coping. On the other hand, likely because of these differences, the ACT group was involved with the TPS for a significantly greater number of weeks and had significantly more medical visits to the TPS than the no ACT group.
By the last TPS visit, both groups showed significant reductions in pain intensity, pain interference, pain catastrophizing, anxiety, and opioid use. Compared to the no ACT group, the ACT group showed greater reductions in opioid use and pain interference. Moreover, only the ACT group showed a significant reduction in depressed mood by the last TPS visit. These results indicate that patients who are referred for psychology services upon admission to the TPS are a higher-risk group of patients. Nevertheless, learning the ACT approach to behavioral pain management enabled them to wean off opioid medications to a greater extent than the lower-risk, no ACT group while at the same time reporting greater improvements in mood and less pain interference. However, differences in treatment duration between the ACT and no ACT groups raise the question as to whether the no ACT group would have achieved additional reductions in opioid weaning, pain interference, and depressive symptomology given an equivalent follow-up time. With these caveats in mind, these results provide preliminary support for the ACT-based intervention in targeting and successfully managing TPS patients at risk of CPSP and persistent opioid use.
TPS opioid consumption and opioid weaning rates
The second clinical practice–based study65 compared opioid consumption and opioid weaning rates an average of 6 months after surgery between TPS patients who were opioid naïve or opioid experienced presurgically. Opioid consumption in daily morphine milligram equivalents (MME/day) was examined on admission to the TPS, at hospital discharge after surgery, and on the day of their last TPS visit an average of 6 months after surgery.
Opioid-experienced patients (n = 137) were taking a mean of 78.8 ± 100.2 MME/day prior to surgery. The daily dose almost doubled to 140.5 ± 124.0 MME/day by the time they were discharged from hospital after surgery. At the last TPS visit ~6 months after surgery they were taking 78.3 ± 113.9 MME/day (44.3% decrease), which was essentially the same dose they had been taking prior to surgery (Figure 1). In terms of weaning rates, by the 6-month mark, 35 opioid-experienced patients (25%) had been completely weaned, 50 (36%) had reduced their opioid use by >50% of the hospital discharge dose, 27 (19%) had reduced their opioid use by <50%, and 27 (19%) had increased opioid use since hospital discharge.
Figure 1.
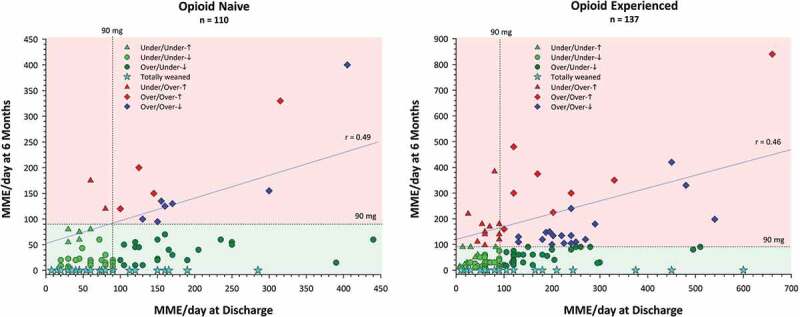
Mean daily opioid use in daily morphine milligram equivalents (MME/day) at the end of TPS treatment shown as a function of daily morphine milligram equivalents at hospital discharge (i.e., prior to TPS treatment) for opioid-naïve and opioid-experienced patients. Also shown is the 90 MME/day maximum dose recommended by the U.S.69 and Canadian70 opioid guidelines (dashed lines). Based on the 90 MME/day, patients in the two lower quadrants (green shading) represent treatment successes and those in the two upper quadrants (red shading) represent treatment failures. Green triangles represent patients who were under 90 MME/day on both occasions and higher at 6 months than at hospital discharge; green circles represent patients who were under 90 MME/day on both occasions and lower at 6 months than at hospital discharge; dark green circles represent patients who were over 90 MME/day at hospital discharge and under at 6 months; cyan stars represent patients who were totally weaned (MME/day = 0) by 6 months; red triangles represent patients who were under 90 MME/day at hospital discharge and over at 6 months; red diamonds represent patients who were over 90 MME/day on both occasions and higher at 6 months than at hospital discharge; blue diamonds represent patients who were over 90 MME/day on both occasions and lower at 6 months than at hospital discharge. Left panel adapted with permission from Clarke et al.65.
In contrast, opioid-naïve patients, who nonetheless were identified as high risk for postsurgical pain (n = 110), were taking a mean of 106.7 ± 80.6 MME/day at the time of hospital discharge after surgery, which was reduced to 37.3 ± 61.1 MME/day at the final TPS visit (65% decrease) ~6 months after surgery. In terms of weaning rates, by 6 months after surgery, 51 (46%) had been successfully weaned from opioids, 39 (35%) had reduced opioid use by >50% of their hospital discharge dose, 11 (10%) had reduced opioid use by <50%, and 9 (8%) had increased their opioid use from hospital discharge.
Figure 1 presents a more fine-grained picture of opioid use for the two groups, showing the daily morphine milligram equivalents dose at hospital discharge after surgery and at the last TPS visit an average of 6 months later based on the 2016 U.S.69 and 2017 Canadian70 opioid guideline recommendations not to exceed a maximum of 90 MME/day. Not surprising, the scale is doubled for the opioid-experienced group. The two lower quadrants (green shading) show treatment successes and the two upper quadrants (red shading) show failures. The lower left quadrants show patients who were below the recommended 90 MME/day at both time points split into those who were totally weaned by 6 months, those who were below 90 MME/day at discharge and even lower at 6 months, and those whose 6-month dose, though lower than 90 MME/day, was higher than their hospital discharge dose. The lower right quadrant shows patients who were above 90 MME/day at hospital discharge and who were totally weaned by 6 months or had managed to reduce their opioid dose to the recommended dosage or lower. As noted above, these patients represent treatment successes. In contrast, the upper left quadrant shows patients whose discharge dose was 90 MME/day or less but were taking more 6 months later. The upper right quadrant shows patients who were above the 90 MME/day limit on both occasions split into those whose dose was above or below the hospital discharge dose. Figure 2 shows a similar display for the opioid-experienced patients but plotting the hospital discharge dose as a function of the preoperative dose, retaining the symbols and legend from the previous figure depicting the 6-month outcome. Thus, the two upper quadrants indicate that there are at least 14 patients (cyan stars) whose preoperative and discharge doses were between 90 and 600 MME/day but who nevertheless were able to totally wean off opioids by the last TPS visit. Finally, note that the correlation coefficient for opioid-experienced patients shown in Figure 1 (r = 0.46) is lower than that in Figure 2 (r = 0.59), providing evidence for the efficacy of TPS treatment, which likely was responsible for reducing the magnitude of the relationship (i.e., breaking the link) between hospital discharge and end of TPS treatment opioid use.
Figure 2.
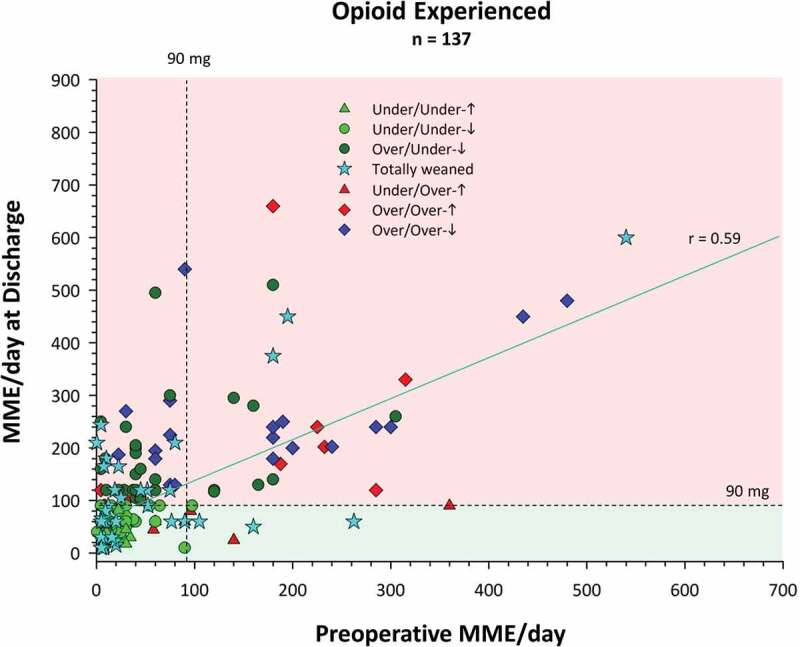
Mean daily opioid use in daily morphine milligram equivalents at hospital discharge shown as a function of daily morphine milligram equivalents preoperatively for opioid-experienced patients. Also shown is the 90 MME/day maximum dose recommended by the U.S. and Canadian opioid guidelines (dashed lines). Legend as in Figure 1.
Importantly, Brief Pain Inventory (BPI) pain intensity and interference scores at the last TPS visit were significantly lower than their respective post–hospital discharge scores (pre-TPS treatment) in both opioid-naïve and opioid-experienced groups, with the former group reporting larger improvements in pain interference than the latter group (Figure 3). These results indicate that the significant reduction in opioid use is not occurring at the expense of pain and pain-related interference.
Figure 3.
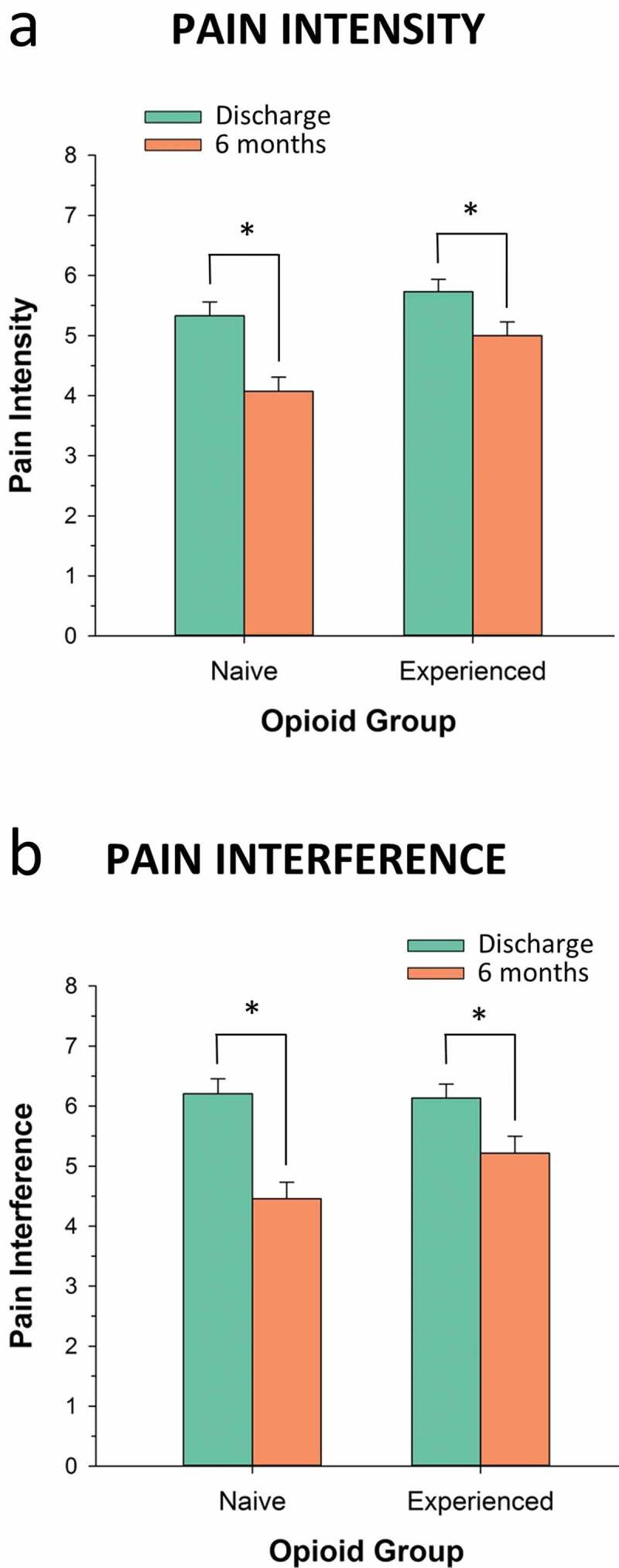
Pain intensity (0–10 Numeric Rating Scale) and pain interference (0–10 Numeric Rating Scale) scores at hospital discharge and at the end of TPS treatment 6 months later shown for opioid-naïve and opioid-experienced patients. Data from Clarke et al.65. *P < 0.009.
As noted above, 19% percent of opioid-experienced patients increased opioid use from hospital discharge to the 6-month time point. This subgroup is clearly of interest because these patients comprise our treatment failures, having faced the greatest challenge weaning from opioids. When compared with opioid-experienced patients who successfully reduced opioid consumption, this subgroup was more likely to be male, an organ transplant patient, and to have been diagnosed with a mental health disorder. For the entire sample of opioid-experienced patients, the predictors of opioid dose reduction from hospital discharge to the last TPS visit included lower pain catastrophizing scores, lower prevalence of neuropathic pain, and a negative history of recreational drug use. In contrast, the only predictor of dose reduction for opioid-naïve patients was the daily morphine milligram equivalent at hospital discharge.65
To our knowledge, this is the first study to report detailed data on opioid weaning rates and opioid doses in patients receiving comprehensive, targeted care for postsurgical pain and opioid use in the months after surgery. A multidisciplinary Acute Pain Service outpatient clinic similar to the TPS reported on a sample of 200 patients after surgery.71 Fifty-four percent and 32% were discharged from hospital on “weak” and “strong” opioids, respectively. At the end of the outpatient program 3 months later, 20% and 6% of patients were taking weak and strong opioids, respectively. Information on preoperative opioid use and daily morphine milligram equivalent doses before and after surgery were not reported, making it difficult to compare these findings with the TPS results presented above.
Conclusions
To date, the major known risk factors for CPSP are perioperative pain, opioid use, and negative affect, including depression, anxiety, pain catastrophizing, and posttraumatic stress disorder–like symptoms. The Toronto General Hospital TPS was specially developed to target patients at high risk of developing CPSP based on the above risk factors. Patients are identified early and provided comprehensive care by a multidisciplinary team consisting of pain physicians, advanced practice nurses, psychologists, and physical therapists. Preliminary results from two nonrandomized controlled trials indicate that the TPS effectively reduces pain intensity, pain-related interference, pain catastrophizing, symptoms of anxiety and depression, and opioid use. Almost half of opioid-naïve patients and one in four opioid-experienced patients were opioid free by the 6-month point. A multicenter randomized controlled trial of the TPS in several Ontario hospitals is currently ongoing.
Funding Statement
Funding for the Transitional Pain Service was provided by the Ontario Ministry of Health and Long-Term Care. J.K. is supported by a Canadian Institutes of Health Research Canada Research Chair in Health Psychology at York University. H.C. is supported by a Merit Award from the Department of Anesthesia, University of Toronto.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Wall PD, Jones M.. Defeating pain: the war against a silent epidemic. New York (NY): Plenum Press; 1991. [Google Scholar]
- 2.Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population-level survey and linked health care administrative data. Pain. 2017;158(3):408–16. doi: 10.1097/j.pain.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 3.Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Incremental health care costs for chronic pain in Ontario, Canada: a population-based matched cohort study of adolescents and adults using administrative data. Pain. 2016;157(8):1626–33. doi: 10.1097/j.pain.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 4.Parsons B, Schaefer C, Mann R, Sadosky A, Daniel S, Nalamachu S, Stacey BR, Nieshoff EC, Tuchman M, Anschel A. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res. 2013;6:459–69. doi: 10.2147/JPR.S44939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94(3):201–9F. doi: 10.2471/BLT.15.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher D, Stamer UM, Pogatzki-Zahn E, Zaslansky R, Tanase NV, Perruchoud C, Kranke P, Komann M, Lehman T, Meissner W, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32(10):725–34. doi: 10.1097/EJA.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 7.Pagé MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–80. doi: 10.2147/JPR.S40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76:167–71. [PubMed] [Google Scholar]
- 9.Martin AL, McGrath PA, Brown SC, Katz J. Children with chronic pain: impact of sex and age on long-term outcomes. Pain. 2007;128(1–2):13–19. doi: 10.1016/j.pain.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 2010;112(3):514–15. doi: 10.1097/ALN.0b013e3181cf423d. [DOI] [PubMed] [Google Scholar]
- 11.Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, Markman JD, Oaklander AL, Polydefkis MJ, Raja SN, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain. 2015;156(7):1184–97. doi: 10.1097/j.pain.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilron I, Vandenkerkhof E, Katz J, Kehlet H, Carley M. Evaluating the association between acute and chronic pain after surgery: impact of pain measurement methods. Clin J Pain. 2017;33(7):588–94. doi: 10.1097/AJP.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 13.Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, Edwards RR, Ji RR, Katz J, Kavelaars A, Levine JD, et al. Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci. 2018. doi: 10.1038/s41583-018-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke H. Transitional Pain Medicine: novel pharmacological treatments for the management of moderate to severe postsurgical pain. Expert Rev Clin Pharmacol. 2016;9(3):345–49. doi: 10.1586/17512433.2016.1129896. [DOI] [PubMed] [Google Scholar]
- 15.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. [DOI] [PubMed] [Google Scholar]
- 16.Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113(1):1–4. doi: 10.1093/bja/aeu012. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 18.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. J Pain Res. 2015;15:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 20.Cohen L, Fouladi RT, Katz J. Preoperative coping strategies and distress predict postoperative pain and morphine consumption in women undergoing abdominal gynecologic surgery. J Psychosom Res. 2005;58(2):201–09. doi: 10.1016/j.jpsychores.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Holtzman S, Clarke HA, McCluskey SA, Turcotte K, Grant D, Katz J. Acute and chronic postsurgical pain after living liver donation: incidence and predictors. Liver Transpl. 2014;20(11):1336–46. doi: 10.1002/lt.23949. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Asmundson GJ, McRae K, Halket E. Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. Eur J Pain. 2009;13(8):870–78. doi: 10.1016/j.ejpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. [DOI] [PubMed] [Google Scholar]
- 24.Pagé MG, Katz J, Curtis K, Lutzky-Cohen N, Escobar EM, Clarke HA. Acute pain trajectories and the persistence of post-surgical pain: a longitudinal study after total hip arthroplasty. J Anesth. 2016;30(4):568–77. doi: 10.1007/s00540-016-2183-4. [DOI] [PubMed] [Google Scholar]
- 25.Pagé MG, Katz J, Romero Escobar EM, Lutzky-Cohen N, Curtis K, Fuss S, Clarke HA. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain. 2015;156(3):460–68. doi: 10.1097/01.j.pain.0000460327.10515.2d. [DOI] [PubMed] [Google Scholar]
- 26.Pagé MG, Campbell F, Isaac L, Stinson J, Katz J. Parental risk factors for the development of pediatric acute and chronic postsurgical pain: a longitudinal study. J Pain Res. 2013;6:727–41. doi: 10.2147/JPR.S51055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz J. Perioperative predictors of long-term pain following surgery. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. Proceedings of the 8th world congress on pain, progress in pain research and management. Vol. 8. Washington (DC): IASP Press; 1997. p. 231–40. [Google Scholar]
- 28.Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesth Analg. 2011;113(5):1242–53. doi: 10.1213/ANE.0b013e31822c9a59. [DOI] [PubMed] [Google Scholar]
- 29.Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 2017;11(4):169–77. doi: 10.1177/2049463717720636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steyaert A, Lavand’homme P. Prevention and treatment of chronic postsurgical pain: a narrative review. Drugs. 2018;78(3):339–54. doi: 10.1007/s40265-018-0866-x. [DOI] [PubMed] [Google Scholar]
- 31.Katz J. Pain begets pain: predictors of long-term phantom limb pain and post-thoracotomy pain. Pain Forum. 1997;6(2):140–44. doi: 10.1016/S1082-3174(97)70048-5. [DOI] [Google Scholar]
- 32.VanDenKerkhof EG, Hopman WM, Goldstein DH, Wilson RA, Towheed TE, Lam M, Harrison MB, Reitsma ML, Johnston SL, Medd JD, et al. Impact of perioperative pain intensity, pain qualities, and opioid use on chronic pain after surgery: a prospective cohort study. Reg Anesth Pain Med. 2012;37(1):19–27. doi: 10.1097/AAP.0b013e318237516e. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Guyatt GH, Kennedy SA, Romerosa B, Kwon HY, Kaushal A, Chang Y, Craigie S, de Almeida CP, Couban RJ, et al. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. Cmaj. 2016;188(14):E352–E61. doi: 10.1503/cmaj.151276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, Peelen LM, Kappen TH, van Wijck AJ, Kalkman CJ, Meissner W. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120(5):1237–45. doi: 10.1097/ALN.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 35.Choinière M, Watt-Watson J, Victor JC, Baskett RJ, Bussieres JS, Carrier M, Cogan J, Costello J, Feindel C, Guertin MC, et al. Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: a 2-year prospective multicentre study. Cmaj. 2014;186(7):E213–23. doi: 10.1503/cmaj.131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto PR, McIntyre T, Araujo-Soares V, Almeida A, Costa P. Psychological factors predict an unfavorable pain trajectory after hysterectomy: a prospective cohort study on chronic postsurgical pain. Pain. 2018. doi: 10.1097/j.pain.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 37.Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, Bittner R, Kehlet H. Predictive risk factors for persistent postherniotomy pain. Anesthesiology. 2010;112(4):957–69. doi: 10.1097/ALN.0b013e3181d31ff8. [DOI] [PubMed] [Google Scholar]
- 38.Chidambaran V, Ding L, Moore DL, Spruance K, Cudilo EM, Pilipenko V, Hossain M, Sturm P, Kashikar-Zuck S, Martin LJ, et al. Predicting the pain continuum after adolescent idiopathic scoliosis surgery: A prospective cohort study. Eur J Pain. 2017;21(7):1252–65. doi: 10.1002/ejp.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. Jama. 2009;302(18):1985–92. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 40.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. 1999;43:563–67. [DOI] [PubMed] [Google Scholar]
- 41.Tiippana E, Nilsson E, Kalso E. Post-thoracotomy pain after thoracic epidural analgesia: a prospective follow-up study. Acta Anaesthesiol Scand. 2003;47:433–38. [DOI] [PubMed] [Google Scholar]
- 42.Attal N, Masselin-Dubois A, Martinez V, Jayr C, Albi A, Fermanian J, Bouhassira D, Baudic S. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain. 2014;137(Pt 3):904–17. doi: 10.1093/brain/awt354. [DOI] [PubMed] [Google Scholar]
- 43.Brandsborg B, Nikolajsen L. Chronic pain after hysterectomy. Curr Opin Anaesthesiol. 2018;31(3):268–73. doi: 10.1097/ACO.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 44.Pinto PR, McIntyre T, Araujo-Soares V, Almeida A, Costa P. Psychological factors predict an unfavorable pain trajectory after hysterectomy: a prospective cohort study on chronic postsurgical pain. Pain. 2018;159(5):956–67. doi: 10.1097/j.pain.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 45.Kleiman V, Clarke H, Katz J. Sensitivity to pain traumatization: a higher-order factor underlying pain-related anxiety, pain catastrophizing and anxiety sensitivity among patients scheduled for major surgery. Pain Res Manag. 2011;16:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilliard PE, Waljee J, Moser S, Metz L, Mathis M, Goesling J, Cron D, Clauw DJ, Englesbe M, Abecasis G, et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. JAMA Surg. 2018;153(10):929–37. doi: 10.1001/jamasurg.2018.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozet I, Nishio I, Robbertze R, Rotter D, Chansky H, Hernandez AV. Prolonged opioid use after knee arthroscopy in military veterans. Anesth Analg. 2014;119(2):454–59. doi: 10.1213/ANE.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112(6):991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 49.Rivat C, Ballantyne J. The dark side of opioids in pain management: basic science explains clinical observation. Pain Rep. 2016;1(2):e570. doi: 10.1097/PR9.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111(3):657–77. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 51.Khan RS, Ahmed K, Blakeway E, Skapinakis P, Nihoyannopoulos L, Macleod K, Sevdalis N, Ashrafian H, Platt M, Darzi A, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122–31. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Katz J, Buis T, Cohen L. Locked out and still knocking: predictors of excessive demands for postoperative intravenous patient-controlled analgesia. Can J Anaesth. 2008;55(2):88–99. doi: 10.1007/BF03016320. [DOI] [PubMed] [Google Scholar]
- 53.Baratta JL, Schwenk ES, Viscusi ER. Clinical consequences of inadequate pain relief: barriers to optimal pain management. Plast Reconstr Surg. 2014;134(4 Suppl 2):15S–21S. doi: 10.1097/PRS.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 54.Gurusamy KS, Vaughan J, Toon CD, Davidson BR. Pharmacological interventions for prevention or treatment of postoperative pain in people undergoing laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD008261. doi:10.1002/14651858.CD008261.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones NL, Edmonds L, Ghosh S, Klein AA. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia. 2013;68(2):179–89. doi: 10.1111/anae.12067. [DOI] [PubMed] [Google Scholar]
- 56.Robinson KP, Wagstaff KJ, Sanghera S, Kerry RM. Postoperative pain following primary lower limb arthroplasty and enhanced recovery pathway. Ann R Coll Surg Engl. 2014;96(4):302–06. doi: 10.1308/003588414X13946184900525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S, Nallamothu BK. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, Brummett CM. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259–65. doi: 10.1097/j.pain.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang A, Azam A, Segal S, Pivovarov K, Katznelson G, Ladak SS, Mu A, Weinrib A, Katz J, Clarke H. Chronic postsurgical pain and persistent opioid use following surgery: the need for a transitional pain service. Pain Manag. 2016;6(5):435–43. doi: 10.2217/pmt-2016-0004. [DOI] [PubMed] [Google Scholar]
- 60.Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97–105. doi: 10.2217/pmt.15.7. [DOI] [PubMed] [Google Scholar]
- 61.Katz J, Weinrib A, Fashler SR, Katznelzon R, Shah BR, Ladak SS, Jiang J, Li Q, McMillan K, Mina DS, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695–702. doi: 10.2147/JPR.S91924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinrib A, Azam MA, Latman VV, Janmohamed T, Clarke H, Katz J. Manage My Pain: A patient-driven mobile platform to prevent and manage chronic postsurgical pain. In: El MC, editor. Novel Applications of Virtual Communities in Healthcare Settings. Hershey (PA): IGI Global; 2018. p. 93–126. [Google Scholar]
- 63.Weinrib AZ, Burns LC, Mu A, Azam MA, Ladak SS, McRae K, Katznelson R, Azargive S, Tran C, Katz J, et al. A case report on the treatment of complex chronic pain and opioid dependence by a multidisciplinary transitional pain service using the ACT Matrix and buprenorphine/naloxone. J Pain Res. 2017;10:747–55. doi: 10.2147/JPR.S124566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azam MA, Weinrib AZ, Montbriand J, Burns LC, McMillan K, Clarke H, Katz J. Acceptance and Commitment Therapy to manage pain and opioid use after major surgery: preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain. 2017;1(1):37–49. doi: 10.1080/24740527.2017.1325317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke H, Azargive S, Montbriand J, Nichols J, Sutherland A, Valeeva L, Boulis S, McMillan K, Ladak S, Ladha K, et al. Opioid weaning and pain management in postsurgical patients at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2018;2(1):236–47. doi: 10.1080/24740527.2018.1501669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katz J, Fashler SR, Wicks C, Page MG, Roosen KM, Kleiman V, Clarke H. Sensitivity to Pain Traumatization Scale: development, validation, and preliminary findings. J Pain Res. 2017;10:1297–316. doi: 10.2147/JPR.S134133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–32. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 68.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention . CDC guideline for prescribing opioids for chronic pain—United States; 2016 [Accessed October 24, 2018]. https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm.
- 70.Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper L, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659–E66. doi: 10.1503/cmaj.171389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12:19–24. doi: 10.1016/j.sjpain.2016.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . CDC guideline for prescribing opioids for chronic pain—United States; 2016 [Accessed October 24, 2018]. https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm.


