Abstract
Background
The optimal management of patients with incidental meningiomas remains unclear. The aim of this study was to characterize the radiologic and neurological outcomes of expectant and stereotactic radiosurgery (SRS) management of asymptomatic meningioma patients.
Methods
Using data from 14 centers across 10 countries, the study compares SRS outcomes to active surveillance of asymptomatic meningiomas. Local tumor control of asymptomatic meningiomas and development of new neurological deficits attributable to the tumor were evaluated in the SRS and conservatively managed groups.
Results
In the unmatched cohorts, 727 meningioma patients underwent SRS and were followed for a mean of 57.2 months. In the conservatively managed cohort, 388 patients were followed for a mean of 43.5 months. Tumor control was 99.0% of SRS and 64.2% of conservatively managed patients (P < .001; OR 56.860 [95% CI 26.253-123.150]). New neurological deficits were 2.5% in the SRS and 2.8% of conservatively managed patients (P = .764; OR 0.890 [95% CI 0.416-1.904]). After 1:1 propensity matching for patient age, tumor volume, location, and imaging follow-up, tumor control in the SRS and conservatively managed cohorts was 99.4% and 62.1%, respectively (P < .001; OR 94.461 [95% CI 23.082-386.568]). In matched cohorts, new neurological deficits were noted in 2.3% of SRS-treated and 3.2% of conservatively managed patients (P = .475; OR 0.700 [95% CI 0.263-1.863]).
Conclusions
SRS affords superior radiologic tumor control compared to active surveillance without increasing the risk of neurological deficits in asymptomatic meningioma patients. While SRS and active surveillance are reasonable options, SRS appears to alter the natural history of asymptomatic meningiomas including tumor progression in the majority of patients treated.
Keywords: asymptomatic, meningioma, stereotactic radiosurgery, surveillance
Key Points.
In asymptomatic meningioma patients, stereotactic radiosurgery (SRS) affords superior radiologic tumor control over conservative management.
SRS for asymptomatic meningiomas does not appear to increase the risk of new neurological deficits as compared to patients managed with active surveillance.
Importance of the Study.
Incidental meningiomas are commonly diagnosed. The optimal management of these tumors in terms of tumor control and neurological preservation remains unclear. Many physicians and patients with asymptomatic meningiomas choose expectant management over stereotactic radiosurgery (SRS). Although longer follow-up and ideally prospective validation of these results seems warranted, the current study provides a benchmark for SRS treatment of asymptomatic meningioma patients; it suggests that SRS affords superior radiologic tumor control over conservative management without increasing the risk of new neurological deficits. Further study identifying specific subgroups of asymptomatic meningioma patients at presentation who most benefit from SRS is required to prevent tumor growth and development of tumor-related neurological signs or symptoms while minimizing the risk of SRS sequelae.
Meningiomas comprise 13%-26% of all primary intracranial tumors.1,2 Most meningiomas are histologically benign (WHO grade I). The prevalence of asymptomatic meningiomas appears to be increasing in part through diagnosis secondary to increased use of brain imaging for minor head injuries and other nonspecific neurological symptoms.3–6 Once diagnosed, the optimal management of an asymptomatic meningioma remains controversial.5,7–11 In one population-based survey from the United States, observation with longitudinal surveillance is a commonly chosen approach for smaller meningiomas (<2 cm).12
However, over periods of 4 or more years, 24%-92% of small to moderately sized meningiomas will demonstrate a linear increase in the diameter of the tumor.3–5,7,8,13–15 Volumetric assessments of meningioma change vary from 18.8% to 88% over a 4+ year time interval.16 With growth, the risk of tumor-related signs and symptoms developing in patients varies from 0% to 10%.17 Also, the anxiety and mood changes associated with an untreated meningioma may affect a patient’s quality of life.18 Surgical treatment can also incur long-term considerable limitations in health-related quality of life.19
While resection remains a treatment of choice for many symptomatic meningiomas, the benefit to risk profile for removing an asymptomatic meningioma has led many to choose expectant management.20 Stereotactic radiosurgery (SRS) has become increasingly used for treating meningiomas; its minimally invasive nature has also led to SRS treatment of asymptomatic meningiomas.21,22 This international Multicenter Matched Cohort Analysis of Incidental Meningioma Progression During Active Surveillance or After Stereotactic Radiosurgery (IMPASSE) study is a multicenter matched cohort analysis that aims to evaluate the efficacy and safety of SRS in the treatment of patients with asymptomatic meningiomas.
Materials and Methods
Study Design and Population
The study included SRS-treated patients with incidental and asymptomatic meningiomas (SRS cohort) from participating centers through the International Radiosurgery Research Foundation (IRRF) and supported as study IRRF-07-31-2020. These centers obtained approval for the study from their respective institutional review boards and also had approval for sharing of deidentified data with the IRRF coordinating office. Baseline patient, tumor, and treatment variables as well as longitudinal follow-up data for patients in the SRS and observation cohorts were collected by study investigators and sent to the IRRF coordinating office. The IRRF coordinator subsequently verified the data for completeness and forward deidentified datasets to the study coordinating investigator. IRRF data were obtained from 14 centers across 10 countries. Comparable data fields were obtained for the observation group of asymptomatic meningiomas subject to active clinical and radiologic surveillance (observation cohort) from an IRRF site (the University of Virginia) and the Walton Centre NHS Foundation Trust, a stand-alone neuroscience hospital in the United Kingdom which serves a region of approximately 3.5 million people and partners with 18 other hospitals.23
The meningioma was diagnosed on the basis of neuroimaging findings including extra-axial location, uniform contrast enhancement, the presence of calcification, and the absence of known cancer in the patient. Histology if available was limited to only WHO grade I meningiomas. The study was limited to patients >16 years old. Patients with multiple meningiomas were excluded from the analysis. At the time of diagnosis, patients did not exhibit signs or symptoms attributable to the identified meningioma. In the observation and SRS groups, patients underwent longitudinal evaluations with serial neuroimaging and clinical assessments during the duration of follow-up. Time to tumor progression as defined by Response Assessment in Neuro-Oncology (RANO) criteria was noted during the longitudinal follow-up period.
Intervention
SRS was described according to a consensus definition, and, in this study, radiosurgery was delivered in 1 session to the asymptomatic meningioma. Radiosurgery was performed using the Gamma Knife (Elekta AB, Stockholm, Sweden). SRS utilized brain MRI and/or CT with contrast for stereotactic targeting; radiosurgical planning using a multi-isocentric approach. The SRS technique and dose selection were selected as per the local clinical team and model of radiosurgical technology available at that site.
Outcomes
Comparing the impact of SRS vs observation, the primary endpoint of the analysis was to determine local control defined as stable or regressed tumor on neuroimaging studies based upon RANO24 criteria. A tumor was defined as stable if its volume changed by less than 25% of the baseline volume and regressed if the volume decreased by ≥25% of the baseline volume. Tumor progression was defined as tumor volume increase of ≥25% of its baseline. Secondary outcomes included tumor regression, tumor progression, tumor progression-free survival, and development of a new neurological deficit. A new neurological problem was a focal deficit (eg, a cranial nerve deficit, sensory disturbance, or motor dysfunction) attributable to the tumor. Tumor progression-free survival was measured in months based on radiological follow-up. Patients were censored at the time of death or loss to follow-up. New neurological deficit was defined as changes in global or focal neurological status attributable to the tumor and compared to initial diagnosis for the observation group and prior to treatment in the SRS cohort.
Statistical Analysis
All statistical analyses were performed using Stata (version 16.1; StataCorp, College Station, TX, USA). Baseline patient and meningioma characteristics were compared between the observation and SRS cohorts. Continuous variables were compared using Student t or Mann-Whitney U tests, and categorical variables were compared using Pearson χ 2 or Fisher exact tests, where appropriate.
Patient age at presentation, duration of follow-up, and tumor volume have been previously shown to impact the natural history of meningiomas.6,14 To control for potential confounders of treatment outcome, the 2 cohorts were matched, without replacement, in a 1:1 ratio with a caliper of 0.01 using propensity scores derived from patient age, meningioma volume, tumor location, and duration of neuroimaging follow-up. The matching process was performed using the PSMATCH2 package developed for Stata. An absolute standardized difference of <0.10 between observation and SRS cohorts for the matched covariates was considered adequate balance. Univariate comparisons of the unmatched and matched cohorts were performed for outcome measures using binary logistic regression analysis. Time-dependent analyses for progression-free survival were performed using Kaplan-Meier and actuarial methods. Differences between function curves were analyzed using the log-rank test. Statistical significance was defined as P < .05, and all tests were 2-tailed. Missing data were not imputed.
Results
Unmatched Patient and Tumor Attributes
In the unmatched cohorts, data were collected on 727 SRS patients and 388 conservatively managed patients (Table 1). The mean age of the SRS group was 56.9 years, and the observation group’s mean age was 62.6 years (P < .001). Mean initial Karnofsky Performance Score (KPS) was 90 in the SRS group and 100 in the conservatively managed group. Mean meningioma volume of the SRS and conservatively managed cohorts was 4.3 and 3.7 cc, respectively (P = .055). Mean radiologic follow-up durations for the SRS and conservatively managed cohorts were 57.2 and 43.5 months, respectively (P < .001). Mean clinical follow-up for the SRS and observation cohorts were 56.3 and 43.5 months, respectively (P < .001). The mean dose to the tumor was 13.0 Gy in the SRS-treated cohort.
Table 1.
Comparison of Baseline Characteristics Between Unmatched SRS and Conservative Management Cohorts
| Total (n = 1117) | SRS (n = 727) | Conservative Management (n = 388) | P Value | |
|---|---|---|---|---|
| Age, mean yr (SD) | 58.8 (13.4) | 56.9 (13.7) | 62.6 (12.0) | <0.001 |
| Male, n (%) | 242/1095 (22.1) | 160/725 (22.1) | 82/288 (22.2) | 0.972 |
| Baseline KPS, median (IQR) | 95 (90-100) | 90 (90-100) | 100 (90-100) | 0.014 |
| Diameter, mean mm (SD) | 19.6 (9.2) | 20.0 (9.4) | 18.9 (8.8) | 0.061 |
| Volume, mean cm3 (SD) | 4.1 (4.9) | 4.3 (4.0) | 3.7 (6.0) | 0.055 |
| Laterality, n (%) | ||||
| Right | 517/1099 (47.0) | 330/711 (46.4) | 187/388 (48.2) | 0.107 |
| Left | 336/1099 (47.7) | 336/711 (47.3) | 188/388 (48.5) | |
| Midline | 58/1099 (5.3) | 45/711 (6.3) | 13/388 (3.4) | |
| Location, n (%) | <0.001 | |||
| Skull base | 438/1112 (39.4) | 335/724 (46.3) | 103/388 (26.6) | |
| Convexity | 641/1112 (57.6) | 374/724 (51.7) | 267/388 (68.8) | |
| Other | 33/1112 (3.0) | 15/724 (2.1) | 18/388 (4.6) | |
| Margin dose, mean Gy (SD) | –– | 13.0 (1.9) | –– | –– |
| Maximum dose, mean Gy (SD) | –– | 26.0 (5.1) | –– | –– |
| Isocenters, median (IQR) | –– | 9 (6–15) | –– | –– |
| Treatment volume, mean cm3 (SD) | –– | 5.4 (4.7) | –– | –– |
| Imaging follow-up, mean mo (median; SD) | 52.6 (42; 39.6) | 57.2 (48; 43.7) | 43.5 (36; 27.9) | <0.001 |
| Clinical follow-up, mean mo (median; SD) | 51.9 (42; 39.2) | 56.3 (45; 43.3) | 43.5 (36; 27.9) | <0.001 |
Abbreviations: IQR, interquartile range; SD, standard deviation; SRS, stereotactic radiosurgery.
Bold values are those with P < 0.05.
Matched Patient and Tumor Attributes
After propensity score matching for patient age, duration of imaging follow-up, tumor location, and tumor volume, 311 patients remained in each cohort (Table 2). In the SRS cohort, the mean age was 61.1 years, and it was 60.7 years for the conservatively managed cohort (P = .723). Median KPS in the SRS and observation groups was 90 and 100, respectively (P < .001). Mean tumor volumes in the SRS and observation groups were 3.8 and 3.9 cc, respectively (P = .804). Regarding laterality of the meningioma, the SRS group and observation cohorts had no appreciable differences in tumor side or midline location (P = .451). Mean clinical and imaging follow-up durations in the SRS and conservatively managed groups were also not significantly different between the 2 cohorts.
Table 2.
Comparison of Baseline Characteristics Between Matched SRS and Conservative Management Cohorts
| SRS (n = 311) | Conservative Management (n = 311) | P Value | |
|---|---|---|---|
| Age, mean yr (SD) | 61.1 (12.9) | 60.7 (11.7) | 0.723a |
| Male, n (%) | 72/311 (23.2) | 63/311 (20.3) | 0.381 |
| Baseline KPS, median (IQR) | 90 (90-100) | 100 (90-100) | <0.001 |
| Diameter, mean mm (SD) | 19.0 (9.3) | 19.1 (8.9) | 0.876 |
| Volume, mean cm3 (SD) | 3.8 (3.3) | 3.9 (6.5) | 0.804a |
| Laterality, n (%) | 0.451 | ||
| Right | 140/307 (45.6) | 151/311 (48.6) | |
| Left | 149/307 (48.5) | 148/311 (47.6) | |
| Midline | 18/307 (5.9) | 12/311 (3.9) | |
| Location, n (%) | 0.798a | ||
| Skull base | 93/311 (29.9) | 95/311 (30.6) | |
| Convexity | 206/311 (66.2) | 207/311 (66.6) | |
| Other | 12/311 (3.9) | 9/311 (2.9) | |
| Margin dose, mean Gy (SD) | 12.9 (1.8) | –– | –– |
| Maximum dose, mean Gy (SD) | 26.0 (4.7) | –– | –– |
| Isocenters, median (IQR) | 9 (6–15) | –– | –– |
| Treatment volume, mean cm3 (SD) | 4.9 (4.1) | –– | –– |
| Imaging follow-up, mean mo (median; SD) | 46.5 (36; 35.5) | 46.6 (42; 28.3) | 0.961a |
| Clinical follow-up, mean mo (median; SD) | 47.4 (36; 36.1) | 46.6 (42; 28.3) | 0.767 |
Abbreviations: IQR, interquartile range; SD, standard deviation; SRS, stereotactic radiosurgery.
aMatched covariates.
Bold values are those with P < 0.05.
Radiologic and Neurologic Outcomes for Unmatched Cohorts
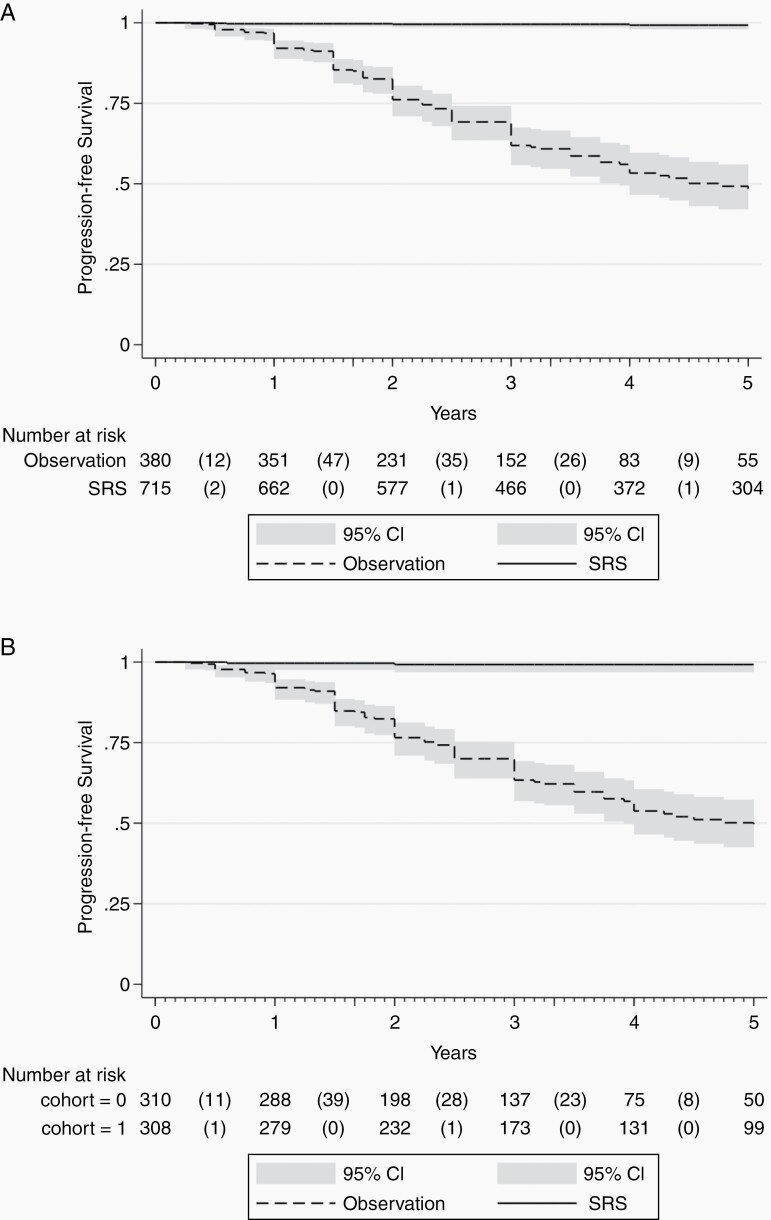
In the unmatched cohorts, tumor control (ie, stability or regression) was observed in 99.0% of SRS-treated patients and 64.2% of patients in the observation cohort (P < .001; OR 56.86 [95% CI 26.253-123.150]) (Table 3). Tumor progression-free survival between the unmatched SRS and conservatively managed cohorts was appreciably different over time and favored SRS (Figure 1a; log-rank test, P < .001). Tumor regression was observed in 45.4% of SRS-treated patients and 0.8% of patients in the observation cohort (P < .001; OR 106.781 [95% CI 33.966-335.694]). No patients in the SRS cohort had evidence of radiation-associated intracranial malignancy.
Table 3.
Comparison of Neurological and Radiologic Outcomes Between Unmatched SRS and Conservative Management Cohorts
| SRS (n = 727) | Conservative Management (n = 388) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Tumor control, n (%) | 713/720 (99.0) | 249/388 (64.2) | 56.860 (26.253-123.150) | <0.001 |
| Tumor regression, n (%) | 327/720 (45.4) | 3/388 (0.8) | 106.781 (33.966-335.694) | <0.001 |
| Tumor progression, n (%) | 7/720 (1.0) | 141/388 (36.3) | 0.017 (0.008-0.037) | <0.001 |
| New neurological deficit, n (%) | 18/711 (2.5) | 11/388 (2.8) | 0.890 (0.416-1.904) | 0.764 |
Abbreviations: CI, confidence interval; OR, odds ratio; SRS, stereotactic radiosurgery.
Bold values are those with P < 0.05.
Fig. 1.
a. Kaplan-Meier plot comparing tumor progression-free survival between unmatched SRS and conservative management cohorts. b. Kaplan-Meier plot comparing tumor progression-free survival between matched SRS and conservative management cohorts. Abbreviation: SRS: stereotactic radiosurgery.
New neurological deficits attributable to the meningioma were observed in 2.5% of SRS and 2.8% of patients in the observation cohort (P = .764; OR 0.890 [95% CI 0.416-1.904]). Of those with a new neurological deficit related to the meningioma, tumor progression was reported in 10/11 (90.9%) in the observation cohort and 2/18 (11.1%) in the SRS cohort. At last follow-up, the combined endpoint of tumor and neurological deficit progression-free survival for the unmatched cohorts is illustrated in Supplementary Figure 1.
Radiologic and Neurologic Outcomes Between Matched Cohorts
In the matched cohort analysis (Table 4), tumor control in the SRS and observation cohorts was observed in 99.4% and 62.1%, respectively (P < .001; OR 94.461 [95% CI 23.082-386.568]). Tumor progression-free survival between the unmatched SRS and conservatively managed cohorts was appreciably different over time favored SRS (Figure 1b; log-rank test, P < .001). Tumor regression at last follow-up was seen in 44.4% of the SRS cohort and 1% of the conservatively managed cohort (P < .001; OR 81.896 [95% CI 25.702-260.950]). Similar to the unmatched cohort, no patients in the SRS cohort had evidence of radiation-induced intracranial malignancy.
Table 4.
Comparison of Neurological and Radiologic Outcomes Between Matched SRS and Conservative Management Cohorts
| SRS (n = 311) | Conservative Management (n = 311) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Tumor control, n (%) | 309/311 (99.4) | 193/311 (62.1) | 94.461 (23.082-386.568) | <0.001 |
| Tumor regression, n (%) | 138/311 (44.4) | 3/311 (1.0) | 81.896 (25.702-260.950) | <0.001 |
| Tumor progression, n (%) | 2/311 (1.0) | 118/311 (37.9) | 0.011 (0.003-0.043) | <0.001 |
| New neurological deficit, n (%) | 7/308 (2.3) | 10/311 (3.2) | 0.700 (0.263-1.863) | 0.475 |
Abbreviations: CI, confidence interval; OR, odds ratio; SRS, stereotactic radiosurgery.
Bold values are those with P < 0.05.
New neurological deficits attributable to the meningioma were observed in 2.3% of SRS-treated and 3.2% of the observation cohort (P = .475; OR 0.700 [95% CI 0.263-1.863]).Of those with a new neurological deficit related to the meningioma, tumor progression was demonstrated in 9/10 (90.0%) in the observation cohort and 1/7 (14.2%) in the SRS cohort. At last follow-up, the combined endpoint of tumor and neurological deficit progression-free survival for the matched cohorts is illustrated in Supplementary Figure 2.
Discussion
The current study is an international, multicenter analysis of outcomes of 1117 patients with asymptomatic meningiomas managed with SRS (N = 727) or observation (N = 388). After SRS, RANO-based tumor control in the unmatched cohorts was observed in 99.0% of the SRS-treated and 64.2% of the observed patients at last follow-up (P < .001). New neurological deficits attributable to the tumor were observed in 2.5% in the SRS cohort and 2.8% of patients in the observation cohort. Thus, not every tumor that progressed by RANO criteria led to development of a new neurological deficit at that time. However, over time, tumor progression may of course lead to new or worsening neurological deficits particularly if the tumor growth continues over many years.6 In SRS-treated patients, there were no cases of radiation-induced intracranial malignancy, but late sequelae of SRS in patients with asymptomatic meningiomas require longer-term study. This finding is consistent with prior reports for patients treated with SRS for meningiomas and other intracranial pathology.25
In the second portion of the analysis, factors associated with meningioma growth including patient age, tumor volume, tumor location, and duration of follow-up were matched.6,11 In a 1:1 propensity score-matched cohort analysis of 311 patients in each arm with a mean follow-up of approximately 46.5 months, tumor control was observed in 99.4% of SRS-treated patients as compared to 62.1% of observed patients (P < .001). New neurological deficits attributable to the tumor were noted in 2.3% of SRS-treated and 3.2% of observed patients (P = .475). While the option for active surveillance of an asymptomatic meningioma followed by SRS at the time of radiologic and/or clinical progression remains a reasonable one, the current study results are impressive in demonstrating that SRS affords local tumor control defined as RANO stability or regression while not increasing the risk of neurological deficits in this population.
Studies have demonstrated the long-term durability of tumor control of meningiomas treated with SRS. In such studies, progression-free survival of WHO grade I meningiomas of 95%-97% and 82%-87% have been reported at 5 and 10 years, respectively, after SRS.22,26 Moreover, these studies demonstrate a low risk of new or worsening neurological deficits in SRS-treated meningioma patients. However, such studies have generally focused on recurrent or residual meningiomas after resection or in meningioma patients who have demonstrated tumor-related signs or symptoms prior to treatment.
Asymptomatic or incidentally found meningiomas while increasingly common are often expectantly managed.12 While the natural history of incidental meningiomas varies in part due to the heterogeneity of progression definitions and follow-up duration, meningiomas typically demonstrate slow progression.27 In a 5-year prospective study of 70 untreated patients, 75% demonstrated progression by 15%. In another study of 65 patients with incidental meningiomas, 35.4% had an increase in size by >2 mm during a mean follow-up of 74 months, and progression occurred in 50% by 10 years and 75% at 15 years for meningiomas <2 cm in initial size.14 In a meta-analysis of meningiomas, Sughrue et al noted that 44% of all untreated meningiomas and 49% tumors ≤2.5 cm demonstrated growth on serial imaging over a median follow-up period of 4.6 years.6 Rapid exponential growth of an incidental meningioma is less common with 7%-10% of untreated incidental meningiomas demonstrating such behavior.6 Similarly, the risk of the development of a neurological deficit at 5 years is low and ranges from 0% to 10%.10 In the setting of progressive growth leading to worsening mass effect upon neural structures, neurological symptoms or signs may be more likely to be manifested in patients presenting with initially asymptomatic meningiomas.
Altering the trajectory of growth of asymptomatic meningiomas in a minimal access approach with SRS confers the benefits of tumor control while avoiding the risks of a resection in those with progression.27,28 Moreover, within the study observation period of approximately 5 years, SRS to asymptomatic meningiomas appears to confer no increased risk of neurological deficits over observation alone. As the mean age of patients in the matched cohorts was 61 years and considering the average life expectancy in North American and the United Kingdom, the at-risk period for patients developing radiological and neurological progression from an initially asymptomatic meningioma is likely a decade or more for most patients in the current study. Thus, altering the trajectory of growth of meningiomas with radiosurgery may confer benefit to patients with asymptomatic meningiomas over the long term including lessening the need for invasive surgery to resect the tumor after it has progressed and/or caused neurological deficits. Further longitudinal study would be required to determine if SRS offers a long-term neurological protective effect in patients initially presenting with an asymptomatic meningioma.
On balance, the decision to treat or to observe an incidental meningioma is multifactorial and should take into account the predicted natural history weighed against the patient’s age, comorbidity burden, and importantly personal preference.29 Considering that the vast majority of surgically removed tumors are WHO grade I,11 the results of this study present an argument for SRS treatment in patients deemed higher risk of progression or for whom the period at risk is substantial in duration.
Strengths and Limitations
The study has several strengths including the large size of the SRS and observation cohorts and the participation from centers in various countries improving the generalizability of the findings. The statistical methodology including propensity matching to control for multiple prognostic factors represents an additional strength.
The study also has several limitations. The retrospective nature of the design may result in bias and confounding factors in the analysis. Decisions for SRS treatment and observation could not be discerned based on the collected data and could be a source of bias. Hence, there may be inherent selection bias that may not be accounted for in the statistical matching. Not all included patients had pathological diagnosis, and thus potential inclusion of patients with WHO grades II and III meningiomas or other tumors resembling meningiomas on neuroimaging studies such as hemangioperictyomas may confound the results. However, very few patients required resection so this incidence is unlikely. Also, while the mean follow-up duration in the unmatched and matched cohorts was approximately 4 years, longer surveillance is needed to determine the long-term risk of tumor growth and associated neurological deficits.
In addition, the current study findings may not apply to pediatric patients, but presentation with an asymptomatic meningioma is more common in adulthood rather than childhood age. The current study design does not permit assessments of quality of life and cost-effectiveness of care. Also, the RANO criteria for assessing response in treated meningiomas is not a validated tool for this particular purpose but has been validated for other brain tumor applications.
Despite best attempts at controlling for measured baseline variables, unmeasured variables that may confound the results may not be accounted for. Although neurological outcome was captured in the form of development of new neurological deficit, validated performance status and quality of life assessments were not performed as part of this study. Moreover, the eventual outcome of the neurological deficit, which may have triggered steroid or surgical treatment, was not fully recorded. Therefore, conclusions regarding overall functional outcomes could not be drawn. As the primary outcome was radiologic tumor control, secondary outcomes analyzed were considered exploratory as the multiple tests were not adjusted and may be subject to an elevated false detection rate. While RANO criteria were applied at each site, neuroimaging review and tumor volume quantification were performed by respective participating centers, and there was no centralized imaging core or adjudication of these findings. Therefore, inter- and intra-rater reliability could not be ascertained. Also, surveillance protocols for radiologic and clinical assessment were dependent on institutional practices. Beyond traditional SRS parameters including dose and tumor volume, we did not collect specific data on variations in SRS techniques or devices utilized.
Conclusions
In this large, multicenter matched cohort analysis, SRS afforded superior radiologic control of asymptomatic meningiomas over observation for all time points evaluated. SRS did not increase the risk of neurological deficit development as compared to observation. For meningioma patients presenting without symptoms, SRS was associated with less risk of CNS progression of the tumor and no increase in the development of a new neurological deficit as compared to those managed with observation. SRS appears to alter the natural history of asymptomatic meningioma growth. However, whether tumor control achieved with SRS treatment translates into preservation or improvement of functional outcome remains to be determined over a longer follow-up period; SRS and active surveillance are reasonable options. We believe that these data for SRS outcomes support considering SRS to treat patients presenting with an asymptomatic meningioma and no imminently threatening medical issues. A randomized clinical trial of active surveillance vs SRS can help to further define the optimal management of asymptomatic meningiomas.
Supplementary Material
Acknowledgments
The authors appreciate the assistance of Ulas Yener, MD, and Kenneth Bernstein, MS. They assisted in data collection for this study.
Funding
No funding was received for this study.
Author contributions. J.S., C.-J.C., S.P., A.I.I., M.D.J., and L.D.L. participated in the study design. S.P., A.I.I., A.B., S.P., Y.S., A.M.N., W.A.R., S.R.T., A.M.N.E., K.A., R.M.E., V.D., D.M., C.-C.L., H.-C.Y., R.L., J.H., R.M.A., D.P., D.K., N.M.M., M.T., H.S., C.A., G.N.B., and R.J.B. participated in data collection. J.S., C.-J.C., A.B., and S.P. verified and analyzed the data. J.S., C.-J.C., A.B., S.P., L.D.L., and D.K. assisted with data interpretation. J.S., S.P., C.-J.C., and A.B. wrote the manuscript. All authors reviewed the report and gave final approval to submit it for publication.
Conflict of interest statement. L.D.L. is a shareholder in Elekta AB, the manufacturer of some radiosurgical devices. All other authors have no competing interests.
References
- 1. Bondy M, Lee Ligon B. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996; 29(3):197–205. [DOI] [PubMed] [Google Scholar]
- 2. Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004; 363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 3. Hashiba T, Moto NH, Izumoto S, et al. . Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas: clinical article. J Neurosurg. 2009; 110(4):675–684. [DOI] [PubMed] [Google Scholar]
- 4. Kuratsu JI, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000; 92(5):766–770. [DOI] [PubMed] [Google Scholar]
- 5. Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningiomas: clinical article. J Neurosurg. 2011; 114(5):1250–1256. [DOI] [PubMed] [Google Scholar]
- 6. Sughrue ME, Rutkowski MJ, Aranda D, et al. . Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. 2010; 113(5):1036–1042. [DOI] [PubMed] [Google Scholar]
- 7. Jo KW, Kim CH, Kong DS, et al. . Treatment modalities and outcomes for asymptomatic meningiomas. Acta Neurochir (Wien). 2011; 153(1):62–67. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura M, Roser F, Michel J, et al. . The natural history of incidental meningiomas. Neurosurgery. 2003; 53(1):62–71. [DOI] [PubMed] [Google Scholar]
- 9. Yano S, Kuratsu JI. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006; 105(4):538–543. [DOI] [PubMed] [Google Scholar]
- 10. Behbahani M, Skeie GO, Eide GE, et al. . A prospective study of the natural history of incidental meningioma – hold your horses! Neurooncol Pract. 2019; 6(6):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Islim AI, Mohan M, Moon RDC, et al. . Incidental intracranial meningiomas: a systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol. 2019; 142(2):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal V, McCutcheon BA, Hughes JD, et al. . Trends in management of intracranial meningiomas: analysis of 49,921 cases from modern cohort. World Neurosurg. 2017; 106:145–151. [DOI] [PubMed] [Google Scholar]
- 13. Yoneoka Y, Fujii Y, Tanaka R. Growth of incidental meningiomas. Acta Neurochir (Wien). 2000; 142(5):507–511. [DOI] [PubMed] [Google Scholar]
- 14. Jadid KD, Feychting M, Höijer J, et al. . Long-term follow-up of incidentally discovered meningiomas. Acta Neurochir (Wien). 2015; 157(2):225–230. [DOI] [PubMed] [Google Scholar]
- 15. Kim KH, Kang SJ, Choi JW, et al. . Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg. 2018. doi: 10.3171/2017.12.JNS171943 [DOI] [PubMed] [Google Scholar]
- 16. Amelot A, van Effenterre R, Kalamarides M, Cornu P, Boch AL. Natural history of cavernous sinus meningiomas. J Neurosurg. 2018. doi: 10.3171/2017.7.JNS17662 [DOI] [PubMed] [Google Scholar]
- 17. Romani R, Ryan G, Benner C, Pollock J. Non-operative meningiomas: long-term follow-up of 136 patients. Acta Neurochir (Wien). 2018; 160(8):1547–1553. [DOI] [PubMed] [Google Scholar]
- 18. Bunevicius A, Deltuva VP, Tamasauskas A. Association of pre-operative depressive and anxiety symptoms with five-year survival of glioma and meningioma patients: a prospective cohort study. Oncotarget. 2017; 8(34):57543–57551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nassiri F, Price B, Shehab A, et al. . Life after surgical resection of a meningioma: a prospective cross-sectional study evaluating health-related quality of life. Neuro Oncol. 2019; 21(Suppl 1):i32–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Näslund O, Skoglund T, Farahmand D, Bontell TO, Jakola AS. Indications and outcome in surgically treated asymptomatic meningiomas: a single-center case-control study. Acta Neurochir (Wien). 2020; 162(9):2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salvetti DJ, Nagaraja TG, Levy C, Xu Z, Sheehan J. Gamma Knife surgery for the treatment of patients with asymptomatic meningiomas. J Neurosurg. 2013; 119(2):487–493. [DOI] [PubMed] [Google Scholar]
- 22. Sheehan JP, Starke RM, Kano H, et al. . Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study: clinical article. J Neurosurg. 2014; 120(6):1268–1277. [DOI] [PubMed] [Google Scholar]
- 23. Islim AI, Kolamunnage-Dona R, Mohan M, et al. . A prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro Oncol. 2020; 22(2):278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang RY, Bi WL, Weller M, et al. . Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019; 21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf A, Naylor K, Tam M, et al. . Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 2019; 20(1):159–164. [DOI] [PubMed] [Google Scholar]
- 26. Kondziolka D, Mathieu D, Lunsford LD, et al. . Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008; 62(1):53–58. [DOI] [PubMed] [Google Scholar]
- 27. Reinert M, Babey M, Curschmann J, et al. . Morbidity in 201 patients with small sized meningioma treated by microsurgery. Acta Neurochir (Wien). 2006; 148(12):1257–1266. [DOI] [PubMed] [Google Scholar]
- 28. Kolakshyapati M, Ikawa F, Abiko M, et al. . Multivariate risk factor analysis and literature review of postoperative deterioration in Karnofsky Performance Scale score in elderly patients with skull base meningioma. Neurosurg Focus. 2018; 44(4):E14. [DOI] [PubMed] [Google Scholar]
- 29. Lee EJ, Kim JH, Park ES, et al. . A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017; 127(5):971–980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.