Abstract
Objective:
To describe and synthesize the literature on adult traumatic brain injury (TBI) family caregiver and dyad intervention. TBI is a common injury that has a significant long-term impact, and is sometimes even characterized as a chronic condition. Informal (ie, unpaid) family caregivers of adults with TBI experience high rates of burnout, depression, fatigue, anxiety, lower subjective well-being, and poorer levels of physical health compared to noncaregivers. This study addresses the critical gap in the understanding of interventions designed to address the impact of TBI on adult patients and their family caregivers.
Data Sources:
PubMed and MEDLINE.
Study Selection:
Studies selected for review had to be written in English and be quasi-experimental or experimental in design, report on TBI caregivers, survivors with heavy involvement of caregivers, or caregiver dyads, involve moderate and severe TBI, and describe an intervention implemented during some portion of the TBI care continuum.
Data Extraction:
The search identified 2171 articles, of which 14 met our criteria for inclusion. Of the identified studies, 10 were randomized clinical trials and 4 were nonrandomized quasi-experimental studies. A secondary search to describe studies that included individuals with other forms of acquired brain injury in addition to TBI resulted in 852 additional titles, of which 5 met our inclusion criteria.
Data Synthesis:
Interventions that targeted the caregiver primarily were more likely to provide benefit than those that targeted caregiver/survivor dyad or the survivor only. Many of the studies were limited by poor fidelity, low sample sizes, and high risk for bias based on randomization techniques.
Conclusions:
Future studies of TBI caregivers should enroll a more generalizable number of participants and ensure adequate fidelity to properly compare interventions.
Keywords: Brain injuries, traumatic, Caregivers, Rehabilitation
In the United States, 2.5 million people each year sustain traumatic brain injuries (TBI), and more than 5.3 million people with TBI live with long-term physical, cognitive, and psychological disabilities.1–3 Due to its long-term impact, TBI is considered a chronic condition.4 After moderate and severe TBI, individuals are unable to make their own decisions. Families of adults with TBI are generally not prepared for their new complex role as a caregiver. Unlike many chronic diseases, TBI affects people of all ages, with many young patients requiring decades, and potentially a lifetime, of specialized, highly involved care. In a large national dataset from Canada, adults with TBI were among the youngest in home care, nursing home care, and complex continued care settings when compared to both other neurological and non-neurological conditions.5 Much research has focused on factors that might influence outcomes from moderate and severe TBI; fewer studies have investigated the role of caregivers and families in outcomes after moderate and severe TBI in adults.
With TBI, disabilities persist for months or years post injury.6,7 On returning home from inpatient care, individuals with TBI often must rely on informal and untrained caregivers for support and advocacy. These caregivers include spouses, children, other family members, or friends, and not trained caregivers. Patients with TBI may find it difficult to find and access the resources that support their choice to live at home rather than in an institution.
Compared to patient-oriented interventions, fewer interventions have targeted untrained caregivers of adults with TBI. Caregivers of adults with chronic medical conditions suffer from depression, fatigue, burden, burnout, anxiety, lower subjective well-being, and poorer levels of physical health compared to non-caregivers.8–17 Family caregivers of such chronic conditions are typically unpaid, and caregiver satisfaction with life worsens over time, especially when caring for individuals with severe TBI.18 Caregiver impaired health status and burden correlated with global disability after severe TBI.17 Thus, interventions that reduce strain are important both for patients and caregivers. At this time, no guidelines or recommendations from official governing are available to clinicians who wish to offer guidance to caregivers of adults with TBI. The purpose of this systematic review was to describe and synthesize the existing literature of published adult TBI family caregiver and dyad intervention studies.
Methods
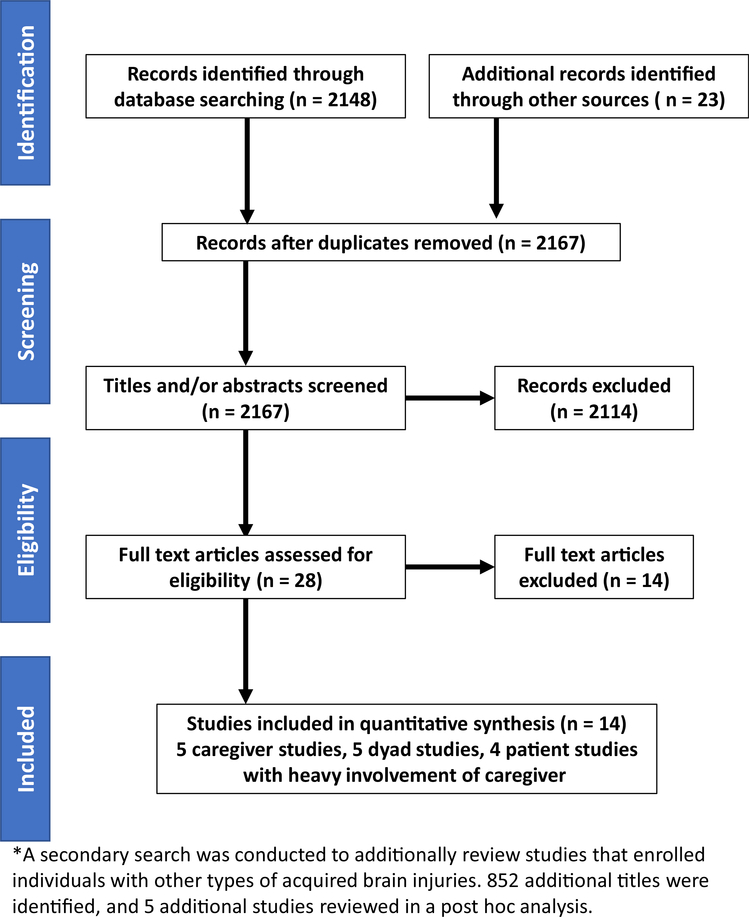
We conducted a critical analysis of studies of adult TBI family caregiver and dyad intervention. Studies had to meet the following inclusion criteria: (1) be written in English; (2) use a quasi-experimental or experimental design with a comparison group and intervention group; (3) describe an intervention in TBI caregivers, TBI survivors with heavy involvement of caregivers, or TBI caregiver dyads; (4) involve moderate or severe TBIs; (5) include an intervention that was implemented during some portion of the care continuum; (6) enroll adult patients as participants. We excluded studies that (1) involved patients with only mild TBI; (2) included patients with other forms of acquired brain injury (ie, stroke); (3) did not involve caregivers in the study or did not report outcomes of caregivers in the study; (4) enrolled pediatric patients as participants. We later conducted a post-hoc search to describe and reference additional interventions that enrolled individuals with other forms of acquired brain injury. We defined moderate and severe TBI by using the Glasgow Coma Scale, or, if the scale was not reported, by using consensus from authors of the respective studies.19 We excluded from our review dissertations, books, abstracts, ongoing unpublished studies, and conference proceedings. We searched PubMed and MEDLINE for the following keywords: traumatic brain injury and family; traumatic brain injury caregiver; traumatic brain injury caregiver interventions; traumatic brain injury and caregiver experimental studies; traumatic brain injury caregiver quasi-experimental studies. We included articles published before the date of the search (October 22, 2017). We tracked the search process with a Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram (fig 1). In table 1, we provide summaries of the articles meeting our inclusion criteria, and we describe their interventions in table 2 and their outcomes in table 3. All authors reached consensus was reached on article inclusion. For each study, 1 team member completed the data extraction using a pre-developed electronic form. Table 4 includes information extracted from each study. A second team member verified all extracted data, and disagreements were resolved through discussion or third party consultation when consensus could not be reached. Two authors (N.K., T.B.) categorized the interventions, and a third author (B.G.K.) adjudicated any disagreements in categorization of the interventions.
Fig 1.
We excluded 14 articles from our systematic review of literature on interventions for individuals with TBI and their caregivers. We found that 8 studies were not quasi-experimental or experimental in design; 1 study include nonfamily caregivers who were paid and preselected; and 5 studies included patients with other forms of acquired brain injury (eg, stroke, aneurysm).
Table 1.
Summary description of studies in systematic review for literature on interventions for individuals with TBI and their caregivers
| Study | Study Size | Time From TBI to Enrollment | Enrolled | Intervetion Target | Intervention Type | Tailored vs OSFA | Mode of Delivery | Sessions (No.) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 Rivera27 | 67 | NA | CG | CG | SB | Tailored | IP | 12 |
| 2 Moriarty35 | 162 | 11.17 y | Dyad | Dyad | SB | OSFA | IP & phone | 8 |
| 3 Powell31 | 153 | NA | CG | Dyad | SB | Tailored | Phone | 10 |
| 4 Hanks36 | 158 | NA | Dyad | CG | Support | Tailored | Phone | 5.4 |
| 5 Brown37 | 514 | >1 y | Dyad | Dyad | SB & support | OSFA | IP | 4 |
| 6 McLaughlin29 | 201 | 20% >1 y | CG | CG | SB | OSFA | Web | 10 |
| 7 Morris40 | 34 | 66% >1 y | CG | CG | SB | OSFA | Written | 2 |
| 8 Semlyen34 | 112 | <4 wk | Dyad | Survivor | SB | Tailored | IP | Variable |
| 9 Thornton28 | 54 | >6 mo | Dyad | Survivor | SB | OSFA | IP | 18 |
| 10 Bell39 | 171 | <1 y | S | Survivor | SB | Tailored | Phone | 7 |
| 11 Bell32 | 433 | <1 y | S | Survivor | SB | Tailored | Phone | 11 |
| 12 Togher33 | 88 | >9 mo | Dyad | Dyad | SB | Tailored | IP | 10 |
| 13 Bowen38 | 96 | Variable | CG | CG | SB | Tailored | IP | Variable |
| 14 Sinnakaruppan30 | 99 | 3 y | Dyad | Dyad | SB | OSFA | IP | 8 |
NOTE: Study size indicates number enrolled in each study. Enrolled column indicates if survivors, caregivers, or both (dyads) were included in this number.
Abbreviations: CG, caregiver; IP, in person; NA, not applicable; OSFA, one-size-fits-all method; S, survivor; SB, skill building.
Table 2.
Brief description of interventions and specific outcome measures found in review of literature on TBI individuals and their caregivers
| Author | Intervention | Design | Survivor Outcomes (P<.05) | Caregiver Outcomes (P<.05) |
|---|---|---|---|---|
|
| ||||
| Rivera27 | Problem-solving vs written materials | RCT | None significant | Depressive symptoms (favored I) Dysfunctional problem solving (favored I) Caregiver health complaints (favored I group) |
| Moriarty35 | Cognitive, behavioral, interpersonal, and home environment strategies | RCT | NA | Depressive symptoms: favored I Burden: favored I |
| Thornton28 | Balance retraining: conventional vs virtual reality | Quasi RCT | No significant difference | Qualitative outcome only: virtual reality group had more enjoyment and confidence |
| Togher33 | Conversation skills: dyad vs patient only training vs control | RCT | I group: improved conversation | Caregiver ability to acknowledge and reveal competence of survivor most improved in dyad group |
| Powell31 | Education and mentored problem-solving & written materials vs usual care | RCT | None significant | Composite and BSI favored I group |
| Hanks36 | Mentor vs no mentor | RCT | I group with better behavioral control, less chaos in the living environment, lower alcohol use, less emotion-focused and avoidance coping, and good physical quality of life compared to control group. | I group had greater community integration |
| Brown37 | Curriculum-based or self-directed advocacy training group (via ABRS) | RCT | Pre- and post-improvement in ABRS. | None |
| Bell39 | Telephone call vs usual care after discharge | RCT | Primary composite in favor of I group. | None measured |
| Bell32 | Telephone call vs usual care after discharge | RCT | None | None |
| Morris40 | Informational booklet for caregivers | Longitudinal, mixed variable, within- and between-subject design. | None | The book was readable, useful, and they read the entire book. |
| McLaughlin29 | Web site focused on advocacy, communication skills, and resources for families. | RCT | None tested | Skill application, intentions, knowledge better in I group. |
| Bowen38 | Multidisciplinary team approach compared 3 groups of patients: (1) early, (2) late and (3) control | Non-blinded trial | None obtained | No differences |
| Semlyen34 | Multidisciplinary service compared to single discipline. | Quasi-experimental design | Multidisciplinary group experienced improvement in all measures as time went on | Lowered level of distress favoring multidisciplinary group |
| Sinnakaruppan30 | Community-based educational program | RCT; within and between subject comparison | I group had improved depressive symptoms, anxiety, somatic symptoms, social dysfunction, and self-esteem within group. COPE-I (acceptance), COPE-J (focus on and venting emotion), COPE-N (alcohol or drug use) were significant improvement in the between group comparison. The caregivers rating of the FIM scores of the patients was statistically significant different in the experimental group compared to control. | Lower depressive symptoms, improved coping using social support |
Abbreviations: ABRS, Advocacy Behavioral Rating Scale; C, Control group; I, Intervention group.
Table 3.
Summary table of instruments used to measure outcomes in caregiver and dyad TBI intervention studies
| Outcome (Global or Broad) | Studies That Tested These Outcomes | Instruments Used |
|---|---|---|
|
| ||
| Caregiver life changes and appraisal | Powell,31 Moriarty35 | Composite of Bakas Caregiving Outcomes Scale,31 Caregiving Appraisal Scale35 |
| Depressive symptoms | Powell,31 Rivera,27 Moriarty,35 Hanks,36 Bowen,38 Sinnakaruppan30 | Brief Symptom Inventory 18,31,36 Center for Epidemiological Studies Depression Scale,27,35 Wimbeldon self-reported scale,38 HADS30 |
| Coping | Powell,31 Hanks,36 Sinnakaruppan30 | Brief COPE,31 coping inventory,36 COPE scale30 |
| Well-being | Rivera,27 McLaughlin29 | Satisfaction with Life Scale27,29 |
| Caregiver burden | Rivera27,35 | Caregiver Burden Scale27,35 |
| Social problem-solving abilities | Rivera27 | Social problem solving scale27 |
| Community integration | Hanks36 | Community integration measure36 |
| Family assessment | Hanks36 | Family assessment device36 |
| Health-related quality of life | Hanks36 | SF-1236 |
| Alcohol use | Hanks36 | Short Michigan alcoholism screening test36 |
| Advocacy | Brown37 | Advocacy Behavioral Rating Scale37 |
| Skill application, intentions, knowledge about TBI | McLaughlin29 | Skills pertaining to web training29 |
| Level of informed | Bowen38 | Survey of being informed38 |
| Anxiety (or other common psychiatric symptoms) | Morris,40 Semlyen,34 Sinnakaruppan30 | HADS anxiety scale,41 GHQ-2830,34,40 |
| Functional status after TBI | Semlyen,34 Sinnakaruppan30 | Barthal Index,34 FIM,30,34 Newcastle Independence Assessment Form35 |
| Balance | Thornton28 | ABC,28 LEFS28 |
| Composite outcome | Bell32,39 | Composite outcome that included numerous previously described outcomes.32,39 |
| Conversational skills | Togher33 | Adapted measure of participation in conversation33 |
| Self-esteem | Sinnakaruppan30 | Rosenburg self-esteem scale30 |
Abbreviations: ABC, Activities-specific Balance Confidence Scale; COPES, COPE scale; GHQ-28, General Health Questionnaire; HADS, Hospital Anxiety and Depression Scale; LEFS, Lower Extremity Functional Scale; SF-12, Medical Outcomes Study 12-Item Short-Form Health Survey.
Table 4.
Information extracted from each study
| Methodology | Demographics | Intervention |
|---|---|---|
|
| ||
| Title | Sample size | Type of interventions |
| Study design | Age of participants | Materials and procedures used |
| Caregiver and survivor outcomes | Gender of participants | Training of interventionist |
| Instruments used in the study | Study inclusion and exclusion criteria | Mode of delivery |
| Process of study randomization | If demographics table was provided | Location and infrastructure needed |
| Concerns about generalization of study | Study attrition | Total time in session |
| Clinical significance of findings | Target patient population | Number of sessions |
| Bivariate or multivariate analyses | Country of study | Schedule of sessions |
| Methodological strengh of study | NA | If intervention was tailored or not |
| Methodological strengh of study | NA | If intervention was changed during study period |
| Methodological strengh of study | NA | If fidelity and precision was adhered to |
| Methodological strengh of study | NA | If intervention delivery as planned |
Abbreviations: ABC, Activities-specific Balance Confidence Scale; COPES, COPE scale; GHQ-28, General Health Questionnaire; LEFS, Lower Extremity Functional Scale; NA, not applicable; SF-12, Medical Outcomes Study 12-Item Short-Form Health Survey.
To critique the studies, we followed a practice like that followed by Bakas and colleagues20,21 in their reviews of stroke caregiver and dyad interventions. We used criteria from the Consolidated Standards of Reporting Trials (CONSORT) and Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statements and assessing the reports for threats to bias and validity.22–25 We described the samples, interventions, and outcomes, and highlighted the best designed studies highlighted for further discussion. We extracted details of the interventions by the Template for Intervention Description and Replication checklist.26 Inter-study heterogeneity, including severity of TBI, time since TBI, types of interventions, outcome measures, and duration of treatment, was high, precluding a meta-analysis, and, therefore, we used a narrative synthesis of all the included studies.
Post-Hoc Literature Search
We conducted a secondary search of studies that additionally enrolled caregivers of individuals with other forms of acquired brain injury, because some of these interventions targeted components of brain injury recovery that also may be beneficial for caregivers of individuals with TBI. In the rehabilitation period, for instance, similar techniques for caregivers may be applicable across different disease spectrums. Therefore, we also separately reviewed literature that included subjects with other neurological disease processes in addition to TBI to determine best strategies for caregivers. Our original literature search excluded 3 studies that enrolled non-TBI subjects into the study, and a second literature search of articles with the additional keywords brain injury caregiver resulted in 2 more studies, giving us 5 more studies to analyze.
Results
Our primary search identified 2148 journal articles. From other sources, we identified another 23 articles, which gave us 2171 articles. After we removed duplicates, we had 2167 articles, and, from these articles, we excluded 2139 based on relevance of titles or abstracts. As a result, we now had 28 papers from 27 unique studies that merited review of the full text articles. Based on our inclusion and exclusion criteria, we identified 14 of these 28 studies that met our criteria for inclusion (see fig 1).
Our secondary literature search, on February 15, 2018, resulted in 852 additional unique titles for review. The interventions of these 5 studies that enrolled individuals with TBI in addition to other types of acquired brain injuries are summarized in table 5.
Table 5.
Brief description of interventions that enrolled individuals with TBI in addition to other types of acquired brain injuries
| Study (Author and Year of Publication) | Intervention | Design | Number of Survivors With TBI of Total | Survivor Outcome (P<.05) | CG Outcome (P<.05) |
|---|---|---|---|---|---|
|
| |||||
| Backhaus 201044 | Training in coping strategies. Participants were randomized to 12 in person sessions compared to usual care | RCT | 9/20 | Less emotional distress, improved perceived self-efficacy | Less emotional distress, improved perceived self-efficacy |
| Backhaus 201645 | Brain injury coping skills program—manualized cognitive behavioral treatment compared to support group. | RCT | 6/19 | None | None |
| Carnevale 200646 | Natural behavioral setting management (education & individual behavior) vs education vs control group, targeted to participants with behavioral impairment. | 3 arm RCT | 24/37 | Decreased frequency targeted behavioral problems | NA |
| Grill 200748 | 3-y advisory program after acquired brain injury | Nonrandomized prospective before and after study | 426/1534 | FIM and survival | none |
| Kreutzer 201547 | Brain injury family intervention that entailed curriculum-based education, skill building, and support | Prospective controlled repeat measures design | 116/137 | None | Improvement in met needs, satisfaction with services, reduced burden |
Abbreviation: CG, caregiver.
Designs
Of the 14 studies identified for inclusion in our study, 10 were randomized clinical trials (RCTs) of interventions and 4 were nonrandomized quasi-experimental studies. We used CONSORT statement criteria to determine quality and validity of the RCT studies. Randomization was poor in some studies, in which there was no block randomization or attempt to equalize the groups.27,28 Some studies provided little information about how subjects were randomized.28–30 Only 2 studies provided detail about the study design’s randomization methods: Powell et al31 used block randomization based on hospital disposition, and Bell et al32 used stratified randomization based on discharge FIM, location, and block randomization. Neither the RCTs nor the nonrandomized quasi-experimental studies could blind the intervention to the survivor or the caregiver. Nonrandomized quasi-experimental studies had a higher risk of bias.33,34
Samples
Most of the studies provided their sample demographics in tables.27,29,31–33,35–39 Sample sizes ranged from 34 to 514 participants. Depending on the particular study enrollments, the sample size may have reflected the number of caregivers or survivors, or both. Country locations of the studies were the United States (8 studies),27,29,31,32,35–37,39 the United Kingdom (2 studies),34,38 Scotland (2 studies),30,40 Canada (1 study),28 and Australia (1 study).33 Sample sizes for 3 of the studies were <50 caregivers enrolled in the study.28,33,40 In studies reporting age, the mean age of caregivers was 48.25 years. Several studies reported age ranges of survivors and caregivers. Most caregivers in studies that reported sex were female (n=621/751, 83%), and most survivors were male (n=399/1140, 35%), data consistent with that reported in literature on both TBI and caregiving.41,42 Most caregivers were spouses or parents.
Five studies evaluated an intervention specifically for the caregiver,27,29,31,38,40 5 studies for the caregiver dyad,30,33,35,36,43 and 4 studies included heavy involvement of the caregiver, but ultimately the studies were conducted with interventions designed primarily for the TBI survivor.28,32,34,39 In general, studies with interventions for the survivor, but heavily involved the caregiver, did not measure caregiver outcomes at all,32,39 and only 1 such study had significant caregiver outcomes.34 Of the 5 studies that involved the caregiver only, 3 had positive significant findings in the caregivers.27,29,40 Of the 5 dyad studies, 3 showed positive findings in both the survivor and caregiver.30,33,36
Interventions
Like interventions found for poststroke caregivers,20,21 the TBI caregiver and dyad interventions were of 3 main types: (1) support; (2) skill-building; (3) a combination of these 2 types. Skill-building interventions involve strategies that focus on processes that facilitate problem solving, goal setting, and communicating with healthcare professionals; stress management; hands-on training in such skills as lifting and mobility techniques and assistance with activities of daily living; and communication tailored to the needs of the individual with TBI.20 Support interventions are defined as engaging in interactions with peers for support and advice (eg, support groups, online discussion forums).20 Twelve studies were classified as skill building. Skill building encompassed all modalities of delivery, including the Web, in-person, written education, and phone. Another study combined skill building and support.37 Only 1 study involving an intervention that specifically provided support to caregivers as a primary intervention was included, even though several studies listed support as a secondary feature of their study intervention. This study was designed to teach curriculum-based or self-directed advocacy training.37 Unlike the stroke literature, there were no interventions that were primarily psychoeducational (provision of information only).20,21
Modes of Delivery
In the studies we reviewed, study content was delivered to participants in person, by telephone, online, via written information, or through a combination of these methods (see table 1). Interventions that required face-to-face meetings were evaluated in 8 studies,27,28,30,33–35,38,43 most of these studies were conducted in the home setting, but other studies required participants to travel to centralized locations.33,43 Two additional studies required face-to-face meetings to complete multidisciplinary team-based interventions34,38; the 2 interventions discussed in these studies incorporated the skills of therapists, counselors, social workers, psychologists, and nurses.
Four studies31,32,36,39 about interventions delivered by telephone exclusively met criteria for our review (see table 1). Three of these 4 studies31,32,39 were among the strongest methodologically of all studies we reviewed. Two additional studies27,35 used telephone interventions as a component of the study, with additional in-person sessions; the telephone interventions covered several matters, including coping strategies, education, caregiver-specific needs, and rehabilitation advice.
Only 1 study29 described a web-based intervention. This study recruited participants via a website, thereby ensuring participation by individuals likely to use the Web to begin with.29 The fidelity of this study was difficult to determine, because participants were encouraged but not required to access the Web program, and participants’ time spent on the website was not explicitly recorded.
A written intervention was described by only 1 study,40 wherein the researchers provided caregivers with an educational pamphlet. Qualitative evidence indicated that participants thought the pamphlet was helpful, but there were no statistically significant outcomes. However, there was a trend that approached significance for anxiety reduction in caregivers who received the booklet earlier in the course of illness (<9mo) compared to caregivers who received the booklet later in the course of illness (>1y).40
Across the studies we reviewed, the number of intervention sessions varied from 2 to 18, with generally a higher number of sessions if participants were close geographically to the study site, or if sessions were not in person (see table 1). Of all the interventions, 57% (n=8/14) were tailored for the specific caregiver or caregiver dyad and 43% (n=6/14) were not specifically tailored (see table 1).
Outcomes
Outcomes of interest varied across the studies. For individuals with TBI, outcomes included global functioning, physiologic measures, communication abilities, balance, conversation skills, and personal relationships (see table 3). For caregivers, outcomes included measures of fatigue, depressive symptoms, anxiety, caregiver strain, and overall health outcomes specific to caregivers of adults with TBI. The most common outcome tested in both caregivers and survivors was depressive symptoms. Several validated instruments were used, including the Brief Symptom Inventory 18,31,36 the Center for Epidemiological Studies Depression Scale,27,35 the Wimbledon self-reported scale,38 and the Hospital Anxiety and Depression Scale.30 Many had been described previously in caregivers and adults with TBI, such as Satisfaction With Life Scale in caregivers, FIM in survivors, and the Brief Symptom Inventory 18 in both caregivers and survivors. Although many of the measures have known evidence of reliability and validity, their use for TBI likely has limitations (see table 3).
Other outcomes had little to no external reliability or validity, and 4 outcomes were endpoints designed specifically for a particular study.33,34,36,37 Some studies tested participants’ knowledge of the intervention, in addition to, or instead of, pre-validated outcome measures.28,29 Such endpoints may emphasize statistical significance of findings, with little attention to effect sizes or clinical significance.
The reliability and validity of outcome measures in TBI survivors and caregivers either were not described or were incomplete for 6 studies.28,29,33,36–38 For example, studies commonly did not describe whether the survivor or the caregiver completed the survivors’ data collection forms. For studies in which data were collected from survivors, studies did not report the cognitive and language skills as well as the ability to respond to questionnaires.28,36,37 Many studies used bivariate statistics rather than multivariate analyses to report findings, and only 1 study reported an intention-to-treat analysis.36 Some studies used well validated, appropriate outcomes.27,31,35 Three studies used a composite outcome derived from numerous previously well-validated outcome measures on the basis of a couple of rationales: for one, the numerous separate endpoints could lead to false positive results, and, for another, the needs of the patient population after TBI are heterogeneous.31,32,39
In determining the outcome of the intervention, most studies did not follow their participants longer than a year.27–29,31–33,35,37–40 Three studies enrolled participants for 2 years.32,34,36 Without data on long-term outcomes, it is difficult to know whether the interventions might have had enduring benefits for caregivers or for individuals with TBI.
Summary and Best Designed Studies
Based on our critique, 3 studies demonstrated positive significant results, and were likely to have limited risk of bias. The study by Powell31 was a high fidelity RCT with outcomes that were well validated. The study evaluated a telehealth-based mentored problem solving intervention and demonstrated that caregivers in the intervention group had an improved composite score consisting of a combination of coping, wellbeing as a caregiver, depressive symptoms, community participation, and caregiving mastery.31
The study by Moriarty et al35 was also a high fidelity RCT of an in-home and phone intervention designed to improve family knowledge and support, while assuring that modifiable in-home environmental factors were improved. The study concluded that caregivers in the intervention group had significantly lower depressive symptoms and caregiving burden.35 However, both Powell31 and Moriarty35 each used the same occupational therapist throughout the study, and, therefore, the conclusion may not be generalizable to other institutions.
Bell32 conducted a high fidelity RCT evaluating a telephone intervention, using a composite score in a heterogeneous group of patients.39 When the study was expanded later to a multicenter trial, the results were not statistically significant, perhaps because the cumulative endpoint was obtained in survivors rather than in the caregivers who participated heavily in the program.32
Secondary Findings
In table 5, we describe a summary of additional interventions that enrolled subjects with other forms of acquired brain injury (such as strokes or brain tumors). Of these studies, 4 were high fidelity RCTs that focused on caregiver coping strategies, behavioral management, education, and support.44–47 The fifth study was a large pre- and post-intervention study that evaluated an advisory program for individuals with acquired brain injury and their caregivers.48 There were 4 dyad studies.44–47 Grill et al48 did not enroll caregivers but included heavy involvement of the caregiver. In the 4 dyad studies, all interventions took place in person, and 1 study added telephone mode of delivery.48 Interventions described in the studies ranged from 5 to 16 sessions, and a wide range of 38 to 1534 subjects were enrolled in the interventions. All interventions focused on skill building, and 1 study included support and psychoeducation in the intervention.44 Backhaus et al44 and Kreutzer et al47 demonstrated significant benefit to caregivers (see table 5). Grill,44 Backhaus,46 and Carnavale48 and colleagues discussed the significant benefit of the interventions to survivors (see table 5).
Discussion
We critiqued and described the 14 studies selected for review based on basic features of each study as well as common elements described in the TREND and CONSORT statements. These include descriptions of study design, study samples, intervention fidelity and precision, types of intervention, method of delivery, tailoring vs one-size-fits-all approach, time spent in intervention, outcome assessment, and generalizability. Because the field of interventions for caregivers of adult TBI is nascent in comparison to that of stroke or pediatric TBI, we compared and contrasted these 2 similar fields in certain instances. Our recommendations are presented in table 6.
Table 6.
Recommendations for interventions for TBI individuals and their caregivers based on a systematic review of the literature
| Component | Recommendation |
|---|---|
|
| |
| Caregiver vs dyad vs heavily involved caregiver in a survivor intervention | Interventions that are specifically designed to help the survivor, but require heavy input of the caregiver are unlikely to be of benefit for the caregiver. |
| Interventions | As most interventions are skill building, it is unknown if other kinds of interventions, such as psychosocial or support based, are as beneficial. Future studies are needed. If interventions are skill building in nature, they should be tailored to the individual’s disability or functional status |
| Tailored vs one size fits all | Interventions that are tailored are better for both survivor and caregiver. |
| Mode of delivery | Face to face delivery methods are more likely to confer benefit, and are preferred. Telephone interventions may provide help to caregivers, but are less likely to have significant benefits for survivors. At this time, there is not enough information to determine if web based studies will provide benefit. |
| Number of sessions | 5 to 9 sessions likely confer the most benefit to survivors and caregivers |
Study Designs
We reviewed 4 nonrandomized comparison studies,33,34,38,40 using the TREND statement criteria for our evaluation. The nonrandomized nature of these studies increases risk for study bias. Nonrandomized studies based the groupings on geography, convenience, or time since injury. For example, Bowen et al38 and Morris et al40 based the early vs late intervention on the time since TBI. Other groups were formed by convenience based on geographical proximity or willingness to participate in the intervention.33,34 This presents difficulty in making further clinical recommendations based on conclusions from these nonrandomized studies. Future studies evaluating interventions for caregivers of individuals with TBI should randomize participants to decrease the risk for bias.
Samples
An important criterion in CONSORT and TREND guidelines is assessing for baseline differences on key demographics (eg, caregiver sex, relationship, social class) and other characteristics, but 3 studies28,34,40 provided none of this information or minimal information to this effect. Inclusion criteria for survivors differed among the studies for time since TBI and severity of TBI. These differences make recommending improvements to interventions difficult, particularly for the optimal timing and caregiver selection criteria, for a couple of reasons: for one, the needs of caregivers after critical illness are known to change over time, and, for another, the adaptive and coping skills learned by caregivers in the inpatient environment may not translate to the home setting.49 In general, studies did not describe modifying the intervention to accommodate for potential cognitive impairments. Future studies of caregivers of individuals with TBI should enroll participants at the same time frame and standardize outcomes to account for TBI severity or cognitive impairments of the individual with TBI.
Intervention Fidelity and Precision
The CONSORT and TREND statements advocate for details about the interventions and how they are delivered. Treatment fidelity consists of 5 components: (1) treatment design; (2) training; (3) delivery of treatment; (4) receipt of treatment; and (5) enactment.50 Treatment fidelity was well described in only 6 of the 14 studies.27,31,32,35,37,39Treatment design includes the theoretical background of the intervention and information about the dosage for both the treatment and control groups (length, number, content, and duration of contacts). Training for the interveners should be described, as well as how the intervention is delivered and evaluated (eg, evaluation checklists). Only 5 studies described the intervention well enough to be considered high fidelity.27,32,35,37,39 Further, imprecision of the intervention was high in many studies, which presents a challenge when trying to make clinical recommendations based on study outcomes.34,38 Future interventions should strive to have high treatment intervention fidelity built into study designs by maintaining the number, length, and frequency of intervention sessions.50
Types of Interventions
Most studies that met criteria for this review were classified as skill building. Because only 1 study discussed an intervention specifically for caregivers, we cannot make definitive clinical recommendations about the utility of support groups as opposed to skill building interventions for caregivers of individuals with TBI. Many stroke-related aftercare interventions highlight the importance of psychoeducational interventions,51–57 but, in our study sample, we found no reported interventions that were psychoeducational only. While psychoeducational intervention alone is not recommended for stroke,20,21 studies have reported that combining psychoeducational strategies with skill building interventions can reduce anxiety depressive symptoms among caregivers and lead to improved quality of life for both caregivers and TBI survivors.20,21 Future studies involving caregivers of individuals with TBI should explore the role of the combination of psychoeducational and skill building interventions.
Method of Delivery
We categorized the interventions performed in the 14 selected studies into 5 broad categories for method of delivery: (1) face to face; (2) written; (3) telephone delivery; (4) Web; (5) a combination of these methods (see table 1). Studies describing face-to-face delivery27,28,30,34 reported favorable outcomes for caregivers in the realms of depressive symptoms, functional problem solving, health complaints, and distress levels. The favorable outcomes must be weighed against the cost and resources needed for in-person meetings, especially in rural settings or when studies involve a multidisciplinary team intervention.34,38 Face-to-face meetings seem an appropriate modality of delivery in teaching specific skills (eg, balance training, conversational tools),28,33 but may be less beneficial for teaching skills of advocacy.38
Telephone and Web interventions offer certain conveniences. Caregivers may access Web interventions from their home or work at any time during the day. Caregivers may also connect with others who are at a distance. A disadvantage of telephone and Web interventions is that caregivers and TBI individuals may not have access to a telephone, computer, or high-speed Internet service, or may lack the appropriate skills for using these methods. Even so, RCTs in pediatric TBI literature report benefit from Web-based caregiver interventions, especially in poorer populations, thus supporting the notion that Web-based interventions may be generalizable to participants of all socioeconomic statuses.58–60 At the same time, the pediatric TBI caregiver literature discusses the need for individualized computer skills training to fully benefit from computer and Web-based intervention.61
Web access can be inconsistent, especially in rural areas, and may not be accessible to all caregivers. Our review included 1 Web-based study, but fidelity can be inconsistent. If Web-based interventions are developed, we recommend that time spent in various portions are able to be tracked, and that participants are engaged in the intervention. This review did not describe interventions delivered by teleconferencing. An upcoming teleconferencing intervention for caregivers of children with TBI may give us an opportunity to study how this delivery method could work for adults.62
Tailoring Vs One Size Fits All
Many of the studies used a tailored approach rather than a one-size-fits-all approach.27,31,36,38 Tailored approaches are difficult to generalize outside of the study. For instance, in Bowen et al,38 the treatment team determined when and whether the caregiver or patient required interventions. In studies with a one-size-fits-all approach, an outline of the treatment intervention is beneficial if the study is to be replicated. Moriarty35 provides a table listing the goals for both caregiver and patient at each session, such that the intervention could be reproduced. Nonetheless, tailored studies seem to offer the most benefit for caregivers of individuals with TBI when compared to a one-size-fits-all approach, particularly for depressive symptoms.
Time Spent and Number of Sessions in Interventions
We could not determine from this review whether the amount of time spent in an intervention was proportional to positive results. Therefore, we cannot make clinical recommendations cannot about the time necessary to spend in the intervention. Future studies should consider that caregivers generally do not have ample free time, and time spent in interventions should be efficient in order to maximize benefits.
Outcomes
Despite the heavy burden placed on caregivers of TBI, no outcome specifically measures caregiving burden in families with a TBI survivor. Therefore, we used caregiving outcomes that have been validated in other disease processes, including stroke, including the Composite of Bakas Caregiving Outcomes Scale,31 the Caregiving Appraisal Scale,35 and the Caregiver Burden Scale.27,35 Many of the caregiving outcome measures have been used across several different types of medical populations, but it may be worthwhile to consider testing these existing measures in the TBI population.
The stroke caregiver literature shows that interventions are preferred for best caregiver outcomes and that dyad interventions are preferred for survivor outcomes.20 We did not find a similar relation in the TBI studies reviewed. Indeed, 4 of 5 of the dyad studies30,33,35,36 reported positive findings among the caregivers. Of the 4 studies that provided an intervention to the survivor that required heavy involvement of the caregiver, only 1 study reported positive results in the caregiver; the study required intense multidisciplinary rehabilitation using a family-focused team approach.34 Although clinical implications cannot be made at this time, future studies involving caregivers should target caregivers and provide outcomes specific to needs of caregivers only when caregivers are heavily involved in the intervention.
Generalizability
Many studies had limited generalizability, thus making it challenging to provide clinical recommendations based on this review. Studies that enrolled only spouse caregivers may not generalize to adult children or other unpaid caregivers. Because we reviewed only studies written in English, generalizability is limited to countries with drastically different healthcare systems and cultural norms around illness recovery and caregiving.
Critique of Studies Included in Secondary Analysis
Five studies in our secondary analysis enrolled individuals with other forms of acquired brain injury in addition to TBI. We critiqued these interventions using the CONSORT and TREND statements. Overall, these 5 studies described high fidelity interventions. The 4 RCT studies in the secondary analysis described the randomization process appropriately.44–47 Like the studies enrolling individuals with only TBI, the 4 RCT studies included no obvious correlation between outcome and number of sessions. Two of the RCT studies required the survivor to have passed cognitive testing prior to consenting for the study to determine whether survivors could respond to outcome measures, thus improving the validity of outcomes.44,45 Three of these studies enrolled <50 subjects,44–46 and all studies except Carnevale et al46 recruited subjects from the same institution, making these samples less generalizable to a broader population. However, the interventions were likely broadly generalizable to a wider group of brain injured individuals themselves, because they used tailored approaches (eg, cognitive behavioral therapy) or targeted specific behaviors (eg, aggression). Grill48 had the largest participant sample of all studies we analyzed in both our primary and secondary reviews; it was also the only study to demonstrate survival benefit in its intervention group. This particular study described an extensive 2-year post-discharge rehabilitation program. Four of the 5 studies used well validated outcome measures.44,45,47,48
If interventions are developed to target specific rehabilitative outcomes (eg, behavioral impairments, caregiver coping, specific skillsets), further research should consider TBI interventions that also enroll individuals with other acquired brain injuries. However, for interventions targeting TBI specific concerns (eg, injury prevention, TBI related education, TBI support), we recommend referencing studies that enroll TBI individuals only.
Study Limitations
To minimize publication bias, we conducted an extensive search of multiple databases, but we may have missed papers beyond the scope of the databases we searched. We searched solely English language publications. The overall quality of the studies was heterogeneous, with studies of varying quality and bias based on the CONSORT and TREND guidelines.
Our systematic review is limited by our exclusion of studies in the primary search that enrolled individuals with other types of brain injury. Because individuals who have sustained a TBI may be younger than those with other neurologic disorders, their caregivers also may be younger. However, depending on the specific need addressed by an intervention, it is likely that data can be extrapolated from studies that enrolled subjects with other disease processes (see table 5). Of the 4 published RCTs that included subjects with other acquired brain injuries overall, none were powered to detect whether the intervention worked better specifically in individuals and their caregivers after TBI when compared to other acquired brain injuries (see table 5).44–46,48
We could not conduct a meta-analysis of the data reviewed for several reasons. The measured outcomes of studies, even for similar interventions, were in multiple broad categories, resulting in significant heterogeneity. The intervention types, targets, modes of delivery, and number of sessions also differed considerably.
Conclusions
Deficiencies in the literature make it difficult to develop definitive clinical recommendations for caregiver guidance. Future research should include more rigorous study design; pay particular attention to fidelity of interventional delivery, sustainability of outcome, dosage of interventions, and feasibility of the interventions; and consider timing of the study relative to injury as well as feasibility and accessibility of interventions. It may be difficult to generalize findings from tailored studies that are patient- or caregiver-centered, because of the many different measures and outcomes discussed in these studies; however, using outcome measures with stronger evidence of reliability and validity would allow for better comparisons of these studies. Caregiver interventions within practice settings could improve outcomes not only for caregivers (eg, mental and physical health, quality of life) but also for patients (eg, reduced readmission rates, less chance of institutionalization, reduced disability, improved quality of life).
List of abbreviations:
- CONSORT
Consolidated Standards of Reporting Trials
- RCT
randomized controlled trial
- TBI
traumatic brain injury
- TREND
Transparent Reporting of Evaluations with Nonrandomized Designs
References
- 1.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Traum Rehabil 2008;23:394–400. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil 1999;14:602–15. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CA. Traumatic brain injury—related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveillance Summaries 2017;66:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma 2010;27:1529–40. [DOI] [PubMed] [Google Scholar]
- 5.Colantonio A, Hsueh J, Petgrave J, Hirdes JP, Berg K. A profile of patients with traumatic brain injury within home care, long-term care, complex continuing care, and institutional mental health settings in a publicly insured population. J Head Trauma Rehabil 2015; 30:E18–29. [DOI] [PubMed] [Google Scholar]
- 6.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Ternkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil 2009;24:439–51. [DOI] [PubMed] [Google Scholar]
- 7.Jourdan C, Bayen E, Pradat-Diehl P, et al. A comprehensive picture of 4-year outcome of severe brain injuries. Results from the PariS-TBI study. Ann Phys Rehabil Med 2016;59:100–6. [DOI] [PubMed] [Google Scholar]
- 8.Gillen R, Tennen H, Affleck G, Steinpreis R. Distress, depressive symptoms, and depressive disorder among caregivers of patients with brain injury. J Head Trauma Rehabil 1998;13:31–43. [DOI] [PubMed] [Google Scholar]
- 9.Marsh NV, Kersel DA, Havill JA, Sleigh JW. Caregiver burden during the year following severe traumatic brain injury. J Clin Exp Neuropsych 2002;24:434–47. [DOI] [PubMed] [Google Scholar]
- 10.Saban KL, Griffin JM, Urban A, Janusek MA, Pape TL, Collins E. Perceived health, caregiver burden, and quality of life in women partners providing care to veterans with traumatic brain injury. J Rehabil Res Dev 2016;53:681–92. [DOI] [PubMed] [Google Scholar]
- 11.Mellick D, Gerhart KA, Whiteneck GG. Understanding outcomes based on the post-acute hospitalization pathways followed by persons with traumatic brain injury. Brain Injury 2003;17:55–71. [DOI] [PubMed] [Google Scholar]
- 12.Schmotz C, Richinger C, Lorenzl S. High burden and depression among late-stage idiopathic Parkinson disease and progressive supranuclear palsy caregivers. J Geriatr Psychiatry Neurol 2017;30: 267–72. [DOI] [PubMed] [Google Scholar]
- 13.Takai M, Takahashi M, Iwamitsu Y, et al. The experience of burnout among home caregivers of patients with dementia: relations to depression and quality of life. Arch Gerontol Geriatr 2009;49:e1–5. [DOI] [PubMed] [Google Scholar]
- 14.Johansen S, Cvancarova M, Ruland C. The effect of cancer patients’ and their family caregivers’ physical and emotional symptoms on caregiver burden. Cancer Nurs 2017;4:91–9. [DOI] [PubMed] [Google Scholar]
- 15.Leibach GG, Trapp SK, Perrin PB, et al. Family needs and TBI caregiver mental health in Guadalajara, Mexico. NeuroRehabilitation 2014;34:167–75. [DOI] [PubMed] [Google Scholar]
- 16.Pinquart M, Sorensen S. Differences between caregivers and non-caregivers in psychological health and physical health: a meta-analysis. Psychol Aging 2003;18:250–67. [DOI] [PubMed] [Google Scholar]
- 17.Bayen E, Pradat-Diehl P, Jourdan C, et al. Predictors of informal care burden 1 year after a severe traumatic brain injury: results from the PariS-TBI study. J Head Trauma Rehabil 2013;28:408–18. [DOI] [PubMed] [Google Scholar]
- 18.Manskow US, Friborg O, Roe C, Braine M, Damsgard E, Anke A. Patterns of change and stability in caregiver burden and life satisfaction from 1 to 2 years after severe traumatic brain injury: a Norwegian longitudinal study. NeuroRehabilitation 2017;40:211–22. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81–4. [DOI] [PubMed] [Google Scholar]
- 20.Bakas T, Clark PC, Kelly-Hayes M, King RB, Lutz BJ, Miller EL. Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association and American Stroke Association. Stroke 2014;45:2836–52. [DOI] [PubMed] [Google Scholar]
- 21.Bakas T, McCarthy M, Miller ET. Update on the state of the evidence for stroke family caregiver and dyad interventions. Stroke 2017;48:e122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 23.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148: 295–309. [DOI] [PubMed] [Google Scholar]
- 24.Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004;94:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston: Wadsworth Cengage Learning; 2002. [Google Scholar]
- 26.Hoffmann TC, Glasziou PP, Boutron I, et al. [Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide] [German]. Gesundheitswesen 2016;78:175–88. [DOI] [PubMed] [Google Scholar]
- 27.Rivera PA, Elliott TR, Berry JW, Grant JS. Problem-solving training for family caregivers of persons with traumatic brain injuries: a randomized controlled trial. Arch Phys Med Rehabil 2008;89:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton M, Marshall S, McComas J, Finestone H, McCormick A, Sveistrup H. Benefits of activity and virtual reality based balance exercise programmes for adults with traumatic brain injury: perceptions of participants and their caregivers. Brain Injury 2005;19: 989–1000. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin KA, Glang A, Beaver SV, Gau JM, Keen S. Web-based training in family advocacy. J Head Trauma Rehabil 2013;28:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinnakaruppan I, Downey B, Morrison S. Head injury and family carers: a pilot study to investigate an innovative community-based educational programme for family carers and patients. Brain Injury 2005;19:283–308. [DOI] [PubMed] [Google Scholar]
- 31.Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil 2016; 31:180–90. [DOI] [PubMed] [Google Scholar]
- 32.Bell KR, Brockway JA, Hart T, et al. Scheduled telephone intervention for traumatic brain injury: a multicenter randomized controlled trial. Arch Phys Med Rehabil 2011;92:1552–60. [DOI] [PubMed] [Google Scholar]
- 33.Togher L, McDonald S, Tate R, Power E, Rietdijk R. Training communication partners of people with severe traumatic brain injury improves everyday conversations: a multicenter single blind clinical trial. J Rehabil Med 2013;45:637–45. [DOI] [PubMed] [Google Scholar]
- 34.Semlyen JK, Summers SJ, Barnes MP. Traumatic brain injury: efficacy of multidisciplinary rehabilitation. Arch Phys Med Rehabil 1998;79:678–83. [DOI] [PubMed] [Google Scholar]
- 35.Moriarty H, Winter L, Robinson K, et al. A randomized controlled trial to evaluate the veterans' in-home program for military veterans with traumatic brain injury and their families: report on impact for family members. PM R 2016;8:495–509. [DOI] [PubMed] [Google Scholar]
- 36.Hanks RA, Rapport LJ, Wertheimer J, Koviak C. Randomized controlled trial of peer mentoring for individuals with traumatic brain injury and their significant others. Arch Phys Med Rehabil 2012;93: 1297–304. [DOI] [PubMed] [Google Scholar]
- 37.Brown AW, Moessner AM, Bergquist TF, Kendall KS, Diehl NN, Mandrekar J. A randomized practical behavioural trial of curriculum-based advocacy training for individuals with traumatic brain injury and their families. Brain Injury 2015;29:1530–8. [DOI] [PubMed] [Google Scholar]
- 38.Bowen A, Tennant A, Neumann V, Chamberlain MA. Neuropsychological rehabilitation for traumatic brain injury: do carers benefit? Brain Injury 2001;15:29–38. [DOI] [PubMed] [Google Scholar]
- 39.Bell KR, Temkin NR, Esselman PC, et al. The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Arch Phys Med Rehabil 2005;86: 851–6. [DOI] [PubMed] [Google Scholar]
- 40.Morris KC. Psychological distress in carers of head injured individuals: the provision of written information. Brain Injury 2001;15: 239–54. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Service: Centers for Disease Control and Prevention. Report to Congress: traumatic brain injury in the United States. Washington (DC): U.S. Government Printing Office; 1999. [Google Scholar]
- 42.Stone R, Cafferata GL, Sangl J. Caregivers of the frail elderly: a national profile. Gerontologist 1987;27:616–26. [DOI] [PubMed] [Google Scholar]
- 43.Brown R, Pain K, Berwald C, Hirschi P, Delehanty R, Miller H. Distance education and caregiver support groups: comparison of traditional and telephone groups. J Head Trauma Rehabil 1999;14: 257–68. [DOI] [PubMed] [Google Scholar]
- 44.Backhaus SL, Ibarra SL, Klyce D, Trexler LE, Malec JF. Brain injury coping skills group: a preventative intervention for patients with brain injury and their caregivers. Arch Phys Med Rehabil 2010;91: 840–8. [DOI] [PubMed] [Google Scholar]
- 45.Backhaus S, Ibarra S, Parrott D, Malec J. Comparison of a cognitive-behavioral coping skills group to a peer support group in a brain injury population. Arch Phys Med Rehabil 2016;97:281–91. [DOI] [PubMed] [Google Scholar]
- 46.Carnevale GJ, Anselmi V, Johnston MV, Busichio K, Walsh V. A natural setting behavior management program for persons with acquired brain injury: a randomized controlled trial. Arch Phys Med Rehabil 2006;87:1289–97. [DOI] [PubMed] [Google Scholar]
- 47.Kreutzer JS, Marwitz JH, Sima AP, Godwin EE. Efficacy of the brain injury family intervention: impact on family members. J Head Trauma Rehabil 2015;30:249–60. [DOI] [PubMed] [Google Scholar]
- 48.Grill E, Ewert T, Lipp B, Mansmann U, Stucki G. Effectiveness of a community-based 3-year advisory program after acquired brain injury. Eur J Neurol 2007;14:1256–65. [DOI] [PubMed] [Google Scholar]
- 49.Adams D, Dahdah M. Coping and adaptive strategies of traumatic brain injury survivors and primary caregivers. NeuroRehabilitation 2016;39:223–37. [DOI] [PubMed] [Google Scholar]
- 50.Borrelli B, Sepinwall D, Ernst D, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol 2005;73: 852–60. [DOI] [PubMed] [Google Scholar]
- 51.Clark MS, Rubenach S, Winsor A. A randomized controlled trial of an education and counselling intervention for families after stroke. Clin Rehabil 2003;17:703–12. [DOI] [PubMed] [Google Scholar]
- 52.Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. J Adv Nurs 2005;52:640–50. [DOI] [PubMed] [Google Scholar]
- 53.Dennis M, O’Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomised controlled trial. BMJ (Clinical research ed) 1997;314:1071–6 [discussion: 1076–7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilling K, Coshall C, McKevitt C, Daneski K, Wolfe C. A family support organiser for stroke patients and their carers: a randomised controlled trial. Cerebrovasc Dis 2005;20:85–91. [DOI] [PubMed] [Google Scholar]
- 55.Forster A, Dickerson J, Young J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet 2013;382:2069–76. [DOI] [PubMed] [Google Scholar]
- 56.Lincoln NB, Francis VM, Lilley SA, Sharma JC, Summerfield M. Evaluation of a stroke family support organiser: a randomized controlled trial. Stroke 2003;34:116–21. [DOI] [PubMed] [Google Scholar]
- 57.Braithwaite V, McGown A. Caregivers’ emotional well-being and their capacity to learn about stroke. J Adv Nurs 1993;18: 195–202. [DOI] [PubMed] [Google Scholar]
- 58.Antonini TN, Raj SP, Oberjohn KS, et al. A pilot randomized trial of an online parenting skills program for pediatric traumatic brain injury: improvements in parenting and child behavior. Behav Ther 2014;45:455–68. [DOI] [PubMed] [Google Scholar]
- 59.Petranovich CL, Wade SL, Taylor HG, et al. Long-term caregiver mental health outcomes following a predominately online intervention for adolescents with complicated mild to severe traumatic brain injury. J Pediatr Psychol 2015;40:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wade SL, Kurowski BG, Kirkwood MW, et al. Online problem-solving therapy after traumatic brain injury: a randomized controlled trial. Pediatrics 2015;135:e487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carey JC, Wade SL, Wolfe CR. Lessons learned: the effect of prior technology use on Web-based interventions. Cyberpsychol Behav 2008;11:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor K, Catroppa C, Godfrey C, et al. Managing challenging behaviour in preschool children post-traumatic brain injury with online clinician support: protocol for a pilot study. Pilot Feasibility Stud 2017;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]