Abstract
Background
Prediction of endoscopic postoperative recurrence (POR) and prophylactic treatment based on clinical risk profile have thus far been inconclusive. This study aimed to examine the association between clinical risk profile and the development of endoscopic POR in a Crohn’s disease population without postoperative treatment and to identify individual risk factors of endoscopic POR.
Methods
Medical records of 142 patients with Crohn’s disease during follow-up after ileocecal or ileocolonic resection without prophylactic treatment at 3 referral centers were reviewed. Endoscopic POR was defined as a modified Rutgeerts score ≥i2b. Clinical risk profiles were distilled from current guidelines. Both uni- and multivariate logistic regression analysis were used to assess the relationship between risk profiles and endoscopic POR.
Results
Endoscopic POR was observed in 68 out of 142 (47.9%) patients. Active smoking postsurgery (odds ratio [OR], 3.01; 95% confidence interval [CI], 1.24-7.34; P = 0.02), a Montreal classification of A3 (OR, 3.05; 95% CI, 1.07-8.69; P = 0.04), and previous bowel resections (OR, 2.58; 95% CI, 1.07-6.22; P = 0.03) were significantly associated with endoscopic POR. No significant association was observed between endoscopic POR and any guideline defined as a high-/low-risk profile. However, patients with a combination of any 3 or more European Crohns & Colitis Organisation– (OR, 4.87; 95% CI, 1.30-18.29; P = 0.02) or British Society of Gastroenterology–defined (OR 3.16; 95% CI, 1.05-9.49; P = 0.04) risk factors showed increased odds of developing endoscopic POR.
Conclusions
Our results suggest that patients with a combination of any 3 or more European Crohns & Colitis Organisation– or British Society of Gastroenterology–defined risk factors would probably benefit from immediate prophylactic treatment.
Keywords: natural history, Crohn disease, endoscopic recurrence, risk stratification
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) characterized by a relapsing and remitting course of inflammation in the gastrointestinal tract.1 Despite ongoing drug development and expansion of therapeutic options over the last 3 decades, more than 50% of patients still require surgery within 10 years of diagnosis.2 Ileocolonic resection (ICR) is the most commonly used surgical intervention, however, rarely leads to cure of the disease with recurrence usually appearing in the neoterminal ileum.3 Within 6 months postsurgery, approximately 70% of patients show endoscopic postoperative recurrence (POR), with approximately 18% of patients progressing to symptomatic POR within 1 year.4, 5 After 3 years, endoscopic POR rates increase to approximately 80%, with symptomatic POR requiring treatment intensification in 45% of all patients at 5 years.4, 5 As for surgical POR (the need for another resection), rates are 0.6% at 1 year, 10.9% at 5 years, 18.6% at 10 years, and 28.3% at 20 years.4, 6
Previous research has shown that the postoperative clinical course is best predicted by assessing the severity of endoscopic lesions within the first year after surgery.7-9 For this reason, current guidelines, including the American College of Gastroenterology (AGA), the European Crohn’s and Colitis Organisation (ECCO), and the British Society of Gastroenterology (BSG), support the use of endoscopy as the gold standard to assess recurrent disease, which can be quantified with the Rutgeerts score.7, 10, 11 A score of ≥i2 or a modified score of ≥i2b is generally defined as endoscopic POR, requiring step-up of treatment (Table 1).11, 12
TABLE 1.
Modified Rutgeerts Score12
| i0 | No lesions in the distal ileum |
|---|---|
| i1 | <5 aphthous lesions in the distal ileum |
| i2a | Lesions confined to the ileocolonic anastomosis (including anastomotic stenosis) |
| i2b | ≥5 aphthous lesions or larger lesions, with normal mucosa inbetween, in the neoterminal ileum (with or without anastomotic line) |
| i3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| i4 | Large ulcers with diffuse mucosal inflammation or nodules or stenosis in the neoterminal ileum |
Many efforts have been investigated to prevent severe endoscopic POR. Current practice and guidelines are based upon clinical characteristics to label patients as either high- or low-risk and recommend postoperative treatment in high-risk patients in an attempt to avoid endoscopic POR.7, 11 However, to date, clinical risk models to preoperatively predict which patients are at an increased risk of endoscopic POR and therefore require early postoperative treatment have not been validated.13 The postoperative recurrence model of CD has been considered a unique model to study the natural history of this disease, because recurrence represents the earliest phase of inflammation.14-17 Numerous studies have evaluated potential risk factors for POR, yet results have been inconclusive and inconsistent, predominantly because of outcome and population heterogeneity.18-26 Furthermore, the majority of these studies are affected by the use of postoperative medical treatment as an important confounder. In addition, evidence regarding the benefit of early prophylactic treatment in high-risk patients is limited.7, 11, 27, 28 So far, only 3 studies assessing risk factors for POR in patients with CD without the use of postoperative medical treatment have been published.29-31 No natural history studies assessing risk factors for endoscopic POR using the modified Rutgeerts cutoff of ≥i2b have been performed.
Hence the question remains whether patients with a high clinical risk profile according to current guidelines,7, 10, 11 left untreated until their first endoscopy after surgery, show significantly higher rates of endoscopic POR than low-risk patients and thus may benefit from early postoperative treatment intervention, ultimately preventing disease progression.
Therefore, this study aims to examine the associations between clinical risk profiles and the development of endoscopic POR within 12 months after surgery in a postoperative CD population without prophylactic anti-inflammatory treatment. In addition, endoscopic POR rates and potential risk factors that could influence the rate of recurrence in these patients using the modified Rutgeerts score were evaluated.
MATERIALS AND METHODS
This study is a multicenter, retrospective cohort study performed in 3 referral centers specializing in IBD in Europe: the Amsterdam University Medical Center (UMC) location AMC (the Netherlands), the Ghent University Hospital (Belgium), and the University Medical Centre Ljubljana (Slovenia).
All patients with historically established CD who underwent ileocecal resection or resection of the neoterminal ileum with primary anastomosis between October 2007 and July 2019 were reviewed for the use of any anti-inflammatory treatment or intervention until their first endoscopy after resection. We excluded patients who did not undergo a follow-up endoscopy within the first year after surgery, those who were lost to follow-up, those in whom not all macroscopically diseased bowel was removed, and those who received any anti-inflammatory treatment during follow-up.
Collection of Clinical Variables
Medical records and details of the outpatient clinic follow-up were reviewed for the following patient characteristics: sex, age at surgery, disease duration, previous IBD-related bowel surgery, Montreal classification, smoking behavior after surgery, type of anastomosis (side-to-side, end-to-side, side-to-end), surgical indication (stenosis, perforation, or therapy-refractory disease), length of resected ileum, and IBD-related medication use 6 months before surgery. Active smoking was defined as patients currently smoking ≥5 cigarettes/week, postoperative cessation was defined as patients who stopped smoking after surgery at least until their follow-up endoscopy, and nonsmokers were defined as patients who had never smoked or only smoked occasionally. Finally, IBD-related medications used before surgery included adalimumab, infliximab, vedolizumab, ustekinumab, azathioprine, methotrexate, 6-tioguanine, 6-mercaptopurine, budesonide, corticosteroids, and mesalazine.
For a subset of patients (Amsterdam UMC cohort, n = 95), both resection specimens and pathology reports were available and reviewed by a single IBD pathologist (AM) regarding the presence of granulomas. The modified Rutgeerts score was assessed from detailed reports and high-quality endoscopic photos that were taken during all endoscopic procedures, reviewed by a single expert gastroenterologist (GD).
Endpoints
The primary outcome of interest was the association of endoscopic POR with clinical risk profiles as defined in current guidelines.7, 10, 11 Three different definitions (AGA, ECCO, and BSG) of high-risk patients were evaluated for their association with endoscopic POR (Table 2).
TABLE 2.
Definitions of High Risk According to Current Guidelines
| Guidelines | High-Risk Definition | |
|---|---|---|
| ≥1 of Following Factors | ≥2 of Following Factors | |
| AGA11 | Age ≤30 y | — |
| Active smoking | ||
| ≥2 prior surgeries for penetrating disease, with or without perianal disease | ||
| ECCO7 | Current smoking | — |
| Prior intestinal surgery | ||
| Penetrating disease at index surgery | ||
| Perianal location | ||
| Granulomas in resection specimen | ||
| Myenteric plexitis | ||
| BSG10 | — | Active smoking |
| Penetrating disease | ||
| Multiple resections | ||
| Perianal fistulae | ||
| Extensive small bowel disease (≥50 cm ileum) | ||
| Residual active disease | ||
| Granulomas or myenteric plexitis |
Secondary outcomes of this study were the overall endoscopic POR of CD, defined as a modified Rutgeerts score of ≥i2b without the use of postoperative anti-inflammatory treatment, and the association of baseline patient characteristics with endoscopic POR, unbiased by the use of prophylactic medical therapy.
Statistical Analysis
Descriptive statistics were used to examine the baseline characteristics of all included patients. Continuous data are expressed as median (interquartile ratio [IQR]), and categorical data as frequencies and percentages. For each guideline definition, multiple cutoffs were generated based on the number of risk factors present. Subsequently, the association between the amount of risk factors present per guideline definition with endoscopic POR was assessed using univariate logistic regression analyses.
In addition, the association between each of the baseline characteristics and the probability of endoscopic POR was assessed using univariate logistic regression analyses. A multivariate logistic regression model was constructed using only the covariates showing a significant (P ≤ 0.05) association with endoscopic POR on univariate analysis. Missing values in covariates were imputed using multivariate imputation by chained equations in R.32 Imputation was performed for covariates with <15% missing values (smoking behavior after surgery, family history of IBD, type of anastomosis, and length of resected ileum). Imputation of these variables was performed 5 separate times, generating 5 separate datasets. The multivariate regression analysis was performed on all 5 imputed datasets and the results were pooled and are expressed as odds ratios (ORs) with 95% percent confidence intervals (CI).
Two-tailed probabilities were used with a P value ≤0.05 considered as statistically significant. All analyses were performed using IBM SPSS statistics version 26 and R 3.6.1.
Ethical Considerations
This study was waived from review of the medical ethics board.
RESULTS
One hundred and forty-two patients with established CD at the AMC, Ghent University Hospital, and University Medical Centre Ljubljana who underwent ICR between October 2007 and July 2019 were enrolled. Of these 142 patients, 56.3% were female and 43.7% were male, with a median age of 33 years. There were no patients with disease located exclusively in the colon, as expected. Disease behavior showed 48.6% with structuring disease, 44.4% with penetrating disease, and 21.8% with perianal disease. The indication for surgery was stenosis in the majority of patients (52.8%). Other indications were refractory disease (21.1%) and perforation (26.1%).
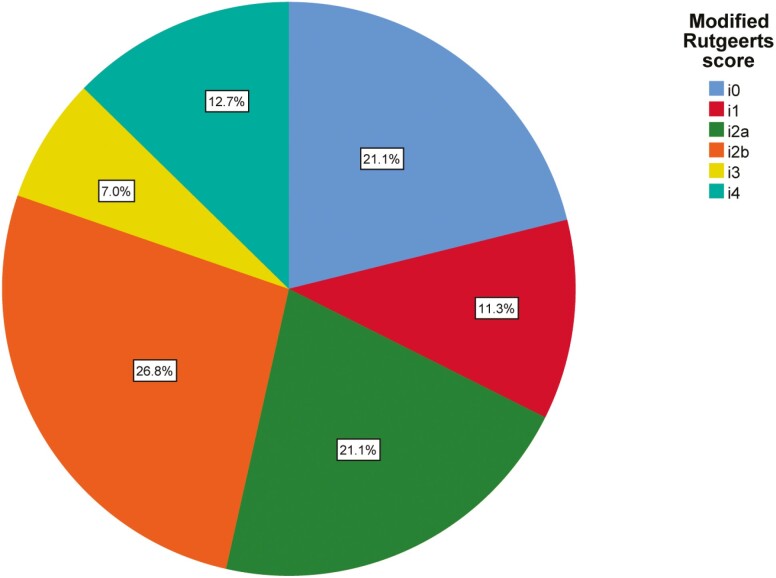
The median time between diagnosis of CD and surgery was 5 years and 224 days, and the median time between resection and endoscopy was 203 days (IQR, 180-253 days). The majority of patients were nonsmokers (61.2%). The main differences between the IBD centers were for disease duration (P = 0.02), type of anastomosis (P = 0.00), surgical indication (P = 0.01), disease location (P = 0.02), and medication use 6 months before surgery (P = 0.00). No significant difference in endoscopic POR was seen between the centers (P = 0.50). Demographic and clinical features are shown in Table 3. The overall endoscopic POR rates are shown in Fig. 1.
TABLE 3.
Patient Characteristics
| Characteristics | Amsterdam (n = 95) | Ghent (n = 15) | Ljubljana (n = 32) | Total (N = 142) | |
|---|---|---|---|---|---|
| Sex | Female, n (%) | 60 (63.2) | 7 (46.7) | 13 (40.6) | 80 (56.3) |
| Age, y (at time of surgery) | Median y (IQR) | 33 (24-48) | 29 (25-45) | 33 (26-45.5) | 33 (25-47) |
| Disease duration | Median y (IQR) | 7 (1-17) | 3 (0-8) | 2.5 (0-9) | 5 (1-14.3) |
| Family history of IBD | Yes, n (%) | 21 (25.6) | 5 (41.7) | 3 (10.3) | 29 (23.6) |
| Previous IBD-related abdominal surgery | Yes, n (%) | 23 (24.3) | — | 8 (25.0) | 31 (21.8) |
| Age at diagnosis, y, n (%) | ≤16 (Montreal classification A1) | 8 (8.4) | 1 (6.7) | 2 (6.3) | 11 (7.7) |
| 17-40 (Montreal classification A2) | 72 (75.8) | 11 (73.3) | 25 (78.1) | 108 (76.1) | |
| ≥40 (Montreal classification A3) | 15 (15.8) | 3 (20.0) | 5 (15.6) | 23 (16.2) | |
| Disease location, n (%) | Ileal disease (L1) | 59 (62.1) | 8 (53.3) | 11 (34.3) | 78 (54.9) |
| Ileocolonic disease (L3) | 36 (37.9) | 7 (46.7) | 21 (65.6) | 64 (45.1) | |
| Upper GI involvement (L4) | 4 (4.2) | 2 (13.3) | — | 6 (4.2) | |
| Disease behavior, n (%) | Nonstricturing/penetrating (B1) | 8 (8.4) | 2 (13.3) | — | 10 (7.0) |
| Stricturing (B2) | 46 (48.4) | 8 (53.3) | 15 (46.9) | 69 (48.6) | |
| Penetrating (B3) | 41 (43.2) | 5 (33.3) | 17 (53.1) | 63 (44.4) | |
| Perianal disease (p) | 21 (22.1) | 4 (26.7) | 6 (18.8) | 31 (21.8) | |
| Smoking postsurgery, n (%) | Active smoker | 20 (21.5) | 2 (16.7) | 8 (26.7) | 30 (22.4) |
| Nonsmoker | 54 (58.1) | 8 (66.7) | 20 (66.7) | 82 (61.2) | |
| Postoperative cessation | 19 (20.4) | 1 (8.3) | 2 (6.7) | 22 (16.4) | |
| Type of anastomosis, n (%) | Side-to-side anastomosis | 80 (89.9) | 15 (100) | 2 (8.3) | 97 (72.4) |
| End-to-side anastomosis | 4 (4.5) | — | 15 (62.5) | 19 (14.2) | |
| End-to-end anastomosis | 4 (4.5) | — | 7 (29.2) | 11 (8.2) | |
| Surgery indication, n (%) | Perforation | 33 (34.7) | 3 (20.0) | 1 (3.1) | 37 (26.1) |
| Stenosis | 42 (44.2) | 10 (66.7) | 23 (71.9) | 75 (52.8) | |
| Refractory disease | 20 (21.1) | 2 (13.3) | 8 (25.0) | 30 (21.1) | |
| Length of resected ileum (cm) | mean (±SD) or median (IQR) | 25.8 (14.3) | 22.4 (9.9) | 21.3 (16.3) | 22 (15–30) |
| Extended resection (≥50 cm ileum resected) | Yes, n (%) | 8 (8.7) | — | 1 (4.8) | 9 (7.1) |
| Granulomas in resection specimen | Yes, n (%) | 28 (30.1) | NA | NA | 28 (30.1) |
| Medication use 6 months before surgery, n (%) | Any | 79 (83.2) | 8 (53.3) | 18 (56.3) | 105 (73.9) |
| Biologics | 43 (45.3) | 5 (33.3) | 4 (12.5) | 52 (36.6) | |
| Anti-TNFs | 41 (43.2) | 5 (33.3) | 4 (12.5) | 50 (35.2) | |
| Immunomodulators | 33 (34.7) | 3 (20.0) | 12 (37.5) | 48 (33.8) | |
| Corticosteroids | 44 (46.3) | 4 (26.7) | 4 (12.5) | 52 (36.6) | |
| Endoscopic recurrence (i2b, i3, i4) | Yes, n (%) | 47 (49.5) | 7 (46.7) | 12 (37.5) | 68 (47.9) |
Percentages shown are valid percentages. GI indicates gastrointestinal.
FIGURE 1.
Rates of different scores according to Rutgeerts score for all 142 patients. Each slice represents the percentage of patients within that subcategory of the modified Rutgeerts score.
The majority of patients had a Rutgeerts score of i2 (47.9%, n = 68) when using the original Rutgeerts’ score. After subdividing i2 into i2a and i2b, 38 patients had a Rutgeerts’ score i2b (26.8%). Endoscopic POR, defined as a Rutgeerts score of ≥i2b, was seen in 46.5% of patients (n = 66), whereas using a cutoff score of ≥i2 resulted in 67.6% of patients (n = 96) with endoscopic POR. Modification of the Rutgeerts score by subdividing i2 into i2a and i2b thus resulted in a decrease of 21.1% of patients classified as having endoscopic disease recurrence.
High- vs Low-Risk Patient Association With Endoscopic Recurrence
Ninety-eight (69%), 99 (69.7%), and 52 (36.6%) patients were considered at high risk of developing POR according to current AGA, ECCO, and BSG consensus.7, 10, 11 No significant association with endoscopic POR was observed when comparing high- vs low-risk patients using univariate logistic regression analysis for any of these high-risk definitions (AGA: OR, 1.06; 95% CI, 0.52-2.17; P = 0.87; ECCO: OR, 1.50; 95% CI, 0.72-3.10; P = 0.28; and BSG: OR, 1.25; 95% CI, 0.63-2.48; P = 0.52, respectively).
However, the presence of any ≥3 risk factors compared to <3 risk factors was the only cutoff resulting in a significantly increased odds of developing endoscopic POR for both the ECCO and BSG high-risk definitions (OR, 4.87; 95% CI, 1.30-18.29; P = 0.02 and OR, 3.16; 95% CI, 1.05-9.49; P = 0.04), as shown in Table 4. Increasing the number of risk factors present according to AGA consensus did not result in a significant association with endoscopic POR.
TABLE 4.
Univariate Associations of High-Risk Patients Who Have Surgery With Endoscopic Recurrence (Rutgeerts score ≥i2b)
| OR (95% CI) | P | ||
|---|---|---|---|
| Without histology (N = 142) | |||
| AGA definition | ≥1 factors present (n = 98, 69%) | 1.06 (0.52-2.17) | 0.87 |
| ≥2 factors present (n = 29, 20.4%) | 1.85 (0.81-4.23) | 0.15 | |
| All 3 factors present (n = 7, 4.9%) | 1.57 (0.34-7.29) | 0.57 | |
| ECCO definition | ≥1 factors present (n = 99, 69.7%) | 1.50 (0.72-3.10) | 0.28 |
| ≥2 factors present (n = 43, 30.3%) | 1.50 (0.73-3.07) | 0.27 | |
| ≥3 factors present (n = 14, 9.9%) | 4.87 (1.30-18.29) | 0.02a | |
| BSG definition | ≥2 factors present (n = 52, 36.6%) | 1.25 (0.63-2.48) | 0.52 |
| ≥3 factors present (n = 17, 12%) | 3.16 (1.05-9.49) | 0.04a | |
| With histology (n = 95) | |||
| ECCO definition | ≥1 factors present (n = 78, 82.2%) | 1.50 (0.52-4.35) | 0.45 |
| ≥2 factors present (n = 35, 36.9%) | 1.96 (0.84-4.58) | 0.12 | |
| ≥3 factors present (n = 18, 19%) | 3.29 (1.07-10.13) | 0.04a | |
| BSG definition | ≥2 factors present (n = 42, 44.2%) | 1.74 (0.77-3.94) | 0.19 |
| ≥3 factors present (n = 19, 20%) | 3.65 (1.19-11.15) | 0.02a |
aIndicates significance.
When we added the presence of granulomas to factors contributing to the risk profile (found in 30% of resection specimens), 78 (82.2%) patients according to the ECCO definition and 42 (44.2%) patients according to the BSG definition out of 95 patients (AMC cohort alone) were classified as having a high risk of endoscopic POR.
When we looked at the outcomes, we did not observe any difference in the incidence of endoscopic POR among high- vs low-risk patients using univariate logistic regression analysis for both definitions (ECCO: OR, 1.50; 95% CI, 0.52-4.35; P = 0.45 and BSG: OR, 1.74; 95% CI, 0.77-3.94; P = 0.19).
When we looked at cumulative risk factors, again the presence of any ≥3 risk factors was significantly associated with an increased odds of endoscopic POR (ECCO: OR, 3.29; 95% CI, 1.07-10.13; P = 0.04 and BSG: OR, 3.65; 95% CI, 1.19-11.15; P = 0.02).
Our findings were comparable when we performed the same analysis with a Rutgeerts score ≥i2 (instead of i2b) as a cutoff for both the full cohort and the Amsterdam UMC cohort alone (Supplementary Table 1).
Risk Factors for Recurrence
Univariate analysis showed that active smoking after surgery (OR, 3.84; 95% CI, 1.56-9.44; P = 0.003), age at surgery (OR, 1.03; 95% CI, 1.04-1.06; P = 0.02), and a Montreal classification of A3 (OR, 3.78; 95% CI, 1.39-10.26; P = 0.01) were significantly associated with endoscopic POR (Table 5). Interestingly, postoperative cessation of smoking showed a marginally increased odds of patients developing endoscopic POR (OR, 2.38; 95% CI, 0.91-6.21; P = 0.08).
TABLE 5.
Uni- and Multivariate Analysis of the Association Between Clinical Variables and Endoscopic Recurrence (Rutgeerts score ≥i2b)
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Sex; ref: male | 1.36 (0.70-2.65) | 0.36 | ||
| Age at surgery, y | 1.03 (1.04-1.06) | 0.02a | ||
| Disease duration, y | 1.00 (0.96-1.03) | 0.78 | ||
| Smoking postsurgery; ref: no | ||||
| Active smoker | 3.84 (1.56-9.44) | 0.003a | 3.01 (1.24-7.34) | 0.02a |
| Postoperative cessation | 2.38 (0.91-6.21) | 0.08 | 1.68 (0.65-4.32) | 0.29 |
| Family history of IBD; ref: no | 1.61 (0.69-3.74) | 0.27 | ||
| Montreal classification age at diagnosis | ||||
| A1 vs A2 and A3 | 0.60 (0.17-2.14) | 0.43 | ||
| A2 vs A1 and A3 | 0.48 (0.22-1.05) | 0.07 | ||
| A3 vs A1 and A2 | 3.78 (1.39-10.26) | 0.01a | 3.05 (1.07-8.69) | 0.04a |
| Montreal classification location; ref: L3 | 0.96 (0.50-1.86) | 0.91 | ||
| Upper GI disease: yes vs no | 1.09 (0.21-5.61) | 0.92 | ||
| Montreal classification behavior | ||||
| B1 vs B2 and B3 | 0.52 (0.13-2.18) | 0.37 | ||
| B2 vs B1 and B3 | 1.39 (0.72-2.70) | 0.33 | ||
| B3 vs B1 and B2 | 0.98 (0.51-1.90) | 0.95 | ||
| Perianal disease; ref: no | 1.43 (0.64-3.18) | 0.38 | ||
| Medication 6 months before surgery | ||||
| Yes vs no | 1.11 (0.52-2.36) | 0.65 | ||
| Biologics; ref: no | 0.70 (0.35-1.40) | 0.31 | ||
| Anti-TNFs; ref: no | 0.69 (0.35-1.39) | 0.30 | ||
| Immunomodulators; ref: no | 0.69 (0.34-1.38) | 0.29 | ||
| Corticosteroids; ref: no | 1.29 (0.65-2.56) | 0.47 | ||
| History of IBD surgery, yes vs no | 2.39 (1.05-5.45) | 0.04a | 2.58 (1.07-6.22) | 0.03a |
| Type of anastomosis | ||||
| SSA vs ESA and EEA | 0.73 (0.36-1.48) | 0.38 | ||
| ESA vs SSA and EEA | 0.45 (0.16-1.27) | 0.13 | ||
| EEA vs SSA and ESA | 3.16 (0.80-12.43) | 0.10 | ||
| Surgical indication | ||||
| Perforation vs stenosis and refractory disease | 0.84 (0.40-1.77) | 0.65 | ||
| Stenosis vs refractory disease and perforation | 1.13 (0.58-2.19) | 0.72 | ||
| Refractory disease vs stenosis and perforation | 1.02 (0.45-2.31) | 0.96 | ||
| Length of resected ileum, cm | 1.03 (0.99-1.05) | 0.06 | ||
| Extensive resection (≥50 cm); ref: no | 1.11 (0.46-2.66) | 0.82 | ||
| Granulomas in resection specimen; ref: no | 0.84 (0.35-2.04) | 0.70 |
a P values < 0.05. EEA indicates end-to-end anastomosis; ESA, end-to-side anastomosis; GI, gastrointestinal; SSA, side-to-side anastomosis.
All other parameters showed no significant relationship with endoscopic POR.
When endoscopic POR was defined as a Rutgeerts score ≥i2 rather than i2b, active smoking after surgery compared with no smoking (OR, 3.56; 95% CI, 1.13-11.19; P = 0.03) and the use of anti-tumor necrosis factor (TNF) drugs before surgery (OR, 0.47; 95% CI, 0.23-0.99; P = 0.05) showed a significant association with endoscopic POR (Supplementary Table 2).
Further, we performed a multivariate logistic analysis, excluding age at surgery because of multicollinearity. Of the remaining variables that were significant at univariate analysis, active smoking after surgery, a Montreal classification of A3, and a history of previous IBD-related bowel resection were significantly associated with endoscopic POR of CD after ileocecal resection in the multivariate analysis (Table 5), with odds ratios of 3.0 (95% CI, 1.24-7.34; P = 0.02), 2.6 (95% CI, 1.07-6.22; P = 0.03), and 3.1 (95% CI, 1.07-8.69; P = 0.04) respectively.
Multivariate analysis with a Rutgeerts score of i2 as the cutoff showed active smoking after surgery (OR, 3.07; 95% CI, 1.06-8.86; P = 0.04) and the use of anti-TNF drugs 6 months before surgery (OR, 0.48; 95% CI, 0.22-1.01; P = 0.05) to be significantly associated with a higher or lower risk of endoscopic POR, respectively (Supplementary Table 2).
DISCUSSION
Our retrospective analyses show endoscopic POR rates of 67.6% for a Rutgeerts score ≥i2 and 46.5% for a Rutgeerts score ≥i2b with a median follow-up time of 7 months (203 days; IQR, 180-253 days) in patients who did not receive any prophylactic anti-inflammatory treatment after ICR to prevent endoscopic POR of CD. These results are similar to previously reported endoscopic recurrence rates. In 1990, Rutgeerts, Geboes, Vantrappen, et al8 reported an endoscopic POR rate of 61% for a Rutgeerts score ≥i2 and 44% for a Rutgeerts score ≥i3. A recent update from the same center, now 30 years later, reported a endoscopic POR of 70% for a Rutgeerts score ≥i2 and 45.4% for a Rutgeerts score ≥i2b at a median time of 6.2 months.33 Surprisingly and despite novel medical treatments that have become available (20% of patients received immediate preventive therapy after surgery), the authors could not show significant progress in changing the disease course after ICR.
Furthermore, our results could not confirm the presumed association of clinical risk profiles (high- versus low-risk, based on current guidelines) with the incidence of endoscopic POR.7, 10, 11 Only when ≥3 of the “established” risk factors were present, seen in 9.9% (ECCO definition) and 12% (BSG definition) of our cohort, did we observe a significant association with endoscopic POR. This effect was constant across the different subanalyses (Supplementary Table 2). The recently published BSG guideline already suggests a stricter cutoff, with at least 2 or more factors required to be present as compared to other guidelines.10 Taken together, our results suggest that an even stricter stratification of patients at high risk (needing prophylactic treatment) seems desirable.
Both active smoking after surgery and previous IBD-related resections are known as factors associated with endoscopic POR.18, 34, 35 Replication of these variables as risk factors in our cohort population, unaffected by postoperative anti-inflammatory treatment, supports their independent association with endoscopic POR.
Somewhat surprisingly, our results suggest that older age at diagnosis (Montreal A3) is significantly associated with endoscopic POR. Older age at diagnosis refers to montreal A3 which is >40 years at diagnosis. Out of the 142 included patients within our cohort, 23 (16.2%) classified as Montreal A3. In these 23 patients, a striking 17 (73.9%) showed a Rutgeerts score of ≥i2b with an estimated 3-fold increased odds of developing endoscopic recurrence.
Despite ongoing debate regarding age at diagnosis as a risk factor for recurrence, our results contrast with previously reported outcomes indicating a decreased risk when patients are diagnosed at an older age.36 It is usually accepted that patients with CD diagnosed at a younger age represent a more severe, genetically influenced phenotype, whereas onset of CD at an older age may rather be the result of predominantly environmental exposures, progressing more slowly.37 As a result, the age at diagnosis represented in the Montreal classification could actually be past the “true” age at diagnosis and is just not recognized earlier because of the slow progressing of the disease. Therefore, one might think that these patients would present a more advanced disease stage at index surgery. Nonetheless, our results show only a significant different distribution in active smoking (47.8% vs 25.47%; P = 0.01) and disease duration (median 2 years vs median 6 years; P = 0.03) between the Montreal A3 group vs the A1 and A2 groups, both associated with an increased risk of recurrence.34, 36 Despite correcting for these observed differences in our multivariate model, we found that the Montreal A3 group remained significantly associated with endoscopic recurrence. We believe that the exclusion of patients receiving postoperative prophylactic treatment, mostly affecting the Montreal A1 group, may have altered the comparisons made in these already small groups of patients. These results should therefore be interpreted with caution.
Notably, anti-TNF treatment 6 months before surgery was not associated with endoscopic POR in our cohort (OR, 0.69; 95% CI, 0.35-1.39; P = 0.30). A similar result was observed in Wasmann et al,38 in 106 patients undergoing primary ICR between 2002 and 2009 (hazard ratio, 1.68; 95% CI, 0.6-5.1) with comparable use of anti-TNF drugs before surgery (31% vs 35.2%). Moreover, recent data from a retrospective series of 823 patients with CD undergoing primary ICR between 2000 and 2019 again suggests a nonsignificant association (hazard ratio, 1.21; 95% CI, 0.87-1.67; P = 0.26) with endoscopic POR (Rutgeerts score ≥ i2b).39 Although the failure of anti-TNF treatment before surgery suggests a more refractory group of patients with a potential increased risk of developing POR, this assumption could not be confirmed in both our cohort without prophylactic treatment and 2 recent studies in which up to 47% of patients did receive postoperative prophylactic treatment.
Current guidelines propose prophylactic treatment with either anti-TNFs or thiopurines within 4 weeks after surgery for patients with a high-risk profile.7, 11 In addition, all patients should undergo endoscopic monitoring at 6 months and be treated accordingly based on the presence of endoscopic recurrence.7, 11
If we had followed the guidelines in our cohort, 85 patients (60%) according to the ECCO definition and 35 patients (25%) according to the BSG definition would have potentially been overtreated, which is associated with potential adverse effects and high cost.
Moreover, immediate prophylactic treatment does not guarantee endoscopic or clinical remission. Data from the PREVENT trial40 showed that the 6-month endoscopic POR rates in high-risk patients were 45% in patients receiving thiopurines and 21% in patients receiving anti-TNF treatment. In addition, the authors concluded that prophylactic treatment with infliximab is not superior compared to placebo for the prevention of clinical recurrence. We therefore suggest that only patients with any combination of at least 3 ECCO- or BSG-defined risk factors should receive immediate prophylactic treatment, justifying the associated high cost, increased patient burden, and possibility of adverse effects.
Strengths and Weaknesses
Our study is one of the few studies including only patients who did not receive anti-inflammatory therapy after surgery until follow-up endoscopy, allowing us to assess the natural behavior of CD after surgery without medical interference. The nature of this cohort also provided the opportunity to examine the difference in endoscopic recurrence between patients classified as high- or low-risk according to the literature.7, 10, 11 Providing clinicians with a clinically relevant risk profile may result in a more accurate estimation of the number of risk factors associated with endoscopic recurrence and lowers the proportion of patients being potentially overtreated, thereby reducing patient burden and health care costs.
There are, however, some limitations to our study, predominantly because of its retrospective nature. First, we selected only those patients without prophylactic anti-inflammatory treatment postsurgery from 3 referral centers. As a consequence, our cohort reflects a relatively small part of IBD referral center patients, limiting the generalizability of our results to the total IBD population.
Second, the scoring of endoscopies was mainly based on endoscopic reports and pictures from multiple endoscopists. However, all available reports and high-quality images were reviewed by a single expert endoscopist (GD).
Third, after stratification by endoscopic recurrence or clinical high- vs low-risk, the relatively small number of patients limited the accuracy of the analyses. We were therefore underpowered to detect small differences between groups. Nonsignificant outcomes thus only mean that large differences between groups were not found.
Last, we limited our study to endoscopic outcomes. We were therefore unable to assess the impact of high- vs low-risk profiles on long-term clinical and surgical outcomes.
CONCLUSIONS
Active smoking after surgery, older age at diagnosis (ages >40 years) and previous IBD-related bowel resections were identified as risk factors for endoscopic POR. No difference in endoscopic POR between clinically high- and low-risk patients, according to current guideline definitions, was observed. Our results suggest that patients with a combination of any ≥3 ECCO- and BSG-defined risk factors would probably benefit from immediate prophylactic treatment. Additional prospective studies designed to assess the benefit of prophylactic treatment based on this composite risk profile with endoscopic, clinical, and surgical endpoints must be conducted to provide a more conclusive answer to this important question.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Elien Glorieus for assisting with patient data extraction.
Author contributions: Conceptualization: VJ, MD, GD. Methodology: VJ, MD, GD. Formal analysis: VJ, NM. Data acquisition: VJ, GN, MK, PH, CB, WB, AM. Writing—original draft: VJ. Writing—review and editing: MD, GD, WB, PH, GN, AM, NM. Approval of final manuscript: all authors.
REFERENCES
- 1. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology . Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. [DOI] [PubMed] [Google Scholar]
- 2. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 3. Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128:856–861. [DOI] [PubMed] [Google Scholar]
- 4. de Buck van Overstraeten A, Eshuis EJ, Vermeire S, et al. Short- and medium-term outcomes following primary ileocaecal resection for Crohn’s disease in two specialist centres. Br J Surg. 2017;104:1713–1722. [DOI] [PubMed] [Google Scholar]
- 5. O’Connor A, Hamlin PJ, Taylor J, et al. Postoperative prophylaxis in Crohn’s disease after intestinal resection: a retrospective analysis. Frontline Gastroenterol. 2017;8:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beelen EMJ, van der Woude CJ, Pierik MJ, et al. Decreasing trends in intestinal resection and re-resection in Crohn’s disease: a nationwide cohort study. Ann Surg. 2021;273:557–563. [DOI] [PubMed] [Google Scholar]
- 7. Gionchetti P, Dignass A, Danese S, et al. ; ECCO . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–149. [DOI] [PubMed] [Google Scholar]
- 8. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. [DOI] [PubMed] [Google Scholar]
- 9. Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen GC, Loftus EV Jr, Hirano I, et al. ; AGA Institute Clinical Guidelines Committee . American Gastroenterological Association Institute Guideline on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017;152:271–275. [DOI] [PubMed] [Google Scholar]
- 12. Gecse K, Lowenberg M, Bossuyt P, et al. Sa1198 agreement among experts in the endoscopic evaluation of postoperative recurrence in Crohn’s disease using the Rutgeerts score. Gastroenterology 2014;146:S-227. [Google Scholar]
- 13. Campbell JP, Vaughn BP. Optimal delivery of follow-up care after surgery for Crohn’s disease: current perspectives. Clin Exp Gastroenterol. 2016;9:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Achkar JP, Hanauer SB. Medical therapy to reduce postoperative Crohn’s disease recurrence. Am J Gastroenterol. 2000;95:1139–1146. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed T, Rieder F, Fiocchi C, et al. Pathogenesis of postoperative recurrence in Crohn’s disease. Gut. 2011;60:553–562. [DOI] [PubMed] [Google Scholar]
- 16. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed SG. The role of infection in the pathogenesis of vaso-occlusive crisis in patients with sickle cell disease. Mediterr J Hematol Infect Dis. 2011;3:e2011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riss S, Schuster I, Papay P, et al. Repeat intestinal resections increase the risk of recurrence of Crohn’s disease. Dis Colon Rectum. 2013;56:881–887. [DOI] [PubMed] [Google Scholar]
- 19. Unkart JT, Anderson L, Li E, et al. Risk factors for surgical recurrence after ileocolic resection of Crohn’s disease. Dis Colon Rectum. 2008;51:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manser CN, Frei P, Grandinetti T, et al. ; Investigators of the Swiss IBD Cohort Study . Risk factors for repetitive ileocolic resection in patients with Crohn’s disease: results of an observational cohort study. Inflamm Bowel Dis. 2014;20:1548–1554. [DOI] [PubMed] [Google Scholar]
- 21. Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008;103: 196–205. [DOI] [PubMed] [Google Scholar]
- 22. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang W, Tang Y, Nong L, et al. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: a meta-analysis of observational studies. J Crohns Colitis. 2015;9:293–301. [DOI] [PubMed] [Google Scholar]
- 24. Ellis L, Calhoun P, Kaiser DL, et al. Postoperative recurrence in Crohn’s disease. The effect of the initial length of bowel resection and operative procedure. Ann Surg. 1984;199:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolff BG. Factors determining recurrence following surgery for Crohn’s disease. World J Surg. 1998;22:364–369. [DOI] [PubMed] [Google Scholar]
- 26. Simillis C, Purkayastha S, Yamamoto T, et al. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn’s disease. Dis Colon Rectum. 2007;50:1674–1687. [DOI] [PubMed] [Google Scholar]
- 27. Cohen-Mekelburg S, Schneider Y, Gold S, et al. Risk stratification for prevention of recurrence of postoperative Crohn’s disease. Gastroenterol Hepatol (N Y). 2017;13:651–658. [PMC free article] [PubMed] [Google Scholar]
- 28. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute Technical Review on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017;152:277–295.e3. [DOI] [PubMed] [Google Scholar]
- 29. Margagnoni G, Aratari A, Mangone M, et al. Natural history of ileo-caecal Crohn’s disease after surgical resection. A long term study. Minerva Gastroenterol Dietol. 2011;57:335–344. [PubMed] [Google Scholar]
- 30. Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Renna S, Cammà C, Modesto I, et al. Meta-analysis of the placebo rates of clinical relapse and severe endoscopic recurrence in postoperative Crohn’s disease. Gastroenterology. 2008;135:1500–1509. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riviere P, Vermeire S, Irles-Depe M, et al. Rates of post-operative recurrence of Crohn’s disease and effects of immunosuppressive and biologic therapies. Clin Gastroenterol Hepatol. Published online ahead of print April 6, 2020. doi: 10.1016/j.cgh.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 34. Reese GE, Nanidis T, Borysiewicz C, et al. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213–1221. [DOI] [PubMed] [Google Scholar]
- 35. Hellers G. Crohn’s disease in Stockholm County 1955-1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl. 1979;490:1–84. [PubMed] [Google Scholar]
- 36. Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol. 2005;11:3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Essers JB, Lee JJ, Kugathasan S, et al. ; NIDDK IBD Genetics Consortium . Established genetic risk factors do not distinguish early and later onset Crohn’s disease. Inflamm Bowel Dis. 2009;15:1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wasmann K, van Amesfoort J, van Montfoort ML, et al. The predictive value of inflammation at ileocecal resection margins for postoperative Crohn’s recurrence: a cohort study. Inflamm Bowel Dis 2020;26:1691–1699. [DOI] [PubMed] [Google Scholar]
- 39. Arkenbosch J, Beelen E, Hoentjen F. P0635: postoperative prophylactic thiopurines or biologicals are protective for clinical and endoscopic recurrence at short term follow-up after primary ileocecal resection in Crohn’s disease, but not associated with a long-term beneficial effect on the risk of re-resection. UEG J. 2020;8:491. [Google Scholar]
- 40. Regueiro M, Feagan BG, Zou B, et al. ; PREVENT Study Group . Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150:1568–1578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.