Abstract
The p53 protein is subject to Mdm2-mediated degradation by the ubiquitin-proteasome pathway. This degradation requires interaction between p53 and Mdm2 and the subsequent ubiquitination and nuclear export of p53. Exposure of cells to DNA damage results in the stabilization of the p53 protein in the nucleus. However, the underlying mechanism of this effect is poorly defined. Here we demonstrate a key role for c-Abl in the nuclear accumulation of endogenous p53 in cells exposed to DNA damage. This effect of c-Abl is achieved by preventing the ubiquitination and nuclear export of p53 by Mdm2, or by human papillomavirus E6. c-Abl null cells fail to accumulate p53 efficiently following DNA damage. Reconstitution of these cells with physiological levels of c-Abl is sufficient to promote the normal response of p53 to DNA damage via nuclear retention. Our results help to explain how p53 is accumulated in the nucleus in response to DNA damage.
During cancer development there is a strong selection for the loss of p53 function. This occurs primarily via mutation in the p53 gene, or through inactivation of the p53 protein by viral and cellular oncogenes (45). Stimulation of p53 by oncogenes or stress conditions induces cell growth arrest, senescence, or apoptosis (reviewed in references 12, 40, and 45). In normal cells, the p53 protein is tightly regulated at multiple levels. These include the level of protein stability, posttranslational modifications, and subcellular localization (1, 20). The key negative regulator of p53 is the proto-oncogene mdm2. Mdm2 inhibits the transcriptional activity and growth suppression ability of p53 (32). The most important mechanism by which Mdm2 negatively regulates p53 is by promoting its degradation through the ubiquitin-proteasome pathway (16, 24), by acting as an E3 ligase (18). This activity of Mdm2 requires physical interaction between the two proteins. Inhibition of this interaction by antibodies or peptides directed to the interaction site results in the accumulation of p53 (4). The nuclear export of p53 is important for its degradation by Mdm2 (35). Blocking this nuclear export of p53 by the drug leptomycin B results in the accumulation of p53 in the nucleus (10). In addition to Mdm2, several other proteins have been shown to promote p53 for degradation, including the human papillomavirus (HPV) E6 protein (44).
The expression of the high-risk HPV E6 leads to various anogenital and some oral cancers (44). The E6 protein (HPV type 16 [HPV-16] and HPV-18) promotes p53 for degradation by recruiting a cellular E3 ligase, E6-associated protein (E6-AP), which interacts with p53 only in the presence of E6 (reviewed in references 17 and 44). E6 binds p53 in the C terminus and in the core domain; the latter is essential for p53 degradation (8, 27). E6 can also inhibit p53 activities without promoting its degradation, implicating the involvement of additional inhibitory mechanisms (reviewed in reference 44). This efficient silencing of p53 by E6 explains why in cervical tumors, in contrast to most other tumors, p53 remains wild type. However, once the cervical tumor cells metastasize, there is a selection for p53 mutations (9).
The negative regulation of p53 can be neutralized by the action of partner proteins and by specific modifications. In response to oncogene expression, the p14ARF protein protects p53 from Mdm2-mediated degradation (reviewed in reference 40). In addition, specific modifications of p53, such as phosphorylation on serines 15 and 20 and on threonine 18, activate p53 by reducing the affinity of p53 for Mdm2 (reviewed in reference 32). Of these, phosphorylation of serine 20 by Chk2, a target for ataxia telangiectasia mutant protein (ATM) activation, has an important physiological role in the activation and stabilization of p53 in response to DNA damage (7, 41). Thus, the ATM-Chk2 DNA damage signaling pathway appears to be important in p53 regulation. This is further supported by the identification of Chk2 mutations in Li-Fraumeni syndrome patients (3).
Interestingly, in response to ionizing radiation ATM activates another important regulator of p53, c-Abl, which is a nonreceptor tyrosine kinase (2, 38). c-Abl and p53 respond to similar genotoxic stresses (28). Expression of c-Abl induces G1 cell growth arrest in a p53-dependent manner (47, 50). Fibroblasts lacking c-Abl are impaired in their G1 arrest response to ionizing irradiation (50). The induction of c-Abl-dependent apoptosis in response to DNA damage involves collaboration with p73 (reviewed in reference 39) and to lesser extent with p53 (49). However, DNA damage-induced cell killing may also occur in the absence of c-Abl (28). c-Abl and p53 interact in vitro, and this interaction is further enhanced by DNA damage in vivo (48). Thus, a number of studies support a role for c-Abl in the cellular response to DNA damage, in which p53 is a key player.
Recently, we found that c-Abl neutralizes the ability of Mdm2 to promote p53 for degradation and to inhibit the transcriptional and apoptotic activities of p53 (46). This finding prompted us to examine the role of c-Abl in the accumulation of p53 by DNA damage, a major trigger of p53 activation. We report that c-Abl is important for the signaling pathway inducing p53 in response to DNA damage. The mechanisms underlying this important role of c-Abl were investigated. Our results support a key role for c-Abl in the activation of p53 by stress, by regulating the nuclear export of p53 and the extent of its ubiquitination.
MATERIALS AND METHODS
Cells and transfection assays.
HeLa cells and the fibroblastic cell lines c-Abl−/−+LacZ (Abl−/− fibroblasts reconstituted with LacZ) and c-Abl−/−+c-Abl (Abl−/− fibroblasts reconstituted with c-Abl) (46) were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum at 37°C. H1299 cells and Saos-2 cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum at 37°C. The Saos-2 cell line was derived from an osteosarcoma, and the H1299 cell line was derived from lung adenocarcinoma; neither of these lines expresses p53. HeLa cells were derived from cervical carcinoma infected with HPV-18. The c-Abl−/− +c-Abl fibroblasts expressed physiological levels of c-Abl (46). Transfections by the calcium phosphate precipitation method were carried out as previously described (15). The amount of expression plasmids used in each experiment is indicated in the corresponding figure legend. A constant amount of plasmid DNA in each sample was maintained by adding empty vector. Sf9 insect cells were grown in Grace's medium supplemented with yeastolate and lactalbumin hydrolysate solutions and 10% heat-inactivated fetal calf serum. Cells were grown and infected at 27°C. For the activation of p53, fibroblasts were treated with 3 or 10 μg of mitomycin C (Sigma) per ml or 3 μg of doxorubicin (Sigma) per ml or exposed to γ-irradiation (10 Gy).
Western blot analysis and luciferase assay were carried out as previously described (15). For immunofluorescent staining, cells were plated on glass coverslips. Twenty-four hours posttransfection, cells were treated for 2 h with the proteasome inhibitor ALLN (150 μM; Calbiochem) in order to prevent the degradation of p53 and Mdm2 in the cytoplasm. Cells were fixed in cold methanol and stained with anti-p53 antibodies (PAb1801 and DO1) followed by Cy3-conjugated goat anti-mouse immunoglobulin secondary antibody. Cells were stained simultaneously for DNA using DAPI (4′,6′-diamidino-2-phenylindole). Stained cells were observed with a confocal microscope (Zeiss).
The antibodies used were anti-human p53 monoclonal antibodies PAb1801 and DO1, anti-mouse p53 PAb248 and PAb421, anti-c-Abl ABL-148 (Sigma), anti-α-tubulin (DM1A; Sigma), anti-histone 2B (LG2-2; kindly provided by Dan Eilat, Hadassah University Hospital, Jerusalem; Israel), Cy3-conjugated goat anti-mouse immunoglobulin, and horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratory).
Ubiquitination assay in vivo and in vitro.
The ubiquitination of p53 in vivo was detected by transfecting HeLa cells with 0.5 μg of human p53 expression plasmid, with or without 4 μg of expression plasmid for c-abl. Twenty-two hours posttransfection, cells were treated with 150 μM ALLN for 2 h. Following treatment, cells were subjected to nuclear cytoplasmic fractionation. To prepare the cytoplasmic fraction, the cell pellets were resuspended in cytoplasmic buffer (10 mM Tris HCl [pH 8.0], 10 mM KCl). Cells were allowed to swell for 2 min, and then NP-40 was added to 0.4%, followed by centrifugation. The supernatant contained the soluble cytoplasmic fraction. The pellets were washed once more with the cytoplasmic buffer before proceeding to nuclear fractionation. For the preparation of the nuclear fraction, the remaining cell pellet was resuspended in high-salt radioimmunoprecipitation assay buffer (50 mM Tris [pH 8.0], 5 mM EDTA, 400 mM NaCl, 1% NP-40, 1% deoxycholate, and 0.025% sodium dodecyl sulfate [SDS]). The purity of the cytoplasmic fraction was verified by probing with anti-α-tubulin, while that of the nuclear fraction was verified with anti-histone 2B. Nuclear extracts were subjected to Western blot analysis using the indicated antibodies.
The in vitro reconstitution assay for the ubiquitination of p53 by E6–E6-AP was carried out essentially as described by Gonen et al. (14). Human p53 was translated in vitro by TNT reaction in wheat germ extract. The conjugation reaction mixture contained 0.2 μg of human E1 (affinity purified on a ubiquitin column), 1 μg of His-tagged UbcH5c (purified on a nickel column), glutathione S-transferase (GST)–HPV-16 E6 (purified on a glutathione Sepharose column), 0.3 μl of Sf9 cell extract expressing E6-AP, 2 μl of in vitro-translated p53, and different amounts of extract from Sf9 cells that were either infected with baculoviral vector encoding c-Abl or noninfected. The reaction was carried out in 12.5 μl containing 40 mM Tris (pH 7.5), 2 mM dithiothreitol, 5 mM MgCl2, 10 μg of ubiquitin, 5 mM ATPγS, and 0.5 μg of ubiquitin-aldehyde. The reaction was performed at 30°C for 50 min. The mixture was then resolved by SDS–10% polyacrylamide gel electrophoresis (10% PAGE).
The in vitro reconstitution assay for p53 ubiquitination by Mdm2 contained the following components: 0.5 μg of human E1 (affinity purified on a ubiquitin column), 0.5 μg of His-tagged UbcH5c (purified on a nickel column), 0.3 μg of GST-Mdm2 (purified on a glutathione Sepharose column), 0.5 μl of in vitro-translated p53 in wheat germ extract (Promega), and 0.5 μg of Sf9 extracts (from control and c-Abl-expressing cells). The reaction mixture contained 40 mM Tris (pH 7.6), 2 mM dithiothreitol, 5 mM MgCl2, 10 μg of ubiquitin, and 1 mM ATPγS. The reaction was performed at 30°C for 1 h. The mixture was then resolved by SDS–10% PAGE and subjected to Western blotting using anti-human p53 antibodies (PAb1801 and DO1).
Plasmids.
The expression plasmids used were those encoding human wild-type p53 (pRC/CMV wtp53), HPV-16 E6 (pCB6 HPV16E6; a generous gift from K. Vousden), His-tagged E6-AP in a baculoviral vector (a generous gift from M. Scheffner), human E1 and His-tagged UbcH5c (14), GST–HPV-16 E6, mouse wild-type c-abl (pCMV c-abl IV) and kinase-defective c-abl (pCMV c-abl K290H), mouse mdm2, and human Hdm2 (15, 46). The green fluorescent protein (GFP) plasmids used were the enhanced GFP plasmid (pEGFP Clontech) and pEGFP fused to the farnesylation signal of Ha-ras (pEGFPF; a generous gift from W. Jiang and T. Hunter). c-Abl was fused to the N terminus of GFP. Mouse c-abl was amplified by PCR using the following oligonucleotides: a 5′ primer, GCGAATTCCACCATGGGGCAGCAGCCTGG, containing an EcoRI restriction site and a 3′ primer, CAGGATCCCTCCGGACAATGTCGCTGA, containing a BamHI restriction site. The PCR product of c-abl was digested with these sites and cloned into the same sites in pEGFP. The reporter plasmid used was the cyclin G luciferase plasmid (15). For expression of c-Abl in baculovirus, c-Abl IV cDNA was fused to a six-His tag by PCR amplification. The 5′ oligonucleotide was GCGGATCCCATGCATCATCATCATCATCATGGGCAGCAGCCTGGAAAAGT, and the 3′ oligonucleotide was CCGAATTCACCTCCGGACAATGTCGTCGCTGAT. The PCR product was digested with BamHI and EcoRI and ligated into a baculovirus expression vector digested with the same restriction enzymes (pVL1393; Pharmingen). The baculovirus was generated and amplified in cells according to the manufacturer's instructions.
RESULTS
c-Abl is critical for the efficient accumulation of p53 in response to DNA damage.
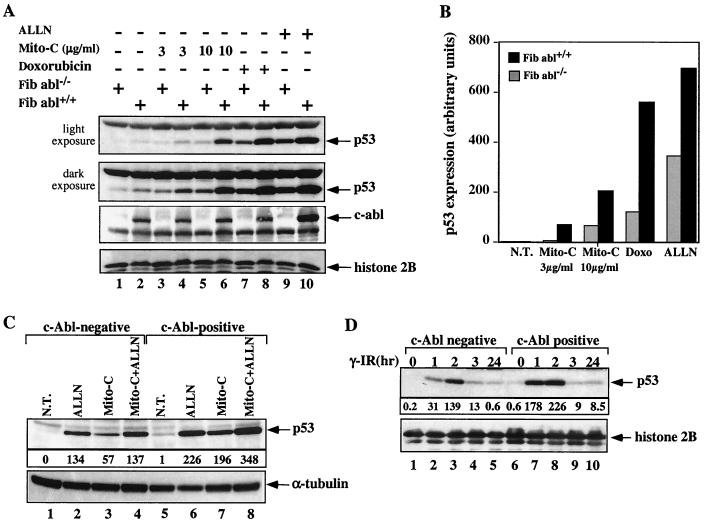
Cooperation between c-Abl and p53 in the cellular response to stress has been implicated by a number of studies (reviewed in reference 22). However, the underlying molecular mechanism for this cooperation has not been defined. It has previously been shown that overexpression of c-Abl can neutralize the degradation of p53 by Mdm2 (46). This finding encouraged us to propose that c-Abl may play a role in the accumulation of p53 in cells exposed to stress. Since both c-Abl and p53 are activated by double-strand DNA breaks, the role of c-Abl in the accumulation of p53 in response to such DNA damage was investigated. For this purpose, mouse embryo fibroblasts (MEFs) were generated from normal and c-abl null mice. Cells were exposed to mitomycin C for 6 h before harvest. The steady-state levels of endogenous p53 were determined by subjecting the cell extracts to Western blot analysis using anti-p53 antibodies (PAb421 and PAb248). As shown in Fig. 1A, the accumulation of p53 in cells exposed to 3 μg of mitomycin C per ml is greatly impaired in fibroblasts lacking c-Abl compared with normal fibroblasts (lanes 3 and 4, dark exposure). A similar effect was observed after exposure to 10 μg of mitomycin C per ml (lanes 5 and 6, light exposure). To ensure that this effect is not specific only to mitomycin C, cells were also exposed to another DNA-damaging agent, doxorubicin (3 μg/ml). Again, the accumulation of p53 was more efficient in the presence of c-Abl (lanes 7 and 8, light exposure). The expression of p53 was quantified by densitometry, and the results are summarized in Fig. 1B.
FIG. 1.
c-Abl enhances the accumulation of endogenous p53 in response to DNA damage. (A) MEFs from a c-abl null mouse (Fib abl−/−) or from a normal mouse (Fib abl+/+) were either untreated (lanes 1 and 2) or treated as indicated. Cells were incubated with ALLN (150 μM) for 4 h prior to harvest or were treated with mitomycin C (Mito-C) at the indicated concentrations for 6 h or with doxorubicin at 3 μg/ml for 6 h. At the end of the treatment, cell extracts were subjected to Western blot analysis using anti-p53 antibodies (PAb248 and PAb421). Two exposures of the enhanced chemiluminescence-treated blot showing p53 are presented in order to reveal the levels of basal and activated p53. The same membrane was reprobed with anti-c-Abl, and the amounts of protein loaded were monitored by reprobing with anti-histone 2B. (B) The intensity of the bands obtained in the light exposure was quantified by densitometry and the values are plotted on the graph. N. T., no treatment; Doxo, doxorubicin. (C) Fibroblasts null for c-Abl (c-Abl negative) and fibroblasts reconstituted with c-Abl (c-Abl positive) were either not treated (N.T.), incubated with ALLN (150 μM) for 4 h before harvest, treated with mitomycin C (Mito-C) (3 μg/ml) for 6 h, or subjected to both treatments together. At the end of the treatment, cell extracts were subjected to Western blot analysis using anti-p53 antibodies (PAb248 and PAb421). The intensities of the p53 bands were quantified by densitometry and are presented in arbitrary units. The amounts of protein loaded were monitored by reprobing with anti-α-tubulin. (D) Fibroblasts null for c-Abl (c-Abl negative) or reconstituted with c-Abl (c-Abl positive) were either untreated (lanes 1 and 6) or exposed to γ-irradiation (γ-IR) for the periods indicated. The intensity of the bands was quantified as in panel B. The protein levels were determined as for panel C.
It should be noted that the basal level of the p53 protein is higher in normal cells than in cells lacking c-Abl (Fig. 1A, lanes 1 and 2), consistent with c-Abl protecting p53 even in the absence of stress. Similarly, the accumulation of p53 by treatment with the proteasome inhibitor ALLN was enhanced by the presence of c-Abl (Fig. 1A, lanes 9 and 10; light exposure). The similarity in the level of the p53 protein between cells treated with ALLN (lane 10) and normal cells, but not c-abl null cells (lane 7), exposed to doxorubicin (lane 8), demonstrates the requirement for c-Abl in order to achieve maximal accumulation of p53 in response to DNA damage.
As an additional approach, the role of c-Abl in the accumulation of p53 in response to DNA damage was tested in a different experimental system. For this purpose we used two fibroblastic cell lines, c-Abl−/−+LacZ (c-abl null fibroblasts infected with LacZ retrovirus) and c-Abl−/− +c-Abl (c-abl null fibroblasts reconstituted with c-Abl at levels within the physiological range) (46). The accumulation of p53 in response to mitomycin C (3 μg/ml for 6 h) was greater in cells reconstituted with c-Abl than in c-abl null cells (Fig. 1C, lanes 3 and 7). Here too, the basal level of the p53 protein, and its accumulation in the presence of ALLN was higher in cells expressing c-Abl (Fig. 1C, lanes 1 and 5 and lanes 2 and 6). Further, the two cell lines were exposed to γ-irradiation (10 Gy), and the steady-state level of p53 was measured by Western blot analysis using anti-p53 antibodies (PAb421 and PAb248). In the c-Abl-expressing cells, the p53 protein was elevated by 1 h and remained high for a subsequent hour (Fig. 1D, lanes 6 to 10). On the other hand, in c-abl null cells, the elevation by 1 h was less than 20% of that seen in c-Abl-expressing cells. Even at 2 h the levels of p53 expression in c-abl null cells reached only 60% that of c-Abl-expressing cells (lanes 1 to 5). These results are in accord with those obtained with the primary cells. Together, these findings support an important role for c-Abl in the rate and extent of p53 accumulation in response to different DNA-damaging agents.
c-Abl enhances the nuclear accumulation of p53.
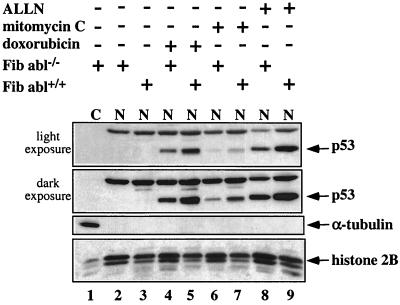
Treatment of cells with an inhibitor of nuclear export, leptomycin B, results in the accumulation of p53 (10). It was therefore tempting to suggest that c-Abl may protect p53 by preventing its nuclear export in stressed cells. To test this conjecture, the effect of c-Abl on the accumulation of p53 within the nuclei of cells exposed to DNA damage was examined. The c-abl null MEFs and their normal counterparts were exposed to DNA-damaging agents. Nuclear fractions were prepared, and the extracts were subjected to Western blot analysis using anti-p53 antibodies (PAb248 and PAb421). Upon exposure to doxorubicin (3 μg/ml for 6 h), the accumulation of p53 within the nuclei of c-abl null MEFs was markedly lower than in the nuclei of normal MEFs (Fig. 2, lanes 4 and 5, light exposure). A consistent difference, albeit at lower expression levels, was observed after treatment with mitomycin C (3 μg/ml for 6 h) (Fig. 2, lanes 7 and 8). Similar results were obtained when c-Abl-reconstituted fibroblastic cells were compared to c-abl null fibroblasts after exposure to doxorubicin and mitomycin C (data not shown). Overall, these results demonstrate that the physiological levels of c-Abl are essential for the efficient accumulation of p53 within the nuclei of cells exposed to DNA damage. It should be noted that the elevation of the p53 protein in the nucleus by blocking its proteasomal degradation was again largely dependent on c-Abl (Fig. 2, lanes 8 and 9). This implicates c-Abl as an important regulator of p53 expression within the nucleus in nonstressed cells as well.
FIG. 2.
Role for c-Abl in the nuclear accumulation of p53 in response to DNA damage. MEFs from a c-abl null mouse (Fib abl−/−) or from a normal mouse (Fib abl+/+) were either not treated (lanes 1 and 2) or treated with doxorubicin at 3 μg/ml for 6 h (lanes 4 and 5), with mitomycin C at 3 μg/ml for 6 h (lanes 6 and 7), or with with ALLN at 150 μM for 4 h (lanes 8 and 9). Nuclear fractions were prepared, and extracts were subjected to Western blot analysis for p53 expression using anti-p53 antibodies (PAb421 and PAb248). Extract from the cytoplasmic fraction of untreated cells was included as a control (C). The purity of the nuclear and cytoplasmic fractionation was monitored by reprobing the membrane with anti-histone 2B and anti-α-tubulin, respectively.
The nucleocytoplasmic shuttle of p53 by Mdm2 is inhibited by c-Abl.
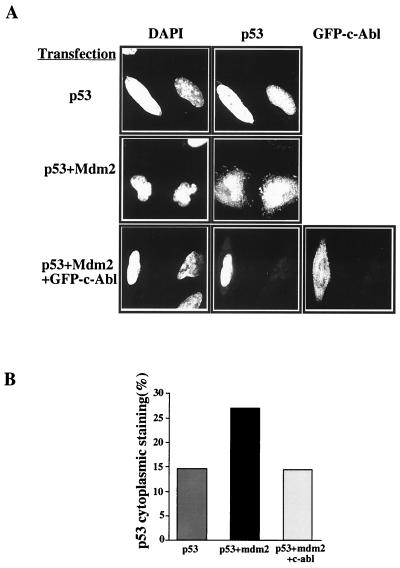
Our findings above demonstrating a role for c-Abl in the accumulation of p53 in the nucleus raised the possibility that c-Abl may interfere with the nuclear export of p53. The nuclear export of p53 by Mdm2 has been previously shown to be essential for the ability of Mdm2 to promote p53 for degradation (10, 35). This raised the possibility that c-Abl may protect p53 in the nucleus by preventing its nuclear export by Mdm2. This notion is particularly attractive in light of our previous findings showing that c-Abl protects p53 from Mdm2-mediated degradation and that the protected p53 is functionally active. This possibility was tested by monitoring the effect of c-Abl on Mdm2-mediated nuclear export of p53. The p53-deficient Saos-2 cells were transfected with an expression plasmid for p53 alone, or in combination with expression plasmids for mdm2 and c-abl-GFP. Twenty-four hours posttransfection, cells were treated with the proteasome inhibitor ALLN for 2 h to prevent p53 degradation. Cells were then fixed in methanol and stained for p53 using anti-p53 monoclonal antibodies (PAb1801 and DO1) followed by a Cy3-conjugated goat anti-mouse immunoglobulin secondary antibody. c-Abl–GFP expression was monitored by GFP fluorescence, and the nuclei were visualized by staining the DNA with DAPI. Stained cells were examined under the confocal microscope. As shown in Fig. 3, the proportion of cells with nuclear staining only compared with cells with nuclear and cytoplasmic staining was scored for each combination. Cotransfection of Mdm2 with p53 increased the proportion of cells with cytoplasmic staining almost twofold, consistent with previous findings (10, 35). Importantly, in the presence of c-Abl and Mdm2, the Mdm2-mediated nuclear export of p53 was diminished and the proportion of cells with cytoplasmic staining was reduced to the level observed with p53 alone (Fig. 3). Thus, c-Abl prevents the nuclear export of p53 induced by Mdm2.
FIG. 3.
c-Abl overcomes the nuclear export of p53 by Mdm2. (A) Saos-2 cells were transfected with the expression plasmids p53 (0.2 μg), mdm2 (0.5 μg), and GFP-c-abl (4 μg). Twenty-four hours posttransfection, cells were treated with ALLN (150 μM) for 2 h and then fixed in cold methanol. Fixed cells were stained for p53 using anti-p53 antibodies (PAb1801 and DO1) followed by Cy3-conjugated goat anti-mouse immunoglobulin and simultaneously stained for DNA using DAPI. The GFP and GFP–c-Abl fluorescence is shown in the rightmost panel. Stained cells were examined with a confocal microscope. Magnification, ×800. (B) Summary of p53 localization in stained cells (100 to 850 cells) from three independent experiments. The staining phenotype was categorized in two groups, one with nuclear p53 staining only and one with nuclear and cytoplasmic staining. The graph shows the percentage of cells with cytoplasmic staining.
c-Abl inhibits the nuclear export of p53 by E6 in HeLa cells.
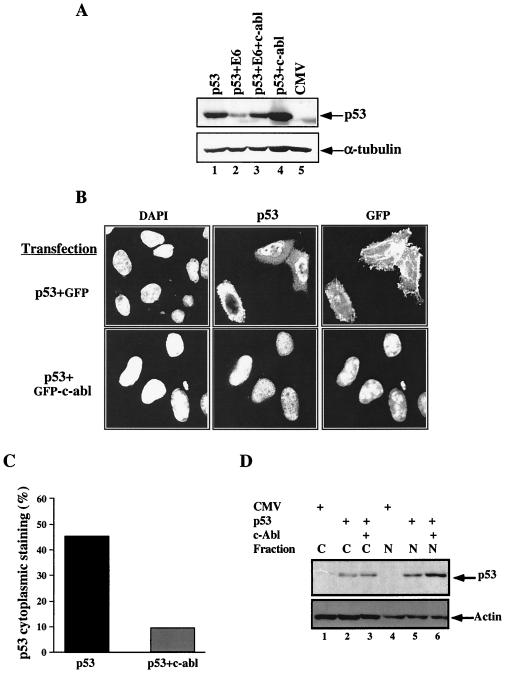
As with Mdm2, the degradation of p53 by the HPV E6 protein requires, at least to a large extent, the nuclear export of p53 (10). It was of interest to determine whether c-Abl can also protect p53 from E6-mediated degradation and, if so, whether it blocks the nuclear export of p53 in HPV-infected cells. To test this hypothesis, Saos-2 cells were transfected with an expression plasmid for wild-type p53 alone, or together with an expression plasmid for E6, with or without an expression plasmid for c-abl. Twenty-four hours posttransfection, cells were harvested and subjected to Western blot analysis using anti-p53 antibodies. The level of p53 expression was reduced in the presence of E6 (Fig. 4A, lanes 1 and 2), consistent with previous findings (37). Importantly, coexpression of c-Abl protected p53 from E6-mediated degradation (Fig. 4A, lane 3). The same result was obtained in H1299 cells, a lung carcinoma-derived cell line lacking p53 expression (data not shown). Thus, c-Abl can block the ability of E6 to destabilize p53, and this effect is not cell type specific. It should be noted that coexpression of c-Abl with p53 in the absence of E6 also elevated the level of p53 expression (Fig. 4A, lane 4), probably by overcoming Mdm2-mediated destabilization (46). The protection of p53 from E6 was effective also in the absence of c-Abl kinase activity (data not shown), as is the case with the protection of p53 from Mdm2 (46).
FIG. 4.
c-Abl protects p53 from degradation and inhibits its nuclear export by E6. (A) c-Abl protects p53 from HPV E6-mediated degradation. Saos-2 cells were transfected with the indicated combination of expression plasmids: p53 (50 ng), HPV E6 (0.5 μg), and c-abl (3 μg). Twenty-four hours posttransfection, cells were harvested and cell extracts were subjected to Western blot analysis using a mixture of anti-p53 antibodies (PAb1801 and DO1). The same blot was reprobed with anti-α-tubulin antibody. (B) HeLa cells were transfected with the indicated expression plasmids for p53 (1 μg) together with either farnesylated pEGFP (pEGFPF; 1 μg) or pEGFP-c-Abl (4 μg). Twenty-four hours posttransfection, cells were treated with ALLN, fixed, and stained for p53 as described for Fig. 3A. Stained cells were examined with a confocal microscope. Magnification, ×800. (C) Summary of the p53 staining in panel A. Over 300 stained cells from three independent experiments were counted, and the staining phenotype was categorized as in Fig. 3B. (D) HeLa cells were transfected and treated as for panel B with the exception that mouse instead of human p53 was used. Following treatment cytoplasmic (lanes 1 to 3) and nuclear (lanes 4 to 6) fractions were prepared and subjected to Western blot analysis using anti-p53 antibody (CM5). Equal loading between the different transfections was monitored by probing with antiactin.
On the basis of this result, the effect of c-Abl on the nuclear export of p53 by E6 was examined. This question was addressed with HeLa cells, a cell line derived from an HPV-18-infected cervical carcinoma expressing wild-type p53. HeLa cells were transfected with small amounts of wild-type p53 expression plasmid together with expression plasmids for either GFP or c-Abl–GFP fusion protein. Twenty-four hours posttransfection, cells were treated with ALLN, fixed, and stained for p53 as described above. In approximately one-half of the transfected cells, p53 was expressed both in the nucleus and in the cytoplasm, and in some cells it was expressed in the cytoplasm only (Fig. 4B). In contrast, a dramatic shift in p53 localization to the nucleus was observed when p53 and c-Abl–GFP were coexpressed (Fig. 4B). Quantitative analysis of several hundred p53 and c-Abl–GFP-coexpressing cells indicated that in over 90% of these cells, p53 was confined to the nucleus (Fig. 4C). To further quantify this finding, the effect of c-Abl on the nuclear export of p53 was examined by Western blot analysis of nuclear and cytoplasmic fractions. HeLa cells were transfected with expression vector for mouse p53, in order to distinguish it from the endogenous human p53, with or without an expression vector for c-Abl. Twenty-four hours posttransfection, nuclear and cytoplasmic fractions were prepared and the levels of p53 in each fraction were monitored by Western blot analysis using anti-p53 polyclonal antibody (CM5; Novocastra). The presence of c-Abl enhanced the accumulation of the p53 protein in the nucleus but not in the cytoplasm. Overall, these results supports the notion that c-Abl prevents the nuclear export of p53 by E6 and promotes the accumulation of p53 within the nucleus.
Ubiquitination of p53 by Mdm2 is attenuated by c-Abl in vivo and in vitro.
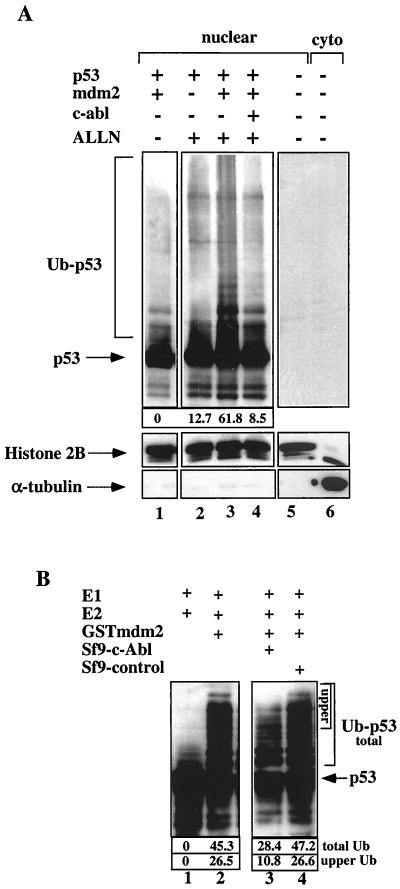
The results presented here show that c-Abl promotes p53 accumulation within the nucleus and prevents its nuclear export by Mdm2 and E6. Since Mdm2 is an E3 ligase and since E6 promotes the ubiquitination of p53 by E6-AP, it was of great interest to determine whether c-Abl interferes with the ubiquitination of p53 by Mdm2 or E6 to E6-AP. From the current literature, it is unclear whether the ubiquitination of p53 occurs in the nucleus or the cytoplasm. To address this question, the effect of c-Abl on the ubiquitination of p53 by Mdm2 was examined. To test the effect in vivo, H1299 cells were transfected with p53 alone, p53 and mdm2, or both together with c-abl. Twenty-four hours posttransfection, cells were treated with ALLN for 2 h, to prevent p53 degradation, prior to harvesting nuclear and cytoplasmic fractions. Nuclear extracts were resolved by SDS-PAGE, and p53 ubiquitin conjugates were detected by Western blot analysis using anti-p53 antibodies (PAb1801 and DO1). Within the nuclear fraction, the ubiquitination of p53 was enhanced when Mdm2 was coexpressed with p53, compared with the expression of p53 alone (Fig. 5A, lanes 2 and 3). Importantly, the addition of c-Abl prevented the in vivo ubiquitination of p53 by Mdm2 both of the lower conjugates and of the smear at a high molecular weight (lane 4). This result supports the notion that c-Abl protects p53 within the nucleus by impairing the efficiency of its ubiquitination. It should be noted that the pattern of p53 conjugation (lane 3) disappeared in the absence of ALLN (lane 1), supporting the identification of the smeared and clear bands above p53 as p53 conjugates. These bands were shown to contain ubiquitin molecules by coimmunoprecipitation assay using the ubiquitin-Ha tag (data not shown).
FIG. 5.
The effect of c-Abl on the ubiquitination of p53 by Mdm2 in vivo and in vitro. (A) HI299 cells were transfected with the indicated expression plasmids for p53 (1 μg), mdm2 (2 μg), and c-abl (4 μg). Twenty-four hours after transfection, cells were incubated with ALLN (150 μM) for 2 h. Nuclear and cytoplasmic (cyto) fractions were prepared, and the extracts were resolved by SDS-PAGE followed by blotting with anti-p53 antibodies (PAb1801 and DO1). The purity of the nuclear and cytoplasmic fractionation was monitored by reprobing the membrane with anti-histone 2B and anti-α-tubulin, respectively. The positions of the p53-ubiquitin (Ub) conjugates are indicated. The intensity of the ubiquitinated p53 bands was quantified by densitometry and is presented as arbitrary units (10−2) below the blots. The level of ubiquitination of p53 in the absence of ALLN (lane 1) was taken as background and was given the value 0. The intensity in the other lanes was calculated relative to lane 1. (B) Ubiquitination of p53 by Mdm2 in an in vitro reconstitution assay. An in vitro-synthesized human p53 was incubated with E1, E2 (UbcH5c), and GST-Mdm2 (lane 2). Incubation without Mdm2 was used as a control (lane 1). Extract from Sf9 cells infected with baculovirus encoding c-Abl (lane 3) or from noninfected Sf9 cells (lane 4) was added to the reaction mixture, and the reaction was carried out at 30°C for 1 h. The mixture was subjected to Western blotting using anti-p53 antibodies (PAb1801 and DO1). The intensity of the ubiquitinated p53 bands is presented as in panel A. The intensity of the high-molecular-weight p53 conjugates (“upper”) was measured separately.
To gain further support for this conclusion, the effect of p53 conjugation was examined in an in vitro reconstitution assay. In vitro-synthesized p53 was incubated with purified E1, His-tagged purified UbcH5c as the E2, and purified GST-Mdm2 as the E3 ligase, as described in Materials and Methods. p53-ubiquitin conjugates appeared only in the presence of all three components, not in the absence of Mdm2 (Fig. 5B, lanes 1 and 2). The effect of c-Abl on the ubiquitination of p53 was tested by including in the ubiquitination reaction extracts from control Sf9 cells or from c-Abl-expressing cells. The overall efficiency of ubiquitination was impaired in the presence of extracts containing c-Abl by 40% (Fig. 5B, lane 3), while the control extract had no inhibitory effect (lane 4). The inhibitory effect of c-Abl on the generation of the high-molecular-weight p53 conjugates was more significant, reaching 60% inhibition. Overall, these ubiquitination assays support the notion that c-Abl impairs the ubiquitination of p53 by Mdm2.
c-Abl impairs the ubiquitination of p53 by E6 in vivo and in vitro.
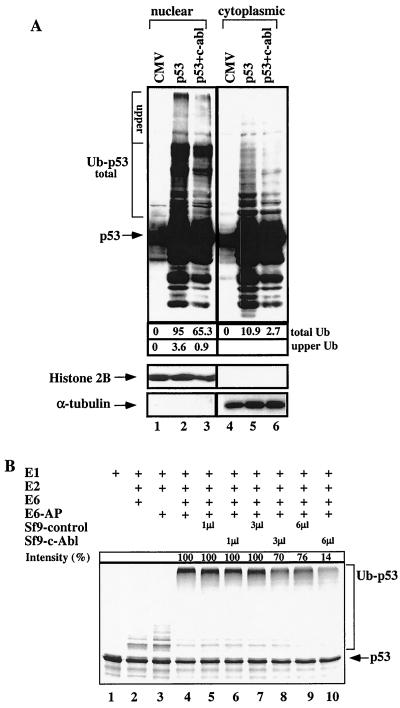
The effect of c-Abl on the ubiquitination of p53 by Mdm2 encouraged us to test its effect on the ubiquitination of p53 by E6. This is of particular importance since some degradation of p53 by E6–E6-AP appears to occur in the nucleus (10). The effect of c-Abl on the ubiquitination of p53 in vivo was examined in HeLa cells. Cells were transfected with an expression plasmid for wild-type p53 with or without an expression plasmid for c-abl. Twenty-four hours after the transfection, cells were treated with ALLN, nuclear and cytoplasmic fractions were prepared, and extracts were subjected to Western blot analysis using an anti-p53 antibody. Transfection of HeLa cells with p53 alone resulted in extensive ubiquitination of p53 in the nucleus as measured by the accumulation of high-molecular-weight bands of p53 (Fig. 6A, lane 2). In the absence of ALLN, these p53 conjugates were not observed (data not shown). Importantly, in the presence of c-Abl there was a significant decrease in the amount and molecular weight of the p53 conjugates in the nucleus (Fig. 6A, compare lanes 2 and 3). A reduction of 30% was seen in the total ubiquitination, and more importantly, with the high-molecular-weight p53 conjugates the reduction reached 75%. c-Abl also reduced the amount of p53 conjugates in the cytoplasm by approximately 75% (Fig. 6A, compare lanes 5 and 6). This observation demonstrates that c-Abl impairs the efficiency and extent of E6–E6-AP-dependent ubiquitination of p53 within the nucleus. It cannot be excluded that Mdm2 also contributed to the ubiquitination of p53 in HeLa cells; the extent of this contribution is difficult to assess.
FIG. 6.
c-Abl impairs the ubiquitination of p53 by E6–E6-AP in vivo and in vitro. (A) HeLa cells were transfected with an expression plasmid for p53 (1 μg), either alone or together with an expression plasmid for c-Abl (4 μg). Twenty-four hours posttransfection, cells were treated with ALLN for 2 h. Nuclear and cytoplasmic fractions were prepared and subjected to Western blot analysis using anti-p53 antibodies (PAb1801 and DO1). The intensity of the ubiquitinated conjugates of p53 (Ub-p53) was quantified and is presented as arbitrary units (10−2) below the blot. The intensity of the high-molecular-weight p53 conjugates (“upper”) was also measured separately. The levels of ubiquitination in the presence of vector alone (lanes 1 and 4) were taken as background and given the value 0. The intensity in the other lanes was calculated relative to the corresponding background. (B) Ubiquitination of p53 by E6–E6-AP in vitro. Radioactively labeled p53 was synthesized in vitro and incubated with purified E1, UbcH5C as an E2, HPV E6, and E6-AP (lanes 4 to 9). As controls, p53 was incubated in the absence of one or more components (lanes 1 to 3). Extracts from cells infected with baculovirus encoding c-Abl and from noninfected cells were added to the reaction mixture. The reaction was carried out at 30°C for 50 min, and the mixture was subjected to SDS-PAGE followed by exposure to an X-ray film. The intensity of the high-molecular-weight band of ubiquitinated p53 was quantified by densitometry. Lane 4 was taken as 100%, from which the relative intensity of ubiquitinated p53 bands was calculated.
To address the same question more directly we examined the effect of c-Abl on the ubiquitination of p53 in an in vitro reconstitution assay. The ubiquitination of p53 in vitro was performed essentially as previously described (14). The p53 protein was synthesized in vitro in the presence of [35S]methionine and incubated with purified E1, UbcH5C as an E2, HPV E6, and E6-AP. The appearance of p53 ubiquitin conjugates was obtained only when all the components were present (Fig. 6B, lane 4) but not in the absence of E6 or E6-AP (lanes 1 to 3). The effect of c-Abl on the ubiquitination of p53 was tested by adding to the ubiquitination reaction extracts of Sf9 cells that were infected with baculovirus expressing c-Abl or of noninfected cells used as a control. The efficiency of the ubiquitination of p53 was significantly impaired in the presence of extract from c-Abl-infected Sf9 cells in a dose-dependent manner, reaching 86% inhibition (Fig. 6B, lanes 6, 8, and 10), while the extracts from control cells had only a minor effect, with inhibition reaching only 24% (lanes 5, 7 and 9). The extent of inhibition by Sf9 expressing c-Abl correlated with the integrity of the c-Abl protein in the Sf9 extracts. Overall, these in vivo and in vitro assays suggest that c-Abl impairs the ubiquitination of p53 by E6–E6-AP, thereby providing an explanation for the accumulation of p53 in the nucleus.
c-Abl neutralizes the inhibitory effects of HPV E6 on p53 transcriptional activity.
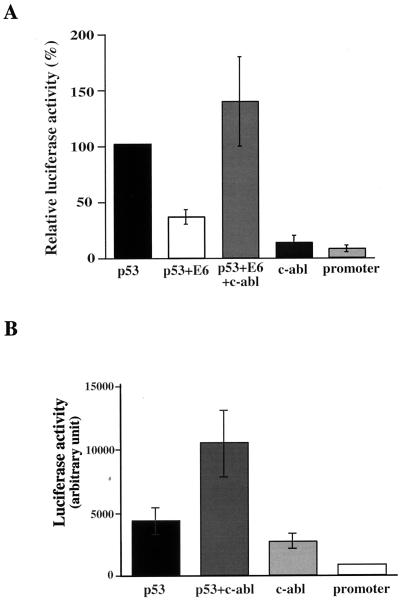
The findings that c-Abl protects p53 from degradation and nuclear export by E6 (Fig. 4) and impairs its ubiquitination by E6–E6-AP (Fig. 6) prompted us to ask whether p53 that is protected by c-Abl from E6 remains functionally active. This was of particular interest, since the HPV E6 protein can also inhibit p53 activity without promoting it for degradation (23, 25, 26, 43). To test this notion, the effect of c-Abl on the inhibitory effect of E6 on p53 transcriptional activity was measured. Saos-2 cells were transiently transfected with a reporter plasmid containing the luciferase gene under the control of the cyclin G promoter. The induction of the luciferase activity by p53 was reduced by 60% in the presence of E6 (Fig. 7A). Significantly, coexpression of c-Abl completely neutralized the inhibition by E6, and p53 activity was increased to a higher level than that obtained with p53 alone (Fig. 7A). Expression of c-Abl alone had a minor effect on the cyclin G promoter (Fig. 7A); hence, this effect of c-Abl was specific for p53. This result suggests that c-Abl stabilizes p53 in an active form despite the presence of E6.
FIG. 7.
c-Abl neutralizes the inhibitory effect of E6 on p53 transcriptional activity. Saos-2 cells (A) or HeLa cells (B) were transfected with the cyclin G luciferase reporter plasmid alone or with expression plasmids for the various combinations as indicated. The amounts of plasmid DNA per transfection were 50 ng (A) or 20 ng (B) for p53, 0.5 μg for E6, 3 μg for c-Abl, and 0.5 μg for the reporter plasmid. Twenty-four hours posttransfection, cells were harvested and the luciferase activity in the cell extracts was measured. Data include standard deviations of triplicates from one of four independent experiments with consistent results.
These results raised the question of whether c-Abl protects p53 from E6 only when E6 is expressed alone or also when it is expressed in the context of HPV-infected cell. To explore this, HeLa cells were transfected with the cyclin G luciferase reporter plasmid alone, and low transcriptional activity was observed, presumably reflecting the activation of endogenous p53 by the transfection conditions (34). Expression of c-Abl together with the reporter gene induced a significant level of luciferase activity (Fig. 7B), supporting the notion that c-Abl enhances the transcriptional activity of endogenous wild-type p53 in the presence of E6. This activation reached already 60% of the activity that was obtained by the expression of exogenous p53 in these cells (Fig. 7B). Co-expression of p53 together with c-Abl increased p53 activity even further, presumably reflecting the ability of c-Abl to overcome not only the inhibition by E6 but also by other negative regulators, such as Mdm2 (46). Overall, these results support a role for c-Abl in the activation of p53 in HPV-infected cells.
DISCUSSION
c-Abl is important for efficient accumulation of p53 in response to DNA damage.
Under normal growth conditions, the p53 protein is kept as a labile and inactive protein. But upon exposure to DNA damage and other stress signals, it accumulates in the nucleus and becomes transcriptionally active. Here we demonstrated that physiological levels of c-Abl are crucial for the efficient accumulation of endogenous p53 in response to various DNA-damaging agents (Fig. 1). c-Abl enhances both the rate and the extent of p53 accumulation in response to stress (Fig. 1). In contrast, the DNA damage-induced accumulation of p53 in c-abl-deficient fibroblasts is weak and slow. This implicates c-Abl as an important regulator in the stabilization of p53 in response to DNA damage. Moreover, our results support a role for c-Abl in the regulation of p53 levels in nonstressed cells. First, the basal level of the p53 protein is significantly higher in cells expressing c-Abl than in cells lacking it. Second, the extent of p53 stabilization by blocking its proteasomal degradation is c-Abl dependent (Fig. 1). Hence, c-Abl is an important factor regulating the maintenance of p53 protein levels under normal conditions. This action may assure a rapid and efficient stabilization of p53 upon exposure to stress.
These findings provide a mechanistic explanation for the cooperation between p53 and c-Abl in response to genotoxic stresses. c-Abl enhances the transcriptional activity of p53 (13, 47, 48) and is required for the down-regulation of Cdk2 activity in cells exposed to DNA damage. p53 is important for the ability of c-Abl to promote G1 growth arrest and apoptosis (13, 47, 49, 50), suggesting a synergistic action of both proteins in the growth-inhibitory response to genotoxic stress. c-Abl is activated by ATM in response to DNA damage (2, 38). This provides one of the multiple pathways by which ATM protects p53 from the inhibitory effect of Mdm2. ATM activates p53 by direct phosphorylation on serine 15 of p53, and it activates Chk2 to phosphorylate p53 on serine 20. The latter modification has the most dramatic effect on the accumulation of p53 in response to DNA damage (7, 41). More recently, a direct phosphorylation of Mdm2 by ATM has been shown to contribute to this effect (30). Presumably, the combined activation of multiple parallel pathways is essential for securing an efficient and rapid activation of p53 in response to DNA damage.
c-Abl regulates p53 nuclear export and ubiquitination.
The mechanisms involved in the enhanced accumulation of p53 in the presence of c-Abl following DNA damage have not been defined. Since the nuclear export of p53 is essential for its degradation by both Mdm2 and E6 (10, 35), it was tempting to propose that c-Abl protects p53 by regulating its subcellular localization. Indeed, c-Abl is required for the efficient accumulation of p53 in the nuclei of cells exposed to DNA damage (Fig. 2A). Specifically, c-Abl prevents the nuclear export of p53 by Mdm2 and by HPV E6 (Fig. 2 and 3). It is not yet clear whether the nucleocytoplasmic shuttle of p53 requires the nuclear export signal (NES) of Mdm2 (35), that of p53 (42), or the combined signals of both. c-Abl may affect the accessibility of the NES of p53. c-Abl binds the C terminus of p53 and stabilizes its interaction with DNA (33). This interaction is likely to stabilize the tetrameric form of p53 and consequently mask the NES of p53 and prevent its nuclear export. This notion is supported by the fact that the interaction between p53 and c-Abl is enhanced by DNA damage (48). However, other mechanisms operating in parallel cannot be excluded at this stage.
It has recently been suggested that the ubiquitination of p53 by Mdm2 in the nucleus facilitates its nuclear export (5, 11). It is conceivable that the nuclear export of p53 by E6 operates through a similar mechanism, in particular since both Mdm2 and E6 promote the ubiquitination and nuclear export of p53 (10). c-Abl impairs both Mdm-2- and E6–E6-AP-mediated p53 ubiquitination within the nucleus (Fig. 5A and 6A) and in an in vitro reconstituted degradation assay (Fig. 5B and 6B). This explains the effect of c-Abl on the accumulation of p53 in the nuclei of stressed cells. The ability of c-Abl to interfere with both processes is consistent with this conjecture.
How does c-Abl impair the ubiquitination of p53 by E6–E6-AP and by Mdm2? To date, little is known about the regulation of p53-ubiquitin conjugation. While c-Abl does not inhibit the p53-Mdm2 interaction (46), it may affect the E3 ligase activity of Mdm2 (Fig. 5). Similarly, the binding between p53 and E6 is not inhibited by c-Abl (data not shown), but p53–E6-AP binding, which is essential for the ubiquitination of p53 (19), could be affected. In fact, the binding site of c-Abl in p53 (residues 363 to 393 [33]) overlaps with one of the two binding sites for E6 (residues 376 to 384 [27]). Moreover, the stabilization of the p53-DNA complex by c-Abl (33) may render p53 more resistant to degradation by E6 (31). By stabilizing the tetrameric form of p53 (33), c-Abl may also impair efficient ubiquitination of p53 by Mdm2 or E6-AP. The action of c-Abl on p53 stabilization may also involve indirect mechanisms, such as through p300 (51). Unraveling the mechanism by which c-Abl impairs the ubiquitination of p53 is a subject for further investigation.
Role for c-Abl in the protection of p53 from HPV E6.
c-Abl protects p53 from degradation by HPV E6 (Fig. 4A). This degradation is essential for E6 to inhibit p53-mediated apoptosis (43) but refractory for the inhibition of other activities of p53, such as DNA binding (25), transcriptional activity, and the induction of growth arrest (23, 26, 43). Indeed, inhibition of E6-mediated p53 degradation by a proteasome inhibitor is insufficient for the full activation of p53. An additional stimulation of p53, such as exposure to DNA damage, appears to be required (29). Importantly, the p53 protein that is protected by c-Abl remains functionally active (Fig. 7). Therefore, c-Abl not only protects p53 from degradation by E6 but also overcomes the degradation-independent inhibitory effects of E6. Mechanisms underlying the latter inhibitory effects are partially understood. E6 blocks the interaction between p53 and the transcriptional coactivators CBP and p300, thereby impairing the transcriptional activity of p53 (52). Further, the nuclear localization of p53 is perturbed in the presence of E6, resulting in the accumulation of inactive p53 in the cytoplasm (29).
In the majority of cervical tumors, p53 remains wild type (8, 36), and its signaling pathways are intact in HPV-infected cells (6). While there is no direct evidence for the regulation of E6 in HPV-infected cells, p53 can be stabilized and activated in response to mitomycin C in some HPV-infected cells (29). Interestingly, the same drug activates c-Abl (21) and enhances its interaction with p53 (48). Our findings suggest that c-Abl plays a cardinal role in the activation of p53 in HPV-infected cells in response to stress; in this case the transfected DNA triggers p53 activation (34). Presumably, under normal conditions, the presence of c-Abl is insufficient to protect p53 from HPV E6. In principle, the c-Abl protein may provide an effective means of activating p53 in HPV-infected cells.
ACKNOWLEDGMENTS
R.V.S., S.C., and Z.G. contributed equally to this work.
We thank M. Oren, K. Vousden, W. Jiang, and T. Hunter for the generous gift of plasmids, D. Lane and D. Eilat for the generous gift of antibodies, and Y. Reiss for purified E1. We are grateful to S. Moody-Haupt for critical comments.
This work was supported by a Center for Excellence grant of the Israel Science Foundation awarded to Y.B.-N., A.C., and Y.H., by a Research Career Development Award from the Israel Cancer Research Fund awarded to Y.H., and by the Lejwa Fund for Biochemistry and was supported in part by research grant 1-FY01-177 from the March of Dimes Birth Defects Foundation.
REFERENCES
- 1.Ashcroft M, Vousden K H. Regulation of p53 stability. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 2.Baskaran R, Wood L D, Whitaker L L, Canman C E, Morgan S E, Xu Y, Barlow C, Baltimore D, Wynshawboris A, Kastan M B, Wang J Y J. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–519. doi: 10.1038/387516a0. [DOI] [PubMed] [Google Scholar]
- 3.Bell D W, Varley J M, Szydlo T E, Kang D H, Wahrer D C, Shannon K E, Lubratovich M, Verselis S J, Isselbacher K J, Fraumeni J F, Birch J M, Li F P, Garber J E, Haber D A. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286:2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 4.Böttger A, Böttger V, Sparks A, Liu W L, Howard S F, Lane D P. Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr Biol. 1997;7:860–869. doi: 10.1016/s0960-9822(06)00374-5. [DOI] [PubMed] [Google Scholar]
- 5.Boyd S C, Tsai K Y, Jacks T. An intact Hdm2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;2:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 6.Butz K, Shahabeddin L, Geisen C, Spitkovsky D, Ullmann A, Hoppe Seyler F. Functional p53 protein in human papillomavirus-positive cancer cells. Oncogene. 1995;10:927–936. [PubMed] [Google Scholar]
- 7.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook T, Tidy J A, Vousden K H. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 9.Crook T, Vousden K H. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J. 1992;11:3935–3940. doi: 10.1002/j.1460-2075.1992.tb05487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman D A, Levine A J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer R K, Yu Z K, Maki C G. The Mdm2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 12.Giaccia A J, Kastan M B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 13.Goga A, Liu X, Hambuch T M, Senechal K, Major E, Berk A J, Witte O N, Sawyers C L. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791–799. [PubMed] [Google Scholar]
- 14.Gonen H, Bercovich B, Orian A, Carrano A, Takizawa C, Yamanaka K, Pagano M, Iwai K, Ciechanover A. Identification of the ubiquitin carrier proteins, E2s, involved in signal-induced conjugation and subsequent degradation of IkBa. J Biol Chem. 1999;274:14823–14830. doi: 10.1074/jbc.274.21.14823. [DOI] [PubMed] [Google Scholar]
- 15.Haupt Y, Barak Y, Oren M. Cell type-specific inhibition of p53-mediated apoptosis by mdm2. EMBO J. 1996;15:1596–1606. [PMC free article] [PubMed] [Google Scholar]
- 16.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 17.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 18.Honda R, Yasuda H. Association of p19ARF with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huibregtse J M, Scheffner M, Howley P M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez G S, Khan S H, Stommel J M, Wahl G M. p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene. 1999;18:7656–7665. doi: 10.1038/sj.onc.1203013. [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda S, Ren R, Pandey P, Shafman T D, Feller S M, Weichselbaum R R, Kufe D W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 22.Kharbanda S, Yuan Z M, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 23.Kiyono T, Hiraiwa A, Ishii S, Takahashi T, Ishibashi M. Inhibition of p53-mediated transactivation by E6 of type 1, but not type 5, 8, or 47, human papillomavirus of cutaneous origin. J Virol. 1994;68:4656–4661. doi: 10.1128/jvi.68.7.4656-4661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubbutat M H G, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 25.Lechner M S, Laimins L A. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol. 1994;68:4262–4273. doi: 10.1128/jvi.68.7.4262-4273.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechner M S, Mack D H, Finicle A B, Crook T, Vousden K H, Laimins L A. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J. 1992;11:3045–3052. doi: 10.1002/j.1460-2075.1992.tb05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70:4509–4516. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z G, Baskaran R, Lea Chou E T, Wood L D, Chen Y, Karin M, Wang J Y. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani F, Banks L. Inhibition of E6 induced degradation of p53 is not sufficient for stabilization of p53 protein in cervical tumour derived cell lines. Oncogene. 1999;18:3309–3315. doi: 10.1038/sj.onc.1202688. [DOI] [PubMed] [Google Scholar]
- 30.Maya R, Balass M, Kim S T, Shkedy D, Leal J-F M, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, Kastan M B, Katzir E, Oren M. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molinari M, Milner J. p53 in complex with DNA is resistant to ubiquitin-dependent proteolysis in the presence of HPV-16 E6. Oncogene. 1995;10:1849–1854. [PubMed] [Google Scholar]
- 32.Momand J, Wu H H, Dasgupta G. MDM2-master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 33.Nie Y, Li H H, Bula C M, Liu X. Stimulation of p53 DNA binding by c-Abl requires the p53 C terminus and tetramerization. Mol Cell Biol. 2000;20:741–748. doi: 10.1128/mcb.20.3.741-748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renzing J, Lane D P. p53-dependent growth arrest following calcium phosphate-mediated transfection of murine fibroblasts. Oncogene. 1995;10:1865–1868. [PubMed] [Google Scholar]
- 35.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheffner M, Munger K, Byrne J C, Howley P M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines Proc. Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 38.Shafman T, Khanna K K, Kedar P, Spring K, Kozlov S, Yen T, Hobson K, Gatei M, Zhang N, Watters D, Egerton M, Shiloh Y, Kharbanda S, Kufe D, Lavin M F. Interaction between ATM protein and c-Abl in response to DNA damage. Nature. 1997;387:520–522. doi: 10.1038/387520a0. [DOI] [PubMed] [Google Scholar]
- 39.Shaul Y. c-Abl: activation and nuclear targets. Cell Death Differ. 2000;7:10–16. doi: 10.1038/sj.cdd.4400626. [DOI] [PubMed] [Google Scholar]
- 40.Sherr C J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 41.Shieh S Y, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 42.Stommel J M, Marchenko N D, Jimenez G S, Moll U M, Hope T J, Wahl G M. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas M, Matlashewski G, Pim D, Banks L. Induction of apoptosis by p53 is independent of its oligomeric state and can be abolished by HPV-18 E6 through ubiquitin mediated degradation. Oncogene. 1996;13:265–273. [PubMed] [Google Scholar]
- 44.Thomas M, Pim D, Banks L. The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 45.Vogt Sionov R, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999;18:6145–6157. doi: 10.1038/sj.onc.1203130. [DOI] [PubMed] [Google Scholar]
- 46.Vogt Sionov R, Moallem E, Berger M, Kazaz A, Gerlitz O, Ben Neriah Y, Oren M, Haupt Y. c-Abl neutralizes the inhibitory effect of Mdm2 on p53. J Biol Chem. 1999;274:8371–8374. doi: 10.1074/jbc.274.13.8371. [DOI] [PubMed] [Google Scholar]
- 47.Wen S-T, Jackson P K, Van Etten R A. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 1996;15:1583–1595. [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan Z-M, Huang Y, Fan M M, Sawyers C, Kharbanda S, Kufe D. Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J Biol Chem. 1996;271:26457–26460. doi: 10.1074/jbc.271.43.26457. [DOI] [PubMed] [Google Scholar]
- 49.Yuan Z-M, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci USA. 1997;94:1437–1440. doi: 10.1073/pnas.94.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Z-M, Huang Y, Whang Y, Sawyers C, Weichselbaum R, Kharbanda S, Kufe D. Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Z M, Huang Y, Ishiko T, Nakada S, Utsugisawa T, Shioya H, Utsugisawa Y, Yokoyama K, Weichselbaum R, Shi Y, Kufe D. Role for p300 in stabilization of p53 in the response to DNA damage. J Biol Chem. 1999;274:1883–1886. doi: 10.1074/jbc.274.4.1883. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann H, Degenkolbe R, Bernard H U, O'Connor M J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]