Abstract
Angiotensin-converting enzyme 2 (ACE2) acts as a key receptor for the spike of SARS-CoV-2. Two main microRNAs (miRs), miR-200c-3p and miR-421-5p, are considered to modulate the expression of ACE2 gene and alterations in the expression of these miRNAs may influence the outcomes of COVID-19 infection. Accordingly, we examined whether miRNAs directing ACE2 expression altered in the SARS-CoV-2 infection. 30 patients with COVID-19 included in the study. At the time of admission and discharge, the expression of miR-200c-3p and miR-421-5p, inflammatory cytokine IL-6, and regulatory T cells' expression profiles (CD4, CD25, and Foxp3) were examined using quantitative real-time PCR method. At the time of admission, the expression levels of miR-200c-3p and miR-421-5p as well as CD4, CD25, and Foxp3 significantly decreased while IL-6 expression notably enhanced. However, by the time of discharge, the expression levels of the genes were opposite to the time of admission. Moreover, Pearson correlation analysis indicated that IL-6 expression negatively correlated with Foxp3 and miR-200c-3p expressions despite miR-421-5p and miR-200c-3p positively correlated at admission time. By manipulating miR-200c-3p and miR-421-5p expressions and controlling the ACE2 level, it is plausible to modulate the inflammation by reducing IL-6 and maintenance tolerance hemostasis during COVID-19 infection.
Keywords: COVID-19, ACE-2, miR-200c-3p, miR-421-5p, IL-6
1. Introduction
The novel coronavirus disease 2019 (COVID-19) has been dramatically affected 220 countries in the world (Organization, W.H, 2020). Vigorous efforts to control the outbreak of COVID-19 have not been practical yet, and prognostic factors which can support therapeutic approaches are still elusive. Similar to previous coronaviruses, SARS-CoV and the Middle East respiratory syndrome (MERS), the novel coronavirus named SARS-CoV-2, manifests severe symptoms such as acute respiratory distress syndrome (ARDS) and multiple organ failure in almost 20% infected patients (Guan et al., 2020; Hajivalili et al., 2020).
Notably, angiotensin-converting enzyme 2 (ACE2) has been identified as a substantial entry receptor for the spike protein of SARS-CoV-2, which is downregulated (Iwasaki et al., 2020) during the COVID-19 infection (Hoffmann et al., 2020). ACE2 acts to maintain the balance of blood pressure and electrolyte in human bodies by playing a role in the renin-angiotensin–aldosterone system (RAAS) and reducing the angiotensin II levels in the circulation (Lim et al., 2020; Ames et al., 2019). It has been reported that higher ACE2 concentrations lead to increased vulnerability to SARS-CoV-2 infection (Iwasaki et al., 2020) as a predominant expression of ACE2 occurs in the epithelial cells of the lung and intestine. These two organs act as the primary entry site for the SARS-CoV-2 (Iwasaki et al., 2020). Therefore, the extent of COVID-19 infection gets more prominent by ACE2 in patients with severe COVID-19.
Several studies have reported that microRNAs (miRNAs) could modulate ACE2 expression in various cell types and diseases (Wang et al., 2015). miRNAs are highly conserved small non-coding RNAs that can negatively regulate gene expressions. Growing evidence showed that serum miRNAs could serve as a novel prognostic marker for SARS-CoV-2 infection (Zhou et al., 2016). Among various miRNAs subjected to ACE2 regulator (Nersisyan et al., 2020), the miR-200 family, especially hsa-miR-200c-3p directly targets 3’ UTR of ACE2 mRNA leading to decreased ACE2 expression. Expression of miR-200c-3p is induced through the NF-κB pathway during infection with the pandemic flu strain H5N1 and is associated with acute respiratory distress syndrome. In particular, the NF-κB signaling pathway is hyper-activated in SARS-CoV infections, suggesting that miR-200c-3p could also be up-regulated in patients with COVID-19, which subsequently result in decreased levels of ACE2 protein (Onabajo et al., 2020). Additionally, ACE2 is subjected to post-transcriptional regulation by miR-421-5p which is implicated in the development of thrombosis (Lambert et al., 2014). miR-421-5p can also target hypoxia-responsive repressors (HRR) to disturb a balance between inflammatory and anti-inflammatory processes and maintain constant overexpression of HIF-mediated inflammatory genes, leading to inflammation-induced lung damage (Abul Bashar Mir Md Khademul and Khan, 2020).
IL-6 is a potent pro-inflammatory cytokine playing a substantial role in inflammatory responses, cancers, and viral infections (Soltani-Zangbar et al., 2021; Kany et al., 2019). IL-6 is well-recognized in the development of COVID-19, which further affects clinical manifestations of the disease by enhancement of T helper 17 (Th17) differentiation and inhibition of regulatory T (Treg) cells (Kimura and Kishimoto, 2010; Gubernatorova et al., 2020).
Regulatory T cells (Tregs) are a subpopulation of T cells that suppress the immune response, thereby preserving self-tolerance and homeostasis. Studies have shown that Tregs are able to repress cell proliferation and cytokine secretion and play a crucial role in preventing autoimmunity (Balandya et al., 2012). Tregs expressing CD4, FOXP3, and CD25 are thought to be derived from the naïve CD4+ cells (Curiel, 2007). Effector T cells also express CD4 and CD25, which are difficult to distinguish from each other (Chen, 2011). Tregs secret A collection of inhibitory cytokines. Including TGFbeta, (Read et al., 2000) and IL-10, (Collison et al., 2007). It has also been demonstrated that Tregs can stimulate other cell types to express IL-10 (Kearley et al., 2005).
On the contrary, Th17, CD4+ CD25+ Foxp3+ Treg cell accounts for modulation of the inflammation by producing the anti-inflammatory factors and suppressing both innate and adaptive immune responses. Treg cells take part in maintaining immune tolerance and balance between inflammatory and regulatory responses. The dysfunction of Treg cells is one of the causative mechanisms of the immune system dysregulation and hyper inflammation in COVID-19 (Qin et al., 2020; Chen et al., 2020).
Regarding previous studies, high expression of ACE2 was associated with innate and adaptive immune response and enhanced inflammatory response induced by cytokine secretions such as IL-1, IL-8, and IL-6 (Huang et al., 2020). Thus, we speculated that ACE2 directing miRNAs might be affected by inflammation and tolerance changes during COVID-19 disease. To this aim, we investigated the expression of two main ACE2 directing miRNAs, miR-200c-3p and miR-421-5p, in peripheral bloods of COVID-19 patients at admission and discharge time. In addition, we evaluated the correlation between ACE2 regulator miRNAs and immune response related factors in SARS-COV2 infection.
2. Materials and methods
2.1. Study subjects
Thirty adult patients with confirmed COVID-19 admitting into Taleghani hospital at Shahid Beheshti University of Medical Sciences, Tehran, Iran, were enrolled in this study from May until August 2020. The patients were monitored in two steps, at the time of hospitalization and the time of discharge. Routine serological tests analyzed all subjects, and COVID-19 infection was confirmed by positive SARS-COV2 real-time polymerase chain reaction (real-time-PCR) results of throat swab samples and chest C.T. scan. The Ethics committee approved this study of Shahid Beheshti University of Medical Sciences (IR-SBU.RIGLD.REC1199.006). Written informed consent was obtained by the Ethics Commission of the designated hospital for emerging infectious diseases.
2.2. Quantitative real-time PCR
According to the manufacturer's instructions, peripheral blood samples of patients and healthy volunteers were collected and total RNA isolated using a total RNA isolation kit (Yekta Tajhiz Azama, Tehran, Iran). To analyze mature miRNA, cDNA was generated by stem-loop R.T. primer, and subsequently, quantitative real-time PCR applying SYBR Green Master Mix (Ampliqon, Herlev, Denmark) was conducted. For mRNA analyses, cDNA was synthesized from 2 μg total RNA per sample using a cDNA synthesis kit (Yekta Tajhiz, Tehran, Iran). The quantitative real-time PCR (qPCR) assays were performed using the Rotor-Gene Q system (Qiagen, Hilden, Germany) and the Fast SYBR Green Master Mix (Ampliqon). GAPDH and U6 genes were used as endogenous controls for the normalization of mRNA and miRNA gene expressions. Relative quantification was calculated by the 2(−ΔΔCt) method and expressed as relative to endogenous controls. The sequences of oligonucleotides used as qPCR primers were designed using the OLIGO v.7.56 software (Molecular Biology Insights, Inc., USA) and listed in Table 1 .
Table 1.
Quantitative Real-time PCR primers.
| Gene name | Sequences | Accession number |
|---|---|---|
| hsa-miR-200c-3p | Fw: 5´-GCGGCGGTGGCAGTGTCTTAGC-3′ Rv: 5´-ATCCAGTGCAGGGTCCGAGG-3′ |
|
| hsa-miR-421-5p | Fw: 5´-GCTTCGGCAGCACATATACTAAAAT-3′ Rv: 5´-CGCTTCACGAATTTGCGTGTCAT-3´ |
|
| snRNA U6 | Fw: 5´-AGCAGATCTTGAGGTTACGGA-3′ Rv: 5´-GGCATGAGGCAAACAGTCT-3´ |
|
| CD4 | Fw:5’-ACATCAAGGTTCTGCCCAC-3′ Rv:5’-TGGCAGGTCTTCTTCTCAC-3’ |
NM_001382705.1 |
| CD25 | Fw:5’-ACTTCCTGCCTCGTCACAAC-3′ Rv:5’-ACTCTTCCTCTGTCTCCGCT-3 |
NM_000417.3 |
| IL-6 | Fw:5’- GATTCAATGAGGAGACTTGCC-3’ Rv: 5’- GGTCAGGGGTGGTTATTGC-3’ |
NM_001371096.1 |
| GAPDH | FW: 5’-TGAAGGTCGGAGTCAACGGATTTGGT-3′ Rv: 5’-CATGTGGGCCATGAGGTCCACCAC-3’ |
NM_001357943.2 |
2.3. Enzyme-linked immunosorbent assay (ELISA)
Serum concentrations of IL-6 were determined by ELISA, we used a Human IL-6 kit from Karmania Pars Gene (Cat. No. KPG-HIL6).
2.4. Statistical analysis
Inter-group comparisons were made by student t-test parametric analysis. All data were reported as mean ± SD and p < 0.05 was considered as statistically significant. Statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, CA, USA). The Pearson's correlation coefficient was applied to assess the linear relationship between continuous variables.
3. Results
3.1. The expressions of miR-200c and miR-421 in blood samples of COVID-19 patients
In this study, 30 patients with COVID-19 confirmed by PCR and C.T. scan were admitted to the infectious ward of Taleghani Hospital, Shahid Beheshti University of Medical Sciences. 18 healthy control participants were recruited. The mean ages of patients were 59.67, and 68% and 32% were male and female, respectively. The mean ages of control were 32.7, and 42% and 58% were male and female, respectively. All healthy controls have been confirmed with no infection with pathogenic microorganisms or respiratory viruses other than SARS-CoV-2. All participants were included in the study only with written consent.
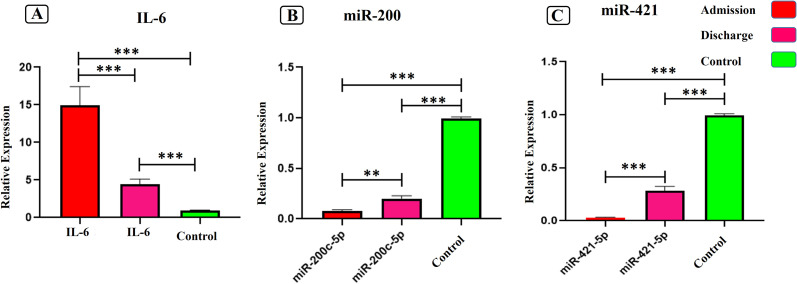
The expression levels of ACE 2 regulators (miR-200c-3p and miR-421-5p) and IL-6 were evaluated at the time of admission and discharge in the Patient's blood samples. A significant increase in the expression of IL-6 in peripheral bloods of SARS-COV2 infected patients compared to the control group was identified (Fig. 1A), P < 0.0001. Moreover, the expression of IL-6 showed a significant decrease at discharge time compared to admission time (Fig. 1A), P < 0.0001. On the other hand, a significant downregulation of miR-200c-3p (Fig. 1B) and miR-421-5p (Fig. 1C) expression (8- and 9-fold changes, respectively) were observed among COVID-19 infected patients compared to healthy controls (P-value <0.0001). In contrast, at the time of discharge from the infectious ward, a significant upregulation of miR-200c-3p (2-fold change) and miR-421-5p (3-fold change) was observed compared to admission time. However, it still has less expression than control samples (P-value <0.0123 and < 0.0001 respectively). (Fig. 1A, B).
Fig. 1.
The relative expressions of IL-6, miR-200c-3p, and miR-421-5p in peripheral blood of COV ID-19 patients at admission and discharge time. (A) The relative expression of IL-6. (B) The relative expression of miR-200c-3p. (C) The relative expression of miR-421-5p. The data are presented as mean ± S.D. ** indicate P < 0.01, *** P < 0.001. One-way ANOVA was used to make comparisons between groups.
3.2. The expressions of inflammatory cytokine IL-6 and Treg-designated genes
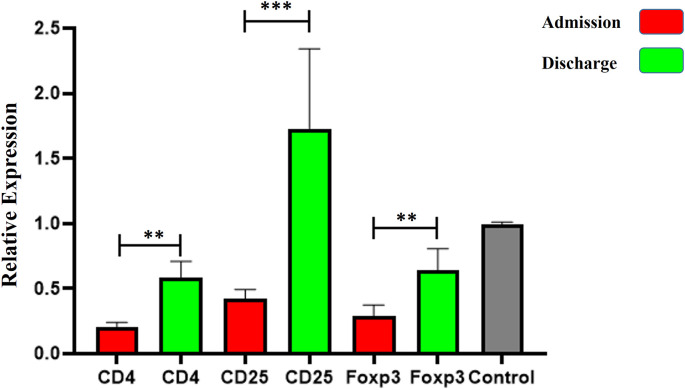
As illustrated in Fig. 1a, the expression levels of IL-6 in peripheral bloods of SARS-COV-2 infected patients were significantly elevated compared to the control group at both times of admission and discharge (P < 0.0001). Nevertheless, the expression of IL-6 was notably diminished by the time of discharge from the hospital when compared to admission time (p < 0.0001). However, the evaluation of expression levels of genes designating regulatory T cells (CD4, CD25, and Foxp3) in peripheral blood of COVID-19 patients revealed that the levels of CD4, CD25, and Foxp3 at the time of admission (0.2 ± 0.08, 0.42 ± 0.1, 0.28 ± 0.11, respectively), were significantly up-regulated compared to those at the time of discharge (0.58 ± 0.14, 1.72 ± 0.68, 0.64 ± 0.18 respectively) (p < 0.001). healthy control group (Fig. 2 ).
Fig. 2.
The relative expressions of regulatory T cells markers in peripheral blood of COVID-19 patients at admission and discharge time. The relative expression of CD4, CD25, and Foxp3 showed a significant increase at discharge time compared to admission time. The data are presented as mean ± S.D. ** indicate P < 0.01, *** P < 0.001. One-way ANOVA was used to make comparisons between groups.
3.3. The concentration of IL-6
IL-6 protein level was evaluated as one of the critical regulators of severe COVID-19 patients. The mean concentration of IL-6 in the peripheral blood of SARS-COV-2 infected patients was 197 pg/ml, which 32-fold were higher than the healthy control group (P < 0.0001) (Fig. 3 ).
Fig. 3.
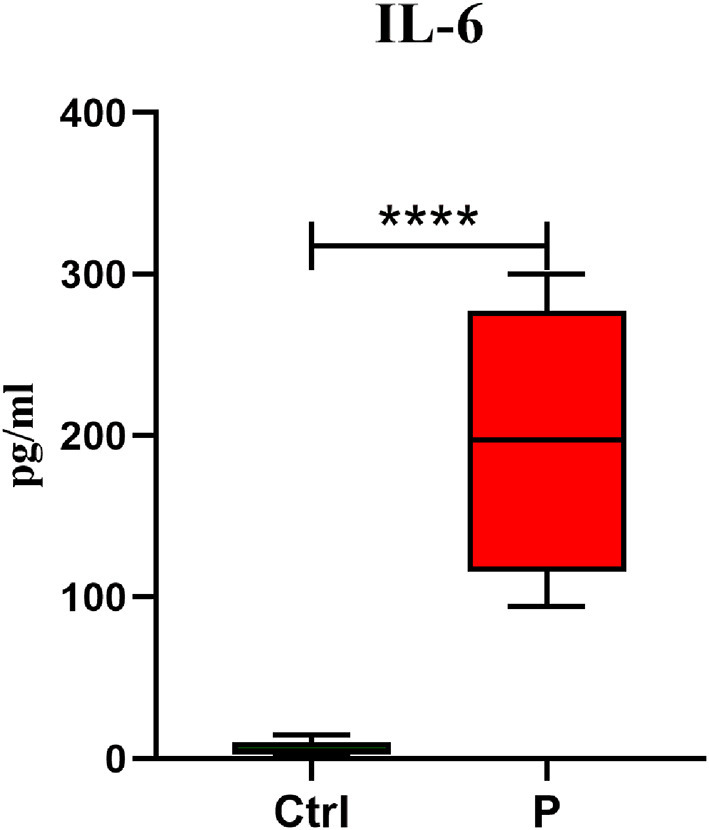
IL-6 concentration in serum sample of COVID-19 patients. The serum was harvested and an ELISA assay for IL-6 was performed. The data are presented as mean ± S.D. **** indicate P < 0.0001. A t-test was used to make comparisons between groups. Ctrl control individuals and P Positive patients for COVID-19.
3.4. Correlation analysis of Foxp3, IL-6, miR-200c-3p, and miR-421 expression in COVID-19 patients at admission time
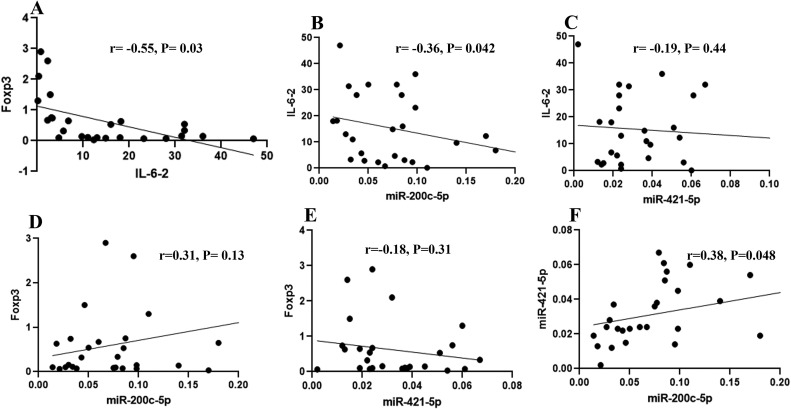
Pearson correlation analysis was performed to evaluate Foxp3, IL-6, miR-200c-3p, and miR-421 associations in peripheral blood samples of SARS-COV-2 patients. As shown in Fig. 4 , the relative expression of Foxp3, a key marker for Treg cells (Ziegler and Buckner, 2009), was negatively correlated with that of IL-6 in patients with COVID-19 (r = −0.55, P = 0.03, Fig. 4A), although the correlation of Foxp3 with either miR-200c or miR-421 was not statistically meaningful (Fig. 4D–E). Moreover, there was a significantly negative correlation between IL-6 and miR-200c-3p but not miR-421 at admission time (r = −0.36, P = 0.042, Fig. 4B–C). Finally, there was a noticeably positive correlation between the relative expression of miR-421-5p and miR-200c-3p in infected individuals (r = −0.38, P = 0.048) (Fig. 4F).
Fig. 4.
Scatter plots representing the correlation between miRNAs expression and immune response-related factors in SARS-COV2 infection. (A) Pearson correlation analysis between Foxp3 and IL-6 in peripheral blood samples of SARS-COV2 patients. (B) Correlation between IL-6 and miR-200c-3p. (C) Correlation between IL-6 and miR-421. (D) Correlation between Foxp3 expression and miR-200c-3p. (E) Correlation between Foxp3 and miR-421. (F) Correlation between miR-421 and miR-200c-3p expression. The regression line is also represented (Pearson correlation coefficient (r), P-value (P)).
4. Discussion
miRNAs express ubiquitously across species and play significant roles in the development and perform diverse regulatory functions in cells, including viral infections (Głobińska et al., 2014; Rossi et al., 2015). In many cases, miRNAs have been proposed as minimally invasive markers for the early detection of diseases, and human encoded miRNAs could be the finest candidates for the development of miRNA-based therapeutics in the management of SARS-CoV-2 infection (Bozgeyik, 2021). In this study, we purposed to investigate the levels of miR-200c-3p and miR-421-5p in admitted and recovered COVID-19 patients and seeking their correlations with inflammation and tolerance-related gene expressions. Our results illustrated a significant decrease in the levels of miR-200c-3p and miR-421-5p in COVID-19 patients at the time of admission, which was compensated at discharge time. In addition, a positive correlation between miR-200c-3p and miR-421-5p was identified implying the concurrent decrease of miR-200c-3p and miR-421-5p in SARS-CoV-2 infection when patients admitted to the hospital. Although, the expression levels at both times notably diminished in comparison to healthy controls proposing it may take time to receive miR-200c-3p and miR-421-5p expression levels in infected people to those in healthy individuals.
miR-200c and miR-421 are important modulators of ACE2, the specific functional receptor for SARS-CoV-2cells (Papannarao et al., 2021; Trojanowicz et al., 2019). Despite the indicated levels of ACE2 mRNA and protein across human tissues, Wang et al. reported no evidence of ACE2 mRNA and protein in the typical blood cells. Nevertheless, they demonstrated the concentration of ACE2 protein in plasma could be approximately 85 ng/l (Wang et al., 2020a). Therefore, it might be logical to evaluate the expressions of miRNAs regulating ACE2 in the whole blood as an indicator of ACE2 expression during the infection. Previously, it has been elucidated that the expressions of miR-200c and miR-421 were correlated with SARS-CoV-2 infection through the overexpression of the miRNAs as mentioned above simultaneously with repression of ACE2 expression in both rat and human cardiomyocytes (Lu et al., 2020; Joshi et al., 2019). Considering miRNAs are the earliest molecular regulators, circulating such miRNAs could be a potential biomarker in the early identification of those at the risk of severe COVID-19 (Papannarao et al., 2021).
According to studies, the infection of cells by SARS viruses that bind ACE2 results in inhibition of ACE2 activity and decreased ACE2 expression in infected cells (Glowacka et al., 2010; Haga et al., 2008; Kuba et al., 2005; Zhang et al., 2020). Lung samples from patients with idiopathic pulmonary fibrosis (IPF) have decreased ACE2 expression associated with lung injury and fibrosis (Li et al., 2007). Regarding our results, by the time of hospitalization, the expressions of miR-200c and miR-421 dramatically reduced and then enhanced at the recovered time, proposing the levels of ACE2 expression continuously decreased from the first time of admission to the end of discharge and possibly never achieve the normal level for a long time. Despite limiting data of miRNA expression relative to ACE2, in convinced with our data, Hoffmann et al. reported that increased ACE2 expression might confer increased susceptibility to host cell entry of SARS-CoV-2 (Hoffmann et al., 2020). This may be concluded that during the infection by COVID-19, the expressions of miRNAs regulating ACE2 elevated the expression of ACE2 downregulated and so improve the process of healing. Furthermore, presumably, ACE2 downregulation by SARS-CoV-2 results in a decreased opportunity for further viral cell entry, thereby limiting viral spread. Meanwhile, SARS-CoV-2-induced downregulation of ACE2 could impair clearance of Ang II and hence lead to aggravation of tissue damage (Bourgonje et al., 2020).
Liu Q et al., showed that transfection of HEK293T cells with miR-200c-3p inhibited ACE2 expression (Liu et al., 2017). On the other hand, studies conducted by Trojanowicz et al. showed that increasing the level of miR-421-5p in the bloodstream leads to a decrease in ACE2 levels. There is an inverse relationship between miR-421-5p and ACE2 expression levels (Trojanowicz et al., 2019; Trojanowicz et al., 2017). Therefore, the results obtained in this study are in line with previous studies; with the onset of the disease and subsequent deterioration of patients, a decrease in the level of two miR-200c-3p, 421 was observed, and, after recovery, significantly increased compared to admission time. Removal of ACE2 inhibition can increase the expression of this gene in these patients (Wang et al., 2020). Contrary to our findings Ruan Pimenta et al., reported that miR-200c-3p levels increase in COVID-19 patients with severe conditions (Pimenta et al., 2021), while Lu et al., and Liu Q et al., in separate studies showed that miR-200c mainly targets ACE2 and reduces the expression of this gene (Patel and Verma, 2020; Bao et al., 2021).Furthermore, studies showed high expression of ACE2 in young or older people, which along with IL-6 is effective in causing cytokine storms (Patel and Verma, 2020; Bao et al., 2021). According to our results and other studies, the low level of miR-200c did not inhibit the expression of ACE2, which in COVID-19 patients is associated with severity of the disease. In addition, we showed that mir-421, which is a negative regulator of ACE2, was reduced, which is in line with other findings regarding cytokine storm and Tregs inhibition, which is in contrast to the results of Ruan Pimenta et al. Therefore, studies are needed to elucidate exact molecular mechanisms.
Cytokine storm is mainly observed in COVID-19 patients, and the crucial cytokine playing a role, in this case, is IL-6 (Mohebbi et al., 2020). IL-6 is a pleiotropic cytokine that could be induced due to the deregulated expression of ACE2 in COVID-19 patients. The changes in ACE2 expression lead to immune system dysfunction and cause cytokine release syndrome (CRS) (Wang et al., 2020). This study showed that IL-6 in protein and mRNA levels increased significantly in COVID-19 infected patients (Fig. 3). Similar to our findings, L. Bergantini et al. And Jing Liu et al. showed upregulation of IL-6 levels compared to mild cases in a separate study. This increase has caused lymphopenia and a pro-inflammatory cytokine storm in severe COVID-19 patients compared to mild cases (Liu et al., 2020; Bergantini et al., 2021).
The evaluation of the RNAseq data of SARS-COV2-infected and uninfected cell lines has shown that the expression of IL-6 significantly increased in infected cell lines (Bao et al., 2021). Moreover, it has been elucidated that patients with high ACE2 expression are more likely to develop cytokine storms dominated by IL-6 (Bao et al., 2021). In this regard, correlation analysis demonstrated a significantly negative association between the expression of IL-6 and miR-200c-3p, so that patients with the highest levels of IL-6 had the lowest expression of miR-200-3p. These results suggest that decreased miR-200c-3p expression in SARS-COV2 infection removes its inhibitory effects on ACE2, subsequently promoting cytokine storms by inducing the expression of IL-6. Infection of human epithelial cells by SARS-COV2 induces high levels of IL-6 compared to parainfluenza 2 virus and influenza A virus (Okabayashi et al., 2006). As reported by Xu et al., a dramatic increase in IL-6 could justify the presence of T helper 17 (Th17) in COVID-19 patients due to an essential role of IL-6 in the differentiation of Th17 cells as interacting with dendritic cells (Xu et al., 2020). Correspondingly, IL-6 is a vital agent participating in regulating inflammatory Th17 cells/regulatory T (Treg) cells balance. Elevated circulating IL-6 levels contribute to the deregulation of the Treg/Th17 cell ratio toward an increase in Th17 cells, leading to high production of inflammatory cytokines by Th17 cell activation.
Accumulating body of data revealed that severe COVID-19 patients had a notable decline in Treg cell levels (Qin et al., 2020; Wang et al., 2020) meanwhile increased levels of Th17 cells culminating in diminished the Treg/Th17 cell ratio (Xu et al., 2020; Wu and Yang, 2020). CD4+ CD25+ Foxp3+ Treg cells are critical modulators of the inflammation by producing the anti-inflammatory factors maintaining immune tolerance and balance between inflammatory and regulatory responses (Qin et al., 2020; Chen et al., 2020). Consistently, we showed the adverse correlation between IL-6 and FOXp3 expressions, indicating the opposite relationship between inflammation/tolerance in our study subjects. Furthermore, we demonstrated significant alteration in CD25 marker in admission and discharge patients which hypothesized these changes in the level of IL-6 and other cytokines involved in the cytokine storm cause a change in cell arrangement to further activate the immune response, which after recovery and suppression of the hyperactive immune system, this cell arrangement probably changes to inhibit the immune system, which causes inhibitory markers to increase.
The proportion of Treg cells and Th17 cells was affected by SARS-CoV-2 in different ways. Several studies showed severe COVID-19 patients had a significant decrease in Treg cell levels, (Wang et al., 2020; Qin et al., 2020) and increased Th17 cells, (Wu and Yang, 2020) subsequence to decrease in the Treg/Th17 cell ratio IL-6 induces the generation of Th17 cells from naïve T cells and inhibits Treg (iTreg) differentiation (Bettelli et al., 2006). Accordingly, increased serum level of IL-6 contribute to imbalance of the Treg/Th17 ratio (Chi et al., 2020). Our result showed with decrease level of IL-6 in discharge patients its ratio of Treg / Th17 increased which facilitates the establishment of immunological tolerance.
Additionally, a body of recent data has been illustrated that the reduced frequency and dysfunction of Treg cells in the course of SARS-CoV2 infection lead to disease progression, respiratory system injury, and ARDS (Qin et al., 2020; Sadeghi et al., 2020). In convinced with these studies, our results depicted that the expression levels of CD4, CD25, and Foxp3 markers relative to Treg cells were reduced in admitted COVID-19 patients while those levels elevated once patients recovered. However, the Pearson correlation analysis of Foxp3 with miR-200c-3p and miR-421 was not statistically meaningful. This lacks of association can be justified by either the indirect effects of miR-200c-3p and miR-421 on Foxp3 expression or the low sample size (Fig. 5 ). Emerging researches are needed to exclusively explain how ACE2 directing miRNAs are associated with inflammation and likely tolerance state in COVID-19 patients.
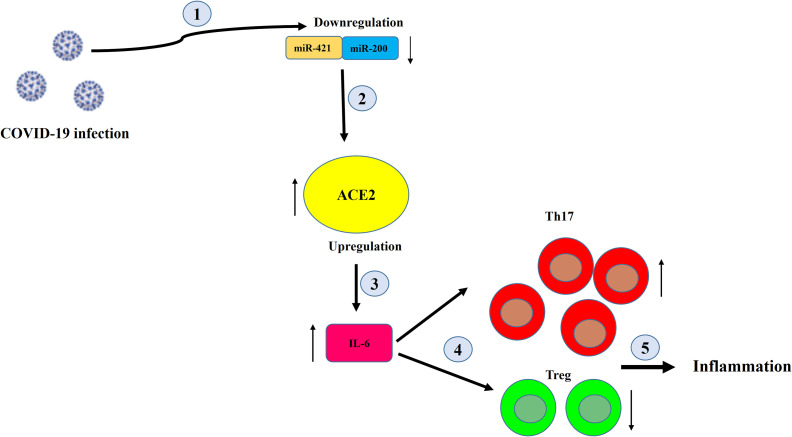
Fig. 5.
Schematic of the effect of reducing the level of miR-200c-3p miR-421-5p on the rate of inflammation in COVID-19 patients.
In conclusion, miRNAs could be considered an early detection of infection in blood streams of COVID-19 patients which dysregulation of miR-200c-3p and miR-421are correlated by the expression of ACE2 and inflammatory cytokine IL-6. Thus, it is speculated that targeting these miRNAs can maintain the level of ACE2 affecting the inflammation/tolerance balance and be promising in the treatment of severe COVID-19 patients. Further studies are needed to elucidate these pathways and warrant our results.
Author contribution
Study design: K Baghaei. Methodology development: S Abdolahi, M Hosseini. Facility provided: M R Zali, H A Aghdaei. Analysis and interpretation of data: S Abdolahi, R Rezaei, S R Mohebbi, M Rostami-Nejad. Writing and reviewing the manuscript: S Abdolahi, M Hosseini, H Mirjalali, A Yadegar. Study supervision: K Baghaei.
CRediT authorship contribution statement
Shahrokh Abdolahi: Methodology, Validation, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Maryam Hosseini: Methodology, Validation, Investigation, Writing – original draft, Writing – review & editing. Ramazan Rezaei: Data curation, Formal analysis. Seyed Reza Mohebbi: Data curation, Formal analysis. Mohammad Rostami-Nejad: Data curation, Formal analysis. Hamed Mirjalali: Writing – original draft, Writing – review & editing. Abbas Yadegar: Writing – original draft, Writing – review & editing. Hamid Asadzadeh Aghdaei: Project administration. Mohamad Reza Zali: Project administration. Kaveh Baghaei: Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors would like to thank the Research Institute of Gastroenterology and Liver Diseases of the Shahid Beheshti Medical University for its financial support of this study (Grant No. 1101).
References
- Abul Bashar Mir Md Khademul I., Khan M. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci. Rep. 2020;10(1):19395. doi: 10.1038/s41598-020-76404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames M.K., Atkins C.E., Pitt B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019;33(2):363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandya E., et al. Human seminal plasma fosters CD 4+ regulatory T-cell phenotype and transforming growth factor-β1 expression. Am J Reprod Immunol. 2012;68(4):322–330. doi: 10.1111/j.1600-0897.2012.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z., et al. High expression of ACE2 in the human lung leads to the release of IL6 by suppressing cellular immunity: IL6 plays a key role in COVID-19. Eur Rev Med Pharmacol Sci. 2021;25(1):527–540. doi: 10.26355/eurrev_202101_24425. [DOI] [PubMed] [Google Scholar]
- Bergantini L., et al. Prognostic bioindicators in severe COVID-19 patients. Cytokine. 2021;141 doi: 10.1016/j.cyto.2021.155455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH 17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bourgonje A.R., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozgeyik I. Therapeutic potential of miRNAs targeting SARS-CoV-2 host cell receptor ACE2. Meta gene. 2021;27 doi: 10.1016/j.mgene.2020.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.J.I. Tregs in immunotherapy: opportunities and challenges. Immunotherapy. 2011;3(8):911–914. doi: 10.2217/imt.11.79. [DOI] [PubMed] [Google Scholar]
- Chen G., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z., et al. Antagonist Tocilizumab man be the Key to Reduce the Mortality. 2020. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison L.W., et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Curiel T.J. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117(5):1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głobińska A., Pawełczyk M., Kowalski M.L. MicroRNAs and the immune response to respiratory virus infections. Expert. Rev. Clin. Immunol. 2014;10(7):963–971. doi: 10.1586/1744666X.2014.913482. [DOI] [PubMed] [Google Scholar]
- Glowacka I., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernatorova E., et al. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajivalili M., Hosseini M., Haji-Fatahaliha M. Gaining insights on immune responses to the novel coronavirus, COVID-19 and therapeutic challenges. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M., et al. Inflammation triggered by sars-cov-2 and ace2 augment drives multiple organ failure of severe covid-19: molecular mechanisms and implications. Inflammation. 2020:1–22. doi: 10.1007/s10753-020-01337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., et al. Hypoxic regulation of angiotensin-converting enzyme 2 and mas receptor in human CD34+ cells. J. Cell. Physiol. 2019;234(11):20420–20431. doi: 10.1002/jcp.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J., et al. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+ CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., et al. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. 2014;127(4):243–249. doi: 10.1042/CS20130420. [DOI] [PubMed] [Google Scholar]
- Li X., et al. Attenuation of bleomycin-induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Curr. Pharm. Des. 2007;13(12):1257–1268. doi: 10.2174/138161207780618867. [DOI] [PubMed] [Google Scholar]
- Lim S., et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2020:1–20. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., et al. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discovery. 2017;3(1):1–17. doi: 10.1038/celldisc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., et al. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J. Mol. Cell. Cardiol. 2020;148:46–49. doi: 10.1016/j.yjmcc.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebbi S.R., et al. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterology and hepatology from bed to bench. 2020;13(4):388. [PMC free article] [PubMed] [Google Scholar]
- Nersisyan S., et al. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T., et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 2006;78(4):417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onabajo O.O., et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020:1–11. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H . Vol. 182. 2020. Coronavirus Disease (COVID-19): Situation Report. [Google Scholar]
- Papannarao J.B., et al. Upregulated miR-200c may increase the risk of obese individuals to severe COVID-19. medRxiv. 2021 [Google Scholar]
- Patel A.B., Verma A.J.J. Nasal ACE2 levels and COVID-19 in children. JAMA. 2020;323(23):2386–2387. doi: 10.1001/jama.2020.8946. [DOI] [PubMed] [Google Scholar]
- Pimenta R., et al. MiR-200c-3p expression may be associated with worsening of the clinical course of patients with COVID-19. Mol Biol Res Commun. 2021;10(3):141. doi: 10.22099/mbrc.2021.40555.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmström V., Powrie Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G.A., Silvestri M., Colin A.A. Respiratory syncytial virus infection of airway cells: role of microRNAs. Pediatr. Pulmonol. 2015;50(7):727–732. doi: 10.1002/ppul.23193. [DOI] [PubMed] [Google Scholar]
- Sadeghi A., et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2020;236(4):2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- Soltani-Zangbar M.S., et al. Application of newly developed SARS-CoV2 serology test along with real-time PCR for early detection in health care workers and on-time plasma donation. Gene Reports. 2021;23 doi: 10.1016/j.genrep.2021.101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowicz B., et al. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrology Dialysis Transplantation. 2017;32(2):287–298. doi: 10.1093/ndt/gfw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowicz B., et al. Circulating miR-421 targeting leucocytic angiotensin converting enzyme 2 is elevated in patients with chronic kidney disease. Nephron. 2019;141(1):61–74. doi: 10.1159/000493805. [DOI] [PubMed] [Google Scholar]
- Wang X., et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell. Signal. 2015;27(3):443–452. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Wang Y., et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020;17(11):1522. doi: 10.7150/ijms.46695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10) doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., et al. miR-421 is a diagnostic and prognostic marker in patients with osteosarcoma. Tumor Biol. 2016;37(7):9001–9007. doi: 10.1007/s13277-015-4578-5. [DOI] [PubMed] [Google Scholar]
- Ziegler S.F., Buckner J.H. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11(5):594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]