Abstract
SARS-CoV-2, the causative agent for COVID-19, an ongoing pandemic, engages the ACE2 receptor to enter the host cell through S protein priming by a serine protease, TMPRSS2. Variation in the TMPRSS2 gene may account for the disparity in disease susceptibility between populations. Therefore, in the present study, we have used next-generation sequencing (NGS) data of world populations from 393 individuals and analyzed the TMPRSS2 gene using a haplotype-based approach with a major focus on South Asia to study its phylogenetic structure and their haplotype sharing among various populations worldwide. Our analysis of phylogenetic relatedness showed a closer affinity of South Asians with the West Eurasian populations therefore, host disease susceptibility and severity particularly in the context of TMPRSS2 will be more akin to West Eurasian instead of East Eurasian. This is in contrast to our prior study on the ACE2 gene which shows South Asian haplotypes have a strong affinity towards East Eurasians. Thus ACE2 and TMPRSS2 have an antagonistic genetic relatedness among South Asians. Considering the significance of the TMPRSS2 gene in the SARS-CoV-2 pathogenicity, COVID-19 infection and intensity trends could be directly associated with increased expression therefore, we have also tested the SNPs frequencies of this gene among various Indian state populations with respect to the case fatality rate (CFR). Interestingly, we found a significant positive association between the rs2070788 SNP (G Allele) and the CFR among Indian populations. Further our cis eQTL analysis of rs2070788 shows that the GG genotype of the rs2070788 tends to have a significantly higher expression of TMPRSS2 gene in the lung compared to the AG and AA genotypes thus validating the previous observation and therefore it might play a vital part in determining differential disease vulnerability. We trust that this information will be useful in understanding the role of the TMPRSS2 variant in COVID-19 susceptibility and using it as a biomarker may help to predict populations at risk.
Keywords: COVID-19, TMPRSS2, India, rs2070788, Haplotype, Linkage disequilibrium
1. Introduction
COVID-19 is an ongoing pandemic that has cost millions of lives worldwide, caused by the SARS-CoV-2 virus of the Beta Family. Along with ACE2 (Angiotensin-converting enzyme 2) which acts as a receptor, TMPRSS2 (Transmembrane protease, serine 2), a serine protease, is also involved in virus entry into the host cell through S Protein priming (Hoffmann et al., 2020; Zhou et al., 2020). The impact of the COVID-19 crisis is not uniform across ethnic groups. Patients from different ethnic backgrounds suffer disproportionately (Webb Hooper et al., 2020). Discrepancies in infection, as well as CFR, could be due to multiple reasons e.g., existing comorbidities, differences in quarantine and social distancing policies, access to medical care, reliability & coverage of epidemiological data, and population age structure, which shows that mortality is greater among the elderly and those with comorbidity (Ejaz et al., 2020; Sanyaolu et al., 2020). However, many young and healthy people have also lost their lives due to rapid cytokine storms (Muschitz et al., 2021). It is important to note that these factors do not appear to account for all the disparities noticed among groups, and there are significant knowledge gaps. However, countries with strict standards for the collection and presentation of epidemiological data suggest that human variation in genetic makeup may account for differential susceptibility and severity in disease outcomes among different populations (SeyedAlinaghi et al., 2021). There is evidence that supports the role of ACE2 gene variations in COVID-19 susceptibility among Indian populations (Srivastava et al., 2020a; Srivastava et al., 2020b). However, little is known regarding the genetic structure of the TMPRSS2 gene among South Asian populations, a detailed analysis of the sequence data of the TMPRSS2 gene from world populations may unveil its haplotype sharing, which may help understand the role of TMPRSS2 in disease susceptibility globally.
TMPRSS2 has been linked to a variety of physiological and pathological processes. Along with SARS-CoV-2 and Influenza virus, various other human coronaviruses such as HCoV-229E, MERS-CoV, and SARS-CoV, have been also identified to utilize this protein for cell entrance (Shen et al., 2017). Androgenic hormones were shown to upregulate this gene in prostate cancer cells, while androgen-independent prostate cancer tissue was found to downregulate it (Mollica et al., 2020). Northern blots analysis has revealed that in mice TMPRSS2 is mainly expressed in the kidney and prostate, whereas in humans, TMPRSS2 is largely expressed in the prostate, salivary gland, stomach, and colon (Vaarala et al., 2001). TMPRSS2 is also expressed in the epithelia of the respiratory, urogenital and gastrointestinal tracts according to in-situ hybridization investigations performed on mice embryos and adult tissues (Vaarala et al., 2001). Given the relevance of the TMPRSS2 gene in the SARS-CoV-2 infection process, COVID-19 infection and severity pattern may be directly linked to elevated TMPRSS2 gene expression, resulting in varying disease susceptibility outcomes in various communities. However, the role of TMPRSS2 polymorphism in disease susceptibility in the Indian populations is largely unexplored. Therefore, in the current study, we analyzed the haplotype structure of the TMPRSS2 gene focusing on South Asia and its genetic variants that could be responsible for changes in the gene expression in the lungs tissue and, tested its correlation with epidemiological data available on COVID-19, for any existing association among Indian population.
2. Material and methods
The Haplotype analysis of the TMPRSS2 gene among various world populations was performed using NGS data from Pagani et al. (Pagani et al., 2016). PLINK 1.9 was used to extract complete coordinates of the TMPRSS2 gene from the dataset for different populations (Purcell et al., 2007). Based on Principal component analysis (PCA) results performed by Pagani et al., ethnic outliers samples from Sahul and Africa were excluded, as well relatives up till second-degree, from which a total of 393 samples and 795 SNVs (Single nucleotide variants) were observed and used subsequently for our study (Supplementary Table 1 and 2). The plink file was converted to fasta (ped to IUPAC) by a customized script (Budak, 2020). For phasing, Fst calculation, and generation of Network and Arlequin input file, DNAsp (ver 6.0) was used (Rozas et al., 2017). MEGA X was used to construct an Fst based Neighbour-joining tree (Kumar et al., 2018). To calculate Nei's genetic and average pairwise distance, Arlequin 3.5 was used and plotted on a graph by R V3.1 (Excoffier and Lischer, 2010; R: The R Project for Statistical Computing [Internet]. [cited, 2021). Network v5 and network publisher were employed to draw the median-joining network while total and prevalent haplotypes in the TMPRSS2 gene for each population were calculated using XML file generated through Arlequin 3.5 (Excoffier and Lischer, 2010; Bandelt et al., 1999).
For the association study, we searched for the studies on TMPRSS2 gene variants reported in the literature elsewhere in relation to COVID-19 susceptibility (Mollica et al., 2020; Andolfo et al., 2021; Asselta et al., 2020; Bhattacharyya et al., 2020; Darbani, 2020; Hou et al., 2020; Irham et al., 2020; Iyer et al., 2020; Jeon et al., 2020; Kim and Jeong, 2021; Latini et al., 2020; Paniri et al., 2021; Piva et al., 2021; Ragia and Manolopoulos, 2020; Senapati et al., 2020; Sharma et al., n.d.; Singh et al., 2021; Strope et al., 2020; Torre-Fuentes et al., 2021; Vargas-Alarcón et al., 2020; Wang et al., 2020; Wulandari et al., 2021; Saih et al., 2021; Schönfelder et al., 2021; Kehdy et al., 2021) from this a total of 5 SNPs (rs2070788, rs734056, rs12329760, rs2276205, and rs3787950) was observed in our data and studied subsequently in detail. Data from the Estonian Biocentre (Chaubey et al., 2017; Pathak et al., 2018; Re3data, 2014; Tätte et al., 2019), phase 3 of the 1000 Genomes Project (Durbin et al., 2010), and our new genotyped samples from several Indian states were used to calculate the frequency of each of these SNPs among various Indian populations using plink 1.9. We obtained state-wise frequency of cases and deaths from https://www.mygov.in/corona-data/covid19-statewise-status/. State-wise frequency maps for rs2070788 and COVID-19 CFR among the Indian population were made using https://www.datawrapper.de/. and worldwide spatial distribution of rs2070788 was generated from the PGG.SNV toolkit using 1000 genome samples (Zhang et al., 2019). The linear regression calculations for statewise allele frequency Vs the CFR were done using Microsoft excel and results were further validated by SPSS (ver 26) at a 95% confidence interval and 1000 bootstrapping (2,000,000 seeds) for a two-tailed significance. The composite plots for all variables were produced using a customized R script (ver 4) (R: The R Project for Statistical Computing [Internet]. [cited, 2021). Expression quantitative trait loci (eQTL) were examined utilizing the GTEx database (http://www.gtexportal.org/home/) to determine the relationship between genetic variations and TMPRSS2 gene expression profile (THE GTEX CONSORTIUM et al., 2015). Haploview was used to generate a Linkage disequilibrium (LD) map and to calculate aggregate haplotypes frequency carrying rs2070788 (G allele) in each population (Barrett et al., 2005).
3. Result and discussion
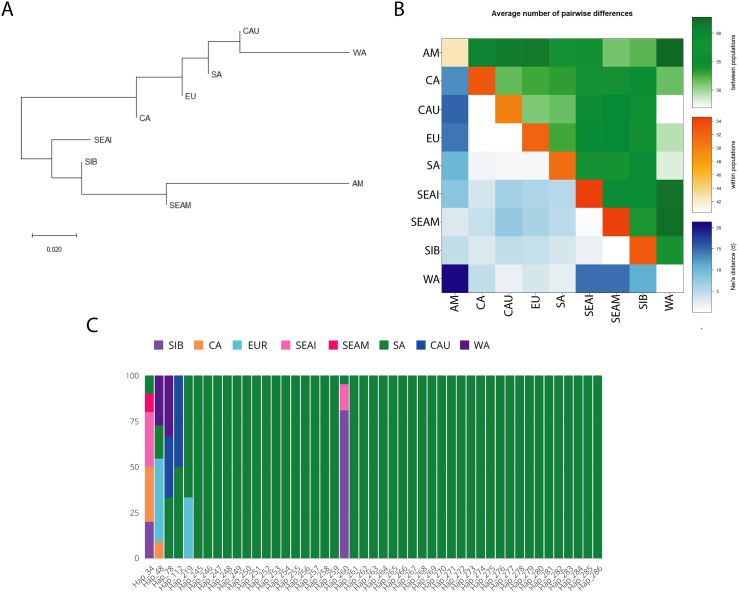
TMPRSS2 is a serine protease enzyme that is encoded in humans by the TMPRSS2 gene located on chromosome 21q22.3. (Paoloni-Giacobino et al., 1997). This protein aids in the entry of various viruses into the host cells, like the influenza virus, and human coronaviruses such as HCoV-229E, MERS-CoV, SARS-CoV, and SARS-CoV-2 by proteolytically cleaving and then activating the viral envelope glycoproteins (Huggins, 2020), and thus can be inhibited by TMPRSS2 inhibitor (Hoffmann et al., 2020). Genetic variation in this gene may account for differential vulnerability for COVID-19 disease among diverse populations. Therefore, in the present study with our major focus on South Asia, we examine the genetic relatedness, using TMPRSS2 gene sequence data among world populations by haplotype-based approach for comparison among the various groups. Fst based Neighbour-Joining (NJ) tree showed the clustering of South Asians with the West Eurasian populations (Caucasus, West Asia, Europe, and Central Asia) therefore, suggesting the closer affinity of South Asians with West Eurasians populations for the TMPRSS2 gene. (Fig. 1A). Similarly, the Average Pairwise differences analysis showed smaller diversity and genetic distance between populations, among East and West Eurasians, while greater diversity and genetic distance was observed between East and West Eurasian populations. The lowest diversity was found in West Asia & the American populations (Fig. 1B). A median-joining (MJ) network analysis of the TMPRSS2 gene revealed that there are 499 haplotypes throughout this gene among the examined populations, with five prevalent haplotypes (Hap 34, Hap 48, Hap 75, Hap 98, and Hap 260), each having ≥10 individuals. Haplotypes 48 and 75 were found to be more common in Europe, while haplotypes 98 and 260 were frequent in Siberia. Haplotype 34 was predominant in Southeast Asia, followed by Central Asia (Supplementary Table 3A and Supplementary Fig. 1). South Asian populations carry 47 haplotypes, among which 6 are shared (Hap_34, Hap_48, Hap_78, Hap_112, Hap_219, and Hap_260) with other continental populations while the rest are unique to South Asia. Among the shared haplotypes, five are shared majorly with the West Eurasian populations i.e. (Hap_34, Hap_48, Hap_78, Hap_112, Hap_219), whereas only a single haplotype (Hap_260) is majorly shared with the East Eurasian populations. (Fig. 1C and Supplementary Table 3B). Thus haplotype sharing, as well as Fst analysis, are consistent with the West Eurasian affiliation of the majority of South Asian TMPRSS2 haplotypes (Fig. 1C and Fig. 1A). Therefore, the host susceptibility of SARS-CoV-2 for the TMPRSS2 gene among South Asians is most likely expected to be similar to West Eurasians rather than that of East Eurasians. In contrast with this, our previous study on the ACE2 gene has shown the strong genetic affinity of South Asian haplotypes with the East Eurasians (Srivastava et al., 2020a; Srivastava et al., 2020b). Thus, for the South Asians, ACE2 and TMPRSS2 have an antagonistically genetic relatedness. As a result, it is worth proposing that the South Asian population's susceptibility to SARS-CoV-2 will fall somewhere between West and East Eurasian people, which is most likely the cause of the moderate susceptibility seen in first and second waves.
Fig. 1.
(A) Neighbour-Joining (NJ) tree based on Fst distance, showing genetic relationship for TMPRSS2 gene among the studied population. (B) Matrix showing average paired variation for TMPRSS2 gene, between the population (green) in the upper triangle, within-population (orange) along diagonal, and Nei's distance between populations are shown (blue) in the lower triangle. The obtained value for different variables is directly proportional to the color gradient. (C) The stacked bar-plot represents 47 haplotypes observed in TMPRSS2 Gene among South Asian populations. Frequency and sharing for each haplotype with South Asia and to other geographic regions are indicated with different colored bars.
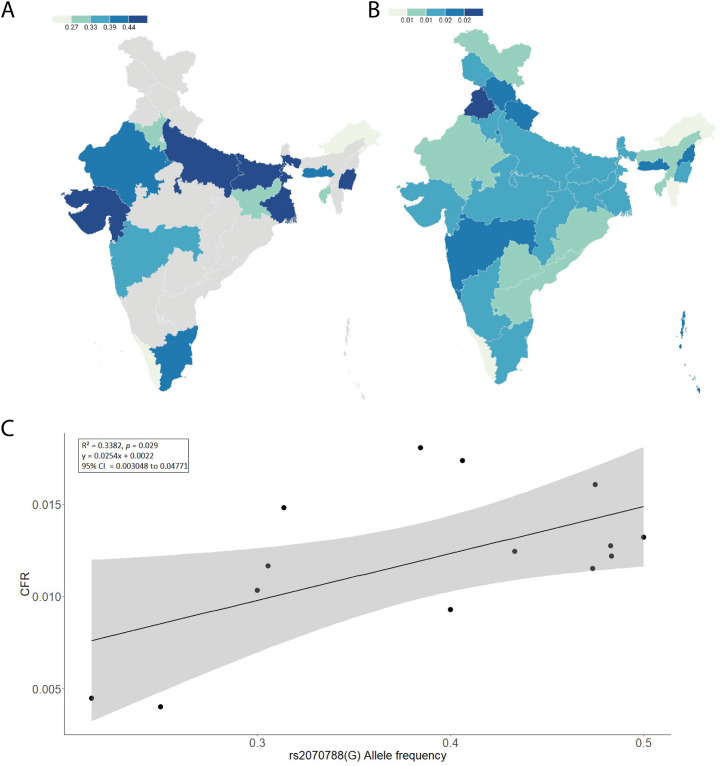
Recognizing the importance of the TMPRSS2 gene in the SARS-CoV-2 infection cycle, the disparity in COVID-19 infection and severity could be attributed to enhanced TMPRSS2 gene expression thus, variation in this gene may play such part and therefore may result in a difference in disease susceptibility in different communities around the world. The effect of TMPRSS2 polymorphism in COVID-19 susceptibility in Indian people is largely unknown and this needs to be investigated. Therefore, we calculated state-wise allele frequencies in Indian populations for all the 5 SNPs (rs2070788, rs734056, rs12329760, rs2276205, and rs3787950) observed in our data. The linear regression analysis was carried out to test the association of these SNP's frequency with COVID-19 CFR among various Indian states (Supplementary Table 4 A, B, and 5). The linear regression analysis performed for rs2070788 (G allele) allele frequency showed a significant positive correlation with CFR among Indian populations;i.e.- p = 0.029 (at significance threshold of p < 0.05). Among the Indian populations, higher CFR was observed where the allele frequency is higher and vice versa (Fig. 2A and B). The goodness of fit (R2) explained 33.82% of the variation (Figs. 2C) with an effect size (β) of 0.582, suggesting a large effect of this allele in the Indian population. As this is an active pandemic with changing numbers of infected and dead patients, we confirmed our findings at different timelines (latest up to August 2021). The recent data reciprocate the previous observation with no substantial difference between the outcomes. Thus, supporting the previous observation of strong positive association (Table 1 ).
Fig. 2.
(A) frequency map (%) showing the spatial distribution of allele rs2070788 among Indian populations. Grey color marks the absence of data. (B) The Map of state-wise frequency (%) of case-fatality rate (CFR) (updated till 30th August 2021). (C) The linear regression graph shows the relationship between rs2070788 (G) allele frequency and COVID-19 CFR of the TMPRSS2 gene.
Table 1.
Results of tests conducted for statistical significance at different timelines of the pandemic in India.
| Observation |
Linear regression analysis parameters |
|||
|---|---|---|---|---|
| rs2070788 | R2 | p-value | Effect size (β) | Std. Error (Se) |
| June 2021_CFR | 0.338 | 0.029 | 0.582 | 0.080467 |
| July 2021_CFR | 0.310 | 0.039 | 0.557 | 0.082181 |
| August 2021_CFR | 0.289 | 0.047 | 0.537 | 0.083416 |
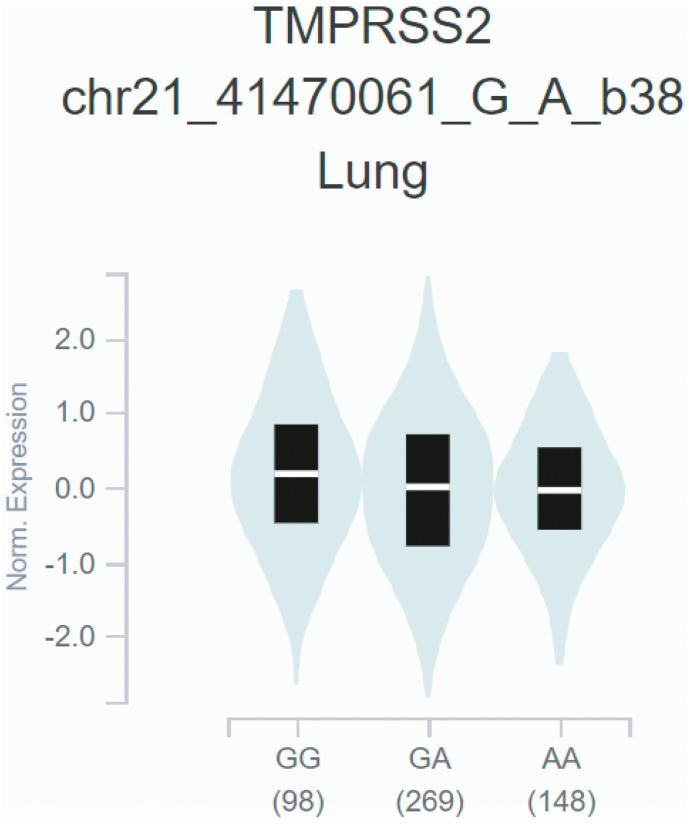
rs2070788 was previously reported to have significantly associated with the susceptibility to influenza infection, therefore, have clinical significance in infectious diseases (Cheng et al., 2015). We used the publicly accessible GTEx database to confirm our associations between rs2070788 (G) allele and TMPRSS2 expression in the lungs. We obtained expression of the TMPRSS2 gene across different genotypes in eQTL from 515 samples, which shows that the rs2070788 GG genotype had significant higher TMPRSS2 expression (p -value threshold <0.05) in the lung as compared to the AG and AA genotypes, i.e. GG > AG > AA (p-value = 8.9e-9, A allele normalized effect size = −0.115) (Fig. 3 ). Thus, the G allele may contribute to severe consequences in SARS-CoV-2 infection in populations with high frequency. We found that G allele frequency in India ranges from 20% to 50%, with the mean frequency of 39%, lowest being in Arunachal Pradesh and highest in Bihar which is in accordance as per data observed which clearly shows Arunachal Pradesh is among those states that show lowest CFR while Bihar and other states are among higher CFR (Supplementary Table 4A and B). Thus this may explain the disparity in severity of the COVID-19 pandemic among various Indian states (Fig. 2 B). Being an androgen-sensitive gene TMPRSS2 is known to mediate sex-related effects and rs2070788 SNP is known to play an important role (Alshahawey et al., 2020). Higher expression of TMPRSS2 in males might make them more prone to virus fusion and could explain high COVID-19 mortality in males (Lamy et al., 2020; Peckham et al., 2020).
Fig. 3.
Violin plot of TMPRSS2 expression for cis eQTL rs2070788 in Lung tissue. The allelic effect of rs2070788 on normalized TMPRSS2 gene expression levels is presented by boxplots inside violin plots. G and A alleles indicate the major and minor allele types, respectively with the number of subjects shown under each genotype. The plots indicate the density distribution of the samples in each genotype. The white stripe in the box plot (black) illustrates the median value of the gene expression at each genotype.
For Linkage disequilibrium (LD) analysis, LD plots were made for each population focussing on rs2070788 and nearby SNPs on that haplotype. LD blocks of various sizes were observed among Central Asians, Caucasians, Europeans, South Asians, Siberians, and West Asians. The highest LD level was found in Americans (Supplementary Fig. 2). We also calculated aggregate haplotypes frequency carrying rs2070788 (G allele), in each population presented in (Supplementary Table 6). Considerable levels of variation in haplotype frequency were observed among the populations. The highest haplotype frequency was observed in America (0.654), while the lowest haplotype frequency was recorded in Southeast Asia Island (0.322), these findings are consistent with epidemiological data available on COVID-19 which clearly shows that the American population has the most number of cases and death while Southeast Asians are much below in the list. We also looked for worldwide distribution of rs2070788 (G allele) from 1000 genome and gnomAD database (Supplementary Table 7 and Supplementary Fig. 3) and found consistent with the previous observation, rs2070788 (G allele) frequency was highest in Americans, while lowest in African and East Asians populations, this may explain high fatality among Americans populations while African and East Asians being least affected. Low severity among East Asians could be due to adaptation at many genes that engage with coronaviruses, also including the SARS-CoV-2, which began 25,000 years back for coronaviruses, or a related virus outbreak in East Asia at that time (Souilmi et al., 2021).
We caution that this SNP (rs2070788) to be one of the several factors affecting COVID-19 severity; however, the major limitations of the present study is the lack of additional data on covariates such as age, sex, existing comorbidity, virus strain, access to healthcare facilities, and precautionary measures which can alter the CFR.
4. Conclusion
In conclusion for the first time, we have shown a closer affinity of South Asians with the West Eurasian populations for the TMPRSS2 gene. Hence, host disease susceptibility in the context of the TMPRSS2 gene will be more likely similar to West Eurasian populations. This is in contrast to our prior study on the ACE2 gene, which showed a closer genetic affinity of South Asian haplotypes with East Eurasians. Thus, for South Asians, ACE2 and TMPRSS2 have an antagonistic genetic relationship. So, it is worth proposing that the susceptibility of the South Asian population to SARS-CoV-2 will fall somewhere between West and East Eurasian populations, which may be most likely the cause of the moderate susceptibility. We also found a significant genetic association between the rs2070788 (G) allele and COVID-19 CFR among Indian populations. Cis eQTL of rs2070788 across different genotypes from the GTEx database further confirms associations between rs2070788(G) allele and TMPRSS2 expression, which shows G allele is associated with higher expression in the lungs. This information could be used as a genetic biomarker to predict susceptible populations, which may be very useful during the epidemic in policymaking and making better resource allocation.
Funding
This research is supported by ICMR India (grant number- 2021-6389).
Data availability statement
All datasets generated for this study are included in the article/Supplementary Material.
Author contributions
GC and RKP conceived and designed this study. RKP, AS, and PPS analyzed the data. RKP, AS, PPS, and GC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by ICMR India (grant number- 2021-6389). GC is supported by Faculty IOE grant BHU (6031). RKP is supported by the UGC- RET fellowship, AS is supported by UGC-CAS fellowship, and PPS is supported by CSIR fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.meegid.2022.105206.
Appendix A. Supplementary data
Supplementary material Supplementary Fig. 1 The median-joining network of TMPRSS2 gene. The circle size determines the number of samples with a certain haplotype. The five most common haplotypes are marked.
Supplementary Fig. 2 LD (linkage disequilibrium) maps of the TMPRSS2 gene, focusing on rs2070788 and its haplotype, in world populations. Shading from white to red indicates the intensity of r2 from 0 to 1. Strong LD is represented by a high percentage (>80) in darker red squares.
Supplementary Fig. 3 The spatial distribution of SNP rs2070788 from 1000 genome data.
References
- Alshahawey M., Raslan M., Sabri N. Sex-mediated effects of ACE2 and TMPRSS2 on the incidence and severity of COVID-19; the need for genetic implementation. Curr Res Transl Med. 2020 Nov;68(4):149–150. doi: 10.1016/j.retram.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo I., Russo R., Lasorsa V.A., Cantalupo S., Rosato B.E., Bonfiglio F., et al. Common variants at 21q22.3 locus influence MX1 and TMPRSS2 gene expression and susceptibility to severe COVID-19. iScience. 2021 Apr 23;24(4) doi: 10.1016/j.isci.2021.102322. 102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging. 2020 Jun 5;12(11):10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelt H.J., Forster P., Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999 Jan 1;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005 Jan 15;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya C., Das C., Ghosh A., Singh A.K., Mukherjee S., Majumder P.P., et al. 2020 May. Global spread of SARS-CoV-2 subtype with spike protein mutation D614G is shaped by human genomic variations that regulate expression of TMPRSS2 and MX1 genes [Internet] [cited 2021 Aug 28] p. 2020.05.04.075911. Available from: [DOI] [Google Scholar]
- Budak G. 2020. ped2fasta - PED to FASTA converter [Internet]https://github.com/gungorbudak/ped2fasta/blob/32c30f7755be7d07f924aa4d37e082eda0e3fa49/ped2fasta.pl [cited 2021 Nov 26]. Available from: [Google Scholar]
- Chaubey G., Ayub Q., Rai N., Prakash S., Mushrif-Tripathy V., Mezzavilla M., et al. “Like sugar in milk”: reconstructing the genetic history of the Parsi population. Genome Biol. 2017 Jun 14;18(1):110. doi: 10.1186/s13059-017-1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Zhou J., To K.K.-W., Chu H., Li C., Wang D., et al. Identification of TMPRSS2 as a susceptibility gene for severe 2009 pandemic a(H1N1) influenza and a(H7N9) influenza. J. Infect. Dis. 2015 Oct 15;212(8):1214–1221. doi: 10.1093/infdis/jiv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbani B. The expression and polymorphism of entry machinery for COVID-19 in human: juxtaposing population groups, gender, and different tissues. Int. J. Environ. Res. Public Health. 2020 Jan;17(10):3433. doi: 10.3390/ijerph17103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin R.M., Altshuler D., Durbin R.M., Abecasis G.R., Bentley D.R., Chakravarti A., et al. A map of human genome variation from population-scale sequencing. Nature. 2010 Oct;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020 Dec 1;13(12):1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L., Lischer H.E.L. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and windows. Mol. Ecol. Resour. 2010;10(3):564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Zhao J., Martin W., Kallianpur A., Chung M.K., Jehi L., et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020 Jul 15;18(1):216. doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins D.J. Structural analysis of experimental drugs binding to the SARS-CoV-2 target TMPRSS2. J Mol Graph Model. 2020 Nov;1(100) doi: 10.1016/j.jmgm.2020.107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irham L.M., Chou W.-H., Calkins M.J., Adikusuma W., Hsieh S.-L., Chang W.-C. Genetic variants that influence SARS-CoV-2 receptor TMPRSS2 expression among population cohorts from multiple continents. Biochem. Biophys. Res. Commun. 2020 Aug 20;529(2):263–269. doi: 10.1016/j.bbrc.2020.05.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer G.R., Samajder S., Zubeda S., Dsn S., Mali V., Pv S.K., et al. Infectivity and progression of COVID-19 based on selected host candidate gene variants. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Blazyte A., Yoon C., Ryu H., Jeon Y., Bhak Y., et al. Ethnicity-dependent allele frequencies are correlated with COVID-19 case fatality rate [internet] Preprints. 2020 doi: 10.14348/molcells.2021.2249. https://www.authorea.com/users/367817/articles/487091-ethnicity-dependent-allele-frequencies-are-correlated-with-covid-19-case-fatality-rate?commit=92f9ba974af4c5e0ff312d7dd9994aa1b1589975 Oct [cited 2021 Aug 28]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehdy F.S.G., Pita-Oliveira M., Scudeler M.M., Torres-Loureiro S., Zolini C., Moreira R., et al. Human-SARS-CoV-2 interactome and human genetic diversity: TMPRSS2-rs2070788, associated with severe influenza, and its population genetics caveats in Native Americans. Genet Mol Biol [Internet] 2021 Aug;25:44. doi: 10.1590/1678-4685-GMB-2020-0484. http://www.scielo.br/j/gmb/a/LZ5N4QRCYzHRHwhXGFmQCJB/abstract/?lang=en [cited 2021 Nov 26]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-C., Jeong B.-H. Strong correlation between the case fatality rate of COVID-19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon-induced transmembrane protein 3 (IFITM3) gene at the population-level. Genes. 2021 Jan;12(1):42. doi: 10.3390/genes12010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018 Jun;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy P.-J., Rébillard X., Vacherot F., de la Taille A. Androgenic hormones and the excess male mortality observed in COVID-19 patients: new convergent data. World J. Urol. 2020 Jun;2:1–3. doi: 10.1007/s00345-020-03284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini A., Agolini E., Novelli A., Borgiani P., Giannini R., Gravina P., et al. COVID-19 and genetic variants of protein involved in the SARS-CoV-2 entry into the host cells. Genes. 2020 Sep;11(9):1010. doi: 10.3390/genes11091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica V., Rizzo A., Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020 Sep 1;16(27):2029–2033. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschitz C., Trummert A., Berent T., Laimer N., Knoblich L., Bodlaj G., et al. Attenuation of COVID-19-induced cytokine storm in a young male patient with severe respiratory and neurological symptoms. Wien Klin Wochenschr [Internet] 2021 Apr 27 doi: 10.1007/s00508-021-01867-2. [cited 2021 Aug 28]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L., Lawson D.J., Jagoda E., Mörseburg A., Eriksson A., Mitt M., et al. Genomic analyses inform on migration events during the peopling of Eurasia. Nature. 2016 Oct;538(7624):238–242. doi: 10.1038/nature19792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniri A., Hosseini M.M., Akhavan-Niaki H. First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations. J. Biomol. Struct. Dyn. 2021 Jul 3;39(10):3576–3593. doi: 10.1080/07391102.2020.1767690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoloni-Giacobino A., Chen H., Peitsch M.C., Rossier C., Antonarakis S.E. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics. 1997 Sep 15;44(3):309–320. doi: 10.1006/geno.1997.4845. [DOI] [PubMed] [Google Scholar]
- Pathak A.K., Kadian A., Kushniarevich A., Montinaro F., Mondal M., Ongaro L., et al. The genetic ancestry of modern Indus Valley populations from Northwest India. Am. J. Hum. Genet. 2018 Dec 6;103(6):918–929. doi: 10.1016/j.ajhg.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020 Dec 9;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva F., Sabanovic B., Cecati M., Giulietti M. Expression and co-expression analyses of TMPRSS2, a key element in COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021 Feb 1;40(2):451–455. doi: 10.1007/s10096-020-04089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007 Sep 1;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R: The R Project for Statistical Computing [Internet]. [cited 2021 Aug 28. https://www.r-project.org/ Available from:
- Ragia G., Manolopoulos V.G. Assessing COVID-19 susceptibility through analysis of the genetic and epigenetic diversity of ACE2-mediated SARS-CoV-2 entry. Pharmacogenomics. 2020 Dec 1;21(18):1311–1329. doi: 10.2217/pgs-2020-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re3data Org. Estonian Biocentre Public Data. 2014. http://service.re3data.org/repository/r3d100010986 [cited 2021 Aug 28]; Available from:
- Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S.E., et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017 Dec 1;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- Saih A., Bouqdayr M., Baba H., Hamdi S., Moussamih S., Bennani H., et al. Computational analysis of missense variants in the human transmembrane protease serine 2 (TMPRSS2) and SARS-CoV-2. Biomed. Res. Int. 2021 Oct;19(2021) doi: 10.1155/2021/9982729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020 Aug 1;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder K., Breuckmann K., Elsner C., Dittmer U., Fistera D., Herbstreit F., et al. Transmembrane serine protease 2 polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus type 2 infection: a German case-control study. Front. Genet. 2021 Apr;21(12) doi: 10.3389/fgene.2021.667231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S., Kumar S., Singh A.K., Banerjee P., Bhagavatula S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS-CoV-2 infection in human. J. Genet. 2020;99:53. doi: 10.1007/s12041-020-01217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeyedAlinaghi S., Mehrtak M., MohsseniPour M., Mirzapour P., Barzegary A., Habibi P., et al. Genetic susceptibility of COVID-19: a systematic review of current evidence. Eur. J. Med. Res. 2021 May 20;26(1):46. doi: 10.1186/s40001-021-00516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Singh I, Haider S, Malik MZ, Ponnusamy K, Rai E. ACE2 Homo-dimerization, Human Genomic variants and Interaction of Host Proteins Explain High Population Specific Differences in Outcomes of COVID19 [Internet]. 2020 Apr [cited 2021 Aug 28] p. 2020.04.24.050534. Available from: 10.1101/2020.04.24.050534v1. [DOI]
- Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017 Nov 1;(142):1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Choudhari R., Nema V., Khan A.A. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021 Jan;1(150) doi: 10.1016/j.micpath.2020.104621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souilmi Y., Lauterbur M.E., Tobler R., Huber C.D., Johar A.S., Moradi S.V., et al. An ancient viral epidemic involving host coronavirus interacting genes more than 20,000 years ago in East Asia. Curr. Biol. 2021 Aug 23;31(16):3504–3514.e9. doi: 10.1016/j.cub.2021.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Bandopadhyay A., Das D., Pandey R.K., Singh V., Khanam N., et al. Genetic association of ACE2 rs2285666 polymorphism with COVID-19 spatial distribution in India. Front. Genet. 2020;11:1163. doi: 10.3389/fgene.2020.564741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Pandey R.K., Singh P.P., Kumar P., Rasalkar A.A., Tamang R., et al. Most frequent south Asian haplotypes of ACE2 share identity by descent with east Eurasian populations. PLoS One. 2020 Sep 16;15(9) doi: 10.1371/journal.pone.0238255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope J.D., PharmD C.H.C., Figg W.D. TMPRSS2: potential biomarker for COVID-19 outcomes. J. Clin. Pharmacol. 2020 May 21 doi: 10.1002/jcph.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tätte K., Pagani L., Pathak A.K., Kõks S., Ho Duy B., Ho X.D., et al. The genetic legacy of continental scale admixture in Indian Austroasiatic speakers. Sci. Rep. 2019 Mar;7(9):3818. doi: 10.1038/s41598-019-40399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THE GTEX CONSORTIUM, Ardlie K.G., Deluca D.S., Segrè A.V., Sullivan T.J., Young T.R., et al. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015 May 8;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Fuentes L., Matías-Guiu J., Hernández-Lorenzo L., Montero-Escribano P., Pytel V., Porta-Etessam J., et al. ACE2, TMPRSS2, and Furin variants and SARS-CoV-2 infection in Madrid, Spain. J. Med. Virol. 2021 Feb;93(2):863–869. doi: 10.1002/jmv.26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarala M.H., Porvari K.S., Kellokumpu S., Kyllönen A.P., Vihko P.T. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J. Pathol. 2001;193(1):134–140. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH743>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Vargas-Alarcón G., Posadas-Sánchez R., Ramírez-Bello J. Variability in genes related to SARS-CoV-2 entry into host cells (ACE2, TMPRSS2, TMPRSS11A, ELANE, and CTSL) and its potential use in association studies. Life Sci. 2020 Nov;1(260) doi: 10.1016/j.lfs.2020.118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Huang S., Gao R., Zhou Y., Lai C., Li Z., et al. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. 2020 Nov 10;6(1):1–16. doi: 10.1038/s41421-020-00231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020 Jun 23;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulandari L., Hamidah B., Pakpahan C., Damayanti N.S., Kurniati N.D., Adiatmaja C.O., et al. Initial study on TMPRSS2 p.Val160Met genetic variant in COVID-19 patients. Hum Genomics. 2021;15:29. doi: 10.1186/s40246-021-00330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Gao Y., Ning Z., Lu Y., Zhang X., Liu J., et al. PGG.SNV: understanding the evolutionary and medical implications of human single nucleotide variations in diverse populations. Genome Biol. 2019;20:215. doi: 10.1186/s13059-019-1838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Fig. 1 The median-joining network of TMPRSS2 gene. The circle size determines the number of samples with a certain haplotype. The five most common haplotypes are marked.
Supplementary Fig. 2 LD (linkage disequilibrium) maps of the TMPRSS2 gene, focusing on rs2070788 and its haplotype, in world populations. Shading from white to red indicates the intensity of r2 from 0 to 1. Strong LD is represented by a high percentage (>80) in darker red squares.
Supplementary Fig. 3 The spatial distribution of SNP rs2070788 from 1000 genome data.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.