Abstract
Background
High-grade meningioma (HGM) is difficult to treat, and recurrent HGM after radiotherapy has an especially poor prognosis. We retrospectively analyzed the cases of 44 consecutive patients with recurrent and refractory HGM who were treated by reactor-based boron neutron capture therapy (BNCT).
Methods
In 2005–2019, we treated 44 recurrent and refractory HGMs by reactor-based BNCT. We analyzed the patients’ tumor shrinkage, overall survival (OS) after initial diagnosis, OS after BNCT, progression-free survival (PFS) post-BNCT, and treatment failure patterns.
Results
The median OS (mOS) after BNCT and mOS after initial diagnosis were 29.6 (95% CI: 16.1–40.4) and 98.4 (95% CI: 68.7–169.4) months, respectively. The median follow-up after BNCT was 26 (6.4–103) months. The grade 2 (20 cases) and 3 (24 cases) post-BNCT mOS values were 44.4 (95% CI: 27.4–not determined) and 21.55 (10.6–30.6) months, respectively (P = .0009). Follow-up images were obtained from 36 cases at >3 months post-BNCT; 35 showed tumor shrinkage during the observation period. The post-BNCT median PFS (mPFS) of 36 cases was 13.7 (95% CI: 8.3–28.6) months. The post-BNCT mPFS values in patients with grade 2 and 3 disease were 24.3 (95% CI: 9.8–not determined) and 9.4 (6.3–14.4) months, respectively (P = .0024). Local recurrence was observed in only 22.2% of cases. These results showed good local tumor control and prolonged survival for recurrent HGM cases.
Conclusions
Most of these cases had relatively large tumor volumes. The proportion of grade 3 patients was extremely high. Our patients thus seemed to have poor prognoses. Nevertheless, reactor-based BNCT exerted relatively good local control and favorable survival for recurrent and refractory HGMs.
Keywords: boron neutron capture therapy, high-grade meningioma, nuclear reactor, radiotherapy
Key Point.
1. Reactor-based boron neutron capture therapy (BNCT) showed relatively good local control, tumor shrinkage, and favorable survival along with acceptable safety for recurrent and refractory high-grade meningioma (HGM) patients in poor condition.
Importance of the Study.
High-grade meningiomas (HGMs), especially recurrent and refractory HGMs, are difficult to control and there is no standard treatment for them. Boron neutron capture therapy (BNCT) is an ideal particle radiation modality that can theoretically exert cytocidal effects selectively on tumor cells and is biological cell-targeting radiotherapy. We previously reported the effectiveness of BNCT for HGMs by analyzing survival and tumor shrinkage, albeit over a relatively short observation period. In this study we retrospectively analyze 44 consecutive patients with recurrent and refractory HGM who were treated with reactor-based BNCT and followed up for a longer period of time. The cases had already been treated with intensive treatments such as repetitive surgeries and stereotactic radiosurgery (SRS). Nonetheless, we demonstrate that BNCT achieved better survival, tumor shrinkage, and local tumor control than other radiation modalities. Based on these observations, we have begun an investigator-lead, randomized, controlled trial of accelerator-based BNCT for recurrent and refractory HGM.
Boron neutron capture therapy (BNCT) is a targeted radiation approach that enables the selective killing of malignant cells and the sparing surrounding normal cells. BNCT is a binary approach: a boron-10 (10B)-labeled compound must deliver higher concentrations of 10B to the target tumor cells relative to the surrounding normal tissues. This is followed by low-energy thermal neutron irradiation. When a neutron collides with 10B, α and recoiling 7Li particles are released within a diameter equivalent to that of a tumor cell by the 10B(n, alpha)7Li neutron capture reaction.1 These particles are high linear energy transfer (LET) radiation and can destroy a sufficient amount of 10B-containing cells without exerting hazardous effects on the adjacent normal cells. Accordingly, if sufficient quantities of boron compounds can be made to accumulate selectively in tumor cells with enough contrast to surrounding normal cells, BNCT becomes an ideal particle radiotherapy.
BNCT is suitable for tumors with a highly infiltrative nature, such as malignant gliomas. As described below, we also aggressively apply BNCT for refractory high-grade meningiomas (HGMs, World Health Organization [WHO] grade 2 or 3), since HGMs also tend to infiltrate the dura matter and brain parenchyma.
It is difficult to treat HGMs, and HGMs that recur after radiotherapy have a particularly poor prognosis. The reported median overall survival (mOS) and the median progression-free survival (mPFS) values of patients with recurrent HGM after radiotherapy are 24.6 and 5.2 months, respectively.2 Although treatments for recurrent HGM have been reported (including chemotherapeutic regimens), no standard treatment has been established.3 We have applied reactor-based BNCT for refractory HGM cases that recurred after intensive treatments, and we reported its effects with special reference to tumor shrinkage.4,5 We also reported the survival benefit afforded by BNCT in 20 recurrent and refractory HGM cases with relatively short observation periods; the median length of follow-up in the previous report was 13.1 months (95% CI: 9.4–40.4) for all cases.6 In the present study, we attempted to further clarify the respective OS and PFS values of grade 2 and grade 3 cases, the tumor shrinkage, the local control, and the patterns of treatment failure after BNCT was applied to refractory and recurrent HGMs, with a larger number of patients and longer follow-up periods.
Patients and Methods
Patients
Forty-four patients with recurrent and refractory HGM were treated with nuclear reactor-based BNCT from June 2005 to February 2019 at our institution. Twenty cases were WHO grade 2 (45.5%) and the other 24 cases were WHO grade 3 (54.5%). Prior to BNCT, each of the 44 patients underwent a craniotomy. Among all of the patients, 114 surgeries were performed (mean number of surgeries per case: 2.91, range 1–6). Prior to BNCT, some radiotherapies (RT) were applied in 40 cases (90.9%): stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) was applied a total of 77 times (mean SRS or SRT: 1.75 times per case, range 1–8). External beam fractionated radiotherapy (EBRT) was applied in 10 cases. In only 4 cases (9.1%), no RT was applied. The patients’ characteristics (eg, age, gender, tumor location, and Karnofsky Performance Status) are summarized in Table 1. In Japan, SRS and SRT tend to be favored over EBRT as the radiation therapy for refractory high-grade meningiomas; this trend toward selecting SRS and SRT for high-grade meningiomas appears to be more pronounced in Japan than other countries. Many of the patients in the present series were residents of other regions in Japan and were referred to our institution for a final selection of treatment(s) for recurrent and refractory HGM.
Table 1.
Characteristics of the 44 Patients With Recurrent and Refractory HGM
| Age, years | Mean, SD | 59.5 | 12.3 |
| Median, range | 62.5 | 29–79 | |
| n | % | ||
| Sex | Female | 29 | 65.9% |
| Male | 15 | 34.1% | |
| Histology | WHO 2 | 20 | 45.5% |
| WHO 3 | 24 | 54.5% | |
| Location | Convexity | 14 | 31.8% |
| Falx/parasagittal | 17 | 38.6% | |
| Skull base | 13 | 29.5% | |
| KPS | ≥80 | 32 | 72.7% |
| 60≤, <80 | 12 | 27.3% | |
| Previous operation | n | % | |
| Total | 114 | 100% | |
| No. of times | |||
| Mean, SD | 2.9 | 1.4 | |
| Previous radiotherapy | n | % | |
| Total | 40 | 90.9% | |
| No. of times | |||
| EBRT | Mean, SD | 0.23 | 0.42 |
| SRS + SRT | 1.75 | 1.64 | |
| Tumor volume at BNCT, ml | Mean, SD | 42.7 | 52.9 |
| Median, range | 24.5 | 1.5–299.2 |
Abbreviations: BNCT = boron neutron capture therapy; EBRT = external beam fractionated radiotherapy; HGM = High-grade meningioma; KPS = Karnofsky Performance Status; SRS = stereotactic radiosurgery; SRT = stereotactic radiotherapy, WHO = World Health Organization.
Clinical Regimen of BNCT for HGM
This protocol was approved by the Ethical Committee of Osaka Medical College, and each candidate was also discussed and approved by the board of reviewers at the Institute for Integrated Radiation and Nuclear Science, Kyoto University. The clinical regimen of BNCT for HGMs was modified slightly from that for malignant gliomas.7 Briefly, patients were typically administered 500 mg/kg of boronophenylalanine (BPA; purchased mainly from Interpharma Praha, Prague, Czech Republic). In a few cases, the boron carrier sodium borocaptate (BSH) was simultaneously used. Further details of the clinical regimen are reported elsewhere.6
BPA was administered in the 2 hours (200 mg/kg/h) just prior to the neutron irradiation and then during the neutron irradiation (100 mg/kg/h). The boron concentration in the patient’s blood was monitored by sampling every 1 hour after the boron compound administration until the neutron irradiation was completed. The boron concentrations from BPA in the tumor and normal brain were estimated from the tumor/normal brain (T/N) ratio of 18F-BPA on positron emission tomography (PET),8 if this PET was available. When the PET was not available, the putative T/N ratio of 3.5 was adopted. In the current series, 32 patients underwent F-BPA-PET for the estimation of T/N ratio. The compound biological effectiveness and the relative biological effectiveness of neutron beams and compounds have been described previously.9
The neutron fluence rate was simulated by the dose-planning system SERA (Idaho National Engineering and Environmental Laboratory, Idaho Falls, ID), and the total doses to the tumor and normal brain were estimated. The neutron irradiation time was set to not exceed 15 Gy-Eq to the normal brain. Here, Gy-Eq (Gy: Gray) means an X-ray dose that can exert effects that are biologically equivalent to those of the total BNCT radiation. After the treatment, the precise doses administered were reestimated.
Assessment of Effectiveness
The radiologic best response was evaluated based on serial brain magnetic resonance imaging (MRI) scans, according to the two-dimensional Macdonald criteria.10 We used the Macdonald criteria to evaluate the radiologic response as it has typically been assessed in prospective clinical trials for recurrent meningiomas.11,12 Briefly, a complete response (CR) was defined as the complete disappearance of enhancing tumor; a partial response (PR) was defined as a ≥50% reduction in the product of perpendicular diameters (including the longest diameter), and progressive disease (PD) was defined as a ≥25% increase in the product of perpendicular diameters or clinical deterioration consistent with progression. Patients whose responses did not fulfill any of the CR, PR, or PD criteria were defined as having stable disease (SD). Progression-free survival (PFS) was defined as freedom from local tumor progression, distant tumor progression, and death.
Since many of the patients had been referred to Osaka Medical College from far away in Japan, follow-up images were periodically sent to us from the referring physicians (usually every 2–3 mo) for as long as possible. Follow-up images >3 months after BNCT were obtained for 36 of the 44 patients (grade 2: n = 17; grade 3: n = 19), and these images were processed for the tumor shrinkage (radiological best response) and PFS analyses.
Survival Analysis
The patients’ overall survival (OS) was defined in two ways: according to the number of months they survived after their initial diagnosis, and according to the number of months they survived after the application of BNCT. In all cases, the survival information was obtained from the patient or their family by phone inquiry or from documents provided by the referring physicians. The cutoff date of this survival analysis was the end of June 2020. The patients’ PFS was analyzed as described above, and the OS and PFS data were processed for statistical analysis using the log-rank test. Statistical significance was defined as a P-value < .05.
Results
Prescribed Dose of BNCT for Tumor Tissue
The irradiation time was planned not to exceed 15 Gy-Eq in the normal brain tissue, as noted above. The absorbed dose to the tumor tissue was dependent on both the boron concentration in the tumor tissue and the neutron irradiation time, which varied in each case. Therefore, the absorbed dose to the tumor tissue in our protocol was not uniform from case to case. The maximum and minimum absorbed doses in the current series were 68.7 ± 18.2 (mean ± SD) Gy-Eq and 36.7 ± 16.4 Gy-Eq, respectively (Table 2). These values are almost the same as those obtained in our earlier study.6
Table 2.
The BNCT Tumor Doses, OS, PFS, Treatment Failure Rate, and CTCAE Data of HGM Patients
| Tumor dose of BNCT, Gy-Eq: | |||
| Max | Mean, SD | 68.7 | 18.2 |
| Median, range | 72 | 22.7–111.5 | |
| Min | Mean, SD | 36.7 | 16.4 |
| Median, range | 33.7 | 9.5–71.4 | |
| OS from BNCT (44 cases), months: | |||
| WHO grade 2 + 3 | Median, range | 29.6 | 6.4–103 |
| WHO grade 2 | 44.4 | 6.4–103 | |
| WHO grade 3 | 21.55 | 7.5–60.4 | |
| OS from diagnosis (44 cases), months: | |||
| WHO grade 2 + 3 | Median, range | 98.4 | 25.6–344.8 |
| WHO grade 2 | 224.4 | 29.3–325.3 | |
| WHO grade 3 | 69.6 | 25.6–344.8 | |
| PFS (36 cases), months: | |||
| WHO grade 2 + 3 | Median, range | 13.7 | 1.4–90.5 |
| WHO grade 2 | 24.3 | 5.1–90.5 | |
| WHO grade 3 | 9.4 | 1.4–39.4 | |
| Treatment failure (36 cases), n, % | 36 | ||
| Local | 8 | 22.2% | |
| Out of field | 11 | 30.6% | |
| Systemic metastasis | 4 | 11.1% | |
| Dissemination | 3 | 8.3% | |
| Others | 2 | 5.6% | |
| CNS necrosis (44 cases), n, %: | |||
| CTCAE grade | |||
| Grade 2 | 15 | 34.1% | |
| Grade 3 | 6 | 13.6% |
Abbreviations: BNCT = boron neutron capture therapy; CNS = central nervous system; CTCAE = Common Terminology Criteria for Adverse Events; HGM = High-grade meningioma; OS = overall survival; PFS = progression-free survival; WHO = World Health Organization.
Tumor Shrinkage
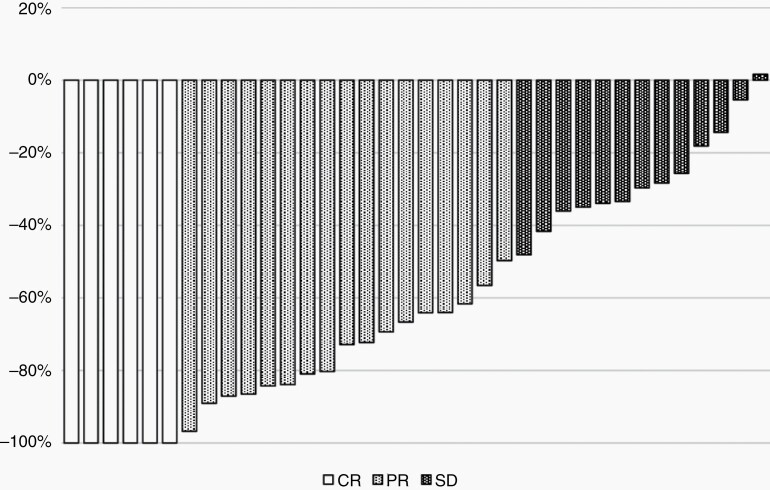
Tumor shrinkage analyses were applied for the 36 patients for whom follow-up MRI findings were available ≥3 months after BNCT. The tumor shrinkage values of the best response (waterfall plot) are shown in Figure 1. Thirty-five of the 36 cases showed tumor shrinkage. Based on the patients’ baseline images, CR (n = 6), PR (n = 17), and SD (n = 13) were observed.
Fig. 1.
Tumor shrinkage analysis using a waterfall plot. The best response was assessed from the baseline image and using the Macdonald criteria. Of the 36 cases, 25 showed tumor shrinkage. Six cases (16.7%) achieved a CR, 17 (47.2%) showed a PR, and 13 (36.1%) had SD. Abbreviations: CR = complete response; PR = partial response; SD = stable disease.
OSs After BNCT and After the Initial Diagnosis
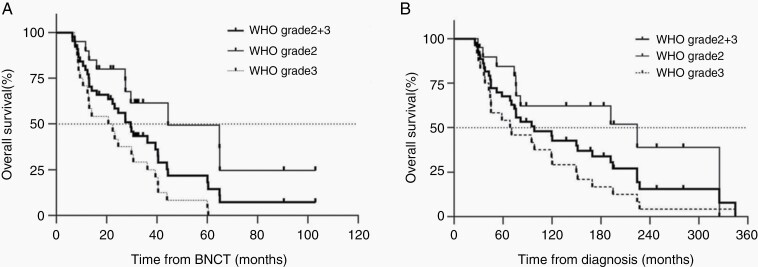
The median follow-up period (defined for all cases from BNCT to the date of death or censorship) for OS was 26.0 months (range 6.4–103 mo). The Kaplan–Meier survival curves after the BNCT and after the initial diagnosis are shown in Figure 2A and B. The mOS from the BNCT for the sum of the grade 2 and 3 cases was 29.6 months (95% CI: 16.1–40.4). The mOS from the BNCT of the grade 2 patients was 44.4 months (95% CI: 27.4–not determined), which was significantly longer than that of the grade 3 patients at 21.6 months (95% CI: 10.6–30.6) (P = .0009).
Fig. 2.
Survival analysis after BNCT and after HGM diagnosis. (A, B) Kaplan–Meier curves after BNCT and after diagnosis. (A) The mOS after BNCT for the sum of the grade 2 and 3 cases with 95% CI was 29.6 (95% CI: 16.1–40.4) months. The mOS after BNCT for the grade 2 cases was 44.4 (95% CI: 27.4–not determined) months, and that for the grade 3 cases was 21.6 (10.6–30.6) months (P = .0009, log-rank test). (B) The mOS value for the sum of the grade 2 and 3 groups after the diagnosis was 98.4 (95% CI: 68.7–169.4) months. The mOS after diagnosis for the grade 2 group was 224.4 (95% CI: 75.9–325.3) months, and that for the grade 3 group was 69.6 (42.8–120.3) months (P = .0111, log-rank test). Abbreviations: BNCT = boron neutron capture therapy; HGM = high-grade meningioma; mOS = median overall survival.
The mOS after diagnosis for the sum of the grade 2 and 3 patients was 98.4 months (95% CI: 68.7–169.4). The mOS after diagnosis for the grade 2 patients was 224.4 months (95% CI: 75.9–325.3), which was significantly longer than that of the grade 3 patients at 69.6 months (42.8–120.3) (P = 0.0111).
PFS After BNCT
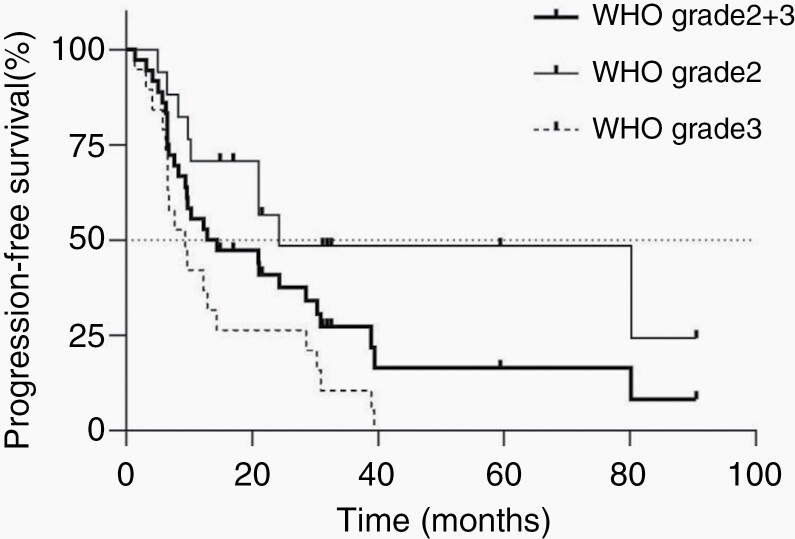
Follow-up images ≥3 months after BNCT were obtained for 36 of the 44 patients. We analyzed the PFS of these 36 patients using Kaplan–Meier curves (Figure 3). The mPFS after BNCT was 13.7 (95% CI: 8.3–28.6) months. The respective mPFS after BNCT values of the grade 2 and 3 patients were 24.3 months (95% CI: 9.8–not determined) and 9.4 months (6.3–14.4), respectively, with significantly longer mPFS for the grade 2 patients (P = .0024).
Fig. 3.
Progression-free survival (PFS) analysis after boron neutron capture therapy (BNCT). A Kaplan–Meier curve was applied for the estimation of PFS.
Treatment Failure Patterns
The patients’ treatment failure patterns are summarized in Table 2. Treatment failure is defined in this study as the cause of the classification of the patient’s status as PD. The percentages of patients exhibiting each treatment failure pattern were as follows: local recurrence (22.2%), out-of-field recurrence (30.6%), systemic metastasis (11.1%), and cerebrospinal fluid (CSF) dissemination (8.3%). In comparison with our previous report,6 the rate of local recurrence increased somewhat, which seemed to be due to the longer-term follow-up in our present series. These results showed good local tumor control.
Toxicity
All 44 cases were assessed for brain radiation necrosis after BNCT, judging from follow-up images and clinical follow-up not only from our database but also from the referring physicians. Grade 2 toxicity was observed in 15 patients (34.1%), and these patients were treated with steroids. Grade 3 toxicity was observed in 6 patients (13.6%), who were treated temporarily with bevacizumab (Table 2).
Next, we analyzed the relationship between the total radiation dose and the incidence of radiation necrosis. The equivalent total dose in 2-Gy fractions (EQD2) of the preceding radiotherapy for the normal brain adjacent to the lesions was available in 35 cases out of 44. The total EQD2 of the preceding radiotherapy and maximum brain dose by BNCT and Common Terminology Criteria for Adverse Events (CTCAE) grade of radiation necrosis of the brain are summarized in Supplementary Table 1. Among the 35 cases for which the EQD2 was available, 4, 13, 13, and 5 patients had CTCAE grades of 0, 1, 2, and 3, respectively. The total EQD2 of the preceding radiotherapy and BNCT for the normal brain adjacent to the lesions was compared between the patients with radiation necrosis grades 0 + 1 (treatment unnecessary) and radiation necrosis grades 2 + 3 (treatment necessary). The mean EQD2 ± SD values for the two groups were 77.2 ± 32.6 and 109.6 ± 30.5, respectively, and these values were significantly different by Wilcoxon rank sum test (P = .0153).
Representative Case Presentation
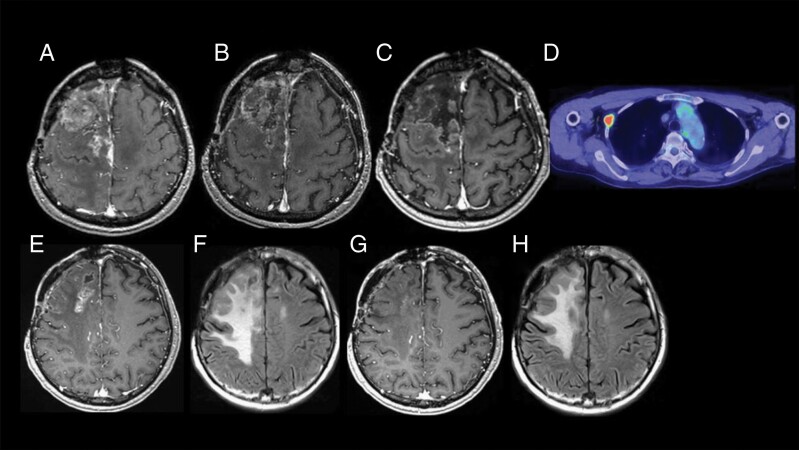
A 68-year-old male had undergone three surgeries for right frontal parasagittal meningioma since 2006. The histological diagnosis after the first surgery was anaplastic meningioma (WHO grade 3). He received EBRT in 2010, SRS on two occasions in 2013 and 2014, and intensity-modulated radiation therapy (IMRT) in 2015. Irrespective of these aggressive treatments, the recurred mass progressed and the patient was referred to us as a candidate for BNCT. He suffered from left hemiparesis and used a wheelchair.
F-BPA-PET was not available for the patient, so the putative T/N ratio of 3.5 was adopted in BNCT administered in October 2017. Figure 4 shows the good local control of the mass. A follow-up MRI examination at 14 months post-BNCT revealed the patient’s CR (Figure 4C). His hemiparesis recovered temporarily but became aggravated 14 months after BNCT. The MRI showed brain radiation necrosis (Figure 4E and F). He was treated with two cycles of bevacizumab, and his symptoms and MRI then showed a response (Figure 4G and H). Unfortunately, 21 months after the patient’s BNCT, fluorodeoxyglucose-positron emission tomography (FDG-PET) showed a pleural lesion (Figure 4D), which was confirmed as anaplastic meningioma by surgery. The PET scan also revealed other metastatic lesions. He is doing well at the time of this manuscript preparation with no recurrence of tumor in the head and no progressive aggravation of systemic metastasis.
Fig. 4.
Representative clinical course. The best response was judged as a CR. The treatment failure pattern was systemic metastasis. (A–C) The Gd-MRI taken just prior to BNCT, 4.5 months after BNCT, and 14 months after BNCT, respectively. (E, F) Gd and FLAIR-MRI taken 14 months after BNCT. (G, H) Gd-MRI and FLAIR-MRI taken 16 months after MRI, 2 cycles after bevacizumab treatment. (D) FDG-PET scan taken 21 months after BNCT, demonstrating pleural metastasis of meningioma. Abbreviations: BNCT = boron neutron capture therapy; CR = complete response; Gd-MRI = gadolinium-enhanced magnetic resonance imaging; FLAIR-MRI = fluid-attenuated inversion recovery-magnetic resonance imaging; FDG-PET = fluid-attenuated inversion recovery-magnetic resonance imaging.
Discussion
Our previous investigations revealed the effects of reactor-based BNCT for recurrent and refractory HGM, chiefly with respect to tumor shrinkage, local tumor control, and survival benefit.4–6 In the latest of these reports, the post-BNCT mOS for 20 patients with HGM was 14.1 months, with a median follow-up of 13.1 months.6 The main purpose of the present study was to determine the mOS after BNCT with a much larger number of cases and longer-term follow-up. As the present study was also a retrospective clinical study, we obtained all of the patients’ survival data and collected the patient follow-up MR images for as long a term as possible for the PFS analysis. The median follow-up of the 44 patients used for the OS analysis was 26.0 months, and as a result, the post-BNCT mOS improved to 29.6 months based on all 44 grade 2 and grade 3 cases. Our analyses also gave an mPFS of 13.7 months based on the 36 available cases. The MRIs of these 36 cases were collected until the date of PD, death, or censorship.
No standard medical treatments—and indeed, no effective treatments—have been established for recurrent and refractory HGMs,13 and the natural course of these cases remains uncertain. Three recent phase II clinical trials using new medical agents (including octreotide analogues and tyrosine kinase inhibitors) for HGM yielded approximately equivalent posttreatment mPFS and mOS values of 6 months and 2 years, respectively.2,14,15 These results thus seem to be close to the natural course of recurrent and refractory HGM. Compared to these prior studies’ mOS and mPFS values, the values in our present study were far better. Kaley et al. proposed that new agents which achieve a PFS of ≥6 months in ≥35% of patients should be considered to have clinical potential.13 Our present data also fulfill this requirement.
Moreover, our cases were treatment-refractory, and the patients were extensively treated with repetitive surgeries and repetitive RT. The proportion of grade 3 patients in our series was thus much higher (54.5%) than the proportions in the three reports on recurrent and refractory HGM mentioned above.2,14,15 Our patients tended to have poor prognoses, but despite their poor conditions, reactor-based BNCT exerted good local control and achieved relatively good survival rates for these patients with recurrent and refractory HGM.
Regarding RT, EBRT was first used to treat HGM, but with unsatisfactory results.16 Some investigators reported the same tendency of limited clinical benefits of EBRT for HGM.17–19 SRS is the modality most often chosen for HGM.20–22 The mPFS after re-irradiation using SRS for recurrent and refractory HGM was reported as 8–12 months,23,24 but SRS is generally used for small-sized HGM such as those with a mean tumor volume of 4.8–7.4 ml.25,26 In the present HGM series, the mean tumor volume was 42.2 ml.
Lin et al. reported very good PFS for HGM patients at 6 months after treatment (84%) with the use of mainly SRS as re-irradiation.27 It is rather difficult to compare our present findings regarding the efficacy of BNCT to the report of Lin et al. because their grade 2 and 3 case numbers were 24 and 5, respectively, and the median tumor volume of their cases was only 3.8 ml. With regard to radiographic response, they reported that 2 (8%) and 3 (12%) patients achieved a CR and PR, respectively, among their 29 grade 2 and 3 cases; in our present series BNCT achieved a CR in 6 (16.7%), PR in 17 (47.2%), and SD in 13 (36.1%) of the total 36 cases with available follow-up images. This potent tumor shrinkage may be ascribed to the higher level of LET particles in BNCT compared to photon and proton irradiation.28,29
Our BNCT data should be compared with the data for proton and carbon therapy in patients with refractory and recurrent HGM, because not only proton and carbon therapies but also BNCT is a particle radiation modality. Unfortunately, only a few papers about proton or carbon particle therapy for recurrent HGM have been published. Among them, El Shafie et al. recently reported a very good mPFS of 25.7 months in their analysis of grade 2 cases (n = 25) and grade 3 cases (n = 6) of HGM recurrence after RT; however, the OS after treatment was not reported. In their report, the majority (81%; 34/42) of patients were treated by carbon ion therapy, which is characterized by a high LET and high relative biological effectiveness.30 This may have been the reason for their quite good results. Regarding treatment failure, all recurrent cases were locoregional in the report by El Shafie et al. Lin et al. reported a similar trend in treatment failure using SRS as the re-irradiation modality.27 Generally speaking, systemic metastasis and CSF dissemination rarely occur even in HGM.31,32
On the other hand, the treatment failure patterns in the present series were local recurrence (22.2%), out-of-field recurrence (30.6%), systemic metastasis (11.1%), and CSF dissemination (8.3%). Our results showed good local tumor control, which is the major difference between BNCT and other radiation modalities. IMRT, SRS, SRT, and proton and carbon radiation exert an excellent and strict spatial focus of the radiation to the lesion, whereas BNCT is not required to strictly focus neutrons, and thus infiltrative tumor cells as well as the main tumor mass may be damaged simultaneously.
Regarding adverse events (AEs), we lost 1 of our 44 patients with HGM due to disseminated intravascular coagulation syndrome, as reported previously.4 This may have been because this patient was given a long-term overdose of steroids for the treatment of radiation injury-induced edema. In the present series, 16 patients (36.4%) were treated with steroids and 6 patients (13.6%) were treated with bevacizumab for brain edema that was probably caused by radiation injury. Brain edema requiring steroids would be classified as CTCAE grade 2, and that requiring treatment with bevacizumab would be classified as CTCAE grade 3 (Table 2). However, bevacizumab has not yet been approved for the treatment of radiation necrosis in Japan under the national public health insurance. In our series, therefore, only patients with economic means were able to use bevacizumab. We thus analyzed the EQD2 dose and CTCAE grade of radiation necrosis by combining grades 0 + 1 and grades 2 + 3, as described above. The rates of these grade 2 and 3 AEs seem relatively high. This may be ascribed to the patients’ extensive preceding treatment with RT and large target volume. There were 4 cases in the present study who received no RT prior to BNCT. In 1 of these 4 cases, no brain radiation necrosis appeared and a CR status was maintained throughout the 7-year-observation period. The other patients who had no preceding RT showed no brain radiation necrosis. As described above, there is a significant difference in the total preceding radiotherapy and BNCT maximum dose for the normal brain just adjacent to the tumor between those patients who require treatment for radiation necrosis and those patients who do not.
Bevacizumab can control brain edema due to radiation injury after BNCT very well, as we reported elsewhere.33,34 We are currently negotiating to have this agent approved for radiation injury under Japan’s public health insurance system.
Several limitations of our study bear mention. This study was retrospective in nature, with a heterogeneous patient population and sometimes limited records (ie, with some important data missing). A further prospective randomized controlled trial (RCT) is necessary, as described below.
In Japan today, all BNCT activities are shifting from reactors to accelerators in hospital settings.35 In June 2020, approval was granted for the accelerator-based BNCT treatment of refractory or advancing head and neck cancers under public health insurance coverage.36,37 We are currently negotiating for public health insurance coverage for the on-label use of accelerator-based BNCT for recurrent malignant gliomas. Based on the experiences with reactor-based BNCT for HGMs described herein, we are now performing an investigator-lead, clinical RCT using accelerator-based BNCT for radiation-refractory and recurrent HGM for the on-label use of accelerator-based BNCT.
Conclusion
Reactor-based BNCT achieved good local control, tumor shrinkage, and favorable survival along with acceptable safety for recurrent and refractory HGM patients in poor condition.
Supplementary Material
Funding
This work was partly supported by Grants-in-Aid for Scientific Research (B) (16390422 and 19390385) from the Japanese Ministry of Education, Science and Culture to S.-I.M. and by the Takeda Science Foundation for Osaka Medical College.
Conflict of interest statement. None of the authors have any conflict of interest to disclose.
Authorship statement. Design: S.-I.M., S.K. Implementation: S.T., S.-I.M., S.K., K.O., M.S., Y.S. Analysis of the data: S.T., M.W., and S.-I.M. Interpretation of the data: S.T., M.W., and S.-I.M.
References
- 1. Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151(1):1–18. [PubMed] [Google Scholar]
- 2. Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyatake S, Tamura Y, Kawabata S, Iida K, Kuroiwa T, Ono K. Boron neutron capture therapy for malignant tumors related to meningiomas. Neurosurgery. 2007;61(1):82–90; discussion 90–91. [DOI] [PubMed] [Google Scholar]
- 5. Tamura Y, Miyatake S, Nonoguchi N, et al. Boron neutron capture therapy for recurrent malignant meningioma. Case report. J Neurosurg. 2006;105(6):898–903. [DOI] [PubMed] [Google Scholar]
- 6. Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake SI. Boron neutron capture therapy for recurrent high-grade meningiomas. J Neurosurg. 2013;119(4):837–844. [DOI] [PubMed] [Google Scholar]
- 7. Miyatake S, Kawabata S, Kajimoto Y, et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg. 2005;103(6):1000–1009. [DOI] [PubMed] [Google Scholar]
- 8. Imahori Y, Ueda S, Ohmori Y, et al. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med. 1998;39(2):325–333. [PubMed] [Google Scholar]
- 9. Morris GM, Coderre JA, Hopewell JW, Micca PL, Fisher C. Boron neutron capture irradiation of the rat spinal cord: effects of variable doses of borocaptate sodium. Radiother Oncol. 1996;39(3):253–259. [DOI] [PubMed] [Google Scholar]
- 10. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 11. Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shih KC, Chowdhary S, Rosenblatt P, et al. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol. 2016;129(2):281–288. [DOI] [PubMed] [Google Scholar]
- 13. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norden AD, Ligon KL, Hammond SN, et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. 2015;84(3):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raizer JJ, Grimm SA, Rademaker A, et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(1):93–101. [DOI] [PubMed] [Google Scholar]
- 16. Mahmood A, Caccamo DV, Tomecek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33(6):955–963. [DOI] [PubMed] [Google Scholar]
- 17. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57–61. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro-oncology. 2006;8(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchetti M, Bianchi S, Milanesi I, et al. Multisession radiosurgery for optic nerve sheath meningiomas—an effective option: preliminary results of a single-center experience. Neurosurgery. 2011;69(5):1116–1122; discussion 1122–1123. [DOI] [PubMed] [Google Scholar]
- 20. Hakim R, AlexanderE, 3rd, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42(3):446–453; discussion 453–444. [DOI] [PubMed] [Google Scholar]
- 21. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas: treatment results on the basis of a 22-year experience. Cancer. 2012;118(4):1048–1054. [DOI] [PubMed] [Google Scholar]
- 22. Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49(5):1029–1037; discussion 1037–1028. [DOI] [PubMed] [Google Scholar]
- 23. Kim M, Lee DH, Kim Rn HJ, Cho YH, Kim JH, Kwon DH. Analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg. 2017;153:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Wojcieszynski AP, Ohri N, Andrews DW, Evans JJ, Dicker AP, Werner-Wasik M. Reirradiation of recurrent meningioma. J Clin Neurosci. 2012;19(9):1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Khatib M, El Majdoub F, Hoevels M, et al. Stereotactic LINAC radiosurgery for incompletely resected or recurrent atypical and anaplastic meningiomas. Acta Neurochir (Wien). 2011;153(9):1761–1767. [DOI] [PubMed] [Google Scholar]
- 26. Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53–58; discussion 58–60. [DOI] [PubMed] [Google Scholar]
- 27. Lin AJ, Hui C, Dahiya S, et al. Radiologic response and disease control of recurrent intracranial meningiomas treated with reirradiation. Int J Radiat Oncol Biol Phys. 2018;102(1):194–203. [DOI] [PubMed] [Google Scholar]
- 28. Mozes P, Dittmar JO, Habermehl D, et al. Volumetric response of intracranial meningioma after photon or particle irradiation. Acta Oncol. 2017;56(3):431–437. [DOI] [PubMed] [Google Scholar]
- 29. Rieken S, Habermehl D, Haberer T, Jaekel O, Debus J, Combs SE. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): early treatment results and study concepts. Radiat Oncol. 2012;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Shafie RA, Czech M, Kessel KA, et al. Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol. 2018;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103(7):1427–1430. [DOI] [PubMed] [Google Scholar]
- 32. Enam SA, Abdulrauf S, Mehta B, Malik GM, Mahmood A. Metastasis in meningioma. Acta Neurochir (Wien). 1996;138(10):1172–1177; discussion 1177–1178. [DOI] [PubMed] [Google Scholar]
- 33. Furuse M, Nonoguchi N, Kuroiwa T, et al. A prospective multicenter single-arm clinical trial of bevacizumab for patients with surgically untreatable symptomatic brain radiation necrosis. Neurooncol Pract. 2016;3(4):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyatake S, Kawabata S, Hiramatsu R, Furuse M, Kuroiwa T, Suzuki M. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol. 2014;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyatake SI, Wanibuchi M, Hu N, Ono K. Boron neutron capture therapy for malignant brain tumors. J Neurooncol. 2020;149(1):1–11. [DOI] [PubMed] [Google Scholar]
- 36. Sumitomo Heavy Industries, Ltd. Obtains Medical Device Approval for Manufacturing and Sales of Accelerator Based BNCT System and the Dose Calculation Program in Japan. World’s First BNCT System as Medical Device. https://www.shi.co.jp/english/info/2019/6kgpsq0000002ji0.html. Accessed May 19, 2020. [Google Scholar]
- 37. Stella Pharma Receives Marketing and Manufacturing Approval in Japan for Steboronine® Intravenous Drip Bag 9000 mg/300 mL. World’s First BNCT Drug. https://stella-pharma.co.jp/cp-bin/wordpress5/wp-content/uploads/2020/03/Press-releaseSteboronine-approvalENG.pdf. Accessed May 19, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.