ABSTRACT
Background
Abuse of prescription opioids is a serious problem in North America.
Aims
The aim of this study was to conduct a systematic review of peer-reviewed and grey literature to examine existing strategies aimed at improving the appropriate use of prescription opioids and/or reducing the misuse, abuse, and diversion of these drugs.
Methods
The following electronic databases were searched to September 2015 without language restrictions: MEDLINE, EMBASE, PsycINFO, and CINAHL; the grey literature was searched to May 2014. Reference lists of retrieved papers were also searched. Studies were eligible if a strategy was implemented and its impact on at least one of the primary outcomes of interest (appropriate prescription opioid use; misuse, abuse, opioid use disorder, diversion; overdose) was measured. Standardized, prepiloted forms were used for relevance screening, quality appraisal, and data extraction.
Results
A total of 65 studies that assessed 66 distinct strategies were identified. Due to the heterogeneity of the strategies, a qualitative synthesis was conducted. Many studies combined more than one type of strategy and measured various types of outcomes. The strategies with most promising results involved education, clinical practices, collaborations, prescription monitoring programs, public campaigns, opioid substitution programs, and naloxone distribution. We also found strategies that had some unintended consequences after implementation.
Conclusions
Our review identified successful strategies that have been implemented and evaluated in various jurisdictions. There is a need to replicate and disseminate these strategies where the problem of prescription opioid misuse and abuse has taken a toll on society.
KEYWORDS: systematic review, opioids: strategies: policies, programs, practices
RÉSUMÉ
Contexte: L’abus d’opïodes sur ordonnance est un problème grave en Amérique du Nord.
But: Effectuer une revue systématique de la littérature examinée par des pairs eet de la littérature grise afin d’étudier les stratégies existantes pour améliorer l’usage approprié des opioïdes sur ordonnance ou réduire la mauvaise utilisation, l’abus ou le détournement de ces médicaments.
Méthodes: Des recherches ont été effectuées sans aucune restriction de langue dans les bases de données électroniques suivantes : MEDLINE, EMBASE, PsycINFO et CINAHL jusqu’en septembre 2015 et dans la littérature grise jusqu’en mai 2014. Des recherches ont aussi été effectuées dans les listes de références des articles retenus. Les études étaient admissibles si une stratégie avait été mise en oeuvre et que son effet sur au moins un des principaux résultats étudiés (usage approprié des opioïdes sur ordonnance; mauvaise utilisation, abus, trouble de consommation ou détournement des opioïdes; surdose) avait été mesuré. Des formulaires normalisés et préalablement mis à l’essai ont été utilisés pour déterminer la pertinence et évaluer la qualité des études, et en extraire les données.
Résultats: Au total, 65 études évaluant 66 stratégies distinctes ont été répertoriées. En raison de l’hétérogénéité des stratégies, une synthèse qualitative a été effectuée. De nombreuses études combinaient plus d’un type de stratégie et mesuraient divers types de résultats. Les stratégies dont les résultats étaient les plus prometteurs portaient sur l’éducation, les pratiques cliniques, les collaborations, les programmes de surveillance des ordonnances, les campagnes publiques, les programmes de substitution des opioïdes et la distribution de naloxone. Nous avons également trouvé des stratégies qui avaient eu des conséquences imprévues après leur mise en oeuvre.
Conclusions: Notre revue a recensé des stratégies fructueuses mises en oeuvre et évaluées dans diverses juridictions. Ces stratégies doivent être reproduites et diffusées là où le problème de la mauvaise utilisation et de l’abus d’opioïdes sur ordonnance a eu un effet néfaste sur la société.
Introduction
Abuse of prescription opioids is a serious health and safety problem in North America. In the United States, more than 165 000 people died of overdose related to opioid pain medications between 1999 and 2014.1 In Canada, after a record-breaking year of apparent opioid-related deaths in 2016 (2861 deaths), the Public Health Agency of Canada predicts the number of Canadians that died from opioid overdoses will surpass 4000 by the end of 2017.2,3
Overprescribing of opioids by health care professionals has been implicated as the root cause of the current epidemic. In Canada, there were more than 21.5 million opioid prescriptions filled in 2016 alone, with an increasing proportion of strong opioids among all opioids dispensed.4 On the other hand, the Position Statement from the Canadian Pain Society recognizes that essential tools for managing moderate to severe pain involves pharmacotherapy, which may include opioids among other analgesics, in combination with physical and psychological approaches.5 It seems that the underlying cause for overprescribing of opioids is poor understanding and management of acute and chronic pain itself,6 and it has been suggested that opioid prescribing is a surrogate for inadequate pain management resources.7
An area that has not been the subject of many systematic reviews relates to strategies to promote the appropriate use of prescription opioids and reduce their harms, including abuse, opioid use disorder, and diversion. The knowledge users interested in this topic are not limited to health care professionals but rather are representatives of diverse groups within our communities, including public health, prevention services, government, law enforcement, regulators, and insurance payers, all of whom are interested in programs, strategies, policies, and regulations to solve the problem of inappropriate opioid use. A few recent systematic reviews have synthesized the evidence for narrow and specific types of strategies, including primary care delivery models for treating opioid use disorders,8 supervised dosing versus off-site consumption of opioid substitution treatment,9 community overdose prevention and naloxone distribution programs,10–13 clinical strategies for reducing aberrant drug-related behavior (e.g., treatment agreements, urine drug testing),14,15 and prescription opioid policies (namely, guidelines and legislation).16
Our goal was to conduct a comprehensive systematic review to more broadly identify existing strategies, programs, policies, and practices aimed at improving the appropriate use of prescription opioids and/or reducing the misuse, abuse, and deaths related to these drugs, with a focus on strategies that can be implemented in North America, the epicenter of the current crisis.
Methods
We followed the PRISMA checklist, and the methods for this review have been previously published in Prospero.17
The research question addressed in this review included the following components:
What: What are the existing strategies, programs, policies and practices aimed at (1) improving the appropriate use of prescription opioids and/or (2) reducing the misuse, abuse, and diversion of these drugs? Misuse was defined as taking a medication in a manner or dose other than prescribed; taking someone else’s prescription, even if for a legitimate medical complaint such as pain; or taking a medication to feel euphoria (i.e., to get high). The term nonmedical use of prescription drugs also refers to these categories of misuse.18
Who: There are many organizations and agencies with a keen interest in promoting the appropriate use of and/or reduction of inappropriate use of opioids. However, given limited resources and time to conduct this review, we narrowed the sources of material to four major sectors: (1) health-related professions and regulatory authorities; (2) government, public health/health promotion agencies, prevention and treatment organizations; (3) insurance organizations, including workers’ compensation; and (4) law enforcement agencies. Therefore, we excluded materials produced by military organizations, the pharmaceutical industry, or for-profit organizations. In addition, we excluded Internet-based or media-related strategies.
Where: We were interested in materials that are particularly applicable to the current Canadian context.
When: In North America, abuse of prescription opioids began to rise with the introduction of Oxycontin in 1996.19,20 Therefore, we focused on studies published in the 20 years after the release of Oxycontin.
Searches
To identify peer-reviewed publications, we searched the following electronic bibliographic databases from inception to September 2015 with no language restrictions: MEDLINE, EMBASE, PsycINFO, and CINAHL. All search strategies were developed by the research team in consultation with the knowledge users group and executed by an experienced librarian. The search strategies were adapted from the P.I.C.O. structure (Patient, Intervention, Comparison, and Outcomes) of reviews of effects of interventions. The controlled vocabulary differs significantly across peer-reviewed databases. Therefore, search terms were customized for each database. The search strategies are shown in Appendix 1.
It was also anticipated many relevant studies would not be published in peer-reviewed journals. We addressed this gap by also systematically searching the grey literature. The grey literature is a rich source of material that is not controlled by commercial publishing but offers advantages of usually being more current, free, relevant, unique, and on nonmainstream topics or aspects.21 For grey literature, Canadian websites for the following groups were searched (Appendix 2): regulatory authorities for health-related professionals (e.g., colleges for physicians, pharmacists, and nurses), government (federal and provincial), public health and health promotion agencies, prevention and treatment organizations, workers’ compensation boards, private insurance companies, and law enforcement agencies. There were no language restrictions. However, for feasibility, only materials uploaded, updated, or available in the previous 20 years were searched.
Eligibility criteria
The following primary opioid-related outcomes were considered eligible for inclusion:
Appropriate prescription of opioids for pain measured by pain intensity or functional improvement, number of high-dose opioid prescriptions, intermittent use of long-acting opioids, combination with benzodiazepines, appropriate education provided to patient, appropriate selection of patients for opioids, and appropriate monitoring of patients on opioids.
Misuse, abuse, opioid use disorder, and diversion of prescription opioids.
Fatal or nonfatal opioid-related overdoses.
A secondary outcome of interest was unintended consequences of the implemented strategy. These could be adverse consequences to participants (e.g., being harassed by the police because they were carrying naloxone; additional burden on the clinical staff) or to society (e.g., shifting the opioid crisis to a neighbor region where the strategy had not been implemented).
Only studies with empirical data evaluating the effectiveness of strategies on our outcomes of interest were included in this review. These could be quantitative (observational or experimental), qualitative, or mixed-method studies. For grey literature, we included data evaluations, foundation reports, government reports, grantee publications, noncommercially published conference papers, reports, special reports, and working papers, committee reports, testimony, and conference proceedings.
All strategies that have been developed, implemented, and evaluated in North America, Europe, and Australia/New Zealand were eligible for inclusion. Strategies that have been implemented outside of these regions were only included if they were applicable or useful to the opioid-related issues in Canada (i.e., if the country had trends in prescription opioid use and misuse similar to those in Canada and/or the country has a health care system similar to Canada).
Relevancy screening
Titles and/or abstracts of the studies retrieved were screened independently by rotating pairs of reviewers using the full set of inclusion/exclusion criteria and a standardized, prepiloted form using Distiller SR software. The full text of studies meeting all criteria or where there was uncertainty were retrieved and assessed for relevancy by rotating pairs of reviewers. Any disagreements were resolved through discussion with reviewer pairs and a third reviewer was consulted when consensus could not be reached.
Data extraction
A standardized, prepiloted form was used to extract data from the included studies for evidence synthesis. Data were extracted according to the variables that have been agreed upon by the team members for all papers included in this review, which included country, settings, target population, group that developed the strategy, components of the strategy, duration, outcomes, and results. During the process of data extraction, we met regularly to resolve issues related to locating the data in the text, establishing the nature and type of the data, ascertaining reliability of data extraction, and checking data extraction in preparation for analysis.
Quality appraisal
To assess the quality of each included study, we first applied a classification by methodological design:
Group A: Evidence from randomized studies.
Group B: Evidence from controlled experimental studies without randomization or from epidemiological studies (cross-sectional, cohort, or case–control analytic studies).
Group C: Evidence from comparisons between times or places with or without the intervention; dramatic results in uncontrolled experiments or qualitative statements could be included here.
Second, a critical appraisal checklist was used to assess risk of selection, performance, detection, attrition, and reporting bias in each study (Appendix 3). The risk of bias assessments was entered in RevMan software version 5.3.22
Data analysis and synthesis
Empirical data included both quantitative and qualitative evaluations of the impact of the strategy on any of the outcomes described above. Quantitative data were analyzed as differences between groups (for studies in groups A and B) or within groups (for studies in group C). We calculated standardized effect sizes of interventions that yielded statistically significant results: Cohen’s d for main differences,23 Cohen’s d from t statistics,24 Cohen’s d from F test,25 Cohen’s h for differences in proportion,26 Hedge’s g from t statistics when sample size was less than 30,27 and logit d from odds ratio.26,28,29 (Appendix 4).
For studies with multiple outcomes, we reported the measured outcome with the largest effect size for each of the three outcomes of interest. The effect size was expressed as a negative or positive value, indicating that the intervention either had a smaller or greater effect than the control, respectively. The effect sizes were grouped into categories of no (0–0.19), small (0.20–0.49), medium (0.50–0.79), or large (0.8 or larger) effect.30 In the graphical representations, no effect was assigned a value of 0, small was assigned 1, medium 2, and large 3. When the data were only reported as a qualitative statement, we assigned its impact factor (0, 1, 2, or 3) based on similar studies from which we had calculated the effect size. The association between number of contents in each strategy and the impact factor was calculated using correlation coefficient for each outcome. Rather than a meta-analysis, we conducted a narrative synthesis to describe the interventions and a quantitative analysis to assess the impact of each intervention.
Results
The searches of electronic databases yielded 5169 titles and abstracts and searches of the grey literature yielded 72 studies (Figure 1). A total of 557 full texts were obtained from the electronic databases and grey literature. Of these, 65 met the inclusion criteria and were included: 9 randomized trials31–39 and 56 nonrandomized studies.40–95 One study described two strategies and provided separate results.38 The characteristics of population, strategies, outcomes, and unintended consequences are shown in Appendix 5. The target population for the strategies was grouped into three groups: (1) patients and opioid users, (2) health care providers, and (3) the general public.
Figure 1.
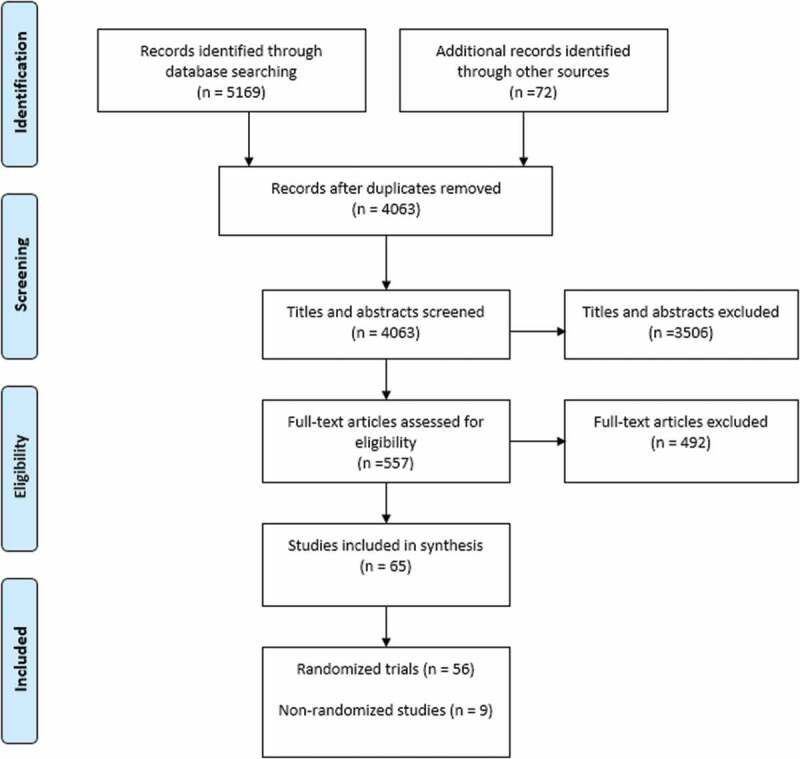
PRISMA flow diagram.
Critical appraisal of the studies
Overall, randomized trials and nonrandomized studies had significant methodological shortcomings. Among the nine randomized trials, the most common types of bias were performance (blinding of participants and personnel) and detection bias (blinding of outcome assessment; Figure 2). There was a potential for selection bias in approximately half of the trials due to unclear methods of randomization and allocation concealment. One study had a high risk of attrition bias due to a 39% drop out rate31 and one study had a high risk of bias due to potential for conflict of interest.32 There was no indication of reporting bias in any of the trials included.
Figure 2.
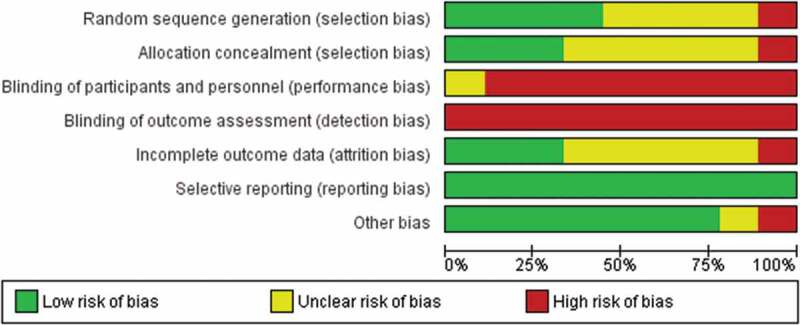
Risk of bias summary of randomized controlled trials.
Among the 56 nonrandomized studies, the most frequent methodological flaws were lack of a separate control group, lack of description of how groups were formed, lack of description of pre-intervention characteristics, lack of examination of whether important differences existed between the remaining and dropout participants, lack of documentation of participation (compliance with the intervention), poor reporting of main outcomes measurements at baseline, lack of adjustments for pre-intervention differences, and statistical methods of analysis that were not optimal (Figure 3). The majority of studies had a clear research question, a clear description of the strategy (or intervention process), a clear documentation of the effects of the intervention on some of the exposure parameters, and length of follow-up of 3 months or greater. For some methodological indicators, most studies lacked clear description and therefore the judgments were “unclear”: whether participation rate was at least 50%, whether loss to follow-up was less than 35%, whether the analysis considered the participants in the groups they were originally allocated, and whether there was a direct between-group comparison or not.
Figure 3.

Risk of bias summary of nonrandomized studies.
Target population of the strategies
Forty-eight strategies were aimed at only one target group, 15 were aimed at two target groups, and three were aimed at all three target groups of interest (Figure 4). The most common target group was health care professionals: as a single target in 33 strategies and combined with opioid users in 11 strategies.
Figure 4.
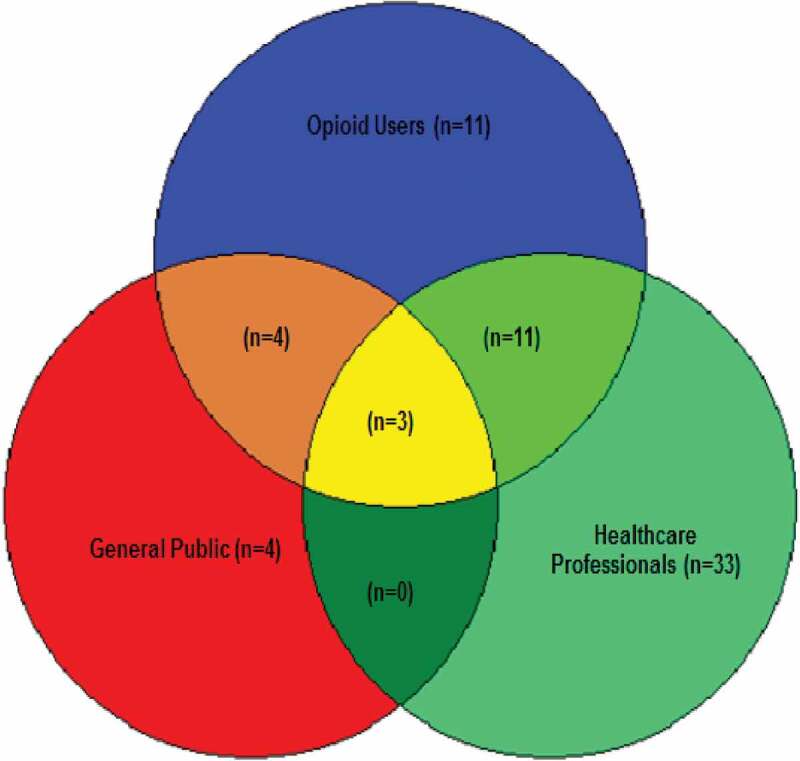
Target populations.
Content of the strategies
The content of the strategies was categorized into educational, clinical practice, naloxone distribution, opioid substitution therapies, prescription monitoring, campaigns to return unused opioids (take-back programs), regulations, policies, and public campaigns. A strategy could have more than one type of content: one strategy had five contents,77 3 strategies had four,44,67,81 13 strategies had three,37,41,43,48,51,53,60,63,66,70,76,79,85 29 strategies had two,31,32,34,38–40,42,45,46,49,52,55,59,62,65,69,72–74,80,82–84,87,88,91,93,94 and 20 strategies had a single content.33,35,37,47,50,54,56–58,61,63,68,71,78,86,89,90,92,95,96 The association between the number of contents and the impact factors was small for all three outcomes of interest: 0.32 for appropriate use, 0.08 for misuse, and 0.14 for overdose. The contents of each strategy are explained below:
Education. These strategies involved formal teaching to improve knowledge or training to impart specific skills. Examples of educational strategies were attending a workshop or a continuing medical educational event.65 Thirty-five strategies involved education.31,32,34,35,37–39,41–44,48,49,52,53,55,60,63,65,67,70,72,74,76,77,79,81,82,85,88,90,91,93,94,97
Clinical practice. These strategies involved changes in how health care was delivered, such as implementation of recommendations from clinical practice guidelines,87 using a tool to improve opioid prescribing,86 implementing urine drug tests,85 or using disease management programs.46 Thirty-two strategies involved clinical practice changes.32–34,39,40,42–46,48,51,56,57,61,65–67,69,72,74,77–80,85–88,91,92,95,97
Reversal of overdose with naloxone involved distribution of naloxone to caregivers or bystanders with the potential to reverse an opioid-related overdose. An example was an intervention that included not only distribution of naloxone but also an educational campaign to identify high-risk individuals.55 Nine strategies involved naloxone distribution.41,52,53,55,60,63,76,82,93
Opioid substitution therapies were carried out by healthcare professionals who prescribe methadone or buprenorphine for opioid use disorder and dependence. This type of strategy was usually combined with clinical or educational strategies.43,80 Six strategies involved opioid substitution therapies.43,51,69,77,80,89
Take-back program involved reducing the amount of unused opioids in households and preventing diversion by providing drop-boxes and incentives for safe disposal of the medications. Only one study was included.62
Prescription monitoring or review programs (PMPs) are strategies that use an electronic system to keep track of opioid prescriptions by physicians or opioid dispensing by pharmacists. They were usually combined with education,44 changes in clinical practices,48 or regulations.70 Eighteen strategies involved PMPs.31,40,41,44,48,50,54,58,64,66,67,70,73,77,81,83,84,87
Regulations and policies are strategies that use legislation or policies to correct or incentivize certain behaviors. It could be the sole content of a strategy47 or it could be combined with education,49 prescription monitoring program,73 or public campaigns.81 Sixteen strategies involved regulations or policies.47,49,53,59,60,62,63,66,68,70,71,73,75,81,83,84,96
Community or public health campaigns included strategies aimed at prevention or public health strategies. Seven strategies (reported in five studies) involved community or public health campaigns.37,38,76,81,94
Collaborative strategies involved bringing diverse groups of people together to solve a common issue or to improve a situation, such as an interprofessional or multidisciplinary team. Ten strategies included collaboration as a key component of their strategies.37,44–46,51,59,67,77,79,85
Impact of the strategies
Impact on appropriate use of opioids
Twenty-six studies measured the impact of a strategy on improving or ensuring appropriate use of opioids (Table 1). The impact factors ranged from −2 to 3. The only strategy with a negative impact involved a comparison of a PMP between two states in the United States.64 Researchers found that in suspected diversion or doctor shopping, the health care providers using PMPs were 53% less likely to discuss the concerns with the patient and 73% less likely to state to the patient that they were out of stock of the medication. Eight strategies had no effect on appropriate use of opioids,54,58,59,65,66,74,79,84 seven strategies had a small positive effect,32,33,42,46,70,77,89 six strategies had a moderate positive effect,39,43,48,80,85,88 and four strategies had a large positive effect, which included (1) Project Lazarus, a community activation and coalition-building, monitoring, and surveillance data, prevention of overdoses, and use of rescue medication for reversing overdoses and evaluation of the program41; (2) implementation of a treatment agreement developed with cooperation of anesthesiologists, psychologists, nurses, rehabilitation specialists, and clinical pharmacists45; (3) a clinic-wide strategy including opioid prescribing policies and protocols, guidelines to address depression and substance abuse screening, drug selection, dose titration, urine toxicology testing, review of the PMP database and agreement violations, in addition to a monthly meeting with a multidisciplinary committee to review protocols and discuss cases and provider education67; and (4) a multifaceted education initiative regarding pethidine, tramadol, and morphine prescriptions consisting of in-services and feedback by clinical pharmacists, literature discussion, and posters.91
Table 1.
Outcomes, effect sizes, and impact factors.
| Study | Measured outcome | Category of outcome | Derived effect size measure | Effect size | Impact factor |
|---|---|---|---|---|---|
| Lamb et al. 2007 | Pain ratings | Appropriate use | Extrapolationa | — | 0b |
| Wiedemer et al. 2007 | Resolution of aberrant behaviors | Misuse, abuse, and addiction | Cohen’s hc | 0.57 | 2 |
| Bujold et al. 2012 | Narcotics confiscated | Misuse, abuse, and addiction | Cohen’s hc | 0.75 | 2 |
| Cochella and Bateman 2012 | Prescribing long-acting opioids for acute pain | Appropriate use | Cohen’s hc,d | 0.61 | 2 |
| Cochella and Bateman 2012 | Overdose deaths | Overdose and deaths | Cohen’s hc | 0.21 | 1 |
| Dormuth et al. 2012 | Inappropriate opioid prescriptions | Appropriate use | Cohen’s d from mean difference | 0.03 | 0 |
| Manchikanti et al. 2006 | Opioid prescription abuse | Misuse, abuse, and addiction | Logit d | 0.43 | 1 |
| Gonzalez and Kolbasovsky 2012 | Number of opioid prescribers | Misuse, abuse, and addiction | Cohen’s d from mean difference | 0.16 | 0 |
| Spoth et al. 2013 | Lifetime narcotic misuse | Misuse, abuse, and addiction | Cohen’s d from t statistic (independent) | 0.30 | 1 |
| Thomas et al. 2013 | Prescription alterations | Misuse, abuse, and addiction | Cohen’s d from t statistic (correlated) | 0.29 | 1 |
| Pade et al. 2012 | Change in pain scores | Appropriate use | Cohen’s d from t statistic (correlated) | 0.68 | 2 |
| Pade et al. 2012 | Percentage of patients relapsed | Misuse, abuse, and addiction | Cohen’s hc | 0.66 | 2 |
| Stover 2010 | Social functioning perception | Appropriate use | Cohen’s d from t statistic (independent) | 0.26 | 1 |
| Stover 2010 | Reduction in cravings | Misuse, abuse, and addiction | Not statistically significant | — | 0 |
| Wheeler et al. 2012 | Overdose reversal | Overdose and death | Cohen’s hc | 0.28 | 1 |
| Piper et al. 2007 | Number of drug users alive | Overdose and death | Cohen’s hc | 0.89 | 3 |
| Albert et al. 2011 | Opioid prescriptions | Appropriate use | Cohen’s h | 1.62 | 3 |
| Albert et al. 2011 | Overdose | Overdose and death | Cohen’s h | 0.01 | 0 |
| Walley et al. 2013 | Overdose reversal | Overdose and death | Cohen’s d from mean difference | 0.01 | 0 |
| Cicero et al. 2005 | Tramadol abuse rates | Misuse, abuse, and addiction | Not statically significant | — | 0b |
| Ablaihed et al. 2014 | Opioid prescriptions | Misuse, abuse, and addiction | Hedge’s g from t statistic (correlated) | 0.54 | 2 |
| Schlicher 2015 | Opioid prescriptions | Misuse, abuse, and addiction | Cohen’s hc | 0.32 | 1 |
| Andrews et al. 2013 | Intermittent hydromorphone use | Appropriate use | Cohen’s hc | 0.49 | 1 |
| Barry et al. 2015 | Pain interference | Appropriate use | Cohen’s d from mean differences | 0.67 | 2 |
| Barry et al. 2015 | Urinary opioids | Misuse, abuse, and addiction | Cohen’s d from mean differences | 1.13 | 3 |
| Davis 2015 | Overdose reversals | Overdose and death | Extrapolatione | — | 0 |
| DiPaula and Menachery 2015 | Urinary opioids | Misuse, abuse, and addiction | Cohen’s hc | 1.03 | 3 |
| Dwyer et al. 2013 | Performed rescue measures | Overdose and death | Cohen’s d from t statistic (independent) | 0.57 | 2 |
| Fulton-Kehoe et al. 2015 | Methadone poisoning | Overdose and death | Extrapolationf | –1.27 | –3 |
| Furlan et al. 2014 | Patients referred to methadone treatment | Appropriate use | Logit dc | 0.18 | 0 |
| Furlan et al. 2014 | Reduction of pentazocine PO | Misuse, abuse, and addiction | Cohen’s h | 0.54 | 2 |
| Green et al. 2013 | Prescribers discussing concerns with patients | Appropriate use | Logit d | –0.40 | –2 |
| Green et al. 2013 | Using PMP | Misuse, abuse, and addiction | Logit d | 1.78 | 3 |
| Green et al. 2015 | Deaths | Overdose and death | Cohen’s d from mean differences | 1.87 | 3 |
| Gugelmann et al. 2013 | Pain complaints | Appropriate use | Logit d | 0.07 | 0 |
| Gugelmann et al. 2013 | Discharged with opioids | Misuse, abuse, and addiction | Logit d | 0.31 | 1 |
| Johnson et al. 2014 | High-volume oxycodone providers | Misuse, abuse, and addiction | Cohen’s hc | 1.57 | 3 |
| Johnson et al. 2014 | Death rates from opioids | Overdose and death | Logit d | 0.18 | 0 |
| Kanate et al. 2015 | Medical evaluations | Overdose and death | Cohen’s h | 1.16 | 3 |
| Katzman et al. 2014 | Morphine equivalents of opioids dispensed | Appropriate use | Cohen’s hc | 0.20 | 1 |
| Katzman et al. 2014 | Deaths | Overdose and death | Cohen’s h | 0.00 | 0 |
| Keast et al. 2015g | Average number of opioid prescription claims per member | Misuse, abuse, and addiction | Extrapolationa | — | 0 |
| Keast et al. 2015 | Mortality rates | Overdose and death | Cohen’s h | 0.00 | 0 |
| Ketcham et al. 2014 | Number of ER visits per opioid user | Misuse, abuse, and addiction | Cohen’s d from mean differences | 3.26 | 3 |
| Kim et al. 2014 | Number of opioid pills | Misuse, abuse, and addiction | Cohen’s d from mean differencesh | 0.07 | 0 |
| Kunins 2015/Larochelle et al. 2015 | Milligrams of opioids | Misuse, abuse, and addiction | Cohen’s d from mean differences | 0.00 | 0 |
| Kunins 2015/Larochelle et al. 2015 | Overdose from prescription opioids | Overdose and death | Cohen’s d from mean differences | 0.02 | 0 |
| Paone et al. 2015 | High-dose opioid prescriptions | Misuse, abuse, and addiction | Cohen’s h | 1.02 | 3 |
| Paone et al. 2015 | Mortality | Overdose and death | Cohen’s h | 1.14 | 3 |
| Saitz et al. 2014 | Self-reported opioid misuse | Misuse, abuse, and addiction | Not statistically significant | — | 0 |
| Saitz et al. 2014 | Drug use consequences | Overdose and death | Not statistically significant | — | 0 |
| Sandoo et al. 2011 | Prescription of inappropriate drugs | Misuse, abuse, and addiction | Cohen’s h | 0.15 | 0 |
| Summers et al. 2014 | Violated treatment agreement | Misuse, abuse, and addiction | Logit df | –0.32 | −1 |
| James et al. 2014 | ED patients on extremely high opioid dose (>1000/mg MEQ) | Appropriate use | Logit d | 0.96 | 3 |
| Husk et al. 2014 | Percentage of ED patients not receiving opioids | Appropriate use | Cohen’s h | 0.06 | 0 |
| Husk et al. 2014 | Opioid prescriptions | Misuse, abuse, and addiction | Logit d | 0.04 | 0 |
| Saenger et al. 2013 | Urinary drug screens completed | Appropriate use | Cohen’s h | 0.57 | 2 |
| Saenger et al. 2013 | Monthly prescription refills | Misuse, abuse, and addiction | Cohen’s ha | 0.36 | 1 |
| Gray et al. 2015 | Units of opioid disposals | Misuse, abuse, and addiction | Extrapolationa | — | 1 |
| Gray et al. 2015i | Deaths from overdose | Overdose and death | Cohen’s h | 0.32 | 1 |
| Ringwalt et al. 2015 | Incidence of opioid prescriptions | Appropriate use | Logit d | 0.08 | 0 |
| Garcia et al. 2014 | Average daily dose of opioids | Appropriate use | Extrapolation | — | 0j |
| Garcia et al. 2014 | Number of opioid users | Misuse, abuse, and addiction | Logit d | 0.11 | 0 |
| Leece et al. 2013 | Naloxone administration | Overdose and death | Cohen’s hc | 0.17 | 0 |
| Delcher et al. 2015 | Deaths from oxycodone | Overdose and death | Cohen’s d from mean differencesk | 0.26 | 1 |
| Naliboff et al. 2011 | Pain ratings | Appropriate use | Cohen’s d from mean differences | 0.40 | 1 |
| Naliboff et al. 2011 | Patients discharged due to opioid misuse | Misuse, abuse, and addiction | Not statistically significant | — | 0 |
| Reifler et al. 2012 | Intentional opioid exposures | Misuse, abuse, and addiction | Cohen’s h | 0.19 | 0 |
| Franklin et al. 2012 | Workers on 120 mg/day MEQ | Misuse, abuse, and addiction | Cohen’s hc | 0.40 | 1 |
| Franklin et al. 2012 | Opioid-related deaths | Overdose and death | Cohen’s hc | 0.52 | 2 |
| Taylor et al. 2007 | Patients on pethidine | Appropriate use | Logit d | 0.82 | 3 |
| Doe-Simkins et al. 2014 | Opioid use within last 30 days | Misuse, abuse, and addiction | Not statistically significant | — | 0 |
| Doe-Simkins et al. 2014 | Actions taken during overdose | Overdose and death | Not statistically significant | — | 0 |
| Doe-Simkins et al. 2009 | Successful rescues | Overdose and death | Cohen’s hc | 0.22 | 1 |
| Gaston et al. 2009 | Appropriate actions in case of overdose | Overdose and death | Cohen’s d from t statistic (correlated) | 1.39 | 3 |
| McCarty et al. 2004 | Positive attitude toward buprenorphine | Appropriate use | Not statistically significant | — | 0 |
| Srivastava et al. 2012 | Physician difficulty with dosing | Appropriate use | Cohen’s d from t statistic (correlated) | 0.54 | 2 |
| Sullivan 2006 | Methadone prescriptions | Appropriate use | Cohen’s d from F test | 0.63 | 2 |
| Otto et al. 2009/Zahradnik et al. 2009 | Defined drug dosage | Misuse, abuse, and addiction | Logit d | 0.51 | 2 |
| Jamison et al. 2010 | Average pain ratings | Appropriate use | Cohen’s d from mean differences | 0.38 | 1 |
| Jamison et al. 2010 | Drug misuse index | Misuse, abuse, and addiction | Logit d | 1.14 | 3 |
| Lofwall et al. 2011 | Adherence to maximum dose | Appropriate use | Cohen’s h | 0.46 | 1 |
| Lofwall et al. 2011 | Percentage of doctors giving 7 days or less of buprenorphine | Misuse, abuse, and addiction | Logit d | 0.46 | 1 |
| Spoth et al. 2008 (Study I) |
Lifetime narcotic misuse | Misuse, abuse, and addiction | Cohen’s h | 0.44 | 1 |
| Spoth et al. 2008 (Study II) |
Lifetime nonprescribed medication use | Misuse, abuse, and addiction | Cohen’s h | 0.16 | 0 |
| Spoth et al. 2013 (Study III) |
Lifetime prescription opioid misuse | Misuse, abuse, and addiction | Cohen’s h | 0.13 | 0 |
| Burchman and Pagel 1995 | Good response to treatment | Appropriate use | Cohen’s hc | 0.89 | 3 |
| Chelminski et al. 2005 | Pain disability index | Appropriate use | Cohen’s d from t statistic (correlated) | 0.44 | 1 |
| Goldberg et al. 2005 | Total opioid consumption | Misuse, abuse, and addiction | Cohen’s d from mean differences | 0.12 | 0 |
aExtrapolation was based on interpretation of qualitative data.
bThe aim of study was to demonstrate noninferiority rather than superiority of the intervention.
cThe calculation for effect size assumes that the expected change in the control is 50% of the measured intervention change.
dThe lowest value in the reported range was used to calculate a conservative estimate of the effect size.
eThe impact factor is assigned comparatively, using other calculated impact factors of similar strategies as a framework.
fThe relative risk is used an approximation of odds ratio for logit d.
gUnintentional Poisoning Deaths in Oklahoma, https://www.ok.gov/health2/documents/UP_Deaths_2007-2012.pdf.
hThe pooled standard deviation assumes equal sample size in the comparison groups.
iDrug Overdose Deaths, Tennessee Department of Health, https://www.tn.gov/assets/entities/health/attachments/Drug_Overdose_Deaths_county_level_summary_through_2015_PDF.pdf.
jThe study showed mixed results.
kThe standard deviation of the intervention group was used as an approximation of the pooled standard deviation.
PO = by mouth; PMP = prescription monitoring program; ER = emergency room; ED = emergency department.
Impact on misuse, abuse, opioid use disorder (addiction), and diversion
Forty studies measured the impact of the strategy on outcomes of misuse, abuse, opioid use disorder, and/or diversion (Table 1). The impact factors ranged from −1 to 3. The only strategy with a negative impact on this outcome was a 1-h educational group session on the nature, theories, and treatment of pain provided by a clinical psychologist for new patients. The aim of the intervention was to reduce violations of the opioid treatment agreement. The results showed that those who attended the educational session were 1.8 times more likely to be discharged due to violation of the treatment agreement, and the explanation was that participants in the educational session could perceive a false sense of privilege becaus they had participated in their care beyond a typical first physician office visit.90 Sixteen strategies had no effect on this outcome,31,33,35,37,38,47,52,59,61,66,71,73,75,83,86,89 10 strategies had a small positive effect,37,38,56,62,65,77,78,85,87,92 six strategies (seven studies) had a moderate positive effect,34,40,44,58,80,95,97 and seven had a large positive effect, which included (1) a standard protocol for buprenorphine with naloxone for patients with low-back pain and opioid use disorder43; (2) a collaborative practice, with prescription of buprenorphine and naloxone, plus weekly urine drug testing51; (3) a prescription monitoring program with easy access at the point of care64; (4) a structured cognitive behavioral training program for prevention of substance misuse32; (5) a law enforcement change in the state of Florida68; (6) a recidivism program with staff education for high emergency department users72; and (7) a public health initiative involving clinical practice guidelines, media, town halls, public campaigns, and announcements.81
Impact on overdose and deaths
Twenty-two studies measured the impact of the strategy on outcomes of overdose and death (Table 1). The impact factors ranged from −3 to 3. The strategy with the largest negative impact included the implementation of an opioid dosing guideline (maximum 120 morphine equivalents per day) where there was a marked increase in mortality due to methadone.57 Ten strategies had a negligible or no impact on overdoses and deaths.35,41,49,52,68,70,75,76,81,93 Five strategies had a small positive effect,48,50,53,62,94 two had a moderate positive effect,55,56 and four had a large positive effect,60,63,69,82 which included (1) overdose prevention training and naloxone distribution, plus a change in the legal status of naloxone to permit its administration by any member of the public60; (2) pharmacy-based naloxone distribution plus education and training63; (3) First Nations healing strategies plus opioid substitution and primary care involvement69; and (4) take-home naloxone and training program.82
Most promising strategies by content and target audience
Figure 5a and 5b show the impact of the strategies by content and target population. It suggests that the most promising strategies to improve appropriate use of opioids are (1) educational strategies aimed at health care professionals; (2) clinical strategies aimed at patients, opioid users, and health care professionals; and (3) collaborations. The most promising strategies to reduce misuse, abuse, opioid use disorder, and diversion of opioids are (1) educational strategies aimed at patients, opioid users, and health care providers; (2) clinical strategies aimed at patients, opioid users, and health care providers; (3) PMPs; (4) collaborations,; (5) public health; and (6) opioid substitution. The most promising strategies to reduce overdoses and deaths are (1) education aimed at patients and opioid users and (2) naloxone distribution.
Figure 5.
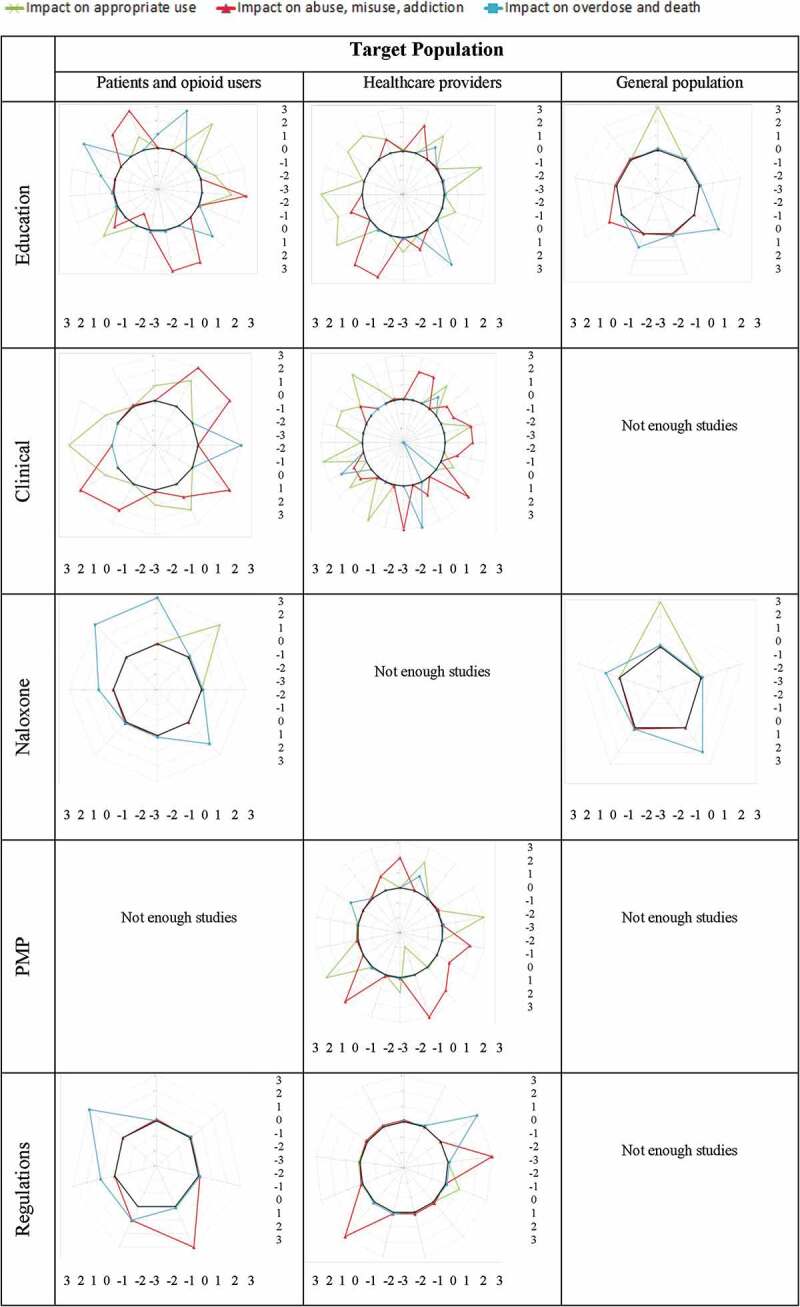
Impact factors by content and target population.
Figure 5.
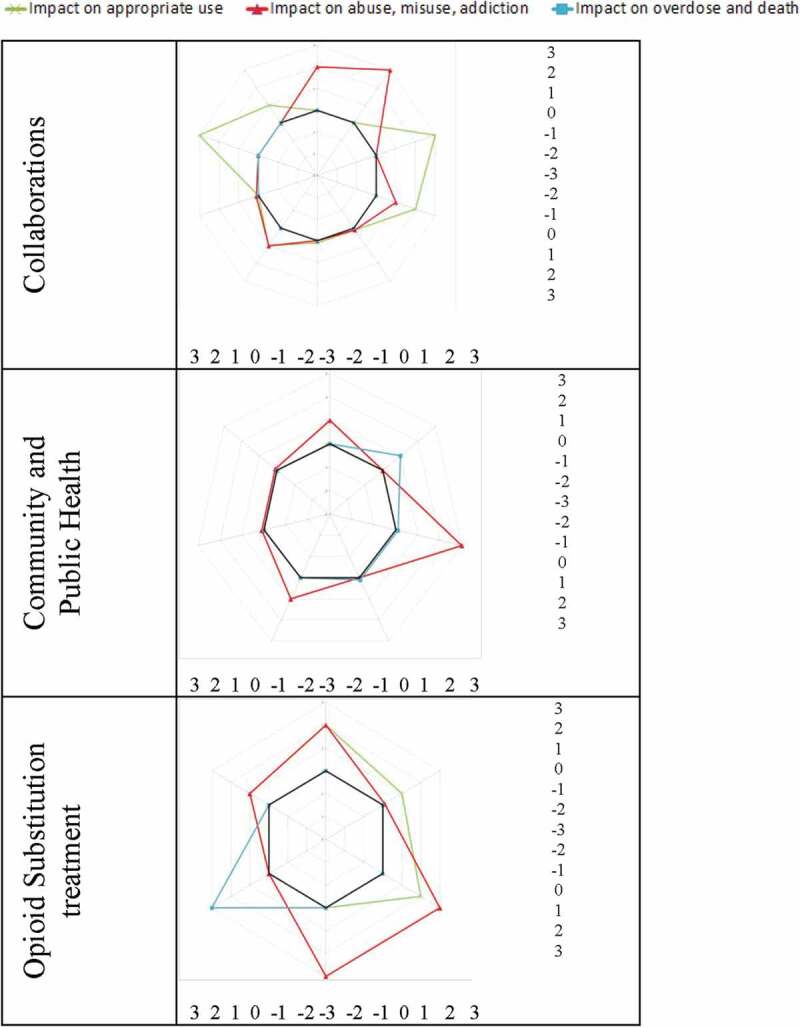
(Continued).
Unintended consequences of implemented strategies
Among the 66 strategies described, 19 (29%) had some type of unintended consequence41,42,44–46,49,53,57,59,60,68,71,73–75,79,82,84,90 and 4 reported no unintended consequence.47,55,64,65 It was unclear in 43 studies whether there were any unintended consequences or not.
Consequences that affected the target population of the strategy were reported, such as patients not receiving necessary prescriptions71; patients paying for their prescriptions out of their own pockets71; overdose due to rotation from other opioids to methadone57,74; more overdose by morphine, hydromorphone, and heroine68,75; stolen naloxone kits53,60,82; being harassed by police over possession of naloxone82; stigma associated with carrying a naloxone kit60; stigma associated with receiving a prescription for addiction79; a paradoxical increase in overdose because suspension of physicians who were prescribing improperly led to patients on withdrawal and overdosing from other sources41; patients had a false sense of privilege because of participation in an educational session, leading to more opioid abuse90; and possible beliefs that naloxone access enables addiction to opioids.49
There were consequences to the staff involved in the implementation of strategies: extra burden on the clinical staff (pharmacists and/or physicians)42,71; burden on pharmacy staff who had to assemble intranasal naloxone kits; or risks of needle stick injury to staff who had to assemble intramuscular naloxone.49
Societal consequences included shifting the opioid crisis to a neighboring region where the strategy had not been implemented44; higher costs49; increase in the proportion of prescriptions of opioids73,84; increase in dose of opioids prescribed46,59; shifting from one opioid to another59; increase in use of benzodiazepines and barbiturates52; and more patients developing opioid tolerance or filling prescriptions from other sources.45 (For details about the unintended consequences reported in each study, see Appendix 5.)
Discussion
Interpretation
We searched the peer-reviewed and grey literature for studies that implemented and evaluated strategies, programs, policies, and practices to improve the appropriate use of opioids and reduce misuse, abuse, opioid use disorder, diversion, overdoses, and deaths related to opioids. We found 65 studies reporting on 66 distinct strategies. Though the majority of the studies were at high risk of bias, there is some indication that the most promising strategies involved education, clinical practices, collaborations, PMPs, public campaigns, opioid substitution programs, and naloxone distribution. Twenty-nine percent of strategies reported some sort of unintended consequence.
Significance
Misuse and abuse of opioids have become a widespread problem in Canada, but many areas do not yet have the necessary measures in place to address this. This systematic review benefits from the diversity of strategies and outcomes that were implemented and evaluated in various jurisdictions similar to the Canadian context. Knowledge users can refer to this systematic review in the planning stages of implementing interventions to improve the appropriate use of prescription opioids and/or reduce the misuse, abuse, and diversion of these drugs. Knowledge users can also appraise the interventions of interest to make contextually appropriate modifications and combine various strategies to achieve the desired effects. As such, this comprehensive compilation of studies provides a concrete foundation for knowledge users to build upon. Lastly, by appraising the quality of the evidence, we highlight the deficits and need for improvement in this body of literature. We encourage knowledge users to engage with the suggestions for future studies to improve the quality of evidence in this field, while incorporating economic feasibility into the growing body of literature.
Limitations
Limitations of the existing literature
The quality of evidence in this body of literature contains many methodological flaws. The majority of studies are observational in nature, with only nine randomized controlled trials among the 65 studies. In addition, most grey literature publications did not provide empirical data. Another limitation was that a minority of studies reported unintended consequences associated with the strategies.
Limitations of the methods used in this review
One limitation of our review is that the literature search was last updated in September 2015. Since then, there have been publications of studies that could potentially be included in this review. We updated the electronic searches up to March 2018 and there were 1427 titles and abstracts. After screening by two authors, there were 182 remaining titles and abstracts that could potentially meet the inclusion criteria for this current review. Another limitation is using assumptions to calculate an effect size (ES). Fourteen studies did not have a separate control for comparison, and this was particularly common in studies on regulation changes. In an effort to avoid overestimating the ES, we assumed the expected change in the control to be 50% of the measured intervention change in these 14 studies. Another limitation is that the impact factor was extrapolated when studies were qualitative in nature or did not provide sufficient data to calculate an ES; this was applied to four studies. Extrapolation relied on both clinical expertise and the completed framework of impact factors as a reference, which introduced some degree of subjectivity into the analysis. Lastly, studies could not be combined for best evidence synthesis or meta-analysis due to distinct differences in the strategies, populations, or outcomes between each study.
Strengths
This review examined a full spectrum of strategies that were implemented and empirically evaluated to tackle the opioid crisis in North America and to maintain the appropriate use of opioids in improving pain and function among patients with chronic pain. Few systematic reviews in this field have conducted comprehensive grey literature searches. In doing this, we compiled a comprehensive repository of relevant publications that included peer-reviewed articles and empirical evidence from grey literature. We conducted a narrative synthesis to describe the interventions and a quantitative analysis to assess the effectiveness of each intervention, and we were able to calculate effect sizes and map these strategies using radar charts to visualize the data and make conclusions about the most promising strategies. In addition, we produced a framework that stratifies each intervention by impact factor and type of outcome assessed. This unique framework emphasizes the importance of both elements; for example, even a small impact on overdose and death holds remarkable significance. Decision makers can prioritize the categories of outcome according to their objectives and use the impact factors to determine relative effectiveness of an intervention for a particular outcome. Our study capitalizes on the heterogeneity of interventions, populations, and outcomes found in the literature, so that decision makers can appraise the various interventions in context and tailor their modifications accordingly.
Similar studies
Strategies and interventions to address the sweeping opioid crisis have been the subject of several narrative reviews,98–101 providing a broad overview of existing strategies, as well as drawing attention to more novel interventions. Narrative syntheses, however, lack comprehensive and systematic literature search strategies and, thus are prone to publication bias.
We are aware of a handful of recent systematic reviews that have synthesized the evidence for specific types of strategies, including primary care delivery models for treating opioid use disorders,8 supervised dosing versus off-site consumption of opioid substitution treatment,9 community overdose prevention and naloxone distribution programs,10–13 supervised consumption sites,102,103 clinical strategies for reducing aberrant drug-related behavior (e.g., treatment agreements, urine drug testing),14,15 and prescription opioid policies (namely, guidelines and legislation).16 Consistent across four reviews was the finding that naloxone and overdose prevention programs are associated with a reduction in overdose mortality and increased odds of recovery.10–13 A recent scoping review also found mixed evidence for the effectiveness of prescription drug monitoring programs.104 These findings are consistent with the findings of our review.
However, most of these prior reviews employed restrictive search strategies and/or had inadequate or nonexistent quality appraisal. Only one review considered some degree of grey literature.9
Only one published systematic review, to our knowledge, has considered the effectiveness of a broad range of strategies.105 Haegerich and colleagues examined the impact of state policy and systems-level interventions on prescriber and patient behavior and health outcomes (e.g., overdoses), finding low-quality evidence of positive effects for PMPs, insurer strategies, pain clinic legislation, clinical guidelines, and naloxone distribution.105 There was also little evidence of effect for safe storage and disposal strategies and patient education. The review by Haegerich et al.,105 though comprehensive in scope, also had limitations, including limiting searches to Medline, searches up to 2014, including only English-language studies, and primarily relying on studies from the United States.
Future research
Many promising strategies have already been implemented in the past couple of years in North America, such as naloxone distribution. There is a need to conduct empirical studies of more novel interventions. We found many publications that described various novel strategies, but they were excluded because they lacked an empirical measure to assess the impact on any of the outcomes of interest. A list of the excluded studies can be obtained upon request.
Future studies should aim at high methodological standards. In nonexperimental studies where randomization and separate control groups are not possible, it would be ideal to conduct interrupted time series or controlled before and after as the study design. Interrupted time series and controlled before and after are particularly useful in the context of public health interventions. Nonexperimental or observational studies should also discuss concurrent interventions or policy changes that may affect the population or region of interest. If no other concomitant interventions were introduced, studies should report either the measured outcome at the pre-intervention time point or the literature value that is relevant to the population or region of interest. Rather than only reporting the final change in the outcomes, we recommend that future studies report the measured outcomes at both the pre- and postintervention time points. Studies should also report the sample size or population size, so that it is possible to calculate the variance of the effect sizes. Lastly, we recommend that future studies include a cost–benefit analysis of an intervention, so that decision makers and policymakers can better assess the relative cost-effectiveness and feasibility of the interventions.
Conclusions
This broad-scope systematic review found some promising strategies to tackle the opioid crisis in North America. The content of these strategies included education, clinical practice changes, naloxone distribution, PMPs, regulations, collaborations, public health, and opioid substitution treatments. The most common target population of these strategies was health care professionals, followed by patients/opioid users and the general public. Twenty-nine percent of the strategies described some type of unintended consequence, which affected the target population, the health care professionals involved in the implementation of the strategy, or the public in general. There is a need for high-quality studies in this area to assess the impact of novel strategies on various outcomes, including appropriate use of opioids and reduction of misuse, abuse, opioid use disorder, diversion, overdoses, and deaths related to opioids.
Supplementary Material
Acknowledgments
We thank the following individuals for their support in obtaining the funding and/or for volunteering their time to conduct grey literature searches, article screening, and quality appraisal: Heather Dickinson, Regie Caverson, Heather Divine, R. Thomas Girling, Marty McLeod, Amy Porah-Waller, Anita Srivastava, Norman Buckley, Diane Vermilyea, Arun Radhakrishnan, Soudeh Taghdiri, Joanna Liu, Andrew Wong, Melanie Fortune, Fernanda Miyumi, Abdul Hamad, and Cynthia Chen.
Funding Statement
We acknowledge funding from the Canadian Institutes for Health Research to conduct this systematic review (#KSU-126318).
Supplementary information
Supplemental data for this article can be accessed here.
Declaration of interest statement
The authors have no conflict of interests to declare.
References
- 1.Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada . Statement from the co-chairs of the federal, provincial and territorial special advisory committee on the epidemic of opioid overdoses; 2017 [accessed 2017 Nov 27]. https://www.canada.ca/en/publichealth/news/2017/09/statement_from_theco-chairsofthefederalprovincialandterritorials.html.
- 3.Public Health Agency of Canada . Statement from the co-chairs of special advisory committee on the epidemic of opioid overdoses on updates to opioid-related mortality data; 2017 Dec 18 [accessed 2017 Dec 30]. http://www.newswire.ca/news-releases/statement-from-the-co-chairs-of-special-advisory-committee-on-the-epidemic-of-opioid-overdoses-on-updates-to-opioid-related-mortality-data-664993543.html.
- 4.Canadian Institute for Health Information . Pan-Canadian trends in the prescribing of opioids, 2012 to 2016. 2017 [accessed 2017 Dec 26]. https://www.cihi.ca/sites/default/files/document/pan-canadian-trends-opioid-prescribing-2017-en-web.pdf.
- 5.Canadian Pain Society. Position statement on opioid analgesics in pain management—2015 update. Pain Res Manag. 2015;20(6):287. doi: 10.1155/2015/251464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch ME, Fischer B.. Prescription opioid abuse: what is the real problem and how do we fix it? Can Fam Physician. 2011;57:1241–1242, e403–e405. [PMC free article] [PubMed] [Google Scholar]
- 7.Finestone HM, Juurlink DN, Power B, Gomes T, Pimlott N.. Opioid prescribing is a surrogate for inadequate pain management resources. Can Fam Physician. 2016;62:465–468. [PMC free article] [PubMed] [Google Scholar]
- 8.Lagisetty P, Klasa K, Bush C, Heisler M, Chopra V, Bohnert A. Primary care models for treating opioid use disorders: what actually works? A systematic review. PLoS One. 2017;12(10):e0186315. doi: 10.1371/journal.pone.0186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saulle R, Vecchi S, Gowing L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database Syst Rev. 2017;4:CD011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177–1187. doi: 10.1111/add.v111.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Subst Abuse. 2015;36(2):240–253. doi: 10.1080/08897077.2015.1010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giglio RE, Li G, DiMaggio CJ. Effectiveness of bystander naloxone administration and overdose education programs: a meta-analysis. Inj Epidemiol. 2015;2(1):10. doi: 10.1186/s40621-015-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153–163. doi: 10.1097/ADM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 14.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–720. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Argoff CE, Kahan M, Sellers EM. Preventing and managing aberrant drug-related behavior in primary care: systematic review of outcomes evidence. J Opioid Manag. 2014;10(2):119–134. doi: 10.5055/jom.2014.0201. [DOI] [PubMed] [Google Scholar]
- 16.Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109–118. doi: 10.5055/jom.2016.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furlan A, Irvin E, Van Eerd D, Carnide N, Munhall C, Kim J, Mahood, MacDonald S. Strategies to support the appropriate use of prescription opioids: a systematic review using narrative and best evidence synthesis methods. PROSPERO. 2014;CRD42014009675. http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42014009675. [Google Scholar]
- 18.Substance Abuse and Mental Health Services Administration . Results from the 2016 national survey on drug use and health: detailed tables. 2017 [accessed 2018 Mar 7]. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.htm.
- 19.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181(12):891–896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson A, Normandin S. Grey matters! Finding grey literature. Ottawa (Canada): Canadian Agency for Drugs and Technologies in Health; 2008. [Google Scholar]
- 22.Review manager (RevMan) [Computer program]. Version 5.3. Copenhagen (Denmark): The Nordic Cochrane Centre: The Cochrane Collaboration; 2014. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=9675 [Google Scholar]
- 23.Borenstein M. Effect sizes based on means. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons; 2009. p. 21–32. [Google Scholar]
- 24.Rosenthal R. Meta-analytic procedures for social research. Newbury Park (CA): Sage; 1991. [Google Scholar]
- 25.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The hand-book of research synthesis. New York (NY): Sage; 1994. p. 231–244. [Google Scholar]
- 26.Rosnow RL. Effect sizes for experimenting psychologists. Can J Exp Psychol. 2003;57(3):221–237. doi: 10.1037/h0087427. [DOI] [PubMed] [Google Scholar]
- 27.Hedges LV, Olkin I. Statistical methods for meta-analysis. 1st ed. San Diego (CA): Academic Press; 1985. [Google Scholar]
- 28.Glass G, Smith ML, McGaw B. Meta-analysis in social research. Bevery Hills (CA): Sage; 1981. [Google Scholar]
- 29.Borenstein M. Converting among effect sizes. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons; 2009. p. 45–49. [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 31.Gonzalez AM III, Kolbasovsky A. Impact of a managed controlled-opioid prescription monitoring program on care coordination. Am J Manag Care. 2012;18:516–524. [PubMed] [Google Scholar]
- 32.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150(3):390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naliboff BD, Wu SM, Schieffer B, Bolus R, Pham Q, Baria A, Aragaki D, Van Vort W, Davis F, Shekelle P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain. 2011;12(2):288–296. doi: 10.1016/j.jpain.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Otto C, Crackau B, Löhrmann I, Zahradnik A, Bischof G, John U, Rumpf HJ. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105(3):221–226. doi: 10.1016/j.drugalcdep.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spoth R, Redmond C, Clair S, Shin C, Greenberg M, Feinberg M. Preventing substance misuse through community–university partnerships: randomized controlled trial outcomes 4 1/2 years past baseline. Am J Prev Med. 2011;40(4):440–447. doi: 10.1016/j.amepre.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M, Feinberg M. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am J Public Health. 2013;103(4):665–672. doi: 10.2105/AJPH.2012.301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spoth R, Trudeau L, Shin C, Redmond C. Long-term effects of universal preventive interventions on prescription drug misuse. Addiction. 2008;103(7):1160–1168. doi: 10.1111/j.1360-0443.2008.02160.x. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med. 2006;21(4):360–362. doi: 10.1111/j.1525-1497.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ablaihed L, Barrueto J, Pimentel L, Comer A, Browne BJ, Hirshon JM. High risk care plans effectively decrease emergency department resource utilization. Ann Emerg Med. 2014;64(4 Suppl. 1):S4. doi: 10.1016/j.annemergmed.2014.07.033. [DOI] [Google Scholar]
- 41.Albert S, Brason FW II, Sanford CK, Dasgupta N, Graham J, Lovette B. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 2011;12(Suppl, 2):S77–S85. doi: 10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- 42.Andrews LB, Bridgeman MB, Dalal KS, Abazia D, Lau C, Goldsmith DF, John DS. Implementation of a pharmacist-driven pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342–1349. doi: 10.1111/ijcp.12311. [DOI] [PubMed] [Google Scholar]
- 43.Barry DT, Cutter CJ, Beitel M, Liong C, Schottenfeld RS. Cognitive-behavioral therapy and educational counseling for chronic pain and opioid dependence. Drug Alcohol Depend. 2015;146:e218–e219. [Google Scholar]
- 44.Bujold E, Huff J, Staton EW, Pace WD. Improving use of narcotics for nonmalignant chronic pain: a lesson from Community Care of North Carolina. J Opioid Manag. 2012;8(6):363–367. doi: 10.5055/jom. [DOI] [PubMed] [Google Scholar]
- 45.Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage. 1995;10(7):556–563. doi: 10.1016/0885-3924(95)00085-D. [DOI] [PubMed] [Google Scholar]
- 46.Chelminski PR, Ives TJ, Felix KM, Prakken SD, Miller TM, Perhac JS, Malone RM, Bryant ME, DeWalt DA, Pignone MP. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5(1):3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Muñoz A. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf. 2005;14(12):851–859. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- 48.Cochella S, Bateman K. Provider detailing: an intervention to decrease prescription opioid deaths in Utah. Pain Med. 2011;12(Suppl. 2):S73–S76. doi: 10.1111/j.1526-4637.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 49.Davis C, Walley A, Bridger C. Lessons learned from the expansion of naloxone access in Massachusetts and North Carolina. J Law Med Ethics. 2015;43(1):19–22. doi: 10.1111/jlme.12208. [DOI] [PubMed] [Google Scholar]
- 50.Delcher C, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-Molina MM. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s Prescription Drug Monitoring Program. Drug Alcohol Depend. 2015;150:63–68. doi: 10.1016/j.drugalcdep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 51.DiPaula BA, Menachery E. Physician–pharmacist collaborative care model for buprenorphine-maintained opioid-dependent patients. J Am Pharm Assoc (2003). 2015;55(2):187–192. doi: 10.1331/JAPhA.2015.14177. [DOI] [PubMed] [Google Scholar]
- 52.Doe-Simkins M, Quinn E, Xuan Z, Sorensen-Alawad A, Hackman H, Ozonoff A, Walley AY. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health. 2014;14:297. doi: 10.1186/1471-2458-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009;99(5):788–791. doi: 10.2105/AJPH.2008.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dormuth CR, Miller TA, Huang A, Mamdani MM, Juurlink DN. Effect of a centralized prescription network on inappropriate prescriptions for opioid analgesics and benzodiazepines. CMAJ. 2012;184(16):E852–E856. doi: 10.1503/cmaj.120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dwyer K, Walley AY, Langlois BK, Mitchell PM, Lin SC, Nelson KP, Cromwell J, Bernstein E. Opioid education and nasal naloxone rescue kitsin the emergency department. Western Journal of Emergency Medicine. 2015. May; 16(3):381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–331. doi: 10.1002/ajim.v55.4. [DOI] [PubMed] [Google Scholar]
- 57.Fulton-Kehoe D, Sullivan MD, Turner JA, Garg RK, Bauer AM, Wickizer TM, Franklin GM. Opioid poisonings in Washington state medicaid: Trends, dosing, and guidelines. Medical care. 2015;53(8): 679–685. [DOI] [PubMed] [Google Scholar]
- 58.Furlan AD, MacDougall P, Pellerin D, Shaw K, Spitzig D, Wilson G, Wright J. Overview of four prescription monitoring/review programs in Canada. Pain Res Manag. 2014;19(2):102–106. doi: 10.1155/2014/634171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447–454. doi: 10.18553/jmcp.2014.20.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaston RL, Best D, Manning V, Day E. Can we prevent drug related deaths by training opioid users to recognise and manage overdoses? Harm Reduct J. 2009;6:26. doi: 10.1186/1477-7517-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldberg KC, Simel DL, Oddone EZ. Effect of an opioid management system on opioid prescribing and unscheduled visits in a large primary care clinic. J Clin Outcomes Manag. 2005;12:621–628. [Google Scholar]
- 62.Gray J, Hagemeier N, Brooks B, Alamian A. Prescription disposal practices: a 2-year ecological study of drug drop box donations in Appalachia. Am J Public Health. 2015;105(9):e89–e94. doi: 10.2105/AJPH.2015.302689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green TC, Dauria EF, Bratberg J, Davis CS, Walley AY. Orienting patients to greater opioid safety: models of community pharmacy-based naloxone. Harm Reduct J. 2015;12:Art–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green TC, Mann MR, Bowman SE, Zaller N, Soto X, Gadea J, Cordy C, Kelly P, Friedmann PD. How does use of a prescription monitoring program change pharmacy practice? J Am Pharm Assoc. 2013;53(3):273–381. doi: 10.1331/JAPhA.2013.12094. [DOI] [PubMed] [Google Scholar]
- 65.Gugelmann H, Shofer FS, Meisel ZF, Perrone J. Multidisciplinary intervention decreases the use of opioid medication discharge packs from 2 urban EDs. Am J Emerg Med. 2013;31(9):1343–1348. doi: 10.1016/j.ajem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Husk G, Nguyen T, Jellinek-Cohen S, Li M, Heller M. The effect of a statewide prescription monitoring program on ed prescribing of controlled substances. Acad Emerg Med. 2014;21:S310. [Google Scholar]
- 67.James J, Klein JW, Chew L, Merrill J, Jackson SL. A comprehensive clinic-based strategy to promote safe opioid prescribing for chronic non-cancer pain in the primary care setting. J Gen Intern Med. 2014;29:S472. [Google Scholar]
- 68.Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes—Florida, 2010–2012. MMWR Morb Mort Wkly Rep. 2014;63:569–574. [PMC free article] [PubMed] [Google Scholar]
- 69.Kanate D, Folk D, Cirone S, Gordon J, Kirlew M, Veale T, Bocking N, Rea S, Kelly L. Community-wide measures of wellness in a remote First Nations community experiencing opioid dependence: evaluating outpatient buprenorphine–naloxone substitution therapy in the context of a First Nations healing program. Can Fam Physician. 2015;61(2):160–165. [PMC free article] [PubMed] [Google Scholar]
- 70.Katzman J, Comerci GD, Landen M, Loring L, Jenkusky SM, Arora S, Kalishman S, Marr L, Camarata C, Duhigg D. Rules and values: a coordinated regulatory and educational approach to the public health crises of chronic pain and addiction. Am J Public Health. 2014;104(8):1356–1362. doi: 10.2105/AJPH.2014.301881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keast SL, Nesser N, Farmer K. Strategies aimed at controlling misuse and abuse of opioid prescription medications in a state Medicaid program: a policymaker’s perspective. Am J Drug Alcohol Abuse. 2015;41(1):1–6. doi: 10.3109/00952990.2014.988339. [DOI] [PubMed] [Google Scholar]
- 72.Ketcham EM, Fredella A, Malone M, Ketcham C, Larkin GL. A novel Six-Sigma-based multidisciplinary administrative intervention reduces opioid-seeking patient emergency department recidivism. Ann Emerg Med. 2014;64(4 Suppl. 1):S99. doi: 10.1016/j.annemergmed.2014.07.306. [DOI] [Google Scholar]
- 73.Kim E, Genes N, Coupet M. Effect of mandatory prescription drug monitoring program on emergency department prescribing of scheduled drugs. Ann Emerg Med. 2014;64(4 Suppl. 1):S81–S82. doi: 10.1016/j.annemergmed.2014.07.254. [DOI] [Google Scholar]
- 74.Lamb L, Pereira JX, Shir Y. Nurse case management program of chronic pain patients treated with methadone. Pain Manag Nurs. 2007;8(3):130–138. doi: 10.1016/j.pmn.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med. 2015;175(6):978–987. doi: 10.1001/jamainternmed.2015.0914. [DOI] [PubMed] [Google Scholar]
- 76.Leece P, Hopkins S, Marshall C, Orkin A, Gassanov M, Shahin R. Development and implementation of an opioid overdose prevention and response program in Toronto, Ontario. Can J Public Health. 2013;104(3):e200–e204. doi: 10.17269/cjph.104.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lofwall MR, Wunsch MJ, Nuzzo PA, Walsh SL. Efficacy of continuing medical education to reduce the risk of buprenorphine diversion. J Subst Abuse Treat. 2011;41(3):321–329. doi: 10.1016/j.jsat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Manchikanti L, Manchukonda R, Damron KS, Brandon D, McManus CD, Cash K. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician. 2006;9:57–60. [PubMed] [Google Scholar]
- 79.McCarty D, Rieckmann T, Green C, Gallon S, Knudsen J. Training rural practitioners to use buprenorphine: using the Change Book to facilitate technology transfer. J Subst Abuse Treat. 2004;26(3):203–208. doi: 10.1016/S0740-5472(03)00247-2. [DOI] [PubMed] [Google Scholar]
- 80.Pade PA, Cardon KE, Hoffman RM, Geppert CMA. Prescription opioid abuse, chronic pain, and primary care: a co-occurring disorders clinic in the chronic disease model. J Subst Abuse Treat. 2012;43(4):446–450. doi: 10.1016/j.jsat.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Paone D, Tuazon E, Kattan J, Nolan ML, O'Brien DB, Dowell D, Farley TA, Kunins HV. Decrease in rate of opioid analgesic overdose deaths—Staten Island, New York City, 2011–2013. MMWR Morb Mortal Wkly Rep. 2015;64(18):491–494. [PMC free article] [PubMed] [Google Scholar]
- 82.Piper TM, Rudenstine S, Stancliff S, Sherman S, Nandi V, Clear A, Galea S. Overdose prevention for injection drug users: lessons learned from naloxone training and distribution programs in New York City. Harm Reduct J. 2007;4:3. doi: 10.1186/1477-7517-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reifler LM, Droz D, Bailey JE, Schnoll SH, Fant R, Dart RC, Bucher Bartelson B. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13(3):434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 84.Ringwalt C, Garrettson M, Alexandridis A. The effects of North Carolina’s prescription drug monitoring program on the prescribing behaviors of the state’s providers. J Prim Prev. 2015;36(2):131–137. doi: 10.1007/s10935-014-0381-0. [DOI] [PubMed] [Google Scholar]
- 85.Saenger M, Duva IM, Abraham C, Porter R, D’Antignac V. Inter-professional “community of practice” to implement “universal precautions” for opioid safety within the patient centered medical home. J Gen Intern Med. 2013;28:S440–S441. [Google Scholar]
- 86.Sandoo VW, Mcaughtry P, Al-Hadad A, Mavani F, Zheng T, Conroy S. Stop start tool for improving prescribing amongst older inpatients: results from a three year audit cycle. Age Ageing. 2011;40:ii25. [Google Scholar]
- 87.Schlicher N. Reforming emergency care: experts put focus on value, better alignment. ED Manag. 2015;27:73–79. [PubMed] [Google Scholar]
- 88.Srivastava A, Kahan M, Jiwa A. Prescription opioid use and misuse: piloting an educational strategy for rural primary care physicians. Can Fam Physician. 2012;58:e210–e216. [PMC free article] [PubMed] [Google Scholar]
- 89.Stover H. Barriers to opioid substitution treatment access, entry and retention: a survey of opioid users, patients in treatment, and treating and non-treating physicians. Eur Addict Res. 2010;17(1):44–54. doi: 10.1159/000320576. [DOI] [PubMed] [Google Scholar]
- 90.Summers P, Martin C, Quidgley-Nevares A. The impact of an educational group session on opioid treatment agreement violations. J Pain. 2014;15(Suppl 2):S40. [Google Scholar]
- 91.Taylor SE, Braitberg G, Lugt J. Multifaceted education initiative minimizes pethidine prescribing in the emergency department. Emerg Med Australas. 2007;19(1):25–30. doi: 10.1111/emm.2007.19.issue-1. [DOI] [PubMed] [Google Scholar]
- 92.Thomas CP, Kim M, Kelleher SJ, Nikitin RV, Kreiner PW, McDonald A, Carrow GM. Early experience with electronic prescribing of controlled substances in a community setting. J Am Med Inform Assoc. 2013;20(e1):e44–e51. doi: 10.1136/amiajnl-2012-001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wheeler E, Davidson PJ, Stephen Jones T, Irwin KS. Community-based opioid overdose prevention programs providing naloxone—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:101–105. [PMC free article] [PubMed] [Google Scholar]
- 95.Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573–584. doi: 10.1111/j.1526-4637.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 96.Kunins HV. Abuse-deterrent opioid formulations: part of a public health strategy to reverse the opioid epidemic. JAMA Intern Med. 2015;175(6):987–988. doi: 10.1001/jamainternmed.2015.0939. [DOI] [PubMed] [Google Scholar]
- 97.Zahradnik A, Otto C, Crackau B, Löhrmann I, Bischof G, John U, Rumpf HJ. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104(1):109–117. doi: 10.1111/j.1360-0443.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- 98.Fischer B, Jones W, Murphy Y, Ialomiteanu A, Rehm J. Recent developments in prescription opioid-related dispensing and harm indicators in Ontario, Canada. Pain Physician. 2015;18:E659–E662. [PubMed] [Google Scholar]
- 99.Hawk KF, Vaca FE, D’Onofrio G. Reducing fatal opioid overdose: prevention, treatment and harm reduction strategies. Yale J Biol Med. 2015;88:235–245. [PMC free article] [PubMed] [Google Scholar]
- 100.Nadelmann E, LaSalle L. Two steps forward, one step back: current harm reduction policy and politics in the United States. Harm Reduct J. 2017;14(1):37. doi: 10.1186/s12954-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soelberg CD, Brown RE Jr, Du Vivier D, Meyer JE, Ramachandran BK. The U.S. opioid crisis: current federal and state legal issues. Anesth Analg. 2017;125(5):1675–1681. doi: 10.1213/ANE.0000000000002403. [DOI] [PubMed] [Google Scholar]
- 102.Potier C, Laprevote V, Dubois-Arber F, Cottencin O, Rolland B. Supervised injection services: what has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;145:48–68. doi: 10.1016/j.drugalcdep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 103.Kennedy MC, Karamouzian M, Kerr T. Public health and public order outcomes associated with supervised drug consumption facilities: a systematic review. Curr HIV/AIDS Rep. 2017;14(5):161–183. doi: 10.1007/s11904-017-0363-y. [DOI] [PubMed] [Google Scholar]
- 104.Finley EP, Garcia A, Rosen K, McGeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17(1):420. doi: 10.1186/s12913-017-2354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


