Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) involves multiple organs and shows increased inflammatory markers. Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, several studies have reported the association between severe COVID-19 and MIS-C. Reversible cerebral vasoconstriction syndrome (RCVS) presents with thunderclap headaches and multifocal reversible vasoconstriction on imaging. RCVS is very rare in children. This article reports two cases of pediatric COVID-19 with severe MIS-C and clinical and imaging features indicative of RCVS.
Methods
Clinical, laboratory, and imaging data of the patients were reviewed. The diagnosis of RCVS was confirmed based on clinical symptomatology and brain magnetic resonance imaging findings.
Results
Two pediatric patients with clinical findings compatible with severe MIS-C and hemodynamic compromise presented to the hospital. During their hospitalization course, they developed thunderclap headaches and neurological deficits. Both were receiving vasoactive agents, intravenous immunoglobulin, and immunosuppressants. Imaging studies showed marked multifocal cerebral vasoconstriction in both cases and infarcts in one. The course and management of the patients will be presented. After controlling inflammation and elimination of triggers, both patients were ultimately symptom free upon discharge. Cerebral vasoconstriction had completely resolved on follow-up imaging.
Conclusions
Although a variety of symptoms including headaches may be seen in pediatric COVID-19 patients with MIS-C, RCVS should be considered as a differential diagnosis in cases of thunderclap headache accompanied by neurological signs in these patients. Imaging findings and follow-up are also key in establishing the diagnosis.
Keywords: Multisystem inflammatory syndrome in children, Reversible cerebral vasoconstriction syndrome, COVID-19, Thunderclap headache
Background
Multisystem inflammatory syndrome in children (MIS-C) can present as an inflammatory response to coronavirus disease 2019 (COVID-19) involving multiple organs such as the heart, gastrointestinal tract, lung, kidneys, and nervous system along with increased inflammatory markers.1 , 2 Numerous cases of involvement of the central and peripheral nervous systems have been reported in MIS-C with a variety of clinical presentations including seizures, encephalitis, encephalopathy, stroke, acute disseminated encephalomyelitis, and focal central nervous system involvement.3 , 4 Reversible cerebral vasoconstriction syndrome (RCVS) emerges with severe sudden, recurrent, and reversible headaches accompanying multifocal, often bilateral, vasoconstriction on imaging with or without focal neurological signs.5 This syndrome is quite rare in children, particularly young children, and has only been reported in case reports or very small case series.6 Recently two adult case reports have been published that reported RCVS in COVID-19 patients.7 , 8 This article reports two pediatric COVID-19 patients with severe MIS-C in association with thunderclap headaches and findings of RCVS.
Case 1
A 10-year-old girl presented with fever, dysentery, and hypovolemia without upper respiratory symptoms and without any known previous medical history. Her blood tests showed cytopenia, high troponin levels, and increased inflammatory markers. She had been in contact with a positive case of COVID-19 one week before admission (Table ). The patient was diagnosed with severe MIS-C and treated with pulsed methylprednisolone (30 mg/kg, 2 doses) and intravenous immunoglobulin (IVIG) (2 g/kg), which was continued with 2 mg/kg of methylprednisolone. She received epinephrine and milrinone due to hemodynamic instability and left ventricular dysfunction seen on echocardiography.
TABLE.
Patients’ Demographics and Characteristics
| Patient Number/Sex/Age (Years) | System Involvements | Link to COVID-19 | Inflammatory Markers | Admission Laboratory Data | Neurological Signs | Echocardiogram | Brain MRI and MRA | Immune Therapy | ICU Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|
| 1/F/10 | Cardiovascular, gastrointestinal, nervous, blood | Sick contact | ESR: 50 mm LDH: 1447 U/L Ferritin: >1650 ng/mL CRP: 108 mg/L D-dimer: 2821 ng/mL Albumin: 2.5 g/dL |
WBC: 9200 cells/μL (Lymph: 11%) Hb: 7.6 g/dL Platelet: 239,000 cells/μLT roponin: 1449 ng/LC reatinine: 0.7 mg/dL INR: 1.2 PTT: 31 seconds FDP: 24 μg/mL Fibrinogen: 354 mg/dL |
Thunderclap headache, seizure, photophobia, blurred vision, hemiplegia, proximal weakness of lower extremity | LVEF = 51%, mitral regurgitation, mild dilated LA and LV, no coronary arteriopathy | Hemodynamic infarction, RCVS | IVIG (2 g/kg, 2 dose), IVMP (30 mg/kg, 4 doses then 2 mg/kg), infliximab (10 mg/kg) | 21 |
| 2/M/6 | Cardiovascular, gastrointestinal, nervous, blood, mucocutaneous, renal | Positive serum IgG | ESR: 60 mm LDH: 793 U/L Ferritin: 649 ng/mL CRP: 154 mg/L D-dimer: 2100 ng/mL Albumin: 4.1 g/dL |
WBC: 9400 cells/μL (Lymph: 20%) Hb:13.2 g/dLP latelet: 120,000 cells/μLT roponin: 17.3 ng/LC reatinine: 0.8 mg/dL INR: 1.1 PTT: 28 seconds FDP: 15 μg/fibrinogen: 391 mg/dL |
Thunderclap headache, photophobia | LVEF = 55%, mild pericardial effusion, small aneurysm in right coronary artery | RCVS | IVIG (2 g/kg, 1 dose), IVMP (30 mg/kg, 3 doses then 2 mg/kg) | 6 |
Abbreviations:
COVID-19 = Coronavirus disease 2019
CRP = C-reactive protein
ESR = Erythrocyte sedimentation rate
F = Female
FDP = Fibrin degradation products
Hb = Hemoglobin
ICU = Intensive care unit
INR = International normalized ratio
IVIG = Intravenous immunoglobulin
IVMP = Intravenous methylprednisolone
LA = Left atrium
LDH = Lactate dehydrogenase
LV = Left ventricle
LVEF = Left ventricular ejection fraction
Lymph = Lymphocyte
M = Male
MRA = Magnetic resonance angiography
MRI = Magnetic resonance imaging
PTT = Partial thromboplastin time
RCVS = Reversible cerebral vasoconstriction syndrome
WBC = White blood cell
On the fourth day of hospitalization, she experienced an abrupt severe thunderclap frontal headache, nausea, vomiting, epigastric pain, chest pain, and a suspected seizure with upward gaze. She also complained of diplopia and visual impairment, and ST elevation was observed in her electrocardiogram. Further tests showed elevated ferritin, troponin, amylase, and lipase levels, and ultrasound confirmed pancreatitis. Cardiac computed tomographic angiography showed normal coronary arteries. IVIG (2 g/kg) was administered again along with levetiracetam as an antiepileptic drug.
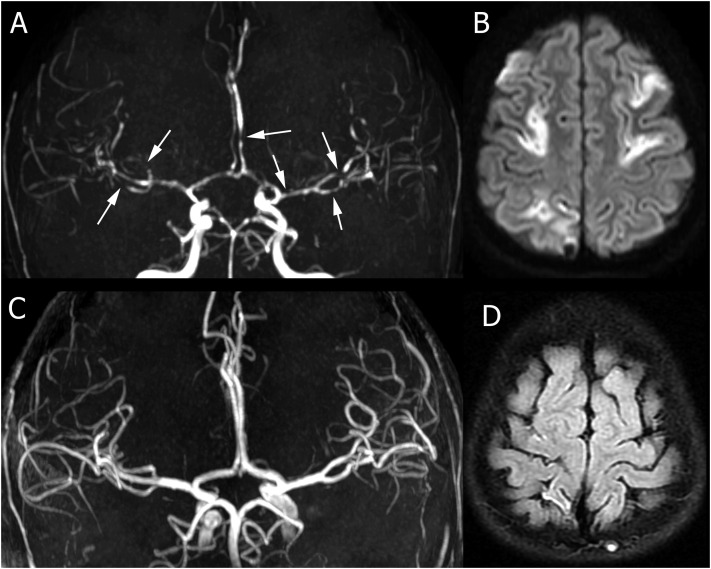
She continued to suffer from intermittent abrupt and severe headaches alongside nausea and hypotension. The patient had an episode of seizure with upward gaze and urinary incontinence. The dose of levetiracetam was therefore increased, and carbamazepine and gabapentin were added. After four days, brain magnetic resonance imaging (MRI) showed multifocal infarcts (Fig 1 A). Brain magnetic resonance angiography (MRA) showed widespread multifocal foci of arterial narrowing bilaterally (Fig 1B). No subarachnoid hemorrhage was identified. High levels of inflammatory markers such as ferritin were still observed in laboratory investigations. An additional dose of methylprednisolone pulse (20 mg/kg) and a single dose of infliximab (10 mg/kg) were administered to control inflammation. The severity and frequency of headaches, symptoms of autonomic dysfunction, and involvement of the other organs decreased. Two weeks later, a second dose of infliximab was administered given the insignificant decrease in inflammatory markers, especially ferritin. Inflammatory markers ultimately decreased, lower extremity weakness and cardiac symptoms improved, and headache attacks and autonomic dysfunction were completely alleviated. After 27 days, the patient was discharged in good general condition with fludrocortisone, aspirin, and oral prednisolone (2 mg/kg). A two-month follow-up revealed no residual neurological deficits, and medications were reduced and ultimately discontinued. Follow-up brain MRI and MRA five months later showed residual small foci of chronic infarct and resolution of the areas of vasoconstriction (Fig 1C and D).
FIGURE 1.
Initial and follow-up imaging for patient 1. (A) Magnetic resonance angiography (MRA) demonstrates multifocal areas of vasoconstriction in multiple vascular territories (arrows). (B) Diffusion-weighted images a week after presentation show multifocal acute infarcts in different vascular territories. (C) Follow-up imaging after five months demonstrates complete resolution of the foci of vasoconstriction on MRA. (D) Fluid-attenuated inversion recovery images demonstrate small residual areas of chronic infarct in the cortical regions.
Case 2
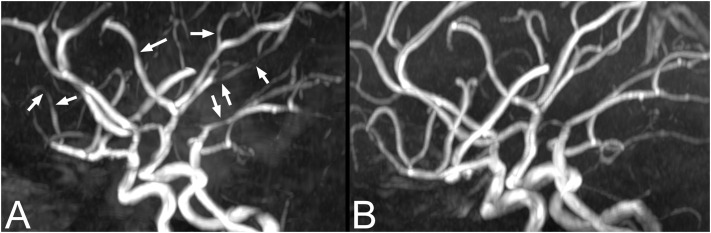
A six-year-old previously healthy male presented with fever, diarrhea, vomiting, severe abdominal pain, peripheral edema, erythematous lips, and bilateral conjunctivitis. Serologic tests were positive for COVID-19 and showed increased inflammatory markers (Table). Symptoms of shock that developed during his hospitalization were treated with fluid therapy, epinephrine, and milrinone. Also, the patient received packed cells very early in the first hours of admission because of low hemoglobin. Echocardiography revealed myocardial dysfunction. The patient was diagnosed with MIS-C and Kawasaki shock syndrome and underwent pulse methylprednisolone therapy (30 mg/kg, two doses) and IVIG therapy (2 g/kg) for five days. On the fifth day of hospitalization, the patient developed an abrupt, severe, and thunderclap headache followed by nausea and vomiting. A brain MRI and MRA was performed the following day, showing multifocal bilateral foci of arterial vasoconstriction (Fig 2 A) but no infarcts.
FIGURE 2.
Initial and follow-up imaging for patient 2. (A) Magnetic resonance angiography (MRA) demonstrates multifocal areas of vasoconstriction in multiple vascular territories (arrows). (B) Follow-up imaging after six months demonstrates complete resolution of the foci of vasoconstriction on MRA.
Vasoactive agents were tapered after improvements in his cardiac function. The patient's headaches resolved around the ninth to tenth day of hospitalization. On the eleventh day of hospitalization, he developed gross hematuria, proteinuria, dysmorphic red blood cell (60%), and hypertension and was diagnosed with nephritis and therefore underwent a third pulse steroid therapy (30 mg/kg). After 11 days, he was discharged in good general condition, with oral prednisolone (2 mg/kg), aspirin, and amlodipine. A one-month clinical follow-up revealed no neurological deficits. A six-month follow-up brain MRI and MRA showed complete resolution of vasoconstriction (Fig 2B).
Discussion
We present the first report of two pediatric COVID-19 patients with severe MIS-C who developed severe thunderclap headaches during their treatment course. Brain MRA was consistent with RCVS, and one patient had multifocal infarcts. Follow-up imaging also demonstrated complete resolution of vasoconstriction. Both patients met the criteria of the Centers for Disease Control and Prevention definition for MIS-C associated with COVID-19 infection as evidenced by their clinical and laboratory indicators.9 MIS-C was treated in these patients using IVIG, corticosteroids, and other immunosuppressant agents. Vasoactive agents were administered during their hospitalization due to shock and cardiac dysfunction. Medications were decreased and discontinued after observing improved organ function and inflammatory markers. Headaches and neurological signs were not present in the patients at the time of discharge or on follow-up.
Current diagnostic criteria for RCVS are (1) acute severe headache, (2) monophasic course without new symptoms more than one month after onset, (3) segmental cerebral arterial vasoconstriction, (4) exclusion of aneurysmal subarachnoid hemorrhage, (5) normal or near-normal cerebrospinal fluid, and (6) complete or marked normalization of arterial changes after 12 weeks, although some patients may not meet all criteria. The pathogenic mechanism is thought to be a transient dysregulation of arterial tone secondary to sympathetic overactivity.5 Both of our patients fulfill the diagnostic criteria for RCVS. Our patients did not undergo catheter angiography, but that is not a necessary study for the diagnosis of RCVS, and the MRA examinations were adequately informative. Recently, a new scoring system named RCVS2 score (range: −2 to 10) has been developed to distinguish RCVS from other intracranial arteriopathies. Although the RCVS2 score has been targeted for young adults, both of our cases had a score higher than five according to this scale, which purportedly confirms the diagnosis of RCVS with 94% specificity and 86% sensitivity using the new scoring system.10 The sudden vasoconstriction may cause daily-to-weekly thunderclap headaches with an approximate duration of one minute.11 Nausea, vomiting, and transient hypertension can present during the course of the disease, which were observed in all our patients. Focal neurological signs are observed in about 43% of cases, including visual impairment, photophobia, blindness, dysarthria, and ataxia. Generalized seizures are also reported in 17% of the patients.12 , 13 These focal neurological signs were observed in our patients, and seizure was also noted in one. Ischemic events in RCVS were mostly observed as weakness of the limbs and face typically in the second week,13 which was seen transiently (less than 24 hours) in one of our cases (case No.1).
Several triggers have been proposed for RCVS such as exercise, swimming, exposure to vasoactive agents and immunosuppressants, transfusion of blood products including IVIG, and various brain diseases.14 , 15 To avoid these triggers, efforts were made to reduce and discontinue the vasoactive agents used to treat hemodynamic instability of the patients immediately after the improvement of their shock and cardiac function. The infusion rate of IVIG was also decreased as well as the intervals between injections because their RCVS symptoms had emerged one to two days after IVIG administration in the course of the disease. Shen et al. reported RCVS in an elder patient following IVIG administration for Guillain-Barré syndrome. They speculated that two factors could medicate RCVS in the patient, first, dysfunction of cerebral autoregulation in Guillain-Barré syndrome, and second, IVIG can independently increase serum viscosity, platelet activation, intravascular hypercoagulopathy, and necrotizing microangiopathy, which may cause posterior reversible encephalopathy syndrome and stroke.16 However, in spite of the common use of IVIG and vasoactive substances in pediatrics, RCVS is exceedingly rare in the age group of our patients. Therefore, it is unlikely that IVIG was the cause primary cause of RCVS in our patients. Nevertheless, the question whether COVID-19 and MIS-C could predispose pediatric vasculature to be more susceptible to cerebral vasoconstriction as a result of medication use remains a possibility. Some research suggests that corticosteroids may worsen the prognosis of RCVS.17 Nevertheless, corticosteroids were continued due to the underlying disease severity. On the other hand, RCVS is also associated with other inflammatory diseases, including lupus18 and Takayasu arteritis.19 Vascular and endothelial damage caused by immune complexes and autoantibodies in these diseases increases the blood-brain barrier permeability. These changes have also been suggested as contributing to RCVS.20 , 21 Therefore, managing inflammation with corticosteroids or immunosuppressants may be effective in controlling inflammation, endothelial damage, and possibly subsequent RCVS in MIS-C, although this remains speculative at this time. RCVS is treated based on symptomatic treatment and elimination of triggers,12 , 13 both of which were performed for our patients to the extent possible. Calcium channel blockers have also been suggested.22 However, it was not feasible to use them in these patients owing to their initial hypotension and cardiac dysfunction.
COVID-19 may induce vasoconstriction,23 and spikes in blood pressure may lead to loss of cerebral autoregulation, which is the proposed mechanism of RCVS. Vasoconstriction is not limited to brain vessels in MIS-C. Gomez et al. showed that vasoconstriction plays an important role in causing gastrointestinal complications in pediatric patients with MIS-C associated with COVID-19.24 COVID-19 can lead to downregulation of angiotensin-converting enzyme 2, which can cause overactivation of the classic renin-angiotensin axis and lead to vasoconstriction.25 Only two cases with COVID-19 and RCVS were reported in the literature, albeit in adults.7 , 8 Dakay et al. reported a female with respiratory symptoms of COVID-19, an abrupt headache, and bilateral frontal subarachnoid hemorrhage on imaging. Vertebral artery dissection was observed on cerebral angiogram. Mild-to-moderate bilateral vasospasm suggestive of RCVS was observed on her imaging seven days later.7 Mansoor et al. reported a 31-year-old female with RCVS in the setting of mild COVID-19 who was successfully treated with nimodipine and aspirin.8
Other arterial abnormalities have been reported in pediatric COVID-19 cases, including focal cerebral arteriopathy (FCA). This entity has been described in two children with COVID-19 and focal neurological signs in the absence of MIS-C.26 , 27 The first was a 12-year-old male with generalized seizures, right hemiplegia, dysarthria, positive nasopharyngeal swab and cerebrospinal fluid for COVID-19, and FCA of the proximal left middle cerebral artery. The second patient was a 13-year-old female with positive nasopharyngeal polymerase chain reaction for COVID-19, persistent headaches, speech impairment, and right-sided hemiparesis, without the MIS-C criteria. Imaging revealed an infarct and moderate focal stenosis in the left middle cerebral artery, with wall thickening and contrast enhancement on vascular wall imaging, confirming the inflammatory type of FCA.27 Given the clinical signs and characteristic imaging findings in these two cases, our patients do not fit the diagnosis of FCA. Thunderclap headache was the main initial neurological symptom in our patients. Multifocal and bilateral vasoconstriction was observed on imaging and was shown to be transient and reversible on follow-up, as required for the confirmation of RCVS. On the other hand, FCA is associated with focal neurological signs, altered levels of consciousness, and sometimes seizures rather than with severe and abrupt headaches. The pattern of vascular involvement did not match the pattern seen in FCA.28 , 29 Similarly, the clinical presentation of severe thunderclap headaches in RCVS, which may recur daily, is not typical for primary central nervous system vasculitis.
Numerous neurological manifestations have been reported in patients with MIS-C. Headaches30 and restlessness31 with incidences of 26% and 57%, respectively, have been reported as the most prevalent symptoms. A study on 186 patients with MIS-C reported neurological signs, including encephalitis, seizures, and altered levels of consciousness in 11% of the cases.32 Moreover, encephalopathy, headache, lethargy, ataxia, and dysarthria were reported in 4 of 27 patients with MIS-C associated with abnormal signal intensities of the splenium of the corpus callosum suggestive of focal myelin edema.4 , 33 We have not found a prior report of RCVS in children with COVID-related MIS-C in the literature.
This article is the first report of RCVS in pediatric COVID-19 patients with severe MIS-C. Inflammation in severe MIS-C may cause endothelial vascular damage, arterial vasospasm, and increased vascular tone. Furthermore, autonomic dysfunction may cause cerebrovascular dysregulation and the emergence of RCVS symptoms. IVIG, corticosteroid pulses, and vasoactive agents used to treat MIS-C have been known as the triggers of RCVS. Both patients were eventually symptom-free upon discharge and during follow-up. In conclusion, RCVS should be considered in pediatric patients with COVID-19-related MIS-C with thunderclap headaches with or without focal neurological signs. Further studies are needed to establish the exact pathophysiology and management guidelines in these patients.
Footnotes
A.S. and Z.P. have contributed equally to this article.
Conflict of Interest: None.
Declaration of interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schupper A.J., Yaeger K.A., Morgenstern P.F. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese L.H., Dodick D.W., Schwedt T.J., Singhal A.B. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 6.Coffino S.W., Fryer R.H. Reversible cerebral vasoconstriction syndrome in pediatrics: a case series and review. J Child Neurol. 2017;32:614–623. doi: 10.1177/0883073817696817. [DOI] [PubMed] [Google Scholar]
- 7.Dakay K., Kaur G., Gulko E., et al. Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Dis. 2020;29:105011. doi: 10.1016/j.jstrokecerebrovasdis.2020.105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansoor T., Alsarah A.A., Mousavi H., Khader Eliyas J., Girotra T., Hussein O. COVID-19 associated reversible cerebral vasoconstriction syndrome successfully treated with nimodipine and aspirin. J Stroke Cerebrovasc Dis. 2021;30:105822. doi: 10.1016/j.jstrokecerebrovasdis.2021.105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) | CDC n.d. https://www.cdc.gov/mis-c/hcp/ Available at:
- 10.Rocha E.A., Topcuoglu M.A., Silva G.S., Singhal A.B. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92:e639–e647. doi: 10.1212/WNL.0000000000006917. [DOI] [PubMed] [Google Scholar]
- 11.Chen S.-P., Fuh J.-L., Wang S.-J. Reversible cerebral vasoconstriction syndrome: an under-recognized clinical emergency. Ther Adv Neurol Disord. 2010;3:161–171. doi: 10.1177/1756285610361795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal A.B., Hajj-Ali R.A., Topcuoglu M.A., et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68:1005–1012. doi: 10.1001/archneurol.2011.68. [DOI] [PubMed] [Google Scholar]
- 13.Sattar A., Manousakis G., Jensen M.B. Systematic review of reversible cerebral vasoconstriction syndrome. Expert Rev Cardiovasc Ther. 2010;8:1417–1421. doi: 10.1586/erc.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S.-P., Fuh J.-L., Wang S.-J. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother. 2011;11:1265–1276. doi: 10.1586/ern.11.112. [DOI] [PubMed] [Google Scholar]
- 15.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y.-Y., Cheng Z.-J., Zhou C.-G., Dai T.-M., Nie H.-B. Reversible cerebral vasoconstriction syndrome following Guillain-Barré syndrome: a rare complication. Chin Med J. 2018;131:2237–2238. doi: 10.4103/0366-6999.240793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal A.B., Topcuoglu M.A. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88:228–236. doi: 10.1212/WNL.0000000000003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrleman C., Naggara O., Grevent D., et al. Reversible cerebral vasoconstriction syndrome in paediatric patients with systemic lupus erythematosus: implications for management. Dev Med Child Neurol. 2019;61:725–729. doi: 10.1111/dmcn.14031. [DOI] [PubMed] [Google Scholar]
- 19.Uchida Y., Matsukawa N., Oguri T., et al. Reversible cerebral vasoconstriction syndrome in a patient with Takayasu's arteritis. Intern Med. 2011;50:1611–1614. doi: 10.2169/internalmedicine.50.5185. [DOI] [PubMed] [Google Scholar]
- 20.Lee M.J., Cha J., Choi H.A., et al. Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: implications for pathophysiology and diagnosis. Ann Neurol. 2017;81:454–466. doi: 10.1002/ana.24891. [DOI] [PubMed] [Google Scholar]
- 21.Wu C.-H., Lirng J.-F., Wu H.-M., et al. Blood-brain barrier permeability in patients with reversible cerebral vasoconstriction syndrome assessed with dynamic contrast-enhanced MRI. Neurology. 2021;97:e1847–e1859. doi: 10.1212/WNL.0000000000012776. [DOI] [PubMed] [Google Scholar]
- 22.Ducros A., Boukobza M., Porcher R., Sarov M., Valade D., Bousser M.-G. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091–3101. doi: 10.1093/brain/awm256. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardila Gómez I.J., López P.P., Duque D.C., et al. Abdominal manifestation of multisystemic inflammatory syndrome in children. J Pediatr Surg Case Rep. 2021;74:102042. doi: 10.1016/j.epsc.2021.102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divani A.A., Andalib S., Di Napoli M., et al. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29:104941. doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297:E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulko E., Overby P., Ali S., Mehta H., Al-Mufti F., Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41:2348–2350. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerretsen P., Kern R.Z. Reversible cerebral vasoconstriction syndrome or primary angiitis of the central nervous system? Can J Neurol Sci. 2007;34:467–477. [PubMed] [Google Scholar]
- 29.Mandell D.M., Matouk C.C., Farb R.I., et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke. 2012;43:860–862. doi: 10.1161/STROKEAHA.111.626184. [DOI] [PubMed] [Google Scholar]
- 30.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toubiana J., Poirault C., Corsia A., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kontzialis M., Soares B.P., Huisman T.A.G.M. Lesions in the splenium of the corpus callosum on MRI in children: a review. J Neuroimaging. 2017;27:549–561. doi: 10.1111/jon.12455. [DOI] [PubMed] [Google Scholar]