Summary:
Systemic sclerosis (SSc; scleroderma) has the highest individual mortality of all rheumatic diseases and interstitial lung disease (ILD) is among the leading causes of SSc-related death. Two drugs are now approved by the Food & Drug Administration (FDA) and indicated for slowing the rate of decline in pulmonary function in patients with SSc-ILD: nintedanib (a tyrosine kinase inhibitor) and tocilizumab (the first biologic agent targeting the interleukin-6 pathway in SSc). In addition, two generic drugs with cytotoxic and immunoregulatory activity, mycophenolate mofetil and cyclophosphamide, have shown comparable efficacy in a Phase II trial but are not FDA-approved for SSc-ILD. In light of the heterogeneity of the disease, the optimal therapeutic strategy in the management of patients with SSc-ILD is still to be determined. The objectives of this review are two-fold: (1) review the body of research focused on the diagnosis and treatment of SSc-ILD; and (2) propose a practical approach for diagnosis, stratification, management, and therapeutic decision-making in this clinical context. This review presents a practical classification of SSc patients in terms of disease severity (subclinical vs. clinical ILD) and associated risk of progression (low vs. high risk). The pharmacological and non-pharmacological options as first and second-line therapy, as well as potential combination approaches, are discussed in light of the recent approval of tocilizumab for SSc-ILD.
Keywords: Systemic sclerosis, interstitial lung disease, lung fibrosis, tocilizumab, nintedanib, mycophenolate mofetil, cyclophosphamide, stem cell transplant
Introduction
Systemic sclerosis (SSc; scleroderma) is a heterogeneous chronic autoimmune disease characterized by vascular damage, inflammation, and fibrosis of the skin and internal organs (1). SSc is the rheumatic disease with the highest individual mortality and has a detrimental impact on quality of life (1,2). Two main subsets of SSc are described based on the distribution of skin involvement: limited cutaneous systemic sclerosis (lcSSc) characterized by distal skin thickening, and diffuse cutaneous systemic sclerosis (dcSSc) with widespread distal and proximal cutaneous changes (3,4). SSc is also characterized by the detection of specific and often mutually exclusive serum autoantibodies (5). A composite classification of SSc patients based on the combination of degree of skin involvement and antibody subtype is now considered more helpful in predicting disease course as scleroderma-specific antibodies are predictive of internal organ involvement (6). Patients who develop progressive SSc-associated interstitial lung disease (SSc-ILD) are more likely to be positive for the anti-topoisomerase I antibody (anti-Scl-70 antibody) and anti-nuclear antibody with nucleolar pattern (notably including anti-PM/Scl-75, anti-PM/Scl-100, anti-Th/To, anti-U3-RNP/Fibrillarin, anti-RNA-polymerase I, or anti-NOR-90 antibodies), regardless of the cutaneous subset (6–9).
ILD is among the leading causes of SSc-related death (10). The prevalence of SSc-ILD varies depending on the assessment method (X-Rays, high resolution computed tomography [HRCT]), the screening strategy (systematic HRCT versus selection of patients based on the results of pulmonary function tests [PFTs]), the targeted populations (dcSSc versus lcSSc), and differences in geographic location or expertise of the medical center (11,12). In national observational registries and international cohorts, approximately 65% of SSc-patients have or will develop ILD in the course of their disease (11–14). The high mortality related to SSc-ILD has led to recent randomized controlled trials (RCTs) forging substantial progress in the management of this manifestation (15). Conventional immune-modulatory agents such as cyclophosphamide (CYC) and mycophenolate mofetil (MMF) are evidence-based treatments typically implemented in clinical practice (16,17). More recently, well-conducted phase III RCTs have led to the approval of two targeted therapies for SSc-ILD by the U.S. Food and Drug Administration (FDA) (18–22). Nintedanib is a tyrosine kinase inhibitor; in 2019 it became the first medication approved to slow the rate of decline in pulmonary function in patients with SSc-ILD, based on the results of the SENSCIS trial (NCT02597933) (18,19). Tocilizumab is a monoclonal antibody targeting the interleukin (IL)-6 receptor; in 2021 it became the first biologic medication approved for the same indication, based on the results of the faSScinate (NCT01532869) and focuSSced (NCT02453256) trials (20–22).
Despite these recent FDA approvals, the optimal therapeutic strategy for the management of patients with SSc-ILD is yet to be determined, especially given the heterogeneity of the disease (23). The objectives of this review are two-fold: (1) to review the body of research focused on diagnosis and treatment of SSc-ILD and (2) to propose a practical approach for diagnosis, stratification, management, and therapeutic decision-making in this clinical context. The management strategy proposed in this review reflects the authors’ opinions, experience, and clinical practice.
Pathogenic considerations and rationale for available therapeutic options in SSc-ILD.
The pathogenesis of SSc-ILD is not fully understood but includes a triad of pathogenic events: endothelial dysfunction, early inflammatory features, and excessive deposition of extracellular matrix (ECM) components produced by activated myofibroblasts (9,24). ECM deposits induce an increased stiffness of lung tissue with reduction of pulmonary compliance and volumes. These pathogenic events can lead to a restrictive ventilatory defect captured by spirometry alongside impairment in gas exchange; some patients may remain asymptomatic despite evidence of disease on HRCT, whereas the consequences of severe and advancing disease include dyspnea and death.
The direct inhibition of myofibroblast activation or the targeting of other cellular subsets participating in the production of key mediators responsible for myofibroblast activation provide the rationale for candidate drugs in SSc-ILD. Early inciting factors include epithelial and endothelial damage that may be promoted by abberant innate and adaptive immunity that can produce pro-fibrotic and pro-inflammatory mediators inducing myofibroblast activation. Through the production of IL-13 and IL-4, Th2-lymphocytes have a direct impact on fibroblasts and can induce the activation of alternative pro-fibrotic M2 macrophages that notably produce high levels of transforming growth factor β (TGFβ), platelet-derived growth factor (PDGF), and factors from the fibroblast growth factor (FGF) family favoring myofibroblast activation (25–27). The tyrosine kinase inhibitor, nintedanib, inhibits the receptors of vascular endothelial growth factor (VEGF), PDGF, and FGF family with subsequent anti-fibrotic properties (28). Acute phase reactants, and specifically IL-6, play an important role in the pathogenesis of SSc-ILD. IL-6 is produced by B-cells, M1 macrophages, and myofibroblasts (29,30). In vitro studies suggest that IL-6 can favor the expression of IL-4 and IL-13-receptors with subsequent increase of pro-fibrotic M2 macrophage polarization (31). The inhibition of the IL-6 receptor by tocilizumab can directly impact myofibroblast activation and M2 macrophage polarization with potential anti-fibrotic properties (29,32). Through their impact on the proliferation of fibroblasts, B-cells, and T-helper lymphocytes, conventional immunomodulatory agents such as MMF, an inhibitor of de-novo synthesis of guanosine nucleotides, or the alkylating agent cyclophosphamide can also have anti-fibrotic effects (33,34).
Key parameters for the diagnosis, screening and assessment of SSc-ILD
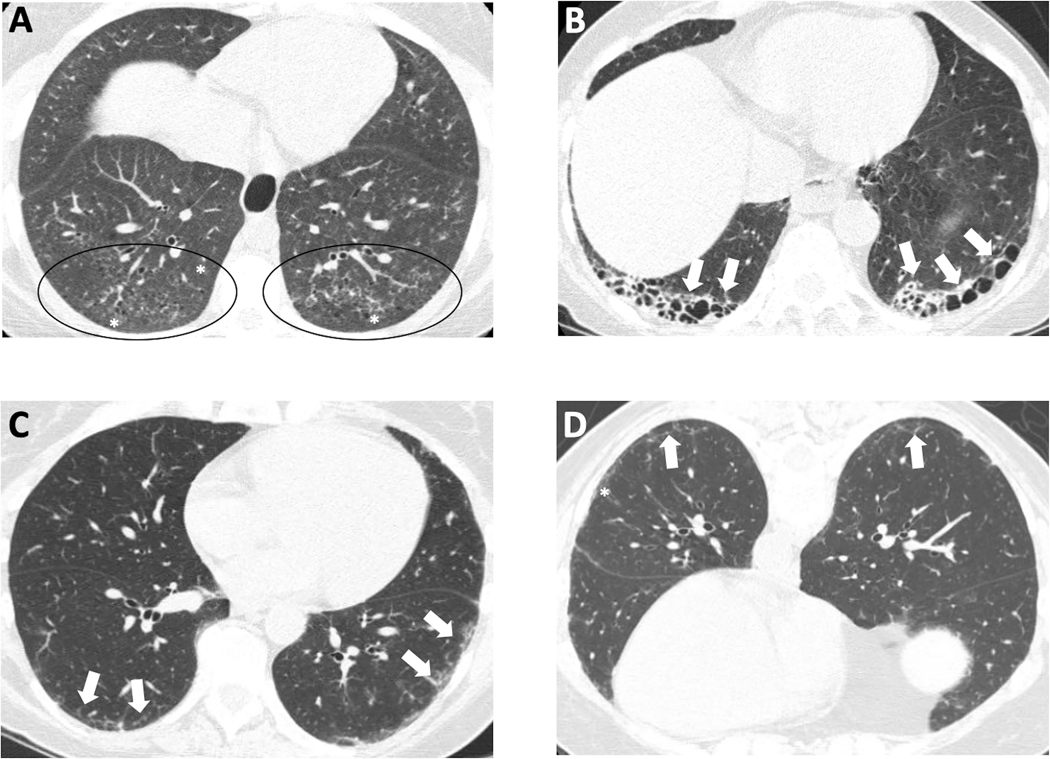
HRCT is the reference standard for early diagnosis of SSc-ILD (12,35,36). In the majority of patients (70%−80%), SSc-ILD is characterized by a pattern of nonspecific interstitial pneumonia (NSIP) that includes parenchymal changes classically located in bi-basal and posterior regions of the lungs, and defined by the presence of reticular abnormalities with peri-bronchovascular extension and subpleural sparing with absence of honeycombing and frequent ground-glass attenuations (Figure 1A) (13,37,38). Ground glass opacity in early SSc may represent either inflammation or fibrosis that is below the resolution of the HRCT technique at the level of intralobular septa and interstitium surrounding alveoli. Early radiologic-pathologic correlation studies using HRCT have demonstrated that bronchiectasis or bronchiolectasis within areas of ground glass are strong indicators of fibrosis, whereas ground glass without bronchiectasis is strong evidence of inflammation (39). The presence of traction bronchiectasis with minimal ground glass opacifications is thus more specifically consistent with fibrotic NSIP. Approximately 10% of patients with SSc-ILD have an HRCT pattern of usual interstitial pneumonia (UIP) defined by subpleural and basal predominant lesions including honeycombing (mandatory criterion) with or without peripheral traction bronchiectasis or bronchiolectasis (Figure 1B). In patients with connective tissue disease (CTD)-ILD, especially rheumatoid arthritis-ILD, UIP predicts a worse prognosis compared with NSIP; the specific prognostic value of HRCT patterns in SSc-ILD is more controversial (40). Patient survival in SSc-ILD does not differ between NSIP and UIP according to the histopathological patterns on lung biopsy (41). Considering the sensitivity and specificity of HRCT for SSc-ILD and the lack of predictive value of histopathological patterns in SSc-ILD, lung biopsy is thus not recommended for the diagnosis and assessment of SSc-ILD. A prone HRCT acquisition is recommended to rule out early ILD, as the predominant bi-basal and posterior localization of HRCT findings in SSc-ILD may produce false-positives due to position-induced changes (i.e., atelectasis) (Figure 1 C,D) (42). Quantitative HRCT allows precise quantification of SSc-ILD lung involvement (QILD, or the sum of lung involvement with ground glass opacities, fibrotic reticulations, and honeycombing) and of fibrotic changes (quantification of lung fibrosis [QLF], or fibrotic reticulations alone) (43,44). The extent of lung involvement has been demonstrated to have prognostic value; accurately assessing the degree of lung involvement provides a valuable tool for stratifying disease severity and risk of progression (45,46).
Figure 1: HRCT images of three different patients with SSc-interstitial lung disease.
Nonspecific interstitial pneumonitis (NSIP) with a lower lobe subpleural predominant distribution of primarily ground glass opacity (* and circles) (A). Definite usual interstitial pneumonitis (UIP) with subpleural lower lobe honeycombing (arrows) (B). Mild interstitial lung disease on the supine image (arrows) (C) which could be interpreted as dependent atelectasis, however it persists on the prone image (D), confirming the presence of interstitial lung disease; the pattern of septal thickening (arrows) and ground glass opacity (*) without bronchiectasis is most consistent with NSIP in a patient with scleroderma.
Spirometry and gas exchange are the reference standard measurements for the assessment of lung physiology. The impact of SSc-ILD on forced vital capacity (FVC), total lung capacity (TLC), and diffusion capacity of the lungs for carbon monoxide (DLco) is a marker of disease severity. In terms of screening and diagnosis, SSc-ILD may initially have only mild or no impact on PFT parameters; normal values of FVC, TLC and DLco do not rule out early SSc-ILD (12). In a US multicenter study of patients with early dcSSc, FVC<80% (%predicted) had a sensitivity of 63% and a negative predictive value (NPV) of 61% for the detection of SSc-ILD. The combination of FVC<80% or DLco <80% had a sensitivity and NPV of 85% and 70% respectively, demonstrating that PFTs alone are an inadequate screening tool for the diagnosis of SSc-ILD (12). A European study also demonstrated similar results and highlighted that among patients with normal FVC (%predicted) but with SSc-ILD on HRCT, 50% had extensive ILD (>20% of parenchymal involvement) (47). In addition, the range of FVC% predicted in healthy volunteers ranges between 80–120% predicted, which can miss clinically meaningful decline in a patient who declines within the FVC% predicted “normal range,” e.g., from 110% to 80%. Therefore, it is now accepted that both PFT and HRCT should be performed for initial screening and diagnosis of SSc-ILD (35). We recommend performing HRCT and PFT for baseline ILD screening in all early SSc patients (early relates to the onset of their symptoms that are specific for SSc), regardless of the cutaneous or autoantibody subtypes (36). Every patient with a new diagnosis of SSc-ILD based on HRCT should have initial full PFTs (i.e., spirometry, lung volumes, and DLco) for baseline reference and a 6-minute walk test (6MWT) to assess the impact on gas exchange and exercise capacity. Although 6MWT can be influenced by different organ involvement in SSc such as pulmonary vascular disease and cardiac involvement,for example, we use 6MWT in clinical practice to document baseline distance and oxygen saturation and follow it annually (or more frequently for new or worsening of symptoms) to assess for decline in both of these parameters (48,49). Clinical scales such as the modified Medical Research Council dyspnea scale or the New York Heart Association functional classification of dyspnea are simple to incorporate in clinical practice and can provide important information to assess for SSc-ILD progression (50,51).
Progression of SSc-ILD : definitions, risk factors and monitoring.
There are different definitions for the progression of SSc-ILD. OMERACT (Outcome Measures in Rheumatology) has proposed the definition of “clinically meaningful progression” of CTD-ILD based on the evolution of PFT parameters; this definition can be applied to SSc-ILD. OMERACT defines progression as ≥10% relative decline in FVC(%predicted) or 5 to <10% relative decline in FVC (%predicted) and ≥15% relative decline in DLco(%predicted). The INBUILD trial, which focused on fibrotic ILDs, has also proposed a composite definition of “progressive fibrosing ILD” as an inclusion criterion, that was notably applied to patients with SSc-ILD (19). In this trial, one of the following criteria was required to fulfill the definition of progression within the prior 24 months: a) ≥10% relative decline in FVC(%predicted), or b) 5 to <10% relative decline in FVC(%predicted) and worsening of respiratory symptoms or an increased extent of fibrosis on HRCT, or c) worsening of respiratory symptoms and an increased extent of fibrosis on HRCT, regardless of the evolution of FVC(% predicted).
The results from the focuSSced trial demonstrate that early treatment should be considered in patients with SSc-ILD at high-risk of progression, regardless of the actual progression rate and/or before decline of lung function or progression is identified through close monitoring (21). This approach constitutes a paradigm shift in the field of SSc-ILD and emphasizes the need for reliable and accessible predictive markers of SSc-ILD progression. The predictive value of such markers in observational studies and RCTs varies according to the targeted populations and the definition of SSc-ILD progression (Table 1) (36,52). Serum markers used in clinical practice such as anti-topoisomerase I antibodies and higher C-reactive protein (CRP) values are associated with SSc-ILD progression (53,54). Other biomarkers such as KL-6, CCL2, CCL18, CXCL4, or SP-D may predict the progression of SSc-ILD but are not available in routine practice and are currently used in the context of exploratory clinical research (36,52,55,56). Negative anti-centromere antibody and history of smoking may also constitute as risk factors for progressive ILD although the data are less consistent (6,57).
Table 1:
Parameters available in clinical practice and associated with progressive SSc-ILD
| Parameters |
|---|
|
|
| Demographical and clinical parameters |
| Advanced age |
| Male gender |
| African-American ethnicity |
| dcSSc |
| Pulmonary Function Tests |
| Low baseline FVC (%predicted)* |
| Low baseline DLco (%predicted)* |
| HRCT findings |
| Extent of ILD on HRCT (cut-off value >20% of lung parenchyma for total lung involvement) |
| Serum markers |
| Anti-Scl70/Topoisomerase I antibody |
| Nucleolar pattern (especially including anti-Th/To and U3-RNP) |
| Elevated acute phase reactants, including serum CRP levels greater than upper limit of normal |
cut-off values vary across studies
ILD=Interstitial Lung Disease; dcSSc=diffuse cutaneous systemic sclerosis; FVC=forced vital capacity ; DLco=diffusion capacity of the lungs for carbon monoxide; HRCT= high resolution computed tomography; CRP=C-reactive protein
The heterogeneous rates of disease progression and treatment response underscore the need for close monitoring of patients with SSc-ILD after initial diagnosis or treatment initiation (35,58). The majority of patients who will develop severe SSc-ILD will do so in the first 5 years after the onset of the disease, although late progression may also occur (52). After initial diagnosis of SSc-ILD with baseline HRCT and PFT, the follow-up of all SSc-ILD patients should include PFT (FVC and DLco) at least every 6 months for the first 3 to 5 years from onset of the first non-Raynaud’s phenomenon manifestation (Table 1) in order to monitor for progression (36,52). Although substantial progress has been made in HRCT techniques, allowing high-quality HRCT with low dose radiation (typically 1.5–2.5 mSv), the systematic follow-up and monitoring of all SSc-ILD patients with sequential chest HRCT is not currently recommended (35,36). In case of worsening symptoms or clinically meaningful progression (as defined in the INBUILD trial), a follow-up HRCT can be considered to assess for progressive ILD. Other causes of progressive symptoms such as pulmonary vascular disease or cardiac involvement should also be considered due to the multifactorial nature of SSc-associated manifestations. In SSc patients without ILD or with stable or controlled ILD after the first 3 to 5 years, annual PFTs are useful to monitor both onset or progression of SSc-ILD and to screen for SSc-associated pulmonary arterial hypertension (PAH) (7,59).
Classification of SSc-ILD and sub-groups of patients depending on initial severity and risk of progression.
SSc-ILD trajectories are divided into two large subsets, depending on the initial clinical presentation. Subclinical ILD is classified by the presence of ILD with minimal extent on HRCT (usually 5– 10% based on visual or computer quantification) and no ILD-related clinical symptoms (such as dyspnea and cough) and normal initial PFT (including FVC and DLco) or no clinically meaningful decline in PFT, if serial PFTs are available. With the institution of HRCT for screening and diagnosis of SSc-ILD, this subgroup is likely to increase over time. Clinicians also need to use their judgment to assess if symptoms such as cough are related to ILD or to other causes such as silent gastric aspiration or upper airway cough syndrome.
The remaining patients with ILD are classified as clinical ILD (which is the majority of current cases of SSc-ILD due to lack of universal screening in SSc patients); they are classified by the presence of mild to severe ILD on HRCT and one or more of the following features: abnormal initial PFT (including FVC and/or DLco) and/or clinically meaningful decline of PFT parameters (including FVC and/or DLco). Clinical ILD is associated with ILD-related symptoms or impact of ILD on daily life.
Within these subsets, patients can be further divided into low risk of progressive ILD (no elevated acute phase reactants, positive anti-centromere antibody) and high risk of progressive ILD (Table 1). The subgroups of subclinical ILD patients at high risk of progression (as shown in the focuSSed trial), as well as all patients with clinical ILD, would benefit from early therapeutic intervention for SSc-ILD. Close monitoring (at least every 6 months) is also necessary in patients with subclinical ILD with low risk of progression to confirm stability.
Clinical evidence for the management of SSc-ILD based on Phase II and III trials
The main therapeutic agents for the treatment of SSc-ILD have immunomodulatory properties, anti-fibrotic properties, or both (23). The results from the main phase II and III RCTs and their targeted populations are detailed in Table 2.
Table 2:
Inclusion criteria and targeted population in key Phase II and III trials including SSc-ILD patients
| Trials | Drug | Targeted population (main criteria) | Controlled group | Background therapy | N assigned Arm | % of patients with SSc ILD | Pulmonary outcome used for efficacy | Main results on this pulmonary outcome |
|---|---|---|---|---|---|---|---|---|
| SLS I | CYC | -Patients with diffuse or limited cutaneous subset -SSc-ILD defined by active alveolitis or GGO on CT -disease duration of less than 7 years -FVC between 45 and 85(%pred) -at least grade 2 exertional dyspnea*. |
Placebo | Potentially disease-modifying medications excluded and prednisone in doses >10 mg/day excluded |
N=158 CYC=79 PCB=79 |
CYC=100% PCB=100% |
FVC (%pred) at 12 months adjusted for baseline FVC |
Mean absolute difference in adjusted 12-month FVC was 2.53 percent (95%CI, 0.28 to 4.79), favoring CYC (P<0.03) |
| SLS II | MMF | -Patients with diffuse or limited cutaneous subset -SSc-ILD defined GGO on CT (with reticulations or not) -disease duration of less than 7 years -FVC between 45 and 80(%pred) -at least grade 2 exertional dyspnea*. |
CYC | Potentially disease-modifying medications excluded and prednisone in doses >10 mg/day excluded |
N=142 MMF=69 CYC=73 |
CYC=100% MMF=100% |
Course of FVC (%pred) over time from 3 months to 24 months |
The course of the % FVC did not differ significantly between the two treatment groups. (P=0.24) The adjusted % predicted FVC improved from baseline to 24 months by 2.19% in the MMF group (95% CI 0.53–3.84) and 2.88% in the CYC group (1.19–4.58) |
| SENCIS | NINT | -Patients with diffuse or limited cutaneous subset -SSc-ILD with CT showing fibrosis affecting at least 10% of the lungs -FVC of at least 40% |
Placebo | Prednisone (up to 10 mg per day) or MMF/MTX at a stable dose for at least 6 months before randomization could participate in the trial |
N=580 NINT=288 PCB=288 (+ 3 randomized despite non-eligibility and 1 withdrawal) |
NINT=100% PCB=100% |
Annual rate of decline in FVC (milliliters per year), assessed over a 52-week period |
The adjusted annual rate of change in FVC was −52.4 ml per year in the NINT group and −93.3 ml per year in the PCB group (difference, 41.0 ml per year; (95% CI;2.9 to 79.0) (P = 0.04)) |
| faSScinate | TCZ | -Patients with dcSSc with or without ILD -with active disease‡ -disease duration < 5 years |
Placebo | No background immunomodulatory therapies were allowed | N=87 TCZ=43 PCB=44 |
Not available | FVC (milliliters) declined at week 24 and 48 (secondary outcome) And % of patients experiencing worsening of FVC (%pred) in each arm |
Smaller decrease in FVC for TCZ than for PCB from baseline to 24 weeks (TCZ –34 mL vs PCB –171 mL; least square mean difference 136 mL, 95% CI 9 to 264; p=0.0368) but from baseline to 48 weeks no significant difference (TCZ –117 mL vs PCB –237 mL; 120 mL, 95% CI –23 to 262; p=0.0990). Fewer patients in the TCZ group than in the placebo group had worsening of FVC (%pred) at 24 weeks (p=0.009) or at 48 weeks (p=0.037) |
| focuSSed | TCZ | -Patients with dcSSc with or without ILD -with active disease‡ -disease duration < 60 months |
Placebo | No background immunomodulatory therapies were allowed | N=212 TCZ=105 PCB=107 |
TCZ=67% PCB=65% |
difference in distribution of change from baseline to week 48 in FVC% predicted (Key secondary outcome) |
There was a shift in the distribution of change from baseline in FVC (%pred) at week 48 favoring TCZ (van Elteren nominal p=0.002 versus placebo) In patients with SSc ILD at baseline the LSM of FVC (% pred) change from baseline was −6.4 in the PCB group and 0.1 in the TCZ with LSM difference between treatment groups of 6.5 (95%CI 3.4–9.5) p<0.0001. |
on the Magnitude of Task component of the Mahler Baseline Dyspnea Index;
a relative decline in the FVC of at least 10% of the predicted value, a relative decline in the FVC of 5% to less than 10% of the predicted value and worsening of respiratory symptoms or an increased extent of fibrosis on high-resolution CT, or worsening of respiratory symptoms and an increased extent of fibrosis
an increase of at least 3 on the modified Rodnan skin score at screening compared with the last visit within the previous 1–6 months or new-onset systemic sclerosis diagnosed within 1 year before screening, involvement of one new body area with an increase of modified Rodnan skin score of at least 2 or two new body areas with increase of at least 1, documentation of worsening of skin thickening in the previous 6 months, or at least one tendon friction rub plus at least one laboratory criterion (C-reactive protein ≥10.0 mg/L, erythrocyte sedimentation rate ≥28 mm/h, or platelets ≥330 000/μL)
MMF: mycophenolate mofetil; TCZ=tocilizumab; NINT=Nintedanib; SLS=Scleroderma Lung study; LSM=least square mean; ILD=Interstitial Lung Disease; dcSSc=diffuse cutaneous systemic sclerosis; FVC=forced vital capacity ; DLco=diffusion capacity of the lungs for carbon monoxide; HRCT= high resolution computed tomography; QLF= quantification of lung fibrosis; QILD=quantitative interstitial lung disease CRP=C-reactive protein; GGO=ground glass opacities
The Scleroderma Lung Study I (SLS-I) evaluated the effects of oral cyclophosphamide (CYC) versus placebo in SSc-ILD. SLS-I demonstrated that the mean absolute difference in adjusted 12 month FVC (%predicted) was 2.53% favoring CYC (p<0.03) (16). CYC also improved dyspnea and quality of life compared to placebo. SLS-I is a pivotal study which demonstrated for the first time that SSc-ILD is responsive to immunosuppressive treatment in a clinical trial setting. The Scleroderma Lung Study II (SLS-II) demonstrated that the treatment of SSc-ILD with MMF for 2 years or CYC for 1 year was associated with statistically significant improvement of FVC(%predicted) in both arms at 24 months, without a between-arm difference (P=0.24) (17). Significant favorable transitions from ground-glass and/or lung fibrosis HRCT patterns to a normal pattern were observed in both arms of SLS-II (44,60). MMF and CYC also improved the modified Rodnan skin score (mRSS) course over 24 months in participants with dcSSc (61). In SLS-II, MMF was associated with less toxicity and was better tolerated than CYC. For these reasons, MMF is now considered the standard of care as first-line therapy for the treatment of SSc-ILD (62).
The SENSCIS trial, a Phase III RCT, evaluated the efficacy of nintedanib compared to placebo for patients with SSc-ILD. Patients receiving a stable dose of MMF or methotrexate for at least 6 months prior to randomization were permitted to enroll. The intergroup difference of the annual rate of change in FVC was 41.0 mL per year (95% CI 2.9 to 79.0) in favor of nintedanib (p=0.04) (18). The treatment effect of nintedanib on the annual rate of change in FVC was numerically, but not statistically significantly, lower in participants who were taking MMF at baseline than in those not taking MMF (difference of nintedanib versus placebo of 26.3 ml per year (95%CI −27.9 to 80.6) and 55.4 ml per year (95%CI 2.3–108.5) in the groups taking and not taking MMF, respectively). In addition, there were marked geographic differences in the background use of MMF. In North America, where the majority of patients were receiving MMF, the difference between treatment arms was even smaller at 10.3 ml per year (95%CI −27.9 to 80.6), but still in favor of nintedanib. As a result, the SENSCIS data suggest a possible additive or synergistic effect from combining MMF and nintedanib but the details of such a combination require further clarification (63).
The phase II faSScinate and phase III focuSSced trials evaluated the safety and efficacy of tocilizumab in patients with early active dcSSc (20,21). The primary endpoint was the difference in mean change from baseline in mRSS at week 24 and 48 in faSScinate and focuSSced, respectively. Despite a numerical difference in favor of tocilizumab in change in mRSS, neither trial reached statistical significance at p< 0.05 for their primary endpoints. However, the key secondary endpoint showed statistically significant and clinically meaningful differences in change from baseline in FVC (% predicted) at week 48 in favor of tocilizumab. In faSScinate, patients treated with tocilizumab had a smaller decrease in FVC from baseline to 24 weeks (least square mean difference 136 mL, 95% CI 9 to 264; p=0.04 in favor of tocilizumab) with a numerical effect in favor of tocilizumab also observed at week 48 (least square mean difference 120 mL, 95% CI –23 to 262; p=0.099 in favor of tocilizumab) (20). At both time points, fewer patients in the tocilizumab group than in the placebo arm had worsening of FVC (% predicted). In the focuSSced trial, 68 patients in each arm had SSc-ILD on HRCT (representing 67% and 65% of the patients in the tocilizumab and placebo arms, respectively). In these patients, risk factors for SSc-ILD progression were similar in the tocilizumab and placebo arms, including (mean (SD)) disease duration (23 months (17.2) vs. 22.6 (16.6)), proportion with positive anti-topoisomerase antibodies (68.7% vs. 68.8%), C-reactive protein levels (11.2 milligram/liter (17.4) vs. 8.0 (13.1)), baseline FVC (%pred; 77.7 (13.9) vs. 81.5 (14.9)) and baseline Quantitative ILD (20.5% (12.8) vs. 16.8% (8.8)) in the tocilizumab and placebo arms respectively (22). In the focuSSced trial, the least square mean difference of FVC (% predicted) in patients with SSc-ILD showed a change from baseline of −6.4% for placebo and +0.1 for tocilizumab (least square mean difference between groups of 6.5% (95%CI 3.4–9.5) p<0.0001) (21). Post-hoc analysis showed that early SSc-ILD was not synonymous with minimal ILD on HRCT as 41% had total lung involvement of >10 to 20% and 36% had total lung involvement >20% using a computer-generated algorithm. These data highlighted that the stabilization of lung function in the tocilizumab arm was consistent across all severity groups of SSc-ILD, demonstrating that the effects of tocilizumab were observed in all subgroups (22).
Other targeted biologics such as rituximab (anti-CD20 antibody) and abatacept (CTLA4 immunoglobulin fusion protein) have shown some beneficial effects on FVC in patients with SSc-ILD (64). In a phase II trial, abatacept showed a non-significant reduction of FVC decline at 12 months (least square mean FVC(%predicted) 2.79%, 95%CI (−0.69, 6.27), favoring abatacept compared to placebo) (64). A similar trend was observed in the open-label extension at month 18 (65). In an open-label trial comparing rituximab to CYC, mean FVC (% predicted) improved from 61.30% (SD=11.28) at baseline to 67.52% (SD 13.59) at 6 months in the rituximab arm, but declined from 59.25% (SD 12.96) to 58.06% (11.23) in the CYC arm, with a mean difference in FVC (% predicted) at 6 months of 9.46 (95% CI: 3.01–15.90; p=0.003) (66). A recent Japanese Phase II trial evaluating the impact of rituximab on skin involvement also showed promising results on FVC progression, as FVC (% predicted) change from baseline to week 24 was 0.09% in the rituximab group compared with –2.87% in the placebo group (difference 2.96% [95% CI 0.08–5.84]; p=0.04 favoring rituximab) (67).
The phase II Scleroderma: Cyclophosphamide Or Transplantaion (SCOT) trial has demonstrated the efficacy of myeloablative chemotherapy with radiation and hematopoietic stem cell transplantation (HSCT) to improve survival in a population of severe SSc patients. Among the included patients, 100% had SSc-ILD in the transplantation group and 95% in the CYC control group (68). In this RCT, 36% of patients in the HSCT arm had improvement of FVC ≥10% compared to 23% in the CYC arm. The proportion of the patients with decreased FVC ≥10% was lower in the HSCT arm than in the CYC arm (17 versus 41% respectively). Observational before-and-after HSCT studies also suggest an improvement of ILD extent on HRCT, although the small sample size precludes firm conclusions (69).
Lung transplant could be considered for patients with SSc-ILD, especially when other available treatments have failed (70,71). Referral for lung transplant should notably be considered in cases of progressive FVC and DLco decline despite combination of immunosuppressive and anti-fibrotic therapies, worsening symptoms such as dyspnea on exertion (without any other identifiable cause), and/or increasing oxygen requirement (72). In carefully selected patients with mild- to- moderate extra-pulmonary manifestations related to SSc, lung transplant for SSc-ILD has shown similar outcomes as in other fibrotic lung diseases or in PAH (73).
Points to consider when interpreting the nintedanib and tocilizumab SSc-ILD RCTs
When interpreting the results of SENSCIS and foscuSSced, it is important to underscore that the study populations were different in these trials (early active dcSSc in focuSSced, progressive ILD regardless of the cutaneous subset in SENSCIS) with potential impact on the natural progression rate in the placebo arms. Moreover, background therapies were allowed in SENSCIS, which could have contributed to limiting the FVC decline in both arms and could have impacted the results on extra-pulmonary manifestations. The expected FVC decline in the general population after age 25 years is 25–30 ml/year which is another point to consider in interpreting the FVC decline in these phase III trials, notably in the placebo arms (74). In SENSCIS, the FVC decline in the placebo arm was 93.3 mL (119.3 mL in patients not taking MMF in the placebo group), a 3- to 4-fold greater decline compared with the healthy population (18,63). In focuSSced, the placebo arm showed an absolute FVC decline of 255 mL which corresponds to a 10-fold greater decline compared to the healthy population, highlighting that included patients were at high risk of severe decline (21). This difference in rate of FVC decline between the two trials can be explained by the natural history of SSc-ILD and the underlying pathogenic mechanisms. In focuSSced, the patients included had early dcSSc, with more prominent immune-inflammatory features that were captured at a very early phase, without significant SSc-ILD during the screening phase prior to randomization and baseline HRCT (75). These patients were rarely included in previously designed SSc-ILD studies because significant and/or progressive clinical ILD was a required inclusion criterion. Thus the early treatment of this specific population of inflammatory SSc patients at high risk of progression may represent a window of opportunity to prevent the decline of pulmonary function in SSc-ILD. The patients included in the SENSCIS trial had clinical ILD where we can hypothesize that fibrotic pathways were more established with an FVC decline more predictable and similar to what was expected based on previous SSc-ILD studies (16,17). Both tocilizumab and nintedanib, nonetheless, showed biological effects that can be considered disease-modifying in SSc-ILD.
A proposed strategy for the management of SSc-ILD
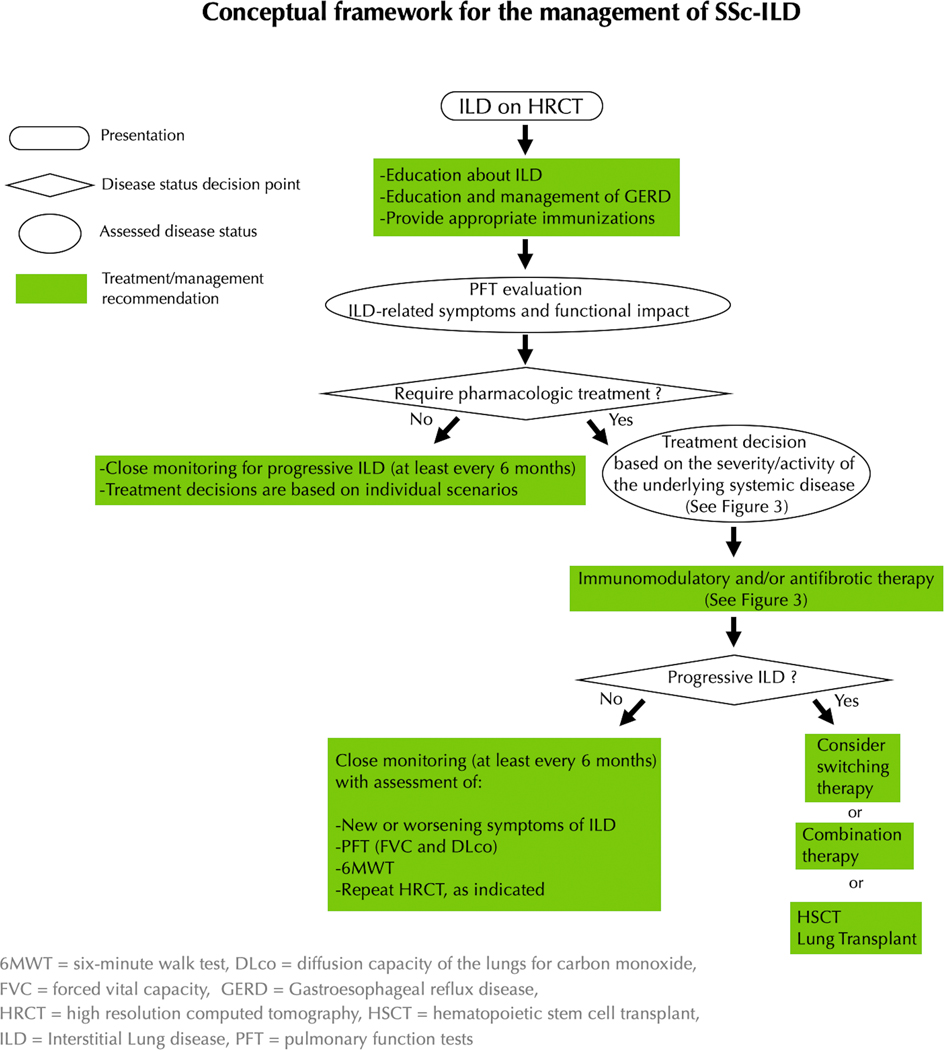
All patients with SSc-ILD deemed appropriate for pharmacologic treatment should be initiated on immunomodulatory treatment, as the pathogenesis of early ILD includes immune dysfunction and inflammation resulting in fibrosis (Figure 2). Our treatment decision algorithm for SSc-ILD is provided in Figures 2 and 3. The first step in the treatment decision algorithm is the classification of the patient along the dimension of disease severity (subsets of subclinical ILD or clinical ILD), based on ILD-specific symptoms and clinical impact, extent of ILD on HRCT, and functional impact based on FVC and/or DLco (58,70). All patients with clinical ILD should be considered for immunomodulatory treatment (15,35). If a patient has subclinical ILD, further stratification regarding risk of progressive disease determines if a given patient is a candidate for pharmacologic treatment. Treatment options may be further stratified based on the severity or activity of the extra-pulmonary manifestations of SSc.
Figure 2:
Conceptual framework for the management of SSc-ILD
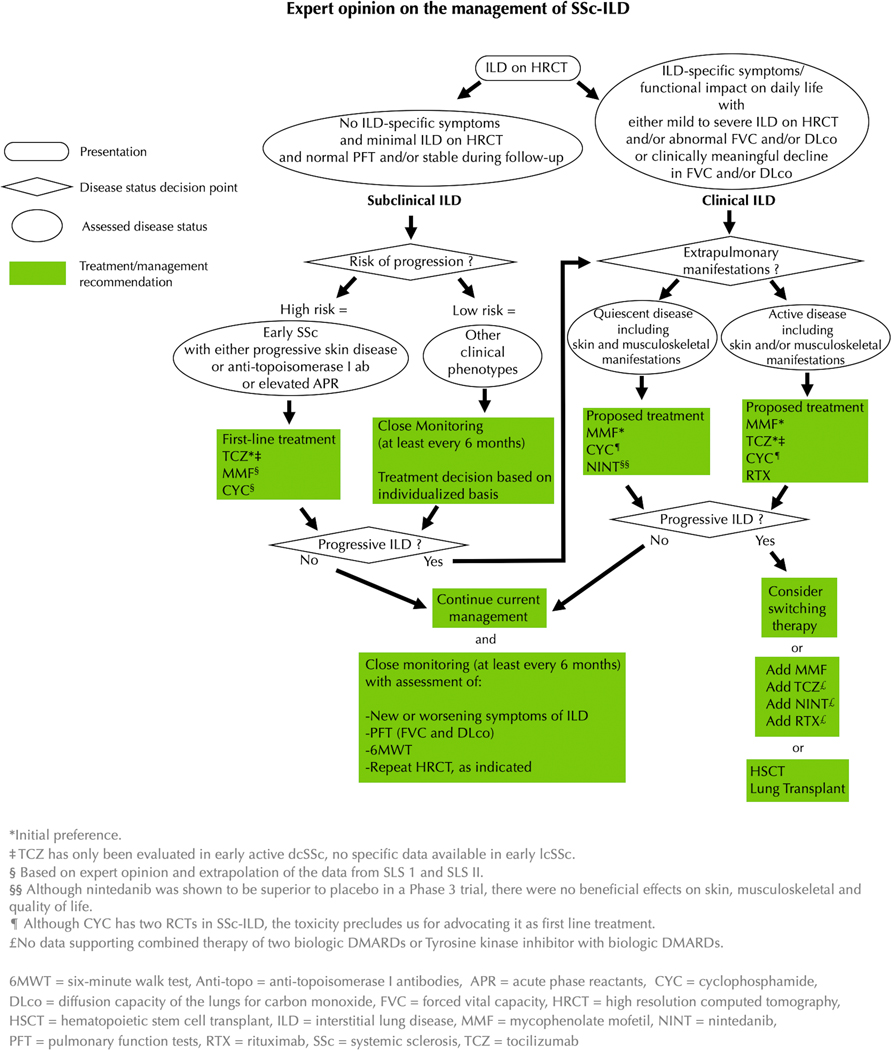
Figure 3:
Expert opinion on the management of SSc-ILD
Non-Pharmacological measures
All patients should be educated about ILD, symptom monitoring, and non-pharmacologic management. Non-pharmacological treatments include receipt of appropriate vaccinations such as influenza, pneumococcal, and COVID vaccines, pulmonary rehabilitation, and oxygen therapy if indicated. Pulmonary rehabilitation should be offered to those patients with SSc-ILD in whom dyspnea and other aspects of ILD are limiting functional capacity (76). Oxygen therapy should be considered in cases of hypoxemia (spO2<88%). The 6MWT is useful to evaluate cardiopulmonary exercise desaturation that would require oxygen therapy.
Patients should be educated about silent aspiration; optimal care of gastroesophageal reflux disease should be undertaken with early initiation of proton-pump inhibitors. Any inhalation of recreational drugs such as tobacco, marijuana, vaping, and other products should be discontinued. Recent studies have highlighted the importance of fostering a good nutritional status to maintain respiratory function in chronic respiratory disorders, especially in patients with gastrointestinal symptoms (77–79). Annual screening for immunosuppressant-induced non-melanoma skin cancers is also recommended.
Pharmacological treatment
Data emerging from the recent RCTs of tocilizumab suggest that early immunomodulatory treatments should be considered for patients with subclinical ILD with a high risk of progression (i.e., early SSc with progressive skin disease, or anti-topoisomerase antibody or elevated acute phase reactants). Tocilizumab may be proposed as initial treatment based on Phase II and III trials; patients should be advised to administer the weekly subcutaneous injections in parts of the body spared or minimally involved with skin thickening, typically the upper, outer/posterior region of the arm (21,80). MMF and CYC remain alternative options, although they lack RCT data in the context of subclinical ILD. In patients with subclinical ILD and low risk of progression, close monitoring of PFT every 6 months in early SSc is needed and case-by-case treatment decisions may be considered.
As mentioned above, all patients with clinical ILD should be considered for immunomodulatory treatment (15,35). In case of quiescent skin and musculoskeletal manifestations, MMF is the initial treatment from the authors’ perspective, with CYC and nintedanib as other acceptable first-line options that might be considered. In case of active disease including skin and/or musculoskeletal manifestations, tocilizumab, CYC, or MMF should be introduced, considering their effects on extra-pulmonary manifestations in focuSSced, SLS-I and II, respectively. Rituximab may also be an option although we usually reserve this as second-line treatment given the absence of randomized double-blind controlled trials for this drug in SSc-ILD (Figure 3). Up-front combination of nintedanib with MMF in patients with active extra-pulmonary and rapidly progressive disease is also acceptable first-line therapy (such patients may also be candidates for autologous hematopoietic stem cell transplant). We do not recommend nintedanib alone as first-line therapy in patients with SSc-ILD with active extra-pulmonary disease given the absence of impact on these manifestations in SENSCIS (63).
After treatment initiation, clinical monitoring with FVC and DLco at least every 6 months is recommended, although in those with progressive ILD, we may consider FVC and DLCo every 4 months until stabilization is documented (58). In case of stabilization, first-line treatment should be continued. In case of worsening respiratory symptoms, other diagnoses, such as cardiac involvement or pulmonary vascular disease, should be explored. If worsening parenchymal disease is suspected, a repeat HRCT should be performed to confirm progression of ILD. In the event of advancing disease despite first-line therapy, a second-line therapeutic strategy should be employed.
Three main options are proposed as second-line therapeutic strategies (Figure 3): 1) switching to another treatment, 2) considering combination of an immunomodulatory agent with an antifibrotic agent or combining two immunomodulatory agents (e.g., MMF and tocilizumab, or MMF and rituximab; although there are no data supporting the efficacy and/or safety of these combination therapies), 3) considering HSCT. Lung transplant is usually reserved for those with progressive ILD despite trials of different therapies and requires referral to a lung transplant center.
Long-term management
The follow-up of patients from SLS-I, SLS-II, and the CYC arm of SCOT have suggested that the benefit of immunomodulation was not maintained after discontinuation of the immunomodulatory agent (68,81,82). Although the optimal duration of treatment has not been determined to date, we would recommend at least 5 years of treatment, although many require longer-term treatment. This duration should take into account the initial severity of ILD, the evaluation and stabilization of ILD-related symptoms, the extra-pulmonary manifestations of SSc, and the risk of ILD progression/relapse once the treatment is stopped. In our practice, approximately 20–30% of patients experience relapse of skin and/or lung involvement once immunomodulatory therapy is discontinued. To date, there are no clinical data to support dose adjustments, such as decreased MMF dosage, after stabilization of the disease. Lower dosage may limit the risk of long-term side effects, including risk of malignancy, but such adjustments should be based on individual patient preferences and should take into account initial severity and subsequent impact of progression in case of relapse. As an example, a patient with moderate ILD and FVC% of 70% may have adequate pulmonary reserve to consider dose down-titration but someone with an FVC% of 40% who requires supplemental O2 therapy would likely not be an appropriate candidate for medication down-titration.
In case of stabilization on treatment, and/or after treatment discontinuation, PFT should continue to be performed at least every 6 months in all SSc patients for 1–2 years. After this period of close monitoring, all patients should undergo annual PFT, as late progression may occur despite long-term stabilization. The screening for other visceral manifestations, especially PAH, should also be continued according to the published screening algorithms (59).
Perspectives: early introduction of combination therapies and new combinations
Recent RCTs in PAH have demonstrated that substantial progress could be obtained through an early combination of existing drugs (83,84). The combination of bDMARDS with cDMARDS is widely used and recommended for the treatment of extra-pulmonary manifestations in other CTDs, such as rheumatoid arthritis. The complex and overlapping pathobiology involved in SSc-ILD, which involves inflammation, fibrosis, and vascular changes, also supports the potential for combination therapies, as does the finding that a diverse range of drugs has clinical utility. As such, there are many reasons to consider combination therapy as a viable approach for treating SSc-ILD.
The combination of MMF and nintedanib demonstrated a reasonable safety profile in SENSCIS, although the benefit of the combination of the two active drugs compared with monotherapy alone could not be fully demonstrated in this trial (63). In the focuSSced trial, patients taking cDMARDS were excluded, precluding any conclusion regarding the safety or efficacy of tocilizumab in combination with MMF or methotrexate (21). Nonetheless, with their differing mechanisms of action, MMF and tocilizumab may have complementary effects (85). However, we need additional data to assess for trade-offs between efficacy and safety of this combination. The efficacy and safety of the combination of a biologic such as tocilizumab with a tyrosine kinase inhibitor such as nintedanib, is still to be determined. This combination may be especially relevant considering the anti-inflammatory properties of tocilizumab and the potential more specific anti-fibrotic effects of nintedanib through PDGF and FGF-R inhibition, as well as its potential impact on vasculopathy through VEGF-R inhibition (28). The ongoing SLS-III study is investigating the impact of pirfenidone, another anti-fibrotic agent indicated for the treatment of idiopathic pulmonary fibrosis (NCT03221257), as an upfront combination treatment with MMF versus placebo and MMF in patients with SSc-ILD (86).
Conclusion
The current review provides a state-of-art practical overview of the management of SSc-ILD. As therapeutic options expand, expert perspective remains an important source of treatment guidance. The recent addition of two FDA approved medications for SSc-ILD have broadened the cache of available treatments; management should be determined by stratifying patients in terms of disease severity, risk of progression, and activity of extra-pulmonary disease. Patients with subclinical ILD and a high risk for progression should be provided therapy to prevent lung function loss; tocilizumab has demonstrated benefit in those with high risk for progression. As shown in the focuSSed trial, early ILD is not necessarily mild ILD. Tocilizumab is effective in attenuating lung function loss along a wide spectrum of lung involvement on HRCT, suggesting it can be utilized in clinical ILD with a spectrum of degree of underlying lung involvement. Nintedanib can be considered as first-line therapy in SSc-ILD but preferentially in those with limited extra-pulmonary disease (a rare scenario in early SSc) or as part of upfront combination therapy for progressive SSc-ILD in patients who are candidates for HSCT. Immunosuppressive therapy with MMF should also be considered as a primary treatment approach for clinical ILD and particularly in those with other active manifestations. In this setting, MMF has the potential to improve pulmonary function over time in the majority of patients and is similarly active with respect to improvements over time in skin disease, dyspnea, and health-related quality of life (87). Current immunomodulatory and anti-fibrotic interventions attenuate the impact of SSc-ILD but have yet to demonstrate long-lasting benefit on how patients feel, function, or survive. Further questions of upfront or sequential combination therapy with immunosuppressives and anti-fibrotics, or addition of bDMARDs, as done in other rheumatic diseases, remain areas of further research.
Acknowledgments
Funding:
Dr. Khanna was supported by the NIH/NIAMS K24-AR063120 and R01- AR-070470
Dr. Lescoat was funded by the French network of the University Hospitals HUGO (Hôpitaux Universitaire du Grand Ouest) (AAP JCM2020) and a grant from Rennes University Hospital (CORECT Visiting Grant 2020).
Dr. Roofeh was funded by the NIH/NIAMS T32-AR007080
Dr Bernstein was supported by NIH/NIAMS K23-AR075112
Conflict of Interest:
DK: Dr. Khanna reports consulting fees from Acceleron, Actelion, Bayer, BMS, Boehringer Ingelheim, CSL Behring, Horizon, Prometheus and Genentech/Roche, grants from Bayer, BMS, and Horizon; and Dr Khanna is the Chief Medical officer of Eicos Sciences Inc and has stock options.
AL: Nothing to disclose
DR: Nothing to disclose
EJB: Dr. Bernstein reports grants and personal fees from Boehringer Ingelheim, grants from Kadmon, grants from Eicos Sciences, grants from Corbus, grants from Pfizer, outside the submitted work.
EAK: Nothing to disclose
MDR: Dr. Roth reports grants and non-financial support from Genentech, Inc, outside the submitted work
FM: Dr. Martinez reports personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, non-financial support from ProterrixBio, personal fees and non-financial support from GlaxoSmithKline, personal fees from MD Magazine, personal fees from Methodist Hospital Brooklyn, personal fees and non-financial support from Miller Communications, personal fees and non-financial support from National Society for Continuing Education, personal fees from New York University, personal fees and non-financial support from PeerView Communications, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sunovion, personal fees from UpToDate, personal fees from WebMD/MedScape, other from Afferent/Merck, non-financial support from Gilead, non-financial support from Nitto, personal fees from Patara/Respivant, other from Biogen, other from Veracyte, non-financial support from Zambon, personal fees from American Thoracic Society, grants from NIH, personal fees and non-financial support from Physicians Education Resource, personal fees from Rockpointe, other from Prometic, grants from Rare Disease Healthcare Communications, personal fees and other from Bayer, other from Bridge Biotherapeutics, personal fees and non-financial support from Canadian Respiratory Network, grants from ProMedior/Roche, personal fees and non-financial support from Teva, personal fees from CME Outfitters, personal fees and non-financial support from Csl Behring, personal fees from Dartmouth University, personal fees from DevPro, from Gala, personal fees from Integritas, personal fees from IQVIA, personal fees from Projects in Knowledge, personal fees and non-financial support from Sanofi/Regeneron, from twoXAR, personal fees from Vindico, other from AbbVie, personal fees from Academy for Continuing Healthcare Learning, personal fees from United Therapeutics, personal fees from Novartis, outside the submitted work
KRF: Dr. Flaherty reports grants and personal fees from Boehringer Ingelheim, personal fees from Bellerophon, personal fees from Respivant, personal fees from Blade Therapeutics, personal fees from Roche/Genentech, personal fees from Shionogi, personal fees from DevPro, personal fees from Astra Zeneca, personal fees from Pure Health, personal fees from Horizon, personal fees from Fibrogen, personal fees from Sun Pharmaceuticals, personal fees from Pliant, personal fees from United Therapeutics, personal fees from Arrowhead, personal fees from Lupin, personal fees from Polarean, personal fees from PureTech, outside the submitted work
CPD: Dr. Denton reports grants and personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Bayer, personal fees from Sanofi, grants and personal fees from Inventiva, personal fees from Boehringer Ingelheim, personal fees from Roche, grants and personal fees from CSL Behring, personal fees from Corbus, grants from Servier, grants from Arxx Therapeutics, personal fees from Acceleron, during the conduct of the study
References
- 1.Denton CP, Khanna D. Systemic sclerosis. The Lancet 2017;390:1685–1699. [DOI] [PubMed] [Google Scholar]
- 2.Frantz C, Avouac J, Distler O, Amrouche F, Godard D, Kennedy AT, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Seminars in Arthritis and Rheumatism 2016;46:115–123. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–205. [PubMed] [Google Scholar]
- 4.LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–1576. [PubMed] [Google Scholar]
- 5.Domsic RT, Medsger TA. Autoantibodies and Their Role in Scleroderma Clinical Care. Curr Treat Options in Rheum 2016;2:239–251. [Google Scholar]
- 6.Nihtyanova SI, Sari A, Harvey JC, Leslie A, Derrett-Smith EC, Fonseca C, et al. Using Autoantibodies and Cutaneous Subset to Develop Outcome-Based Disease Classification in Systemic Sclerosis. Arthritis Rheumatol 2020;72:465–476. [DOI] [PubMed] [Google Scholar]
- 7.Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan EKL, Damoiseaux J, Carballo OG, Conrad K, Melo Cruvinel W de, Francescantonio PLC, et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015. Front Immunol 2015;6:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Etiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease. Am J Respir Crit Care Med 2020;201:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–1905. [DOI] [PubMed] [Google Scholar]
- 11.Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger TA. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol 2007;34:104–109. [PubMed] [Google Scholar]
- 12.Bernstein EJ, Jaafar S, Assassi S, Domsic RT, Frech TM, Gordon JK, et al. Performance Characteristics of Pulmonary Function Tests for the Detection of Interstitial Lung Disease in Adults With Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol 2020;72:1892–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann-Vold A-M, Molberg Ø, Midtvedt Ø, Garen T, Gran JT. Survival and causes of death in an unselected and complete cohort of Norwegian patients with systemic sclerosis. J Rheumatol 2013;40:1127–1133. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty DH, Kwakkenbos L, Carrier M-E, Salazar G, Assassi S, Baron M, et al. The Scleroderma Patient-Centered Intervention Network Cohort: baseline clinical features and comparison with other large scleroderma cohorts. Rheumatology (Oxford) 2018;57:1623–1631. [DOI] [PubMed] [Google Scholar]
- 15.Roofeh D, Distler O, Allanore Y, Denton CP, Khanna D. Treatment of systemic sclerosis–associated interstitial lung disease: Lessons from clinical trials. Journal of Scleroderma and Related Disorders 2020;5:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus Placebo in Scleroderma Lung Disease. New England Journal of Medicine 2006;354:2655–2666. [DOI] [PubMed] [Google Scholar]
- 17.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. New England Journal of Medicine 2019;380:2518–2528. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. New England Journal of Medicine 2019;381:1718–1727. [DOI] [PubMed] [Google Scholar]
- 20.Khanna D, Denton CP, Jahreis A, Laar JM van, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016;387:2630–2640. [DOI] [PubMed] [Google Scholar]
- 21.Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Respiratory Medicine 2020;0. Available at: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30318-0/abstract. Accessed September 3, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Roofeh D, Lin CJF, Goldin J, Kim GH, Furst DE, Denton CP, et al. Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease. Arthritis Rheumatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Distler O. Assessment of recent evidence for the management of patients with systemic sclerosis-associated interstitial lung disease: a systematic review. ERJ Open Res 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkmann ER, Varga J. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat Rev Rheumatol 2019;15:208–224. [DOI] [PubMed] [Google Scholar]
- 25.Lescoat A, Ballerie A, Augagneur Y, Morzadec C, Vernhet L, Fardel O, et al. Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lescoat A, Ballerie A, Jouneau S, Fardel O, Vernhet L, Jego P, et al. M1/M2 polarisation state of M-CSF blood-derived macrophages in systemic sclerosis. Ann Rheum Dis 2019;78:e127. [DOI] [PubMed] [Google Scholar]
- 27.Valenzi E, Bulik M, Tabib T, Morse C, Sembrat J, Bittar HT, et al. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Annals of the Rheumatic Diseases 2019;78:1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J 2015;45:1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denton CP, Ong VH, Xu S, Chen-Harris H, Modrusan Z, Lafyatis R, et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 2018;77:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numajiri H, Kuzumi A, Fukasawa T, Ebata S, Yoshizaki-Ogawa A, Asano Y, et al. B cell depletion inhibits fibrosis via suppressing pro-fibrotic macrophage differentiation in a mouse model of systemic sclerosis. Arthritis Rheumatol 2021. [DOI] [PubMed] [Google Scholar]
- 31.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 2014;15:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Jia G, Guttman A, DePianto DJ, Morshead KB, Sun K-H, et al. Osteopontin Links Myeloid Activation and Disease Progression in Systemic Sclerosis. Cell Rep Med 2020;1:100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrova DT, Brandhorst G, Koch C, Schultze FC, Eberle C, Walson PD, et al. Mycophenolic acid reverses TGF beta-induced cell motility, collagen matrix contraction and cell morphology in vitro. Cell Biochem Funct 2015;33:503–508. [DOI] [PubMed] [Google Scholar]
- 34.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47:85–118. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Barake R, Barsotti S, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. The Lancet Rheumatology 2020;2:e71–e83. [DOI] [PubMed] [Google Scholar]
- 36.Roofeh D, Jaafar S, Vummidi D, Khanna D. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol 2019;31:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, Bois RM du, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. European Respiratory Journal 2015;46:976–987. [DOI] [PubMed] [Google Scholar]
- 38.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse infiltrative lung disease: pathologic-CT correlation. Radiology 1993;189:693–698. [DOI] [PubMed] [Google Scholar]
- 40.Takei R, Arita M, Kumagai S, Ito Y, Tokioka F, Koyama T, et al. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease. Respirology 2018;23:385–391. [DOI] [PubMed] [Google Scholar]
- 41.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002;165:1581–1586. [DOI] [PubMed] [Google Scholar]
- 42.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44–e68. [DOI] [PubMed] [Google Scholar]
- 43.Kim HG, Tashkin DP, Clements PJ, Li G, Brown MS, Elashoff R, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol 2010;28:S26–35. [PMC free article] [PubMed] [Google Scholar]
- 44.Goldin JG, Kim GHJ, Tseng C-H, Volkmann E, Furst D, Clements P, et al. Longitudinal Changes in Quantitative Interstitial Lung Disease on Computed Tomography after Immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc 2018;15:1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh NSL, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248–1254. [DOI] [PubMed] [Google Scholar]
- 46.Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 2014;146:422–436. [DOI] [PubMed] [Google Scholar]
- 47.Suliman YA, Dobrota R, Huscher D, Nguyen-Kim TDL, Maurer B, Jordan S, et al. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis & Rheumatology 2015;67:3256–3261. [DOI] [PubMed] [Google Scholar]
- 48.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–1446. [DOI] [PubMed] [Google Scholar]
- 49.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 50.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. In: Vol 9th. Little. Boston: Brown & Co; 1994:253–256. [Google Scholar]
- 51.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–586. [DOI] [PubMed] [Google Scholar]
- 52.Distler O, Assassi S, Cottin V, Cutolo M, Danoff SK, Denton CP, et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J 2020;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Mayes MD, Pedroza C, Draeger HT, Gonzalez EB, Harper BE, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res (Hoboken) 2013;65:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis & Rheumatology (Hoboken, NJ) 2014;66:1625–1635. [DOI] [PubMed] [Google Scholar]
- 55.Stock CJW, Hoyles RK, Daccord C, Kokosi M, Visca D, De Lauretis A, et al. Serum markers of pulmonary epithelial damage in systemic sclerosis-associated interstitial lung disease and disease progression. Respirology 2021;26:461–468. [DOI] [PubMed] [Google Scholar]
- 56.Salazar GA, Kuwana M, Wu M, Estrada-Y-Martin RM, Ying J, Charles J, et al. KL-6 But Not CCL-18 Is a Predictor of Early Progression in Systemic Sclerosis-related Interstitial Lung Disease. J Rheumatol 2018;45:1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaeger VK, Valentini G, Hachulla E, Cozzi F, Distler O, Airó P, et al. Brief Report: Smoking in Systemic Sclerosis: A Longitudinal European Scleroderma Trials and Research Group Study. Arthritis & Rheumatology (Hoboken, NJ) 2018;70:1829–1834. [DOI] [PubMed] [Google Scholar]
- 58.Roofeh D, Khanna D. Management of systemic sclerosis: the first five years. Curr Opin Rheumatol 2020;32:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young A, Moles VM, Jaafar S, Visovatti S, Huang S, Vummidi D, et al. Performance of the DETECT Algorithm for Pulmonary Hypertension Screening in a Systemic Sclerosis Cohort. Arthritis Rheumatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim GHJ, Tashkin DP, Lo P, Brown MS, Volkmann ER, Gjertson DW, et al. Using Transitional Changes on High-Resolution Computed Tomography to Monitor the Impact of Cyclophosphamide or Mycophenolate Mofetil on Systemic Sclerosis-Related Interstitial Lung Disease. Arthritis Rheumatol 2020;72:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Namas R, Tashkin DP, Furst DE, Wilhalme H, Tseng C-H, Roth MD, et al. Efficacy of Mycophenolate Mofetil and Oral Cyclophosphamide on Skin Thickness: Post Hoc Analyses From Two Randomized Placebo-Controlled Trials. Arthritis Care Res (Hoboken) 2018;70:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaafar S, Lescoat A, Huang S, Gordon J, Hinchcliff M, Shah AA, et al. Clinical characteristics, visceral involvement, and mortality in at-risk or early diffuse systemic sclerosis: a longitudinal analysis of an observational prospective multicenter US cohort. Arthritis Research & Therapy 2021;23:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Highland KB, Distler O, Kuwana M, Allanore Y, Assassi S, Azuma A, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. [DOI] [PubMed] [Google Scholar]
- 64.Khanna D, Spino C, Johnson S, Chung L, Whitfield ML, Denton CP, et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis & Rheumatology 2020;72:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chung L, Spino C, McLain R, Johnson SR, Denton CP, Molitor JA, et al. Safety and efficacy of abatacept in early diffuse cutaneous systemic sclerosis (ASSET): open-label extension of a phase 2, double-blind randomised trial. The Lancet Rheumatology 2020;2:e743–e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) 2018;57:2106–2113. [DOI] [PubMed] [Google Scholar]
- 67.Ebata S, Yoshizaki A, Oba K, Kashiwabara K, Ueda K, Uemura Y, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. The Lancet Rheumatology 2021;0. Available at: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(21)00107-7/abstract. Accessed June 1, 2021. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med 2018;378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Launay D, Marjanovic Z, Bazelaire C de, Florea L, Zohar S, Keshtmand H, et al. Autologous hematopoietic stem cell transplant in systemic sclerosis: quantitative high resolution computed tomography of the chest scoring. J Rheumatol 2009;36:1460–1463. [DOI] [PubMed] [Google Scholar]
- 70.Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol 2021;33:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernstein EJ, Peterson ER, Sell JL, D’Ovidio F, Arcasoy SM, Bathon JM, et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol 2015;67:1314–1322. [DOI] [PubMed] [Google Scholar]
- 72.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1–15. [DOI] [PubMed] [Google Scholar]
- 73.Jablonski R, Dematte J, Bhorade S. Lung transplantation in scleroderma: recent advances and lessons. Curr Opin Rheumatol 2018;30:562–569. [DOI] [PubMed] [Google Scholar]
- 74.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khanna D, Denton CP. Integrating new therapies for systemic sclerosis-associated lung fibrosis in clinical practice. Lancet Respir Med 2021;9:560–562. [DOI] [PubMed] [Google Scholar]
- 76.Dowman LM, McDonald CF, Hill CJ, Lee AL, Barker K, Boote C, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax 2017;72:610–619. [DOI] [PubMed] [Google Scholar]
- 77.Nakayama K, Nakajima Y, Tanaka R, Hirata K-I, Emoto N. Predictors of Long-term Outcomes in Patients With Connective Tissue Disease Associated With Pulmonary Arterial Hypertension. J Clin Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanjrawi AA, Mathers L, Webster S, Corte TJ, Carey S. Nutritional status and quality of life in interstitial lung disease: a prospective cohort study. BMC Pulm Med 2021;21:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jouneau S, Crestani B, Thibault R, Lederlin M, Vernhet L, Valenzuela C, et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir Res 2020;21:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suleman Y, Clark KEN, Cole AR, Ong VH, Denton CP. Real-world experience of Tocilizumab in systemic sclerosis: potential benefit on lung function for anti-topoisomerase (ATA) positive patients. Rheumatology 2021. Available at: 10.1093/rheumatology/keab273. Accessed April 4, 2021. [DOI] [PubMed] [Google Scholar]
- 81.Khanna D, Furst DE, Clements PJ, Tashkin DP, Eckman MH. Oral cyclophosphamide for active scleroderma lung disease: a decision analysis. Med Decis Making 2008;28:926–937. [DOI] [PubMed] [Google Scholar]
- 82.Volkmann ER, Tashkin DP, Sim M, Li N, Goldmuntz E, Keyes-Elstein L, et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis 2019;78:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lajoie AC, Lauzière G, Lega J-C, Lacasse Y, Martin S, Simard S, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 2016;4:291–305. [DOI] [PubMed] [Google Scholar]
- 84.Khanna D, Zhao C, Saggar R, Mathai SC, Chung L, Coghlan JG, et al. Long-Term Outcomes in Patients With Connective Tissue Disease-Associated Pulmonary Arterial Hypertension in the Modern Treatment Era: Meta-Analyses of Randomized, Controlled Trials and Observational Registries. Arthritis Rheumatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandran S, Leung J, Hu C, Laszik ZG, Tang Q, Vincenti FG. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: A randomized controlled trial. Am J Transplant 2020. [DOI] [PubMed] [Google Scholar]
- 86.Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, et al. An Open-label, Phase II Study of the Safety and Tolerability of Pirfenidone in Patients with Scleroderma-associated Interstitial Lung Disease: the LOTUSS Trial. J Rheumatol 2016;43:1672–1679. [DOI] [PubMed] [Google Scholar]
- 87.Volkmann ER, Tashkin DP, LeClair H, Roth MD, Kim G, Goldin J, et al. Treatment With Mycophenolate and Cyclophosphamide Leads to Clinically Meaningful Improvements in Patient-Reported Outcomes in Scleroderma Lung Disease: Results of Scleroderma Lung Study II. ACR Open Rheumatology 2020;2:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]