Abstract
Autoimmunity following COVID-19 vaccination has been reported. Herein, a 79-year-old man with clinical and immunological features of autoimmune hepatitis type 1 after ChAdOx1 nCoV-19 vaccination is presented. Clinical manifestations rapidly remitted after the instauration of immunomodulatory management. This case, together with a comprehensive review of the literature, illustrates the association between COVID-19 vaccines and the development of autoimmune conditions.
Keywords: Autoimmune hepatitis, COVID-19, SARS-CoV-2, AstraZeneca, ChAdOx1 nCoV-19, AZD1222
1. Introduction
Infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been commonly associated with liver disorders. The overall frequency of acute liver injury in COVID-19 is about 26.5%, and up to 41.1% of patients present with transaminitis [1]. Levels of glutamic-oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) [1], and elevated total bilirubin are considered biomarkers of deleterious COVID-19 outcomes [2]. Although a direct assault of SARS-CoV-2 on hepatocytes leading to abnormal liver enzyme levels has been suggested as the main mechanism of liver injury, hepatocytes do not express high numbers of receptors for angiotensin converting enzyme 2 (ACE-2) [2], and direct infection of hepatocytes has not been found [3]. However, residual viral antigens has been detected in hepatic tissue during the convalescent phase, up to 6 months after recovery [4]. Other mechanisms, such as ischemia and cholangiopathy, have recently come under scrutiny [5,6]. In addition, an autoimmune phenomenon could be associated with hepatopathy following SARS-CoV-2 infection [7].
COVID-19 vaccination has been broadly associated with diverse autoimmune conditions. These include autoimmune hepatitis (AIH) [8], myocarditis [9,10], immune thrombotic thrombocytopenia [11], Guillain-Barré syndrome [12], and giant cell arteritis [13]. Interestingly, patients with autoimmune diseases (ADs) prior to vaccination may exhibit flares following the administration of the biologic [10].
All the above data indicate the possibility of autoimmune phenomena related to SARS-CoV-2 infection or post-vaccination [14]. Although the mechanisms have not been elucidated yet, molecular mimicry is considered one of the major explanation of autoimmunity after vaccination [15,16]. In addition, bystander activation and epitope spreading may also contribute to autoimmunity in those susceptible individuals with ADs or with pre-existing latent autoimmunity [17]. Herein, we present a patient with AIH after AstraZeneca-ChAdOx1 nCoV-19 (AZD1222) vaccination, and a review of similar cases reported in the literature.
2. Case presentation
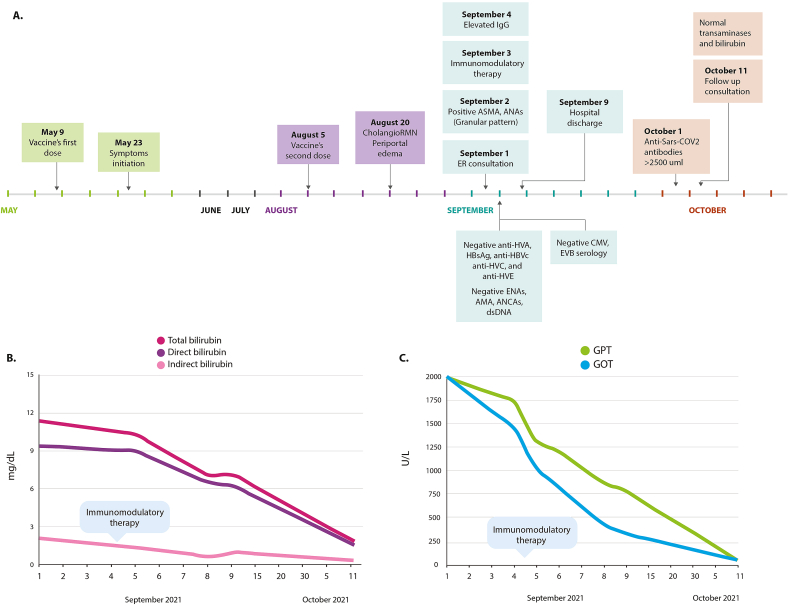
A 79-year-old man came to the emergency room in September 2021 with a 3-month history of right upper quadrant abdominal pain associated with jaundice, pruritus, acholia, and choluria (Fig. 1A). He denied chronic alcohol abuse, previous hepatotropic virus infections, familial autoimmunity, or additional comorbidities. The patient was not on concomitant medications. He received the ChAdOx1 nCoV-19 vaccine (AZD1222) on May 9th, 2021, developing the above-mentioned symptomatology 15 days after the first dose. Despite clinical manifestations, he received the second dose on August 5, 2021 (Fig. 1A). The patient was referred to our center on September 1, 2021, from a primary care center.
Fig. 1.
Clinical timeline and liver function. A. Clinical evolution of autoimmune hepatitis. B. Levels of total, direct, and indirect bilirubin. C. Levels of GOT and GPT. CholangioRMN: magnetic cholangioresonance, anti-HVA: hepatitis A total antibodies; HBsAg: hepatitis B surface antigen; anti-HBc: hepatitis B core antibody; anti-HCV: hepatitis C total antibodies; ENAs: extractable nuclear antigens; AMA: Antimitochondrial antibodies; ANCAs: antineutrophil cytoplasmic antibodies; dsDNA: anti-double-stranded DNA antibodies; ER: emergency room; ASMA: anti-smooth muscle antibody; ANAs: antinuclear antibodies; IgG: Immunoglobulin G; CMV: cytomegalovirus; EBV: Epstein-Barr virus, GOT: glutamic-oxaloacetic transaminase; GPT: glutamic pyruvic transaminase.
Examination revealed conjunctival and soft palate jaundice as well as generalized yellow pigmentation of the skin, and mild abdominal tenderness in the right upper quadrant without other significant findings. Blood tests showed mixed hyperbilirubinemia (11.9 mg/dL) with direct bilirubin predominance (9.39 mg/dL) (Fig. 1B), elevated transaminases (GOT 2003 U/L, GPT 1994 U/L) (Fig. 1C), negative hepatotropic viruses’ profile (i.e., hepatitis A, B, C, and E), and non-reactive IgG and IgM serology for cytomegalovirus and Epstein-Barr. In addition, the patient had mild lymphopenia (0.910 10x^3/μL), with normal leukocyte (5.06 10x^3/μL) and platelet counts (253 10x^3/μL). Fibrinogen, erythrocyte sedimentation rate (ESR), and C reactive protein were within the normal range.
Given the direct hyperbilirubinemia, an obstructive biliary disease was suspected. Abdominal ultrasound showed edema of the gallbladder walls with a pattern described in acute hepatitis without cholelithiasis. The spleen was normal in size and shape, despite a venous doppler showing increased portal diameter and elevated portal flow velocity (15 mm and 22 cm/s, respectively). The upper gastrointestinal endoscopy showed esophagitis (Forrest III) and chronic antral gastritis. In addition, cholangioresonance showed no biliary obstruction nor additional relevant findings.
After the initial approach, a hepatitis of autoimmune origin was suspected. The patient presented positivity for anti-smooth muscle antibodies (ASMA) (71.0 U, QUANTA Flash – INOVA, San Diego, California), low titer anti-nuclear antibodies (ANAs) (1:80 granular pattern, by indirect immunofluorescence on Hep-2 cells), and elevated IgG (2058 mg/dL, normal range: 650–1600 mg/dL). Other autoantibodies, including anti- RNP, -Sm, -Ro, -La, -mitochondrial antibodies (AMA), -dsDNA, and anti-neutrophil cytoplasmic antibodies, were negative. Anti-SARS-CoV-2 total antibodies after receiving both doses of vaccine were high (>2500 U/mL, threshold for positivity ≥0.8 U/mL, by electrochemiluminescence immunoassay, Roche Diagnostics International AG, Rotkreuz, Switzerland).
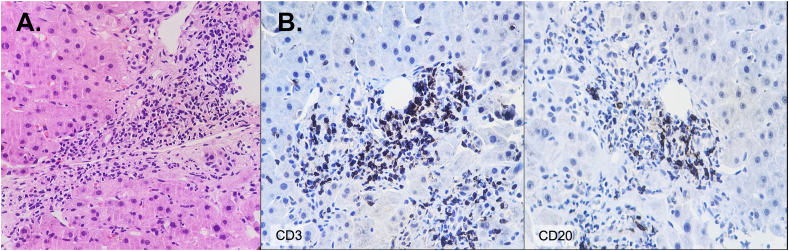
Liver biopsy, performed on September 20th (after discharge and initiation of immunomodulatory management), showed focal cholestasis and lobulation of the parenchyma, marked ductular proliferation, as well as lymphocytic infiltrate in the portal spaces with the presence of eosinophils, corresponding to a necroinflammatory hepatitis grade 2 stage 2 with focal cholestasis as previously classified [18] (Fig. 2A). Immunohistochemistry staining revealed numerous T CD3+ cells interspersed with a few B CD20+ cells (Fig. 2B). Altogether, the data indicate fulfillment of the International Autoimmune Hepatitis Group (IAIHG) criteria for type 1 AIH [19], and suggest that it was developed after COVID-19 vaccination, meeting most of the Bradford Hill causality criteria [20,21].
Fig. 2.
Liver biopsy histological findings. A. The portal tract shows ductular proliferation, lymphocytic infiltrate, some eosinophils, and interface hepatitis. H&E; X40. B. Immunohistochemistry. Numerous lymphocytes T CD3+ cells interspersed with few lymphocytes B CD20+ cells. Immunohistochemistry; X40.
Due to these findings, therapy with hydrocortisone (100mg per day for 3 days) was initiated, switched to prednisone (50mg per day). Azathioprine (50mg per day) was initiated. During the nine days following the establishment of treatment, the patient presented a progressive decrease in the level of liver enzymes (Fig. 1C), and a marked improvement in symptoms, including jaundice, pruritus, and fatigue. Because of favorable clinical evolution, the patient was discharged from the hospital on September 9th. Ambulatory management, including tapering prednisolone of 10 mg per week, was prescribed. In a follow-up consultation on October 11th, the patient's clinical condition continued to improve, as did his liver enzymes (i.e., GOT 39 U/L, GPT 35 U/L), and bilirubin levels (i.e., 1.77 mg/dL).
3. Discussion
Herein, a patient with typical type 1 AIH after the ChAdOx1 nCoV-19 vaccine is described, illustrating the association while causation remains to be confirmed.
AIH is defined as the presence of chronic progressive hepatitis of inflammatory origin [22]. Walderstörm described it for the first time in 1951. Currently, mechanisms for AIH development are not fully understood. However, the combination of genetic predisposition and environmental triggers are crucial players in its appearance [22]. Abdominal pain, jaundice, general malaise, and abnormal liver function tests are the most common clinical manifestations. This condition is classified into two groups: Type 1 AIH is positive for ANAs and ASMA, whereas Type 2 AIH is positive for anti-liver and kidney microsome antibodies type 1 (LKM1) and liver cytosol type 1 antibody (LC1), and both types have high IgG levels [22,23].
In the study by Rela et al. [8], two patients developed AIH after the AstraZeneca-ChAdOx1 nCoV-19 (AZD1222) vaccination. Both patients presented with liver profile alterations 2 weeks after the administration of the vaccine and further tests confirmed the diagnosis (i.e., liver biopsy: lymphocyte infiltration, multiacinar hepatic necrosis, periportal neocholangiolar proliferation). However, only one patient presented reactivity to ANAs (1:80) and none of them presented positivity for ASMA, LKM1 nor LKM2. Thus, suggesting that vaccination may induce an acute inflammatory reaction, but production of autoantibodies could take longer.
Findings in liver biopsy correlate with reports of AIH following SARS-CoV-2 vaccination. Necroinflammatory hepatitis was observed in all cases of AIH following vaccination with Moderna mRNA-1273 COVID-19 [[24], [25], [26], [27], [28], [29]], Pfizer-BioNTech mRNA vaccine [[30], [31], [32], [33]], and AstraZeneca ChAdOx1 nCoV-19 vaccine [8,34]. It has been suggested that portal infiltration with eosinophils correlates with drug-induced liver injury, including vaccines (i.e., Moderna mRNA-1273 and AstraZeneca ChAdOx1 nCoV-19 vaccines) [8,27,28]. This could partially explain the existence of hepatitis following vaccination without positivity for liver-associated autoantibodies (Table 1) [8,10,12,13,[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]].
Table 1.
Reported cases of autoimmunity diseases related to COVID -19 vaccination.
| Author | Autoimmune disease | Type of vaccine | Time of appearance | Laboratory findings |
|---|---|---|---|---|
| Iremli et al. [35] | Subacute Thyroiditis | Sinovac – Coronavac vaccine | Case 1: 4 days after 2 doses | Case 1: |
| TSH 0.473 mIU/L, fT4 14.1 pmol/L | ||||
| Case 2: 4 days after 1 dose | Negative TPO, Tg and TRAB autoantibodies | |||
| CRP 100.5 mg/dL | ||||
| Case 3: 7 days after 2 doses | ESR 53 mm/hr | |||
| Thyroid USG bilateral focal hypoechoic areas with decreased blood flow | ||||
| Case 2: | ||||
| TSH 0.01 mIU/L, fT4 5.2 pmol/L, | ||||
| Negative TPO, Tg and TRAB autoantibodies | ||||
| CRP 6 mg/dL, ESR 19mm/hr | ||||
| Thyroid USG bilateral focal hypoechoic areas with decreased blood flow | ||||
| Case 3: | ||||
| TSH 0.9 mIU/L, fT4 13.85 pmol/L, | ||||
| Negative TPO, Tg and TRAB autoantibodies | ||||
| CRP 2.4 mg/dL, ESR 25 mm/hr | ||||
| Thyroid USG bilateral hypoechoic areas with decreased blood flow | ||||
| Rubinstein et al. [63] | Flare Graves' disease: Thyroid eye disease | Pfizer- BioNTech mRNA vaccine | 3 days after 2 doses | Normal TSH, T4, and free T3, |
| Elevated thyroid stimulating immunoglobulin | ||||
| ANA 1:320. | ||||
| Orbit CT scan enlarged inferior and medial recti | ||||
| muscles without tendon involvement or sinus | ||||
| Disease | ||||
| Lui et al. [49] | Graves' disease | Pfizer- BioNTech mRNA vaccine | 5 weeks after 2 doses | TSH <0.02 mIU/L, fT4 66.6 pmol/L, fT3 30.50 |
| pmol/L | ||||
| Thyroid stimulating immunoglobulin level 420% | ||||
| Positive TPO and Tg | ||||
| Kim et al. [51] | Immune Thrombocytopenia | AstraZeneca ChAdOx1 nCoV-19 vaccine | 2 days after 1 dose | Hemoglobin 14.0 g/dL, white blood cell count |
| 7500/μL, platelet count 4000/μL. | ||||
| Peripheral blood smear: isolated marked | ||||
| thrombocytopenia without schistocytes. | ||||
| Normal clotting times | ||||
| Negative hepatitis B virus surface antigen, anti- | ||||
| hepatitis C virus antibody, VIH, ANAS, dsDNA and | ||||
| urea breath test. | ||||
| Negative anti-heparin/platelet factor 4 IgG | ||||
| Chittal et al. [50] | Thrombotic Thrombocytopenia | Moderna mRNA-1273 COVID-19 | 3 days after 2 dose | Platelet count 65,000 platelets/mcL with further |
| decline to 29,000 platelets/mcL | ||||
| Mildly elevated aPTT | ||||
| Positive lupus anticoagulant | ||||
| Positive anti-Platelet factor 4 | ||||
| Jawed et al. [36] | Flare acute immune thrombocytopenia | Pfizer- BioNTech mRNA vaccine | 18 Days after the first dose | |
| Platelet count 1000/mcL | ||||
| Peripheral smear thrombocytopenia and normal red blood cell morphology | ||||
| Reticulocyte count 2.2% | ||||
| Prothrombin time 16.2 secs | ||||
| INR 1.5 mg/dL, LDH 310 U/L | ||||
| Negative ANAs | ||||
| Gaignard et al. [37] | Acute immune thrombocytopenia | Moderna mRNA-1273 COVID-19 | Case 1 (IPT): 3 days after 1 dose | Case 1: |
| Platelet count 3000/mcL | ||||
| Autoimmune hemolytic anemia | Hemoglobin 15.1g/dL | |||
| Case 2 (AIHA): 5 days after 1 dose | White cells count 6.600/L | |||
| Case 2: | ||||
| Hemoglobin 8.6g/dL, reticulocyte count 310 × 109/L, white cells count 11.700/L, platelets count 344000/mcL | ||||
| Elevated transaminases, LDH and bilirubin and decreased haptoglobin | ||||
| MPO, p-ANCA, and c-ANCA negative | ||||
| IAT, IgG- and C3- DAT Positive | ||||
| Gadi et al. [38] | Autoimmune Hemolytic Anemia | Moderna mRNA-1273 COVID-19 | 7 days after 1 dose | Hemoglobin 7.1 g/dL, MCV 93 fL, reticulocyte count 15.5%, |
| Total bilirubin 3.7 mg/dL, direct bilirubin 0.8 mg/dL | ||||
| Haptoglobin <8 mg/dL, LDH 746 U/L | ||||
| DAT was negative for IgG and C3d. However, eluate prepared from the patient's red blood cell was reactive against all test lockups | ||||
| Aoun et al. [46] | Cold agglutinin disease | Pfizer- BioNTech mRNA vaccine | 3 days after 1 dose | Hemoglobin of 57 g/L, 10% reticulocytes |
| LDH 671 iu/l | ||||
| Total bilirubin 46.4 lmol/l and direct bilirubin 12.5 lmol/l | ||||
| Peripheral blood film showed marked agglutination | ||||
| Direct antiglobulin test - positive for C3. | ||||
| Negative ANAS, cytomegalovirus, Epstein–Barr virus, HIV, parvovirus, mycoplasma | ||||
| Negative flow cytometric immunophenotyping and protein electrophoresis | ||||
| Tabata et al. [45] | Aplastic Anemia | Pfizer- BioNTech mRNA vaccine | 4 days after 2 doses | White blood cell count 1.6 × 109/L and platelet count 11 × 109/l |
| Positive IgG cytomegalovirus and Epstein-Barr - not indicative of virus reactivation | ||||
| Negative hepatitis B, hepatitis C, and VIH | ||||
| Bone marrow biopsy: a hypocellular marrow | ||||
| An et al. [39] | Reactive arthritis | Sinovac – Coronavac vaccine | 3 days after 1 dose | ESR 32 mm/h, CRP 15.0 mg/L |
| Synovial fluid analysis: leukocytes were 20–25/HP, neutrophils 90%, lymphocytes 4%, monocytes 6%, no crystals. | ||||
| Negative gram stain, Negative bacterial cultures | ||||
| Negative ANAs, RF, anti-CCP antibody, and HLA-B27 | ||||
| Mücke et al. [40] | Immune complex vasculitis | Pfizer- BioNTech mRNA vaccine | 12 days after 2 doses | ESR 42 mm/h, CRP 8.69 mg/dL, IL-6104 pg/mL |
| IgG 1549 mg/dL | ||||
| C3 87mg/dL, C4 16.1 mg/L | ||||
| Positive ANAs 1:80, and negative c-ANCA, and p-ANCA | ||||
| Maye et al. [47] | Flare IgA vasculitis | Pfizer- BioNTech mRNA vaccine | 24 hours before dose | Urinalysis: red cell count of 165 cells/mm3, urinary albumin to creatinine ratio of 4.9 mg/mmol. |
| Serum creatinine 112μmol/L | ||||
| Normal serum urea and electrolytes | ||||
| Elevated IgA titres at 3.10 g/L | ||||
| Negative ANAS, ANCA, and rheumatoid factor. Viral antibody titres were negative - acute or chronic infection. | ||||
| Leber et al. [41] | Acute Thyroiditis and Bilateral Optic Neuritis | Sinovac – Coronavac vaccine | 12 hours after 2 doses | Positive TPO and Tg antibodies |
| Negative ANAs, RF, anti-dsDNA, ENAs, C3/C4, ACE IgA, IgG, IgM and anticardiolipin IgM and IgG antibodies, reactive MOG-IgG (1/320 dilution) | ||||
| TSH 13.2 mUI/L, and normal levels of fT4 | ||||
| Nasuelli et al. [12] | Guillain-Barré syndrome | AstraZeneca ChAdOx1 nCoV-19 vaccine | 10 days after 1 dose | Electromyography compatible with demyelinating motor polyneuropathy |
| Lumbar puncture + CSF with normal white blood cell count and glycorrhachia | ||||
| Negative Anti-GM1 IgG-IgM, GQIb IgG-IgM, GM2 IgG-IgM, anti MAG, and anti-GAD | ||||
| Tagliaferri et al. [42] | Myasthenia Gravis Crisis | Moderna mRNA-1273 COVID-19 | 7 days after 2 doses | Authors did not performed autoantibodies |
| Ghielmetti et al. [25] | Acute autoimmune-like hepatitis | Moderna mRNA-1273 COVID-19 | 7 days after de 1 dose | Hemoglobin 14.8 g/dL, white cell count 6.300/L |
| Platelets 309000/L | ||||
| INR 1.16, GOT 1127 U/L, GPT 1038 U/L, | ||||
| GGT 536 U/L, ALP 192 U/L, Total bilirubin 204.8 μmoL/l | ||||
| Hepatotropic virus infections profile negative | ||||
| IgG 19.96 g/L | ||||
| Positive ANAs (1:640, fine speckled pattern), anti-gastric parietal cells, Anti-β-2 glycoprotein IgA, and atypical AMA antibodies | ||||
| Negative anti-β-2 glycoprotein IgM and IgG | ||||
| ASMA, ANCA, SLA, anti-LKM1, anti-LC1, U1-snRNP, SSA/Ro, SS-B/La, CENP-B, Scl-70, Jo-1, Sm, dsDNA, Fibrillarin, RNA Polymerase III, Rib-P, PM-Scl, PCNA, and Mi-2 | ||||
| HLA DRB1*01:01 11:01, HLA DQA1*01:01 05:01, and HLA DQB1*03:01 05:01 | ||||
| Avci et al. [48] | Autoimmune hepatitis | Pfizer- BioNTech mRNA vaccine | 30 days after doses | Total bilirubin 11.8 mg/dL, direct bilirubin 9.18 mg/dL |
| GPT 455 IU/ml, GOT 913 IU/ml, GGT 292 IU/ml, ALP 436 IU/ml | ||||
| Hemoglobin 13.3 g/dL, white cell counts 8.530/L | ||||
| Platelets 1999000/mm3 | ||||
| ANAS 1/100 | ||||
| ASMA 1/100 | ||||
| IgG 4260 mg/dL | ||||
| Hepatotropic virus infections profile negative | ||||
| Normal ceruloplasmin and serum copper levels. | ||||
| Londoño et al. [24] | Autoimmune hepatitis | Moderna mRNA-1273 COVID-19 | After 1 dose | GOT 993 IU/L, GPT 1312 IU/L, GGT 209 IU/L |
| Total bilirubin 2.3 mg/dL (peak of 8.5 mg/dL) | ||||
| ALP 190 IU/L | ||||
| Positive ANAs (1:80), ASMA (1:40), anti-SLA, anti-LC1 | ||||
| Liver ultrasound was normal. | ||||
| Rela et al. [8] | Autoimmune hepatitis | AstraZeneca ChAdOx1 nCoV-19 vaccine | Case 1: 8 days after 1 dose | Case 1: |
| Bilirubin of 14.9 mg/dL, GPT 1025 IU/L | ||||
| Case 2: 16 after 1 dose | GOT 1101 IU/L, INR 2.96 | |||
| Positive ANAs (1:80 - speckled pattern) | ||||
| Negative ANCAs, SLA, ASMA, LKM-1 antibodies | ||||
| Case 2: | ||||
| Total bilirubin 19.2 mg/dL, GOT 1361 IU/L, GPT 1094 IU/L | ||||
| Negative ANAs, ANCAs, ASMA, LKM-1 antibodies | ||||
| Rocco et al. [30] | Autoimmune hepatitis | Pfizer- BioNTech mRNA vaccine | 7 days after 2 doses | Total Bilirubin 10.5 mg/dL, direct Bilirubin 7.5 mg/dL, ALP 243 IU/L, GGT 524 IU/L, GOT 1401 IU/L, GPT 1186 IU/L |
| Positive ANAs (1:160 - speckled pattern) | ||||
| Negative ASMA, AMA, and anti-LKM-1 | ||||
| Total IgG 3500 mg/dL | ||||
| Patil et al. [52] | Systemic lupus erythematosus | AstraZeneca ChAdOx1 nCoV-19 vaccine | 14 days after 1 dose | Hemoglobin 9.3 g/dL, hematocrit 26.8%, MCV 73.2, white cells count 4640/mm3, neutrophils 60%, lymphocytes 32.9%, platelets count 134000/mcL |
| CRP 2.8 mg/L, ESR 92 mm/h | ||||
| ANAS 1:320 | ||||
| Positive antigen for dsDNA, nucleosomes, histones, and AMA m2. | ||||
| Elevated IgG, IgM, IgA | ||||
| Coombs test weakly positive. | ||||
| Urinalysis: 1+ albuminuria and 3–4 RBC per high power field. | ||||
| Chest X-ray was normal. | ||||
| McShane et al. [28] | Autoimmune hepatitis | Moderna mRNA-1273 COVID-19 | 4 days after 1 dose | Bilirubin 270 μmol/L, GOT 217U/L, GPT 1067U/L |
| Positive ASMA antibodies | ||||
| Total IgG 21.77g/L | ||||
| Vuille-Lessard et al. [26] | Autoimmune hepatitis | Moderna mRNA-1273 COVID-19 | 2 days after 1 dose | Total bilirubin 65 μmol/L, GOT 811 U/L, GPT 579 U/L, ALP 124 U/L, GGT 361 U/L, INR 1.23 |
| Albumin 28 g/L | ||||
| IgG 39.4 g/L | ||||
| Positive ANAs (1:1280), and ASMA | ||||
| Negative Anti-AMA-M2, anti-LKM and anti-LSA antibodies | ||||
| Bril et al. [31] | Autoimmune hepatitis | Pfizer- BioNTech mRNA vaccine | 7 days after 1 dose | Bilirubin 4.8 mg/dL, GOT 754 U/L, GPT 2001 U/L |
| ALP 170 U/L | ||||
| Positive ANAs/1:1280- homogeneous pattern) and dsDNA autoantibodies. | ||||
| Negative AMA, ASMA, LKM-1, and ANCAs | ||||
| Total IgG 1081 mg/dL | ||||
| Tun et al. [27] | Autoimmune hepatitis | Moderna mRNA-1273 COVID-19 | 3 days after 1 dose | Bilirrubin 190 μmol/L, GPT 1048 U/L, GOT 229 U/L |
| Albumin 41 g/L | ||||
| IgG 25.1 g/L, IgM 2.2 g/L, Positive ANAs | ||||
| Garrido et al. [29] | Autoimmune hepatitis | Moderna mRNA-1273 COVID-19 | 15 days after 1 dose | Total bilirubin 1.14 mg/dL GOT 1056U/L, GPT 1092U/L, GGT 329U/L, ALP 24U/L |
| ANAS 1:100 speckled pattern | ||||
| Elevated IgG | ||||
| Negative AMA, ASMA, LKM, SLA, ANCA | ||||
| Hepatotropic virus infections profile negative | ||||
| Clayton-Chubb et al. [34] | Autoimmune hepatitis | AstraZeneca ChAdOx1 nCoV-19 vaccine | 26 days after 1 dose | Bilirubin 17 lmol/L, GPT 1774 U/L, GOT 633 U/L, GGT 136 U/L, ALP 118 U/L |
| Albumin 45 g/L, INR 1.1. | ||||
| ANAS 1:160 speckled pattern | ||||
| IgG normal | ||||
| Negative LKM, ASMA, AMA, SLA | ||||
| Hepatotropic virus infections profile negative | ||||
| Palla et al. [32] | Autoimmune hepatitis | Pfizer- BioNTech mRNA vaccine | 30 days after 2 dose | Serum transaminases 4xupper limit |
| ANAS 1:640 | ||||
| Total IgG 2400mg/dL | ||||
| Negative AMA, ASMA, LKM | ||||
| Hepatotropic virus infections profile negative | ||||
| Lodato et al. [33] | Acute cholestatic hepatitis | Pfizer- BioNTech mRNA vaccine | 15 days after 1 dose | Total bilirubin 17.54 mg/dL, Direct bilirubin 12.94 mg/dL, GTP 52 U/L, GOT 51 U/L. |
| Normal IgG | ||||
| Negative ANAS, ASMA, LKM1, AMA, ENAS | ||||
| Hepatotropic virus infections profile negative | ||||
| Capassoni et al. [43] | Polymyositis | AstraZeneca ChAdOx1 nCoV-19 vaccine | 4 days after | White cell count 13000, 76% neutrophils, CRP elevation |
| Procalcitonin negative. | ||||
| Aldolase raise (10,3 U/L NV < 7.6) | ||||
| ANAS 1:160, borderline positivity Anti-Pm/scl-75 antibodies | ||||
| Electromyography: moderate severity subacute myositis without muscle damage and polyneuropathy. | ||||
| Conticini et al. [44] | Relapse of microscopic polyangiitis | Pfizer- BioNTech mRNA vaccine | Few days after 1 dose | Arterial blood pO2 48 mm Hg) |
| CRP 3.7 mg/dL, creatinine 1.55 mg/dL | ||||
| Negative autoimmunity blood tests | ||||
| High‐resolution computed tomography: diffuse “ground‐glass” opacities with superimposed septal thickening and subpleural consolidations | ||||
| Sauret et al. [13] | Giant Cell Arteritis | AstraZeneca ChAdOx1 nCoV-19 vaccine | Few days after 1 dose | GGT 112 U/L, ALP 40 U/L |
| Authors did not performed autoantibodies | ||||
| Ishay et al. [10] | Pfizer- BioNTech mRNA vaccine | Case 1: 3 days after 1 dose | Case 1: Negative ANAs, RF, and anti-CCP antibodies | |
| Case 1: Symmetric polyarthritis | Case 2: 10 days after 1 dose | Case 2: No autoantibodies | ||
| Case 2: Exacerbation of Beçet disease | Case 3: 1 day after 1 dose | Case 3: No autoantibodies | ||
| Case 3: Pericarditis | Case 4: 3 days after 1 dose | Case 4: No autoantibodies | ||
| Case 4: Termporal arteritis like disease | Case 5: Few hours after 1 dose | Case 5: No autoantibodies | ||
| Case 5: | Case 6: 14 days after 2 doses | Case 6: No autoantibodies | ||
| Fever of unknown origin | Case 7: 10 days after 1 dose | Case 7: No autoantibodies | ||
| Case 6: Oligoarthritis | Case 8: 2 weeks after 2 doses | Case 8: White cells count 12,700/μL, CRP 1.4 mg/dL, | ||
| Case 7: Pericarditis | Troponin 103 ng/L, CPK 2380 U/L, no autoantibodies | |||
| Case 8: Myocarditis |
TSH: Thyroid-stimulating hormone; fT4: free thyroxine; TPO: thyroid peroxidase; Tg: Thyroglobulin; TRAB: thyrotropin receptor antibody; CRP: C-Reactive Protein, ESR: erythrocyte sedimentation rate; USG: ultrasonography; INR: international normalized ratio; LDH: lactate dehydrogenase; ANAs: antinuclear antibody; MPO: myeloperoxidase; p-ANCA: perinuclear anti-neutrophil cytoplasmic antibodies; c-ANCA: centrally antineutrophil cytoplasmic antibodies; IAT: indirect antiglobulin test; DAT: direct antiglobulin test; MCV: mean corpuscular volume; IgG: immunoglobulin G; C3: complement C3; RF: rheumatoid factor; CCP: cyclic citrullinated peptide; HLA: human leukocyte antigens; IL-6: interleukin-6; C4: complement C4; dsDNA: double-stranded deoxyribonucleic acid; ENAs: extractable nuclear antigen; ACE: angiotensin-converting enzyme; IgA: immunoglobulin A; IgM: immunoglobulin; MOG: myelin oligodendrocyte glycoprotein; CSF: cerebrospinal fluid; GM1: gangliosidosis-1; GQIb: Ganglioside GQIb; GM2: gangliosidosde-2; MAG: myelin associated glycoprotein; GAD: glutamic acid decarboxylase; GOT: glutamic-oxaloacetic transaminase; GPT: glutamic pyruvic transaminase. GGT: gamma-glutamyl transferase; ALP: alkaline phosphatase; ASMA: anti-smooth muscle antibody; SLA: soluble liver antigen; LKM1: liver kidney microsomal type 1; LC1: liver cytosolic antigen type 1; AMA: antimitochondrial antibodies; U1-snRNP: U1 small nuclear ribonucleoprotein particle; CENP: centromere protein B; Rib-P:ribosomal P protein; PCNA: Proliferating cell nuclear antigen; AMA-M2: Anti-mitochondrial M2 antibody, CPK: creatine phosphokinase.
Although the intramuscular route of administration is safe for lipid nanoparticle (LNP) RNA vaccines, it has been shown that intravenous administration may allow antigen presentation in the liver and lungs, promoting T cell infiltration and damage [53]. Further analyses of LNPs and their likely influence on liver damage are warranted.
Vuille-Lessard et al. [26] reported a case of a 76-year-old woman who developed severe AIH with positive ASMA and elevated IgG after Moderna-mRNA-1273 vaccine administration. In this case, as in ours, it is likely that patients already had an autoimmune background prior to vaccination given the early positivity for IgG autoantibodies. These cases resemble the reported flares following vaccination in patients with overt ADs [10]. In further reports on autoimmunity following COVID-19 vaccination, most of the patients presented with autoimmunity in the first two weeks after vaccination, and some of them presented with early positivity for pathogenic autoantibodies (Table 1). This indicates that vaccines may trigger overt autoimmunity in susceptible individuals or in patients with latent autoimmunity.
This conjecture was also raised by Talotta et al. [54] and Akinosoglou et al. [17] who propose that vaccination does not generate new autoimmune diseases, but rather triggers long-lasting latent autoimmunity. This should call attention to the evaluation of a profile risk prior to the administration of vaccines, including latent and overt autoimmunity, familial autoimmunity, and genetic risk scores [55].
Although the mechanisms associated with vaccination and autoimmunity are still unknown, molecular mimicry has emerged as the most likely process associated with this phenomenon [15,56]. Molecular mimicry is defined as “the similarity between a host epitope and an epitope of a microorganism or environmental agent” [56]. This similarity allows the development of cross-reactivity mediated by T and B cells, which promotes the production of pathogenic autoantibodies that may present different specificities for the human proteome. In addition, intermediate, but not complete homology, is considered the most critical factor for cross-reactivity [56].
The AstraZeneca-ChAdOx1 nCoV-19 (AZD1222) vaccine consists of a replication-impaired chimpanzee adenoviral vector ChAdOx1 that contains the spike (S) protein gene of the virus [57]. Recent studies have shown massive heptapeptide sharing between S protein and the human proteome [15], and other in silico studies have suggested the possible influence of viral nucleocapsid protein in the development of cross-reactivity [58].
These studies have found that the S protein may exhibit similarities with neurological, endocrinological, and gastrointestinal proteins. For the latter, it was found that SARS-CoV-2 exhibits homology with the “Hepatitis A virus cellular receptor 2” [59]. However, none of the reported in silico studies found homology related to classic pathogenic epitopes already reported in the literature (Table 1).
Epitope spreading, defined as the “development of an immune response to epitopes distinct from, and noncross-reactive with, the disease-causing epitope” [60], may be an alternative explanation for the autoimmune response associated with COVID-19 vaccines.
On the other hand, since vaccines could be a trigger rather than a cause of autoimmunity [61], bystander activation may be another mechanism for autoimmunity following vaccination. This is characterized by “auto-reactive B and T cells that undergo activation in an antigen-independent manner, influencing the development and course of autoimmunity. Activation occurs due to a combination of an inflammatory milieu, co-signaling ligands, and interactions with neighboring cells” [62]. Given the early development of overt autoimmunity following vaccination (Table 1), it is likely that COVID-19 vaccines primed autoreactive T and B cells in susceptible individuals, accelerating the development of overt autoimmunity.
4. Conclusion
The COVID-19 vaccine may induce the development of ADs, including AIH. The liver injury could be secondary to either bystander activation or epitope spreading, rather than molecular mimicry. However, autoimmunity prior to vaccination cannot be excluded as a risk factor. As a corollary, high-risk profiles and latent autoimmunity should be considered before vaccination.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all Diana Monsalve for her contributions and fruitful discussions during the preparation of the manuscript.
References
- 1.Sharma A., Jaiswal P., Kerakhan Y., Saravanan L., Murtaza Z., Zergham A., Honganur N.-S., Akbar A., Deol A., Francis B., Patel S., Mehta D., Jaiswal R., Singh J., Patel U., Malik P. Liver disease and outcomes among COVID-19 hospitalized patients – a systematic review and meta-analysis. Ann. Hepatol. 2021;21 doi: 10.1016/j.aohep.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha M.H., Regueiro M., Sandhu D.S. Gastrointestinal and hepatic manifestations of COVID-19: a comprehensive review. World J. Gastroenterol. 2020;26:2323–2331. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Guo Q.N., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Pan G.Q., Li Q.R., Huang X., Cui Y., Liu X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 4.Cheung C.C.L., Goh D., Lim X., Tien T.Z., Lim J.C.T., Lee J.N., Tan B., Tay Z.E.A., Wan W.Y., Chen E.X., Nerurkar S.N., Loong S., Cheow P.C., Chan C.Y., Koh Y.X., Tan T.T., Kalimuddin S., Tai W.M.D., Ng J.L., Low J.G.-H., Yeong J., Lim K.H. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71:226–229. doi: 10.1136/gutjnl-2021-324280. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., Lv T., Liang J., Zhang Q., Xu W., Xie Y., Wang X., Yuan Z., Liang J., Zhang R., Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth N.C., Kim A., Vitkovski T., Xia J., Ramirez G., Bernstein D., Crawford J.M. Post–COVID-19 cholangiopathy: a novel entity. Am. J. Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 7.Bartoli A., Gitto S., Sighinolfi P., Cursaro C., Andreone P. Primary biliary cholangitis associated with SARS-CoV-2 infection. J. Hepatol. 2021;74:1245–1246. doi: 10.1016/j.jhep.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rela M., Jothimani D., Vij M., Rajakumar A., Rammohan A. Auto-immune hepatitis following COVID vaccination. J. Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 9.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., Zoabi M., Aisman M., Goldschmid N., Berar Yanay N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishay Y., Kenig A., Tsemach-Toren T., Amer R., Rubin L., Hershkovitz Y., Kharouf F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharm. 2021;99 doi: 10.1016/j.intimp.2021.107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elalamy I., Gerotziafas G., Alamowitch S., Laroche J.-P., Van Dreden P., Ageno W., Beyer-Westendorf J., Cohen A.T., Jimenez D., Brenner B., Middeldorp S., Cacoub P. SARS-CoV-2 vaccine and thrombosis: an expert consensus on vaccine-induced immune thrombotic thrombocytopenia. Thromb. Haemostasis. 2021;121:982–991. doi: 10.1055/a-1499-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasuelli N.A., De Marchi F., Cecchin M., De Paoli I., Onorato S., Pettinaroli R., Savoini G., Godi L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol. Sci. 2021;42:4747–4749. doi: 10.1007/s10072-021-05467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauret A., Stievenart J., Smets P., Olagne L., Guelon B., Aumaître O., André M., Trefond L. Case of giant cell arteritis after SARS-CoV-2 vaccination: a particular phenotype? J. Rheumatol. 2021 doi: 10.3899/jrheum.210724. jrheum. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanpanah N., Rezaei N. Autoimmune complications of COVID‐19. J. Med. Virol. 2021;94:54–62. doi: 10.1002/jmv.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucchese G., Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones. 2020;25:731–735. doi: 10.1007/s12192-020-01145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinosoglou K., Tzivaki I., Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin. Immunol. 2021;226 doi: 10.1016/j.clim.2021.108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Yeoman A.D., Westbrook R.H., Al-Chalabi T., Carey I., Heaton N.D., Portmann B.C., Heneghan M.A. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50:538–545. doi: 10.1002/hep.23042. [DOI] [PubMed] [Google Scholar]
- 20.Hill A.B. The environment and disease: association or causation? Proc. Roy. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. https://pubmed.ncbi.nlm.nih.gov/14283879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas R.M., McMichael A.J. Association or causation: evaluating links between “environment and disease”. Bull. World Health Organ. 2005;83:792–795. [PMC free article] [PubMed] [Google Scholar]
- 22.Floreani A., Restrepo-Jiménez P., Secchi M.F., De Martin S., Leung P.S.C., Krawitt E., Bowlus C.L., Gershwin M.E., Anaya J.-M. Etiopathogenesis of autoimmune hepatitis. J. Autoimmun. 2018;95:133–143. doi: 10.1016/j.jaut.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka A. Autoimmune hepatitis: 2019 update. Gut Liver. 2020;14:430–438. doi: 10.5009/gnl19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Londoño M.-C., Gratacós-Ginès J., Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination – still casualty? J. Hepatol. 2021;75:1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., Terziroli Beretta-Piccoli B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J. Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuille-Lessard É., Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tun G.S., Gleeson D., Dube A., Al-Joudeh A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.09.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McShane C., Kiat C., Rigby J., Crosbie Ó. The mRNA COVID-19 vaccine – a rare trigger of autoimmune hepatitis? J. Hepatol. 2021;75:1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrido I., Lopes S., Simões M.S., Liberal R., Lopes J., Carneiro F., Macedo G. Autoimmune hepatitis after COVID-19 vaccine – more than a coincidence. J. Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casualty. J. Hepatol. 2021;75:728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID‐19 vaccination. A rare adverse effect? Hepatology. 2021 doi: 10.1002/hep.32156. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodato F., Larocca A., D'Errico A., Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J. Hepatol. 2021;75:1254–1256. doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J. Hepatol. 2021;75:1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.İremli B.G., Şendur S.N., Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J. Clin. Endocrinol. Metab. 2021;106:2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jawed M., Khalid A., Rubin M., Shafiq R., Cemalovic N. Acute immune thrombocytopenia (ITP) following COVID-19 vaccination in a patient with previously stable ITP. Open Forum Infect. Dis. 2021;8 doi: 10.1093/ofid/ofab343. ofab343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaignard M.-E., Lieberherr S., Schoenenberger A., Benz R. Autoimmune hematologic disorders in two patients after mRNA COVID-19 vaccine. HemaSphere. 2021;5:e618. doi: 10.1097/HS9.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.V Gadi S.R., Brunker P.A.R., Al‐Samkari H., Sykes D.B., Saff R.R., Lo J., Bendapudi P., Leaf D.E., Leaf R.K. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion. 2021;61:3267–3271. doi: 10.1111/trf.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An Q.-J., Qin D.-A., Pei J.-X. Reactive arthritis after COVID-19 vaccination. Hum. Vaccines Immunother. 2021;17:2954–2956. doi: 10.1080/21645515.2021.1920274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mücke V.T., Knop V., Mücke M.M., Ochsendorf F., Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect. Dis. 2021;21:958. doi: 10.1186/s12879-021-06655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leber H.M., Sant'Ana L., Konichi da Silva N.R., Raio M.C., Mazzeo T.J.M.M., Endo C.M., Nascimento H., de Souza C.E. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with CoronaVac: a case report. Ocul. Immunol. Inflamm. 2021:1–7. doi: 10.1080/09273948.2021.1961815. In press. [DOI] [PubMed] [Google Scholar]
- 42.Tagliaferri A.R., Narvaneni S., Azzam M.H., Grist W. A case of COVID-19 vaccine causing a myasthenia gravis crisis. Cureus. 2021;13 doi: 10.7759/cureus.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capassoni M., Ketabchi S., Cassisa A., Caramelli R., Molinu A.A., Galluccio F., Guiducci S. AstraZeneca (AZD1222) COVID‐19 vaccine‐associated adverse drug event: a case report. J. Med. Virol. 2021;93:5718–5720. doi: 10.1002/jmv.27175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conticini E., D'Alessandro M., Bergantini L., Bargagli E., Gentili F., Mazzei M.A., Cantarini L., Frediani B. Relapse of microscopic polyangiitis after vaccination against COVID‐19: a case report. J. Med. Virol. 2021;93:6439–6441. doi: 10.1002/jmv.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabata S., Hosoi H., Murata S., Takeda S., Mushino T., Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J. Autoimmun. 2021;126 doi: 10.1016/j.jaut.2021.102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Aoun S., Motabi I. Cold agglutinin disease after COVID-19 vaccine. Br. J. Haematol. 2021;195:650. doi: 10.1111/bjh.17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maye J.A., Chong H.P., Rajagopal V., Petchey W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-247188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avci E., Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J. Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lui D.T.W., Lee K.K., Lee C.H., Lee A.C.H., Hung I.F.N., Tan K.C.B. Development of graves' disease after SARS-CoV-2 mRNA vaccination: a case report and literature review. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chittal A., Rao S., Lakra P., Nacu N., Haas C. A case of COVID-19 vaccine-induced thrombotic thrombocytopenia. J. Community Hosp. Intern. Med. Perspect. 2021;11:776–778. doi: 10.1080/20009666.2021.1980966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim G., Choi E.-J., Park H.-S., Lee J.-H., Lee J.-H., Lee K.-H. A case report of immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. J. Kor. Med. Sci. 2021;36:e306. doi: 10.3346/jkms.2021.36.e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patil S., Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J. Cosmet. Dermatol. 2021;20:3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castiblanco J., Anaya J.-M. Genetics and vaccines in the era of personalized medicine. Curr. Genom. 2015;16:47–59. doi: 10.2174/1389202916666141223220551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rojas M., Restrepo-Jiménez P., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Leung P.S.C., Ansari A.A., Gershwin M.E., Anaya J.-M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018;95:100–123. doi: 10.1016/j.jaut.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O'Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., Carvalho Y. de M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.-S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., Kfouri R. de Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.-M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O'Brien K., O'Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O'Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanduc D. From anti-SARS-CoV-2 immune responses to COVID-19 via molecular mimicry. Antibodies. 2020;9:33. doi: 10.3390/antib9030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021;20 doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powell A.M., Black M.M. Epitope spreading: protection from pathogens, but propagation of autoimmunity? Clin. Exp. Dermatol. 2001;26:427–433. doi: 10.1046/j.1365-2230.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 61.Salemi S., D'Amelio R. Could autoimmunity Be induced by vaccination? Int. Rev. Immunol. 2010;29:247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 62.Pacheco Y., Acosta-Ampudia Y., Monsalve D.M., Chang C., Gershwin M.E., Anaya J.-M. Bystander activation and autoimmunity. J. Autoimmun. 2019;103 doi: 10.1016/j.jaut.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Rubinstein T.J. Thyroid eye disease following COVID-19 vaccine in a patient with a history graves' disease: a case report. Ophthalmic Plast. Reconstr. Surg. 2021;37:e221–e223. doi: 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]