Graphical abstract
Abbreviations: ACE2, Angiotensin-converting enzyme receptor 2; GISAID, Global Initiative on Sharing All Influenza Data; CDC, Centre for Disease Control and Prevention; VOI, Variant of Interest; VOC, Variant of Concern; VOHC, Variant of High Consequence; CMV, Cytomegalovirus; NIAID, National Institute of Allergy and Infectious Diseases; COVE, Coronavirus Efficacy; PHE, Public Health Emergency; EUAL, Emergency Use Assessment and Listing; RCT, Randomised clinical trials; SAE, Serious adverse events; CNBG, China National Biotec Group; BIBP, Beijing Institute of Biological Products; WIBP, Wuhan Institute of Biological Products; ADDE, antibody dependent disease enhancement; NERVTAG, New and Emerging Respiratory Virus Threats Advisory Group; WGS, Whole-genome sequencing; SIADP, Sharing all influenza data platform; HPVV, Human papillomavirus vaccine; RVZV, Recombinant varicella-zoster vaccine; HBV, Hepatitis B virus vaccine; NTD, N-terminal domain; RBD, Receptor-binding domain
Keywords: Covid-19, Mutations, Variant strains, Vaccine efficacy, Clinical trials
Abstract
Background
The SARS-CoV-2 coronavirus epidemic is hastening the discovery of the most efficient vaccines. The development of cost-effective vaccines seems to be the only solution to terminate this pandemic. However, the vaccines’ effectiveness has been questioned due to recurrent mutations in the SARS-CoV-2 genome. Most of the mutations are associated with the spike protein, a vital target for several marketed vaccines. Many countries were highly affected by the 2nd wave of the SARS-CoV-2, like the UK, India, Brazil and France. Experts are also alarming the further COVID-19 wave with the emergence of Omicron, which is highly affecting the South African populations. This review encompasses the detailed description of all vaccine candidates and COVID-19 mutants that will add value to design further studies to combat the COVID-19 pandemic.
Methods
The information was generated using various search engines like google scholar, PubMed, clinicaltrial.gov.in, WHO database, ScienceDirect, and news portals by using keywords SARS-CoV-2 mutants, COVID-19 vaccines, efficacy of SARS-CoV-2 vaccines, COVID-19 waves.
Results
This review has highlighted the evolution of SARS-CoV-2 variants and the vaccine efficacy. Currently, various vaccine candidates are undergoing several phases of development. Their efficacy still needs to check for newly emerged variants. We have focused on the evolution, multiple mutants, waves of the SARS-CoV-2, and different marketed vaccines undergoing various clinical trials and the design of the trials to determine vaccine efficacy.
Conclusion
Various mutants of SARS-CoV-2 arrived, mainly concerned with the spike protein, a key component to design the vaccine candidates. Various vaccines are undergoing clinical trial and show impressive results, but their efficacy still needs to be checked in different SARS-CoV-2 mutants. We discussed all mutants of SARS-CoV-2 and the vaccine’s efficacy against them. The safety concern of these vaccines is also discussed. It is important to understand how coronavirus gets mutated to design better new vaccines, providing long-term protection and neutralizing broad mutant variants. A proper study approach also needs to be considered while designing the vaccine efficacy trials, which further improved the study outcomes. Taking preventive measures to protect from the virus is also equally important, like vaccine development.
Introduction
The SARS-CoV-2 is the new name assigned to the novel coronavirus, previously called 2019-nCoV [1,3]. It emerged in late 2019 and caused a disease designated as COVID-19 (Corona Virus Disease 19). On 11 March 2020 is the date when WHO officially stated the SARS-CoV-2 pandemic [2]. Till now, it affected 269 million cases and 5.3 million deaths in 224 countries and territories. With the emergence of Omicron, the COVID-19 cases grew up on daily basis up to 0.6 million cases and thousands of deaths [4,5]. COVID-19 and its complications are most common in older adults, front-line workers, and people with certain coexisting conditions. Historically, the first SARS-CoV was discovered in China by the end of February 2003. At the moment, at least seven coronavirus types have been linked to human disease, including OC43, NL63, 229E, HKU1, SARS-CoV, MERS-CoV, furthermost newly discovered SARS-CoV-2 [6]. Based on their genomic structure and evolutionary connection, these seven kinds are divided into four categories: alphacoronavirus (NL63, 229E), beta coronavirus (HKU1, SARS-CoV, MERS-CoV), gamma coronavirus, and delta coronavirus. Coronaviruses (CoVs) are part of the Coronaviridae family of viruses and owns a massive single-stranded RNA genome of 26–32 kb [7]. RNA viruses have a significantly higher mutation rate (about million times) than their hosts, which is associated with changes in evolvability and virulence, both of which are regarded favorable for viral adaptability [8]. The chief reason for its global spread is their associated mutations that are linked to the spike protein, crucial for the attachment and virus entry in host cells via ACE2 [9]. According to GISAID, three main SARS-CoV-2 clades have been found so far, which are clade G (a variant of spike protein having S-D614G), clade V (NS3-G251, ORF3a coding protein), and clade S (ORF8–L84S variant) [10]. Mercatelli et al. looked at 48,635 SARS-CoV-2 highlights and matched them with Wuhan Reference Genome (NC 045512.2), finding 353,341 mutation occurrences [11]. Wang et al. recently recognized 13 variation sites in the SARS-CoV-2 genome, highly prone to the mutation. These include ORF1ab, S, ORF8, ORF3a, and N terminal region. They found the highest mutation rate in positions 8782 in ORF1a and 28,144 in ORF8 with 9.47% and 30.53%, respectively [12].
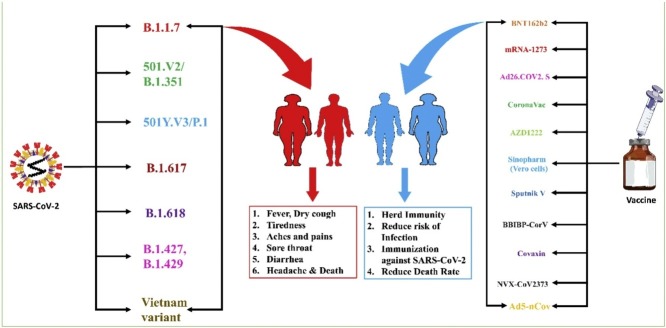
Emerging variants of SARS-CoV-2
As the COVID-19 broke out in China, the rise of various variants of the virus started appearing from different global locations like B.1.1.7 from UK and B.1.351 from South Africa [13]. In the South African variant, the transmission between individuals was high regarding population immunity, which might have facilitated the rise and spread [13]. These variants had mutations in the RBD of S-protein, responsible for a high transmission rate among individuals [14]. The transmission rate was 40–70% more than the original virus [15]. The South African variant had two more mutations in the S-protein that made the virus escape from neutralizing antibodies. In Manaus, Brazil, another set of mutations in the new strain P.1 lineage was identified [16]. Because the number of new SARS-CoV-2 mutations is constantly growing, the clinical effect is unknown. Many variants of SARS-CoV-2 are emerging, but will they have different clinical effects? Almost all viruses mutate; however, the SARS-CoV-2 virus mutation has made life uncomfortable throughout the globe, being its symptoms, deaths, economy, quality of life, etc. The NERVTAG from the UK, on 21 January 2021, published an article describing the results from many preliminary outcomes of the B.1.17 variant. This variant from the UK was detected in the south of England in September of 2020, and it was reported that the transmission rate is very high [17]. Because of the high transmission rate, it got transmitted to dozens of other countries and still spreading. The B.1.17 contains about seventeen mutations, of which eight are in spike protein. The main problem of these mutations is that the vaccines approved in the UK are based on spike protein only to pose effects on efficacy. It was also proposed that the B.1.1.7 has the possibility of an increased mortality rate compared to the non-mutated virus, as reported by NERVTAG [17].
The other variant of SARS-CoV-2 includes P.1 that has emerged from Brazil since mid of 2020. This variant was highly infectious, which made Brazil the third affected country in the world. The surge of infections was massive, struck Manaus city, and led to the Brazilian healthcare system [17]. The other variant of SARS-CoV-2 includes B.1.351, which was identified in South Africa in 2020. The South African variant has led slight reduction in the production of neutralizing antibodies [17].
The different variants of SARS-CoV-2 are emerging, and the infectivity rate is also increasing. The fact is that how the virus is mutating needs to be understood. The generation of different variants in the laboratory and then investigating those variants against the approved vaccines can provide answers regarding the efficacy of vaccines, infectivity rate, antibody titers, and various other prospects that can be thought of. However, an initiative was taken by a newly formed G2K-UK Virology Consortium, investigating how the different known variants will impact the global pandemic. It is collaborative research led by ten other institutes in the United Kingdom that will work parallelly with the COVID-19 genomics. The United Kingdom has produced 400 thousand genomic sequences of SARS-CoV-2 to investigate the effect of different variants on the vaccine efficacy, pathophysiology, severity of infection, and what else is possible. The main goals will be to investigate the mutation impact on the transmission rate between subjects, the efficacy of vaccines, and different treatment by using various cell-based and animal-based models in experimental investigations to decipher the effect of mutations on the marketed vaccines.
As the virus is continuously changing since it emerged in the Wuhan city of China [2] people desperately waiting for the vaccine, so it is essential to determine how it is changing. The potential of vaccine escape is crucial; however, Hibberd points out that the present variants have evolved concerning transmission rate and responsiveness towards the vaccines. The SARS-CoV-2 variants with or without mutations will be focused on consortium work and investigating the change of virus behaviour with each mutation. Our understanding of vaccinations will be dramatically influenced when epidemiological and experimental data on strains and mutations are obtained.
SARS-CoV-2 evolution
There is hardly any part of the world that has not seen the SARS-CoV-2 pandemic; surprisingly, the development of vaccines was also parallelly in line with the rising cases. Presently several vaccines have been marketed, and countries worldwide are immunizing their people rapidly, keeping in mind that it is the only solution for the COVID-19 pandemic neutralization. The vaccines that have been approved are mostly spiked protein-based or mRNA developed by Pfizer-BioNTech vaccines and Moderna. The other vaccines are adenovirus vector-based, developed by Oxford Astra Zeneca, Cansino and Johnson and Johnson, etc. The WGS has provided around 36,000 SARS-CoV-2 sequences on the global initiative on SAIDP for rapid vaccine development [19]. The sequence provided a directional path for the scientists to track the spread of different lineages throughout the globe [20,21].
Several research studies have claimed an association between SARS-CoV-2 genomic changes and the subject’s immune reactivity. In this scenario, we discuss that the changes that occurred had any impact on the vaccine efficacy? The previously developed vaccines like mumps, rubella, measles, rotavirus, sabin oral poliovirus, influenza, hepatitis A, rabies, and yellow fever present the whole virus either as inactivated or live-attenuated, which results in the polyclonal B-cell response against many epitopes. This may be the reason that the diversity of humoral and cell-mediated immunity leads to the explanation of why no vaccine escape strains have been demonstrated for these viruses [22]. In the case of the influenza virus, which is an exception, the mutation in the haemagglutinin and neuraminidase antigens is causing structural alterations in the epitopes that are not recognized by the immune system. So, the vaccine might be failing to prevent new mutant strains [22]. Reports suggests that the human convalescent plasma like in human coronavirus 229E is matching with influenza, and with time the human coronavirus 229E renders the subjects ineffective to neutralize the novel mutant strains [23,24].
The influenza virus data could tell us that evolution of the corona virus might lead to the compromission of vaccine efficacy. Immunocompromised patients are highly susceptible to SARS-CoV-2 infection, result in the generation of SARS-CoV-2 mutants [25]. It has been reported that the length of antigen that is used by presently marketed SARS-CoV-2 vaccines is short, comprising of around 1270 AAs. The antibody response is restricted to RBD and NTD only [61]. Due to drift in the antigens might cause mutations because of many possible reasons like naturally occurring infection, natural selection, or the vaccine itself. The mutations in the sequence of an antigen will lead to a loss of antibody neutralization. So, the monoclonal antibodies against a particular vaccine sequence led to a decrease in the efficacy. These events have been demonstrated by previous vaccines like RSV, polio, measles, and present one SARS-CoV-2 [26,27].
The other report is that the polyclonal antibodies can also mutate, and as a result, they are unable to neutralize the target. These findings have been reported in SARS-CoV-2 subjects. T virus was allowed to grow in the convalescent plasma of the recovered subjects [28]. The mutation in the RBD and NTD after serial passages were generated, which allowed the virus to escape from antibody neutralization. The convalescent plasma from twenty subjects could not neutralize the SARS-CoV-2 when the virus grew in the plasma. The AAA was selected to be mutated Glu484Lys in the RBD as was found in the SARS-CoV-2 variant-B.1.351, spreading fast in South Africa [29,30]. It was also reported that the SARS-CoV-2 variants like 501Y.V2 with substitutions in Glu484Lys, Lys417Asn, and Asn501Tyrin the RBD and, Asp215Gly, Asp80Ala, Leu18Phe, 242–244del and Arg246Ile in the NTD are less prone to antibody neutralization by the Plasma sera from the patients which were exposed to previous different variants [31]. One investigation has claimed a complete loss of neutralizing efficacy in the high number of samples [32].
The investigation on the variants of SARS-CoV-2 has suggested that the coronavirus can evolve with high resistance to immune system neutralization. There is a lot of variability among different individuals as a response to S-protein [28]. There was a one to a three-fold reduction in the antibody neutralizing titers in the sera of vaccinated subjects against Asn501Tyr, Lys417Asn, and Glu484Lys in contrast to the extensive decrease glimpse in the selection study, and no subject demonstrated loss of antibody neutralizing efficacy [28]. So, in the vaccinated subjects, it has been [34] common concern regarding virus neutralization. Thus, a thorough study is in demand to investigate the efficacy of antibody neutralization in the licensed vaccines against SARS-CoV-2 variants.
Mutation associated with SARS-CoV-2 and new wave of SARS infectivity
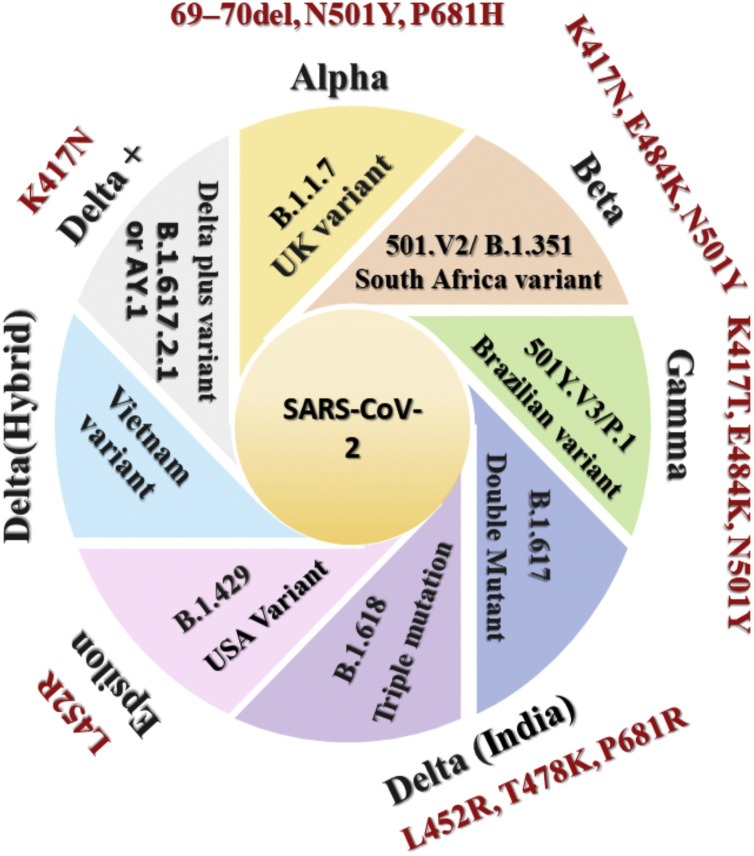
Scientists around the world are tracing variations in the SARS-CoV-2. These assist researchers in exploring the mechanism behind why specific COVID variants are spreading faster than the other variants, how they affect people’s health, and how different efficacious vaccines are in combating these strains. Thousands of variants have been found after disclosure of the SARS-CoV-2 genomic sequences [35]. In most cases, these variants result from mutations originating from random changes in SARS-CoV-2 genomic copies within the infected person. Now, numerous variants of SARS-COV-2 are circulating globally [35]. The CDC classified these into three categories that need to be monitored, namely Variant of Interest (VOI), Variant of Concern (VOC), and Variant of High Consequence (VOHC) [35]. Various reported variants are demonstrated in Fig. 1 .
Fig. 1.
Demonstrates all the variants of the SARS-CoV-2 (Black) with the associated key mutations (Brown).
Studies conducted to determine the variation in the SARS-CoV-2 genomic sequence identified several regions in the genome responsible for the increased variation [36]. Most of the spike protein-associated mutation includes E484K, P681H, 144 Del, N501Y, H69-V70 Del, D614G, T19R, K77T, G142D, E154K, N440K, L452R, T478K, E484Q, P681R, D950N, Q1071H, H1101D, H69–V70 Del, etc. results in conformationally changed spike protein. These mutations in spike protein raises the alarm concerning its disease-causing ability, transmissibility, and impact on vaccine efficacy by variants. Due to the emergence of the new South Africa and UK versions, an unexpected increase in cases of COVID-19 was recorded at the end of 2020. Both versions a common mutation (N501Y) associated with the RBD of spike protein, which increased its transmission in the range of 40%–70% [37,38].
Alpha/UK variant
The first variant of SAR-CoV-2, also named B.1.1.7, was reported to be found in southeast England in September 2020. Several nations, including the United States, have reported with B.1.1.7 lineage since 20 December 2020. At this time, its leading VOC accounting for most of the COVID-19 cases of Europe (92%) and the USS (59%) as of April 2021. In this variant, the Asparagine (N) at position 501 is replaced with the amino acid Tyrosine. Studies suggest this variant is more infection rate of having than the previous one with an increment in the death rate of 60% [39,40]. This variant contains approx. Seventeen mutations, most of them are associated with spike protein, including E484K, P681H, 144 Del, N501Y, H69–V70 Del, D614G, etc. Among them, N501Y was found to help the virus tightly bound with the ACE2 receptor [41,42]. E484K is the escape mutation that helps to evade the immune system (Escape Antibodies); deletion of Y144 reduces the antibody’s binding affinity. When compared to other variants, no differences were found in reported symptoms or duration of disease in individuals infected via B.1.1.7, registered in the study published in The Lancet [43]. Another cohort study on 341 patients, among them 198 and 143 are positive and negative for the B.1.1.7 infection, respectively, also suggested no association among severity of disease and death and lineage [44]. Sub-lineages of these variants have also been detected, containing additional mutation M:V70L and D178H, which first appeared in February 2021 up to April 2021 swiftly rose to account for 1.8% and 36.8% in the United States and Washington, respectively. Sub-lineage B.1.1.7-M:V70L-S: D178H is dominantly found in the US as of 8 May 2021. The majority of instances were discovered in Washington, California, and Ohio [45]. Various studies are ongoing to find out the actual transmission and the vaccine efficacy against this variant.
Beta/South Africa variant (SAA) 501.V2/B.1.351
This variant at first discovered in South Africa at the end of October 2020. Spike protein of this variant features numerous mutations, comprising K417N, E484K, and N501Y. Contrary to B.1.1.7 lineage found in the United Kingdom, B.1.351 lacks deletion at 69/70. Mutations like K417N and E484K are accountable for immunological escape in the 501Y.V2 variant [46].
Gamma/Brazilian variant 501Y.V3/P.1
BVV, also known as B.1.1.28. This clade has become the most disseminating lineage in Brazil and started in February 2020. Variant contains N501Y, E484K, and K417T mutations in a new P.1 lineage (501Y.V3), found out in Brazil [47]. This is the earliest variant with the S protein mutated at E484K and discovered in October 2020 from the patients in Rio de Janeiro. However, phylogenetic analysis indicates that the variant most probable arose in July 2020 [48]. Reduction in the neutralizing capacity of convalescent and mRNA vaccine-provoked blood serum results from these alterations [49]. Since then, the 484 K.V2 variety has expanded to the UK, US, Singapore, Norway, Denmark, Argentina, and many more. The E484K mutation, getting so much interest from the researcher because evidence suggested that it could allow immunological escape [50]. As of March 2021, preliminary studies indicated about 10× more viral load and transmissibility rate 1.4×–2.2× higher with P.1 infection than other COVID-19-infected people. Younger humans are more prone to infection without gender difference. Lethality increases by 10–80% and can evade 25–61% of previous Corona immunity. Several vaccines were found to be much less efficient than others [51,52]. Two subvariants of P.1, 28-AM-1 and 28-AM-2 having K417T, N501Y, E484K mutations and thought to be autonomously originated in the same Brazilian Amazonas region (Toovey et al., 2021).
Delta/India/double mutant (B.1.617) variant
B.1.617 is the official designation for the double mutant, which contains 13 mutations, seven of which are in the spike protein. In this strain first time, both the L452R and E484Q mutation appeared together. The E484Q mutation is noteworthy because it close to the E484K mutations previously reported in the SVV and BVV B.1.351 and P.1 [53].
Delta/India/triple mutation (B.1.618)
Triple mutant simply means a variant of the COVID which formed when three different mutants combined [54]. The variant was first found in Maharashtra (October) then subsequently in some other state of India. It evolved from the double mutant COVID. The deletion of H146 and Y145 distinguishes the spike protein and mutations E484K and D618G [55]. WHO classified this strain/variant as “VOC” and “VOI” according to the CDC [35,56].
AL.20C-B.1.427 and B.1.429
In recent months, the US reported several variants. Among these variants, one is registered in California found with the L452R mutation, deemed a variant of concern. There are two versions of the CAL.20C variant: B.1.427 and B.1.429. These variants thought to produce a more solid adhesion, preventing neutralizing antibodies from interfering with the process. Shreds of evidence are required to determine the severity and transmission of this variant [41]. L452R mutation-carrying California variety could be up to 20% more transmissible than wild-type strains [57].
Vietnam variant
On 29 May 2021, a new COVID-19 variant is discovered in Vietnam. This variant is the hybrid of variants found in India and the UK and seems to be spread through the air very quickly. The genome data of these variants is not published yet, but it will be posted very soon and by the Health Minister of Vietnam [58].
Omicron (B.1.1.529)
On November 09, 2021, the first case of B.1.1.529 was reported in Botswana (South Africa), subsequently to WHO on November 24, 2021, which was further declared as a VOC on November 26, 2021 [59]. The emergence of this variant again put the scientific community in big trouble. It is still a question whether this new variant has high infectivity or transmissibility than previous variants. Omicron sequence revealed that it is associated with S-gene target failure (can be used as a marker) and has 69−70del which seems similar to the alpha variant [44]. It also has more than 30 mutations, including T95I, K417N, N501Y, T478K, N679K, G142D/143−145del, and P681H. These mutations are already reported in previous VOC (alpha, beta, gamma, and delta) [60]. Past genome sequencing suggested that these deletions and mutations are associated with increased viral transmissibility, higher antibody escape, and viral binding affinity [61,62] but still required confirmation for Omicron. Genomic analysis also revealed that omicron spike’s protein comprises 26 amino acids, which makes it quite different from past variants. Another quite different insertional mutation (ins214EPE) was also reported in Omicron [63]. There is a need to explore the omicron transmissibility compared to delta variants. With continuous growing cases in South Africa (SA), this variant might succeed the delta variant in SA. Currently available data from SA patients suggest that most people affected with Omicron are younger and represent the same symptoms as past variants [64]. Current mutations’ explorations indicate that Omicron might be spread rapidly and might be escape antibodies at far more rate than previous SARS-CoV-2 variants [59,65]. Genovese performed mutational analysis of Omicron and suggested that (1) Omicron have more ACE binding than delta variant, results in increased infectivity (2) way of the interaction of Omicron with ACE is also different from the existing one, the reason behind the different type interaction is the presence of AK mutation in the RBD, which further enhance its binding with ACE and antibody escape [66,67]. As Omicron share so many common mutations with past variants so, it is expected that available vaccine may be helpful for the prevention of further burden of SARS-CoV-2 cases, but still studies required to confirm this assumption.
SARS-CoV-2 vaccines efficacy (VE)
VE is defined as the disease risk in the participants who received the vaccine relative to the risk of disease for unvaccinated individuals in a controlled study. The 0% efficacy indicates that the vaccine is ineffective. If vaccine efficacy is 50%, it means half of the peoples having the risk of infection. Because the studies were conducted with diverse populations, locations, and viral types, comparing the efficacy of the various vaccinations is difficult [68].
In this current global pandemic of COVID, safe and effective vaccines are the most cost-effective solution to reduce the further global burden of COVID-19 [69]. According to the center of vaccine at the London School of Hygiene & Tropical Medicine [70,71] as of 13 May 2021 total of 317 vaccines candidates are in a different phase of development in 94 clinical trial centres comprising 29 in Phase I, 28 in Phase I–II, 8 in Phase II and 22 in Phase III of the development. More than 1.35 billion shots of the vaccine were administered, which is the world’s most extensive vaccination campaign ever in history. 22.4 million doses are administered daily basis [72] yet there is still some hesitation in the population about the vaccine efficacy. According to the WHO vaccine, “hesitancy — refers to a person’s hesitation or inability to get vaccinated even though vaccines are available” is one of the significant health threat globally [73]. Due to this hesitation, the prevalence of COVID-19 is continuously increasing. The herd immunity threshold for SARS-CoV-2 lies in between 60%–83% [74]. One way to reduce this hesitation is to educate the general population by communicating with the scientific community to acquire the people’s trust, ensure increased vaccine uptake, and ultimately boost herd immunity.
One reason people hesitate about the vaccine’s efficacy is rapidity in the development, production, and approvals. When compared to the conventional vaccine approval process, the approval is five times faster in the case of the COVID-19 vaccines. On the other hand, the use of novel technologies in vaccine development, such as the DNA and RNA vaccine, which will be transformed into vaccine antigen protein in the host body, instead of the traditional vaccine, where the protein is purified and then directly delivered, will help to accelerate the development of these vaccines. Although mRNA vaccines are new but not unknown, researchers also studied Zika, rabies, flu, and CMV [75]. After discovering the SARS-CoV-2 genetic sequence in January 2020, multiple mRNA vaccines for COVID were developed to target spike protein [76]. The development of the mRNA-based vaccine to prevent COVID-19 infection was a success story with no significant health consequences. Only minor side effects like; redness, pain, and swelling have been reported with these vaccine candidates. Apart from this, a systemic symptom of fatigue, fever, headache, and myalgia and arthralgia also observed in the window period of the first 24–48 h of vaccination [77]. Several vaccine candidates are being developed against SARS-CoV-2 they have begun or will begin in large-scale, RCT and placebo-controlled clinical trials soon. There are various vaccine candidates in Phase 3 on a variety of vaccine platform:
The whole SARS-CoV-2 virus as a vaccine and how is its efficacy?
The SARS-CoV2 Pandemic is one of the deadliest viruses that the universe has ever seen, impacting every domain of society. The other uniqueness of this virus is that about 155 lead vaccine candidates were developed globally, and many were marketed within nine months, which is not less than a miracle. Several research groups have published results from phase one and two clinical trial of vaccine candidates generated from vaccine platforms [78]. As this pandemic virus is novel to the human race, it is tough to predict the possible adverse effects, whether short-term or long-term, because it is critical to add clinical outcome patient data to toxicity and immunogenicity of vaccines SARS-CoV-2 [78]. An investigation led by Xia et al. provided evidence for the safety and immunogenicity of the whole virion of SARS-CoV-2 inactivated by a β-propiolactone emulsified with Al(OH)3, which was developed by CNBG and BIBP [79]. This vaccine candidate was evaluated in randomized, double-blind, placebo-controlled phase 1/2 trials in healthy subjects around 18 or older. This investigation was one of the first examined research on whole inactivated SARS-CoV-2 virus vaccine candidates on older people with more than sixty, which were highly susceptible to infection. This vaccine was given at a two-dose strategy at three concentrations of 2 μg, 4 μg, and 8 μg/dose. It was observed that both the younger and older aged subjects tolerated well, and surprisingly the younger had more solicited adverse effects than older ones. After vaccination, the overall rates of these adverse effects were observed in 47% of subjects in 72 number of 18–59 and 19% in more than 60 years of age within 28 days [78]. The vaccine candidate was showed similar immunogenicity in both aged groups. The geometric mean of antibody titers against SARS-CoV-2 was measured by 50 neutralization assay fourteen days after the second dose were 81,211 and 229 in 18–59 aged group and 81,132 and 171 in >60-year-old group at the doses of 2 μg, 4 μg, and 8 μg concentration. The researchers also investigated the cross-reactivity of the neutralizing antibodies against virus many isolates, and it was demonstrated that the vaccine showed protection and appear in the blood. In phase-II of the vaccine in the 18–59 aged group, the effect of decreasing the time gap between two doses from 28 days to 14/21 days on the immunogenicity potential was investigated. It was reported that 4 μg had high immunogenic potential at a 21-day time gap with an antibody titter of 283. Still, when the time was reduced to 14 days, there was a significant decrease in immunogenicity with a neutralizing antibody titer of 170 [80]. So, these results suggested that the immunogenicity will decrease when the time gap of fewer than 3 weeks between two doses should be less than 3 weeks.
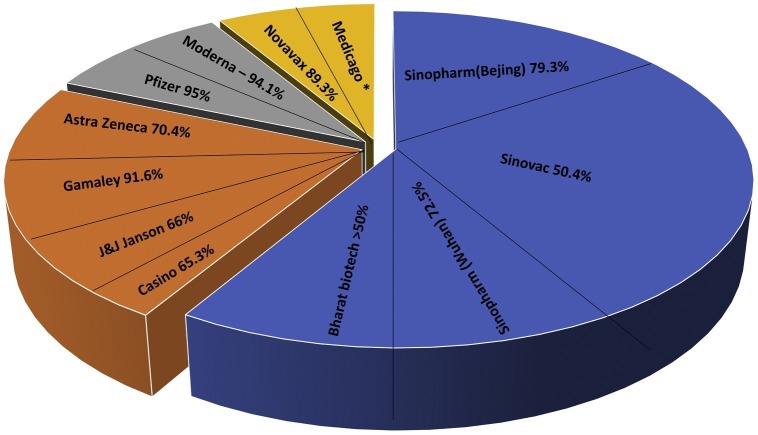
The two vaccines developed by WIBP and the other by BIBP demonstrated similar adverse effects and the antibody titers after vaccination, indicating the reproducibility of patient/clinical trials of the same vaccination methods produced by different manufacturers [79]. However, the primary concern of using the whole virus as a vaccine ensures almost all immunogenic epitopes, which is crucial for considering the safety and efficacy of vaccines. The improper inactivation processes of vaccines can change the properties of the epitopes, as previous inactivated vaccines have demonstrated, which led to the production of non-neutralizing antibodies, causing disease induction rather than prevention [78]. So, the proper inactivation of vaccines can often result in a better prognosis, like keeping this strategy in mind. The CNBG-BBNG developed vaccine showed no disease enhancement in pre-clinical animal models with better protection [81]. The important point is that all pre-clinical animal models have not been worked out for stimulating the ADDE in human subjects. The long-term vigilance of qualitative and quantitative characteristics of the antibodies against SARS-CoV-2 post-vaccine administration is crucial to determine for claiming protection. The generation of memory B-cells and T-cells is critical to producing long-lasting protection against this pandemic. The virus-specific T-cells like CD4 will help the B-cells produce the optimal antibody response. The cytotoxic T-cells are also crucial to wipe out the virus from the body when the anybody generation is undergoing [82]. If the vaccines successfully generate memory B-cells and T-cells and high affinity neutralizing antibodies, it will be a wonder. It will give considerable relief to people all over the world. Approved vaccine efficacies are listed and demonstrated in Table 1 and Fig. 2 .
Table 1.
Vaccines authorized for emergency use or approved for full use.
| Sr. no | Vaccine name | Manufacturer | Types of vaccine | Country of origin | Dose | Storage (°C) | Efficacy | References |
|---|---|---|---|---|---|---|---|---|
| 1. | BNT162b2 (Community) | Pfizer-BioNTech | mRNA | Germany, United States | 2 | −80 to −60 | 95% | [117] |
| 2. | mRNA-1273 | Moderna | mRNA | United States | 2 | −25 to −15 | 94.5% | [118] |
| 3. | Ad26.COV2. S | Janssen (Johnson & Johnson) | Viral vector | United States, Netherlands | 1 | 2–8 | 66.3% | [75] |
| 4. | AZD1222 (Vaxzevria) | Oxford-AstraZeneca | Viral vector | United Kingdom, Sweden | 2 | 2–8 | 81.3% | [93] |
| (Covishield) | ||||||||
| 5. | Ad5-nCov | CanSino | Viral vector | China | 1 | 2-8 | 65.28% | [120] |
| 6. | Sputnik V | Gamaleya | Viral vector | Russia | 2 | −18.5 (2 year) | 91.6% | [102] |
| 2–8 (6 month) | ||||||||
| 7. | Covaxin | Bharat Biotech | Inactivated | India | 2 | 2–8 | 80.6% | [109] |
| 8. | BBIBP-CorV | Sinopharm (Beijing) | Inactivated | China | 2 | 2–8 | 79.34% | [89,121] |
| 9. | NVX-CoV2373 | Novavax | Protein subunit | United States | 2 | 2–8 (6 month) | 96.4% | [116] |
| −20 (2 year) | ||||||||
| 10. | Inactivated (Vero Cell) | Sinopharm (Wuhan) | Inactivated | China | 2 | 2–8 | 72.51% | [69] |
| 11. | CoronaVac | Sinovac | Inactivated | China | 2 | 2–8 | 50.38% | [109] |
| 12. | CoviVac (KoviVac) | Chumakov Center | Inactivated | Russia | 2 | 2–8 | Unknown | [123] |
| 13. | QazCovid-in (QazVac) | Kazakhstan RIBSP | Inactivated | Kazakhstan | 2 | 2–8 | Unknown | [124] |
| 14. | RBD-dimer | Anhui Zhifei Longcom | Protein subunit | China | 3 | 2–8 | Unknown | [125] |
| 15. | EpiVacCorona | FBRI | Protein subunit | Russia | 2 | 2–8 | Unknown | [126] |
Fig. 2.
Demonstrates the efficacy of approved SARS-CoV-2 vaccines.
Pfizer-BioNTech mRNA vaccine (BNT162b2)
BioNTech vaccine is a Lipid Nanoparticle Particle (LNP) formulated vaccine designed by modifying the SARS-CoV-2 spike protein mRNA so that its expression is increased with a mutation in two Proline residues helps in locking into pre-fused conformation [83]. The MHRA (United Kingdom) was the first regulatory body to give EMU for Pfizer-BioNTech mRNA vaccine (BNT162b2) in the vaccination program of the UK for healthcare staff and the elderly. Meanwhile, anaphylaxis was observed in two elderly ladies (40 and 49 years old) who had known food and drug allergies and were carrying epinephrine auto-injectors [90]. Phase I investigations of BNT162b2 revealed 100% anti-spike seropositivity by day 21, followed by the booster dose at 28 to increase to titer more than convalescent participants [84]. The increase of spike-specific CD8+ and Th1 subtype CD4+ T-cell responses was also observed in a follow-up paper, with a large portion generating interferon-γ [85]. Total 43,448 participated in the safety study of Pfizer-BioNTech mRNA vaccine conducted in the US and Germany; among them, 21,720 received BNT162b2, and others received placebo. BNT162b2 group reported the pain at the injection site (mild-moderate) just after the seven days, and 1% of participants reported severe pain, more frequent in the peoples below the age of 55. Approx. 16%, 11% younger and older recipients reported fever respectively post-second dose. Some BNT162b2 recipients have experienced 4 major adverse events associated with the vaccine: shoulder injury, adenopathy, PSVT, and paraesthesia in the right leg. BNT162b2 (dose 30 g, given 21 days gap) was proven safe with 95% efficacious against COVID-19 in a two-dose regimen [86]. According to one study, after the first dose of the BNT162b2 vaccine, infection rates reduced 58%, 69%, and 72% after 12–20, 21–44, and 45–59 days respectively when compared to controls (unvaccinated) [87].
Moderna (mRNA-1273 vaccine)
Safety and efficacy were recently demonstrated for Moderna vaccine candidate mRNA-1273 at the NIAID. Phase I study of mRNA 1673 revealed the 100% seropositivity for anti-spike independent of dose which retained above to convalescent controls up to 90 days after the second shot of immunization, meanwhile there were increase the S-specific Th1 subtype CD4+ T-cell but low level of CD8+ were observed (Widge et al., 2021). Coronavirus Efficacy (COVE) named to the phase 3 trial conducted to access the mRNA-1273 safety and efficacy and result showed that vaccine is having 94.1% efficacious in the prevention of the Infection of SARS-CoV-2 with mild to moderate local reactions and some moderate-severe reaction like fatigue, arthralgia (joint pain), myalgia (muscle pain) and headache reported by the 50% of the participants after the second dose and all these are self-resolved [88,89]. CDC has also reported that anaphylaxis is reported within 15 min after injection in 2.5 cases/million administered doses [90].
Oxford–AstraZeneca COVID-19 vaccine (Covishield)
The AstraZeneca–Oxford University and SII develop Covishield. A viral vector-based vaccine in which adenovirus ChAdOx1, a modified vector from chimpanzee, is used. In phase I/II studies, the vaccine proved to be safe with an increase in antibody rate after the first shot additional increases on the second shot. Anti-IgA and IgG antibodies against SARS-CoV-2 spike protein were readily found in sera from vaccinated participants following immunization, indicating that B-cells were activated and increased in large numbers [91]. Flow cytometry confirmed the predominant secretion of Th1 cytokines (IL-2, IFN-γ, and TNF-α) by CD4+ T-cells instead of Th2 (IL-5 and IL-13) [92]. On the first dose, the efficacy of this vaccine is reported 76.0% after 22 days and 81.3% after the second shot of vaccine) [93]. It shows 75% efficacy against UK variant, and for other lineages, efficacy is 84% [93]. This vaccine has a good safety profile except for some mild side effects, including pain at the injection site, headache which are self-resolved after some time. MHRA also reported some cases of anaphylaxis [94]. European Medicines Agency recently reported a rare, very serious side effect of a blood clot associated with the low platelet counts approx. 1 case in 1 lakh vaccinated people [95].
Sinopharm COVID-19 vaccine
The first vaccine to approved in China for widespread use, and Sinopharm developed it. It is inactivated virus vaccine also known by the name of Sinopharm BBIBP-CorV. In phase I/II randomized clinical trials, the vaccine was safe and well-tolerated when tested on two different age groups with two doses (4 μg) on 0 and 21 (28) days. On day 42, all vaccination recipients developed antibody responses to SARS-CoV-2. The study concluded that the neutralizing antibody titer was stronger with one dose of 8 μg vaccinated participants and two doses of 4 μg at day 0 and 14 compared to the participants who received two doses on day 0 and 21 (28) [96]. The aluminium used with this vaccine is found to elicit the Th2 response [83]. A total of 60,000 people worldwide took part in the vaccine’s phase III clinical studies [98]. According to the data provided by the WHO regarding phase III, the vaccine is 78.1% effective against the COVID-19 (symptomatic) [99] before that UAE’s MOHAP announced its efficacy 86% [100]. The WHO authorized EUA for use in COVAX on 7 May 2021 [101].
Gamaleya Sputnik V (Gam-COVID-Vac)
Sputnik V is a human adenovirus vector-based vaccine in which rAd26 and rAd5 are used. The “V” in the name stands for the vaccine. In Phase II trials, all participants produced anti-spike protein antibodies. Moreover, after 42 days, all participants showed neutralizing Ab response which was earlier found only in 61% of participants in the phase I study. The Ab titer was found 1.5× higher than the convalescent patients. After 28 days of Phase II trials in all participants, the Th-cells (CD4+) and killer T-cells (CD8+) response increased up to 2.5% and 1.3% of Th and Killer T-cells, respectively, with the frozen formulation.
The phase 3 trial of Sputnik V exhibited 91.6% effectiveness against COVID-19 and well-tolerated when studied in the broad cohort [81]. Further, with freeze-dried formulation, it was 1.3% and 1.1% [102]. Discomfort at the injection, headache), asthenia and myalgia, and arthralgia were the most prevalent side effects in Phase 1/2 trials [102].
Covaxin (BBV152)
Covaxin is also a virus-based inactivated Vaccine against COVID-19, developed by the Bharat Biotech in collaboration with the ICMR. The technology used in the development of the Covaxin is similar to previously used in the development of the polio vaccine. BBV152 elicited substantial neutralizing antibody responses in the phase 1 study, which persisted up to 3 months following the second vaccination shot. In contrast to the phase 1 study, BBV152 displayed higher reactogenicity and safety and increased humoral and cell-mediated immune responses in the phase 2 trial. Phase 3 efficacy study published in Lancet reported that the vaccine shows 81% efficacy. Covaxin also shows promising results in neutralizing the new UK strain B.1.1.7 [104]. Recently ICMR reported that Covaxin is also effective against the triple mutant strain B.1.617 [105]. Studies also demonstrate that the vaccine has neutralizing effect approx. 3 and 2.7 fold for the new variants B.1.351 and B.1617.2 respectively [106,107]. After two doses of vaccination, the IgG and neutralizing efficacy of the vaccine is boosted against the B.1.1.28.2 and D614G compared to the natural infection. In vaccine recipients, there was a reduction of the neutralizing titer up to 1.92 fold for B.1.1.28.2 variant in comparison to the D614G variants [108].
Sinovac (CoronaVac vaccine)
CoronaVac is a potential inactivated COVID-19 vaccine developed by Sinovac Life Sciences, Beijing, China. It was made from Vero cells obtained from African green monkey kidney cells infected with SARS-CoV-2 (CN02 strain). The vaccine is safe without any SAE, and the seroconversion is more than 90% in phase I/II trials but lower titer and T-cell responses than convalescent patients [109]. It was 67% efficacious in preventing symptomatic SARS-CoV-2, 85% reduction in the hospitalization of the patients, and 80% reduced death [110]. Participants who got this vaccine had fewer fever reports than those who received the Pfizer-BioNTech, Oxford–AstraZeneca, or CanSino vaccinations [111].
Ad26.COV2.S/Janssen (Johnson & Johnson)
Jansen vaccine is the human adenovirus-based vaccine developed by Janssen, a subsidiary of Johnson & Johnson. It completed its Phase 3 of clinical trials with 43,000 peoples after a single shot immunization, Ad26-S. PPP generated robust neutralizing antibody responses and offered complete protection against SARS-CoV-2 challenge in five out of six macaques; despite one macaque having low virus levels in nasal swabs 29 days, the seroconversion rate was found 83%–100% in the dose and age-dependent manner. On the other hand, there were also cell-mediated responses. 80–83% of participants shows Th1-skewed CD4+ T-cell responses while CD8+ T-cell responses were found in 51–64% of individuals [112] 28 days completion of vaccination Janssen announced vaccine is 66% effective in one dose regimen in the prevention of the symptomatic COVID-19 [113]. According to research, the vaccination is highly successful, with an 85 percent success rate against severe COVID-19 and a 100% success rate against hospitalization or death [114].
Novavax COVID-19 vaccine
This vaccine is also assigned the name SARS-CoV-2 rS. It is a recombinant nanoparticle-associated adjuvant Matrix M1 vaccine developed by Novavax and CEPI. It is currently being tested in India under the brand name Covovax. When given with vaccine, it elicits higher neutralizing Ab titer than Covid-19 convalescent serum. All the participants received shows Th1-type CD4+ T-cell responses [115]. Novavax revealed on 12 March 2021, that their COVID-19 vaccine candidate was 96.4% and 86% effective against the original strain and Lineage B.1.1.7, respectively. In patients without HIV/AIDS, it was found to be 55% effective against the 501.V2 strain. In addition, it was 100 percent effective in preventing serious sickness [116].
Above mentioned vaccine and safe and productive against COVID-19 prevention. These all are approved vaccines either in one country or in multiple countries. They are also undergoing clinical trials in many countries. Vaccination is the only way that might be helpful to combat the further global pandemic. The variation in the SARS-CoV-2 after a short time interval is the cause for concern. Several additional escape variants could be seen in the future, leading to a catastrophic epidemic reflection, as already observed in South Africa. Increment in viral transmission gives more opportunity for SARS-CoV-2 mutations to arise. As a result, the only way to halt the pandemic is for safe and effective vaccines against circulating variations to be disseminated evenly worldwide. The current scenario is like all high-income countries rush to immunize their populations as soon as possible. They expose themselves to the possibility of SARS-CoV-2 emerging with a new mutation that vaccines might be incapable of defending against. To combat new SARS-CoV-2 variations, it may be necessary to develop new vaccinations regularly. There is a need for higher vaccination coverage to attain herd immunity [38].
Wave of SARS-CoV-2
COVID-19 waves simply understand by assuming the waves of the sea. Where, the number of infections rises and subsequently falls — each cycle depicts one “wave” of coronavirus. The first instance of COVID-19 in China was recorded one and half years ago. Within a week, it had spread throughout the entire world. The impact of the pandemic on the economies of diverse societies was significant, resulting in a sharp drop in the production of numerous items [127]. Some segments of society believed the epidemic was gone, dismissing the early announcement of the incoming second wave, due to low infection numbers during the summer months. Meanwhile, new virus variations emerged, increasing the risk of infection and, as a result, an increased number of patients admitted to ICUs [128]. As a result, unemployment rates have risen, as have other societal issues. Mortality rate during first and second waves are listed in Table 2 .
Table 2.
Mortality rate due to SARS-CoV-2 waves.
| Mortality rate | ||
|---|---|---|
| Age group | 1st wave | 2nd wave |
| <10 | 0.27% | 0.34% |
| 10–20 | 0.53% | 0.31% |
| 20–30 | 2.08% | 1.72% |
| 30–40 | 5.27% | 5.39% |
| 40–50 | 11.98% | 10.82% |
| 50–60 | 23.29% | 21.23% |
| 60–70 | 28.76% | 28.21% |
| 70–80 | 19.99% | 22.17% |
| 80+ | 7.82% | 9.81% |
First wave
The initial wave (first) wreaked havoc on practically every part of the globe, albeit because of seasonal changes; the southern hemisphere was hit later, but no less severely [129]. The second wave arrived, and it was far stronger than the first, as predicted by several experts. The first wave of the COVID-19 affected multiple nations, but the story did not end here; the second wave might be even more deadly [129].
Second wave
Medical definition of the second wave “An infectious phenomenon that can emerge during a pandemic. The disease first infects a specific set of people. Infections appear to be on the decline. Then, in a separate portion of the population, infections rise, resulting in a second wave of infections”. A Lancet article published in April 2020 warned of the likelihood of a second wave COVID-19 pandemic [129]. In addition, according to a study published in Nature, “a second wave pandemic presents an impending threat to society, with an enormous toll in terms of human deaths and a terrible economic impact” [130]. The second wave of COVID-19 is affected India very severely and other countries as well, and shows more potential threat of COVID-19 than the first wave. With 8944 instances as of 17 May 2021, India has the highest number of serious or critical COVID-19 cases globally [131]. Subsequently then the slope of the cases again downfall. Experts believed that the story is not the end here and gives indication about third wave that might be more dangerous than the previous waves as the virus emerged with the new mutation. Table 2 compare the mortality rate in first and second wave among different age groups.
Third-wave
The daily tally of Coronavirus cases in the countries appears to decline since the first week of May. The daily count of COVID-19 cases has dropped to 2.6 lakh, down from a peak of 4.14 lakh. The number of active cases has also continued to reduce, with the total number reducing from 37.45 lakh to around 32.25 lakh. According to the Indian Express piece, if the downward trend continues, cases could reach the February 2020 level by the end of July. Following a jump in the cases numbers and deaths during the second wave, many experts expect that the third COVID-19 wave more deadly.
Fourth wave
On December 03, 2021, South Africa’s health minister Joe Phaahla declared that SA is facing the 4th wave of COVID-19. The tally of daily cases in SA increased by 13 times (1184% in 10 days) between November 24 to December 04 [132]. Germany also reported 50,000 cases daily, which is the highest number since the initiation of the COVID-19 pandemic. The report suggests that the huge number of unvaccinated people in Germany is the reason behind this 4th wave surge of COVID-19 in Germany [133]. Additionally, Russia also faces the 4th surge of COVID-19 with 40,000 daily cases. Further, it is expected that the 4th wave will affect all continents with a huge number of cases. There is a need to increase the vaccination rate that might be a reason to combat this ongoing Pandemic. With vaccination, there is a critical need to adhere to the other precautions like limiting unnecessary movement, wearing the mask, using sanitizers, etc.
Study design of SARS-CoV-2 vaccine efficacy trials
The vaccine candidates go through several developmental phases. At the same time, the discrepancies between the trials at different developmental phases may seem blurry. Initially, trials started with a small population around 30–100 healthy volunteers (Phase 1) concerning assess safety and immunogenicity (it comprises studying several doses and vaccination schedules, mainly focus on safety and its tolerability, as well as planned to deliver preliminary assessments of immunogenicity), then further carried out to a higher number and targeted population (Phase 2) to acquire data of safety and immunogenicity and finally measuring the protection from the disease by the vaccine (Phase 3). The demonstration of vaccine effectiveness (VE), usually done through a Phase 3 study, is essential for licensure and informing policymakers about vaccine use. VE is the ability of the vaccine to reduce the incidence of the infection on vaccination concerning controls [68]. Adequate safety and efficacy data in context vaccine is the key element considered when considering vaccine licensure [134].
The West Africa Ebola outbreak from 2013 to 2016 broke new boundaries in terms of pace and study design, setting a model for vaccine evaluation during Public Health Emergency (PHE). Based on the lesson acquired during that outbreak, WHO’s Blueprint Plan of Action intends to decrease the time between the announcement of a PHE and the provision of medical countermeasures, such as vaccines. The main purpose of conducting clinical efficacy studies during PHEs is to acquire data that will allow a vaccine to be used more widely within a defined regulatory framework. Efficacy evidence from a trial conducted while disease transmission is continuing is crucial for regulatory approval. The vaccine must be used under the supervision of a functional National Regulatory Authority (NRA), In line with the WHO's Emergency Use Assessment and Listing (EUAL) for vaccines. If a valid surrogate of protection exists, then licensure decisions may be based on immunological evidence. Biomarkers that correlate with the clinical endpoint assessing vaccination efficacy are also known as Correlates of risk. On the other hand, validated surrogates are those biomarkers that help in the vaccine efficacy prediction [135]. Validated surrogates of protection could be useful in guiding the development of vaccines, lowering the size and time of definitive trials, and assisting the transition of a vaccine discovered in a trial to a new setting [136].
The COVID-19 vaccines necessitate clinical benefit and safety evidence in Placebo-controlled trials for regulatory approval. To promote consistent assessment and comparison of the efficacy of the vaccines, a basic set of clinical objectives is proposed, together with considerations to guide the selection of the primary endpoints based on clinical and statistical reasons set a cut-off of an endpoint of a minimum of 50% in the vaccinated group relative to placebo along with a 95% CI offering warrant not less than 30% reduction [137] this is reliable with the Solidarity Vaccines Trial design of WHO [138]. Two of the endpoints, clinically confirmed symptomatic infection of SARS-CoV-2, independent of the severity of the symptoms, and confirmed infection of SARS-CoV-2 with severe symptoms, will almost certainly be utilized worldwide because they suit conventional endpoints used in almost all vaccine efficacy trials [139]. In general, vaccine trials are divided into two main categories, immunogenicity trials, and VFT trials; in both, safety is evaluated.
Vaccine immunogenicity trials
The critical factor for vaccine developments is immunogenicity. The vaccines’ primary mechanism is to give the human body exposure to inactivated or live microorganisms; on subsequent exposure to this natural microorganism, either viruses or bacteria protect against them. Endpoints based on the immunological response, such as antibody level in serum, rate of the immune response, and so on, are a great deal of interest and used to determine doses of vaccines and their ideal schedules in the early stages. Immunogenicity outcomes are still the most significant endpoints in vaccine research, including bridging trials, combination trials, concurrent studies, and lot-to-lot consistency trials, and play an essential role in the process of vaccine development [140]. If a vaccine is calming that prevents the infection by SARS-CoV-2, it needs to induce cellular and humoral response against the target virus (SARS-CoV-2). Based on this response, the immunological response suitable endpoints are set.
Vaccine field trials (VFT) (individual randomized controlled trials)
VFT includes VE and vaccine effectiveness trials. VE is measured in Phase II or Phase III by considering the reduced risk of inpatients received vaccines compared to subjects who don’t receive vaccine. In contrast, vaccine effectiveness is typically examined post-marketing retrospectively at the population level via observational studies [141]. In VFT, Participants are assigned randomly to receive either the vaccine or placebo. After months the difference in the clinical outcomes and infection rates are compared, which indicates the vaccine efficacy [142].
Finally, the data from the clinical trials concerning the perseverance immune response induced by the vaccine are crucial when we evaluate the vaccine efficacy. This response also affects the vaccine dose and schedule of the administration. To tackle this pandemic, we required a vaccine with long-lasting immunity with a low and single-dose administration that will ultimately lead to prolonged vaccine supply.
Conclusion
Vaccination is the only way that might be helpful to combat the further global pandemic. The variation in the SARS-CoV-2 after a short time interval is the cause for concern. Several additional escape variants could be seen in the future, leading to a catastrophic epidemic reflection, as already observed in South Africa. Increment in viral transmission gives more opportunity for SARS-CoV-2 mutations to arise. As a result, the only way to halt the pandemic is for safe and effective vaccines against circulating variations to be disseminated evenly worldwide. Currently, there is no vaccine data against the new variants classified as a variant of concern. There is a need to develop vaccines that will be efficacious against the newly arisen mutants circulating worldwide and lead to an increased death rate. There is also a need to exile the production of the vaccines so more populations get vaccinated as soon as possible. This review discussed all up-to-date information regarding the various mutants of SARS-CoV-2, vaccines, and their efficacy against the original variants with some new variants.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Data availability
Raw data and other supplementary material are available at the following repository: osf.io/7zpcj.
No data was used for the research described in the article.
Data will be made available on request.
References
- 1.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik J.A., Maqbool M. COVID-19: an overview of current scenario. CELLMED. 2020;10:21.1–21.8. doi: 10.5667/CELLMED.2020.0021. [DOI] [Google Scholar]
- 3.Malik J.A., Mulla A.H., Farooqi T., Pottoo F.H., Anwar S., Rengasamy K.R.R. Targets and strategies for vaccine development against SARS-CoV-2. Biomed Pharmacother. 2021;137 doi: 10.1016/J.BIOPHA.2021.111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weekly epidemiological update on COVID-19 — 4 May 2021. n.d. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---4-may-2021. [Accessed 10 May 2021].
- 5.Coronavirus cases worldwide by country | Statista. n.d. https://www.statista.com/statistics/1043366/novel-coronavirus-2019ncov-cases-worldwide-by-country/. [Accessed 10 May 2021].
- 6.Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaway E. The coronavirus is mutating — does it matter? Nature. 2020;585:174–177. doi: 10.1038/d41586-020-02544-6. [DOI] [PubMed] [Google Scholar]
- 10.Forster P., Forster L., Renfrew C., Forster M. Vol. 117. 2020. Phylogenetic network analysis of SARS-CoV-2 genomes; pp. 9241–9243. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercatelli D., Giorgi F.M. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol. 2020;11:1–13. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C., Liu Z., Chen Z., Huang X., Xu M., He T., et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92:667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu H., Chen Q., Yang G., He L., Fan H., Deng Y.Q., et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369 doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Hopkins S., et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. MedRxiv. 2021 doi: 10.1101/2020.12.30.20249034. 2020.12.30.20249034. [DOI] [Google Scholar]
- 16.Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings – SARS-CoV-2 coronavirus/nCoV-2019 genomic epidemiology – virological. n.d. https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586. [Accessed 16 June 2021].
- 17.Burki T. Understanding variants of SARS-CoV-2. Lancet (London, England) 2021;397:462. doi: 10.1016/S0140-6736(21)00298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? Lancet Respir Med. 2021;9:333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.043. 812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? Lancet Respir Med. 2021;9:333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? Lancet Respir Med. 2021;9:333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eguia R.T., Crawford K.H.D., Stevens-Ayers T., Kelnhofer-Millevolte L., Greninger A.L., Englund J.A., et al. A human coronavirus evolves antigenically to escape antibody immunity. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanò L., Paladini S., Galli C., Raimondo G., Pollicino T., Zanetti A.R. Hepatitis B vaccination: are escape mutant viruses a matter of concern? Hum Vaccines Immunother. 2015;11:53–57. doi: 10.4161/hv.34306. Landes Bioscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/nejmc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mas V., Nair H., Campbell H., Melero J.A., Williams T.C. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine. 2018;36:6660–6673. doi: 10.1016/j.vaccine.2018.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C., et al. Escape from neutralizing antibodies 1 by SARS-CoV-2 spike protein variants. eLife. 2020;9:1. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I., et al. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. bioRxiv. 2020 doi: 10.1101/2020.12.28.424451. 2020.12.28.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29 doi: 10.1016/j.chom.2021.01.014. 477–488.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020 doi: 10.1101/2020.12.21.20248640. 10:2020.12.21.20248640. [DOI] [Google Scholar]
- 31.Cele S., Gazy I., Jackson L., Hwa S.H., Tegally H., Lustig G., et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SARS-CoV-2 variant classifications and definitions. n.d. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html. [Accessed 13 May 2021].
- 36.Kartawidjaja J. No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共分散構造分析. Orphanet J Rare Dis. 2020;21:1–9. [Google Scholar]
- 37.The Lancet Physician burnout: a global crisis. Lancet. 2019;394:93. doi: 10.1016/S0140-6736(19)31573-9. [DOI] [PubMed] [Google Scholar]
- 38.Barrett J.C. 2021. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. [Google Scholar]
- 39.Jewell B.L. Monitoring differences between the SARS-CoV-2 B.1.1.7 variant and other lineages. Lancet Public Health. 2021;6 doi: 10.1016/s2468-2667(21)00073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coronavirus mutations and variants: what does it mean? | SRHD. n.d. https://srhd.org/news/2021/coronavirus-mutations-and-variants-what-does-it-mean [Accessed 13 May 2021].
- 42.Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.012. 1295–1310.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham M.S., Sudre C.H., May A., Antonelli M., Murray B., Varsavsky T., et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6 doi: 10.1016/s2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frampton D., Rampling T., Cross A., Bailey H., Heaney J., Byott M., et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021;21(9):1246–1256. doi: 10.1016/s1473-3099(21)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen L., Bard J.D., Triche T.J., Judkins A.R., Jaclyn A., Gai X. 2021. Spike protein D178H and membrane protein V70L mutations; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inside the B.1.1.7 coronavirus variant — The New York Times. n.d. https://www.nytimes.com/interactive/2021/health/coronavirus-mutations-B117-variant.html. [Accessed 21 May 2021].
- 47.Candido D.S., Claro I.M., de Jesus J.G., Souza W.M., Moreira F.R.R., Dellicour S., et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/SCIENCE.ABD2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voloch C.M., da Silva Francisco R., de Almeida L.G.P., Cardoso C.C., Brustolini O.J., Gerber A.L., et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol. 2021;95(10) doi: 10.1128/jvi.00119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592 doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonaka C.K.V., Franco M.M., Gräf T., de Lorenzo Barcia C.A., de Ávila Mendonça R.N., de Sousa K.A.F., et al. Genomic evidence of SARS-CoV-2 reinfection involving E484K spike mutation, Brazil. Emerg Infect Dis. 2021;27 doi: 10.3201/eid2705.210191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein – SARS-CoV-2 coronavirus/nCoV-2019 genomic epidemiology – virological. n.d. https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-from-amazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-and-n501y-in-the-spike-protein/585. [Accessed 21 May 2021].
- 52.SARS-CoV-2 reinfection by the new Variant of Concern (VOC) P.1 in Amazonas, Brazil – SARS-CoV-2 coronavirus/nCoV-2019 genomic epidemiology – virological. n.d. https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596. [Accessed 21 May 2021].
- 53.This “double mutant” variant is adding fuel to India’s COVID-19 crisis; n.d. https://www.nationalgeographic.com/science/article/this-double-mutant-variant-is-adding-fuel-to-indias-covid-19-crisis. [Accessed 22 May 2021].
- 54.What is the triple mutant coronavirus variant in India? n.d. https://www.womenshealthmag.com/health/a36232744/what-is-triple-mutant-variant-coronavirus/. [Accessed 13 May 2021].
- 55.Prakash S.J., Mishra A.P., Samal K.C. Biotica Research Today; 2021. Triple mutant Bengal strain (B.1.618) research of coronavirus and the worst COVID outbreak in India; pp. 1–5. [Google Scholar]
- 56.Cherian S., Potdar V., Jadhav S., Yadav P., Gupta N. 2021. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng X., Garcia-Knight M.A., Khalid M.M., Servellita V., Wang C., Morris M.K., et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184 doi: 10.1016/j.cell.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vietnam says new Covid variant is hybrid of India and UK strains | Coronavirus | The Guardian. n.d. https://www.theguardian.com/world/2021/may/29/vietnam-discovers-new-hybrid-covid-variant-state-media-reports. [Accessed 31 May 2021].
- 59.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;0 doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.GISAID — hCov19 variants. n.d. https://www.gisaid.org/hcov19-variants/. [Accessed 10 December 2021].
- 61.Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29 doi: 10.1016/J.CHOM.2020.11.007. 44–57.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/S41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatakrishnan AJ, Anand P, Lenehan PJ, Suratekar R. Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin. n.d.;529.
- 64.Frequently asked questions for the B.1.1.529 mutated SARS-CoV-2 lineage in South Africa — NICD. n.d. https://www.nicd.ac.za/frequently-asked-questions-for-the-b-1-1-529-mutated-sars-cov-2-lineage-in-south-africa/. [Accessed 10 December 2021].
- 65.Pulliam J. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. MedRxiv. 2021:1–43. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genovese L. Investigating the mutational landscape of the SARS-CoV-2 Omicron variant via ab initio quantum mechanical modeling. bioRxiv. 2021:1–16. [Google Scholar]
- 67.Ford C.T., Machado D.J., Janies D.A. Predictions of the SARS-CoV-2 omicron variant (B.1.1.529) spike protein receptor-binding domain structure and neutralizing antibody interactions. bioRxiv. 2021 doi: 10.1101/2021.12.03.471024. 2021.12.03.471024. [DOI] [Google Scholar]
- 68.Halloran M.E., Haber M., Longini I.M., Struchiner C.J. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol. 1991;133:323–331. doi: 10.1093/oxfordjournals.aje.a115884. [DOI] [PubMed] [Google Scholar]
- 69.Alkandari D., Herbert J.A., Alkhalaf M.A., Yates C., Panagiotou S. SARS-CoV-2 vaccines: fast track versus efficacy. Lancet Microbe. 2021;2:e89–e90. doi: 10.1016/s2666-5247(21)00034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.COVID-19 vaccine tracker. n.d. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/. [Accessed 13 May 2021].
- 71.Shrotri M., Swinnen T., Kampmann B., Parker E.P.K. An interactive website tracking COVID-19 vaccine development. Lancet Glob Health. 2021;9:e590–e592. doi: 10.1016/s2214-109x(21)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.More than 1.35 billion shots given: Covid-19 vaccine tracker. n.d. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/. [Accessed 13 May 2021].
- 73.A year of challenges and change. MEDICC Rev. 2019;21:3. doi: 10.37757/MR2019.V21.N1.1. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 75.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baden L.R., el Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isakova-Sivak I., Rudenko L. A promising inactivated whole-virion SARS-CoV-2 vaccine. Lancet Infect Dis. 2021;21:2–3. doi: 10.1016/S1473-3099(20)30832-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isakova-Sivak I., Rudenko L. A promising inactivated whole-virion SARS-CoV-2 vaccine. Lancet Infect Dis. 2021;21:2–3. doi: 10.1016/S1473-3099(20)30832-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.008. 713–721.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang J.K., Zhang T., Wang A.Z., Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14 doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/nejmoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans | medRxiv. n.d. https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1. [Accessed 29 May 2021].
- 86.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;3099:1–11. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.WHO . WHO; 2021. Background document on the mRNA vaccine. 2. [Google Scholar]
- 90.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. Am J Transplant. 2021;21:1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 93.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.UK Gov. Coronavirus vaccine — weekly summary of Yellow Card reporting. 2021.
- 95.AstraZeneca’s COVID-19 vaccine: benefits and risks in context | European Medicines Agency. n.d. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context. [Accessed 15 May 2021].
- 96.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.China Sinopharm’s coronavirus vaccine taken by about a million people in emergency use | Reuters. n.d. https://www.reuters.com/article/us-health-coronavirus-vaccine-sinopharm-idUSKBN27Z0PY. [Accessed 15 May 2021].
- 99.Immunization ON. Evidence assessment: Sinopharm/BBIBP COVID-19 vaccine. n.d.
- 100.UAE: Ministry of Health announces 86 per cent vaccine efficacy | Health – Gulf News. n.d. https://gulfnews.com/uae/health/uae-ministry-of-health-announces-86-per-cent-vaccine-efficacy-1.1607490555571. [Accessed 15 May 2021].
- 101.Sinopharm coronavirus vaccine: WHO grants emergency use authorization for Chinese-made vaccine — The Washington Post. n.d. https://www.washingtonpost.com/world/2021/05/07/who-sinopharm-emergency-use/. [Accessed 15 May 2021].
- 102.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sapkal G.N., Yadav P.D., Ella R., Deshpande G.R., Sahay R.R., Gupta N., et al. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Travel Med. 2021;28 doi: 10.1093/jtm/taab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sapkal Gajanan N., Yadav Pragya D. 4th ed. Vol. 28. Oxford University Press; 2021. (Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B 1.1.7 variant of SARS-CoV-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yadav P.D., Sapkal G.N., Ella R., Sahay R.R., Nyayanit D.A., Patil D.Y., et al. Neutralization against B.1.351 and B.1.617.2 with sera of COVID-19 recovered cases and vaccinees of BBV152. bioRxiv. 2021 doi: 10.1101/2021.06.05.447177. 2021.06.05.447177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yadav P.D., Sapkal G.N., Abraham P., Ella R., Deshpande G., Patil D.Y., et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis. 2021;ciab411 doi: 10.1093/cid/ciab411. [DOI] [PubMed] [Google Scholar]
- 108.Sapkal G., Yadav P.D., Ella R., Abraham P., Patil D.Y., Gupta N., et al. Neutralization of B.1.1.28 P2 variant with sera of natural SARS-CoV-2 infection and recipients of BBV152 vaccine. bioRxiv. 2021 doi: 10.1101/2021.04.30.441559. 2021.04.30.441559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Immunization ON. Evidence assessment: Sinovac/CoronaVac COVID-19 vaccine. n.d.
- 111.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Covid vaccine: Single-dose Johnson & Johnson jab is 66% effective — BBC News. n.d. https://www.bbc.com/news/health-55857530. [Accessed 16 May 2021].
- 114.Johnson & Johnson single-shot vaccine 85% effective against severe COVID-19 disease — ABC News. n.d. https://abcnews.go.com/Health/johnson-johnson-single-shot-vaccine-85-effective-severe/story?id=75557358. [Accessed 16 May 2021].
- 115.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/nejmoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Novavax vaccine 96% effective against original coronavirus, 86% vs British variant in UK trial | Reuters. n.d. https://www.reuters.com/article/uk-health-coronavirus-vaccines-novavax-idUSKBN2B32ZI. [Accessed 17 May 2021].
- 117.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21 doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.CanSinoBIO’s COVID-19 vaccine 65.7% effective in global trials, Pakistan official says | Reuters. n.d. https://www.reuters.com/article/us-health-coronavirus-vaccine-pakistan/cansinobios-covid-19-vaccine-657-effective-in-global-trials-pakistan-official-says-idUSKBN2A81N0. [Accessed 2 June 2021].
- 121.Farooqi T., Malik J.A., Mulla A.H., Al Hagbani T., Almansour K., Ubaid M.A., et al. An overview of SARS-COV-2 epidemiology, mutant variants, vaccines, and management strategies. J Infect Public Health. 2021;14:1299–1312. doi: 10.1016/J.JIPH.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russia approves its third COVID-19 vaccine, CoviVac | Reuters. n.d. https://www.reuters.com/article/us-health-coronavirus-russia-vaccine-idUSKBN2AK07H. [Accessed 2 June 2021].
- 124.Immunogenicity, efficacy and safety of QazCovid-in® COVID-19 vaccine – full text view – ClinicalTrials.gov. n.d. https://clinicaltrials.gov/ct2/show/NCT04691908. [Accessed 2 June 2021].
- 125.Yang S., Li Y., Dai L., Wang J., He P., Li C., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Study of the tolerability, safety, immunogenicity and preventive efficacy of the EpiVacCorona vaccine for the prevention of COVID-19 – full text view – ClinicalTrials.gov. n.d. https://clinicaltrials.gov/ct2/show/NCT04780035. [Accessed 2 June 2021].
- 127.Zhang S.X., Arroyo Marioli F., Gao R. A second wave? What do people mean by COVID waves? — a working definition of epidemic waves. MedRxiv. 2021;2021(14):3775–3782. doi: 10.2147/RMHP.S326051. 2021.02.21.21252147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu J., Li C., Wang S., Li T., Zhang H. Genetic variants are identified to increase risk of COVID-19 related mortality from UK Biobank data. MedRxiv. 2020;2020:11–21. doi: 10.1101/2020.11.05.20226761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu S., Li Y. Beware of the second wave of COVID-19. Lancet. 2020;395:1321–1322. doi: 10.1016/S0140-6736(20)30845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cacciapaglia G., Cot C., Sannino F. Second wave COVID-19 pandemics in Europe: a temporal playbook. Sci Rep. 2020;10 doi: 10.1038/s41598-020-72611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Critical coronavirus (COVID-19) cases most impacted countries worldwide 2021 | Statista. n.d. https://www.statista.com/statistics/1109971/serious-critical-coronavirus-2019ncov-cases-worldwide-most-impacted-countries/. [Accessed 20 May 2021].
- 132.Omicron: South Africa health minister declares 4th wave of COVID-19. n.d. https://www.downtoearth.org.in/news/africa/omicron-south-africa-health-minister-declares-4th-wave-of-covid-19-80556. [Accessed 10 December 2021].
- 133.Unvaccinated people lead Covid 4th wave in Germany: report | World News — Hindustan Times. n.d. https://www.hindustantimes.com/world-news/unvaccinated-people-lead-covid-4th-wave-in-germany-report-101636690003241.html. [Accessed 10 December 2021].
- 134.Russek-Cohen E., Rubin D., Price D., Sun W., Cox E., Borio L. A US Food and Drug Administration perspective on evaluating medical products for Ebola. Clin Trials. 2016;13:105–109. doi: 10.1177/1740774515620613. [DOI] [PubMed] [Google Scholar]
- 135.Qin L., Gilbert P.B., Corey L., McElrath M.J., Self S.G. A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis. 2007;196:1304–1312. doi: 10.1086/522428. [DOI] [PubMed] [Google Scholar]
- 136.Hudgens M.G., Gilbert P.B., Self S.G. Endpoints in vaccine trials. Stat Methods Med Res. 2004;13:89–114. doi: 10.1191/0962280204sm356ra. [DOI] [PubMed] [Google Scholar]
- 137.Parker F.R. CRC Press; 2021. Department of health and human services, U.S. Food and Drug Administration: authority and responsibility. FDA administrative enforcement manual; pp. 21–60. [DOI] [Google Scholar]
- 138.Krause P., Fleming T.R., Longini I., Henao-Restrepo A.M., Peto R., Dean N.E., et al. COVID-19 vaccine trials should seek worthwhile efficacy. Lancet. 2020;396:741–743. doi: 10.1016/S0140-6736(20)31821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clements-Mann M.L., Harro C.D. Lessons for AIDS vaccine development from non-AIDS vaccines. AIDS Res Hum Retroviruses. 1998;14:S197–S203. [PubMed] [Google Scholar]
- 140.Wang W.W.B., Mehrotra D.V., Chan I.S.F., Heyse J.F. Statistical considerations for noninferiority/equivalence trials in vaccine development. J Biopharm Stat. 2006;16:429–441. doi: 10.1080/10543400600719251. [DOI] [PubMed] [Google Scholar]
- 141.Dean N.E., Gsell P.S., Brookmeyer R., De Gruttola V., Donnelly C.A., Halloran M.E., et al. Design of vaccine efficacy trials during public health emergencies. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eyal N., Lipsitch M. Testing SARS-CoV-2 vaccine efficacy through deliberate natural viral exposure. Clin Microbiol Infect. 2021;27:372–377. doi: 10.1016/j.cmi.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data and other supplementary material are available at the following repository: osf.io/7zpcj.
No data was used for the research described in the article.
Data will be made available on request.