Abstract
Gut-related metabolites have been linked with respiratory disease. The crosstalk between the gut and lungs suggests that gut health may be compromised in COVID-19. The aims of the present study were to analyze a panel of gut-related metabolites (acetyl-L-carnitine, betaine, choline, L-carnitine, trimethylamine, and trimethylamine N-oxide) in patients with COVID-19, matched with healthy individuals and patients with non-COVID-19 respiratory symptoms. As results, metabolites from this panel were impaired in patients with COVID-19 and were associated with the symptoms of breathlessness and temperature, and it was possible to differentiate between COVID-19 and asthma. Preliminary results showed that lower levels of betaine appeared to be associated with poor outcomes in patients with COVID-19, suggesting betaine as a marker of gut microbiome health.
Keywords: Gut microbiota, COVID-19, Respiratory, Metabolomics
Introduction
Increasing evidence suggests that the gut may be compromised by COVID-19 [1,2]. The gut microbiome is essential to health and well-being, whereas gut microbiota perturbations are linked with many diseases, included respiratory diseases [3]. Despite the anatomic distinctions between the gut and lungs, recent evidence into the lung microbiota has identified crosstalk with the gut microbiota [4]. One mechanism of gut-lung crosstalk is by the distribution of gut metabolites. A gut-derived metabolite, trimethylamine N-oxide (TMAO), has been identified as a novel marker of gut health and disease, in particular in cardiometabolic diseases, and associations have been shown with disease severity and outcome, diet, ethnicity, sex, and lifestyle; it would therefore be suitable to investigate the associations of the gut with COVID-19 [5]. The aims of the present study were to investigate the association of circulating levels of gut-related metabolites (acetyl-L-carnitine, betaine, choline, L-carnitine, trimethylamine, and TMAO) in patients with COVID-19. We hypothesized that patients with COVID-19 would have different levels of metabolites compared with healthy individuals owing to compromised gut health. Furthermore, we compared patients who had COVID-19 with patients affected by other acute respiratory diseases (i.e., acute asthma and pneumonia).
Methods
Forty-one patients with acute COVID-19 were recruited on admission at Glenfield General Hospital, Leicester, UK, and plasma samples were matched by age and sex with samples from 28 healthy patients, 30 patients with acute asthma, and 24 patients with pneumonia. Each patient consented to have blood samples taken and outcomes surveyed. Gut-related metabolites were measured using liquid chromatography with tandem mass spectrometry [6].
Statistical analyses were performed using IBM SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA). Distribution of metabolites between the groups and their association with clinical features were analyzed using Spearman rank correlation and the Kruskal-Wallis test. Comparisons between COVID-19 and non-COVID-19 groups were performed using the Mann-Whitney test for continuous variables and the chi-square test for categorical variables. A one-way analysis of variance was used to compare metabolite levels across each of the study groups. A P value <0.05 was considered statistically significant.
Results
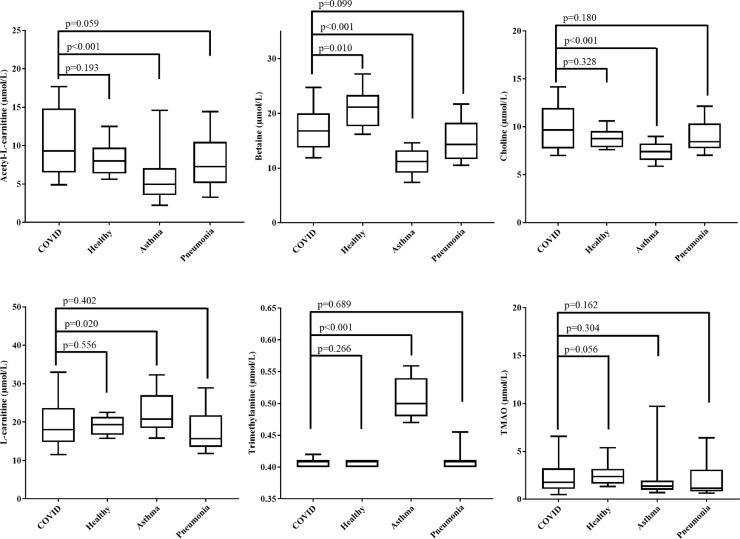
Demographics of the study population showed that the COVID-19 cohort was mostly male (68%) with a median age of 54 years. The majority of patients reported breathlessness (90%) and a new or continuous cough (76%) (Table 1 ). Distribution of metabolites showed no significant difference between healthy patients and those with COVID-19 for acetyl-L-carnitine, choline, L-carnitine, trimethylamine, and TMAO (P ≥ 0.056). There was a difference in betaine levels between these groups (P = 0.010), with higher levels in the healthy cohort, but not after Bonferroni correction (adjusted P = 0.058). No differences between patients with COVID-19 and those with pneumonia were observed (P ≥ 0.059; adjusted P ≥ 0.352). Furthermore, patients with asthma had lower levels of acetyl-L-carnitine, betaine, and choline and higher levels of trimethylamine when compared with patients who had COVID-19 (adjusted P < 0.001) (Table 2 ). Similar patterns were observed when patients with asthma and healthy patients were compared (adjusted P ≤ 0.030). There was an overall significant difference in metabolite distribution between the study groups (P ≤ 0.028), with a notable difference in distribution of acetyl-L-carnitine, betaine, choline, L-carnitine, and trimethylamine between patients with COVID-19 and those with asthma (P ≤ 0.020) (Fig. 1 ). The distribution of metabolites among COVID-19 symptoms (breathlessness, fatigue, loss of smell and/or taste, new, continuous cough, and runny nose) was analyzed and showed elevated levels of acetyl-L-carnitine and L-carnitine in patients who reported breathlessness. There were no other significant observations (Table 2). Additionally, betaine and TMAO were negatively correlated with temperature (r s = –0.395 and –0.344, respectively; P ≤ 0.028), whereas betaine also was correlated with O2 saturation levels (r s = 0.312; P = 0.050) (Table 2). Comorbidities were separated into three groups; diabetes, cardiac disease, and chronic pulmonary disease. We found no significant differences in metabolite levels for 10 patients who reported having chronic pulmonary disease (P ≥ 0.191) or 9 who had diabetes (P ≥ 0.295). However, in 6 patients who had cardiac disease, we found elevated levels of betaine, choline, L-carnitine, and TMAO (P ≤ 0.049). Furthermore, there was no significant difference in metabolite levels when at least one comorbidity (e.g., diabetes + cardiac disease + chronic pulmonary disease) was present (P ≥ 0.101). Kaplan-Meier survival analysis showed that when betaine levels were split by the median, a trend was observed between lower betaine levels and reduced survival (more than 2-fold lower survival than among patients with higher betaine levels) (P = 0.407).
Table 1.
Study demographic characteristics
| Characteristic | COVID-19 cohort (n = 41) | Non-COVID-19 cohort (n = 82)* | P value |
|---|---|---|---|
| Age, y | 54 (43–65) | 57 (47–66) | 0.520 |
| Male | 68% | 65% | 0.840 |
| Heart rate | 91 (75–104) | 81 (68–97) | 0.113 |
| Respiratory rate | 19.5 (16–20) | 18 (14–20) | <0.001 |
| O2 saturation | 96 (94–98) | 97 (95–98) | 0.061 |
| Temperature,°C | 36.7 (36.3–37.3) | 36.6 (36.2–37.0) | <0.001 |
| Lymphocyte | 1.3 (0.9–1.8) | 1.5 (1.1–2.2) | <0.001 |
| Eosinophil | 0.10 (0.03–0.23) | 0.16 (0.08–0.29) | <0.001 |
| C-reactive protein | 54 (24–171) | 47 (16–124) | 0.049 |
| Breathlessness | 90% | ||
| Fatigue | 51% | ||
| New, continuous cough | 76% | ||
| Runny nose | 15% | ||
| Loss of smell and/or taste | 15% | ||
| Mortality | 17% | ||
| Metabolites (µmol/L) | |||
| Acetyl-L-carnitine | 7.4 (5.4–11.1) | 6.6 (5.0–9.8) | 0.001 |
| Betaine | 15.9 (12.3–19.9) | 14.6 (11.4–19.9) | 0.067 |
| Choline | 8.5 (7.5–9.9) | 8.1 (7.4–9.2) | 0.001 |
| L-carnitine | 19.2 (15.8–22.7) | 19.3 (16.0–22.1) | 0.318 |
| Trimethylamine | 0.41 (0.40–0.46) | 0.41 (0.41–0.49) | 0.003 |
| Trimethylamine n-oxide | 1.6 (1.1–3.0) | 1.6 (1.1–3.0) | 0.853 |
Data are reported as the median (interquartile range) for continuous variables and as a percentage for categorical variables.
The non-COVID-19 group included patients with asthma, healthy patients, and patients with pneumonia.
Table 2.
Distribution of metabolites by illness and COVID-19 signs and symptoms and correlations with clinical features
| Acetyl-L-carnitine | Betaine | Choline | L-carnitine | TMA | TMAO | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Illness | ||||||||||||
| COVID-19 vs. asthma | <0.001 | <0.001 | <0.001 | 0.020 | <0.001 | 0.304 | ||||||
| COVID-19 vs. pneumonia | 0.059 | 0.099 | 0.180 | 0.402 | 0.689 | 0.162 | ||||||
| COVID-19 vs. healthy | 0.193 | 0.010 | 0.328 | 0.556 | 0.266 | 0.056 | ||||||
| Healthy vs. asthma | 0.005 | <0.001 | <0.001 | 0.114 | <0.001 | 0.006 | ||||||
| Healthy vs. pneumonia | 0.548 | <0.001 | 0.706 | 0.196 | 0.177 | 0.003 | ||||||
| Asthma vs. pneumonia | 0.037 | 0.004 | 0.002 | 0.005 | <0.001 | 0.681 | ||||||
| COVID-19 signs and symptoms | ||||||||||||
| Breathlessness | 0.007 | 0.130 | 1.000 | 0.009 | 0.352 | 0.160 | ||||||
| Fatigue | 0.048 | 0.454 | 0.752 | 0.083 | 0.396 | 0.654 | ||||||
| Loss of smell and/or taste | 0.578 | 0.449 | 0.558 | 0.383 | 0.521 | 0.081 | ||||||
| New, continuous cough | 0.790 | 0.262 | 0.396 | 0.476 | 0.625 | 0.134 | ||||||
| Runny nose | 0.292 | 0.782 | 0.507 | 0.218 | 0.564 | 0.507 | ||||||
| Correlations with clinical features | rs | P value | rs | P value | rs | P value | rs | P value | rs | P value | rs | P value |
| Temperature | –0.020 | 0.897 | –0.395 | 0.011 | –0.139 | 0.387 | 0.212 | 0.183 | –0.171 | 0.285 | –0.344 | 0.028 |
| Heart rate | –0.135 | 0.445 | –0.147 | 0.408 | –0.120 | 0.499 | –0.088 | 0.620 | 0.091 | 0.610 | –0.284 | 0.103 |
| Respiratory rate | –0.264 | 0.151 | –0.227 | 0.220 | –0.078 | 0.678 | –0.080 | 0.667 | –0.045 | 0.812 | –0.099 | 0.596 |
| O2 saturation | –0.096 | 0.555 | 0.312 | 0.050 | –0.159 | 0.327 | –0.077 | 0.635 | 0.069 | 0.671 | 0.054 | 0.741 |
| Lymphocytes | 0.035 | 0.827 | 0.026 | 0.870 | –0.020 | 0.899 | –0.099 | 0.539 | 0.080 | 0.621 | 0.052 | 0.745 |
| Eosinophils | –0.114 | 0.478 | 0.263 | 0.097 | 0.289 | 0.067 | –0.077 | 0.633 | –0.025 | 0.874 | 0.241 | 0.129 |
| C-reactive protein | –0.203 | 0.223 | 0.066 | 0.695 | 0.301 | 0.066 | –0.127 | 0.447 | –0.004 | 0.980 | –0.136 | 0.414 |
| Temperature | –0.020 | 0.897 | –0.395 | 0.011 | –0.139 | 0.387 | 0.212 | 0.183 | –0.171 | 0.285 | –0.344 | 0.028 |
TMA, trimethylamine; TMAO, trimethylamine N-oxide
Fig. 1.
Box and whisker plot showing the distribution of gut-related metabolites between patients with COVID-19, asthma, and pneumonia and healthy patients. Whiskers show the 10th to 90th percentiles. Statistical significance between the COVID-19 cohort and healthy, asthma, and pneumonia groups is also displayed.
Discussion
To the best of our knowledge, this is the first study investigating associations of gut-related metabolites with COVID-19. The main finding from the present study is that metabolites from the choline-TMAO and carnitine-TMAO pathways are associated with COVID-19 symptoms (breathlessness and temperature) and severity (O2 saturation levels), and we were able to differentiate between COVID-19 and acute asthma.
Growing evidence indicates key gut-lung crosstalk that allows maintaining homeostasis and disease evolution. The concept of the gut-lung axis, however, is not completely understood despite recent evidence identifying host-microbe as well as microbe-microbe interactions [7]. Patients with respiratory infections typically have gut dysfunction or secondary gut dysfunction complications, suggesting a gut-lung interaction [3]. The influence of gut microbiota on lung microbial composition and disease has been proposed by two crosstalk mechanisms: 1) direct seeding of the respiratory tract with bacteria and 2) the distribution of bacterial metabolites [8]. Increasing evidence supports a “common mucosal response” wherein the effects of the gut microbiota on the mucosal immunity may influence an immune response on distal mucosal sites including the lungs, whereas gut bacterial cells and metabolites may also elicit an immune response at distal sites [9]. An alternative mechanism of the gut-lung interaction is by systemic dissemination of metabolites. Current research has investigated the role of short-chain fatty acids, which can suppress lung inflammation through the activation of G protein–coupled receptors and exert antiinflammatory properties [10].
In all, we investigated whether the distribution of metabolites was associated with COVID-19. We focused on the choline and carnitine pathways, which are derived from dietary sources of red meat, fish, and eggs and converge into a common metabolite, TMAO. TMAO is formed from the bacterial cleavage of trimethylamine [6]. Differences in metabolite levels were only observed between patients with COVID-19 and those with asthma. Previous studies have shown that choline, betaine, and L-carnitine are lower in asthmatic patients and suggest the possibility of supplementation to improve symptoms of asthma. Mechanistically, these metabolites are thought to modulate immune inflammation, suppress oxidative stress, and improve airway inflammation [11]. Findings in individuals with asthma have shown significantly elevated and decreased metabolites, including trimethylamine, from tracheal wash and exhaled breath samples [12]. Among microbial-derived metabolites, evidence suggests that short-chain fatty acids, bile acids, polyunsaturated fatty acids, and now those from this investigation, gut-derived metabolites, are contributors to asthma pathophysiology [10]. Markers of inflammation have also been associated with COVID-19 symptoms, use of respiratory support, and mortality, whereas TMAO has been reported to be a mediator in systemic inflammation via the NLRP3 inflammasome by activating NF-kB and signaling reactive oxygen species. By this mechanism, it is suggested that TMAO levels would correlate with inflammation exacerbated by the COVID-19 infection [13].
Limitations of this study include the proof-of-concept design, with a small sample size coupled with low mortality numbers that could influence the results. As a consideration, the metabolites in this study are related to the gut microbiota; it is therefore necessary to take into account dietary data, treatment (i.e., antibiotics), and whether patients take supplements or not, because the detected levels of metabolites not only could come from the metabolism of the intestinal microbes but also could be directly influenced by these sources.
In conclusion, this study showed that gut-related metabolites—in particular, betaine—were associated with COVID-19 symptoms. Lower levels of metabolites, and in particular, betaine, in patients with asthma or COVID-19 suggests a role for betaine as a surrogate marker for gut health in COVID-19, and we hypothesize that dietary intervention against the gut microbiome could improve outcomes and enhance immunity. Validation studies are warranted.
Acknowledgements
The authors acknowledge the invaluable efforts of the research nurses responsible for the in-clinic sample collection as well as the input from the wider East Midlands Breathomics Pathology Node consortium (a list of members can be found at https://ember.le.ac.uk/web).
Footnotes
This research was funded by the Medical Research Council (MRC), the Engineering and Physical Sciences Research Council Stratified Medicine Grant for Molecular Pathology Nodes (Grant No. MR/N005880/1), the Midlands Asthma and Allergy Research Association, and the British Lung Foundation (Grant No. BLFPHD17–1). This work was also supported by the National Institute for Health Research (NIHR) (Leicester Biomedical Research Centre) and the MRC UK Consortium on MetAbolic Phenotyping (to T.S.). The work was carried out at the university hospitals of Leicester National Health Service (NHS) Trust, University of Leicester, and Loughborough University, supported by the NIHR Leicester Biomedical Research Centre and the NIHR Leicester Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. A.S. receives research grant support from CardioPath, Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy, and UniNA and Compagnia di San Paolo in the frame of the STAR (Sostegno Teritoriale alla Attività di Ricerca) program. The remaining authors have no conflicts of interest to declare.
References
- 1.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: New evidence. J Immunol Res. 2020;2020 doi: 10.1155/2020/2340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 5.Salzano A, Cassambai S, Yazaki Y, Israr MZ, Bernieh D, Wong M, et al. The gut axis involvement in heart failure: Focus on trimethylamine N-oxide. Heart Fail Clin. 2020;16:23–31. doi: 10.1016/j.hfc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Israr MZ, Bernieh D, Salzano A, Cassambai S, Yazaki Y, Heaney LM, et al. Association of gut-related metabolites with outcome in acute heart failure. Am Heart J. 2021;234:71–80. doi: 10.1016/j.ahj.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsland BJ, Trompette A, Gollwitzer ES. The gut–lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 9.Ipci K, Altıntoprak N, Muluk NB, Senturk M, Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Eur Arch Otorhinolaryngol. 2017;274:617–626. doi: 10.1007/s00405-016-4058-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Sarwar KA, Lasky-Su J, Kelly RS, Litonjua AA, Weiss ST. Gut microbial-derived metabolomics of asthma. Metabolites. 2020;10:97. doi: 10.3390/metabo10030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta AK, Singh BP, Arora N, Gaur SN. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. 2010;215:527–534. doi: 10.1016/j.imbio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Bazzano M, Laghi L, Zhu C, Magi GE, Serri E, Spaterna A, et al. Metabolomics of tracheal wash samples and exhaled breath condensates in healthy horses and horses affected by equine asthma. J Breath Res. 2018;12 doi: 10.1088/1752-7163/aade13. [DOI] [PubMed] [Google Scholar]
- 13.Terruzzi I, Senesi P. Does intestinal dysbiosis contribute to an aberrant inflammatory response to SARS-CoV-2 in frail patients? Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.110996. [DOI] [PMC free article] [PubMed] [Google Scholar]